Abstract
Exposure to hypoxia increases pulmonary vascular resistance, leading to elevated pulmonary arterial pressure and, potentially, right heart failure. Vascular remodeling is an important contributor to the increased pulmonary vascular resistance. Hyperproliferation of smooth muscle, endothelial cells, and fibroblasts, and deposition of extracellular matrix lead to increased wall thickness, extension of muscle into normally non-muscular arterioles, and vascular stiffening. This review highlights intrinsic and extrinsic modulators contributing to the remodeling process.
Keywords: hypoxia, heart failure, remodeling
Introduction
In the normal adult lung, the pulmonary circulation is a low-resistance vasculature with thin-walled vessels. For over 100 years, it has been appreciated that acute challenge with hypoxia causes pulmonary vessels to contract, a phenomenon termed hypoxic pulmonary vasoconstriction (reviewed in Ref. 155), which optimizes gas exchange by diverting perfusion from areas of inadequate ventilation. Decades later, the migration of low-altitude dwelling individuals into high-altitude regions uncovered the deleterious effects of prolonged hypoxia on the pulmonary vasculature (22a, 146). It is now recognized that long-term exposure to hypoxia, resulting from residence at high altitude or chronic lung disease, causes sustained contraction and vascular remodeling in the lung, leading to increased pulmonary arterial pressure (Ppa) and pulmonary vascular resistance (PVR), subsequent right heart enlargement, and, if severe enough, right ventricular failure and death (4, 8, 22a, 53, 101, 112, 113, 118, 152). Given the important contribution of pulmonary vascular remodeling to the increase in PVR observed with chronic hypoxia (CH), substantial efforts have been invested in understanding the cellular basis for these changes.
Hypoxia-Induced Changes in the Pulmonary Vascular Wall
Following the initial findings of altitude-induced pulmonary dysfunction in the early 1960s, research focused on characterizing the effects of CH on pulmonary vascular structure. Histological analysis of postmortem or postoperative tissue specimens from native high-altitude populations in South America, Tibet, and the U.S. revealed that long-term exposure to hypoxia was associated with thickening of the walls of the small arteries due to pulmonary arterial smooth muscle cell (PASMC) hyperplasia and muscle extension into precapillary arterioles (8, 53, 101, 112, 152). These human anatomic data provided compelling evidence of vascular remodeling in response to hypoxia. Subsequent studies in a variety of species confirmed and extended our understanding of the effect of long-term hypoxia on the structure of the pulmonary circulation (159). For example, experiments conducted in the high mountains of Colorado identified altitude as the primary cause of “brisket disease,” or heart failure, in cattle (176). Susceptible cattle exhibited extensive vascular remodeling, characterized by collagen deposition, thickened adventitia, increased intimal and medial thickness, and extension of muscle in small pulmonary arteries (126, 149, 151), which was particularly robust in neonatal calves (148, 149). Like humans or cattle experiencing hypoxia due to altitude, pigs also develop significant remodeling with altitude exposure (reviewed in Ref. 126).
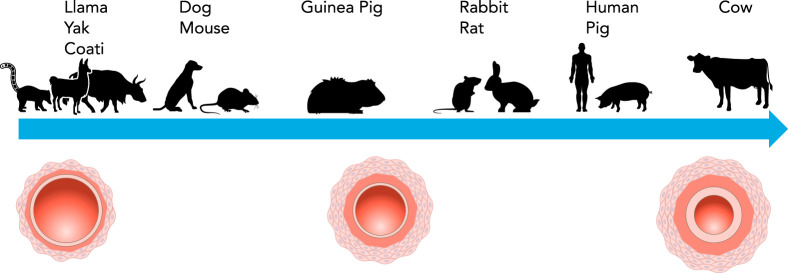
Although early studies used large animals in a high-altitude setting, in the laboratory, just a few weeks in an experimental hypoxic environment (typically 10% O2) predictably and reproducibly increased vascular wall thickness in rats due to PASMC hypertrophy, and hyperplasia and muscularization of typically non-muscular arterioles (30, 93, 124). In contrast, wall thickening is minimal in mice, and remodeling more often takes the form of distal muscularization (reviewed in Ref. 151) and functional stiffening of proximal, conduit arteries (171). Some species, such as llamas, coati, and yaks, are completely protected from hypoxia-induced vascular remodeling (35, 51, 52). The explanation for species-specific differences in degree of remodeling (FIGURE 1) is still under investigation, although with respect to cattle, genetic mutations that confer susceptibility and/or the greater presence of PASMCs in peripheral arteries of normotensive animals may underlie some of these differences (64, 106).
FIGURE 1.
Relative effect of hypoxia on pulmonary vascular remodeling in various species
Hyperproliferation of PASMCs and fibroblasts can account for medial and adventitial thickening, respectively; however, extension of muscle along the vascular tree likely includes a component of cell migration as well. Supporting this concept, cells exposed to hypoxia in vitro or isolated from CH animals are hypermotile (39, 73, 163, 178). Elegant fate-mapping experiments indicate that the cells surrounding the small arterioles following exposure to hypoxia derive from a smooth muscle lineage and are suggestive of cell migration followed by clonal expansion (135, 136). Unfortunately, the lack of specific markers for migration and inability to track single cells over time in vivo create practical difficulties in measuring the exact contribution of cell migration to the remodeling process.
In addition to wall thickening resulting from cellular hyperproliferation, enhanced deposition and/or cross-linking of extracellular matrix components (i.e., collagen) causes vascular stiffening of the large, conduit pulmonary arteries (34, 105, 170, 171). Reduced distensibility of the proximal vasculature contributes to increased Ppa via loss of right ventricle-pulmonary artery coupling, increased right ventricular afterload, and increased pulsatility of flow in the distal vasculature (34, 156).
Given the substantial CH-induced structural changes observed in the pulmonary circulation (FIGURE 2) and that conventional vasodilators had little acute effect on Ppa (10, 19, 97, 108), it was widely regarded that “fixed” remodeling was the underlying cause of increased PVR. Indeed, imaging studies in chronically hypoxic lungs often used vascular infusion of agents to visualize vessels and appeared to show reduced luminal diameter and decreased numbers of small-diameter vessels, or vascular pruning, suggesting remodeling occurred in an inward fashion to occlude vessels (55, 123). However, it is possible that these observations may have reflected robust vasoconstriction or variable perfusion (12, 91, 150), since careful morphological examination in fully vasodilated lungs revealed medial expansion was directed outward and was associated with angiogenesis (58). Later studies demonstrating that inhibitors of Rho kinase (ROCK), a cytoskeletal regulator that promotes contraction, acutely normalized Ppa in chronically hypoxic rats (40, 102, 103) suggested that constriction was not permanent and that remodeling instead may augment contraction secondary to increased muscularity of the small pulmonary arteries.
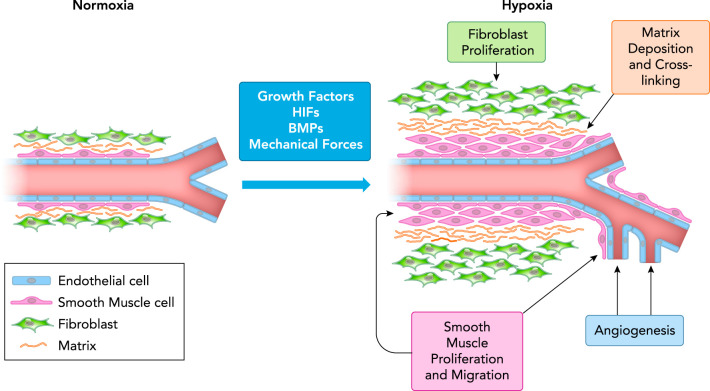
FIGURE 2.
