Abstract
The role of the ASIC1a in evoking the exercise pressor reflex in rats with simulated peripheral artery disease is unknown. This prompted us to determine whether ASIC1a plays a role in evoking the exaggerated exercise pressor reflex in decerebrated rats with simulated peripheral artery disease. To simulate peripheral artery disease, we ligated the left femoral artery 72 h before the experiment. The right femoral artery was freely perfused and used as a control. To test our hypothesis, we measured the effect of injecting two ASIC1a blockers into the arterial supply of the triceps surae muscles with and without the femoral artery ligated on the reflex pressor responses to 1) static contraction of the triceps surae muscles, 2) calcaneal tendon stretch, and 3) intra-arterial injection of diprotonated phosphate (pH 6.0). We found that the ASIC1a blockers psalmotoxin-1 (200 ng/kg) and mambalgin-1 (6.5 μg/kg) decreased the pressor responses to static contraction as well as the peak pressor responses to injection of diprotonated phosphate when these responses were evoked from the freely perfused hindlimb. In contrast, ASIC1a blockers only decreased the peak pressor responses evoked by injection of diprotonated phosphate in the hindlimb circulation with simulated peripheral artery disease. This inhibitory effect was less than the one measured from the healthy hindlimb. Independently of the hindlimb of interest, ASIC1a blockers had no effect on the pressor responses to tendon stretch. Our results do not support the hypothesis that ASIC1a play a role in evoking the exercise pressor reflex arising from a hindlimb with simulated peripheral artery disease.
NEW & NOTEWORTHY The role of ASIC1a in evoking the metabolic component of the exercise pressor reflex in peripheral artery disease is unknown. Using a within-rat experimental design, we found that the contribution of ASIC1a decreased in a rat model of peripheral artery disease. These results have key implications to help finding better treatments and improve morbidity, quality of life, and mortality in patients with peripheral artery disease.
Keywords: acidosis, autonomic control, inorganic phosphate, mechanoreflex, metaboreflex
INTRODUCTION
Peripheral artery disease affects more than 200 million people worldwide (33). It is characterized by insufficient blood and oxygen supply to the lower-limb muscles (15) that causes acute pain during mild exercise, such as walking (i.e., intermittent claudication). In addition, peripheral artery disease exaggerates the sympathetic and arterial blood pressure responses to exercise, effects that greatly increase the risk of cardiovascular morbidity and mortality (26). Elucidating the mechanisms that cause this exaggerated pressor response is thus key to finding better treatments and improving life expectancy and quality of life of patients with peripheral artery disease.
The hemodynamic response to exercise arises from coordinated and redundant neural mechanisms that function to improve blood flow to the exercising muscles (1, 28). Several findings showed that among these mechanisms the exercise pressor reflex played a key role in evoking the exaggerated pressor response to exercise in humans with peripheral artery disease (27, 29a). The afferent arm of this reflex comprises mechano- and metabosensitive group III and IV afferent fibers (18, 25), whose endings originate in the interstitial space of contracting myocytes and their accompanying vessels and tendons (12, 35). The responses of group III and IV afferents to static contraction are significantly greater in rats with simulated peripheral artery disease than in rats with freely perfused hindlimb muscles (36). Thus, the mechanical and metabolic components of the exercise pressor reflex are presumed to be exaggerated in rats with simulated peripheral artery disease (36).
The metabolic stimuli and their corresponding receptors evoking the exaggerated metabolic component of the exercise pressor reflex in patients with peripheral artery disease are not known, but several findings suggest that accumulation of cyclooxygenase byproducts (22, 42), ATP (37), and acidic compounds play key roles (40). For the latter, lactate, inorganic phosphate, and H+ ions have been found to activate and/or sensitize a family of proton-sensitive channels, the acid-sensing ion channels (ASICs; see Refs. 16 and 17). ASICs are composed of seven receptors, namely ASIC1a and -b, ASIC2a and -b, and ASIC3, -4, and -5, which open when exposed to a broad range of pHs (from 7.0 to < 4.5; see Refs. 4, 14, and 41). Tsuchimochi et al. (40) found that ASIC3 played a role in the exaggerated pressor response to exercise in a rat model of peripheral artery disease by showing that the selective ASIC3 antagonist APETx2 reduced the pressor response evoked by static contraction. Other ASICs might also be involved, but their roles remain undocumented.
ASIC1a appears to be a good candidate because it opens at a pH of 7.0 and has a pH50 of 6.6 (2). Moreover, its current is potentiated by the presence of lactate ions (16, 17). Recently, our laboratory found that ASIC1a played a key role in sensing lactic acid and diprotonated phosphate to evoke the metabolic component of the exercise pressor reflex in rats whose contracting muscles were freely perfused (11). We noted with interest that occluding the circulation of the contracting muscles of humans functions to increase the production of acidic metabolites compared with that measured when the contracting muscles are freely perfused (20, 21, 30), findings that raise the possibility that the contribution of ASIC1a in evoking the metabolic component of the exaggerated exercise pressor reflex increases in rats with simulated peripheral artery disease. This prompted us to test the hypothesis that ASIC1a plays a role in evoking the exaggerated metabolic component of the exercise pressor reflex evoked in rats whose femoral arteries were ligated, a maneuver that simulated peripheral artery disease.
METHODS
Ethical Approval
The Institutional Care and Use Committee of the Pennsylvania State University College of Medicine approved all of the procedures (PRAMS200946708). The authors understand and conformed to the ethics guidelines of the journal for animal use in research.
Animal Characteristics, Wellness, and Sample Size
Experiments were conducted at constant room air temperature (21°C) on 35 male Sprague-Dawley rats (Charles River) weighing 350–550 g. Rats were housed within the central animal facility of the Pennsylvania State University College of Medicine, with access to food and water ad libitum and exposure to a 12-h:12-h light-dark cycle. All attempts were made to minimize animal discomfort and pain.
Surgical Procedure for Ligating the Femoral Artery
Seventy-two hours before the experiment, we ligated the left femoral artery using a 6.0 silk suture. The rat was anesthetized by inhalation of 4% isoflurane in oxygen. The femoral artery was isolated from its vein and nerve and was then ligated approximately 5 mm below the inguinal ligament. Finally, the wound was sutured, and 1–2 mg/kg of bupivacaine was injected subcutaneously. This surgical procedure simulated the blood flow pattern to skeletal muscle seen in peripheral artery disease. Specifically, muscle metabolic demand is met at rest but is not met during exercise. Likewise, femoral artery ligation decreases maximal blood flow to ∼10–20% of normal (29, 43).
General Surgical Procedure
Each rat was anesthetized initially by inhalation of 4% isoflurane in oxygen. The surgical procedure was started only when the corneal reflex was abolished and when pinching the hindpaw did not produce a withdrawal reflex. The rat’s trachea was cannulated, and its lungs were mechanically ventilated (683; Harvard Apparatus, Inc., Holliston, MA). The amount concentration of isoflurane was reduced to 2% for the rest of the surgery. The left and right common carotid arteries and right jugular vein were cannulated using RenaPulse High Fidelity Pressure Tubing (RPT040; Braintree Scientific, Inc., Braintree, MA) to record arterial blood pressure (P23XL; Gould-Statham Instruments Inc., Los Angeles, CA), draw arterial blood samples, and inject drugs into the systemic circulation, respectively. We cannulated the left and right superficial epigastric arteries (SUBL-140; Braintree Scientific, Inc.), which is a side branch of the femoral artery. A snare (2.0 silk suture) was placed around the left and right femoral arteries and vein ∼0.5–1 cm upstream from the superficial epigastric catheter. When tightened, the snare partially trapped injections of diprotonated phosphate in the hindlimb circulation (see below).
