Abstract
Although cell therapy-mediated cardiac repair offers promise for treatment/management of heart failure, lack of fundamental understanding of how cell therapy works limits its translational potential. In particular, whether reparative cells from failing hearts differ from cells derived from nonfailing hearts remains unexplored. Here, we assessed differences between cardiac mesenchymal cells (CMC) derived from failing (HF) versus nonfailing (Sham) hearts and whether the source of donor cells (i.e., from HF vs. Sham) limits reparative capacity, particularly when administered late after infarction. To determine the impact of the donor source of CMCs, we characterized the transcriptional profile of CMCs isolated from sham (Sham-CMC) and failing (HF-CMC) hearts. RNA-seq analysis revealed unique transcriptional signatures in Sham-CMC and HF-CMC, suggesting that the donor source impacts CMC. To determine whether the donor source affects reparative potential, C57BL6/J female mice were subjected to 60 min of regional myocardial ischemia and then reperfused for 35 days. In a randomized, controlled, and blinded fashion, vehicle, HF-CMC, or Sham-CMC were injected into the lumen of the left ventricle at 35 days post-MI. An additional 5 weeks later, cardiac function was assessed by echocardiography, which indicated that delayed administration of Sham-CMC and HF-CMC attenuated ventricular dilation. We also determined whether Sham-CMC and HF-CMC treatments affected ventricular histopathology. Our data indicate that the donor source (nonfailing vs. failing hearts) affects certain aspects of CMC, and these insights may have implications for future studies. Our data indicate that delayed administration of CMC limits ventricular dilation and that the source of CMC may influence their reparative actions.
NEW & NOTEWORTHY Most preclinical studies have used only cells from healthy, nonfailing hearts. Whether donor condition (i.e., heart failure) impacts cells used for cell therapy is not known. We directly tested whether donor condition impacted the reparative effects of cardiac mesenchymal cells in a chronic model of myocardial infarction. Although cells from failing hearts differed in multiple aspects, they retained the potential to limit ventricular remodeling.
Keywords: cardiac repair, cell therapy, fibrosis, ventricular remodeling
INTRODUCTION
The inability of the infarcted heart to repair itself underlies its trajectory to failure. Despite initial promise to replace directly the lost contractile units, “stem cell” therapy has failed to deliver on direct myocardial regeneration. Yet multiple laboratories report that cell therapy can improve cardiac function without significant production of new myocytes (7, 18, 19, 24). Indeed, cell therapy attenuates ventricular remodeling and improves ventricular function, at least when administered soon after infarction (14, 27, 35). It remains unresolved, however, whether delayed administration of cell therapy could improve cardiac function and limit fibrosis. This question, along with other questions related to timing, dose, route, and, of course, which cell to use, has occupied the attention of numerous laboratories.
In addition to such questions, there are other questions that relate to eventual translation. If one presumes that the source of cells for cell therapy will be autologous, questions arise regarding potential limitations of the donor cells. That is, patient candidates for cell therapy will most likely have heart failure, but it is unclear whether cells harvested from failing hearts improve cardiac function. This limitation in the field has been fueled by the near-exclusive reliance on healthy donors in preclinical studies. Thus, the question of efficacy (or lack thereof) of heart failure-derived cardiac cell therapy remains unresolved.
One conspicuous unanswered question is how cell therapy works. One of the most consistent observations in cell therapy studies is the reduction in fibrosis. Given this common observation, we queried whether there were aspects of therapeutic cell biology that may confer at least part of the cells’ reparative capacity. We further queried whether differences in the donor source (i.e., those derived from failing hearts) had identifiable differences in broad characteristics.
Thus, we addressed several questions regarding the potential translational capacity of cell therapy. First, we examined whether donor conditions impact characteristics of CMCs. Second, we examined whether delayed administration (i.e., 1-mo post-MI) of cells would improve cardiac function. Third, we assessed whether heart failure-derived cells were inferior to normal heart-derived cells when administered late after MI. Our hypothesis was that heart failure-derived cells were inferior to normal heart-derived cells and that this reparative defect related to their altered metabolic remodeling profile.
METHODS
All animal procedures were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Isolation of Cardiac Mesenchymal Cells
Male, C57BL6/J mice (12–16 wk) were subjected to 60 min of ischemia and then reperfused for 28 days; male mice from the same cohort were subjected to sham surgery (i.e., thoracotomy but no myocardial infarction). These mice served as the donors for the heart failure-derived cardiac mesenchymal cells (HF-CMC) and the sham heart-derived cardiac mesenchymal cells (Sham-CMC), respectively. Slowly adherent CMCs were segregated based on their relatively late adherence to polystyrene culture plates, as we described previously (37). Here, cells from inbred C57BL6 mice were used. Hence, the experiments are effectively autologous from a genetic perspective.
RNA-Sequencing
Total RNA was isolated from Sham-CMC and HF-CMC, as previously described (5, 22, 34). RNA was quantified and assessed using NanoDrop ONEC (Thermo Scientific), and 10 μg of RNA was sent to Novogene Corporation, Inc., for RNA sequencing. For analysis, raw fastq files were trimmed with trimmomatic (version 0.36) by using the command line arguments “ILLUMINACLIP:all_fastq_primers.fa:2:30:10 LEADING:7 TRAILING:5 SLIDINGWINDOW:4:15 MINLEN:36” (1). Trimmed reads were aligned and assembled using Hisat2 (version 2.0.4) and Stringtie (version 1.3.4d) (15, 23). Differentially expressed genes were determined using Cuffdiff (version 2.2.1) (30). Finally, differentially expressed genes were deposited on the Gene expression Omnibus (GEO) with the accession number GSE144010. Heat maps were generated as previously described (10).
Real-Time PCR Gene Array
Total RNA was isolated using an RNA extraction kit according to the manufacturer’s instructions (Qiagen). RNA quality was determined using a spectrophotometer and reverse transcribed using a cDNA conversion kit. The cDNA was used on the real-time RT2 profiler PCR Array (no. PAMM-018Z; Qiagen) in combination with RT2 SYBR Green qPCR Mastermix (no. 330529; Qiagen). Fold change was calculated using ΔΔCt method, in which ΔCt was calculated between gene of interest (GOI) and an average of reference gene, β2-microglobulin, followed by ΔΔCt calculations (ΔCt [test group] − ΔCt [control group]).
Isolation of Extracellular Vesicles
Extracellular vesicles (EVs) derived from CMCs were isolated as previously described (20, 33). Briefly, Sham-CMC and HF-CMCs were maintained in growth media until they were 70–80% confluent. Next, growth media were removed, and CMCs were washed with PBS and suspended in conditioned media (Basal DMEM-F-12 media supplemented with 0.5% BSA) for 24 h. The next day, conditioned media were collected and centrifuged at 3,000 g for 15 min at 4°C to eliminate dead cells. Supernatant-containing EVs were precipitated with polyethylene glycol buffer [33.4% wt/vol PEG 4000, 50 mM HEPES (pH 7.4), 1 mM NaCl] and incubated overnight at 4°C. The resulting EV pellets were resuspended in 1× PBS and centrifuged at 100,000 g for 1 h at 4°C (Beckman Coulter Optima L-90 K ultracentrifuge). Finally, resulting pellets were resuspended in RIPA buffer for protein isolation or TRIzol for RNA extraction.
Myocardial Infarction
Adult female C57BL6/J mice were subjected to 60 min of regional myocardial ischemia and allowed to reperfuse for 35 days, as previously described (9, 13). Briefly, mice were anesthetized interperitoneally using 60 mg/kg of pentobarbital sodium. Following anesthesia, mice were ventilated, and the chest was opened through a midline sternotomy. Next, an 8-0 nylon suture was passed under left coronary artery ∼2 mm below the left auricle, and a nontraumatic balloon occluder was applied on the artery. Ischemia-reperfusion (I/R) was induced by tightening and inflating the occluder and then deflating and removing it. To minimize the impact of blood loss, blood from a donor mouse was given at serial times during surgery. Electrocardiogram and rectal temperature were carefully monitored throughout the experiment. Mice were then allowed to recover in a cage with oxygen in a temperature-controlled area. To the extent possible, the surgeon was blinded to treatment.
