Abstract
Disruption of the normal gut microbiome (dysbiosis) is implicated in the progression and severity of myriad disorders, including hypercholesterolemia and cardiovascular disease. Probiotics attenuate and reverse gut dysbiosis to improve cardiovascular risk factors like hypertension and hypercholesterolemia. Lactobacillus reuteri is a well-studied lactic acid-producing probiotic with known cholesterol-lowering properties and anti-inflammatory effects. In the present study, we hypothesized that L. reuteri delivered to hypercholesterolemic low-density lipoprotein receptor knockout (LDLr KO) mice will reduce cholesterol levels and minimize cardiac injury from an ischemic insult. L. reuteri [1 × 109 or 50 × 106 colony-forming units (CFU)/day] was administered by oral gavage to wild-type mice and LDLr KO for up to 6 wk followed by an ischemia-reperfusion (I/R) protocol. After 4 wk of gavage, total serum cholesterol in wild-type mice receiving saline was 113.5 ± 5.6 mg/dL compared with 113.3 ± 6.8 and 101.9 ± 7.5 mg/dL in mice receiving 1 × 109 or 50 × 106 CFU/day, respectively. Over the same time frame, administration of L. reuteri at 1 × 109 or 50 × 106 CFU/day did not lower total serum cholesterol (283.0 ± 11.1, 263.3 ± 5.0, and 253.1 ± 7.0 mg/dL; saline, 1 × 109 or 50 × 106 CFU/day, respectively) in LDLr KO mice. Despite no impact on total serum cholesterol, L. reuteri administration significantly attenuated cardiac injury following I/R, as evidenced by smaller infarct sizes compared with controls in both wild-type and LDLr KO groups. In conclusion, daily L. reuteri significantly protected against cardiac injury without lowering cholesterol levels, suggesting anti-inflammatory properties of L. reuteri uncoupled from improvements in serum cholesterol.
NEW & NOTEWORTHY We demonstrated that daily delivery of Lactobacillus reuteri to wild-type and hypercholesterolemic lipoprotein receptor knockout mice attenuated cardiac injury following ischemia-reperfusion without lowering total serum cholesterol in the short term. In addition, we validated protection against cardiac injury using histology and immunohistochemistry techniques. L. reuteri offers promise as a probiotic to mitigate ischemic cardiac injury.
Keywords: cholesterol; gut microbiota, Lactobacillus reuteri; myocardial infarction; probiotics
INTRODUCTION
Despite a recent rise in popularity, probiotics have a relatively long history in human health research. Defined as microorganisms that confer health benefits to the host when ingested in adequate amounts (43), probiotics have been formally studied since the 1920s (41), with formal research on probiotics and normal gut flora, or microbiota, appearing by the mid-20th century (29). The gut microbial population interacts with host, including metabolism of polysaccharides, bile acids, and drugs, as well as production of amino acids, vitamins, and short-chain fatty acids (35, 36, 40, 49). Because of these extensive interactions, the gut microbiota is now a common target for research and therapeutics.
Lactobacillus reuteri, a gram-positive commensal strain in the intestinal tract of both humans and mice (13, 25), is a well-studied probiotic with purported cardiovascular disease (CVD) benefits (10, 14, 18). Although results are mixed for weight loss (7, 15, 30, 39), studies are more consistent for lowering total cholesterol in both rodents (15, 46) and humans (17, 27). L. reuteri is proposed to impart this benefit though its bile salt hydrolase activity, which leads to an increase in bile acid excretion in the feces and subsequent lowering of total cholesterol (47, 48)
With a consideration of its direct interaction with host intestinal cells and production of bioactive agents, it is not surprising that the gut microbiota influences both the development and functionality of the intestinal immune system (40, 45). Disruption of gut-host interaction, or dysbiosis, is associated with an inflammatory-prone state and several metabolic disorders, including obesity, diabetes, and CVD. Inflammation plays an integral role in the progression and initiation of CVD and is central to proinflammatory events like myocardial infarction (MI; see Ref. 11). Still, a majority of research has focused on the ability of the gut to affect risk factors for MI, such as hypertension and atherosclerosis, and not necessarily on mitigating injury caused by MI (19, 28, 53). Cardiac remodeling post-MI is a complex coordinated inflammatory response and is adversely affected by excessive inflammation (32) and conditions that promote excessive inflammation such as gut dysbiosis (54).
Considering the established role of cholesterol as a risk factor for CVD, we hypothesized that L. reuteri delivered to hypercholesterolemic low-density lipoprotein receptor knockout (LDLr KO) mice will reduce cholesterol levels and minimize cardiac injury from an ischemic insult. Total cholesterol in LDLr KO ranges from 200 to 400 mg/dL on a normal chow diet. Given the elevated cholesterol levels on low-fat diets, LDLr KO mice are viewed as a model for familial hypercholesterolemia (38), and therefore allowed us to investigate the effects of L. reuteri on elevated cholesterol levels outside of a diet-induced obesity setting. Toward this end, we demonstrate that administration of L. reuteri attenuated cardiac injury from MI to a similar degree in wild-type and LDLr KO mice without having a significant impact on total serum cholesterol.
MATERIALS AND METHODS
Animals, diet, and probiotic administration.
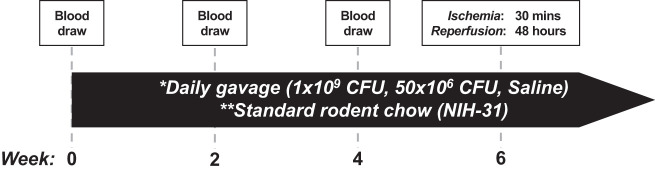
Experiments followed protocols that were approved by the Institutional Animal Care and Use Committee at the University of Arizona and to 2011 National Institutes of Health (NIH) guidelines for care and use of laboratory animals. Twelve-month-old LDLr KO male mice (B6.129S7-Ldlr<tm1Her>/J; Jackson Laboratories, Bar Harbor, ME) and wild-type male mice (C57BL/6J; Jackson Laboratories) were randomized to one of the following three groups: L. reuteri 1 billion (1 × 109) colony-forming units (CFU)/day, L. reuteri 50 million (50 × 106) CFU/day, or saline control (Fig. 1). L. reuteri (UAS Laboratories, Madison, WI) was administered through daily gavage, using 300 μL of saline as vehicle. Control groups received 300 μL of saline alone through daily gavage. Gavage protocol continued for up to 6 wk, before the ischemia-reperfusion (I/R) protocol was initiated (Fig. 1). Mice were fed standard rodent chow (NIH-31; 18% fat, 59% carbohydrate, 23% protein). Body weights were measured weekly.
Fig. 1.
