Abstract
Sudden Unexpected Death in Epilepsy (SUDEP) is the most common cause of death in refractory epilepsy patients. Human studies and animal models suggest that respiratory arrest is the initiating event leading to death in many cases of SUDEP. It has previously been reported that the onset of apnea can coincide with the spread of seizures to the amygdala, and apnea can be reproduced by electrical stimulation of the amygdala. The aim of the current work was to determine if the amygdala is required for seizure-induced respiratory arrest (S-IRA) in a mouse model of SUDEP. Experiments were performed on DBA/1 mice that have audiogenic seizures with a high incidence of fatal postictal respiratory arrest. Electrolytic lesions of the amygdala significantly reduced the incidence of S-IRA without altering seizures, baseline breathing or the hypercapnic ventilatory response. These results indicate that the amygdala is a critical node in a pathway to the lower brainstem that is needed for seizures to cause respiratory arrest.
Keywords: SUDEP, amygdala, respiration, seizures
1. Introduction
SUDEP is second only to stroke among neurological causes in years of potential life lost [1]. It is defined as the “sudden, unexpected, witnessed or unwitnessed, non-traumatic, and non-drowning death of a patient with epilepsy with or without evidence of a seizure, excluding documented status epilepticus, and in which postmortem examination does not reveal a structural or toxicological cause of death” [2]. It has long been known that seizures can impair cardiorespiratory function [3, 4]. It was recently confirmed that this can be fatal, when the MORTEMUS study reported physiological recordings from SUDEP cases in epilepsy monitoring units [5]. A consistent pattern of events occurred prior to death, in which a generalized tonic-clonic seizure led first to terminal apnea followed by terminal asystole. Although SUDEP is likely to occur from a combination of impaired respiratory and cardiac function [6, 7], and decreased arousal [8, 9], importance has been placed on mechanisms that lead to seizure induced respiratory arrest (S-IRA) since terminal apnea has occurred before terminal asystole when physiological recordings have been made during SUDEP in a small number of human cases, as well as during seizure induced death in animal models [5, 10, 11]
Recent work has implicated the amygdala as an important structure in the anatomical pathway linking seizures to S-IRA [12-15]. Central apnea and O2 desaturation were found to occur with, and only with, seizure spread to the amygdala during intracranial EEG recording from an intractable epilepsy patient [12]. Apnea can be reproduced in patients with electrical stimulation of the amygdala, suggesting that there is an anatomical connection from the amygdala to the brainstem respiratory network that causes inhibition of breathing [12, 13, 15].
DBA/1 and DBA/2 mouse strains have a high incidence of fatal respiratory arrest following seizures. It has proven to be a productive model to study the mechanisms of S-IRA and SUDEP [16-20] as generalized seizures can be induced by an auditory stimulus, and this reliably leads to S-IRA followed by cardiac arrest and sudden death [20-22]
In the current study, we used the DBA/1 mouse model of SUDEP to examine the role that the amygdala plays in S-IRA. Electrolytic lesions of the amygdala were made to determine if the amygdala is a necessary structure in the descending pathway activated by seizures to cause S-IRA.
2. Materials and Methods
2.1. Mouse husbandry and genotyping
All procedures and experiments involving mice were carried out with approval of the University of Iowa Institutional Animal Care and Use Committee, and in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, 8th edition [23]. The minimum possible number of animals was used, and care was taken to reduce any discomfort.
DBA/1 mice were obtained from Envigo Bioproducts (Madison, WI). All mice were housed and bred in the animal facility at University of Iowa Carver College of Medicine in a temperature and humidity controlled environment with a 12-h light/dark cycle. Mice were provided with food (standard NIH mouse diet) and water ad libitum.
DBA/1 mice were “primed” [22] to have audiogenic seizures (AGSz) starting at the age of P21. In this priming procedure mice were exposed to acoustic stimulation using an alarm bell (Twin metal bell analog quartz alarm clock, La Crosse Technology, La Crosse, WI) once daily for a maximum of 60 secs. This priming procedure was continued for a minimum of three days until mice had full seizures, defined as generalized tonic-clonic seizures terminating in hindlimb extension and respiratory arrest, for at least two consecutive days. After induction of full seizures mice were resuscitated using a rodent ventilator (Harvard Apparatus, Germany) set at a stroke volume of 150 μL at 150 strokes per minute. P rimed adult mice were later used in experiments at ages P40-P65, at which time they were tested to confirm that they were still susceptible to S-IRA from AGSz and resuscitated as outlined above.
2.2. Amygdala lesions
Mice were anesthetized with 0.5-3% isoflurane delivered by face mask from a precision vaporizer (Summit Anesthesia Solutions) and positioned in a stereotaxic frame (Stoelting, 51730U). Stereotaxic coordinates for the amygdala (from bregma: −1.3 mm caudal, ± 2.9 lateral, −4.3 ventral) were derived from an atlas of the mouse brain [24]. A midline incision was made, two small holes were drilled in the skull, and a unipolar electrode (World Precision Instruments, Sarasota, FL) was lowered into the amygdala where DC current (1 mA for 15 sec) was delivered using an Isolated Pulse Generator (Model 2100, A-M Systems, Carlsborg, WA) to induce electrolytic lesions. For EKG monitoring, two leads were tunneled under the skin and sutured to the anterior thoracic wall in an approximate Lead II configuration, one near the heart apex on the left and the other near the right shoulder. An EKG headmount was attached to the skull with an anchoring screw and dental cement. The skin was sutured closed, leaving only the headmount socket exposed. Animals received post-operative analgesia with meloxicam (0.5 mg/kg, i.p.) for 4 days, and were allowed to recover for 4-5 days before physiological recordings were made and seizures were induced.
