Abstract
Pressure-induced constriction (PIC) is an inherent response of small arteries and arterioles in which increases in intraluminal pressure evoke vasoconstriction. It is a critical mechanism of blood flow autoregulation in the kidney and brain. Degenerin (Deg) and transient receptor potential (Trp) protein families have been implicated in transduction of PIC because of evolutionary links to mechanosensing in the nematode and fly. While TrpC6 has been suggested to contribute to PIC signaling, direct supporting evidence is contradictory. Therefore, the aim of this study was to determine the importance of TrpC6 in PIC signaling using a mouse model lacking TrpC6. To address this aim, we evaluated graded pressure (20–90 mmHg), depolarization (4–80 mM KCl)-, and adrenergic receptor (phenylephrine; PE 10−7–10−4 M)-mediated constriction of isolated middle cerebral artery (MCA) segments from 9-wk-old male wild-type (TrpC6+/+, n = 7) and homozygous null (TrpC6−/−, n = 9) TrpC6 mice (Jackson Laboratories). Isolated MCA segments were cannulated and pressurized with physiological salt solution using pressure myography (Living Systems). Vasoconstrictor responses to KCl and PE were identical in TrpC6−/− and TrpC6+/+ mice. In contrast, PIC responses were totally abolished in TrpC6−/− mice. At 90 mmHg, the calculated myogenic tone was −0.8 ± 0.5 vs. 10.7 ± 1.7%, P = 0.0002 in TrpC6−/− and TrpC6+/+ mice, respectively. Additionally, there were no changes in mechanical properties of circumferential wall strain and stress or morphological properties of wall thickness and wall-to-lumen ratio at 50 mmHg between TrpPC6−/− and TrpC6+/+ mice. Although these results demonstrate that TrpC6 is critical for the integrated PIC response, they do not identify whether TrpC6 acts as a mechanosensor or a downstream signaling component.
NEW & NOTEWORTHY Pressure-induced, but not agonist-induced, vasoconstriction is abolished in the middle cerebral artery (MCA) of TrpC6 null mice. TrpC6 localization in dissociated cerebral vascular smooth muscle cells is primarily cytoplasmic and not associated with the surface membrane where a mechanoelectrical coupler might be expected. These findings suggest that TrpC6 is required for transduction of pressure-induced constriction in the MCA; however, its role as a mechanoelectrical coupler or downstream signal amplifier remains unresolved.
Keywords: degenerin, mechanotransduction, myogenic
INTRODUCTION
Small arteries and arterioles in certain organs, including the brain and kidney, have an inherent ability to sense and react to changes in intraluminal pressure: dilating in response to decreases, and constricting in response to increases in intraluminal pressure (9, 13, 32). This response is called myogenic constriction and/or pressure-induced constriction (PIC) (1). It is an inherent property of vascular smooth muscle cells (VSMCs) and is a critical mechanism of local blood flow autoregulation, matching local blood flow to metabolic needs. This mechanism also protects delicate microvessels from exposure to damaging high systemic blood pressure (2, 8, 31).
PIC signaling is mechano-dependent. Signaling is initiated when increases in intraluminal pressure result in a longitudinal stretch of VSMCs oriented circumferentially around the vessel. VSMC stretch activates a mechanoreceptor current (MRC), eventually leading to VSMC membrane depolarization, Ca2+ influx, smooth muscle contraction, and vasoconstriction (7, 11–13). Numerous molecules have been proposed to contribute to mechanotransduction including, but not limited to, the extracellular matrix, integrins, cytoskeleton, second messenger systems, and ion channels (14, 19). Among ion channels, members of the degenerin (Deg) and transient receptor potential (Trp) families have received attention as candidate sensors.
Both Deg and Trp channels have an evolutionary link to mechanosensing in sensory neurons in model organisms (nematode and fly). Consistent with the concept of evolutionary conservation of function, numerous in vitro and in vivo studies show certain Deg channel proteins (βENaC, γENaC, ASIC2) that are required for normal transduction of PIC in mammalian VSMCs in small arteries and arterioles of the brain and kidney (16, 20, 23, 24, 35, 39, 40). At least one study demonstrates the importance of βENaC as a mediator of mechanoreceptor currents in renal VSMCs (7).
Trp channels comprise a large family of ion channels with several subfamilies including TrpM, TrpV, TrpA, TrpN, and TrpC (canonical) classes of channels (45). Among other Trp channel subfamilies, TrpC channels are expressed in VSMCs (15). TrpC channels form homo- or heterotetramer complexes and are activated by G protein-coupled receptors (GPCRs) (41, 56). Among the TrpC channels, TrpC6 acts as a key mediator of vascular smooth muscle function (15, 29, 40, 43, 50, 54, 59). The role of TrpC6 channels has been proposed in myogenic constriction as well as in autoregulation of cerebral blood flow (CBF) in normotensive and hypertensive animals by recent studies (52, 53). Several lines of evidence suggest that TrpC6 contributes to mechanosignaling responses. First, transient silencing of TrpC6 in isolated middle cerebral and posterior cerebral arteries using antisense oligo DNA or small interfering (si) RNA, respectively, abolishes PIC (30, 59). Second, coexpression of TrpC6 with GPCRs encodes sensitivity to osmotic stretch in isolated heterologous systems (39, 40). However, not all studies support a role for TrpC6 in PIC signaling. Deitrich et al. (15) generated a TrpC6 knockout mouse model characterized by an enhanced generalized vasoconstrictor ability and intact PIC in large-diameter cerebral arteries. The specific identity of those cerebral vessels was not disclosed (15). Our laboratory is interested in determining whether and how Deg and Trp signaling in PIC may be linked. However, to address the conflicting role of TrpC6 in PIC, we examined PIC in the TrpC6−/− model using traditional approaches in a small-artery model, which is an excellent ex vivo model to study the PIC responses of the cerebral small-resistance arteries (6, 24, 55). Therefore, the purpose of this study was to clarify the importance of TrpC6 to PIC- and agonist-induced constriction in middle cerebral artery segments. To address this, we examined PIC, depolarization-induced contraction, α-adrenergic receptor agonist-induced contraction, and vessel morphology in adult male TrpC6 knockout and wild-type mice.
