Abstract
Vasodilatory effects of insulin support the delivery of insulin and glucose to skeletal muscle. Concurrently, insulin exerts central effects that increase sympathetic nervous system activity (SNA), which is required for the acute maintenance of blood pressure (BP). Indeed, in a cohort of young healthy adults, herein we show that intravenous infusion of insulin increases muscle SNA while BP is maintained. We next tested the hypothesis that sympathoexcitation evoked by hyperinsulinemia restrains insulin-stimulated peripheral vasodilation and contributes to sustaining BP. To address this, a separate cohort of participants were subjected to 5-s pulses of neck suction (NS) to simulate carotid hypertension and elicit a reflex-mediated reduction in SNA. NS was conducted before and 60 min following intravenous infusion of insulin. Insulin infusion caused an increase in leg vascular conductance and cardiac output (CO; P < 0.050), with maintenance of BP (P = 0.540). As expected, following NS, decreases in BP were greater in the presence of hyperinsulinemia compared with control (P = 0.045). However, the effect of NS on leg vascular conductance did not differ between insulin and control conditions (P = 0.898). Instead, the greater decreases in BP following NS in the setting of insulin infusion paralleled with greater decreases in CO (P = 0.009). These findings support the idea that during hyperinsulinemia, SNA-mediated increase in CO, rather than restraint of leg vascular conductance, is the principal contributor to the maintenance of BP. Demonstration in isolated arteries that insulin suppresses α-adrenergic vasoconstriction suggests that the observed lack of restraint of leg vascular conductance may be attributed to sympatholytic actions of insulin.
NEW & NOTEWORTHY We examined the role of sympathetic activation in restraining vasodilatory responses to hyperinsulinemia and sustaining blood pressure in healthy adults. Data are reported from two separate experimental protocols in humans and one experimental protocol in isolated arteries from mice. Contrary to our hypothesis, the present findings support the idea that during hyperinsulinemia, a sympathetically mediated increase in cardiac output, rather than restraint of peripheral vasodilation, is the principal contributor to the maintenance of systemic blood pressure.
Keywords: autonomic nervous system, blood flow, insulin, muscle sympathetic nerve activity
INTRODUCTION
Beyond its metabolic effects, insulin has vasodilatory actions that increase delivery of insulin and glucose to target tissues, including skeletal muscle (4–7, 15, 32, 40, 57, 61, 69). In isolated arteries, the vasomotor effects of insulin are dependent on the balance between endothelial-derived nitric oxide (NO)-mediated vasodilation and endothelin-1-mediated vasoconstriction, with the net effect typically being vasodilation in the healthy state (16, 17, 31). However, in vivo, endothelin-1 is not the sole opposing force to insulin-stimulated NO-mediated vasodilation. Indeed, the hemodynamic effects of insulin reflect an integrated response of neurohumoral and endothelial-derived vasoactive signals (47, 48). That is, insulin also has centrally mediated effects that activate the sympathetic nervous system, which can limit insulin-induced vasodilation via α-adrenergic receptor-mediated vasoconstriction in healthy young adults (1, 51, 62). In support of this, patients with regional sympathectomy exhibit a more rapid NO-mediated vasodilation in response to insulin in the denervated limb than in the innervated limb (55). Accordingly, via central and peripheral mechanisms, insulin mediates several opposing hemodynamic actions that may serve to concurrently increase skeletal muscle blood flow (and subsequent delivery of insulin and glucose) and induce vasoconstriction in select vascular beds to maintain blood pressure.
However, the extent to which sympathetic nerve activity (SNA) limits insulin-stimulated skeletal muscle blood flow remains largely unknown. Herein our approaches for studying the role of SNA in modulating vasodilatory responses to insulin involved direct measures of SNA using the gold standard technique of microneurography (59), as well as a transient “silencing” of SNA via application of neck suction (NS), which selectively loads the carotid baroreceptors (i.e., simulated carotid hypertension). Neck suction is a method commonly used in human physiology research to study carotid baroreflex control of blood pressure (11) and was employed here, for the first time, as a tool to examine the role of SNA in modulating insulin-stimulated leg blood flow. We hypothesized that sympathoexcitation evoked by hyperinsulinemia would restrain insulin-stimulated leg blood flow and conductance, contributing to the maintenance of blood pressure. More specifically, we hypothesized that during systemic insulin infusion, reducing SNA via neck suction would produce greater peripheral vasodilation and reduction in blood pressure relative to neck suction under basal (nonhyperinsulinemic) conditions. Furthermore, to examine the role of insulin stimulation in modulating α-adrenergic receptor-mediated vasoconstriction, a follow-up proof-of-concept experiment was performed in mouse isolated arteries.
METHODS
Human participants.
All participants were young adults (<40 yr of age), healthy, nonobese [body mass index (BMI) <30 kg/m2], normoglycemic (fasting glucose <100 mg/dL), and nonsmokers without chronic diseases and taking no medications known to affect endocrine, cardiovascular, or autonomic function. Women were not pregnant (confirmed by negative pregnancy test on the morning of the study visit) and were studied in the self-reported early follicular phase of the menstrual cycle (days 1–7) or placebo phase of oral contraceptive use. Participants were asked to refrain from alcohol, caffeine, and exercise for 24 h and fast for 12 h before the study visit following recently published guidelines (36). Written informed consent was obtained from all participants, and all experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic (protocol 1, no. 13-001237) and the University of Missouri (protocol 2, no. 2013126) and conformed to the Declaration of Helsinki.
Experimental protocol 1 in humans: insulin-stimulated muscle SNA.
A subset of data from protocol 1 were from previously published work (2, 37); however, the analyses and results included here are specific to the unique hypothesis raised. Participants were admitted to the Clinical Research and Trials Unit at the Mayo Clinic, and the study day began with instrumentation at 0800. Participants rested supine during instrumentation. An intravenous catheter was placed in the dominant arm for insulin and glucose infusion, and an arterial catheter (20 gauge, 5 cm) was placed in the nondominant arm using aseptic technique under local anesthesia (2% lidocaine). Beat-to-beat heart rate and blood pressure were continuously monitored using a lead II electrocardiogram (GE Datex-Ohmeda Cardiocap/5; GE Healthcare, Chicago, IL) and brachial arterial catheter (TruWave Pressure Transducer; Edwards Lifescience, Irvine, CA), respectively.
