Abstract
Macular degeneration (MD) often leads to the loss of the fovea and surrounding central visual field. This type of visual loss is very common and can present particular challenges for oculomotor tasks that may rely on the fovea. For certain tasks, individuals develop a new, eccentric fixational locus. Our previous work has shown that smooth pursuit is impaired in MD. However, extent of retinal lesion size and eccentricity of fixation do not directly contribute to changes in smooth pursuit gain. Oculomotor limitations due to eccentric eye position in the orbit may be another culprit. Here we test the hypothesis that deficits in smooth pursuit in MD would be reduced under head-unrestrained conditions. To that end, we examined eye, head, and gaze movements in eight individuals with MD and seven age-matched controls in response to a step-ramp pursuit stimulus. We found that despite variability across participants, both groups had similar smooth pursuit head movements (P = 0.76), while both had significantly higher pursuit gains in the head-restrained condition (P < 0.0001), suggesting that in older populations, head movements may lead to a decrease in pursuit gain. Furthermore, we did not find a correlation between eccentricity of fixation and amount of head displacement during the trial (P = 0.25), suggesting that eccentric eye position does not lead to higher reliance on head movements in smooth pursuit. Our finding that individuals with MD have lower pursuit gains, despite similar head movements as controls, suggests a difference in how MD affects mechanisms underlying eye versus head movements in smooth pursuit.
NEW & NOTEWORTHY This article is the first to look at eye and head movements in observers with macular degeneration. It is the first to show that in older individuals, regardless of central field defect, freedom of head movement may reduce pursuit gain. Despite oculomotor limitations due to eccentric fixation, individuals with macular degeneration do not rely on head movements more than age-matched controls, with both groups having a similarly heterogenous eye and head movement strategy for pursuit.
Keywords: eccentric viewing, eye/head coordination, head-free, macular degeneration, smooth pursuit
INTRODUCTION
Age-related macular degeneration (AMD) can often lead to the loss of the fovea and the surrounding central visual field. This type of vision loss is very common, affecting nearly 7% of individuals over 40, and nearly 15% of those over 60, in the United States alone (Klein et al. 2011). Along with juvenile macular degeneration, these present the most common cause of central visual field loss and can pose particular challenges for oculomotor tasks that rely on the high-acuity foveal retina. For certain tasks, individuals develop a new, eccentric fixational area – the preferred retinal locus (PRL). In addition to reduced visual acuity, depending on the extent of the scotoma, this new PRL can lead to a highly eccentric position of the eye in the orbit.
We have previously shown that smooth pursuit is impaired in individuals with macular degeneration, and the degree of impairment depends on the direction of target motion relative to the location of the damaged retina and the fixational PRL (Shanidze et al. 2016a). One might consider that it is the lack of the fovea that causes deficit in smooth pursuit performance. However, we have also shown that scotoma size and PRL location do not directly contribute to variations in smooth pursuit gain (Shanidze et al. 2017). Another consideration could be that individuals’ eccentric fixation leads to an eccentric eye position that might impede smooth pursuit when the head is restrained, especially for target directions along fovea-PRL axis (Stahl, 2001). Thus head movements could become particularly important for successful pursuit in this population. In fact, although several studies in nonhuman primates have shown that there is no significant improvement in pursuit when the head is unrestrained (e.g., Dubrovsky and Cullen 2002; Lanman et al. 1978), the authors in the first study suggest that the reason head movements are used when possible in pursuit is to maintain a relatively central position of the eye in the orbit (Dubrovsky and Cullen 2002). This preference for a limited range of eye motion in the orbit may become a necessity for those with eccentric fixation.
Human studies have largely mirrored those in nonhuman primates. These studies suggest that a small, nonsignificant advantage is conferred by head movements, under a number of conditions (for review, see Barnes 1993), although it is possible that improvements in smooth pursuit in the head-unrestrained condition are limited by the already high pursuit gains seen in normal observers. In a more recent study, Ackerley and Barnes (2011a) looked at the effects of target extinction during pursuit. Again, they found a consistent increase in gains and more sustained smooth pursuit when individuals’ heads were unrestrained (Ackerley and Barnes 2011a). In a follow-up study, the authors suggest that head movements do not require continued visual input but instead rely on extraretinal (internal) information during pursuit (Ackerley and Barnes 2011b), making them a potentially important compensatory mechanism for people with a fragmented retina.
In this study we set out to determine whether smooth pursuit gains are improved under head-unrestrained conditions (where eye and head movements contribute to an overall gaze pursuit velocity) in individuals with central visual field loss due to macular degeneration. We hypothesized that there will be a significant improvement in pursuit in individuals with MD, as compared with their age-matched controls. Furthermore, we expect that smooth pursuit head movements would be greatest for target trajectories most affected by PRL location and therefore placement of the eyes in the orbit. We used a head-mounted infrared eye-tracking and inertial head-tracking system to assess eye and head movements in individuals with central field loss due to macular degeneration and age-matched controls. We presented observers with a modified step-ramp pursuit stimulus (Rashbass 1961), with a target moving along the four cardinal directions at a speed of 10°/s. We found that smooth pursuit gains were higher in the head-restrained condition for both groups. Furthermore, regardless of head restraint, individuals with macular degeneration had lower gains than controls. Interestingly, this difference appears to be entirely driven by changes in the eye, not head movements between groups. The extent of head movement in head-free smooth pursuit varied greatly across individuals in both groups; however, on the whole, there was no difference in the range of head movements between observers with macular degeneration and controls. On the other hand, eye movement velocities were significantly lower in MD participants. These findings suggest that mechanisms underlying eye and head movements in smooth pursuit in this population may be differently affected by the disease.
METHODS
Participants
All research was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Smith-Kettlewell Eye Research Institute. We recruited eight participants with central field loss (ages: 56–89 yr, 4 men) and seven controls (ages: 61–78 yr, 1 man). All participants provided written, informed consent. All controls had no vision or eye movement disorders. All participants with central field loss had macular degeneration (6 with age-related macular degeneration and two with Stargardt’s disease, a form of juvenile macular degeneration) in one or both eyes (see Table 1).
Table 1.
Participant summary
| Participant | Sex | Age | Dx | Visual Acuity (logMAR) | Mars Contrast Sensitivity | PRL Distance, ° |
|---|---|---|---|---|---|---|
| P1 | M | 56 | Stargardt’s | 1.55 | ||
| P2 | F | 79 | AMD (left) | 0 | 0.92 | 0.0 |
| P3 | F | 77 | AMD | 0.3 | 1.36 | 2.3 |
| P4 | M | 78 | AMD | 1.1 | 1.36 | 14.7 |
| P5 | F | 87 | AMD | 0.2 | 1.40 | 6.2 |
| P6 | F | 77 | AMD | 1.2 | 1.28 | 7.7 |
| P7 | M | 89 | AMD | 1.2 | 0.88 | 13.0 |
| P8 | M | 58 | Stargardt’s | 0.9 | 1.64 | 5.3 |
| C1 | M | 61 | 0.9 | 1.8 | 0.0 | |
| C2 | F | 75 | 0.26 | 1.84 | 0.0 | |
| C3 | F | 72 | 0.3 | 1.8 | 0.0 | |
| C4 | F | 77 | 0.1 | 1.76 | 0.0 | |
| C5 | F | 78 | −0.1 | 1.8 | 0.0 | |
| C6 | F | 74 | 0.72 | 1.72 | 0.0 | |
| C7 | F | 70 | 0.22 | 1.8 | 0.0 |
Preferred retinal locus (PRL) distance is reported for the dominant eye. Visual acuity is reported without optical correction. AMD, age-related macular degeneration; Dx, diagnosis; F, female participant; M, male participant.
