Abstract
Shifts in the gut microbiome play a key role in blood pressure regulation, and changes in the production of gut microbial metabolites are likely to be a key mechanism. Known gut microbial metabolites include short-chain fatty acids, which can signal via G-protein-coupled receptors, and trimethylamine-N oxide. In this review, we provide an overview of gut microbial metabolites documented thus far to play a role in blood pressure regulation.
Keywords: blood pressure, G-protein-coupled receptors, gut microbial metabolite, hypertension
Introduction
It is now well-appreciated that the gut microbiota has the ability to influence the physiology of the host organism (6, 9, 19, 61, 71, 81). One way that this is accomplished is via metabolites produced by the gut microbiota, which can be absorbed into the blood stream of the host and thus can influence host proteins at distant sites. Recently, multiple studies have outlined connections between changes in gut microbial metabolites and hypertension. Hypertension is correlated with shifts in the gut microbiota (known as gut dysbiosis) in humans (59, 93, 116) and in animal models (3, 28, 64, 66, 101, 116). As a consequence, microbial metabolite production is altered in hypertension (21). Different gut microbial metabolites have been reported to have both positive (35, 69) and negative (55, 59, 105, 118) effects on cardiovascular function. In this review we will discuss current knowledge regarding specific gut microbial metabolites, which are reported to influence blood pressure regulation.
Short-Chain Fatty Acid Metabolites
Among the more studied species of gut microbial metabolites are short-chain fatty acids (SCFAs), and thus SCFAs will be a primary focus of this review. The term “SCFAs” refers primarily to straight-chain 2–4 carbon compounds: acetate, propionate, and butyrate. SCFAs are byproducts of dietary fiber digestion by the gut microbiota in the colon and cecum. Gut microbial production of SCFAs is quite robust; the concentration of SCFAs in the colonic lumen has been reported to be ~100 mM (18).
SCFAs are absorbed into the bloodstream of the host by diffusion as well as by monocarboxylate transporters (23). Although the host can produce SCFAs, the vast majority of circulating SCFAs are microbial in origin. This is evidenced by the fact that SCFAs are virtually undetectable in the plasma of germ-free mice (which lack gut microbiota) (78). Acetate is the most abundant of the SCFAs in the circulation, generally reported to be at least 100 μM. However, acetate levels can be much higher with different diets, and the exact proportion of acetate:propionate:butyrate also varies depending on dietary manipulations (31, 58). However, acetate is consistently the most abundant of the three SCFAs, both in colonic lumen and in circulation (25, 31, 58, 96).
SCFA-Mediated Cell Signaling: G-Protein-Coupled Receptors
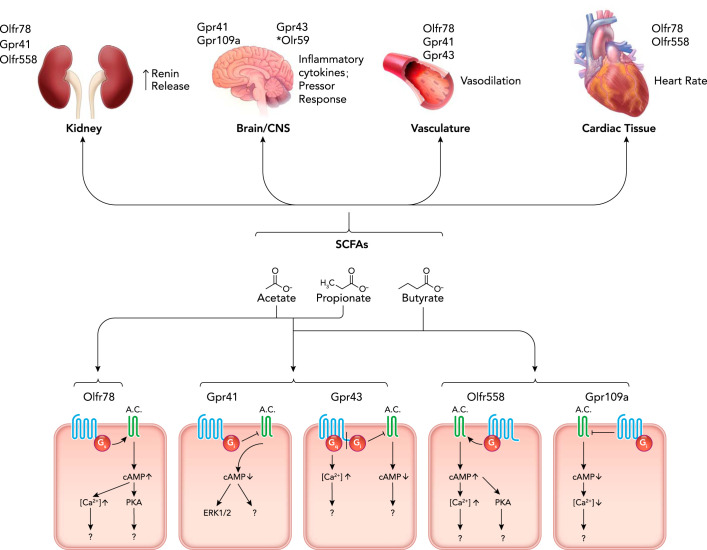
SCFAs in circulation can affect host physiology in a number of ways, including by acting as ligands for G-protein-coupled receptors (GPCRs). Here, we will briefly discuss each of the GPCRs that are known to be activated by SCFAs: Gpr41, Gpr43, Gpr109a, Olfr78, and Olfr558. The relevant sites of expression, ligands, and mechanism (if known) are summarized in FIGURE 1.
FIGURE 1.
Mechanisms and sites of microbial SCFA-mediated blood pressure regulation
Production of short-chain fatty acids (SCFAs) in the gut leads to absorption in the distal gut through diffusion and active transport. SCFAs then travel through the circulation to activate receptors in the kidney, brain, sympathetic nervous system, vasculature, and heart, leading to blood pressure effects. (*Note: Olr59 is the rat ortholog of Olfr78.) Although each organ system shows known receptor expression, these effects have not all been proven to be due to receptor activation. Acetate, propionate, and butyrate are all ligands for Gpr41 and Gpr43, whereas acetate and propionate activate Olfr78. Butyrate acts on Olfr558 and Gpr109a. Niacin can also activate GPR109a. Activation of these GPCRs leads to downstream effects that are yet to be elucidated.