MATERIALS AND METHODS
Animals
All studies were conducted in genetically modified TrpC6 knockout (TrpC6−/−; n = 9) and wild-type (TrpC6+/+; n = 7) male mice, 8–10 wk of age. Body weight was higher in TrpC6 knockout than wild-type mice (Table 1). Mice were provided with standard rodent chow (0.4% Na+, Teklad) and water ab libitum and maintained in a vivarium with a 12:12-h light-dark cycle. Male knockout mice were obtained from The Jackson Laboratory (stock no. 37345) and crossed with female wild-type mice of mixed genetic background within our mouse colony, repeatedly selecting for knockout allele, for about six to eight generations. Heterozygous mating pairs were then used to generate TrpC6 homozygous null mice. Thereafter, mating pairs were maintained as homozygote × homozygote. Offspring were genotyped at 3 wk of age using DNA isolated from tail samples (DirectTail PCR, Viagen), and genotypes were reconfirmed from liver samples following phenotypic analysis. Genotypic analysis was performed using PCR. Oligonucleotide sequences and amplification conditions were obtained from The Jackson Laboratory instructions. PCR products were separated on agarose gels and visualized using GelCodeRed. All protocols and procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Table 1.
Age, body weight, wall thickness, and wall-to-lumen ratio of wild-type and TrpC6−/− mice
| TrpC6 Genotype |
P Value | ||
|---|---|---|---|
| +/+ | −/− | ||
| n | 9 | 7 | |
| Age, wk | 8.8 ± 0.3 | 9.0 ± 0.3 | 0.29 |
| Body weight, g | 30.2 ± 0.8 | 25.8 ± 0.4 | 0.0005 |
| Wall thickness, µm | 13.4 ± 0.8 | 14.3 ± 1.5 | 0.55 |
| Wall-to-lumen ratio | 0.3 ± 0.02 | 0.3 ± 0.04 | 0.69 |
Values are means ± SE; n = number of mice. Data were compared using independent 2-tailed t tests.
Immunolabeling of TrpC6 in Enzymatically Dissociated Cerebral VSMCs
Enzymatic dissociation of cerebral VSMCs.
To immunolabel TrpC6 in cerebral VSMCs, male TrpC6+/+ and TrpC6−/− mice were anesthetized with isoflurane, cervically dislocated, and given a pneumothorax. After the skull was removed, the brain was placed in cold Hanks' balanced salt solution (HBSS) and surface cerebral vessels were dissected and maintained on an ice bath. Cerebral vessels were gently spun to pellet, and the HBSS was decanted. The vessels were then incubated at 37°C for 15 min in 4 mL of an enzymatic solution containing 78 U papain and 3 mg dithioerythritol (DTT). Following incubation, the vessels were pelleted and the enzymatic solution was discarded. A second incubation in 4 mL of an enzymatic solution containing 6 U collagenase type II, 3 mg soybean trypsin inhibitor type II, and 3 mg elastase followed at 37°C for 12 min. Vessels were collected by gentle centrifugation and washed twice in 10 mL of HBSS. Vessels were resuspended in 200 μL of HBSS, then gently triturated to release VSMCs with a series of fire-polished, plugged Pasteur pipettes in decreasing diameter, then passed over a 70-μm filter to remove any undigested debris. The collected VSMCs were fixed in 4% paraformaldehyde for 10 min before being pipetted onto slides, and the liquid was allowed to evaporate and stored at room temperature until staining.
Immunolabeling of enzymatically dissociated VSMCs.
Samples were rehydrated in distilled water, rinsed in PBS three times, 5 min each, blocked in 5% normal donkey serum (NDS) for 1 h, and then incubated with primary antibody targeted to the C-terminal region of human TrpC6 (rabbit anti-TrpC6, US Biologicals catalog no.146114, 1:100, overnight at room temperature) or TrpA1 (rabbit anti-TrpA1, Abcam no.68847, 1:100, overnight at room temperature) in 5% NDS. Samples were colabeled with mouse anti-α-smooth muscle actin (1:100, Sigma) as a VSMC marker. Samples were rinsed in PBS three times and then incubated with secondary antibody solution of donkey anti-mouse Alexa 488 (1:1000, Molecular Probes) and donkey anti-rabbit Alexa 546 (1:500, Invitrogen) for 1 h at room temperature. Following final rinses in PBS, samples were coverslipped and imaged on a Leica TCS SP8 confocal microscope using a ×60 objective and ×5 optical zoom. Individual channels were scanned sequentially, and laser power, gain, and offset were adjusted to permit a faint signal for TrpC6 in the knockout samples and TrpA1 in no primary antibody controls. All samples comparing TrpC6 were scanned under identical conditions and imaged side by side. All samples comparing TrpA1 were also scanned under identical conditions and imaged side by side. Quantitation of TrpA1 mean relative fluorescence, presented as relative gray values, was obtained in regions of interest (ROIs) drawn around single cells using Leica software. The TrpA1 signal was normalized to α-smooth muscle actin (relative gray values) and/or cell area (µm2). Images were prepared identically for publication in Adobe Photoshop.
Vascular Reactivity
Isolation of middle cerebral arteries.
Mice were anesthetized with isoflurane and decapitated. Brains were immediately removed and placed in ice-cold physiological salt solution (PSS) composed of (in mM/L) 130.0 NaCl, 4.0 KCl, 1.8 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 6.0 glucose, 4.0 NaHCO3, 10 HEPES, and 0.03 EDTA, which was equilibrated with a gas mixture of 95% O2 and 5% CO2, at pH ∼7.4. Middle cerebral arteries (MCAs; ~50.0-μm inner diameter at 50 mmHg) were isolated using microsurgical instruments and a boom-stand stereo microscope (Zeiss).
Cannulation of isolated MCAs.
After isolation, MCAs were cannulated onto glass cannulas in a pressure-flow chamber, and equilibrated at 0 mmHg for 30 min, then at 50 mmHg for 15 min at 37°C. Inflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VT). The inner and outer diameters were measured by video microscopy with a microangiometer using MetaMorph 6.1 software.
Depolarization- and agonist-induced constriction.
Increasing concentrations of KCl (4, 20, 40, and 80 mM, 3-min incubations) were used to test the depolarization-induced constrictor responses at the beginning of the experiments, and α1-receptor agonist phenylephrine (PE; 10−7–10−4 M), was used to test the viability of the vessels at the end of the experiment at 50 mmHg, ensuring that samples lacking myogenic responsiveness were still viable.
Myogenic responses of the MCA.
Active diameter changes of MCAs were measured to stepwise increases in intraluminal pressure (15–90 mm Hg with 15-mmHg increments for 5 min) in Ca2+-containing PSS. Then, the bath solution was exchanged for Ca2+-free PSS composed of (in mM/L) 130.0 NaCl, 4.0 KCl, 1.2 MgSO4, 1.2 KH2PO4, 6.0 glucose, 4.0 NaHCO3, 10 HEPES, 0.03 EDTA, and 2.0 EGTA, which was equilibrated with a gas mixture of 95% O2 and 5% CO2, at pH ∼7.4 (24).