Standard microneurography procedures and techniques (24) were used to record multiunit postganglionic muscle SNA from the peroneal nerve, posterior to the fibular head. The peroneal nerve was localized with transcutaneous stimulation and two-dimensional ultrasonography (12). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses and no afferent neural response was evoked by skin stimuli. The recorded signal was amplified 100,000-fold, band-pass filtered (700–2,000 Hz), rectified, and integrated (resistance-capacitance integrator circuit, time constant 0.1 s) using a nerve traffic analyzer (662C-3; Bioengineering of University of Iowa).
Following a period of quiet rest, insulin (Novolin R) was infused intravenously at a steady rate of 1.0 mU·kg fat-free mass−1·min−1, an infusion rate that produces circulating levels of insulin that mimic the postprandial state. Fat-free mass was determined via DEXA. Blood glucose was determined every ~5 min at bedside using the glucose oxidase method (GM9 Glucose Analyzer, Analox Instruments, Stourbridge, UK; 2900D Biochemistry Analyzer, Yellow Springs Instruments, Yellow Springs, OH) and maintained at baseline levels, achieved by variable infusion rates of a dextrose solution. Plasma was also obtained and stored at −80°C for analysis of insulin, epinephrine, and norepinephrine. Analyses were performed by the Immunochemistry Core Laboratory of the Clinical Research and Trials Unit and the Department of Laboratory Medicine and Pathology at the Mayo Clinic. Plasma insulin was assessed using a two-site immunoenzymatic assay performed on the Dxl automated immunoassay system (Beckman Instruments, Chaska, MN). Plasma catecholamines were measured by reverse-phase high-performance liquid chromatography with electrochemical detection after extraction with activated alumina. Steady-state measures were collected at baseline and after 60 min of the insulin infusion.
Experimental protocol 2 in humans: insulin-stimulated blood flow and mitigation of muscle SNA by neck suction.
Before participation, individuals were familiarized with the study procedures including the neck suction device. On the study day, participants were admitted to the Clinical Research Center at the University of Missouri at 0800. Participants rested supine during instrumentation, which included a three-lead electrocardiogram (Bio Amp FE132; ADInstruments, Colorado Springs, CO) and noninvasive beat-to-beat blood pressure via finger photoplethysmography (Human NIBP Controller ML282; ADInstruments, Colorado Springs, CO) calibrated to upper arm blood pressure (automated sphygmomanometry). Femoral artery diameter and blood velocity were measured using Doppler ultrasound (iE33; Philips Medical Systems, Bethesda, MD). An 11-MHz linear array transducer was placed over the superficial femoral artery. Simultaneous diameter and velocity signals were obtained in duplex mode at a pulsed frequency of 5 MHz and corrected with an insonation angle of 60°. Sample volume was adjusted to encompass the entire lumen of the vessel without extending beyond the walls, and the cursor was set midvessel.
An intravenous catheter was placed in the antecubital vein of both arms, one for insulin and glucose infusion and the other for periodic blood sampling. Insulin (Humulin R) was infused intravenously to reach a steady rate of 40 mU·m−2 body surface area·min−1, an infusion rate that elicited similar circulating levels of insulin as were evoked in protocol 1. As in protocol 1, blood glucose was determined every ~5 min at bedside (YSI 2300 STAT PLUS glucose analyzer) and maintained at baseline levels by infusing a dextrose solution at a variable rate. Plasma was also obtained and stored at −80°C for analysis of insulin, which was assessed using a commercially available kit (cat no. 80-INSHU-E10.1; ALPCO, Salem, NH).
Following instrumentation, participants were fitted with a malleable neck collar that encircled the anterior two-thirds of the neck for the application of neck suction (BaroCollar; Physiology Research Instruments, Austin, TX). Appropriate neck chamber placement was ensured by first fitting the subject on the basis of observed neck size and then performing a calibration trial. During neck suction testing, 5-s pulses of −60 mmHg of suction were applied to selectively load (simulated carotid hypertension) the carotid baroreceptors. The 5-s period of neck suction provides reflex activation of carotid baroreceptors and allows for the examination of peripheral vascular responses during a baroreflex-mediated fall in SNA (11, 13, 14, 18, 29, 30, 42, 43, 45, 50, 52, 64). A variable pressure source was used to generate the changes in neck collar pressure, and these changes were delivered to the collar through large-bore two-way solenoid valves. To accurately quantify the stimulus applied, a pressure transducer was connected to a port on the collar. To minimize respiratory-related modulation of heart rate, the 5-s pulses of neck suction were delivered to the carotid sinus during controlled breathing. Specifically, participants were instructed to breathe along to a metronome set at ~15 breaths/min, consistent with work published previously (23, 26, 39, 49). Respiration was assessed by use of a Piezo Respiratory Belt Transducer (MLT1132; ADInstruments, Colorado Springs, CO).
Following instrumentation and a period of quiet rest, baseline blood samples and cardiovascular measurements were collected. During the recording period, five trials of neck suction (sustained 5-s pulses at −60 mmHg) were delivered, each separated by ~2 min, and results from the five trials were averaged (11, 42). This approach has been shown previously to transiently reduce muscle SNA (11, 13, 14, 18, 29, 30, 42, 45, 50). Following baseline neck suction, insulin infusion began. Sixty minutes following the start of the insulin infusion, five trials of neck suction (sustained 5-s pulses at −60 mmHg) were repeated, again separated by ~2 min.
Analysis in protocols 1 and 2.