Experimental time line. Study began with 40 total mice: 20 wild-type (C57BL/6J) and 20 low-density lipoprotein receptor knockout mice. After the first blood draw and weight measurement, mice were randomized within the 2 models to the following 3 treatment groups: 1 billion (1 × 109) colony-forming units (CFU)/day of Lactobacillus reuteri, 50 million (50 × 106) CFU/day of L. reuteri, and saline (control). **Mice were fed a standard chow diet (NIH-31; 18% fat, 59% carbohydrates, 23% protein). *Mice were administered treatments daily by oral gavage in 300 μL of saline, and body weights were measured weekly. Submental blood draws were taken at 0, 2, and 4 wk for collection of total serum. In the 5th wk, mice were randomly chosen from each experimental group and subjected to 30 min of ischemia followed by 48 h of reperfusion. Mice were then euthanized, and hearts were prepared for analysis.
Blood collection and cholesterol determination.
Blood was collected at baseline, 2 wk, and 4 wk. Mice were fasted for 5 h prior, and blood was collected via submental bleed. Whole blood was allowed to clot for 15–60 min, after which it was centrifuged (1,000–2,000 g) for 10 min at 4°C. Serum was removed and flash-frozen in liquid nitrogen. Total serum cholesterol (Wako Chemical, Richmond, VA) was determined using colorimetric enzymatic assays on a BioTek Synergy 2 plate reader.
Surgical procedures.
After 5 to 6 wk of oral gavage of the respective treatment, mice were anesthetized with an intraperitoneal injection of 250 mg/kg of tribromoethanol (Sigma), intubated, and ventilated with 0.5–2% isoflurane (Phoenix Pharmaceuticals). The heart was then exposed, and the left anterior descending artery was occluded using 8-0 suture compressing a small piece of tubing (PE-10) to prevent vessel damage during occlusion. Occlusion was maintained for 30 min, after which the animal was allowed 2 days of reperfusion.
Determination of infarct size.
Following 48 h of reperfusion, mice were placed in a chamber and anesthetized via isoflurane inhalation. Mice were then euthanized via cervical separation. After being euthanized, the chest cavity was opened, and the left anterior descending artery was reoccluded at the same site as the original occlusion. The heart was then perfused with 1% Evans blue dye, staining the whole heart, except the left coronary bed. This perfusion outlines the ischemic zone, or area at risk (AAR). Hearts were then excised and frozen for 15 min at −20°C, after which they were transversely sectioned at 1 mm of thickness. The slices were then incubated in 1% 2,3,5-triphenyltetrazoliumchloride stain (TTC, No. T8877; Sigma, St. Louis, MO). After incubation with TTC, necrotic tissue stains white [area of necrosis (AON)], viable tissue within the AAR stains red, and fully perfused tissue will remain blue. AAR and AON were measured for each section, both sides, using ImageJ, and infarct size was reported as a percentage of AON to AAR. Sections were then fixed in 10% formalin overnight and paraffin embedded (5).
Histological staining and immunohistochemistry.
Following paraffin embedment, formalin-fixed tissues were transversely sectioned at 5 μm and placed on microscope slides. Slides were stained with hematoxylin and eosin (H&E), anti-interleukin-6 (IL-6, 1:300, No. ab6672; Abcam), and anti-intercellular adhesion molecule-1 (ICAM-1, CD54, 1:50, No. 4915; Cell Signaling Technology) antibodies. Anti-IL-6-stained sections were exposed to HRP-conjugated goat anti-rabbit (1:500, RRID: AB_2313567; Jackson ImmunoResearch Laboratories) secondary antibody and visualized with diaminobenzidine (DAB, No. SK-4105; Vector Laboratories). Anti-ICAM-stained sections were exposed to an anti-rabbit/mouse avidin/biotin-based peroxidase secondary kit (Vectastain Elite ABC HRP Kit) and visualized with DAB (No. SK-4105; Vector Laboratories). Unstained sections (secondary only) were used as negative control. Infiltration of immunohistochemical markers was measured using ImageJ. First, area of infiltration was normalized to the total area of the heart (infiltration/total area). Next, this value was normalized to AAR, as previously determined by Evans blue and TTC staining and presented as a percentage. Last, these values were correlated with the AON/AAR, as determined by Evans blue and TTC staining.
RT-PCR.
Total RNA was isolated from liver tissue of mice from each experimental group using the Qiagen (Germantown, MD) RNeasy Midi kit (No. 75144) and converter to cDNA using the transcriptor first-strand synthesis kit (04 379 012 001; Roche, Mannheim, DE). Real-time PCR was performed with the Roche (Indianapolis, IN) Lightcycler 480 and Roche LightCycler 480 SYBR Green I Master mix (No. 04707516001). Primer sets for carnitine palmitoyltransferase 1A (CPT1A; forward: 5′-GCACTGCAGCTCGCACATTACA-3′; reverse: 5′-CTCAGACAGTACCTCCTTCAGGAAA-3′), IL-6 (forward: 5′-TAGTCCTTCCTACCCCAATTTCC-3′; reverse: 5′-TTGGTCCTTAGCCACTCCTTC-3′), and 18S (forward: 5′-ACTGAGGCCATGATTAAG-3′; reverse: 5′-GCTATCAATCTGTCAATCC-3′), were obtained from qPrimerDepot (44a).
Data and statistical analysis.
All data are presented as means ± SE. An unpaired t test was used to compare baseline total serum cholesterol. A two-way repeated-measures ANOVA followed by a Bonferroni’s multiple-comparison test was used to compare changes in body weight and cholesterol levels in each experimental group over the experimental time frame. Infarct sizes (AON/AAR%) among treatment groups were compared by two-way ANOVA followed by a Bonferroni’s multiple-comparison test. To determine the correlation between histological and immunohistochemical staining (H&E, IL-6, and ICAM) with infarct size, Pearson’s r correlation coefficient was calculated followed by linear regression analysis to determine if the slopes were significantly different from zero and to compare regression lines from each stain.
RESULTS
Body weight.
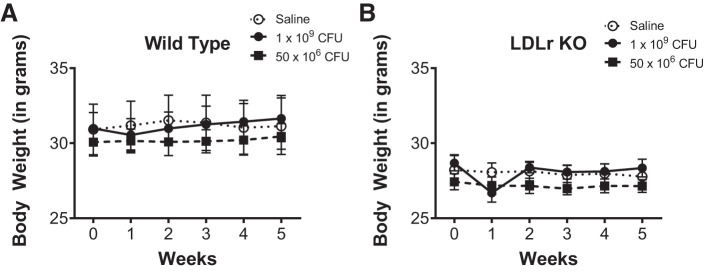
LDLr KO mice are a model for familial hypercholesterolemia with altered serum lipoprotein profiles similar to human disease (20). Wild-type and LDLr KO mice were fed standard rodent chow ad libitum, and body weight was monitored over the course of the study protocol. We chose a standard nonatherogenic rodent chow to eliminate diet as a confounding variable. At the outset of the study, wild-type mice weighed significantly more than their LDLr KO counterparts, regardless of treatment group. L. reuteri supplementation at either 1 × 109 CFU/day or 50 × 106 CFU/day had no effect on body weight in wild-type or LDLr KO mice (Fig. 2, A and B). Interestingly, administration of L. reuteri instigated a brief decline in body weight in the first week, although this body weight loss was not significant. Body weight data are summarized in Table 1.
