Keywords: atopic dermatitis, itch, neurogenic inflammation, pruritus, quality of life
Abstract
Itch is a topic to which everyone can relate. The physiological roles of itch are increasingly understood and appreciated. The pathophysiological consequences of itch impact quality of life as much as pain. These dynamics have led to increasingly deep dives into the mechanisms that underlie and contribute to the sensation of itch. When the prior review on the physiology of itching was published in this journal in 1941, itch was a black box of interest to a small number of neuroscientists and dermatologists. Itch is now appreciated as a complex and colorful Rubik’s cube. Acute and chronic itch are being carefully scratched apart and reassembled by puzzle solvers across the biomedical spectrum. New mediators are being identified. Mechanisms blur boundaries of the circuitry that blend neuroscience and immunology. Measures involve psychophysics and behavioral psychology. The efforts associated with these approaches are positively impacting the care of itchy patients. There is now the potential to markedly alleviate chronic itch, a condition that does not end life, but often ruins it. We review the itch field and provide a current understanding of the pathophysiology of itch. Itch is a disease, not only a symptom of disease.
Scratching an itch can be intensely pleasurable, yet chronic itch is a disease that impacts quality of life to the same extent as pain.
Signals between the environment, skin, immune cells, and afferent nerve fibers funnel into the spinal circuitry and brain to relay the sensation of itch, which promotes scratching.
The mechanisms that underlie itch and opportunities for targeting this unmet need are reviewed.
I. INTRODUCTION
‟Itching is an unpleasant sensation which provokes the desire to scratch.ˮ So began the previous review on the physiology of itching in this journal in 1941, written by the father of investigative dermatology, Stephen Rothman. This definition was penned by Samuel Hafenreffer in 1660, with pruritus, an identical term, in place of itch. The definition continues to appear regularly at the beginning of manuscripts. Why such reinforcement occurs, unique to this topic, is indicative of the fascination that surrounds itch. Scratching an itch can also be pleasurable (269). The poet Ogden Nash wrote that happiness is to have a scratch for every itch. The sensation may be unpleasant, the scratching response rewarding, but patients who suffer from acute or chronic itch, which affects 15% of the population, are miserable.
Rothman’s review was prescient. The complete overlap between areas in which itch and pain were felt was indicative of shared neuronal pathways but could not explain the distinct motor responses of scratching an itch versus withdrawal from a painful stimulus. The triple response of Sir Thomas Lewis, manifest by vasodilatation, flare, edema and associated itch attributed to a ‟H-substanceˮ not necessarily exclusive to histamine, was consistent with mediators aside from histamine. Itch associated with systemic processes including cholestasis and hematological conditions was noted to be distinct from histamine, consistent with current understanding that clinical itches are driven primarily by mediators and pathways that are independent from histamine. The concepts of central inhibitory pathways and central sensitization were introduced.
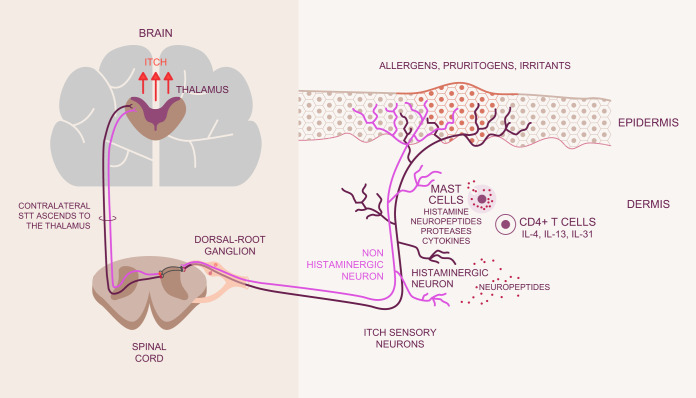
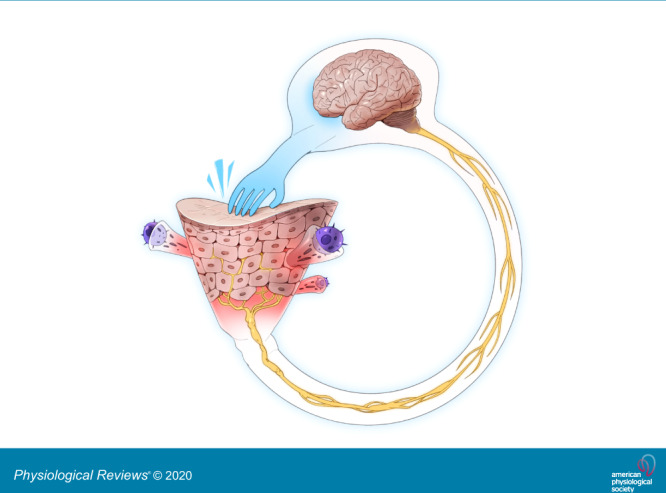
Why do we have the sensation of itch? Conventional thinking has been that the major role of the sensation of itch and the subsequent motor response of scratching is to remove environmental insults from the skin, especially arthropods. Hence, itch is considered a protective sensation and response to the environment. More recent thinking is that in addition, scratching damages the epidermal barrier and facilitates an appropriate physiological cascade associated with sensing the insult (FIGURE 1). This cascade includes multidirectional communication between the nervous and immune systems within the skin, perhaps primarily directed against components of the microbiota, which can evoke itch and pain (FIGURE 1). The pleasure that accompanies scratching may serve to reinforce the motor activity to further ensure neuroimmune communication.
FIGURE 1.
Initiation of itch. Allergens, pruritogens, and irritants are exogenous substances which interface with the skin in acute and chronic itch. Branching terminal fibers of afferent neurons which sense these substances reach the epidermis, the uppermost viable layer of skin immediately below the stratum corneum barrier. The sensory neurons are considered histaminergic or non-histaminergic. Neural activity drives the recruitment of immune cells, including mast cells and CD4+ T cells among others. These cells release mediators that activate cognate receptors on sensory neurons to release neuropeptides to contribute to the itch-scratch cycle. Messages are relayed from the peripheral afferents to their cell bodies in dorsal root or trigeminal ganglia followed by synapsing with second-order neurons in the spinal cord. The thalamus then assists in the interpretation of messages encoding itch. IL, interleukin; STT, spinothalamic tract.
Depending on the insult, such as a contact allergen, or in association with a disease, for example atopic dermatitis or cholestasis, a pathophysiological itch may develop.
Our goal is to provide an understanding of the overall physiology and associated pathophysiology of itch. We focus on neuronal aspects, as a nervous system is needed to have the sensation of itch, as well as the multidirectional connections between the nervous and immune systems that impact itch transmission from the skin into the spinal cord. These areas are the ones most supported by objective data indicative of mechanisms, and it is appreciated that an understanding of central itch circuitry in the mouse is advancing rapidly.
Itch is rarely a pure sensation. Instead, itch typically includes degrees of burning, prickling, and stinging. This delineation is of importance because experimental stimuli as well as clinical conditions manifest such differences, which can overlap with pain. Atopic dermatitis (AD), for which itch is a sine qua non, often has a degree of pain, and Staphylococcus aureus, which is resident in AD skin, can evoke pain and itch. Our peripheral neurosensory-immune system together with central circuitry is able to differentiate between itch and pain. This observation leads to the long-standing question of whether or not there is specific neuronal coding for itch. An extension of this topic is whether the sensation of itch is restricted to skin or if other organs get itchy. We are accustomed to associating pain with visceral organs, but there is a developing consensus that processes associated with removal of material from any epithelia, be it the bladder, gut, or respiratory system, including cough, may be equivalent to itch (205).
A plethora of discoveries is shaping our understanding and helping us to understand itch. A critical caveat of ongoing research is the need to use experimental species and the challenge to translate findings from mice, canines, and non-human primates into the human condition. Scratching behavior in animals is considered to relate to human itch, although there is no objective method of asking if a non-human itches, nor is there an objective measure of human itch. If itch depends on the ability to scratch, then from an evolutionary standpoint, it can be argued that itch developed in conjunction with receptors that first appear in tetrapods (29). However, if scratching is considered a behavioral response manifest by rubbing following injection of a channel activator, then fish may have the sensation of itch (80).
In the clinic, itch is considered either acute or chronic. Acute itch is defined as an itch that lasts less than 6 wk, whereas chronic itch persists for 6 wk or longer and is experienced by 15% of the population. A variety of animal models are considered proxies for chronic itch by some investigators, but given the brevity of these models, we have difficulty making that connection. Information is lost in translation between models and human physiology, modulating the perspectives between the bench and bedside, while promising findings in mouse models (104) have often failed in the clinic (28). At the same time, appreciation of the impact of itch together with the fascinating physiology underlying this sensation is driving therapeutic success and improved quality of life for those who itch.
II. GLOSSARY
A. Types of Itches
Acute itch: itch lasting less than 6 wk.
Chronic itch: itch lasting 6 wk or longer (under causes, note that it can be a chronic acute mechanism).
Neurogenic itch: induced by mediators but in the absence of neural damage. As the mediators of neurogenic itch are defined, it is likely that neurogenic itch will fall under pruritoceptive itch.
Neuropathic itch: associated with damaged neurons, e.g., post-herpetic neuralgic itch or small fiber neuropathy.
Pruritoceptive itch: itch associated with pruritogen activation of sensory fibers.
Psychogenic itch: having a psychosomatic or psychiatric origin, e.g., delusions of parasitosis.
B. Selected Definitions and Terminology
Allodynia: pain or itch from what is normally a nonpainful or itchy stimulus. It is associated with central sensitization.
Alloknesis: triggering of itch from what is normally a nonpruritogenic stimulus includes itch.
Atmoknesis: itch that occurs when skin is exposed to air, such as when clothing is removed.
Central sensitization: increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input. It is often associated with peripheral injury or inflammation, such that persistent stimulation of nociceptors or pruriceptors leads to increased excitability of central pathways, decreased activity of inhibitory pathways, and chronic pain or itch.
Dysesthesia: an abnormal sensation, often including some components of burning, itch, pain, pins and needles, and touch. It can occur anywhere on the skin but is often associated with the scalp.
Neurogenic inflammation: inflammation associated with the release of mediators, particularly substance P (SP) or calcitonin gene-related peptide (CGRP), from peripheral afferent neurons, which then impact the immune system.
Sensitive skin: a widely used term that has defied an objective definition or pathophysiology. It can occur in the setting of specific diagnoses, including atopic dermatitis, less well-defined conditions such as fibromyalgia, or it can refer to itching, burning, pricking sensations, or dysesthesias of unknown cause.
III. SENSORY NEURONS
Itch is sensed by cutaneous nerve fibers called pruriceptors. These primary afferent fibers serve as antennae and constantly sample the environment of the skin to detect and respond to cues. Signals are propagated along neural pathways to the spinal cord and brain for interpretation and response. Analogous to itch, algogenic (painful) stimuli, heat, and cold are sensed by nociceptors. It is possible that all pruriceptors function as nociceptors in humans but whether the reverse is true remains to be clarified. There are data in mice that support the concept of a small number of uniquely pruriceptive fibers. Nociceptors are categorized into two biophysically distinct major afferent classes, thinly myelinated Aδ and unmyelinated C-fibers. Aδ are 2–5 µm in diameter and have a conduction velocity of up to 8 m/s. C-fibers average 0.2–1.5 µm in diameter and have a conduction velocity less than 2 m/s. Sensory nerves do not function in isolation but are part of an interactive milieu. This milieu includes a plethora of mediators generated from the sensory neurons themselves, neighboring cells, the barrier, and the microbiome. These mediators range in size from ions to proteins, include pH, and may include localized cell or tissue tension. Each neuronal population is heterogeneous and polymodal, meaning that it has more than one detection property, often associated with specific ion channels that detect, for example, heat, cold, acid, and whether they respond to cellular stretch or a light touch from a cellular probe, being mechanically sensitive, or insensitive, and the yet ill-defined milieu that is generated by tissue inflammation. These neurons are pseudounipolar, with cell bodies residing in dorsal root (or trigeminal) ganglia. The peripheral branch of the axon extends to the skin while the central branch synapses with first-order neurons in the dorsal horn of the spinal cord. These proceed to interact with second-order neurons that comprise excitatory and inhibitory circuits that project to the parabrachial nucleus of the thalamus for processing, interpretation in additional brain regions, and a resultant motor response of scratching. C-fibers, and to a lesser extent Aδ-fibers, are responsible for itch sensing. It is not yet known if the recently described specialized glial cells that form a meshlike network near the dermal-epidermal junction and initiate the sensation of pain contribute to the sensation of itch (1).
Itch fibers comprise fewer than 10% of the C-fibers in skin. Diversity in the C-fiber population has long been recognized, is manifest in several ways, is dynamic, and evolves postnatally in mice and possibly in humans. Rather than being strictly absolute, C-fibers can be distinguished by markers and by function, often illustrated via Venn diagrams. Single-cell RNA sequencing has led to even further subdivisions, but whether these are static is not known. ‟Silentˮ nociceptors constituting almost a quarter of C-fibers in human skin have been described (246). These mechanically insensitive but heat-responsive C-afferents or silent nociceptors biophysically transition to mechanically responding fibers in the setting of injury. A conventional distinction between C-fibers, although not absolute, is by peptidergic versus nonpeptidergic markers. Peptidergic markers include SP and CGRP which are released by these fibers. They also express the nerve growth factor (NGF) receptor and tropomyosin (or tyrosine) receptor kinase A (TrkA) and somatostatin (SOM). Nonpeptidergic markers include the c-Ret neurotrophin receptor as well as artemin. In general, the c-Ret+ neurons express the isolectin IB4, a lectin that binds to nonpeptidergic neurons.
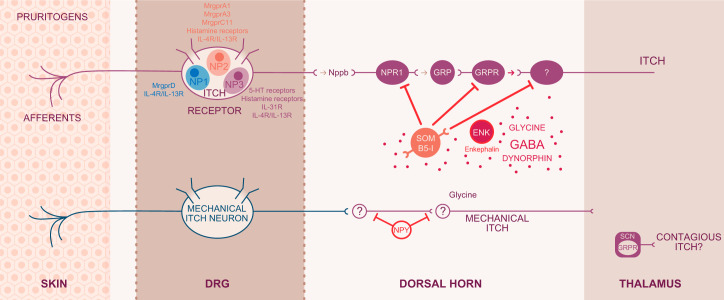
With an unbiased RNA profiling analysis that combined neuronal information with functional impact, four nonpeptidergic (NP 1–4) itch-specific classes were identified. NP1 is considered to mediate neuropathic pain and itch. NP2 and NP3 are also involved in itch sensation with NP3 responsible for the transduction of inflammatory itch (293). These data suggest that there is a neuronal segregation between chemical and acutely induced and inflammatory-mediated itch (FIGURE 2).
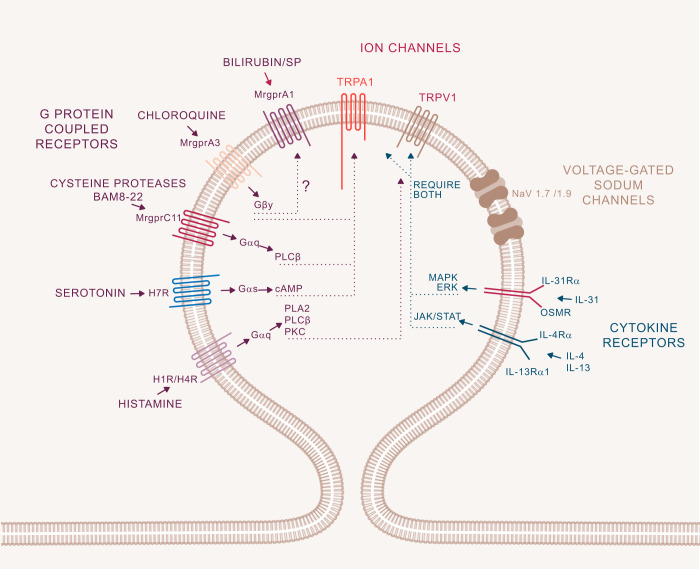
FIGURE 2.
Itch circuitry from the periphery to the brain. Itch-related G protein-coupled receptors (GPCRs) and cytokine receptors on sensory neurons from the skin to the spinal cord are categorized as NP1, NP2, and NP3 neurons. Some receptors are expressed in all NP1–3 neurons, whereas MrgprA3, an itch specific marker, is present only in the NP2 population. The natriuretic peptide Nppb acts on its receptor NPR1 to link transmission between dorsal root ganglion (DRG) and spinal neurons. Complex inhibitory and excitatory interneuron activity modulates the signal that will be projected to the brain. Gastrin releasing peptide (GRP) and GRP receptor (GRPR) are each key to excitatory itch circuits in the spinal cord. Inhibitory basic helix loop helix 5 interneurons (B5-I) and somatostatin (SOM) interneurons have inhibitory function. Loss of inhibition either by neuronal depletion of B5-I interneurons or the inhibitory neurotransmitters GABA, glycine, or dynorphin results in intensified itch. This pattern most likely occurs in chronic itch conditions. Enkephalinergic interneurons are important in the gate control between intense pain and itch sensation. Mechanical itch is transmitted through a different, so far unclassified, neuron population and second-order neurons in the spinal cord. The circuitry does not involve excitatory GRP but includes the inhibitory neuropeptide NPY (neuropeptide Y). Contagious itch in mice is relayed through GRPR+ neurons in the suprachiasmatic nucleus. [Modified from Dong and Dong (71), with permission from Elsevier.]
Another, and particularly useful discriminator between itch sensory neuron function, is between fibers that are either mechanosensitive or mechanoinsensitive (151). The two prototypic substances that evoke itch in human subjects are histamine and spicules of the bean plant Mucuna pruriens. The plant and its spicules are colloquially referred to as cowhage and informally known as itching powder. The sensations elicited by cowhage were known even to Rothman to be independent of histamine. Electrophysiological recordings from human and mouse primary afferents revealed that histamine acts on a subset of mechanoinsensitive C-fibers that also respond to heat and capsaicin. In contrast, cowhage activates mechanosensitive fibers in humans. Possible differences of signal intensity carried via mechano-sensitive or -insensitive neurons may be accounted for by the diameter of the respective cutaneous receptive fields. A cutaneous receptive field is defined as the area within the skin activated by a stimulus and concurrently evoking action potential (132). The activation of the receptive fields by histamine or cowhage spicules seem to differ from each other, which may be explained by distinct and overlapping C-fibers that convey different stimuli, the pruritogens themselves, or the mode of application, spicules for cowhage, and injection for histamine.
A. Transmission of Messages
All primary afferents project into the dorsal horn of the spinal cord. Peptidergic and nonpeptidergic neurons target distinct areas of the superficial laminae in the dorsal horn. The peptidergic C-afferents project into the outer region of lamina I and lamina II, whereas the nonpeptidergic subset targets the inner region of lamina II. This segregation within the spinal cord maintains functional distinction of the C-afferents. Aδ nociceptors target lamina I and deeper lamina V of the spinal cord. Low-threshold C-mechanoreceptors venture to the inner ventral part of lamina II where protein kinase C (PKC)-γ-expressing interneurons are concentrated. Deeper dorsal horn contains the wide-range dynamic projection neurons that respond to innocuous and nociceptive stimuli. Projection neurons that carry the messages beyond the spinal cord comprise 2–5% of the neuronal population, which mostly projects to the parabrachial (PB) nucleus of the dorsolateral brain stem. Within this small population, 80% express the neurokinin 1 receptor (NK1R) and likely connect with SP+/GABA– interneurons (46, 180), a neuronal population considered a fine-tuning gate of messages. This neuronal circuit is (68, 283) implicated in coping behavior associated with sustained pain and itch (120).
The above-mentioned interneurons are the gate-keepers of neuronal activity that modulate afferent input carried to the projection neurons. Morphologically, interneurons show heterogeneous features from islet, central, radial, and vertical cells (41, 99). Readers are referred to excellent articles that discuss interneuron morphology and function in detail (317, 318). Biochemical characterization somewhat simplified the interneurons into the excitatory interneurons which express vesicular glutamate transporter VGLUT2 (20, 243) and neurochemical markers including PKC-γ, neurotensin, and somatostatin, which also serve to define certain subpopulations of excitatory interneurons (228, 229, 282). The testicular orphan nuclear receptor 4 (TR4) is part of the excitatory interneuron circuitry involved in the transmission of itch and pain (298). Selective deletion of TR4 in the central nervous system resulted in loss of excitatory interneurons in the superficial dorsal horn. Phenotypically, TR4 mutant mice had increased mechanical responses but reduced responses to heat and lack of responses to various pruritogens. These findings favor the theory of converging itch and pain circuitry in the spinal cord. Gastrin releasing peptide (GRP), considered an itch-specific neurotransmitter and its corresponding receptor GRPR, are in the excitatory interneuron population (271, 272, 298) and may label an itch-specific circuit, at least in mice. Ablation of GRPR+ interneurons affected itch behavior but not pain responses, suggesting a distinction between pain and itch circuitry. Peptidergic C-fiber input to the excitatory interneurons appears to engage the neurotransmitter natriuretic polypetide b (Nppb) that is present in the TRPV1+ afferent neuronal subset (186). Initially, GRP was identified as an itch-specific neuropeptide released upon activation of peptidergic C-afferents by pruritogens which led to activation of its cognate receptor GRPR in the spinal cord (271, 272). The relative roles of Nppb and GRP in the periphery have led to a dynamic discussion in field of itch research. Currently, the integrated concept is that Nppb is released from peripheral afferents and induces GRP to be transmitted from spinal cord interneurons. In accordance with its itch-specific role, Nppb is expressed in the TRPV1+ subset that also coexpressed the members of the itch-associated mas-related G protein-coupled receptor family members MrgprA3 and MrgprC11 (170, 171). Hence, the neurons that express the receptor for Nppb, namely, natriuretic peptide receptor (Npr), are the first level of spinal cord neurons responsible for the neural itch circuitry. In summary, the excitatory itch circuit includes the peripheral release of Nppb, spinal GRP and GRPR activation in spinal interneurons that are TR4+. Complementary to the TR4+ interneurons, inhibitory interneurons express the transcription factor Bhlhb5. Loss of Bhlhb5 in basic helix loop helix 5 interneurons (B5-I) inhibitory interneurons results in excessive scratching without alteration of pain responses (229). The majority of inhibitory Bhlhb5 interneurons produce the neurotransmitter γ-aminobutyric acid (GABA), which is diminished in chronic conditions of itch and pain (FIGURE 2).
IV. LABELED LINE FOR ITCH IN MICE
In mice, acute itch is transmitted via pruritoceptive neurons that respond to chemical stimuli. These neurons express TRPV1+ and MrgprA3+ and fall predominately into the NP2 and to a small extent the NP3 populations (FIGURE 2). Acute and chronic itch are both relayed through this neuronal populations (71) consistent with a specific “itch” labeled line in this species. Whether the labeled line transmission continues in pathological conditions has not been addressed. In general, itch-specific receptors discussed above are all present in the NP2/NP3 population, including those for interleukin (IL)-31, IL-4, and IL-13 (FIGURE 2). Capsaicin activates all TRPV1+ neurons, including the itch-specific MrgprA3+/TRPV1+ neurons. The NP1 population, which is positive for MrgprD, is polymodal, responding to itch, pain, mechanical, and thermal stimuli. That this population does not show overlap with NP2 or NP3 suggests the existence of an additional line that imparts itch. In the central nervous system, as discussed above, complex circuits encode itch. Briefly, this circuitry includes Vglut2+ neurons. Itch signaling is associated with the release of Nppb, at least in the NP3 population. Since Nppb was not detected in NP2 itch neurons, NP2 and NP3 populations engage separate or overlapping circuitry for itch. In NP3 neurons, Nppb engages with its receptor NPRA followed by communication to GRPR via GRP release in the spinal cord (FIGURE 2). Mechanical itch is most likely transmitted through low-threshold mechanoreceptors in which skin structures such as Merkel cells might be involved (FIGURE 2). The mechanical itch-sensitive neurons have been shown to connect to spinal NPY+ inhibitory interneurons that receive low-threshold mechanical input. The circuitry of contagious itch requires the presence of GRPR+ neurons as discussed later (see sect. XVII).