Gpr41 (free fatty acid receptor 3) and Gpr43 (free fatty acid receptor 2) were first reported as SCFA receptors in 2003 by two separate groups (15, 56). Gpr43 couples to both Gi and Gq proteins, whereas Gpr41 couples to Gi (15, 56). However, for both Gpr41 and Gpr43, activation has been reported to lead to both inhibition of cAMP and increases in intracellular calcium (56). Both Gpr41 and Gpr43 are activated by three to six ligands, depending on the assay used. Propionate is the best ligand for both receptors, with acetate, butyrate, and isobutyrate also being relatively strong ligands. Although the exact EC50 values reported vary, Gpr41 and Gpr43 are activated by SCFAs in the μM range. In addition to SCFA ligands, β-hydroxybutyrate has been reported to be both an agonist (111) and an antagonist (54) for Gpr41. With regard to blood pressure regulation, Gpr41 is known to be expressed in the vascular endothelium (69) and in the autonomic ganglia (54, 73), where activation promotes ERK1/2 phosphorylation (54). Gpr43 is also expressed in blood vessels (although the cell type of expression has not yet been reported) (79). Gpr41 KO mice have isolated systolic hypertension, implying that Gpr41 plays an important role in setting basal vascular tone (69). This is consistent with the fact that acute delivery of SCFAs causes vasodilation ex vivo (69) and that acute delivery of SCFAs also causes a brief hypotensive response in vivo (79). Although the hypotensive response to SCFAs is not absent in Gpr41 KO mice, the dose response is shifted (79). This implies that Gpr41 is not the sole player in SCFA-mediated changes in vascular tone. Further studies are needed to elucidate the role of SCFAs in this context.
Gpr109A is a SCFA receptor that is activated by butyrate (EC50 ~1 mM) but not by acetate or propionate (94). Gpr109A is also a receptor for β-D-hydroxybutyrate and for niacin (94, 97, 110). Gpr109A signaling leads to Gi activation and thus to decreases in intracellular cAMP (91). With regard to blood pressure regulation, Gpr109A is expressed in the rostral ventrolateral medulla (RVLM), where it plays a role in the central control of blood pressure in response to activation by niacin, which leads to an increase in L-glutamate and ROS production (80); to our knowledge, however, the role of Gpr109a in blood pressure regulation has not been tied to butyrate.
Olfr78 and Olfr558 are both olfactory receptors, and both are well-known to be expressed in numerous tissues other than the nose where they act as chemosensory GPCRs for SCFAs (26, 37). Of note, in this review, we will primarily use the murine nomenclature for these receptors (Olfr78 and Olfr558), but the human orthologs of Olfr78 and Olfr558 utilize a different nomenclature (OR51E2 and OR51E1), and the rat orthologs have yet a different nomenclature (Olr59 and Olr63). As olfactory receptors, both Olfr78 and Olfr558 are thought to signal by increasing cAMP (in the olfactory epithelium, this occurs via coupling to Golf; presumably, these receptors can also couple via Gs).
Olfr78 was initially deorphanized in 2009 by two groups: one group found that the human ortholog, OR51E2, responded to propionate (82), whereas the other group reported that OR51E2 was activated by β-ionone (70). A third group in 2013 reported that both Olfr78 and OR51E2 were activated by acetate/acetic acid and propionate/propionic acid, but not by butyrate/butyric acid or β-ionone (79). However, it is worth noting that β-ionone activation of OR51E2 has been consistently reported by others (43, 70). β-Ionone activation is typically reported by studies utilizing a calcium signaling readout for Olfr78 activation, whereas the study that failed to see β-ionone activation used a cAMP assay; thus it is possible that β-ionone is an example of biased agonism. OR51E2 has also been reported to respond to androstenone derivatives (1, 70). In 2015, it was reported that Olfr78 was activated by acetate, propionate, and lactate (20). However, a separate report in 2016 failed to see lactate activation for OR51E2 (4), and two studies reported Olfr78 activation by lactate only at very high levels (4, 117). For SCFA activation of Olfr78, EC50s are in the low millimolar range. Finally, a report in 2020 identified the corresponding esters of acetate and propionate as Olfr78 ligands by screening for activation in the olfactory epithelium in situ (90). Intriguingly, these authors suggest that the esters may not be ligands themselves but may be converted into their corresponding acids by enzymes in the nasal mucus.
With regard to blood pressure regulation, Olfr78 is expressed in blood vessels (smooth muscle cells), as well as in the renal afferent arteriole (79), where it has been shown to impact renin release (79). Specifically, it was shown that isolated juxtaglomerular apparati from wild-type mice release renin in response to a SCFA but that this response is markedly attenuated in juxtaglomerular apparati from Olfr78 KO mice (79). In agreement with this, it was also reported that Olfr78 KO mice have lower circulating plasma renin (79). As for the role of Olfr78 in vascular smooth muscle, the acute hypotensive response to SCFAs is not absent in Olfr78 KO mice, but (like in Gpr41 KO mice) the dose-response is shifted (79).
