The output of an RLK-MAPK signaling pathway maintains cytokinin homeostasis, thereby determining spikelet number per panicle in rice.
Abstract
Grain number is a flexible trait that strongly contributes to grain yield. In rice (Oryza sativa), the OsMKKK10-OsMKK4-OsMPK6 cascade, which is negatively regulated by the dual-specificity phosphatase GSN1, coordinates the trade-off between grain number and grain size. However, the specific components upstream and downstream of the GSN1-MAPK module that regulate spikelet number per panicle remain obscure. Here, we report that ERECTA1 (OsER1), a negative regulator of spikelet number per panicle, acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade and that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is required to maintain cytokinin homeostasis. OsMPK6 directly interacts with and phosphorylates the zinc finger transcription factor DST to enhance its transcriptional activation of CYTOKININ OXIDASE2 (OsCKX2), indicating that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway shapes panicle morphology by regulating cytokinin metabolism. Furthermore, overexpression of either DST or OsCKX2 rescued the spikelet number phenotype of the oser1, osmkkk10, osmkk4, and osmpk6 mutants, suggesting that the DST-OsCKX2 module genetically functions downstream of the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway. These findings reveal specific crosstalk between a MAPK signaling pathway and cytokinin metabolism, shedding light on how developmental signals modulate phytohormone homeostasis to shape the inflorescence.
INTRODUCTION
Rice (Oryza sativa), one of the most important staple cereal crops worldwide, feeds more than half the world’s population. Increasing grain yield is a continual focus of rice breeding and improvement. Rice yield is a complicated trait determined by tiller number, grain weight, and grain number per panicle. Of these, grain number per panicle is a more flexible component, which depends on the number of primary and secondary branches, and thus plays a major role in determining grain yield (Xing and Zhang, 2010). Rice has a determinate inflorescence in which meristems differentiate into primary branch meristems attached to a central rachis, which then form several secondary branch meristems (Zhang and Yuan, 2014). During panicle morphogenesis, the multiple inflorescence meristems that form and give rise to spikelets, flowers, and glumes are pivotal determinants of grain number and size. These spatiotemporally programmed cellular processes contribute interactively to specify rice panicle architecture. To date, the genetic basis underlying the determination of spikelet number per panicle has been studied by mapping quantitative trait loci (QTLs) and identifying mutants; these studies have found that the maintenance of meristem size and activity is closely associated with panicle branching and the number of spikelets (Komatsu et al., 2003; Ashikari et al., 2005; Kurakawa et al., 2007; Huang et al., 2009a; Tabuchi et al., 2011; Li et al., 2013; Wu et al., 2016; Huo et al., 2017), thereby supporting the hypothesis that the activity of the reproductive meristem plays an essential role in determining rice grain production (Li et al., 2013).
Emerging compelling evidence has suggested that the phytohormone cytokinin plays a crucial role in maintaining shoot apical meristem (SAM) activity (Ashikari et al., 2005; Kurakawa et al., 2007; Zhao et al., 2010; Perales and Reddy, 2012). Either reduced levels of endogenous cytokinin or the suppression of cytokinin signaling inhibits SAM activity (Werner et al., 2001, 2003; Higuchi et al., 2004; Riefler et al., 2006; Kurakawa et al., 2007), whereas increasing cytokinin levels enhances SAM activity (Rupp et al., 1999; Ashikari et al., 2005). In rice, the important QTL Grain number 1a encodes the cytokinin oxidase/dehydrogenase CYTOKININ OXIDASE2 (OsCKX2), which catalyzes the degradation of active cytokinin. This QTL negatively regulates grain number per panicle (Ashikari et al., 2005). Accordingly, a decrease in OsCKX2 expression results in the overaccumulation of cytokinin in inflorescence meristems, leading to increased grain production (Ashikari et al., 2005). Molecular evidence indicates that the transcription factor DROUGHT AND SALT TOLERANCE (DST; Huang et al., 2009b) controls the activity of the reproductive SAM by directly binding to the promoter of OsCKX2 and positively regulating its expression; therefore, disrupting DST significantly increases cytokinin levels and grain production (Li et al., 2013). Nevertheless, the signaling pathway that regulates cytokinin metabolism during inflorescence development remains elusive.
Mitogen-activated protein kinase (MAPK) cascades are highly conserved, ubiquitous signaling pathways in eukaryotes. The sequential phosphorylation of proteins in these cascades leads to altered substrate activities and regulates cell proliferation and differentiation and the coordination of responses to environmental inputs (Widmann et al., 1999; Xu and Zhang, 2015). MAPK cascades play essential roles in multiple processes in plants, including defense, stress responses, and developmental programs (Meng and Zhang, 2013; Komis et al., 2018; Zhang et al., 2018). In rice, the OsMKKK10-OsMKK4-OsMPK6 cascade is negatively regulated by the dual-specificity phosphatase GRAIN SIZE AND NUMBER1 (GSN1), which directly dephosphorylates OsMPK6, thereby coordinating the trade-off between grain number per panicle and grain size (Guo et al., 2018; Xu et al., 2018a, 2018b; Wang et al., 2019). Moreover, we previously identified a potential association between the GSN1-MAPK module and phytohormone signaling in determining the plasticity of panicle architecture in rice (Guo et al., 2018). However, the precise genetic and molecular mechanism underlying how the GSN1-MAPK module and cytokinin metabolism control spikelet number per panicle is currently unclear. Identifying the components upstream and downstream of the GSN1-MAPK module could reveal unrecognized molecular mechanisms.
Here, we characterized the rice mutant oser1, which displays a markedly increased number of spikelets per panicle compared with the wild type, indicating that OsER1, encoding an ERECTA family protein, is a negative regulator of spikelet number per panicle. We demonstrate that OsER1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control the number of spikelets produced per panicle. Moreover, we show that OsMPK6 interacts with and phosphorylates DST to enhance its transcriptional activation of OsCKX2, indicating that the OsMKKK10-OsMKK4-OsMPK6 cascade controls the number of spikelets per panicle by regulating cytokinin metabolism. These findings advance our understanding of how perceived developmental signals control phytohormone metabolism to shape panicle morphology. In addition, they provide a framework for understanding the role of receptor and signal conversion in inflorescence development, offering a potential means to improve crop yields.
RESULTS
OsER1 Is Responsible for Rice Panicle Morphogenesis and Plays a Negative Role in Determining Spikelet Number Per Panicle
The ERECTA (ER) gene, which encodes a receptor-like protein kinase (RLK), has been extensively studied in Arabidopsis (Arabidopsis thaliana), where it regulates numerous developmental processes including stomatal formation and patterning, inflorescence architecture, and ovule development (Torii et al., 1996; Shpak et al., 2003; Meng et al., 2012; Pillitteri and Torii, 2012; Shpak, 2013). Although emerging evidence indicates that the overexpression of ER confers thermotolerance to rice (Shen et al., 2015), the explicit function of ER in controlling inflorescence development in rice remains unclear. We therefore investigated the function of OsER1 (LOC_Os06g10230) using CRISPR/Cas9 gene editing (Ma et al., 2015). Strikingly, the oser1 mutant displayed increased spikelet number per panicle and reduced grain size without altered plant architecture (Figures 1A to 1C). Moreover, the average spikelet number per panicle of the oser1 mutant was markedly increased, with reduced grain length but enhanced grain width compared with the wild type (Figures 1F to 1H). However, the average grain yield per plant was comparable to that of the wild-type Fengaizhan-1 (FAZ1; Oryza indica) due to a reduced setting percentage in oser1 (Figures 1I and 1J). Consistent with these findings, the oser1 mutant in the O. japonica variety Zhonghua-11 (ZH11) background, which carries the same mutant allele of OsER1 as that in FAZ1, also showed increased spikelet number per panicle (Supplemental Figures 1A to 1C and 2A). These results suggest that OsER1 is responsible for panicle morphogenesis in rice.
Figure 1.
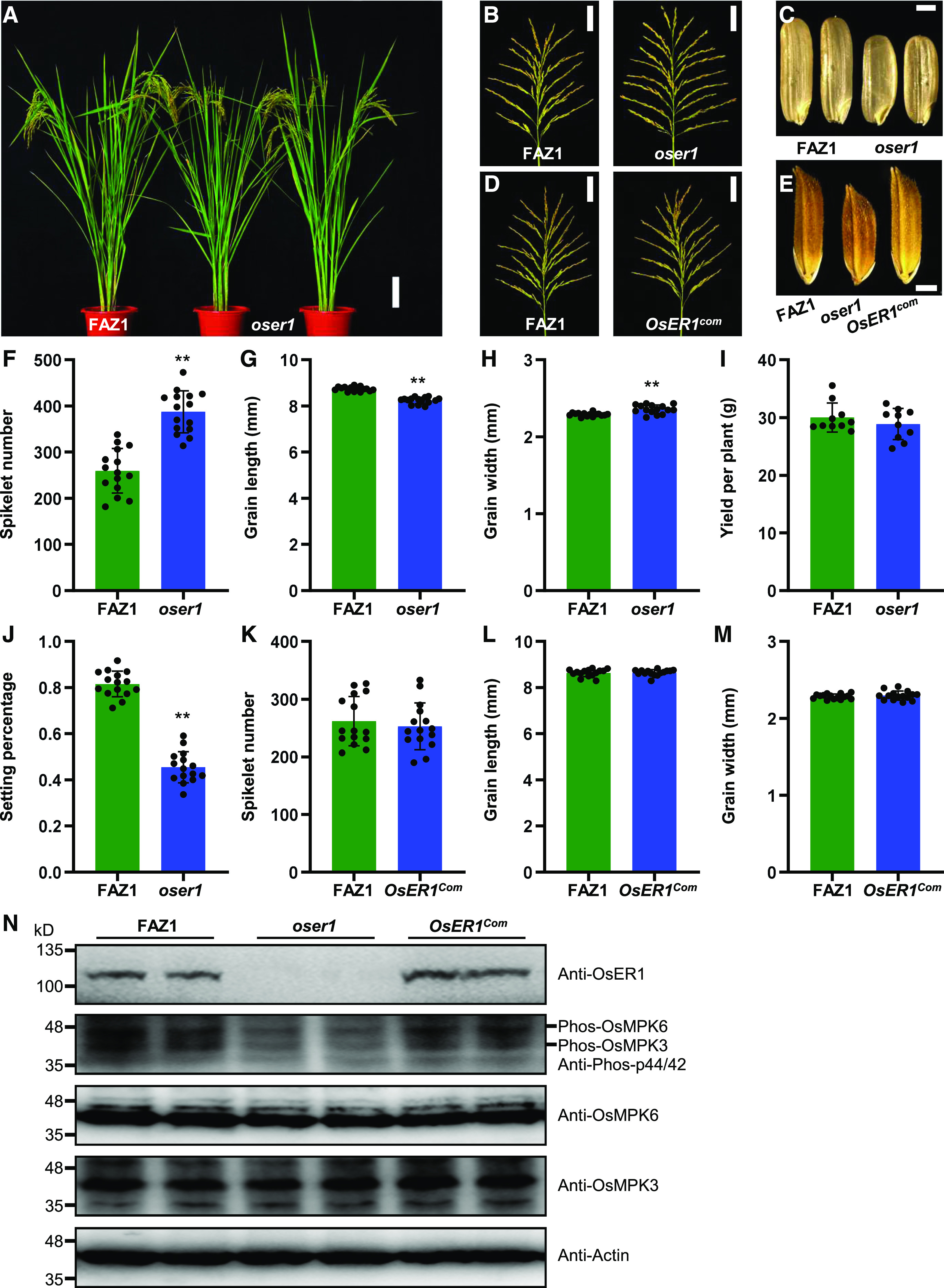
OsER1 Is Responsible for Rice Panicle Morphogenesis and Negatively Regulates Spikelet Number per Panicle.
(A) Plant architecture of wild-type FAZ1 and mutant oser1 plants at the reproductive stage. Scale bar, 10 cm.
(B) Rice panicles from FAZ1 and oser1 plants. Scale bar, 5 cm.