Fig. 2.
Body weights were not impacted by Lactobacillus reuteri administration. A: line graph summary of body weight (in g) measured weekly for wild-type mice administered daily by gavage saline (open circles; n = 5), L. reuteri at 1 × 109 colony-forming units (CFU)/day (closed circles; n = 6), or L. reuteri at 50 × 106 CFU/day (closed squares; n = 5). B: line graph summary of body weight (in g) measured weekly for low-density lipoprotein receptor knockout (LDLr KO) mice administered daily by gavage saline (open circles; n = 5), L. reuteri at 1 × 109 CFU/day (closed circles; n = 4), or L. reuteri at 50 × 106 CFU/day (closed squares; n = 6). Data are presented as means ± SE.
Table 1.
Body weight, total serum cholesterol, and cardiac injury
| Saline | 1 × 109 CFU | 50 × 106 CFU | |
|---|---|---|---|
| Wild type | |||
| n | 5 | 8 | 6 |
| Body wt, g | 31.1 ± 1.9 | 31.6 ± 1.5 | 30.5 ± 0.8 |
| Total cholesterol, mg/dL | 113.5 ± 5.6 | 113.3 ± 6.8 | 101.9 ± 7.5 |
| AON/AAR, % | 13.8 ± 1.9 | 7.2 ± 0.8* | 8.0 ± 1.2* |
| LDLr KO | |||
| n | 5 | 6 | 7 |
| Body wt, g | 27.8 ± 0.6 | 28.3 ± 0.6 | 27.1 ± 0.4 |
| Total cholesterol, mg/dL | 283.0 ± 11.1 | 263.3 ± 5.0 | 253.1 ± 7.0 |
| AON/AAR, % | 13.7 ± 0.7 | 6.6 ± 0.8* | 6.9 ± 1.2* |
Values are means ± SE; n, number of mice. CFU, colony-forming units; AON, area of necrosis; AAR, area at risk; LDLr KO, low-density lipoprotein receptor knockout. Body weights were measured weekly, and submental blood was drawn at 0 (baseline), 2, and 4 wk for determinations of total serum cholesterol. Body wt values listed above were final measures before euthanasia. Total serum cholesterol values listed above were determined at 4 wk. Total serum cholesterol before randomization of LDLr KO mice (224.2 ± 6.3 mg/dL, n = 18) was significantly elevated over wild-type mice (104.0 ± 2.8 mg/dL, n = 19) by standard unpaired t test (P < 0.0001). AON normalized to AAR converted to a percentage was compared by 2-way ANOVA followed by a post hoc Bonferroni’s multiple-comparison test.
P < 0.001 from respective saline controls.
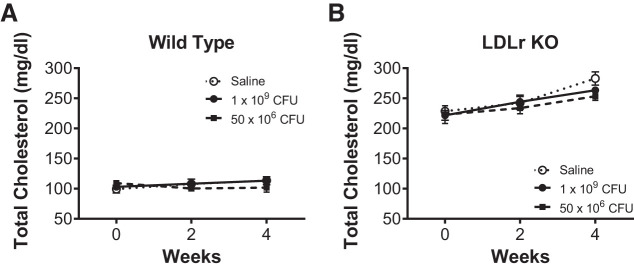
Total serum cholesterol.
Blood samples were collected at 0, 2, and 4 wk and analyzed for total serum cholesterol. As expected, total serum cholesterol before randomization of LDLr KO mice (224.2 ± 6.3 mg/dL, n = 18) was significantly elevated over wild-type mice (104.0 ± 2.8 mg/dL, n = 19) by standard unpaired t test (P < 0.0001). Using a two-way repeated-measures ANOVA followed by a Bonferroni’s multiple-comparison test, neither time nor L. reuteri supplementation impacted total serum cholesterol in wild-type mice (Fig. 3A). At final blood draw (4 wk), total serum cholesterol in wild-type mice receiving saline was 113.5 ± 5.6 mg/dL compared with 113.3 ± 6.8 and 101.9 ± 7.5 mg/dL in mice administered L. reuteri. Genetic deletion of the LDLr in LDLr KO mice induced a gradual and significant (P < 0.001) increase in total serum cholesterol over the experimental time course (Fig. 3B). Although L. reuteri supplementation in LDLr KO mice attenuated total serum cholesterol at 4 wk, cholesterol levels from the final blood draw were not impacted (P = 0.226, treatment effect; P = 0.608, treatment × time interaction) by L. reuteri treatment at 1 × 109 CFU (263.3 ± 5.0 mg/dL) or 50 × 106 CFU (253.1 ± 7.0 mg/dL) compared with LDLr KO mice administered saline (283.0 ± 11.1 mg/dL).
Fig. 3.
Total serum cholesterol was not decreased by Lactobacillus reuteri administration. Total serum cholesterol from a submental blood draw after a 5-h fast was averaged at 0, 2, and 4 wk from wild-type (A) and low-density lipoprotein receptor knockout (LDLr KO, B) mice administered daily by gavage saline [open circles, n = 5 (A and B)], L. reuteri at 1 × 109 colony-forming units (CFU)/day [closed circles, n = 6 (A) and 4 (B)], or L. reuteri at 50 × 106 CFU/day [closed squares, n = 5 (A) and 6 (B)]. Data are presented as means ± SE.
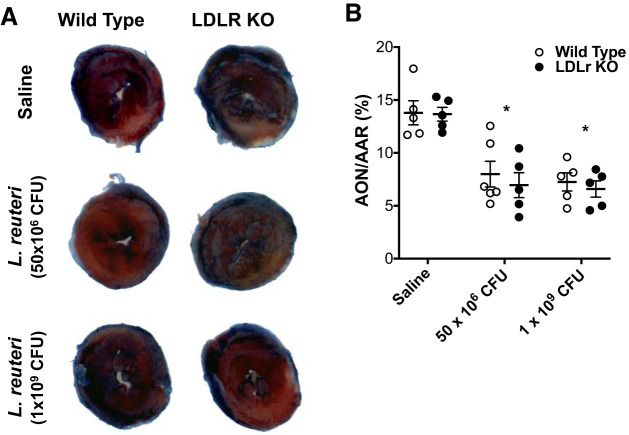
Infarct size: Evans blue and TTC staining.