Data from both protocols were collected using the PowerLab data acquisition system (analog-to-digital converter; ADInstruments, Colorado Springs, CO) with a sampling rate of 1,000 Hz. Stroke volume was estimated from the arterial blood pressure waveform using the Modelflow method through LabChart (LabChart; ADInstruments, Sydney, NSW, Australia), which incorporates age and sex. Cardiac output and total peripheral resistance were calculated. In protocol 1, sympathetic neurograms were analyzed using a semiautomated program (Ensemble-C; Elucimed, Ltd.) by a single investigator (D. W. Jacob), and muscle SNA was expressed as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), and normalized burst area [arbitrary units (AU)/min]. In protocol 2, ultrasound recordings were analyzed off-line using specialized edge detection software (Cardiovascular Suite; Quipu srl, Pisa, Italy). Blood flow was calculated from continuous diameter and mean blood velocity recordings using the following equation: 3.14 × [diameter (cm)/2]2 × mean blood velocity (cm/s) × 60 and reported as milliliters per minute. Vascular conductance was calculated as blood flow (mL/min) ÷ mean arterial blood pressure (mmHg) × 100 and reported as milliliters per minute per 100 mmHg. Hemodynamic variables were analyzed second by second and data time-aligned for averages across trials. Main outcome variables include a relative (%) change in hemodynamic parameters following acute neck suction, where a 10-s average immediately before the onset of NS was equal to 100%. Data are from the initiation of neck suction and include the 5-s stimulus and 5-s poststimulus recovery.
Experimental protocol 3 in isolated arteries from mice: insulin-induced modulation of α-adrenergic receptor-mediated vasoconstriction.
On the basis of findings from protocol 2 in humans, we designed a follow-up proof-of-concept experiment in isolated arteries from mice to test the hypothesis that vascular exposure to insulin can suppress α-adrenergic receptor-mediated vasoconstriction. Furthermore, we tested whether this phenomenon is endothelium dependent and reliant on the phosphatidylinositol 3-kinase (PI3K)/Akt arm of the insulin signaling pathway.
All animal study procedures received prior approval by the University of Missouri Institutional Animal Care and Use Committee. The University of Missouri is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. For this experiment, isolated aortic rings were used from 20 wild-type [28.4 ± 0.6 g body wt, mean ± SE; C57BL/6J background bred at the University of Missouri] and 4 diabetic db/db (48.3 ± 0.7 g body wt, no. 00697; The Jackson Laboratory, Bar Harbor, ME) 8-wk-old male mice. Aortas were harvested and cleaned of perivascular adipose tissue in ice-cold physiological saline solution (pH 7.40) and cut into four 2-mm segments, such that experiments could be performed in duplicate within each experimental condition. The ability to obtain multiple rings from a single aorta represents a strength of this model. Aortic rings were then mounted in wire myograph organ bath chambers (620M; Danish Myo Technology, Hinnerup, Denmark) containing warmed physiological saline solution gassed with 95% O2-5% CO2 and maintained at 37°C as previously described (21, 66). Aortic rings were treated with 80 mM KCl to ensure viability. Next, aortas were incubated with or without insulin (100 nM, Humulin R; Eli Lilly, Indianapolis, IN) for 30 min before stimulation with increasing concentrations of phenylephrine (an α1-adrenergic receptor agonist; 10−9 to 10−5 M) while insulin (or lack thereof) remained in the bath. Additional mechanistic experiments were completed in aortic rings that underwent endothelium denudation, aortic rings treated with an NO synthase inhibitor [Nω-nitro-l-arginine methyl ester (l-NAME), 300 µM, no. N5751; Millipore-Sigma, St. Louis, MO], and aortic rings treated with a PI3K inhibitor (wortmannin, 100 nM, no. 19545-26-7; Millipore-Sigma). Aortic rings were mechanically denuded by gently rubbing the intima of the blood vessel against the myograph pins. Endothelial denudation was confirmed by the lack of a relaxation response to acetylcholine (10−5 M) at the end of the phenylephrine dose-response curve. Vasoconstriction responses to phenylephrine were normalized to KCl-induced constriction. Area under the curve (AUC) for each phenylephrine dose-response curve was also calculated using the trapezoidal rule. It should be acknowledged that aortic rings were selected as model of choice for this proof-of-concept experiment and that vessels were exposed to a high range of phenylephrine concentrations. To most appropriately establish the role of insulin in mitigating SNA-induced peripheral vasoconstriction, experiments should be recapitulated in isolated resistance arteries as well as in vivo under more physiological conditions (e.g., using sympathoexcitatory maneuvers).
Statistical analysis.
In protocols 1 and 2, the effect of insulin (i.e., baseline vs. hyperinsulinemia) and neck suction on main outcome variables was assessed using a one-way or two-way repeated measures analysis of variance (ANOVA), as appropriate, and pairwise multiple comparisons were made using Student–Newman–Keuls method. No sex differences were detected on primary outcome variables in protocols 1 and 2; therefore, data from male and female participants were pooled for analysis. In protocol 3, a two-way repeated measures ANOVA was used to assess the influence of insulin on the vasoconstrictor responses to increasing phenylephrine concentrations under each different condition. Within condition, comparisons for AUC were made using a paired two-tailed Student’s t test. An α of P < 0.05 was considered statistically significant. Data are reported as means ± SE.
RESULTS
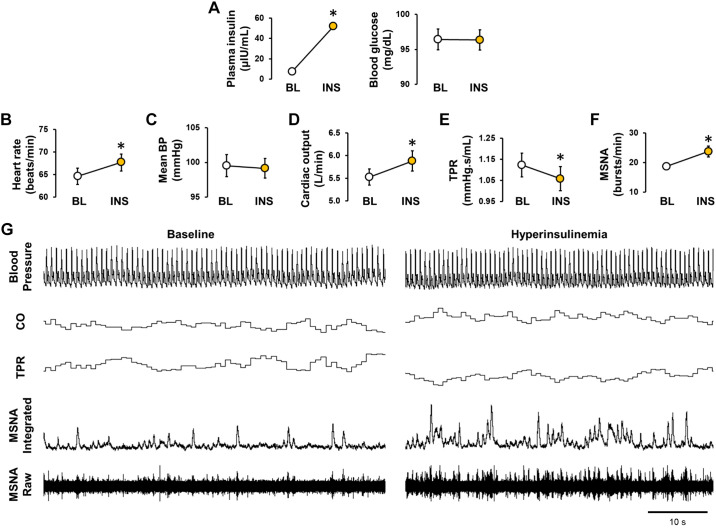
Hyperinsulinemia robustly increases muscle SNA without impacting mean arterial blood pressure.
