Abstract
The aim of the study was to examine whether the sustained increases in the excitability of afferent fibers traversing the dorsal columns evoked by their polarization depend on the branching points of these fibers. To this end, the effects of epidural polarization were compared in four spinal regions in deeply anesthetized rats; two with the densest collateralization of muscle afferent fibers (above motor nuclei and Clarke’s column) and two where the collateralization is more sparse (rostral and caudal to motor nuclei, respectively. The degree of collateralization in different segments was reconstructed in retrogradely labeled afferent fibers in the rat. Nerve volleys evoked in peripheral nerves by electrical stimulation of the dorsal columns within these regions were used as a measure of the excitability of the stimulated fibers. Potent increases in the excitability were evoked by polarization above motor nuclei and Clarke’s column, both during constant direct current (DC) polarization (1 µA for 1 min) and for at least 30 min following DC polarization. Smaller excitability increases occurred during the polarization within other regions and were thereafter either absent or rapidly declined after its termination. The postpolarization increases in excitability were counteracted by the GABAA receptor antagonist bicuculline and the α5GABAA extrasynaptic receptor antagonist L655708 and enhanced by the GABAA receptor agonist muscimol and by ionophoretically applied GABA. As extrasynaptic α5GABAA receptors have been found close to Na channels within branching points, these results are consistent with the involvement of branching points in the induction of the sustained postpolarization increases in fiber excitability.
NEW & NOTEWORTHY Polarization of sensory fibers traversing dorsal columns of the spinal cord may considerably increase the excitability of these fibers. We show that this involves the effects of current at branching points of afferent fibers and depends on extrasynaptic effects of GABA. These results contribute to our understanding of the mechanism underlying plasticity of activation of nerve fibers and may be used to increase the effectiveness of epidural stimulation in humans and recovery of spinal functions.
Keywords: branch points, extrasynaptic, GABAA receptor, myelinated nerve fibers, plasticity, rat, spinal cord
INTRODUCTION
Changes in the excitability of nerve fibers following their polarization have previously been found to be short lasting and mainly small (Anastassiou et al. 2011; Faber and Korn 1989; Gutnick et al. 1975; Nelson 1966). However, sustained and large increases in the excitability have recently been reported (Bolzoni and Jankowska 2015; Jankowska et al. 2016), and both their duration and extent appeared to depend on the site of polarization. That is, when myelinated afferent fibers traversing the dorsal columns of the spinal cord were polarized by direct current (DC) applied epidurally, the excitability of these fibers increased multi-old and the increase outlasted the short period (≤1 min) of polarization for at least 1–2 h. In contrast, the polarization of muscle afferent fibers within their terminal projection areas (within hindlimb motor nuclei or in the dorsal horn) only resulted in a moderate increase (up to twofold) and declined within a couple of minutes. One possible reason for this discrepancy might have been differences in the properties of preterminal nerve branches, lacking myelin sheaths, and myelinated portions of nerve fibers within the dorsal columns. However, the aftereffects of DC on myelinated fibers in peripheral nerves and dorsal roots were found to be as weak as those following intraspinally applied DC (Bolzoni et al. 2017, 2019). We, therefore, hypothesized that the stronger and longer lasting postpolarization increase in the excitability of nerve fibers traversing the dorsal columns is less dependent on the myelinization than on the specific fiber properties at their branching points within the dorsal columns, specifically at the branch points where the collaterals depart the dorsal columns to enter the spinal gray matter and where the thresholds of excitation are at their lowest (Danner et al. 2011; Struijk et al. 1992). The first aim of this study was to verify this hypothesis.
Morphological studies demonstrated that the regions of the primary branching points of afferent fibers are within or just ventral to the dorsal columns (Hongo et al. 1987; Ishizuka et al. 1979; Lucas-Osma et al. 2018; Walmsley et al. 1985, 1995) and would thereby be readily depolarized by DC applied to the spinal cord surface. These studies also revealed differences in the density of collaterals issued along the lumbosacral enlargement, making it possible to compare effects of DC within the regions with the high and the low density, an approach we take here. However, the available data on the collateralization of primary afferents are primarily for the feline group Ia afferents, while no comparison of the number of collaterals issued in different spinal segments has yet been done in other species. The differences in afferent branching density in the rat used in this study might be either similar or lower than in the cat as the rat spinal segments are much shorter. Therefore, we combined the analysis of effects of polarization of dorsal column fibers at different segmental levels in the rat with a morphological study of the density of collaterals of large muscle afferents within the thoracic T12 to lumbar L5 segments. It will be shown that the most potent postpolarization effects are evoked within segments containing motor nuclei and dorsal spinocerebellar tract (DSCT) neurons and are fully compatible with DC-evoked sustained increases in excitability within afferent branching points.
We further hypothesized that these postpolarization sustained increases in excitability depend on sodium channels in close proximity to extrasynaptic α5GABAA receptors (Delgado-Lezama et al. 2013; Paul et al. 2012; Perez-Sanchez et al. 2017) which have been recently found within branching points (Lucas-Osma et al. 2018) where they may be targeted by extrasynaptic GABA (Farrant and Nusser 2005; Kullmann et al. 2005; Olsen and Sieghart 2009).
To verify this hypothesis, the second aim of this study, we examined the effects of the synaptic and extrasynaptic GABAA antagonists bicuculline (Curtis 1973) and L655708 (Delgado-Lezama et al. 2013; Lucas-Osma et al. 2018), respectively, as well as the GABAA receptor agonist muscimol (Curtis and Lodge 1982), administered either systemically or ionophoretically, on DC-evoked changes in excitability within the regions with the high and the low collateral density.
The results of both series of experiments were fully consistent with the involvement of branching points in the induction of the sustained postpolarization increases in fiber excitability. After having pinpointed the sites at which the most effective DC actions on nerve fibers are evoked, one may expect faster progress in elucidating how they are evoked and how they may be used to enhance the beneficial effects of epidural and transcutaneous stimulation in humans. The results of the present study may also be relevant for the interpretation of clinical effects of transcranial and epidural stimulation and polarization, by supplementing the discussion on mechanisms and functional consequences of brain and spinal cord polarization hitherto primarily focused on its effects on somata, dendrites, and terminals of axons of nerve cells while abstaining from polarization-evoked changes in the axons themselves (see, e.g., Morya et al. 2019). However, the main conclusions concerning primary effects of epidural stimulation, i.e., that they are evoked by stimulation of myelinated afferent fibers (Capogrosso et al. 2013, 2018a), are in full agreement with our conclusions, although our results concern fibers stimulated within the dorsal columns and not those in the dorsal roots.
MATERIALS AND METHODS
The electrophysiological experiments were performed at the Department of Physiology, University of Gothenburg, Sweden. The morphological analysis was done at the Neuroscience and Mental Health Institute and Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, AB, Canada. The topography of effects of polarization and actions of GABA antagonists were analyzed by Y. Li and E. Jankowska and actions of Muscimol and GABA by K. Hari, I. Hammar, and E. Jankowska while collateralization at different segmental levels was compared by K. Hari, A. M. Lucas-Osma, K. K. Fenrich, and D. J. Bennett.
Ethical Approval
All experiments were approved by the Regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and by the Health Sciences University of Alberta Animal Care and Use Committee and followed European Union, Canadian Council on Animal Care, and NIH guidelines for animal care. The animals were housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy (40 adult rats of both sexes; Sprague-Dawley, 200–330 g) and at the Health Sciences University of Alberta Animal Care Facility (4 adult female rats; Sprague-Dawley, 200–330 g) Particular measures were taken to minimize both the animal discomfort and the number of animals used.
Electrophysiological Experiments
Preparation.
The experiments were performed using the same protocol as in the previous series of experiments on the effects of DC (e.g., Jankowska et al. 2017). Anesthesia was induced with isoflurane (Baxter Medical AB, Kista, Sweden) (4.5% in air) followed by intraperitoneal administration of pentobarbital sodium (Apoteksbolaget, Göteborg, Sweden; 30 mg/kg) together with α-chloralose (Acros Organics, Geel, Belgium, 30 mg/kg). During the experiment, the anesthesia was supplemented with additional doses of α-chloralose (3 mg/kg, up to 40 mg/kg) at 3–4 h intervals. Neuromuscular transmission was blocked by gallamine triethiodide (Sigma Aldrich, G8134) injected intravenously (via the tail vein) at an initial dose of 10 mg/kg supplemented with 5 mg/kg. Gallamine was not administered until ~3 h after anesthesia induction, by which time deep and stable anesthesia was established. Artificial ventilation was applied by a respiratory pump (CWE; model SAR-830/P) to maintain the end-tidal CO2 level at ~3–4%. Core body temperature was kept at ~38°C with servo-controlled heating lamps. To compensate for fluid loss, 10–20 ml of acetate buffer were injected subcutaneously at the beginning of the experiments. The experiments were terminated by a lethal injection of an anesthetic causing cardiac arrest.
Following tracheal intubation and tail vein cannulation, the peroneal (Per) nerve including nerve branches to tibialis anterior (TA) and the tibial (Tib) nerve including nerve branches to medial and lateral gastrocnemius (G) were dissected free and mounted on pairs of electrodes in a paraffin oil pool at rooms temperature, and Th13–L4 spinal segments were exposed by laminectomy after the vertebral column had been stabilized by a clamp.
Experimental design.
Changes in the size and the latency of nerve volleys in peripheral nerves were used as a measure of the excitability of the fibers stimulated in the dorsal columns using the setup illustrated in Fig. 1A. The fibers were stimulated epidurally (see Stimulation), thereby ensuring an optimal circulation of the spinal cord and maintaining its protection by the cerebrospinal fluid.
Fig. 1.