The left and right ankles were secured using zip ties to prevent movement during the contraction procedure. The hip was also secured with a metal clamp. The head of the rat was secured in a Kopf stereotaxic unit. The calcaneus bones from both legs were severed, and their attached Achilles tendons were connected to a force transducer (FT03; Grass Instrument Co., Quincy, MA) and a rack and pinion device. The left and right tibial nerves were isolated and hooked with a bipolar stainless-steel electrode.
Using a blunted spatula, we decerebrated the rat by sectioning the brain <1 mm rostral to the superior colliculus (10). Isoflurane was then discontinued, and the lungs were ventilated with room air. Blood arterial Po2 (100–150 mmHg), Pco2 (35–40 mmHg), and [] (22–26 mmol/L) were kept within physiological range. Because the ASIC1a antagonist psalmotoxin-1 has been found to potentiate ASIC1b at a conditioning pH of >7.40 (5), particular attention was made to maintain arterial blood pH between 7.35 and 7.40. Body temperature was maintained around 37°C using a heating lamp. At the end of the experiment, the decerebrated rats were euthanized by intravenous injection of a supersaturated KCl solution.
Experimental Protocols
Contraction of the triceps surae muscles.
Baseline tension of the triceps surae muscles was set at 60–100 g. The stimulator output was set at a current intensity that evoked a twitch tension approximately equal to 90% of the maximum (∼1.50 times motor threshold). During static contraction, the tibial nerve was stimulated for 30 s at 40 Hz (0.01-ms pulse duration) to reflexively increase arterial blood pressure (34). The left and right legs were contracted alternately with ≥10 min of recovery.
Once a pressor reflex was evoked from both legs, the snares around the left (i.e., ligated side) and right (i.e., the freely perfused side) femoral arteries and veins were tightened, after which PcTx-1 (200 ng/kg, 100 μL) or Mamb-1 (6.5 μg/kg, 100 μL) was injected only into the left femoral artery via the left superficial epigastric artery catheter. The snares were released 5 min after the drug was injected. Following a reperfusion period of 5 min (i.e., 10 min after the ASIC1a antagonists were injected), the left triceps surae muscles were contracted for 30 s. To quantify the recovery from ASC1a blockade, a second contraction from the left side was evoked 10 min later (i.e., 20 min after the ASIC1a antagonists were injected). After a recovery period of ≥10 min, the baseline exercise pressor reflex of the right hindlimb was evoked by contracting the right triceps surae muscles. The ASIC1a blockade protocol was then repeated, except this time the antagonists were injected into the right superficial epigastric artery. The interval between the ASIC1a antagonist injection into the left and right arterial circulation was ≥30 min.
Stretch of the triceps surae muscles.
In rats paralyzed by intravenous injection of pancuronium bromide (0.2 mg/mL, 0.2 mL), we used the same protocol for the experiments with tendon stretch as we used for the experiments with static contraction. The triceps surae muscles were stretched for 30 s by turning a rack and pinion attached to the Achilles tendon.
Intra-Arterial Injections of Diprotonated Phosphate
In all experiments in which we injected diprotonated phosphate, we first paralyzed the rat with an intravenous injection of pancuronium bromide (0.2 mg/mL, 0.2 mL). The timeline of the stimulus presentation for the experiments with injection of diprotonated phosphate was the same as that for our experiments with static contraction or tendon stretch. Before injecting the diprotonated phosphate solution, we tightened the snare placed around the femoral artery and vein to partially trap the solution in the hindlimb circulation. Then, we injected diprotonated phosphate (86 mM, pH = 6.0, 100 μL) into the superficial epigastric artery to reflexively increase blood pressure. We released the snare when blood pressure returned to baseline, which took <2 min.
Control Experiments
Control for electrical activation of group III and IV axons.
To show that tibial nerve stimulation did not electrically activate the axons of the group III and IV afferents, we paralyzed the rat with pancuronium bromide (1 mg/mL, 0.2 mL iv) and stimulated the tibial nerves for 30 s at 40 Hz with the highest current used to evoke contraction. If an increase in blood pressure was observed, the data were excluded from the data set. On two occasions, we found that electrical stimulation of the tibial nerve evoked a pressor response when the freely perfused triceps surae muscles were paralyzed, whereas it had no effect on the ligated hindlimb. The data from the freely perfused hindlimb were then excluded, but the data from ligated hindlimb were not.
Control for blockade of ASIC1a within the central nervous system.
To verify that injecting the ASIC1a antagonists did not have effect on the ability of the brainstem to generate the exercise pressor reflex, we contracted the right triceps surae muscles 15 min after injecting the ASIC1a blockade in the left superficial epigastric artery.
Control that the drug was injected in the triceps surae muscles.
To determine that injections into the hindlimb arterial circulation accessed the triceps surae muscles, we injected 0.2 mL of Evans Blue dye into the superficial epigastric artery of each rat tested. We considered that the solution entered into the muscle when its belly was stained blue. If the color of the muscle did not change, we excluded the data from the study. On one occasion, the blue dye control did not stain the belly of the freely perfused muscles, whereas it stained the triceps surae muscles with ligated arterial circulation. The data from the freely perfused hindlimb were then excluded, but the data from ligated hindlimb were not.
Drug Preparation
PcTx-1 and Mamb-1 (Alomone Laboratories) were dissolved in 0.9% saline and stored in 0.2-mL aliquots at −20°C. The dose of PcTx-1 (200 ng/kg, 100 μL) and Mamb-1 (6.5 μg/kg, 100 μL) was chosen based on previous findings from our laboratory showing that these doses effectively blocked the pressor response in freely perfused rats (11). Diprotonated phosphate solution was made by dissolving 153 mg (43 mM) of Na2HPO4 and 129 mg (43 mM) of NaH2PO4 into 25 mL of 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]. The pH of the solution was decreased to 6.0 by adding HCl and, if necessary, adjusted on a weekly basis.
Data Analysis
Tension and arterial blood pressure signals were amplified using Gould Universal amplifiers (Gould-Statham Instruments, Inc., CA). All signals were displayed and recorded at 1 kHz using an A/D converter (Micro1401 mkII; Cambridge Electronic Design Limited, Cambridge, UK) and its associated commercially available software (Spike2, 7.20, RRID: SCR_000903; Cambridge Electronic Design Limited). Heart rate was determined beat by beat from the arterial pressure pulse signal and expressed as beats per minute.
To determine the effect of diprotonated phosphate injections, we calculated the peak increase in blood pressure. To determine the effect of static contraction or tendon stretch, we calculated the peak increase in blood pressure and the increase in the blood pressure index. The latter was calculated by integrating the area under the curve during the 30-s contraction or stretch period and then subtracting from this value the area under the curve measured during baseline (i.e., 1 s before the contraction or stretch and multiplied by 30). This variable provides an index of the entire pressor response, unlike the peak pressor response, which represents the instantaneous maximal value. Using a similar method, we calculated the change in peak tension produced by the contraction or stretch as well as the tension-time index (i.e., the equivalent of the blood pressure index for tension). The time course of the pressor response to static contraction or tendon stretch was plotted by averaging the mean arterial blood pressure signal every 2 s.
