Abstract
Exosomes comprise extracellular vesicles (EVs) with diameters between 30 and 150 nm. They transfer proteins, RNA, and other molecules from cell to cell, playing an important role in the interactions between cells. The tumor microenvironment (TME) has been found to contain various cells and molecules that have an important impact on tumor development. In the TME, macrophages have been found to have an important relationship with tumor cells, with tumors recruiting and inducing macrophages to become tumor-associated macrophages (TAMs), which promote tumor development. Recently, exosomes have been found to play a critical role in the interaction between tumor cells and macrophages. Thus, in this review, we summarize the roles and mechanisms of exosomes in the interaction between tumor cells and macrophages and the potential methods by which exosomes are used to target the communication between tumor cells and macrophages to treat cancer.
Keywords: tumor, macrophage, exosome, therapeutic target
Graphical Abstract
Exosomes play a role in bridging the interaction between tumor cells and macrophages. Guo et al. summarize the roles and mechanisms of exosomes in the interaction between tumor cells and macrophages and the potential methods by which exosomes are used to target the interaction to treat cancer.
Main Text
Exosomes are the smallest extracellular vesicles (EVs) with diameters between 30 and 150 nm and play an important role in the interactions between cells.1,2 Exosomes mainly contain proteins and RNAs and can also include glycoconjugates, lipids, and DNAs. Exosomal proteins include integral exosomal membrane proteins, lipid-anchored outer membrane proteins, peripheral surface proteins, lipid-anchored inner membrane proteins, inner peripheral membrane proteins, exosomal enzymes, and soluble proteins.1 Exosomal RNAs consist of mRNA and noncoding RNAs such as microRNAs and long noncoding RNAs (lncRNAs).3 Regarding the biogenesis of exosomes, it is generally thought that vesicles bud into endosomes, which then mature into multivesicular bodies (MVBs) and fuse with the plasma membrane (PM), from which the exosomes are released.1 The specific process is as follows. During the process of maturation of early endosomes into late endosomes, the early endosomal membrane encapsulates specific proteins, lipids, and cytosol to form MVBs. Most MVBs fuse with lysosomes, which degrade their contents, while some MVBs fuse with the PM, through which their contents are released into the extracellular environment.4 Early endosomes form MVBs through many pathways, and the most common pathway depends on endosomal sorting complexes required for transport (ESCRT).2
In recent years, the tumor microenvironment (TME) has been found to have an important impact on tumor development. The TME is generated by tumor cells, containing a variety of molecules and cells that promote tumor metastasis, angiogenesis, drug resistance, and so on.5 In the TME, macrophages have been found to have an important relationship with tumors, with tumors recruiting and inducing macrophages to become tumor-associated macrophages (TAMs) that promote tumor development.
Macrophages are immune cells that play an important role in tissue hemostasis, inflammation, and pathology.6 Macrophage polarization is the process by which macrophages are activated into one of two different phenotypes at specific times and locations via multiple signals.7 Macrophages can be polarized into classically activated M1 macrophages or alternatively activated M2 macrophages.8 These phenotypes have different molecular characteristics and different functions. M1 macrophages appear in an inflammatory environment dominated by Toll-like receptors (TLRs) and interferon (IFN) signaling pathways and are often associated with immune responses to bacterial and intracellular pathogens. M2 macrophages are found in an environment dominated by TH2 responses, such as immune responses to worms and asthma- and allergy-inducing pathogens.7
Some studies have observed that TAMs can present the M1 phenotype, which is shown to inhibit tumorigenesis,9 while others have observed that TAMs present the M2 phenotype, which is shown to promote tumorigenesis,10 and other studies have also observed that TAMs behave as an intermediate state not committed to either the M1 or M2 phenotype. In fact, evolution of the phenotypes and functions of TAMs is a dynamic process. In the early stage, TAMs present the M1 phenotype, after which they are reprogrammed into the M2 phenotype by the regulation of tumor cells and are significantly associated with poor prognosis in human cancer.11, 12, 13 M2-like TAMs can promote tumor metastasis, treatment resistance, and angiogenesis and inhibit tumor immunity.12,14,15 Therefore, the interaction between macrophages and tumor cells plays an important role in the development of tumors.16 In recent years, exosomes have been found to act as bridges connecting macrophages and tumor cells.17
In this review, we summarize the roles and mechanisms of exosomes in the interaction between tumor cells and macrophages as well as the potential methods that use exosomes to target the communication between tumor cells and macrophages to treat cancer.
Cancer Cell-Derived Exosomes Internalized by Macrophages
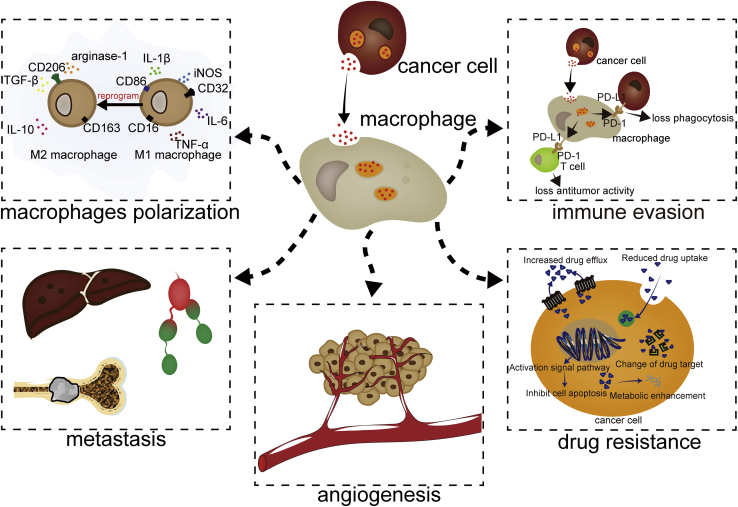
Studies have shown that, in a variety of cancers, such as gastric cancer, breast cancer, hepatocellular carcinoma (HCC), and nasopharyngeal carcinoma, exosomes can be secreted into the TME to regulate the function of neighboring cells, thus creating an environment conducive to tumor development.18, 19, 20, 21 Recent studies have shown that macrophage uptake of exosomes secreted by tumor cells can be regulated in a number of ways.22 For example, intercellular cell adhesion molecule-1 (ICAM-1) is enriched in exosomes derived from pancreatic ductal adenocarcinoma (PDAC) and interacts with CD11c exposed on the surface of macrophages to mediate exosomes docking to macrophages,23 while regenerating islet-derived protein 3β (REG3β), released by paracarcinoma tissue, binds to the glycoproteins exposed on the surface of EVs, interfering with macrophage uptake of these EVs.24 After macrophages internalize exosomes derived from cancer cells, molecules enriched in the exosomes enter the macrophages and regulate their polarization, thereby influencing metastasis, angiogenesis, drug resistance, and immune evasion (Figure 1; Table 1).
Figure 1.
The Activities of Cancer Cell-Derived Exosomes on Macrophages
Exosomes derived from cancer cells are internalized by macrophages to regulate the polarization of macrophages, liver metastasis, bone metastasis, lymphatic metastasis, angiogenesis, drug resistance, and immune evasion.
Table 1.