Before testing, all participants were assessed using a standard battery of tests to measure their acuity, contrast sensitivity, and stereoacuity. For MD participants, scotomata were mapped monocularly using standard microperimetry approaches, at 0 dB, in the scanning laser ophthalmoscope (Optos OCT/SLO). PRL eccentricity was measured from the fovea using the procedure outlined in Verghese et al. (2016). Briefly, the foveal pit location was estimated, when possible, from optical coherence tomography, and if the foveal pit could not be visualized, the location of the fovea was estimated using normative data of foveal distance from the center of the optic disk (Kabanarou et al. 2006). For individuals with macular degeneration, we assume similar eccentricity for binocular viewing as their better/dominant eye (Kabanarou et al. 2006; Tarita-Nistor et al. 2012). Due to fixational instability and very poor visual acuity, monocular microperimetry in the SLO was not possible for participant P1. Ocular dominance was assessed using Miles’ “hole-in-the-hand” method (Roth et al. 2002).
Equipment
Participants were seated 1 m away from an LCD monitor (124 × 72cm, TCL Corp., Huizhou, China). Eye movements were recorded binocularly using head-mounted infrared eye-tracking goggles (PupilLabs, Munich, Germany) sampling at 200 Hz. Head movements were recorded using a nine-axis inertial measurement unit (IMU; LP-Research, Tokyo, Japan), sampling at 400 Hz (100 Hz for participants P6 and P8).
Eye movement recordings.
The PupilLabs eye tracker consists of two eye cameras that record images of each eye (200 Hz) and a scene camera (60 Hz) placed on the forehead and directed toward the monitor. From the eye camera images, pupil contours are detected and parameters of three-dimensional (3D) eye models are estimated for each eye (Swirski and Dodgson 2013). An online calibration provided by PupilLabs (see Calibration) is used to establish the transformation function between the eye cameras and the scene camera coordinate frames by aligning the optical axis of each eye to intersect the target location detected in the scene camera image projected on a sphere camera model. The transformation function is used to calculate the eye gaze vectors in the scene camera 3D coordinate frame. These gaze vectors and additional eye model parameters are logged at the time of the experiment for offline analysis.
To compensate for potential slippage of the eye-tracking goggles on the face, the PupilLabs PupilCapture software continues to optimize the model parameters throughout the recording (Dierkes et al. 2018). However, as continuous modifications to the eye model parameters throughout the recordings can introduce noise in the eye-in-head velocity estimation, we fixed the eye model during our recordings. To mitigate effects of slippage, we firmly anchored the goggles to the head with an athletic eyewear retainer. After a stable, viable eye model was determined before online calibration, the eye model parameters were fixed. An eye model was deemed viable if the eye model parameters were stable while the participant systematically moved his or her eyes around the entire extent of the screen and the distance parameters (pupil size, eye center position from the eye camera, etc.) were physiologically appropriate. In addition, to consider the model acceptable for use, the model confidence reported by Pupil Capture software had to consistently remain above 0.9.
Head movement recordings.
The IMU sensor was positioned on the forehead of the participant such that its local coordinate frame was aligned with the eye tracker’s scene camera. With the use of its gyroscope and accelerometer data, the IMU can measure the angular velocity of the head in the world coordinate frame. By positioning the IMU as mentioned above, we were able to map the scene camera coordinate frame to the world coordinate frame, allowing us to directly compare motion of the eyes, head, and smooth pursuit targets.
Eye movement data were recorded on a dedicated computer (Dell Inspiron 7559 Laptop). A separate computer was used to record head-movement data and for stimulus presentation (Dell Inspiron I7567 Laptop). The computers were connected via a local area network and synchronized using custom software (Python Language Reference, version 2.7, Python Software Foundation).
Head movements were recorded for both head restraint conditions. To verify the efficacy of our head restraint, we examined head movements across all head-restrained trials in two ways. First, we looked at the peak horizontal and vertical head speeds (absolute value of the velocity output of the IMU). We found that the average for each group had a peak head speed of 0.97 ± 0.64°/s for MD participants and 0.91 ± 0.51°/s for controls on the horizontal and 0.86 ± 0.63°/s for MD participants and 0.81 ± 0.56°/s for controls on the vertical. There was no difference in peak head speeds between the two participant groups (two-tailed t test, P = 0.56). We also looked at the duration of any head movements with velocity that exceeded a 2°/s threshold. We found that out of a total of 800 trials, 30 had head movements that exceeded the 2°/s threshold in the horizontal direction and 32 in the vertical. Within the 30 trials where horizontal velocity was >2°/s, these head movements lasted an average of 2.7% of the trial. For the vertical movements, the average head movement duration was 4.0% of the trial.
Calibration
To calibrate the eye tracker, we used the PupilLabs built-in marker calibration routine, at 1-m viewing distance, on the stimulus screen. Calibration was performed after the head tracking goggles were placed on the participant’s head and each eye camera was adjusted for optimal detection of the pupil. The participants were seated such that the nasion was aligned with the center of the screen along the vertical and horizontal dimensions. The scene camera was aligned such that the monitor was entirely within its field of view. Subsequently, participants were presented with five black bullseye markers, shown one at a time, against a white background, in the center and the four corners of the screen.
Subsequently, we performed an additional custom calibration/validation routine that provided a denser mapping of the pursuit area and allowed us to account for the viewing distance in the offline data analysis. Participants were asked to look at targets (same bullseye used during calibration) that appeared in 13 locations, evenly positioned across the entire screen. Starting with the center target, participants were asked to look at each target as it appeared and subsequently to locate the next one. With the use of a standard homography technique to reduce measurement error (fitgeotrans, MATLAB, MathWorks, Cambridge, MA), this validation procedure allowed us to map the gaze vectors provided by the eye tracker in the scene camera coordinate frame onto the world coordinates at the validation viewing distance (1 m). To ascertain the accuracy of our approach, for each validation, we calculated the distance between the predicted and measured eye position for each marker. We found that for individuals with macular degeneration median validation error in the head-unrestrained trials was 3.56° [interquartile range (IQR) = 1.72–5.58°] and in head-restrained trials it was 3.15° (IQR = 2.64°-–3.88°). For control participants, median validation error was 0.78° (IQR = 0.73–1.84°) in head-unrestrained trials and 1.07° (IQR = 0.72–1.41°) in head-restrained trials. Our measurements for control participants are consistent with previous studies looking at the accuracy of the PupilLabs eye-tracking goggles (Ehinger et al. 2019; Macinnes et al. 2018) but are higher in those with macular degeneration. This outcome is unsurprising given the eccentric fixation and increased fixational instability in this population (Crossland et al. 2004).
Eye model parameter determination, calibration, and validation were performed at the beginning of each block (consisting of 60 trials of a given head-restraint condition). During the head-restrained condition, each participant’s head was placed comfortably in a chin and forehead rest, which was positioned in front of the participant. All participants were first tested in the head-unrestrained condition, followed by the head-restrained condition. Between conditions participants were allowed a short break, at which time the eye-tracking goggles were removed and the participant was free to move around.
Smooth Pursuit
For each trial, participants viewed a 1° radius white annulus (0.2° radius black center) that appeared in the center of a black screen and were asked to follow a target that moved in a modified step-ramp paradigm (Rashbass 1961). Once fixation was acquired for a requisite amount of time, the experimenter initiated the trial. The central target disappeared and reappeared at one of four possible locations, 6° from center after a short delay of random duration between 1 and 1.5 s. The target then moved in the opposite direction of the initial step (0°, 90°, 180°, and 270°) until it reached the edge of the screen, moving through screen center (for a total excursion of 31.9° in the horizontal and 17.9° in the vertical). Targets moved at 10°/s, and each trajectory combination was repeated 15 times, for a total of 60 trials for each pursuit condition (head restrained and unrestrained). Target trajectories were randomized within each block. Eye and head movement and stimulus data were saved for offline analysis.