Statistical Analysis
Data are presented as the means ± SD. Using a Kolmogorov-Smirnoff test, we verified that our samples respected a normal distribution. The pre- to poststimulus (i.e., static contraction, tendon stretch, and diprotonated phosphate) change in peak pressor response, peak heart rate, blood pressure index, peak tension, and tension-time index were evaluated using Student’s paired t test. We also used Student’s paired t test to evaluate the effect of ligating the femoral artery or the pre- to postinjection effect of ASIC1a blockade on the different variable tested. In the case when serial measures were made after ASIC1a blockade (i.e., during diprotonated phosphate and contraction experiments), a one-way repeated-measures ANOVA was used. Finally, differences in the time course of blood pressure were evaluated using two-way repeated-measures ANOVA (treatment × time). The level of significance was set at P < 0.05. When a statistical difference was found, post hoc multiple-comparison analysis was performed using Tukey’s honestly significant difference test. Effect size was calculated using Cohen’s d for Student’s t test or partial η2 for ANOVAs (6). A Cohen’s d index for effect size was considered as small, medium, or large when d was close to 0.2, 0.5, or 0.8, respectively (6). An η2 for effect size was considered as small, medium, or large when η2 was close to 0.02, 0.13, or 0.26, respectively (6). When individual data are not presented, effect size was also calculated using 95% confidence intervals (9). Confidence intervals are presented as the lower and upper limit of the interval that should, if this experiment is repeated, contain 95% of the time the true value of the treated effect (9). Statistical analyses were conducted using Statistica 8.0 (RRID: SCR_014213; StatSoft, Inc., Tulsa, OK).
RESULTS
Effects of Left Femoral Artery Ligation
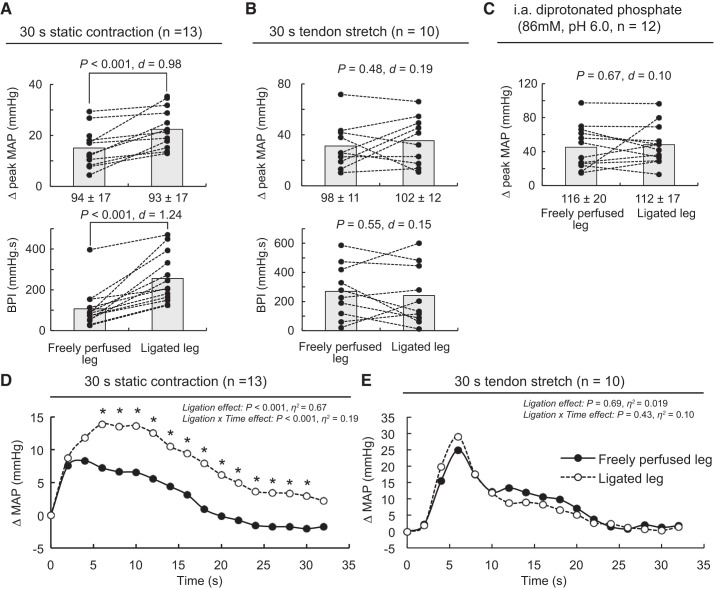
Independently of the hindlimb of interest, static contraction, tendon stretch, and injection of diprotonated phosphate into the arterial circulation of the triceps surae muscles increased mean arterial blood pressure and heart rate above their baseline levels (Table 1). Static contraction of the triceps surae muscles with the femoral artery ligated evoked a significantly greater peak pressor response and blood pressure index than did static contraction of the contralateral triceps surae muscles with a freely perfused femoral artery (n = 13; Fig. 1A). Analysis of the time course of these pressor responses revealed that the exercise pressor reflex evoked from triceps surae muscles with their femoral arteries ligated was significantly greater than the exercise pressor reflex evoked from triceps surae muscles with freely perfused femoral arteries 6–8 s after the onset of contraction (Fig. 1D). On the contrary, the peak pressor response, blood pressure index, and cardioaccelerator responses evoked by stretching the calcaneal tendon of the triceps surae muscle with ligated femoral arteries were not different than the responses evoked from the triceps surae muscles with freely perfused femoral arteries (n = 10; Fig. 1, B and E). Likewise, the peak pressor and cardioaccelerator responses evoked by injection of diprotonated phosphate (86 mM, pH = 6.0) into the arterial circulation of the triceps surae muscles with ligated femoral arteries were not different from the responses evoked from the triceps surae muscles with freely perfused femoral arteries (n = 12; Fig. 1C). Importantly, the peak tension or tension time index evoked during the contracting or stretching procedures was not different between the two limbs.
Table 1.
Pressor and cardioaccelerator responses to intra-arterial injection of diprotonated phosphate, static contraction, or tendon stretch of the triceps surae muscles
| Indexes | Baseline | Peak | 95% CI | P Value | Cohen’s d |
|---|---|---|---|---|---|
| Static contraction | |||||
| MAP, mmHg | |||||
| Freely perfused | 94 ± 17 | 110 ± 22 | [11:19] | <0.001 | 1.52 |
| Ligated | 93 ± 17 | 115 ± 21 | [18:27] | <0.001 | 2.07 |
| HR, beats/min | |||||
| Freely perfused | 333 ± 52 | 337 ± 53 | [2:6] | <0.001 | 0.96 |
| Ligated | 341 ± 57 | 347 ± 56 | [3:8] | <0.001 | 1.05 |
| BPI, mmHg·s | |||||
| Freely perfused | 2,697 ± 503 | 2,816 ± 505 | [49:165] | 0.001 | 0.86 |
| Ligated | 2,653 ± 513 | 2,918 ± 530 | [183:328] | <0.001 | 1.67 |
| Tension, g | |||||
| Freely perfused | 84 ± 8 | 884 ± 541 | [474:1,127] | <0.001 | 1.48 |
| Ligated | 82 ± 10 | 708 ± 215 | [496:754] | <0.001 | 2.91 |
| TTI, kg·s | |||||
| Freely perfused | 2.4 ± 0.2 | 20.2 ± 11.7 | [10.8:24.9] | <0.001 | 1.53 |
| Ligated | 2.3 ± 0.3 | 17.2 ± 6.0 | [11.2:18.5] | <0.001 | 2.45 |
| Tendon stretch | |||||
| MAP, mmHg | |||||
| Freely perfused | 98 ± 11 | 129 ± 15 | [18:44] | <0.001 | 1.67 |
| Ligated | 102 ± 12 | 138 ± 22 | [22:49] | <0.001 | 1.62 |
| HR, beats/min | |||||
| Freely perfused | 341 ± 54 | 344 ± 55 | [2:5] | 0.001 | 1.14 |
| Ligated | 340 ± 54 | 344 ± 55 | [2:6] | 0.004 | 1.11 |
| BPI, mmHg·s | |||||
| Freely perfused | 2,804 ± 314 | 3,074 ± 287 | [138:402] | <0.001 | 1.09 |
| Ligated | 2,952 ± 340 | 3,194 ± 389 | [97:386] | <0.001 | 0.93 |
| Tension, g | |||||
| Freely perfused | 86 ± 21 | 761 ± 22 | [656:695] | <0.001 | 22.6 |
| Ligated | 90 ± 14 | 769 ± 31 | [656:706] | <0.001 | 20.6 |
| TTI, kg·s | |||||
| Freely perfused | 0.4 ± 0.1 | 18.0 ± 0.6 | [15.3:16.1] | <0.001 | 28.2 |
| Ligated | 0.3 ± 0.1 | 18.3 ± 1.1 | [15.1:16.7] | <0.001 | 17.1 |
| Diprotonated phosphate | |||||
| MAP, mmHg | |||||
| Freely perfused | 116 ± 20 | 161 ± 43 | [29:61] | <0.001 | 1.60 |
| Ligated | 112 ± 17 | 160 ± 37 | [33:63] | <0.001 | 1.83 |
| HR, beats/min | |||||
| Freely perfused | 406 ± 35 | 415 ± 29 | [2:16] | 0.016 | 0.64 |
| Ligated | 404 ± 41 | 411 ± 38 | [2:12] | 0.007 | 0.73 |
Values are means ± SD. BPI, blood pressure index; CI, confidence interval; HR, heart rate; MAP, mean arterial blood pressure; TTI, tension-time index. A 95% CI is presented as the lower and upper boundary of the interval containing the true value of the effect of stimulus on the corresponding index.