Olfr558, the most closely related olfactory receptor to Olfr78, has been reported to respond to butyric acid/butyrate (2, 46). One group has also reported that Olfr558 can respond to nonanoic acid (82), although other groups have not seen activation with this compound (45, 46). A study in 2019 identified a total of 18 ligands for Olfr558 and OR51E1, with butyrate acting as the strongest activator of downstream signaling (cAMP) for both the murine and human ortholog (EC50 of ~0.5 mM) (46). Although a role for Olfr558 in blood pressure regulation has not been investigated, Olfr558 is expressed in the renal cortex (46), and OR51E1 is expressed in the heart (50). A microarray study has also identified Olfr558 expression in isolated juxtaglomerular cells (16).
Finally, it is important to note that GPCR-mediated SCFA signaling has been well-conserved evolutionarily, with orthologs in different species responding similarly for Gpr41 (15), Olfr78 (79), and Olfr558 (46). In fact, although many murine olfactory receptors do not have clear orthologs in other species, both Olfr78 and Olfr558 are extremely well-conserved among placental mammals, having complete one-to-one orthologous relationships among at least 13 different species (72).
SCFA-Mediated Cell Signaling: Other Pathways
In addition to GPCR signaling, SCFAs can also affect cell biology by affecting cell proliferation. SCFAs have been reported to both increase (34, 84) and decrease (4, 14, 34, 38, 52, 87) cell proliferation. It has been suggested that butyrate stimulates the growth of colonocytes in the absence of the Warburg effect but inhibits proliferation when the Warburg effect is in play (34). SCFAs also have effects on apoptosis (5, 22, 39) and on histone deacetylases (HDACs) (24, 30, 47, 65, 99). These effects on cell proliferation, apoptosis, and HDACs are generally thought to be independent of GPCRs, although Gpr41 has been suggested to play a role in mediating HDAC inhibition (112). Although these pathways are no doubt important, they have not as of yet been implicated in blood pressure regulation and thus will not be a focus of this review.
The Microbiota, SCFAs, and Blood Pressure Regulation
The interplay between gut microbial metabolites and blood pressure regulation has been established in animal models (11, 64, 69, 75, 79, 95). In studies involving mice and rats, fecal microbiota transplantation (FMT) into germ-free mice has become the gold standard for definitively identifying key roles of the microbiome in disease states such as obesity, irritable bowel disorders, and hypertension (29, 44, 53, 66, 81). As the name implies, FMT involves the transplantation of fecal microbiome samples from a donor into the intestinal tract of a recipient. Using FMT, multiple studies have linked gut dysbiosis and shifts in SCFA-producing bacteria with hypertension in both human patients and animal hypertension models (3, 59, 66, 116). Mell et al. performed FMT after antibiotic treatment and found that blood pressure increased in salt-sensitive hypertensive Dahl rats that received FMT from salt-resistant Dahl rats (66). This hypertensive phenotype was correlated with higher levels of plasma acetate (66). Allelic variations were found in olfactory receptor genes between the salt-sensitive and salt-resistant Dahl rat strains, suggesting a potential mechanism for the difference in hypertensive response (66). However, this study did not look at levels of other SCFAs such as propionate or butyrate. These results are corroborated in another study in Dahl salt-sensitive rats that found that CRISPR excision of G-protein-coupled estrogen receptor 1 (Gper1) resulted in lower blood pressure, and FMT with microbiota from WT hypertensive counterparts reversed the protective blood pressure effect of Gper1 KO and increased plasma acetate levels (101). High-salt-diet-induced hypertension in salt-sensitive rats also increased fecal acetate and propionate levels in correlation with increased blood pressure (13). Another study in spontaneously hypertensive rats (SHR) also showed increased cecal butyrate levels at baseline compared with normotensive WKY controls, with a corresponding decrease in circulating butyrate, indicating a possible defect in SCFA transport and absorption in this context (114).
Despite these studies, there is conflicting evidence as to whether increased SCFAs positively or negatively correlate with hypertension (3, 116). A study by Yang et al. found that hypertensive rats had a significantly decreased population of acetate and butyrate-producing bacteria compared with their normotensive Wistar Kyoto counterparts (116). This is corroborated by Adnan et al., who also saw a decrease in butyrate-producing bacteria in a similar hypertensive rat model (3); both of these studies were done in a SHR model. Yang et al. performed similar studies in an angiotensin II (Ang II) rat model of hypertension and demonstrated that Ang II-induced hypertension led to similar reductions in bacterial diversity (116). Notably, a study from Karbach et al. showed that germ-free mice are resistant to Ang II-induced hypertension, demonstrating a pivotal role of the microbiota in this model of hypertension (51). This finding is especially impactful given that Ang II infusion is the most common animal hypertension model used in NIH-sponsored studies (40). Finally, a key study by Li et al. performed FMT into a germ-free mouse model and found that blood pressure increased in mice that received FMT from hypertensive human donors (59). This finding demonstrated that a hypertensive phenotype arises from a particular gut microbial composition and that the phenotype can be transferred via the microbiota (59). Although correlative links between gut dysbiosis and changes in SCFA levels have been established by these studies, they do not establish that these effects are mediated by direct effects of SCFAs on blood pressure regulation and/or hypertension. It is also worth noting that the correlation between gut microbiota and blood pressure regulation appears to be bidirectional—there is clear evidence both that the gut microbiota are remodeled by hypertension and that altering the gut microbiota can alter blood pressure regulation.