Data Analysis
The 3D position of the pupil center in world coordinates was transformed offline into the eye/gaze angle using the gaze normal values calculated by the eye tracker model and adjusted using the homography described in Calibration. The horizontal and vertical projections of the eye angle were considered the raw eye position data to be used for further analysis. The head horizontal and vertical projections of the rotation angle were calculated by integrating the angular velocity signal of the IMU. The raw head and eye position data and head angular velocity were evenly resampled at 1,000 Hz. Raw eye velocity was obtained from differentiation of the position data. The eye velocity data were filtered with a two-pole Butterworth noncausal filter (cutoff = 25 Hz) before saccade detection analysis.
Potential saccades were detected offline using the criteria of eye velocity exceeding 40°/s, or variance exceeding 150(°/s)2 in a 10-sample window (Heinen et al. 2005). All saccades were subsequently verified by one of the authors. All velocity traces were visually inspected, and those with excessive data loss due to artifacts or blinks were rejected for future gain analysis. Data for both eyes were processed as described above. For each participant, the dominant eye (where possible: 9/15 participants, 5 MD) or the eye with the least number of artifacts or lost trials was chosen for further analysis.
Participants with macular degeneration rarely maintained a consistent gaze velocity throughout the smooth pursuit trial, often showing variation during the epoch conventionally defined as the steady-state interval of pursuit. Within a trial, individuals’ pursuit gain could vary between virtually 0 to gains much closer to 1. Therefore, we used the period of longest continuous pursuit velocity to calculate the pursuit gaze gain and the eye and the head horizontal and vertical projections of the angular velocity in the world coordinate frame. This approach is described in detail in Shanidze et al. (2016a). Briefly, we used an 80-ms sliding window, with 20-ms overlap to sample the total (magnitude) velocity time series. The windows were compared, and all consecutive windows with gains within 0.2 of each other were combined; the longest segment after pursuit onset was used for further analysis. Overlapping segments with gains whose medians were within the 25th and 75th percentiles of each other were combined to create larger segments. The gaze gain was calculated as the sum of eye-in-head and head-in-space average velocities, divided by the target velocity for each window and combined as described above. During the period of continuous pursuit, the head and eye mean velocities and the head peak velocity were calculated and reported. This approach was chosen to systematically select the most representative pursuit performance for each trial, although shorter periods of higher (or lower) pursuit gain may have been present in the trial. The average gain for the longest segment (gray rectangles in Fig. 1) is reported in Fig. 2. The values obtained using this analysis are consistent with those obtained using other approaches in older adults (Shanidze et al. 2017; Sharpe and Sylvester 1978).
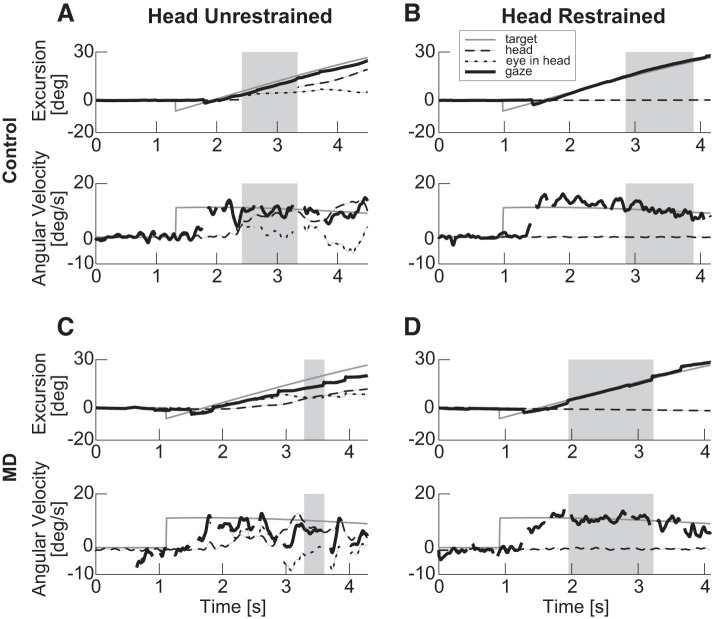
Fig. 1.
Example eye and head position and velocity data for 1 control (A and B, participant C2) and 1 participant with age-related macular degeneration (MD) (C and D, participant P5). A and C: head-unrestrained condition. B and D: head-restrained condition. The target ramp was in the rightward direction (0°) on all 4 trials. Top: position. Bottom: velocity data. Dashed black line: head, dotted black line: eye in head, solid black line: eye in space (gaze), and gray line: target. Regions designated by gray rectangles are those chosen as periods of longest continuous pursuit for the particular trial. All metrics are along the horizontal direction.
Fig. 2.
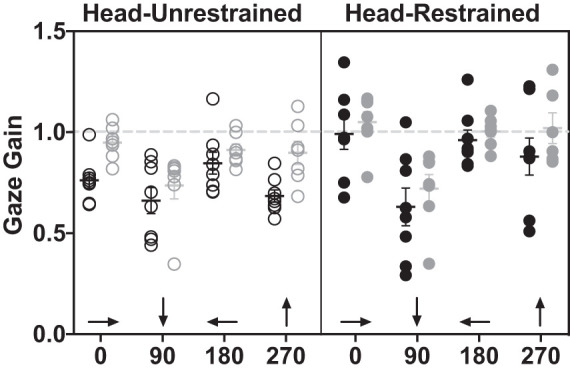
Smooth pursuit gaze gain of individuals with macular degeneration (black circles) and controls (gray circles) under head-unrestrained (outlined, left) and head-restrained (filled, right) conditions. Light gray dashed line represents gain = 1 (gaze velocity = target velocity). Error bars represent SE. Arrows along x-axis show target trajectories.
Due to the large width of the monitor, the distance from the target to the observer was not constant across the entire target trajectory; since the monitor is flat, its edges are slightly farther from the observer than the center. Therefore, the target velocity expressed in visual degrees per second was corrected for the target’s apparent velocity as it reached the edge of the screen (gray trace in Fig. 1, A–D, bottom.
Eye and head displacements in horizontal and vertical directions were evaluated for the whole period the target moved on the screen (pursuit section). The eye-in-head excursions due to pursuit only were calculated by subtracting the total eye-in-head excursion due to saccades from total eye excursion during pursuit. During the pursuit section, the median and the peak value of the head angular velocity in the two directions (horizontal and vertical) were calculated.
A number of our participants tended to have small head movements that were not associated with the smooth pursuit task. These were uncorrelated and are likely associated with a reduction in postural stability associated with aging (Woollacott and Shumway-Cook 2002). Therefore, for individual analyses of eye and head movements we looked at displacement and velocity only along the direction of target motion (i.e., horizontal for 0° and 180° and vertical for 90° and 270°). However, for gaze gain calculation we were able to extract this data directly from our eye and head movement sensors and it represents the magnitude of the eye-in-space velocity. This simplification is possible because nontask-related head movements are typically accompanied by compensatory eye movements, which are therefore canceled out in gaze velocity.
All time-series analyses were performed using custom software written in MATLAB (Mathworks, Boston, MA).
Statistical analyses.
All statistical analyses were performed using Prism 8 software (Graphpad Software LLC, San Diego, CA). Due to recording artifact, participant C3 did not have data for the 270° target trajectory in the head-restrained condition. Therefore, in lieu of a three-way ANOVA with repeated measures, we used a mixed-effects analysis (using a maximum likelihood approach) of gain and mean eye velocity, standard in Prism 8. The fixed effects were: target direction, head-restraint condition, and participant type (MD versus control), as well as the interaction terms. We used the Tukey adjustment for multiple comparisons to account for the four target directions. Geisser-Greenhouse correction was applied for analysis where appropriate to adjust for differences in variability between effects. Only P values for significant interaction terms are reported. Alpha was set at 0.05 throughout.