(C) Brown rice grains from FAZ1 and oser1. Scale bar, 2 mm.
(D) Comparison of panicles between FAZ1 and the complementation line OsER1com. Scale bar, 5 cm.
(E) Comparison of mature paddy rice grains between FAZ1, oser1, and OsER1com. Scale bar, 2 mm.
(F) to (J) Comparisons of average spikelet number per panicle (n = 15 plants) (F), grain length (n = 15 plants) (G), grain width (n = 15 plants) (H), yield per plant (n = 10 plants) (I), and seed setting percentage (n = 15 plants) (J) between FAZ1 and oser1.
(K) to (M) Comparisons of average spikelet number per panicle (n = 15 plants) (K), grain length (n = 15 plants) (L), and grain width (n = 15 plants) (M) between FAZ1 and the complementation line OsER1com. Values are given as the mean ± sd. **P < 0.01 compared with the wild type using Student’s t test.
(N) The phosphorylation level of OsMPK6 was reduced in the oser1 mutant but restored in OsER1com. Samples were prepared from 0.1-cm to 0.5-cm young panicles pooled from different plants and subjected to immunoblot analysis with the anti-OsER1, anti-Phospho-p44/42, anti-OsMPK6, and anti-OsMPK3 antibodies. The anti-Actin antibody was used as the loading control.
To further confirm the phenotype of oser1, we performed a genetic complementation test in which the full-length OsER1 gene from FAZ1 was introduced into the oser1 mutant via Agrobacterium tumefaciens-mediated transformation. A positive transgenic line harboring OsER1 displayed completely wild-type phenotypes with respect to spikelet number per panicle and grain size (Figures 1D, 1E, and 1K to 1M), but overexpression of OsER1 in the wild-type FAZ1 background had no effect on spikelet number per panicle (Supplemental Figure 3). These results indicate that OsER1 is not only required for rice panicle development, but also sufficient for regulating spikelet number per panicle.
In Arabidopsis, MAPK cascades function downstream of RLK signaling (Meng and Zhang, 2013; Xu and Zhang, 2015). Therefore, we assayed the level of MAPK phosphorylation in young panicles of the oser1 mutant and found that the loss of function of OsER1 suppressed the phosphorylation of both OsMPK6 and OsMPK3 without altering their abundance (Figure 1N). Moreover, we analyzed the cell division rate in younger panicles using flow cytometry and found that the percentage of G2/M phase cells with 4c DNA content was markedly increased in the oser1 mutant, but that the percentage of G1 phase cells with 2c DNA content was reduced compared with the wild type (Supplemental Figures 4A to 4C). Accordingly, the expression levels of cell-cycle–related genes were significantly elevated in young panicles of oser1 compared with wild-type FAZ1 (Supplemental Figure 4D), suggesting that localized cell proliferation occurs more rapidly in oser1 during panicle morphogenesis. Overall, these results suggest that OsER1 controls localized cell proliferation to negatively regulate spikelet number by activating a MAPK cascade in rice.
OsER1 Acts Upstream of the OsMKKK10-OsMKK4-OsMPK6 Cascade to Negatively Regulate Spikelet Number per Panicle
We previously proposed that the GSN1-MAPK module coordinates the trade-off between grain number and grain size by integrating localized cell differentiation and proliferation (Guo et al., 2018). Further investigation confirmed that the gsn1 osmpk6 double mutant produces significantly more spikelets per panicle than gsn1 but fewer than osmpk6 in the FAZ1 genetic background (Supplemental Figure 5), which carries a mutant OsMPK6 allele from the dsg1 mutant (Liu et al., 2015). These results strongly indicate that the GSN1-MAPK module plays a crucial role in spikelet formation, with GSN1 functioning as a positive regulator of this process; however, the OsMKKK10-OsMKK4-OsMPK6 cascade acts as a negative regulator of spikelet formation (Guo et al., 2018). To further investigate the relationship between OsER1 and the OsMKKK10-OsMKK4-OsMPK6 cascade in determining panicle morphology, we compared the number of spikelets per panicle and observed that the oser1, osmkkk10, osmkk4, and osmpk6 mutants showed similar panicle architectures and produced many more spikelets than wild-type FAZ1 (Supplemental Figure 6). These results imply that both OsER1 and the OsMKKK10-OsMKK4-OsMPK6 cascade play negative roles in determining the number of spikelets per panicle.
Moreover, crossing with oser1 rescued the decreased spikelet number phenotype of the gsn1 mutant (Figures 2A and 2E), suggesting that the phenotypes observed in the gsn1 mutant are genetically dependent on OsER1. In addition, overexpression of GSN1 in the wild-type background resulted in the production of more spikelets per panicle, similar to the phenotype of the oser1 mutant (Figures 2B and 2F). Constitutively active OsMKK4 and OsMKKK10 both suppressed the increased number of spikelets when expressed in the oser1 mutant (Figures 2C, 2D, 2G, and 2H). These results indicate that OsER1 acts upstream of the GSN1-MAPK module to regulate spikelet formation in rice.
Figure 2.
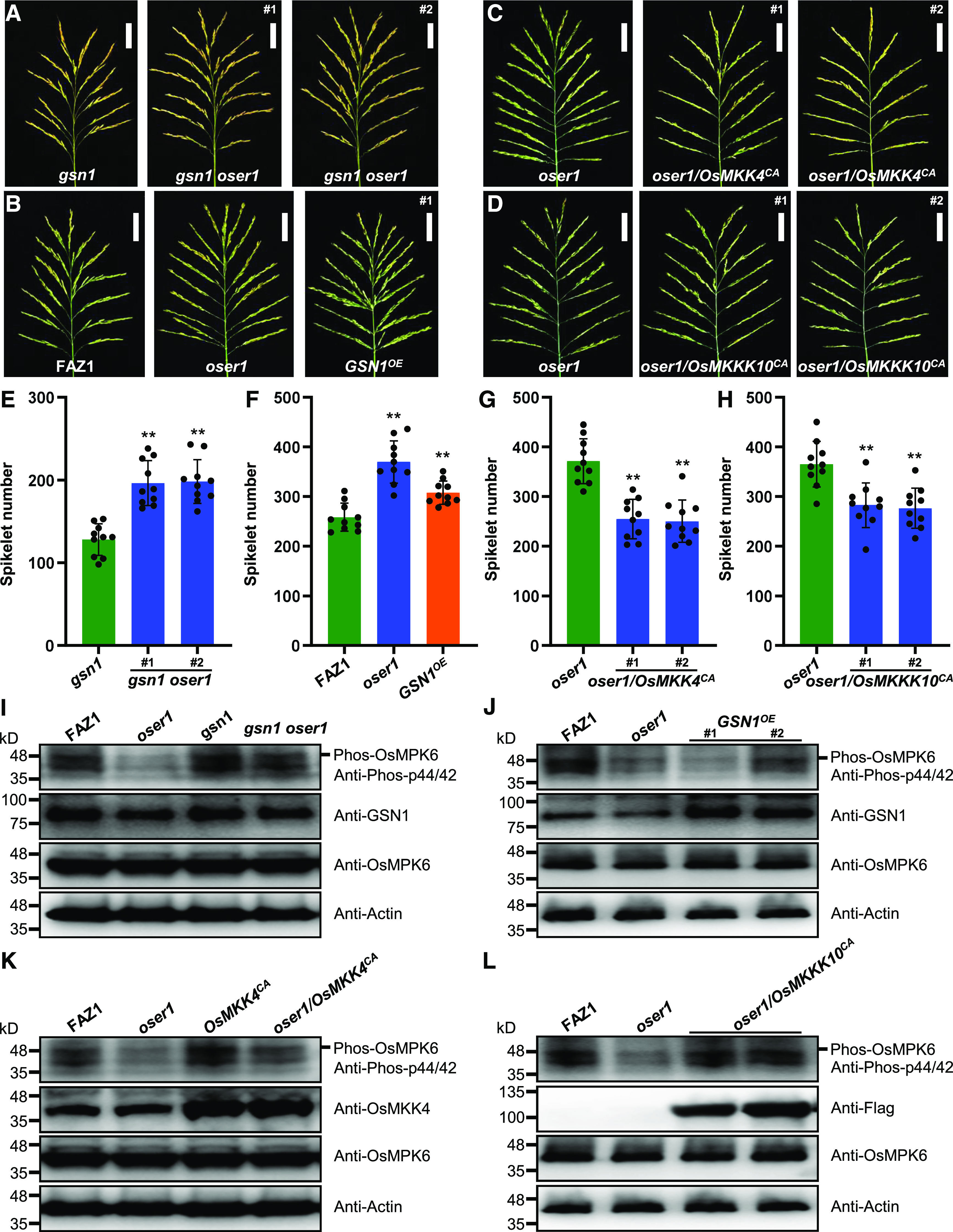
OsER1 Acts Upstream of the OsMKKK10-OsMKK4-OsMPK6 Cascade to Negatively Regulate Spikelet Number per Panicle.
(A) Panicles from gsn1 mutant and gsn1 oser1 double mutant rice plants. Scale bar, 5 cm.
(B) Comparison of panicles between FAZ1 and oser1 and GSN1 overexpression line (GSN1OE). Scale bar, 5 cm.
(C) Panicles from oser1 mutant and oser1 plants expressing constitutively active OsMKK4 (oser1/OsMKK4CA). Scale bar, 5 cm.
(D) Panicles from oser1 mutant and oser1 plants expressing constitutively active OsMKKK10 (oser1/OsMKKK10CA). Scale bar, 5 cm.
(E) to (H) Comparisons of average spikelet number per panicle for gsn1 and gsn1 oser1 double mutant plants (n = 10 plants) (E), FAZ1 and oser1 and GSN1OE (n = 10 plants) (F), oser1 and oser1/OsMKK4CA lines (n = 10 plants) (G), and oser1 and oser1/OsMKKK10CA lines (n = 10 plants) (H). Values are given as the mean ± sd. **P < 0.01 compared with the wild type using Student’s t test.
(I) to (L) Comparisons of the phosphorylation levels of OsMPK6 in oser1 mutant and gsn1 oser1 double mutant (I), in the oser1 and GSN1OE lines (J), in the oser1 and oser1/OsMKK4CA lines (K), and in the oser1 and oser1/OsMKKK10CA lines (L). Proteins extracted from pooled 0.1-cm to 0.5-cm young panicles were subjected to immunoblot analysis with anti-Phospho-p44/42, anti-OsMPK6, anti-OsMKK4, anti-GSN1, or anti-Flag antibodies. The anti-Actin antibody was used as the loading control.
Next, we assayed the phosphorylation levels of OsMPK6 in young panicles of different transgenic plants and found that the loss of function of OsER1 in the gsn1 oser1 double mutant reduced the phosphorylation level of OsMPK6 compared with gsn1 (Figure 2I). The same decrease in phosphorylated OsMPK6 levels was observed in plants overexpressing GSN1 (Figure 2J). Consistent with this finding, constitutively activated OsMKK4 and OsMKKK10 significantly enhanced the phosphorylation levels of OsMPK6 in oser1 (Figures 2K and 2L), implying that OsER1 associates with OsMKKK10 and OsMKK4 to modulate the phosphorylation status of OsMPK6. Overall, these results suggest that OsER1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to negatively regulate the number of spikelets per panicle.
The OsER1-OsMKKK10-OsMKK4-OsMPK6 Pathway Is Required to Modulate Cytokinin Metabolism
Although the above evidence supports the hypothesis that OsER1 and the OsMKKK10-OsMKK4-OsMPK6 cascade act in a common pathway to control spikelet number per panicle, the downstream components responsible for spikelet formation remains unknown. Notably, we previously reported that OsCKX2 is expressed at significantly higher levels in gsn1 compared with FAZ1 during young panicle development, but the cytokinin-activating gene LONELY GUY is expressed at dramatically lower levels in this mutant (Guo et al., 2018). In agreement with this finding, the levels of several cytokinins were significantly lower in the gsn1 mutant than in FAZ1, thereby supporting the hypothesis that cytokinin activity is attenuated in the panicle meristem of the gsn1 mutant, which contributes to the reduction in spikelet number per panicle (Guo et al., 2018). Therefore, we hypothesized that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is closely associated with the role of cytokinin homeostasis in determining the number of spikelets in rice.