Cell Injections
At 35 days post-I/R procedure, slowly adherent CMCs pooled from three independent isolations (at passage 7) were injected into the lumen of the left ventricle via echocardiography-guided intracavitary injection, as previously described (9). Briefly, mice were anesthetized with isoflurane (3% for induction and 1.5% for maintenance). The left ventricle was scanned in the short-axis view from apex to base to determine the optimal site for needle insertion a site that avoided the infarct scar and major coronary vessels. Under the guidance of a real-time B-mode view, a 30-gauge injection needle attached to a 1.0-mL syringe was then carefully inserted percutaneously into the center of the left ventricular cavity; 106 cells in 200 μl of PBS or the same volume of PBS medium as vehicle control were delivered over 90-s intervals. After another 35 days of recovery, mice were subjected to a final echocardiogram. A total of 64 mice were originally enrolled for myocardial I/R procedure. Among enrolled mice, nine mice died after infarction, and five mice were excluded according to exclusion criteria (LVEF > 35% before treatment). The remaining mice were randomly assigned into three groups of 16 mice for the HF-CMC group, 18 mice for the Sham-CMC group, and 16 mice for vehicle group. Following cell injections, two mice died in cell-treated groups.
Echocardiography
Transthoracic echocardiography of the left ventricle was performed using the Vevo 2011 Imaging System (VisualSonics, Inc.), similar to previously described methods (2, 4, 8, 12, 25, 36). All echocardiography measurements were performed under isoflurane anesthesia (3% for induction and 1% for maintenance). Body temperature was carefully maintained at 37.0 ± 0.2°C throughout the study. The parasternal long-axis and parasternal short-axis views were used to obtain two-dimensional (2D) mode images for the measurement of LV mass, end-diastolic (LVEDV) and end-systolic LV volume (LVESV), stroke volume (SV), and ejection fraction (EF). At least three measurements were taken and averaged for each parameter. Digital images were analyzed by blinded observers using the Vevo 2100 workstation software. Measurements were performed according to the American Society for Echocardiography. Left ventricular ejection fraction (LVEF) was calculated by using the formula [(EDV-ESV)/EDV] × 100.
Hemodynamics Assessment
Two to five days after final echocardiography, mice were subjected to terminal pressure-volume loop assessments. Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg ip), intubated, and ventilated. Rectal temperature was kept at 37 ± 0.2°C. A 1.0-Fr pressure-volume (PV) catheter (PVR-1035; Millar Instruments) was inserted into the left ventricle via the right carotid artery. The position of the catheter was carefully adjusted until typical and stable PV loop signals were acquired. After 30 min of stabilization, the PV signals were recorded continuously with a MPVS ULTRA Pressure-Volume Unit (Millar Instruments). Inferior vena cava occlusion was performed with external compression to produce variably loaded beats for determination of the end-systolic PV relation and other derived constructs of LV performance. Parallel conductance from surrounding structures was calculated by a bolus injection of 5 µl of 30% NaCl through the jugular vein. Echocardiography-derived SV was used as outside reference in α-calibration for LV volume. All hemodynamic data analyses were performed offline, using LabChart 7.0 software, by an investigator blinded to the treatment.
Pathology
Following final echocardiography, hearts were excised and arrested in diastole with 2% KCl. Hearts were then sectioned into 4-µm sections for histology and immunofluorescence staining. A midventricular section was fixed with 10% formalin; the samples were subsequently embedded, cut, and mounted. Later, the slides were deparaffinized and rehydrated as needed for the appropriate stain, including HABP, trichrome, Isolectin B4, and CD45. For all analyses described below, the microscopist was blinded to group assignment.
Cardiomyocyte hypertrophy.
Cardiac sections were stained with wheat germ agglutinin (WGA; AlexaFluor 555 conjugate; Invitrogen) to identify cell borders and stained with DAPI to detect nuclei. WGA-stained cells were visualized using a Nikon TE-2000E2 microscope interfaced with a Nikon A1 confocal system. Cell areas were measured using Nikon Elements software [64-bit version 3.22.00 (Build 710)]. Cardiomyocytes where chosen based on their circularity and whether they had centrally located nuclei. Circularity was calculated using the shape factor feature in NIS-Elements AR 4.0. Cardiomyocytes were chosen based on a shape factor between 1.0 and 0.895 (radius ratio of 1:1 to 1:1.4).
Capillary density.
Cardiac sections were stained with isolectin B4 (fluorescein labeled Griffonia simplicifolia Lectin I; Vector Laboratories), as we have described previously (5). Capillary density was determined by dividing the total number of isolectin B4-positive vessels by the area of the image (no. of capillaries/mm2).
Cardiac fibrosis.
Cardiac sections were stained with Masson’s trichrome and picrosirius stain to determine fibrosis and total collagen content according to published protocols (28, 29, 37). Here, total sizes of the scar and the viable myocardium were expressed as percentage of the mass of the risk area with respect to the entire left ventricle. The risk area was defined as the transmural area between the furthest outer lateral edges of the scar, which was defined by Masson’s trichrome staining. Viable myocardium in the risk area was determined as the difference between risk and scar areas. Images were acquired digitally and areas measured using Nikon software (NIS-Elements AR Analysis 4.13.05 64-bit).
Hyaluronan-binding protein staining.
Hyaluronan levels were determined as previously described (32). Briefly, hyaluronan-binding protein (HABP) staining was performed using biotinylated hyaluronan binding protein (b-HABP) primary antibody (no. 385911; Sigma-Aldrich) and a streptavidin-conjugated Alexa Fluor 568 as secondary antibody (no. S11226; ThermoFisher). Sections were also stained with DAPI (no. D3571; ThermoFisher) to identify nuclei. Images and quantitation were performed using a fluorescent microscope BZ-X810 (Keyence).
Reverse Transcriptase PCR and Real-Time PCR
The total RNA from Sham-CMC and HF-CMC was extracted and used to make cDNA, as described previously (5, 22, 34). The relative levels of mRNA transcripts were quantified by real-time PCR using Power SYBR Green (Thermo Fisher Scientific) on a real-time PCR system (ABI 7900 HT; Applied Biosciences). Most primers were made using NCBI Primer Blast, except for hypoxanthine phosphoribosyltransferase (HPRT) primers (PPM03559E-200; Qiagen). The data were normalized to mouse HPRT mRNA threshold cycle (Ct) values by using the ΔΔCt comparative method.
Hyaluronan Isolation and Sizing
Hyaluronan was isolation, as previously described (21), with slight modifications. Briefly, 1 mL of 10× proteinase K (PK) (no. 25530015; Invitrogen) was added to 10 mL of conditioned media and incubated at 60°C for 4 h. Next, glycosaminoglycans were precipitated by adding four volumes of prechilled (−20°C) 200 proof ethanol and incubated at −20°C overnight. The next day (day 2), samples were centrifuged at 14,000 g for 20 min. Resulting pellets were washed by adding four volumes of prechilled (−20°C) 75% ethanol and centrifuged at 14,000 g for 20 min. Supernatants were discarded, and residual ethanol was removed by pipetting. Pellets were air-dried at room temperature for 20 min and dissolved by adding 200 μL of 100 mM ammonium acetate (pH 7.0), followed by boiling at 100°C for 5 min to inactivate PK. Samples were chilled on ice for 5 min. Nucleic acid digestion was performed by adding 4 μL of benzonase (no. 70664-3; EMD Millipore), and samples were incubated at 37°C overnight. The next day (day 3), samples were heated at 100°C for 5 min to inactivate benzonase, followed by a second ethanol precipitation using 1 mL of 200 proof ethanol and incubated at −20°C overnight. The next day (day 4), samples were centrifuged at 14,000 g for 10 min, and pellets were washed with 1 mL of cold 75% ethanol and centrifuged, as described earlier. Pellets were resuspended in 50 μL of 100 mM ammonium acetate (pH 7.0). Samples were divided into two parts (25 μL each). One part was treated with hyaluronidase (no. 360249; Santa Cruz Biotechnology) to serve as a negative control, and the other part was left untreated. Samples were incubated at 37°C overnight. The next day (day 5), enzymes were heat inactivated (as described earlier), and samples were lyophilized using an Eppendorf vacufuge concentrator (Concentrator plus, model 5305). Samples were resuspended in 10 M formamide (no. 11814320001; Sigma-Aldrich) and allowed to sit overnight at 4°C. The next day (day 6), samples were run on a 1% agarose gel (prerun for 6 h at 80 V) at 100 V for 2 h. Next, the gel was equilibrated in 30% ethanol for 1 h at room temperature (RT) before staining with Stains-All (no. E9379; Sigma-Aldrich) solution in the dark. On the next day (day 7), we decanted the Stains-All solution, and gels were equilibrated in water for 1 h and then imaged using a scanner (Epson Perfection V500 photo).