Effects of chronic hypoxia on the pulmonary arterial wall
Under normoxic conditions, main and resistance level arteries consist of a single layer of endothelial cells, and thin layers of smooth muscle and fibroblasts. Exposure to prolonged hypoxia causes vessels to be exposed to elevated levels of local and circulating growth factors, and increased mechanical forces such as shear stress. Hypoxia also stimulates production of bone morphogenetic proteins (BMPs) and activates hypoxia-inducible factors (HIFs). The resulting changes in the vascular wall include proliferation of fibroblasts and smooth muscle cells, smooth muscle extension into non-muscular arterioles, increased deposition and cross-linking of extracellular matrix components (i.e., collagen), and angiogenesis.
Endothelial cells (ECs) exposed to in vitro hypoxia, especially severe levels (i.e., ≤1% O2), exhibit increased proliferation (50, 158, 178). However, since intimal thickening is rarely observed with CH outside of some cattle, the relevance of this response to vascular remodeling and development of hypoxia-induced pulmonary hypertension in most species is unclear. One possibility is that microvascular EC proliferation in response to hypoxia may contribute to angiogenesis (58, 114, 158), perhaps as a compensatory mechanism to increase gas exchange.
Extrinsic Factors That Promote Pulmonary Vascular Remodeling
The mechanisms underlying smooth muscle and fibroblast hyperproliferation include both intrinsic and extrinsic factors. As noted earlier, one of the first pulmonary responses to hypoxia is vasoconstriction. Reduced diameter increases the mechanical forces exerted on the pulmonary vascular wall, both perpendicular strain from elevated pressure and increased shear stress (or drag) along the endothelium. A role for altered hemodynamic stress influencing remodeling is supported by the fact that augmenting pulmonary blood flow, alone or in combination with hypoxia, increased remodeling (125). Moreover, supernumerary vessels, small-diameter arteries that branch from the parent artery at a 90° angle and contain strictures at the opening that protect against high hemodynamic stress, do not exhibit wall thickening in chronically hypoxic rats (109).
As the interface with the bloodstream, ECs serve a critical barrier function and are ideally positioned to respond to changes in mechanical forces (i.e., shear stress). Under normal conditions, excess endothelial production of anti-proliferative vasodilators, such as nitric oxide and prostacyclin, maintain low PVR. With hypoxia and increased shear stress, levels of endothelial-derived pro-proliferative circulating factors (i.e., endothelin-1) are increased (134, 142), whereas synthesis of anti-proliferative factors is reduced (reviewed in Ref. 148). When applied in vitro, ET-1 induces migration and proliferation of PASMCs (60, 92, 134), and proliferation and collagen production in lung fibroblasts (3). In vivo, ET receptor inhibitors prevented vascular remodeling in several animal models (29). Platelets can also respond to changes in shear stress with release of serotonin (5-HT), possibly accounting for elevated circulating levels observed in CH (5, 22). Similar to ET-1, 5-HT uptake promotes PASMC growth and vascular remodeling (37, 84, 85). Some of these responses can be attributed to internalization of 5-HT via the serotonin transporter (SERT) and receptor-independent signaling (85, 87), although inhibiting 5-HT1B receptors by silencing or pharmacological inhibitors reduced PASMC proliferation and CH-induced remodeling (85), suggesting potential cooperativity between uptake and receptor signaling.
A major signaling pathway regulating PASMC growth involves bone morphogenetic protein (BMP) signaling. BMPs are part of the transforming growth factor-β (TGF-β) super family and bind to complexes containing type I and type II receptors (145) to activate SMAD1/5/8 signaling and downstream transcriptional responses (79). BMPs also induce SMAD-independent signaling, involving MAP kinases (MAPKs), phosphatidylinositol 3-kinase/AKT, and protein kinase C (179). Most work examining the role of BMPs in pulmonary vascular remodeling has focused on ligand (BMP2, BMP4, and BMP7) and receptor (BMPR1 and BMPR2) binding. Importantly, BMPs exert differential effects on PASMC growth depending on the location within the vasculature; BMP4 was anti-proliferative in PASMCs from proximal vessels (99) but induced proliferation (180) in PASMCs from distal vessels. Mice with partial deficiency for BMP2 and BMP4 were more susceptible to and protected against hypoxia-induced vascular remodeling, respectively (6, 44). With respect to lung fibroblasts, BMP4 may play a protective role by antagonizing TGF-β-induced proliferation and collagen production (111), whereas, in ECs, activation of BMPR2 by BMP2 or BMP4 was linked to nitric oxide production (45), providing a paracrine effect whereby normal BMP signaling exerts anti-proliferative effects on PASMCs and/or fibroblasts.
Infiltrating immune cells provide another source of paracrine factors that can promote vascular remodeling. Recruited by pro-inflammatory signals from adventitial fibroblasts (38, 75), macrophage accumulation around pulmonary vessels in chronically hypoxic animals in turn stimulates fibroblast growth, collagen production, and secretion of growth factors that promote smooth muscle proliferation (75, 119, 129).
In both PASMCs and fibroblasts, hypoxia induces the expression and activity of lysyl oxidases (Loxs) (105), enzymes that oxidize lysine residues in elastin and collagen to promote formation and repair of the extracellular matrix. Upregulation of Loxs contributes to vascular stiffness via promoting extracellular matrix deposition and collagen cross-linking. Recently, galectin-3, a β-galactoside-binding lectin, was found to be induced in the hypoxic lung and to contribute to collagen production from adventitial fibroblasts (83). Increased deposition and/or cross-linking of extracellular matrix components not only increases vascular stiffness but may also induce intracellular signaling that promotes growth/motility via matrix-cell interactions.
Hypoxia Alters Expression and Activity of Membrane Channels/Transporters
A variety of intrinsic cellular mechanisms are activated by hypoxia to promote a pro-proliferative, pro-migratory phenotype in PASMCs and/or fibroblasts. One of the earliest changes noted in PASMCs from chronically hypoxic animals was membrane depolarization (154), subsequently identified to be due to repressed expression/activity of several types of K+ channels (94, 140, 166, 169). Augmenting PASMC K+ channel expression/activity reduced remodeling in chronically hypoxic rats (16, 94, 116), but the exact mechanism by which depressed K+ channel activity and/or depolarization causes remodeling is still being explored. One possibility is that increased intracellular K+ confers resistance to apoptosis (21). Another possibility is that depolarization might drive activation of voltage-gated calcium channels (VGCC) in the L-type channel family, leading to elevated basal intracellular calcium concentration ([Ca2+]i). Consistent with this hypothesis, increased [Ca2+]i is required for PASMC growth and migration (47, 72, 73) and has been documented in cells from hypoxic animals (143, 168). However, inhibitors of L-type channels have little effect on PASMCs from hypoxic animals (76, 143, 168). Rather, the elevated basal [Ca2+]i appears to occur primarily via upregulation of canonical transient receptor potential (TRPC) proteins (76, 168), which form Ca2+-permeable nonselective cation channels that are not activated by depolarization but can be modulated by phosphorylation, receptor activation, or store depletion (reviewed in Ref. 36). Nonselective cation channels are required for elevated [Ca2+]i (73, 76, 168), migration (73), and proliferation (88) in PASMCs exposed to hypoxia in vitro or isolated from CH animals. Interestingly, VGCCs in hypoxic PASMCs can be activated by mitogenic agonists (82, 164) and contribute to stimulated proliferation (128), suggesting a potential role in the setting of excessive growth factors. Whether [Ca2+]i is increased in hypoxic adventitial fibroblasts is unknown, but hypoxia-induced growth requires activation of Ca2+-dependent PKC isoforms (28), suggesting elevated [Ca2+]i might also be a feature of hypoxic exposure in these cells.