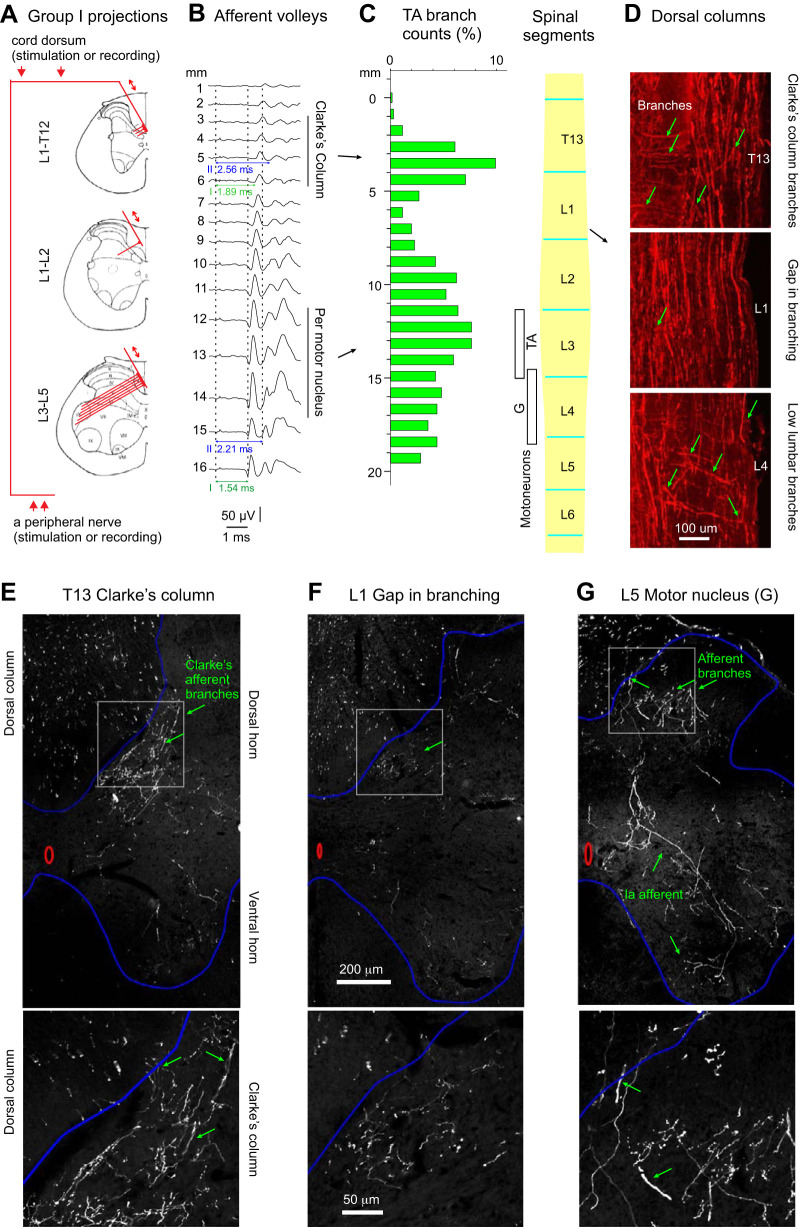
Rostrocaudal distribution of branching points of largest muscle afferent fibers traversing the dorsal columns. A: diagrams indicating the predicted degree of density of collaterals of group I and group II peroneal (Per) muscle afferents issued from the dorsal columns within the Th13–L5 segments. Double arrows above the 3 diagrams indicate the direction of impulses, either toward the peripheral nerves following stimulation of the dorsal columns (2 arrows above the top diagram) or toward the spinal cord following stimulation of the peripheral nerve (2 arrows below the bottom diagram). B: afferent volleys evoked by stimulation of the common peroneal nerve at 5T. They were recorded from the surface of the spinal cord (cord dorsum) at sites 1 mm apart over a distance of 16 mm between the Th13 and the L5 segments. The 1st component, attributed to group I muscle afferents, appeared at stimulus intensities of 1T and was maximal at 2T. The 2nd component, attributed to group II afferents, appeared at 2.2T and was maximal at 5T. The dotted vertical lines indicate the onset of the stimulus artifact and the positive peak of afferent volleys in group I and group II afferents, respectively. The horizontal lines indicate the latencies of group I and group II afferent volleys with respect to the stimulus artefacts. The vertical lines to the right indicate the estimated location of Per motor nuclei in L3–L4 segments and the caudal extent of the rat Clarke’s column. C: histogram of the number of tibialis anterior (TA) afferent branches arising from the dorsal columns per millimeter along the T13–L5 segments of the spinal cord, normalized to the total branch count in one of the rats with viral TA muscle labeling with AAV9-CAG-tdTom. Location of TA and gastrocnemius (G) motoneurons labeled in this rat is indicated with white boxes, next to the schematic of the lumbosacral enlargement with segments lengths drawn to the scale used for the afferent count histogram. D: example sagittal sections including images of TA (afferent branches arising from the dorsal columns within T13, L1, and L4 segments (green arrows) in 1 of the rats. Epifluorescence microscopy using a ×10 objective. E–G: examples of transverse sections illustrating G afferent branches arising from the dorsal columns within T13, L1, and L5 segments (green arrows). Confocal microscopy with a ×25 water immersion objective. Boxed parts in top images are enlarged in bottom images.
To relate the effects of DC to the degree of the collateralization of the dorsal column fibers, DC was applied at different segmental levels with respect to the location of hindlimb motor nuclei. The location of the peroneal and\or tibial motor nuclei was defined at the beginning of each experiment as corresponding to the 3- to 4-mm length of the spinal cord over which the largest afferent volleys were evoked when Per and Tib nerves were stimulated at intensities supramaximal for group I afferents, as illustrated in Fig. 1B. The tungsten electrode used for the subsequent epidural stimulation was positioned at the center of this region, about half-way between the posterior spinal vein and the dorsal root entry zone. Two or three stimulation intensities, just at and slightly above threshold for the volleys, were selected for testing, after verification that much larger volleys could be evoked by increasing the stimulus intensity, i.e., allowing a sufficient range for the demonstration of any facilitatory effects.
To examine effects of GABA or its antagonists or agonists on DC-evoked changes in the excitability at various segmental levels, nerve volleys evoked by stimulation of afferent fibers in the dorsal column at these levels were compared under four circumstances: first, during a control period, lasting for a minimum of 5 min or until the responses of the stimulated fibers were stable; second, following intravenous administration of the GABA antagonists or agonist, or during GABA ionophoresis, until any changes evoked by drug application appeared to reach a plateau, usually for a period of 10–20 min; third, during 1 min of polarization; and fourth, during the postpolarization period for up to 30 min.
We collected data for each of the experimental series in at least three to four rats, with a minimum of six to eight series of records, allowing estimates of statistical significance. To increase the outcome of the experiments and to minimize the number of the experimental animals, the nerve volleys were, as a rule, recorded in parallel in Per and Tib nerves thereby doubling the number of the experimental series in a sequence.
Recording.
Nerve volleys evoked by epidural stimulation were recorded bipolarly, via a pair of silver-silver chloride electrodes ~3–4 mm apart. Afferent volleys evoked by peripheral nerve stimulation were recorded using a silver-silver chloride ball electrode in contact with the cord dorsum close to the dorsal root entry points against a reference electrode inserted into one of the back muscles. Both single records and averages of 10 nerve volleys were stored online using a sampling frequency of 33 kHz (resolution of 0.03 ms), with a low-pass filter set to 15 or 1 Hz and high-pass filter set to 5 or 3 kHz. The effects of DC on epidurally evoked nerve volleys were recorded continuously during the first 30 s of DC application as well as immediately after it was terminated and once a minute during the pre- and postpolarization periods.
Multicomponent volleys were recorded from the peripheral nerves but the analysis was restricted to the earliest components of the averaged records. These components were evoked at similar latencies as afferent volleys recorded at the dorsal root entry zone following near-threshold stimulation of the nerves and were therefore attributed to group Ia afferents.
Stimulation.
The dorsal column was stimulated monopolarly via tungsten needle electrodes insulated except at the tip for 20–30 µm (microneurography active needle, UNA35FNM, FHC, Bowdoin, ME; impedance 30–400 kΩ) against a 2-cm long wire reference electrode inserted in back muscles at the midline just rostral to the laminectomy. Single 0.2-ms rectangular stimuli were delivered at intensities up to 50 µA. Peripheral nerves were stimulated bipolarly at two to five times threshold using constant voltage stimuli.
DC polarization.
DC was applied using a custom-designed, battery-driven, constant current stimulator (D. Magnusson, University of Gothenburg) or a DS3 Isolated Current Stimulator (Digitimer, UK). The current was passed via the same tungsten electrode that was used for stimulation of the fibers, as we have previously demonstrated that DC does not interfere with the stimulating current pulses under our experimental conditions (see Fig. 2 in Bączyk and Jankowska 2014). One-microampere cathodal current was applied to the surface of the spinal cord as described by Jankowska et al. (2017). Even though a few seconds of epidural polarization suffices for inducing postpolarization increases in fiber excitability (Bączyk and Jankowska 2018; Jankowska et al. 2017), the reported results were routinely evoked by 1-min polarization.
Fig. 2.