RESULTS
Head-Unrestrained and Head-Restrained Smooth Pursuit
Figure 1 shows examples of smooth pursuit in the head-unrestrained and -restrained conditions in an individual with AMD (P5; Fig. 1, C and D) and a control (C2; Fig. 1, A and B). In the head-unrestrained data of the control participant (Fig. 1A), one can see the typical pattern of oculomotor pursuit initiation, followed by a contribution by the head, several hundred milliseconds later. Outside of the initial saccade placing the eye on the target (blank region in the velocity eye trace), participant C2 largely relies on a combination of smooth eye and head movements to maintain her eye on target. Note at the end of the trial where the head velocity exceeds that of the target velocity and a compensatory eye movement is generated. In the head-restrained case (Fig. 1B), the participant is able to maintain the eye on the target using smooth pursuit eye movements alone.
The strategy is not dissimilar in the case of the participant with AMD (P5; Fig. 1, C and D). This participant again initiates pursuit with the eyes after an initial saccade placing the eye on the pursuit target. In the head-unrestrained case (Fig. 1C), she subsequently also uses a combination of eye and head movements to follow the target. Again, she is able to compensate for a faster-than-target head velocity with a counterrotation of the eye. However, this participant’s eye movements are a combination of smooth pursuit and saccades. The majority of the saccades appear to be catch-up saccades compensating for smooth pursuit gaze velocity that is slower than the target velocity, particularly in the head-unrestrained case (Fig. 1C, position). Of note are the higher gaze velocities in the head-restrained condition for both participants. This observation is borne out further in the group gain analysis below.
Pursuit Gain
Looking at overall gaze (eye relative to space) velocities, individuals with macular degeneration had consistently and significantly lower gains than controls in both head-restrained and head-unrestrained conditions [P = 0.007, F(1,52) = 7.85, Fig. 2]. Furthermore, there was a significant effect of the head movement condition [restrained versus unrestrained, P < 0.0001, F(1,51) = 22.02] and target direction [P < 0.0001, F(3,52) = 9.49]. For target direction, smooth pursuit gain of the target moving along the downward (90°) trajectory was significantly lower than for all other directions (P < 0.01, Tukey’s multiple comparisons test). Additionally, there was a significant interaction of target direction and head restraint condition [P = 0.01, F(2,51) = 4.18], where smooth pursuit gain for the downward (90°) trajectory was not greater with the head restrained, unlike all other target directions. There were no other significant interactions (P ≥ 0.1). The above analysis was performed using a mixed effects analysis of repeated measures. For both groups, gains were lowest in the 90° (target moving downward) condition regardless of head restraint status. This horizontal versus vertical pursuit asymmetry is consistent with previous studies that have shown vertical/horizontal asymmetries in smooth pursuit gain, in several species, such as cat and human (e.g., Evinger and Fuchs 1978; Ke et al. 2013, respectively). Interestingly, gains were overall higher in the head-restrained condition for both groups for all but the downward target direction.
Eye-in-Head Velocity
To determine whether eye-in-head velocity amplitude changed between MD and control participants, we looked at mean eye-in-head velocity during the period of longest continuous pursuit, averaged across all trials for each target direction and participant (Fig. 3). Because pursuit consisted of eye and head velocity in the head-unrestrained condition, we also looked at mean head velocity during the same period for the head-unrestrained condition. For the purposes of statistical analysis, to account for opposite target directions having opposite sign, we multiplied all eye and head velocity values in response to targets in the 180° and 270° directions by −1. We found significantly higher eye velocities for control versus MD participants in the head-unrestrained (P = 0.003) and head-restrained conditions (P < 0.013). Mean head velocities were not significantly different between the two groups [P = 0.999, F(5,173) = 19.13, nested one-way ANOVA with Sidak adjustment for multiple comparisons, Fig. 3A].
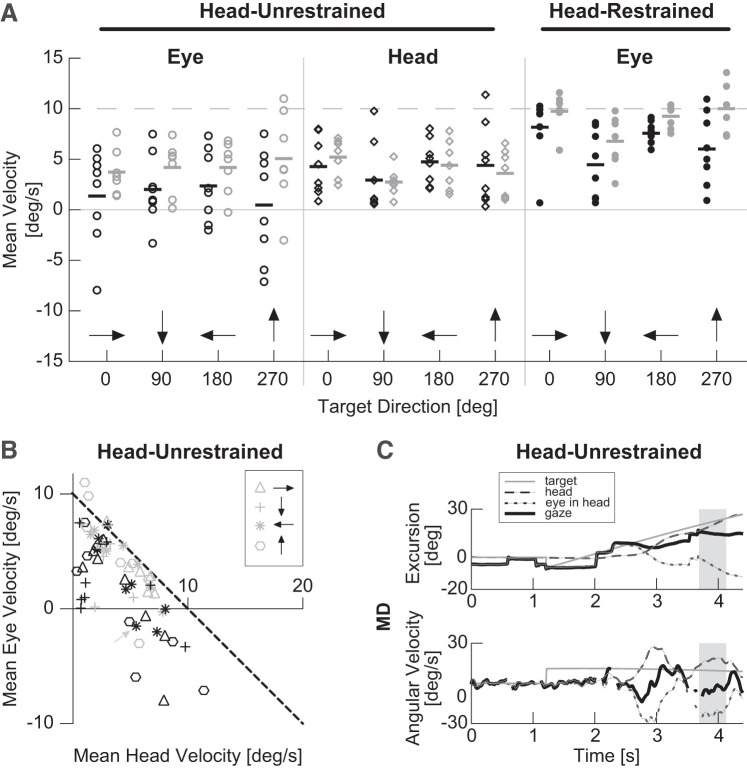
Fig. 3.
Mean eye and head velocity during longest period of continuous pursuit plotted across target directions. A: mean eye and head velocity is plotted for the head-unrestrained condition (columns 1 and 2) and mean eye velocity is plotted for the head restrained condition (column 3). Gray dashed line indicates target velocity. Black and gray horizontal bars indicate median velocity for each group/condition. B: mean eye and head velocity data from Head-Unrestrained columns in A are plotted against each other. Black dashed line represents perfect smooth pursuit gaze velocity. Each dot represents a control (gray) or macular degeneration (MD) participant (black). Data for trials in the head-unrestrained condition are designated with open symbols. Black arrows indicate target direction. C: single head-unrestrained horizontal smooth pursuit trial for participant P6 (gray dashed arrow in B). Top: position. Bottom: velocity data. Dashed black line: head, dotted black line: eye in head, solid black line: eye in space (gaze), and gray line: target. Region designated by gray rectangles was chosen as period of longest continuous pursuit for the given trial.
During head-unrestrained pursuit, eye and head velocity together (gaze) make up smooth pursuit velocity. Therefore, to better visualize how changes in eye, but not head velocity, would affect pursuit, we plotted mean eye velocity during periods of continuous pursuit versus corresponding mean head velocities (Fig. 3B). In the figure, the black dashed line indicates all combinations of ideal eye and head velocities for smooth pursuit of a 10°/s target. Overall, control participants (gray symbols) tend to be closer to this line, although there is a broad range of eye and head velocity combinations across all participants. Furthermore, the figure makes clear deficits in pursuit of downward (90°) targets, compared with other directions, as indicated by the pursuit gain (Fig. 2). Consistent with this observation, when we performed simple linear regressions for each target direction and group, we found all to have a significant relationship between eye-in-head and head velocities except those for the downward (90°) target direction (Table 2). For ideal pursuit, we expect a linear relationship with slope of −1 and intercept of 10°/s, where all eye and head velocity combinations add up to a gaze velocity of 10°/s. The results in Table 2 are generally consistent with this model.
Table 2.
Simple linear regression results for data in Fig. 3B
| Target Direction |
||||
|---|---|---|---|---|
| 0° | 90° | 180° | 270° | |
| MD | F(1,6) = 20.31 | F(1,6) = 1.985 | F(1,6) = 18.06 | F(1,6) = 19.65 |
| Slope | −1.461 | −0.485 | −1.377 | −1.203 |
| y-Intercept | 7.612 | 3.450 | 8.894 | 5.757 |
| P value | 0.004 | 0.209 | 0.005 | 0.004 |
| R2 | 0.772 | 0.249 | 0.751 | 0.766 |
| Control | F(1,5) = 26.21 | F(1,5) = 1.52 | F(1,5) = 40.60 | F(1,5) = 13.93 |
| Slope | −1.107 | −0.927 | −0.999 | −1.722 |
| y-Intercept | 9.500 | 6.737 | 8.606 | 11.28 |
| P value | 0.004 | 0.272 | 0.001 | 0.014 |
| R2 | 0.840 | 0.233 | 0.890 | 0.736 |
MD, macular degeneration.