Effects of SCFA Supplementation at Baseline and in Hypertensive Models
Before we consider the effects of direct SCFA supplementation on models of hypertension, it is important to briefly touch on what is known about the interplay between SCFAs and blood pressure in normal physiology. Studies suggesting that SCFAs can cause hypotensive effects have existed for decades (12, 27, 67, 69, 74). Direct application of SCFAs have been shown to cause vasorelaxation in mouse, rat, and human isolated vascular tissue ex vivo (27, 67, 69, 74). SCFAs are also known to acutely reduce blood pressure when delivered as a bolus intravenously, intraperitoneally, or intracolonically (75, 79, 86). A recent study implied that central nervous system activation may play a role, since intracerebroventricular injection of butyrate led to not only a decrease in mean arterial pressure but also activation of cardioregulatory brain regions (114). This activation was attenuated in a SHR model. This study also showed decreased expression of Gpr41 and Olr59 (the rat ortholog of Olfr78) in the hypothalamus of the rat, hinting at a potential mechanism (114). In separate studies, acute delivery of SCFAs has also been shown to increase heart rate when given intraperitoneally (54), but, when administered intracolonically, another study showed heart rate decreased (75).
Given the observation of dysbiosis-dependent changes of SCFAs in hypertension, several studies have directly manipulated SCFAs via supplementation in animal hypertension models. A study from Wang et al. found that intramedullary butyrate infusion in Ang II-treated rats reduced blood pressure and urinary excretion of renin and markers of renin angiotensin aldosterone system (RAAS) activation (59). Butyrate treatment of astrocytes isolated from a SHR model also showed differential regulation of bioenergetics and neuroinflammatory genes compared with normotensive astrocytes (115). Another study using telemetry-implanted mice showed butyrate supplementation via IP injection reduced Ang II-induced hypertension, improved gut barrier function, and restored gut barrier hypoxia (103). Marques et al. found that acetate supplementation in drinking water of deoxycorticosterone acetate (DOCA)-salt hypertensive mice also showed reduction in systolic and diastolic pressures, fibrosis, and hypertrophy (64). Recently, a study from Bartolomeus et al. found that propionate supplementation in drinking water attenuated hypertension, cardiac hypertrophy, and fibrosis in the Ang II hypertension mouse model (11). These cardioprotective effects were abrogated in regulatory T-cell-depleted Ang II mice, suggesting that propionate acts through regulatory T-cells (11). Although the effects of SCFA supplementation in animal models of hypertension are extremely promising, it remains to be seen whether it would be effective in treating human models of hypertension.
Clinical Data
Confirming established animal model findings in clinical research remains a significant hurdle in elucidating the role of SCFAs in hypertensive settings. However, some clues can be gleaned from clinical studies from the past few years. Two clinical studies looking at hypertensive and pre-hypertensive patients both found significantly decreased microbial diversity and levels of SCFA-producing bacteria compared with healthy patients by fecal metabolite analysis (59, 113). A separate study conducted by Gomez-Arango et al. reported that butyrate production was negatively associated with blood pressure in a clinical study of pregnancy-induced hypertension (including gestational hypertension, preeclampsia, and HELLP syndrome) (44). In obese pregnant women, elevated systolic and diastolic blood pressure correlated with a decrease in butyrate-producing bacteria and bacterial expression of the butyrate-producing buk enzyme (44). In contrast, two clinical studies from de la Cuesta-Zuluaga et al. and Huart et al. demonstrated a correlation between increased fecal levels of SCFAs (acetate, propionate, and butyrate) and hypertension (28, 48). Higher levels of fecal SCFAs could correlate with lower circulating SCFAs due to a defect in transport or absorption, as has been suggested in animal models (114). None of these studies directly looked at circulating SCFA levels; however, a clinical cohort in a study from Kim et al. confirmed that hypertensive human patients showed distinct microbiome composition and that higher blood pressure levels observed in these patients correlated with lower plasma butyrate levels (53).
Although these clinical data primarily show a correlative effect of gut dysbiosis, further clinical studies are currently recruiting or in progress to further evaluate the role of the microbiome in hypertension. Additionally there are a host of variables that can complicate interpretation of clinical data, such as medication, diet (100), age (17), gender (63), and racial background (102). Our knowledge of the impact of these variables on microbiome composition under normal and dysbiosis conditions is still limited. For example, a study by Walejko et al. (102) showed distinct gut microbiota taxonomy and serum metabolite profiles between hypertensive and normotensive white or African-American patients (102). Although this study was a small sample size and did not measure acetate, propionate, or butyrate, it highlights the need for a fuller understanding of racial differences in the development of microbiome-based therapies for hypertension.
Hypertension Origins/Mechanisms
Obstructive sleep apnea (OSA) is significantly more prevalent in hypertensive patients and even more widespread in drug-resistant hypertension (60, 76, 88). Accordingly, multiple animal studies have linked gut dysbiosis to both sleep apnea and hypertension (3, 41). Durgan et al. performed FMT from OSA rats into normotensive rats, which resulted in increased blood pressure levels and lowered levels of butyrate-producing bacteria (3). Ganesh et al. used a rat model of OSA to demonstrate that gut dysbiosis can cause hypertension. Analysis of the gut microbiome in these OSA mice revealed a decrease in SCFA-producing bacteria and a corresponding decrease in plasma acetate (41).