In our previous publication, we demonstrated that Bifidobacterium animalis subsp. lactis 420 but not L. salivarius 33 (Ls-33) protected against cardiac injury from I/R (5). Accordingly, we tested whether L. reuteri, a probiotic from the same genus but different species as Ls-33, was cardioprotective against cardiac injury from I/R. Following up to 6 wk of gavage, mice from each experimental group described above were subjected to an I/R protocol. Myocardial injury was quantified by comparing the AON with the AAR. Percent infarct size is defined as AON (white) relative to the AAR (pink) (Fig. 4A). Comparing wild-type and LDLr KO mice untreated and treated with 50 × 106 or 1 × 109 CFU L. reuteri, we discovered a significant treatment effect (P < 0.0001) by two-way ANOVA that was not impacted by genetic deletion of the LDLr (genotype), nor was there a significant interaction between treatment and genotype. Post hoc analysis clearly identified that L. reuteri treatment in wild-type mice at 50 × 106 CFU (8.0 ± 1.2%) or 1 × 109 CFU (7.2 ± 0.8%) significantly decreased infarct size following I/R from wild-type saline-treated controls (13.8 ± 1.1%), as measured by Evans blue and TTC staining. Similarly, L. reuteri treatment in LDLr KO mice at 50 × 106 (7.0 ± 1.2%) or 1 × 109 (6.6 ± 0.8%) CFU reduced infarct size following I/R from LDLr KO saline-treated controls (13.6 ± 0.6%). These data are summarized in Table 1 and a scatter plot (Fig. 4B).
Fig. 4.
Infarct size was attenuated by Lactobacillus reuteri administration. During the 5th wk of L. reuteri administration, mice were subjected to the ischemia-reperfusion (I/R) protocol as described in materials and methods. A: representative images of Evans blue/triphenyltetrazoliumchloride (TTC) staining of hearts following I/R in wild-type and low-density lipoprotein receptor knockout (LDLr KO) mice treated with saline, L. reuteri at 50 × 106 colony-forming units (CFU)/day, and L. reuteri at 1 × 109 CFU/day. B: scatter plot summary of area of necrosis (AON; white-stained area) was normalized to area at risk (AAR; red-stained area) and represented as a percentage summarized in Table 1. Experimental groups are as follows: wild-type saline, n = 5; wild-type L. reuteri at 50 × 106 CFU/day, n = 5; wild-type L. reuteri at 1 × 109 CFU/day, n = 6; LDLr KO saline, n = 5; LDLr KO L. reuteri at 50 × 106 CFU/day, n = 5; and LDLr KO L. reuteri at 1 × 109 CFU/day, n = 6. There was a significant treatment effect (P < 0.0001) by 2-way ANOVA. *P < 0.001 from respective saline controls by post hoc Bonferroni’s multiple-comparison test. Data are presented as means ± SE.
Infarct size: histological and immunohistochemical staining.
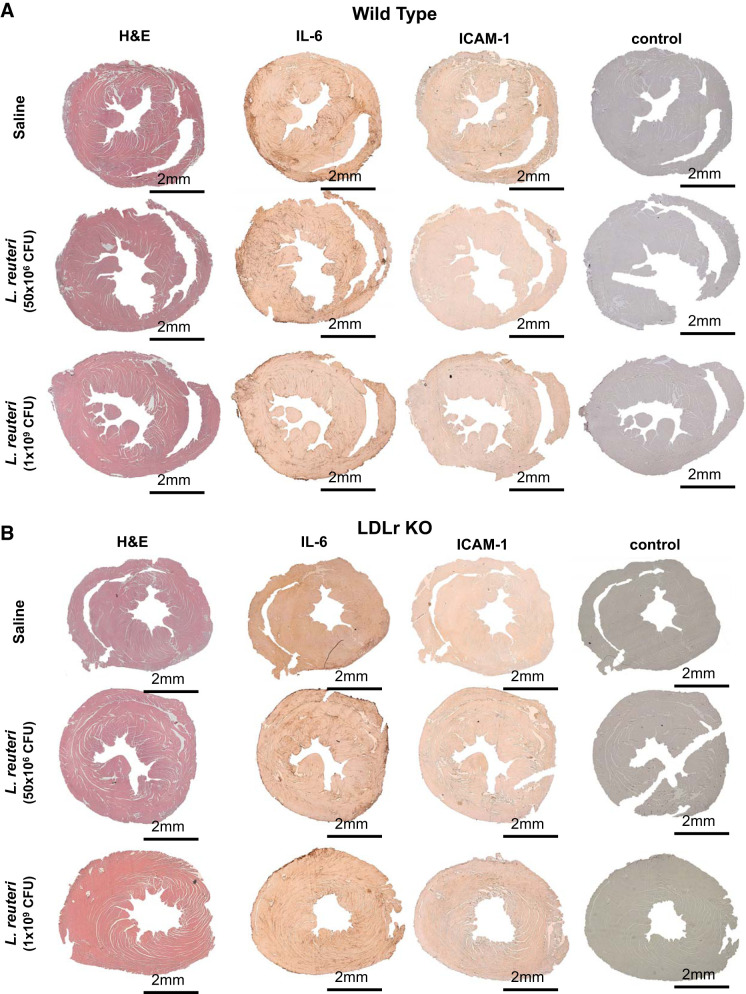
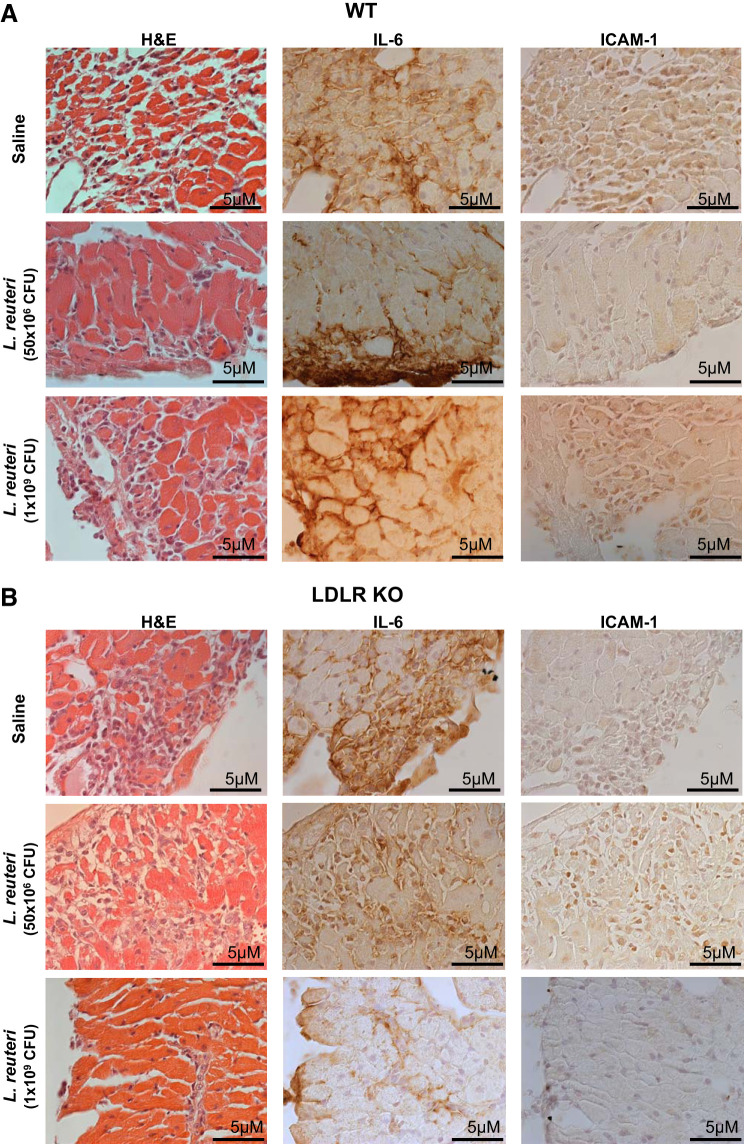
Cardiac injury from an ischemic event is instigated by a coordinated response of proinflammatory cytokines, chemokines, and immune response cells (9, 32, 37). Accordingly, we performed histology and immunohistochemistry (IHC) on serial sections for H&E, IL-6 (an inflammatory cytokine post-MI; see Ref. 34), and ICAM-1 (induced by IL-6 promoting leukocyte adherence and infiltration; see Ref. 8). Representative histological images are shown in Fig. 5A (×5) and at higher magnification in Fig. 6 (×63).