As stated above, as a group, individuals with macular degeneration tend to have similar head velocities but smaller eye velocities (Fig. 3A). Eye velocity deficits are particularly evident for higher head velocities, where individuals with MD often seem to have eye movements in the opposite direction of the target (Fig. 3B). Figure 3C shows an example trial for an individual whose eyes frequently moved in the opposite direction of target motion. It is evident from the figure that although this participant is able to generate appropriate head movements for pursuit of the target (dashed lines), she is unable to cancel the vestibulo-ocular reflex (VOR) response for the majority of the trial (dotted lines) and therefore cannot maintain her gaze on target. The participant and target direction are indicated with a gray dashed arrow in Fig. 3B. It is important to note, however, that while Fig. 3C illustrates an example trial, the symbols in Fig. 3B represent average eye and head velocities across all trials for a given direction and participant.
Head Movement
Overall, there was large variability across all participants in their use of head movements during pursuit. There was no difference in head movement across participant groups. None of the participants had head movements in the opposite direction of the target. Figure 4A shows total head displacement across the entire ramp portion of the trial. For each target trajectory, each point represents the mean displacement for a given participant. Since total target motion was different for vertical versus horizontal target trajectories, the data are normalized by the target trajectory and direction, which yields a positive displacement between 0 and 1 (head displacement was always less than target displacement). There is no significant difference in total head displacement across groups [P = 0.97, F(1,13) = 0.001]. However, there was a significant effect of target direction, with participants of both groups having smaller proportion of head displacement to target displacement in the vertical directions [P < 0.0001, F(3,39) = 11.12, two-way ANOVA with repeated measures and Tukey-Kramer adjustment for multiple comparisons]. The outcomes are similar when we look at peak head velocity across the trial (Fig. 4B). Unlike for eye velocity, and consistent with mean head velocity (Fig. 3A), individuals in both groups had a similar range of peak head velocities (P = 0.764, F(1,13) = 0.094]. Additionally, target trajectory had a significant effect on head velocity [P = 0.002, F(1.96,25.45) = 7.916, Geisser-Greenhouse's epsilon = 0.65, two-way ANOVA with repeated measures and Tukey-Kramer adjustment for multiple comparisons]. Note: to account for opposite target directions having opposite sign, we multiplied all head velocity values in response to targets in the 180° and 270° directions by −1 for the purposes of statistical analyses (Fig. 4B).
Fig. 4.
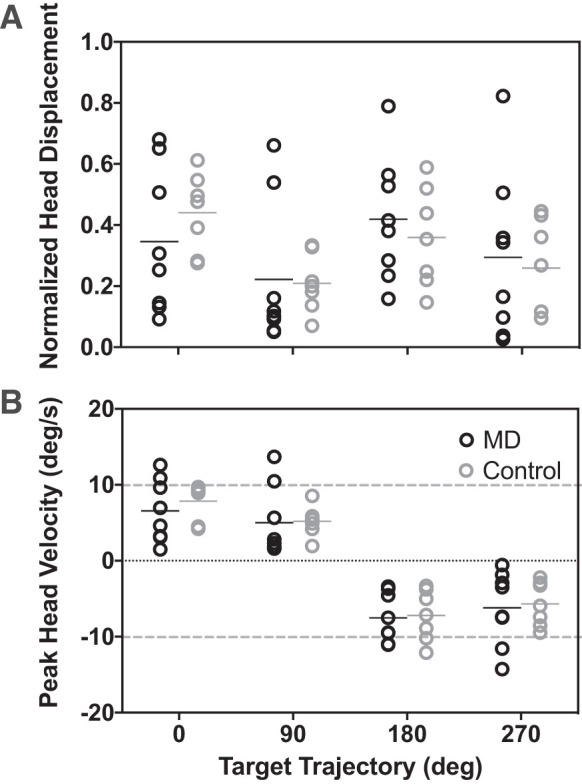
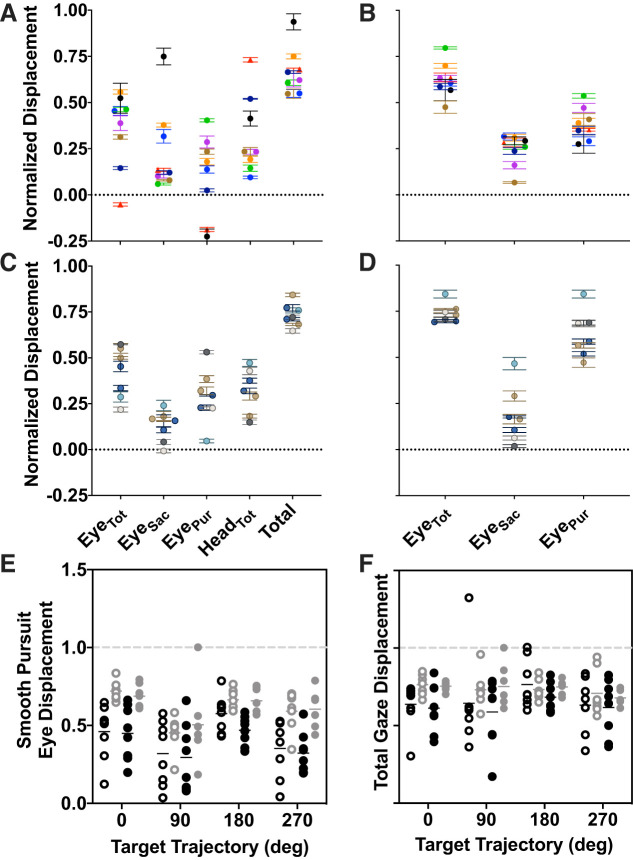
Head movement during smooth pursuit. A: total mean head displacement across the entire trial normalized by total target displacement. B: mean peak head velocity during the period of longest continuous pursuit. Sign corresponds to target direction; gray dashed lines demarcate target velocity. Black circles: participants with macular degeneration; gray: controls. Black and gray horizontal bars mark median values for each distribution.
Eye/Head Contributions to Smooth Pursuit
To determine how eye and head movements contribute to overall pursuit of the target, we looked at displacement of the eye and head across the duration of the pursuit trial (ramp). Figure 5, A–D summarizes how participants used saccadic and smooth pursuit eye movements, and head movements to maintain eyes on the target. Each symbol color corresponds to a given participant and shows the mean displacement for the corresponding column. Overall, there is a variation in strategies across individuals, both those with macular degeneration and controls. Note that because there is no head movement in the head-restrained condition (Fig. 5, B and D) the total displacement of the eye (column 1) corresponds to the total eye and head displacement in Fig. 5, A and C (gaze displacement). Despite the variability in strategy across individuals (use of head versus eye movements and saccades versus smooth pursuit) both MD and control groups adopted similar total gaze displacements strategies across the trial. For example, in the MD group, participant P3 (green) relies largely on smooth pursuit eye movements to maintain eyes on the target in the unrestrained condition (Fig. 5A). On the other hand, participant P8 (dark blue) makes very small eye movements (saccades or smooth pursuit) and instead follows the target largely with the head in the head-unrestrained condition. However, when the head is restrained, he uses a combination of smooth pursuit and saccadic eye movements throughout the trial. Similar patterns can be found in the control group, where participant C3 (turquoise) relies largely on head movements in the head unrestrained case but uses smooth pursuit eye movements when the head is restrained. On the other hand, participant C5 (charcoal) relies on smooth pursuit eye movements when the head is unrestrained.
Fig. 5.