Statistical Analysis
Results are shown as means ± SD. Statistical analysis was conducted using a two-tailed Student’s t test to compare two groups or by one-way ANOVA followed by multiple-comparison test when appropriate. Differences in the outcomes by treatment groups and preinjection versus postinjection were tested using repeated-measures ANOVA. Interaction terms between groups and pre-/postinjection were included in models to determine whether pre-/postdifferences were modified by group type. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC) or GraphPad Prism version 8 (GraphPad Software, La Jolla, CA). Differences were considered statistically significant if P < 0.05.
RESULTS
Donor Condition Impacts Transcriptional Signature of Cardiac Mesenchymal Cells
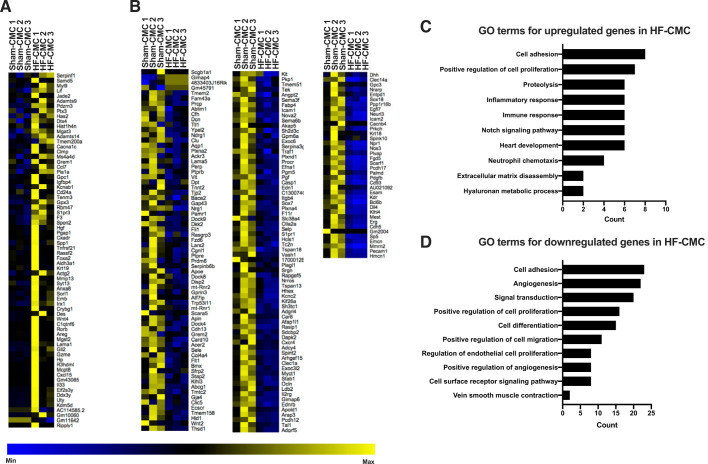
To determine whether donor condition impacts cardiac mesenchymal cells, we characterized transcriptional profiles of heart failure-derived cardiac mesenchymal cells (HF-CMC) compared with sham heart-derived cardiac cells (Sham-CMC) by unbiased RNA-seq analysis. Out of 53,461 Ensembl genes, 247 genes were differentially expressed at the threshold of FDR (false discovery rate)-adjusted P value of <0.05 (data available on Gene Expression Omnibus: GSE144010). Compared with Sham-CMCs, 70 genes were upregulated (Fig. 1A), and 177 genes were downregulated in HF-CMCs (Fig. 1B). Gene Ontology (GO) enrichment analysis of differentially expressed genes indicates that compared with Sham-CMCs, HF-CMCs upregulate genes involved in cellular response to cell adhesion, proliferation, proteolysis, inflammatory and immune response, Notch signaling, heart development, neutrophil chemotaxis, extracellular matrix disassembly, and hyaluronan metabolic process (Fig. 1C), among others (Supplemental Table S1; Supplemental Material for this article is available at https://doi.org/10.6084/m9.figshare.11862660), whereas genes involved in cell adhesion, angiogenesis, signal transduction, regulation of cell proliferation and differentiation, and endothelial cell migration were downregulated in HF-CMCs (Fig. 1D), among others (Supplemental Table S2). Taken together, these data suggest that donor conditions impact transcriptional signatures of CMCs.
Fig. 1.
Donor condition impacts transcriptional signature of cardiac mesenchymal cells (CMCs). Total RNA was isolated from CMCs derived from sham (Sham-CMC; n = 3) and heart failure mice (HF-CMC; n = 3) and was subjected to unbiased RNA-seq analysis to characterize global transcriptional changes. A: heat map analysis showing upregulated genes. B: heat map analysis showing downregulated genes. C: Gene Ontology (GO) enrichment analysis showing top upregulated genes. D: GO enrichment analysis showing top downregulated genes. A test statistic was used to determine significance of differentially expressed genes in Sham-CMC and HF-CMC groups. A Fisher’s exact test was used to determine significantly enriched GO terms.
Because one of the most consistent observations in cell therapy studies is reduction of fibrosis, we additionally assessed specific changes to fibrotic genes by real-time polymerase chain reaction gene arrays. Results show that a number of genes were significantly dysregulated in HF-CMC compared with Sham-CMC (Supplemental Table S3). HF-CMC upregulates fibrotic genes that promote recruitment of immune cells (Ccl12) to the site of inflammation and in extracellular matrix remodeling (Mmp8), whereas some downregulated genes are involved in anti-inflammation (Il10) and profibrotic (Il13) responses, among other genes (Supplemental Table S3).
To further characterize how heart failure-derived CMCs may differ from sham heart-derived CMCs, extracellular vesicles (EVs) were isolated and characterized. Compared with Sham-CMCs, we did not observe a significant difference in extracellular vesicle abundance (Supplemental Fig. S1A) or expression of EV-associated genes involved in various metabolic processes and extracellular matrix remodeling (Supplemental Fig. S1B); however, one technical limitation was the addition of BSA to the media used for our EV study. Because BSA may contain exogenous exosomes, the presence of any exogenous exosomes may impact the aforementioned observations. Collectively, these data suggest that although donor conditions impact transcriptional, fibrotic, and Toll-like receptor profiles of CMCs, extracellular vesicle abundance and expression of selected EV-associated genes are not different. Given these observations, we next sought to determine whether Sham-CMC and HF-CMCs differ in their reparative capacity.
Delayed Administration of Cardiac Mesenchymal Cells Limits Ventricular Dilation
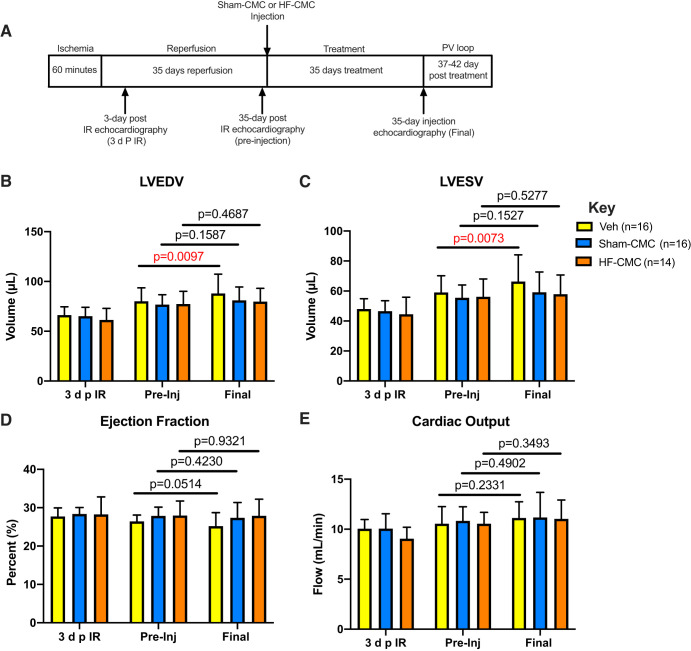
To determine whether delayed administration of CMCs is beneficial during heart failure, Sham-CMC and HF-CMC were isolated and transplanted into the ventricular lumen of I/R mice. Echocardiography analyses (Fig. 2) was performed at 3 days post-I/R, 35 days post-I/R (preinjection), and 35 days after injection (final). Following I/R, left ventricular end-diastolic (LVEDV) and systolic (LVESV) volumes increased from 3 days post-I/R to preinjection across all groups (vehicle-, Sham-CMC-, and HF-CMC-treated mice), indicating that cardiac dysfunction was similar across all groups before CMC transplantation (Fig. 2, B and C). For vehicle-treated hearts, LVEDV and LVESV were significantly increased from preinjection to final (Fig. 2, B and C). Interestingly, there was no significant increase in LVEDV and LVESV in Sham-CMC and HF-CMC treated hearts, indicating that delayed administration of either Sham- or HF-derived CMC limits ventricular dilation. Next, we looked at indices of cardiac function in vehicle-, Sham-CMC-, and HF-CMC-treated hearts. We did not observe significant changes in ejection fraction (EF) or cardiac output (CO) from preinjection to final for vehicle-, Sham-CMC-, and HF-CMC-treated hearts (Fig. 2, D and E). Furthermore, no significant differences were observed in stroke volume (SV) or left ventricular diastolic (LVA;D) and systolic (LVA;S) areas (Table 1).