2.3. Physiological monitoring during seizures
Simultaneous recordings were made of EKG, plethysmography and video during induction of seizures using a custom built mouse epilepsy monitoring system, and data were saved on computer using Matlab software (Mathworks Co.; Natick, MA) [11]. A preamplifier (8406-SE31M-C, Pinnacle Technology Inc., Lawrence, KS) was inserted into the headmount connector to record EKG. Breathing frequency and tidal volume were measured with standard open-flow, whole-body plethysmography using a chamber with a volume of 815 ml. There were ports for airflow in and out, and for pressure and temperature/humidity monitors. The plethysmography chamber was supplied with continuous flow of room air at ~400 ml/min via supply and exhaust air pumps (MK-1504 Aquarium Air Pump; AQUA Culture, Bentonville, AR) balanced to maintain chamber pressure near atmospheric. Animals within the chamber were provided food and water ad libitum. Chamber pressure, which changes with breathing, was measured using a digital differential pressure transducer (SDP610-25Pa; Sensirion AG, Switzerland). Signal outputs from the pressure transducer were connected to a microcontroller (Teensy 3.2; PJRC.com, Sherwood, OR) using a custom-designed printed circuit board. The digital transducers and microcontroller communicated through an I2C interface via the Arduino platform (ARDUINO 1.6.9; Arduino.cc, and Teensyduino 1.28; PJRC.com, Sherwood, OR) to upload configuration code of the digital sensors to the microcontroller board. Chamber temperature, relative humidity and pressure were acquired continuously at 100 Hz through a USB port with data acquisition software custom-written in MATLAB. EKG leads were tethered to a commutator (#8408; Pinnacle Technology Inc; Lawrence, KS) and connected to an analog amplifier (Model 440 Instrumentation Amplifier; Brownlee Precision Co.; San Jose, CA) where signals were further amplified by 10x and band-pass filtered from 1 to 500 Hz). These signals were digitized with an analog-to-digital converter (PCI-6225, National Instruments Corp., Austin, TX) installed in a desktop computer and acquired with the same custom-written MATLAB data acquisition software. The EKG sampling rate was 1 kHz, while plethysmography was sampled at 100 Hz.
Analyses of respiratory rate and heart rate were performed using software custom-written in MATLAB and/or Clampfit software (Molecular Devices, Sunnyvale, CA). Inspiratory peaks were detected automatically from plethysmography data using threshold detection on the the first derivative of the pressure signal after applying a bandpass filter of 1–10 Hz, and then finding the local maximum of the original pressure trace. Accuracy of peak detection was verified manually. R-wave detection of ECG data was performed by applying a continuous wavelet transform and peak detection in MATLAB. The time interval between breath peaks (interbreath interval) and the R-R interval from detected R waves were computed as the mean for 6-second epochs.
2.4. Ventilatory response to hypercapnia and hypoxia
To measure the HCVR, a commercially available plethysmograph chamber (Buxco, Wilmington, NC) with an open flow system controlled at 700 ml/min was used to determine frequency (f), tidal volume (VT) and minute ventilation (VE). Temperature of the chamber, humidity and pressure were measured as described above. The HCVR protocol involved exposure of mice to 50% O2 (balance N2) for 30 min as baseline. Gas combinations of 3, 5 and 7% CO2 with 50% O2 (balance N2) were then applied for 5 minutes each. To measure the HVR, mice were exposed to 21% O2 (balance N2) for 30 minutes and then to 10% O2 (balance N2) for 5 minutes.
The HCVR and HVR were measured in mice before and after amygdala lesions. Body temperature was recorded with telemetry probes (IPTT-300, BDMS, Seaford, DE) inserted intrascapularly 5 days before experiments. Chamber temperature was maintained at room temperature (25 C) by a heat lamp and a feedback controller (TCAT-2AC, Physitemp Instruments, Clifton,NJ). All data were acquired with custom-written Matlab software, and analyzed as above.
2.5. Seizure Induction
To induce seizures, mice were placed into the plethysmography chamber and the headmount described above was connected to the commutator. Baseline plethysmography and EKG were recorded for 15 minutes after which a small rubber plug (2 cm in diameter) was removed from the side wall of the chamber. The alarm bell used in AGSz priming was activated and placed at the opening of the chamber for a maximum of 60 seconds or until the mouse had a full seizure terminating in tonic hindlimb extension, at which time the rubber plug was reintroduced to seal the plethysmograph chamber. If the mouse did not have a full seizure terminating in tonic hindlimb extension by the end of the 60 sec of exposure to the alarm, the same procedure was repeated every 24 hours until the mouse had a full seizure or for a maximum of 3 days. If the mouse did not have a seizure after 3 consecutive trials they were not included in the primary analysis.
2.6. Histology
Animals that survived the seizure trial and did not succumb to S-IRA were deeply anesthesized with isoflurane (5%) and brains were harvested and drop-fixed in 4% PFA. For animals with S-IRA, mice were left in the whole body plethysmography chamber and recording was continued until asystole occurred. Brains were then removed and drop-fixed in 4% PFA. Brains were kept in 4% PFA for 24 hours and then in 30% sucrose for 1-2 days. Brains were frozen, and 40 μm sections were prepared with a Cryostat (model CM3050 S; Leica Biosystems) and mounted on microscope slides. Slides were Nissl stained with cresyl violet and cover-slipped. Slides were then examined with light microscopy to determine the location of lesions.