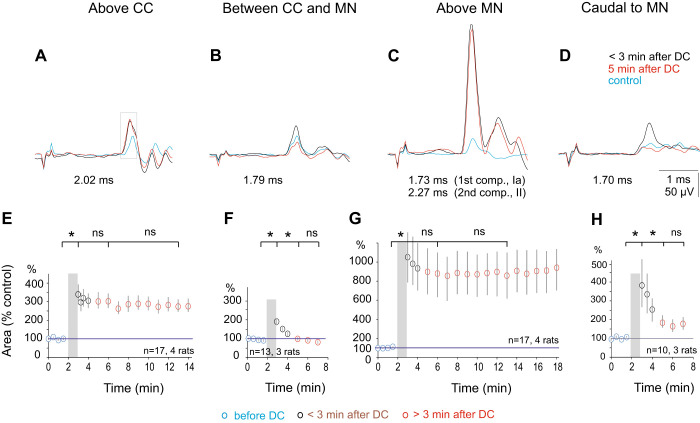
Comparison of postpolarization effects of direct current (DC) applied at 4 rostrocaudal levels. A–D: examples of nerve volleys in the peroneal nerve following stimulation of the dorsal columns over Clarke’s column (CC), caudal to Clarke’s column, above peroneal (Per) motor nuclei (MN), and caudal to these nuclei. Averages of 10 single records obtained at ~1 Hz. The volleys were evoked by epidural stimulation of 22, 15, 24, and 16 µA, respectively. In A–D, the superimposed traces were obtained before DC application (blue), at the end of the cathodal polarization with 1 µA (black) and 5 min after the polarization was discontinued (red). The areas of the first components of the volleys were measured within a time window indicated by the rectangle in A. Note in A and C that nerve volleys recorded immediately after and 5 min after the termination of the polarization were similar while the corresponding nerve volleys in B and D declined to the level of control records. E–H: time course of effects of polarization in the same four regions as in A–D. Ordinate, normalized areas of the earliest components of the nerve volleys (means ± SE) evoked by stimulation of the dorsal columns, measured within time windows of 0.45, 0.45, 0.54, and 0.58 ms, respectively. Note a different ordinate scale in G. Abscissa, time in min. Gray columns indicate periods of polarization (1 min). Statistical significance is as indicated (*P < 005; ns, not significant).
Ionophoresis.
GABA and muscimol were ejected from glass micropipettes (tip 2.5–3 µm; 12–40 MΩ) filled with a solution of 0.25 M (GABA, Sigma Aldrich, O3835) or 0.05 M muscimol (Sigma Aldrich, M1523), respectively. In both cases, 30- to 60-nA positive current was used. The micropipette was inserted into the dorsal column through a small opening (<0.4 mm2) made in the dura and pia matters, to the depth 100–150 µm from the surface, at a distance of 0.5–1 mm from the tip of the tungsten electrode. Before the beginning of the ionophoresis, a retaining current of −10 nA was passed to prevent drug diffusion. Ionophoresis was initiated when stable control responses had been recorded for 5–10 min. It was continued for 10 or 20 min and maintained for the duration (1 min) of the dorsal column polarization. The ionophoresis was either continued throughout the postpolarization period or was discontinued by passing a retaining current (10 nA, see results). The parameters of current used for the ionophoresis were within the ranges used previously for testing effects of GABA and muscimol on terminals of group Ia muscle afferents (20–80 nA; Curtis and Lodge 1982).
Drug solutions.
The following solutions were administered either intraperitoneally or intravenously: chloralose at 3 mg/ml (in distilled H2O), gallamine at 20 mg/ml, and pentobarbital at 10 mg/ml in 0.9% NaCl, bicuculline methochloride (Sigma Aldrich, B7686) dissolved in saline to 2 or 4 mg/ml, and L655708 (Sigma, 130477-52-0) at 1 mg/ml first dissolved in a minimum amount of ethanol and then diluted in distilled H2O.
The solutions used for ionophoresis were muscimol 0.05 M (Sigma Aldrich, Sweden) and GABA (Sigma) 0.25 M, both in 0.9% NaCl.
Analysis.
The nerve volleys were compared by measuring their areas, using an analysis program designed by E. Eide (the University of Göteborg; see Jankowska et al. 1997). The areas were expressed in arbitrary units and measured within a time window of 0.4–0.7 ms from the onset of the earliest components of nerve volleys to exclude a nonlinear summation with later components. These early components were routinely verified to be evoked at latencies corresponding to the latencies of afferent volleys in Ia muscle spindle afferents in muscle nerves. Thereby, volleys recorded from mixed muscle nerves could be attributed to group Ia or Ib muscle afferents as group II and cutaneous afferents in these nerves display 0.7–0.8 ms longer conduction time. The differences in latencies of afferent volleys in group Ia and II afferents are indicated in Fig. 1B.
The results are illustrated with data from single experiments and with data pooled from several experiments. As no differences were found between the effects on Per and Tib afferents, data for these afferents were pooled together for statistical analysis. The statistical significance was analyzed in Sigma Plot (Jandel Scientific, San Rafael, CA). It was estimated using repeated measures analysis of variance (RM ANOVA) with the post hoc comparisons to control by Dunnett's test and Student’s t test for paired samples or for unpaired samples assuming equal variance. The normality of data sets was verified by the Shapiro-Wilk normality test (P > 0.05). Most data sets were found to be normally distributed. For those that were not normally distributed, a Mann-Whitney rank-sum test was used. The significance level indicated by a level a set at *P < 0.05.
Viral Tracing and Proprioceptive Afferent Branch Quantification
Proprioceptive afferents of the Per and Tib nerves were labeled by bilateral injections of AAV9-tdTom and AAV9-GFP into the TA and G (both lateral and medial heads) muscles. To improve transduction efficiencies, viral vectors (AAV9-CAG-tdTom, 5.9 × 1012 vg/ml; AAV9-CAG-GFP, 2 × 1012 vg/ml; UNC Vector Core) were incubated with LAH4 peptide [200 µM] at 37°C for >45 min immediately before injection (Liu et al. 2014). With the use of sterile techniques, rats were anaesthetized using isoflurane (2.5% in 95:5 oxygen:carbon dioxide mixture), the hindlegs were shaved and disinfected with 10% chlorhexidine gluconate, and bilateral TA and G were surgically exposed. For each leg, muscles were injected with either AAV9-CAG-tdTom or AAV-CAG-GFP at different locations (6 locations, 2–4 µl per injection) throughout each of the muscle to increase muscle afferent transduction efficiency (total AAV = 10 µl per TA and 20 µl per G). To differentiate between extensor and flexor muscle afferents in histological analysis, AAV9-CAG-tdTom was injected into left TA and right G, whereas AAV9-CAG-GFP was injected into right TA and left G. However, as only AAV9-CAG-tdTom consistently labeled afferents, we only quantified afferents from these injections. Following injection, the skin was sutured using suture staples, and the animals were placed in a heated cage until recovered from anesthesia. Buprenorphine was injected (0.03 mg/kg sc) before suturing and again 8–12 h later (0.02 mg/kg). Animals showed no signs of discomfort, changes in grooming, or changes in locomotion following AAV injections. After waiting 2–3 mo to allow adequate fluorophore expression (tdTomato and GFP) rats were euthanized with euthanyl (BimedaMTC; 700 mg/kg) and perfused intracardially with 100 ml of saline containing sodium nitrite (1 g/l; Fisher) and heparin (300 IU/l, from 1,000 U/ml stock; Leo Pharma) for 3–4 min, followed by 400 ml of 4% paraformaldehyde (PFA; in phosphate buffer at room temperature), over 15 min. Spinal cords were excised and postfixed in PFA overnight at 4°C, cryoprotected in 30% sucrose in phosphate buffer, frozen, and cut on a cryostat NX70 (Fisher Scientific) in sagittal (n = 3 rats) or transverse (n = 1 rat) 25-µm sections. Spinal cord sections were mounted on slides and rinsed with phosphate-buffed saline (PBS; 50 mM) containing 0.3% Triton X-100 (PBS-TX, 3× 15-min rinses used for all PBS-TX rinses). To amplify the viral labeling, sections were incubated overnight at room temperature with antibodies to the tdTom (rabbit anti-RFP 1:500; PM005, MBL International) and GFP (chicken anti-GFP 1:500; ab13970, Abcam). The slides were rinsed again in PBS-TX. To visualize the labeling, the fluorescent secondary antibodies including goat anti-rabbit Alexa Fluor 555 (1:500; Abcam, ab150078) and goat anti-chicken DyLight 488 (1:500, Abcam, ab96947) (in PBS-TX) were applied on slides for 2 h at room temperature. After being rinsed with PBS-TX (2× 15 min) and PBS (2× 15 min), the slides were coverslipped in Fluoromount G (00-4958-02, ThermoFisher Scientific, Waltham, MA).
Image acquisition for collateral counting was performed by epifluorescence (Leica DM 6000 B) microscopy using a 10x objective lens (NA = 0.3), with a 0.9-mm length of cord in each sagittal image, progressively scanning images from rostral to caudal. The sagittal images were stitched together into a single sagittal image of the entire length of the spinal cord using Leica Tilescan software (Leica Application Suite X software; Leica Microsystems CMS GmbH, Germany). Images of transverse sections for figure preparation were acquired using a confocal microscope (Leica SP8) with a ×25 water immersion objective lens (NA = 0.95).
Axon branches emerging from the dorsal columns and projecting toward the dorsal horn were counted in each 1-mm image and pooled across all sagittal sections into a total axon count at that level. These branch counts were normalized by the total number of labeled primary axons. The location of the spinal segments was estimated from the location of the viral labeling of the motoneurons since our viral injections labeled the motoneurons as well as sensory axons (TA and G motoneuron pools at L3–L5 and L4–L6, respectively (Mohan et al. 2015; Molander et al. 1984; Nicolopoulos-Stournaras and Iles 1983; Panneton et al. 2005). We only counted axon branches that had diameters of ~2 µm, penetrated the gray matter, and branched from large diameter axons (~4 µm) located in the dorsal column.
RESULTS
The Topography of Effects of Epidural Polarization
DC-evoked sustained increases in the excitability of afferent fibers traversing the dorsal columns were hypothesized to critically depend on the depolarization of the branching points of these fibers (see introduction). If so, the most potent postpolarization increases in the excitability of group Ia afferents of the Per and Tib nerves should be evoked within the segments where the majority of axon collaterals are densely given off within the dorsal columns, from where they project to either the motor nuclei or the DSCT in the caudal part of Clarke’s column as indicated in Fig. 1A. Accordingly, weaker DC effects would be evoked within the interposed segments with more sparse branching.