V. NEUROANATOMY
Primary sensory neurons absorb information from the periphery to inform the spinal cord where somatosensory processing continues. In general, the circuitry is spatially organized, with nociceptive and thermosensitive afferents entering the superficial spinal cord whereas the cutaneous and proprioceptive afferents target the ventral regions of the spinal cord. Here, attention is directed towards embryological development and organization in the spinal cord as related primarily to pruritoceptive primary afferents and spinal cord segments responsible for transmission and modulation of itch.
A. Development of the Spinal Anatomy
The spinal cord is generated from the vertebrate neural plate that also gives rise to neural crest cells from which most neurons and glia originate. The neural plate thickens and invaginates, resulting in the rostro-caudal neural tube formation. Expansion of the neural tube in the head region leads to formation of the brain, whereas more caudally the same neural tube expansion forms the spinal cord. The neural tube closes along the entire body axis and neural crest cells transition from epithelial to mesenchymal cells migrating into distal parts of the embryo (159). The peripheral nervous system (PNS) is formed by cranial neural crest cells and ectodermal placodes (30). Neural crest cells give rise to dorsal root ganglia (DRG) neurons and sympathetic ganglia of the PNS. The DRG neurons form bilaterally along the developing spinal cord to innervate the skin and various organs. Remaining neural tube elements generate the truncal spinal cord which consists of only a single cell layer (10). These cells of the epithelium divide and become progenitor cells for the neural and glial cells within the spinal cord which subsequently undergo cell differentiation to form the grey and white matter of the spinal cord.
1. Laminar organization of the spinal cord
At different positions of the spinal cord, distinct neuronal types are present along the dorsal-ventral axis, which results in the laminar organization of specific neurons according to their functional and physiological properties (41, 99). Laminar segregation of the neuronal functions within the spinal cord segregate cells of motor function more ventrally while cells mediating sensory information are present in the dorsal horn. Lamina I–X in the adult spinal cord are determined by cytoarchitectonic parameters. Broadly, pain, thermosensitive afferents, so called C- and Aδ-fibers from the dorsal root ganglion, innervate the lamina I–II, touch-sensitive afferents enter the lamina II inner (i) to lamina V, and lastly, proprioceptive afferents such as Aβ and Aδ target the more ventral spinal cord including the motor neuron (MN) region. A different labeling for Rexed laminae regions are as following: marginal layer (ML) for lamina I, substantia gelatinosa (SG) for lamina II, nucleus propius (NP) for lamina III–V, and MN for lamina IX (281). Various transcription factors (TF) define during development the cytoarchitecture and diversity of neurons in the spinal cord. The itch-specific C-pruritoceptor fibers represent a subpopulation of the C-nociceptors.
2. Spinal transcription factors and neuronal cell development
The distinct combination and interplay of signaling pathways with homeodomain and basic helix-loop-helix (bHLH) transcription factors (229) regulate neurogenesis and define cellular identity in a tightly timed choreography during development of the spinal cord (150). In the ventral neural tube homeodomain, TFs are the drivers of neuronal fate mapping during development (150). The expression and presence of TFs during developmental stages is dynamic and often transient, i.e., bHLH factors ASCL1 and ATOH1 and NEUROG1 appear in proliferating progenitors but disappear during differentiation and once cells become postmitotic. The lineage of pruritoceptors in the spinal cord is presumably dependent on the proneural NEUROG1 which has been described for their DRG development (175). In the spinal cord, the same proneural lineage might represent the precursors of itch-specific neurons. For itch circuits, the bhlhb5 TF is developmentally crucial in determining the itch-inhibitory interneurons (229). The transcription factors PAX2 and TLX3 are postmitotic and remain in postnatal stages and are used as lineage markers for neuron populations. A recent publication suggested that most interneurons that receive pruritoceptive input lack PAX2 labeling (100). Sonic hedgehog is a morphogen that is crucial for the patterning of the dorsoventral axis of the spinal cord by either activating or repressing TFs (43, 97). Notch signaling maintains cell proliferation while bHLH influences cell differentiation. A concentration gradient of sonic hedgehog regulates the TF expression which is later cross-regulated by the bone morphogenetic proteins (BMP) and WNT signaling for the generation of the dorsal cell types (79, 241). BMP and WNT are instrumental in the generation of dorsal interneurons. The rostrocaudal axis is patterned and positioned via the interplay of graded concentrations of fibroblast growth factor (FGF), retinoic acid (RA), and transforming growth factor (TGF)-β member GDF11 which regulate the expression of homeobox (HOX) TFs in progenitor and postmitotic cells. Detailed discussions of the developmental fate and signaling pathways influencing the neuronal map during development are available (45, 128, 161, 187, 292). Terminal neuronal phenotypes are regulated by the expression of transcription factors such as LHX2 and LHX9 which also promote axonal guidance. Transiently expressed TFs such as bHLH members ATOH1, PTF1A, and ASCL1 directly influence gene regulation for the terminal fate of neuronal phenotypes (37, 236). GABAergic neurons, enzymes, and inhibitory neuron circuits, for example, are regulated by PTFA1 (37). These inhibitory neurons are fundamental to the processing of itch signals in the developed spinal cord. Neurons derived from the dorsal and ventral horns remain in their original segments although the laminar structure is modified as the spinal cord neuronal map is finalized.
VI. HUMAN VERSUS MOUSE NEURONS
Comparisons between mouse and human neurons have now been made with respect to the peptidergic population of nociceptors (231). Given that pruritoceptive neurons form a small subset within the nociceptors, it may be reasonable to consider that the similarities and differences are transferable to the itch-specific population. Somata size and neurochemical characterization by TrkA, a small diameter neuron marker, as well as TrkB and TrkC which label larger diameter neurons were similar between mouse and human. In contrast, 50% of human neurons express TrkA+ and Ret during adulthood, whereas the expression profile for Ret and TrkA undergoes developmental changes in mice. Additionally, human TrkA+ neurons are colabeled with TRPV1. Perhaps these double positive neurons have an evolutionary advantage, to provide protection coverage during heat or inflammation. It is speculated that the TrkA/TRPV1 neuron cluster responds to acute and highly localized painful stimuli. Perhaps a small subset of these double positive neurons is responsible for itch transmission in humans. Human and mouse DRG neurons differ in their expression profile and number of positive neurons responsible for the transmission of itch and pain.
VII. CIRCUITRY AND GATING OF ITCH
The circuitry that controls itch transmission encompasses a dense network of neurons within the superficial dorsal horn, which is innervated by C- and Aδ-fibers. Noxious and pruritic stimuli are processed mostly in the superficial dorsal horn, whereas deeper dorsal horn neurons receive nociceptive and pruritic input via polysynaptic innervation. Interplay between inhibitory and excitatory interneurons sets the tone for the spinal input/output and sensitivity of the message. The interneurons are defined by their neurochemical and neuropeptidergic signatures (281), including the expression of marker molecules SOM, neuropeptide Y (NPY), and parvalbumin (PV) (317, 318). Generally, the gross differentiation of interneurons into excitatory or inhibitory subsets is according to neurochemical and neuropeptidergic characterization. For example, SOM+ interneurons excite neurons in the mechanical pain circuit by two distinct mechanisms: 1) the superficial SOM+ interneurons receive monosynaptic Aδ- and C-fiber input to process acute mechanical pain, and 2) the second SOM+ population in the deeper layers of the spinal cord receives polysynaptic input that is modulated by intermediate inhibitory interneurons. These are engaged by Aβ afferents, which are responsible for the transmission of nonpainful mechanosensation. For this reason, activation of SOM+ interneurons directly results in spontaneous pain and decreased mechanical pain thresholds (58, 73). Additionally, SOM+ interneurons broadly overlap with the expression of excitatory mediators such as vesicular glutamate transporter 3 (VGluT3) and calretinin (CR) which are known to amplify nociceptive messaging (257). In contrast to the function of the second SOM+ population, inhibitory interneurons in the superficial dorsal horn are essential for controlling sensitivity to painful and itchy stimuli. Approximately 40% of interneurons located in lamina I–III contain the inhibitory neurotransmitter GABA, and defined as GABAergic. In a subpopulation, the GABAergic neurons coexpress, and most likely co-release glycine, the other major inhibitory neurotransmitter. Within the deeper lamina of the spinal cord, GABAergic interneurons are key factors in the Gate Control Theory (183).
The Gate Control Theory introduced by (183) explains the mechanism of control mediated by nociceptive and non-nociceptive neuronal input. Lifting of this control leads to peripheral and central sensitization processes seen in inflammation and pathological conditions of itch and pain. The theory provides that non-nociceptive large-diameter neurons “close” the gate by activating interneurons in the spinal cord which in turn “inhibit the activation of projection neurons as crucial neuronal connectors to the supraspinal nervous system” (41, 99). Noxious input is transmitted by the nociceptors which are mostly peptidergic or nonpeptidergic, unmyelinated, slow conducting C-fiber afferents and by a subset of myelinated Aδ-fibers. Activation of the nociceptors “opens” the gate and excites the projection neurons and “turns off” the inhibitory interneurons. Essential players in the Gate Control Theory are morphologically and neurochemically diverse inhibitory interneurons that release inhibitory neurotransmitters to modulate pain messages (41, 99). It has long been acknowledged that loss of inhibitory circuitry due to cellular and molecular changes results in chronic pain and amplification of pain signals to the brain. More recently, inhibitory interneurons were reported to also control itch messages (58, 73). One of the major neurotransmitters is GABA, which controls the sensory gating of itch and pain transmission in the spinal cord and marks the population of inhibitory interneurons (FIGURE 2). Besides GABA, inhibitory interneurons express NPY, dynorphin (DYN), and galanin (155, 301). A subset of DYN+ interneurons which also express glycine+ and GABA+ might modulate the signal transmission by co-release of these neurotransmitters. However, it is unclear if these neurotransmitters are differentially employed for the inhibition of itch and pain messages.
The role of GABA in itch transmission has gained attention in the last few years. Briefly, GABA signals via two distinct types of receptors. GABA-A receptors are ligand-gated ion channels that exist in pentameric structures. These also serve as receptors for benzodiazepines and ethanol. GABA-B receptors are metabotropic glutamate receptors and belong to the group C family of G protein-coupled receptors (GPCRs) (39, 40). The initial work on the role of GABA and glycine in itch circuitry suggested that strychnine, a glycine-receptor antagonist, was more effective than GABA receptor antagonists in disinhibiting the spontaneous firing in the paws of itchy mice (6). A different approach suggests GABA as a major inhibitory transmitter in a mouse model of neuropathic itch as well as inflammatory chronic itch. With a cellular approach in which medial ganglionic eminence (MGE) cells of the forebrain are transplanted into the mouse spinal cord, the group tested whether rescue of GABAergic control impacted different itch phenotypes. In bhlhb5 mutant mice, MGE transplantation of GABAergic cells resulted in improvement of neuropathic itch (42). When tested in IL-31TG mice, the MGE-GABAergic cell transplants also successfully rescued the GABAergic tone and subsequently reduced the phenotypical severe itch (54).
The above-mentioned bhlhb5+ interneurons (B5-I) are crucial in the control of itch. B5-I neurons receive input from neurons that are activated by itch counterstimuli such as menthol and capsaicin and act rather anti-pruritic. B5-I neurons inhibit the excitation of the itch specific GRPR+ interneurons (discussed further below), which are activated by pruritogen-specific circuitry. Dynorphin, a kappa-opioid agonist, marks the B5-I interneurons (139). Dynorphin might be the neurotransmitter that blocks itch mediated by chemical counterstimuli, whereas GABA might be relevant in blocking itch counterstimuli associated with scratching (mechanical input).
In terms of excitatory circuitry specific for itch, the identification of GRP has gained attention. The recent attention to GRP builds on work from Alan Cowan who demonstrated in 1983 that bombesin, a peptide with tight sequence homology to GRP, caused scratching when injected intrathecally (96). GRP has been reported to be expressed in peptidergic DRGs, released upon pruritic stimuli, and then communicates via GRPR in the spinal cord (271, 272). GRP was thus identified as the first itch-specific neuropeptide engaged in the transmission from the periphery to the central nervous system. The details of this role have been questioned (259). Newer studies describe the release of Nppb from DRG neurons which induces GRP circuitry in the spinal cord in response to multiple pruritogens (186). Moreover, the GRP+ neurons are presumably excitatory interneurons. In summary, these findings led to the current modality-specific circuit from Nppb, Nppb-receptor (Npra), GRP, and finally GRPR. In a different study, GRP-positive interneurons were found to receive noxious input and gate nociceptive information in the superficial dorsal horn. However, intense nociceptive stimulation of the GRP+ interneurons recruits enkephalinergic interneurons which in turn results in inhibition of the intense nociception (270). A “leaky gate model” was described in which GRP+ interneurons code itch but also receive nociceptive input and are thus strongly activated by intense pain, triggering enkephalin release which in turn inhibits pain.
Insight into the functional segregation of peripherally and spinally expressed somatostatin uncovered the role of SOM in itch modulation (119). It was demonstrated that optogenetic stimulation of SOM+ sensory neurons was sufficient to evoke itch. In addition, SOM in combination with Nppb or GRP potentiated the itch induced by pruritogens. Itch induced by intrathecal SOM was attenuated by the release of DYN from B5-I interneurons. This finding led to the suggestion that itch induced by SOM was mediated via inhibition of DYN+ neurons and subsequent disinhibition of GRPR+ neurons. Additionally, SOM released by primary afferents suppresses pain, leading to the possibility that at least SOM-modulated forms of itch might also inhibit pain, extending the concept of somatosensory regulation by counterstimuli.
VIII. GLIA NEURON INTERACTION
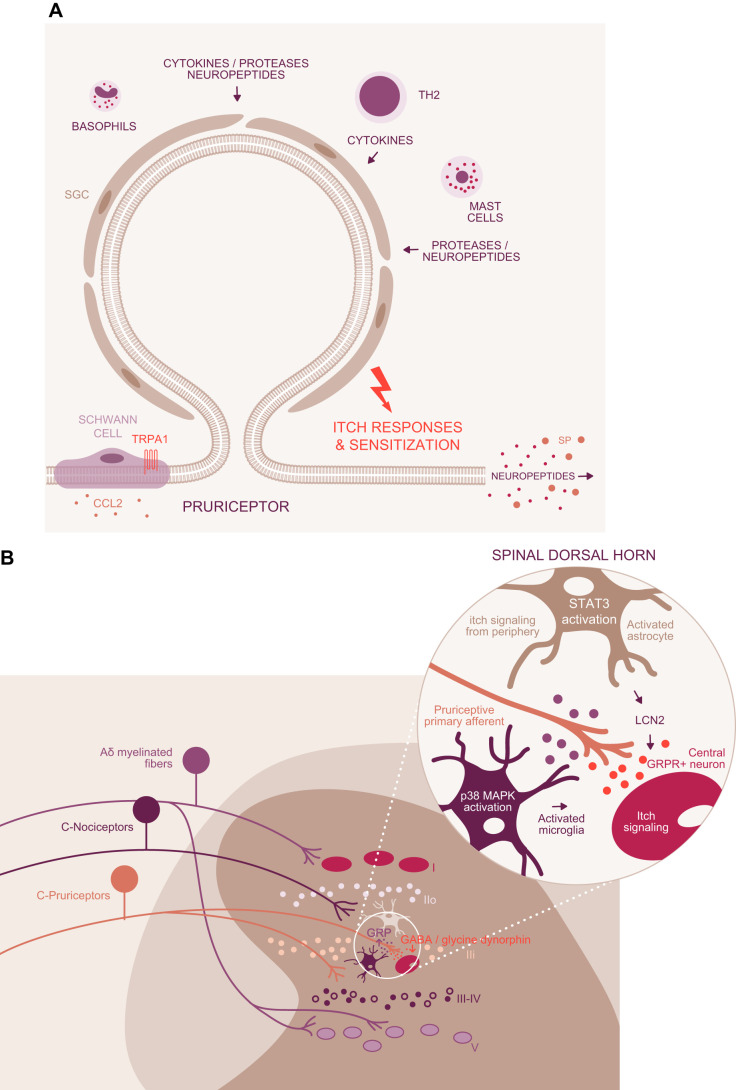
Glia are implicated in chronic itch, consistent with their involvement in inflammatory and neuropathic pain (13, 286–288). Glia, from the Greek, meaning glue, were discovered by Rudolph Virchow, the German anatomist. Virchow suggested that glia provided a matrix for neurons to be embedded in the brain. Glia have gained attention as important modulators and communicators within the nervous system in healthy and diseased tissue. In the periphery, Schwann cells, or neurilemma, produce myelin sheets around neuronal axons. Schwann glial cells (SGC) express transient receptor potential (TRP) channels and modulate neurogenic inflammation, pain, and eventually itch. Schwann cells express TRPA1, which is linked to neuroinflammation (146). Silencing of TRPA1 in nociceptors yielded mechanical allodynia attenuation without influencing microglia infiltration, whereas TRPA1 silenced in SGC resulted in reduction of allodynia and signs of neuroinflammation. The underlining mechanism of SGC-expressed TRPA1 is speculated to occur via CCL2 release, which serves as a chemoattractant for macrophages. SGC-specific TRPA1 may function separately from TRPA1 on itch-specific neurons. Further studies are required to detail the impact of SGC on inflammatory and chronic itch (FIGURE 3A). Such studies may be of particular importance in light of the identification of a mesh network comprised of specialized cutaneous Schwann-glial cells that are essential for sensing noxious (1).
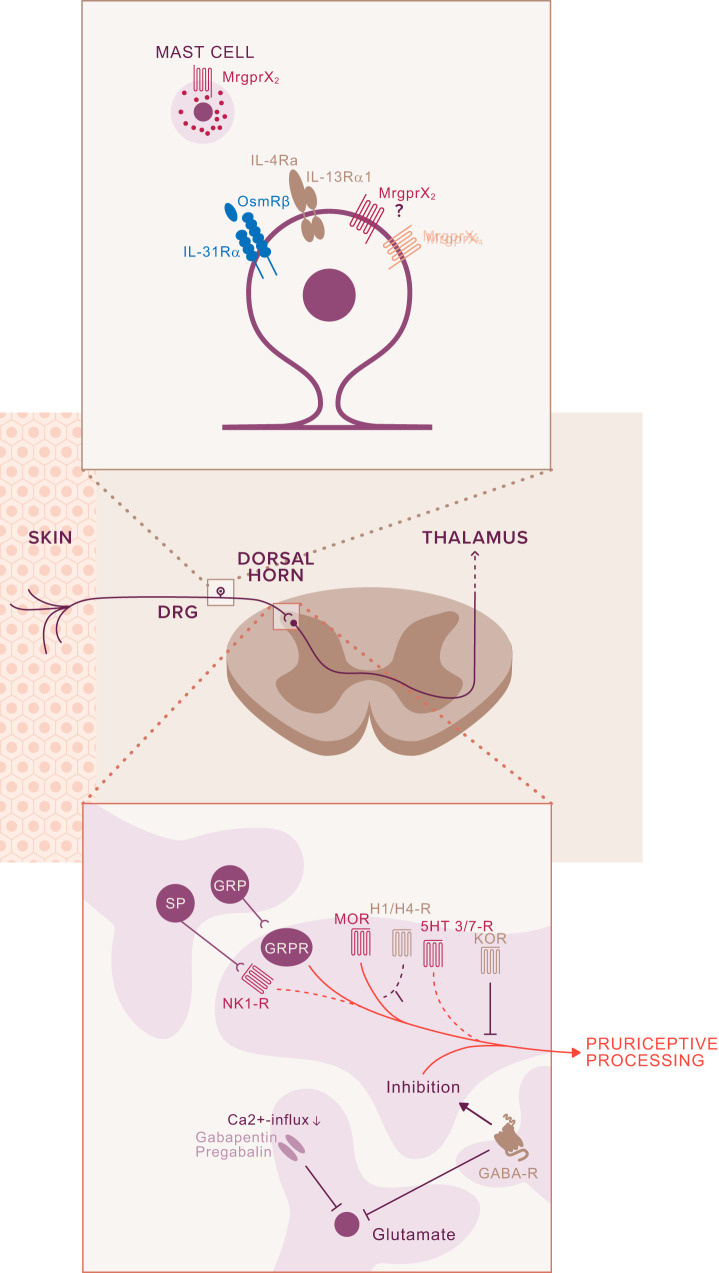
FIGURE 3.
Glia-neuron interaction. A: in the periphery, Schwann cells are thought to modulate itch via TRPA1 channels and the release of CCL2. While expressed in neurons and Schwann glial cells (SGC), silencing of TRPA1 in the latter resulted in reduction of sensitization and signs of inflammation. B: spinal cord glia comprises microglia, astrocytes, and oligodendrocytes. Pruriceptors, a subpopulation of nociceptors, target lamina II inner (IIi). Activated microglia as detected by increased p38 mitogen-activated protein kinase (MAPK) signaling (323) might modulate the pruriceptors and the gastrin releasing peptide receptor (GRPR) neurons (itch signaling second-order neurons). The close proximity of GRPR and gastrin releasing peptide (GRP) in spinal cord neurons enables direct interaction. Itch signaling and amplification of the itch signal might result in a decrease of the inhibitory neurotransmitters (GABA, dynorphin, glycine) and simultaneously increase the excitatory activity via GRP. Activated astrocytes (astrogliosis), detected by increased signal transducers and activators of transcription 3 (STAT3) immunolabeling, release lipocalin 2 (LCN2) which plays a major role in itch progression and maintenance of chronic itch.
Microglia, astrocytes, and oligodendrocytes are the neuroglia in the central nervous system (232). As with pain, activation of central glial cells has been reported to contribute to itch in Nc/Nga mice, a mouse strain available in Japan that is prone to develop itch under non-pathogen-free housing conditions (252). Astrogliosis, an abundance of reactive astrocytes, was a feature involved in chronic itch. To explore the hypothesis of glial contribution to chronic itch, morphological changes of astrocytes have been observed over time. As compared with healthy, non-itchy mice, the astrocytes in the itchy mice were characterized by enlarged bodies and aberrant arborized processes, a sign of astrogliosis.
Lipocalin (LCN), akin to some other glial transmitters such as ATP, is linked to neuronal inflammation and in the modulation of neuronal excitability. In addition, intrathecal co-injection of LCN2 together with GRP enhanced GRP-elicited itch, suggesting that LCN2 plays a role in itch progression and the maintenance of chronic itch (FIGURE 3B). Aligned with these findings, serum LCN2 levels are high in atopic dermatitis and psoriasis patients (290), suggesting that LCN2 might serve as a biomarker.
Toll-like receptor-4 (TLR4) expressed in astrocytes has also been linked to chronic itch (172). TLR4 is a member of the TLR family responsible for innate immunity, recognizing exogenous ligands and pathogen-associated molecular patterns (PAMP) in the course of viral or bacterial infections (173, 174). They react also to endogenous ligands and danger-associated signals during tissue injury. Liu et al. (172) showed that TLR4 knockout (KO) mice are characterized by reduced spontaneous scratching and touch-evoked scratching in models of dry skin and contact dermatitis. The reduced behavior in the mice correlated also with less activated GFAP+ astrocytes in the spinal dorsal horn in chronic itch mice. Of note, acute itch as induced by compound 48/80 and chloroquine (CQ) were unaffected in TLR4 KO mice. Activation of TLR4 by intrathecal lipopolysaccharide indeed evoked pain and suppressed itch but still enhanced alloknesis and chronic itch, accompanied by reduced astrogliosis. Astrocyte-expressed TLR4 may thus contribute to the maintenance and sensitization of chronic itch.