Fig. 1.
The effects of 72-h femoral artery ligation on the pressor responses to static contraction (A and D), tendon stretch (B and E), or intra-arterial injection of diprotonated phosphate (C). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP) and blood pressure index (BPI). D: averaged time course of the pressor response evoked by static contraction from the freely perfused or ligated leg. E: averaged time course of the pressor response evoked by tendon stretch from the freely perfused or ligated leg. The averaged time course of blood pressure includes 2 s of baseline, 30 s of contraction, and 2 s after the end of contraction. For clarity, error bars were omitted. The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. Static contraction (n = 13), tendon stretch (n = 10), and diprotonated phosphate (86 mM, pH 6.0; n = 12) experiments were conducted on separated rats. Diprotonated phosphate solution was injected in the superficial epigastric artery, which is a side branch of the femoral artery. Baseline values for blood pressure are presented below the x-axes. *P < 0.001, significant difference compared with the response evoked from the triceps surae muscles with freely perfused femoral artery.
Effects of ASIC1a Blockade
Contraction experiments.
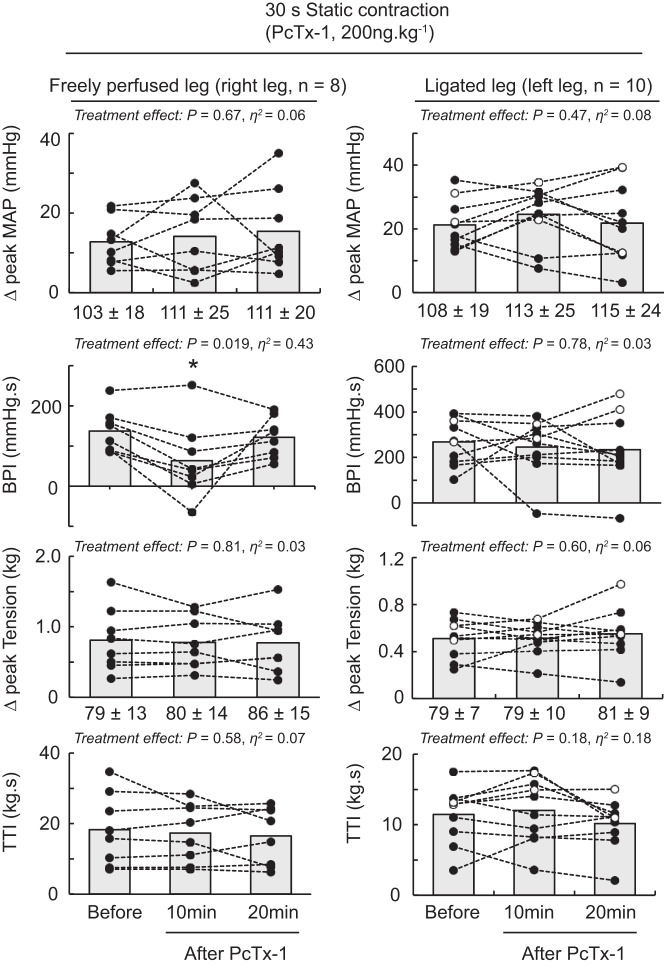
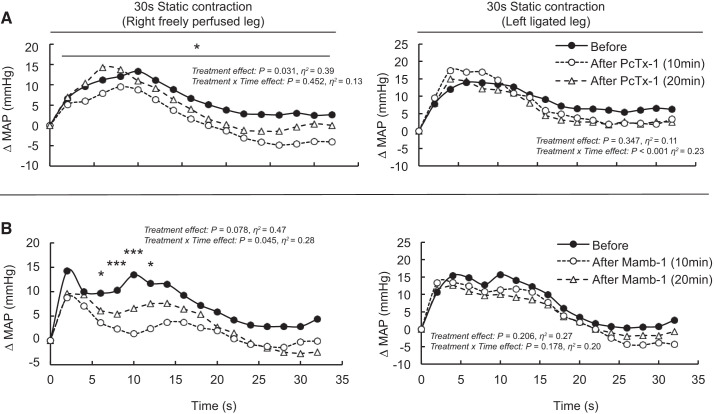
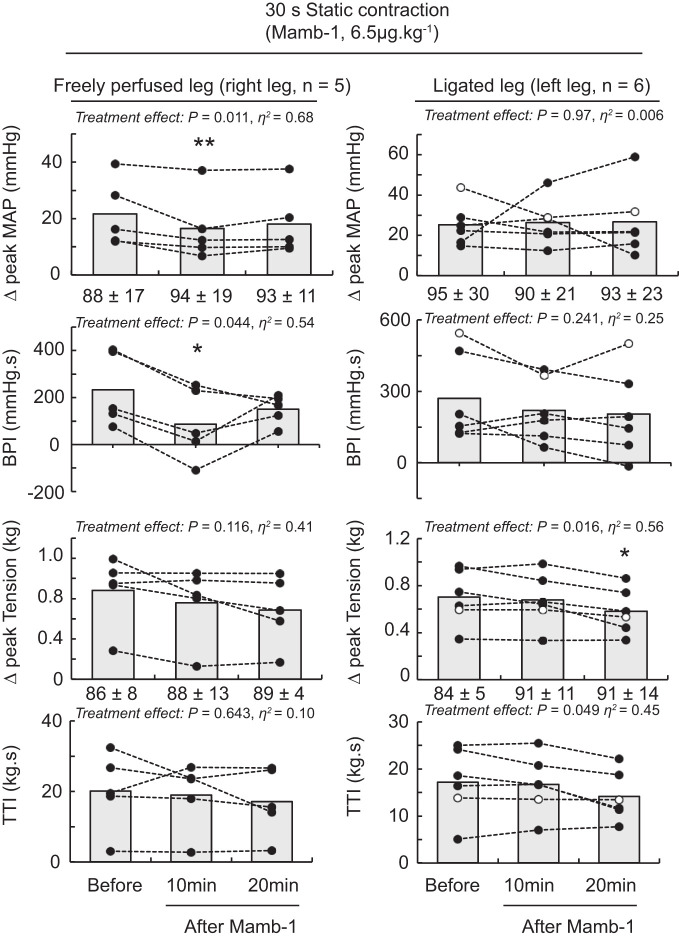
Blocking ASIC1a by injecting PcTx-1 (n = 10) or Mamb-1 (n = 6) into the arterial supply of the triceps surae muscles with their femoral arteries ligated did not significantly decrease the peak pressor responses, blood pressure index, and cardioaccelerator responses evoked by static contraction (Table 2 and Figs. 2–4). In contrast, PcTx-1 (n = 8) and Mamb-1 (n = 5), when injected into the arterial supply of the triceps surae muscles with freely perfused femoral arteries, significantly decreased the blood pressor index evoked by static contraction (Figs. 2–4). In addition, Mamb-1 significantly decreased the peak pressor response evoked by static contraction of the triceps surae muscles with freely perfused femoral arteries. Analysis of the time course of these pressor responses revealed that blocking ASIC1a with Mamb-1 significantly reduced blood pressure 10 s after the onset of the contraction (Fig. 4). A treatment × time interaction effect was found only for Mamb-1 experiments, so it was not possible to perform these analyses for the experiments using PcTx-1. After 10 min of recovery, the attenuating effects of PcTx-1 or Mamb-1 on the blood pressure index, peak pressor response, or time course of blood pressure evoked by static contraction were no longer significant (Figs. 2–4). Importantly, injecting PcTx-1 or Mamb-1 into the arterial circulation of the triceps surae muscles with the femoral artery ligated had no effect on the peak pressor response, blood pressure index, or cardioaccelerator response evoked by static contraction of the contralateral triceps surae muscles with freely perfused femoral arteries (Table 3). PcTx-1 or Mamb-1 had no effect on the peak tension or tension-time index evoked by static contraction (Figs. 2 and 3). However, a significant decrease in peak tension was found 20 min after Mamb-1 was injected when static contraction was evoked from the triceps surae muscles with ligated femoral arteries. The TTI tended to be lower as well (P = 0.056).