Twenty healthy men (n = 13) and women (n = 7) completed protocol 1. Participant characteristics are reported in Table 1. Plasma insulin and blood glucose concentrations during systemic infusion of insulin are presented in Fig. 1A and demonstrate successful achievement of hyperinsulinemia (P < 0.001) with maintenance of fasted blood glucose levels (P = 0.491). Resting heart rate increased in response to insulin infusion (P = 0.005, Fig. 1B), whereas mean arterial blood pressure remained unchanged (P = 0.685, Fig. 1C). Data from Modelflow showed an increase in cardiac output (P = 0.006, Fig. 1D) and reduction in total peripheral resistance (P = 0.009, Fig. 1E) during insulin infusion. No change in stroke volume was observed (87 ± 3 to 88 ± 3 mL/beat; P = 0.473). Muscle SNA burst frequency (P = 0.002, Fig. 1F), burst incidence (29.9 ± 1.9 to 37.3 ± 3.3 bursts/100 heartbeats, P = 0.007), and normalized burst area (4,121 ± 227 to 5,028 ± 372 AU/min, P = 0.002) all increased from baseline in response to insulin infusion. Similarly, insulin infusion increased plasma catecholamine levels (norepinephrine, 164 ± 15 to 208 ± 16 pg/mL, P < 0.001; epinephrine, 47 ± 5 to 61 ± 6 pg/mL, P = 0.015; n = 18 participants).
Table 1.
Participant demographics
| Protocol 1 | Protocol 2 | |
|---|---|---|
| Men, women, n | 13, 7 | 5, 5 |
| Age, yr | 28 ± 1 | 29 ± 2 |
| Height, cm | 178 ± 3 | 170 ± 2 |
| Weight, kg | 78 ± 3 | 69 ± 3 |
| Body mass index, kg/m2 | 25 ± 1 | 24 ± 1 |
Values are means ± SE; n = no. of participants.
Fig. 1.
Hyperinsulinemia robustly increases muscle sympathetic nerve activity without impacting mean arterial blood pressure. In protocol 1, steady-state measures were collected at baseline (BL) and after 60 min of the insulin infusion (INS). Plasma insulin and blood glucose concentrations (A), heart rate (B), mean blood pressure (BP; C), cardiac output (CO; D), total peripheral resistance (TPR; E), and muscle sympathetic nerve activity (MSNA; F). Representative original traces from a male participant (22 yr old, body mass index 25.3 kg/m2; G). Data are reported as means ± SE (n = 20 participants). In A and F, error bars are within symbols. *Significance (P < 0.05) from BL.
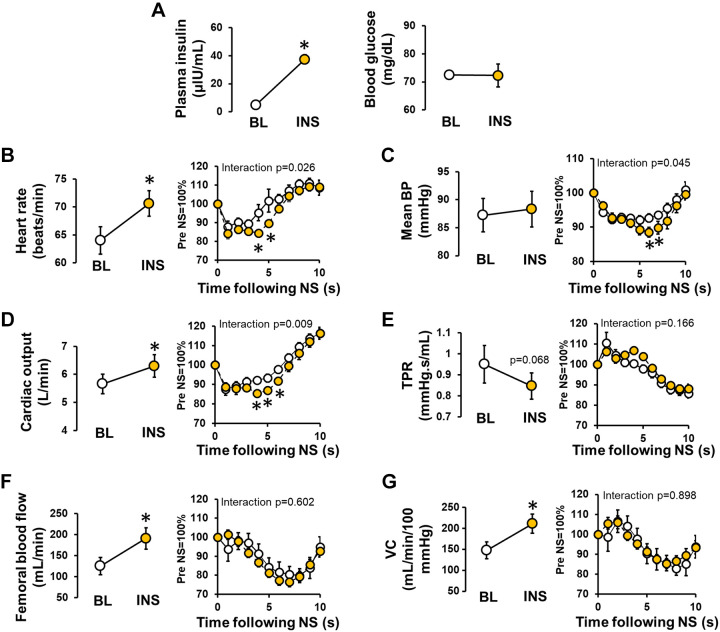
Sympathetically mediated increases in cardiac output, rather than restraint of peripheral vasodilation, contribute to the maintenance of blood pressure during hyperinsulinemia.
Ten healthy men (n = 5) and women (n = 5) completed protocol 2. Participant characteristics are reported in Table 1. Plasma insulin and blood glucose concentrations during systemic infusion of insulin are presented in Fig. 2A, and as in protocol 1, support the successful achievement of hyperinsulinemia (P < 0.001) with maintenance of fasted blood glucose levels (P = 0.718). Consistent with protocol 1, insulin infusion increased resting heart rate (P < 0.001, Fig. 2B), whereas mean arterial blood pressure was maintained (P = 0.540, Fig. 2C). Also in alignment with protocol 1, insulin infusion increased cardiac output (P = 0.002, Fig. 2D) and tended to reduce total peripheral resistance (P = 0.068, Fig. 2E), with no change in stroke volume (92 ± 6 to 93 ± 6 mL/beat, P = 0.659). Femoral blood flow (Fig. 2F) and vascular conductance (Fig. 2G) increased from baseline in response to insulin infusion (P = 0.005 and P = 0.016, respectively).
Fig. 2.
Sympathetically mediated increases in cardiac output, rather than restraint of peripheral vasodilation, contribute to the maintenance of blood pressure during hyperinsulinemia. In protocol 2, cardiovascular measures were continuously collected in response to 5-s pulses of neck suction (NS) to simulate carotid hypertension and elicit a reflex-mediated reduction in sympathetic activation. NS was conducted before [i.e., baseline (BL)] and 60 min following the start of the insulin infusion (INS). During the recording period, five trials of NS (sustained 5-s pulses at −60 mmHg) were delivered, each separated by a 2-min washout. Results from the five trials were averaged. Plasma insulin and blood glucose concentrations (A), heart rate (B), mean blood pressure (BP; C), cardiac output (D), total peripheral resistance (TPR; E), femoral blood flow (F), and vascular conductance (VC; G). For B–G, panels at left display the effect of insulin infusion, and panels at right display the relative effect of NS over a 10-s period at BL (○) vs. during INS (yellow circles). Data are reported as means ± SE (n = 10 participants). In A, error bars are within symbols. *Significance (P < 0.05) from BL.