Following a rise in [Ca2+]i, several downstream signal transduction pathways and transcription factors are activated that could be involved in hypoxia-induced proliferation (reviewed in Ref. 70) and/or migration (73, 178). In particular, Ca2+ activates nuclear factor of activated T cells (NFAT), which in turn reduces K+ channel expression and increases proliferation (18), providing a link between alterations in [Ca2+]i, dysregulated K+ channel expression/activity, and PASMC growth. Accordingly, remodeling was reversed when NFAT was pharmacologically inhibited (18) or genetically deleted (13). Moreover, Ca2+-dependent increases in the expression of the water channel, aquaporin 1 (AQP1), are required for hypoxia-induced migration of PASMCs (73). In endothelial and tumor cells, increased AQP1 levels may result in localized control of water flux across the cell membrane, possibly allowing for directed cell movement (131); however, in PASMCs, the actions of AQP1 appear to be independent of water transport (71) and instead require the COOH-terminal tail portion of the protein, which regulates the levels of β-catenin, a dual-function protein, to control both migration and proliferation (185). AQP1 may also participate in cytoskeletal rearrangement (98) to facilitate cell movement.
Another contributor to control of migration and proliferation in PASMCs is the Na+/H+ exchanger (NHE), a main contributor to pH homeostasis (86, 122). NHE isoform 1 (NHE1) is upregulated in PASMCs by hypoxia, leading to increased NHE activity and an alkaline shift in pH (127, 139). Activation of NHE is required for growth factor-induced proliferation (120), whereas loss of NHE activity (121) or genetic deletion of NHE1 (183, 184) attenuated hypoxia-induced vascular remodeling and PASMC proliferation and migration. NHE1 activation represses a growth inhibitor pathway by increasing p27, a cyclin-dependent kinase inhibitor, while simultaneously stimulating proliferation by decreasing the nuclear transcription factor E2F1 (183). NHE1 may also facilitate cell growth and migration via regulation of cytoskeletal arrangement (31); in fibroblasts, NHE1 binds to actin filaments through the adaptor protein, ezrin, providing a link between NHE1 and cytoskeletal rearrangement (31). Preliminary studies indicate similar NHE1-actin interactions occur in PASMCs (90). Hypoxia increased NHE1 expression in ECs (26), but whether NHE1 contributes to hypoxia-induced EC migration, proliferation, or angiogenesis is unknown.
Reactive Oxygen Species and Altered Metabolism
Changes in mitochondrial-derived reactive oxygen species (ROS) are well documented in response to hypoxia and are essential for initiation of vasoconstriction (reviewed in Ref. 155); thus it is not unreasonable to suspect that mitochondrial ROS might also play a role in the remodeling process. Indeed, mitochondrial fission, which is typically associated with increased ROS production from mitochondria (177), has been reported in PASMCs from CH rats, with inhibitors of fission attenuating remodeling (89). However, observations that ROS levels were reduced and MitoQ, a mitochondrial-targeted antioxidant, had no effect on hypoxia-induced remodeling (110) would appear to argue against a role for mitochondrial ROS in CH-induced remodeling. Cytosolic ROS are also generated by NADPH oxidase (Nox). Nox1 (25, 161), Nox2 (7, 77, 100), and Nox4 (11, 48, 96) are expressed in pulmonary vascular tissue and have been studied with respect to hypoxia-induced remodeling. For example, female mice with genetic deficiency for Nox1 were protected from the development of CH-induced pulmonary vascular remodeling (57), whereas male mice instead developed PH in the absence of hypoxia, highlighting the importance of sex hormones in the pulmonary vasculature. Indeed, Nox1 mediates the effects of estrogen and serotonin on PASMC proliferation (56). Deficiency of the Nox2 subunit, gp91phox, also protected against medial wall thickening in CH mice (77). Of note, since Nox2 is highly expressed in immune cells and these studies were conducted in global knockout animals, whether Nox2 activity in phagocytic cells, vascular cells, or both is necessary for hypoxia-induced vascular remodeling is unclear. Abundant data demonstrate hypoxia increases Nox4 expression in PASMCs, pulmonary arteries, or lungs from CH mice and rats (48, 50, 96, 107, 172), where Nox4 activation is necessary for hypoxia-induced reduction of Kv currents (95) and mediates BMP4-induced increases in protein expression of nonselective cation channels (66). Nox4 activation increases H2O2 production and cyclin D1 expression, a factor important in the control of cell cycle (173), and enhances proliferation by inhibiting peroxisome proliferator-activated receptor gamma (PPARγ) (80, 81). Since Nox4 is repressed by PPARγ (49), downregulation of PPARγ sets up a feed-forward mechanism to further enhance Nox4 activity. NF-κB has been identified as both an upstream regulator (14, 81, 173) and a downstream effector (15, 172) of Nox4, suggesting an additional NF-κB/Nox4 feed-forward loop.
Despite substantial evidence implicating Nox4 interactions with several pathways critical to pulmonary vascular remodeling during hypoxia, results from in vivo Nox4 inhibition have proved equivocal. GKT137831, which inhibits both Nox4 and Nox1, attenuated hypoxia-induced wall thickening but not muscularization of small arteries (50), yet Nox4 deficiency had no effect on pulmonary vascular remodeling in CH mice (57, 162). Whether these data suggest that Nox4 is not necessary for CH-induced pulmonary vascular remodeling in vivo or plays a less important role in mice than other species remains to be determined.
Signaling Pathways That Form Nodes of Interaction
Many of the pathways outlined above are linked through regulation by the oxygen-sensitive transcription factors, hypoxia-inducible factor 1 and 2 (HIF-1 and HIF-2, respectively). HIF-1 was originally identified as a heterodimeric transcription factor consisting of a constitutively expressed β (HIF-1β) subunit and an oxygen-sensitive α (HIF-1α) subunit (132), with subsequent studies identifying HIF-2α as structurally similar to HIF-1α and also binding HIF-1β to form the HIF-2 transcription factor (117). Both α subunits are hydroxylated on two proline residues via prolyl hydroxyalse domain (PHD) proteins using molecular oxygen as a substrate, allowing binding to the von Hippel-Lindau protein, ubiquitination, and proteasomal degradation (reviewed in Refs. 133, 117). Reductions in oxygen levels limit hydroxylation, allowing HIF-1α/HIF-2α accumulation.
HIF-1α is found in all cells, whereas HIF-2α expression is more restricted. In the lung, hypoxia induces HIF-1 (39, 41, 68, 69, 115, 157, 181) and HIF-2 (39, 69, 157) in PASMCs, fibroblasts, and ECs. Initial evidence that HIFs contribute to pulmonary responses to CH came from transgenic animals, where complete loss of HIF-1α or HIF-2α was lethal (23, 63), whereas heterozygosity for the null alleles (Hif1a+/− and Hif2a+/−) impaired development of hypoxia-induced PH and vascular remodeling (20, 182). Targeting HIF-1 (2) or HIF-2 (27, 59) activity pharmacologically or with knockdown approaches also reduced CH-induced vascular remodeling. Use of inducible Cre recombinase strategies to selectively delete HIFs in adult animals revealed that complete loss of smooth muscle Hif1a (9) or endothelial Hif2a (157), or partial global loss of Hif2a (59), attenuated hypoxia-induced vascular remodeling. Similar benefits were achieved with constitutive HIF-2α homozygous deletion targeted to ECs (67, 147). Consistent with these findings, HIF-1 mediates hypoxia-induced PASMC proliferation (138) and HIF-2 mediates both migration and proliferation in fibroblasts (39) in vitro. Surprisingly, mice with constitutive homozygous deletion of HIF-1α targeted to vascular smooth muscle cells (68) or complete global (59) or EC-targeted deletion of HIF-2α (147) exhibited enhanced PH or death during hypoxia, suggesting that, in these mice, HIFs played a beneficial role. Inconsistency in results across these studies highlight the complexity of HIF signaling and indicate further investigation will be required to determine whether homozygous versus heterozygous genetic modifications, long-term (constitutive) versus short-term (inducible) deletions, off-target effects of the drivers used, and/or sex- or strain-dependent differences account for the variability observed.