Calculations
Myogenic tone (%) was calculated using the formula [(DP−DA)/DP] × 100, where DP is passive diameter, and DA is active diameter of the vessels at a given intraluminal pressure value. Circumferential strain of the vessel wall was calculated using the formula [(DP–D15)/D15], where DP is the passive diameter at a given intraluminal pressure, and D15 is the passive diameter at 15 mmHg under Ca2+-free conditions. Circumferential stress was calculated using the formula P × DP/(2 × WT), where DP is passive diameter, WT is wall thickness, and P is intraluminal pressure (where 1 mmHg = 1.334 × 102 N/m2) under Ca2+-free conditions.
Statistics
All data are expressed as means ± SE and analyzed using an independent two-tailed t test or two-way repeated-measures ANOVA where appropriate. Differences among groups were determined using Sidak’s multiple comparison post hoc test. Statistics were performed using GraphPad Prism 7.02 software. The specific statistical test applied is stated in the figure legends. Statistical significance was considered at P < 0.05.
RESULTS
TrpC6 Is Expressed in Dissociated Cerebral VSMCs
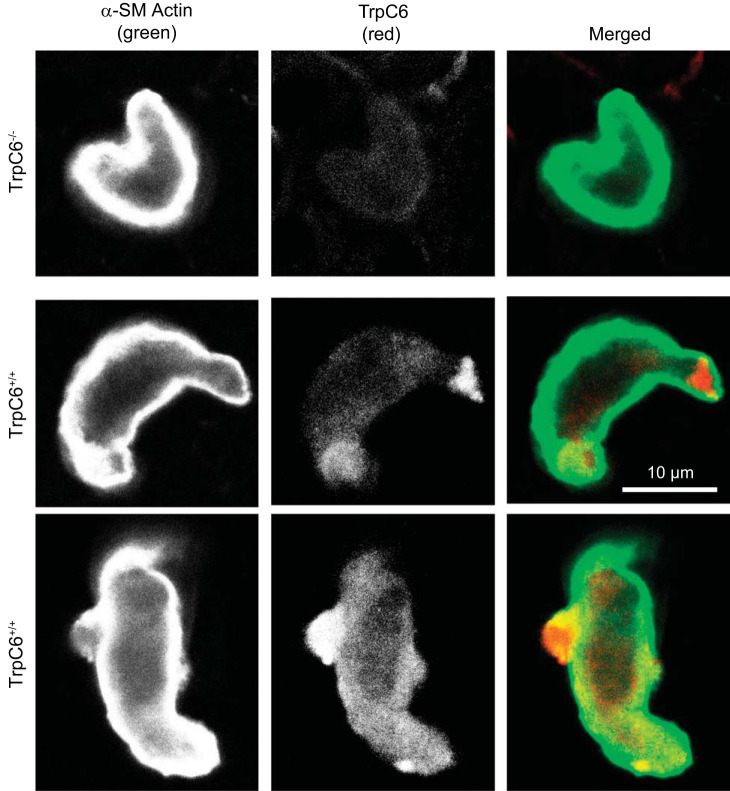
Smooth muscle cells, identified by intense labeling with anti-α-smooth muscle actin (green), also expressed TrpC6 (red) (Fig. 1). The signal for TrpC6 was fairly weak with a mostly cytoplasmic distribution pattern that was distinctly different than the near membrane-associated pattern of α-smooth muscle actin. Similar images were obtained in TrpC6−/− (n = 5) and TrpC6+/+ (n = 7) VSMCs.
Fig. 1.
Transient receptor potential family member (TrpC6) is expressed in the cytoplasm of dissociated cerebral vascular smooth muscle cells (VSMCs). VSMCs were enzymatically dissociated from a mixed pool of surface cerebral vessels, and their branches from a wild-type and TrpC6−/− animal were then labeled with mouse anti-α-smooth muscle (SM) actin (green; far left) and rabbit anti-TrpC6 (red; middle). The merged image is shown at right. A faint signal is present in the TrpC6−/− VSMCs; however, a greater signal, mostly cytoplasmic, is observed in the TrpC6+/+ VSMCs.
Agonist-Induced Constrictor Responses in Isolated MCAs
Changes in the inner diameter in response to KCl (4-80 mM) and PE (10−7–10−4 M) are shown in Fig. 2, A and B, respectively. Normalized vasoconstriction responses to the highest doses of KCl (45.5 ± 1.1 vs. 40.9 ± 1.2%, P = 0.2) were similar between TrpC6−/− and TrpC6+/+ mice, respectively (Fig. 2A). Vasoconstriction responses to PE (10−4 M, 37.4 ± 1.7 vs. 38.7.0 ± 1.2%, P = 0.8) were identical between TrpC6−/− and TrpC6+/+ mice, respectively (Fig. 2B).
Fig. 2.
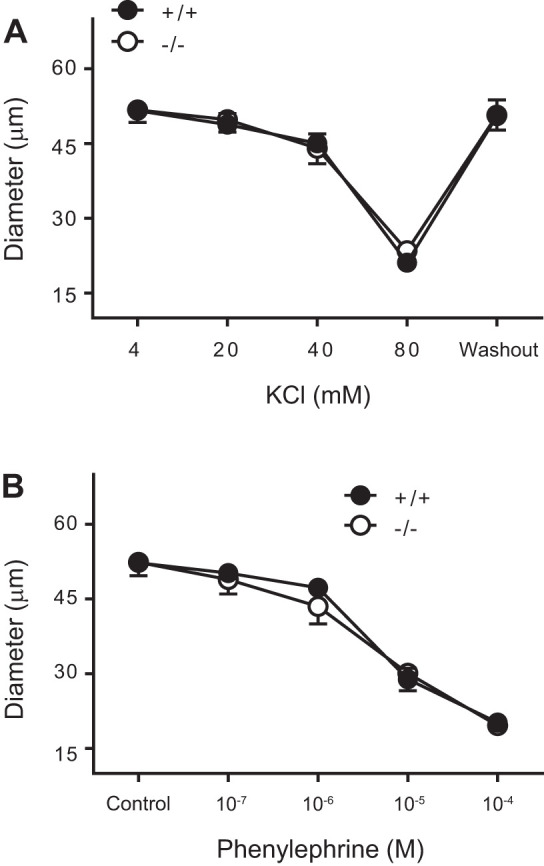
Vasomotor responses of isolated middle cerebral arteries (MCAs) to depolarizing, and α-adrenoreceptor agonist agents are not different. A: vasoconstriction responses of isolated MCAs from TrpC6+/+ (n = 7) and TrpC6−/− (n = 9) mice to KCl were identical. B: vasoconstriction responses of isolated MCAs from TrpC6+/+ and TrpC6−/− mice to phenylephrine (PE) were similar.