Another potential mechanism of hypertension induction includes overactivation of the gut-brain-microbiome axis. Overactivation of the sympathetic nervous system and adrenergic receptor signaling has been linked to hypertension (62, 85) and gut dysbiosis (83). A study from Toral et al. showed decreased blood pressure and noradrenaline levels on FMT between normotensive and spontaneously hypertensive rats (95). Additionally, FMT induced changes in expression of SCFA receptors Olr59, Gpr41, and Gpr43 in the paraventricular nucleus of the brain, suggesting a possible mechanism (95). In a separate study, Bartley et al. showed an increase in colonic concentrations of SCFAs in β1- and β2-adrenergic receptor knockout mice. SCFAs also have been shown to induce parasympathetic vagal outflow (78) and sympathetic tone (54). These studies highlight the potential interplay between the gut microbiome and the CNS, and provide a potential mechanism for SCFA-mediated hypertension effects.
TMAO/TMA Biosynthesis and Clinical Significance
In addition to SCFAs, another gut microbial metabolite that has been linked to cardiovascular disease is trimethylamine-N oxide (TMAO) (21). However, it is to be noted that the influence of this metabolite is studied more in the context of atherosclerosis rather than on blood pressure regulation. TMAO is a small organic compound that is formed in the liver by the oxidation of trimethylamine (TMA). TMA is produced by gut microbiota from dietary components, including carnitine and choline, which are highly abundant in red meat, fish, and eggs (36). The plasma concentration of TMAO in healthy individuals was measured as 3 µM (92). TMA and TMAO accumulate in plasma under pathophysiological conditions such as end-stage renal disease and chronic kidney disease (10, 92). In healthy individuals, about half of the TMA and TMAO is excreted unchanged from the circulation within 24 h, primarily via urine, respiration, and sweat (7, 8, 89). The remaining half of TMAO reduces back into TMA via the action of TMAO reductase in human gut (36).
Recent studies in animals and humans have elucidated a role for TMAO in metabolic (42), cerebrovascular (118), and cardiovascular (106) diseases. In particular, as noted above, TMAO has been implicated in atherosclerosis (55). Wang et al. have shown a relationship between gut microbial-dependent metabolism of choline and atherosclerosis in an apolipoprotein e knockout (Apoe−/−) mouse model (105). Moreover, TMAO levels predict risk for cardiovascular disease in a clinical cohort (105). Another recent study demonstrated that TMAO induced alterations in bile acid profiles and that this led to an increased rate of atherosclerotic lesion formation in the Apoe−/− mouse model (32). Mechanistically, it was suggested that changes in bile acids may lead to activation of farnesoid X receptor and small heterodimer partner (32). Ultimately, this would inhibit bile acid synthesis by reducing Cyp7a1 expression (32).
A recent dietary intervention study with a crossover design recruited both omnivorous and vegan/vegetarian human subjects and examined changes in TMAO in response to red meat, white meat, or non-meat protein sources. The study showed that chronic red meat consumption increases systemic TMAO by both increasing dietary precursors and increasing TMA/TMAO production from carnitine, as well as reduced renal excretion (104). Increased TMAO has been reported to correlate with poor outcomes, including mortality and cardiovascular disease events, in individuals with Type 1 diabetes (109), and TMAO levels are increased in women with preeclampsia (108). Indeed, there is also evidence in animal models that TMAO can worsen existing disease: TMAO infusion in rats prolongs hypertension in a low-dose Ang II model but did not alter blood pressure in normotensive rats (98).
Other Metabolites
Although the gut microbial metabolites reviewed above are relatively well-studied in terms of blood pressure control, it is likely that there are many other metabolites that may also play a role. For example, gut microbial fermentation also produces gaseous compounds such as hydrogen sulfide (H2S) and methane (CH4) (68). In fact, gut microbial production gives rise to ~50% of total circulating and fecal H2S, and H2S is a known vasodilator (33, 49, 77, 107). In a recent metabolomics study (21), untargeted metabolomics were used to compare conventional (with gut microbiota) or germ-free mice (without gut microbiota) receiving infusions of either saline or Ang II. In conventional animals, there were 12 plasma metabolites and 96 fecal metabolites, which were significantly different in Ang II-treated mice compared with saline-treated mice. Surprisingly, none of these metabolites were similarly altered in germ-free mice treated with Ang II, implying that the majority of the metabolomic changes that occur in this model are dependent on the gut microbiota. Further studies are required to determine whether these metabolites play direct roles in the presentation of hypertension, and what mechanisms may be involved.
Gaps in the Knowledge
Recent advances showing the wide-ranging effects of microbial metabolites on blood pressure regulation are intriguing, although many mechanistic questions remain to be answered. For instance, SCFA receptors such as Gpr41, Gpr43, and Gpr109a have been found to be expressed in the CNS (54, 80, 95), and there are links between gut dysbiosis and increased sympathetic drive (83), but to date the precise mechanism of the cross talk between gut and CNS remains an open question. Another key pathway, the RAAS and in particular renin release, is also known to be impacted by metabolites via Olfr78 in juxtaglomerular cells (79), but other downstream effects on the RAAS have not been determined. However, it is also known that SCFA supplementation leads to a decrease in blood pressure and vasodilation (75, 79, 114). Differing levels of GPCR expression across tissues as well as disparate EC50 of GPCRs for SCFAs (15, 79) could be a potential explanation, but further studies are required to rectify apparently conflicting physiological effects of SCFAs and other metabolites. Studies including tissue-specific GPCR knockouts and germ-free mouse models will be informative in providing a definitive link between activation of these receptors and microbial metabolite effects on RAAS, CNS, and other novel pathways. Finally, the role of microbial metabolites in other systems that impact blood pressure and hypertension, such as the heart and vasculature, have been hinted at but are yet to be fully explored.