Fig. 5.
Histology and immunohistochemistry validated attenuation of cardiac injury by Lactobacillus reuteri administration. Representative images at ×5 magnification of hematoxylin and eosin (H&E), interleukin-6 (IL-6), and intercellular adhesion molecule-1 (ICAM-1) in wild-type (A) or low-density lipoprotein receptor knockout (LDLr KO, B) mice administered daily by gavage saline [n = 5 (A and B)], L. reuteri at 1 × 109 colony-forming units (CFU)/day [n = 6 (A) and 4 (B)], or L. reuteri at 50 × 106 CFU/day [n = 5 (A) and 6 (B)].
Fig. 6.
Cellular morphology patterned cellular infiltration within area of necrosis (AON). Representative images of hematoxylin and eosin (H&E), interleukin-6 (IL-6), and intercellular adhesion molecule-1 (ICAM-1) at ×63 magnification captured from overlapping regions within AON in wild-type (WT, A) or low-density lipoprotein receptor knockout (LDLr KO, B) mice administered daily by gavage saline [n = 5 (A and B)], Lactobacillus reuteri at 1 × 109 colony-forming units (CFU)/day [n = 6 (A) and 4 (B)], or L. reuteri at 50 × 106 CFU/day [n = 5 (A) and 6 (B)].
Cellular infiltration was evident in H&E-stained hearts following I/R and matched infarct size and location of AAR and AON. L. reuteri treatment in both wild-type (Fig. 5A) and LDLr KO (Fig. 5B) mice at either dose significantly and equally reduced cellular infiltration (H&E), indicating an attenuation of I/R injury, similarly to infarct size measured by Evans blue and TTC; wild-type mouse hearts were not different from treated or untreated LDLr KO counterparts. IHC staining for IL-6 and ICAM-1 paralleled H&E and Evans blue/TTC staining; infarct area by IL-6 and ICAM-1 staining was significantly reduced in hearts of mice treated with L. reuteri compared with hearts of mice treated with saline. Genetic deletion of LDLr did not impact infarct size compared with wild-type mice. Furthermore, imaging AON at higher magnification (×63) revealed cellular morphology and staining patterns consistent with I/R injury in wild-type (Fig. 6A) and LDLr KO (Fig. 6B) mice.
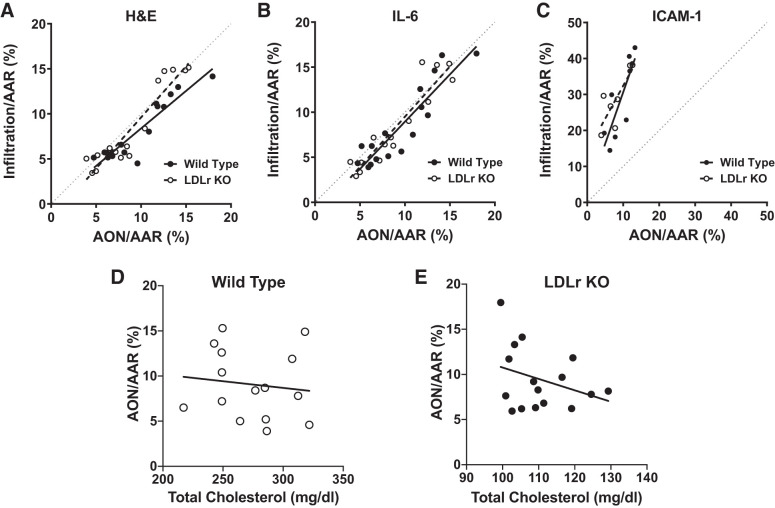
To further validate determinations of infarct size and assess extent of border zone infiltration, we correlated IHC staining with Evans blue/TTC staining (AON/AAR%). Normalized H&E, IL-6, and ICAM-1 staining was plotted against AON/AAR% followed by linear regression analysis; the line of identity indicating a 1:1 correlation between the two measures was included for reference (Fig. 7, A–C). As indicated by the Pearson’s r correlation coefficient, IHC and AON/AAR% were highly and significantly correlated, and the relationship was linear (Table 2). Moreover, the slopes for H&E/AON/AAR% and IL-6/AON/AAR% relationships overlaid the line of identity, whereas the ICAM-1/AON/AAR% relationship deviated from the line of identity with slopes that were significantly (P < 0.0001) greater than H&E/AON/AAR% and IL-6/AON/AAR% slopes.
Fig. 7.
Histology and immunohistochemistry correlated with infarct size measured by Evans blue and triphenyltetrazoliumchloride (TTC). Correlation between hematoxylin and eosin (H&E, A), interleukin-6 (IL-6, B), and intercellular adhesion molecule-1 (ICAM-1, C) staining (quantified as described in materials and methods) and area of necrosis (AON)/area at risk (AAR) % was determined [wild type, n = 16; low-density lipoprotein receptor knockout (LDLr KO), n = 15]. Correlation between AON/AAR% and total cholesterol (mg/dL) for wild-type (n = 16, D) and LDLr KO (n = 15, E) mice. Pearson’s r correlation coefficient was calculated followed by linear regression analysis to determine if lines were linear (R2); values are summarized in Table 2.
Table 2.