Pressure-Induced Constriction of Isolated MCAs
Vasoconstriction responses and calculated myogenic tone of isolated MCAs to stepwise increases in intraluminal pressure are shown in Fig. 3. The active (Ca2+-containing) and passive (Ca2+-free) inner diameters of MCAs from TrpC6+/+ (n = 7) and TrpC6−/− (n = 9) mice are shown in Fig. 3, A and B, respectively. There was no difference in passive responses between TrpC6+/+ and TrpC6−/− mice. Calculated myogenic tone for both groups is shown in Fig. 3C. MCAs from TrpC6+/+ mice developed increasing myogenic tone from 15 to 90 mmHg. In contrast, myogenic tone remained unchanged despite increases in intraluminal pressure and did not increase above −0.8 ± 0.5% (at 90 mmHg) in MCAs from TrpC6−/− mice. At 90 mmHg, myogenic tone was abolished in TrpC6−/− mice (−0.8 ± 0.5 vs. 10.7 ± 1.7% in TrpC6+/+, P = 0.0002, Fig. 2C). The log-pressure vs. myogenic tone relationship, and the slope of this relationship are shown in Fig. 4, A and B, respectively. The slope of the log-pressure vs. myogenic tone was significantly lower in the TrpC6−/− compared with TrpC6+/+ MCAs (1.12 ± 0.6 vs. 8.0 ± 1.9, P = 0.002, respectively, Fig. 4B). This finding provides further support that PIC was abolished in the TrpC6−/−. Moreover, the slope of this relationship in TrpC6−/− mice was statistically not different from zero (2-tailed independent t test, Fig. 4B), confirming that the PIC response was abolished.
Fig. 3.
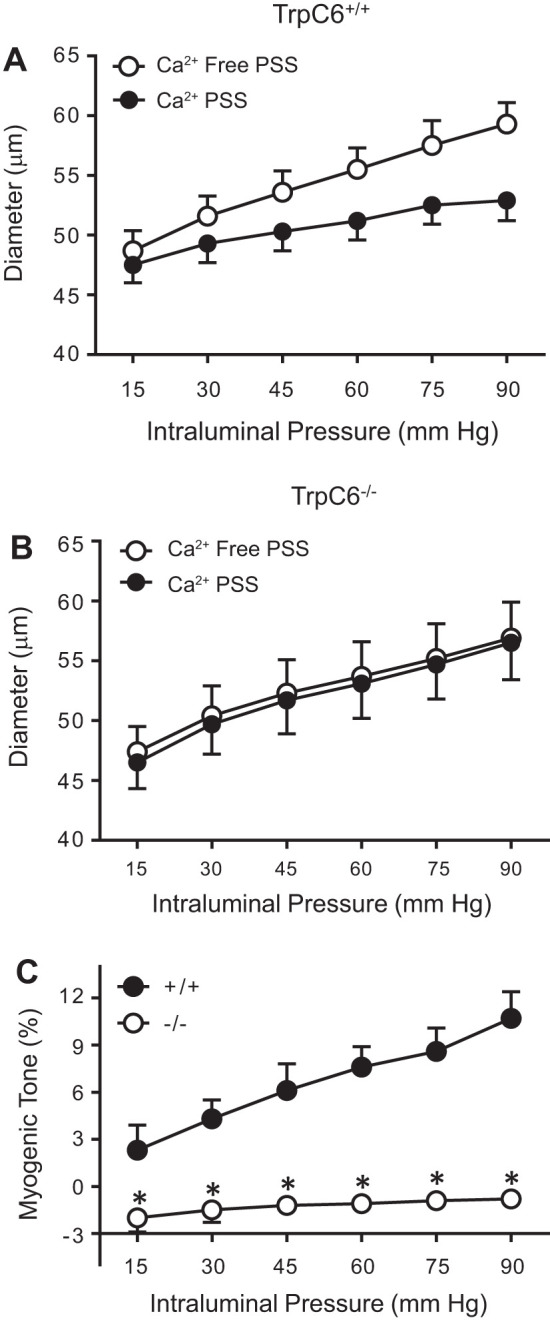
Pressure-induced vasoconstriction of isolated middle cerebral arteries (MCAs). A: pressure-induced constriction of isolated MCAs from TrpC6+/+ (n = 7) mice in response to stepwise increases in intraluminal pressure under active (Ca2+-containing) conditions is lower compared with that of under passive (Ca2+-free) conditions. B: pressure-induced vasoconstriction of isolated MCAs from TrpC6−/− (n = 9) mice in response to stepwise increases in intraluminal pressure under active (Ca2+-containing) conditions is similar to that of under passive (Ca2+-free) conditions. Two-way repeated-measures ANOVA showed an effect of intraluminal pressure (P < 0.0001) and passive dilation (P = 0.0022) and an interaction between the Ca2+-free condition and intraluminal pressure (P < 0.0001). C: myogenic tone of isolated MCAs from TrpC6+/+ (n = 7) and TrpC6−/− mice (n = 9). Two-way repeated-measures ANOVA showed an effect of intraluminal pressure (P < 0.0001) and genotype (P = 0.0123) and an interaction between genotype and intraluminal pressure (P = 0.0002). Sidak’s post hoc test showed a significant effect of genotype on myogenic tone at 15 (P = 0.0237), 30 (P = 0.0009), 45 (P < 0.0001), 60 (P < 0.0001), 75 (P < 0.0001), and 90 (P < 0.0001) mmHg pressure values.
Fig. 4.