The Functions and Mechanisms of Cancer-Derived Exosomes on Macrophages
| Cancer | Molecule | Mechanism | Function | References |
|---|---|---|---|---|
| Hepatocellular carcinoma | miR-146a | – | promoting M2 polarization, immune evasion | 25,26 |
| miR-23a-3p | PTEN/PI3K | promoting M2 polarization, immune evasion | 27 | |
| Ovarian cancer | miR-222-3p | – | promoting M2 polarization | 28 |
| miR-1246 | Cav1/p-gp | promoting M2 polarization, resistance against paclitaxel | 29 | |
| Head and neck cancer | miR-21 | – | promoting M2 polarization | 30 |
| Pancreatic cancer | miR-301a-3p | PTEN/PI3K | promoting M2 polarization | 31 |
| MIF | – | promoting pre-metastatic niches formation and metastasis | 32 | |
| Prostate cancer | – | integrin signaling | promoting M2 polarization | 33 |
| MFG-E8 | – | promoting M2 polarization | 34 | |
| OSCC | miR-29a-3p | SOCS1/STAT6 | promoting M2 polarization | 35 |
| Colon cancer | miR-1246 | – | promoting M2 polarization | 36 |
| Colorectal cancer | IRF-2 | inducing macrophage-releasing VEGFC | promoting lymphatic metastasis | 37 |
| Large B cell lymphoma | NSE | inhibiting NF-κB activity | promoting M2 polarization | 38 |
| avβ5 | – | promoting liver metastasis | 39 | |
| Lung adenocarcinoma | miR-21 | targeting Pdcd4 | promoting bone metastasis | 40 |
| NSCLC | AREG | EGFR pathway | promoting bone metastasis | 41 |
| Esophageal squamous cell carcinoma | HMGB1 | – | promoting immune evasion | 42 |
| Melanoma | – | inducing endothelial GM-CSF to induce HIF-1α in M1 or HIF-2α in M2 | promoting angiogenesis | 43 |
–, unknown.
Cancer Cell-Derived Exosomes Regulate the Polarization of Macrophages
Exosomes derived from cancer cells can be internalized by macrophages where they regulate polarization. In most cases, exosomes induce M2 polarization,44, 45, 46 while some studies have confirmed that cancer-derived exosomes can induce M1 polarization.47 The polarization induced may depend on the stage of the cancer.47 Different cell lines from the same type of tumor secrete different exosomes to activate macrophage polarization. In prostate cancer, for example, exosomes secreted by African-American prostate cancer (PCa) e006aa-ht cells strongly induce the pro-inflammatory M2 phenotype.48 Cancer cells deliver molecules such as microRNAs (miRNAs) and proteins to regulate macrophage polarization.
miRNAs
Numerous studies have shown that exosomes secreted by tumors can carry miRNAs and transfer miRNAs into macrophages to regulate the polarization of macrophages. Different tumor-derived exosomes carry different miRNAs. In HCC, miR-146a enrichment in exosomes can promote M2 polarization and inhibit T cell function.25 In ovarian cancer, exosomal miR-222-3p derived from ovarian cancer cells is an effective regulator of M2 polarization that promotes cancer development.28 Further studies have shown that exosomal miRNAs secreted by tumor cells can regulate the polarization of macrophages through a variety of pathways.
PTEN/PI3K/AKT Pathway
Researches have reported that the activation of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway is the important way for M2 polarization, while phosphatase and tensin homolog (PTEN) inhibits the PI3K/AKT pathway, suppressing the upregulation of M2-related genes, such as CD68, CD204, and arginase-1.49, 50, 51, 52 In HCC, exosomal miR-23a-3p downregulates PTEN expression and then upregulates phosphorylated AKT and PD-L1 expression in macrophages, suggesting that macrophages expressing PD-L1 are regulated by exosomes derived from HCC cells via an exosomal miR-23a/PTEN/AKT pathway.27 In pancreatic cancer, hypoxia-related exosomal miR-301a-3p promotes M2 polarization by inhibiting PTEN and activating the PI3Kγ signaling pathway.31
Integrin Signaling
STAT1 is the main transcriptional factor that regulates M1 macrophage polarization, while STAT6 promotes M2 macrophage polarization.53,54 Integrin β3 activates STAT1 signaling and suppresses STAT6 signaling, thereby promoting macrophage polarization into the M1 type. However, the expression of integrin β3 is promoted by STAT6 and inhibited by STAT1 to maintain a balance between STAT1 and STAT6 signal transduction. Thus, loss of integrin β3 disrupts this balance and promotes M2 polarization.55 In prostate cancer, the most abundant miRNAs in PC3 exosomes significantly downregulate integrin β3 expression, inducing macrophage M2 polarization.33
SOCS1/STAT6 Pathway
It is known that STAT6 signaling plays a critical role in promoting M2 polarization.53 SOCS1 contains an SH2 domain that induces proteasomal degradation, which can suppress STAT6 signaling.56 In oral squamous cell carcinoma (OSCC), OSCC-derived exosomes with captured miR-29a-3p directly target SOCS1 and downregulate its expression, thereby stimulating the STAT6 signal and promoting M2 macrophage polarization.35
The process by which cancer cells secrete exosomal miRNAs is regulated by many factors, including the physicochemical features of the TME and some transcriptional factors. One of the physicochemical features of the TME is hypoxia, and the harmful TME interferes with the ability of the endoplasmic reticulum (ER) to fold proteins, causing ER stress.57,58 Both of them can promote cancer cells to secrete exosomal miRNAs. In epithelial ovarian cancer (EOC), hypoxia induces miRNAs in EOC cell-derived exosomes to promote M2 macrophage polarization via hypoxia-inducible factors (HIFs).17 In HCC, ER-stressed HCC cells release exosomal miR-23a-3p to upregulate PD-L1 expression in macrophages.27
In addition, some transcription factors can affect the process of cancer cell secretion of exosomal miRNA, such as spalt-like transcription factor 4 (SALL4), epithelial-to-mesenchymal transition-activating transcription factors (EMT-TFs), and p53.26,30,36 SALL4 is an oncofetal protein that is associated with poor HCC prognosis.59 It can bind to the promoter of mir-146a-5p to directly regulate HCC cell secretion of exosomal mir-146q-5p to promote M2 polarization.26 Increasing evidence supports that EMT-TFs, such as SNAIL, TWIST, and ZEB families, can induce the expression of certain miRNAs, playing an important role in tumorigenesis.60,61 Overexpressed SNAIL can directly activate the transcription of miR-21 that is enriched in tumor-derived exosomes to promote M2-like polarization.30
Proteins
In addition to miRNAs, cancer cell-derived exosomes can carry proteins to promote the polarization of macrophages. Neuron-specific enolase (NSE) is a glycolytic enzyme present in neurons that is produced in significant amounts by all types of neuroendocrine neoplasia cells (APUDomas).62 In large B cell lymphoma, lymphoma-derived exosomes mediate NSE into macrophages to promote M2 polarization by enhancing nuclear p50 translocation, which causes the loss of classical nuclear factor κB (NF-κB) activity.38 Milk fat globule-epidermal growth factor (EGF) factor 8 (MFG-E8), a member of the discoidin family, binds to apoptotic cells via phosphatidylserine and mediates the engulfment of apoptotic cells by macrophages.63,64 It has been confirmed that exosomes derived from prostate cancer have higher expression levels of MFG-E8, which can induce prostate cancer-associated macrophages.34 In colorectal cancer (CRC), the proteome of CRC cell exosomes that educates tumor-favorable macrophages primarily focuses on promoting cytoskeletal rearrangement.65