Table 2.
Effect of ASIC1a blockade on the cardioaccelerator responses to diprotonated phosphate injections, static contraction, or passive stretch of the triceps surae muscles with or without the femoral artery ligated
| Experiment (Leg Arterial Circulation) | n | Before | 10 min After Injection | 20 min After Injection | 95% CI | P Value | η2/Cohen’s d |
|---|---|---|---|---|---|---|---|
| Contraction | |||||||
| PcTx-1 | |||||||
| Freely perfused | 8 | 5 ± 2 | 2 ± 2 | 3 ± 3 | [−4:0] | 0.121 | η2 = 0.26 |
| Ligated | 10 | 4 ± 3 | 5 ± 2 | 5 ± 4 | [−1:3] | 0.667 | η2 = 0.04 |
| Mamb-1 | |||||||
| Freely perfused | 5 | 7 ± 5 | 5 ± 2 | 4 ± 2 | [−8:4] | 0.352 | η2 = 0.23 |
| Ligated | 6 | 3 ± 4 | 4 ± 1 | 3 ± 2 | [−3:3] | 0.973 | η2 = 0.005 |
| Stretch | |||||||
| PcTx-1 | |||||||
| Freely perfused | 5 | 3 ± 2 | 1 ± 3 | Not tested | [−2:0] | 0.319 | d = 0.52 |
| Ligated | 5 | 3 ± 2 | 4 ± 3 | Not tested | [0:3] | 0.104 | d = 0.89 |
| Mamb-1 | |||||||
| Freely perfused | 5 | 5 ± 3 | 4 ± 2 | Not tested | [−2:0] | 0.148 | d = 0.48 |
| Ligated | 5 | 4 ± 2 | 8 ± 3 | Not tested | [−1:3] | 0.224 | d = 0.52 |
| Diprotonated phosphate | |||||||
| PcTx-1 | |||||||
| Freely perfused | 6 | 8 ± 7 | 5 ± 7 | 8 ± 8* | [−5:−1] | 0.043 | η2 = 0.47 |
| Ligated | 6 | 14 ± 14 | 8 ± 10 | 5 ± 2 | [−11:0] | 0.136 | η2 = 0.33 |
| Mamb-1 | |||||||
| Freely perfused | 6 | 4 ± 4 | 2 ± 1 | 3 ± 2 | [−6:2] | 0.389 | η2 = 0.17 |
| Ligated | 6 | 6 ± 3 | 4 ± 6 | 3 ± 2 | [−5:2] | 0.239 | η2 = 0.24 |
Values are means ± SD; n = sample size. ASIC1a, acid-sensing ion channels; CI, confidence interval; Mamb-1, mambalgin-95%; PcTx-1, psalmotoxin-1. Increase in heart rate evoked by static contraction, passive stretch, or diprotonated phosphate injections (86 mM, pH 6.0); 95% CI is presented as the lower and upper boundary of the interval containing the true value of the effect of ASIC1a blockade at 10 min. Effect size is presented as partial η-squared (η2) or Cohen’s d, depending on whether the statistical test was a 1-way repeated-measures ANOVA or Student’s paired t test, respectively.
P < 0.05, significant difference compared with 10 min after injection.
Fig. 2.
The effect of psalmotoxin-1 (PcTx-1) on the pressor response-evoked byto static contraction of the triceps surae muscles with freely perfused (n = 8; left) or ligated femoral arteries (n = 10; right). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP), blood pressure index (BPI), peak increase in tension, and time-tension index (TTI) to static contraction. The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. ○, Data points that could be obtained in only 1 leg. Baseline values for blood pressure and tension (in g) are presented below the x-axes of their respective graphs. Contractions were evoked before and at 10 and 20 min following PcTx-1 (200 ng/kg, 100 μL) injection in the superficial epigastric artery. *P < 0.05, significant difference compared with before ASIC1a blockade.
Fig. 4.
Average time course of the pressor response to static contraction following acid-sensing ion channel 1a (ASIC1a) blockade. ASIC1as were antagonized by injecting psalmotoxin-1 (PcTx-1; n = 8 and 10, 200 ng/kg, 100 μL; A) or mambalgin-1 (Mamb-1; n = 5 and 6, 6.5 μg/kg, 100 μL; B) into the superficial epigastric artery. The pressor responses to contraction were evoked at 10 and at 20 min after injection of the ASIC1a antagonist. The averaged time course of blood pressure includes 2 s of baseline, 30 s of contraction, and 2 s after the end of contraction. The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. Sample sizes are different because in 2 and 1 of the experiments with PcTx-1 and Mamb-1, respectively, we could not obtain a pressor response from the freely perfused leg. For clarity, error bars were omitted. MAP, mean arterial pressure. *P < 0.05, significant difference between before and 10 min after ASIC1a blockade; ***P < 0.001.
Table 3.