However, these predictions were made based on the data on the collateralization of feline afferent fibers. In the cat lower lumbar segments, group Ia axon collaterals were found to be issued from axons in the dorsal columns, at distances between branches ranging between 0.3 and 3 mm (1.2 ± 0.6 mm, mean and SD) or 0.3 and 1.5 mm (0.8 ± 0.3 mm) for collaterals of ascending or descending branches, respectively (Ishizuka et al. 1979 see also Brown and Fyffe 1978). Rostral to this area, the branching density along the illustrated ascending branches decreased initially (albeit to an undefined degree) but then increased again at the level of DCST neurons at the lower thoracic and upper lumbar cord where the distances between the subsequent branching sites of feline group Ia afferent over Clarke’s column were reported to be two to three times greater than over motoneurons (0.5–5.5 mm, 2.4 ± 1.1 mm; Hongo et al. 1987) in keeping with smaller numbers of afferents that contact individual DSCT neurons (Nicol and Walmsley 1991). As no similar data on rodent afferent fibers were available, the number of axon collaterals issued by proprioceptive afferent fibers from the Per and Tib nerves were estimated following retrograde labeling by intramuscular injections of viral tracers into the TA and G muscles, respectively (bilaterally AAV9-tdTom in left G and right TA, n = 8 muscles in 4 rats), and we counted the large diameter branches (>2 µm) arising from the large (~4 µm) axons in the dorsal columns. They are tentatively classified as proprioceptive group I and possibly group II afferent fibers as axonal branches of such fibers were traced to motor nuclei, both previously (Lucas-Osma et al. 2018) and in the present sample (see Fig. 1G). Group Ia afferent fibers are also the main peripheral source of input to DSCT neurons in Clarke’s column.
As anticipated, branch point density peaked near the motoneuron pools for TA and G muscles (L3 for TA and L4 for G) and then again near Clarke’s column (T13/L1), whereas there was a scarcity of branches between these regions in the L1/L2 segments with a significantly lower average branching density. Minimum TA and G afferent branching densities were, respectively, 13.6 ± 8.1% and 17.9 ± 14.6% of the peak branching density in the L3–L4 lumbar region (averages ± SD for 4 rats, P < 0.05; Student’s t test). There were more branches over a wider range in the L3–L4 lumbar region than in Clarke’s column, but the peak branching density was not different (peak Clarke’s column TA and G branching densities 85.8 ± 43.0% and 65.2 ± 23.0% of the branching density in L3–L4 lumbar region, respectively, P > 0.05). The average distance between these peak branching densities in Clarke’s column and the L3/L4 lumbar region was 9.0 ± 0.5 mm and 9.0 ± 0.9 mm for TA and G afferents, respectively. This provided a substantial gap with far fewer branches. Branches usually arose from the lateral part of the dorsal columns, projected in the ventrolateral direction, and entered gray matter at the medial edge of the dorsal horn.
As the Per and Tib motor nuclei in the rat extend over 3–4 mm (Molander et al. 1984; Nicolopoulos-Stournaras and Iles 1983), the location of these nuclei and their associated afferent innervation was estimated to coincide with the 3- to 4-mm-long region of the spinal cord within which the largest afferent volleys from group I afferents were recorded close to the entry of the dorsal roots. In Fig. 1B, these volleys are represented by the earliest components of the illustrated cord dorsum potentials. The records also show that the afferent volleys from slower conducting afferents (i.e., the second components of these potentials) followed the largest afferent volleys from the group I afferents. Both the threshold (>2T) and the longer latency (0.6–0.7 ms) of the second components are compatible with those in group II muscle afferents (see Edgley and Jankowska 1987; Vincent et al. 2017). They appeared in parallel with an even longer latency third components, representing cord dorsum potentials evoked by group II and skin afferents in the peroneal nerve and reflecting synaptically evoked dorsal horn field potentials evoked by these afferents. The latency of both the group I and II volleys increased by 0.35 ms over 10 mm, corresponding to a conduction velocity of 28.6 mm/ms of the tapering ascending collaterals of these fibers. The caudal part of Clarke’s column was estimated to coincide with the most rostrally recorded afferent volleys within the L1–Th13 segments, typically ~10 mm rostral to the motor nuclei, consistent with our anatomical estimates.
To verify that the long-lasting effects during the postpolarization period are related to the degree of collateralization of afferent fibers, we compared the effects of DC applied at four rostrocaudal levels: 1) within the region over motor nuclei where group I afferent volleys were maximal, 2) 7–10 mm more rostrally, over the caudal region of Clarke’s column, 3) at sites between 1 and 2, and 4) in the caudal part of the lumbosacral enlargement, where fewer branches arise from the dorsal columns. The results illustrated in Fig. 2 confirmed that the largest postpolarization increases in the excitability of epidurally stimulated Per group I afferent fibers were evoked at the level of the Per motor nuclei (Fig. 2, C and G). The nerve volleys recorded within the first 10 s following the termination of the DC were severalfold larger than the control nerve volleys (on average 1,051 ± 263%) and remained increased over the whole period of recording. In previous studies, the increase was maintained for at least 1–2 h (Jankowska et al. 2017). A partial decline occurred within the first 1–3 min but was not statistically significant. Thereafter, the mean size of the nerve volleys was stable (871 ± 223% of the control after 5 min and 876 ± 231% after 10 min), without declining further. During this period, the latency of the nerve fibers, another expression of the increase in the excitability at the site of the stimulation remained shorter (Fig. 2C, cf. black and red traces).
The increases in the excitability of fibers stimulated above Clarke’s column (Fig. 2, A and E) were weaker, to 337 ± 53% just after termination of the polarization, displaying a similarly small initial decline, and were sustained (to 286 ± 42% after 5 min and 273 ± 33% after 10 min, t test failing to indicate a statistically significant difference between them; P = 0.0602). The increase in the amplitude of the nerve volleys was associated with a decrease in their latency (Fig. 2A).
In contrast, the increases in fiber excitability at stimulation sites between the Per motor nuclei and Clarke’s column (Fig. 2, B and F) were both weaker (to 188 ± 19%) and transient. Within 5 min after termination of the polarization, the nerve volleys had returned to the original size or were even further reduced (81 ± 8%). The difference between the effects observed immediately following DC and 5 min later was highly statistically significant (P = 6.5E-0.5). The excitability of fibers stimulated within the caudal part of the lumbosacral enlargement, most likely caudal to the Per motor nuclei but overlapping with Tib motor nuclei, was also weaker (Fig. 2, D and H). It similarly declined within 5 min (from 382 ± 124% to 177 ± 32%) where it was not statistically significantly different from baseline (P = 0.0556).
Long-lasting increases in the excitability of some nonmyelinated fibers within their terminal projection area were reported to be activity dependent (Schaffer axon collaterals in hippocampal slices; McNaughton et al. 1994). In contrast, long-lasting increases in the excitability of myelinated afferent fibers following epidural polarization did not require that the fibers were activated during the polarization period (Bączyk and Jankowska 2018; Jankowska et al. 2016) and were thus activity independent, as were the postpolarization effects evoked by DC pulses (Bączyk and Jankowska 2018). To allow a comparison with these observations, the series of experiments illustrated in Fig. 2 were carried out under conditions where the fibers were stimulated before and after but not during the period of the polarization.
GABA Antagonists Prevent Induction of DC-Evoked Sustained Increase in the Excitability of Afferent Fibers
To examine whether GABA contributes to the long-lasting increase in the afferent excitability evoked by DC, we analyzed effects of two GABA antagonists, L655708, which blocks the extrasynaptic α5GABAA receptors and bicuculline, which blocks all GABAA receptors.
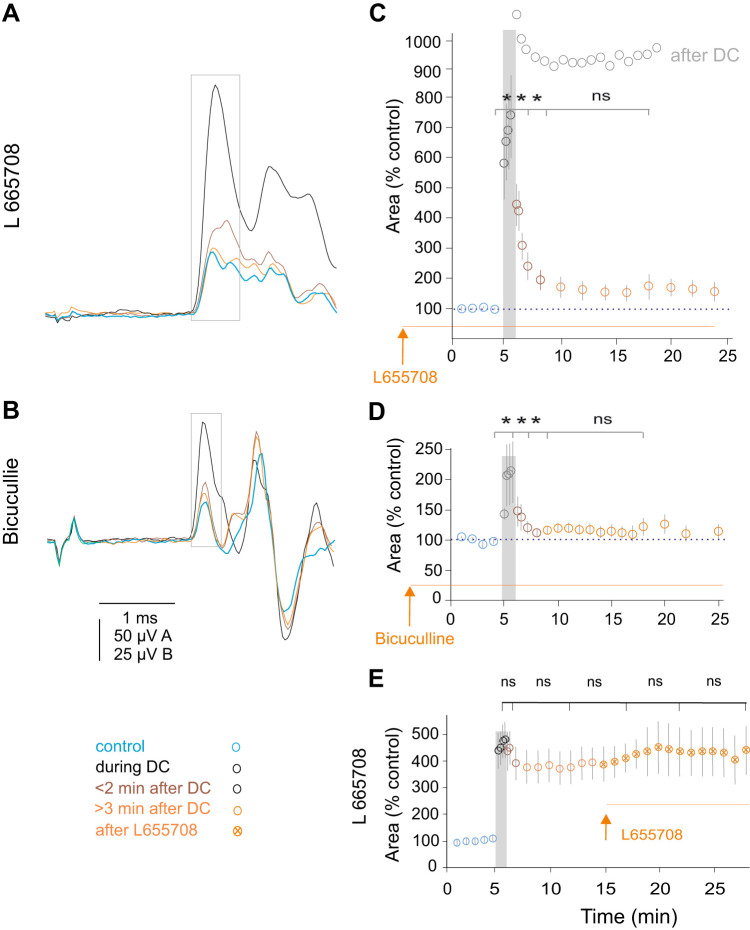
When the antagonists were administered before DC polarization, the increase in the nerve volleys evoked by polarization strongly declined as soon as the polarization was terminated (Fig. 3, A–D), in contrast to the sustained increase in untreated preparations. Plots in Fig. 3, C and D, show that the decline took place already during the first minute of the postpolarization period and approached control levels after 2–3 min. The decline was more potent after pretreatment with bicuculline (to 50–150% of control) than with L665708 (to 150–200% of control).