Cancer Cell-Derived Exosomes Influence Tumor Metastasis via Macrophages
Liver Metastasis
According to the “seed and soil” hypothesis,66 tumors can form a microenvironment conducive to tumor development in distant organs before distant metastasis occurs, and this microenvironment is called a premetastatic niche.67 It has been demonstrated that, in a variety of tumors, exosomes secreted by cancer cells can reach liver and promote premetastatic niche formation through a multistep process to promote liver metastasis, and this process includes uptake of exosomes by liver Küpffer cells, which are a unique type of macrophage, to create a fibrotic microenvironment with immune cells that is conducive to metastasis.68 PDAC-derived exosomes induce Küppfer cells to secrete transforming growth factor β (TGF-β) and induce hepatic stellate cells to express fibronectin to enhance the recruitment of bone marrow-derived macrophages. Macrophage migration inhibitory factor (MIF) is highly expressed in PDAC-derived exosomes having a vital role in that process to promote premetastatic niche formation and metastasis.32 Integrins are the main cellular adhesion receptors involved in the process of primary tumor development of metastasis.69 Results from exosomal proteomics analyses revealed that exosomal integrin avβ5 is involved in liver metastasis.22 Tumor-derived exosomes expressing integrin avβ5 specifically bind to Küppfer cells, which recognize the liver as a target organ for the formation of premetastatic niches.39
Bone Metastasis
Osteoclasts are also a unique type of macrophage. Osteoclastogenesis is a vital step in bone metastasis. Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis by targeting programmed cell death 4 (PDCD4).40 Amphiregulin (AREG) is a member of the EGF family that promotes cancer cell growth and survival.70 Non-small-cell lung cancer (NSCLC)-derived exosomes enriched in AREG increase the expression of receptor activator of NF-κB ligand (RANKL) in preosteoclasts through the EGF receptor (EGFR) pathway. RANKL can induce the expression of proteolytic enzymes, thereby promoting osteolytic bone metastasis.41
Lymphatic Metastasis
Similarly to liver metastasis, premetastatic niches are also very important to lymphatic metastasis. One of the features of the premetastatic niches in the lymph node is lymphatic network remodeling, which involves lymphatic enlargement and lymphangiogenesis.71,72 CRC exosomal IRF-2 induces the release of vascular endothelial growth factor C (VEGFC) from macrophages, thereby remodeling the lymphatic network in a sentinel lymph node, serving as a biomarker for predicting the development of CRC lymph node metastasis.37
Cancer Cell-Derived Exosomes Influence Angiogenesis via Macrophages
Macrophages are important for inducing angiogenesis during tumor growth because they produce angiogenic factors.73 Angiogenesis is mediated by many factors, such as tumor necrosis factor α (TNF-α) and interleukin 8 (IL-8).74,75 Endothelial cell expression of granulocyte-macrophage colony stimulating factor (GM-CSF) is induced by melanoma exosomes that stimulate HIF-1α in M1 or HIF-2α in M2 polarized macrophages. HIF-1α promotes neoangiogenesis, while HIF-2α facilitates morphogenic normalization of the neovasculature. Thus, HIFs expressed by M1 or M2 macrophages can be stimulated by GM-CSF induced by melanoma cell exosomes to promote angiogenesis.43
Cancer Cell-Derived Exosomes Influence Drug Resistance via Macrophages
As mentioned above, cancer cell-derived exosomes regulate the polarization of macrophages, and M2 macrophages have been shown to promote drug resistance through multiple pathways.76,77 Therefore, the current study found that cancer cell-derived exosomes mainly promoted drug resistance by regulating macrophage M2 polarization. In ovarian cancer, exosomal miR-1246 confers paclitaxel (PTX) resistance via targeting Cav1/p-gp/M2-type macrophage axis.29 Interestingly, macrophages can also secrete exosomes to promote drug resistance by regulating the cancer cell phenotype,78 indicating that exosomes may be involved in a positive feedback loop between cancer cells and macrophages to enhance tumor drug resistance.
Cancer Cell-Derived Exosomes Influence Tumor Immune Evasion via Macrophages
Cancer cell-derived exosomes can promote tumor immune evasion.25 One way for this to occur is through macrophages.79 Alternatively, cancer cell-derived exosomes can affect the function of macrophages to directly promote immune evasion. In a variety of tumors, cancer cell-derived exosomes can promote the expression of PD-1 by macrophages.44,42 The increased expression of PD-1 significantly reduces the phagocytosis of macrophages against cancer cells, thereby inducing immune evasion.12 Alternatively, cancer cell-derived exosomes can enable macrophages to regulate the anti-tumor function of other immune cells, thereby inducing immune evasion. Tumor-derived exosomes can upregulate the expression of PD-L1 in macrophages, inhibiting the function of T cells, leading to immune evasion.27,80
Macrophage-Derived Exosomes Influence Tumor Cells
Macrophage-Derived Exosomes Regulate Tumor Invasion and Metastasis
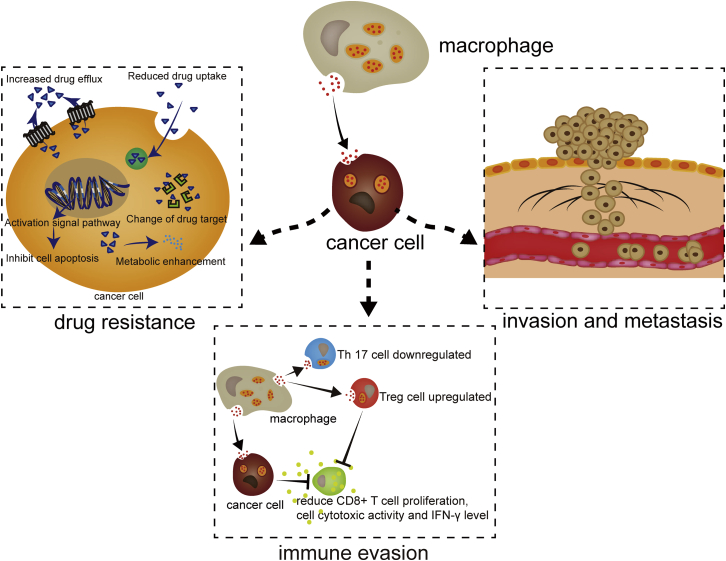
In most cases, TAMs show an M2 phenotype and thus promote tumor invasion and metastasis (Figure 2; Table 2). In HCC, miR-125a and miR-125b are expressed at low levels in exosomes, thus promoting HCC invasion.81 TGF-β receptor 3 (TGFβR3) is a transmembrane proteoglycan that binds TGF-β in a cell type-specific manner and presents TGF-β to TGFβR1 or TGFβR2.82 PDAC macrophages secrete miR-501-3p, which inhibits TGFBR3, leading to activation of TGF-β signal transduction to promote PDAC cell invasion.83 Brahma-related gene 1 (BRG1) is an ATPase subunit of SWI/SNF complexes that has been implicated in different human cancers.84,85 In colon cancer, miR-21 and miR-155 are highly expressed in macrophage-derived exosomes and downregulate the expression of BRG1 by binding to the BRG1 coding sequence, promoting metastasis.86 Myocyte enhancer factor 2C (MEF2C) is a member of the MADS-box transcription factor family and is an important regulator of skeletal muscle development.87 Reduced MEF2C expression is associated with nuclear accumulation of β-catenin and cell migration.88 Exosomal miR-233 derived from macrophages promotes breast cancer invasion through the MEF2C-β-catenin pathway.88
Figure 2.