Eye displacement during pursuit. A and C: macular degenerations (MDs; A) and controls (C) show eye displacement across the entire ramp portion of the trial during head-unrestrained smooth pursuit. Columns correspond to: total eye displacement (EyeTot), eye displacement due to saccades only (EyeSac), eye displacement due to smooth pursuit only (EyePur), head displacement (HeadTot), and total displacement due to eye and head motion (Total), respectively. B and D: MDs (B) controls (D) show eye displacement during head-restrained smooth pursuit. Columns correspond to: total eye displacement, eye displacement due to saccades only, and eye displacement due to smooth pursuit only. Each color corresponds to a given participant. Data are averaged across all trials, all directions; error bars are SE. E: comparison of total gaze (eye and head) displacement due to smooth pursuit alone during head-unrestrained (open) and head-restrained (solid) conditions across target trajectories for individuals with macular degeneration (black) and controls (gray). F: comparison of total eye-in-space displacement (smooth pursuit + saccades), organized as described in E. All data are normalized by target displacement. Dashed gray lines in E and F indicate equal eye and target displacement.
One potential difference between the individuals with macular degeneration and controls is the use of saccades. Figure 5, A–D, suggest that, on average, those with macular degeneration use saccades more than their age-matched controls. To probe this difference further, we examined the contribution of smooth pursuit eye movements versus saccades more closely in Fig. 5, E and F. Figure 5E compares total displacement between head restraint conditions (open vs. solid symbols) and participant groups (black vs. gray). In the head-unrestrained condition (open symbols) we computed the total displacement due to smooth pursuit eye and head movements. Here, we analyzed gaze displacement due to pursuit velocity alone (having removed saccades as described in Data Analysis). We compared these to those due to smooth pursuit only in the head-restrained condition. We found a significant difference between participant groups [P < 0.0001, F(1,52) = 37.62] but not head restraint conditions [P = 0.38, F(1,51) = 0.78]. Additionally, there was a significant effect of target trajectory [P = 0.0002, F(3,52] = 8.02, mixed effects analysis of repeated measures). These data suggest that regardless of whether the head was allowed to move, individuals cover a similar proportion of the target trajectory using available means (eye or head smooth pursuit movements). However, individuals with macular degeneration do not follow the target as accurately as controls.
In Fig. 5F, we extended this analysis to saccadic eye movements in addition to smooth pursuit. The data are plotted analogously to those in Fig. 5E. Here, there is no difference between target directions [P = 0.30, F(3,52) = 1.24] or head restraint conditions [P = 0.42, F(1,51) = 0.67]. We did find a significant effect of participant type [P = 0.003, F(1,52) = 9.613, mixed effects analysis of repeated measures]. Given that eye displacements due to saccades and smooth pursuit are in the same direction as target displacement, the data in Fig. 5 suggest that although individuals with macular degeneration do not especially benefit from the ability to move their heads in the head-unrestrained condition, catch-up saccades are instrumental for both groups for targets that are moving along the vertical trajectories.
PRL Eccentricity and Head Movement Strategy
Finally, we were interested whether the extent of macular degeneration affects use of head movement in smooth pursuit. Given that eye eccentricity in the orbit is a potential motivation for head movement in macular degeneration, we chose PRL distance from the former fovea as a metric for disease progression, as it is a direct measure of eccentricity of fixation. Figure 6 shows the relationship between PRL eccentricity and total head displacement across the trials, as well as the total gaze displacement (both averaged across all trials and normalized by target displacement). It is evident from the figure that there is no relationship between PRL distance and these two metrics (head: P = 0.25, R2 = 0.25; eye + head: P = 0.69, R2 = 0.03, Pearson correlation).
Fig. 6.
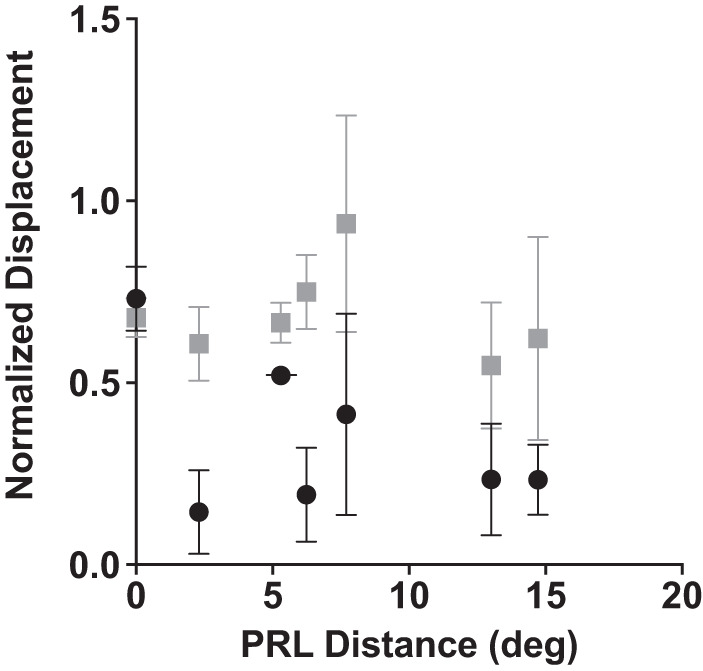
Relationship between preferred retinal locus (PRL) eccentricity and head (black circle) and total eye and head (gray square) displacement across the trial. Error bars are SD.
DISCUSSION
Overall, we find that individuals with macular degeneration have a similar range of head movement strategies during smooth pursuit as age-matched controls. These participants do not seem to benefit from head movements, contrary to our hypothesis. In fact, we find that both participants with macular degeneration and age-matched (older) controls tend to have lower pursuit gains in the head-unrestrained condition (Fig. 2). This finding is interesting, given that previous studies have suggested that there might be no change or a modest benefit to having an unrestrained head (Waterston and Barnes 1992). The latter outcome may be especially likely in the case where a target might disappear (Ackerley and Barnes 2011a), since head movements do not require direct visual input for pursuit (Ackerley and Barnes 2011b) and vestibulo-ocular reflex (VOR) cancellation is possible without the constant presence of the visual stimulus (Barnes and Grealy 1992). This experimental condition is particularly relevant in macular degeneration, where the target may disappear as it enters the central scotoma (Pidcoe and Wetzel 2006; Shanidze et al. 2016a). A potentially important difference between the studies are the instructions given to the participants. Ackerley and Barnes (2011a) specifically instructed their participants to pursue with the eyes and head. Our participants, on the other hand, were instructed to pursue in a manner that was most natural to them. This outcome may result not only in the greater variability we see in head movement velocities across participants but also in lower gaze velocities due to the lower head velocities in some participants. In their study (Ackerley and Barnes 2011a), the authors found that gaze velocities when the head was unrestrained were a summation of the gaze velocity in the head-restrained trials and 15% of the head velocity (which always exceeded target velocity).
Smooth Pursuit in Macular Degeneration
Of note is that despite these possible advantages of head movement in smooth pursuit, we did not see an improvement in participants with macular degeneration. First, since this is the first study looking at smooth pursuit in macular degeneration in a more natural context, we can conclude that the impairments previously reported in head-fixed smooth pursuit in MD (González et al. 2018b; Shanidze et al. 2016a, 2017) can be generalized to patients’ everyday lives. Furthermore, this study addresses the possibility that smooth pursuit is impaired in macular degeneration due to the eccentric resting eye position when a peripheral retinal locus is used for fixation (Whittaker et al. 1988). Both the finding that head movements do not offer an advantage for pursuit in this population (Fig. 5) and a lack of correlation between head and gaze movement and PRL eccentricity (Fig. 6) suggest that individuals with macular degeneration do not rely on head movements to compensate for physical oculomotor limitations present when fixation is asymmetric. The question then remains: what causes poor smooth pursuit performance in macular degeneration?
