Abstract
Objective
This study was aimed to evaluate the antibacterial activity of the latex of three species members of Jatropha (J. curcas, J. gossypilofia Linn., and J. multifida) against methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase- (ESBL-) producing Escherichia coli and ESBL-producing Klebsiella pneumonia, carbapenemase-resistant Enterobacteriaceae (CRE)-E. coli, K. pneumoniae-carbapenemase (KPC), and carbapenemase-resistant Pseudomonas aeruginosa (CRPA).
Method
The antibacterial activities were calculated based on the inhibition zones using the Mueller–Hinton agar diffusion method, minimum inhibitory concentration (MIC) using Mueller–Hinton broth in a microdilution method, and minimum bactericidal concentration (MBC) using blood agar plate.
Results
The latex of Jatropha showed antibacterial activities against the MRSA and CRPA. All latex of Jatropha appeared to have the antibacterial activities against MRSA and CRPA in the diffusion method (20.4–23.7 mm and 12–15 mm), MIC (0.19–6.25%, and 25%), and MBC (0.39–12.5% and 50%). Phytochemical screening of latex indicated the presence of flavonoids.
Conclusions
The latex of J. curcas, J. gossypilofia Linn., and J. multifida has the potential to be developed as antibacterial agents, especially against MRSA and CRPA strain, but further in vivo research and discovery of the mode of its action are required to shed the light on the effects.
1. Introduction
Bacterial resistance to antibiotics has become a serious problem in the world, including in Indonesia. This is a factor in the high mortality rate and continues to increase every year [1]. Inappropriate use of antibiotics is one of the causes of antibiotic resistance [2]. The emergence of multidrug-resistant (MDR) bacteria causes treatment ineffectiveness [3]. This has a devastating effect on patients suffering from MDR bacterial infections, increasing the treatment costs, which become increasingly high and difficult to afford and causing prolonged illness and even death [4]. One alternative to overcome these problems is to use natural ingredients such as materials from fungi [5] and plants [6], one of which is Jatropha.
Our study aimed to investigate the antibacterial activities of Jatropha latex against MDR bacteria. In this study, we used three species of Jatropha plants to measure the antibacterial activities against MDR bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli, ESBL-producing Klebsiella pneumonia, carbapenemase-resistant Enterobacteriaceae (CRE)-E. coli, K. pneumoniae-carbapenemase (KPC), and carbapenemase-resistant Pseudomonas aeruginosa (CRPA). Three types of latex used in this study were Jatropha curcas, Jatropha gossypilofia Linn., and Jatropha multifida.
2. Methods
2.1. Collection of Latex
Jatropha was collected from the field of Universitas Muhammadiyah Semarang in Semarang at the rainy season in January 2019. The collected plants were identified and classified according to the types of Herbarium Semarangense at the Department of Biology, Universitas Negeri Semarang. The latex of J. curcas, J. gossypilofia Linn., and J. multifida was collected by cutting the stems. The latex was stored in sterile dark bottles.
2.2. Materials
The used materials were Autoclave (Hirayama HICLAV HV-25), Incubator (WTC Binder), biological safety cabinet (Labconco Purifier Class II Biosafety Cabinet), freezer (Electrolux), densimat (Biomerieux), Mueller Hinton Agar (MHA), Mueller Hinton Broth (MHB), Blood Agar Plate (BAP), McFarland, MRSA, ESBL-producing Escherichia coli, ESBL-producing Klebsiella pneumonia, CRE-E. coli, KPC, and CRPA that were obtained from the Medical Microbiology Laboratory of dr. Kariadi Central Hospital, Semarang, Central Java, Indonesia. MDR isolates were identified, and the susceptibility patterns were obtained using Vitek®MS (BioM´erieux).
2.3. Bacterial Preparation
The tested organisms were subcultured BAP for 24 h at (35 ± 2)°C. The colonies were inoculated in a normal saline solution. The bacteria cell suspensions were homogenized and adjusted to 0.5 McFarland standards (1.5 × 108 CFU/mL) using a densimat.
2.4. Antibacterial Assay of Latex
2.4.1. Agar Well Diffusion Assay
The antibacterial activities of latex were evaluated using agar well diffusion assay [7, 8]. In this method, 100 μL of each test organism, which was equivalent to a 0.5 McFarland standard, was inoculated on the MHA. Then, it was spread on to the surface of the agar using a sterilized glass spreader. After 10 minutes of inoculation, the wells were prepared using a sterilized steel cork borer (1 cm in diameter). Wells were made on each plate, out of which three wells were loaded with each latex. Each test was done in triplicate. All the plates were then incubated aerobically at 35 ± 2°C for 16–20 h. The antibacterial activities were evaluated by measuring the diameters of zones of inhibition (mm) against the test organism.