2.7. Experimental Design and Statistical Analysis
Bilateral lesions were attempted in approximately equal numbers of male and female mice. Littermates were used for sham control surgeries. For sham lesions, the same procedure used to lesion the amygdala outlined previously in section 2.2 was followed, and the electrode was lowered into the amygdala except no current was delivered to the electrode, and no electrolytic lesion was made. As there was no significant differerence in rates of S-IRA between mice with Sham lesions and mice in which lesions were not accurately placed in the amygdala on either side (84% vs 100% respectively, p=0.481) these groups were pooled together in the Control group. Those mice with a lesion in the amygdala on at least one side were included in the Lesion group. All statistics were performed using Prism 7 (GraphPad Software, La Jolla, CA, USA). Numerical data are reported as mean ± standard deviation (SD). Fisher’s exact test (twotailed) was used to compare incidence of S-IRA between the two experimental groups. A oneway ANOVA was used when 3 or more groups were being compared. Statistical significance was inferred if p < 0.05.
3. Results
3.1. Amygdala lesions did not affect baseline breathing or chemoreception in DBA/1 mice.
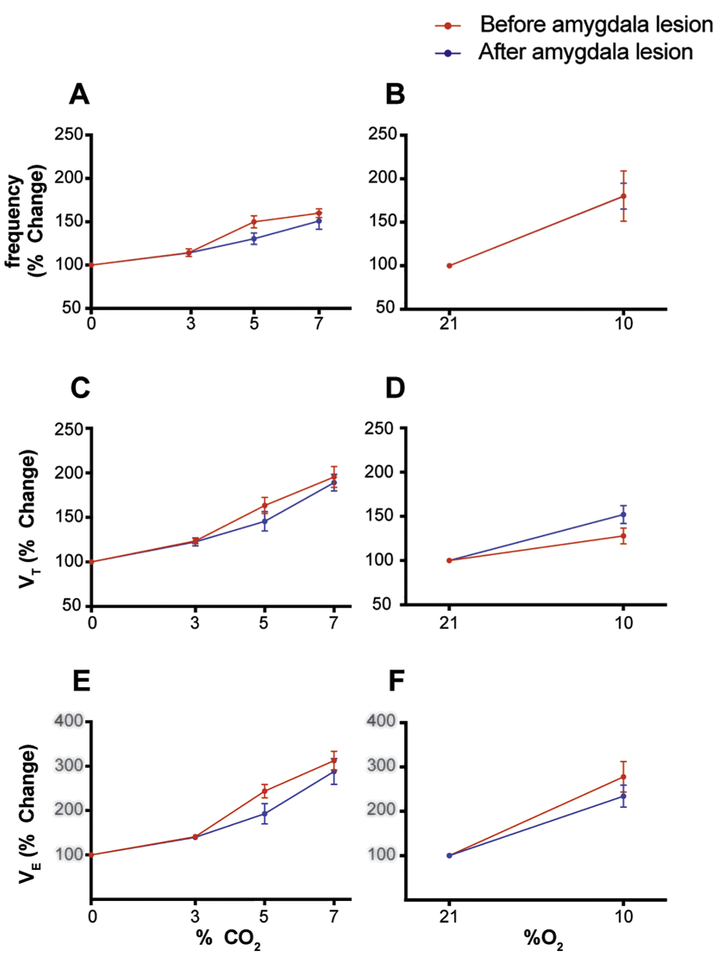
Amygdala ablation did not affect baseline breathing in DBA/1 mice. VE without lesion 1.86 ± 0.43, with lesion 1.73 ± 0.24 (μl/min/g); f without lesion 194.36 ± 23.56, with lesion 194.12 ± 23.01 (breaths/min); VT without lesion 10.86 ± 0.5, with lesion 11.38 ± 2.01 (μl/g) (p>0.05, Wilcoxon test (Figure 1B,D,F). The HCVR was also not affected by amygdala lesions in DBA/1 mice. The difference in curves of frequency (p=0.179), VT (p=0.419), and VE (p=0.228) were not different with or without amygdala lesions (2 way ANOVA, Bonferroni post Hoc test) (Figure 1A,C,E). Similarly, the HVR curves were also not different for frequency (p=0.990), VT (p=0.096), or VE (p=0.318) with or without amygdala lesions (2 way ANOVA, Bonferroni post Hoc test) (Figure 1B,D,F). These results suggest that the amygdala does not participate in generation of baseline breathing or chemosensory modulation of respiratory output.
Figure 1-. Amygdala lesions did not affect baseline breathing or chemoreception in DBA/1 mice.
A, C, E- CO2 chemoreception as measured by the HCVR was not significantly different in DBA/1 mice pre- and post-amygdala lesion. Plotted are the percentage increase in frequency (p=0.179), VT (p=0.419) and VE (p=0.228) in response to hypercapnia. (2 way ANOVA, Bonferroni post Hoc test)
B, D, F- O2 chemoreception as measured by the HVR was also not affected by amygdala lesions. Plotted are the percentage increase in frequency (p=0.990), VT(P=0.096), and VE (P=0.318) in response to hypoxia (2 way ANOVA, Bonferroni post Hoc test).