Microglia constitute another type of glial cell and surpass neurons in number. They are derived from myeloid precursors, constantly screen the environment for danger, and have been implicated in maintenance of neuropathic pain (284). Scratching triggered by compound 48/80 activates microglia in the spinal cord (322). Like neuropathic pain, activation of microglia occurs within minutes. This scratch-induced microglial activation was detected with colabeling for microglia (CD11b) and phosphorylation of p38. Inhibition of the scratching by nalfurafine, a κ-opioid receptor agonist, resulted in diminished microglial p38 phosphorylation. Unlike in pain, reactive microglia are not limited to the dorsal horn of the cervical spinal cord. Glutamate or ATP released by pruritoceptors might activate microglia or itch-specific neurotransmitters such as GRP or Nppb, which may then turn on microglial activation. Furthermore, mast cells are essential to the recruitment of glial cells in the spinal cord in neuropathic and neurodegenerative diseases (95, 108).
IX. SENSITIZATION IN ITCH
Patients suffering from chronic itch, analogous to those with chronic pain conditions, perceive intensified responses to mild pruritic stimuli. This phenomenon is referred to as sensitization. Dysesthesia, the Greek term for abnormal sensation, captures the altered perception and somatosensory changes occurring in itch and pain. Alloknesis in itch and allodynia in pain are comparable terms describing the dysesthesia in patients to non-itchy/painful stimuli such as to fabrics. Exacerbated itch and pain to stimuli are categorized under the terms hyperknesis for itch and hyperalgesia for pain conditions. Some of the molecular and cellular processes described above contribute to the sensitization processes: loss of inhibitory control, activation of glial and immune cells, and increase of excitatory activity in sensory neurons. Touch-evoked pain is a concern in 50% of neuropathic pain patients (127). It is not clear if touch-sensitive neurons are responsible or whether touch-specific C-fibers are switched on in neuropathic pain and increase the sensitivity to message pain for touch.
Alloknesis has been defined as itch occurring to innocuous but dynamic tactile stimuli, which can be mildly painful (208) or above the activation threshold of mechanosensitive C-nociceptors (38). The classic concept of alloknesis by touch has been extended to itch sensitization by innocuous warmth or noxious heat (12). Another phenomenon in AD patients is cross-modality, where painful stimuli can enhance the itch sensation (114, 123). This form of dysesthesia may be termed “algoknesis” (12). Akiyama et al. (5) asked whether pruritogens cross-sensitize with respect to behavioral and neuronal responses of cultured DRG neurons. When bovine adrenal medulla 8–22 (BAM8-22) was injected, the group observed an enhancer effect on scratching behavior when followed by a second injection of BAM8–22 or the pruritogenic hexapeptide SLIGRL. DRG neurons did not respond by mirroring the behavioral responses, suggesting a different cellular mechanism of the ongoing behavioral effects (5). In contrast, SLIGRL application induced cross-sensitization for BAM8–22 which was also reflected in increased neuronal activity of DRGs, not only in the mean peak response but also the increase in the number of responsive cells. SLIGRL reduced serotonin (5-hydroxytryptamine or 5-HT) evoked scratching but did not influence histamine. Histamine cross-sensitized behavioral responses to BAM8–22 but failed to induce increased neuronal activity similar to the effects observed for BAM8-22. One might speculate that neuronal changes as observed for SLIGRL are upon effects on subsequent signaling elements employed by the subsequent pruritogen. For example, activity modulation of the respective receptor and/or the signal transduction receptors, such as TRPA1, on DRG neurons might explain the cross-sensitization triggered by SLIGRL. These findings highlight the concept of peripheral sensitization. However, these are limited to an acute setting (immediate application of pruritogens) in mice, and how these observations translate to humans is not clear.
The effects of continuous exposure to mediators on hyperknesis, alloknesis, and algoknesis remain to be determined. In a dry skin model, Akiyama et al. (3) addressed the question whether chronic itch cause sensitization of itch pathways. In this model, SLIGRL as well as 5-HT injection into the dry skin caused hyperkinetic itch responses. These behavioral responses were also reflected by enhanced neuronal activity of isolated DRG neurons from the itchy mice, suggesting a peripheral sensitization for hyperknesis in the dry skin model (3). Protease activated receptor (PAR)-2 mediated effects could result from other cellular sources than DRGs in the phenomenon of hyperknesis given that SLIRGL-mediated effects in itch versus pain have been disputed (3, 105). Of note, other mediators and neuroactive cytokines such as IL-31 or NGF might impact neuronal activity, receptor distribution, as well as nerve fiber density in an inflammatory setting such as in AD or other inflammatory conditions. For instance, in human surrogate models, intradermal injections of NGF had limited impact on chemical itch sensitization, but were reported to induce hypersensitivity to pinprick (15). Sensitivity to pinprick has been defined as hyperknesis which seems to be relayed by polymodal C-fibers, whereas when occurring secondary to an itch provocation, type I Aδ-fibers relay through central mechanisms the hyperknesis (14). In ultraviolet B-induced inflammation, it is speculated that mechanically insensitive fibers and mechano-nociceptors respond with higher intensity to stimulation, implying that heightened activity has been set through peripheral and central processes. A similar contribution of peripheral and central sensitization is assumed to occur in itchy lesions of AD patients to pinprick-evoked hyperknesis (14). MrgprD-labeled C-fibers innervating the superficial layers of the epidermis and transmitting non-histaminergic itch, in this context of more importance, are sensitized to punctate stimuli in a mouse model of contact dermatitis (215). An underlying cause for pinprick-induced hyperknesis may be the loss of polymodal C-fibers and/or the sensitization of the remaining or a certain subset of them. However, one cannot exclude the central component of sensitized pathways such as loss of descending control from supraspinal levels and enhanced excitement of spinothalamic tract (STT) neurons. For instance, STT neurons respond more intensely to “normal” input from pruritoceptive afferents (12) which results in alloknesis or hyperknesis (12, 151). The discovery that pain-related dysesthesias observed in patients are independent from capsaicin-sensitive C-fibers, segment-restricted, as well as myelinated-fiber modulated suggests a complex interplay and synaptic connections, which might be similar in itch-experienced dysesthesias. Another observation related to sensitization made in chronic itch is that innocuous thermal stimuli affect serotonin-mediated but not histaminergic itch (14). In general, there seems to be limited sensitization to histaminergic itch, but increased responses to cowhage-induced itch in chronic itch patients. Additionally, noxious heat and other algogenic stimuli, i.e., acetylcholine and bradykinin, evoke itch rather than pain in chronically suffering patients (114, 123), suggesting that tachyphylaxis does not occur with itch.
X. ITCH IN THE BRAIN
The brain represents the final station for the somatosensory processing of itch. After the spinal coding of itch under acute or sensitized conditions, the signals are transferred via projection neurons, likely NK1R+ neurons, which connect to the STT. The parabrachial nucleus (PBN) is the next supraspinal itch processing station, which branches into different brain regions. Most activated regions in the human brain are the primary and secondary somatosensory cortex (S1-S2) as detected with functional magnetic resonance imaging. In chronic itch patients, the cingulate and prefrontal cortex reveal a more robust activity as compared with healthy controls when each group is treated with histamine. Descending control from higher brain regions modulates responses in the spinal cord (6). Serotoninergic neurons from the nucleus raphe magnus (NRM) have been shown to directly connect with GRPR+ neurons and to potentiate itch. Noradrenergic neurons in the locus coeruleus modulate inhibitory interneuron activity in the spinal cord. The periaqueductal gray (PAG) is activated during scratching and was considered as an itch suppressing brain area. Tachykinin-1 (Tac1) labeled neurons in the PAG facilitate itch since their ablation significantly abolishes itch behavior in acute and chronic models. Direct activation of the Tac1+ neurons causes itch behavior in mice (92). This descending faciliatory pathway from Tac1+ PAG neurons was not linked to serotoninergic control.
The pleasure of scratching an itch is processed in the midbrain reward center. GABAergic and dopaminergic (DA) neurons in the ventral tegmental area (VTA) partake a role in the central processing of itch. In general, DA neurons are key neuronal elements in motivation and reward systems (66b), whereas GABA neurons are believed to drive aversion and disrupt reward processes (277). VTA GABA neurons are activated in acute itch and show an intensified activation when scratching is prevented, suggesting a function in processing the aversive and unpleasant components of the itch sensation. Intriguingly, DA neurons in the VTA are activated temporally delayed, and the activation is linked to the scratching behavior of the tested animals. These data support the concept that the midbrain VTA DA neurons might transfer the central pleasure component of scratching an itch. Chronic itch processing in the VTA was demonstrated to occur via DA and GABAergic neurons, suggesting a general function of the midbrain region to control acute and chronic itch. Another center in the brain is responsible for the integration of the affective component of itch. As shown for pain (325), the amygdala links anxiety and stress affections to chronic itch (242). Even though both sensory modalities converge in shared central regions, pain and itch are discernable from each other. The central pathways and circuits that differentiate itch from pain overlap anatomically, and the mechanisms by which these sensations are distinguished remain to be determined. Central mechanisms of contagious itch relay via the GRP-GRPR axis in the suprachiasmatic nucleus (SCN) in mice as discussed in section XVII.
XI. G PROTEIN-COUPLED RECEPTORS
7-Transmembrane receptors or GPCRs are exquisitely associated with itch arising in the periphery. Their ligands are direct mediators of itch when the cognate receptors are expressed on neurons and indirect when the receptors are expressed on nonneuronal cells. Extensive tables that list GPCRs associated with itch have been published, yet data supporting the importance of any particular GPCR in any acute or chronic clinical itch are remarkably limited. GPCRs that are most strongly associated with human itch are discussed, including members of the Mas-related G protein-coupled receptor (Mrgpr) family.
Itch arising in the periphery can be divided into two broad categories: histamine dependent and histamine independent. Clinical itches associated with histamine are now considered limited primarily to some patients with urticaria and drug reactions with a lesser role for histamine in AD and other conditions. This limitation accounts for the relative ineffectiveness of antihistamines in itch. It follows that histamine-independent itch is the primary driver of itch that arises in the periphery. The foundation for this understanding is built on a number of observations. These observations include the identification of mechanosensitive C-fibers responsive to cowhage but not histamine (132), that the active component of cowhage stimulates Mrgprs, and that additional substances that evoke itch in humans and scratching in mice stimulate specific GPCRs unrelated to histamine receptors.
A. Mrgprs
Mrgprs have risen to prominence with respect to itch that is independent of histamine. This prominence is manifest in a number of ways and provides a basis for the discussion here. First, Mrgprs respond to a variety of pruritogens, and these links are examined (FIGURE 4). Second, the expression of Mrgprs is limited primarily to sensory neurons implicated in itch as well as mast cells, long implicated in itch and allergy, and is also discussed. Neurons and mast cells are in close proximity and are considered to interact directly in epithelia where neuroimmune interactions are prominent and where neurogenic inflammation takes root.
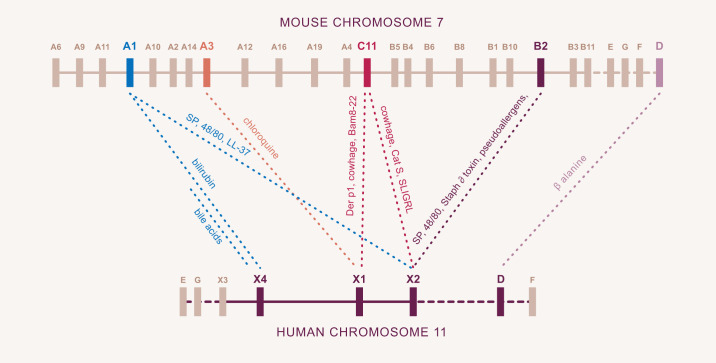
FIGURE 4.
Mrgpr gene loci and receptor ligands in mice and humans. All Mrgpr genes are located on chromosome 7 in the mouse and chromosome 11 in humans. While still considered orphan receptors, substances known to activate specific mouse and human Mrgprs are indicated. Note that some substances which activate a single human receptor interact with more than one mouse Mrgpr. Similarly, some substances which activate a single mouse Mrgpr interact with more than one human receptor.
Mrgprs comprise a family of orphan GPCRs identified early in this century by an academic group led by Xinzhong Dong (72) while a postdoctoral fellow with David Anderson at Caltech and now at Johns Hopkins, and a group led by Paola Lembo and Sultan Ahmad, at Astra Zeneca (163). The respective groups referred to the receptors as Mrgprs and sensory neuron-specific receptors. The encoding of ~50 Mrgprs in the mouse indicated initially that only olfactory receptors were more numerous. A potential role in sensing was appreciated given the additional combinations of tissue distribution limited primarily to sensory neurons and mast cells, together with the appearance of Mrgprs in tetrapods. It is now recognized that the large number of Mrgpr genes in mice, due to a retrotransposon insert was the exception, as other rodents had fewer, and humans have eight. Mrgprs were divided into subclasses based on sequence homology. Mice have A, B, and C subclasses, which correspond loosely to human X1–4 while both species have D, E, F, and G (FIGURE 4). Ironically, canines, considered excellent at scratching, were found to have only a single Mrgpr, homologous to X2. These confounding observations raised the possibility that while most GPCRs were activated by a small number of ligands, perhaps Mrgprs were broad sensors, which turns out to be the case.
A role for Mrgprs in itch was first suggested in 2009 when it was found that CQ induced scratching in mice that was dependent on MrgprA3 and that CQ could also activate human MRGPRX1. The year before, Reddy, Lerner, and co-workers (224) isolated mucunain, a cysteine protease that was the active component of cowhage. While we found that mucunain, and subsequently the endogenous cysteine protease cathepsin S associated with itch and inflammation, could activate protease-activated receptors, further studies revealed that these proteases targeted Mrgprs (222, 224). The surprising finding that a protease could evoke itch via activation of a Mrgpr served to inform further studies that would explain a long-standing discrepancy between mouse and human studies involving SP. Specifically, SP is linked to itch, pain, and neurogenic inflammation. A series of publications revealed that the peripheral actions of SP are mediated via Mrgprs, throwing a wrench into the conventional view that NK1 was the primary receptor for SP. NK1 antagonists were developed in the 1990s and found to be effective in mouse models of itch and inflammation but failed in the treatment of inflammation in humans. Preliminary results from uncontrolled studies suggested that oral administration of the NK1 antagonist aprepitant might be useful for the treatment of chronic itch. These results could not be confirmed in controlled studies (285). Topical application of aprepitant was also ineffective in itch (296). Moreover, NK1 mutant mice still scratched when injected with SP (28). In contrast, Mrgpr cluster Δ−/− mice, which lack 12 Mrgprs but retain mast cell SP-responsive MrgprB2, did not scratch. SP-evoked scratching was found to be dependent on neuronally expressed murine MrgrpA1 while SP activated human MRGPRX2 (27), a finding that has recently been extended to pain (98). The explanation for the conflicting findings between mice and human turns out to be simple: The NK1 antagonists were also antagonizing mouse Mrgprs but had no impact on human Mrgprs. These observations make Mrgprs interesting therapeutic targets for the treatment of itch.
As there are more Mrgprs in mice relative to humans, mapping with respect to ligand specificity would not be expected to be one-to-one, and it is not (FIGURE 4). Several itch mediators as well as substances implicated in allergy, pseudoallergy, and inflammation activate only individual human MRGPRs, but some of these substances activate more than one mouse Mrgpr. Linking a specific endogenous substance with a pathological itch has now occurred. Bile acids activate human MRGPRX4 (182) and can account for cholestatic itch. That cholestatic itch does not occur readily in either mice or canines is consistent with the lack of a functionally homologous receptor in these other species. It is not much of a speculation to think that the itch of chronic kidney disease, which is limited to humans, may also result from an endogenous ligand activating a Mrgpr. Three receptors in humans, MRGPRX1, MRGPRX2, and MRGPRX4, are thus considered to fulfill the roles of the A, B, and C subclasses of Mrgprs while β-alanine activates MrgprD in both species.
With respect to cellular expression, in humans, MRGPRX1 and MRGPRX4 are expressed only on sensory nerves. MRGPRX2 is expressed on mast cells but has also been identified on DRG neurons (226, 311). While MRGPRX2 is not readily identified in neurons based on RNAseq of resting neurons, expression has not been considered during the process of neurogenic inflammation, a critical knowledge deficit in the context of intercellular and mediator crosstalk. With respect to expression in mice, MrgprB2 is limited to mast cells and considered generally homologous to human MRGPRX2. The Mrgprs A1, A3, and C11 are limited to sensory nerves. While MrgprA1 is highly expressed only during development, the low level of expression in adult mice retains function, as mice scratch to bilirubin.
The affinity for ligand receptor interactions is critical. Thus SP activates MrgprB2, MrgprA1, and MRGPRX2 (FIGURES 4 and 5). MrgprB2 is still present in the Mrgpr cluster Δ−/− but SP does not cause scratching in these mice. Thus, in mice, SP causes itch by activating MrgprA1 on sensory nerves rather than activating MrgprB2. In humans, if MRGPRX2 is only on mast cells, then SP evokes itch by stimulating mast cells. In this scenario, the mouse receptors MrgprA1 and MrgprB2 share functional homologies with human MRGPRX2. This concept is consistent with single human receptors sharing function with multiple mouse Mrgprs as noted in FIGURE 4. This concept is supported further by the following observations. SLIGRL, the hexapeptide derived from PAR-2, but which stimulates MrgprC11 on sensory nerves in mice to evoke itch, also activates MRGPRX2, while the potent itch inducer compound 48/80 activates Mrgprs expressed on mast cells and neurons. Returning to cysteine protease activation of Mrgprs, while cathepsin S activates MrgprC11 and MRGPRX2, the dust mite cysteine protease Der p1 associated with allergy and inflammation, but not itch, activates MrgprC11 and MRGPRX1 while cowhage activates human MRGPRX1 and MRGPRX2. Finally, vancomycin which is implicated in red man syndrome and staphylococcal delta toxin which is associated with atopic dermatitis, and thus itch, activate MRGPRX2 (213).
FIGURE 5.
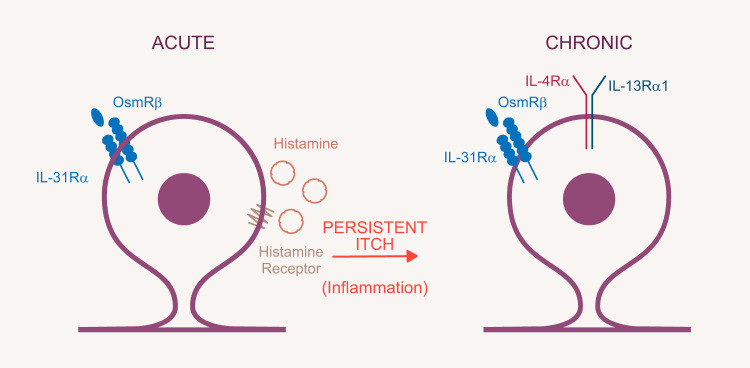
Sensory neuronal receptors and signaling molecules for the transduction of itch. Sensory afferents express a multitude of receptors, providing redundancy to ensure the transmission of acute and chronic itch signaling. A number of these receptors are depicted here, including serotonin-5-hydroxytryptamine receptors (5-HTRs), promiscuous murine itch receptors MrgprA1/A3/C11, cytokine signaling receptor complexes interleukin (IL)-31RA, oncostatin M receptor β (OSMRβ), and IL-4Ra and IL-13R and voltage-gated sodium channels (Nav). A plethora of downstream signaling molecules link receptor activation with generation of action potentials, the details of which remain to be determined. In persistent itch, signaling might increase receptor expression, including the itch-related signaling transducer channels TRPV1 and TRPA1. It remains to be investigated whether downstream signaling mediators such as the mitogen-activated protein kinase (MAPK) and Janus activated kinase (JAK)/signal transducers and activators of transcription (STAT) acutely alter itch-relevant receptors via sensitization or on transcriptional levels. BAM8-22, bovine adrenal medulla 8-22; PKC, protein kinase C; PLA, phospholipase A; PLC, phospholipase C; SP, substance P.
Neuronal expression of Mrgprs has allowed these receptors to serve as markers or fluorescent tags that have been used to trace pathways of itch in mice. Of particular importance, MrgprA3 fibers were found to innervate the epidermis exclusively and respond to mechanical stimuli, noxious heat, and a number of pruritogens (103). These are features of non-histaminergic, polymodal itch fibers. Ablation of these fibers with the aid of diphtheria toxin resulted in decreased scratching behavior from a range of pruritogens while pain sensations were intact. Evaluation of dry skin and allergy in these mice, considered chronic models of itch, were also modulated. Mice were engineered further to express TRPV1 only in MrgprA3 neurons, and then treated with capsaicin. Scratching but no pain behavior was evoked. These studies established the existence of a labeled line for itch, at least in mice, in contrast to the prevailing intensity theory of itch versus pain.
A number of areas of Mrgpr biology in general and itch in particular remain to be clarified. 1) Beyond bile acids, what are the physiological endogenous or exogenous ligands for Mrgprs, and what might that imply about Mrgpr biology in humans versus mice, canines or non-human primates? 2) Do human Mrgprs of the E, F, or G subclasses have relevance to itch? 3) Since MrgprA3 neurons are considered nonpeptidergic but respond to SP, can peptidergic and nonpeptidergic neurons communicate directly? 4) Is expression of neuronal Mrgprs inducible during the course of neurogenic inflammation? 5) While CQ induces scratching in mice and can activate human MRGPRX1, hydroxychloroquine, a derivative, is used widely in the therapy of autoimmune conditions but rarely triggers itch. Therefore, might the itch associated with CQ in Africans be linked to a Mrgpr polymorphism? 6) As elevated levels of MRGPRX2 on mast cells correlate with severe urticaria (87), it may be reasonable to expect that Mrgpr polymorphisms will be associated with, or protect from, acute or chronic itches.
Taken together, Mrgprs on mast cells and neurons provide powerful links between itch, allergy, and neurogenic inflammation. The recent identification of a Mrgpr antagonist that inhibits itch in mice (28) and the demonstration of residues critical for activity via in silico modeling and in vitro activity (157, 223) are consistent with the possibility that drugs that target this class of receptors will be identified and evaluated for therapeutic benefit.
B. NK1R
Tachykinins are a family of neuropeptides. They stimulate rapid contraction of intestinal muscles as compared with the slow effects mediated by bradykinins. The best-known tachykinin with respect to itch is SP. Until recently, the cognate receptor for SP in the periphery has been considered the neurokinin-1 receptor, NK1R. It is now appreciated that while NK1R may have a role in the spinal cord with respect to itch, the relevant receptors for SP in the periphery are members of the Mrgpr family, discussed above. This shift in our understanding parallels the realization that the role of the H1 receptor in clinical itch is remarkably limited. SP is detected in primary sensory nerves that innervate the epidermis, dermis, blood vessels, and various cell types. SP exerts differentiated function on different cell types and cutaneous structures. For example, it has been suggested that SP upregulates NGF in keratinocytes which would affect neuronal regeneration (44) or lead to sensitization. A key role for SP in neurogenic inflammation is via close vicinity to cutaneous blood vessels which is aggravated through the additional feed-forward loop of NGF-dependent upregulation and release of SP in endothelial cells (185). Moreover, these tachykinin-marked sensory neurons are classified as the unmyelinated C-fibers which mark the C-nociceptors and the thinly myelinated Aδ-fibers, both of which conduct nociceptive stimuli and mediate the neurogenic inflammation. In addition to SP, CGRP is equally present and released from perivascular nerve fibers and is responsible for the flare in neurogenic inflammation (94, 211), although CGRP does not cause itch. NK1R+ neurons in the spinal cord appear to mark projection neurons for messaging itch and pain to supraspinal levels. The generation of knockin mouse with a Rosa 1s tdTomato reporter by the Ross group is enlightening (117). Within the skin, the authors detected Cre-mediated recombination in dermal fibroblasts but not in mast cells, endothelial cells, Merkel cells, or Langerhans cells as elsewhere described. The lumbar spinal cord showed tdTomato labeling within lamina I while deeper levels of the spinal cord stained for NK1R. NK1R labeling was detected in most projection neurons, important cross points for the transmission of pain and itch (8). In this context, it was reported that ablation of the NK1R+ cells in the spinal cord of the rat attenuated the scratching responses elicited by 5-HT. The study revealed that NK1R+ and GRPR+ neuron toxic ablation in an animal model of chronic itch with ovalbumin (OVA) treatment showed that SP-SAP resulted in reduction of spontaneous scratching and alloknesis scores. Toxic ablation of GRPR+ interneurons did not affect either scratching responses or alloknesis (49). This finding suggests that the spinal GRP-GRPR axis is not essential for itch sensitization. Intriguingly, the lack of the GRPR neurons as well as the NK1R+ resulted in significant reduction of enhanced responses to CQ. The segregation for this observed function of the spinal GRPR neurons may be linked to peripheral sensitization (hyperknesis) versus spinal action of sensitization (alloknesis). Nevertheless, spinal NK1R+ neurons seem to play a role in both sensitized responses as well as the itch of OVA– mice, possibly due to a selective central sensitization of these spinal neurons and their polysynaptic nature. However, there is contradictory data suggesting functional NK1R in TRPV1+ rat DRG neurons population, implying a peripheral role in nociception, particularly in heat hyperalgesia (319).
