Abstract
Heart failure (HF) is characterized by autonomic imbalance with sympathetic hyperactivity and loss of parasympathetic tone. Intracardiac ganglia (ICG) neurons represent the final common pathway for vagal innervation of the heart and strongly regulate cardiac functions. This study tests whether ICG cholinergic neuron activation mitigates the progression of cardiac dysfunction and reduces mortality that occurs in HF. HF was induced by transaortic constriction (TAC) in male transgenic Long-Evans rats expressing Cre recombinase within choline acetyltransferase (ChAT) neurons. ChAT neurons were selectively activated by expression and activation of excitatory designer receptors exclusively activated by designer receptors (DREADDs) by clozapine-N-oxide (TAC + treatment and sham-treated groups). Control animals expressed DREADDs but received saline (sham and TAC groups). A separate set of animals were telemetry instrumented to record blood pressure (BP) and heart rate (HR). Acute activation of ICG neurons resulted in robust reductions in BP (∼20 mmHg) and HR (∼100 beats/min). All groups of animals were subjected to weekly echocardiography and treadmill stress tests from 3 to 6 wk post-TAC/sham surgery. Activation of ICG cholinergic neurons reduced the left ventricular systolic dysfunction (reductions in ejection fraction, fractional shortening, stroke volume, and cardiac output) and cardiac autonomic dysfunction [reduced HR recovery (HRR) post peak effort] observed in TAC animals. Additionally, activation of ICG ChAT neurons reduced mortality by 30% compared with untreated TAC animals. These data suggest that ICG cholinergic neuron activation reduces cardiac dysfunction and improves survival in HF, indicating that ICG neuron activation could be a novel target for treating HF.
NEW & NOTEWORTHY Intracardiac ganglia form the final common pathway for the parasympathetic innervation of the heart. This study has used a novel chemogenetic approach within transgenic ChAT-Cre rats [expressing only Cre-recombinase in choline acetyl transferase (ChAT) neurons] to selectively increase intracardiac cholinergic parasympathetic activity to the heart in a pressure overload-induced heart failure model. The findings from this study confirm that selective activation of intracardiac cholinergic neurons lessens cardiac dysfunction and mortality seen in heart failure, identifying a novel downstream cardiac-selective target for increasing cardioprotective parasympathetic activity in heart failure.
Keywords: acetylcholine, autonomic, DREADDs, heart failure, parasympathetic
INTRODUCTION
Heart failure (HF) is a widespread and devastating cardiovascular disease affecting nearly 23 million people worldwide and contributing to 270,000 deaths a year in the United States (5). A distinctive hallmark of HF is autonomic imbalance with sympathetic hyperactivity and depressed parasympathetic tone that detrimentally impact the progression of HF, including the development of exercise intolerance, ventricular remodeling, arrhythmias, and premature death (17, 31). In the initial stages of HF, compensatory responses to improve cardiac output, including neurohormonal activation, are beneficial to maintain cardiovascular homeostasis. However, a chronic shift in autonomic balance results in hemodynamic stress and deleterious effects on the heart (24). Impaired parasympathetic control of the heart is a characteristic of HF that is a powerful independent negative prognostic predictor of arrhythmia and disease progression (32, 34, 49).
Although the clinical significance of sympathetic over activity in HF is well established (16), parasympathetic regulation and modulation had received much less attention until recently. Despite recent advancements in parasympathetic modulation by cardiac vagal nerve stimulation, which has been shown to be cardioprotective in animal models of HF (9, 28), this treatment strategy had mixed results in humans (as reported in a recent clinical study NECTAR-HF), most likely due to nonselective activation of both sensory and motor vagal fibers, nonselective stimulation parameters, and ubiquitous activation of the entire vagus nerve (49).
Parasympathetic control of the heart involves convergence and integration of projections from a discrete subpopulation of neurons in the vagal motor nuclei of the brainstem to cholinergic neurons within intracardiac ganglia (ICG). ICG form the final common pathway of the cardiac autonomic nervous system, sending axonal projections to discrete regions of the heart (2). ICG form complex neural networks to regulate regional cardiac functions, including chronotropic, dromotropic, and inotropic actions, as well as coronary blood flow and myocardial perfusion (23). ICG not only relay central efferent preganglionic information but also process sensory afferent information from the myocardium and control efferent postganglionic firing (3). Therefore, the intrinsic cardiac nervous system controls cardiac function on a beat-to-beat basis in the absence of input from the central nervous system (2). Furthermore, previous functional and immunohistochemical studies revealed control of ventricular remodeling via cholinergic nerve projections from ICG neurons to the ventricle walls (33, 44).
ICG system dysfunction has been implicated in atrial and ventricular arrhythmias (20, 39), myocardial ischemia, and HF (8). Both an attenuation in neurotransmission within ICG (6, 7) and altered membrane properties of ICG neurons (14, 15, 41) contribute to withdrawal of parasympathetic activity to the heart in HF. Most relevant to this study is that low-intensity electrical stimulation of the right atrial ganglionic plexus protects against ventricular arrhythmias in canines that have an acute myocardial infarction (19, 43).
The ganglionic plexus and ICG contain not only parasympathetic neurons but also sympathetic postsynaptic neurons, interneurons, sensory neurons, and various noncholinergic nerve inputs that can release a multitude of neurotransmitters and neuropeptides, including nitric oxide, substance P, vasointestinal peptide, and calcitonin gene-regulated peptide (22). Therefore, electrical stimulation of the ICG would be nonselective and would stimulate both parasympathetic and many other populations of neurons that often oppose parasympathetic activation. To overcome this limitation, we used a novel chemogenetic approach within transgenic rats that only express Cre-recombinase in choline acetyl transferase (ChAT) neurons. A viral microinjection of floxed designer receptors exclusively activated by designer receptors (DREADDs) into the ICG of these ChAT-Cre rats enabled selective and controlled activation of ICG cholinergic parasympathetic activity to the heart as cardiac dysfunction developed in a transaortic constriction (TAC) model of HF.
MATERIALS AND METHODS
Ethical approval.
All animal procedures were completed in agreement with the institutional guidelines and with the approval of George Washington University and in compliance with suggestions from the panel of Euthanasia of the American Veterinary Medical Association and National Institutes of Health’s (NIH) Guide for the Care and Use of Laboratory Animals.
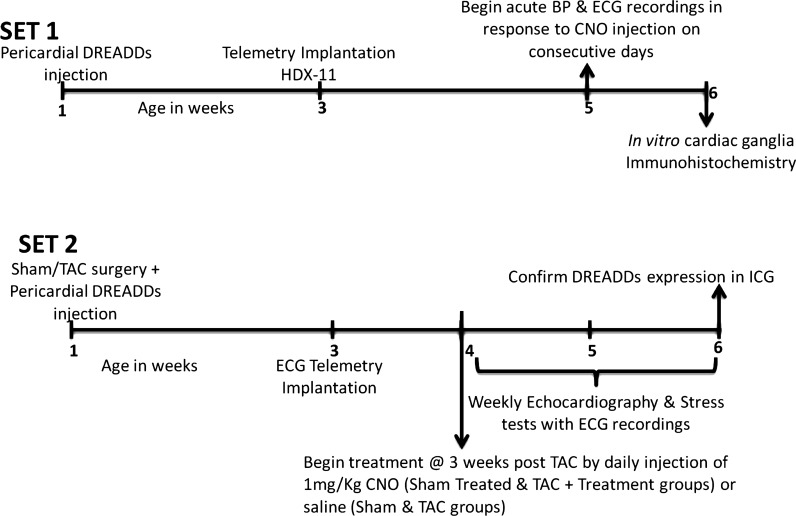
A timeline illustrating the schedule of experiments is shown in Fig. 1.