The Activities of Macrophage-Derived Exosomes on Cancer Cells
Exosomes derived from macrophages are internalized into cancer cells, regulating invasion, metastasis, drug resistance, and immune evasion.
Table2.
The Functions and Mechanisms of Macrophage-Derived Exosomes on Cancer Cells
| Cancer | Molecule | Mechanism | Function | References |
|---|---|---|---|---|
| HCC | miR-125 | – | promoting invasion | 81 |
| miR-142, miR-223 | – | inhibiting proliferation | 89 | |
| PDAC | miR-501-3p | inhibiting TGFBR3, activating TGF-β signal | promoting invasion | 83 |
| miR-365 | – | inducing resistance against gemcitabine | 90 | |
| Colon cancer | miR-21, miR-155 | downregulating BRG1 | promoting metastasis | 86 |
| Breast cancer | miR-233 | Mef2c/β-catenin | promoting invasion | 88 |
| IL-6 | STAT3 pathway | promoting proliferation and metastasis | 91 | |
| ADAM15 | inhibiting avβ3 | inhibiting adhesion, growth and migration | 92 | |
| Recipient gastric cancer | ApoE | PI3K/AKT | promoting migration | 93 |
| miR-21 | – | inducing resistance against DDP | 94 | |
| Glioblastoma | miR-21 | targeting PDCD4 | inducing resistance against temozolomide | 95 |
| modulating PEG3 | inducing immune evasion | 96 | ||
| EOC | miR-223 | PTEN-PI3K/AKT | inducing chemotherapy resistance | 78 |
| miR-29a-3p, miR-21-5p | upregulating Treg/Th17 ratio | inducing immune evasion | 97 |
–, unknown
IL-6 is a pleiotropic cytokine that regulates the immune system and has pathological roles in inflammation, autoimmunity, and cancer.98 Macrophages exposed to apoptotic cancer cells secrete exosomes with increasing amounts of IL-6 to stimulate the phosphorylation of STAT3, thereby promoting breast cancer proliferation and metastasis.91 Apolipoprotein E (ApoE) is a secreted protein involved in lipoprotein metabolism that regulates metastasis.99 Exosomes derived from M2 macrophages transfer ApoE to recipient gastric cancer cells, remodeling cytoskeleton-supported migration via the PI3K-AKT signaling pathway.93
TAMs can also show an M1 phenotype and inhibit invasion and metastasis. M1 macrophage-derived exosomes create a pro-inflammatory environment, enhancing antitumor activity via the caspase-3 pathway.100 Exosomal miR-142 and miR-223 derived from macrophages affect the posttranscriptional regulation of proteins in HCC cells to inhibit their proliferation.89 A disintegrin and metalloprotease 15 (ADAM15) is a member of the ADAM family that regulates cell survival and inflammatory responses.101 Phorbol 12-myristate 13-acetate, a typical protein kinase C activator, stimulates macrophages to release exosomal ADAM15, suppressing vitronectin- and fibronectin-induced tumor growth.92
Exosomes Derived from Macrophages Enhance Drug Resistance
Macrophages can induce chemotherapy resistance in tumor cells (Figure 2).102 One of the mechanisms by which this resistance is conferred involves the release of exosomes (Table 2). Exosomal miR-223 induces chemotherapy resistance EOC cells through a novel exosomal miR-223/PTEN-PI3K/AKT signaling pathway.78 Temozolomide is an alkylating antineoplastic drug that is used to treat patients with glioblastoma (GBM) multiforme.103 GBM-associated macrophages secrete exosomal miR-21 to target PDCD4, which enhances GBM cell resistance against temozolomide.95 For EOC treatment, cisplatin (DDP) is a widely used platinum-based compound with clinical activity against a variety of solid tumors, including testicular, bladder, ovarian, colorectal, lung, and head and neck cancers.104 Exosomes transfer miR-21 from macrophages to gastric cancer cells to confer DDP resistance.94 Gemcitabine is the most important cytidine analog with a strong antitumor effect on a variety of tumors.105 The sensitivity of PDAC cells to gemcitabine is significantly decreased by the transfer of miR-365 through macrophage-derived exosomes.90
Exosomes Derived from Macrophages Promote Tumor Immune Evasion
Macrophages are regulators of tumor immunity.106 Macrophage-derived exosomes can reduce the attack of immune cells on tumor cells by changing the characteristics of tumor cells, thereby inducing immune evasion. Macrophage-derived exosomes can increase the expression of miR-21 in tumor cells, which reduces the expression of paternally expressed gene 3 (PEG-3), resulting in reduced CD8+ T cell proliferation, cell cytotoxic activity, and IFN-γ level, thereby promoting immune evasion of glioma cells.96 In addition, in most cases, exosomes derived from macrophages can induce immune evasion by regulating the function of other immune cells. In EOC, macrophage-derived exosomal miR-29a-3p and miR-21-5p can upregulate the regulatory T cell (Treg)/T helper (Th)17 cell ratio, promoting immune evasion (Figure 2; Table 2).97
The Role of Communication between Tumor Cells and Macrophages in Cancer Treatment
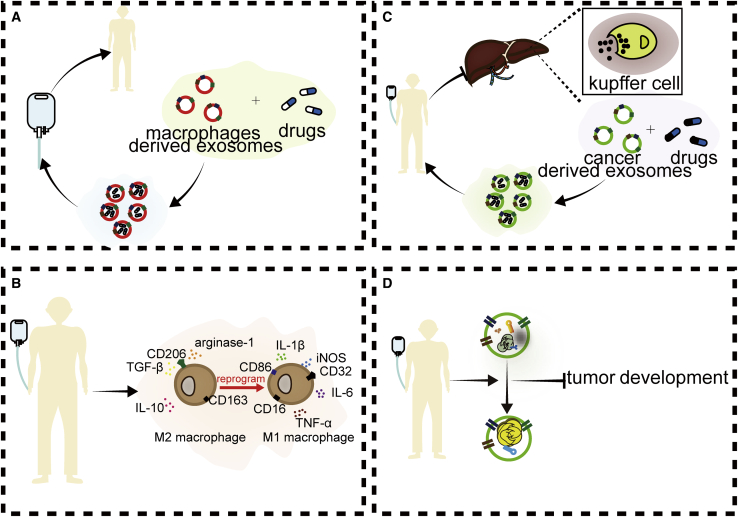
Because of the critical role of exosomes in the communication between tumor cells and macrophages, many studies have attempted to achieve therapeutic goals by altering the state of tumor cells directly or indirectly by remodeling the exosomes that connect tumors and macrophages (Figure 3).