Conclusions
Numerous studies have provided evidence that the gut microbiota and hypertension are linked. However, the nature of this link remains uncertain, since there is clear evidence not only for gut microbes to influence the progression of hypertension but for hypertension to remodel the gut microbiota. We hypothesize that this truly is a bidirectional interaction—for example, hypertension itself may induce changes in gut microbiota, and these changes may then serve to further drive the hypertension. Studies to date have outlined potential mechanistic roles for SCFAs in mediating host-microbe communication in hypertension, often acting via host SCFA G-protein-coupled receptors. Similarly, there are clear roles for TMAO in cardiovascular diseases. However, it seems highly unlikely that SCFAs and TMAO are the only players in mediating communication between microbes and host in the context of blood pressure regulation. Looking forward, in future studies it will be critical to better understand the players involved and to uncover the mechanisms underlying host-microbe communications. This will require not only a more nuanced understanding of SCFA signaling but moving beyond SCFA signaling to understand how other microbial metabolites influence the host and to understand how changes in host physiology result in remodeling of the gut microbiota.
Acknowledgments
This work was supported by National Institutes of Health Grants F31 HL-144061 (to B.P.), R01 HL-128512 (to J.L.P.), and R01 DK-107726 (to J.L.P.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
B.G.P. and J.L.P. prepared figures; B.G.P., M.U.C., and J.L.P. drafted manuscript; B.G.P., M.U.C., and J.L.P. edited and revised manuscript; B.G.P., M.U.C., and J.L.P. approved final version of manuscript.
References
- 1.Abaffy T, Bain JR, Muehlbauer MJ, Spasojevic I, Lodha S, Bruguera E, O’Neal SK, Kim SY, Matsunami H. A testosterone metabolite 19-hydroxyandrostenedione induces neuroendocrine trans-differentiation of prostate cancer cells via an ectopic olfactory receptor. Front Oncol 8: 162, 2018. doi: 10.3389/fonc.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adipietro KA, Mainland JD, Matsunami H. Functional evolution of mammalian odorant receptors. PLoS Genet 8: e1002821, 2012. doi: 10.1371/journal.pgen.1002821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, Natarajan N, Yong HM, De Santiago B, Oh JJ, Yoon AR, Panettieri RA, Homann O, Sullivan JK, Liggett SB, Pluznick JL, An SS. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep 6: 38231, 2016. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26: 653–661, 2010. doi: 10.1016/j.nut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12: 169–181, 2016. doi: 10.1038/nrneph.2015.191. [DOI] [PubMed] [Google Scholar]
- 7.Ascher S, Reinhardt C. The gut microbiota: An emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol 48: 564–575, 2018. doi: 10.1002/eji.201646879. [DOI] [PubMed] [Google Scholar]
- 8.Ayesh R, Mitchell SC, Smith RL. Dysfunctional N-oxidation of trimethylamine and the influence of testosterone treatment in man. Pharmacogenetics 5: 244–246, 1995. doi: 10.1097/00008571-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 21: 1300–1304, 2006. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139: 1407–1421, 2019. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer W, Richards DW Jr. A vasodilator action of acetates. J Physiol 66: 371–378, 1928. doi: 10.1113/jphysiol.1928.sp002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, Leibowitz A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 10: E1154, 2018. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 107: 1337–1344, 2012. doi: 10.1038/bjc.2012.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 16.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5: 80, 2017. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- 19.Cabreiro F, Gems D. Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Mol Med 5: 1300–1310, 2013. doi: 10.1002/emmm.201100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527: 240–244, 2015. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheema MU, Pluznick JL. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to angiotensin II. Hypertension 74: 184–193, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TH, Chen WM, Hsu KH, Kuo CD, Hung SC. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 355: 913–918, 2007. doi: 10.1016/j.bbrc.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 23.Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 557: 719–731, 2004. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. J Biol Chem 254: 1716–1723, 1979. [PubMed] [Google Scholar]
- 25.Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32: 2094–2101, 1979. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- 26.Dalesio NM, Barreto Ortiz SF, Pluznick JL, Berkowitz DE. Olfactory, taste, and photo sensory receptors in non-sensory organs: it just makes sense. Front Physiol 9: 1673, 2018. doi: 10.3389/fphys.2018.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daugirdas JT, Wang X, Nutting C, Swanson V, Agrawal A. Depressant effect of acetate in isolated cardiac tissue. Cardiovasc Res 22: 566–570, 1988. doi: 10.1093/cvr/22.8.566. [DOI] [PubMed] [Google Scholar]
- 28.de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 11: E51, 2018. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S, Pinto-Sanchez MI, Philip V, McLean PG, Hagelsieb MG, Surette MG, Bergonzelli GE, Verdu EF, Britz-McKibbin P, Neufeld JD, Collins SM, Bercik P. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 9: eaaf6397, 2017. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 30.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156: 84–96, 2014. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 31.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding L, Chang M, Guo Y, Zhang L, Xue C, Yanagita T, Zhang T, Wang Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis 17: 286, 2018. doi: 10.1186/s12944-018-0939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donertas Ayaz B, Zubcevic J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: A potential role for hydrogen sulfide. Pharmacol Res 153: 104677, 2020. doi: 10.1016/j.phrs.2020.104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 48: 612–626, 2012. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fechner A, Kiehntopf M, Jahreis G. The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J Nutr 144: 599–607, 2014. doi: 10.3945/jn.113.186858. [DOI] [PubMed] [Google Scholar]