Fig. 1.
A timeline illustrating the schedule of experiments in the study. Animals in set 1 underwent pericardial designer receptors exclusively activated by designer receptors (DREADDs) injections and were used to record blood pressure (BP) and heart rate in response to intraperitoneal CNO injections. Animals in set 2 were assigned to 4 groups that expressed DREADDs within intracardiac ganglia (ICG) and underwent weekly in vivo echocardiography and peak effort stress tests. CNO, clozapine-N-oxide; TAC, transaortic constriction.
Transgenic animals and chemogenetic approach.
Homozygous male transgenic rats expressing the Cre recombinase enzyme selectively within ChAT neurons [strain: LE-Tg (ChAT-Cre) 5.1Deis. RRRC no. 658], where the Cre gene was introduced immediately before the ATG of the choline acetyltransferase (ChAT) gene, were obtained from the Rat Resource and Research Center (University of Missouri). ICG ChAT neurons were selectively activated using DREADDs, engineered G protein-coupled receptors that are selectively activated by a pharmacologically inert clozapine analog, clozapine-N-oxide (CNO) (36). The Cre-Lox recombination system was used to achieve robust and selective expression of DREADDs within cholinergic parasympathetic ICG neurons. Four microliters of viral vector containing floxed excitatory DREADDs [AAV2-hSyn-DIOhM3D(Gq)-mcherry] was surgically microinjected into the ICG within the right atrial fat pads to elicit selective expression of DREADDs in right atrial ICG neurons. Due to the presence of double-floxed inverse open-reading frames, the expression of DREADDs was limited to Cre-expressing ChAT neurons in the ICG. The expression of DREADDs in ChAT neurons was confirmed in each animal at the end of the study by confocal imaging of mCherry expression in an in situ right atrial ICG ganglia preparation, as done previously (15).
Four groups of animals expressing DREADDs in ICG ChAT neurons were used: sham, untreated TAC, TAC + treatment, and sham + treated. TAC + Treatment and sham + treatment groups received daily CNO intraperitoneal injections starting at 3 wk post-sham/TAC surgery for 3 wk to activate DREADDs in cholinergic ICG neurons. Sham and untreated TAC animals received daily saline intraperitoneal injections for the same time interval.
TAC surgeries and intracardiac viral injections.
Transaortic constriction-induced pressure overload was used to generate progressive HF in male transgenic ChAT-Cre Long-Evans rats using a minimally invasive TAC approach, as reported previously (18). Briefly, 1-wk-old rats were anesthetized by hypothermia, a 0.5-cm incision was made at the level of the chest, the chest was opened, and the thymus was retracted to reveal the aorta. A 4-0 silk suture was passed around the ascending aorta, and with a 25-gauge needle temporarily placed adjacent to the aorta, the suture was tied around the aorta and needle. The needle was then removed, leaving the constricting suture around the aorta. Buprenorphine was applied as an analgesic. Immediately after successful completion of the TAC procedure, 4 μL of floxed excitatory DREADD [AAV2-hSyn-DIOhM3D(Gq)-mCherry, 2.01E+13 vg/mL, Virovek] virus was injected into the right atrial fat pads for selective DREADD expression in ICG ChAT neurons. The chest was then closed with several sutures, and animals were monitored for 24 h during recovery, after which they were returned to the cage with the mother. Sham animals underwent similar surgery, except that the aorta was not constricted.
Telemetry implant surgeries.
Two weeks after the TAC surgery and pericardial DREADDs injections, rats were anesthetized (2% isoflurane) and implanted with a transmitter (ETA-F10 or HDX-11 Data Sciences International) to record the ECG, heart rate (HR), and blood pressure (BP). After implantation, animals recovered for 1 wk before daily recording sessions of BP and the ECG were started. For experiments involving acute activation of ICG-ChAT neurons, baseline mean BP and HR were recorded 30 min before and 2 h after DREADD activation by intraperitoneal CNO injection (1 mg/kg). Data were analyzed using DSI Ponemah software (version 5.20; Data Sciences International).
Echocardiography.
Weekly assessments of in vivo cardiac function from 3 wk until 6 wk post-TAC were conducted via small-animal echocardiography to compare disease progression among groups (29, 40). Rats were anesthetized with 2% isoflurane, and motion-mode (M-mode) echocardiograms, Doppler images, and two-dimensional strain images were acquired using a Visual Sonics Vevo 3100 imaging system (Fuji Film) at frame rates of ≤1 kHz with a 25-MHz linear array transducer. A two-dimensional parasternal long-axis view of the LV provided LV ejection fraction (LVEF). LV structural variables and endocardial fractional shortening (FS) were measured using M-mode signals obtained from a parasternal short-axis view of the LV at the level of papillary muscles. Tissue Doppler imaging via an apical four-chamber view provided pulse wave motion of blood inflow through the mitral valves to assess diastolic function, including early diastolic mitral inflow (E wave), late diastolic mitral inflow (A wave), and early-to-late diastolic mitral inflow ratio (E/A ratio). Tissue Doppler mode in continuous wave motion was used to image aortic outflow to measure velocities in the ascending and descending aorta. This was also used to calculate stroke volume and cardiac output. The ECG, respiratory waveform, and body temperature were acquired simultaneously with each echocardiography data set.
Treadmill peak effort tests.
Because heart rate recovery (HRR) is a strong predictor of mortality (10, 13), longitudinal changes in HRR were measured starting 3 wk post-TAC/sham surgery. All rats underwent a weekly treadmill peak effort test to measure HRR (1, 46, 47). One week following telemetry implantation surgery, rats were acclimatized to the treadmill environment and running speeds. On the day of the experiment, rats underwent an initial warmup period of 5 min at a speed of 6 cm/s. Speed then quickly ramped up to 12 cm/s and increased by 6 cm/s every 3 min until exhaustion, which was defined as the speed where rats refused to continue running on the treadmill for ≥1min (Naughton protocol). The ECG was acquired before (30 min), during, and after (30 min) the test. HRR was measured as the HR at peak effort minus HR at 15 s post-peak effort (10, 13).
Immunohistochemistry and confocal fluorescence imaging.