Figure 3.
Therapies Targeting the Communication between Tumor Cells and Macrophages via Exosomes
(A) Using macrophage-derived exosomes to deliver drugs to improve drug delivery efficiency and efficacy. (B) Using drugs to reprogram M2 macrophages to M1 macrophages. (C) Using cancer cell-derived exosomes to deliver drugs to prevent the phagocytosis of macrophages. (D) Using drugs to alter exosomal content.
Target Macrophage-Derived Exosomes
Because of the chemotherapy resistance characteristics of tumors and the antitumor function of M1 macrophages, many studies have sought to use M1 macrophage-derived exosomes to deliver drugs to improve drug delivery efficiency and efficacy. For example, macrophage-derived exosomes carrying acridine orange (Exo-AO) are more potent than free AO against melanoma cells. Exo-AO has longer retention and higher cytotoxicity than does free AO.107 Treatment with M1 macrophage-derived exosomes carrying PTX (PTX-M1-Exos) showed a greater antitumor effect than did treatment with non-PTX-containing M1 macrophage-derived exosomes or with PTX alone.100 Exo-PTX has great potential for delivering a variety of chemotherapeutic drugs and enhancing antitumor effects against drug-resistant cancers.100,108 M1 exosomes can also be used as vaccine adjuvants. They induce Th1 cytokine release, enhance the activity of a lipid calcium phosphate nanoparticle-encapsulated Trp2 vaccine, and induce a stronger antigen-specific cytotoxic T cell response.109 Rayamajhi et al.110 used small EVs (sEVs) from mouse macrophages for hybridization with synthetic liposomes to form hybrid exosomes (HEs). It was found that HEs loaded with water-soluble doxorubicin had enhanced antitumor toxicity and exhibited pH-sensitive drug release under acidic conditions, which facilitated targeted drug delivery to tumors because the TME is acidic.
In addition, people intend to use drugs to regulate the production of macrophage-derived exosomes or use M1 macrophage-derived exosomes to alter macrophage phenotypes to change the composition of macrophage-derived exosomes. In HCC cells, TNF-like weak inducer of apoptosis (TWEAK) increases the level of miR-7 in macrophage-derived exosomes and inhibits tumor metastasis by inhibiting the EGFR/AKT/extracellular signal-regulated kinase (ERK)1/2 pathway.111 Choo et al.112 found that exosome-mimetic nanovesicles derived from M1 macrophages can reprogram M2 macrophages into M1 macrophages and enhance the antitumor activity of checkpoint inhibitors.
Target Cancer-Derived Exosomes
Because exosomal molecules secreted by tumor cells can promote the development of tumors by regulating the polarization of macrophages, researchers have sought to suppress the process and intend to reverse it by modifying the expression of the molecules in exosomes secreted by tumors.113,114 Dahuang Zhechong pill has been documented for the treatment of abdominal masses for thousands of years. It can reduce the expression of CCL2 in CRC-derived exosomes and inhibit CCL2-mediated M2-promoting paradigm to improve the profibrotic microenvironment and inhibit liver metastasis of CRC.115 Exosomes extracted from ECGC-treated breast cancer cells exhibit increased expression of miR-16 and inhibited TAM infiltration and M2 polarization.116
Using nanoparticles to deliver chemotherapy drugs to tumor cells has made great progress in recent years, but a large portion of nanoparticles preferentially enter the liver and are engulfed by macrophages, especially Küpffer cells, which reduces the concentration of the drug in the target organ.117 It is important for nanoparticles to escape elimination by macrophages. Utilizing the unique properties of exosomes can improve the efficiency and efficacy of drug delivery. After exosome-like nanovesicles (ENVs) produced by transforming metastatic breast cancer 4T1 cell-derived exosomes were injected intravenously into mice, they can be preferentially taken up by Küpffer cells. Pretreatment with ENVs leads to changes in gene expression of macrophage phagocytosis because of the translocation of membrane nucleolin from the inner face of the PM to the cell surface and intercellular Ca2+ fluxes, resulting in a decrease in cationtic, 2-dioleoyl trimethylammonium propane (DOTAP)/1, 2-dioleoyl-sn-glycerol-3-phosphoethanolamine (DOPE) liposome (DDL) uptake in the liver. Therefore, more doxorubicin-loaded DDL is transported to the lungs, increasing drug delivery efficiency.118 Other researchers have injected modified peripheral blood-isolated exosomes before intravenous injection of grapefruit nanovectors (GNVs), which reduced the accumulation of GNVs in the liver and redirected GNVs to the lungs, resulting in a higher efficiency of GNV drug delivery.119 Although the carcinogenic components in exosomes have been removed in the study, there are still potential risks in clinical application because the exosomes are isolated from cancer cells. Once the safety of this treatment has been clarified, gradually carrying out relevant clinical trials may be a goal worth pursuing.
The use of exosomes to treat tumors is still in the exploration stage. Scientists are continuously exploring methods to improve the effectiveness of tumor treatment. In addition to the above methods, there are still a variety of methods worth trying. We can use genetic modification to reduce the generation of these carcinogenic exosomes or reduce the expression of carcinogenic components in exosomes, combining them with immunotherapy and radiochemotherapy to improve the prognosis of patients.
Tumor cells interact with not only macrophages but also various cells such as T cells and dendritic cells (DCs). Exosomes play an important role in these interactions. Therefore, clarifying the role of exosomes in the interaction between tumor cells and various cells will help us fully understand the vital role played by exosomes in tumorigenesis and development, and provide theoretical guidance for transformation of exosomes for cancer treatment. In addition, understanding exosome content and function can help with the invention of new drugs to modify the expression of the substances in the exosomes and suppress tumor development. In these areas, there is still great research value.
Currently, there are several methods for isolating exosomes for laboratory research, including differential ultracentrifugation, density gradient ultracentrifugation, ultrafiltration, size-exclusion chromatography (SEC), flow field-flow fractionation (F4), immunoaffinity capture, exosome precipitation, microfluidic devices, and so on.120 However, all of these methods are facing the problem that the output is not large enough for clinical treatment. Using modern bioengineering to obtain sufficient production of exosomes by mass production of cells in vitro or changing cell culture conditions to stimulate cells to secrete exosomes more efficiently may be good ideas to solve this problem. In fact, there are already relevant studies trying to expand the production of exosomes for clinical applications, such as cellular nanoporation and ultrafiltration cartridges and pumps.121,122 Furthermore, the isolation of exosomes also faces other challenges, such as the establishment of recognized standards.123 Once these problems are resolved, a great breakthrough will likely be made in the treatment of tumors using exosomes.
Conclusion
The interaction between tumor cells and macrophages plays a crucial role in cancer development. Exosomes play a role in bridging the interaction between tumor cells and macrophages. Exosomes may be a potential therapeutic tool for targeting the communication between tumor cells and macrophages.
Author Contributions
W.G., Y.L., W.P., and H.S. designed and drafted the manuscript; W.G., Y.L., and H.S. wrote figure legends and revised the article; W.G. and W.P. drew the figures. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 81070362, 81172470, 81372629, 81772627, 81874073, and 81974384), the National Key R&D Program of China (no. 2018YFC1313303), two key projects from the Nature Science Foundation of Hunan Province (nos. 2015JC3021 and 2016JC2037), and by a project from the China Cancer Elite Team Innovative Grant (no. 201606).