Correlation of histology, immunohistochemistry and total cholesterol to infarct size
| Parameter | Slope | Pearson’s r | R2 | P Value |
|---|---|---|---|---|
| H&E AON/AAR | ||||
| Wild type | 0.83 | 0.924 | 0.854 | <0.0001 |
| LDLr KO | 1.11 | 0.940 | 0.884 | <0.0001 |
| IL-6 AON/AAR | ||||
| Wild type | 1.05 | 0.908 | 0.825 | <0.0001 |
| LDLr KO | 1.15 | 0.934 | 0.873 | <0.0001 |
| ICAM-1 AON/AAR | ||||
| Wild type | 2.86 | 0.829 | 0.688 | 0.0057 |
| LDLr KO | 1.83 | 0.802 | 0.645 | 0.0297 |
| AON/AAR total cholesterol | ||||
| Wild type | −0.126 | −0.327 | 0.107 | 0.2161 |
| LDLr KO | 0.015 | −0.122 | 0.645 | 0.6643 |
LDLr KO, low-density lipoprotein receptor knockout; AON, area of necrosis; AAR, area at risk. Histological and immunohistochemistry staining [hematoxylin and eosin (H&E), interleukin-6 (IL-6), and intercellular adhesion molecule-1 (ICAM-1)] was correlated with infarct size determined by Evans blue and triphenyltetrazoliumchloride staining (AON/AAR%). Infarct size (AON/AAR%) was also correlated with total cholesterol. Pearson’s r correlation coefficient was calculated followed by linear regression analysis to determine if lines were linear (R2) and if the slopes were significantly different.
In addition, we correlated Evans blue/TTC staining (AON/AAR%) with total cholesterol levels for wild-type and LDLr KO mice (Fig. 7, D and E). There was no statistically significant correlation between AON/AAR% and total cholesterol in either wild-type or LDLr KO mice. Furthermore, the slopes of the relationships were not significantly different from zero (Table 2). These findings clearly indicated that infarct size was not dependent on cholesterol levels.
Expression of inflammatory and oxidative metabolism genes in the liver.
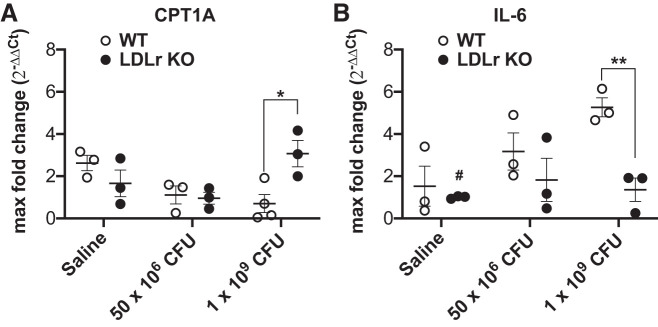
Cholesterol levels impact hepatic lipid homeostasis while loss of the LDL receptor can trigger cellular inflammatory and oxidative stress in the liver (23, 52). Therefore, we measured mRNA expression of CPT1A, the rate-limiting enzyme for fatty acid oxidation in the liver, a liver integral membrane protein. There was a significant (P = 0.009) interaction between L. reuteri treatment and genotype, with a specific significant (P = 0.028) increase measured in LDLr KO mice treated with L. reuteri at 1 × 109 CFU over wild-type mice treated with L. reuteri at 1 × 109 CFU (Fig. 8A). IL-6, an inflammatory cytokine, potently stimulates hepatic fatty acid synthesis through downstream regulation of CPT1A (50). IL-6 mRNA expression (Fig. 8B) was significantly impacted by dose (main effect, P = 0.048) and genotype (main effect, P = 0.0074). In addition, significant decrease in IL-6 expression was detected in saline-treated LDLr KO mice compared with wild-type mice treated with L. reuteri at 1 × 109 CFU (P = 0.021). Finally, IL-6 expression was significantly greater in wild-type mice compared with LDLr KO mice both treated with L. reuteri at 1 × 109 CFU (P = 0.039).
Fig. 8.
Dose-dependent impact of Lactobacillus reuteri on inflammatory and oxidative metabolism genes. Bar graph summary of carnitine palmitoyltransferase 1A (CPT1A, A) and interleukin-6 (IL-6, B) mRNA expression profiles from the liver in wild-type (WT,white circles) and low-density lipoprotein receptor knockout (LDLr KO, black circles) mice administered daily by gavage saline, L. reuteri at 1 × 109 colony-forming units (CFU)/day, or L. reuteri at 50 × 106 CFU/day. *P = 0.028 and #P = 0.0214 from wild type at 1 × 109 CFU/day; **P = 0.00394 (n = 3 for each experimental group). Data are presented as means ± SE.
DISCUSSION
The possible cholesterol-lowering effect of probiotic bacteria was first suggested, early on, by Mann and Spoerry (26) who discovered that high consumption of fermented milk reduced serum cholesterol in African men of the Maasai tribe. Since then, years of research in animal models and human clinical trials consistently show the cholesterol-lowering potential of probiotics (3). In this study, we chose L. reuteri based on established evidence as a cholesterol-lowering probiotic and a proposed anti-inflammatory mechanism through enhanced bile salt hydrolase activity (10, 14, 47, 48). Furthermore, considering the explosion of proprotein convertase subtilisin/kexin type 9 inhibitors as a cholesterol-lowering therapy (2), we chose the LDLr KO mouse as a model of hypercholesterolemia at a 12-mo time point to more closely mimic the human condition.
The most seminal finding of our study was a significant reduction of cardiac damage following I/R mice in either genotype with L. reuteri administration. This reduction measured by multiple techniques was not dependent on dose. For both groups of mice, we saw a significant near 50% reduction in infarct size with L. reuteri treatment compared with controls at either dose. This suggests that perhaps delivery of amounts lower than 50 × 106 CFU/day may be sufficient for a cardioprotective effect.
Staining with H&E, IL-6, and ICAM-1 showed similar patterns as seen with Evans blue/TTC. IL-6 is a proinflammatory cytokine that can be released by myocardial and immune cells. Expression and production of IL-6 is upregulated following MI and plays a central role in resorption of necrotic tissue (6). ICAM-1, whose expression can be induced by IL-6, is an adhesion molecule released by endothelial cells and cardiomyocytes to promote adhesion of leukocytes. When correlating H&E, IL-6, and ICAM-1 with Evans blue/TTC staining, the relationships were highly significant, linear, and nonzero. Whereas H&E and IL-6 matched the line of identity, ICAM-1 staining did not. ICAM-1 staining was generally more diffuse and extended beyond the distinct area of necrosis defined by Evans blue/TTC, H&E, and IL-6 staining. Following MI, ICAM-1 is present in the border zone and areas of cardiac tissue susceptible to necrotic damage (8, 21). Therefore, ICAM-1 staining may provide an estimate of border zone infiltration and potential salvageable tissue following MI and during reperfusion injury.
Although LDLr KO mice weighed less than wild-type mice at 12 mo, L. reuteri administration had little effect on body weight. Previous research with L. reuteri shows mixed evidence for weight control. L. reuteri treatment significantly reduced body weight in Apoe−/− mice fed a high-fat diet (7), whereas a different study found no effect of L. reuteri on body weight in a hamster model of hyperlipidemia (15). It is clear that the impact of L. reuteri on body weight is both strain and study dependent (39).