In vitro preparations of intracardiac ganglia comprising of ChAT-Cre neurons were obtained as explained previously (14, 15). Animals were anesthetized with the inhalation anesthetic isoflurane. After the animals were deeply anesthetized, no longer responded to a toe pinch, and stopped breathing, the chest was opened via a midline incision, and the right atrium was cut. After exsanguination, the right atrium was excised and placed in ice-cold Krebs-Hanseleit solution containing (in mM) 115 NaCl, 3.3 KCl, 2.0 CaCl2, 1.4 MgSO4, 25.0 NaHCO3, 1.0 KH2PO4, 5.0 glucose, and 1.0 lactate. The ganglia were dissected and fixed for 24 h at 4°C in a periodate-lysine-paraformaldehyde fixative (2% paraformaldehyde; Electron Microscopy Sciences) with 115mM l-lysine and 20 mM sodium metaperiodate (30) in phosphate-buffered saline (PBS). Samples were then washed (3 × 2 min, 3 × 15 min, 1 × 24 h) with PBS to remove the fixative. Ganglia were blocked for 6 h in 2% bovine serum albumin (BSA) with 2% Triton X-100 in PBS. Ganglia were then incubated with ChAT and mCherry primary antibodies [2% BSA, 0.5% Triton X-100, 1:100 goat anti-ChAT (AB144P; EMD Millipore), and 1:500 rabbit anti-mCherry (ab167453; Abcam)] in PBS for 24 h with shaking at 4°C. Unbound primary antibody was removed with washes lasting 3 × 2 min, 3 × 15 min, 1 × 1 h, and 1 × 24 h. Following this, samples were incubated in the secondary antibodies [2% BSA, 0.5% Triton X-100, 1:200 donkey anti-goat FITC (AP180F; EMD Millipore), and 1:1000 donkey anti-rabbit Alexa Fluor 594 (ab150076; Abcam)] in PBS. Secondary antibodies were washed in the same manner as the primary antibodies. Finally, ganglia were imaged using a confocal microscope (Leica TCS SP8 MP) with a ×40 water immersion lens.
Data analysis and statistics.
Echocardiography data were analyzed using Visual Sonics software. BP and ECG signals were analyzed using DSI Ponemah. HRR was measured using AD Instruments Labchart. Data were presented as means ± SE and statistically compared using paired t tests. A mixed-effects model (restricted maximum likelihood method) with Tukey’s post-multiple-comparisons test (Graph Pad Prism version 8) was used to compare longitudinal measurements among groups. Mortality data were plotted using Kaplan Meier survival analysis with log rank test. Differences among groups were considered significant if P < 0.05.
RESULTS
Acute activation of ICG-ChAT neurons reduced BP and HR.
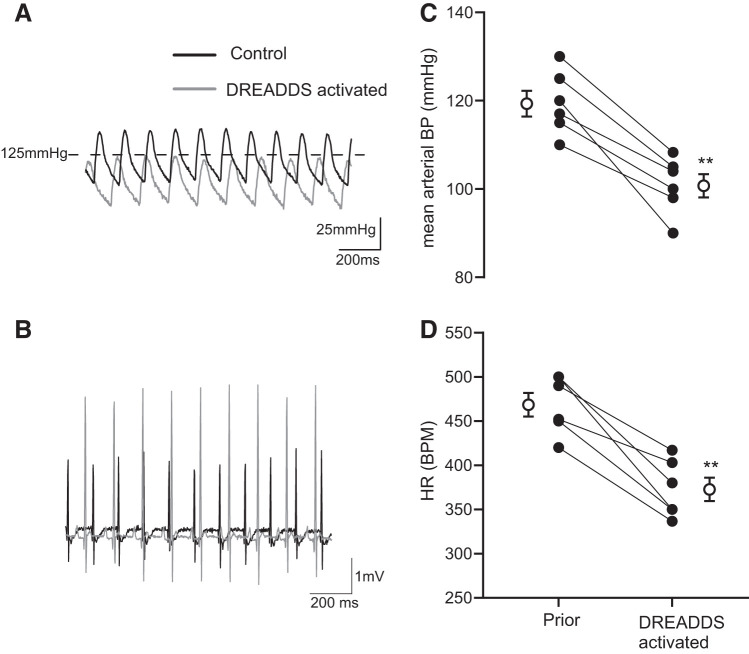
In telemetry-implanted conscious unrestrained rats, acute activation of ICG-ChAT neurons expressing excitatory DREADDs after an intraperitoneal injection of CNO induced a robust reduction in mean arterial BP and HR of ∼20 mmHg and ∼100 beats/min, respectively (Fig. 2). These changes in BP and HR persisted for ≥3 h.
Fig. 2.
Acute activation of intracardiac ganglia (ICG)-choline acetyl transferase (ChAT) neurons reduces blood pressure (BP) and heart rate (HR). In awake telemetry-instrumented rats that received pericardial injections of designer receptors exclusively activated by designer receptors (DREADDs) in cardiac ganglia (set 1), BP (A and C) and HR (B and D) dropped within 30–40 min after administering CNO (1 mg/kg), the ligand for the excitatory DREADDs expressed within the ICG cholinergic neurons. The response was maintained for ≥1 h post-clozapine-N-oxide (CNO) injection given intraperitoneally. ●, Data from individual animals; ○, means ± SE in each group. **P < 0.01, paired t test; n = 6. BPM, beats/min.
Activation of ICG-ChAT neurons improved in vivo cardiac function in TAC animals.
All groups of ChAT-Cre transgenic rats, starting from 3 wk up to 6 wk post-TAC/Sham surgery, underwent weekly echocardiographic assessments. No differences in echocardiographic measurements of function between sham and sham + treatment animals were observed.
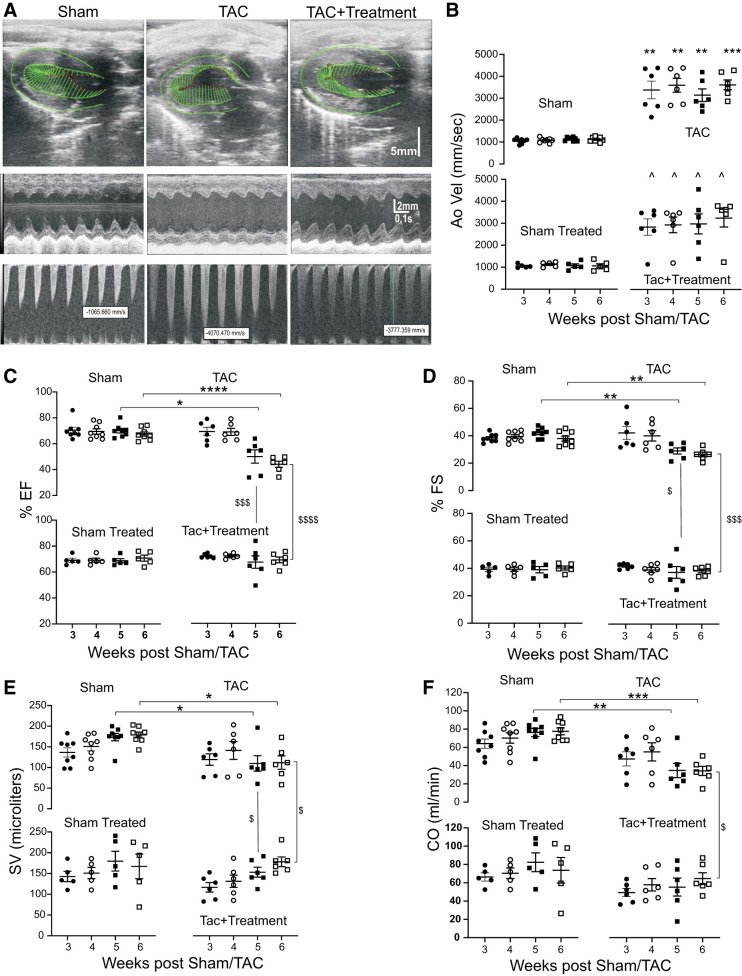
Doppler flow measurements of the descending aorta immediately after the constriction point revealed significantly higher velocities at all time points in TAC and TAC + treatment animals compared with sham and sham +treatment animals (Fig. 3, A and B). Compared with sham animals, TAC animals had reduced HR at 5 and 6 wk post-sham/TAC (Table 1). TAC animals also had significantly reduced left ventricular systolic function, including ejection fraction, fractional shortening, stroke volume, and cardiac output at 5 and 6 wk post-sham/TAC compared with sham animals. However, cardiac function was significantly better at 5 and 6 wk post-TAC in TAC + treatment animals (Fig. 3C).