Interestingly, OsCKX2 was significantly downregulated in the oser1 mutant compared with wild-type FAZ1 during young panicle development (Figure 3A), and OsCKX2 protein levels were also lower in the mutant (Figure 3B), thereby suggesting that loss of function of OsER1 perturbs the regulation of cytokinin metabolism responsible for panicle morphogenesis. We thus assayed the endogenous cytokinin levels in young panicles and found that the levels of multiple cytokinins were markedly elevated in the oser1 mutant (Figure 3C), confirming the notion that OsER1 is required for regulating cytokinin metabolism. In addition, the oser1 mutant exhibited an increased spikelet number per panicle resembling that of the osckx2 mutant (Supplemental Figures 1A to 1C and 2B). Overall, these results indicate that loss of function of OsER1 results in the production of more spikelets due to the disruption of cytokinin metabolism.
Figure 3.
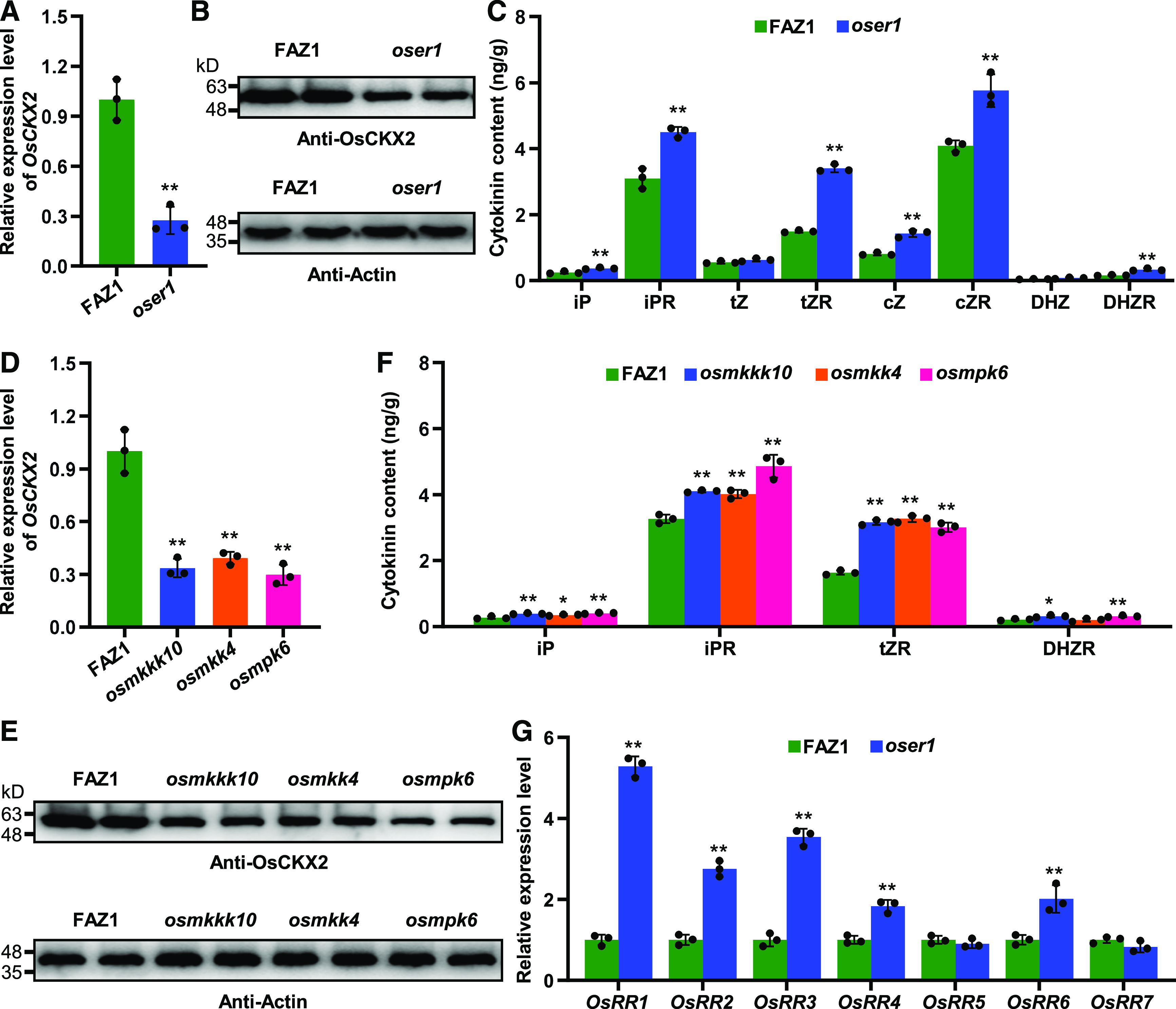
The OsER1-OsMKKK10-OsMKK4-OsMPK6 Pathway Is Required to Modulate Cytokinin Metabolism.
(A) Relative expression levels of OsCKX2 in FAZ1 and the oser1 mutant (n = 3 pools, and five 0.1-cm to 0.5-cm young panicles per pool). The UBQ5 gene was used as the internal reference to normalize gene expression data.
(B) The levels of OsCKX2 protein in young panicles of FAZ1 and the oser1 mutant. Proteins extracted from pooled 0.1-cm to 0.5-cm young panicles were subjected to immunoblot analysis using the anti-OsCKX2 antibody. The anti-Actin antibody was used as the loading control.
(C) Comparison of endogenous cytokinin (iP, iPR, tZ, tZR, cZ, cZR, DHZ, and DHZR) levels in young panicles (0.1 cm to 0.5 cm) between FAZ1 and the oser1 mutant (n = 3 pools, and 20 young panicles per pool).
(D) Relative expression levels of OsCKX2 in FAZ1, osmkkk10, osmkk4, and osmpk6 (n = 3 pools, and five 0.1-cm to 0.5-cm young panicles per pool). The UBQ5 gene was used as the internal reference to normalize gene expression data.
(E) The levels of OsCKX2 protein in young panicles of FAZ1, osmkkk10, osmkk4, and osmpk6. Proteins extracted from pooled 0.1-cm to 0.5-cm young panicles were subjected to immunoblot analysis using the anti-OsCKX2 antibody. The anti-Actin antibody was used as the loading control.
(F) Comparison of endogenous cytokinin (iP, iPR, tZR, and DHZR) levels in young panicles (0.1 cm to 0.5 cm) between FAZ1 and the osmkkk10, osmkk4, and osmpk6 mutants (n = 3 pools, and 20 young panicles per pool).
(G) Relative expression levels of the cytokinin signal transduction-related RESPONSE REGULATOR genes in FAZ1 and the oser1 mutant (n = 3 pools, and five 0.1-cm to 0.5-cm young panicles per pool). The UBQ5 gene was used as the internal reference to normalize gene expression data. Values are given as the mean ± sd. *P < 0.05; **P < 0.01 compared with the wild type using Student’s t test.
Furthermore, the transcript and protein levels of OsCKX2 in the osmkkk10, osmkk4, and osmpk6 mutants were also lower than those in FAZ1 (Figures 3D and 3E). The endogenous cytokinin levels were also higher, as expected (Figure 3F), indicating that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is required for the regulation of cytokinin metabolism. Additionally, the expression levels of RESPONSE REGULATOR genes, which serve as markers for the cytokinin response, were significantly elevated to varying degrees in the oser1 mutant (Figure 3G), indicating that loss of function of OsER1 enhances cytokinin signaling, ultimately leading to the formation of more spikelets. Taken together, these findings suggest that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is required to modulate cytokinin metabolism, which is responsible for panicle development in rice.
OsMPK6 Interacts with and Phosphorylates DST
To further explore the molecular mechanism by which the GSN1-MAPK module and cytokinin metabolism regulate spikelet number, we performed a yeast two-hybrid assay to screen for proteins that physically interact with OsMPK6, which interacts with and phosphorylates its substrates to modulate multiple biological processes in rice (Hu et al., 2015; Ueno et al., 2015; Tian et al., 2017). Intriguingly, one of the OsMPK6 interactors, the zinc finger transcription factor DST, was previously shown to regulate drought and salt tolerance (Huang et al., 2009b), and to modulate grain production by controlling the expression of OsCKX2 in rice (Li et al., 2013). We then performed a yeast two-hybrid assay to confirm the interaction of DST with OsMPK6 (Figure 4A). Moreover, we performed a pull-down assay to confirm the interaction between OsMPK6 and DST in vitro. His-tagged DST was pulled down by MBP-OsMPK6, but not by MBP alone (Figure 4B), indicating that DST directly binds to OsMPK6 in vitro.
Figure 4.
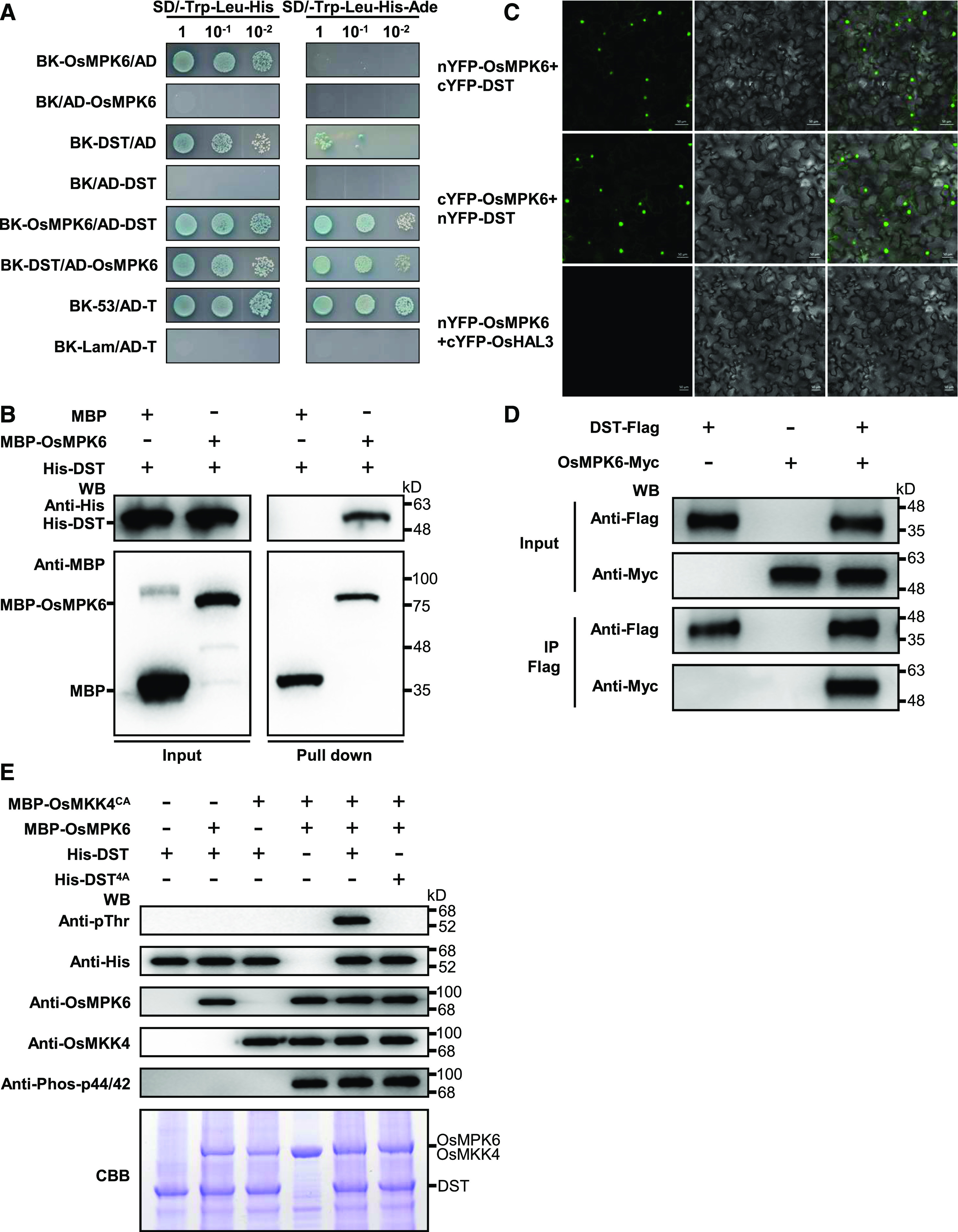
OsMPK6 Interacts with and Phosphorylates DST.