Fig. 3.
Effects of synaptic and extrasynaptic GABA receptor antagonists on postpolarization excitability of afferent fibers. A and B: examples of nerve volleys induced by stimulation of the dorsal columns above the peroneal motor nucleus before and after application of direct current (DC; 1 µA for 1 min) following intravenous administration of L665708 and bicuculline. Note that the increase in the volley size was not associated with a shortening of the latency. Average records (n = 10) of nerve volleys recorded from the peroneal nerve during the indicated periods. The volleys in A and B were evoked 15 min and 60 min after injection of L665708 (1 mg/kg) and bicuculline (2 mg/kg) by 27.5 µA and 5 µA, respectively. The areas of the first components of the volleys were measured within time windows indicated by rectangles in A and B. C and D: the time course of DC evoked changes in nerve volleys following administration of L665708 and bicuculline. Pooled data for 11 and 7 series of records exemplified in A and B. The statistical comparison using t test was made between the last control records and last records during DC (P = 0.0035) (P = 0.0378), during and 1 min after DC (P = 0.0063) (P = 0.0467), between 1 and 3 min (P = 0.0033) (P = 0.1621), and between 3 and 10 min (P = 0.0968) (P = 0.2426) after DC, respectively. To aid the comparison with data in untreated preparations, the plot from Fig. 2G is redrawn (in light gray) and superimposed in C. E: the time course of DC-evoked changes in nerve volleys when DC application preceded the administration of L665708 (1 mg/kg). Pooled data from 7 series of records in 3 rats. The statistical analysis (t test) failed to reveal significant differences between records obtained during DC application and during the postpolarization period. *P < 0.05; ns, not significant.
However, the antagonists failed to prevent the increase of nerve volleys during DC polarization, although the degree to which they were increased was reduced. Following administration of L655708 and bicuculline, the nerve volleys were increased by DC applied at the level of the Per and Tib motor nuclei to 741 ± 149% and 212 ± 51%, respectively, while in untreated preparations the increases frequently exceeded 1,000% as illustrated in Fig. 2, C and G (1,061 ± 64%). In addition, after administration of the antagonists, the shortening of the latencies of the nerve volleys induced by DC was inconsistent and, if seen, only moderate. DC failed to shorten latencies of 6/16 (38%) and 0/57 (0%) volleys in preparations treated and untreated with the GABA antagonists, respectively. For volleys that increased to 300–500%, the latencies were shortened by 0.036 ± 0.001 ms (n = 16) in the treated, as compared with 0.099 ± 0.001 ms (n = 57) in the untreated preparations (P = 7.06E-09, t test).
In contrast to effects of GABA, antagonists applied before DC administration of L655708 and bicuculline did not decrease the fiber excitability once the sustained increase in the excitability had stabilized during the postpolarization period (Fig. 3E). Therefore, the comparison of the plots in Fig. 3, C–E, leads to the conclusion that GABAA receptor antagonists blocked the induction but not the expression of the sustained increases in the excitability by DC polarization.
GABA and the GABA-Agonist Muscimol Only Weakly Enhance the DC-Evoked Increase in the Excitability of Afferent Fibers
The conclusion that GABAA antagonists interfere with the DC-evoked increase in the excitability of nerve fibers in the dorsal columns suggested that GABA effects may be critical for this phenomenon. We, therefore, first investigated whether the GABAA agonist muscimol and GABA would affect the excitability of the afferents when applied alone (without concomitant dorsal column depolarization).
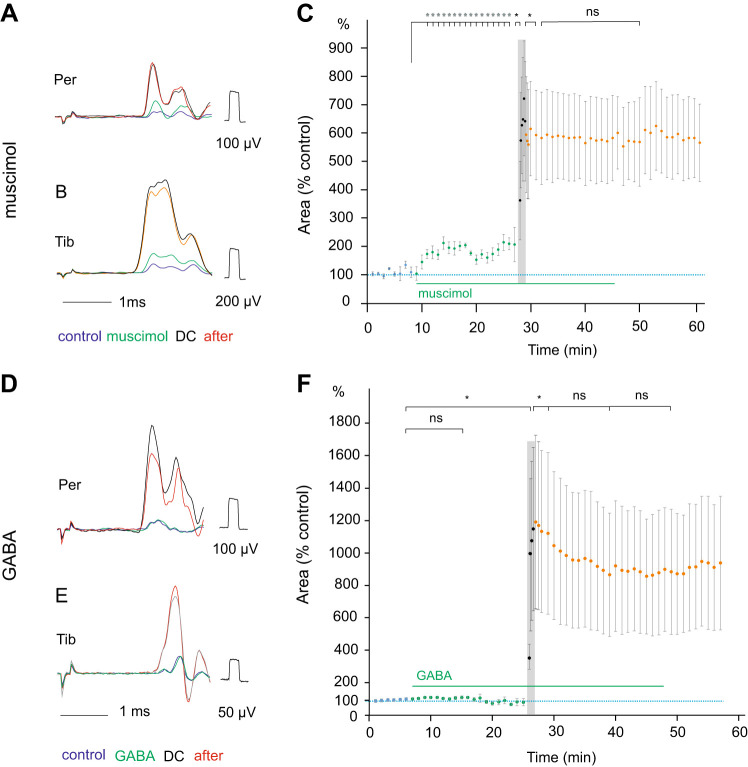
Following systemic administration, muscimol (0.5 mg/kg iv) increased the nerve volleys evoked by epidural stimulation within the region overlying motor nuclei to a moderate (170 ± 42%) but statistically significant degree (Fig. 4C) and shortened the latencies of these volleys (by 0.046 ± 0.001 ms). The dose of 0.5 mg/kg was chosen based on previously having been reported to evoke near-maximal behavioral effects (Poncelet et al. 1987). The effects of ionophoretically applied GABA (30–40 nA) were inconsistent as the size of the nerve volleys was increased in only 3/8 series in 4 rats), and the mean effects were negligible (Fig. 4F) with no changes in the latency of the nerve volleys detected.
Fig. 4.
Effects of muscimol and GABA on the development and expression of direct current (DC) modulatory actions. A and B: examples of nerve volleys evoked in the peroneal (Per) and tibial (Tib) nerves by stimulation of the dorsal column above their motor nuclei. The nerve volleys (averages of ten single records) were evoked by 23 µA and 25 µA, respectively, and recorded during the control period, following the intravenous administration of muscimol (0.5 mg\kg), during 1-µA DC application for 1 min and 10 min after its termination are superimposed. C: Increase in the area of nerve volleys evoked by stimulation of the dorsal columns (ordinate) following muscimol administration, during 1 min polarization and during the postpolarization period (abscissa). Pooled data (means ± SE) from 10 series of records from Per and Tib in 3 rats, including those illustrated in A and B. The differences were statistically significant between the volleys evoked at the end of the control period and following muscimol (P = 0.0032), between the nerve volleys evoked before and at the onset of DC (P = 0.0036), and between the nerve volleys at the end of DC and 2 min after its termination (P = 0.0068) but not between 2 and 10 min of the postpolarization period. A t test for paired samples was used (n = 11). D and E: examples of nerve volleys evoked by stimulation of the dorsal column as in A and B. The nerve volleys were evoked by 26 µA and 35 µA during the control period, 3 min after the beginning of GABA ionophoresis (30 nA), during 1-µA DC application and 10 min after its termination. F: time course of changes in the area of nerve volleys evoked by stimulation of the dorsal columns before and during GABA ionophoresis and during and following a 1-min 1-µA polarization. Pooled data (means ± SE) from 13 series of records in 3 rats. Using t test for paired samples, the differences between the volleys were statistically significant for those at the end of the control period and during DC (P = 0.0271), between the nerve volleys at the end of DC and 2 min after its termination (P = 0.0133), but not between 2 and 10 min of the postpolarization period (P = 0.0655) nor between 10 and 20 min of this period (P = 0.9790). *P < 0.05; ns, not significant.
When muscimol injection or GABA iontophoresis preceded the dorsal column depolarization, the nerve volleys evoked both during the polarization and the postpolarization period appeared to be increased within the same range as in preparations in which DC was not preceded by muscimol (Fig. 4, A–C) or was not combined with GABA (Fig. 4, D–F), but the material was too small for statistical comparisons.
The effects of muscimol and GABA administered during the postpolarization period were inconclusive even though increases in the size of nerve volleys to 120 −130% were seen during the postpolarization period in some experiments. During the first 5 min following muscimol injection, the mean size of nerve volleys increased with respect to the volleys recorded immediately preceding the injection to 124, 118, and 124% after 0.5, 1, and 1.5 mg/kg, respectively, in four rats although the number of the observations was too small to allow estimates of their statistical significance. A similar effect was found during GABA ionophoresis when the volleys increased to 132, 136, 155, and 139% compared with those evoked before ionophoresis (at 30-, 40-, 50-, and 60-nA ionophoresis current, respectively, in 1 rat). By using the size of the nerve volleys as a measure of the DC-evoked increase in the excitability of the dorsal column afferent fibers, any additional increase in the excitability of these fibers by muscimol or GABA was thus difficult to evaluate.