Fig. 3.
Chronic chemogenetic activation of intracardiac ganglia (ICG)-choline acetyl transferase (ChAT) neurons improves cardiac function. A: representative B-mode longitudinal axis images (2D; top), M-mode short-axis signals (middle), and descending aorta doppler flow recordings (bottom) for echocardiographic studies of sham, untreated transaortic constriction (TAC), and designer receptors exclusively activated by designer receptors (DREADDs) activated TAC animals at 6 wk post-TAC/sham surgery. Green lines denote the left ventricular epicardium and endocardium during mid-systole. The vector display reveals that left ventricular contractions are improved in TAC + treatment compared with untreated TAC. B–F: echocardiographic longitudinal data from sham (n = 8), untreated TAC (n = 6), TAC + treatment (n = 6), and sham-treated (n = 5) animals at 3, 4, 5, and 6 wk post-TAC/sham surgery reveal differences in aortic velocity (B), ejection fraction (EF; C), fractional shortening (FS, D), stroke volume (SV; E), and cardiac output (CO; F). Data were compared using mixed-model analysis with Tukey’s multiple-comparison test. *P < 0.05, sham vs. untreated TAC; **P < 0.01; ***P < 0.001; ****P < 0.0001; $untreated TAC vs. TAC + treatment; ^sham vs. TAC+ treatment (B); $$$P < 0.001, untreated TAC vs. TAC + treatment; $$$$P < 0.0001, untreated TAC vs. TAC + treatment.
Table 1.
Echocardiographic data for sham, TAC, TAC + T, and sham-treated groups at 3, 4, 5 and 6 wk post-sham/TAC surgery
| 3 wk Post |
4 wk Post |
5 wk Post |
6 wk Post |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | TAC | TAC + T | Sham treated | Sham | TAC | TAC+T | Sham treated | Sham | TAC | TAC + T | Sham treated | Sham | TAC | TAC + T | Sham treated | |
| LV mass, mg | 373.0 ± 27.7 | 606.5 ± 72.0 | 424.4 ± 70.6 | 385.0 ± 30.7 | 419.6 ± 36.5 | 684.8 ± 98.7 | 573.7 ± 58.8 | 385.0 ± 30.7 | 544.7 ± 30.9 | 711.8 ± 77.3 | 735.0 ± 80.4 | 517.2 ± 42.6 | 631.6 ± 32.5 | 1012.0 ± 142.7** | 742.0 ± 58.8‡ | 561.3 ± 65.0 |
| LVID,d, mm | 6.1 ± 0.1 | 5.5 ± 0.3 | 5.0 ± 0.2 | 6.2 ± 0.3 | 6.3 ± 0.1 | 6.1 ± 0.3 | 5.5 ± 0.3 | 6.2 ± 0.3 | 6.6 ± 0.2 | 6.8 ± 0.3 | 6.4 ± 0.4 | 6.4 ± 0.2 | 7.1 ± 0.1 | 7.7 ± 0.5 | 7.3 ± 0.2 | 7.0 ± 0.2 |
| LVID,s, mm | 3.8 ± 0.1 | 3.2 ± 0.3 | 2.9 ± 0.1 | 3.8 ± 0.2 | 3.8 ± 0.1 | 3.7 ± 0.2 | 3.3 ± 0.2 | 3.8 ± 0.2 | 3.8 ± 0.2 | 4.9 ± 0.3* | 4.0 ± 0.4† | 3.9 ± 0.2 | 4.4 ± 0.2 | 5.7 ± 0.5** | 4.5 ± 0.2‡ | 4.2 ± 0.1 |
| LVPW,d, mm | 1.5 ± 0.1 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.3 ± 0.1 | 1.6 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.3* | 2.0 ± 0.2 | 1.6 ± 0.1 |
| IVS,d, mm | 1.3 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.8 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.0 | 1.9 ± 0.2 | 2.2 ± 0.3 | 1.5 ± 0.1 | 1.5 ± 0.0 | 2.2 ± 0.2* | 2.3 ± 0.2* | 1.6 ± 0.1 |
| HR, beats/min | 471.1 ± 9.3 | 399.5 ± 28.0 | 424.6 ± 5.5 | 467.7 ± 11.0 | 463.9 ± 6.3 | 391.3 ± 25.7 | 441.8 ± 19.0 | 467.7 ± 11.0 | 438.6 ± 8.9 | 322.5 ± 29.0* | 346.8 ± 44.3 | 460.5 ± 11.5 | 434.0 ± 12.8 | 325.6 ± 34.5** | 365.4 ± 34.7 | 433.8 ± 15.9 |
| E/A ratio | 1.8 ± 0.14 | 1.75 ± 0.11 | 1.93 ± 0.24 | 1.68 ± 0.2 | 1.78 ± 0.13 | 1.87 ± 0.2 | 2.1 ± 0.24 | 1.68 ± 0.2 | 1.76 ± 0.07 | 1.57 ± 0.1 | 1.7 ± 0.12 | 1.65 ± 0.11 | 1.72 ± 0.1 | 1.97 ± 0.1 | 2.27 ± 0.4 | 1.65 ± 0.2 |
| PR interval, s | 0.04 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.04 ± 0.0 | 0.03 ± 0.0 | 0.03 ± 0.0 | 0.04 ± 0.0* | 0.04 ± 0.0 | 0.03 ± 0.0 | ||||
Values are means ± SE; n, number of subjects. HR, heart rate; IVS,d, interventricular septal thickness in diastole; LVID,d, left ventribular (LV) internal diameter in diastole; LVID,s LV internal diameter in systole; LVPW,d, LV posterior wall thickness in diastole; TAC, transaortic constriction; TAC + T, TAC + treatment. No. of animals in each group are as follows: sham (n = 8), TAC (n = 6), TAC + T (n = 6), and Sham treated (n = 5). Ratio of early (E) to late (A) mitral inflow (E/A).