References
- 1.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi A.A., Desai N.N., Qureshi M.Z., Librelotto D.R.N., Gasparri M.L., Bishayee A., Nabavi S.M., Curti V., Daglia M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018;36:328–334. doi: 10.1016/j.biotechadv.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Sato-Kuwabara Y., Melo S.A., Soares F.A., Calin G.A. The fusion of two worlds: non-coding RNAs and extracellular vesicles—diagnostic and therapeutic implications (Review) Int. J. Oncol. 2015;46:17–27. doi: 10.3892/ijo.2014.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 5.Nagarsheth N., Wicha M.S., Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.Z.W., Kozaki T., Ginhoux F. Studying tissue macrophages in vitro: are iPSC-derived cells the answer? Nat. Rev. Immunol. 2018;18:716–725. doi: 10.1038/s41577-018-0054-y. [DOI] [PubMed] [Google Scholar]
- 7.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 8.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Zhu Y., Xu W., Xu J., Yang M., Chen P., Zhao J., Geng L., Gong S. PKCα in colon cancer cells promotes M1 macrophage polarization via MKK3/6-P38 MAPK pathway. Mol. Carcinog. 2018;57:1017–1029. doi: 10.1002/mc.22822. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W., Ke S.Q., Huang Z., Flavahan W., Fang X., Paul J., Wu L., Sloan A.E., McLendon R.E., Li X. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N., Gupta R., Tsai J.M., Sinha R., Corey D. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaynagetdinov R., Sherrill T.P., Polosukhin V.V., Han W., Ausborn J.A., McLoed A.G., McMahon F.G., Gleaves L.A., Degryse A.L., Stathopoulos G.T. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J. Immunol. 2011;187:5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J., Yao Y., Gong C., Yu F., Su S., Chen J., Liu B., Deng H., Wang F., Lin L. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 16.Cassetta L., Pollard J.W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Zhou J., Li X., Wang X., Lin Y., Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Shi H., Yuan X., Jiang P., Qian H., Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer. 2018;17:146. doi: 10.1186/s12943-018-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luga V., Zhang L., Viloria-Petit A.M., Ogunjimi A.A., Inanlou M.R., Chiu E., Buchanan M., Hosein A.N., Basik M., Wrana J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 20.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y., Zhuang S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68:1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Xia L., Lin J., Wang H., Oyang L., Tan S., Tian Y., Su M., Wang H., Cao D., Liao Q. Exosomes in nasopharyngeal carcinoma. J. Cancer. 2018;9:767–777. doi: 10.7150/jca.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linton S.S., Abraham T., Liao J., Clawson G.A., Butler P.J., Fox T., Kester M., Matters G.L. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS ONE. 2018;13:e0206759. doi: 10.1371/journal.pone.0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonjoch L., Gironella M., Iovanna J.L., Closa D. REG3β modifies cell tumor function by impairing extracellular vesicle uptake. Sci. Rep. 2017;7:3143. doi: 10.1038/s41598-017-03244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Q., Zhao H., Jiang Y., Yin C., Zhang J. HCC-derived exosomes: critical player and target for cancer immune escape. Cells. 2019;8:E558. doi: 10.3390/cells8060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin C., Han Q., Xu D., Zheng B., Zhao X., Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology. 2019;8:1601479. doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Fan L., Yu H., Zhang J., He Y., Feng D., Wang F., Li X., Liu Q., Li Y. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 2019;70:241–258. doi: 10.1002/hep.30607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying X., Wu Q., Wu X., Zhu Q., Wang X., Jiang L., Chen X., Wang X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanlikilicer P., Bayraktar R., Denizli M., Rashed M.H., Ivan C., Aslan B., Mitra R., Karagoz K., Bayraktar E., Zhang X. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018;38:100–112. doi: 10.1016/j.ebiom.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh C.H., Tai S.K., Yang M.H. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering miR-21-abundant exosomes. Neoplasia. 2018;20:775–788. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 32.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson S., Kim S., Lee C., Deci M., Nguyen J. The phenotypic effects of exosomes secreted from distinct cellular sources: a comparative study based on miRNA composition. AAPS J. 2018;20:67. doi: 10.1208/s12248-018-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soki F.N., Koh A.J., Jones J.D., Kim Y.W., Dai J., Keller E.T., Pienta K.J., Atabai K., Roca H., McCauley L.K. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J. Biol. Chem. 2014;289:24560–24572. doi: 10.1074/jbc.M114.571620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai J., Qiao B., Gao N., Lin N., He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 2019;316:C731–C740. doi: 10.1152/ajpcell.00366.2018. [DOI] [PubMed] [Google Scholar]
- 36.Cooks T., Pateras I.S., Jenkins L.M., Patel K.M., Robles A.I., Morris J., Forshew T., Appella E., Gorgoulis V.G., Harris C.C. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun B., Zhou Y., Fang Y., Li Z., Gu X., Xiang J. Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int. J. Cancer. 2019;145:1648–1659. doi: 10.1002/ijc.32196. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M.Y., Liu W.J., Wang H., Wang W.D., Liu N.W., Lu Y. NSE from diffuse large B-cell lymphoma cells regulates macrophage polarization. Cancer Manag. Res. 2019;11:4577–4595. doi: 10.2147/CMAR.S203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paolillo M., Schinelli S. Integrins and exosomes, a dangerous liaison in cancer progression. Cancers (Basel) 2017;9:E95. doi: 10.3390/cancers9080095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z., Liu X., Wang H., Li J., Dai L., Li J., Dong C. Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis. Gene. 2018;666:116–122. doi: 10.1016/j.gene.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Taverna S., Pucci M., Giallombardo M., Di Bella M.A., Santarpia M., Reclusa P., Gil-Bazo I., Rolfo C., Alessandro R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017;7:3170. doi: 10.1038/s41598-017-03460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B., Song T.N., Wang F.R., Yin C., Li Z., Lin J.P., Meng Y.Q., Feng H.M., Jing T. Tumor-derived exosomal HMGB1 promotes esophageal squamous cell carcinoma progression through inducing PD1+ TAM expansion. Oncogenesis. 2019;8:17. doi: 10.1038/s41389-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood J.L. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med. Hypotheses. 2016;94:118–122. doi: 10.1016/j.mehy.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F., Li B., Wei Y., Zhao Y., Wang L., Zhang P., Yang J., He W., Chen H., Jiao Z., Li Y. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41. doi: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vrij J., Maas S.L., Kwappenberg K.M., Schnoor R., Kleijn A., Dekker L., Luider T.M., de Witte L.D., Litjens M., van Strien M.E. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer. 2015;137:1630–1642. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- 46.Ham S., Lima L.G., Chai E.P.Z., Muller A., Lobb R.J., Krumeich S., Wen S.W., Wiegmans A.P., Möller A. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front. Immunol. 2018;9:871. doi: 10.3389/fimmu.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao M., Zhang J., Chen W., Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018;37:143. doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panigrahi G.K., Praharaj P.P., Kittaka H., Mridha A.R., Black O.M., Singh R., Mercer R., van Bokhoven A., Torkko K.C., Agarwal C. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med. 2019;8:1110–1123. doi: 10.1002/cam4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lian G., Chen S., Ouyang M., Li F., Chen L., Yang J. Colon cancer cell secretes EGF to promote M2 polarization of TAM through EGFR/PI3K/AKT/mTOR pathway. Technol. Cancer Res. Treat. 2019;18 doi: 10.1177/1533033819849068. 1533033819849068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocher C., Singla D.K. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS ONE. 2013;8:e84009. doi: 10.1371/journal.pone.0084009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 52.Bao L., Li X. MicroRNA-32 targeting PTEN enhances M2 macrophage polarization in the glioma microenvironment and further promotes the progression of glioma. Mol. Cell. Biochem. 2019;460:67–79. doi: 10.1007/s11010-019-03571-2. [DOI] [PubMed] [Google Scholar]
- 53.Rahal O.M., Wolfe A.R., Mandal P.K., Larson R., Tin S., Jimenez C., Zhang D., Horton J., Reuben J.M., McMurray J.S., Woodward W.A. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:1034–1043. doi: 10.1016/j.ijrobp.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 54.Gan Z.S., Wang Q.Q., Li J.H., Wang X.L., Wang Y.Z., Du H.H. Iron reduces M1 macrophage polarization in RAW264.7 macrophages associated with inhibition of STAT1. Mediators Inflamm. 2017;2017:8570818. doi: 10.1155/2017/8570818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su X., Esser A.K., Amend S.R., Xiang J., Xu Y., Ross M.H., Fox G.C., Kobayashi T., Steri V., Roomp K. Antagonizing integrin β3 increases immunosuppression in cancer. Cancer Res. 2016;76:3484–3495. doi: 10.1158/0008-5472.CAN-15-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritz O., Guiter C., Dorsch K., Dusanter-Fourt I., Wegener S., Jouault H., Gaulard P., Castellano F., Möller P., Leroy K. STAT6 activity is regulated by SOCS-1 and modulates BCL-XL expression in primary mediastinal B-cell lymphoma. Leukemia. 2008;22:2106–2110. doi: 10.1038/leu.2008.85. [DOI] [PubMed] [Google Scholar]
- 57.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 58.Cubillos-Ruiz J.R., Bettigole S.E., Glimcher L.H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yong K.J., Gao C., Lim J.S., Yan B., Yang H., Dimitrov T., Kawasaki A., Ong C.W., Wong K.F., Lee S. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N. Engl. J. Med. 2013;368:2266–2276. doi: 10.1056/NEJMoa1300297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 61.Hwang W.L., Jiang J.K., Yang S.H., Huang T.S., Lan H.Y., Teng H.W., Yang C.Y., Tsai Y.P., Lin C.H., Wang H.W., Yang M.H. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat. Cell Biol. 2014;16:268–280. doi: 10.1038/ncb2910. [DOI] [PubMed] [Google Scholar]
- 62.Tapia F.J., Polak J.M., Barbosa A.J., Bloom S.R., Marangos P.J., Dermody C., Pearse A.G. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981;1:808–811. doi: 10.1016/s0140-6736(81)92682-9. [DOI] [PubMed] [Google Scholar]
- 63.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 64.Peng Y., Elkon K.B. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J. Clin. Invest. 2011;121:2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Z., Yang L., Cui Y., Zhou Y., Yin X., Guo J., Zhang G., Wang T., He Q.Y. Cytoskeleton-centric protein transportation by exosomes transforms tumor-favorable macrophages. Oncotarget. 2016;7:67387–67402. doi: 10.18632/oncotarget.11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 67.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y., Wang X.F. A niche role for cancer exosomes in metastasis. Nat. Cell Biol. 2015;17:709–711. doi: 10.1038/ncb3181. [DOI] [PubMed] [Google Scholar]
- 69.Hamidi H., Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z., Chen J., Gu Y., Hu C., Li J.L., Lin S., Shen H., Cao C., Gao R., Li J. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene. 2014;33:3869–3877. doi: 10.1038/onc.2013.348. [DOI] [PubMed] [Google Scholar]
- 71.Stacker S.A., Caesar C., Baldwin M.E., Thornton G.E., Williams R.A., Prevo R., Jackson D.G., Nishikawa S., Kubo H., Achen M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 72.Skobe M., Hawighorst T., Jackson D.G., Prevo R., Janes L., Velasco P., Riccardi L., Alitalo K., Claffey K., Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 73.Chen X., Zhang L., Zhang I.Y., Liang J., Wang H., Ouyang M., Wu S., da Fonseca A.C.C., Weng L., Yamamoto Y. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74:7285–7297. doi: 10.1158/0008-5472.CAN-14-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leibovich S.J., Polverini P.J., Shepard H.M., Wiseman D.M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 75.Koch A.E., Polverini P.J., Kunkel S.L., Harlow L.A., DiPietro L.A., Elner V.M., Elner S.G., Strieter R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Chen Y., Hao L., Hou A., Chen X., Li Y., Wang R., Luo P., Ruan Z., Ou J. Macrophages induce resistance to 5-fluorouracil chemotherapy in colorectal cancer through the release of putrescine. Cancer Lett. 2016;381:305–313. doi: 10.1016/j.canlet.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Dong N., Shi X., Wang S., Gao Y., Kuang Z., Xie Q., Li Y., Deng H., Wu Y., Li M., Li J.L. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br. J. Cancer. 2019;121:22–33. doi: 10.1038/s41416-019-0482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu X., Shen H., Yin X., Yang M., Wei H., Chen Q., Feng F., Liu Y., Xu W., Li Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marton A., Vizler C., Kusz E., Temesfoi V., Szathmary Z., Nagy K., Szegletes Z., Varo G., Siklos L., Katona R.L. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol. Lett. 2012;148:34–38. doi: 10.1016/j.imlet.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Gabrusiewicz K., Li X., Wei J., Hashimoto Y., Marisetty A.L., Ott M., Wang F., Hawke D., Yu J., Healy L.M. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. OncoImmunology. 2018;7:e1412909. doi: 10.1080/2162402X.2017.1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Wang B., Xiao S., Li Y., Chen Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2019;120:3046–3055. doi: 10.1002/jcb.27436. [DOI] [PubMed] [Google Scholar]
- 82.Sun F., Li X., Duan W.Q., Tian W., Gao M., Yang J., Wu X.Y., Huang D., Xia W., Han Y.N. Transforming growth factor-β receptor III is a potential regulator of ischemia-induced cardiomyocyte apoptosis. J. Am. Heart Assoc. 2017;6:e005357. doi: 10.1161/JAHA.116.005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin Z., Ma T., Huang B., Lin L., Zhou Y., Yan J., Zou Y., Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Figura G., Fukuda A., Roy N., Liku M.E., Morris Iv J.P., Kim G.E., Russ H.A., Firpo M.A., Mulvihill S.J., Dawson D.W. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2014;16:255–267. doi: 10.1038/ncb2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCarthy N. Pancreatic cancer: spotlight on BRG1. Nat. Rev. Cancer. 2014;14:213. doi: 10.1038/nrc3709. [DOI] [PubMed] [Google Scholar]