(A) Yeast two-hybrid assays indicating that OsMPK6 interacts with DST. The yeast cells were cultured on SD/-Trp-Leu-His (medium without Trp, Leu, and His) or SD/-Trp-Leu-His-Ade (medium without Trp, Leu, His, and Ade) containing X-ɑ-gal. BK (pGBKT7) and AD (pGADT7) are the bait and prey vectors, respectively.
(B) In vitro pull-down assays of His-tagged DST using MBP-tagged OsMPK6. Pull-down was verified by immunoblotting with anti-His and anti-MBP antibodies. WB, Western Blot.
(C) OsMPK6 associates with DST, as shown by BiFC assays in N. benthamiana leaf cells. OsMPK6 and DST were both fused to the N-terminal fragment of YFP (nYFP) and the C-terminal fragment of YFP (cYFP). nYFP-OsMPK6 and cYFP-DST or cYFP-OsMPK6 and nYFP-DST were then co-expressed in N. benthamiana leaves. The nYFP-OsMPK6 and cYFP-OsHAL3 fusion proteins were used as negative controls.
(D) Co-IP assays indicating that OsMPK6 interacts with DST in planta. Pro35S:DST-Flag and Pro35S:OsMPK6-Myc fusions were co-expressed in N. benthamiana leaves. Proteins were extracted (Input) and immunoprecipitated (IP) with Flag beads. The immunoblot assays were performed using anti-Flag and anti-Myc antibodies. WB, Western Blot.
(E) OsMPK6 phosphorylates DST in vitro. The MBP-OsMKK4CA, MBP-OsMPK6, His-DST, and His-DST4A fusion proteins were expressed in E. coli and purified. The in vitro phosphorylation reactions were performed using the purified proteins. DST phosphorylation was detected with the anti-Phospho-Thr antibody. Recombinant OsMKK4, OsMPK6, and DST were detected using anti-OsMKK4, anti-OsMPK6, and anti-His, respectively. Phosphorylated OsMPK6 was detected with anti-Phospho-p44/42. The gel stained with Coomassie brilliant blue (CBB) was used as a loading control. WB, Western Blot.
Next, to investigate the direct physical interaction between OsMPK6 and DST in planta, we performed a biomolecular florescence complementation (BiFC) assay in which OsMPK6 and DST were fused with the N-terminal or C-terminal parts of split yellow fluorescent protein (YFP); reconstitution of YFP fluorescence indicates interaction between OsMPK6 and DST. The nYFP-OsMPK6 fusion protein interacted with cYFP-DST, and cYFP-OsMPK6 interacted with nYFP-DST, as expected, but no signal was detected between nYFP-OsMPK6 and the negative control, cYFP-OsHAL3 (homolog of the halotolerance yeast protein HAL3; Su et al., 2016), suggesting that OsMPK6 associates with DST in vivo (Figure 4C). Furthermore, we confirmed the interaction between OsMPK6 and DST in a co-immunoprecipitation (Co-IP) experiment in which the DST-Flag and OsMPK6-Myc fusion proteins were co-expressed in Nicotiana benthamiana leaves and the proteins were immunoprecipitated with anti-Flag beads and detected by immunoblot analysis with anti-Flag and anti-Myc antibodies. A band with the expected mobility of OsMPK6-Myc was successfully detected in the anti-Flag immunoprecipitates from leaves expressing DST-Flag and OsMPK6-Myc; by contrast, no OsMPK6-Myc was detected in the absence of DST-Flag expression (Figure 4D). Taken together, these results suggest that OsMPK6 interacts with DST in vitro and in vivo.
Because OsMPK6 interacts with DST, we hypothesized that DST might be a substrate of OsMPK6. Therefore, we performed phosphorylation assays with fusion proteins to determine whether OsMPK6 can phosphorylate DST in vitro. The recombinant MBP-MPK6 strongly phosphorylated His-DST after being activated by a constitutively activated version of OsMKK4 known as OsMKK4CA that carries the Thr238Asp and Ser244Asp mutations (Yang et al., 2001); however, OsMPK6 failed to phosphorylate DST in the absence of constitutively activated OsMKK4CA (Figure 4E). ERK MAP kinases specifically phosphorylate substrates with Ser or Thr residues followed by a proline (S/TP; Jacobs et al., 1999). We analyzed the amino acid sequence of DST and found four potential MAPK phosphorylation sites (Thr88, Thr103, Thr112, and Thr274; Huang et al., 2005). After mutating all four Thr residues to Ala (DST4A), DST was no longer phosphorylated by OsMPK6 (Figure 4E), suggesting that these residues are required for the phosphorylation of DST by OsMPK6. Overall, these findings suggest that OsMPK6 interacts with and phosphorylates DST.
OsMPK6-Mediated Phosphorylation Enhances the Transcriptional Activity of DST and Positively Regulates OsCKX2 Expression
To determine how OsMPK6-mediated phosphorylation affects DST function and to understand the biological significance of this phosphorylation, we investigated whether OsMPK6-mediated phosphorylation increases the transcriptional activation of OsCKX2 by DST. We performed transient transactivation assays using the OsCKX2 promoter fused to a luciferase (LUC) reporter gene (Figure 5A). The DST, OsMPK6, and OsMKK4 effector constructs were expressed under the control of the CaMV 35S promoter (Figure 5A) and co-transfected with the reporter construct into rice protoplasts. Consistent with a previous study, we found that DST activated the expression of OsCKX2 (Figure 5B; Li et al., 2013). Notably, co-expression of OsMPK6 and constitutively activated OsMKK4 (OsMKK4CA) increased DST-activated OsCKX2 expression; by contrast, co-expression with only OsMPK6 or OsMKK4CA failed to enhance DST-activated OsCKX2 expression (Figure 5B), implying that the OsMKK4-OsMPK6 cascade increases the transcriptional activity of DST through phosphorylation. Consistent with this notion, expression of the constitutively activated form of OsMPK6, OsMPK6CA, which carries the Tyr151Cys mutation (Berriri et al., 2012; Xu et al., 2018b), directly increased the DST-induced transcriptional activation to OsCKX2, which depends on the four phosphorylation sites (Figure 5C). Moreover, the quadruple phosphomimetic DST4D (Thr88Asp, Thr103Asp, Thr112Asp, and Thr274Asp) displayed markedly enhanced transcriptional activity (Figure 5C). These results suggest that OsMPK6-mediated phosphorylation enhances the transcriptional activation of OsCKX2 by DST.
Figure 5.
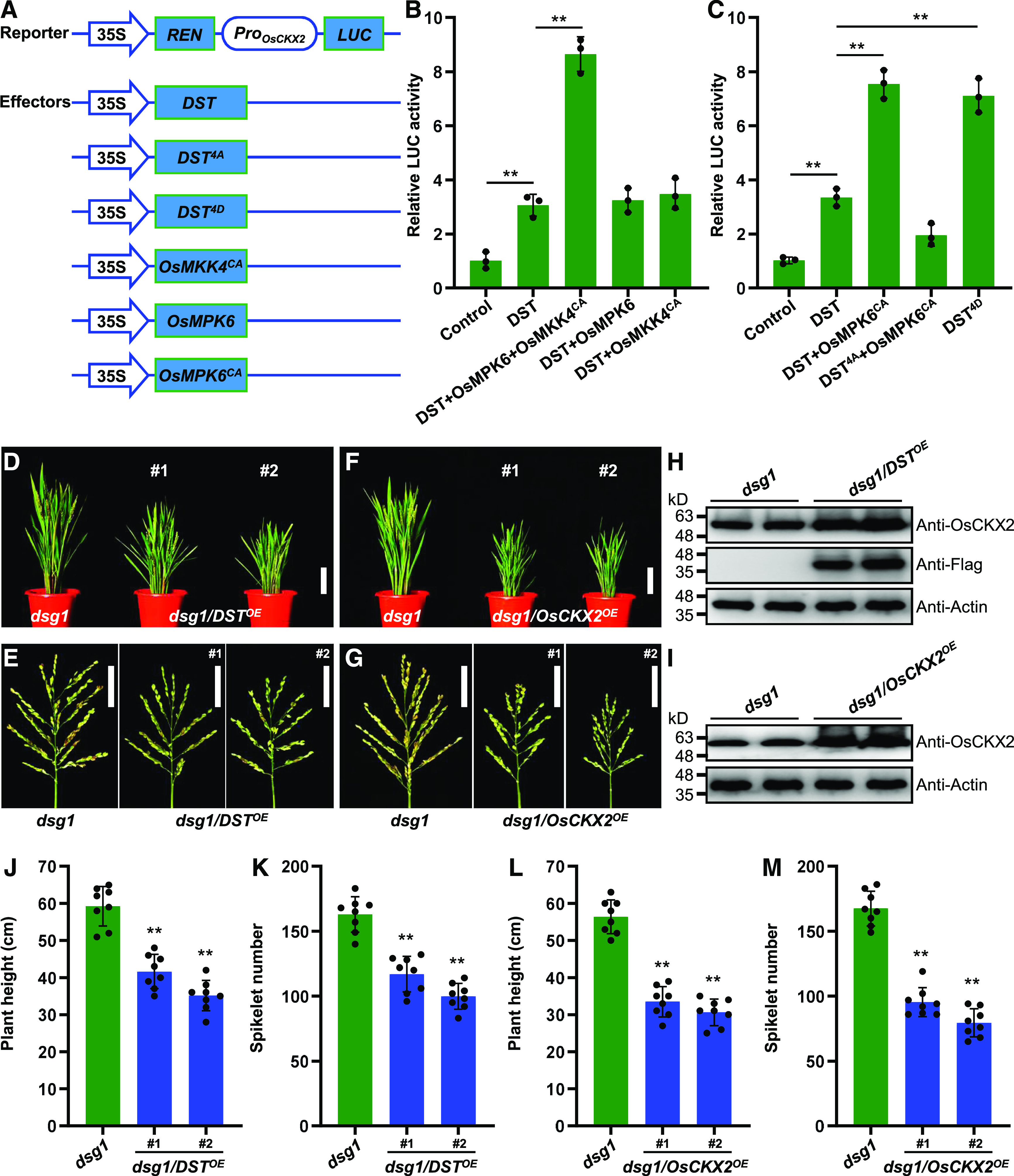
OsMPK6-Mediated Phosphorylation of DST Enhances Its Transcriptional Activity and Positively Regulates OsCKX2 Expression.
(A) Schematic diagram of the reporter and effector constructs used in the dual-LUC assay system.
(B) and (C) Transcriptional activation of OsCKX2 is enhanced by OsMKK4-OsMPK6 cascade-mediated DST phosphorylation (B), and constitutively activated DST (DST4D) (C) in rice protoplasts. Protoplasts were co-transformed with the reporter and different effector constructs and kept in the dark for 16 h. LUC/REN activity is shown (n = 3 independent biological repeats). Values are given as the mean ± sd. **P < 0.01 compared with the control using Student’s t test.
(D) Plant architecture of dsg1 mutant and dsg1 plants overexpressing DST (dsg1/DSTOE) at the reproductive stage. Scale bar, 10 cm.
(E) Panicle architecture of dsg1 mutant and dsg1/DSTOE plants at the reproductive stage. Scale bar, 5 cm.
(F) Plant architecture of dsg1 mutant and dsg1 plants overexpressing OsCKX2 (dsg1/OsCKX2OE) at the reproductive stage. Scale bar, 10 cm.