P < 0.05, sham vs. TAC;
P < 0.01, sham vs. TAC;
P < 0.05, untreated TAC vs. TAC + treatment;
P < 0.01, untreated TAC vs TAC + treatment.
An increase in interventricular septal diameter in diastole (IVS,d), LV posterior wall diameter (LVPW,d), LV internal diameter in systole (LVID,s), and LV mass was observed for untreated TAC animals compared with sham at 6 wk post-sham/TAC, indicating LV hypertrophy (Table 1). Interestingly, activation of ICG ChAT neurons mitigated the increases in LV mass and LVID that were observed for untreated TAC animals (Table 1).
Activation of ICG-ChAT neurons improved cardiac autonomic balance and reduced mortality.
HRR, a clinical index of cardiac autonomic function, was significantly lower in untreated TAC animals compared with sham animals at all time points 4 to 6 wk post-sham/TAC surgery. Activation of DREADDs in ICG-ChAT neurons significantly improved HRR at 6 wk post-TAC in TAC + treatment animals (Fig. 4A). Consistent with these data, reductions in HR, observed in response to acute activation of DREADDs expressing ICG ChAT neurons, were maintained at all time points of treatment (Fig. 4B). The ECG of TAC animals displayed greater PR intervals at 6 wk post-sham/TAC compared with sham animals, which was not affected by activation of DREADDs in ICG-ChAT neurons (Table 1). Importantly, activation of DREADDs in ICG ChAT neurons more than doubled the survival rate of treated animals (from 80% mortality to 50% mortality; Fig. 4C).
Fig. 4.
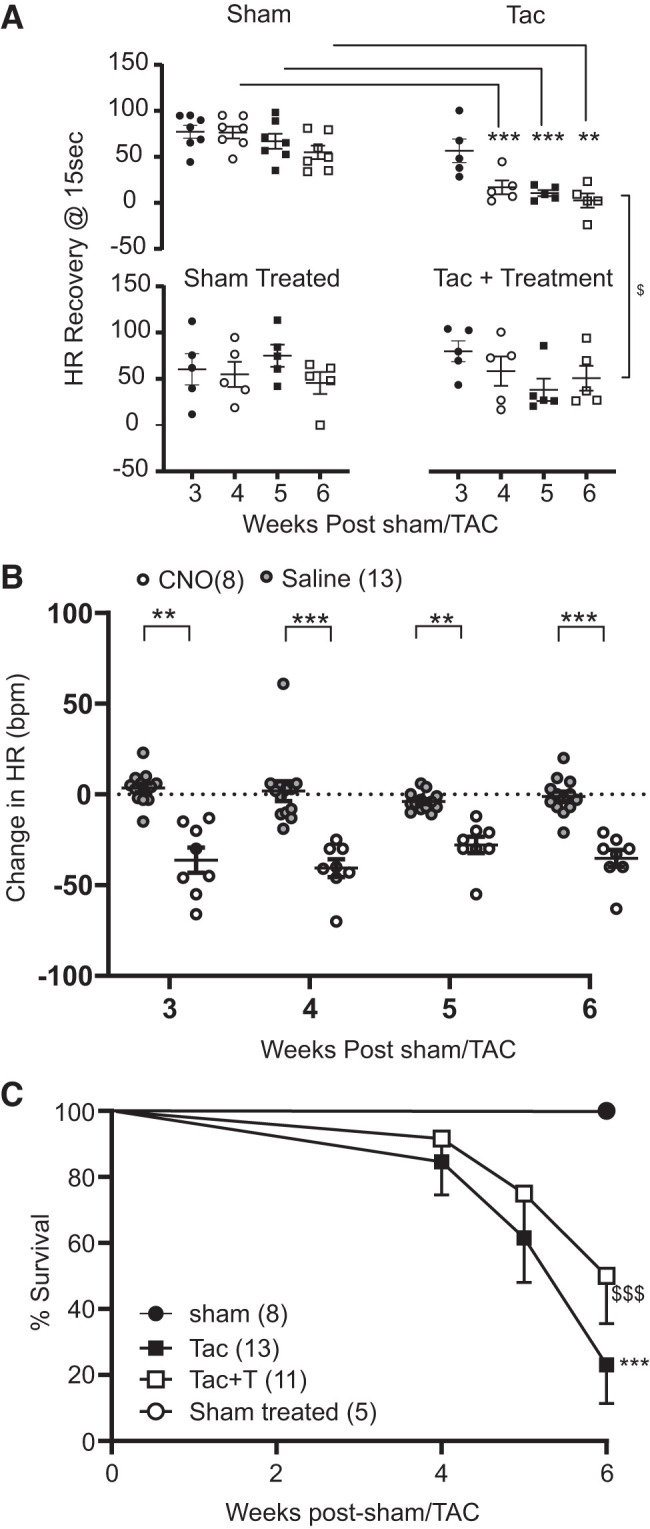
Chronic activation of intracardiac ganglia (ICG)-choline acetyl transferase (ChAT) neurons improves cardiac autonomic function and survival in untreated transaortic constriction (TAC) animals. A: graph depicting longitudinal heart rate (HR) recovery data from sham (n = 7), untreated TAC (n = 5), TAC + treatment (n = 5), and sham-treated (n = 5) telemetry-equipped animals that underwent treadmill stress test at 3, 4, 5, and 6 wk post-TAC/sham surgery. HR recovery was calculated as the difference between peak effort HR and recovery HR at 15 s after cessation of exercise. Results indicate that treated TAC animals had improved HR recovery compared with untreated TAC animals. Data were compared using mixed-model analysis with Tukey’s multiple-comparison test. B: HR responses from telemetry-equipped animals expressing designer receptors exclusively activated by designer receptors (DREADDs) in ICG-ChAT neurons receiving either saline or clozapine-N-oxide (CNO; a ligand for DREADDs activation) recorded at 3–6 wk post-TAC/sham. Saline group includes data from both sham and TAC animals receiving saline, and CNO group includes data from both sham-treated and TAC + treatment animals receiving CNO. Data were compared using mixed-model analysis with Sidak’s post-multiple comparison test, Saline (n = 13) vs. CNO (n = 8). C: Kaplan Meier curves depicting %survival in sham (n = 8), untreated TAC (n = 13), TAC + treatment (n = 11), and sham-treated (n = 5) animals. Untreated TAC animals had significantly reduced survival rates than sham animals. However, chronic activation of DREADDs expressing ICG-ChAT neurons improved survival rates in TAC animals. **P < 0.01, saline vs. CNO; ***P < 0.001, saline vs. CNO, sham vs. untreated TAC; $P < 0.05, untreated TAC vs. TAC + treatment; $$$P < 0.001, untreated TAC vs. TAC + treatment.
Selective expression of DREADDs in ICG-ChAT neurons.