HCVR= Hypercapnic ventilatory response HVR= Hypoxic ventilatory response VT= tidal volume VE= Minute ventilation
3.2. A unilateral Amygdala lesion was sufficient to significantly reduce S-IRA and death in DBA/1 mice
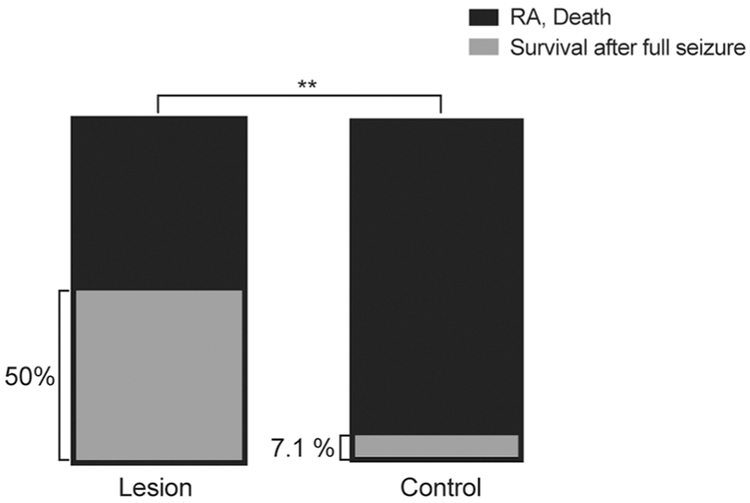
Bilateral amygdala lesions were attempted in primed DBA/1 mice of both sexes at P40-P60 and 4-5 days were allowed for recovery before conducting the audiogenic seizure induction trial. Although bilateral lesions were attempted in all animals, the majority of lesions accurately located in the amygdala were found to be unilateral (n=8/10) upon histologic confirmation of lesion location. For statistical analysis, mice with bilateral lesions outside of the amygdala (n=7) and those that had sham lesion placement (n=10) did not show any difference in S-IRA or death (p=0.4815, Fishers exact test) (Data not shown). Therefore, they were grouped together as Control. Only mice that had full, generalized tonic-clonic Racine level 5 seizures were included in the analysis. Unilateral or bilateral Amygdala lesions significantly reduced the incidence of SIRA and death compared to control animals (Figure 2; 50% for Lesioned (n=10), 92.86% for Control (n=28); p=.0080, Fishers exact test). This indicates that when the amygdalae are intact bilaterally, there is greater likelihood that seizures will cause S-IRA and death, presumably due to activation of direct or indirect projections from the amygdala to the respiratory network in the brainstem.
Figure 2 -. Amygdala lesions significantly reduced S-IRA and death in DBA/1 mice.
Lesioned animals had a significantly higher rate of survival after full seizures compared to Controls. 50% (n=10) compared to 7.14% (n=28), respectively, p=.0080, Fischer’s exact test).
Of note, following lesion surgery, many DBA/1 mice lost their seizure susceptibility. This occurred despite the fact that all mice were successfully primed to have seizures from a young age and seizure susceptibility was confirmed the day prior to surgery. If mice did not have a seizure after attempting the seizure induction trial for 3 days, they were excluded from the survival analysis described above, but histological confirmation of lesion location was still performed. The loss of seizure susceptibility after surgery occurred in mice with amygdala lesions and in Controls (58.33% in amygdala lesioned (n=24) compared to 39.58% in Controls (n=48), p=0.1143, Fishers exact test). Loss of seizure susceptibility was not more likely in mice with bilateral amygdala lesions compared to those with unilateral amygdala lesions (75% of bilateral (n=8) compared to 46.7% of unilateral (n=15), p=0.3788). It is possible that damage to the eardrums from placement of the stereotaxic ear bars during surgery diminished the animals perception of the sound stimulus and thus their seizure susceptibility. It is also possible that the amygdala is a necessary part of the network involved in generating audiogenic seizures, although our results do not support the conclusion that the amygdala is required for audiogenic seizures to be induced as some mice had seizures even after bilateral amygdala lesions. For those mice that did have audiogenic seizures, activation of the amygdala is known to occur during audiogenic seizures and S-IRA of DBA/1 mice [19], consistent with seizures propagating to the brainstem via the amygdala.
3.3. Terminal apnea preceded terminal asystole in death from seizures in DBA/1 mice
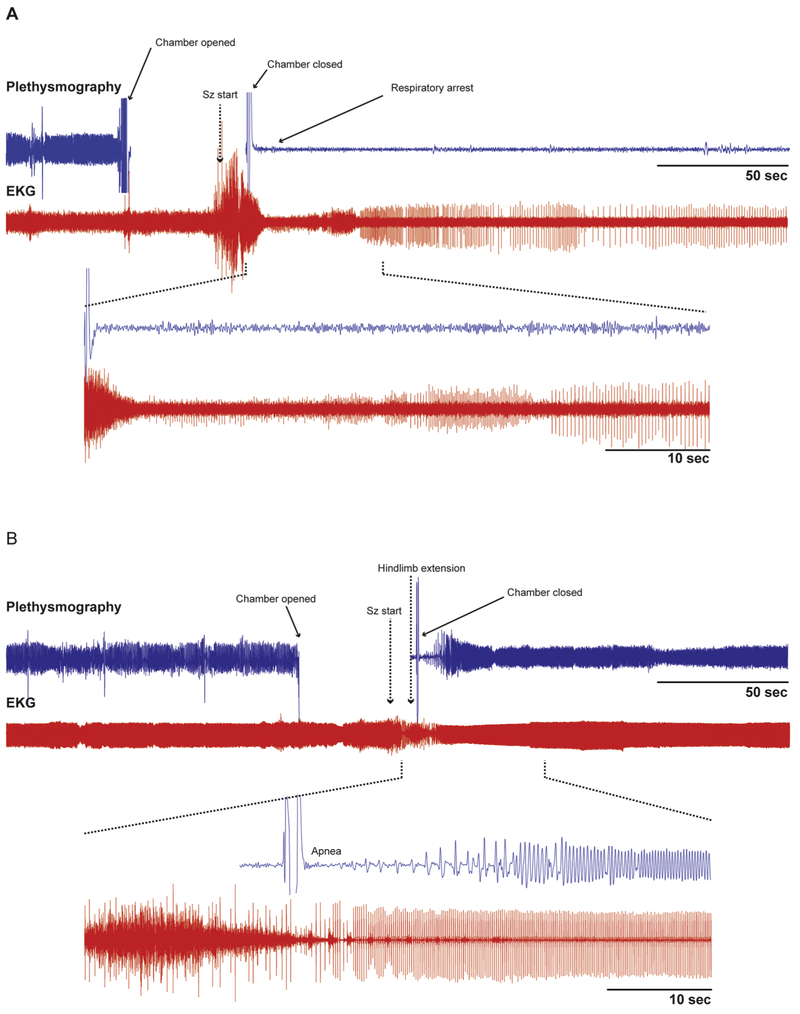
Seizures can cause impaired cardiorespiratory function leading to death. The MORTEMUS study of SUDEP cases in epilepsy monitoring units reported that terminal apnea occurred prior to terminal asystole in all monitored SUDEP cases. It has previously been reported that terminal apnea precedes progressive bradycardia and terminal asystole in DBA/1 mice [22] and Scn1aR1407X/+ mice [11]. Here we confirmed that this sequence of events occurs after fatal seizures in DBA/1 mice [22], where generalized tonic-clonic seizures terminated with tonic hindlimb extension, which occurred nearly simultaneously with respiratory arrest. This was followed by progressive bradycardia and eventual asystole (Figure 3A). After amygdala lesions, DBA/1 mice that survived despite having a full seizure had an interesting respiratory pattern (Figure 3B). Following termination of the seizure with tonic hindlimb extension, there was a brief apnea, after which respiratory rate and amplitude progressively increased until the breathing pattern recovered back to normal.