The preponderance of evidence appears to indicate that HIFs mediate pulmonary vascular remodeling during CH, although the downstream mechanisms are still being defined. In the case of HIF-1, both Ca2+ and pH homeostasis appear to be involved (139, 141). HIF-1 also regulates other factors known to promote hypoxia-induced remodeling, including the expression of K+ channels (17, 140, 175), BMP4 (165), mitochondrial fission (89), and vascular endothelial-derived growth factor (43). Moreover, HIF-1 binds to the Nox4 promoter, leading to Nox4-dependent increases in proliferation and migration (33). Nox4 upregulation further increases HIF-1α transcription via activation of NF-κB (15) and increases HIF activation by interfering with hydroxylation (32), creating a feed-forward loop to augment and/or maintain HIF activity.
Plasma levels of ET-1, a well-known HIF target, were reduced in Hif2a+/− mice (20). Although HIFs induce ET-1 production, ET-1 in turn upregulates HIF-1 selectively in PASMCs by downregulating PHD2 expression (74, 115), creating feed-forward enhancement of HIF-1 not observed in systemic smooth muscle cells (115). Since ECs serve as a primary source of ET-1 in vivo, these data suggest a possible model whereby ET-1 production enhanced by hypoxia-induced HIF-2α expression in ECs subsequently augments HIF-1α in PASMCs, initiating a mechanism for upregulation of HIF-1 and, consequently, HIF target genes to promote PASMC remodeling. HIF-2 in ECs also upregulates arginase 1 (24), an enzyme responsible for converting the NO precursor L-arginine to L-citrulline, perhaps explaining the reduced bioavailability of NO during hypoxia. Arginase 2 is likewise upregulated by hypoxia in pulmonary ECs (158), but whether this response is HIF-dependent is unclear. Finally, the effects of hypoxia on production of pro-inflammatory mediators and growth factors in fibroblasts are also HIF-dependent and serve to both recruit macrophages to the vessels and stimulate fibroblast, PASMC, and possibly EC proliferation (38, 129).
Rho-associated protein kinase (ROCK) is another signaling molecule interconnecting several pathways. A major consequence of ROCK activation is enhancement of the Ca2+ sensitivity of the contractile apparatus (65, 174) to render pulmonary arteries more sensitive to vasoconstrictor stimuli; however, ROCK is also implicated in the remodeling process (42, 137). For example, ROCK activation is necessary for migration and proliferation in a variety of vascular cell types, including PASMCs (46, 60, 78, 137, 163). Upstream activators of ROCK, RhoA and RhoB, mediate cytoskeletal rearrangement, a process necessary for cell movement, and contribute to hypoxia-induced migration and proliferation (178). Consistent with these findings, treatment with ROCK inhibitors reduced neovascularization and remodeling in hypoxia models (1, 40, 62). Activated by various growth factors (144) and by ROS (65), ROCK may thus represent a node of convergence for numerous inputs to augment opening of Ca2+ channels (54, 82, 167) and activity of NHE1 (60, 160).
Last, mammalian target of rapamycin (mTORC) is required for proliferation induced by both in vivo and in vitro hypoxic exposure (69). mTORC2 is activated in CH cells and is a critical regulator of PASMC proliferation and survival via downregulation of AMP-activated protein kinase (AMPK) (48). In PASMCs where HIF-1α levels are increased, depletion of mTORC2 reduced HIF-1α protein, suggesting cross-regulation between these pathways (48). Finally, increased Nox4 expression is required for mTORC2 activation (48), placing Nox4 upstream of another central coordinator of PASMC function.
Future Directions
The aforementioned studies confirm that pulmonary vascular remodeling in response to hypoxia is a multifaceted process (FIGURE 3). Although significant progress has been made in the past century to characterize the structural changes and underlying cellular mechanisms induced by hypoxia, much is yet to be learned in unraveling the complicated process controlling pulmonary vascular cell proliferation and migration. Adding complexity is apparent cross-talk between critical signaling pathways and conflicting data regarding the precise roles HIFs, EC proliferation, and ROS production play in vivo. Many of the differences observed across studies and between in vivo and in vitro results may stem from limitations in current experimental approaches. For example, considerable heterogeneity exists within cell types isolated from different portions of the pulmonary circulation, yielding variable responses to the same stimuli. Thus use of cells from proximal vessels may be applicable for exploring the mechanisms underlying arterial stiffening, whereas cells from distal regions of the lung may be more appropriate for examining mechanisms of migration and/or proliferation. In addition, cells in culture may not entirely reflect the in vivo situation for a variety of reasons, with traditional cell-culture experiments conducted with cells in isolation, on hard cultureware, and under static conditions. In vivo, cell-cell communication, a flexible extracellular matrix, and constant exposure to mechanical forces may elicit very different responses to stimuli. Recent advances with microfluidics (104, 153) and “lung-on-a chip” technology (61) may provide improved approaches for studying interactions between cells under conditions that more faithfully simulate the in vivo environment. And finally, even as new factors are identified for targeting as treatments for pulmonary vascular remodeling, several important obstacles remain in translating this information to therapies. Many of the molecules and signaling pathways recognized as being involved in the remodeling process have other important physiological functions. Thus clinical trials will need to be performed to assess the extent of their benefit in the patient population compared with unwanted side effects. Potentially, use of inhibitors for any of the described pathways to target pulmonary vascular remodeling may only be realized if cell-specific targeting can be achieved. Thus further investigation is clearly needed to identify additional mechanisms involved in the pathogenesis of vascular remodeling and to develop new pharmacological agents aimed at precisely targeting molecules in particular cell populations to reverse the remodeling process.
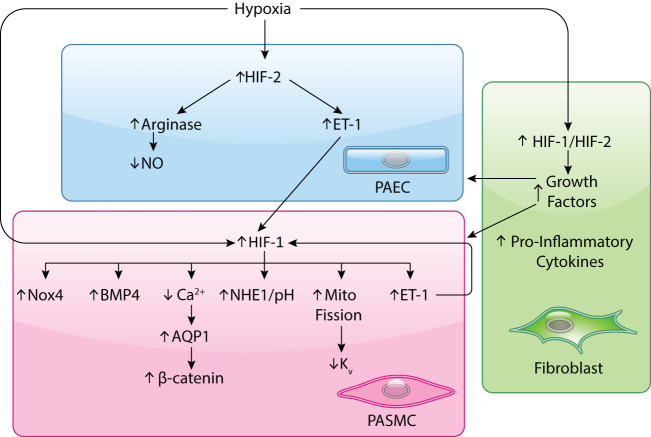
FIGURE 3.
HIF-dependent pathways involved in pulmonary vascular remodeling in response to chronic hypoxia
HIFs have been shown to increase under hypoxic conditions in endothelial and smooth muscle cells and fibroblasts. In endothelial cells (ECs), HIF-2 appears to be the predominant HIF upregulated by hypoxia, leading to upregulation of arginase, and subsequent reduction in nitric oxide (NO) production, and endothelin-1 (ET-1). Both hypoxia and ET-1 can augment HIF-1 levels in pulmonary arterial smooth muscle cells (PASMCs). Downstream consequences of HIF-1 activation in PASMCs include upregulation of Na+/H+ exchanger causing an alkaline shift in pH, induction of NADPH oxidase, bone morphogenetic protein 4 (BMP4) and ET-1, increased mitochondrial (mito) fragmentation and subsequent reduction in voltage-gated K+ channel expression, and upregulation of Ca2+-permeable channels, which facilitate accumulation of aquaporin 1 (AQP1), a water channel that in turn upregulates the pro-growth transcription factor β-catenin. PASMC-derived ET-1 can feed back to augment HIF-1 levels. In fibroblasts, both HIF-1 and HIF-2 levels increase, leading to production of pro-inflammatory cytokines that recruit immune cells and enhanced growth factors that can stimulate EC and PASMC proliferation.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-073859 and HL-126514.