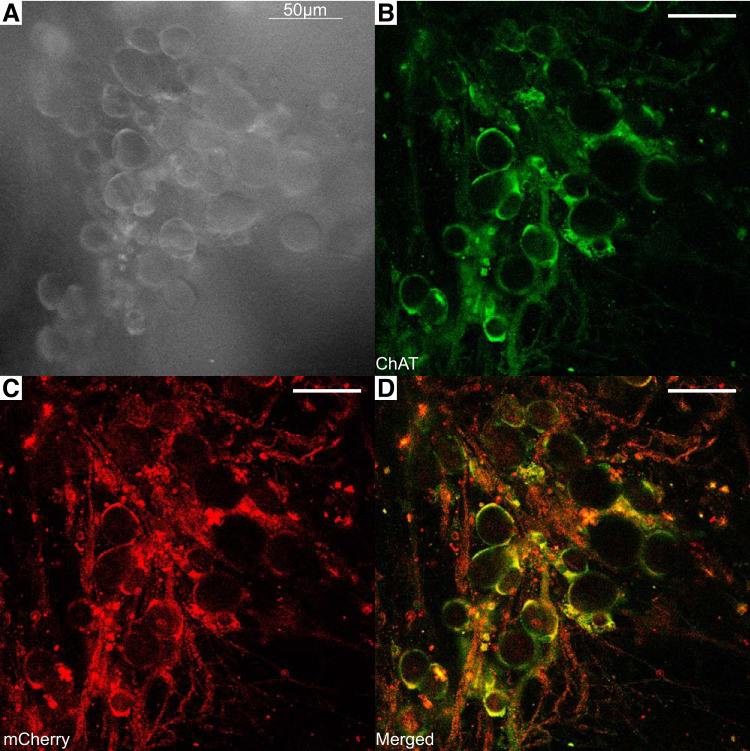
At the completion of all studies, selective and robust expression of DREADDs within right atrial ICG-ChAT neurons was confirmed using standard immunohistochemistry protocols, as shown in Fig. 5. DREADDs tagged with mCherry were colocalized with nearly 99% of ChAT neurons within the right ICG, thus confirming selectivity of DREADD expression within the ChAT neurons of the ICG. The selectivity of DREADD expression was examined, and there was virtually no DREADD expression in non-ChAT neurons in the cardiac ganglia and other tissue, as anticipated. Whereas the number of non-ChAT neurons expressing DREADDs was not quantified, visual examination of tissue from each animal showed that nonselective expression in non-ChAT neurons was limited to <5% of the total DREADDs expressing neurons.
Fig. 5.
Fluorescence images showing selective expression of designer receptors exclusively activated by designer receptors (DREADDs) in the cholinergic neurons of a right atrial intracardiac ganglia (ICG). A: white light image of ICG neurons in the ganglion. B: neurons (green) in the ganglion expressing choline acetyl transferase (ChAT) were labeled with a goat anti-ChAT primary antibody and a donkey anti-goat FITC conjugated secondary antibody. C: the DREADDs-linked-mCherry signal (red) was amplified with a rabbit anti-mCherry primary antibody and a donkey anti-rabbit Alexa Fluor 594-conjugated secondary antibody. D: ChAT and mCherry are colocalized (yellow) in the ICG neurons of the ganglia.
DISCUSSION
The primary conclusions of this study are that 1) acute activation of DREADDs expressed within ICG cholinergic neurons reduces mean arterial blood pressure and heart rate, and 2) activation of ICG cholinergic neurons significantly improved cardiac systolic function, heart rate recovery, and survival in a rat model of pressure overload induced heart failure.
Activation of ICG cholinergic neurons reduced heart rate and blood pressure.
Diverse central and peripheral elements within the cardiac nervous system act in harmony to regulate cardiac functions; direct stimulation of intracardiac neurons occurs through central efferent neuronal inputs from the vagi or stellate ganglia (2). Atrial ganglionated plexi not only serve as a relay station transmitting central parasympathetic impulses to the heart but also function as the integration center of the extrinsic and intrinsic cardiac autonomic nervous system (2). Neurons in each major ganglionated plexus exert control over electrical and mechanical events in all cardiac chambers (48). The negative chronotropic effect due to the activation of right atrial ICG matches the reductions in heart rate we observed in response to acute selective activation of DREADDs expressed in right atrial ICG cholinergic neurons. These findings agree with previous results from canines demonstrating that atrial ganglionic plexus stimulation exerts ventricular electrophysiological effects similar to vagus nerve stimulation (19).
Chemogenetic approach and selectivity of ICG cholinergic neuron activation.
ICG-ChAT neurons were activated using a novel chemogenetic approach whereby DREADDs, engineered Gq protein-coupled receptors, were selectively expressed within cholinergic ICG neurons and activated by clozapine-N-oxide (CNO) (36). The use of Gq-activating DREADDS is a widely adopted approach to selectively activate neurons involved in systems that regulate feeding, sleep wake cycles, and other behaviors (26, 38). The Cre-Lox recombination system targeted the expression of DREADDs within cholinergic ICG neurons. Because of the presence of double-floxed inverse open-reading frames, the expression of DREADDs was only initiated selectively in ChAT neurons that expressed Cre. This was confirmed by immunohistochemical analysis of right atrial ICG that revealed selective expression of mCherry/DREADDs in ChAT Cre neurons. The puncta staining of ChAT and mCherry indicate that DREADDs may also be expressed in nerve terminals in the ICG. Thus, an effect of DREADD activation in synaptic neurotransmission within the ICG, although unlikely, cannot be ruled out. The lack of any significant differences in cardiac function (echocardiography and heart rate recovery) between sham and sham-treated groups demonstrated that off-target effects of CNO or its biologically active metabolites at the concentrations used in this study were unlikely. This agrees with our earlier findings where CNO was used to activate DREADDs in a similar model of HF (18).
Cardioprotective actions of ICG cholinergic neuron activation and underlying mechanisms.
TAC-induced pressure overload led to progressive HF in transgenic ChAT-Cre rats, similar to the outcomes of our previous studies of TAC-induced heart failure (11, 18). Impediment of aortic flow commenced from as early as 3 wk post-TAC in both TAC and TAC + treatment animals, confirming consistent TAC between both groups. Systolic dysfunction and the progression of HF were evident in later stages, particularly at 5 wk post-TAC and beyond. An increase in LV mass and LV systolic diameter in untreated TAC animals demonstrated myocardial hypertrophy in response to aortic banding. These increases were blunted in treated TAC animals. The increased LV systolic diameter indicates reduced fractional shortening and, therefore, reduced ejection fraction with accompanying reductions of stroke volume, cardiac output, and contractility.
TAC-induced myocardial ischemia causes a loss of vital myocardium, which would activate neurohormonal systems and increase chronic stress on the remaining vital myocardium, ultimately causing progressive myocardial remodeling and progressive reduction in ejection fraction. Vagal-mediated activation of cholinergic anti-inflammatory pathways has been shown to elicit downregulation of inflammatory mediators and collagen that are upregulated in heart failure (45). Our results indicate that activation of ICG-ChAT neurons slowed the development of detrimental reduction in cardiac function that was observed in untreated TAC animals. In addition to reducing LV hypertrophy, other mechanisms involving reduced expression of inflammatory mediators, fibrosis, and/or collagen could also be responsible for the observed cardioprotective outcomes. Consistent with our results, previous studies using guinea pigs have shown that vagal nerve stimulation mitigates HF-induced remodeling of the intracardiac nervous system, preserving ventricular function via both neural and cardiomyocyte-dependent actions (4).