Injecting ASIC1a antagonists in the arterial circulation of the left hindlimb has no effect on the pressor response to static contraction evoked from the right hindlimb
| n | Before | After | 95% CI | P Value | Cohen’s d | |
|---|---|---|---|---|---|---|
| PcTx-1 | 8 | |||||
| ∆MAP, mmHg | 14 ± 6 | 13 ± 6 | [−5:4] | 0.805 | 0.08 | |
| ∆HR, beats/min | 4 ± 3 | 3 ± 2 | [−3:2] | 0.548 | 0.17 | |
| BPI, mmHg·s | 121 ± 66 | 152 ± 62 | [−4:65] | 0.077 | 0.56 | |
| ∆Tension, g | 834 ± 662 | 768 ± 448 | [−354:221] | 0.602 | 0.16 | |
| TTI, kg·s | 18.7 ± 12.7 | 17.3 ± 1.0 | [5.7:2.9] | 0.478 | 0.21 | |
| Mamb-1 | 5 | |||||
| ∆MAP, mmHg | 19 ± 7 | 20 ± 12 | [−7:9] | 0.774 | 0.12 | |
| ∆HR, beats/min | 4 ± 3 | 5 ± 4 | [−3:4] | 0.772 | 0.10 | |
| BPI, mmHg·s | 164 ± 126 | 177 ± 144 | [−105:131] | 0.774 | 0.10 | |
| ∆Tension, g | 789 ± 398 | 839 ± 355 | [−78:178] | 0.338 | 0.36 | |
| TTI, kg·s | 18.4 ± 10.5 | 18.0 ± 10.4 | [−6.9:6.2] | 0.882 | 0.05 |
Values are means ± SD; n = sample size. ASIC1a, acid-sensing ion channels; CI, confidence interval; Mamb-1, mambalgin-95%; PcTx-1, psalmotoxin-1; TTI, tension-time index. Ninety-five percent confidence interval is presented as the lower and upper boundary of the interval containing the true value of the effect of ASIC1a blockade. Effect size is presented as Cohen’s d.
Fig. 3.
The effect of mambalgin-1 (Mamb-1) on the pressor response to static contraction of the triceps surae muscles with freely perfused (n = 5; left) or ligated femoral arteries (n = 6; right). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP), blood pressure index (BPI), peak increase in tension, and time-tension index (TTI) to static contraction. The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. ○, Data points that could be obtained in only 1 leg. Baseline values for blood pressure and tension (in g) are presented below the x-axes of their respective graphs. Contractions were evoked before and at 10 and 20 min following Mamb-1 (n = 5; 6.5 μg/kg, 100 μL) injection in the superficial epigastric artery. *P < 0.05, significant difference compared with before ASIC1a blockade; **P < 0.01.
Tendon stretch experiments.
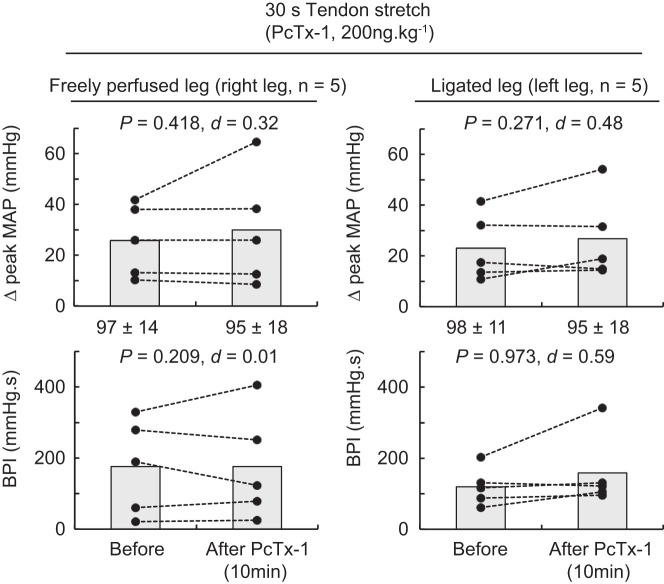
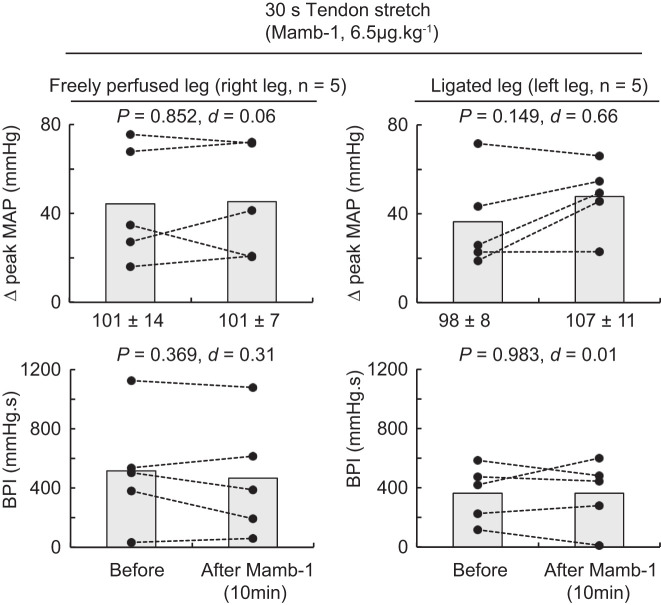
Blocking ASIC1a had no effect on the peak pressor responses, the blood pressure index, or the cardioaccelerator responses to tendon stretch whether they were evoked from the triceps surae muscles with either ligated or freely perfused arteries (Figs. 5 and 6). No difference in peak tension and tension-time index was found between stretches.
Fig. 5.
The effect of psalmotoxin-1 (PcTx-1) on the pressor response to tendon stretch of the triceps surae muscles with freely perfused (n = 5; left) or ligated femoral arteries (n = 5; right). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP) and blood pressure index (BPI) evoked by tendon stretch. The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. Baseline values for blood pressure are presented below the x-axes of the graphs. Tendon stretches were evoked before and 10 min following PcTx-1 (200 ng/kg, 100 μL) injection in the superficial epigastric artery.
Fig. 6.
The effect of mambalgin-1 (Mamb-1) on the pressor response to tendon stretch of the triceps surae muscles with freely perfused (n = 5; left) or ligated femoral arteries (n = 5; right). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP) and blood pressure index (BPI) evoked by tendon stretch. The pressor responses evoked from the freely perfused and ligated legs were obtained within the same animals. Baseline values for blood pressure are presented below the x-axes of the graphs. Tendon stretches were evoked before and at 10 following Mamb-1 (6.5 μg/kg−1, 100 μL) injection in the superficial epigastric artery.
Diprotonated phosphate experiments.
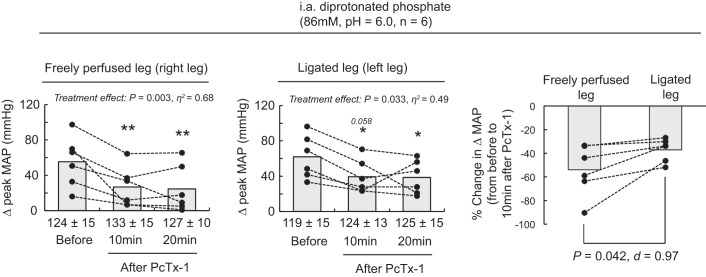
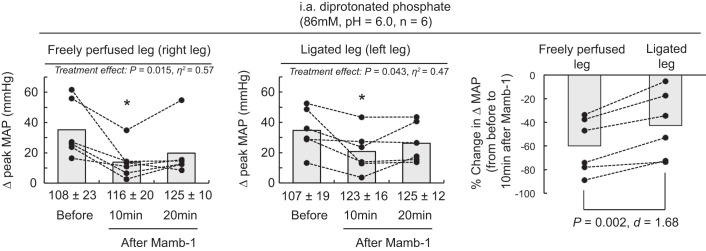
Blocking ASIC1a significantly reduced the peak pressor response to injections of diprotonated phosphate whether it was evoked from triceps surae muscles with a ligated or a freely perfused arterial supply (Figs. 7 and 8). We found that PcTx-1 and Mamb-1 had a greater inhibitory effect when they were injected into the circulation of the triceps surae muscles with freely perfused arterial supply than when they were injected into the circulation of the triceps surae muscles with ligated arterial supply (Figs. 7 and 8).