Figure 3 -. A DBA/1 mouse with an amygdala lesion survived a seizure while a control mouse died of seizure-induced respiratory arrest.
A - Raw data traces of plethysmography and EKG from a control DBA/1 mouse in which terminal apnea occurred during a seizure. The heart rate (EKG) was disrupted during and shortly after the seizure, but continued for a prolonged period before it eventually slowed to terminal asystole (not shown). Inset below shows expanded traces from the time indicated by the dashed lines.
B- Raw data traces of plethysmography and EKG in a DBA/1 mouse with a unilateral R sided amygdala lesion that survived an audiogenic seizure. Note the brief apnea followed by slow increase in amplitude and frequency of breathing which eventually returned to baseline. There was also brief bradycardia that recovered more rapidly. Inset below shows expanded traces from the time indicated by the dashed lines.
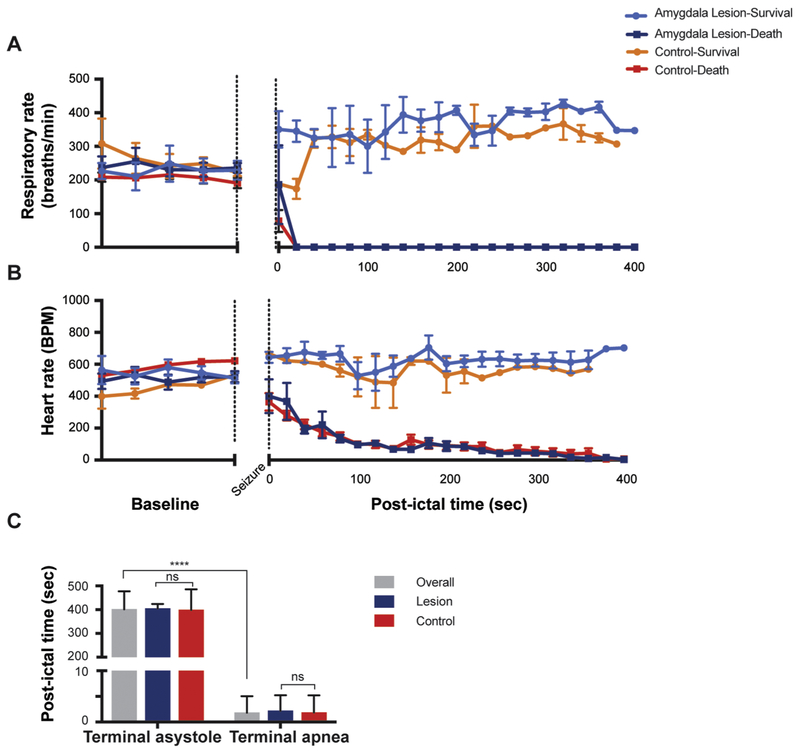
In DBA/1 mice that died from audiogenic seizures, terminal apnea occurred 3.19±1.78 sec after hindlimb extension (n=29). In contrast, terminal asystole occurred 400.5 ± 77.48 sec after hindlimb extension (n=29) (Figure 4), which was significantly different (unpaired t-test, p=0.0001). In amygdala-lesioned mice that survived a full seizure, there was transient apnea with a duration of 2.8 ± 1.79 secs (n=5), after which respiratory rate and heart rate eventually increased to levels higher than baseline (Figure 4).
Figure 4 -. Terminal apnea preceded terminal asystole during seizure-induced death in DBA/1 mice with or without amygdala lesions.
A - Mean values of respiratory rate (breaths/min) in DBA/1 mice before and after seizure induction. There was no difference in the changes in respiratory rate between animals that died with or without lesions, or between animals that survived with or without lesions.
B - Mean values of heart rate (beats/min) in mice before and after seizure induction. Similar to part A, there was no independent effect of amygdala lesions. The changes in heart rate were the same for animals that died, and for animals that lived, regardless of whether they had amygdala lesions.
*Lesion-survival (n=5), lesion-death (n=5), control-survival (n=2), control-death (n=26).