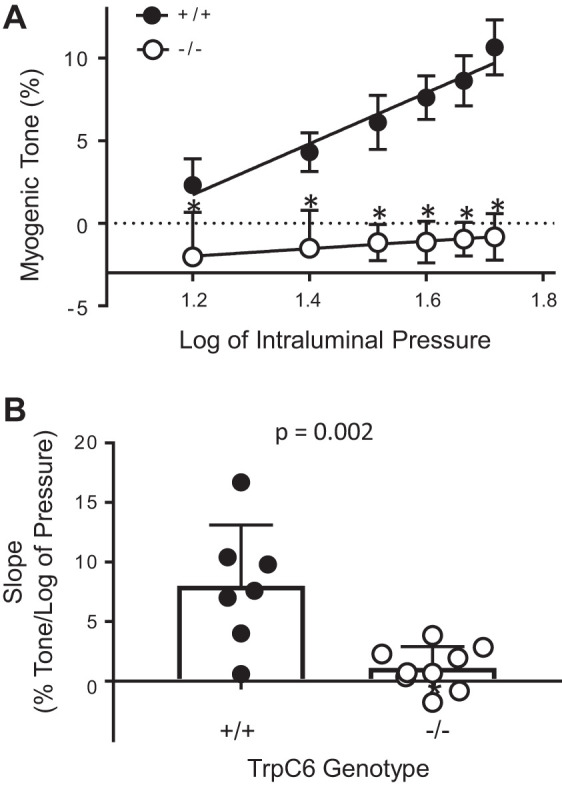
Log of intraluminal pressure and myogenic tone relationship and the slope of the myogenic tone of isolated middle cerebral arteries (MCAs) from TrpC6+/+ and TrpC6−/− mice. Log of pressure and myogenic tone relationship (A) and slope (or sensitivity) of the myogenic tone (B) were significantly reduced in TrpC6−/− compared with TrpC6+/+ mice. The slope of the myogenic tone of TrpC6−/− mice was significantly smaller (P = 0.0002, independent 2-tailed t test). The slope of the log of pressure vs. myogenic tone relationship in TrpC6−/− mice was not different from zero (independent 2-tailed t test).
Mechanical Properties of Isolated MCAs
To determine whether differences in mechanical properties might contribute to altered PIC responses, we calculated the circumferential wall strain and stress under Ca2+-free conditions. The circumferential strain (0.2 ± 0.02 vs. 0.2 ± 0.02, P = 0.8, Fig. 5A) and stress (2.7 ± 0.1 vs. 3.1 ± 0.4, P = 0.4, Fig. 5B) at 90 mmHg, and the circumferential stress were identical in TrpC6−/− and TrpC6+/+ mice, respectively. This finding suggests that mechanical properties of the isolated MCAs do not account for differences in PIC responses in the TrpC6−/− mice. To determine whether structural remodeling occurs in TrpC6−/− mice, we calculated the wall thickness and wall-to-lumen ratio and found them identical between TrpC6−/− and TrpC6+/+ mice (Table 1).
Fig. 5.
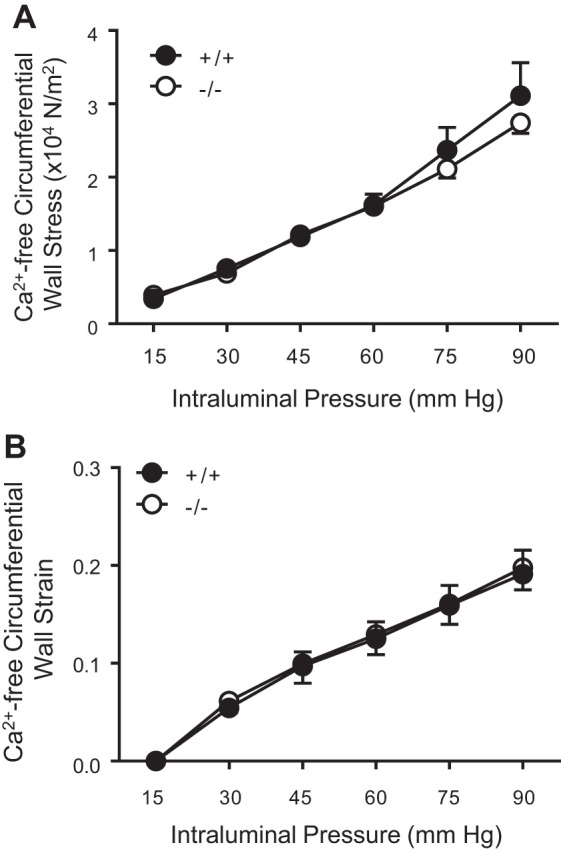
Mechanical properties of isolated middle cerebral arteries (MCAs) from TrpC6+/+ and TrpC6−/− mice. Circumferential wall strain (A) and stress (B) under Ca2+-free conditions are identical between TrpC6−/− and TrpC+/+ mice.
Expression of TrpA1 Is Not Different in Cerebral VSMCs Isolated from TrpC6−/− and TrpC6+/+ Mice
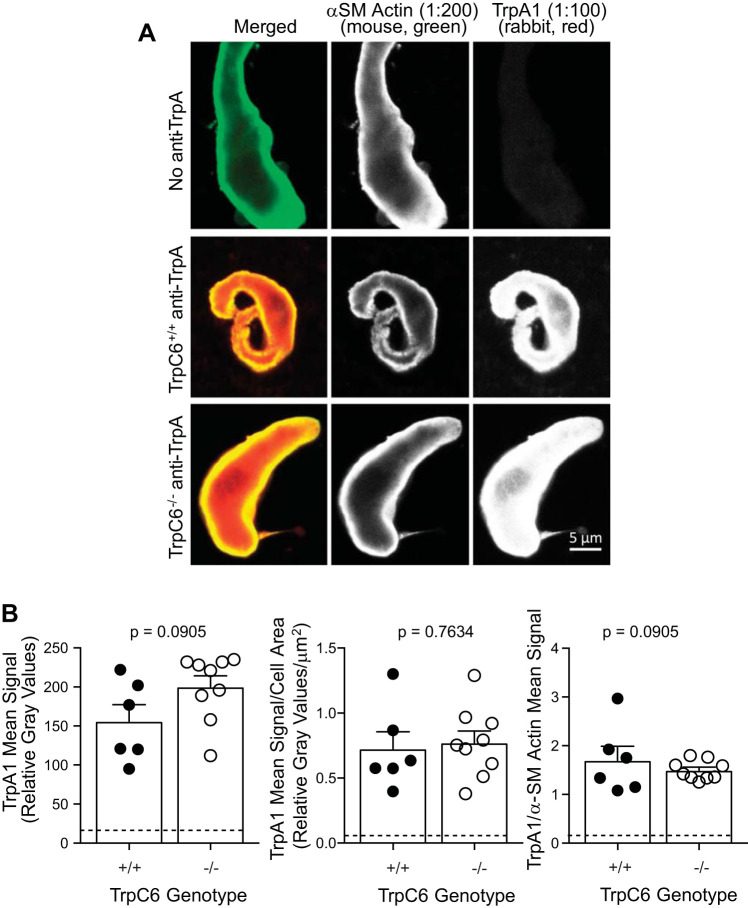
To determine whether TrpA1 expression might be altered in cerebral VSMCs from TrpC6−/− mice, we used semiquantitative immunolabeling (Fig. 6, A and B). Representative images are provided in Fig. 6A, and quantitative group data are provided in Fig. 6B. We found no significant difference in the TrpA1 signal or TrpA1 signal normalized to cell area or α-smooth muscle actin labeling (Fig. 6B).
Fig. 6.