Fig. 2.
Delayed administration of cardiac mesenchymal cells limits ventricular dilation. Female C57BL6/J mice subjected to 60 min of regional myocardial ischemia and allowed to reperfuse for 35 days were treated with either saline (vehicle; n = 16) or cardiac mesenchymal cells (CMCs) isolated from sham (Sham-CMC; n = 16) and heart failure (HF-CMC; n = 14) mice. On day 35, treated mice were subjected to final echocardiography. A: schematic illustrating timeline for ischemia-reperfusion (I/R) procedure, CMC injection, and echocardiography time points [at 3 days post-I/R (3 d p I/R), 35 days post-I/R (preinjection), and 35 days postinjection (final)]. B: left ventricular end diastolic volume (LVEDV) from preinjection to final for hearts treated with vehicle, Sham-CMC, and HF-CMC. C: left ventricular end-systolic volume (LVESV) from preinjection (pre-Inj) to final for hearts treated with vehicle, Sham-CMC, and HF-CMC. D: ejection fraction (EF) from pre-Inj to final for hearts treated with vehicle, Sham-CMC, and HF-CMC. E: cardiac output (CO) from pre-Inj to final for hearts treated with vehicle, Sham-CMC, and HF-CMC. Data are represented as means ± SD. Differences in the outcomes by treatment groups and pre-Inj vs. postinjection were tested using repeated-measures ANOVA. Interaction terms between groups and pre-/postinjection were included in models to determine whether pre-/postdifferences were modified by group type.
Table 1.
Echocardiography data from I/R mice treated with either saline or CMCs isolated from sham and heart failure
| 3 days post-I/R |
Preinjection |
Final |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Sham-CMC | HF-CMC | Vehicle | Sham-CMC | HF-CMC | Vehicle | Sham-CMC | HF-CMC | |
| HR (beats/min) | 552 ± 31 | 548 ± 35 | 532 ± 28 | 502 ± 39 | 510 ± 27 | 498 ± 42 | 514 ± 29 | 488 ± 41 | 505 ± 43 |
| LVA;D, mm2 | 24.2 ± 2.1 | 23.9 ± 2.0 | 23.2 ± 2.5 | 26.6 ± 2.4 | 26.2 ± 1.9 | 26.6 ± 2.6 | 27.9 ± 3.3 | 26.8 ± 2.5 | 26.8 ± 2.5 |
| LVA;S, mm2 | 19.2 ± 1.7 | 18.9 ± 1.8 | 18.5 ± 2.6 | 21.4 ± 2.2 | 20.9 ± 1.8 | 21.2 ± 2.2 | 22.9 ± 3.5 | 21.5 ± 2.7 | 21.5 ± 2.5 |
| SV, µL | 18.2 ± 1.9 | 18.3 ± 2.3 | 16.9 ± 2.0 | 21.0 ± 2.7 | 21.2 ± 2.2 | 21.3 ± 2.3 | 21.6 ± 2.5 | 21.6 ± 1.7 | 21.8 ± 2.7 |
Values are means ± SD; n = 16 saline (vehicle), n = 16 cardiac mesenchymal cells (CMCs) isolated from sham, and n = 14 heart failure (HF)-CMC mice. I/R, ischemia-reperfusion; final, 35 days after injection; HF-CMC, or heart failure-derived cardiac mesenchymal cells; Sham-CMC, heart-derived cardiac mesenchymal cells. No significant difference was observed in various end points related to cardiac function. Outcomes by treatment groups, and preinjection vs. postinjection were tested using repeated-measures ANOVA.
Two to five days after the final echocardiography, (i.e., 37–42 days postinjection), hemodynamic parameters were assessed by pressure-volume loop. Compared with vehicle control, only Sham-CMC improved indices of cardiac function such as EF, maximum (Vmax) and minimum (Vmin) volumes, PowMax, and end-systolic elastance (Ees). HF-CMC cells failed to improve cardiac function (Table 2). Taken together, these data suggest that delayed administration of CMCs limits ventricular dilations without improving left ventricular function, as suggested by our echocardiographic data. Nevertheless, our pressure-volume loop assessment suggest that only CMCs derived from nonfailing heart (i.e., Sham-CMC) improved cardiac function in a model of delayed therapy compared with vehicle control.
Table 2.
Hemodynamic data from mice treated with either saline or CMCs isolated from sham or heart failure
| Vehicle | Sham-CMC | HF-CMC | |
|---|---|---|---|
| SW, mmHg·µL | 1,133 ± 149 | 1,277 ± 181 | 1,184 ± 282 |
| SV, µL | 20.7 ± 2.6 | 21.9 ± 2.1 | 20.9 ± 2.3 |
| Vmax, µL | 93.3 ± 15.9 | 80.7 ± 12.1* | 83.3 ± 13.5 |
| Vmin, µL | 72.6 ± 14.6 | 58.8 ± 11.8* | 62.4 ± 14.0 |
| EF, % | 23.3 ± 3.2 | 28.8 ± 4.5* | 26.7 ± 5.1 |
| Ea, mmHg/µL | 4.17 ± 0.8 | 4.04 ± 0.5 | 4.05 ± 0.6 |
| PowMax, mmHg/s | 23,153 ± 7,958 | 35,338 ± 11,301* | 26,001 ± 13,149 |
| dP/dtmax, mmHg/s | 4,472 ± 807 | 5,083 ± 804 | 5,051 ± 1,029 |
| dP/dtmin, mmHg/s | −4,641 ± 679 | −5,115 ± 874 | −5,070 ± 871 |
| P at dP/dtmax, mmHg | 53.4 ± 5.4 | 57.1 ± 3.2 | 54.8 ± 8.8 |
| τ, ms | 11.0 ± 1.3 | 10.3 ± 1.5 | 10.8 ± 1.9 |
| Ees, mmHg/µL | 2.0 ± 0.5 | 2.8 ± 1.0* | 2.5 ± 0.6 |
| Wet lung wt, mg | 183.9 ± 21.6 | 179.4 ± 19.6 | 176.0 ± 11.8 |
Values are means ± SD; n = 16 saline (vehicle), n = 16 cardiac mesenchymal cells (CMCs) isolated from sham, and n = 13 heart failure (HF)-CMC mice. Ea, arterial elastance; Ees, end-systolic elastance; EF, ejection fraction; HF-CMC, heart failure-derived cardiac mesenchymal cells; Sham-CMC, sham heart-derived cardiac mesenchymal cells; SV, stroke volume; SW, stroke work; Vmax, maximum volume; Vmin, minimum volume. Mice were treated at 35 days following ischemia-reperfusion. One-way ANOVA followed by Sidak’s multiple-comparison test was used to determine significant differences between Sham-CMC- or HF-CMC-treated hearts compared with vehicle.
P < 0.05 compared with vehicle.