One simple possibility is that motion perception is impaired in the damaged retina, providing a noisy or unreliable signal to the smooth pursuit system. However, our recent study suggests that this is not the case, as individuals with macular degeneration are able to discriminate speed and direction of motion (in the same velocity range as was used in this and our previous studies) as well as their age-matched controls (Shanidze and Verghese 2019). Another possibility is that the tolerance for retinal slip is higher in the periphery and therefore pursuit gains can be lower when pursuing peripherally (Winterson and Steinman 1978). However, we have shown previously that pursuit gain is not correlated with PRL eccentricity or scotoma extent (Shanidze et al. 2017). Furthermore, as is evident from Fig. 6, gaze displacement in the direction of the target is also not correlated with PRL eccentricity during head-unrestrained smooth pursuit.
Finally, it is possible that as the oculomotor system is recalibrated for the PRL to be the new oculomotor reference (White and Bedell 1990), behaviors such as smooth pursuit might show asymmetries relative to the PRL’s location from the original fovea. In fact, such asymmetries in gain have been shown in other oculomotor behaviors, such as the vestibulo-ocular reflex (González et al. 2018a). However, although we have previously reported an interaction of target trajectory and scotoma location in macular degeneration (Shanidze et al. 2016a), these changes appeared to be more complex than a simple lateral asymmetry. Additionally, in that study we also showed that individuals might use more than one retinal locus for pursuit, even within a single trial, or no locus at all. This latter result was consistent with our findings in controls that the fovea need not be regularly used for pursuit for a large annular target (similar to the one used here) (Shanidze et al. 2016b). The question then remains: what is the specific underlying cause of smooth pursuit deficit in macular degeneration and how can it be addressed in a rehabilitative context?
The lack of smooth pursuit improvement in the head-unrestrained condition could suggest another possibility in MD. Figure 3A shows mean eye-in-head velocity across participants and conditions. One might note that there are several individuals whose eye velocity was in the opposite direction of the pursuit target. The data in Fig. 3 are only for the period of continuous pursuit (see gray rectangles in Fig. 1); however, this observation is further confirmed in Fig. 5A, where participants P2 (red triangle) and P6 (black circle) have eye displacements due to smooth eye movements in the opposite direction than the target (EyePur) and some of the greatest head displacements. These same individuals have eye velocities in the opposite direction of target velocity in Fig. 3A. In Fig. 3B, we can see that these negative eye-in-head velocities are associated with head velocities ≥5°. Thus we hypothesized that for these individuals the observed eye-in-head velocities are, in fact due to the VOR and not smooth pursuit. Looking at individual trials confirmed our suspicion. In Fig. 3C we show an example trial where participant P6 made a large head movement in pursuit of the target. However, she is clearly unable to follow the target with her gaze for the majority of the trial as the eyes rotate in the opposite direction of the head motion. These data suggest that a future study of VOR cancellation in the MD population may reveal impairments of eye-head coordination that might make the use of head movements disadvantageous in smooth pursuit.
Saccades, Smooth Pursuit, and Head Movement
It is unsurprising that we see a rise in saccadic intrusions in smooth pursuit in our MD participants. On the whole, they have lower pursuit gains and poorer fixational stability, both of which are associated with an increase in saccades. It is interesting, however, that regardless of oculomotor strategy, the overall gaze displacement is similar.
Previously, others have suggested that the reason for using the head (a slower more costly effector) for smooth pursuit might be to maintain a more central location of the eye in the orbit (e.g., Dubrovsky and Cullen 2002). However, we do not find an increase in gaze movement or decrease in saccade-based displacement in the head-unrestrained condition. This consideration is particularly interesting in the context of individuals who have a limited oculomotor range due to macular degeneration and often a more disorderly saccadic behavior (White and Bedell 1990).
Furthermore, we found a significant difference between participants with macular degeneration and controls in the amount of gaze displacement due to smooth pursuit movements alone. In parallel, we did not see a change in the amount of head movement between groups (Fig. 4). This finding suggests that whatever causes the decrease in eye movement velocity in macular degeneration does not extend to head movements. Previous work suggests that there is a shared mechanism that controls eye and head movements in pursuit (Dubrovsky and Cullen 2002). However, it is not one that is equally affected for eye and head movements in our population. One possibility is that smooth pursuit in macular degeneration is akin to the pursuit of an appearing and disappearing target. Prior work suggests that under certain stimulus conditions, the eye and head may be under independent control and visual feedback is required for synchronization of the two movements (Collins and Barnes 1999). The question then remains of why individuals with macular degeneration do not rely more on head movements in smooth pursuit? One possibility to be investigated further is the postural and vestibular instability associated with normal aging (Agrawal et al. 2009; Woollacott and Shumway-Cook 2002). Macular degeneration is largely a disorder of senescence. It is possible that as head movements become of greater utility to this population, they also become more challenging to control and generate accurately, potentially requiring greater cognitive resources, and thus can actually become disadvantageous. Furthermore, as eye movements become noisier in MD, a more precise control of head movements may be required. One potential outcome of this line of investigation may be the development of rehabilitative approaches for this population that focus on more en bloc strategies for certain oculomotor behaviors.
Limitations
There are several limitations in this study. First, the participant population with macular degeneration is highly heterogenous, with large variations in disease progression, scotoma size, PRL location, and effects of aging on other factors (e.g., mobility) to name a few. Therefore, generalizing across the population can be difficult. In this study, each participant was asked to perform multiple repetitions of each condition and we used four target directions to address possible effects of PRL location. Although we did observe an overall decrease in gain for the downward direction, we could not confirm that scotoma location also played an effect. Three participants had gain changes consistent with the findings of Shanidze et al. (2016a), where gains were highest when the target moved away from the scotoma and lowest for targets moving toward the scotoma (P1, P4, and P8). However, all three participants have scotomas along the vertical axis (2 upper and 1 lower visual field), meaning that two had gain decreases in the downward (90°) direction. We also saw lower gains in the downward direction for individuals with small or no binocular scotomas (P2 and P3), right visual field scotoma (P5), disperse scotomas (P6 and P7), and controls. We also did not find that the specifics of the scotoma or PRL had bearing on the chosen head movement strategy. For example, the participant who relied most on head movements for pursuit was the one with no binocular visual field loss (P2, black triangles in Fig. 5, A and B); however, the two others who used eye movements extensively had large central scotomas (P8 and P6). Future studies that use artificial scotomas in addition to individuals with central visual field loss could help further address the natural heterogeneity among MD participants. However, artificial scotomas have their own limitations as they do not develop naturally and over time, allowing individuals to potentially adjust.
Second, because smooth pursuit in individuals with macular degeneration tends to vary across and within trials, it is not possible to analyze the entire steady state portion of pursuit for all individuals. To address this problem, we chose to use the period in the trial of longest continuous pursuit as a snapshot of that individual’s behavior on a given trial. Although overall effective (Shanidze et al. 2016a, 2017), this approach does create the possibility that these periods might vary in duration across participant types or conditions. Head movements could be a particular source of variability. We address this problem in two ways. First, each trial was examined by at least one experimenter and trials where the algorithm was ineffective were flagged and either excluded (for example if the was no period of pursuit that was long enough) or the next longest period was picked instead. On the whole these events were highly uncommon, happening on less than 10% of trials. Second, in addition to the period of longest continuous pursuit, we chose to use displacement metrics across the entire trial. Looking at head velocity metrics for these two approaches (Fig. 3A and Fig. 4B), we find close agreement in the overall similarities across groups. These metrics allowed us to look at how effectively individuals used eye, head and gaze to track targets from the beginning to the end of the trial.
Conclusions
Macular degeneration is a highly prevalent and debilitating disorder that affects many tasks of daily living. Although it has been studied extensively in the context of high-acuity tasks in the laboratory setting, far less is known about how it affects function in other aspects of daily life. This paper takes a step in this direction, looking at smooth pursuit in a more natural, head-unrestrained setting. We find that in individuals with macular degeneration, the ability to move the head does not offer an additional advantage nor does it affect pursuit any more adversely than their age-matched controls. In fact, we find that both groups have a slight, but significant, increase in pursuit gain when the head is restrained. Overall, control participants have higher pursuit gains than those with macular degeneration, regardless of the head restraint condition. The extent of central retinal lesion, addressed here in terms of PRL eccentricity, is not correlated with head movement strategy or overall gaze excursion across the trial.