Neck suction elicited a reduction in mean arterial blood pressure that was greater in the presence of hyperinsulinemia compared with control (main effect of neck suction, P < 0.001; interaction of insulin and neck suction, P = 0.045, Fig. 2C). Neck suction also resulted in larger decreases in heart rate (main effect of neck suction, P < 0.001; interaction of insulin and NS; P = 0.026, Fig. 2B) and cardiac output (main effect of neck suction, P < 0.001; interaction of insulin and neck suction, P = 0.009, Fig. 2D) during hyperinsulinemia compared with control. The effect of neck suction on stroke volume did not differ between insulin and control conditions (main effect of neck suction, P < 0.001; interaction of insulin and neck suction, P = 0.563). Furthermore, the effect of neck suction on total peripheral resistance, femoral blood flow, and femoral vascular conductance did not differ between insulin and control conditions (main effect of neck suction, P < 0.001, P < 0.001, and P < 0.001, respectively; interaction of insulin and neck suction, P = 0.166, P = 0.602, and P = 0.898, respectively, Fig. 2, E–G).
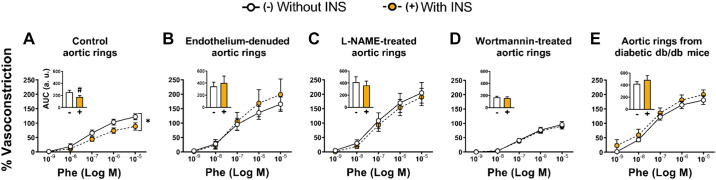
Insulin buffers α-adrenergic vasoconstriction, and this effect is endothelium dependent and reliant on an intact insulin signaling pathway.
In naïve (i.e., untreated) aortic rings, insulin exposure blunted phenylephrine-induced vasoconstriction (AUC, P = 0.047, Fig. 3A). This insulin-suppressing effect on phenylephrine-induced constriction was lost in aortic rings devoid of endothelium (AUC, P = 0.633, Fig. 3B), in aortic rings treated with NO synthase inhibitor l-NAME (AUC, P = 0.212, Fig. 3C), in aortic rings treated with PI3K inhibitor wortmannin (AUC, P = 0.702, Fig. 3D), and in aortic rings from diabetic db/db mice (AUC, P = 0.412, Fig. 3E).
Fig. 3.
Insulin buffers α-adrenergic vasoconstriction, and this effect is endothelium dependent and reliant on an intact insulin signaling pathway. In protocol 3, aortic rings were incubated with (+) or without (−) insulin (INS, 100 nM) for 30 min before stimulation with increasing concentrations of phenylephrine (Phe) while insulin (or lack thereof) remained in the bath. Experiments were performed in control (i.e., untreated) aortic rings (n = 5 mice; A), aortic rings devoid of endothelium (n = 5 mice; B), aortic rings treated with NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, n = 5 mice; C), aortic rings treated with phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin (n = 5 mice; D), and aortic rings from diabetic db/db mice (n = 4 mice; E). Data are reported as means ± SE. a.u., arbitrary units; AUC, area under the curve. *Significant interaction (P = 0.018); #significance (P = 0.047) from Without INS (−).
DISCUSSION
Herein we sought to investigate the role of sympathetic activation in restraining vasodilatory responses to hyperinsulinemia and consequently sustaining blood pressure in healthy adults. Blood pressure is a highly regulated variable by which acute, beat-to-beat changes are achieved via sympathetic innervation of the peripheral vasculature as well as combined sympathetic/parasympathetic activity at the level of the heart. As such, the maintenance of blood pressure in the presence of insulin must result from changes in total peripheral resistance and/or cardiac output (i.e., heart rate and stroke volume). Contrary to our hypothesis, the present findings support the idea that during hyperinsulinemia, neurally mediated increase in cardiac output, rather than restraint of peripheral vasodilation, is the principal contributor to the maintenance of systemic blood pressure.
During the postprandial state, insulin secretion causes vasodilator actions, which are of paramount importance for nutrient delivery to metabolically active tissues, particularly skeletal muscle, and ensuing glycemic control (4–7, 15, 32, 40, 57, 61, 69). Mechanistically, at the endothelial cell level, insulin binds the insulin receptor (IR) and activates downstream IR substrate 1/2/PI3K signaling, ultimately leading to phosphorylation of endothelial NO synthase, increased NO production, and subsequent vasodilation (31, 57, 68). However, in the face of such vasodilator effects of insulin, countercurrent mechanisms are required for preservation of blood pressure during hyperinsulinemia. Indeed, as Loring Rowell (54) once proclaimed in the context of exercise-induced hyperemia, “skeletal muscle is a ‘sleeping giant’ whose blood flow must be under tonic vasoconstrictor constraint if hypotension is to be averted.” Here we reasoned that this principle would be germane to the setting of hyperinsulinemia. That is, we posited that sympathoexcitation caused by hyperinsulinemia likely limits vasodilatory responses in skeletal muscle via α-adrenergic receptor-mediated vasoconstriction, thus contributing to the maintenance of blood pressure. Congruently with this notion, we first corroborated in protocol 1 that systemic infusion of insulin robustly increases muscle SNA while blood pressure remains stable. Next, we examined the degree to which silencing of SNA during insulin stimulation would favor peripheral vasodilation. Specifically, in protocol 2 we posited that during systemic insulin infusion, acute reductions in SNA via neck suction would produce greater peripheral vasodilation and a reduction in blood pressure relative to neck suction under the basal, noninsulinized control state.