2.4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC of Jatropha latex was determined using Mueller–Hinton broth microdilution [9]. MIC determination was performed by a serial dilution technique using 12-well microtiter plates. MHB (100 μL) was placed into the well/plate and Jatropha latex (100 μL) in the dilution series. 10 μL bacterial cell suspensions were placed in each well/plate. Microplates were incubated aerobically at 35 ± 2°C for 16–20 h. The lowest concentrations without visible growth completely inhibited the bacteria (MIC) [10]. The MBC was defined as the lowest concentration of the extract that did not allow any growth [10]. The MBC of Jatropha latex was determined following the methods described by [11] with slight modifications. Wells were subcultured using a 10 μL inoculating loop on to a 5% sheep BAP at (35 ± 2)°C for 16–20 h incubation.
2.5. Phytochemical Screening
The Jatropha latex was screened for the presence of different classes of secondary metabolites, including alkaloids and flavonoids, using previously described methods [12].
3. Results
3.1. Agar Well Diffusion Assay
The antibacterial activities of three Jatropha species were examined in vitro using the diffusion and dilution of six MDR bacteria. Figure 1 shows that all latex exhibited inhibition zones against MRSA and CRPA. J. curcas showed a greater inhibitory zone diameter of 23.7 mm against MRSA and 15 mm against CRPA (Table 1).
Figure 1.
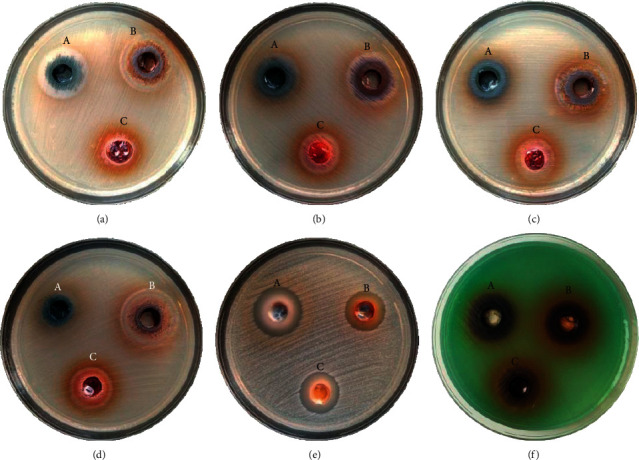
Zones of inhibition of Jatropha latex against MDR bacteria: (a) ESBL-Klebseilla pneumonia, (b) ESBL-E. coli, (c) CRE E. coli, (d) KPC, (e) MRSA, (f) CRPA, (A) J. curcas, (B) J. gossypilofia Linn., and (C) J. multifida.
Table 1.
Diameters of zones of inhibition of Jatropha latex against MDR bacteria (mm).
| Sample | ESBL-producing K. pneumoniae | ESBL-producing E. coli | CRE-E. coli | KPC | MRSA | CRPA |
|---|---|---|---|---|---|---|
| J. curcas | 0 | 0 | 0 | 0 | 23.7 | 15 |
| J. gossypilofia Linn. | 0 | 0 | 0 | 0 | 20.6 | 13 |
| J. multifida | 0 | 0 | 0 | 0 | 20.4 | 12 |
3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The antibacterial activities of the Jatropha latex (J. curcas, J. gossypilofia Linn., and J. multifida) were assayed in vitro by the agar microdilution method against MRSA and CRPA. Table 2 demonstrates that among the three Jatropha latex, J. multifida exhibited the smallest value of MIC and MBC against MRSA (0.19% and 0.39%). Figures 2–4 show that J. curcas and J. gossypilofia Linn. had the same activities against MRSA. Table 2 displays that all latex exhibited the same value of MIC and MBC against CRPA (25% and 50%). Figures 5–7 exhibit that J. curcas, J. gossypilofia Linn., and J. multifida had the potential to be developed as antibacterial agents for MRSA and CRPA strains.
Table 2.
MIC of Jatropha latex against MDR bacteria (%).
| Sample | MIC | MBC | ||
|---|---|---|---|---|
| MRSA | CRPA | MRSA | CRPA | |
| J. curcas | 3.12 | 25 | 6.25 | 50 |
| J. gossypilofia Linn. | 6.25 | 25 | 12.5 | 50 |
| J. multifida | 0.19 | 25 | 0.39 | 50 |
Figure 2.