Donor Condition May Impact Cardiac Mesenchymal Cells
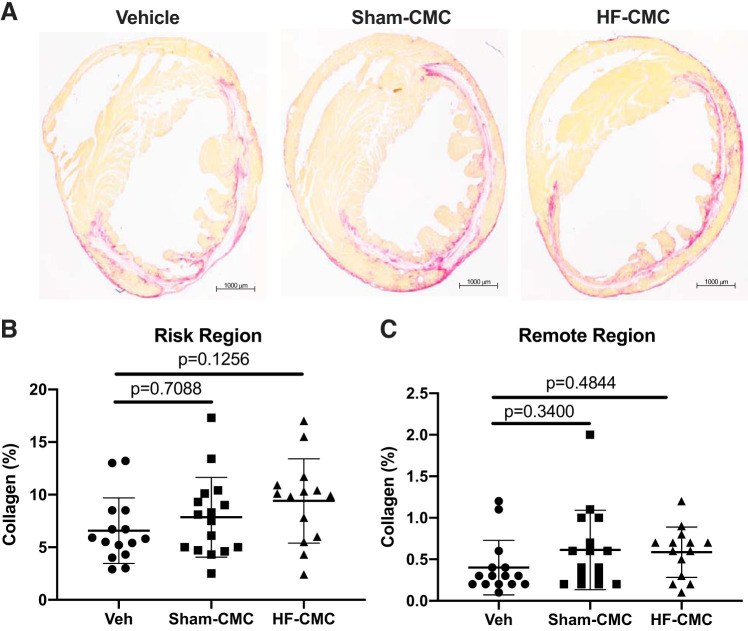
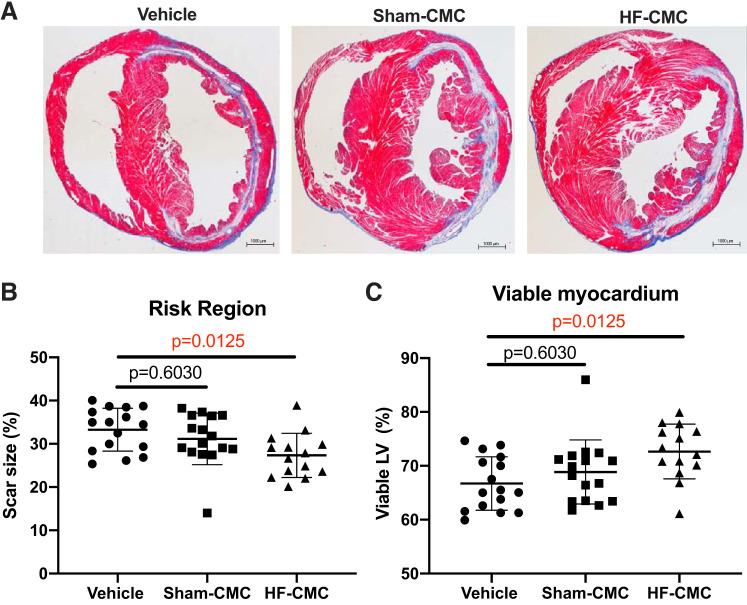
To determine whether donor conditions influence the reparative capacity of CMCs, we assessed collagen content and fibrosis in CMC-treated hearts by picrosirius and trichrome staining. Compared with vehicle control, we did not observe any significant change in total collagen in the risk and remote region of hearts treated with either HF-CMC or Sham-CMCs (Fig. 3, A–C). Interestingly, HF-CMC-treated hearts showed significantly reduced scar size in the risk region of the heart (Fig. 4, A and B) and limited loss of viable myocardium compared with vehicle-treated hearts (Fig. 4, A and C). Scar size and viable myocardium were not changed in Sham-CMC-treated hearts compared with vehicle-treated hearts (Fig. 4, A–C), suggesting that donor conditions may impact the ability of CMCs to regulate scar size.
Fig. 3.
Collagen content is not changed in cardiac mesenchymal cell (CMC)-treated hearts. Sections from saline [vehicle (Veh); n = 15]-, sham (Sham-CMC; n = 16)-, and heart failure (HF-CMC; n = 14)-treated hearts were stained with picrosirius red to determine total collagen content. A: representative images of Veh-, Sham-CMC-, and HF-CMC-treated hearts. B: quantification of total collagen content in the risk region of Veh, Sham-CMC, and HF-CMC treated hearts. C: quantification of total collagen content in the remote region of Veh-, Sham-CMC-, and HF-CMC-treated hearts. Data are represented as means ± SD. One-way ANOVA followed by Sidak’s multiple-comparison test was used to determine significant differences between Sham-CMC- or HF-CMC-treated hearts compared with vehicle.
Fig. 4.
Heart failure-derived cardiac mesenchymal cells (HF-CMC) significantly reduced scar size and limited loss of viable myocardium. Sections from saline (vehicle; n = 16)-, sham (Sham-CMC; n = 16)-, and heart failure (HF-CMC; n = 14)-treated hearts were stained with Masson’s trichrome to determine scar size. A: representative images of vehicle, Sham-CMC, and HF-CMC treated hearts. B: quantification of scar size in vehicle-, Sham-CMC-, and HF-CMC-treated hearts. C: quantification of viable myocardium in vehicle, Sham-CMC, and HF-CMC treated hearts. Data are represented as means ± SD. One-way ANOVA followed by Sidak’s multiple-comparison test was used to determine significant differences between Sham-CMC- or HF-CMC-treated hearts compared with vehicle.
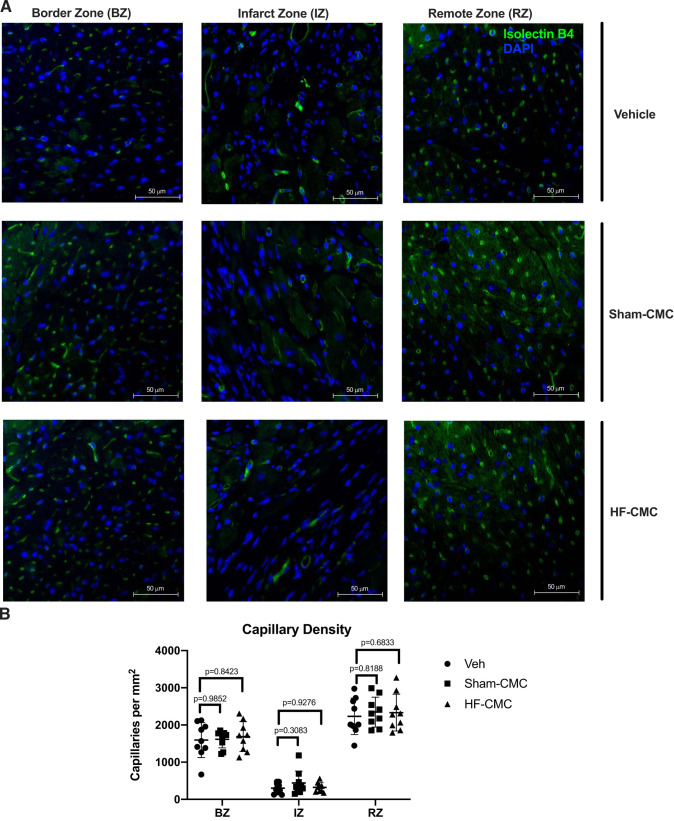
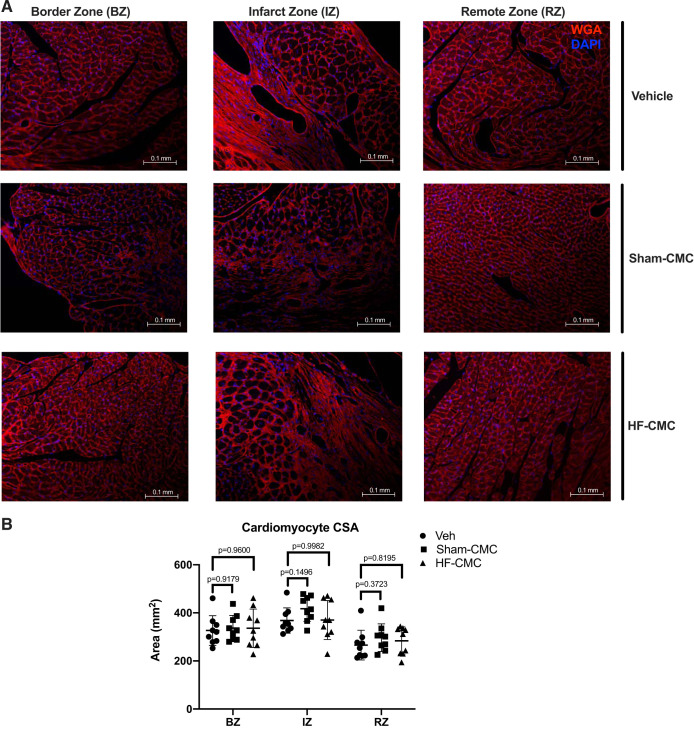
We next assessed the impact of donor conditions on the ability of CMCs to regulate angiogenesis (as a measure of capillary density) and cardiac hypertrophy by isolectin B4 and wheat germ agglutinin staining. We did not observe any significant difference in capillary density in the border zone (BZ), infarct zone (IZ), or remote zone (RZ) of hearts treated with Sham-CMC or HF-CMCs compared with vehicle-treated hearts (Fig. 5, A and B). Similarly, we did not observe any significant difference in myocyte cross-sectional area (MSA) in border the zone (BZ), infarct zone (IZ), or remote zone (RZ) of hearts treated with Sham-CMC or HF-CMCs compared with vehicle treated hearts (Fig. 6, A and B), suggesting that donor conditions may not impact the ability of CMCs to regulate cardiac angiogenesis and cardiac hypertrophy.