We found that following neck suction, the reduction in blood pressure was greater during systemic insulin infusion compared with that in the absence of insulin, supporting a role for sympathetic activation in sustaining blood pressure during acute hyperinsulinemia. However, in contrast to our hypothesis, the effect of neck suction on femoral blood flow and vascular conductance did not differ between insulin and control conditions, nor was there an observable effect on total peripheral resistance. Instead, we report that the greater decreases in blood pressure following neck suction, while in the setting of high insulin levels, were accompanied by greater decreases in cardiac output. This finding, combined with the observation that cardiac output was markedly increased during insulin infusion in both protocols, supports the view that sympathetically mediated increases in cardiac output (primarily via an increase in heart rate), as opposed to restraint of peripheral vasodilation, are critical to the preservation of blood pressure during hyperinsulinemia. Insulin-mediated increases in heart rate are consistent with prior work in humans (1, 9, 10, 63) and are associated with a relative cardiac sympathetic predominance in the setting of lower vagal influence (8, 44, 60). From a translational standpoint, this increase in cardiac sympathetic tone is necessary to maintain blood pressure after a meal (53, 58). For example, patients with autonomic failure exhibit a significant fall in blood pressure after a meal that is attenuated by indomethacin (a β-adrenergic receptor agonist; 53).
Because hyperinsulinemia provokes a prominent increase in muscle SNA (e.g., Fig. 1; 1, 9, 35, 63, 67), we were surprised by the finding that skeletal muscle blood flow responses to insulin were not under a substantial SNA-mediated vasoconstrictor constraint. Next, we put forth three ideas that may reconcile discrepancies between the present and expected findings: 1) nonuniform increases in SNA in response to hyperinsulinemia, 2) sympatholytic actions of insulin, and/or 3) sympathetically mediated vasodilation in the setting of high insulin levels.
First, it is possible that during acute hyperinsulinemia, other vascular beds beyond skeletal muscle (e.g., splanchnic) may play a more critical role in sympathetically mediated control of blood pressure. In this regard, there is evidence for nonuniform increases in SNA in response to hyperinsulinemia. For example, preclinical animal models show no change in renal SNA with high insulin levels despite significant increases in lumbar SNA, and similar observations have been made in humans (3, 22, 38). Second, insulin signaling, besides exerting direct endothelium-dependent NO-mediated vasodilatory effects, may attenuate sympathetic vasoconstriction, a term known as sympatholysis. As such, it is conceivable that the lack of SNA-mediated peripheral vasoconstrictor constraint apparent in the present study in healthy, insulin-sensitive individuals could be attributed to insulin-induced “lysing” of α-adrenergic receptor-mediated vasoconstriction. To begin to explore this idea, we designed a proof-of-concept experiment (i.e., protocol 3) to determine whether, indeed, vascular exposure to insulin suppresses α-adrenergic vasoconstriction. In agreement with this postulate, we show that insulinization of isolated aortic rings blunts constriction to phenylephrine, an α1-adrenergic receptor agonist. Notably, this buffering effect of insulin was only noted in arteries with an intact endothelium and an unrestricted insulin signaling pathway. That is, either endothelium denudation or inhibition of PI3K via wortmannin [a pharmacological model of selective insulin resistance (41, 46)] eliminated the effect of insulin. The insulin-suppressing effect on phenylephrine-induced constriction was also lost in arteries from db/db mice, a well-established murine model of insulin resistance and diabetes (19, 56, 65), as well as in arteries treated with an NO synthase inhibitor. Collectively, these findings are consistent with the idea that vascular insulin signaling can blunt sympathetic vasoconstriction (33) and that the sympatholytic factor in this context is endothelial derived and NO dependent. In line with this, previous work in other models has led to the conclusion that the endothelium is an important modulator of sympathetic vasoconstriction (25) and that NO may in fact be sympatholytic (27, 28). Consistent with the data from db/db mice, the effects of hyperinsulinemia on the mechanisms studied herein may be altered when in a diseased state (e.g., type 2 diabetes). Third, another explanation that likely coexists with that stated above is that insulin augments β-adrenergic receptor-mediated vasodilation, a concept with precedence in the literature (20, 33, 34). Accordingly, it is possible that any increase in muscle SNA potentiates insulin-stimulated vasodilation through β-adrenergic receptor activation and consequent downstream NO production (20).
In aggregate, our findings in insulin-sensitive adults do not support the role of SNA-mediated vasoconstriction as a mechanism to restrain insulin-induced skeletal muscle vasodilation. Both insulin-induced lysing of α-adrenergic vasoconstriction and insulin-induced potentiation of β-adrenergic vasodilation likely explain this finding. Importantly, because of such observed lack of SNA-mediated restraint of peripheral vasodilation, healthy individuals must heavily rely on increases in cardiac output to support blood pressure during hyperinsulinemia. Indeed, our data do support the role of SNA-mediated increases in cardiac output as the primary mechanism in place to maintain blood pressure in the face of peripheral vasodilation caused by hyperinsulinemia. Further research is needed to determine whether this cardiac output dependency for the maintenance of blood pressure during hyperinsulinemia is absent in patients with insulin resistance who display chronic sympathetic overactivity and impaired vasodilatory responses to insulin.
GRANTS
This work was supported in part by the Margaret W. Mangel Faculty Research Catalyst Fund (J.K.L. and J.P.), American Heart Association Grant 15SDG 25080095 (J.K.L.), and National Heart, Lung, and Blood Institute Grants R00-HL-130339 (J.K.L.) and R01-HL-137769 (J.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L. and J.P. conceived and designed research; J.K.L., J.A.S., R.N.S., J.L.H., K.N.H., D.W.J., M.T.M., Z.I.G., B.D.J., T.B.C., C.M.-A., and J.P. performed experiments; J.K.L., J.A.S., R.N.S., J.L.H., K.N.H., D.W.J., and M.T.M. analyzed data; J.K.L., J.A.S., J.L.H., K.N.H., D.W.J., M.T.M., Z.I.G., B.D.J., T.B.C., T.B., C.M.-A., and J.P. interpreted results of experiments; J.K.L., R.N.S., and J.P. prepared figures; J.K.L. and J.P. drafted manuscript; J.K.L., J.A.S., R.N.S., J.L.H., K.N.H., D.W.J., M.T.M., Z.I.G., B.D.J., T.B.C., T.B., C.M.-A., and J.P. edited and revised manuscript; J.K.L., J.A.S., R.N.S., J.L.H., K.N.H., D.W.J., M.T.M., Z.I.G., B.D.J., T.B.C., T.B., C.M.-A., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the time and effort put in by all research participants. We also acknowledge the research team at the Human Integrative Physiology Laboratory at the Mayo Clinic and the nursing team of the Clinical Research and Trials Unit at the Mayo Clinic and the Clinical Research Center at the University of Missouri. Finally, we thank Elizabeth Schroeder for training on the neck suction device.