TrpA1 expression is not altered in cerebral vessels and vascular smooth muscle cells (VSMCs) from TrpC6−/− mice. Representative (A) and group (B) data for TrpA1 immunolabeling in isolated cerebral VSMCs. A: immunolabeling for TrpA1 in representative dissociated cerebral VSMCs is shown. Single-color images are shown in white to aid in visualization of intensity gradations. The first column represents α-smooth muscle (SM) actin labeling in (green in merged image), the middle column represents TrpA1 labeling (red in merged image), and the last column represents a merged image of α-SM and TrpA1. Top row: dissociated cerebral VSMC from a TrpC6−/− animal labeled with α-SM actin and both secondary antibodies, but no rabbit anti-TrpA1 antibody, served as a negative control. Middle row: VSMC from a TrpC6+/+ animal showing TrpA1 expression is robust and associated with α-SM actin which is localized just below the membrane in freshly isolated VSMCs. Bottom row: VSMC from a TrpC6−/− animal showing similar localization and levels of TrpA1. B: quantitation of TrpA1 signaling alone and normalized to cell area and α-SM actin are shown. No differences in TrpA1 signal were found. Data were analyzed using a 2-tailed independent t test, P values are provided.
DISCUSSION
Several Trp channels have been shown to contribute to VSMC mechanosignaling (15, 40, 59). The evidence for the importance of one Trp family channel member, the TrpC6, is equivocal. Studies using pharmacological and transient gene silencing approaches suggest that TrpC6 plays an important role in PIC responses in the middle cerebral and posterior cerebral arteries of the rat (40, 58). In contrast, studies from a TrpC6 knockout mouse model suggest that cerebral VSMC mechanosensing is largely intact in larger cerebral arteries (internal diameter of 170 µm at 40 mmHg) (15). To address this conflict, our laboratory examined PIC in a model arterial segment, the MCA, using a traditional approach of evaluating internal diameter during increasing pressure steps under active and passive conditions in TrpC6−/− mice. The mice used in our studies were obtained from a Jackson Laboratory strain derived from the TrpC6−/− mice used in Dietrich et al. (15), then backcrossed onto a mixed genetic background of our mouse colony. The TrpC6−/− mice have higher body weight compared with their wild-type littermates (Table 1.). Dietrich et al. found an increase in blood pressure of ~7 mmHg in TrpC6−/− mice; however, recent studies from Wang et al. (58) show no difference in blood pressure between TrpC6−/− and wild-type mice (15, 58). In our current investigation, we found that MCA PIC responses were totally abolished in TrpC6−/− mice (Fig. 3, B and C). We also found that the TrpC6−/− genotype did not change the contractility, and mechanical or morphological properties of the MCA (Figs. 2 and 5, Table 1). These findings suggest that these properties do not account for the abolished PIC response. The underlying reason(s) for the differences in PIC responsiveness in our current study compared with Dietrich et al. (15) are not entirely clear; however, they may be related to differences in the 1) genetic background and 2) cerebral artery segment studied. We favor the latter since the cerebral artery segment studied in Dietrich et al. was not identified, and the mechanism underlying VSMC mechanosensing may vary in different vessels. This is likely since the role of degenerins in mediating PIC in small mesenteric arteries is very different from cerebral and renal arteries (17, 18, 36).
PIC is a well-characterized response, having originated over 100 years ago (1). The response is a powerful mechanism of local blood flow autoregulation in small arteries and arterioles of the brain, kidney, skeletal muscle, and heart (5, 8, 23, 38). While numerous molecules (integrins, extracellular matrix, cytoskeleton, ion channels, etc.) are required for the entire signaling cascade, from force transduction to vasoconstriction, the specific role of certain molecules contributing to the response still remain controversial (19, 34, 46, 48). Despite the historic nature of PIC, the mechanisms underlying initiation of the response remain of current scientific interest because the vasoconstriction response to increases in perfusion pressure play a critical role in protecting small delicate capillaries, particularly in the kidney and brain, from baro-trauma associated with hypertension, a contributing factor in end-stage renal disease and stroke (4, 20, 49). PIC responses are often attenuated or lost in chronic inflammatory diseases, increasing susceptibility to end-organ injury, i.e., changes in vascular structure, molecular composition, and function due to chronic exposure to high pressure (20, 25).
We can gain insight into the identity of these molecules by applying our knowledge of a mechanotransduction signaling heuristic developed in sensory neurons of model organisms (27). This heuristic states that transduction of force into a response can be simplified into basic steps including 1) modal-specific stimulus to activate the mechanoreceptor; 2) mechanoelectrical transduction event (i.e., mechanoreceptor current; MRC); 3) post-mechanoreceptor current signal amplification, gain control, and transmission; and 4) integrated response (27) This heuristic is applicable to PIC VSMCs with a few modifications. One modification is the modal-specific stimulus. In VSMCs, the modal-specific stimulus is a longitudinal stretch while embedded in an extracellular matrix. The integrated response is VSMC shortening or vasoconstriction. Our laboratory is using this heuristic and the concept of evolutionary conservation of function to distinguish the role of proteins contributing to mechanosensing in VSMCs.
Evidence from model organism systems suggest at least three different protein families that likely mediate the mechanoelectrical transduction event, which is quantified as a mechanoreceptor current. These include Piezo, Deg, and Trp protein families. While Piezo proteins (Piezo1 and Piezo2) have not yet been implicated in PIC, GPCR-coupled Trp channels and Deg channels have been linked to VSMC mechanoelectrical transduction (20, 24, 40, 58). Several conclusions can be drawn from studies in lower-order species. First, most model organism mechanoreceptor neurons express both Trp and Deg channels (26, 40). Second, the identity of the MRC channel is population specific; it is either Deg or Trp, but not both. Third, only members of the TrpN family, which are not found in mammals, form major carriers of MRCs (45). Other Trp family members do not form mechanosensors in model organisms. Fourth, in mechanosensors where Deg channels mediate the MRC, Trp channels contribute to post-MRC signaling (27). Since VSMCs express both Trp and Deg channels, and at least one Deg protein, the βENaC, mediates MRCs in VSMCs, it is likely that Trp channels contribute to post-MRC signaling (7). Thus, if the roles of Deg and Trps are evolutionarily conserved in mammalian VSMCs, then Deg and Trp channels probably work together to signal PIC in a similar manner. These points support the argument that in VSMCs, Deg channels likely carry the MRC and Trp channels for the post-MRC signal.