No conflicts of interest, financial or otherwise, are declared by the author(s).
L.A.S. drafted manuscript; L.A.S. edited and revised manuscript; L.A.S. approved final version of manuscript.
References
- 1.Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 48: 280–285, 2006. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- 2.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci USA 109: 1239–1244, 2012. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmedat AS, Warnken M, Seemann WK, Mohr K, Kostenis E, Juergens UR, Racké K. Pro-fibrotic processes in human lung fibroblasts are driven by an autocrine/paracrine endothelinergic system. Br J Pharmacol 168: 471–487, 2013. doi: 10.1111/j.1476-5381.2012.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldashev AA, Sarybaev AS, Sydykov AS, Kalmyrzaev BB, Kim EV, Mamanova LB, Maripov R, Kojonazarov BK, Mirrakhimov MM, Wilkins MR, Morrell NW. Characterization of high-altitude pulmonary hypertension in the Kyrgyz: association with angiotensin-converting enzyme genotype. Am J Respir Crit Care Med 166: 1396–1402, 2002. doi: 10.1164/rccm.200204-345OC. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GH, Hellums JD, Moake J, Alfrey CP Jr. Platelet response to shear stress: changes in serotonin uptake, serotonin release, and ADP induced aggregation. Thromb Res 13: 1039–1047, 1978. doi: 10.1016/0049-3848(78)90232-3. [DOI] [PubMed] [Google Scholar]
- 6.Anderson L, Lowery JW, Frank DB, Novitskaya T, Jones M, Mortlock DP, Chandler RL, de Caestecker MP. Bmp2 and Bmp4 exert opposing effects in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 298: R833–R842, 2010. doi: 10.1152/ajpregu.00534.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA 96: 7944–7949, 1999. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias-Stella J, Saldana M. The terminal portion of the pulmonary arterial tree in people native to high altitudes. Circulation 28: 915–925, 1963. doi: 10.1161/01.CIR.28.5.915. [DOI] [PubMed] [Google Scholar]
- 9.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med 189: 314–324, 2014. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barberà JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 347: 436–440, 1996. doi: 10.1016/S0140-6736(96)90011-2. [DOI] [PubMed] [Google Scholar]
- 11.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg JT. Chronic hypoxia-induced pulmonary hypertension does/does not lead to loss of pulmonary vasculature. J Appl Physiol (1985) 103: 1455, 2007. doi: 10.1152/japplphysiol.00739.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Bosc LV. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 301: L872–L880, 2011. doi: 10.1152/ajplung.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bijli KM, Kang BY, Sutliff RL, Hart CM. Proline-rich tyrosine kinase 2 downregulates peroxisome proliferator-activated receptor gamma to promote hypoxia-induced pulmonary artery smooth muscle cell proliferation. Pulm Circ 6: 202–210, 2016. doi: 10.1086/686012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Görlach A. Reactive oxygen species activate the HIF-1α promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 27: 755–761, 2007. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 104: 11418–11423, 2007. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SE, Linden GS, King RR, Blair GP, Stansbury DW, Light RW. Effects of verapamil on pulmonary haemodynamics during hypoxaemia, at rest, and during exercise in patients with chronic obstructive pulmonary disease. Thorax 38: 840–844, 1983. doi: 10.1136/thx.38.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 111: 1519–1527, 2003. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol 153, Suppl 1: S99–S111, 2008. doi: 10.1038/sj.bjp.0707635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callebert J, Esteve JM, Hervé P, Peoc’h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther 317: 724–731, 2006. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]
- 22a.Canepa A, Chavez R, Hurtado A, Rotta A, Velasquez T. Pulmonary circulation at sea level and at high altitudes. J Appl Physiol 9: 328–336, 1956. doi: 10.1152/jappl.1956.9.3.328. [DOI] [PubMed] [Google Scholar]
- 23.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002. doi: 10.1038/nm721. An erratum for this article is available at https://doi.org/10.1038/nm1102-1329b. [DOI] [PubMed] [Google Scholar]
- 24.Cowburn AS, Crosby A, Macias D, Branco C, Colaço RD, Southwood M, Toshner M, Crotty Alexander LE, Morrell NW, Chilvers ER, Johnson RS. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci USA 113: 8801–8806, 2016. doi: 10.1073/pnas.1602978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutaia MV, Parks N, Centracchio J, Rounds S, Yip KP, Sun AM. Effect of hypoxic exposure on Na+/H+ antiport activity, isoform expression, and localization in endothelial cells. Am J Physiol 275: L442–L451, 1998. doi: 10.1152/ajplung.1998.275.3.L442. [DOI] [PubMed] [Google Scholar]
- 27.Dai Z, Zhu MM, Peng Y, Machireddy N, Evans CE, Machado R, Zhang X, Zhao YY. Therapeutic targeting of vascular remodeling and right heart failure in pulmonary arterial hypertension with HIF-2α inhibitor. Am J Respir Crit Care Med 198: 1423–1434, 2018. doi: 10.1164/rccm.201710-2079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das M, Stenmark KR, Ruff LJ, Dempsey EC. Selected isozymes of PKC contribute to augmented growth of fetal and neonatal bovine PA adventitial fibroblasts. Am J Physiol 273: L1276–L1284, 1997. doi: 10.1152/ajplung.1997.273.6.L1276. [DOI] [PubMed] [Google Scholar]
- 29.Davie NJ, Schermuly RT, Weissmann N, Grimminger F, Ghofrani HA. The science of endothelin-1 and endothelin receptor antagonists in the management of pulmonary arterial hypertension: current understanding and future studies. Eur J Clin Invest 39, Suppl 2: 38–49, 2009. doi: 10.1111/j.1365-2362.2009.02120.x. [DOI] [PubMed] [Google Scholar]
- 30.Davies P, Maddalo F, Reid L. Effects of chronic hypoxia on structure and reactivity of rat lung microvessels. J Appl Physiol (1985) 58: 795–801, 1985. doi: 10.1152/jappl.1985.58.3.795. [DOI] [PubMed] [Google Scholar]
- 31.Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na–H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell 6: 1425–1436, 2000. doi: 10.1016/S1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 32.Diebold I, Flügel D, Becht S, Belaiba RS, Bonello S, Hess J, Kietzmann T, Görlach A. The hypoxia-inducible factor-2α is stabilized by oxidative stress involving NOX4. Antioxid Redox Signal 13: 425–436, 2010. doi: 10.1089/ars.2009.3014. [DOI] [PubMed] [Google Scholar]
- 33.Diebold I, Petry A, Hess J, Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010. doi: 10.1091/mbc.e09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler ES, Bischoff JE, Slifka AJ, McCowan CN, Quinn TP, Shandas R, Ivy DD, Stenmark KR. Stiffening of the extrapulmonary arteries from rats in chronic hypoxic pulmonary hypertension. J Res Natl Inst Stand Technol 113: 239–249, 2008. doi: 10.6028/jres.113.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durmowicz AG, Hofmeister S, Kadyraliev TK, Aldashev AA, Stenmark KR. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J Appl Physiol (1985) 74: 2276–2285, 1993. doi: 10.1152/jappl.1993.74.5.2276. [DOI] [PubMed] [Google Scholar]
- 36.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res 84: 329–336, 1999. doi: 10.1161/01.RES.84.3.329. [DOI] [PubMed] [Google Scholar]
- 38.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193: 597–609, 2014. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eul B, Rose F, Krick S, Savai R, Goyal P, Klepetko W, Grimminger F, Weissmann N, Seeger W, Hänze J. Impact of HIF-1α and HIF-2α on proliferation and migration of human pulmonary artery fibroblasts in hypoxia. FASEB J 20: 163–165, 2006. doi: 10.1096/fj.05-4104fje. [DOI] [PubMed] [Google Scholar]
- 40.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 41.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firth AL, Choi IW, Park WS. Animal models of pulmonary hypertension: Rho kinase inhibition. Prog Biophys Mol Biol 109: 67–75, 2012. doi: 10.1016/j.pbiomolbio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005. doi: 10.1161/01.RES.0000181152.65534.07. [DOI] [PubMed] [Google Scholar]