C - Terminal apnea occurred significantly earlier than terminal asystole for all animals that died (3.19±1.78 secs and 400.5 ± 77.48 secs; n=29; p<0.001, paired T-test). There was also no significant difference in time to terminal asystole or terminal apnea between the lesion and control groups (p=0.4571, paired T-test).
There was no significant difference in the time to post-ictal asystole (unpaired t-test, p=0.9314) or apnea (unpaired t-test, p=0.8342) in mice that died with, compared to those that died without, amygdala lesions (Figure 4). This suggests that the postictal bradycardia and asystole seen here was a reflexive effect of respiratory arrest, and not due to a direct effect of the amygdala. The same conclusion was made for postictal death in Scn1aR1407X/+ mice, in which case bradycardia was due to hypoxia that resulted from respiratory arrest [11]. Taken together, our results in DBA/1 mice support the conclusion that the primary initiating event in some cases of SUDEP is terminal apnea [9].
3.4. Laterality of amygdala lesions was not associated with a difference in survival from seizures
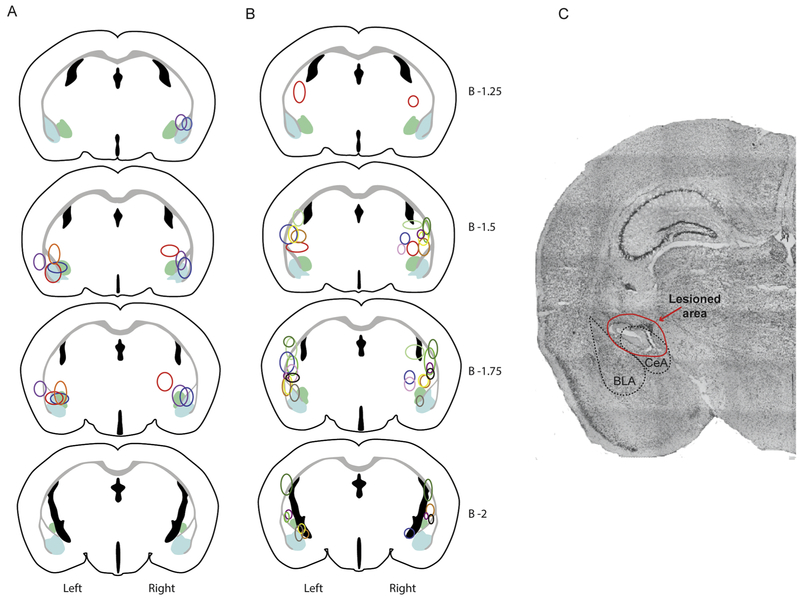
Unilateral amygdala lesions were sufficient to significantly reduce S-IRA and death in DBA/1 mice (Figure 5). For left unilateral amygdala lesions, 1/3 mice survived the seizure induction trial compared to 3/5 mice with R unilateral amygdala lesions. Of those with bilateral amygdala lesions, 1/2 mice survived the trial. These results indicate that laterality of the amygdala lesion did not have a significant effect on the likelihood of S-IRA and death.
Figure 5-. Lesion locations in mice based on seizure induction trial outcome.
Lesion locations in mice that had full generalized tonic-clonic seizures. All mice had at least unilateral lesions of the amygdala.
A- Lesion locations in mice that survived audiogenic seizure induction trial after having full generalized tonic-clonic seizures.
B- Lesion locations in mice that succumbed to seizure induced respiratory arrest during the audiogenic seizure induction trial.
C- Example of lesion appearance on histologic examination of a slice stained w/ cresyl violet.
*Note that each color corresponds to the lesion locations on an individual mouse. For each drawing lesions are plotted on the correct side of the brain (left or right). Light blue represents the area of the basolateral amygdala (BLA) and light green represents the area of the central amygdala (CeA).
4. Discussion
Here we demonstrated that unilateral amygdala lesions significantly reduced the incidence of S-IRA and death in the DBA/1 mouse model of SUDEP. This suggests that the amygdala is an important anatomical structure in the pathway by which seizures cause S-IRA and death. Prevention of S-IRA was not due to a reduction in severity of seizures as we only included data from mice with amygdala lesions that had full generalized tonic-clonic seizures (Racine level 5) compared to controls with full seizures. We also confirmed that respiratory arrest is the initiating event leading to death from seizures in the DBA/1 mouse strain [22]
Seizure spread to, or electrical stimulation of, the amygdala has been shown to cause apnea in epilepsy patients [12-15] suggesting that there is a neural pathway from the amygdala to the brainstem respiratory network. With electrical stimulation of the amygdala, patients were unaware that they were apneic and reported no dyspnea or other discomfort [12]. Furthermore, a recent multicenter study prospectively observed patients in epilepsy monitoring units and found that ictal central apnea occurred significantly more with temporal lobe seizures compared to extratemporal seizures [13] which supports the hypothesis that the amygdala, which is located in the medial temporal lobe, is important for seizure propagation to the brainstem.
Although SUDEP is likely to involve heterogeneous mechanisms, it has been widely believed that most cases are due to sudden cardiac death. However, recent evidence from both human [5] and animal studies [10, 11, 16, 17, 21] has shown that terminal apnea occurs prior to terminal asystole in many cases of SUDEP. It is now believed that SUDEP is likely a combination of impaired respiratory function, cardiac function, and arousal [8, 9], but a role of central respiratory failure as the initiating event may be more common than previously believed. Since seizure spread to, or electrical stimulation of the amygdala has been shown to cause apnea without awareness of the resultant oxygen desaturation in humans [12], electrical activation of the amygdala appears to be sufficient for causing prolonged apnea. The current study demonstrates that the amygdala may also be necessary for S-IRA, as unilateral lesions of the amygdala in DBA/1 mice significantly reduced death from S-IRA. Interestingly, it was only necessary to have a unilateral amygdala lesion to prevent S-IRA. We were not able to determine whether a bilateral lesion was more effective, but a lesion in either the left or right amygdala was effective.