The contribution of muscimol and GABA to the shortening of the latency of nerve volleys evoked during the polarization and postpolarization periods could be more reliably evaluated. The evaluation was based on the findings illustrated in Figs. 2 and 4, i.e., on the demonstration that the latency of nerve volleys evoked by stimulation of the dorsal columns following polarization of the stimulated fibers is shortened for fibers polarized above their branching regions (Fig. 2, A and C) but not for fibers polarized outside of these regions (Fig. 2, B and D) and that the latency is shortened during the polarization as well as during the postpolarization periods (Fig. 4, A, B, D, and E). We, therefore, compared latencies of nerve volleys evoked during the postpolarization period in preparations in which only DC was applied to enhance the effects of epidural stimulation and in preparations in which DC was combined with the administration of muscimol or GABA.
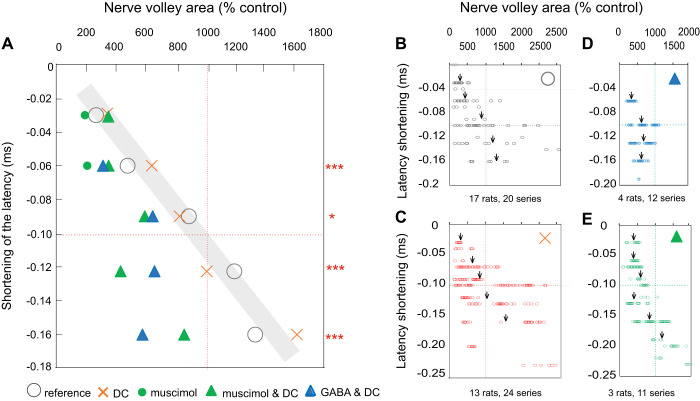
Circles in Fig. 5A illustrate the relationship between the size of nerve volleys evoked by increasing stimulus intensity between near and considerably suprathreshold values (20–50 µA) and the shortening of the latencies of the resulting volleys (shaded area in Fig. 5A) in preparations in which no DC was applied. A similar relationship was found between latencies and amplitudes of nerve volleys when these volleys were increased during the postpolarization period (Fig. 5A, crosses). Using these sets of data as a reference, we next compared the postpolarization latency-size relationship in preparations in which DC application was, or was not, associated with muscimol and GABA administration. Triangles in Fig. 5A show that the shortening of the latencies, by −0.06 to −0.2 ms, was consistently associated with smaller nerve volleys when the polarization was preceded by muscimol intravenous or was associated with GABA ionophoresis. These differences were all highly statistically significant (evaluated with t-test for unpaired samples assuming equal variance). The plots in Fig. 5, D and E show the corresponding data points concentrated to the left of the reference latency-size relationship in Fig. 2, A and B.
Fig. 5.
Relationship between the latency and size of nerve volleys in group I afferents evoked by stimulation of the dorsal column during the postpolarization period. A: the relationship between the shortening of latencies and the increase in the size of the nerve volleys (expressed as the mean latency shift and the mean area of nerve volleys evoked during the postpolarization period) in % of the control area. Circles, nerve volleys evoked by increasing stimulus intensities (near-threshold to 50 µA); they are taken as the reference. Crosses, data for nerve volleys evoked by a constant intensity near-threshold stimuli that were increased by the preceding polarization (1 µA for 1 min). Triangles, as crosses but when direct current (DC) effects were associated with effects of muscimol or GABA. Asterisks indicate statistically significant differences in latencies of volleys shortened by 0.5–0.7, 0.8–0.11, 0.12–0.14, and 0.15–0.18 ms by GABA or muscimol, as compared with volleys represented by crosses. *P = 0.05–0.001, ***P ≤ 0.001. The estimates are based on the t test and apply to effects of both GABA and muscimol. B–D: detailed plots from which the mean values in A were derived. Arrows indicate mean values within the above-indicated ranges. Note the reduced scales. Dotted lines correspond to a 1,000% increase in the size and 0.1-ms shortening of the latencies.
In summary, our results are compatible with the facilitation by GABA of the sustained increase in the DC-evoked excitability of dorsal column fibers. They also indicate that the shortening of the latency of nerve volleys evoked by stimulation of the dorsal columns may be a more sensitive measure of the enhancement of effects of DC by either muscimol or GABA.
DISCUSSION
The results of this study are fully consistent with the hypothesis that the DC-evoked long-lasting increase in the excitability of afferent fibers traversing the dorsal columns critically depends on the effects of epidural polarization of branching points of these fibers and on membrane mechanisms sensitive to GABA.
The Topography of the Regions in Which Polarization of Afferent Fibers in the Dorsal Columns Evokes Long-Lasting Increases in Their Excitability
The spinal cord regions within which the most potent sustained increases in the excitability of afferent fibers were evoked by epidural polarization corresponded to the regions within which the highest density of axon collaterals was found in the present as well as in previous studies. They also corresponded to the regions within which the thresholds for activation of the dorsal column fibers by both epidural and transcutaneous stimulation would be lowest, as indicated by modeling studies (Danner et al. 2011; Struijk et al. 1992). Individual group Ia, Ib, and II muscle afferents target neurons within narrow strips of the gray matter (a fraction of a millimeter rostrocaudally) and are nonevenly distributed within the spinal cord. For instance, the most caudal target of group Ia afferents from hindlimb muscles are motoneurons located in the lower and midlumbar segments while their most rostral target cells are dorsal spinocerebellar tract neurons in Clarke’s column, several segments away. While this has been most extensively documented in the cat, similar segmental distributions of motor nuclei and axonal projections have been found in the rat spinal cord (Arber 2012; McHanwell and Biscoe 1981; Mohan et al. 2015; Molander et al. 1984; Nicolopoulos-Stournaras and Iles 1983; Panneton et al. 2005; Pecho-Vrieseling et al. 2009).
Given the same relationships in the rodent spinal cord, the densest projections of Ia afferents to rat Per and Tib motoneurons would be within the L3–L5 segments (Capogrosso et al. 2013; Mohan et al. 2015; Molander et al. 1984; Nicolopoulos-Stournaras and Iles 1983; Panneton et al. 2005). Group Ia afferents would synapse with DSCT neurons within the caudal part of Clarke’s column at the border between the L1 and Th13 segments (Matsushita and Hosoya 1979; Molander et al. 1984).
The distribution of collaterals of afferents from the TA and G muscles found in this study fully agrees with the data on gross rodent spinal cord anatomy and the labeled motoneuron locations as well as with the degree of collateralization of functionally identified feline group Ia collaterals. For the conclusions of this study, it has been of particular importance that the reconstructions illustrated in Fig. 1 show a gap in their density within the 3- to 4-mm-long region between the hindlimb motor nuclei and Clarke’s column. As indicated in the materials and methods and The Topography of Effects of Epidural Polarization, only collaterals branching from of the largest dorsal column fibers were included in our reconstruction and we consider that most of these fibers are likely to belong to group Ia muscle spindle afferents. However, we cannot exclude that our sample did also include some group Ib or group II afferent fibers targeting neurons at a similar location as that of motoneurons innervated by Ia afferents. If so, it would be possible that the gap in the density of collaterals of group Ia afferents is even more marked than shown in Fig. 2. Alternatively, our data would be compatible with a similar topographic distribution of the group Ib and II as of group Ia afferent fibers.
The most potent sustained polarization-evoked increases in the afferent excitability found within the L3–L5 and the Th13 segments and their absence within the L2–L1 segments are, therefore, fully compatible with the hypothesis that links the plasticity of afferent fibers traversing the dorsal columns to their branching points. The present results would also explain the range of effects of DC found in previous studies where DC was primarily applied at sites corresponding to the location of Per motor nuclei but where the effects were much weaker within regions 2–3 mm rostral or caudal to these sites.
Given the rapidly declining density of epidurally applied current with distance (see Holsheimer 2002), the effects of epidural polarization may in the first hand be linked to the branching points within or just below the dorsal columns. However, there are also other indications that branching points of the afferents deeper within the gray matter may not be involved in the modulation of fiber excitability to the same degree, e.g., much weaker and much slower developing effects of intraspinally than of epidurally applied DC (Bączyk and Jankowska 2018; Bolzoni and Jankowska 2015; Bolzoni et al. 2019; Jankowska et al. 2017; Kaczmarek and Jankowska 2018).
Indications that Branching Points of Afferent Fibers Are Critical for Their Plasticity Based on Effects of GABAA Receptor Antagonists
If the sustained effects of epidurally applied DC depend on depolarizing the branching points of the stimulated fibers per se, depolarization of the axons closest to as well as further from the dorsal columns might contribute to the effects of DC. However, our results demonstrate a vital role of the depolarization of the branching points closest to the dorsal columns. This conclusion is based on our findings that postpolarization increases in excitability were potently reduced by L655708, a selective antagonist of α5GABAA membrane receptors, and that α5GABAA membrane receptors were primarily found at these branching points (Lucas-Osma et al. 2018). When these receptors were blocked by L655708, the DC-induced increase in the excitability of dorsal column fibers that occurred during the current application quickly declined (within less than 2–3 min) after DC was terminated. In the presence of L655708, the time course of changes in the excitability of the fibers during the postpolarization period thus resembled the time course of aftereffects of DC applied within the regions rostral or caudal to the motor nuclei (Fig. 2F and H) where only a minimal number of axon collaterals leave their parent axons.