In summary, most of the data described suggest an ascending spinothalamic role for NK1R+ neurons in itch. Overall, these findings can explain the limited success of NK1R antagonists in the treatment of itch.
C. PARs
PARs are encoded by four distinct genes, PAR-1 to PAR-4 (63). PAR-1 is the receptor for thrombin. Trypsin was found to activate PAR-2 (202). PAR-3 and PAR-4 were cloned by mRNA screening of rat platelets (310). PAR-1, -3, and -4 are expressed on neuronal, epithelial, astrocytes, and immune cells (204). Protease cleavage of PARs unmasks a sequence which acts as a tethered ligand and binds to conserved sequences to induce transmembrane signaling (204). The sequence of the tethered ligand is characteristic and distinct for each PAR, with SLIGRL derived from mouse PAR-2 being the most studied. PAR-induced cellular signaling engages either G proteins or arrestins. For example, PAR-1, -2, and -4 interact with G proteins, which results in activation of different kinases such as mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-kinase (PI3K) or protein kinases as well as tyrosine kinases depending on the activating ligand and residual cell type (266). The activation of PARs increases transcription of cytokines, chemokines, and growth factors which regulate different cellular processes.
Proteases have long been recognized as mediators of non-histaminergic itch. Itch induced by mucunain, the active ingredient of cowhage, was first described by Arthur and Shelley in 1955 (22). Mucunain induces long-lasting, histamine-independent itch without pain. Arthur and Shelley (22) speculated that mucunain might be a protease and induce itch directly via action on sensory nerves or via an indirect mechanism through the release of pruritic compounds. Protease-induced non-histaminergic itch was investigated, identifying trypsin and chymotrypsin as pruritogens (140). Overexpression of proteases or PAR-2 in mice results in a severe eczematous phenotype (86). Moreover, in a severely itchy mouse strain overexpressing serine protease channel activating protease-1 (CAP-1/Prss8), the phenotype could be rescued by backcrossing into PAR-2 KO animals. The concept of PAR-2-mediated itch was challenged by the discovery that the synthetic peptide SLIGRL also activates MrgprC11. It was found that SLIGRL-induced itch was lost in Mrgpr cluster Δ−/− mice. MrgprC11 relies on the presence of the RL-NH2 sequence of the synthetic peptide. The controversy has not been fully clarified, but the possibility remains that the naturally occurring proteases might trigger itch via PAR-2. Steinhoff and colleagues (140)suggested that trypsin and tryptase activate PAR-2 on DRG neurons to trigger histamine-independent itch and both proteases induce neurogenic inflammation and activate PAR-1 and PAR-4. PAR-2 is also involved in pain, which complicates the picture. Effects were reduced in PAR-2-deficient mice, suggesting that the major neuronal function of PAR-2 is to induce hyperalgesia. Trypsin-mediated itch in mice did not require the presence of either MrgprC11 or PAR-2, implying the involvement of an additional receptor, perhaps PAR-4, which is expressed in rodent DRG neurons (23, 25). Regarding signaling, Patricio et al. (209) employed the PAR-4-specific activating hexapeptide AYPGKF-NH2 (AYP) to show that AYP activated the TRPV1+ neuron population and that AYP-elicited itch was diminished in TRPV1-deficient mice. The TRPA1 antagonist HC-0300310 blocked AYP-elicited itch, suggesting the involvement of this channel. However, TRPA1-deficient mice showed augmented scratching to AYP that was mirrored in calcium studies of TRPA1−/− DRG neurons when stimulated with AYP. A compensatory mechanism might play a role in the heightened responses to AYP in TRPA−/− mice and neurons. Another controversial finding of the study is the colocalization of PAR-4 with GRP in peripheral neurons (209). The GRPR antagonist RC-3095 blocked itch induced by AYP in mice, suggesting the involvement of GRP-GRPR circuitry. Due to its ability to cross the blood-brain barrier (9, 19), RC-3095 might exert its effect centrally to block GRPR consistent with prior findings by Mishra and Hoon (186). Future studies with more specific genetic knockout animals will be needed to fully explain this complex picture and its relevance to humans.
D. Histamine
Histamine is one of the most studied substances in preclinical and clinical research. However, its importance in clinical itch outside of urticaria and drug reactions is now considered limited, as that of histamine-independent itch has gained favor. Histamine is a short-acting, endogenously produced, widely distributed biogenic amine (253). Playing a major role in allergic associated inflammation, histamine regulates the maturation, activation, and chemotaxis of immune cells (278) besides exerting immune regulatory functions on monocytes, T cells, macrophages, and various other immune cells (158). Histamine activates, with different affinities, four distinct GPCRs, H1–4, named chronologically in order of their discovery (2). Histamine, the gold standard for histaminergic itch mechanisms studies (FIGURES 1 and 5), causes intense itch accompanied by vasodilation, redness, flare, and swelling in human when applied to the skin. In mice, histamine is often used as a positive control in the context of investigations that involve other pruritogens. The major source of histamine is differential granule release, together with other bioactive substances, from mast cells, following IgE- or Mrgpr-dependent stimulation. Histamine may be released from additional types of cells, including neurons, basophils, and keratinocytes. H1 and H4 receptors are expressed on DRGs (FIGURE 5), although the expression on the latter is low (293). H1R couples to Gαq/11 G protein and induces signaling via phospholipases A2 (PLA2) and phospholipase C (PLC)-β3 as well as protein kinase C-δ (124). H4R couples to Gαi/o proteins and is also expressed on a variety of immune cells and other cell types from the intestine and lungs (66a). Neuronal activation of itch via histamine is linked to TRPV1 (124) as well as TRPV4 (144).
The first antihistamine directed against the H1R, phenbenzamine, was marketed in 1942. H1 antihistamines act to downregulate constitutive receptor activity and are thus inverse agonists rather than H1-receptor antagonists (164). First-generation antihistamines are known for their sedative side effects. It was not until three decades later that the H2R antagonist cimetidine was put into clinical use for the treatment of gastric acid disorders. The first H3 inverse agonist is in use for narcolepsy (147). Cetirizine is a second-generation H1R antagonist. It is a metabolite of hydroxyzine, a widely used first-generation antihistamine. JNJ 7777120 is the first highly selective H4R antagonist, but its development has been curtailed due to adverse effects. Other H4R antagonists are under clinical evaluation for inflammatory disorders such as AD (300), while serliforant is a H4R antagonist undergoing evaluation for vestibulopathy (24).
Urticaria or hives are recurrent, pruritic, and characterized by central swelling with surrounding erythema in any body region. In contrast to most itches, the urge to scratch may be replaced with an urge to rub. What underlies the response of rub versus scratch remains a mystery. Urticaria lasts less than 6 wk in the acute form and may be associated with medications (nonsteroidal anti-inflammatory drugs, opiates, and narcotics), foods, viral infections, and contact allergens. Fifteen percent of such patients also have angioedema. Chronic urticaria continues for more than 6 wk and may be associated with autoimmune disorders such as systemic lupus erythematous. H1R antagonists have been a mainstay of treatment, although many are now treated with biologics directed to IgE and additional targets. It is increasingly appreciated that SP may be a major player in chronic urticaria as well as chronic spontaneous urticaria (CSU) which is characterized by spontaneous lesion reappearance. High levels of SP have been detected in CSU patients. Emerging concepts suggest that SP activates MRGPRX2, an emerging therapeutic target on mast cells, and participates in urticaria and the release of multiple allergens, including histamine. Antihistamines have been widely used in the treatment of allergic disorders for decades based on findings that histamine promotes inflammation and modulates immune responses. Beyond itch, HR are associated with tumorigenesis, manifest as either promoting or inhibiting tumor growth (181). Histamine was believed to promote neuroinflammation in diseases such as multiple sclerosis; however, this finding has been questioned as blocking H4R enhances inflammation (31). There have been discussions about histamine in the role of airway inflammation based on its activity on lung macrophages and the subsequent induction of pro-inflammatory cytokines such as IL-6. In an animal model of allergic airway inflammation, H4R KO animals showed less pulmonary infiltration of eosinophils and lymphocytes with a concomitant reduction of TH2 immune activation (74). Blocking H4R in a model of pulmonary fibrosis showed beneficial effects in reducing inflammation (176, 177). Histamine activates also H1R on pulmonary epithelial cells and induced TLR3 expression leading to IL-8 secretion to accentuate inflammation. Depending on the cell population that is driving the disease pathology, targeting either HR might be beneficial. In an AD model, H4R blocking attenuated itch responses in mice, presumably through reduction of IL-31 secretion from TH2 cells (230). Blocking H4R showed promising results in the psoriasis-like model, induced with imiquimod, through reduction of TH1 responses. These findings underline the pleiotropic effects of histamine. Of note, histamine exerts a protective role in some other preclinical models such as in the trinitrobenzene sulfonic acid model of colonic inflammation in mice (309). In contrast, when colitis was induced with dextran sodium sulfate, targeting H4R improved the condition in this model (244).
E. Serotonin
5-HT is a monoamine neurotransmitter. Injection of serotonin into skin causes itch in humans (34, 299) and scratching in mice (190). Despite much research, including data linking serotonin to allergic skin disease and to pruritus associated with both uremia and cholestasis (279), the importance of serotonin as either a peripheral or central mediator of itch in humans is unknown. This uncertainly is likely a reflection of the complexity of the serotonergic system. This complexity is exemplified by the existence of 14 serotonin receptors from 7 receptor families (106). All are GPCRs except for 5-HT3, which is a ligand-gated ion channel. The diversity of receptors makes it challenging to know exactly which are important in itch. Most of these receptors are expressed in spinal neurons involved in ascending transmission. In the periphery, serotonin can be released from mast cells, platelets, and endothelial cells during injury and inflammation and acts on small- to large-diameter DRG neurons (307). Serotonin is a component of the inflammatory soup known to excite Aβ- and C-fibers (156). Treatment of pruritus by targeting serotonin receptors adds complexity rather than clarification. SSRIs can help treat pruritus, but they increase serotonin (centrally) by preventing reuptake. Ondansetron, an antagonist at the 5-HT3 channel, anecdotally can be of benefit in the treatment of cholestatic itch, uremic itch, and opioid itch. However, such anecdotes provide limited useful information.
F. Additional GPCRs
Endothelin-1 (ET-1) is a 21-amino acid peptide and potent vasoconstrictor implicated in pruritus (142). Produced by endothelial, immune, and neuronal cells, ET-1 engages with two GPCRs, endothelin-A receptor (ETAR) and endothelin-B receptor (ETBR). In mice, ET-1 mediates itch behavior via ETAR and ERK1/2. ETAR+ neurons are of peptidergic origin and overlap with most of the itch receptors. ET-1 induces robust itch responses and is often used in rodent research as a non-histaminergic itch agent and has also been administered to humans via iontophoresis. Intriguingly, the responses in humans seem to be partially linked to histamine-induced itch, most likely via mast cell release based on the findings that a H1R blocker partially blocked the itch responses (142). It would be of interest to test other HR blockers to delineate whether ET-1 is histamine-related itch inducer in humans.
Prostanoids are derived from membrane lipids. Prostanoids are formed via the cyclooxygenase (COX) pathway from arachidonic acid (AA). Prostaglandins are the unstable forms of prostanoids and have been implicated in itch. Prostaglandin E2 (PGE2) is itch-inducing in AD patients, but this seems to rely on histaminergic itch (113). Despite increased levels of prostanoids or other AA derivatives such as thromboxane in AD, it is not clear whether itch is induced directly by these molecules or whether they function as inflammatory sensitizers in itch. The transmembrane protein TMEM79, which is expressed in keratinocytes and neurons, was reported to be crucial in pathological responses to oxidative stress (78), most likely in AD. TMEM79 loss in keratinocytes triggers mast-cell degranulation and subsequently histaminergic itch with PGE2 and H4R/H1R interdependency. Since histamine is not considered a major pruritic contributor to AD, the relevance of this finding is not clear.
Leukotrienes are derivatives of AA and have been long implicated with itch. In particular, leukotriene (LT) B4, a lipid chemoattractant and activator of granulocytes (238), has gained attention as relevant to itch signaling when released from keratinocytes upon itch stimulation by SP and SLIGRL (18, 139a). IL-31-mediated itch may employ keratinocyte derived LTB4 (16). LTB4 inhibition was achieved either by blocking the synthesis of the chemoattractant or by blocking the chemoattractant with pharmacological antagonists. These data support the notion that itch is a complex interplay of neuronal and immune components within the skin and very likely other organs.
Sphingosine 1-phosphate (S1P) is a bioactive signaling lipid associated with a variety of inflammatory diseases. These include not only AD and psoriasis (126), but also multiple sclerosis, which is often accompanied by itch and pain (206) that may be neuropathic in nature (267).The interaction of S1P with the S1PR3 receptor leads to activation of TRPA1 pruriceptors and TRPV1 nociceptors (110). These data reveal that distinct molecular mechanisms may underpin the discrimination of itch versus pain in the periphery.
That cannabinoids are used recreationally led to the identification of endogenous lipid-based compounds and receptors. Use of cannabinoids to treat inflammatory skin disease has been suggested and reported anecdotally (35). The increased legalization of marijuana is likely to drive therapeutic use, especially for itch and AD. Two cannabinoid GPCRs have been identified (191, 214). In mice, both receptors have been detected in sensory neurons, whereas in humans, only CB2R has been so localized (11, 263). Activation of CBR in sensory neurons may decrease neuronal activity and modulate the axon-flare response. Tetrahydrocannabinol, a bioactive component of marijuana, blocked scratching behavior elicited by compound 48/80 (245). These antipruritic effects were CB1R dependent as revealed by use of antagonists. Upon stimulation with hapten, CB1R mutants were susceptible to the development of features of AD (88). N-acetylethanolamines are beneficial for the treatment of itch through activation of the endocannabinoid system, and topical N-palmotoylethanolamine (PEA) has been reported to decrease uremic itch scores to baseline (273). Topical PEA cream also reduced the itch in a cohort of mild to moderate AD patients when applied twice daily for 4–6 wk (75).
XII. TRP CHANNELS
TRP channels constitute a family of ion channels expressed widely across species. They have been strongly implicated in itch, although their necessity has been questioned (234). TRP channels were first identified in Drosophila in 1969 (62). Their importance in nociception came almost three decades later, when it was determined that capsaicin activated a channel that became known as TRPV1. More than 25 distinct TRP channels have been described in mammals. TRPs respond to various stimuli via an influx of cations. TRPs are expressed in diverse cell types, including immune cells, neurons, and keratinocytes, and participate as sensors to maintain skin homeostasis. Based on their amino acid sequences, TRP channels form six subfamilies. These include TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPV (vanilloid). TRP channels are gated by stimuli ranging from small molecules and ions to thermal as well as mechanical inputs, underlying their role in tissue homeostasis and sensation. Their characteristic structure contains a central channel pore and four subunits built around the pore. TRPs feature six transmembrane domains with short interconnections to each other and their long NH2-terminal domains are paired with short COOH-terminal domains, both of which extend into the cytoplasm. TRPs are generally nonselective mono- or divalent cation-gated channels with no anion permeability. Only a few TRPs have demonstrated specific selectivity towards calcium or magnesium, while most are nonspecific and gated by both cations. The association between TRPs and sensing has made them targets of drug development, but side effects, such as altered thermoregulation with respect to antagonists of TRPV1, have confounded drug approval. Furthermore, polymorphisms in TRP channels that manifest with alterations in pruritus, and which could thus guide drug development, have not been reported.
A. TRPV1
TRPV1 is gated by vanilloids, comprising compounds that have a vanillyl group, of which capsaicin and its analog resiniferatoxin, which is 1,000 times more potent, are members. Additional TRPV1 activators include thermal stimulus more than (50) 42°C (49), extracellular protons and anandamide, a lipid derived from cannabinoids. TRPV1 is found mostly on peptidergic nociceptor neurons and is a marker for the sensory neurons mediating pain and itch (50, 196, 197). TRPV1 is also sensitized by molecules released during inflammation, including bradykinin, prostaglandin, and retinoids (52, 313). Activation via inflammatory mediators results in the downstream signaling by protein kinases C and A as well as phosphatidylinositol bisphosphate (PIP2) (227). In inflammatory pain, TRPV1 expression is upregulated, which may account for the increased heat perception (121, 294, 295, 308). Targeting TRPV1 to treat inflammatory pain seemed like a promising treatment strategy. Unfortunately, antagonizing TRPV1 has side effects such as hyperthermia and loss of noxious heat perception (138).
The role of TRPV1 in the mediation of itch associated with histamine, but not other tested pruritic mediators, was uncovered when it was found that the injection of histamine was attenuated in TRPV1-deficient mice. In contrast, itch elicited from the injection of ET-1 and serotonergic itch from α-Me-5-H were not altered. Seemingly, most pruritogens do not engage the TRPV1 channel for the transduction of the itch signal. IL-31 is one of the pruritogens that mediates itch via both TRPV1 and TRPA1 (FIGURE 5).
Efforts are ongoing to tease apart, if possible, the relative contributions of TRPV1 as compared with TRPA1 with respect to itch versus inflammation. In the squaric acid dibutylester (SADBE) model of allergic contact dermatitis, both channels were required for scratching behavior while genetic or pharmacological ablation of the TRPV1 population promoted inflammation (84). In this model, C-fiber neuronal activity precedes immune responses and regulates to some extent immune functions as suggested elsewhere (33). In contrast, TRPV1 was necessary for inflammation in the imiquimod-induced psoriasis mouse model of inflammation (225).
B. TRPA1
The ankyrin family possesses multiple copies of the ankyrin motif residing in the NH2-terminal domain and is required for their assembly and gating of the channel (50, 51, 188, 221). TRPA1 was found initially to have a role in sensing pain and cold and participating in inflammation, and was activated by a range of noxious or pungent substances, including mustard oil, garlic oil, allyl isothiocyanate, and pollutants as well as ∆(9)-tetrahydrocannabinol (THC) and metabolites of acetaminophen (135, 210, 268). TRPA1 has emerged as the channel most tightly associated with the transduction of acute and chronic itch, although that role has now been questioned (234). TRPA1 is expressed by a subpopulation of small-diameter C-fiber polymodal afferent in the dorsal root, trigeminal, and nodose ganglia marked by the coexpression of the NGF receptor TrkA and TRPV1 (135, 268). TRPA1+ fibers project to peripheral tissues including the skin and the viscera. Their depolarization prompts the release of SP, neurokinins, and CGRP which promote extravasation, vasodilation, and neurogenic inflammation as well as hypersensitization to algesic stimuli. TRPA1 has been suggested to function as a gatekeeper for the neuroinflammatory release of neuropeptides. TRPA1 participates in non-histaminergic acute scratching in mice from CQ and the endogenous enkephalin BAM8–22 (168, 302, 303). CQ directly activates MrgprA3 while BAM8–22 activates MrgprC11, each of which couples to TRPA1 (FIGURE 5). With respect to dry skin induced by acetone-ether-water (AEW), and considered by some to recapitulate dry skin in humans and thus be a model of chronic itch, TRPA1 was necessary as TRPA1 KO mice genes scratched less as compared with their wild-type littermates and phenotypic changes were less impressive (303). TRPA1 was similarly necessary for the full manifestation of the effects of oxazolone and urushiol allergic contact dermatitis in mice (168). However, the requirement for TRPA1 in these processes has been questioned (234).
C. TRPV4
TRPV4 is expressed in keratinocytes, macrophages, and nociceptive neurons (145, 178). It has been considered a polymodal sensory modulator for thermal and chemical stimuli as well as osmotic and mechanical sensory inputs. It is also implicated in serotonin-induced itch (7). Serotonin levels have been associated with different pruritic diseases (141, 258), and there are many GPCRs in the serotonin receptor family. In TRPV4 KO mice, scratching behavior is attenuated in response to 5-HT, but not CQ, SLIGRL, or histamine (7). Single-cell calcium imaging was employed to highlight serotonin activation of HTR2A and coupling to TRPV4. The signals were extinguished in TRPV4 KO DRG neurons but remained unaltered in DRG neurons isolated from TRPV1 or TRPA1 KO mice. A link to the HTR7 receptor and TRPA1 has also been reported (190). By sensory neuron gene expression studies across genetically distinct mouse strains, the authors connected expression levels of HTR7 with non-histaminergic itch in the DBA mouse line. HTR7 was labeled in sensory neurons and colocalized with TRPA1 in hairy skin. Both receptors were required to induce neuronal responses by the HTR7 agonist LP-44. HTR7 was also implicated in persistent itch induced by topical MC903. Integrating both serotonin receptors into setting of allergic and nonallergic chronic itch, it has reported that lineage specific ablation of TRPV4 in macrophages and keratinocytes reduces itch in the AEW-dry skin or SADBE-induced ACD model.
D. TRPM8
The normal function of TRPM8 is to sense cold. In contrast to other TRP channels, TRPM8 is activated by menthol or icilin and inhibits or masks the sensation of itch due to the cooling sensation (275). Menthol-containing emollients provide an approach for temporary relief for itch. Skin cooling attenuates spinal neuron responses to histamine injections (131a). Deficiency of TRPM8 in sensory neurons results in attenuated responses to its activators (59), whereas more intense cold, <10°C, was still detected, most likely by other cold-sensitive channels. TRMP8 agonists have now been evaluated in clinical studies. A pilot study tested two TRPM8 agonists, (1R,2S,5R)-N-[2-(2-pyridinyl)ethyl]-2-ispropyl-5-methylcyclohexancarboxamide and menthoxypropanediol, in a double-blind randomized study with dry skin chronic itch patients (262), with promising reduction of itch. In a study of pedal pruritus in atopic dogs, the TRPM8 agonist 1-diisopropylphosphorylheptane was evaluated but did not improve pruritus (276). The effectiveness of targeting TRPM8 remains to be clarified as do the mechanisms that connect TRPM8+ neurons to spinal itch circuitry.
E. TMEM16a Versus TRP Channels in Itch
The concept of TRP channels as major signal transducers for itch has been challenged, as noted at the beginning of this section. Undem’s group used preparations that included skin with intact DRGs and spinal nerves and demonstrated that the calcium-activated chloride channel TMEM16a, rather than TRPs, are activated following stimulation of histamine or CQ (234). It was suggested that technical differences between the neurophysiology methods and the site of nerve recordings could explain discrepancies with prior findings. How this fascinating study impacts the reported supportive role of TRPs in inflammation will be of interest to the field.