Although the precise mechanisms of SUDEP are still unknown, it is of note that seizure spread to the brainstem and post-ictal brainstem dysfunction have both been connected to respiratory dysfunction. Brainstem spreading depolarization during seizures has been correlated with cardiorespiratory collapse [25]. Electrical recordings from rats during seizures induced by 4-aminopyridine demonstrated that brainstem epileptiform discharges occur shortly after discharges in the cortex and hippocampus, and are associated with respiratory arrest [26]. Importantly, respiratory arrest is only seen in cases where prolonged epileptiform discharges are recorded from the brainstem, not when discharges are recorded only from the cortex or hippocampus. Another group found that decreased firing of medullary raphe 5-HT neurons during ictal and post-ictal periods is coincident with a decrease in respiratory output [27]. Considering this evidence, along with observations from our current study and human studies that have linked seizure spread to, and stimulation of, the amygdala with respiratory arrest, we propose that seizure spread from the cortex to the brainstem, via the amygdala, is necessary for S-IRA in some types of seizures, and is an important pathophysiological mechanism in some cases of SUDEP.
The amygdala may induce S-IRA through connections with several areas of the brainstem. Most projections arise from the central amygdala (CeA), which is the primary output nucleus [28-30]. Direct or indirect projections to brainstem respiratory networks are the most likely to be involved in S-IRA. Prior research has shown that descending projections from the amygdala can alter the timing of the respiratory cycle [31]. Projections from the central amygdala synapse onto a site in the midline medulla which produces apnea upon stimulation [32]. There may be direct, inhibitory projections from the amygdala that are activated during seizures and disrupt respiratory networks leading to apnea, or there may be second-order connections through other regions that modulate cardiorespiratory function and receive projections from the amygdala, such as the midbrain periaqueductal grey (PAG) [33]. There have been several recent studies that have examined the role of PAG in S-IRA in DBA/1 mice [19] that have shown that protective PAG mediated cardiorespiratory modulation in response to seizure may be deficient in DBA/1 mice [34], and that activation of this region w/ fluoxetine may be protective against S-IRA [35] Furthermore, a recent neuroanatomical study of the pathway involved in freezing behavior [36] illustrates a network responsible for this conserved behavior that begins in the CeA and synapses onto neurons in the ventrolateral PAG which then project onto motor neurons in the rostral medulla involved in freezing behavior. It is possible that there is a similar pathway from the CeA to the medullary respiratory network via the PAG that is responsible for apnea and respiratory arrest with electrical stimulation or during seizures.
Brainstem areas that could be targets of descending projections from the amygdala include the Pre-Botzinger complex, and the medullary raphe. The pre-Botzinger complex is involved in respiratory rhythm generation, while increased firing of 5-HT neurons in the medullary raphe stimulates bursting in neurons of the pre-Botzinger complex and enhances respiratory output [37]. Since amygdala lesions did not affect baseline breathing, projections from the amygdala are not necessary for generating or modulating the baseline respiratory output, but may only be active during seizures. Further work will be needed to examine these connections and the specific populations of neurons in the amygdala that are involved in S-IRA.
The current study supports the conclusion that terminal central apnea is the primary event leading to cardiorespiratory failure in some cases of SUDEP. Similar observations have previously been made in both human and animal studies [5, 10, 11, 21]. This conclusion is important for design of preventative strategies to reduce the risk of SUDEP. Of note, in DBA/1 mice that died from S-IRA there was no significant difference in the time to post-ictal asystole in mice with or without amygdala lesions. This suggests that postictal bradycardia and asystole seen here was a secondary result of respiratory arrest and not due to a direct effect of projections from the amygdala. Since evidence suggests that it can be the primary initiating event, efforts to reduce mortality from SUDEP may need to include preventing the initial apnea and respiratory arrest rather than prevention of secondary bradycardia and asystole.
5. Conclusions
In summary, we demonstrated here that a unilateral amygdala lesion eliminated S-IRA in some mice that had seizures of equivalent severity as seizures that caused S-IRA in non-lesioned mice. This suggests that the amygdala is an important intermediate structure in the anatomical pathway that generalized seizures in the forebrain follow to the brainstem to cause S-IRA. Our previous work showed that activation of the amygdala was sufficient for causing apnea [12], and we demonstrated here that it is necessary for S-IRA in DBA/1 mice. We also confirmed that respiratory arrest occurs minutes before asystole in the DBA/1 audiogenic seizure mouse model of SUDEP, which further supports the hypothesis that respiratory arrest occurs before cardiac dysfunction in the sequence leading to some cases of SUDEP.
Significance Statement.
SUDEP is the most common cause of mortality in patients with refractory epilepsy, and S-IRA is thought to be important in the pathophysiology in many cases. In an epilepsy patient, the onset of apnea has been shown to coincide with spread of seizures to the amygdala, and in multiple patients apnea was induced by stimulation of the amygdala. Here, we show that lesions of the amygdala reduced the incidence of S-IRA and death in a mouse model of SUDEP. These results provide evidence that the amygdala may be a critical node in the pathway by which seizures influence the brainstem respiratory network to cause apnea.