GRANTS
This work was supported by National Eye Institute Grant R00-EY-026994 (to N. Shanidze) and the Smith-Kettlewell Eye Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S. conceived and designed research; N.S. and A.V. performed experiments; N.S. and A.V. analyzed data; N.S. and A.V. interpreted results of experiments; N.S. and A.V. prepared figures; N.S. drafted manuscript; N.S. and A.V. edited and revised manuscript; N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. James Coughlan for assistance with the implementation and testing of homography transformations in this data set.
REFERENCES
- Ackerley R, Barnes GR. Extraction of visual motion information for the control of eye and head movement during head-free pursuit. Exp Brain Res 210: 569–582, 2011a. doi: 10.1007/s00221-011-2566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R, Barnes GR. The interaction of visual, vestibular and extra-retinal mechanisms in the control of head and gaze during head-free pursuit. J Physiol 589: 1627–1642, 2011b. doi: 10.1113/jphysiol.2010.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med 169: 938–944, 2009. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- Barnes GR. Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog Neurobiol 41: 435–472, 1993. doi: 10.1016/0301-0082(93)90026-O. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Grealy MA. Predictive mechanisms of head-eye coordination and vestibulo-ocular reflex suppression in humans. J Vestib Res 2: 193–212, 1992. [PubMed] [Google Scholar]
- Collins CJ, Barnes GR. Independent control of head and gaze movements during head-free pursuit in humans. J Physiol 515: 299–314, 1999. doi: 10.1111/j.1469-7793.1999.299ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland MD, Sims M, Galbraith RF, Rubin GS. Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vision Res 44: 1537–1546, 2004. doi: 10.1016/j.visres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Dierkes K, Kassner M, Bulling A. A novel approach to single camera, glint-free 3D eye model fitting including corneal refraction. In: ETRA ’18: 2018 Symposium on Eye Tracking Research and Applications, June 14–17, 2018, Warsaw, Poland. New York: ACM Press, 2018, p. 1–9. doi: 10.1145/3204493.3204525. [DOI] [Google Scholar]
- Dubrovsky AS, Cullen KE. Gaze-, eye-, and head-movement dynamics during closed- and open-loop gaze pursuit. J Neurophysiol 87: 859–875, 2002. doi: 10.1152/jn.00447.2001. [DOI] [PubMed] [Google Scholar]
- Ehinger BV, Groβ K, Ibs I, König P. A new comprehensive eye-tracking test battery concurrently evaluating the Pupil Labs glasses and the EyeLink 1000. PeerJ 7: e7086, 2019. doi: 10.7717/peerj.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger C, Fuchs AF. Saccadic, smooth pursuit, and optokinetic eye movements of the trained cat. J Physiol 285: 209–229, 1978. doi: 10.1113/jphysiol.1978.sp012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EG, Shi R, Tarita-Nistor L, Mandelcorn ED, Mandelcorn MS, Steinbach MJ. Image stabilization in central vision loss: the horizontal vestibulo-ocular reflex. Vision (Basel) 2: 19–15, 2018a. doi: 10.3390/vision2020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EG, Tarita-Nistor L, Mandelcorn E, Mandelcorn M, Steinbach MJ. Mechanisms of image stabilization in central vision loss: smooth pursuit. Optom Vis Sci 95: 60–69, 2018b. doi: 10.1097/OPX.0000000000001161. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Badler JB, Ting W. Timing and velocity randomization similarly affect anticipatory pursuit. J Vis 5: 493–503, 2005. doi: 10.1167/5.6.1. [DOI] [PubMed] [Google Scholar]
- Kabanarou SA, Crossland MD, Bellmann C, Rees A, Culham LE, Rubin GS. Gaze changes with binocular versus monocular viewing in age-related macular degeneration. Ophthalmology 113: 2251–2258, 2006. doi: 10.1016/j.ophtha.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Ke SR, Lam J, Pai DK, Spering M. Directional asymmetries in human smooth pursuit eye movements. Invest Ophthalmol Vis Sci 54: 4409–4421, 2013. doi: 10.1167/iovs.12-11369. [DOI] [PubMed] [Google Scholar]
- Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 129: 75–80, 2011. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- Lanman J, Bizzi E, Allum J. The coordination of eye and head movement during smooth pursuit. Brain Res 153: 39–53, 1978. doi: 10.1016/0006-8993(78)91127-7. [DOI] [PubMed] [Google Scholar]
- Macinnes JJ, Iqbal S, Pearson J, Johnson EN. Wearable eye-tracking for research: automated dynamic gaze mapping and accuracy/precision comparisons across devices (Preprint). bioRxiv 299925, 2018. doi: 10.1101/299925. [DOI] [Google Scholar]
- Pidcoe PE, Wetzel PA. Oculomotor tracking strategy in normal subjects with and without simulated scotoma. Invest Ophthalmol Vis Sci 47: 169–178, 2006. doi: 10.1167/iovs.04-0564. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth HL, Lora AN, Heilman KM. Effects of monocular viewing and eye dominance on spatial attention. Brain 125: 2023–2035, 2002. doi: 10.1093/brain/awf210. [DOI] [PubMed] [Google Scholar]
- Shanidze N, Fusco G, Potapchuk E, Heinen S, Verghese P. Smooth pursuit eye movements in patients with macular degeneration. J Vis 16: 1, 2016a. doi: 10.1167/16.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N, Ghahghaei S, Verghese P. Accuracy of eye position for saccades and smooth pursuit. J Vis 16: 23, 2016b. doi: 10.1167/16.15.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N, Heinen S, Verghese P. Monocular and binocular smooth pursuit in central field loss. Vision Res 141: 181–190, 2017. doi: 10.1016/j.visres.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanidze N, Verghese P. Motion perception in central field loss. J Vis 19: 20, 2019. doi: 10.1167/19.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe JA, Sylvester TO. Effect of aging on horizontal smooth pursuit. Invest Ophthalmol Vis Sci 17: 465–468, 1978. [PubMed] [Google Scholar]
- Stahl JS. Eye-head coordination and the variation of eye-movement accuracy with orbital eccentricity. Exp Brain Res 136: 200–210, 2001. doi: 10.1007/s002210000593. [DOI] [PubMed] [Google Scholar]
- Swirski L, Dodgson NA. A fully-automatic, temporal approach to single camera, glint-free 3D eye model fitting. International Workshop on Pervasive Eye Tracking and Mobile Eye-Based Interaction Lund, Sweden, August 13, 2013. [Google Scholar]
- Tarita-Nistor L, Brent MH, Steinbach MJ, González EG. Fixation patterns in maculopathy: from binocular to monocular viewing. Optom Vis Sci 89: 277–287, 2012. doi: 10.1097/OPX.0b013e318244e8b1. [DOI] [PubMed] [Google Scholar]
- Verghese P, Tyson TL, Ghahghaei S, Fletcher DC. Depth perception and grasp in central field loss. Invest Ophthalmol Vis Sci 57: 1476–1487, 2016. doi: 10.1167/iovs.15-18336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston JA, Barnes GR. Visual-vestibular interaction during head-free pursuit of pseudorandom target motion in man. J Vestib Res 2: 71–88, 1992. [PubMed] [Google Scholar]
- White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Invest Ophthalmol Vis Sci 31: 1149–1161, 1990. [PubMed] [Google Scholar]
- Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Invest Ophthalmol Vis Sci 29: 268–278, 1988. [PubMed] [Google Scholar]
- Winterson BJ, Steinman RM. The effect of luminance on human smooth pursuit of perifoveal and foveal targets. Vision Res 18: 1165–1172, 1978. doi: 10.1016/0042-6989(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16: 1–14, 2002. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]