Despite previous studies showing the ability of L. reuteri to reduce cholesterol levels (17, 18, 27), no significant changes in cholesterol were seen between L. reuteri or control treated wild-type or LDLr KO mice at either dose. However, cholesterol levels in LDLr KO mice continued to climb where there was a significant increase in total serum cholesterol over the duration of the study. There was a trend, albeit not significant, toward attenuation of this cholesterol increase in L. reuteri-treated mice at both doses. On average, L. reuteri-treated LDLr KO mice presented at 4 wk with total serum cholesterol levels ~10% lower than controls, which were similar in both size and time line of reduction previously reported in clinical studies with hypercholesterolemic patients (18).
Again, the cholesterol-lowering ability of L. reuteri from previous work was shown in rodents typically fed a high-fat or high-cholesterol diet to induce hypercholesterolemia (15, 46). We chose a standard over high-fat diet because, on a high-fat diet, LDLr KO mice can see their total cholesterol rise to supraphysiological levels (500–2,000 mg/dL). While on a standard diet, LDLr KO mice generally remain in a hypercholesterolemic range of 200–400 mg/dL (12, 16), which is more applicable to a human population.
As stated previously, L. reuteri enhances bile salt hydrolase activity. Bile salts (acids) are derived from cholesterol metabolism primarily in the liver (24). Bacterial enzymes in the gut can deconjugate bile salts using bile salt hydrolases. Deconjugation of bile salts reduces serum cholesterol by increasing de novo synthesis of bile acids or reducing cholesterol solubility and disposal through the feces. An increase in number of bacteria would be predicted to increase bile salt hydrolase activity and a greater decrease in total cholesterol. Both dosages (1 × 109 and 50 × 106 CFU/day) were within established ranges used in previous studies with L. reuteri (7, 46), and 1 × 109 CFU/day was matched to the dose used in previous I/R studies by our laboratory (5). To our knowledge, one other study looked at the dose response to L. reuteri and did not see significant differences in total cholesterol between three doses in hamsters, but saw a dose responses in other parameters (15).
When considering the cholesterol-lowering efficacy of L. reuteri in this study, the magnitude of the effect is likely limited for multiple reasons. First, bile salt reabsorption is very efficient in the body, with ~95% of bile acids being reabsorbed before they reach the large intestine (1, 4). Consequently, the remaining bile salt available as a substrate for the gut microbiota is limited, which would also limit the cholesterol-lowering effect of L. reuteri supplementation. Second, inconsistencies in the magnitude of reduction with other rodent models may be a function of diet. Previously cited research in rodent models used high-fat or high-cholesterol diets (15, 46). High-fat diets are associated with increases in bile acid concentrations in the cecum and feces (31, 44). Given the increased presence of bile salts in the gut, high-fat diets may provide more substrate for deconjugation by gut microbiota and L. reuteri and a more robust impact on lowering total serum cholesterol.
Study limitations and strengths.
There are two primary limitations of our study considerations (1): instigating comparable ischemic sizes and (2) translation to a human clinical population. I/R injury is very localized such that defining infarct/border zone and viable tissue becomes subjective. To address this issue, we implemented several histology and immunohistochemistry techniques to guide our interpretation followed by statistical correlation analysis. This approach will be further investigated to test border zone infiltration and salvageable myocardium.
Translating findings in murine models to humans presents unique challenges. Morphologically, mice have a larger cecum, which gives mice a larger relative population of gut microbes and capacity for carbohydrate fermentation (33). Accordingly, the mouse gut has a greater potential for production of bioactive compounds, like short-chain fatty acids, which have positive effects on anti-inflammation (42, 51). In addition, the composition of mouse gut microbiome is unique compared with humans. Although both are composed of two major phyla, Bacteriodetes and Firmicutes, there are significant differences at the genus level (22).
Despite these limitations, our study demonstrates that the impact of L. reuteri administration is not dependent on hypercholesterolemia. Wild-type and LDLr KO mice had comparable infarct sizes in the absence of L. reuteri and a similar reduction at either dose with L. reuteri administration. Furthermore, our previous work demonstrated that probiotic administration reduced infarct size in both high-fat-fed and normal diet-fed mice, again, suggesting a more global role for the gut in response to MI (5). In this latter study, we strongly implicate a necessary role for regulatory T cells in cardioprotection directed from the gut, which opens up future mechanistic studies to better define the role of gut in multiple maladies, including cardiovascular disease.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01-HL-098256, a National Mentored Research Science Development Award (K01 AR052840), an Independent Scientist Award (K02-HL-105799) from the NIH and American Heart Association (16GRNT31390006) to J.P.K., an interdisciplinary Training Grant in Computational and Mathematical Modeling of Biomedical Systems (GM-004905), and two Interdisciplinary Training Grants in Cardiovascular Sciences (HL-007249 and HL-00795515). Support was also received from the Sarver Heart Center at the University of Arizona and the Steven M. Gootter Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P.K., M.A.L.-P., and J.P.K. conceived and designed research; M.P.K., M.A.L.-P., R.S., and P.R.H. performed experiments; M.P.K., M.A.L.-P., R.S., and J.P.K. analyzed data; M.P.K., R.S., P.R.H., and J.P.K. interpreted results of experiments; M.P.K., R.S., and J.P.K. prepared figures; M.P.K., R.S., P.R.H., and J.P.K. drafted manuscript; M.P.K., M.A.L.-P., R.S., P.R.H., and J.P.K. edited and revised manuscript; M.P.K., M.A.L.-P., R.S., P.R.H., and J.P.K. approved final version of manuscript.