- 86.Lan J., Sun L., Xu F., Liu L., Hu F., Song D., Hou Z., Wu W., Luo X., Wang J. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 87.Homminga I., Pieters R., Langerak A.W., de Rooi J.J., Stubbs A., Verstegen M., Vuerhard M., Buijs-Gladdines J., Kooi C., Klous P. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19:484–497. doi: 10.1016/j.ccr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aucher A., Rudnicka D., Davis D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binenbaum Y., Fridman E., Yaari Z., Milman N., Schroeder A., Ben David G., Shlomi T., Gil Z. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 91.Yu X., Zhang Q., Zhang X., Han Q., Li H., Mao Y., Wang X., Guo H., Irwin D.M., Niu G., Tan H. Exosomes from macrophages exposed to apoptotic breast cancer cells promote breast cancer proliferation and metastasis. J. Cancer. 2019;10:2892–2906. doi: 10.7150/jca.31241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee H.D., Koo B.H., Kim Y.H., Jeon O.H., Kim D.S. Exosome release of ADAM15 and the functional implications of human macrophage-derived ADAM15 exosomes. FASEB J. 2012;26:3084–3095. doi: 10.1096/fj.11-201681. [DOI] [PubMed] [Google Scholar]
- 93.Zheng P., Luo Q., Wang W., Li J., Wang T., Wang P., Chen L., Zhang P., Chen H., Liu Y. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional apolipoprotein E. Cell Death Dis. 2018;9:434. doi: 10.1038/s41419-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng P., Chen L., Yuan X., Luo Q., Liu Y., Xie G., Ma Y., Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chuang H.Y., Su Y.K., Liu H.W., Chen C.H., Chiu S.C., Cho D.Y., Lin S.Z., Chen Y.S., Lin C.M. Preclinical evidence of STAT3 inhibitor pacritinib overcoming temozolomide resistance via downregulating miR-21-enriched exosomes from M2 glioblastoma-associated macrophages. J. Clin. Med. 2019;8:E959. doi: 10.3390/jcm8070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang F., Wang T., Du P., Fan H., Dong X., Guo H. M2 bone marrow-derived macrophage-derived exosomes shuffle microRNA-21 to accelerate immune escape of glioma by modulating PEG3. Cancer Cell Int. 2020;20:93. doi: 10.1186/s12935-020-1163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou J., Li X., Wu X., Zhang T., Zhu Q., Wang X., Wang H., Wang K., Lin Y., Wang X. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol. Res. 2018;6:1578–1592. doi: 10.1158/2326-6066.CIR-17-0479. [DOI] [PubMed] [Google Scholar]
- 98.Yao X., Huang J., Zhong H., Shen N., Faggioni R., Fung M., Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014;141:125–139. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Tavazoie M.F., Pollack I., Tanqueco R., Ostendorf B.N., Reis B.S., Gonsalves F.C., Kurth I., Andreu-Agullo C., Derbyshire M.L., Posada J. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172:825–840.e18. doi: 10.1016/j.cell.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang P., Wang H., Huang Q., Peng C., Yao L., Chen H., Qiu Z., Wu Y., Wang L., Chen W. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9:1714–1727. doi: 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Babendreyer A., Molls L., Simons I.M., Dreymueller D., Biller K., Jahr H., Denecke B., Boon R.A., Bette S., Schnakenberg U., Ludwig A. The metalloproteinase ADAM15 is upregulated by shear stress and promotes survival of endothelial cells. J. Mol. Cell. Cardiol. 2019;134:51–61. doi: 10.1016/j.yjmcc.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 102.Zhou S.L., Zhou Z.J., Hu Z.Q., Huang X.W., Wang Z., Chen E.B., Fan J., Cao Y., Dai Z., Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 103.Thomas A., Tanaka M., Trepel J., Reinhold W.C., Rajapakse V.N., Pommier Y. Temozolomide in the era of precision medicine. Cancer Res. 2017;77:823–826. doi: 10.1158/0008-5472.CAN-16-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 105.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006;17(Suppl 5):v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 106.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iessi E., Logozzi M., Lugini L., Azzarito T., Federici C., Spugnini E.P., Mizzoni D., Di Raimo R., Angelini D.F., Battistini L. Acridine orange/exosomes increase the delivery and the effectiveness of acridine orange in human melanoma cells: a new prototype for theranostics of tumors. J. Enzyme Inhib. Med. Chem. 2017;32:648–657. doi: 10.1080/14756366.2017.1292263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine (Lond.) 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng L., Wang Y., Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. Ther. 2017;25:1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rayamajhi S., Nguyen T.D.T., Marasini R., Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 111.Hu Y., Li D., Wu A., Qiu X., Di W., Huang L., Qiu L. TWEAK-stimulated macrophages inhibit metastasis of epithelial ovarian cancer via exosomal shuttling of microRNA. Cancer Lett. 2017;393:60–67. doi: 10.1016/j.canlet.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 112.Choo Y.W., Kang M., Kim H.Y., Han J., Kang S., Lee J.R., Jeong G.J., Kwon S.P., Song S.Y., Go S. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 113.Trivedi M., Talekar M., Shah P., Ouyang Q., Amiji M. Modification of tumor cell exosome content by transfection with wt-p53 and microRNA-125b expressing plasmid DNA and its effect on macrophage polarization. Oncogenesis. 2016;5:e250. doi: 10.1038/oncsis.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su M.J., Aldawsari H., Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of microRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci. Rep. 2016;6:30110. doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen C., Yao X., Xu Y., Zhang Q., Wang H., Zhao L., Wen G., Liu Y., Jing L., Sun X. Dahuang Zhechong pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J. Ethnopharmacol. 2019;238:111878. doi: 10.1016/j.jep.2019.111878. [DOI] [PubMed] [Google Scholar]
- 116.Jang J.Y., Lee J.K., Jeon Y.K., Kim C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Litzinger D.C., Brown J.M., Wala I., Kaufman S.A., Van G.Y., Farrell C.L., Collins D. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim. Biophys. Acta. 1996;1281:139–149. doi: 10.1016/0005-2736(95)00268-5. [DOI] [PubMed] [Google Scholar]
- 118.Qiu X., Li Z., Han X., Zhen L., Luo C., Liu M., Yu K., Ren Y. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics. 2019;9:2618–2636. doi: 10.7150/thno.32363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Q.L., Zhuang X., Sriwastva M.K., Mu J., Teng Y., Deng Z., Zhang L., Sundaram K., Kumar A., Miller D. Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways. Theranostics. 2018;8:4912–4924. doi: 10.7150/thno.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lamparski H.G., Metha-Damani A., Yao J.Y., Patel S., Hsu D.H., Ruegg C., Le Pecq J.B. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 123.Abramowicz A., Marczak L., Wojakowska A., Zapotoczny S., Whiteside T.L., Widlak P., Pietrowska M. Harmonization of exosome isolation from culture supernatants for optimized proteomics analysis. PLoS ONE. 2018;13:e0205496. doi: 10.1371/journal.pone.0205496. [DOI] [PMC free article] [PubMed] [Google Scholar]