Link Between Degenerin Channels and PIC Transduction
Multiple lines of evidence support a role for degenerin proteins, particularly βENaC, γENaC, and ASIC2, in myogenic signaling (20, 24, 35). Myogenic constriction, but not agonist-induced constriction, is abolished in renal and cerebral small artery/arteriole segments following pharmacological inhibition with amiloride and benzamil, transient gene silencing using siRNA or dominant negative molecules, and in genetically ASIC2- and βENaC-modified mice (24, 37, 40, 55). Additionally, VSMC MRCs are abolished in mice with reduced expression of βENaC, suggesting that βENaC may mediate the mechanoelectrical coupling event in the mechanosensing heuristic discussed earlier (55).
Trp Channel Family and Its Link to Mechanosensing
Trp channels comprise a large family of ion channels, with several subfamilies including TrpM, TrpV, TrpA, TrpN, and TrpC, classes of channels (45). Most Trp channels are conserved in mammals; however, TrpN channels are the exception, as previously noted (45). In lower-order species, TrpN family members NOMPC (Drosophila melanogaster) and Trp4 (nematode) channels mediate MRCs in sensory neurons (57). TrpA contributes to a minor portion (~10%) of the MRC current in the fly (22). Since TrpN are not expressed in mammals and other Trp families do not mediate mechanoreceptor currents in model organisms, there is no evidence of an evolutionary link of mammalian Trp channels as mediators of MRCs in model systems at this time. Moreover, in model organisms where degenerins mediate MRCs, Trp channels contribute to post-MRC signaling (27). Since Deg channels appear to mediate MRCs in VSMCs, it is likely a similar relationship between Deg and Trp channels occurs in VSMCs.
Evidence that TrpC6 Contributes to PIC
Several Trp channels have been implicated in VSMC mechanosensing (21, 45). This includes TrpC ion channels, such as TrpC6 channels, that are also expressed in cerebral arteries (3). The physiological role of TrpC6 channels has been proposed in myogenic constriction and CBF autoregulation in both normotensive and hypertensive animals (52, 53). Toth et al. (52) found that MCAs of young, but not aged mice adapt to hypertension. Additionally, in young hypertensive mice, the pressure-flow relationship shifts toward higher pressure values, which is not shown in aged hypertensive mice (53). These studies conclude that aging impairs the adaptation to hypertension and likely involves the downregulation of TrpC6 channels. Similarly, several studies suggest that suppression of endogenous vascular TrpC6, using oligodeoxynucleotides or siRNA, inhibits PIC in cerebral arteries (40, 58). Our current findings in TrpC6 knockout mice are consistent and confirm that TrpC6 channels are absolutely required for PIC in the MCA. While the data support an important role for TrpC6, they do not identify the role that TrpC6 channels play. Since PIC is a multistep, serial signaling response, the role of TrpC6 as a sensor or amplifier cannot be determined from these studies. However, the localization pattern of TrpC6 observed in this study is primarily cytoplasmic. A protein acting as a mechanoelectrical coupler might be expected to be concentrated at or near the surface membrane where force is transduced, such as the degenerins (16, 24, 35, 36, 55). TrpA1 is a Trp channel with an evolutionary link to mechanosensing in lower order species and mediates ~10% of the MRC in specific Drosophila sensory neurons (22). TrpA1 has been shown to be implicated in nociception, thermal, and oxygen sensing in endothelial cells, VSMCs, and sensory neurons (10, 47, 51). To determine whether alterations in TrpA1 expression might contribute to our findings, we examined TrpA1 expression in cerebral VSMCs using semiquantitative immunolabeling and found no difference between TrpC6−/− and TrpC6+/+ mice (Fig. 6). Based on our findings that vasoconstriction to depolarizing stimuli and α-adrenergic receptor agonists were identical in MCA segments from Trp6C−/− and TrpC6+/+ mice, it is unlikely that compensatory changes in 1) expression of other Trp channels, such as TrpC3 or 2) Ca2+ mobilization dynamics, important in vascular reactivity, are enhanced in TrpC6−/− MCA segments.
Why Is There a Prevailing Notion that Trp Channels Are the Sensors?
The concept that Trp channels are the sensors has probably evolved from perpetuation of misinterpreted information. First is the identification of a Trp channel, NOMPC, as a transducer of MRCs in Drosophila. NOMPC is a member of the TrpN subfamily, and as noted earlier, TrpN is not conserved in mammals (57). With the exception of TrpA, which can carry a minor current (10% of the MRC) in Drosophila sensory neurons, other Trp subfamilies do not mediate MRCs in model organisms (22). Thus, there is no evolutionary link of mammalian Trp channels as primary mediators of MRCs in model organism systems.
A second contributing factor to the potential misunderstanding of the role of Trp channels in PIC is the concept that all mechanical stimuli are created equal. Many peripheral touch receptors in our skin and muscle/tendons are modal specific; they are most sensitive to a specific type of stimulus (33). Non-modal-specific mechanical stimuli, such as hyposmotic swelling-induced membrane stretch, has often been used as a substitute for elongation/stretch (modal specific for VSMCs) because of its feasibility (28). Based on the concept of modal-specific stimulation, an osmotic stimulus probably activates osmoreceptors (42). Since many nematode Trp channels function as osmoreceptors, such as TrpV channels, it is not surprising that activation of VSMCs or heterologous cells using an osmotic stimulus would activate a Trp channel (44). In model organisms where Deg channels mediate MRCs, Trp channels contribute to post-MRC signaling (27). A similar relationship between Deg and Trp channels likely occurs in VSMCs (27).
Important questions remaining to be addressed include 1) how do VSMC Deg and Trp channels contribute to the PIC (as sensors or amplifiers) and 2) how are Deg and Trp channel signaling coupled/linked? Addressing these questions will require dissection of the different mechanosignaling steps, i.e., MRC and post-MRC events. The development of the in vitro stretch assay is a step forward.
Summary and Conclusion
As our study demonstrates, TrpC6 channels are critical to the overall integrated response of pressure-induced constriction in the MCA, but not vasoconstriction per se. Mechanosignaling is a multimolecule, serial signaling event. However, how and where in the signaling pathway Trp channels contribute, remains to be determined.
GRANTS
This work was supported by National Institutes of Health Grants P20GM104357, P20GM121334, P20121334, P01HL051971, and R01HL1136684.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research services were provided by the Molecular Genomics at the University of Mississippi Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.N., M.J.R., J.P.G., and H.A.D. conceived and designed research; Z.N., E.H., and H.A.D. performed experiments; Z.N., E.H., M.J.R., J.P.G., and H.A.D. analyzed data; Z.N., M.J.R., J.P.G., and H.A.D. interpreted results of experiments; Z.N. and H.A.D. prepared figures; Z.N., E.H., and H.A.D. drafted manuscript; M.J.R., J.P.G., and H.A.D. edited and revised manuscript; M.J.R., J.P.G., and H.A.D. approved final version of manuscript.