REFERENCES
- 1.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72: 1729–1738, 2006. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: A new era of lipid lowering therapy. World J Cardiol 9: 76–91, 2017. doi: 10.4330/wjc.v9.i2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Konhilas JP. Probiotic species on cardiovascular disease: the use of probiotics to reduce cardiovascular disease risk factors. In: Bioactive Food as Dietary Interventions for Cardiovascular Disease, edited by Watson RR, Preedy VR. Boston, MA: Elsevier, 2013, p. 303–317. [Google Scholar]
- 4.Chiang JY. Bile acid metabolism and signaling. Compr Physiol 3: 1191–1212, 2013. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danilo CA, Constantopoulos E, McKee LA, Chen H, Regan JA, Lipovka Y, Lahtinen S, Stenman LK, Nguyen TV, Doyle KP, Slepian MJ, Khalpey ZI, Konhilas JP. Bifidobacterium animalis subsp. lactis 420 mitigates the pathological impact of myocardial infarction in the mouse. Benef Microbes 8: 257–269, 2017. doi: 10.3920/BM2016.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res 55: 329–340, 2002. doi: 10.1016/S0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 7.Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One 7: e46837, 2012. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 9.Freytes DO, Santambrogio L, Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs 195: 171–182, 2012. doi: 10.1159/000331392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao C, Major A, Rendon D, Lugo M, Jackson V, Shi Z, Mori-Akiyama Y, Versalovic J. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. MBio 6: e01358-e15, 2015. doi: 10.1128/mBio.01358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol 1: 297–329, 2006. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 12.Hartvigsen K, Binder CJ, Hansen LF, Rafia A, Juliano J, Hörkkö S, Steinberg D, Palinski W, Witztum JL, Li AC. A diet-induced hypercholesterolemic murine model to study atherogenesis without obesity and metabolic syndrome. Arterioscler Thromb Vasc Biol 27: 878–885, 2007. doi: 10.1161/01.ATV.0000258790.35810.02. [DOI] [PubMed] [Google Scholar]
- 13.Hou C, Zeng X, Yang F, Liu H, Qiao S. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol 6: 14, 2015. doi: 10.1186/s40104-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond) 10: 35, 2013. doi: 10.1186/1743-7075-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WC, Chen YM, Kan NW, Ho CS, Wei L, Chan CH, Huang HY, Huang CC. Hypolipidemic effects and safety of Lactobacillus reuteri 263 in a hamster model of hyperlipidemia. Nutrients 7: 3767–3782, 2015. doi: 10.3390/nu7053767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92: 883–893, 1993. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones ML, Martoni CJ, Parent M, Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107: 1505–1513, 2012. doi: 10.1017/S0007114511004703. [DOI] [PubMed] [Google Scholar]
- 18.Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 66: 1234–1241, 2012. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3: 1245, 2012. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowala MC, Recce R, Beyer S, Gu C, Valentine M. Characterization of atherosclerosis in LDL receptor knockout mice: macrophage accumulation correlates with rapid and sustained expression of aortic MCP-1/JE. Atherosclerosis 149: 323–330, 2000. doi: 10.1016/S0021-9150(99)00342-1. [DOI] [PubMed] [Google Scholar]
- 21.Kukielka GL, Youker KA, Hawkins HK, Perrard JL, Michael LH, Ballantyne CM, Smith CW, Entman ML. Regulation of ICAM-1 and IL-6 in myocardial ischemia: effect of reperfusion. Ann N Y Acad Sci 723: 258–270, 1994. doi: 10.1111/j.1749-6632.1994.tb36732.x. [DOI] [PubMed] [Google Scholar]
- 22.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Hossain MA, Sadat S, Hager L, Liu L, Tam L, Schroer S, Huogen L, Fantus IG, Connelly PW, Woo M, Ng DS. Lecithin cholesterol acyltransferase null mice are protected from diet-induced obesity and insulin resistance in a gender-specific manner through multiple pathways. J Biol Chem 286: 17809–17820, 2011. doi: 10.1074/jbc.M110.180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol 74: 263–302, 2015. doi: 10.1016/bs.apha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston M, Loach D, Wilson M, Tannock GW, Baird M. Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response. Immunol Cell Biol 88: 99–102, 2010. doi: 10.1038/icb.2009.71. [DOI] [PubMed] [Google Scholar]
- 26.Mann GV, Spoerry A. Studies of a surfactant and cholesteremia in the Maasai. Am J Clin Nutr 27: 464–469, 1974. doi: 10.1093/ajcn/27.5.464. [DOI] [PubMed] [Google Scholar]
- 27.Martoni CJ, Labbé A, Ganopolsky JG, Prakash S, Jones ML. Changes in bile acids, FGF-19 and sterol absorption in response to bile salt hydrolase active L. reuteri NCIMB 30242. Gut Microbes 6: 57–65, 2015. doi: 10.1080/19490976.2015.1005474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis 60, Suppl 2: S85–S90, 2015. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- 30.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog 53: 100–108, 2012. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Murakami Y, Tanabe S, Suzuki T. High-fat diet-induced intestinal hyperpermeability is associated with increased bile acids in the large intestine of mice. J Food Sci 81: H216–H222, 2016. doi: 10.1111/1750-3841.13166. [DOI] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech 8: 1–16, 2015. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 336: 1262–1267, 2012. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson JK, Wilson ID. Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2: 668–676, 2003. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 37.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 55: 1629–1638, 2010. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell-Braxton L, Véniant M, Latvala RD, Hirano KI, Won WB, Ross J, Dybdal N, Zlot CH, Young SG, Davidson NO. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat Med 4: 934–938, 1998. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- 39.Qiao Y, Sun J, Xia S, Li L, Li Y, Wang P, Shi Y, Le G. Effects of different Lactobacillus reuteri on inflammatory and fat storage in high-fat diet-induced obesity mice model. J Funct Foods 14: 424–434, 2015. doi: 10.1016/j.jff.2015.02.013. [DOI] [Google Scholar]
- 40.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J, Wang J; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rettger LF. Bacillus acidophilus and its therapeutic application. Arch Intern Med (Chic) 29: 357–367, 1922. doi: 10.1001/archinte.1922.00110030082005. [DOI] [Google Scholar]
- 42.Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 268: 462–464, 1977. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 43.Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46, Suppl 2: S58–S61, 2008. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 44.Sato Y, Furihata C, Matsushima T. Effects of high fat diet on fecal contents of bile acids in rats. Jpn J Cancer Res 78: 1198–1202, 1987. [PubMed] [Google Scholar]
- 44a.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38: D792–D799, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 46.Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci 81: 2336–2340, 1998. doi: 10.3168/jds.S0022-0302(98)70123-7. [DOI] [PubMed] [Google Scholar]
- 47.Taranto MP, Sesma F, Pesce de Ruiz Holgado A, de Valdez GF. Bile salts hydrolase plays a key role on cholesterol removal by Lactobacillus reuteri. Biotechnol Lett 19: 845–847, 1997. doi: 10.1023/A:1018373217429. [DOI] [Google Scholar]
- 48.Taranto MP, Sesma F, Font De Valdez G. Localiation and primary characteriation of bile salt hydrolase from Lactobacillus reuteri. Biotechnol Lett 21: 935–938, 1999. doi: 10.1023/A:1005652501404. [DOI] [Google Scholar]
- 49.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 50.Vida M, Gavito AL, Pavón FJ, Bautista D, Serrano A, Suarez J, Arrabal S, Decara J, Romero-Cuevas M, Rodríguez de Fonseca F, Baixeras E. Chronic administration of recombinant IL-6 upregulates lipogenic enzyme expression and aggravates high-fat-diet-induced steatosis in IL-6-deficient mice. Dis Model Mech 8: 721–731, 2015. doi: 10.1242/dmm.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YM, Zhang B, Xue Y, Li ZJ, Wang JF, Xue CH, Yanagita T. The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis 9: 4, 2010. doi: 10.1186/1476-511X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuidema MY, Zhang C. Ischemia/reperfusion injury: the role of immune cells. World J Cardiol 2: 325–332, 2010. doi: 10.4330/wjc.v2.i10.325. [DOI] [PMC free article] [PubMed] [Google Scholar]