Fig. 5.
Capillary density is not changed in sham heart-derived cardiac mesenchymal cell (Sham-CMC)- or heart failure-derived cardiac mesenchymal cell (HF-CMC)-treated hearts. Sections from saline (vehicle; n = 9)-, sham (Sham-CMC; n = 9)-, and heart failure (HF-CMC; n = 9)-treated hearts were stained with isolectin B4 to assess capillary density. A: representative images of isolectin B4-stained vehicle-, Sham-CMC-, and HF-CMC-treated hearts. B: quantification of capillary density (green) in the border zone (BZ), infarct zone (IZ), and remote zone (RZ) of treated hearts. Data are represented as means ± SD. Two-way ANOVA followed by Dunnett’s multiple-comparison test was used to determine significant differences between Sham-CMC- or HF-CMC-treated hearts compared with vehicle in the BZ, IZ, and RZ.
Fig. 6.
Cardiomyocyte size is not changed in sham heart-derived cardiac mesenchymal cell (Sham-CMC)- or heart failure-derived cardiac mesenchymal cell (HF-CMC)-treated hearts. Sections from saline (vehicle; n = 9)-, sham (Sham-CMC; n = 9)-, and heart failure (HF-CMC; n = 9)-treated hearts were stained with wheat germ agglutinin (WGA) to assess cardiomyocyte size. A: representative images of WGA-stained vehicle, Sham-CMC, and HF-CMC treated hearts. B: quantification of cardiomyocyte size in the border zone (BZ), infarct zone (IZ), and remote zone (RZ) of treated hearts. Data are represented as means ± SD. Two-way ANOVA followed by Dunnett’s multiple-comparison test was used to determine significant differences between Sham-CMC or HF-CMC treated hearts compared with vehicle in the BZ, IZ, and RZ.
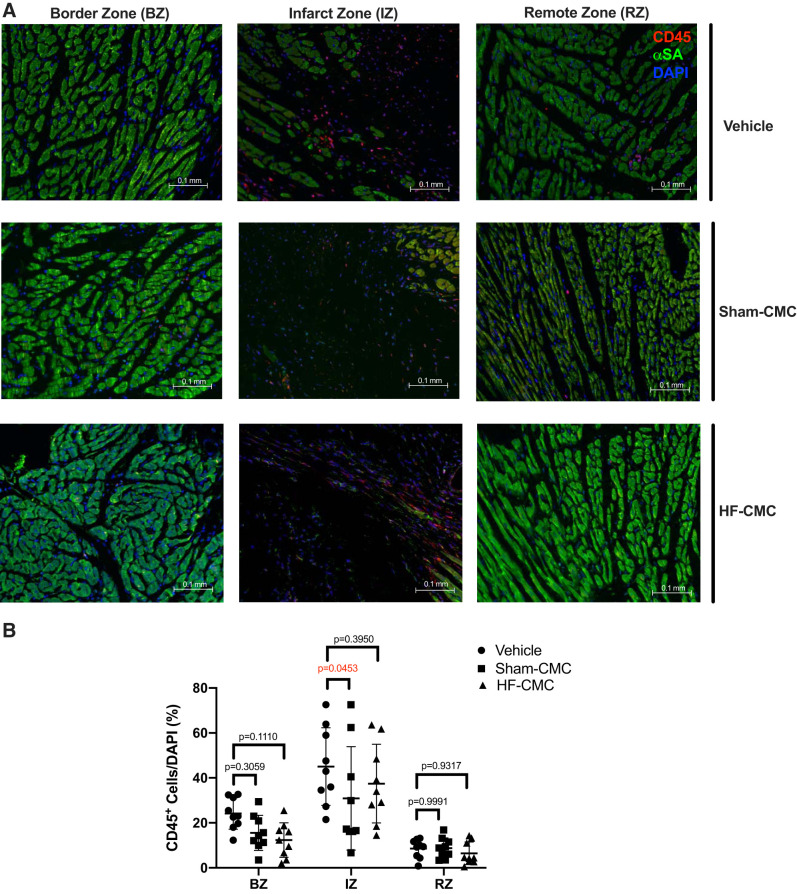
Because immune cells play an important role in development of fibrosis and postinfarct wound healing, we assessed the impact of donor conditions on the ability of CMCs to regulate immune cells by CD45 antigen staining. Compared with vehicle-treated hearts, Sham-CMC treatment significantly reduced CD45-positive cells only in the IZ of the heart (Fig. 7, A and B). There was no reduction of CD45-positive cells in the IZ of HF-CMC treated hearts compared with vehicle-treated hearts. We did not observe any significant difference in the CD45-positive cells in the other regions (RZ and BZ) of Sham-CMC- and HF-CMC-treated hearts compared with vehicle-treated hearts (Fig. 7, A and B). Taken together, these data suggest that although donor conditions may not impact the ability of CMCs to regulated collagen content, angiogenesis, and hypertrophy in treated hearts, donor conditions may impact the ability of CMCs to regulate scar size and immune cell abundance in treated hearts.
Fig. 7.
Administration of heart-derived cardiac mesenchymal cells (Sham-CMCs) reduced immune cell abundance in the infarct zone of the heart. Sections from saline (vehicle; n = 9)-, sham (Sham-CMC; n = 9)-, and heart failure (HF-CMC; n = 9)-treated hearts were stained with CD45 antigen to assess presence of leukocytes. A: representative images of vehicle-, Sham-CMC-, and HF-CMC-treated hearts. B: quantification of CD45 (red) cells in the border zone (BZ), infarct zone (IZ), and remote zone (RZ) of treated hearts. Data are represented as means ± SD. Two-way ANOVA followed by Tukey’s multiple-comparison test was used to determine significant differences between Sham-CMC- or HF-CMC-treated hearts compared with vehicle in the BZ, IZ, and RZ.
Donor Condition Impacts Hyaluronan Metabolism in Cardiac Mesenchymal Cells
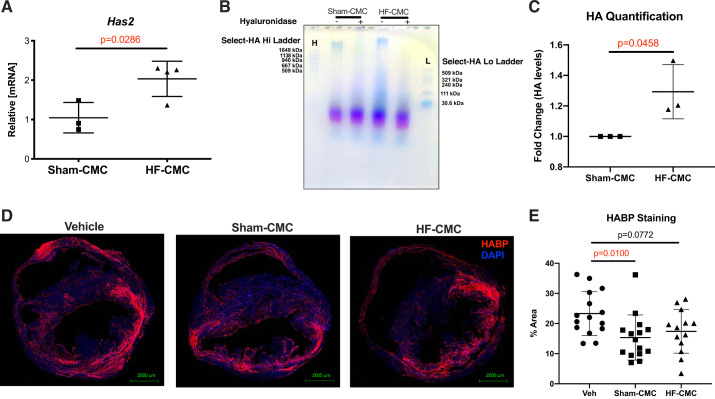
To investigate possible mechanisms by which donor conditions may impact the ability of CMCs to regulate scare size and immune cell abundance in treated hearts, we explored our RNA-seq data to identify differential changes in genes regulating formation of scar and immune cell infiltration in Sham-CMC compared with HF-CMC. Among various changes, some hyaluronan metabolic genes were differentially regulated in Sham-CMC compared with HF-CMC. Hyaluronan metabolism has been implicated in the formation of scar, and its levels correlate with infiltration of immune cells in various disease models (16, 26); to explore the possibility that changes in hyaluronan (HA) metabolism may be associated with reduced levels of immune cells in Sham-CMC treated hearts, we examined our RNA-seq data for expression HA-synthesizing enzymes Has1, Has2, and Has3. Interestingly, GO enrichment analysis from RNA-seq analysis indicated that HF-CMC significantly upregulates genes for the hyaluronan metabolic process (Fig. 1C). Furthermore, Has2 was significantly upregulated in HF-CMCs compared with Sham-CMCs (Fig. 1A); this was confirmed by qPCR analysis (Fig. 8A). Consistent with increased expression of Has2, HA sizing signal was significantly higher in HF-CMC compared with Sham-CMC (Fig. 8, B and C). Other isoforms of HA synthase (Has1 and Has3) were unchanged (Supplemental Fig. S2A).