- 36.Fennema D, Phillips IR, Shephard EA. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos 44: 1839–1850, 2016. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flegel C, Manteniotis S, Osthold S, Hatt H, Gisselmann G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS One 8: e55368, 2013. doi: 10.1371/journal.pone.0055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu H, Shi YQ, Mo SJ. Effect of short-chain fatty acids on the proliferation and differentiation of the human colonic adenocarcinoma cell line Caco-2. Chin J Dig Dis 5: 115–117, 2004. doi: 10.1111/j.1443-9573.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 39.Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L. Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. J Proteome Res 10: 1860–1869, 2011. doi: 10.1021/pr1011125. [DOI] [PubMed] [Google Scholar]
- 40.Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension 61: 757–761, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72: 1141–1150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng 118: 476–481, 2014. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Gelis L, Jovancevic N, Veitinger S, Mandal B, Arndt HD, Neuhaus EM, Hatt H. Functional characterization of the odorant receptor 51E2 in human melanocytes. J Biol Chem 291: 17772–17786, 2016. doi: 10.1074/jbc.M116.734517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M; SPRING Trial Group . Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68: 974–981, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 45.Grison A, Zucchelli S, Urzì A, Zamparo I, Lazarevic D, Pascarella G, Roncaglia P, Giorgetti A, Garcia-Esparcia P, Vlachouli C, Simone R, Persichetti F, Forrest AR, Hayashizaki Y, Carloni P, Ferrer I, Lodovichi C, Plessy C, Carninci P, Gustincich S; FANTOM Consortium . Mesencephalic dopaminergic neurons express a repertoire of olfactory receptors and respond to odorant-like molecules. BMC Genomics 15: 729, 2014. doi: 10.1186/1471-2164-15-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halperin Kuhns VL, Sanchez J, Sarver DC, Khalil Z, Rajkumar P, Marr KA, Pluznick JL. Characterizing novel olfactory receptors expressed in the murine renal cortex. Am J Physiol Renal Physiol 317: F172–F186, 2019. doi: 10.1152/ajprenal.00624.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr 132: 1012–1017, 2002. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 48.Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, Krzesinski JM, Melin P, de Tullio P, Jouret F. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension 74: 1005–1013, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 49.Jankowski J, Westhof T, Vaziri ND, Ingrosso D, Perna AF. Gases as uremic toxins: is there something in the air? Semin Nephrol 34: 135–150, 2014. doi: 10.1016/j.semnephrol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Jovancevic N, Dendorfer A, Matzkies M, Kovarova M, Heckmann JC, Osterloh M, Boehm M, Weber L, Nguemo F, Semmler J, Hescheler J, Milting H, Schleicher E, Gelis L, Hatt H. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res Cardiol 112: 13, 2017. doi: 10.1007/s00395-017-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5: e003698, 2016. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chem Biol Interact 213: 1–12, 2014. doi: 10.1016/j.cbi.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Kim TT, Parajuli N, Sung MM, Bairwa SC, Levasseur J, Soltys CM, Wishart DS, Madsen K, Schertzer JD, Dyck JRB. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am J Physiol Endocrinol Metab 315: E511–E519, 2018. doi: 10.1152/ajpendo.00471.2017. [DOI] [PubMed] [Google Scholar]
- 54.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585, 2013. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 58.Levrat MA, Rémésy C, Demigné C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J Nutr 121: 1730–1737, 1991. doi: 10.1093/jn/121.11.1730. [DOI] [PubMed] [Google Scholar]
- 59.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, Bradley TD. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 21: 241–247, 2003. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 61.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res 114: 1804–1814, 2014. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 63.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339: 1084–1088, 2013. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 64.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135: 964–977, 2017. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 65.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res 57: 3697–3707, 1997. [PubMed] [Google Scholar]
- 66.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31: 1391–1394, 1990. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naito Y, Uchiyama K, Takagi T. Redox-related gaseous mediators in the gastrointestinal tract. J Clin Biochem Nutr 63: 1–4, 2018. doi: 10.3164/jcbn.18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48: 826–834, 2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuhaus EM, Zhang W, Gelis L, Deng Y, Noldus J, Hatt H. Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J Biol Chem 284: 16218–16225, 2009. doi: 10.1074/jbc.M109.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newell PD, Chaston JM, Wang Y, Winans NJ, Sannino DR, Wong AC, Dobson AJ, Kagle J, Douglas AE. In vivo function and comparative genomic analyses of the Drosophila gut microbiota identify candidate symbiosis factors. Front Microbiol 5: 576, 2014. doi: 10.3389/fmicb.2014.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niimura Y, Matsui A, Touhara K. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res 24: 1485–1496, 2014. doi: 10.1101/gr.169532.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nøhr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290: 126–137, 2015. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 74.Nutting CW, Islam S, Daugirdas JT. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol 261: H561–H567, 1991. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- 75.Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 471: 1441–1453, 2019. doi: 10.1007/s00424-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58: 811–817, 2011. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 77.Perna AF, Glorieux G, Zacchia M, Trepiccione F, Capolongo G, Vigorito C, Anishchenko E, Ingrosso D. The role of the intestinal microbiota in uremic solute accumulation: a focus on sulfur compounds. J Nephrol 32: 733–740, 2019. doi: 10.1007/s40620-019-00589-z. [DOI] [PubMed] [Google Scholar]