REFERENCES
- 1.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosa TC, Kaur J, Holwerda SW, Young CN, Curry TB, Thyfault JP, Joyner MJ, Limberg JK, Fadel PJ. Insulin increases ventilation during euglycemia in humans. Am J Physiol Regul Integr Comp Physiol 315: R84–R89, 2018. doi: 10.1152/ajpregu.00039.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol Endocrinol Metab 271: E1067–E1072, 1996. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 5.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73: 637–643, 1991. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- 6.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellavere F, Cacciatori V, Moghetti P, Gemma ML, Dellera A, Tosi F, Negri C, Thomaseth K, Muggeo M. Acute effect of insulin on autonomic regulation of the cardiovascular system: a study by heart rate spectral analysis. Diabet Med 13: 709–714, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia 35: 873–879, 1992. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- 10.Birkett CL, Ray CA, Anderson EA, Rea RF. A signal-averaging technique for the analysis of human muscle sympathetic nerve activity. J Appl Physiol (1985) 73: 376–381, 1992. doi: 10.1152/jappl.1992.73.1.376. [DOI] [PubMed] [Google Scholar]
- 11.Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. doi: 10.1152/ajpheart.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diedrich A, Porta A, Barbic F, Brychta RJ, Bonizzi P, Diedrich L, Cerutti S, Robertson D, Furlan R. Lateralization of expression of neural sympathetic activity to the vessels and effects of carotid baroreceptor stimulation. Am J Physiol Heart Circ Physiol 296: H1758–H1765, 2009. doi: 10.1152/ajpheart.01045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez D, De Pue N, Clement DL. Peripheral vascular responses during carotid baroreceptor stimulation in normotensive and hypertensive subjects. Clin Sci (Lond) 73: 635–640, 1987. doi: 10.1042/cs0730635. [DOI] [PubMed] [Google Scholar]
- 15.Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 36: 104–110, 2013. doi: 10.2337/dc11-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002. doi: 10.1016/S0008-6363(02)00593-X. [DOI] [PubMed] [Google Scholar]
- 17.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 18.Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 280: H1383–H1390, 2001. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- 19.Fellmann L, Nascimento AR, Tibiriça E, Bousquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther 137: 331–340, 2013. doi: 10.1016/j.pharmthera.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Gros R, Borkowski KR, Feldman RD. Human insulin-mediated enhancement of vascular beta-adrenergic responsiveness. Hypertension 23: 551–555, 1994. doi: 10.1161/01.HYP.23.5.551. [DOI] [PubMed] [Google Scholar]
- 21.Grunewald ZI, Jurrissen TJ, Woodford ML, Ramirez-Perez FI, Park LK, Pettit-Mee R, Ghiarone T, Brown SM, Morales-Quinones M, Ball JR, Staveley-O’Carroll KF, Aroor AR, Fadel PJ, Paradis P, Schiffrin EL, Bender SB, Martinez-Lemus LA, Padilla J. Chronic elevation of endothelin-1 alone may not be sufficient to impair endothelium-dependent relaxation. Hypertension 74: 1409–1419, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudbjörnsdottir S, Friberg P, Elam M, Attvall S, Lönnroth P, Wallin BG. The effect of metformin and insulin on sympathetic nerve activity, norepinephrine spillover and blood pressure in obese, insulin resistant, normoglycemic, hypertensive men. Blood Press 3: 394–403, 1994. doi: 10.3109/08037059409102293. [DOI] [PubMed] [Google Scholar]
- 23.Gulli G, Cooper VL, Claydon VE, Hainsworth R. Prolonged latency in the baroreflex mediated vascular resistance response in subjects with postural related syncope. Clin Auton Res 15: 207–212, 2005. doi: 10.1007/s10286-005-0273-8. [DOI] [PubMed] [Google Scholar]
- 24.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearon CM Jr, Dinenno FA. Escape, lysis, and feedback: endothelial modulation of sympathetic vasoconstriction. Curr Opin Pharmacol 45: 81–86, 2019. doi: 10.1016/j.coph.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Hilz MJ, Koehn J, Tillmann A, Riss S, Marthol H, Köhrmann M, Wasmeier G, Schwab S, Stemper B. Autonomic blockade during sinusoidal baroreflex activation proves sympathetic modulation of cerebral blood flow velocity. Stroke 44: 1062–1069, 2013. doi: 10.1161/STROKEAHA.111.680256. [DOI] [PubMed] [Google Scholar]
- 27.Hoiland RL. Is nitric oxide mediated sympatholysis improved with exercise? Yes or nNO? J Physiol 593: 1045–1046, 2015. doi: 10.1113/jphysiol.2014.286815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jendzjowsky NG, Delorey DS. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol 591: 1535–1549, 2013. doi: 10.1113/jphysiol.2012.238998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller DM, Davis SL, Low DA, Shibasaki M, Raven PB, Crandall CG. Carotid baroreceptor stimulation alters cutaneous vascular conductance during whole-body heating in humans. J Physiol 577: 925–933, 2006. doi: 10.1113/jphysiol.2006.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454–H2465, 2011. doi: 10.1152/ajpheart.00772.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 32.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 33.Lembo G, Iaccarino G, Rendina V, Volpe M, Trimarco B. Insulin blunts sympathetic vasoconstriction through the alpha 2-adrenergic pathway in humans. Hypertension 24: 429–438, 1994. doi: 10.1161/01.HYP.24.4.429. [DOI] [PubMed] [Google Scholar]
- 34.Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Hypertension 26: 290–293, 1995. doi: 10.1161/01.HYP.26.2.290. [DOI] [PubMed] [Google Scholar]