Fig. 7.
The effect of psalmotoxin-1 (PcTx-1) on the pressor response to injection of diprotonated phosphate into the arterial circulation of the triceps surae muscles with freely perfused (n = 6; left) or ligated femoral arteries (n = 6; middle). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP) evoked by injection of diprotonated phosphate (86 mM, pH 6.0). Data are also presented as the %change in the peak MAP response evoked by injection of diprotonated phosphate from baseline compared with 10 min after injecting PcTx-1 (right). The pressor responses evoked from the freely perfused and ligated legs were obtained within the same animals. Baseline values for blood pressure are presented below the x-axes of the graphs. The pressor responses were evoked before and 10 and 20 min following PcTx-1 (200 ng/kg, 100 μL) injection in the superficial epigastric artery. *P < 0.05, significant difference compared with before acid-sensing ion channel 1a (ASIC1a) blockade; **P < 0.01. i.a., intra-arterial.
Fig. 8.
The effect of mambalgin-1 (Mamb-1) on the pressor response to injection of diprotonated phosphate into the arterial circulation of the triceps surae muscles with freely perfused (n = 6; left) or ligated femoral arteries (n = 6; middle). Data are presented as individual (●) and group mean (gray bars) for the peak increase in mean arterial pressure (MAP) evoked by injection of diprotonated phosphate (86 mM, pH 6.0). Data are also presented as %change in the peak MAP response evoked by injection of diprotonated phosphate from baseline compared with 10 min after Mamb-1 was injected (right). The pressor responses evoked from the freely perfused and ligated legs were obtained from the same rats. Baseline values for blood pressure are presented below the x-axes of the graphs. The pressor responses were evoked before and at 10 and 20 following Mamb-1 (6.5 μg/kg, 100 μL) injection in the superficial epigastric artery. *P < 0.05, significant difference compared with before ASIC1a blockade. i.a., intra-arterial.
DISCUSSION
We found that static contractions of the triceps surae muscles whose arterial supply was ligated for 72 h evoked a greater pressor response than did static contraction of the contralateral triceps surae muscles whose arterial supply was freely perfused. ASIC1a did not play a role in evoking this exaggerated exercise pressor reflex because blocking ASIC1a with PcTx-1 and Mamb-1, two structurally different ASIC1a antagonists, had no significant effect when the exercise pressor reflex was evoked from the triceps surae muscles with simulated peripheral artery disease. Nevertheless, ASIC1a blockade decreased the blood pressure index evoked by static contraction of the triceps surae muscles with freely perfused femoral arteries as well as the pressor response evoked by injection of diprotonated phosphate. ASIC1a blockade had no effect on the pressor response evoked by tendon stretch. These results show that ASIC1a plays a key role in evoking the metabolic component of the exercise pressor reflex in muscles with a patent arterial supply, but the contribution of ASIC1a decreases in muscles with simulated peripheral artery disease.
The mechanism by which the exercise pressor reflex is exaggerated in peripheral artery disease is uncertain. We found that the exaggeration of the pressor response to static contraction of the triceps surae muscles with ligated femoral arteries started 6–8 s after its onset. This result suggests that the mechanical component of the reflex is not altered by chronic ligation of the femoral artery because the mechanical component of the reflex is thought to intervene early during the contracting period (i.e., when tension is high and the intramuscular metabolic perturbations are low; see Ref. 18). For example, mechanosensitive group III afferent fibers start firing within 500 ms, whereas metabosensitive group IV afferent fibers start firing 5–20 s after the onset of contraction (18). This interpretation is also supported by our data and previous findings (8, 22, 23) showing that the pressor response evoked by tendon stretch was not different whether it was evoked from the triceps surae muscles with ligated or freely perfused arterial supplies. Similarly, we found that the pressor response evoked by injection of diprotonated phosphate into the arterial circulation of the triceps surae muscles with ligated femoral arteries was not different than the one evoked from the triceps surae muscles with freely perfused femoral arteries. Consistent with previous findings (7, 8), these results suggested that the ability of the afferent arm of the reflex to respond to a given metabolic stimulus remains unchanged by chronic ligation of the femoral artery. Alternatively, the exaggerated exercise pressor reflex may have arisen in our preparations from a greater and faster accumulation of metabolic by-products, such as H+, inorganic phosphate, and lactate (20, 30).
The types of receptors responsible for detecting these presumably greater concentrations of metabolites and provoking the exaggerated exercise pressor reflex in peripheral artery disease have yet to be fully identified. ASIC1a was a good candidate because previous findings from our laboratory showed that ASIC1a played a role in evoking the metabolic component of the exercise pressor reflex in rats with freely perfused femoral arteries (11). In the present experiment, we replicated our findings by showing that blocking ASIC1a with two structurally different antagonists significantly reduced the blood pressure index evoked by static contraction of the triceps surae muscles whose arterial supply was freely perfused. However, our data show that ASIC1a blockade had no effect on the peak pressor or blood pressure index evoked by static contraction of the triceps surae muscles whose arterial supply was ligated. This result does not support the hypothesis that ASIC1a plays a role in evoking the exaggerated exercise pressor reflex in rats with simulated peripheral artery disease.
Our experiments do not provide a definite conclusion as to why the contribution of ASIC1a in evoking the metabolic component of the exercise pressor reflex decreased with stimulated peripheral artery disease. We speculate that two mechanisms might play a role. First, previous findings showed that the expression of ASIC1 in the dorsal root ganglia was reduced in rats with simulated peripheral artery disease compared with that in rats with patent arteries (13). This reduction was opposed by an increase in ASIC3 expression (13) that has been found to play a role in evoking the metabolic component of the exercise pressor reflex in rats with simulated peripheral artery disease (40). These results suggest that the number of ASIC1a available for detecting the intramuscular metabolic perturbations induced by exercise was reduced by chronic femoral artery ligation. This hypothesis is also indirectly supported by our data showing that ASIC1a blockade had a reduced inhibitory effect on the pressor response evoked by injection of diprotonated phosphate into the circulation of triceps surae muscles with ligated femoral arteries compared with the inhibitory effect of ASIC1a antagonists injected in the circulation of the triceps surae muscles with freely perfused femoral arteries.
Second, patch clamp experiments showed that ASIC1a remains in an inactivated state (i.e., unresponsive) when the cell is exposed to a prolonged acidic milieu, even when the variation in pH is mild (2). Thus, it is possible that the chronic ischemia of the hindlimb muscles caused by occlusion of their femoral arteries exposed ASIC1a to a prolonged but mild muscle acidosis, decreasing the excitability of ASIC1a on the ligated side. Third, chronic ischemia in peripheral artery disease patients and animals is associated with greater release of cyclooxygenase by-products such as prostaglandin E2 (38). Therefore, the metabolic component of the exercise pressor reflex may be determined both by acidic by-products of contraction and by cyclooxygenase metabolites sensitizing or stimulating of group III and IV afferents (22, 31, 32, 42). The increased contribution of cyclooxygenase metabolites in the triceps surae muscles of rats with simulated peripheral artery disease compared with healthy muscles might have compensated for the loss of contribution of ASIC1a.