(G) Panicle architecture of dsg1 mutant and dsg1/OsCKX2OE plants at the reproductive stage. Scale bar, 5 cm.
(H) and (I) DST protein levels in the young panicles of dsg1/DSTOE (H), and OsCKX2 protein levels in the young panicles of dsg1/OsCKX2OE (I). Proteins extracted from pooled 0.1-0.5 cm panicles were subjected to immunoblot analysis using anti-Flag and anti-OsCKX2 antibodies. The anti-Actin antibody was used as the loading control.
(J) Comparison of average plant height between dsg1 and the dsg1/DSTOE transgenic lines (n = 8 plants).
(K) Comparison of average spikelet number per panicle between dsg1 and the dsg1/DSTOE lines (n = 8 plants).
(L) Comparison of average plant height between dsg1 and the dsg1/OsCKX2OE transgenic lines (n = 8 plants).
(M) Comparison of average spikelet number per panicle between dsg1 and the dsg1/OsCKX2OE lines (n = 8 plants). Values are given as the mean ± sd. **P < 0.01 compared with the control using Student’s t test.
We showed that OsMPK6 is required for rice panicle development and negatively regulates spikelet number (Supplemental Figure 5) and that the expression level of OsCKX2 is significantly reduced in osmpk6 (Figures 3D and 3E), implying that loss of function of OsMPK6 attenuates DST-activated OsCKX2 expression. To further explore the genetic interaction between OsMPK6 and DST, we overexpressed DST in the dsg1 mutant, a null mutant of OsMPK6 (Liu et al., 2015). Overexpressing DST suppressed the plant height and spikelet number per panicle phenotypes of the dsg1 mutant in the ZH11 background (Figures 5D, 5E, 5H, 5J, and 5K), which is consistent with a previous finding on the effect of DST on rice development (Li et al., 2013), supporting the hypothesis that DST is also a negative regulator of spikelet number and acts downstream of OsMPK6. Moreover, overexpressing OsCKX2 in the dsg1 mutant background dramatically decreased plant height and spikelet number per panicle (Figures 5F, 5G, 5I, 5L, and 5M), suggesting that OsCKX2 negatively regulates spikelet number and is a downstream effector in the OsMPK6-DST module. Overall, these results indicate that OsMPK6 acts upstream of DST to positively regulate OsCKX2 gene expression.
The OsER1-OsMKKK10-OsMKK4-OsMPK6 Pathway Controls Spikelet Number per Panicle and Is Genetically Dependent on the DST-OsCKX2 Module
Our findings provide the evidence that OsMPK6 interacts with and phosphorylates DST to enhance the expression of OsCKX2, thereby indicating that direct crosstalk occurs between the OsMKKK10-OsMKK4-OsMPK6 cascade and cytokinin metabolism. To determine whether there is a genetic relationship between the MAPK signaling pathway and the DST-OsCKX2 module, we performed a wide array of transgenic experiments using mutants of the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway. Strikingly, overexpressing DST rescued the spikelet number phenotype of the oser1 mutant (Figures 6A and 6E), indicating that DST is sufficient for OsER1-regulated panicle morphogenesis, which is consistent with that of the dsg1 mutant (Figures 5E and 5K). Overexpressing DST also reduced the number of spikelets in the osmkkk10 mutant (Figures 6B and 6F), which indicates that the regulation of spikelet number by MAPK signaling relies on the action of DST. These results not only imply that the genetic effects of DST on the components of the MAPK cascade are indeed universal, but they also suggest that DST is responsible for MAPK cascade-controlled rice panicle development and genetically acts downstream of the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway.
Figure 6.
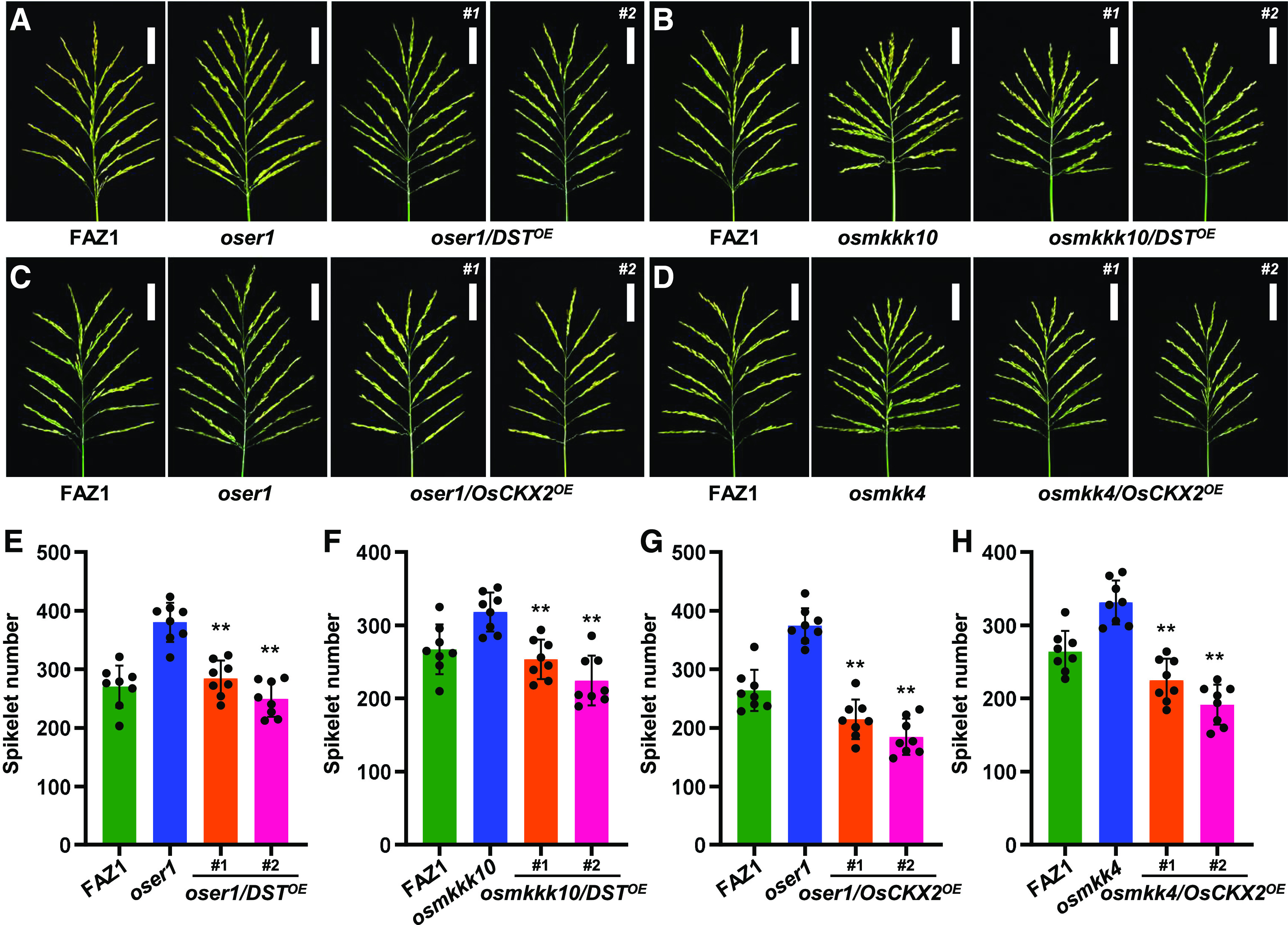
The OsER1-OsMKKK10-OsMKK4-OsMPK6 Pathway Controls DST-OsCKX2 Module-Dependent Regulation of Spikelet Number per Panicle.
(A) Panicle architecture of oser1 mutant and oser1 plants overexpressing DST (oser1/DSTOE) at the reproductive stage. Scale bar, 5 cm.
(B) Panicle architecture of osmkkk10 mutant and osmkkk10 plants overexpressing DST (osmkkk10/DSTOE) at the reproductive stage. Scale bar, 5 cm.
(C) Panicle architecture of oser1 mutant and oser1 plants overexpressing OsCKX2 (oser1/OsCKX2OE) at the reproductive stage. Scale bar, 5 cm.
(D) Panicle architecture of osmkk4 mutant and osmkk4 plants overexpressing OsCKX2 (osmkk4/OsCKX2OE) at the reproductive stage. Scale bar, 5 cm.
(E) to (H) Comparisons of average spikelet number per panicle between oser1 mutant and oser1/DSTOE lines (n = 8 plants) (E), osmkkk10 and osmkkk10/DSTOE lines (n = 8 plants) (F), oser1 and oser1/OsCKX2OE lines (n = 8 plants) (G), and osmkk4 and osmkk4/OsCKX2OE lines (n = 8 plants) (H).
Values are given as the mean ± sd. **P < 0.01 compared with oser1, osmkkk10, or osmkk4 using Student’s t test.
We showed that the abundance of OsCKX2 is markedly reduced in the MAPK signaling pathway mutants during the young panicle stage (Figures 3B and 3E). We further confirmed the genetic effect of OsCKX2 in the oser1 and osmkk4 mutant backgrounds. Overexpressing OsCKX2 in the oser1 mutant background significantly suppressed the production of spikelets and rescued the panicle architecture phenotype (Figures 6C and 6G), suggesting that OsER1-controlled panicle shaping depends on OsCKX2 expression. Moreover, overexpressing OsCKX2 rescued the spikelet number phenotype of osmkk4 (Figures 6D and 6H), which is similar to the findings for dsg1 (Figures 5G and 5M), implying that OsCKX2-regulated cytokinin metabolism, which is required for rice panicle development, acts downstream of the MAPK cascade. These findings strongly support the hypothesis that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is closely associated with cytokinin homeostasis in determining the number of spikelets in rice. Taken together, the results suggest that overexpressing either DST or OsCKX2 rescues the spikelet number phenotypes of the oser1, osmkkk10, and osmkk4 mutants to different extents. They also reveal that the control of spikelet number per panicle by the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway depends on cytokinin metabolism mediated by the DST-OsCKX2 module.
DISCUSSION
Rice is a model plant from the grass family that has evolved a characteristic inflorescence morphology, with complex branches and specialized spikelets. To date, the origin and morphogenesis of the diverse inflorescence architectures of grass family members, which are regulated by complex integrated networks in response to developmental cues and environmental signals, have attracted wide interest (Zhang and Yuan, 2014). Notably, the inflorescence architecture of grass crops is closely associated with the number of spikelets and final grain yield. Increasing molecular evidence suggests that multiple elements play important roles in determining the fate of the reproductive meristem and shaping inflorescence architecture in the grass family (Satoh-Nagasawa et al., 2006; Derbyshire and Byrne, 2013; Li, 2013; Wu et al., 2016; Huo et al., 2017; Jiao et al., 2018; Gao et al., 2019; Guo et al., 2019). For example, in maize, the trehalose-6-phosphate phosphatase RAMOSA3, which is expressed in discrete domains subtending axillary inflorescence meristems, regulates inflorescence branching by modifying sugar signals that move into axillary meristems (Satoh-Nagasawa et al., 2006). In Brachypodium, MORE SPIKELETS1, an APETALA2 transcription factor in the ethylene response factor class, determines meristem fate by regulating the transition to terminal spikelet development (Derbyshire and Byrne, 2013). In rice, upregulating the enoyl-CoA hydratase/isomerase gene NUMBER OF GRAINS1 enhances grain number per panicle by decreasing total fatty acid and linolenic acid contents and endogenous jasmonic acid levels (Huo et al., 2017). In Sorghum, the Teosinte branched/Cycloidea/PCF transcription factor MULTISEEDED1 controls pedicellate spikelet number through the jasmonic acid pathway (Jiao et al., 2018). Nonetheless, the upstream signals that shape inflorescence architecture in crops are unclear. Furthermore, although it is well known that the phytohormone cytokinin promotes cell division and plays a fundamental and conserved role in regulating the size and activity of reproductive meristems in plants (Kyozuka, 2007; Zhang and Yuan, 2014), how cytokinin levels are regulated by signals responsible for inflorescence morphogenesis in grasses remains largely unknown.