Fig. 8.
Administration of heart-derived cardiac mesenchymal cells (Sham-CMCs) reduced hyaluronan (HA) levels in treated hearts. HA metabolism was characterized in CMCs derived from sham (Sham-CMC) and heart failure (HF-CMC) mice by quantitative PCR, agarose gel electrophoresis, and fluorescent microscopy. A: expression of Has2 mRNA in Sham-CMC (n = 3) and HF-CMC (n = 4). B: HA sizing by agarose gel from conditioned media of Sham-CMC (n = 3) and HF-CMC (n = 3). C: quantification of HA sizing by agarose gel from conditioned media of Sham-CMC (n = 3) and HF-CMC (n = 3). D: hyaluronan levels according to hyaluronan-binding protein (HABP; red) staining in vehicle (n = 15)-, Sham-CMC (n = 15)-, and HF-CMC (n = 13)-treated hearts. E: quantification of hyaluronan levels according to HABP (red) staining in vehicle (n = 15)-, Sham-CMC (n = 15)-, and HF-CMC (n = 13)-treated hearts. Data are represented as means ± SD. For 2 groups, an unpaired 2-tailed Student’s t test was used to determine significance between Sham-CMC and HF-CMC. For 3 groups, a 1-way ANOVA followed by Sidak’s multiple-comparison test was used to determine significance between Sham-CMC- or HF-CMC-treated hearts compared with vehicle.
We next examined whether overall abundance of HA was affected in CMC-treated hearts. Compared with vehicle control hearts, HA levels were significantly reduced in hearts treated with Sham-CMCs but not HF-CMCs (Fig. 8, D and E). Because HA catabolism can be regulated by expression of HA-degrading enzymes (38), we assessed our RNA-seq data for expression of the transmembrane hyaluronidase Tmem2. Compared with Sham-CMC, Tmem2 was significantly downregulated in HF-CMCs (Fig. 1B), suggesting that unlike Sham-CMC, HF-CMC may have reduced ability to break down hyaluronan. Next, we assessed whether donor conditions impact hyaluronidase activity of CMCs. We did not observe a significant difference in hyaluronidase activity in Sham-CMC compared with HF-CMC (Supplemental Fig. S2B). Finally, we did not observe significant differences in expression of lysosomal hyaluronidases Hyal1–3 (Supplemental Fig. S2C) and HA receptors (Cd44 and Hmmr) in Sham-CMC compared with HF-CMC by (Supplemental Fig. S2D). Collectively, these data suggest that donor conditions impact CMC ability to synthesis HA but minimally affect HA catabolism.
DISCUSSION
Although cell therapy-mediated cardiac repair offers promise for treatment/management of heart failure (HF), a lack of fundamental understanding of how cell therapy works limits its translational potential. In particular, questions pertaining to sources of cells, whether autologous cells will be used and whether these cells are effective at improving cardiac function, remain elusive. We attempted to answer these questions in the present study. First, we examined whether donor conditions impact characteristics of CMC. Second, we examined whether delayed administration (i.e., 1 mo post-MI) of cells would improve cardiac function. Third, we assessed whether heart failure-derived cells were inferior to normal heart-derived cells in effecting ventricular remodeling when administered late after MI. Our hypothesis was that heart failure-derived cells were inferior to normal heart-derived cells and that this reparative defect related to their altered metabolic remodeling profile.
Previous studies suggest that CMC possesses reparative properties (36, 37). Because most potential patients for cell therapy would have heart failure, we reasoned that it would be important to understand how HF affects CMC. First, we sought to identify the impact of donor conditions on CMC. We showed that CMCs derived from failing hearts (HF-CMC) have distinct transcriptional profiles compared with CMC isolated from a nonfailing (Sham-CMC) heart, as indicated by RNA-seq analysis. Furthermore, GO enrichment analysis of differentially expressed genes suggests that transcriptional changes induced by donor conditions may differentially alter various cellular and metabolic processes in CMC. Collectively, our results suggest that donor conditions impact transcriptional signatures of CMC and may affect functional processes of cardiac mesenchymal cells.
Next, we tested whether changes induced by donor conditions on CMCs affect their reparative capacity when administered late after MI. Our results showed that delayed administration of CMCs limits ventricular dilation without improving left ventricular function. Nevertheless, our pressure-volume loop assessment suggests that only CMCs derived from nonfailing heart (i.e., Sham-CMC) improved cardiac function in a model of delayed therapy. Hence, it is possible that the reparative actions of CMC may be partially impaired in HF-CMC.
We also tested whether donor conditions influence the ability of CMCs to attenuate ventricular remodeling. We showed that, although administering CMC (HF- or Sham-CMC) late after infarction does not significantly affect collagen deposition and hypertrophy, HF-CMC and Sham-CMC differ in their ability to regulate scar size and immune cell abundance in the infarct zone of treated hearts. Because immune cells play a central role in scar formation, we investigated potential mechanisms underlying the differential regulation immune cell and scar size by HF-CMC and Sham-CMC by exploring our RNA-seq data. Among other processes, we found alterations in hyaluronan metabolism in HF-CMC compared with Sham-CMC.
Hyaluronan is one of the most abundant components of the extracellular matrix (ECM). Hyaluronan is a glycosaminoglycan made of repeating units of N-acetylglucosamine and glucuronic acid. Studies have shown that, in other disease models, hyaluronan levels correlates with immune cell infiltration and can influence inflammation (11, 16, 26). We assessed the possibility that changes in HA metabolism conferred by donor conditions in CMC may coincide with immune cell abundance in Sham-CMC treated hearts. We showed that Has2 expression and HA levels were significantly increased in HF-CMC compared with Sham-CMC, suggesting that HF-CMC may promote more HA accumulation, at least when compared with Sham-CMC or vehicle. We also observed signs of reduced hyaluronan degradation in HF-CMC, as indicated by a significant reduction of Tmem2 (hyaluronidase) expression. Consistent with these data, only Sham-CMC significantly reduced HA levels in the heart. Interestingly, this coincides with significantly reduced CD45-positive cells observed in Sham-CMC treated hearts. Previous studies have shown that stimulation of hyaluronan synthesis may contribute to suppression of scar tissue formation (6). Presently, we can only speculate on such an association in our model.
Although we show that donor source affects certain aspects of CMC, these differences do not completely prevent the major salutary effects of CMC treatment. It is possible that donor source is indeed not relevant. That is, any cell delivered to the heart may confer beneficial reparative impact. One explanation could be transient stimulation of acute inflammation associated with wound healing. The therapeutic administration of cells may simply elicit a salutary, proadaptive immune response, as suggested by Vagnozzi et al. (31). Hence, one could speculate that the lack of differences (between CMC-treated groups) could be because cell therapy may in part work through an unintended yet beneficial impact on the immune response in the post-MI heart. It is, however, important to realize that the model of delayed administration reported in our present study differs from that used by Vagnozzi et al. (31). In the Vagnozzi et al. (31) report, the authors administered cells 1 wk after MI; here, we administered cells 1 mo after MI. This significant difference could potentially impact the speculative innate/immune response mechanism we mentioned above.