- 78.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213–217, 2016. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rezq S, Abdel-Rahman AA. Central GPR109A activation mediates glutamate-dependent pressor response in conscious rats. J Pharmacol Exp Ther 356: 456–465, 2016. doi: 10.1124/jpet.115.229146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214, 2013. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a mammalian receptor repertoire. Sci Signal 2: ra9, 2009. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res 120: 312–323, 2017. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheppach W, Bartram P, Richter A, Richter F, Liepold H, Dusel G, Hofstetter G, Rüthlein J, Kasper H. Effect of short-chain fatty acids on the human colonic mucosa in vitro. JPEN J Parenter Enteral Nutr 16: 43–48, 1992. doi: 10.1177/014860719201600143. [DOI] [PubMed] [Google Scholar]
- 85.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 43: 169–175, 2004. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 86.Shubitowski TB, Poll BG, Natarajan N, Pluznick JL. Short-chain fatty acid delivery: assessing exogenous administration of the microbiome metabolite acetate in mice. Physiol Rep 7: e14005, 2019. doi: 10.14814/phy2.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottière HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 46: 507–514, 2000. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax 57: 602–607, 2002. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith JL, Wishnok JS, Deen WM. Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol 125: 296–308, 1994. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- 90.Soelter J, Schumacher J, Spors H, Schmuker M. Computational exploration of molecular receptive fields in the olfactory bulb reveals a glomerulus-centric chemical map. Sci Rep 10: 77, 2020. doi: 10.1038/s41598-019-56863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun 303: 364–369, 2003. doi: 10.1016/S0006-291X(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 92.Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27: 305–313, 2016. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut microbiota composition and blood pressure. Hypertension 73: 998–1006, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 95.Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, Gómez-Guzmán M, Jiménez R, Raizada MK, Duarte J. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol 10: 231, 2019. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 97.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9: 352–355, 2003. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 98.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 30: 1700–1705, 2014. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 3: 111, 2012. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 361: k2179, 2018. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waghulde H, Cheng X, Galla S, Mell B, Cai J, Pruett-Miller SM, Vazquez G, Patterson A, Vijay Kumar M, Joe B. Attenuation of microbiotal dysbiosis and hypertension in a CRISPR/Cas9 gene ablation rat model of GPER1. Hypertension 72: 1125–1132, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walejko JM, Kim S, Goel R, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Gut microbiota and serum metabolite differences in African Americans and white Americans with high blood pressure. Int J Cardiol 271: 336–339, 2018. doi: 10.1016/j.ijcard.2018.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J Hypertens 35: 1899–1908, 2017. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 40: 583–594, 2019. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35: 904–910, 2014. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res 113, Pt A: 300–312, 2016. doi: 10.1016/j.phrs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wen Y, Peng L, Xu R, Zang N, Huang Q, Zhong M. Maternal serum trimethylamine-N-oxide is significantly increased in cases with established preeclampsia. Pregnancy Hypertens 15: 114–117, 2019. doi: 10.1016/j.preghy.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving HH, Hansen TW, Hazen SL, Pedersen O, Rossing P. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with Type 1 diabetes. Diabetes Care 42: 1512–1520, 2019. doi: 10.2337/dc19-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278: 9869–9874, 2003. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 111.Won YJ, Lu VB, Puhl HL III, Ikeda SR. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci 33: 19314–19325, 2013. doi: 10.1523/JNEUROSCI.3102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics 39: 375–384, 2012. doi: 10.1016/j.jgg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 113.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 7: 381, 2017. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang T, Magee KL, Colon-Perez LM, Larkin R, Liao YS, Balazic E, Cowart JR, Arocha R, Redler T, Febo M, Vickroy T, Martyniuk CJ, Reznikov LR, Zubcevic J. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol (Oxf) 226: e13256, 2019. doi: 10.1111/apha.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang T, Rodriguez V, Malphurs WL, Schmidt JT, Ahmari N, Sumners C, Martyniuk CJ, Zubcevic J. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol Rep 6: e13732, 2018. doi: 10.14814/phy2.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou T, Chien MS, Kaleem S, Matsunami H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol 594: 4225–4251, 2016. doi: 10.1113/JP271936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165: 111–124, 2016. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]