XIII. VOLTAGE-GATED SODIUM CHANNELS
The contribution of voltage-gated sodium (Nav) channels to itch is garnering attention (160, 240). Nav channels play a central role in the transmission of sensory information. Peripheral sensory neurons express Nav1.7, Nav1.8, and Nav1.9 (154). Conventionally associated with transmitting pain signals, it is increasingly recognized that Nav channels contribute to itch (FIGURE 5). Loss-of-function mutations in SCN9A, which encodes Nav1.7, results in a congenital deficiency to pain, although itch has not been addressed (64, 198). Gain-of-function mutations cause painful neuropathies (111, 118). Erythromelalgia is an autosomal dominant condition caused by a gain-of-function alteration in Nav1.7 (65) that leads to burning pain and erythema in the extremities (293a) as well as a small fiber neuropathy (82). Gain-of-function changes in Nav1.8 and Nav1.9 also result in small fiber neuropathies (82) and familial episodic pain (321). Impairment of Nav channels impacts depolarization, inactivation, or recovery of neuronal firing and the net hyperexcitability of DRG neurons (280). Nav antagonists are in preclinical and clinical studies (280). With respect to Nav1.9, a woman with a gain-of-function mutation expressed in a heterozygous fashion presented with severe itch and concomitant partial loss of pain sensation (240). To better understand the impact on itch, Nav1.9 and the homologous mutation was studied in mice. Expression was primarily detected in unmyelinated, nonpeptidergic small-diameter DRG neurons, which expressed MrgprA3 or MrgprC11, a subpopulation linked to non-histaminergic itch. Loss of Nav1.9 led to reduction in histamine, BAM8–22, and CQ elicited scratching. Peak neuronal responses for histamine and CQ were similar between KO and wild-type mice, but the percentage of responding neurons was reduced. Like the human Nav1.9 mutation, heterozygous Nav1.9L799p/wt mice scratched more frequently than their wild-type littermates, thought to be due to deactivation or hyperpolarization changes in the channel resulting in excess Na+ influx (162). In line with these findings, a small population of MrgprA3+ neurons from Nav1.9L799p/wt mice was found to be hyperexcitable and may contribute to spontaneous itch activity (240). With respect to Nav1.7, a monoclonal antibody directed against the paddle region of the channel showed efficacy in mouse models of inflammatory and neuropathic pain as well as models for acute and chronic itch (160). Intrathecal injection suppressed itch induced by peripheral administration of compound 48/80, CQ, and GRP while systemic and intrathecal delivery suppressed chronic itch in the dinitrofluorobenzene (DNFB) hapten model of contact sensitivity. Like other Nav channels, Nav1.7 contributes to tetrodotoxin-sensitive sodium channel-mediated excitatory synaptic transmission. The primary afferents expressing Nav channels synapse with lamina IIo neurons and connect to lamina I neurons as part of the circuitry involved in chronic itch and pain.
XIV. CYTOKINES AND ITCH
The immune system, nervous system, and epithelia have evolved to balance homeostasis while protecting against infections, injuries, and threats. The complex interactions between these systems are multifactorial and multidirectional. Danger signals can result in dysregulated responses that are detrimental, with pathophysiological consequences. Direct communication between cells and mediators was implied by the close vicinity of Langerhans cells and mast cells to sensory neurons (21, 115, 265). The simplified version of neuroimmune interaction has been modulated and has gained multiple layers of complexity in which neurons participate actively in the recruitment of immune cells. It is likely that each cell type in skin can communicate directly or indirectly. Since Rothman’s review, the view of a neuroimmune interplay has been widened by understanding the cross-communication between the nervous system and the immune system via small molecules and cytokines. These messenger molecules are released by immune cells and activate receptors on sensory neurons. Key cytokines involved in itch and nociception include IL-2, IL-4, IL-13, and IL-31. While many cytokines are involved in inflammation, none has been implicated directly in pruritus, and they are not discussed further. A variety of small molecules, including histamine and serotonin, are released by controlled and differential granule release from mast cells and interact with neuronal receptors while sensory nerves release the neuropeptides SP and CGRP to activate immune and non-neuronal cells. We focus on the cytokines that interact with cognate receptors on neurons to drive itch and inflammation.
A. IL-31
IL-31 is the cytokine most directly implicated in mediating itch (FIGURE 5). Blocking antibodies to IL-31 or its receptor rapidly decrease itch in mice, canines, and humans (195, 237). The anti-inflammatory effect of such blockade is in flux (200). IL-31 was discovered in 2004. It is a four-helix bundle cytokine with minimal homology to IL-6 but classified as a member of the IL-6 family (109). IL-31 was found initially to be produced by activated CD4+ T lymphocytes but is also made by mast cells, macrophages, basophils, eosinophils, keratinocytes, and dendritic cells (69, 125, 199, 235). Transgenic mice that overexpress IL-31, driven by the Lck promoter in lymphocytes, developed eczematous skin lesions and immune-triggered hair loss. The promoter region includes binding sites for several transcription factors, including NFAT1, STAT6, JunB, AP-1, and NFκB which likely enhance IL-31 expression in TH2 lymphocytes and mast cells (109, 320). IL-31 is not considered a classic TH2 cytokine as the latter are associated with the induction of allergy and eosinophilia, whereas IL-31 tends to be downstream or separate from TH2 processes (109, 136, 207). IL-31 signals through a receptor heterodimer complex consisting of IL-31RΑ and oncostatin-M receptor β (OSMRβ). Both subunits are detected in the skin, brain, lung, trachea, skeletal muscle, testis and ovary, prostate, placenta, spleen, thymus, bone marrow, and blood leukocytes with OSMRβ being the more constitutively expressed subunit (69, 70). Our research has detailed the cellular as well as the molecular mechanism by which IL-31 exerts it effects. IL-31RA has been localized on sensory neurons in mice and humans (32, 55). We analyzed the receptor distribution on sensory neurons and found expression in 3–5% of the total neuron population. MrgprA3, another marker of itch sensory neuron, is expressed in a similar percentage of DRGs. Neurons that respond to histamine, SLIGKV/SLIGRL, and CQ partially overlap with the IL-31RA+ neuron population. There was also overlap of TRPV1 and TRPA1 expression. IL-31 directly induces itch when injected into the murine skin, although questions remain as to whether IL-31 may have nociceptive effects. The expression and distribution of IL-31 receptors on sensory neurons indicate a direct messenger function in sensory aspects of inflammation including itch and possibly pain.
1. IL-31 receptors and downstream signaling
In contrast to other IL-6 cytokines, IL-31 does not interact with the gp130 receptor subunit but binds with low affinity first to IL-31Rα. Following receptor engagement, a plethora of downstream signaling pathways are activated, including Janus activated kinase (JAK)-Stat, Ras/Erk, and the PI3K/AKT (60). Activated TH2 cells are the primary but not exclusive source of IL-31 secretion. Typical AD triggers including Staphylococcus aureus and OVA induce the secretion of IL-31 from TH2 cells (55) while human beta defensin (hBDN) and the antimicrobial peptide cathelicidin-37 (LL-37) induce release from mast cells (199), now recognized to be mediated via Mrgprs. IL-31 is thus ideally suited for the neuroimmune interplay in innate immunity and cutaneous inflammation. Human basophils, which are effectors of allergy, produce IL-31 and are also activated by IL-31 (217). Activation of IL-31RA on basophils provokes the release of IL-4 and IL-13, key cytokine drivers of AD, suggesting a multicellular role for IL-31 to coordinate inflammation and drive itch. Additional insights into the downstream effects of IL-31 have been gained by in vitro studies in which recombinant cytokine is injected into mouse skin. IL-31 influences differentiation and expression of the structural protein filaggrin (61). In AD, impairment of filaggrin results in skin barrier dysfunction (76, 77). These data imply that IL-31 is involved in structural processes in addition to inflammatory ones. The injection of IL-31 daily for 2 wk resulted in keratinocyte proliferation, skin thickening, associated impaired barrier function manifest by increased transepidermal water loss (TEWL), which is a feature of AD, and increased transcription of TRPV2, a signal transducer channel localized on sensory neurons, but not other TRP channels.
2. IL-31 and itch
IL-31 is one of the first cytokines that has been described to directly act on sensory neurons to trigger itch. The initial itch studies were done with osmotic pumps that release IL-31 into wild-type and c-Kit mast cell-deficient mice (69) and transgenic mice overexpressed IL-31. The overexpression resulted in these mice in acanthosis, parakeratosis, and hyperplasia, and an intense immune phenotype infiltrate was present in all and resembled the human disease. Increased numbers of mast cells were noted in the skin of IL-31 transgenic animals, a finding also in human AD. The most direct explanation that accounts for these observations is that while mast cells are not necessary for aspects of the inflammation associated with IL-31, by interacting with its receptor on various cell types, including sensory neurons, this cytokine influences feedback and feed-forward networks between the nervous and immune systems and keratinocytes. In addition, the differential granule release from mast cells, together with pruritogens, exacerbates the itch-scratch cycle. Single injections of IL-31 caused dose-dependent itch in mice that was reduced in mice lacking TRPV1 and TRPA1. These data are consistent with IL-31 acting as a direct pruritogen (55).
It has been suggested that IL-31 elicits acute itch, whereas IL-4 and IL-13 are drivers of some cases of chronic itch. However, due to a constant inflammatory release of IL-31, this cytokine is suitable to target for both acute and chronic itch (FIGURE 6).
FIGURE 6.
Acute and chronic itch in humans. In acute itch, histaminergic itch is induced by histamine via activation of its receptors on sensory neurons. Non-histaminergic itch can be induced by a plethora of molecules, of which some are identified. One such molecule is interleukin (IL)-31 which is released from immune cells including CD4+ TH2 cells and mast cells. Upon release, IL-31 activates a heterodimeric receptor complex consisting of IL-31Rα and oncostatin M receptor β (OSMRβ) on sensory neurons to elicit itch directly. It is possible that this process induces the release of neuropeptides such as substance P (SP) from sensory neurons. SP activates MRGPRX2 on mast cells and may be induced on sensory neurons. Itch triggers degranulation of mast cells and the differential and controlled liberation of various neuropeptides, proteases, and cytokines. A continuous inflammatory milieu may result in chronic itch, which is debilitating in many diseases. IL-31 has been linked to chronic itch skin conditions. During inflammation, immune cells release other cytokines including IL-4 and IL-13 which signal via the heterodimer IL-4Rα and IL-13Rα1 and are reported to function as neuronal enhancers for different itch pathways. Bile acids have recently been reported to activate MRGPRX4 and may thus contribute to cholestatic itch.
The IL-31 responsive neuron population comprises 3–5% of DRG neurons, reflecting transmission via a specific population. The IL-31 population is comprised in the NP3– inflammatory itch sensing neurons which are also activated by histamine (FIGURE 2). The highest overlap of IL-31 responsive neurons was found to be with TRPA1+, consistent with the reduced itch in responses TRPA1 KO mice. IL-31 mediated itch signals through an ERK1/2-dependent mechanism rather than the JAK-STAT pathway. Since ablation of JAK1 in sensory neurons inhibited IL-31 itch (203), the details of signaling in acute versus chronic itch associated with IL-4 and IL-13 require additional clarity. Targeting IL-31 in AD results in significant reduction of itch with no improvement of the inflammation, which raises questions about the connection between itch and inflammation (137). A possible explanation for the missing efficacy in the inflammatory axis might be that a complete depletion of IL-31 is required to block itch and inflammation simultaneously as seen with phototoxic ablation of sensory itch (200) Another neuroimmune-regulatory role of IL-31 is the neuropoietic stimulation on sensory neurons similar to NGF (83) which is consistent with increased nerve fiber density in lesional skin of IL-31 transgenic mice and human AD. Transcriptional profiling of mouse DRGs revealed overlap but also differences between stimulation with IL-31 as compared with NGF. These data should be interpreted with care as NGF is added to the cultured DRG neurons that are stimulated with IL-31. IL-31 stimulation resulted in enhanced expression of neuropoietic genes as compared with NGF (83). Intriguing findings were that the neuropoietic functions exerted by IL-31 were linked to STAT-3 and were TRPV1 independent, which contrasts with the ERK1/2 phosphorylation and presence of TRPV1 associated with itch. IL-31, like other representatives of the IL-6 cytokine family, employs multiple signaling pathways during inflammation, neuropoiesis, and itch.
3. IL-31 in human pruritic diseases
IL-31 levels correlate with the severity of pruritus in AD, prurigo nodularis, and CTCL but not in nonpruritic psoriasis patient samples, consistent with transcriptional upregulation in pruritic AD (53, 194, 256). Expression was induced in human peripheral blood mononuclear cells by staphylococcal superantigen, which is associated with atopy, but not influenced by either influenza or HSV. IL-31 is produced by CTCL T cells, leading to increased IL-31 levels in serum and peripheral blood mononuclear cells, and is correlated with pruritus severity (256). Localization studies with immunofluorescence labeling for IL-31, OSMRβ, and IL-31RA revealed that moderately and severely CTCL skin had higher expression of these components and correlated with visual analogue scale ratings for pruritus. It is possible that IL-31 is the mediator of itch in CTCL patients. The relevance of IL-31 in chronic spontaneous urticaria has been examined (219). IL-31 levels were higher than in negative controls but less than in patients with AD. It has been suggested that in addition to mast cells, IL-31 induces basophil migration and release of IL-4 and IL-13 to contribute to the pathophysiology (218). Overall, the relationship of levels of IL-31, OSMRβ, and IL-31RA in pruritic states, as determined by immunological and molecular approaches, is an active area of investigation. Current observations emphasize the pleiotropic effects of IL-31 and its receptors in inflammatory diseases associated with pruritus.
B. IL-4 and IL-13
The TH2 cytokines IL-4 and IL-13 are major drivers in the proinflammatory orchestration in AD. Both cytokines have been detected at elevated levels in the skin and serum of AD patients. IL-4 is apparent at the onset of AD, whereas IL-13 is associated with chronicity (FIGURE 6). A role for IL-4 and IL-13 in chronic itch, not just AD, has been reported (203). These cytokines activate cultured DRG neurons in mice but do not elicit itch behavior. Rather, IL-4 increases the responsiveness of sensory neurons to other itch mediators, whereas IL-31 activates such neurons and evokes scratching behavior (55). It remains unclear whether IL-13 exerts similar sensitization effects to IL-4. IL-4Rα appears fundamental to chronic itch as conditional knockout mice, lacking IL-4Rα gene in nociceptive neurons, do not develop either chronic itch or histopathological features in the MC903 model of AD-like model. Neither IL-5 nor its receptor had a role in this model. These findings support the cellular and molecular basis for the therapeutic effects of dupilumab, an antibody directed towards the IL-4Rα subunit and the first biologic to treat moderate to severe AD (FIGURES 6 and 7).
FIGURE 7.
Peripheral and central targets of itch. Peripheral itch targets are receptors and mediators of chronic itch including the interleukin (IL)-31 pathway. Targeting IL-4 and IL-13 with dupilumab has been successful in atopic dermatitis. Antagonists of MRGPRX2 might be beneficial in the treatment of inflammation, itch, and urticaria. Antagonism of MRGPX4 has the potential to benefit the treatment of cholestatic itch when driven by bile acids. In neuropathic pain, targeting peripheral GABA-R has proven analgesic properties, which could be translated to itch. Central targets of itch act in the central nervous system and include the use of κ-opioid receptor (KOR) agonists and μ-opioid receptor (MOR) antagonists. Targeting spinal serotonin-pathways via 5-hydroxytryptamine (5-HT) 3 and 7 receptors may achieve clinical success in chronic itch management. Antagonists directed against the NK1R most likely work spinally. Targeting itch-specific circuits such as gastrin releasing peptide receptor (GRPR) might represent a therapeutic avenue. Increasing inhibitory tone via replenishing GABA reduces acute and chronic itch in mice. Gabapentin and pregabalin, widely used to treat neuropathic pain, are prescribed in the clinic to treat chronic itch patients. DRG, dorsal root ganglion; GRP, gastrin releasing peptide; SP, substance P.
XV. OTHER CYTOKINES AND THEIR RELATIONSHIP TO ITCH
IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) are cytokines that function upstream of IL-4, IL-13, and IL-31. They are released from keratinocytes as well as from epithelial cells from other tissues and contribute to the initiation of type 2 inflammation. IL-33 and TSLP evoke scratching behavior in mice and activate mouse sensory neurons (169, 304). None of these cytokines has been shown to evoke itch directly in humans. However, their pleiotropic effects and complex signaling are consistent with at least an indirect impact on itch.
The proinflammatory type 1 cytokine tumor necrosis factor (TNF)-α and its receptor TNFR1 are implicated in acute and chronic itch via peripheral and central sensitization mechanisms in mice (184). Known for its role in chronic pain, cancer, and neuropathy, TNF-α excites and sensitizes primary afferents (130, 233), eventually via TRPV1 upregulation. To evaluate the role of TNF-α in acute itch, the authors employed compound 48/80 and CQ and demonstrated that TNFR1 KO mice showed fewer scratching responses from these itch-inducing agents. Additionally, the dry skin model revealed that TNF-α expression is upregulated in the skin; DRG and the cognate receptor expression is elevated in spinal cord tissue. The findings in mice may correspond to observations in human itch. Thus thalidomide has been effective in many dermatologic disorders, including inflammatory and noninflammatory itches (249). These broad effects likely follow from the impact that thalidomide has on protein ubiquitination (85) and its capacity to decrease TNF-α production (189). Similarly, by regulating neuronal plasticity in pain, TNF-α increases N-methyl-d-aspartate currents in lamina II neurons in the spinal cord and sensitizes spinal cord neurons in chronic itch (180).
A different class of cytokines, namely, chemokines, are regulators of multiple cellular processes (56). Chemokines are expressed in different tissue types, including the central nervous system, and have chemotactic function with implications in neuroinflammation and chronic pain (89–91). CXCL10 signals via CXCR3, a GPCR, both of which have been associated with maintenance of chronic pain post peripheral nerve and chronic constriction injury (57). CXCR3 was recently reported to mediate itch induced by an allergic contact dermatitis mouse model (216). Neuronal responses to CXCL10 were intensified in cultured DRG neurons dissected from mice treated with SADBE as compared with vehicle-treated ones. In a recent study, CXCR3 and its ligand CXCL10 were reported to modulate chronic itch and alloknesis phenomenon induced by the dry skin model and 48/80 injections (131). CXCR3 KO mice exhibited less signs of alloknesis with lower signs of astrocyte activation.
A. JAK Inhibitors
Oclacitinib is a JAK inhibitor (JAKi) approved by the United States Food and Drug Administration in 2013 for the treatment of itch and allergic diseases in canines. The drug was launched in early 2014. The supply was exhausted within 2 mo. This situation exemplified the pent-up demand for effective therapeutics for itch. It raised the profile of the unmet need for both dogs and humans (167). JAKi are emerging as treatments for inflammation in general, including allergic and autoimmune disorders. JAKs were known to be expressed in sensory neurons, which led to the evaluation of a JAKi as a potential therapy for chronic itch (203). The canonical cytokines IL-4 and IL-13 signal via JAKs as do upstream cytokines IL-33 and TSLP. IL-31 transmits itch via MAPK but utilizes JAK signaling to stimulate growth of small fiber neurons. JAK1-JAK3 are also activated by IL-2, IL-7, IL-9, IL-15, and IL-21, whereas IL-6, the prototypic proinflammatory cytokine, signals through JAK1, JAK2, and TYK2 (248).
In the clinic, topical application of tofacitinib, a JAK1/3i, led to itch improvement starting at day 2. Baricitinib, an oral JAK1/2 selective inhibitor, reduced chronic itch and skin inflammation in a phase 2 clinical trial with moderate to severe AD. Genetic depletion of JAK1 in sensory neurons resulted in diminished itch in mice treated with the inflammation inducing vitamin D analogue MC903 (203). Consistent with this finding, a JAK1 gain-of-function point mutation in mice resulted in a pruritic dermatitis phenotype with scratching and stepwise progression into immunological abnormalities (312). The application of petrolatum to the ears masked the damage to the barrier and delayed the immune dysregulation of elevated TH2 cytokines and increased expression of serine proteases, particularly kalikrein-6 and marapsin which are skin barrier regulators. A JAK1 gain-of-function mutation in humans caused severe pruritic dermatitis (67). The JAK inhibitor ruxolitinib led to improvement. Collectively, these data emphasize the role of JAKs in inflammatory conditions and chronic itch. There is a growing interest in developing JAKi as topical treatments for mild-moderate AD to circumvent systemic adverse side effects. Tofacitinib, the first commercially available JAKi, was approved originally for the treatment of rheumatoid arthritis. The ointment formulation of tofacitinib tested in AD patients successfully improved the Eczema Area and Severity Index (EASI) scores to 80% after 4 wk of treatment while showing antipruritic action as early as 24–48 h (36). Similarly, JTE-052 provided itch relief in moderate-to-severe AD patients (192). The relative contributions of neuronal versus immune JAKs in pruritus are being clarified.
XVI. MODELS AND TECHNIQUES
Histamine-induced itch is the most studied and well-understood acute itch despite the findings that most chronic itch forms are of non-histaminergic nature. Preclinical models of non-histaminergic itch are the chemically as well as mechanically induced itch forms broadened with models of dry skin, allergic, atopic, cholestatic, and genetic. Moreover, there has been growing effort to assess itch and the associated dysesthesia in humans with surrogate models. The realization that scratching in animals occurs after algesic and pruritic agents has led to the search for different technical assessments of itch.
A. Measuring Itch in Mice
Behavioral assessment of itch in mice was initially studied by few researchers and was overshadowed by pain research. Behavioral testing to measure itch was undertaken in different ways. Until 2008, pruritogens were assayed by injection into the nape followed by recording and analysis of scratching by bouts over time (149). This approach could not necessarily distinguish pruritic from nociceptive behaviors. An important advance was introduced by Shimada and LaMotte (250). Their cheek model discriminates between itch- and pain-associated behavior (250). Algogens such as capsaicin trigger wiping of the injected ipsilateral cheek with the forelimbs, whereas pruritogens such as histamine evoke scratching via the hindlimbs. This approach is now the standard. Akiyama et al. (4) reported that when mice are injected with algogens such as capsaicin, the mice behavioral responses of wiping were reduced by morphine. In contrast, histamine-evoked scratching was diminished by naltrexone, a μ-opioid antagonist. The authors additionally tested whether spicules, native with cowhage or inactivated, when loaded with histamine or capsaicin would result in wiping or scratching. Cowhage-evoked itch is accompanied by nociceptive sensations such as a stinging, burning sensation (152). Cowhage-evoked scratching peaked between 10 and 15 min while wiping behavior remained through the recording time of 30 min. Even inactivated spicules loaded with histamine elicited more scratch and wiping behavior as compared with the cowhage-loaded ones. Similarly, capsaicin-induced behavior post spicule application was more enhanced than cowhage-elicited responses with more prominent wiping behavior suggesting that the behavioral assessment is more objective than the classical assay. Of note, while cowhage spicules are excellent tools for evaluating itch in humans, mice rapidly remove the spicules, and they are not generally used in mice. By means of the cheek model, the authors uncovered that formalin-induced behavior is more reflective of itch rather than pain or in addition to dose-dependent nociception. Most of the other classical algogens, such as mustard oil and bradykinin, evoked predominantly wiping behavior. LaMotte et al. (153) also introduced another model that provides behavioral differentiation between itch and pain, namely, the calf assay for itch. Processing of somatosensory information for the calf model happens in the lumbar spinal cord region which has been more extensively studied as compared with the trigeminal processing when signals are conveyed from the cheek. An advantage is the better characterized neuronal population in the lumbar spinal cord responding to chemical and mechanical stimuli. Injections of capsaicin evoke licking behavior, whereas histamine injections result in biting responses like paw injections, a behavioral assessment widely used in pain research (316). Profiling neurons from different dermatomes may contribute to more fully understanding similarities and differences between the inputs and the processing of itch and pain.