Heart rate recovery, the decrease in HR following cessation of peak physical exertion, is an important predictor of all-cause mortality and death associated with HF. Low HRR in untreated TAC rats was predictive of the observed increased mortality of those animals. Maximum HR during peak effort was not different among any of the groups, and hence, the lower HRR in untreated TAC animals was most likely due to withdrawal of parasympathetic tone. Increases in PR interval (suggestive of atrioventricular node block) observed in TAC animals were not affected by ICG ChAT neuron activation, suggesting that mechanisms involving changes in conduction through AV node are unlikely to contribute to the cardioprotective action of ICG ChAT neuron activation. Activation of ICG ChAT neurons improved both HRR and survival rates in treated TAC animals. For those animals, HRR reflected the balance of DREADD-meditated reactivation of the parasympathetic nervous system and reduced sympathetic nervous system activity (42). Although our in vivo findings in control animals clearly suggest that activation of ICG ChAT neurons causes reductions in blood pressure and heart rate for at least ∼3 h, such potentially limited activation of these neurons on a daily basis has been shown to have an impressive cardioprotective effect on disease outcomes. Our findings are consistent with previous studies in canines showing that atrial epicardial ganglionated plexus stimulation protects against ischemia-reperfusion-induced ventricular arrhythmias by preserving the expression of cardiac gap junction protein connexin 43. Those studies used a nonselective electrical stimulation approach that, in addition to stimulating parasympathetic neurons, likely also stimulated other neuronal populations that oppose parasympathetic activation (43). We have now further demonstrated that selective chemogenetic activation of ICG cholinergic neurons provides powerful protection of the myocardium during pressure overload HF.
The sympathetic and parasympathetic cardiac autonomic systems have specific stimulatory and inhibitory effects on cardiomyocytes as well as other cardiac neurons. The release of norepinephrine (NE) from the sympathetic nerve varicosities inhibits ACh release from neighboring parasympathetic fibers, whereas ACh released from parasympathetic varicosities can prevent the release of NE from sympathetic fibers (27). Hence, activation of ICG ChAT neurons that release ACh may directly activate muscarinic receptors as well as diminish the release of NE from sympathetic nerve terminals and subsequent downstream adrenergic receptor activation.
Vagal nerve activity dilates coronary arteries by muscarinic receptor activation (25) [independent of left ventricular preload, afterload, and heart rate (35)], and this vagally mediated coronary vasodilation is attenuated in HF (50). ICG neurons have been shown to be critical for autoregulation of coronary flow and myocardial perfusion, even after ablation of extracardiac nerves from CNS control (37). Noncholinergic neurotransmitters and neuropeptides, including nitric oxide (NO) (21) and vasointestinal peptide (VIP), are coreleased with ACh from parasympathetic nerve varicosities to regulate coronary vasomotor tone as well as cardiac contraction and relaxation (12, 24). Reduced bioavailability of NO and VIP contributes to the development of impaired coronary vasomotor tone and flow reserve and negatively impacts myocardial oxygen delivery during HF. Altogether, activation of ICG ChAT neurons could prevent cardiac dysfunction by preserving vital myocardium by preventing the loss of vasomotor tone, reducing inflammation, preventing the loss of intrinsic cardiac cholinergic activity, and inhibiting sympathetic overactivation. Any or all of these mechanisms are likely at play, and further studies are needed to determine their relative dominance within the setting of HF.
Conclusions.
Activation of ICG cholinergic parasympathetic neurons lessens cardiac dysfunction and improves survival in animals with pressure overload-induced HF. In this study, new approaches not previously available in the field were developed and used to identify a novel downstream cardiac-specific target for effectively and selectively increasing cardioprotective parasympathetic activity, thereby improving cardiac function and survival during pressure overload HF.
GRANTS
This work was supported by an American Autonomic Society postdoctoral fellowship (to J.D.) and National Heart, Lung, and Blood Institute Grants HL-133862 and HL-146169.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D., M.W.K., and D.M. conceived and designed research; J.D., A.J.H., J.B.E., J.R.S., M.K.R.D., and J.R. performed experiments; J.D., A.J.H., J.B.E., and D.M. analyzed data; J.D., C.F.S., and D.M. interpreted results of experiments; J.D. prepared figures; J.D. and D.M. drafted manuscript; J.D., M.W.K., and D.M. edited and revised manuscript; J.D., C.F.S., M.W.K., and D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Anastas Popratiloff of the George Washington Nanofabrication Center for expertise in acquiring the immunohistochemistry images.
REFERENCES
- 1.Aaker A, McCormack JG, Hirai T, Musch TI. Effects of ranolazine on the exercise capacity of rats with chronic heart failure induced by myocardial infarction. J Cardiovasc Pharmacol 28: 353–362, 1996. doi: 10.1097/00005344-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 309: H1198–H1206, 2015. doi: 10.1152/ajpheart.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 6.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99: 2958–2963, 1999. doi: 10.1161/01.CIR.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 7.Bibevski S, Dunlap ME. Prevention of diminished parasympathetic control of the heart in experimental heart failure. Am J Physiol Heart Circ Physiol 287: H1780–H1785, 2004. doi: 10.1152/ajpheart.00430.2004. [DOI] [PubMed] [Google Scholar]
- 8.Brack KE, Winter J, Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation–tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev 18: 389–408, 2013. doi: 10.1007/s10741-012-9314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley U, Shivkumar K, Ardell JL. Autonomic Regulation Therapy in Heart Failure. Curr Heart Fail Rep 12: 284–293, 2015. doi: 10.1007/s11897-015-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahalin LP, Arena R, Labate V, Bandera F, Lavie CJ, Guazzi M. Heart rate recovery after the 6 min walk test rather than distance ambulated is a powerful prognostic indicator in heart failure with reduced and preserved ejection fraction: a comparison with cardiopulmonary exercise testing. Eur J Heart Fail 15: 519–527, 2013. doi: 10.1093/eurjhf/hfs216. [DOI] [PubMed] [Google Scholar]
- 11.Cauley E, Wang X, Dyavanapalli J, Sun K, Garrott K, Kuzmiak-Glancy S, Kay MW, Mendelowitz D. Neurotransmission to parasympathetic cardiac vagal neurons in the brain stem is altered with left ventricular hypertrophy-induced heart failure. Am J Physiol Heart Circ Physiol 309: H1281–H1287, 2015. doi: 10.1152/ajpheart.00445.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion HC, Skaf MW, Hare JM. Role of nitric oxide in the pathophysiology of heart failure. Heart Fail Rev 8: 35–46, 2003. doi: 10.1023/A:1022142904202. [DOI] [PubMed] [Google Scholar]