Furthermore, although previous studies utilizing human cardiosphere-derived cells (CDCs) for cell therapy showed that CDCs isolated from diseased human hearts elicited greater reparative benefits compared with that of healthy controls (3), there are several fundamental differences between our study design and that of Cheng et al. (3) that could possibly explain the difference in outcome between these studies. Here, we used a model of reperfused myocardial infarction, whereas, Cheng et al. (3) utilized a permanent ligation model. Also, we used a delayed model of injection, i.e., where cells were injected well after the initial infarct. Conversely, Cheng et al. (3) utilized an early model where cells were injected immediately after MI. Furthermore, we utilized cardiac mesenchymal cells (CMCs) from mice in our study, whereas human CDCs were used in the study by Cheng et al. (3). Finally, Cheng et al. (3) found that some donor cells were more effective than others. This is not too surprising because some donors may have more severe disease than others. The above reasons could explain differences between our findings and that of Cheng et al. (3). There are several elements of our study design that are worth noting. In terms of rigor and reproducibility, this study was planned a priori and carefully controlled. Although it is possible that the surgeons could have been able to discriminate between vehicle and cell (though not which cell), the sonographer was blinded to the identity of the treatment group. Such blinding was made possible by using a cell preparation core (MW laboratory) to prepare the cells in a blinded manner. Exclusions were made only for animals that did not meet the “<50% EF on day 2” requirement, and such exclusions were done without knowledge of group assignment.
In summary, CMCs derived from failing hearts have different transcriptional profiles and functional elements; however, these differences may not completely prevent the major salutary effects of CMCs when administered in a model of delayed therapy. We found that donor conditions impact hyaluronan metabolism in CMCs. Because hyaluronan accumulates in the failing heart (17), we believe that ability of CMCs to metabolize HA and whether that significantly contributes to their reparative potential in other models of HF (i.e., acute HF) is an interesting observation that needs to be further tested as a potential mechanism.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-147844, R01-HL-131647, P30-GM-103492127607, and P01-HL-078825 (to S.P.J.) and R01-HL-141191 and P01-HL-078825 (to M.W.) and American Heart Association Grant 19PRE34380003 (to T.A.).
DISCLOSURES
M.W. has pending intellectual property related to cell therapy. None of the other authors have any relevant conflicts to declare.
AUTHOR CONTRIBUTIONS
M.W. and S.P.J. conceived and designed research; T.N.A., Y.N., A.T., A.J., H.L., X.Z., B.W.L., Y.W.Z., K.R.B., A.M.G., Y.G., and M.W. performed experiments; T.N.A., Y.N., A.T., A.J., H.L., X.Z., B.W.L., Y.W.Z., T.W., K.R.B., D.W.R., A.M.G., S.U., and Y.G. analyzed data; T.N.A. and S.P.J. interpreted results of experiments; T.N.A. prepared figures; T.N.A. drafted manuscript; T.N.A. and S.P.J. edited and revised manuscript; S.P.J. approved final version of manuscript.
REFERENCES
- 1.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120, 2014. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One 8: e83174, 2013. [Erratum in: PLoS One 9: e113944, 2014.] 10.1371/journal.pone.0083174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, Xie Y, Ibrahim A, Aminzadeh MA, Liu W, Li TS, De Robertis MA, Marbán L, Czer LS, Trento A, Marbán E. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail 2: 49–61, 2014. doi: 10.1016/j.jchf.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condorelli G, Roncarati R, Ross J Jr, Pisani A, Stassi G, Todaro M, Trocha S, Drusco A, Gu Y, Russo MA, Frati G, Jones SP, Lefer DJ, Napoli C, Croce CM. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA 98: 9977–9982, 2001. doi: 10.1073/pnas.161120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dassanayaka S, Brainard RE, Watson LJ, Long BW, Brittian KR, DeMartino AM, Aird AL, Gumpert AM, Audam TN, Kilfoil PJ, Muthusamy S, Hamid T, Prabhu SD, Jones SP. Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy. Basic Res Cardiol 112: 23, 2017. doi: 10.1007/s00395-017-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edward M, Quinn JA, Sands W. Keratinocytes stimulate fibroblast hyaluronan synthesis through the release of stratifin: a possible role in the suppression of scar tissue formation. Wound Repair Regen 19: 379–386, 2011. doi: 10.1111/j.1524-475X.2011.00678.x. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer JJ, Kakkar AK, Elrod JW, Watson LJ, Jones SP, Lefer DJ. Low-dose simvastatin improves survival and ventricular function via eNOS in congestive heart failure. Am J Physiol Heart Circ Physiol 291: H2743–H2751, 2006. doi: 10.1152/ajpheart.00347.2006. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 112: 18, 2017. doi: 10.1007/s00395-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe EA, Sinha R, Schlauch D, Quackenbush J. RNA-Seq analysis in MeV. Bioinformatics 27: 3209–3210, 2011. doi: 10.1093/bioinformatics/btr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson P, Arif AA, Lee-Sayer SS, Dong Y. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front Immunol 9: 2787, 2018. doi: 10.3389/fimmu.2018.02787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci USA 100: 4891–4896, 2003. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 116: 572–586, 2015. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 363: 751–756, 2004. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360, 2015. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev 97: 186–203, 2016. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorén CE, Dahl CP, Do L, Almaas VM, Geiran OR, Mörner S, Hellman U. Low Molecular Mass Myocardial Hyaluronan in Human Hypertrophic Cardiomyopathy. Cells 8: 97, 2019. doi: 10.3390/cells8020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martire A, Bedada FB, Uchida S, Pöling J, Krüger M, Warnecke H, Richter M, Kubin T, Herold S, Braun T. Mesenchymal stem cells attenuate inflammatory processes in the heart and lung via inhibition of TNF signaling. Basic Res Cardiol 111: 54, 2016. doi: 10.1007/s00395-016-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieu E, Lamirault G, Toquet C, Lhommet P, Rederstorff E, Sourice S, Biteau K, Hulin P, Forest V, Weiss P, Guicheux J, Lemarchand P. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PLoS One 7: e51991, 2012. doi: 10.1371/journal.pone.0051991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra P, Guo Y, Nong Y, Lorkiewicz P, Nasr M, Li Q, Muthusamy S, Bradley JA, Bhatnagar A, Wysoczynski M, Bolli R, Hill BG. Cardiac mesenchymal cells from diabetic mice are ineffective for cell therapy-mediated myocardial repair. Basic Res Cardiol 113: 46, 2018. doi: 10.1007/s00395-018-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midura RJ, Cali V, Lauer ME, Calabro A, Hascall VC. Quantification of hyaluronan (HA) using a simplified fluorophore-assisted carbohydrate electrophoresis (FACE) procedure. Methods Cell Biol 143: 297–316, 2018. doi: 10.1016/bs.mcb.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Muthusamy S, DeMartino AM, Watson LJ, Brittian KR, Zafir A, Dassanayaka S, Hong KU, Jones SP. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J Biol Chem 289: 29665–29676, 2014. doi: 10.1074/jbc.M114.578682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33: 290–295, 2015. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113: 810–834, 2013. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7: 634–642, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol 23: 475–484, 2000. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 27.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106: 1913–1918, 2002. doi: 10.1161/01.CIR.0000034046.87607.1C. [DOI] [PubMed] [Google Scholar]
- 28.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121: 293–305, 2010. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res 119: 635–651, 2016. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578, 2012. [Erratum in: Nat Protoc 9: 2513, 2014.] 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577: 405–409, 2020. doi: 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, Kayton R, Hascall VC, Lauer ME, Ballabh P. Hyaluronidase and Hyaluronan Oligosaccharides Promote Neurological Recovery after Intraventricular Hemorrhage. J Neurosci 36: 872–889, 2016. doi: 10.1523/JNEUROSCI.3297-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192: 61–69, 2015. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA 107: 17797–17802, 2010. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364: 141–148, 2004. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 36.Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP. A new method to stabilize C-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol 4: 78, 2016. doi: 10.3389/fcell.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wysoczynski M, Guo Y, Moore JB IV, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL, Bolli R. Myocardial reparative properties of cardiac mesenchymal cells isolated on the basis of adherence. J Am Coll Cardiol 69: 1824–1838, 2017. doi: 10.1016/j.jacc.2017.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Yamamoto H, Tobisawa Y, Irie F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol 78-79: 139–146, 2019. doi: 10.1016/j.matbio.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]