B. Genetic Models
Another axis through which to study itch is via genetically modified strains of mice in which the mediation, transmission, or coding of itch has been modified (271, 272). Genetic manipulation of the molecules involved in the transmission of itch either heightens or reduces itch or protects from itch. A variety of models have been introduced in preceding sections. Examples include overexpression of the cytokine IL-31, which results in excessive scratching and self-inflicted lesions and transcription factor bhlhb5 KO animals which have a severe neuropathic itch phenotype. One of the early mouse models for itch is the NC/Nga mouse (129, 251). Spontaneous signs and symptoms include itching, erythema, scaling, dryness, and alopecia at 8 wk of age. However, the NC/Nga mice pathophysiology is not exactly predictable and hence bears a poor rate of reproducibility. Models that have been considered to be proxies for AD and chronic itch include the overexpression of cytokines IL-4, and TSLP, separate from IL-31 (314). A mouse model of CTCL was developed by inoculation of human Myla cells in which levels of microRNA (miRNA) were increased in mouse serum (104). This study uncovered an unusual role for miRNA in chronic itch triggered by the pathology of CTCL. Patients with CTCL suffer from chronic itch for which no treatment paradigms have been established. This model provides a molecular basis to understand the origin of chronic itch in CTCL which was linked to levels of circulating miRNA that were also induced in human patients (220).
C. Itch with Sensitization Models
Most commonly used models for itch are sensitization of the skin by application of either haptens or allergens such as ozaxolone, vitamin D analogues, or OVA (179, 289). In correlation with human AD, the oxazolone model presents an epidermal serine protease signature and a concomitant decrease of antimicrobial peptides such as the β-defensin-3. This model, as well as that using the hapten trinitrochlorobenzene applied to the skin of the hairless mouse strain, shifts the TH1 responses towards TH2 as seen in AD (179). Vitamin D [1α,25-(OH)2D3] or MC903 can be used to induce AD-like skin inflammation (165) MC903 sensitization was linked to the overexpression of TSLP. Epicutaneous sensitization with OVA on shaved skin (102) of different strains of mice induces an eruption that mimics the features of human AD (261). Erotic or dry skin itch can be achieved through repeated application of an AEW solution at any skin location with any mouse strain (201). For psoriatic itch, the topical application of imiquimod, a topical anti-cancer drug, results in spontaneous scratching bouts accompanied by alloknesis (239).
D. Human Itch and Surrogate Models
In humans, itch is often accompanied by nociceptive sensations of burning, pricking, and stinging. Psychophysics is used to measure these sensations using the visual analogue scale while the numerical rating scale is used in the setting of clinical trials to measure itch. Latency, peak, and duration can be incorporated into the itch measurements (254, 255). Cowhage is an ideal tool for evaluating itch in humans. Native cowhage elicits itch, burning, pricking, and stinging. In addition, spicules can be readily inactivated in an autoclave and then ‟reconstitutedˮ with other test agents. The spicules function as microneedles and do not generate nociceptive responses when inactivated. Like murine itch models, pruritogens such as histamine, BAM8-22, and serotonin can be tested in humans via injection, but alternatively by reconstituting spicules. Histaminergic itch is conveyed by the mechanoinsensitive C-fiber population, whereas non-histaminergic itch such as cowhage-elicited itch employs polymodal C-fibers (193). Histamine and mast cell degranulators cause the typical wheal, a manifestation of acute protein extravasation, and flare reaction, caused by neuropeptides.
XVII. CONTAGIOUS ITCH
Contagious or social itch is triggered by visual or other cues in the absence of a conventional pruritogen. In contrast to itch from exposure and infection from a contagious organism, such as the scabies mite, contagious itch is triggered by visual exposure to somebody else scratching or the auditory cues, as in a lecture about pruritic dermatological skin conditions (112). Contagious itch via audiovisual transmission is experienced by humans and non-human primates and has been reported in rodents (112, 247), although the significance of this itch is not clear. The initial description was of a human audience exposed to presentations with pictures of insects, scratch marks, and allergic reactions followed by a presentation with soothing cues with pictures of babies. A significant number of scratching movements was measured during the “itchy” presentation, with increased itch perception reported by the audience, which contrasted with the relaxing portion of the presentation. Mirror neurons were identified initially within the ventral premotor area when monkeys conducted a certain movement or in observation of this movement (122).
An effort has been made to associate the cellular and molecular mechanism of contagious itch with the GRP-GRPR axis (315). Here, naive mice were reported to exhibit contagious itch when observing videos of BRAFNaV1.8 mice, which exhibit exaggerated scratching behavior (324), in the absence of olfactory or auditory stimulation. Dependence on SCN was suggested as this area receives direct visual input; contagious itch was absent in GRP and GRPR mutant mice and mice in which GRPR was conditionally depleted in the SCN, yet was restored by delivery of GRP or by optogenetic stimulation (315). The importance of these findings has been questioned, given that contagious itch was not observed in wild-type mice injected with histamine (166).
XVIII. CLINICAL ITCHES
Itch can arise anywhere along the pathways between the skin and brain. This anatomy encouraged itch to be classified as pruritoceptive when arising in the skin, neuropathic when associated with neuronal pathology, and psychogenic when associated with a psychological condition (291). This approach frames a brief discussion, for which multi-authored reviews are available. A recent report that suggests a descriptive classification of chronic itch (264) has the benefit of additional detail incorporating skin findings together with underlying systemic conditions to assist in establishing itch as a disease while potentially being less informative with respect to mechanism and pathophysiology.
Most itches are pruritoceptive. They arise either from exogenous environmental substances or from the persistent presence of endogenous mediators that are produced in or make their way to the skin. Itches associated with insect bites or poison ivy are examples of acute itches, resulting from allergic responses in each case. Chronic itches are often associated with inflammatory skin disease, such as AD. The co-dependency of these itches and connection with neurogenic inflammation are exemplified by the observation that neural lesions that impact the areas of inflammatory skin disease eliminate the sensation of itch while resulting in resolution of the inflammation (26). Chronic itch can also be a part of noninflammatory systemic diseases, including cholestasis, chronic kidney disease, or even pregnancy. These should be viewed as chronic acute itches as the itch rapidly resolves in most instances shortly after organ transplant or birth. The mediators driving these itches are being determined. With respect to cholestatic itch, bile acids interact with MRGPRX4, while lysophosphatidic acid (LPA) produced by autotaxin (148) interacts with LPA receptors on sensory neurons. With respect to the itch of chronic kidney disease, we have hints that there may be parallels with that of cholestatic itch. As an extension, it is tempting to speculate that the severe itches of primary biliary cirrhosis and polycythemia vera may relate to heme metabolism.
There is no shortage of other challenging itches. The advent of targeted anticancer immunotherapies is frequently associated with therapy-limiting pruritus, for which the mechanism is unknown and no animal models are available (306). Neuropathic and psychogenic itches are chronic and frequently intense. Examples of the former include post-herpetic itch, notalgia paresthetica, and brachioradial pruritus which can be associated with nerve compression. A number of severe itches, including genital itch, which likely include degrees of central sensitization, are not readily categorized. Ocular itch, thought to be of an allergic nature, calls for a topical approach for treatment. Itch from parasites, particularly helminths, including nematodes of the genus Strongyloides, causes misery worldwide, regardless of economic background, and often presents as a diagnostic dilemma and marked delay in diagnosis (274). Again, the mediators are not known.
Not only is the treatment of chronic itch a challenge, but also careful evaluation leads to the determination of an underlying etiology in a fraction of patients (306). When a cause cannot be determined, these patients are considered to have chronic pruritus of unexplained origin or CPUO (143), an acknowledgment of the classic description of fever of unexplained origin or FUO by Petersdorf and Beeson (212). While patients with chronic itch frequently note that they would prefer to commit suicide than continue to suffer (101), documented reports of suicide are rare.
We are often asked if itch differs between the sexes. This question, as well as potential differences in itch that could be associated with mental health, economic differences, or race, which assumes that races can be distinguished, has been considered (66). There are no objective data to support any such differences based on physiology or pathophysiology. There are data to suggest that the processing of itch and pain-related sensations can differ between men and women exposed to native cowhage spicules or cowhage spicules prepared with histamine or capsaicin (107). Differences in the presentation of pruritus based on race and sex have been suggested (133). No difference in the prevalence of itch between sexes has been identified when each has the same disease, in this case, chronic kidney disease (47, 116). In our itch clinic, we sometimes have the impression that on average, woman tolerate severe itch better than men do, but we recognize the subjective nature of this comment. Interpretation of any data that suggest differences must take into account potential differences in interview style. For example, asking if a patient itches or has other nociceptive concerns versus whether a patient spontaneously reports itch can impact findings.
Identical questions have been raised with respect to pain with similar findings. In mice, it has been suggested that male mice utilize microglia in the spinal cord to mediate pain, whereas female mice use T cells, a finding that may be influenced by hormonal differences (260). It is thus possible that therapeutic approaches could be targeted to patient physiology.
XIX. TARGETING ITCH
Just as clinical itches can arise anywhere along the pathways between the skin and brain, interruption of a pathway has the potential to modulate itch (FIGURE 7).
Approaches to treating itch are represented in FIGURES 7 and 8. FIGURE 8 is an acknowledgment of the style of Dr. Seuss.
FIGURE 8.
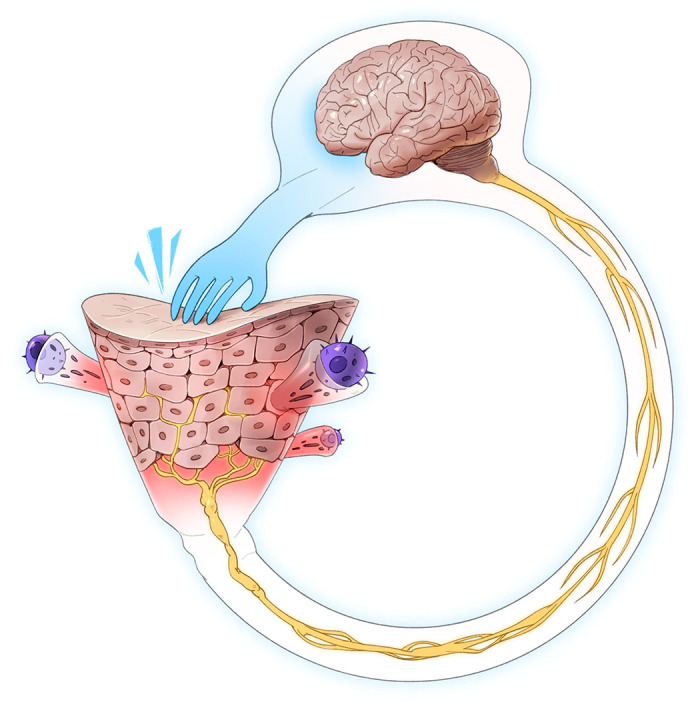
Itch-scratch cycle. The many areas where therapeutic approaches can be directed are depicted. These areas range from the skin barrier, depicted as a funnel with associated immune cells together with afferent fibers that flow into the spinal circuitry and on to the brain.
The skin is viewed as a funnel topped with its barrier and filled with keratinocytes that support a web of sensory fibers. Side chambers topped by inflammatory cells feed into the funnel. The neck of the funnel consists of neuronal fibers that coalesce into the spinal cord. These axons continue to the brain where the signals are interpreted as itch followed by generation of the motor response of scratching which impacts the skin barrier to drive the itch-scratch cycle.
Therapy can thus be directed to protecting or repairing the barrier with topical approaches; blocking sensory neuronal activity or neuropeptide release, immune cell activity, or mediators; or modulating spinal or brain activation or inhibitory pathways. Approaches directed to each of these areas are in clinical use. The combination of fundamental research together with recognition of genetic polymorphisms that impact itch and inflammation may lead to targeted therapeutic approaches with limited off-target effects.
XX. FUTURE DIRECTIONS
Remarkable progress is being made in understanding the physiology of itch. At the same time, we are at the proverbial end of the beginning with respect to having a deep understanding of itch. Questions related to details of mechanisms remain at every level of the itch-scratch cycle, and addressing these questions will provide ongoing opportunities for investigators. The development of humanized mice has the potential to partially overcome the translational gap between preclinical and clinical findings. Why is there so much redundancy with respect to itch signaling pathways? Further to this question, with the recent implication of neutrophils in innervation and itch (297), essentially all cells that populate the skin can contribute to itch. Does itch relate to tickle and touch? Is there communication between free nerve endings, the glial network at the epidermal-dermal junction, Meissner corpuscles, Ruffini endings, and Merkel cells or are these structures independent one from the other? What are the relative and specific contributions of the various receptor and channels involved in itch, including the Piezo channels that are important in touch? It is likely that polymorphisms in receptors and channels, beyond that found already for Nav1.9, will be identified in genetic databases that lead to increased itch. The possibility of finding variants that protect from itch may be elusive as it involves proving a negative.
Imaging techniques are always advancing. The generation of mice in which neurons are functionally labeled with genetically encoded calcium indicators, together with the capacity of genetically labeling cell types in the skin, will allow for detailed visualization of neuroimmune interactions and potentially their bidirectional communication. Can imaging techniques be developed that allow for the visualization of C-fibers in humans in vivo? Will brain and spinal cord imaging in mice and humans eventually lead to resolution on a cellular level and contribute to understanding details of central itch processing and differences with pain?
With respect to itch therapy, as with pain, the placebo effect for drugs or vehicles can exceed 50%. The psychological and psychosomatic factors that contribute in a positive way to the placebo effect are thus important. The inverse, manifest as a negative expectation, or nocebo effect, can be detrimental to therapy (305). How might the learning processes associated with these effects be harnessed to enhance therapeutic benefit? Can we take advantage of inhibitory circuitry to modulate itch? What is the mechanism of action of natural products that may help itch, such as flavonoids? Why are current therapies that target the IL-31 pathway so effective at treating itch but not concurrent inflammation? Besides drugs, physical modalities, such as therapeutic approaches using lasers, already help to relieve itch associated with burn wounds and scars, although the mechanism of efficacy is uncertain. Is such monochromatic light targeting mast cells or something else? Heat and cold can impact neuronal function. Can they be applied to the skin or by point sources in the DRG to target itch? Can objective measures of itch, or other senses, be developed so as to improve upon psychophysical measures and the numerical and visual analog rating scales? Whether itch can be alleviated completely while maintaining other sensory modalities remains to be determined, but we are entering an age in which the suffering from itch may be replaced by the pleasure of scratching.
GRANTS
This work was supported by Pfizer Aspire Program Grant 233429, Dermira, Inc. Grant 231828, and National Institutes of Health Grant 1R21AR067399.
DISCLOSURES
F. Cevikbas holds equity in Dermira, Inc. E. Lerner is on the Scientific Advisory Board of Escient Pharmaceuticals.
ACKNOWLEDGMENTS
Figures 1–7 were designed by Dani Guralnick, DGD LLC. We are most grateful to Jimmy Xia for the whimsical illustration. We thank Anaia Davidson, Lydie Yang, and Malena Ramos for formatting.
Present address of F. Cevikbas: Dermira, Inc., 275 Middlefield Rd., Suite 150, Menlo Park, CA 94025.
Address for reprint requests and other correspondence: E. Lerner, Cutaneous Biology Research Center, Massachusetts General Hospital, 149 13th St., Charlestown, MA 02129 (e-mail: elerner@mgh.harvard.edu).
REFERENCES
- 1.Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang M-D, Usoskin D, El Manira A, Adameyko I, Hjerling-Leffler J, Ernfors P. Specialized cutaneous Schwann cells initiate pain sensation. Science 365: 695–699, 2019. doi: 10.1126/science.aax6452. [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA, Simons FER. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol 533: 69–76, 2006. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 151: 378–383, 2010. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and µ-opoid modulation in mice. Acta Derm Venereol 90: 575–581, 2010. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol 132: 1886–1891, 2012. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Carstens MI, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 6: e22665, 2011. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, Cevikbas F, Kempkes C, Buddenkotte J, Steinhoff M, Carstens E. Involvement of TRPV4 in Serotonin-Evoked Scratching. J Invest Dermatol 136: 154–160, 2016. doi: 10.1038/JID.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Delahanty J, Carstens MI, Carstens E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain 156: 1240–1246, 2015. doi: 10.1097/j.pain.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155: 80–92, 2014. doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol 85: 1–164, 1984. doi: 10.1007/978-3-642-69537-7_1. [DOI] [PubMed] [Google Scholar]
- 11.Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, Anand P. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 138: 667–680, 2008. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Andersen HH, Akiyama T, Nattkemper LA, van Laarhoven A, Elberling J, Yosipovitch G, Arendt-Nielsen L. Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain 159: 1185–1197, 2018. doi: 10.1097/j.pain.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 13.Andersen HH, Arendt-Nielsen L, Gazerani P. Glial Cells Are Involved in Itch Processing. Acta Derm Venereol 96: 723–727, 2016. doi: 10.2340/00015555-2366. [DOI] [PubMed] [Google Scholar]
- 14.Andersen HH, Elberling J, Sølvsten H, Yosipovitch G, Arendt-Nielsen L. Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain 158: 1780–1791, 2017. doi: 10.1097/j.pain.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 15.Andersen HH, Lo Vecchio S, Elberling J, Yosipovitch G, Arendt-Nielsen L. UVB- and NGF-induced cutaneous sensitization in humans selectively augments cowhage- and histamine-induced pain and evokes mechanical hyperknesis. Exp Dermatol 27: 258–267, 2018. doi: 10.1111/exd.13508. [DOI] [PubMed] [Google Scholar]
- 16.Andoh T, Harada A, Kuraishi Y. Involvement of Leukotriene B4 Released from Keratinocytes in Itch-associated Response to Intradermal Interleukin-31 in Mice. Acta Derm Venereol 97: 922–927, 2017. doi: 10.2340/00015555-2697. [DOI] [PubMed] [Google Scholar]
- 18.Andoh T, Kuraishi Y. Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J Dermatol Sci 76: 206–213, 2014. doi: 10.1016/j.jdermsci.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Andoh T, Kuwazono T, Lee J-B, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides 32: 2098–2103, 2011. doi: 10.1016/j.peptides.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Aresh B, Freitag FB, Perry S, Blümel E, Lau J, Franck MCM, Lagerström MC. Spinal cord interneurons expressing the gastrin-releasing peptide receptor convey itch through VGLUT2-mediated signaling. Pain 158: 945–961, 2017. doi: 10.1097/j.pain.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest 62: 626–634, 1990. [PubMed] [Google Scholar]
- 22.Arthur RP, Shelley WB. The role of proteolytic enzymes in the production of pruritus in man. J Invest Dermatol 25: 341–346, 1955. doi: 10.1038/jid.1955.138. [DOI] [PubMed] [Google Scholar]
- 23.Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, Steinhoff M, Chapman K, Zamponi GW, Vergnolle N. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol 150: 176–185, 2007. doi: 10.1038/sj.bjp.0706975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attali P, Gomeni R, Wersinger E, Poli S, Venail F. The Effects of SENS-111, a New H4R Antagonist, on Vertigo Induced by Caloric Test in Healthy Volunteers (HV) Is Related to Plasma Concentrations. Clin Ther 38, 10S: e4, 2016. doi: 10.1016/j.clinthera.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Augé C, Balz-Hara D, Steinhoff M, Vergnolle N, Cenac N. Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil 21: 1189–e107, 2009. doi: 10.1111/j.1365-2982.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 26.Azimi E, Lerner EA, Elmariah SB. Altered manifestations of skin disease at sites affected by neurological deficit. Br J Dermatol 172: 988–993, 2015. doi: 10.1111/bjd.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol 140: 447–453.e3, 2017. doi: 10.1016/j.jaci.2016.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJS, Lerner EA. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 1: e89362, 2016. doi: 10.1172/jci.insight.89362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader M, Alenina N, Andrade-Navarro MA, Santos RA. MAS and its related G protein-coupled receptors, Mrgprs. Pharmacol Rev 66: 1080–1105, 2014. doi: 10.1124/pr.113.008136. [DOI] [PubMed] [Google Scholar]
- 30.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol 232: 1–61, 2001. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 31.Ballerini C, Aldinucci A, Luccarini I, Galante A, Manuelli C, Blandina P, Katebe M, Chazot PL, Masini E, Passani MB. Antagonism of histamine H4 receptors exacerbates clinical and pathological signs of experimental autoimmune encephalomyelitis. Br J Pharmacol 170: 67–77, 2013. doi: 10.1111/bph.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience 142: 1263–1271, 2006. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Bánvölgyi A, Pálinkás L, Berki T, Clark N, Grant AD, Helyes Z, Pozsgai G, Szolcsányi J, Brain SD, Pintér E. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J Neuroimmunol 169: 86–96, 2005. doi: 10.1016/j.jneuroim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol 22, 5 and 6: 390–404, 2011. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- 35.Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci 30: 411–420, 2009. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, Wang C, Purohit V, Mamolo C, Papacharalambous J, Ports WC. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 175: 902–911, 2016. doi: 10.1111/bjd.14871. [DOI] [PubMed] [Google Scholar]
- 37.Borromeo MD, Meredith DM, Castro DS, Chang JC, Tung K-C, Guillemot F, Johnson JE. A transcription factor network specifying inhibitory versus excitatory neurons in the dorsal spinal cord. Development 141: 2803–2812, 2014. doi: 10.1242/dev.105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350: 550–554, 2015. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6: 37–43, 2006. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Bowery NG, Enna SJ. Gamma-aminobutyric acid(B) receptors: first of the functional metabotropic heterodimers [Correction in J Pharmacol Exp Ther 293: 312, 2000.] J Pharmacol Exp Ther 292: 2–7, 2000. [PubMed] [Google Scholar]