- 45.Gangopahyay A, Oran M, Bauer EM, Wertz JW, Comhair SA, Erzurum SC, Bauer PM. Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J Biol Chem 286: 33134–33140, 2011. doi: 10.1074/jbc.M111.274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 47.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 48.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129: 864–874, 2014. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ 2: 483–491, 2012. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson WL, Boggs DF, Kay JM, Hofmeister SE, Okada O, Wagner WW Jr. Pulmonary vascular response of the coati to chronic hypoxia. J Appl Physiol (1985) 88: 981–986, 2000. doi: 10.1152/jappl.2000.88.3.981. [DOI] [PubMed] [Google Scholar]
- 52.Harris P, Heath D, Smith P, Williams DR, Ramirez A, Krüger H, Jones DM. Pulmonary circulation of the llama at high and low altitudes. Thorax 37: 38–45, 1982. doi: 10.1136/thx.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heath D, Smith P, Rios Dalenz J, Williams D, Harris P. Small pulmonary arteries in some natives of La Paz, Bolivia. Thorax 36: 599–604, 1981. doi: 10.1136/thx.36.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbert LM, Resta TC, Jernigan NL. RhoA increases ASIC1a plasma membrane localization and calcium influx in pulmonary arterial smooth muscle cells following chronic hypoxia. Am J Physiol Cell Physiol 314: C166–C176, 2018. doi: 10.1152/ajpcell.00159.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol 57: 542–554, 1976. [PMC free article] [PubMed] [Google Scholar]
- 56.Hood KY, Mair KM, Harvey AP, Montezano AC, Touyz RM, MacLean MR. Serotonin signaling through the 5–HT1B receptor and NADPH oxidase 1 in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 37: 1361–1370, 2017. doi: 10.1161/ATVBAHA.116.308929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, Touyz RM. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells: implications in pulmonary arterial hypertension. Hypertension 68: 796–808, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. J Physiol 547: 133–145, 2003. doi: 10.1113/jphysiol.2002.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu CJ, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, McKeon B, Mouradian G, Li M, Riddle S, Pugliese SC, Brown RD, Wallace EM, Graham BB, Frid MG, Stenmark KR. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J 54: 1900378, 2019. doi: 10.1183/13993003.00378-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huetsch JC, Walker J, Undem C, Lade J, Yun X, Baksh S, Jiang H, Lai N, Shimoda LA. Rho kinase and Na+/H+ exchanger mediate endothelin-1-induced pulmonary arterial smooth muscle cell proliferation and migration. Physiol Rep 6: e13698, 2018. doi: 10.14814/phy2.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- 63.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev 12: 149–162, 1998. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaenke RS, Alexander AF. Fine structural alterations of bovine peripheral pulmonary arteries in hypoxia-induced hypertension. Am J Pathol 73: 377–398, 1973. [PMC free article] [PubMed] [Google Scholar]
- 65.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Q, Fu X, Tian L, Chen Y, Yang K, Chen X, Zhang J, Lu W, Wang J. NOX4 mediates BMP4-induced upregulation of TRPC1 and 6 protein expressions in distal pulmonary arterial smooth muscle cells. PLoS One 9: e107135, 2014. doi: 10.1371/journal.pone.0107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, Shay S, French JL, West J, Haase VH. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36: 1584–1594, 2016. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res 112: 1230–1233, 2013. doi: 10.1161/CIRCRESAHA.112.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J 25: 1922–1933, 2011. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol 302: H1546–H1562, 2012. doi: 10.1152/ajpheart.00944.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai N, Lade J, Leggett K, Yun X, Baksh S, Chau E, Crow MT, Sidhaye V, Wang J, Shimoda LA. The aquaporin 1 C-terminal tail is required for migration and growth of pulmonary arterial myocytes. Am J Respir Cell Mol Biol 50: 1010–1020, 2014. doi: 10.1165/rcmb.2013-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landsberg JW, Yuan JX. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci 19: 44–50, 2004. doi: 10.1152/nips.01457.2003. [DOI] [PubMed] [Google Scholar]
- 73.Leggett K, Maylor J, Undem C, Lai N, Lu W, Schweitzer K, King LS, Myers AC, Sylvester JT, Sidhaye V, Shimoda LA. Hypoxia-induced migration in pulmonary arterial smooth muscle cells requires calcium-dependent upregulation of aquaporin 1. Am J Physiol Lung Cell Mol Physiol 303: L343–L353, 2012. doi: 10.1152/ajplung.00130.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M, Liu Y, Jin F, Sun X, Li Z, Liu Y, Fang P, Shi H, Jiang X. Endothelin-1 induces hypoxia inducible factor 1α expression in pulmonary artery smooth muscle cells. FEBS Lett 586: 3888–3893, 2012. doi: 10.1016/j.febslet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 75.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol 187: 2711–2722, 2011. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 77.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- 79.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 21: 287–298, 2010. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic Biol Med 63: 151–160, 2013. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu X, Murphy TC, Nanes MS, Hart CM. PPARgamma regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-kappaB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luke T, Maylor J, Undem C, Sylvester JT, Shimoda LA. Kinase-dependent activation of voltage-gated Ca2+ channels by ET-1 in pulmonary arterial myocytes during chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 302: L1128–L1139, 2012. doi: 10.1152/ajplung.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo H, Liu B, Zhao L, He J, Li T, Zha L, Li X, Qi Q, Liu Y, Yu Z. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J Am Soc Hypertens 11: 673–683e673, 2017. doi: 10.1016/j.jash.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 84.MacLean MR, Alexander D, Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Morecroft I, Polland K. Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br J Pharmacol 130: 201–204, 2000. doi: 10.1038/sj.bjp.0703314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maclean MR, Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol 661: 309–322, 2010. doi: 10.1007/978-1-60761-500-2_20. [DOI] [PubMed] [Google Scholar]
- 86.Madden JA, Ray DE, Keller PA, Kleinman JG. Ion exchange activity in pulmonary artery smooth muscle cells: the response to hypoxia. Am J Physiol Lung Cell Mol Physiol 280: L264–L271, 2001. doi: 10.1152/ajplung.2001.280.2.L264. [DOI] [PubMed] [Google Scholar]
- 87.Mair KM, MacLean MR, Morecroft I, Dempsie Y, Palmer TM. Novel interactions between the 5-HT transporter, 5-HT1B receptors and Rho kinase in vivo and in pulmonary fibroblasts. Br J Pharmacol 155: 606–616, 2008. doi: 10.1038/bjp.2008.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malczyk M, Veith C, Fuchs B, Hofmann K, Storch U, Schermuly RT, Witzenrath M, Ahlbrecht K, Fecher-Trost C, Flockerzi V, Ghofrani HA, Grimminger F, Seeger W, Gudermann T, Dietrich A, Weissmann N. Classical transient receptor potential channel 1 in hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 188: 1451–1459, 2013. doi: 10.1164/rccm.201307-1252OC. [DOI] [PubMed] [Google Scholar]
- 89.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maylor J, Lu W, Pisarcik S, Walker J, Undem C, Myers A, Shimoda L. Reciprocal regulation of Na+/H+ exchanger isoform 1 (NHE1) and Na+/H+ exchange regulatory factor 1 (NHERF1) in hypoxic pulmonary arterial smooth muscle cells (PASMCs). FASEB J 24: 1023–1024, 2010.19940258 [Google Scholar]