Methodological Considerations/Perspectives
We cannot exclude the possibility that ASIC1a antagonists used in our experiments were less effective when injected into arterial supply of the ligated hindlimb than when injected into the arterial supply of the freely perfused hindlimb. We think, however, that this possibility is unlikely because there is no evidence that the affinity of the antagonists for ASIC1a is affected by ligation of an artery. Moreover, injection of ASIC1a antagonists was effective in attenuating the pressor responses to diprotonated phosphate in the ligated hindlimb. Any difference in the magnitude of the attenuation between the freely perfused and the ligated hindlimb would appear to be better explained by a documented reduction in ASIC1a receptors (13) than by an undocumented decrease in receptor affinity.
Our data showing that femoral artery ligation has no effect on the pressor response evoked by injection of diprotonated phosphate, which is produced by the contracting muscle, contrasts with previous reports showing that the pressor response evoked by injection of lactic acid or capsaicin was exaggerated in rats with simulated peripheral artery disease (13, 19, 22, 24, 39, 40, 42). This might question our results and our interpretation that the ability of the afferent arm of the reflex to respond to a given metabolic stimulus remains unchanged with chronic ligation of the femoral artery. However, several methodological differences between our protocol and the previous reports might explain the discrepancy between our results and need to be considered. First, we made a within-rat comparison, in which each rat acted as its own control, whereas the other studies used a between-animal comparison. Second, capsaicin is a foreign substance and is not produced by contraction. In addition, the pH of the lactic acid injected into the arterial supply of the muscles in these studies (13, 19, 22, 24, 39, 40, 42) was <3.0, a concentration that far exceeds the physiological range. During exercise, the concentration of lactic acid in contracting muscle does not accumulate in its associated state (lactate + H+) because its pKa is 3.86.
Conclusion
Our data show that the contribution of ASIC1a in evoking the metabolic component of the exercise pressor reflex is absent in rats with simulated peripheral artery disease. Nevertheless, our data support the hypothesis that ASIC1a play a key role in evoking the metabolic component of the exercise pressor reflex in rats free of this simulated disease.
GRANTS
Funding for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant R01-AR-059397) and National Heart, Lung, and Blood Institute (Grant P01-HL-134609).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P.D. conceived and designed research; G.P.D. and J.S.K. performed experiments; G.P.D. analyzed data; G.P.D. and M.P.K. interpreted results of experiments; G.P.D. prepared figures; G.P.D. drafted manuscript; G.P.D., J.S.K., J.A.E., and M.P.K. edited and revised manuscript; G.P.D., J.S.K., J.A.E., and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babini E, Paukert M, Geisler H-S, Gründer S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 277: 41597–41603, 2002. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 4.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002. [Erratum in: Proc Natl Acad Sci USA 99: 4752, 2002.] doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Kalbacher H, Gründer S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol 127: 267–276, 2006. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press, 1977. [Google Scholar]
- 7.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594: 641–655, 2016. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 310: H1233–H1241, 2016. doi: 10.1152/ajpheart.00974.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran-Everett D. Explorations in statistics: confidence intervals. Adv Physiol Educ 33: 87–90, 2009. doi: 10.1152/advan.00006.2009. [DOI] [PubMed] [Google Scholar]
- 10.Dobson KL, Harris J. A detailed surgical method for mechanical decerebration of the rat. Exp Physiol 97: 693–698, 2012. doi: 10.1113/expphysiol.2012.064840. [DOI] [PubMed] [Google Scholar]
- 11.Ducrocq GP, Kim JS, Estrada JA, Kaufman MP. ASIC1a plays a key role in evoking the metabolic component of the exercise pressor reflex in rats. Am J Physiol Heart Circ Physiol 318: H78–H89, 2020. doi: 10.1152/ajpheart.00565.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Düring M, Andres KH. Topography and ultrastructure of group III and IV nerve terminals of the cat’s gastrocnemius-soleus muscle. In: The Primary Afferent Neuron, edited by Zenker W and Neuhuber WL. Boston, MA: Springer, 1990, p. 35–41. [Google Scholar]
- 13.Farrag M, Drobish JK, Puhl HL, Kim JS, Herold PB, Kaufman MP, Ruiz-Velasco V. Endomorphins potentiate ASIC currents and enhance the lactic acid-mediated increase in arterial blood pressure-effects amplified in hindlimb ischemia. J Physiol 595: 7167–7183, 2017. doi: 10.1113/JP275058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanukoglu I. ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters. FEBS J 284: 525–545, 2017. doi: 10.1111/febs.13840. [DOI] [PubMed] [Google Scholar]
- 15.Hart CR, Layec G, Trinity JD, Le Fur Y, Gifford JR, Clifton HL, Richardson RS. Oxygen availability and skeletal muscle oxidative capacity in patients with peripheral artery disease: implications from in vivo and in vitro assessments. Am J Physiol Heart Circ Physiol 315: H897–H909, 2018. doi: 10.1152/ajpheart.00641.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 17.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003. doi: 10.1016/S0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Kaufman MP. Stimulation of spinal δ-opioid receptors attenuate the exercise pressor reflex in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 316: R727–R734, 2019. doi: 10.1152/ajpregu.00013.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch H, Okyayuz-Baklouti I, Norris D, Kogler H, Leibfritz D. Correlation of function and energy metabolism in rat ischemic skeletal muscle by 31P-NMR spectroscopy: effects of torbafylline. J Med 24: 47–66, 1993. [PubMed] [Google Scholar]
- 21.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leal AK, McCord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H2140–H2146, 2011. doi: 10.1152/ajpheart.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leal AK, Yamauchi K, Kim J, Ruiz-Velasco V, Kaufman MP. Peripheral δ-opioid receptors attenuate the exercise pressor reflex. Am J Physiol Heart Circ Physiol 305: H1246–H1255, 2013. doi: 10.1152/ajpheart.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 87: 119–128, 1991. doi: 10.1016/0021-9150(91)90014-T. [DOI] [PubMed] [Google Scholar]
- 27.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 29.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 29a.Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M, Baccelli G. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 30.Rexroth W, Semmler W, Gückel F, Stadtlander M, Weicker H, Hild R, van Kaick G. Assessment of muscular metabolism in peripheral arterial occlusive disease using 31P nuclear magnetic resonance spectroscopy. Comparison with metabolite concentrations in femoral blood. Klin Wochenschr 67: 804–812, 1989. doi: 10.1007/BF01725196. [DOI] [PubMed] [Google Scholar]
- 31.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates responses of group IV muscle afferents to static contraction. Am J Physiol 259: H745–H750, 1990. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- 32.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol (1985) 68: 861–867, 1990. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- 33.Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 275: 379–381, 2018. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stacey MJ. Free nerve endings in skeletal muscle of the cat. J Anat 105: 231–254, 1969. [PMC free article] [PubMed] [Google Scholar]
- 36.Stone AJ, Copp SW, McCord JL, Kaufman MP. Femoral artery ligation increases the responses of thin-fiber muscle afferents to contraction. J Neurophysiol 113: 3961–3966, 2015. doi: 10.1152/jn.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone AJ, Yamauchi K, Kaufman MP. Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am J Physiol Heart Circ Physiol 306: H396–H404, 2014. doi: 10.1152/ajpheart.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol (1985) 71: 1837–1842, 1991. doi: 10.1152/jappl.1991.71.5.1837. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 14: 461–471, 2013. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. doi: 10.1113/jphysiol.2012.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang HT, Laughlin MH, Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol Heart Circ Physiol 279: H1890–H1897, 2000. doi: 10.1152/ajpheart.2000.279.4.H1890. [DOI] [PubMed] [Google Scholar]