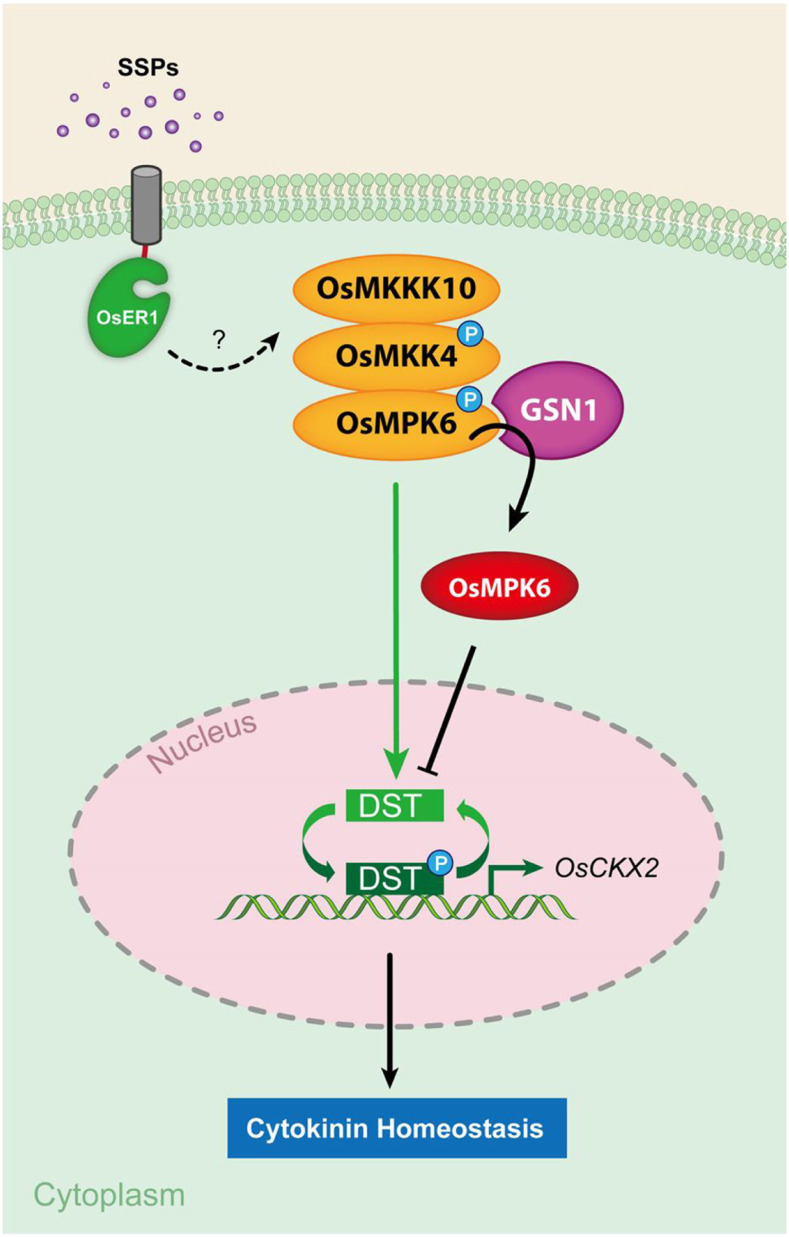
Here, we demonstrated that OsER1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control the number of spikelets per panicle in rice and that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway is required for regulation of cytokinin metabolism. Furthermore, we found that OsMPK6 interacts with and phosphorylates the zinc finger transcription factor DST, enhancing its transcriptional activity and positively regulating the expression of OsCKX2. Several lines of genetic and biochemical evidence strongly support the hypothesis that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway negatively regulates the number of spikelets per panicle by modulating cytokinin metabolism, specifically by increasing the transcriptional activity of DST through phosphorylation. Based on these findings, we propose a working model describing the molecular mechanism underlying the determination of spikelet number per panicle (Figure 7). During panicle morphogenesis, when the active reproductive meristem produces cell-specific signaling molecules such as small secreted peptides (SSPs), this triggers OsER1 to activate the OsMKKK10-OsMKK4-OsMPK6 cascade directly or through unknown mediators. The activated OsMKKK10-OsMKK4-OsMPK6 cascade phosphorylates the zinc finger transcription factor DST to enhance its transcriptional activity and increase the expression level of OsCKX2, which results in the degradation of cytokinin, thereby maintaining cytokinin at levels required for spikelet differentiation and formation. Meanwhile, the spatiotemporally activated GSN1 protein acts like a molecular brake to negatively regulate the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway by inactivating OsMPK6. This attenuates the phosphorylation of DST, thereby decreasing the expression of OsCKX2 and increasing cytokinin levels. Hence, the signaling output of the OsER1-OsMKKK10-OsMKK4-OsMPK6-DST-OsCKX2 pathway negatively regulated by GSN1 maintains cytokinin homeostasis and ultimately determines spikelet number per panicle in rice.
Figure 7.
Proposed Working Model of the Role of the OsER1-OsMKKK10-OsMKK4-OsMPK6-DST-OsCKX2 Pathway in Determining Spikelet Number per Panicle in Rice.
During panicle morphogenesis, when the active reproductive meristem produces cell-specific signaling molecules such as SSPs, the RLK OsER1 binds to the signals and activates the OsMKKK10-OsMKK4-OsMPK6 cascade directly or through unknown mediators. The activated OsMKKK10-OsMKK4-OsMPK6 cascade phosphorylates the transcription factor DST to enhance its transcriptional activity and positively increase the expression level of OsCKX2. This results in the degradation of cytokinin, thereby maintaining proper cytokinin levels for spikelet differentiation and formation. Meanwhile, the spatiotemporally activated GSN1 protein acts like a molecular brake to negatively regulate the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway by inactivating OsMPK6, which attenuates the phosphorylation of DST, thereby decreasing the expression of OsCKX2 and increasing cytokinin levels.
The contribution of both the OsER1 receptor and the OsMKKK10-OsMKK4-OsMPK6 cascade-to-spikelet number per panicle raises the interesting question of how the signaling specificity of the OsMKKK10-OsMKK4-OsMPK6 cascade in different growth and developmental pathways is maintained. In Arabidopsis, the ER receptor kinase gene is strongly expressed in vegetative and reproductive SAMs and in developing leaf and flower primordia, thereby controlling multiple aspects of plant morphology that are critical for efficient plant responses to environmental changes (Kosentka et al., 2019). For example, ER regulates the size of the SAM and the initiation of leaf primordia and plays an essential role in reproductive organ development, which is closely associated with auxin transport and cytokinin homeostasis (Shpak, 2013). Additionally, the mutation of ER resulted in altered hyponastic petiole growth, circadian leaf movements, and the shade avoidance response (Shpak, 2013). Notably, the function of ER and the molecular mechanism of its action are most well understood in stomata development, during which ER negatively modulates cell fate transitions and contributes to the orientation of asymmetric cell divisions (Pillitteri and Torii, 2012). Intriguingly, cell-specific expression of input signaling molecules such as peptide ligands recognized by cell surface receptors could maintain signaling specificity (Lee and Torii, 2012). In Arabidopsis, several secreted cysteine-rich peptides from the EPIDERMAL PATTERNING FACTOR/EPIDERMAL PATTERNING FACTOR-LIKE family have been shown to be ligands of ER (Shpak, 2013; Kosentka et al., 2019). Nevertheless, the secreted peptides responsible for rice inflorescence morphology are largely unknown (Bessho-Uehara et al., 2016). The specificity of signaling can also be determined by the presence of diverse substrates that are differentially expressed and activated by MAPKs.
In Arabidopsis, the well-known MAPKs MPK3 and MPK6 play essential roles in both plant growth and development and biotic and abiotic stress responses by phosphorylating downstream partner proteins, which could contribute to the functional specificity of MAPK cascades (Rodriguez et al., 2010). Although yeast two-hybrid screening and high-throughput methods have been used to identify the putative MAPK substrates, only a small number of MPK6 substrates have been verified by functional evidence in Arabidopsis (Hoehenwarter et al., 2013; Xu and Zhang, 2015; Komis et al., 2018). To date, very few substrates of OsMPK6 have been identified in rice, which are closely associated with the various functions of OsMPK6 in growth and development (Hu et al., 2015; Tian et al., 2017). The loss of function of OsMPK6 results in smaller grain size but increased grain number per panicle (Liu et al., 2015; Guo et al., 2018; Xu et al., 2018b), thereby disordering panicle morphogenesis. Despite the progress in this field, the exact substrates of OsMPK6 involved in determining inflorescence architecture are completely unknown, thus raising the important question of which proteins phosphorylated by OsMPK6 control panicle morphogenesis.
In this study, we identified DST as a substrate of OsMPK6 by yeast two-hybrid screening and confirmed that OsMPK6 interacts with and phosphorylates DST (Figures 4A to 4E). Furthermore, the phosphorylation of DST enhanced its transcriptional activation of OsCKX2 (Figures 5A to 5C). In addition, overexpression of DST rescued the spikelet number per panicle phenotypes of the osmpk6, oser1, and osmkkk10 mutants (Figures 5E, 5K, 6A, 6B, 6E, and 6F). The identification of a substrate that is a direct target of the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway furthers our understanding of how the signals perceived on the cell surface activate the reproductive meristem through nuclear signals. Moreover, the finding that the phosphorylation of DST by OsMPK6 promotes the expression of OsCKX2 (Figures 5A to 5C) indicates that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway directly controls the degradation of cytokinin and maintains cytokinin homeostasis, which is required for inflorescence meristem development. This finding reveals specific crosstalk between a receptor-like protein kinase and cytokinin metabolism in plants.
Cytokinin is a classic phytohormone that plays essential roles in regulating meristem organization and activity and is thus a positive regulator of cell proliferation in the SAM (Zhao et al., 2010; Perales and Reddy, 2012). Notably, the level of cytokinin in the meristem depends on its metabolic degradation, which is catalyzed by multiple CKX enzymes. Thus, an increase in cytokinin levels in the SAM due to a mutation in a CKX gene could enhance meristem activity (Bartrina et al., 2011). However, the signaling pathway upstream of cytokinin metabolism regulation remains elusive. In Arabidopsis, the control of stem-cell fate in the SAM relies on a negative feedback loop involving the secreted peptide CLAVATA3 (CLV3) and the homeodomain transcription factor WUSCHEL (WUS; Zhao et al., 2010; Schaller et al., 2015). In fact, it has been proposed that active cytokinin, together with WUS-induced CLV3 expression, acts as a positional cue in the SAM that regulates the dynamic positioning of WUS expression such that it corresponds to the maximum cytokinin activity (Schaller et al., 2015). Interestingly, an Arabidopsis mutant lacking ER family genes displays significant upregulation of cytokinin-responsive genes in the SAM and an increased stem cell population with hyperinduction of CLV3 expression in response to cytokinin, indicating that ER genes regulate stem cell homeostasis by buffering cytokinin responsiveness (Uchida et al., 2013). These results support the hypothesis that the ER signaling pathway regulates SAM organization by affecting cytokinin accumulation and responses through unknown molecular mechanism.
Here, we demonstrated that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway directly regulates OsCKX2 expression through phosphorylation of DST, illustrating that crosstalk between an ER receptor and cytokinin underlies the activity of the reproductive meristem as well as inflorescence development. Our findings provide a precise mechanistic understanding of how receptor signaling modulates phytohormone metabolism in plant. Nonetheless, the specific molecular mechanism controlled by OsER1 responsible for grain shape remains unclear, which might depend on the diverse downstream substrates of OsMPK6. Hence, more interacting proteins of OsMPK6 that together contribute to panicle architecture plasticity by coordinating grain number per panicle and grain size should be identified in the future.