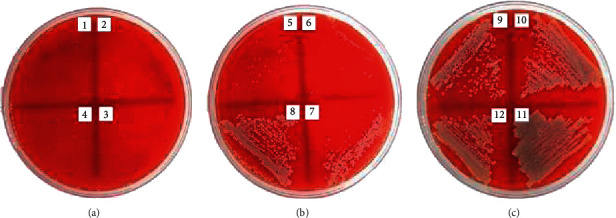
MBC value of J. curcas against MRSA at 6.25%.
Figure 3.
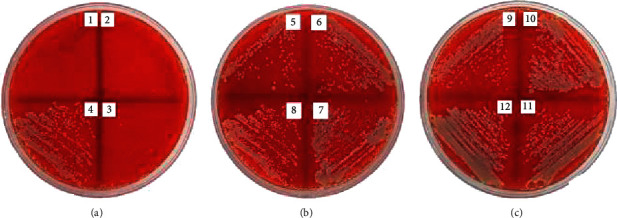
MBC value of J. gossypilofia Linn. against MRSA at 12.5%.
Figure 4.
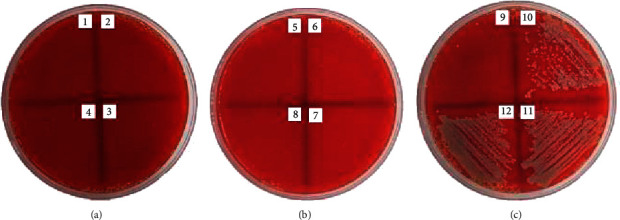
MBC value of J. multifida against MRSA at 0.39%.
Figure 5.
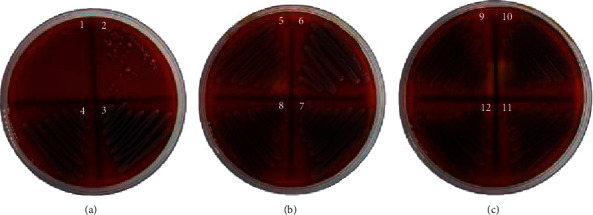
MBC value of J. curcas against CRPA at 50%.
Figure 6.
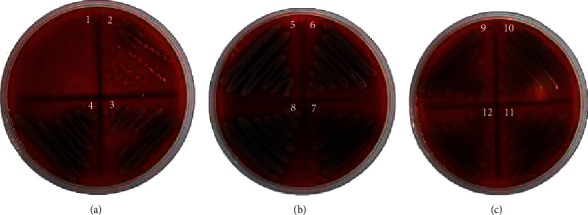
MBC value of J. gossypilofia Linn. against CRPA at 50%.
Figure 7.
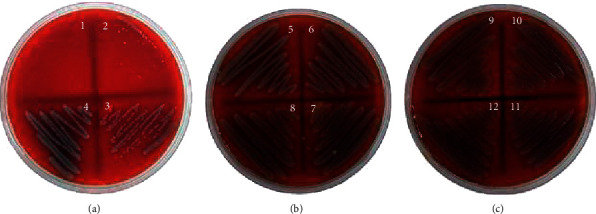
MBC value of J. multifida against CRPA at 50%.
3.3. Phytochemical Analysis
The secondary metabolites are presented in Table 3. Flavonoids are present in all Jatropha latex.
Table 3.
Results of phytochemical analysis of Jatropha latex.
| Sample | Secondary metabolites | ||
|---|---|---|---|
| Flavonoid | Terpenoids | Alkaloids | |
| J. curcas | + | − | − |
| J. gossypilofia Linn. | + | − | − |
| J. multifida | + | − | − |
4. Discussion
The research for new antibacterial agents from a natural or organic source has become a very important endeavor, considering the escalating levels of antibiotic resistance. One of the efforts in this research is focused on the use of Jatropha latex, which is widely available. Traditional medicine has been practiced worldwide, including in Indonesia, for centuries.
The agar well diffusion and microdilution methods were used in the present study due to their routine use as a quantitative method in clinical laboratories. In the agar well diffusion method, 100 μL of each MDR organism, equal to a 0.5 McFarland standard, was inoculated on the MHA. The wells were prepared using a sterilized teel cork borer (1 cm diameter). Wells were made on each plate, out of which three wells were loaded with each of latex. All the plates were then incubated at 35 ± 2°C for 16–20 h. Antibacterial activities were evaluated by measuring the diameters of zones of inhibition (mm) against the MDR organism. In microdilution method, susceptibility test in 12 well microtiter plates contained various concentrations (50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.39, 0.19, 0.09, 0.04, and 0.02%) of Jatropha latex. Then, the standardized numbers of MDR bacteria were inoculated into the wells of the microtiter plates and incubated at 35 ± 2°C for 16–20 h. The MIC value was observed as the lowest concentration where no viability was detected in the wells after incubation. Wells in MIC assays were subcultured using a 10 μL inoculating loop onto a BAP at 35 ± 2°C for 16–20 h incubation. The MBC was defined as the lowest concentration of the extract that did not permit any growth.