- 13.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 14.Dyavanapalli J, Rimmer K, Harper AA. The action of high K+ and aglycaemia on the electrical properties and synaptic transmission in rat intracardiac ganglion neurones in vitro. Exp Physiol 94: 201–212, 2009. doi: 10.1113/expphysiol.2008.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyavanapalli J, Rimmer K, Harper AA. Reactive oxygen species alters the electrophysiological properties and raises [Ca2+]i in intracardiac ganglion neurons. Am J Physiol Regul Integr Comp Physiol 299: R42–R54, 2010. doi: 10.1152/ajpregu.00053.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floras JS. Sympathetic activation in human heart failure: diverse mechanisms, therapeutic opportunities. Acta Physiol Scand 177: 391–398, 2003. doi: 10.1046/j.1365-201X.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 17.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 18.Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, Mendelowitz D, Kay MW. Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc Res 113: 1318–1328, 2017. doi: 10.1093/cvr/cvx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Lu Z, He W, Wu L, Huang B, Yu L, Cui B, Hu X, Jiang H. Effects of low-intensity atrial ganglionated plexi stimulation on ventricular electrophysiology and arrhythmogenesis. Auton Neurosci 174: 54–60, 2013. doi: 10.1016/j.autneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.He B, Lu Z, Jiang H. Atrial ganglionated plexi stimulation may be an effective therapeutic tool for the treatment of heart failure. Med Hypotheses 81: 905–907, 2013. doi: 10.1016/j.mehy.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49: 27–37, 2001. doi: 10.1016/S0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 22.Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Horackova M, Armour JA. Role of peripheral autonomic neurones in maintaining adequate cardiac function. Cardiovasc Res 30: 326–335, 1995. doi: 10.1016/0008-6363(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 24.Klein HU, Ferrari GM. Vagus nerve stimulation: A new approach to reduce heart failure. Cardiol J 17: 638–644, 2010. [PubMed] [Google Scholar]
- 25.Kovach JA, Gottdiener JS, Verrier RL. Vagal modulation of epicardial coronary artery size in dogs. A two-dimensional intravascular ultrasound study. Circulation 92: 2291–2298, 1995. doi: 10.1161/01.CIR.92.8.2291. [DOI] [PubMed] [Google Scholar]
- 26.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res 29: 437–445, 1971. doi: 10.1161/01.RES.29.5.437. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 29.Martinez PF, Okoshi K, Zornoff LA, Oliveira SA Jr, Campos DH, Lima AR, Damatto RL, Cezar MD, Bonomo C, Guizoni DM, Padovani CR, Cicogna AC, Okoshi MP. Echocardiographic detection of congestive heart failure in postinfarction rats. J Appl Physiol (1985) 111: 543–551, 2011. doi: 10.1152/japplphysiol.01154.2010. [DOI] [PubMed] [Google Scholar]
- 30.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson KR Jr, Duscha BD, Hranitzky PM, Kraus WE. Chronic heart failure and exercise intolerance: the hemodynamic paradox. Curr Cardiol Rev 4: 92–100, 2008. doi: 10.2174/157340308784245757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98: 1510–1516, 1998. doi: 10.1161/01.CIR.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 33.Pickard JM, Burke N, Davidson SM, Yellon DM. Intrinsic cardiac ganglia and acetylcholine are important in the mechanism of ischaemic preconditioning. Basic Res Cardiol 112: 11, 2017. doi: 10.1007/s00395-017-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20: 808–816, 2014. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Reid JV, Ito BR, Huang AH, Buffington CW, Feigl EO. Parasympathetic control of transmural coronary blood flow in dogs. Am J Physiol 249: H337–H343, 1985. doi: 10.1152/ajpheart.1985.249.2.H337. [DOI] [PubMed] [Google Scholar]
- 36.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev 63: 291–315, 2011. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouleau JR, Simard D, Rodrigue N, Blouin A, Kingma JG Jr. Myocardial blood flow after chronic cardiac decentralization in anesthetized dogs: effects of ACE-inhibition. Auton Neurosci 97: 12–18, 2002. doi: 10.1016/S1566-0702(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6: e20360, 2011. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherlag BJ, Po S. The intrinsic cardiac nervous system and atrial fibrillation. Curr Opin Cardiol 21: 51–54, 2006. doi: 10.1097/01.hco.0000198980.40390.e4. [DOI] [PubMed] [Google Scholar]
- 40.Sjaastad I, Sejersted OM, Ilebekk A, Bjornerheim R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J Appl Physiol (1985) 89: 1445–1454, 2000. doi: 10.1152/jappl.2000.89.4.1445. [DOI] [PubMed] [Google Scholar]
- 41.Tu H, Liu J, Zhang D, Zheng H, Patel KP, Cornish KG, Wang WZ, Muelleman RL, Li YL. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am J Physiol Cell Physiol 306: C132–C142, 2014. doi: 10.1152/ajpcell.00223.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Vegte YJ, van der Harst P, Verweij N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J Am Heart Assoc 7: e008341, 2018. doi: 10.1161/JAHA.117.008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Li H, Yu L, Chen M, Wang Z, Huang B, Zhou L, Zhou X, Jiang H. Anti-arrhythmic effects of atrial ganglionated plexi stimulation is accompanied by preservation of connexin43 protein in ischemia-reperfusion canine model. Int J Clin Exp Med 8: 22098–22107, 2015. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Miller KE. Characterization of glutamatergic neurons in the rat atrial intrinsic cardiac ganglia that project to the cardiac ventricular wall. Neuroscience 329: 134–150, 2016. doi: 10.1016/j.neuroscience.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu SJ, Li YC, Shi ZW, Lin ZH, Rao ZH, Tai SC, Chu MP, Li L, Lin JF. Alteration of Cholinergic Anti-Inflammatory Pathway in Rat With Ischemic Cardiomyopathy-Modified Electrophysiological Function of Heart. J Am Heart Assoc 6: 6, 2017. doi: 10.1161/JAHA.117.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi F, Kawana K, Tanonaka K, Kamano I, Igarashi T, Gen E, Fujimoto Y, Maki T, Sanbe A, Nasa Y, Takeo S. Improvement of exercise capacity of rats with chronic heart failure by long-term treatment with trandolapril. Br J Pharmacol 126: 1585–1592, 1999. doi: 10.1038/sj.bjp.0702471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshinari K, Yaoita H, Maehara K, Maruyama Y. Different therapeutic responses to treadmill exercise of heart failure due to ischemia and infarction in rats. Cardiovasc Res 65: 457–468, 2005. doi: 10.1016/j.cardiores.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 48.Yuan BX, Ardell JL, Hopkins DA, Armour JA. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc Res 27: 760–769, 1993. doi: 10.1093/cvr/27.5.760. [DOI] [PubMed] [Google Scholar]
- 49.Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Castel MA, D’Onofrio A, Solomon SD, Wold N, Ruble SB. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J 36: 425–433, 2015. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao G, Shen W, Xu X, Ochoa M, Bernstein R, Hintze TH. Selective impairment of vagally mediated, nitric oxide-dependent coronary vasodilation in conscious dogs after pacing-induced heart failure. Circulation 91: 2655–2663, 1995. doi: 10.1161/01.CIR.91.10.2655. [DOI] [PubMed] [Google Scholar]