Taken together, our findings reveal that the OsER1 receptor acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to negatively regulate the number of spikelets per panicle and that the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway controls cytokinin metabolism by phosphorylating DST and enhancing OsCKX2 expression, thereby providing a framework for how perceived developmental signals control phytohormone metabolism to shape the plant inflorescence. Furthermore, these findings provide important insights into the developmental plasticity of the inflorescence and a potential means to breed high-yielding crop varieties with more grains per panicle by genetically manipulating the OsER1-OsMKKK10-OsMKK4-OsMPK6-DST-OsCKX2 pathway. Nevertheless, little is known about the specific SSPs that associate with the OsER1 receptor and are required for panicle development. In addition, information about the specific substrates of OsMPK6 is still lacking, which limits our understanding of the OsMKKK10-OsMKK4-OsMPK6 cascade in rice. Moreover, the molecular mechanism of signal transduction from OsER1 to OsMKKK10 and the genetic association between OsER1 and its close homolog OsER2 (LOC_Os06g03970) remain unclear. Further identification of the proteins associated with the OsER1-OsMKKK10-OsMKK4-OsMPK6 pathway will ultimately uncover the genetics basis of panicle development in rice.
METHODS
Plant Materials and Growth Conditions
The oser1 and osckx2 mutants were isolated by CRISPR/Cas9 gene editing of the elite indica rice (Oryza sativa) variety FAZ1 and the japonica variety ZH11, respectively. All rice plants were cultivated in experimental fields in Shanghai (China) or Lingshui (Hainan Province, China) under natural growth conditions.
Plasmid Construction and Plant Transformation
The full-length genomic sequence of OsER1 was amplified from the wild-type FAZ1 and cloned into the plant binary vector pCAMBIA1300 to produce the complementation construct pCAMBIA1300-gOsER1. To generate overexpression constructs, the full-length coding sequences of OsER1, DST, and OsCKX2 were amplified from FAZ1 and cloned into the plant binary vector pCAMBIA1301 under the control of the Ubiquitin promoter. To produce constitutively activated OsMKK4CA and OsMKKK10CA, the full-length coding sequence of OsMKK4 harboring the Thr238Asp and Ser244Asp mutations and a modified OsMKKK10 sequence harboring a deletion mutation (Xu et al., 2018b) were cloned into pCAMBIA1301. The gene knockout constructs for CRISPR/Cas9 gene editing of OsER1 and OsCKX2 were designed as previously described by Ma et al. (2015). Agrobacterium tumefaciens-mediated transformation in rice with the strain EHA105 was performed as previously described by Hiei et al. (1994). All constructs used in the study were produced using NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs) and were confirmed by sequencing. The PCR primer sets are listed in the Supplemental Dataset.
RNA Extraction and qRT-PCR
Total plant RNA was extracted individually from diverse rice tissues using TRIzol Reagent (Invitrogen). Reverse transcription was performed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo) and 500 ng of total RNA. qRT-PCR analysis was performed with the ABI 7300 Real Time PCR System using Fast Start Universal SYBR Green Master Mix and ROX (Roche). The rice UBQ5 gene was used as an internal reference to normalize gene expression data, which were analyzed using the 2−ΔΔCT method.
Nucleus Isolation and Assessment of Ploidy
Isolation of cell nuclei and ploidy assessment were performed as previously described by Guo et al. (2018). In brief, young rice panicles (0.1 cm to 0.3 cm) were selected and soaked in nuclear isolation solution and then in staining solution (Beckman-Coulter), after which the tissues were chopped with a sharp blade. The nuclei suspension was loaded into a MoFlo (Beckman-Coulter) for flow cytometric analysis after filtering through a 40-μm nylon filter, and the ploidy levels of ∼10,000 nuclei were recorded for each assay. The numbers of diploid and tetraploid nuclei were recorded, and the software FCS Express 4 (https://denovosoftware.com) was used to calculate the relative proportions of cells in the G1, G2/M, and S phases.
Measuring Endogenous Cytokinin Levels
To assay endogenous cytokinin levels, all rice plants were cultivated in experimental fields for approximately five weeks. Next, freshly initiated 0.1-cm to 0.5-cm long panicles were harvested, and ∼1-g samples were pooled for measurements, with three independent biological repeats per sample. Quantification of endogenous cytokinins was performed as described by Wu et al. (2016).
Yeast Two-Hybrid Assays
The Y2H Gold-Gal4 system (Clontech) was used to perform yeast two-hybrid assays. The full-length coding sequences of OsMPK6 and DST were inserted into the pGBKT7 and pGADT7 vectors to prepare the bait and prey constructs, respectively, which were transformed into yeast strain Y2H Gold. For yeast two-hybrid screening of OsMPK6 interacting proteins, the yeast cells were cultured on SD/-Trp medium, and 500-mL cultures were grown to prepare competent cells for transformation with the pGADT7 plasmid library as described by Chen et al. (2019). After transformation, yeast cells containing the pGADT7 plasmid library were cultured on SD/-Trp-Leu-His or SD/-Trp-Leu-His-Ade medium containing X-ɑ-gal at 30°C in the dark for 3 d, after which the positive colonies were selected, and the inserts in the pGBKT7 vectors were sequenced. For the specific yeast two-hybrid assays, the bait and prey constructs were co-transformed into yeast strain Y2H Gold according to the manufacturer’s instructions (Clontech). The yeast cells were cultured on different media for observation. SD/-Trp is yeast culture medium without Trp. SD/-Trp-Leu-His is yeast culture medium without Trp, Leu, and His. SD/-Trp-Leu-His-Ade is culture medium without Trp, Leu, His, and Ade. The PCR primers used for the yeast two-hybrid assays are listed in the Supplemental Data Set.
BiFC Assays
The full-length coding sequences of OsMPK6, DST, and OsHAL3 were cloned into pCAMBIA1300S-YC and pCAMBIA1300S-YN to produce the cYFP-protein and nYFP-protein constructs, respectively. Leaves of five-week–old Nicotiana benthamiana plants were co-infiltrated with A. tumefaciens strain GV3101 carrying the two constructs. After infiltration, the plants were grown in the dark for 48 h, and fluorescence signals were observed under an LSM 880 confocal laser-scanning microscope (Carl Zeiss). PCR primers used for BiFC are listed in the Supplemental Data Set.
Pull-Down Assays
The pull-down assays were performed as described by Chen et al. (2019). In brief, the coding sequence of OsMPK6 was inserted into the pMAL-c5x vector, and the coding region of DST was cloned into the pET-32a vector. The recombinant pMAL-c5x construct was transformed into Escherichia coli TB1-competent cells, and the pET-32a expression plasmid was transformed into E. coli BL21 (DE3)-competent cells, which were cultured at 37°C until the OD600 of the cell culture was 0.5. Recombinant protein production was then induced with 1 mM of IPTG for 36 h at 12°C. Next, E. coli cells expressing MBP-OsMPK6 and MBP were lysed by high-pressure cell disruption (Constant Systems) and centrifuged. The supernatant was incubated with amylose resin (New England Biolabs) for 30 min at 4°C and washed five times with MBP column buffer. Supernatant containing DST-His protein was incubated with approximately the same amount of MBP-OsMPK6 and MBP binding beads for 2 h at 4°C. The beads were washed five times with MBP column buffer, and the eluted mixture was resuspended in SDS loading buffer and boiled for 5 min, separated by 10% (v/v) SDS-PAGE, and immunoblotted with anti-MBP (cat. no. E8032; New England Biolabs) and anti-His (cat. no. AE028; ABclonal) antibodies. PCR primers used for the pull-down assays are listed in the Supplemental Data Set.
Co-IP
The coding sequence of DST was cloned into the pCAMBIA1306-Flag (3x) plasmid to produce the Pro35S:DST-Flag vector. The OsMPK6 coding sequence was cloned into pCAMBIA1301-Myc (7x)-His (6x) to generate the Pro35S:OsMPK6-Myc vector. The Pro35S:DST-Flag and Pro35S:OsMPK6-Myc constructs in A. tumefaciens strain GV3101 were transiently co-expressed in N. benthamiana leaf cells. The protein extraction and immunoblot assays were performed as previously described using anti-Flag (cat. no. 14793; CST) and anti-Myc (cat. no. 2276; CST) antibodies (Guo et al., 2018). The PCR primers used for Co-IP are listed in the Supplemental Data Set.
In Vitro Phosphorylation Assays
The coding sequences of OsMPK6 and OsMPK4CA were inserted into the pMAL-c5x vector, and the coding region of DST was cloned into the pET-32a vector to produce fusion proteins, which were used in the phosphorylation assays. In vitro phosphorylation assays were performed as described by Zhang et al. (2017). In brief, recombinant MBP-OsMPK6 (20 μg) was activated by incubation with recombinant MBP-OsMKK4CA (0.5 μg) in the presence of 50 μM of ATP in 100 μL of reaction buffer (25 mM of HEPES at pH 7.5, 10 mM of MgCl2, and 2 mM of dithiothreitol) at 25°C for 1 h. Activated MBP-OsMPK6 was then used to phosphorylate recombinant His-DST and His-DST4A proteins (1:20 enzyme/substrate) in the same reaction buffer with 200 μM of ATP. The reactions were stopped by adding SDS-loading buffer. After phosphorylation, one half of the protein products was separated by 10% (v/v) SDS-PAGE, followed by staining with Coomassie Brilliant Blue. The other half of the proteins was separated for immunoblotting analysis using anti-Phospho-Thr (cat. no. 9391; CST), anti-His (cat. no. AE028; ABclonal), anti-OsMPK6 (cat. no. AbP80140-A-SE; Beijing Protein Innovation), anti-OsMKK4 (cat. no. AbP80132-A-SE; Beijing Protein Innovation), and anti-Phospho-p44/42 (cat. no. 4370; CST) antibodies. PCR primers used for in vitro phosphorylation assays are listed in the Supplemental Data Set.
Dual-LUC Transient Expression Assays in Rice Protoplasts
The dual-LUC transient expression assays were performed as previously described by Su et al. (2016). The promoter of OsCKX2 was amplified from FAZ1 genomic DNA and cloned upstream of the firefly LUC coding region to form the LUC reporter vector. The reporter vector included two expression cassettes: the target promoter driving the LUC reporter gene and a CaMV 35S promoter driving the Renilla luciferase (REN) gene as an internal control. To construct the different effector vectors, the full-length coding sequences of DST, OsMKK4, and OsMPK6 were amplified and cloned into the vectors driven by the CaMV 35S promoter. The plasmids were purified using a HiSpeed Plasmid Midi Kit (Qiagen) and introduced into rice protoplasts by polyethylene glycol-mediated transformation. Transformed protoplasts were incubated at 22°C for 16 h in the dark. The LUC and REN activities were measured using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. LUC/REN represents relative transcriptional activity. The PCR primers are listed in the Supplemental Data Set.
Plant Protein Extraction and Immunoblot Analysis
Protein extraction and immunoblot assays were performed as described by Guo et al., 2019. Young panicle samples were harvested, and soluble proteins were extracted with a Plant Total Protein Extraction Kit (Sigma-Aldrich) according to the manufacturer’s instructions. In brief, the pooled young panicles were ground to a fine powder in liquid nitrogen. The powder was rinsed with methanol solution, followed by acetone. The supernatant from the final extraction with acetone was removed, and the samples were dried and dissolved. Proteins were denatured by the addition of concentrated SDS loading buffer, followed by boiling for 5 min, and separated by 10% (v/v) SDS-PAGE. The endogenous protein levels of OsER1, OsMKK4, OsMPK6, OsMPK3, OsCKX2, and GSN1 were visualized by immunoblot analysis using anti-OsER1 (cat. no. WG02338; ABclonal), anti-OsMKK4 (cat. no. AbP80132-A-SE; Beijing Protein Innovation), anti-OsMPK6 (cat. no. AbP80140-A-SE; Beijing Protein Innovation), anti-OsMPK3 (cat. no. AbP80147-A-SE; Beijing Protein Innovation), anti-OsCKX2 (cat. no. AbP80080-A-SE; Beijing Protein Innovation), and anti-GSN1 (cat. no. WG01927; ABclonal) antibodies, respectively. Phosphorylated OsMPK6 was visualized by immunoblot analysis using anti-Phospho-p44/42 antibody (cat. no. 4370; CST). The loading control was visualized using anti-Actin antibody (cat. no. M20009; Abmart).