- 41.Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. [Correction in Neuron 82: 1407, 2014.] Neuron 82: 522–536, 2014. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braz JM, Juarez-Salinas D, Ross SE, Basbaum AI. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest 124: 3612–3616, 2014. doi: 10.1172/JCI75214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briscoe J, Small S. Morphogen rules: design principles of gradient-mediated embryo patterning. Development 142: 3996–4009, 2015. doi: 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burbach GJ, Kim KH, Zivony AS, Kim A, Aranda J, Wright S, Naik SM, Caughman SW, Ansel JC, Armstrong CA. The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 117: 1075–1082, 2001. doi: 10.1046/j.0022-202x.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- 45.Butler SJ, Bronner ME. From classical to current: analyzing peripheral nervous system and spinal cord lineage and fate. Dev Biol 398: 135–146, 2015. doi: 10.1016/j.ydbio.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameron D, Polgár E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ. The organisation of spinoparabrachial neurons in the mouse. Pain 156: 2061–2071, 2015. doi: 10.1097/j.pain.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carstens E. Many parallels between itch and pain research. Eur J Pain 20: 5–7, 2016. doi: 10.1002/ejp.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport 21: 303–308, 2010. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 51.Caterina MJ, Pang Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals (Basel) 9: 77, 2016. doi: 10.3390/ph9040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 53.Cedeno-Laurent F, Singer EM, Wysocka M, Benoit BM, Vittorio CC, Kim EJ, Yosipovitch G, Rook AH. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol 158: 1–7, 2015. doi: 10.1016/j.clim.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cevikbas F, Braz JM, Wang X, Solorzano C, Sulk M, Buhl T, Steinhoff M, Basbaum AI. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J Allergy Clin Immunol 140: 454–464.e2, 2017. doi: 10.1016/j.jaci.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 133: 448–460.e7, 2014. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610–621, 2006. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Yin D, Fan B, Zhu X, Chen Q, Li Y, Zhu S, Lu R, Xu Z. Chemokine CXCL10/CXCR3 signaling contributes to neuropathic pain in spinal cord and dorsal root ganglia after chronic constriction injury in rats. Neurosci Lett 694: 20–28, 2019. doi: 10.1016/j.neulet.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Christensen AJ, Iyer SM, François A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, Delp SL. In Vivo Interrogation of Spinal Mechanosensory Circuits. Cell Rep 17: 1699–1710, 2016. doi: 10.1016/j.celrep.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colburn RW, Lubin ML, Stone DJJ Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54: 379–386, 2007. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol 91: 552–566, 2012. doi: 10.1016/j.ejcb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Lüscher-Firzlaff J, Lüscher B, Baron JM. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 129: 426–433.e8, 2012. doi: 10.1016/j.jaci.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 62.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature 224: 285–287, 1969. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 63.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3: 1800–1814, 2005. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 64.Cox JJ, Sheynin J, Shorer Z, Reimann F, Nicholas AK, Zubovic L, Baralle M, Wraige E, Manor E, Levy J, Woods CG, Parvari R. Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations. Hum Mutat 31: E1670–E1686, 2010. doi: 10.1002/humu.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cregg R, Laguda B, Werdehausen R, Cox JJ, Linley JE, Ramirez JD, Bodi I, Markiewicz M, Howell KJ, Chen Y-C, Agnew K, Houlden H, Lunn MP, Bennett DLH, Wood JN, Kinali M. Novel mutations mapping to the fourth sodium channel domain of Nav1.7 result in variable clinical manifestations of primary erythromelalgia. Neuromolecular Med 15: 265–278, 2013. doi: 10.1007/s12017-012-8216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dalgard FJ, Svensson Å, Halvorsen JA, Gieler U, Schut C, Tomas-Aragones L, Lien L, Poot F, Jemec GBE, Misery L, Szabo C, Linder D, Sampogna F, Koulil SS, Balieva F, Szepietowski JC, Lvov A, Marron SE, Altunay IK, Finlay AY, Salek S, Kupfer J. Itch and mental health in dermatological patients across Europe: a cross-sectional study in 13 countries. J Invest Dermatol 140: 568–573, 2020. doi: 10.1016/j.jid.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 66a.De Esch IJP, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci 26: 462–469, 2005. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 66b.De Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, Lammel S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 101: 133–151.e7, 2019. doi: 10.1016/j.neuron.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, Schreiber RA, Prendiville JS, Phang MS, Halparin J, Au N, Dean JM, Priatel JJ, Jewels E, Junker AK, Rogers PC, Seear M, McKinnon ML, Turvey SE. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol 139: 2016–2020.e5, 2017. doi: 10.1016/j.jaci.2016.12.957. [DOI] [PubMed] [Google Scholar]
- 68.Dickie AC, Bell AM, Iwagaki N, Polgár E, Gutierrez-Mecinas M, Kelly R, Lyon H, Turnbull K, West SJ, Etlin A, Braz J, Watanabe M, Bennett DLH, Basbaum AI, Riddell JS, Todd AJ. Morphological and functional properties distinguish the substance P and gastrin-releasing peptide subsets of excitatory interneuron in the spinal cord dorsal horn. Pain 160: 442–462, 2019. doi: 10.1097/j.pain.0000000000001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. [Correction in Nat Immunol 6: 114, 2005.] Nat Immunol 5: 752–760, 2004. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 70.Diveu C, Lelièvre E, Perret D, Lak-Hal A-HL, Froger J, Guillet C, Chevalier S, Rousseau F, Wesa A, Preisser L, Chabbert M, Gauchat J-F, Galy A, Gascan H, Morel A. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem 278: 49850–49859, 2003. doi: 10.1074/jbc.M307286200. [DOI] [PubMed] [Google Scholar]
- 71.Dong X, Dong X. Peripheral and Central Mechanisms of Itch. Neuron 98: 482–494, 2018. doi: 10.1016/j.neuron.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632, 2001. doi: 10.1016/S0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 73.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 159: 1417–1432, 2014. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dunford PJ, O’Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol 176: 7062–7070, 2006. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 75.Eberlein B, Eicke C, Reinhardt H-W, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J Eur Acad Dermatol Venereol 22: 73–82, 2008. doi: 10.1111/j.1468-3083.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 76.Elias PM. Therapeutic Implications of a Barrier-based Pathogenesis of Atopic Dermatitis. Ann Dermatol 22: 245–254, 2010. doi: 10.5021/ad.2010.22.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol 9: 437–446, 2009. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emrick JJ, Mathur A, Wei J, Gracheva EO, Gronert K, Rosenblum MD, Julius D. Tissue-specific contributions of Tmem79 to atopic dermatitis and mast cell-mediated histaminergic itch. Proc Natl Acad Sci USA 115: E12091–E12100, 2018. doi: 10.1073/pnas.1814132115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90: 169–180, 1997. doi: 10.1016/S0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 80.Esancy K, Condon L, Feng J, Kimball C, Curtright A, Dhaka A. A zebrafish and mouse model for selective pruritus via direct activation of TRPA1. eLife 7: e32036, 2018. doi: 10.7554/eLife.32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faber CG, Hoeijmakers JGJ, Ahn H-S, Cheng X, Han C, Choi J-S, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JPH, Waxman SG, Merkies ISJ. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 71: 26–39, 2012. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 83.Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, Hevezi P, Plesser K, Schrumpf H, Krjutskov K, Sergeeva O, Müller HW, Tsoka S, Kere J, Dillon SR, Steinhoff M, Homey B. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol 138: 500–508.e24, 2016. doi: 10.1016/j.jaci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 84.Feng J, Yang P, Mack MR, Dryn D, Luo J, Gong X, Liu S, Oetjen LK, Zholos AV, Mei Z, Yin S, Kim BS, Hu H. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat Commun 8: 980, 2017. doi: 10.1038/s41467-017-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith REJ, Harper JW, Jenkins JL, Thomä NH. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512: 49–53, 2014. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, Charles R-P, Beermann F, Stehle J-C, Breiden B, Sandhoff K, Rotman S, Haftek M, Wilson A, Ryser S, Steinhoff M, Coughlin SR, Hummler E. PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat Commun 2: 161, 2011. doi: 10.1038/ncomms1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, Ra C, Okayama Y. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 134: 622–633.e9, 2014. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Gaffal E, Glodde N, Jakobs M, Bald T, Tüting T. Cannabinoid 1 receptors in keratinocytes attenuate fluorescein isothiocyanate-induced mouse atopic-like dermatitis. Exp Dermatol 23: 401–406, 2014. doi: 10.1111/exd.12414. [DOI] [PubMed] [Google Scholar]
- 89.Gao Y-J, Ji R-R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 126: 56–68, 2010. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y-J, Ji R-R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 7: 482–493, 2010. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y-J, Zhang L, Ji R-R. Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 58: 1871–1880, 2010. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Z-R, Chen W-Z, Liu M-Z, Chen X-J, Wan L, Zhang X-Y, Yuan L, Lin J-K, Wang M, Zhou L, Xu X-H, Sun Y-G. Tac1-Expressing Neurons in the Periaqueductal Gray Facilitate the Itch-Scratching Cycle via Descending Regulation. Neuron 101: 45–59.e9, 2019. doi: 10.1016/j.neuron.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Geppetti P, Capone JG, Trevisani M, Nicoletti P, Zagli G, Tola MR. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain 6: 61–70, 2005. doi: 10.1007/s10194-005-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Girolamo F, Coppola C, Ribatti D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav Immun 65: 68–89, 2017. doi: 10.1016/j.bbi.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Gmerek DE, Cowan A. Bombesin–a central mediator of pruritus? Br J Dermatol 109: 239, 1983. doi: 10.1111/j.1365-2133.1983.tb07091.x. [DOI] [PubMed] [Google Scholar]
- 97.Gouti M, Metzis V, Briscoe J. The route to spinal cord cell types: a tale of signals and switches. Trends Genet 31: 282–289, 2015. doi: 10.1016/j.tig.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 101: 412–420.e3, 2019. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol 540: 189–207, 2002. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gutierrez-Mecinas M, Bell AM, Marin A, Taylor R, Boyle KA, Furuta T, Watanabe M, Polgár E, Todd AJ. Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 158: 440–456, 2017. doi: 10.1097/j.pain.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halvorsen JA, Dalgard F, Thoresen M, Bjertness E, Lien L. Itch and pain in adolescents are associated with suicidal ideation: a population-based cross-sectional study. Acta Derm Venereol 92: 543–546, 2012. doi: 10.2340/00015555-1251. [DOI] [PubMed] [Google Scholar]
- 102.Han H, Headley MB, Xu W, Comeau MR, Zhou B, Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol 190: 904–912, 2013. doi: 10.4049/jimmunol.1201808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han L, Ma C, Liu Q, Weng H-J, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16: 174–182, 2013. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, Luo X, Zhang X, Nackley A, Dokholyan NV, Ji R-R. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99: 449–463.e6, 2018. doi: 10.1016/j.neuron.2018.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han S-K, Simon MI. Intracellular signaling and the origins of the sensations of itch and pain. Sci Signal 4: pe38, 2011. doi: 10.1126/scisignal.2002353. [DOI] [PubMed] [Google Scholar]
- 106.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res 195: 198–213, 2008. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 107.Hartmann EM, Handwerker HO, Forster C. Gender differences in itch and pain-related sensations provoked by histamine, cowhage and capsaicin. Acta Derm Venereol 95: 25–30, 2015. doi: 10.2340/00015555-1894. [DOI] [PubMed] [Google Scholar]
- 108.Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA. Mast cells in neuroinflammation and brain disorders. [Correction in Neurosci Biobehav Rev 83: 774, 2017.] Neurosci Biobehav Rev 79: 119–133, 2017. doi: 10.1016/j.neubiorev.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Hermanns HM. Oncostatin M and interleukin-31: cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev 26: 545–558, 2015. doi: 10.1016/j.cytogfr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Hill RZ, Morita T, Brem RB, Bautista DM. S1PR3 Mediates Itch and Pain via Distinct TRP Channel-Dependent Pathways. J Neurosci 38: 7833–7843, 2018. doi: 10.1523/JNEUROSCI.1266-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoeijmakers JGJ, Faber CG, Merkies ISJ, Waxman SG. Painful peripheral neuropathy and sodium channel mutations. Neurosci Lett 596: 51–59, 2015. doi: 10.1016/j.neulet.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 112.Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proc Natl Acad Sci USA 109: 19816–19821, 2012. doi: 10.1073/pnas.1216160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Honda T, Kabashima K. Prostanoids in allergy. Allergol Int 64: 11–16, 2015. doi: 10.1016/j.alit.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126: 16–23, 2006. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature 363: 159–163, 1993. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 116.Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: a meta-analysis of cross-sectional studies. Medicine (Baltimore) 97: e10633, 2018. doi: 10.1097/MD.0000000000010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang H, Kuzirian MS, Cai X, Snyder LM, Cohen J, Kaplan DH, Ross SE. Generation of a NK1R-CreER knockin mouse strain to study cells involved in Neurokinin 1 Receptor signaling. Genesis 54: 593–601, 2016. doi: 10.1002/dvg.22985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JGJ, Gerrits MM, Tyrrell L, Lauria G, Faber CG, Dib-Hajj SD, Merkies ISJ, Waxman SG; PROPANE Study Group . Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 137: 1627–1642, 2014. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- 119.Huang J, Polgár E, Solinski HJ, Mishra SK, Tseng P-Y, Iwagaki N, Boyle KA, Dickie AC, Kriegbaum MC, Wildner H, Zeilhofer HU, Watanabe M, Riddell JS, Todd AJ, Hoon MA. Circuit dissection of the role of somatostatin in itch and pain. [Correction in Nat Neurosci 21: 894, 2018.] Nat Neurosci 21: 707–716, 2018. doi: 10.1038/s41593-018-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang T, Lin S-H, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565: 86–90, 2019. doi: 10.1038/s41586-018-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci 13: 2105–2114, 2001. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 122.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol 60: 653–670, 2009. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 123.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology 62: 212–217, 2004. doi: 10.1212/WNL.62.2.212. [DOI] [PubMed] [Google Scholar]
- 124.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han S-K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106: 11330–11335, 2009. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ishii T, Wang J, Zhang W, Mascarenhas J, Hoffman R, Dai Y, Wisch N, Xu M. Pivotal role of mast cells in pruritogenesis in patients with myeloproliferative disorders. Blood 113: 5942–5950, 2009. doi: 10.1182/blood-2008-09-179416. [DOI] [PubMed] [Google Scholar]
- 126.Japtok L, Bäumer W, Kleuser B. Sphingosine-1-phosphate as signaling molecule in the skin: relevance in atopic dermatitis. Allergo J Int 23: 54–59, 2014. doi: 10.1007/s40629-014-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13: 924–935, 2014. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 128.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 129.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol 129: 31–40, 2009. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin X, Gereau RW IV. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26: 246–255, 2006. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jing P-B, Cao D-L, Li S-S, Zhu M, Bai X-Q, Wu X-B, Gao Y-J. Chemokine Receptor CXCR3 in the Spinal Cord Contributes to Chronic Itch in Mice. Neurosci Bull 34: 54–63, 2018. doi: 10.1007/s12264-017-0128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131a.Jinks SL, Carstens E. Skin cooling attenuates rat dorsal horn neuronal responses to intracutaneous histamine. Neuroreport 9: 4145–4149, 1998. doi: 10.1097/00001756-199812210-00027. [DOI] [PubMed] [Google Scholar]
- 132.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28: 7659–7669, 2008. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson MK, Kim JK, Risse G. Do alcoholic Korsakoff’s syndrome patients acquire affective reactions? J Exp Psychol Learn Mem Cogn 11: 22–36, 1985. doi: 10.1037/0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- 135.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 136.Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 70: 3–11, 2013. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 137.Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, Etoh T, Mihara R, Nakano M, Ruzicka T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol 142: 1121–1130.e7, 2018. doi: 10.1016/j.jaci.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 138.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 171: 2474–2507, 2014. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82: 573–586, 2014. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139a.Katsube N, Maruyama M, Andoh T, Kuraishi Y. Involvement of leukotriene B4 in substance P-induced itch-associated response in mice. J Invest Dermatol 117: 1621–1626, 2001. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 140.Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in Neuroimmune Communication and Itch. In: Itch: Mechanisms and Treatment, edited by Carstens E, Akiyama T. Boca Raton, FL: CRC/Taylor & Francis, 2014, chapt. 11. https://www.ncbi.nlm.nih.gov/pubmed/24829999. [PubMed] [Google Scholar]
- 141.Kerr PG, Argiles A, Mion C. Whole blood serotonin levels are markedly elevated in patients on dialytic therapy. Am J Nephrol 12: 14–18, 1992. doi: 10.1159/000168411. [DOI] [PubMed] [Google Scholar]
- 142.Kido-Nakahara M, Buddenkotte J, Kempkes C, Ikoma A, Cevikbas F, Akiyama T, Nunes F, Seeliger S, Hasdemir B, Mess C, Buhl T, Sulk M, Müller F-U, Metze D, Bunnett NW, Bhargava A, Carstens E, Furue M, Steinhoff M. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J Clin Invest 124: 2683–2695, 2014. doi: 10.1172/JCI67323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim BS, Berger TG, Yosipovitch G. Chronic pruritus of unknown origin (CPUO): uniform nomenclature and diagnosis as a pathway to standardized understanding and treatment. J Am Acad Dermatol 81: 1223–1224, 2019. doi: 10.1016/j.jaad.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 144.Kim C-H, Lee JM, Yoo JK, Kim J-S, Kim S-U, Chang K-T, Choo Y-K. Inhibitory Effect of Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice by Histamine H4 Receptor Agonist 4-Methylhistamine. Scand J Immunol 83: 409–417, 2016. doi: 10.1111/sji.12420. [DOI] [PubMed] [Google Scholar]
- 145.Kim S, Barry DM, Liu X-Y, Yin S, Munanairi A, Meng Q-T, Cheng W, Mo P, Wan L, Liu S-B, Ratnayake K, Zhao Z-Q, Gautam N, Zheng J, Karunarathne WKA, Chen Z-F. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 9: ra71, 2016. doi: 10.1126/scisignal.aaf1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koivisto A, Jalava N, Bratty R, Pertovaara A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals (Basel) 11: 117, 2018. doi: 10.3390/ph11040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kollb-Sielecka M, Demolis P, Emmerich J, Markey G, Salmonson T, Haas M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med 33: 125–129, 2017. doi: 10.1016/j.sleep.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Kremer AE, Gonzales E, Schaap FG, Oude Elferink RPJ, Jacquemin E, Beuers U. Serum Autotaxin Activity Correlates With Pruritus in Pediatric Cholestatic Disorders. J Pediatr Gastroenterol Nutr 62: 530–535, 2016. doi: 10.1097/MPG.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 149.Kuraishi Y. Methods for preclinical assessment of antipruritic agents and itch mechanisms independent of mast-cell histamine. Biol Pharm Bull 38: 635–644, 2015. doi: 10.1248/bpb.b15-00090. [DOI] [PubMed] [Google Scholar]
- 150.Lai HC, Seal RP, Johnson JE. Making sense out of spinal cord somatosensory development. Development 143: 3434–3448, 2016. doi: 10.1242/dev.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 15: 19–31, 2014. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 101: 1430–1443, 2009. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol 20: 778–782, 2011. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lampert A, Eberhardt M, Waxman SG. Altered sodium channel gating as molecular basis for pain: contribution of activation, inactivation, and resurgent currents. Handb Exp Pharmacol 221: 91–110, 2014. doi: 10.1007/978-3-642-41588-3_5. [DOI] [PubMed] [Google Scholar]
- 155.Landry M, Liu H-X, Shi T-J, Brumovsky P, Nagy F, Hökfelt T. Galaninergic mechanisms at the spinal level: focus on histochemical phenotyping. Neuropeptides 39: 223–231, 2005. doi: 10.1016/j.npep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 156.Lang PM, Moalem-Taylor G, Tracey DJ, Bostock H, Grafe P. Activity-dependent modulation of axonal excitability in unmyelinated peripheral rat nerve fibers by the 5-HT3 serotonin receptor. J Neurophysiol 96: 2963–2971, 2006. doi: 10.1152/jn.00716.2006. [DOI] [PubMed] [Google Scholar]
- 157.Lansu K, Karpiak J, Liu J, Huang X-P, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, Shoichet BK, Roth BL. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 13: 529–536, 2017. doi: 10.1038/nchembio.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.László V, Rothe G, Hegyesi H, Szeberényi JB, Orsoó E, Schmitz G, Falus A. Increased histidine decarboxylase expression during in vitro monocyte maturation: a possible role of endogenously synthesised histamine in monocyte/macrophage differentiation. Inflamm Res 50: 428–434, 2001. doi: 10.1007/PL00000266. [DOI] [PubMed] [Google Scholar]
- 159.Le Douarin NM, Dupin E. The neural crest in vertebrate evolution. Curr Opin Genet Dev 22: 381–389, 2012. doi: 10.1016/j.gde.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 160.Lee J-H, Park C-K, Chen G, Han Q, Xie R-G, Liu T, Ji R-R, Lee S-Y. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 157: 1393–1404, 2014. doi: 10.1016/j.cell.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 161.Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci 4, Suppl: 1183–1191, 2001. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- 162.Leipold E, Liebmann L, Korenke GC, Heinrich T, Giesselmann S, Baets J, Ebbinghaus M, Goral RO, Stödberg T, Hennings JC, Bergmann M, Altmüller J, Thiele H, Wetzel A, Nürnberg P, Timmerman V, De Jonghe P, Blum R, Schaible H-G, Weis J, Heinemann SH, Hübner CA, Kurth I. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 45: 1399–1404, 2013. doi: 10.1038/ng.2767. [DOI] [PubMed] [Google Scholar]
- 163.Lembo PMC, Grazzini E, Groblewski T, O’Donnell D, Roy M-O, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Ström P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci 5: 201–209, 2002. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 164.Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy 32: 489–498, 2002. doi: 10.1046/j.0954-7894.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 165.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA 103: 11736–11741, 2006. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liljencrantz J, Pitcher MH, Low LA, Bauer L, Bushnell MC. Comment on “Molecular and neural basis of contagious itch behavior in mice”. Science 357: eaan4749, 2017. doi: 10.1126/science.aan4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Little PR, King VL, Davis KR, Cosgrove SB, Stegemann MR. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet Dermatol 26: 23–e8, 2015. doi: 10.1111/vde.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27: 3549–3563, 2013. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt S-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci USA 113: E7572–E7579, 2016. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng H-J, Geng Y, Undem BJ, Kollarik M, Chen Z-F, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Q, Weng H-J, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4: ra45, 2011. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu T, Gao Y-J, Ji R-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull 28: 131–144, 2012. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu T, Han Q, Chen G, Huang Y, Zhao L-X, Berta T, Gao Y-J, Ji R-R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 157: 806–817, 2016. doi: 10.1097/j.pain.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu T, Ji R-R. Toll-Like Receptors and Itch. In: Itch: Mechanisms and Treatment, edited by Carstens E, Akiyama T. Boca Raton, FL: CRC/Taylor & Francis, 2014, chapt. 14. https://www.ncbi.nlm.nih.gov/pubmed/24830024. [Google Scholar]
- 175.Lopes C, Liu Z, Xu Y, Ma Q. Tlx3 and Runx1 act in combination to coordinate the development of a cohort of nociceptors, thermoceptors, and pruriceptors. J Neurosci 32: 9706–9715, 2012. doi: 10.1523/JNEUROSCI.1109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lucarini L, Durante M, Lanzi C, Pini A, Boccalini G, Calosi L, Moroni F, Masini E, Mannaioni G. HYDAMTIQ, a selective PARP-1 inhibitor, improves bleomycin-induced lung fibrosis by dampening the TGF-β/SMAD signalling pathway. J Cell Mol Med 21: 324–335, 2017. doi: 10.1111/jcmm.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lucarini L, Pini A, Rosa AC, Lanzi C, Durante M, Chazot PL, Krief S, Schreeb A, Stark H, Masini E. Role of histamine H4 receptor ligands in bleomycin-induced pulmonary fibrosis. Pharmacol Res 111: 740–748, 2016. doi: 10.1016/j.phrs.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 178.Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, Yu W, Qian A, Zhang Y, Liu S, Yin S, Xu A, Cheng J, Liu Q, O’Neil RG, Xia Y, Ma L, Carlton SM, Kim BS, Renner K, Liu Q, Hu H. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol 141: 608–619.e7, 2018. doi: 10.1016/j.jaci.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Man M-Q, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, Leung DYM, Holleran W, Uchida Y, Elias PM. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol 128: 79–86, 2008. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marshall GE, Shehab SA, Spike RC, Todd AJ. Neurokinin-1 receptors on lumbar spinothalamic neurons in the rat. Neuroscience 72: 255–263, 1996. doi: 10.1016/0306-4522(95)00558-7. [DOI] [PubMed] [Google Scholar]
- 181.Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol 177: 516–538, 2020. doi: 10.1111/bph.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meixiong J, Vasavda C, Green D, Zheng Q, Qi L, Kwatra SG, Hamilton JP, Snyder SH, Dong X. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. eLife 8: e44116, 2019. doi: 10.7554/eLife.44116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 184.Miao X, Huang Y, Liu T-T, Guo R, Wang B, Wang X-L, Chen L-H, Zhou Y, Ji R-R, Liu T. TNF-α/TNFR1 Signaling Is Required for the Full Expression of Acute and Chronic Itch in Mice via Peripheral and Central Mechanisms. Neurosci Bull 34: 42–53, 2018. doi: 10.1007/s12264-017-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Milner P, Bodin P, Guiducci S, Del Rosso A, Kahaleh MB, Matucci-Cerinic M, Burnstock G. Regulation of substance P mRNA expression in human dermal microvascular endothelial cells. Clin Exp Rheumatol 22, Suppl 33: S24–S27, 2004. [PubMed] [Google Scholar]