- 91.McLoughlin P, McMurtry I. Counterpoint: Chronic hypoxia-induced pulmonary hypertension does not lead to loss of pulmonary vasculature. J Appl Physiol (1985) 103: 1451–1453, 2007. doi: 10.1152/japplphysiol.00274.2007a. [DOI] [PubMed] [Google Scholar]
- 92.Meoli DF, White RJ. Endothelin-1 induces pulmonary but not aortic smooth muscle cell migration by activating ERK1/2 MAP kinase. Can J Physiol Pharmacol 88: 830–839, 2010. doi: 10.1139/Y10-059. [DOI] [PubMed] [Google Scholar]
- 93.Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest 38: 188–200, 1978. [PubMed] [Google Scholar]
- 94.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 95.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, Weissmann N. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 52: 1033–1042, 2012. doi: 10.1016/j.freeradbiomed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 97.Moinard J, Manier G, Pillet O, Castaing Y. Effect of inhaled nitric oxide on hemodynamics and VA/Q inequalities in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149: 1482–1487, 1994. doi: 10.1164/ajrccm.149.6.8004302. [DOI] [PubMed] [Google Scholar]
- 98.Monzani E, Bazzotti R, Perego C, La Porta CA. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS One 4: e6167, 2009. doi: 10.1371/journal.pone.0006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 100.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Acute hypoxia simultaneously induces the expression of gp91phox and endothelial nitric oxide synthase in the porcine pulmonary artery. Thorax 60: 305–313, 2005. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naeye RL. Children at high altitude: pulmonary and renal abnormalities. Circ Res 16: 33–38, 1965. doi: 10.1161/01.RES.16.1.33. [DOI] [PubMed] [Google Scholar]
- 102.Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 103.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 104.Nashimoto Y, Hayashi T, Kunita I, Nakamasu A, Torisawa YS, Nakayama M, Takigawa-Imamura H, Kotera H, Nishiyama K, Miura T, Yokokawa R. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol 9: 506–518, 2017. doi: 10.1039/C7IB00024C. [DOI] [PubMed] [Google Scholar]
- 105.Nave AH, Mižíková I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadász I, Weissmann N, Seeger W, Brinckmann J, Morty RE. Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34: 1446–1458, 2014. doi: 10.1161/ATVBAHA.114.303534. [DOI] [PubMed] [Google Scholar]
- 106.Newman JH, Holt TN, Cogan JD, Womack B, Phillips JA III, Li C, Kendall Z, Stenmark KR, Thomas MG, Brown RD, Riddle SR, West JD, Hamid R. Increased prevalence of EPAS1 variant in cattle with high-altitude pulmonary hypertension. Nat Commun 6: 6863, 2015. doi: 10.1038/ncomms7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oka M, Morris KG, McMurtry IF. NIP-121 is more effective than nifedipine in acutely reversing chronic pulmonary hypertension. J Appl Physiol (1985) 75: 1075–1080, 1993. doi: 10.1152/jappl.1993.75.3.1075. [DOI] [PubMed] [Google Scholar]
- 109.Oshima K, McLendon JM, Wagner WW Jr, McMurtry IF, Oka M. Chronic hypoxia does not cause wall thickening of intra-acinar pulmonary supernumerary arteries. Physiol Rep 4: e12674, 2016. doi: 10.14814/phy2.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pak O, Scheibe S, Esfandiary A, Gierhardt M, Sydykov A, Logan A, Fysikopoulos A, Veit F, Hecker M, Kroschel F, Quanz K, Erb A, Schäfer K, Fassbinder M, Alebrahimdehkordi N, Ghofrani HA, Schermuly RT, Brandes RP, Seeger W, Murphy MP, Weissmann N, Sommer N. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J 51: 1701024, 2018. doi: 10.1183/13993003.01024-2017. [DOI] [PubMed] [Google Scholar]
- 111.Pegorier S, Campbell GA, Kay AB, Lloyd CM. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-β1 in normal human lung fibroblasts (NHLF). Respir Res 11: 85, 2010. doi: 10.1186/1465-9921-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Penaloza D, Arias-Stella J, Sime F, Recavarren S, Marticorena E. The heart and pulmonary circulation in children at high altitudes: physiological, anatomical, and clinical observations. Pediatrics 34: 568–582, 1964. [PubMed] [Google Scholar]
- 113.Peñaloza D, Sime F. Chronic cor pulmonale due to loss of altitude acclimatization (chronic mountain sickness). Am J Med 50: 728–743, 1971. doi: 10.1016/0002-9343(71)90181-1. [DOI] [PubMed] [Google Scholar]
- 114.Phillips PG, Birnby LM, Narendran A. Hypoxia induces capillary network formation in cultured bovine pulmonary microvessel endothelial cells. Am J Physiol 268: L789–L800, 1995. doi: 10.1152/ajplung.1995.268.5.L789. [DOI] [PubMed] [Google Scholar]
- 115.Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, Semenza GL, Shimoda LA. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am J Physiol Lung Cell Mol Physiol 304: L549–L561, 2013. doi: 10.1152/ajplung.00081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 107: 2037–2044, 2003. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 117.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pryor R, Weaver WF, Blount SG Jr. Electrocardiographic observation of 493 residents living at high altitude (10,150 feet). Am J Cardiol 16: 494–499, 1965. doi: 10.1016/0002-9149(65)90025-1. [DOI] [PubMed] [Google Scholar]
- 119.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quinn DA, Dahlberg CG, Bonventre JP, Scheid CR, Honeyman T, Joseph PM, Thompson BT, Hales CA. The role of Na+/H+ exchange and growth factors in pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 14: 139–145, 1996. doi: 10.1165/ajrcmb.14.2.8630263. [DOI] [PubMed] [Google Scholar]
- 121.Quinn DA, Du HK, Thompson BT, Hales CA. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 157: 1263–1268, 1998. doi: 10.1164/ajrccm.157.4.9704106. [DOI] [PubMed] [Google Scholar]
- 122.Quinn DA, Honeyman TW, Joseph PM, Thompson BT, Hales CA, Scheid CR. Contribution of Na+/H+ exchange to pH regulation in pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 5: 586–591, 1991. doi: 10.1165/ajrcmb/5.6.586. [DOI] [PubMed] [Google Scholar]
- 123.Rabinovitch M, Chesler N, Molthen RC. Point:Counterpoint: Chronic hypoxia-induced pulmonary hypertension does/does not lead to loss of pulmonary vasculature. J Appl Physiol (1985) 103: 1449–1451, 2007. doi: 10.1152/japplphysiol.00274.2007. [DOI] [PubMed] [Google Scholar]
- 124.Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol 236: H818–H827, 1979. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 125.Rabinovitch M, Konstam MA, Gamble WJ, Papanicolaou N, Aronovitz MJ, Treves S, Reid L. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ Res 52: 432–441, 1983. doi: 10.1161/01.RES.52.4.432. [DOI] [PubMed] [Google Scholar]
- 126.Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol (1985) 98: 1092–1100, 2005. doi: 10.1152/japplphysiol.01017.2004. [DOI] [PubMed] [Google Scholar]
- 127.Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L867–L874, 2005. doi: 10.1152/ajplung.00455.2004. [DOI] [PubMed] [Google Scholar]
- 128.Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res 96: 864–872, 2005. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- 129.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, Gassmann M, Seeger W, Hänze J. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: role of hypoxia-inducible transcription factors. FASEB J 16: 1660–1661, 2002. doi: 10.1096/fj.02-0420fje. [DOI] [PubMed] [Google Scholar]
- 131.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434: 786–792, 2005. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 132.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Semenza GL. Pulmonary vascular responses to chronic hypoxia mediated by hypoxia-inducible factor 1. Proc Am Thorac Soc 2: 68–70, 2005. doi: 10.1513/pats.200404-029MS. [DOI] [PubMed] [Google Scholar]
- 134.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res 63: 504–511, 2011. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 135.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep 6: 809–817, 2014. doi: 10.1016/j.celrep.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sheikh AQ, Misra A, Rosas IO, Adams RH, Greif DM. Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med 7: 308ra159, 2015. doi: 10.1126/scitranslmed.aaa9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shimizu T, Fukumoto Y, Tanaka S, Satoh K, Ikeda S, Shimokawa H. Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arterioscler Thromb Vasc Biol 33: 2780–2791, 2013. doi: 10.1161/ATVBAHA.113.301357. [DOI] [PubMed] [Google Scholar]