- 35.Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, Trimarco B, Saccà L. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J Clin Invest 90: 24–29, 1992. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limberg JK, Johnson BD, Mozer MT, Holbein WW, Curry TB, Prabhakar NR, Joyner MJ. Role of the carotid chemoreceptors in insulin-mediated sympathoexcitation in humans. Am J Physiol Regul Integr Comp Physiol 318: R173–R181, 2020. doi: 10.1152/ajpregu.00257.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 304: H1538–H1546, 2013. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marthol H, Brown CM, Zikeli U, Ziegler D, Dimitrov N, Baltadzhieva R, Hilz MJ. Altered cerebral regulation in type 2 diabetic patients with cardiac autonomic neuropathy. Diabetologia 49: 2481–2487, 2006. doi: 10.1007/s00125-006-0368-3. [DOI] [PubMed] [Google Scholar]
- 40.Meijer RI, De Boer MP, Groen MR, Eringa EC, Rattigan S, Barrett EJ, Smulders YM, Serne EH. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19: 494–500, 2012. doi: 10.1111/j.1549-8719.2012.00174.x. [DOI] [PubMed] [Google Scholar]
- 41.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277: 1794–1799, 2002. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 42.Moore DJ, Barlow MA, Gonzales JU, McGowan CL, Pawelczyk JA, Proctor DN. Evidence for the emergence of leg sympathetic vasoconstrictor tone with age in healthy women. Physiol Rep 3: e12275, 2015. doi: 10.14814/phy2.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muenter Swift N, Cutler MJ, Fadel PJ, Wasmund WL, Ogoh S, Keller DM, Raven PB, Smith ML. Carotid baroreflex function during and following voluntary apnea in humans. Am J Physiol Heart Circ Physiol 285: H2411–H2419, 2003. doi: 10.1152/ajpheart.00139.2003. [DOI] [PubMed] [Google Scholar]
- 44.Muscelli E, Emdin M, Natali A, Pratali L, Camastra S, Gastaldelli A, Baldi S, Carpeggiani C, Ferrannini E. Autonomic and hemodynamic responses to insulin in lean and obese humans. J Clin Endocrinol Metab 83: 2084–2090, 1998. doi: 10.1210/jc.83.6.2084. [DOI] [PubMed] [Google Scholar]
- 45.Ogoh S, Fadel PJ, Hardisty JM, Wasmund WL, Keller DM, Raven PB, Smith ML. Does pulsatile and sustained neck pressure or neck suction produce differential cardiovascular and sympathetic responses in humans? Exp Physiol 88: 595–601, 2003. doi: 10.1113/eph8802586. [DOI] [PubMed] [Google Scholar]
- 46.Olver TD, Grunewald ZI, Ghiarone T, Restaino RM, Sales AR, Park LK, Thorne PK, Ganga RR, Emter CA, Lemon PW, Shoemaker JK, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Persistent insulin signaling coupled with restricted PI3K activation causes insulin-induced vasoconstriction. Am J Physiol Heart Circ Physiol 317: H1166–H1172, 2019. doi: 10.1152/ajpheart.00464.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olver TD, Laughlin MH, Padilla J. Exercise and vascular insulin sensitivity in the skeletal muscle and brain. Exerc Sport Sci Rev 47: 66–74, 2019. doi: 10.1249/JES.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padilla J, Olver TD, Thyfault JP, Fadel PJ. Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100: 759–771, 2015. doi: 10.1113/EP085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passino C, Cencetti S, Spadacini G, Quintana R, Parker D, Robergs R, Appenzeller O, Bernardi L. Persistence of baroreceptor control of cerebral blood flow velocity at a simulated altitude of 5000 m. J Hypertens 25: 1862–1870, 2007. doi: 10.1097/HJH.0b013e32826f49a3. [DOI] [PubMed] [Google Scholar]
- 50.Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- 51.Randin D, Vollenweider P, Tappy L, Jéquier E, Nicod P, Scherrer U. Effects of adrenergic and cholinergic blockade on insulin-induced stimulation of calf blood flow in humans. Am J Physiol Regul Integr Comp Physiol 266: R809–R816, 1994. doi: 10.1152/ajpregu.1994.266.3.R809. [DOI] [PubMed] [Google Scholar]
- 52.Rea RF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. Am J Physiol Regul Integr Comp Physiol 253: R929–R934, 1987. doi: 10.1152/ajpregu.1987.253.6.R929. [DOI] [PubMed] [Google Scholar]
- 53.Robertson D, Wade D, Robertson RM. Postprandial alterations in cardiovascular hemodynamics in autonomic dysfunction states. Am J Cardiol 48: 1048–1052, 1981. doi: 10.1016/0002-9149(81)90319-2. [DOI] [PubMed] [Google Scholar]
- 54.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol (1985) 97: 384–392, 2004. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- 55.Sartori C, Trueb L, Nicod P, Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition on vascular actions of insulin in humans. Hypertension 34: 586–589, 1999. doi: 10.1161/01.HYP.34.4.586. [DOI] [PubMed] [Google Scholar]
- 56.Shafrir E. Development and consequences of insulin resistance: lessons from animals with hyperinsulinaemia. Diabetes Metab 22: 122–131, 1996. [PubMed] [Google Scholar]
- 57.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanakaya M, Takahashi N, Takeuchi K, Katayama Y, Yumoto A, Kohno K, Shiraki T, Saito D. Postprandial hypotension due to a lack of sympathetic compensation in patients with diabetes mellitus. Acta Med Okayama 61: 191–197, 2007. doi: 10.18926/AMO/32869. [DOI] [PubMed] [Google Scholar]
- 59.Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 96: 1262–1269, 2004. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 60.Van De Borne P, Hausberg M, Hoffman RP, Mark AL, Anderson EA. Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am J Physiol Regul Integr Comp Physiol 276: R178–R183, 1999. doi: 10.1152/ajpregu.1999.276.1.R178. [DOI] [PubMed] [Google Scholar]
- 61.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 62.Vollenweider L, Tappy L, Owlya R, Jéquier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes 44: 641–645, 1995. doi: 10.2337/diab.44.6.641. [DOI] [PubMed] [Google Scholar]
- 63.Vollenweider P, Tappy L, Randin D, Schneiter P, Jéquier E, Nicod P, Scherrer U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 92: 147–154, 1993. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol Heart Circ Physiol 242: H185–H190, 1982. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10: 131–145, 2014. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res 89: 516–524, 2011. doi: 10.1093/cvr/cvq349. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53: 447–453, 2004. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]