Statistical Analysis
For panicle phenotype analysis, gene expression assays, comparison of endogenous cytokinin levels, and dual-LUC transient expression assays, statistical analysis was performed as described in the figure legends. Significant differences were determined with paired two-tailed Student’s t test. All analyses were performed using the software GraphPad Prism 8 (https://www.graphpad.com/).
Accession Numbers
Sequence data from this article can be found in the MSU Rice Genome Annotation Project Database under the following accession numbers: OsER1, LOC_Os06g10230; OsMKKK10, LOC_Os04g47240; OsMKK4, LOC_Os02g54600; OsMPK6, LOC_Os06g06090; DST, LOC_Os03g57240; OsCKX2, LOC_Os01g10110; GSN1, LOC_Os05g02500; and OsMPK3, LOC_Os03g17700.
Supplemental Data
Supplemental Figure 1. Deletion of OsER1 causes an increase in spikelet number per panicle phenotype resembling that of the osckx2 mutant in the japonica variety ZH11.
Supplemental Figure 2. Genotyping of the oser1 and osckx2 mutants.
Supplemental Figure 3. Overexpression of OsER1 has no effect on spikelet number per panicle.
Supplemental Figure 4. OsER1 regulates localized cell proliferation during young panicle developmental.
Supplemental Figure 5. The GSN1-OsMPK6 module regulates spikelet number per panicle in rice.
Supplemental Figure 6. OsER1 and the OsMKKK10-OsMKK4-OsMPK6 cascade negatively regulate the number of spikelets per panicle.
Supplemental Data Set. Primers used in this study.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We are grateful for the support of the Shanghai Post-doctoral Excellence Program and the SA-SIBS Scholarship Program. We thank Min Shi (Chinese Academy of Science Centre for Excellence in Molecular Plant Sciences) for technical support. We thank Fan Chen (Institute of Genetics and Developmental Biology, Chinese Academy of Science) for the dsg1 mutant. We also thank Greensword Creation Technology Co., Ltd. (Wuhan) for help with cytokinin measurements. This work was supported by the National Natural Science Foundation of China (grant 31788103), the Chinese Academy of Sciences (grants XDB27010104 and QYZDY–SSW–SMC023), the Guangdong Laboratory of Lingnan Modern Agriculture, the Ministry of Science and Technology of China (grant 2016YFD0100902), the China Postdoctoral Science Foundation (grant 2018M642102), the Science and Technology Commission of Shanghai Municipality (grant 18JC1415000), the Chinese Academy of Sciences-Croucher Funding Scheme for Joint Laboratories, and the National Key Laboratory of Plant Molecular Genetics.
AUTHOR CONTRIBUTIONS
H.-X.L. conceived and supervised the project; H.-X.L. and T.G. designed the experiments; T.G. and Z.-Q.L. performed most of the experiments; J.-X.S., W.-W.Y., N.-Q.D., and H.-X.L. performed some of the experiments; T.G. and H.-X.L. analyzed data and wrote the article.
References
- Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M.(2005). Cytokinin oxidase regulates rice grain production. Science 309: 741–745. [DOI] [PubMed] [Google Scholar]
- Bartrina I., Otto E., Strnad M., Werner T., Schmülling T.(2011). Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berriri S., Garcia A.V., Frei dit Frey N., Rozhon W., Pateyron S., Leonhardt N., Montillet J.L., Leung J., Hirt H., Colcombet J.(2012). Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. Plant Cell 24: 4281–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho-Uehara K., et al. (2016). Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proc. Natl. Acad. Sci. USA 113: 8969–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., et al. (2019). Translational regulation of plant response to high temperature by a dual-function tRNAHis guanylyltransferase in rice. Mol. Plant 12: 1123–1142. [DOI] [PubMed] [Google Scholar]
- Derbyshire P., Byrne M.E.(2013). MORE SPIKELETS1 is required for spikelet fate in the inflorescence of Brachypodium. Plant Physiol. 161: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.Q., Wang N., Wang X.L., Zhang X.S.(2019). Architecture of wheat inflorescence: Insights from rice. Trends Plant Sci. 24: 802–809. [DOI] [PubMed] [Google Scholar]
- Guo T., Chen K., Dong N.Q., Shi C.L., Ye W.W., Gao J.P., Shan J.X., Lin H.X.(2018). GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell 30: 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Chen K., Dong N.Q., Ye W.W., Shan J.X., Lin H.X.(2019). Tillering and small grain 1 dominates the tryptophan aminotransferase family required for local auxin biosynthesis in rice. J. Integr. Plant Biol. 62: 581–600. [DOI] [PubMed] [Google Scholar]
- Hiei Y., Ohta S., Komari T., Kumashiro T.(1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6: 271–282. [DOI] [PubMed] [Google Scholar]
- Higuchi M., et al. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 101: 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehenwarter W., Thomas M., Nukarinen E., Egelhofer V., Röhrig H., Weckwerth W., Conrath U., Beckers G.J.(2013). Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol. Cell. Proteomics 12: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Ye M., Li R., Zhang T., Zhou G., Wang Q., Lu J., Lou Y.(2015). The rice transcription factor wrky53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol. 169: 2907–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.D., Lee T.Y., Tzeng S.W., Horng J.T.(2005). KinasePhos: A web tool for identifying protein kinase-specific phosphorylation sites. Nucleic Acids Res. 33: W226–W229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Qian Q., Liu Z., Sun H., He S., Luo D., Xia G., Chu C., Li J., Fu X.(2009a). Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41: 494–497. [DOI] [PubMed] [Google Scholar]
- Huang X.Y., Chao D.Y., Gao J.P., Zhu M.Z., Shi M., Lin H.X.(2009b). A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 23: 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X., Wu S., Zhu Z., Liu F., Fu Y., Cai H., Sun X., Gu P., Xie D., Tan L., Sun C.(2017). NOG1 increases grain production in rice. Nat. Commun. 8: 1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D., Glossip D., Xing H., Muslin A.J., Kornfeld K.(1999). Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13: 163–175. [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lee Y.K., Gladman N., Chopra R., Christensen S.A., Regulski M., Burow G., Hayes C., Burke J., Ware D., Xin Z.(2018). MSD1 regulates pedicellate spikelet fertility in sorghum through the jasmonic acid pathway. Nat. Commun. 9: 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K., Maekawa M., Ujiie S., Satake Y., Furutani I., Okamoto H., Shimamoto K., Kyozuka J.(2003). LAX and SPA: Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 100: 11765–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G., Šamajová O., Ovečka M., Šamaj J.(2018). Cell and developmental biology of plant mitogen-activated protein kinases. Annu. Rev. Plant Biol. 69: 237–265. [DOI] [PubMed] [Google Scholar]
- Kosentka P.Z., Overholt A., Maradiaga R., Mitoubsi O., Shpak E.D.(2019). EPFL signals in the boundary region of the SAM restrict its size and promote leaf initiation. Plant Physiol. 179: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J.(2007). Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655. [DOI] [PubMed] [Google Scholar]
- Kyozuka J.(2007). Control of shoot and root meristem function by cytokinin. Curr. Opin. Plant Biol. 10: 442–446. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Torii K.U.(2012). A tale of two systems: Peptide ligand-receptor pairs in plant development. Cold Spring Harb. Symp. Quant. Biol. 77: 83–89. [DOI] [PubMed] [Google Scholar]
- Li S., et al. (2013). Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 110: 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Hua L., Dong S., Chen H., Zhu X., Jiang J., Zhang F., Li Y., Fang X., Chen F.(2015). OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 84: 672–681. [DOI] [PubMed] [Google Scholar]
- Ma X., et al. (2015). A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8: 1274–1284. [DOI] [PubMed] [Google Scholar]
- Meng X., Wang H., He Y., Liu Y., Walker J.C., Torii K.U., Zhang S.(2012). A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 24: 4948–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Zhang S.(2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51: 245–266. [DOI] [PubMed] [Google Scholar]
- Perales M., Reddy G.V.(2012). Stem cell maintenance in shoot apical meristems. Curr. Opin. Plant Biol. 15: 10–16. [DOI] [PubMed] [Google Scholar]
- Pillitteri L.J., Torii K.U.(2012). Mechanisms of stomatal development. Annu. Rev. Plant Biol. 63: 591–614. [DOI] [PubMed] [Google Scholar]
- Riefler M., Novak O., Strnad M., Schmülling T.(2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.C., Petersen M., Mundy J.(2010). Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61: 621–649. [DOI] [PubMed] [Google Scholar]
- Rupp H.M., Frank M., Werner T., Strnad M., Schmülling T.(1999). Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 18: 557–563. [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N., Nagasawa N., Malcomber S., Sakai H., Jackson D.(2006). A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441: 227–230. [DOI] [PubMed] [Google Scholar]
- Schaller G.E., Bishopp A., Kieber J.J.(2015). The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 27: 44–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., et al. (2015). Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 33: 996–1003. [DOI] [PubMed] [Google Scholar]
- Shpak E.D.(2013). Diverse roles of ERECTA family genes in plant development. J. Integr. Plant Biol. 55: 1238–1250. [DOI] [PubMed] [Google Scholar]
- Shpak E.D., Lakeman M.B., Torii K.U.(2003). Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA Leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell 15: 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Shan J.X., Gao J.P., Lin H.X.(2016). OsHAL3, a blue light-responsive protein, interacts with the floral regulator Hd1 to activate flowering in rice. Mol. Plant 9: 233–244. [DOI] [PubMed] [Google Scholar]
- Tabuchi H., et al. (2011). LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 23: 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li X., Zhou W., Ren Y., Wang Z., Liu Z., Tang J., Tong H., Fang J., Bu Q.(2017). Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture. Plant Physiol. 175: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K.U., Mitsukawa N., Oosumi T., Matsuura Y., Yokoyama R., Whittier R.F., Komeda Y.(1996). The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Shimada M., Tasaka M.(2013). ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant Cell Physiol. 54: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Yoshida R., Kishi-Kaboshi M., Matsushita A., Jiang C.J., Goto S., Takahashi A., Hirochika H., Takatsuji H.(2015). Abiotic stresses antagonize the rice defence pathway through the tyrosine-dephosphorylation of OsMPK6. PLoS Pathog. 11: e1005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., et al. (2019). GRAIN LENGTH AND AWN 1 negatively regulates grain size in rice. J. Integr. Plant Biol. 61: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmülling T.(2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T., Motyka V., Strnad M., Schmülling T.(2001). Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 98: 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C., Gibson S., Jarpe M.B., Johnson G.L.(1999). Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79: 143–180. [DOI] [PubMed] [Google Scholar]
- Wu Y., Wang Y., Mi X.F., Shan J.X., Li X.M., Xu J.L., Lin H.X.(2016). The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 12: e1006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Zhang Q.(2010). Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 61: 421–442. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhang S.(2015). Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20: 56–64. [DOI] [PubMed] [Google Scholar]
- Xu R., Yu H., Wang J., Duan P., Zhang B., Li J., Li Y., Xu J., Lyu J., Li N., Chai T., Li Y.(2018a). A mitogen-activated protein kinase phosphatase influences grain size and weight in rice. Plant J. 95: 937–946. [DOI] [PubMed] [Google Scholar]
- Xu R., et al. (2018b). Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol. Plant 11: 860–873. [DOI] [PubMed] [Google Scholar]
- Yang K.Y., Liu Y., Zhang S.(2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yuan Z.(2014). Molecular control of grass inflorescence development. Annu. Rev. Plant Biol. 65: 553–578. [DOI] [PubMed] [Google Scholar]
- Zhang M., Su J., Zhang Y., Xu J., Zhang S.(2018). Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 45 (Pt A): 1–10. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li J., Li F., Liu H., Yang W., Chong K., Xu Y.(2017). OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and ENHANCES RICE CHILLING tolerance. Dev. Cell 43: 731–743.e5. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Andersen S.U., Ljung K., Dolezal K., Miotk A., Schultheiss S.J., Lohmann J.U.(2010). Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092. [DOI] [PubMed] [Google Scholar]