The latex of Jatropha showed antibacterial activities against the MRSA and CRPA. All latex of Jatropha showed the antibacterial activities against MRSA and CRPA in the diffusion method (20.4–23.7 mm and 12–15 mm), MIC (0.19–6.25% and 25%), MBC (0.39–12.5% and 50%). These results are following the research report by Hernandez-Hernandez [13] that the latex of Jatropha neopauciflora Pax demonstrated inhibition against S. aureus. In another study, the latex of Jatropha curcas displayed potent antimicrobial activity against Pseudomonas aeruginosa [14]. Phytochemical screening of latex showed the presence of flavonoids. These flavonoid compounds have been reported to be used by plants for protection against bacteria and are responsible for antimicrobial activity [15]. The latex of J. curcas, J. gossypilofia Linn., and J. multifida has the potential to be developed as anti-MDR-bacterial agents, especially against MRSA and CRPA strain, but further in vivo research is still needed to explicate on the effects.
5. Conclusion
The latex of J. curcas, J. gossypilofia Linn., and J. multifida is prospective for development as an antibacterial agent, especially against MRSA and CRPA strain, but advanced in vivo research and discovery of the mode of its action are necessary to throw light upon the effects.
Acknowledgments
The authors are grateful to the Department of Medical Laboratory Technology, Universitas Muhammadiyah Semarang, Indonesia, for providing essential facilities for carrying out the study.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Gyles C. The growing problem of antimicrobial resistance Le problème grandissant de l’antibiorésistance Carlton. CVJ. 2011;52(8):817–819. [PMC free article] [PubMed] [Google Scholar]
- 2.Bologa C. G., Ursu O., Oprea T. I., Melançon C. E., Tegos G. P. Emerging trends in the discovery of natural product antibacterials. Current Opinion in Pharmacology. 2013;13(5):678–687. doi: 10.1016/j.coph.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djeussi D. E., et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complementary and Alternative Medicine. 2013;13(164):1–8. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Antimicrobial Resistance Global Report on Surveillance. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 5.Prastiyanto M. E., Setyaningtyas A., Trisnawati L., Syafira A. Antimicrobial activity and identification the compounds of methanol extract from the pleurotus ostreatus fruiting body. El-Hayah. 2016;6(1):29–34. doi: 10.18860/elha.v6i1.4082. [DOI] [Google Scholar]
- 6.Prastiyanto M. E., Wardoyo F. A., Wilson W., Darmawati S. Antibacterial activity of various extracts of averrhoa bilimbi against multidrug resistant bacteria. Biosaintifika. 2020;12(2) [Google Scholar]
- 7.Perez C., Pauli M., Bazerque P. An antibiotic assay by agar well diffusion method. Acta biologiae et medicinae experimentalis. 1990;15:113–115. [Google Scholar]
- 8.Saviano A. M., Lourenço F. R. Using image analysis to determine gentamicin potency by agar diffusion microbiological assay and its measurement uncertainty. Measurement. 2019;146:315–321. doi: 10.1016/j.measurement.2019.06.041. [DOI] [Google Scholar]
- 9.CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing. 29th. Wayne, PA, USA: CLSI; 2019. [Google Scholar]
- 10.Yin C., Xie L., Guo Y. Phytochemical analysis and antibacterial activity of Gentiana macrophylla extract against bacteria isolated from burn wound infections. Microbial Pathogenesis. 2018;114:25–28. doi: 10.1016/j.micpath.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Irobi O. N., Daramola S. O. Bactericidal properties of crude extracts of Mitracarpus villosus. Journal of Ethnopharmacology. 1994;42(1):39–43. doi: 10.1016/0378-8741(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 12.Wadood A. Phytochemical analysis of medicinal plants occurring in local area of mardan. Biochemistry and Analytical Biochemistry. 2013;2(4):2–5. doi: 10.4172/2161-1009.1000144. [DOI] [Google Scholar]
- 13.Hernandez-Hernandez A. . Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. Journal of Ethnopharmacology. 2017;23(204):1–7. doi: 10.1016/j.jep.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Arekemase M. O., Kayode R. M. ., Ajiboye A. . Antimicrobial activity and phytochemical analysis of Jatropha curcas plant against some selected microorganisms. International Journal of Biology. 2011;3(3) doi: 10.5539/ijb.v3n3p52. [DOI] [Google Scholar]
- 15.Kumar S., Pandey A. k. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;2013:16. doi: 10.1155/2013/162750.162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