Differences in the Potency of Effects of GABAA Receptor Antagonists and GABA or the GABAA Receptor Agonist Muscimol
We do not have a ready explanation why the effects of GABAA receptor antagonists are very strong while those of GABA or muscimol are very weak. However, as the extrasynaptic α5GABAA receptors are located in close the proximity to sodium channels, they may contribute to tonic primary afferent depolarization (PAD) (Delgado-Lezama et al. 2013; Lidierth 2006; Lomelí et al. 1998; Rudomin et al. 2004; Russo et al. 2000; Svirskis and Hounsgaard 1998) that assists sodium channel function and thereby increase the excitability of afferent fibers by providing a background depolarization (Lucas-Osma et al. 2018). We may thus hypothesize that a background of tonic actions of GABA on these fibers is required for the increase in the excitability of the polarized dorsal column nerve fibers by DC effects to develop. Consequently, a local increase in the GABA concentration might only have negligible effects while the interference with actions of GABA at the level of its membrane receptors would prevent the DC-evoked changes. The involvement of the tonic GABA-evoked depolarization (for references, see Lidierth 2006; Lucas-Osma et al. 2018; Rudomin 2009) would be enabled by α5GABAA membrane receptors in close proximity to voltage-dependent sodium channels. Such extrasynaptic GABAA receptors were revealed within branching points of group I afferents at a short distance from or within the dorsal columns (Lucas-Osma et al. 2018). The extrasynaptic actions of GABA would thus be likely to be particularly important at the border between the dorsal columns and the dorsal horn where we find most branches emerge, or within the most dorsal laminae of the dorsal horn, i.e., at a short distance from the location of a variety of GABAergic interneurons (see Todd 1996; Yasaka et al. 2010), some of which were found to project to the dorsal columns (D. J. Bennett, A. M. Lucas-Osma, and K. K. Fenrich, unpublished observations). The effects of transsynaptically released GABA would be unlikely to be evoked via axo-axonal synapses in view of the lack of presynaptic boutons at the level of the first branching points. Axo-axonal synapses were found within Clarke’s column where they form enlarged nodal regions with synaptic specializations likely to operate as en passant boutons (Madison et al. 1991) but only at the level of the tertiary axon collaterals (fifth or later branching points within the terminal projection areas of Ia afferents (Walmsley et al. 1995).
Are Postpolarization Increases in the Excitability of Afferent Fibers Linked to the Transsynaptic Release of GABA by DC?
It is conceivable that DC acts on fibers in the dorsal columns by facilitating activation of GABAergic interneurons as well as the release of GABA from terminals of axons of these interneurons close to the dorsal columns. The increase of nerve volleys evoked by epidural stimulation occurring within the first few seconds of the polarization (Jankowska et al. 2017) may speak more in favor of direct, than transneuronal, effects of DC. However, the contribution of effects secondary to the depolarization of terminals of GABAergic neurons, transsynaptic activation of these neurons, and subsequent release of extrasynaptically acting GABA cannot be excluded. Regardless of whether or not DC directly activates GABAergic neurons our results demonstrate that the GABA receptors that these neurons innervate may provide a steady depolarization (PAD) of the branch points of sensory afferents that contributes to the sustained increase in axon excitability by DC and likely more generally promotes axonal conduction at branch points (Lucas-Osma et al. 2018). This topic will require further study to elucidate.
GABA-Modulated Timing of Excitation of Afferent Fibers by Electrical Stimuli
Our results demonstrate that GABA associated with the depolarization of the dorsal columns may modulate the timing of activation of electrically induced nerve impulses within the branching points of the afferent fibers. We failed to detect any changes in the latency of nerve volleys evoked by epidural stimulation by muscimol or GABA alone. However, when the nerve volleys were evoked during or following polarization of the dorsal columns in preparations pretreated by muscimol (iv) or GABA (ionophoretically), their latencies were considerably shorter than in the untreated preparations. As shown in Fig. 5, the shortening was of the order of 50–100 µs. GABA antagonists had, as expected, an opposite effect, counteracting the shortening of the latencies by polarization.
How these changes are related to the effects of GABA and/or DC on sodium channels remains an open question. Shorter latencies of action potentials evoked at a distance from the stimulating electrode when the stimulation intensity is increased are usually explained by the spread of current along the axons whereby the threshold for generating action potentials is reached at a point further away from the stimulating electrode. However, shortening of latencies of nerve volleys in afferent fibers by polarization and by GABA could also reflect a more effective generation of action potentials or conduction. It is thus also of interest for the mechanisms underlying the sustained increases in fiber excitability. If shorter latencies of nerve volleys in the stimulated fibers were due to the generation of action potentials further away from the electrode tip when these fibers were polarized, this would implicate a shorter conduction distance between the dorsal columns and the peripheral nerves or their target cells. However, the results summarized in Fig. 5 only partly support such a possibility because they deviate from the relationship between the latency and the size of nerve volleys evoked by increasing intensities of the stimuli. As judged by the conduction time along group I afferents within the lumbosacral enlargement illustrated in Fig. 1D (0.35 ms over 10-mm distance), the DC-evoked shortening of the latencies by 0.12–0.15 ms (Fig. 5) could correspond to a shortening of the conduction distance by ~4 mm. This would, however, be at variance with the ~1-mm radius of effects of DC in either the rostrocaudal direction (Jankowska et al. 2017) or dorso-ventrally (Bolzoni et al. 2019), which would cause ~0.03 ms shortening. Provided that only a fraction of the observed shortening of the latencies of nerve volleys were due to the reduced conduction distance, the remaining shortening would be compatible with two other phenomena. First, it could be due to a shortening of the latent period of the generation of action potentials. When near-threshold stimuli are used, the latent periods vary, e.g., between 0.17 and 0.33 ms (Jankowska and Roberts 1972) and are shortened by suprathreshold stimuli. Second, an ~0.1-ms difference in the latency could be due to a shortening of the conduction delays across polarized nodes of Ranvier and the branching points within the dorsal columns. The possibility of modifying the nodal delays by intra-axonal polarization has been demonstrated (D. J. Bennett, unpublished data), but the effects of epidurally applied DC will have to be verified.
Functional Consequences
With respect to functional consequences of the shortening of latencies of nerve volleys evoked by stimulation of the dorsal columns, it is essential that changes in the latency would not be restricted to the propagation of nerve impulses toward the periphery but would also modulate central effects of nerve volleys and hence synaptic actions on spinal neurons. The changes in the latency found in this study only amounted to a small fraction of a millisecond, which might not be considered as of functional consequence. However, 0.1–0.2 ms would correspond to a substantial part of the time-to-peak of excitatory postsynaptic potentials (EPSPs). For instance, the time to peak of composite monosynaptic EPSPs in motoneurons is ~1 ms (0.85–1.5 ms) and the rising phase of a high proportion of EPSPs evoked by Ia afferents lasts a fraction of a millisecond (0.25–0.60 ms in fast hindlimb motoneurons (Alstermark and Sasaki 1986; Jack et al. 1971) or 0.2–0.5 ms in DSCT cells (Walmsley 1989; Walmsley and Stuklis 1989). The earlier and more synchronously arriving action potentials in Ia afferents would thus increase the slope of the composite EPSPs and thereby their amplitude and their potency to give rise to action potentials in the postsynaptic neurons. These changes would thus be of consequence for the resulting integrative functions of the spinal neuronal networks.
The results of the present study may also be relevant for the interpretation of clinical effects of transcranial and epidural stimulation and polarization. It is therefore worth noting that under conditions when the peripheral nerves are intact, action potentials in afferent fibers electrically stimulated within the dorsal columns would propagate bidirectionally, both retrogradely, toward the peripheral nerves, and anterogradely, toward their spinal target cells. The retrogradely propagated nerve impulses would collide with those initiated at the level of the receptors and interfere with the transfer of information from the periphery. As pointed out by (Formento et al. 2018), such collision and the resulting loss of the proprioceptive information would be detrimental for the motor control and should be avoided. However, even if propriospinal information collides in a proportion of afferent fibers, e.g., in a small number of fibers activated by epidural stimulation (Capogrosso et al. 2013, 2018b; Holsheimer and Buitenweg 2015), anterogradely propagated nerve impulses induced in such fibers would reach their target cells. Propriospinal information forwarded by other fibers and synaptic actions of these fibers would in this case not be endangered and could be facilitated by combined actions of the stimulated and/or polarized fibers and be beneficial for patients.
GRANTS
Electrophysiological experiments were supported by the University of Gothenburg and Craig Neilson Foundation Grant 546739. Morphological analysis was supported by the Canadian Institutes of Health Research Grants MOP 14697 and PS 165823 and National Institute of Neurological Disorders and Stroke Grant R01-NS-47567.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. conceived and designed research; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. performed experiments; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. analyzed data; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. interpreted results of experiments; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. prepared figures; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. drafted manuscript; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. edited and revised manuscript; Y.L., K.H., A.M.L.-O., K.K.F., D.J.B., I.H., and E.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr Shawn Hochman for the discussion on the results presented in this article. We also thank Leo Sanelli for technical assistance. The electrophysiological experiments were performed at the Department of Physiology, University of Gothenburg, Göteborg, Sweden. The morphological analysis was done at the Neuroscience and Mental Health Institute and Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, AB, Canada.