- 186.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 340: 968–971, 2013. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Molina A, Pituello F. Playing with the cell cycle to build the spinal cord. Dev Biol 432: 14–23, 2017. doi: 10.1016/j.ydbio.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 188.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 108: 595–598, 2002. doi: 10.1016/S0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 189.Mori T, Ito T, Liu S, Ando H, Sakamoto S, Yamaguchi Y, Tokunaga E, Shibata N, Handa H, Hakoshima T. Structural basis of thalidomide enantiomer binding to cereblon. Sci Rep 8: 1294, 2018. doi: 10.1038/s41598-018-19202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, Lyman K, Olsen ASB, Wong JF, Stucky CL, Brem RB, Bautista DM. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 87: 124–138, 2015. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 192.Nakagawa H, Nemoto O, Yamada H, Nagata T, Ninomiya N. Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. J Dermatol 45: 701–709, 2018. doi: 10.1111/1346-8138.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol 100: 2062–2069, 2008. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nattkemper LA, Martinez-Escala M-E, Gelman AB, Singer EM, Rook AH, Guitart J, Yosipovitch G. Cutaneous T-cell Lymphoma and Pruritus: The Expression of IL-31 and its Receptors in the Skin. Acta Derm Venereol 96: 894–898, 2016. doi: 10.2340/00015555-2417. [DOI] [PubMed] [Google Scholar]
- 195.Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, Imayama S, Kato M, Hasebe I, Taira K, Yamamoto M, Mihara R, Kabashima K, Ruzicka T, Hanifin J, Kumagai Y. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol 174: 296–304, 2016. doi: 10.1111/bjd.14207. [DOI] [PubMed] [Google Scholar]
- 196.Nilius B, Mahieu F, Karashima Y, Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem Soc Trans 35: 105–108, 2007. doi: 10.1042/BST0350105. [DOI] [PubMed] [Google Scholar]
- 197.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 198.Nilsen KB, Nicholas AK, Woods CG, Mellgren SI, Nebuchennykh M, Aasly J. Two novel SCN9A mutations causing insensitivity to pain. Pain 143: 155–158, 2009. doi: 10.1016/j.pain.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 199.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, Kajiwara N, Saito H, Nagaoka I, Ogawa H, Okumura K. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol 184: 3526–3534, 2010. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 200.Nocchi L, Roy N, D’Attilia M, Dhandapani R. Interleukin-31-mediated photoablation of pruritogenic epidermal neurons reduces itch-associated behaviours in mice. Nature Biomed Engineering 3: 114–125, 2019. https://www.nature.com/articles/s41551-018-0328-5. [DOI] [PubMed] [Google Scholar]
- 201.Nojima H, Carstens MI, Carstens E. c-fos expression in superficial dorsal horn of cervical spinal cord associated with spontaneous scratching in rats with dry skin. Neurosci Lett 347: 62–64, 2003. doi: 10.1016/S0304-3940(03)00609-8. [DOI] [PubMed] [Google Scholar]
- 202.Nystedt S, Emilsson K, Larsson AK, Strömbeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem 232: 84–89, 1995. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- 203.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh C-S, Gereau RW IV, Miller MJ, Chen Z-F, Hu H, Davidson S, Liu Q, Kim BS. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171: 217–228.e13, 2017. doi: 10.1016/j.cell.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84: 579–621, 2004. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 205.Ostadhadi S, Azimi E, Lerner EA, Dehpour A-R. Are itch and scratching the nausea and vomiting of skin? Exp Dermatol 25: 340–343, 2016. doi: 10.1111/exd.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Osterman PO. Paroxysmal itching in multiple sclerosis. Br J Dermatol 95: 555–558, 1976. doi: 10.1111/j.1365-2133.1976.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 207.Otsuka A, Nomura T, Rerknimitr P, Seidel JA, Honda T, Kabashima K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev 278: 246–262, 2017. doi: 10.1111/imr.12545. [DOI] [PubMed] [Google Scholar]
- 208.Pall PS, Hurwitz OE, King BA, LaMotte RH. Psychophysical measurements of itch and nociceptive sensations in an experimental model of allergic contact dermatitis. J Pain 16: 741–749, 2015. doi: 10.1016/j.jpain.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Patricio ES, Costa R, Figueiredo CP, Gers-Barlag K, Bicca MA, Manjavachi MN, Segat GC, Gentry C, Luiz AP, Fernandes ES, Cunha TM, Bevan S, Calixto JB. Mechanisms Underlying the Scratching Behavior Induced by the Activation of Proteinase-Activated Receptor-4 in Mice. J Invest Dermatol 135: 2484–2491, 2015. doi: 10.1038/jid.2015.183. [DOI] [PubMed] [Google Scholar]
- 210.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 211.Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv 5: 304–311, 2005. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- 212.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 40: 1–30, 1961. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- 213.Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol 9: 3027, 2018. doi: 10.3389/fimmu.2018.03027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pucci M, Pirazzi V, Pasquariello N, Maccarrone M. Endocannabinoid signaling and epidermal differentiation. Eur J Dermatol 21, Suppl 2: 29–34, 2011. doi: 10.1684/ejd.2011.1266. [DOI] [PubMed] [Google Scholar]
- 215.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain 137: 1039–1050, 2014. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain 156: 1737–1746, 2015. doi: 10.1097/j.pain.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Raap U, Gehring M, Kleiner S, Rüdrich U, Eiz-Vesper B, Haas H, Kapp A, Gibbs BF. Human basophils are a source of - and are differentially activated by - IL-31. Clin Exp Allergy 47: 499–508, 2017. doi: 10.1111/cea.12875. [DOI] [PubMed] [Google Scholar]
- 218.Raap U, Weißmantel S, Gehring M, Eisenberg AM, Kapp A, Fölster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol 23: 285–288, 2012. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 219.Raap U, Wieczorek D, Gehring M, Pauls I, Ständer S, Kapp A, Wedi B. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol 19: 464–466, 2010. doi: 10.1111/j.1600-0625.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 220.Ralfkiaer U, Hagedorn PH, Bangsgaard N, Løvendorf MB, Ahler CB, Svensson L, Kopp KL, Vennegaard MT, Lauenborg B, Zibert JR, Krejsgaard T, Bonefeld CM, Søkilde R, Gjerdrum LM, Labuda T, Mathiesen A-M, Grønbæk K, Wasik MA, Sokolowska-Wojdylo M, Queille-Roussel C, Gniadecki R, Ralfkiaer E, Geisler C, Litman T, Woetmann A, Glue C, Røpke MA, Skov L, Odum N. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL). Blood 118: 5891–5900, 2011. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature 440: 1213–1216, 2006. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Reddy VB, Azimi E, Chu L, Lerner EA. Mas-Related G-Protein Coupled Receptors and Cowhage-Induced Itch. J Invest Dermatol 138: 461–464, 2018. doi: 10.1016/j.jid.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Reddy VB, Graham TA, Azimi E, Lerner EA. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J Allergy Clin Immunol 140: 1726–1728, 2017. doi: 10.1016/j.jaci.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28: 4331–4335, 2008. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510: 157–161, 2014. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278: 44400–44404, 2003. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- 227.Rosenbaum T, Simon SA. TRPV1 Receptors and Signal Transduction. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC/Taylor & Francis, 2007, chapt. 5. https://www.ncbi.nlm.nih.gov/pubmed/21204507. [PubMed] [Google Scholar]
- 228.Ross SE, Hachisuka J, Todd AJ. Spinal Microcircuits and the Regulation of Itch. In: Itch: Mechanisms and Treatment, edited by Carstens E, Akiyama T. Boca Raton, FL: CRC/Taylor & Francis, 2014, chapt. 20. https://www.ncbi.nlm.nih.gov/pubmed/24830016. [PubMed] [Google Scholar]
- 229.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65: 886–898, 2010. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rossbach K, Schaper K, Kloth C, Gutzmer R, Werfel T, Kietzmann M, Bäumer W. Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy 71: 189–197, 2016. doi: 10.1111/all.12779. [DOI] [PubMed] [Google Scholar]
- 231.Rostock C, Schrenk-Siemens K, Pohle J, Siemens J. Human vs. Mouse Nociceptors - Similarities and Differences. Neuroscience 387: 13–27, 2018. doi: 10.1016/j.neuroscience.2017.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature 468: 214–222, 2010. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 233.Rozas P, Lazcano P, Piña R, Cho A, Terse A, Pertusa M, Madrid R, Gonzalez-Billault C, Kulkarni AB, Utreras E. Targeted overexpression of tumor necrosis factor-α increases cyclin-dependent kinase 5 activity and TRPV1-dependent Ca2+ influx in trigeminal neurons. Pain 157: 1346–1362, 2016. doi: 10.1097/j.pain.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ru F, Sun H, Jurcakova D, Herbstsomer RA, Meixong J, Dong X, Undem BJ. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol 595: 3651–3666, 2017. doi: 10.1113/JP273795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rüdrich U, Gehring M, Papakonstantinou E, Illerhaus A, Engmann J, Kapp A, Hartmann K, Meyer NH, Gibbs BF, Raap U. Eosinophils Are a Major Source of Interleukin-31 in Bullous Pemphigoid. Acta Derm Venereol 98: 766–771, 2018. doi: 10.2340/00015555-2951. [DOI] [PubMed] [Google Scholar]
- 236.Russ JB, Borromeo MD, Kollipara RK, Bommareddy PK, Johnson JE, Kaltschmidt JA. Misexpression of ptf1a in cortical pyramidal cells in vivo promotes an inhibitory peptidergic identity. J Neurosci 35: 6028–6037, 2015. doi: 10.1523/JNEUROSCI.3821-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, Galus R, Etoh T, Mihara R, Yoshida H, Stewart J, Kabashima K; XCIMA Study Group . Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med 376: 826–835, 2017. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 238.Saeki K, Yokomizo T. Identification, signaling, and functions of LTB4 receptors. Semin Immunol 33: 30–36, 2017. doi: 10.1016/j.smim.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 239.Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain 157: 2536–2543, 2016. doi: 10.1097/j.pain.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Salvatierra J, Diaz-Bustamante M, Meixiong J, Tierney E, Dong X, Bosmans F. A disease mutation reveals a role for NaV1.9 in acute itch. J Clin Invest 128: 5434–5447, 2018. doi: 10.1172/JCI122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JL. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev 14: 2134–2139, 2000. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sanders KM, Sakai K, Henry TD, Hashimoto T, Akiyama T. A Subpopulation of Amygdala Neurons Mediates the Affective Component of Itch. J Neurosci 39: 3345–3356, 2019. doi: 10.1523/JNEUROSCI.2759-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience 158: 189–203, 2009. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schirmer B, Rezniczek T, Seifert R, Neumann D. Proinflammatory role of the histamine H4 receptor in dextrane sodium sulfate-induced acute colitis. Biochem Pharmacol 98: 102–109, 2015. doi: 10.1016/j.bcp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 245.Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacol Exp Ther 329: 314–323, 2009. doi: 10.1124/jpet.108.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci 15: 333–341, 1995. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schut C, Grossman S, Gieler U, Kupfer J, Yosipovitch G. Contagious itch: what we know and what we would like to know. Front Hum Neurosci 9: 57, 2015. doi: 10.3389/fnhum.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. Erratum: JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 17: 78, 2018. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sharma D, Kwatra SG. Thalidomide for the treatment of chronic refractory pruritus. J Am Acad Dermatol 74: 363–369, 2016. doi: 10.1016/j.jaad.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 250.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shiohara T, Hayakawa J, Mizukawa Y. Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci 36: 1–9, 2004. doi: 10.1016/j.jdermsci.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 252.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, Hasegawa A, Nakahara T, Hachisuka J, Akira S, Okano H, Furue M, Inoue K, Tsuda M. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 21: 927–931, 2015. doi: 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- 253.Shore PA, Burkhalter A, Cohn VH Jr. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther 127: 182–186, 1959. [PubMed] [Google Scholar]
- 254.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144: 66–75, 2009. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain 152: 2485–2494, 2011. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, Loren AW, Dentchev T, Wysocka M, Yosipovitch G, Rook AH. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol 133: 2783–2785, 2013. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 257.Smith KM, Boyle KA, Madden JF, Dickinson SA, Jobling P, Callister RJ, Hughes DI, Graham BA. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: implications for spinal pain processing. J Physiol 593: 4319–4339, 2015. doi: 10.1113/JP270855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol 127: 1947–1955, 2007. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- 259.Solorzano C, Villafuerte D, Meda K, Cevikbas F, Bráz J, Sharif-Naeini R, Juarez-Salinas D, Llewellyn-Smith IJ, Guan Z, Basbaum AI. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci 35: 648–657, 2015. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sorge RE, Totsch SK. Sex Differences in Pain. J Neurosci Res 95: 1271–1281, 2017. doi: 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 261.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest 101: 1614–1622, 1998. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ständer S, Augustin M, Roggenkamp D, Blome C, Heitkemper T, Worthmann AC, Neufang G. Novel TRPM8 agonist cooling compound against chronic itch: results from a randomized, double-blind, controlled, pilot study in dry skin. J Eur Acad Dermatol Venereol 31: 1064–1068, 2017. doi: 10.1111/jdv.14041. [DOI] [PubMed] [Google Scholar]
- 263.Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci 38: 177–188, 2005. doi: 10.1016/j.jdermsci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 264.Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, Bergasa NV, Gieler U, Misery L, Wallengren J, Darsow U, Streit M, Metze D, Luger TA, Greaves MW, Schmelz M, Yosipovitch G, Bernhard JD. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 87: 291–294, 2007. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- 265.Steinhoff M, Buddenkotte J, Lerner EA. Role of mast cells and basophils in pruritus. Immunol Rev 282: 248–264, 2018. doi: 10.1111/imr.12635. [DOI] [PubMed] [Google Scholar]
- 266.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 26: 1–43, 2005. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 267.Steinhoff M, Schmelz M, Szabó IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol 17: 709–720, 2018. doi: 10.1016/S1474-4422(18)30217-5. [DOI] [PubMed] [Google Scholar]
- 268.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 269.Su X-Y, Chen M, Yuan Y, Li Y, Guo S-S, Luo H-Q, Huang C, Sun W, Li Y, Zhu MX, Liu M-G, Hu J, Xu T-L. Central Processing of Itch in the Midbrain Reward Center. Neuron 102: 858–872.e5, 2019. doi: 10.1016/j.neuron.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 270.Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X. Leaky Gate Model: Intensity-Dependent Coding of Pain and Itch in the Spinal Cord. Neuron 93: 840–853.e5, 2017. doi: 10.1016/j.neuron.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448: 700–703, 2007. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 272.Sun Y-G, Zhao Z-Q, Meng X-L, Yin J, Liu X-Y, Chen Z-F. Cellular basis of itch sensation. Science 325: 1531–1534, 2009. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat 13: 97–103, 2005. [PubMed] [Google Scholar]
- 274.Tachamo N, Nazir S, Lohani S, Karmacharya P. Strongyloidiasis in the immunocompetent: an overlooked infection. J Community Hosp Intern Med Perspect 6: 32038, 2016. doi: 10.3402/jchimp.v6.32038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157, 2007. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tamamoto-Mochizuki C, Murphy KM, Olivry T. Pilot evaluation of the antipruritic efficacy of a topical transient receptor potential melastatin subfamily 8 (TRPM8) agonist in dogs with atopic dermatitis and pedal pruritus. Vet Dermatol 29: 29–e14, 2018. doi: 10.1111/vde.12486. [DOI] [PubMed] [Google Scholar]
- 277.Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron 73: 1173–1183, 2012. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung W-P, Hofstra CL, Jiang W, Nguyen S, Riley JP, Sun S, Williams KN, Edwards JP, Karlsson L. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther 309: 404–413, 2004. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- 279.Tian B, Wang X-L, Huang Y, Chen L-H, Cheng R-X, Zhou F-M, Guo R, Li J-C, Liu T. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci Rep 6: 36286, 2016. doi: 10.1038/srep36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tibbs GR, Posson DJ, Goldstein PA. Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci 37: 522–542, 2016. doi: 10.1016/j.tips.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 281.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11: 823–836, 2010. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13–27, 2003. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 283.Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci 12: 689–700, 2000. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 284.Tozaki-Saitoh H, Tsuda M. Microglia-neuron interactions in the models of neuropathic pain. Biochem Pharmacol 169: 113614, 2019. doi: 10.1016/j.bcp.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 285.Tsianakas A, Zeidler C, Riepe C, Borowski M, Forner C, Gerss J, Metz M, Staubach P, Raap U, Kaatz M, Urban M, Luger TA, Ständer S. Aprepitant in Anti-histamine-refractory Chronic Nodular Prurigo: A Multicentre, Randomized, Double-blind, Placebo-controlled, Cross-over, Phase-II trial (APREPRU). Acta Derm Venereol 99: 379–385, 2019. doi: 10.2340/00015555-3120. [DOI] [PubMed] [Google Scholar]
- 286.Tsuda M. Spinal dorsal horn astrocytes: new players in chronic itch. Allergol Int 66: 31–35, 2017. doi: 10.1016/j.alit.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 287.Tsuda M. Astrocytes in the spinal dorsal horn and chronic itch. Neurosci Res 126: 9–14, 2018. doi: 10.1016/j.neures.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 288.Tsuda M. Modulation of Pain and Itch by Spinal Glia. Neurosci Bull 34: 178–185, 2018. doi: 10.1007/s12264-017-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tsukumo Y, Harada D, Manabe H. Pharmacological characterization of itch-associated response induced by repeated application of oxazolone in mice. J Pharmacol Sci 113: 255–262, 2010. doi: 10.1254/jphs.10050FP. [DOI] [PubMed] [Google Scholar]
- 290.Turina MC, Landewé R, Baeten D. Lessons to be learned from serum biomarkers in psoriasis and IBD - the potential role in SpA. Expert Rev Clin Immunol 13: 333–344, 2017. doi: 10.1080/1744666X.2017.1244004. [DOI] [PubMed] [Google Scholar]
- 291.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM 96: 7–26, 2003. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 292.Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle 6: 2640–2649, 2007. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- 293.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18: 145–153, 2015. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 293a.Van Genderen PJ, Michiels JJ, Drenth JP. Hereditary erythermalgia and acquired erythromelalgia. Am J Med Genet 45: 530–531, 1993. doi: 10.1002/ajmg.1320450426. [DOI] [PubMed] [Google Scholar]
- 294.Vilceanu D, Honore P, Hogan QH, Stucky CL. Spinal nerve ligation in mouse upregulates TRPV1 heat function in injured IB4-positive nociceptors. J Pain 11: 588–599, 2010. doi: 10.1016/j.jpain.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One 5: e12177, 2010. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wallengren J. Topical aprepitant in clinical and experimental pruritus. Arch Dermatol 148: 957–959, 2012. doi: 10.1001/archdermatol.2012.1018. [DOI] [PubMed] [Google Scholar]
- 297.Walsh CM, Hill RZ, Schwendinger-Schreck J, Deguine J, Brock EC, Kucirek N, Rifi Z, Wei J, Gronert K, Brem RB, Barton GM, Bautista DM. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 8: e48448, 2019. doi: 10.7554/eLife.48448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 78: 312–324, 2013. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Weisshaar E, Dunker N, Röhl F-W, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol 13: 298–304, 2004. doi: 10.1111/j.0906-6705.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 300.Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, Liu W, Lynch V, Asher A, Tsianakas A, Purkins L. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol 143: 1830–1837.e4, 2019. doi: 10.1016/j.jaci.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 301.Wiesenfeld-Hallin Z, Xu XJ. Galanin in somatosensory function. Ann N Y Acad Sci 863: 383–389, 1998. doi: 10.1111/j.1749-6632.1998.tb10708.x. [DOI] [PubMed] [Google Scholar]
- 302.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. J Neurosci 33: 9283–9294, 2013. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155: 285–295, 2013. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wolters F, Peerdeman KJ, Evers AWM. Placebo and Nocebo Effects Across Symptoms: From Pain to Fatigue, Dyspnea, Nausea, and Itch. Front Psychiatry 10: 470, 2019. doi: 10.3389/fpsyt.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wu J, Lacouture ME. Pruritus Associated with Targeted Anticancer Therapies and Their Management. Dermatol Clin 36: 315–324, 2018. doi: 10.1016/j.det.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wu S, Zhu M, Wang W, Wang Y, Li Y, Yew DT. Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund’s adjuvant-induced inflammation. Neurosci Lett 307: 183–186, 2001. doi: 10.1016/S0304-3940(01)01946-2. [DOI] [PubMed] [Google Scholar]
- 308.Wu Z, Yang Q, Crook RJ, O’Neil RG, Walters ET. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154: 2130–2141, 2013. doi: 10.1016/j.pain.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 309.Wunschel EJ, Schirmer B, Seifert R, Neumann D. Lack of Histamine H4-Receptor Expression Aggravates TNBS-Induced Acute Colitis Symptoms in Mice. Front Pharmacol 8: 642, 2017. doi: 10.3389/fphar.2017.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA 95: 6642–6646, 1998. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang S, Liu Y, Lin AA, Cavalli-Sforza LL, Zhao Z, Su B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene 352: 30–35, 2005. doi: 10.1016/j.gene.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 312.Yasuda T, Fukada T, Nishida K, Nakayama M, Matsuda M, Miura I, Dainichi T, Fukuda S, Kabashima K, Nakaoka S, Bin B-H, Kubo M, Ohno H, Hasegawa T, Ohara O, Koseki H, Wakana S, Yoshida H. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J Clin Invest 126: 2064–2076, 2016. doi: 10.1172/JCI82887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yin S, Luo J, Qian A, Du J, Yang Q, Zhou S, Yu W, Du G, Clark RB, Walters ET, Carlton SM, Hu H. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest 123: 3941–3951, 2013. doi: 10.1172/JCI66413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med 202: 541–549, 2005. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yu Y-Q, Barry DM, Hao Y, Liu X-T, Chen Z-F. Molecular and neural basis of contagious itch behavior in mice. Science 355: 1072–1076, 2017. doi: 10.1126/science.aak9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Yuan T, Li J, Shen L, Zhang W, Wang T, Xu Y, Zhu J, Huang Y, Ma C. Assessment of Itch and Pain in Animal Models and Human Subjects. Adv Exp Med Biol 904: 1–22, 2016. doi: 10.1007/978-94-017-7537-3_1. [DOI] [PubMed] [Google Scholar]
- 317.Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol 52: 111–133, 2012. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 318.Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev 92: 193–235, 2012. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhang H, Cang C-L, Kawasaki Y, Liang L-L, Zhang Y-Q, Ji R-R, Zhao Z-Q. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 27: 12067–12077, 2007. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev 19: 347–356, 2008. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhang XY, Wen J, Yang W, Wang C, Gao L, Zheng LH, Wang T, Ran K, Li Y, Li X, Xu M, Luo J, Feng S, Ma X, Ma H, Chai Z, Zhou Z, Yao J, Zhang X, Liu JY. Gain-of-function mutations in SCN11A cause familial episodic pain. Am J Hum Genet 93: 957–966, 2013. doi: 10.1016/j.ajhg.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhang Y, Dun SL, Chen Y-H, Luo JJ, Cowan A, Dun NJ. Scratching activates microglia in the mouse spinal cord. J Neurosci Res 93: 466–474, 2015. doi: 10.1002/jnr.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhang Y, Yan J, Hu R, Sun Y, Ma Y, Chen Z, Jiang H. Microglia are involved in pruritus induced by DNFB via the CX3CR1/p38 MAPK pathway. Cell Physiol Biochem 35: 1023–1033, 2015. doi: 10.1159/000373929. [DOI] [PubMed] [Google Scholar]
- 324.Zhao Z-Q, Huo F-Q, Jeffry J, Hampton L, Demehri S, Kim S, Liu X-Y, Barry DM, Wan L, Liu Z-C, Li H, Turkoz A, Ma K, Cornelius LA, Kopan R, Battey JFJ Jr, Zhong J, Chen Z-F. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest 123: 4769–4780, 2013. doi: 10.1172/JCI70528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, Zhang N, Luo M-H, Tao W, Wang H, Li J, Li J, Qiu B-S, Zhou J-N, Li X, Xu H, Wang K, Zhang X, Liu Y, Richter-Levin G, Xu L, Zhang Z. A neural circuit for comorbid depressive symptoms in chronic pain. [Correction in Nat Neurosci 22: 1945, 2019.] Nat Neurosci 22: 1649–1658, 2019. doi: 10.1038/s41593-019-0468-2. [DOI] [PubMed] [Google Scholar]