A unilateral amygdala lesion significantly reduced seizure-induced respiratory arrest
Baseline breathing and chemoreception were not affected by amygdala lesions
Confirm terminal apnea occurs before asystole in death from seizure in DBA/1 mice
Acknowledgments:
We thank Xiuqiong Zhou for mouse husbandry and genotyping, and Lori Smith-Mellecker for technical contributions. The authors declare no competing financial interests.
Funding: The study was supported by US National Institutes of Health (NIH) grants U01NS090414 and the Beth L Tross Epilepsy Research Fund. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia 2014;55: 1479–85. [DOI] [PubMed] [Google Scholar]
- [2].Nashef L Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997;38: S6–8. [DOI] [PubMed] [Google Scholar]
- [3].Nelson DA, Ray CD. Respiratory arrest from seizure discharges in limbic system. Report of cases. Arch Neurol 1968;19: 199–207. [DOI] [PubMed] [Google Scholar]
- [4].Hughlings-Jackson J On asphyxia in slight epileptic paroxysms: on the symptomatology of slight epileptic fits supposed to depend on discharge-lesions in the uncinate gyrus. Lancet 1899;1: 79. [Google Scholar]
- [5].Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12: 966–77. [DOI] [PubMed] [Google Scholar]
- [6].Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK, Gehlbach BK, Schuele S, Friedman D, Devinsky O, Nei M, Harper RM, Allen L, Diehl B, Millichap JJ, Bateman L, Granner MA, Dragon DN, Richerson GB, Lhatoo SD. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 2018;59: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sainju RK, Dragon DN, Winnike HB, Nashelsky MB, Granner MA, Gehlbach BK, Richerson GB. Ventilatory response to CO2 in patients with epilepsy. Epilepsia 2019;60: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 2016;15: 1075–88. [DOI] [PubMed] [Google Scholar]
- [9].Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 2014;10: 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 2014;592: 4395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR Jr., Richerson GB. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128: 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA, Richerson GB. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J Neurosci 2015;35: 10281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 2017;88: 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, Zelano C. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol 2018;83: 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nobis WP, Gonzalez Otarula KA, Templer JW, Gerard EE, VanHaerents S, Lane G, Zhou G, Rosenow JM, Zelano C, Schuele S. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. J Neurosurg 2019: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang H, Zhao H, Feng HJ. Atomoxetine, a norepinephrine reuptake inhibitor, reduces seizure-induced respiratory arrest. Epilepsy Behav 2017;73: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, Solt K, Feng HJ. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis 2018;110: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao H, Cotten JF, Long X, Feng HJ. The effect of atomoxetine, a selective norepinephrine reuptake inhibitor, on respiratory arrest and cardiorespiratory function in the DBA/1 mouse model of SUDEP. Epilepsy Res 2017;137: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kommajosyula SP, Randall ME, Brozoski TJ, Odintsov BM, Faingold CL. Specific subcortical structures are activated during seizure-induced death in a model of sudden unexpected death in epilepsy (SUDEP): A manganese-enhanced magnetic resonance imaging study. Epilepsy Res 2017;135: 87–94. [DOI] [PubMed] [Google Scholar]
- [20].Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 2006;47: 21–26. [DOI] [PubMed] [Google Scholar]
- [21].Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav 2017;71: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav 2010;17: 436–40. [DOI] [PubMed] [Google Scholar]
- [23].Albus U Guide for the Care and Use of Laboratory Animals (8th edn). In: SAGE Publications Sage UK: London, England; 2012. [Google Scholar]
- [24].Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates: Academic press New York:; 2008. [Google Scholar]
- [25].Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015;7: 282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salam MT, Montandon G, Genov R, Devinsky O, Del Campo M, Carlen PL. Mortality with brainstem seizures from focal 4-aminopyridine-induced recurrent hippocampal seizures. Epilepsia 2017;58: 1637–1644. [DOI] [PubMed] [Google Scholar]
- [27].Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M, Li W, Bo X, Richerson GB, Blumenfeld H. Impaired Serotonergic Brainstem Function during and after Seizures. J Neurosci 2016;36: 2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Benarroch EE. The amygdala: functional organization and involvement in neurologic disorders. Neurology 2015;84: 313–24. [DOI] [PubMed] [Google Scholar]
- [29].Pollak Dorocic I, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlen M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014;83: 663–78. [DOI] [PubMed] [Google Scholar]
- [30].Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011;471: 358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harper RM, Frysinger RC, Trelease RB, Marks JD. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Res 1984;306: 1–8. [DOI] [PubMed] [Google Scholar]
- [32].Verner TA, Pilowsky PM, Goodchild AK. Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res 2008;1208: 128–36. [DOI] [PubMed] [Google Scholar]
- [33].Dampney RA, Furlong TM, Horiuchi J, Iigaya K. Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci 2013;175: 17–25. [DOI] [PubMed] [Google Scholar]
- [34].Kommajosyula SP, Tupal S, Faingold CL. Deficient post-ictal cardiorespiratory compensatory mechanisms mediated by the periaqueductal gray may lead to death in a mouse model of SUDEP. Epilepsy Res 2018;147: 1–8. [DOI] [PubMed] [Google Scholar]
- [35].Kommajosyula SP, Faingold CL. Neural activity in the periaqueductal gray and other specific subcortical structures is enhanced when a selective serotonin reuptake inhibitor selectively prevents seizure-induced sudden death in the DBA/1 mouse model of sudden unexpected death in epilepsy. Epilepsia 2019;60: 1221–1233. [DOI] [PubMed] [Google Scholar]
- [36].Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Luthi A. Midbrain circuits for defensive behaviour. Nature 2016;534: 206–12. [DOI] [PubMed] [Google Scholar]
- [37].Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 2009;29: 3720–37. [DOI] [PMC free article] [PubMed] [Google Scholar]