REFERENCES
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 14. Differential projection to fast and slow motoneurones from excitatory C3-C4 propriospinal neurones. Exp Brain Res 63: 530–542, 1986. doi: 10.1007/BF00237476. [DOI] [PubMed] [Google Scholar]
- Anastassiou CA, Perin R, Markram H, Koch C. Ephaptic coupling of cortical neurons. Nat Neurosci 14: 217–223, 2011. doi: 10.1038/nn.2727. [DOI] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron 74: 975–989, 2012. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Bączyk M, Jankowska E. Presynaptic actions of transcranial and local direct current stimulation in the red nucleus. J Physiol 592: 4313–4328, 2014. doi: 10.1113/jphysiol.2014.276691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bączyk M, Jankowska E. Long-term effects of direct current are reproduced by intermittent depolarization of myelinated nerve fibers. J Neurophysiol 120: 1173–1185, 2018. doi: 10.1152/jn.00236.2018. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Esposti R, Bruttini C, Zenoni G, Jankowska E, Cavallari P. Direct current stimulation modulates the excitability of the sensory and motor fibres in the human posterior tibial nerve, with a long-lasting effect on the H-reflex. Eur J Neurosci 46: 2499–2506, 2017. doi: 10.1111/ejn.13696. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Esposti R, Jankowska E, Hammar I. Interactions between baclofen and DC-induced plasticity of afferent fibers within the spinal cord. Neuroscience 404: 119–129, 2019. doi: 10.1016/j.neuroscience.2019.01.047. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Jankowska E. Presynaptic and postsynaptic effects of local cathodal DC polarization within the spinal cord in anaesthetized animal preparations. J Physiol 593: 947–966, 2015. doi: 10.1113/jphysiol.2014.285940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol 274: 111–127, 1978. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrosso M, Gandar J, Greiner N, Moraud EM, Wenger N, Shkorbatova P, Musienko P, Minev I, Lacour S, Courtine G. Advantages of soft subdural implants for the delivery of electrochemical neuromodulation therapies to the spinal cord. J Neural Eng 15: 026024, 2018a. doi: 10.1088/1741-2552/aaa87a. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Wagner FB, Gandar J, Moraud EM, Wenger N, Milekovic T, Shkorbatova P, Pavlova N, Musienko P, Bezard E, Bloch J, Courtine G. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat Protoc 13: 2031–2061, 2018b. doi: 10.1038/s41596-018-0030-9. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33: 19326–19340, 2013. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR. Bicuculline, GABA and central inhibition. Proc Aust Assoc Neurol 9: 145–153, 1973. [PubMed] [Google Scholar]
- Curtis DR, Lodge D. The depolarization of feline ventral horn group Ia spinal afferent terminations by GABA. Exp Brain Res 46: 215–233, 1982. doi: 10.1007/BF00237180. [DOI] [PubMed] [Google Scholar]
- Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif Organs 35: 257–262, 2011. doi: 10.1111/j.1525-1594.2011.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Loeza-Alcocer E, Andrés C, Aguilar J, Guertin PA, Felix R. Extrasynaptic GABA(A) receptors in the brainstem and spinal cord: structure and function. Curr Pharm Des 19: 4485–4497, 2013. doi: 10.2174/1381612811319240013. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol 385: 393–413, 1987. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev 69: 821–863, 1989. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M, Courtine G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci 21: 1728–1741, 2018. doi: 10.1038/s41593-018-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick M, Rudomín P, Wall PD, Werman R. Is there electrical interaction between motoneurons and afferent fibers in the spinal cord? Brain Res 93: 507–510, 1975. doi: 10.1016/0006-8993(75)90190-0. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation 5: 25–31, 2002. doi: 10.1046/j.1525-1403.2002._2005.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Buitenweg JR. Review: Bioelectrical mechanisms in spinal cord stimulation. Neuromodulation 18: 161–170, 2015. doi: 10.1111/ner.12279. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kudo N, Sasaki S, Yamashita M, Yoshida K, Ishizuka N, Mannen H. Trajectory of group Ia and Ib fibers from the hind-limb muscles at the L3 and L4 segments of the spinal cord of the cat. J Comp Neurol 262: 159–194, 1987. doi: 10.1002/cne.902620202. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Mannen H, Hongo T, Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol 186: 189–211, 1979. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jack JJ, Miller S, Porter R, Redman SJ. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol 215: 353–380, 1971. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Hedén C, Szabo Läckberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci 9: 1375–1387, 1997. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Kaczmarek D, Bolzoni F, Hammar I. Evidence that some long-lasting effects of direct current in the rat spinal cord are activity-independent. Eur J Neurosci 43: 1400–1411, 2016. doi: 10.1111/ejn.13238. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Kaczmarek D, Bolzoni F, Hammar I. Long-lasting increase in axonal excitability after epidurally applied DC. J Neurophysiol 118: 1210–1220, 2017. doi: 10.1152/jn.00148.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Roberts WJ. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol 222: 597–622, 1972. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek D, Jankowska E. DC-evoked modulation of excitability of myelinated nerve fibers and their terminal branches; differences in sustained effects of DC. Neuroscience 374: 236–249, 2018. doi: 10.1016/j.neuroscience.2018.01.036. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol 87: 33–46, 2005. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M. Local and diffuse mechanisms of primary afferent depolarization and presynaptic inhibition in the rat spinal cord. J Physiol 576: 309–327, 2006. doi: 10.1113/jphysiol.2006.110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim YJ, Ji M, Fang J, Siriwon N, Zhang LI, Wang P. Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol Ther Methods Clin Dev 1: 12, 2014. doi: 10.1038/mtm.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomelí J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature 395: 600–604, 1998. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- Lucas-Osma AM, Li Y, Lin S, Black S, Singla R, Fouad K, Fenrich KK, Bennett DJ. Extrasynaptic α5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord. J Neurophysiol 120: 2953–2974, 2018. doi: 10.1152/jn.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci 14: 379–397, 1991. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y. Cells of origin of the spinocerebellar tract in the rat, studied with the method of retrograde transport of horseradish peroxidase. Brain Res 173: 185–200, 1979. doi: 10.1016/0006-8993(79)90620-6. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci 293: 477–508, 1981. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Shen J, Rao G, Foster TC, Barnes CA. Persistent increase of hippocampal presynaptic axon excitability after repetitive electrical stimulation: dependence on N-methyl-D-aspartate receptor activity, nitric-oxide synthase, and temperature. Proc Natl Acad Sci USA 91: 4830–4834, 1994. doi: 10.1073/pnas.91.11.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Tosolini AP, Morris R. Segmental distribution of the motor neuron columns that supply the rat hindlimb: a muscle/motor neuron tract-tracing analysis targeting the motor end plates. Neuroscience 307: 98–108, 2015. doi: 10.1016/j.neuroscience.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol 230: 133–141, 1984. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Morya E, Monte-Silva K, Bikson M, Esmaeilpour Z, Biazoli CE Jr, Fonseca A, Bocci T, Farzan F, Chatterjee R, Hausdorff JM, da Silva Machado DG, Brunoni AR, Mezger E, Moscaleski LA, Pegado R, Sato JR, Caetano MS, Sá KN, Tanaka C, Li LM, Baptista AF, Okano AH. Beyond the target area: an integrative view of tDCS-induced motor cortex modulation in patients and athletes. J Neuroeng Rehabil 16: 141, 2019. doi: 10.1186/s12984-019-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PG. Interaction between spinal motoneurons of the cat. J Neurophysiol 29: 275–287, 1966. doi: 10.1152/jn.1966.29.2.275. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. A serial section electron microscope study of an identified Ia afferent collateral in the cat spinal cord. J Comp Neurol 314: 257–277, 1991. doi: 10.1002/cne.903140205. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol 217: 75–85, 1983. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56: 141–148, 2009. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. The central termination of sensory fibers from nerves to the gastrocnemius muscle of the rat. Neuroscience 134: 175–187, 2005. doi: 10.1016/j.neuroscience.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol 520: 3895–3911, 2012. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature 459: 842–846, 2009. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sanchez J, Lorenzo LE, Lecker I, Zurek AA, Labrakakis C, Bridgwater EM, Orser BA, De Koninck Y, Bonin RP. α5GABAA receptors mediate tonic inhibition in the spinal cord dorsal horn and contribute to the resolution of hyperalgesia. J Neurosci Res 95: 1307–1318, 2017. doi: 10.1002/jnr.23981. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Martin P, Danti S, Simon P, Soubrié P. Noradrenergic rather than GABAergic processes as the common mediation of the antidepressant profile of GABA agonists and imipramine-like drugs in animals. Pharmacol Biochem Behav 28: 321–326, 1987. doi: 10.1016/0091-3057(87)90447-3. [DOI] [PubMed] [Google Scholar]
- Rudomin P. In search of lost presynaptic inhibition. Exp Brain Res 196: 139–151, 2009. doi: 10.1007/s00221-009-1758-9. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Lomeli J, Quevedo J. Tonic differential supraspinal modulation of PAD and PAH of segmental and ascending intraspinal collaterals of single group I muscle afferents in the cat spinal cord. Exp Brain Res 159: 239–250, 2004. doi: 10.1007/s00221-004-1953-7. [DOI] [PubMed] [Google Scholar]
- Russo RE, Delgado-Lezama R, Hounsgaard J. Dorsal root potential produced by a TTX-insensitive micro-circuitry in the turtle spinal cord. J Physiol 528: 115–122, 2000. doi: 10.1111/j.1469-7793.2000.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijk JJ, Holsheimer J, van der Heide GG, Boom HB. Recruitment of dorsal column fibers in spinal cord stimulation: influence of collateral branching. IEEE Trans Biomed Eng 39: 903–912, 1992. doi: 10.1109/10.256423. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol 79: 45–50, 1998. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur J Neurosci 8: 2492–2498, 1996. doi: 10.1111/j.1460-9568.1996.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T, Cope TC. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol 118: 2687–2701, 2017. doi: 10.1152/jn.00497.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B. Synaptic potentials evoked in cat dorsal spinocerebellar tract neurones by impulses in single group I muscle afferents. J Physiol 415: 423–431, 1989. doi: 10.1113/jphysiol.1989.sp017729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Graham B, Nicol MJ. Serial E-M and simulation study of presynaptic inhibition along a group Ia collateral in the spinal cord. J Neurophysiol 74: 616–623, 1995. doi: 10.1152/jn.1995.74.2.616. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Stuklis R. Effects of spatial and temporal dispersion of synaptic input on the time course of synaptic potentials. J Neurophysiol 61: 681–687, 1989. doi: 10.1152/jn.1989.61.4.681. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Wieniawa-Narkiewicz E, Nicol MJ. The ultrastructural basis for synaptic transmission between primary muscle afferents and neurons in Clarke’s column of the cat. J Neurosci 5: 2095–2106, 1985. doi: 10.1523/JNEUROSCI.05-08-02095.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 151: 475–488, 2010. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]