- 138.Shimoda LA. 55th Bowditch Lecture: Effects of chronic hypoxia on the pulmonary circulation: role of HIF-1. J Appl Physiol (1985) 113: 1343–1352, 2012. doi: 10.1152/japplphysiol.00843.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 140.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 141.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183: 152–156, 2011. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimoda LA, Sham JS, Liu Q, Sylvester JT. Acute and chronic hypoxic pulmonary vasoconstriction: a central role for endothelin-1? Respir Physiol Neurobiol 132: 93–106, 2002. doi: 10.1016/S1569-9048(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 143.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2)]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- 144.Shimokawa H, Sunamura S, Satoh K. RhoA/Rho-Kinase in the Cardiovascular System. Circ Res 118: 352–366, 2016. doi: 10.1161/CIRCRESAHA.115.306532. [DOI] [PubMed] [Google Scholar]
- 145.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20: 343–355, 2009. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 146.Singh I, Kapila CC, Khanna PK, Nanda RB, Rao BD. High-altitude pulmonary oedema. Lancet 1: 229–234, 1965. doi: 10.1016/S0140-6736(65)91520-5. [DOI] [PubMed] [Google Scholar]
- 147.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis. Blood 114: 469–477, 2009. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 149.Stenmark KR, Fasules J, Hyde DM, Voelkel NF, Henson J, Tucker A, Wilson H, Reeves JT. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol (1985) 62: 821–830, 1987. doi: 10.1152/jappl.1987.62.2.821. [DOI] [PubMed] [Google Scholar]
- 150.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res 97: 95–98, 2005. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 151.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 152.Sui GJ, Liu YH, Cheng XS, Anand IS, Harris E, Harris P, Heath D. Subacute infantile mountain sickness. J Pathol 155: 161–170, 1988. doi: 10.1002/path.1711550213. [DOI] [PubMed] [Google Scholar]
- 153.Suki B, Hu Y, Murata N, Imsirovic J, Mondoñedo JR, de Oliveira CLN, Schaible N, Allen PG, Krishnan R, Bartolák-Suki E. A microfluidic chamber-based approach to map the shear moduli of vascular cells and other soft materials. Sci Rep 7: 2305, 2017. doi: 10.1038/s41598-017-02659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary hypertensive rats. Am J Physiol 242: H907–H915, 1982. doi: 10.1152/ajpheart.1982.242.5.H907. [DOI] [PubMed] [Google Scholar]
- 155.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4: 560–580, 2014. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, Wang Z, Gupta A, Zhou T, Sun X, Dash S, Wang Z, Balistrieri A, Zheng Q, Cordery AG, Desai AA, Rischard F, Khalpey Z, Wang J, Black SM, Garcia JGN, Makino A, Yuan JX. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 314: L256–L275, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606, 2010. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tucker A, McMurtry IF, Reeves JT, Alexander AF, Will DH, Grover RF. Lung vascular smooth muscle as a determinant of pulmonary hypertension at high altitude. Am J Physiol 228: 762–767, 1975. doi: 10.1152/ajplegacy.1975.228.3.762. [DOI] [PubMed] [Google Scholar]
- 160.Undem C, Rios EJ, Maylor J, Shimoda LA. Endothelin-1 augments Na+/H+ exchange activity in murine pulmonary arterial smooth muscle cells via Rho kinase. PLoS One 7: e46303, 2012. doi: 10.1371/journal.pone.0046303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veit F, Pak O, Egemnazarov B, Roth M, Kosanovic D, Seimetz M, Sommer N, Ghofrani HA, Seeger W, Grimminger F, Brandes RP, Schermuly RT, Weissmann N. Function of NADPH oxidase 1 in pulmonary arterial smooth muscle cells after monocrotaline-induced pulmonary vascular remodeling. Antioxid Redox Signal 19: 2213–2231, 2013. doi: 10.1089/ars.2012.4904. [DOI] [PubMed] [Google Scholar]
- 162.Veith C, Kraut S, Wilhelm J, Sommer N, Quanz K, Seeger W, Brandes RP, Weissmann N, Schröder K. NADPH oxidase 4 is not involved in hypoxia-induced pulmonary hypertension. Pulm Circ 6: 397–400, 2016. doi: 10.1086/687756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Walker J, Undem C, Yun X, Lade J, Jiang H, Shimoda LA. Role of Rho kinase and Na+/H+ exchange in hypoxia-induced pulmonary arterial smooth muscle cell proliferation and migration. Physiol Rep 4: e12702, 2016. doi: 10.14814/phy2.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, Ayon RJ, Choudhury MS, Ko EA, Wang J, Wang C, Makino A, Yuan JX. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol 305: L154–L164, 2013. doi: 10.1152/ajplung.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J, Fu X, Yang K, Jiang Q, Chen Y, Jia J, Duan X, Wang EW, He J, Ran P, Zhong N, Semenza GL, Lu W. Hypoxia inducible factor-1-dependent up-regulation of BMP4 mediates hypoxia-induced increase of TRPC expression in PASMCs. Cardiovasc Res 107: 108–118, 2015. doi: 10.1093/cvr/cvv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007. doi: 10.1152/ajplung.00141.2007. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 169.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol 288: L1049–L1058, 2005. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z, Chesler NC. Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech Model Mechanobiol 11: 279–289, 2012. doi: 10.1007/s10237-011-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, Lakes RS, Eickhoff JC, Chesler NC. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech Model Mechanobiol 12: 1115–1125, 2013. doi: 10.1007/s10237-012-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxid Redox Signal 18: 1765–1776, 2013. doi: 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L196–L203, 2015. doi: 10.1152/ajplung.00097.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- 175.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294: L309–L318, 2008. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
- 176.Will DH, Alexander AF, Reeves JT, Grover RF. High altitude-induced pulmonary hypertension in normal cattle. Circ Res 10: 172–177, 1962. doi: 10.1161/01.RES.10.2.172. [DOI] [PubMed] [Google Scholar]
- 177.Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ. Redox homeostasis and mitochondrial dynamics. Cell Metab 22: 207–218, 2015. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 178.Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, Prendergast GC, Wilkins MR. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 110: 1423–1434, 2012. doi: 10.1161/CIRCRESAHA.112.264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang X, Lee PJ, Long L, Trembath RC, Morrell NW. BMP4 induces HO-1 via a Smad-independent, p38MAPK-dependent pathway in pulmonary artery myocytes. Am J Respir Cell Mol Biol 37: 598–605, 2007. doi: 10.1165/rcmb.2006-0360OC. [DOI] [PubMed] [Google Scholar]
- 180.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 181.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol 275: L818–L826, 1998. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 182.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696, 1999. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu L, Hales CA. Silencing of sodium-hydrogen exchanger 1 attenuates the proliferation, hypertrophy, and migration of pulmonary artery smooth muscle cells via E2F1. Am J Respir Cell Mol Biol 45: 923–930, 2011. doi: 10.1165/rcmb.2011-0032OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 177: 1276–1284, 2008. doi: 10.1164/rccm.200710-1522OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yun X, Jiang H, Lai N, Wang J, Shimoda LA. Aquaporin 1-mediated changes in pulmonary arterial smooth muscle cell migration and proliferation involve β-catenin. Am J Physiol Lung Cell Mol Physiol 313: L889–L898, 2017. doi: 10.1152/ajplung.00247.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]