REFERENCES
- 1.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol 35: 1116–1120, 2008. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol (1985) 111: 1527–1538, 2011. doi: 10.1152/japplphysiol.00895.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng JH, Zhang LF, Gao F, Bai YG, Boscolo M, Huang XF, Zhang X. Mechanics and composition of middle cerebral arteries from simulated microgravity rats with and without 1-h/d -Gx gravitation. PLoS One 9: e97737, 2014. [Erratum in PLoS One 9: e110303, 2014.] doi: 10.1371/journal.pone.0097737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung WS, Weissman JL, Farley J, Drummond HA. βENaC is required for whole cell mechanically gated currents in renal vascular smooth muscle cells. Am J Physiol Renal Physiol 304: F1428–F1437, 2013. doi: 10.1152/ajprenal.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Science, 2009. https://www.ncbi.nlm.nih.gov/books/NBK53081. [Last accessed on 8 June 2020.] [PubMed] [Google Scholar]
- 9.Clifford PS. Local control of blood flow. Adv Physiol Educ 35: 5–15, 2011. doi: 10.1152/advan.00074.2010. [DOI] [PubMed] [Google Scholar]
- 10.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci USA 108: E1184–E1191, 2011. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angelo G, Davis MJ, Meininger GA. Calcium and mechanotransduction of the myogenic response. Am J Physiol 273: H175–H182, 1997. doi: 10.1152/ajpheart.1997.273.1.H175. [DOI] [PubMed] [Google Scholar]
- 12.D’Angelo G, Meininger GA. Transduction mechanisms involved in the regulation of myogenic activity. Hypertension 23: 1096–1105, 1994. doi: 10.1161/01.HYP.23.6.1096. [DOI] [PubMed] [Google Scholar]
- 13.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001. doi: 10.1152/ajpheart.2001.280.4.H1427. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich A, Mederos Y Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989, 2005. [Erratum in Mol Cell Biol 25: 6983, 2005.] doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond HA. βENaC is a molecular component of a VSMC mechanotransducer that contributes to renal blood flow regulation, protection from renal injury, and hypertension. Front Physiol 3: 341, 2012. doi: 10.3389/fphys.2012.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 18.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- 19.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond HA, Stec DE. βENaC acts as a mechanosensor in renal vascular smooth muscle cells that contributes to renal myogenic blood flow regulation, protection from renal injury and hypertension. J Nephrol Res 1: 1–9, 2015. doi: 10.17554/j.issn.2410-0579.2015.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci 92: 394–403, 2013. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gannon KP, McKey SE, Stec DE, Drummond HA. Altered myogenic vasoconstriction and regulation of whole kidney blood flow in the ASIC2 knockout mouse. Am J Physiol Renal Physiol 308: F339–F348, 2015. doi: 10.1152/ajprenal.00572.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008. doi: 10.1152/ajpheart.01380.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ge Y, Gannon K, Gousset M, Liu R, Murphey B, Drummond HA. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced βENaC expression. Am J Physiol Renal Physiol 302: F1486–F1493, 2012. doi: 10.1152/ajprenal.00638.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee TH, Montoya M, Karania S, Garakani AM, Pruitt BL, Goodman MB. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron 71: 845–857, 2011. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geffeney SL, Goodman MB. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 74: 609–619, 2012. doi: 10.1016/j.neuron.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol 586: 5633–5649, 2008. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7: ra49, 2014. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 31.Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70: 687–694, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: implications for local vascular function. Clin Hemorheol Microcirc 34: 67–79, 2006. [PubMed] [Google Scholar]
- 33.Hocker GA. A hypothesis to explain how the sensory cortices respond in the appropriate sensory mode. J R Soc Med 96: 70–73, 2003. doi: 10.1177/014107680309600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 36.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 37.Jernigan NL, LaMarca B, Speed J, Galmiche L, Granger JP, Drummond HA. Dietary salt enhances benzamil-sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol 294: H409–H420, 2008. doi: 10.1152/ajpheart.00571.2007. [DOI] [PubMed] [Google Scholar]
- 38.Johnson PC. Autoregulation of blood flow. Circ Res 59: 483–495, 1986. doi: 10.1161/01.RES.59.5.483. [DOI] [PubMed] [Google Scholar]
- 39.Kim EC, Ahn DS, Yeon SI, Lim M, Lee YH. Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp Physiol 97: 544–555, 2012. doi: 10.1113/expphysiol.2011.062232. [DOI] [PubMed] [Google Scholar]
- 40.Kim EC, Choi SK, Lim M, Yeon SI, Lee YH. Role of endogenous ENaC and TRP channels in the myogenic response of rat posterior cerebral arteries. PLoS One 8: e84194, 2013. doi: 10.1371/journal.pone.0084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobori T, Smith GD, Sandford R, Edwardson JM. The transient receptor potential channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J Biol Chem 284: 35507–35513, 2009. doi: 10.1074/jbc.M109.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Šali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malczyk M, Erb A, Veith C, Ghofrani HA, Schermuly RT, Gudermann T, Dietrich A, Weissmann N, Sydykov A. The role of transient receptor potential channel 6 channels in the pulmonary vasculature. Front Immunol 8: 707, 2017. doi: 10.3389/fimmu.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 45.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 12: 218, 2011. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem 161: 245–254, 2017. doi: 10.1093/jb/mvw082. [DOI] [PubMed] [Google Scholar]
- 47.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev 19: 419–424, 2005. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol 25: 613–618, 2013. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shekhar S, Liu R, Travis OK, Roman RJ, Fan F. Cerebral autoregulation in hypertension and ischemic stroke: a mini review. J Pharm Sci Exp Pharmacol 2017: 21–27, 2017. [PMC free article] [PubMed] [Google Scholar]
- 50.Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 280: 39786–39794, 2005. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- 51.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 52.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137: 1415–1424, 2009. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced βENaC. Am J Physiol Regul Integr Comp Physiol 297: R723–R728, 2009. doi: 10.1152/ajpregu.00212.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev 67: 36–73, 2015. doi: 10.1124/pr.114.009555. [DOI] [PubMed] [Google Scholar]
- 57.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science 287: 2229–2234, 2000. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, doCarmo JM, da Silva AA, Moak SP, Bailey KC, Hall JE. Abstract P192: Trpc6 deficiency causes obesity, impaired glucose tolerance and leptin resistance. Hypertension 72, Suppl_1: 72, 2018. doi: 10.1161/hyp.72.suppl_1.P192. [DOI] [Google Scholar]
- 59.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]