Abstract
The gastrointestinal tract is the only internal organ to have evolved with its own independent nervous system, known as the enteric nervous system (ENS. This Review provides an update on advances that have been made in our understanding of how neurons within the ENS coordinate sensory and motor functions. Understanding this function is critical for determining how deficits in neurogenic motor patterns arise. Knowledge of how distension or chemical stimulation of the bowel evokes sensory responses in the ENS and central nervous system have progressed, including critical elements that underlie mechanotransduction of distension-evoked colonic peristalsis. Contrary to original thought, evidence suggests that mucosal serotonin is not required for peristalsis or colonic migrating motor complexes, although mucosal serotonin can modulate their characteristics. Chemosensory stimuli applied to the lumen can release subtances from enteroendocrine cells, which could subsequently modulate ENS activity. Advances have been made in optogenetic technologies, such that specific neurochemical classes of enteric neurons can be stimulated. A major focus of this Review will be the latest advances in our understanding of how intrinsic sensory neurons in the ENS detect and respond to sensory stimuli and how these mechanisms differ from extrinsic sensory nerve endings in the gut that underlie the gut–brain axis.
Introduction
Over the past decade, there has been extraordinary interest in how the gut and brain communicate with one another, in particular with regards to the microbiome and how substances within the wall of the gut could be responsible for changes in health and wellbeing. Understanding how the gut responds to different substances in the lumen requires an understanding how the different sensory nerves are activated. Discerning how the gut detects sensory stimuli is of supreme importance to understanding the control of the gut-to-brain axis. There have been important advances in our knowledge of the different types of intrinsic and extrinsic sensory neurons that communicate local reflexes within the gut and outside the gut to the spinal cord and brain.
It has been known since the mid-1700s that segments of intestine isolated from vertebrates can respond to particular stimuli, despite the intestine being disconnected from the brain and spinal cord1. Surprisingly, it was not until in the mid-1990s that unequivocal evidence was presented that the enteric nervous system (ENS) contained its own population of sensory neurons2 and these neurons were capable of initiating reflex responses that were mediated entirely via the ENS alone3. Over the past 5–10 years, there have been important advances in our understanding of how chemical and mechanical stimuli applied to the gut wall can elicit neural reflex responses within the ENS. There have also been major advances in our understanding of how extrinsic afferents, with sensory nerve endings in the gut wall (but their cell bodies outside the gut) respond to certain stimuli. Interestingly, studies have shown that even within the same region bowel from the same species, the mechanisms that transduce mechanical or chemical stimuli of the bowel into neural reflexes can differ substantially between intrinsic sensory neurons (in the ENS) and extrinsic sensory nerve endings in the gut, with cell bodies outside gut wall. Understanding how the gut detects sensory stimuli is of supreme importance to understanding the control of the gut-to-brain axis. In this Review, we will discuss the major advances that have been made regarding the mechanisms underlying the transduction of sensory stimuli in the gut wall into neural activity within intrinsic and extrinsic sensory nerves.
Enteric nervous system
Of all the hollow organs in the body, the gastrointestinal tract is the only organ to have evolved with its own complete nervous system, known as the ENS, which can function fully independently of any neural inputs from the central nervous system (CNS), that is the brain and spinal cord1. Perhaps the best evidence to support the autonomous nature of the ENS is exemplified when segments of bowel are removed from vertebrates and studied in isolation ex vivo. Under these conditions, the intestine continues to generate complex propulsive neurogenic motor patterns, despite all extrinsic nerves being severed4–6. The notion that the gut was capable of generating reflex responses independent of the CNS was actually first identified in 1755 by Albert Von Haller, who stated that after removing the intestine from the body “. the intestines in this state after being deprived from all communication with the brain, preserve their peristaltic motion”1 (Figure 1). His discovery was further supported 135 years later by Carl Lüderitz who provided the first clear description that local stimulation of the bowel could evoke polarized responses in isolated segments of intestine7,8. About 10 years later, Bayliss and Starling then confirmed the presence of polarized neural responses in exteriorized segments of dog intestine following local stimulation, leading them to formulate the ‘law of the intestine’, that stated: “Local stimulation of the gut produces excitation above and inhibition below the excited spot”9(Fig.1). However, it was work by Paul Trendelenberg during the first World War that the now common term ‘peristaltic reflex’ was coined, which he used to describe the actual propulsion of content along isolated segments of bowel4. Since these early seminal studies, there has been much progress in identifying the fundamental control mechanisms underlying neurogenic propulsion in the small and large intestine of small vertebrates5,6,10–17, including the small intestine18 and colon19 of humans, confirming that the ENS alone is capable of generating complex neurogenic motor patterns without inputs from the CNS (Fig.2).
Fig. 1: Key developments in the enteric nervous system.
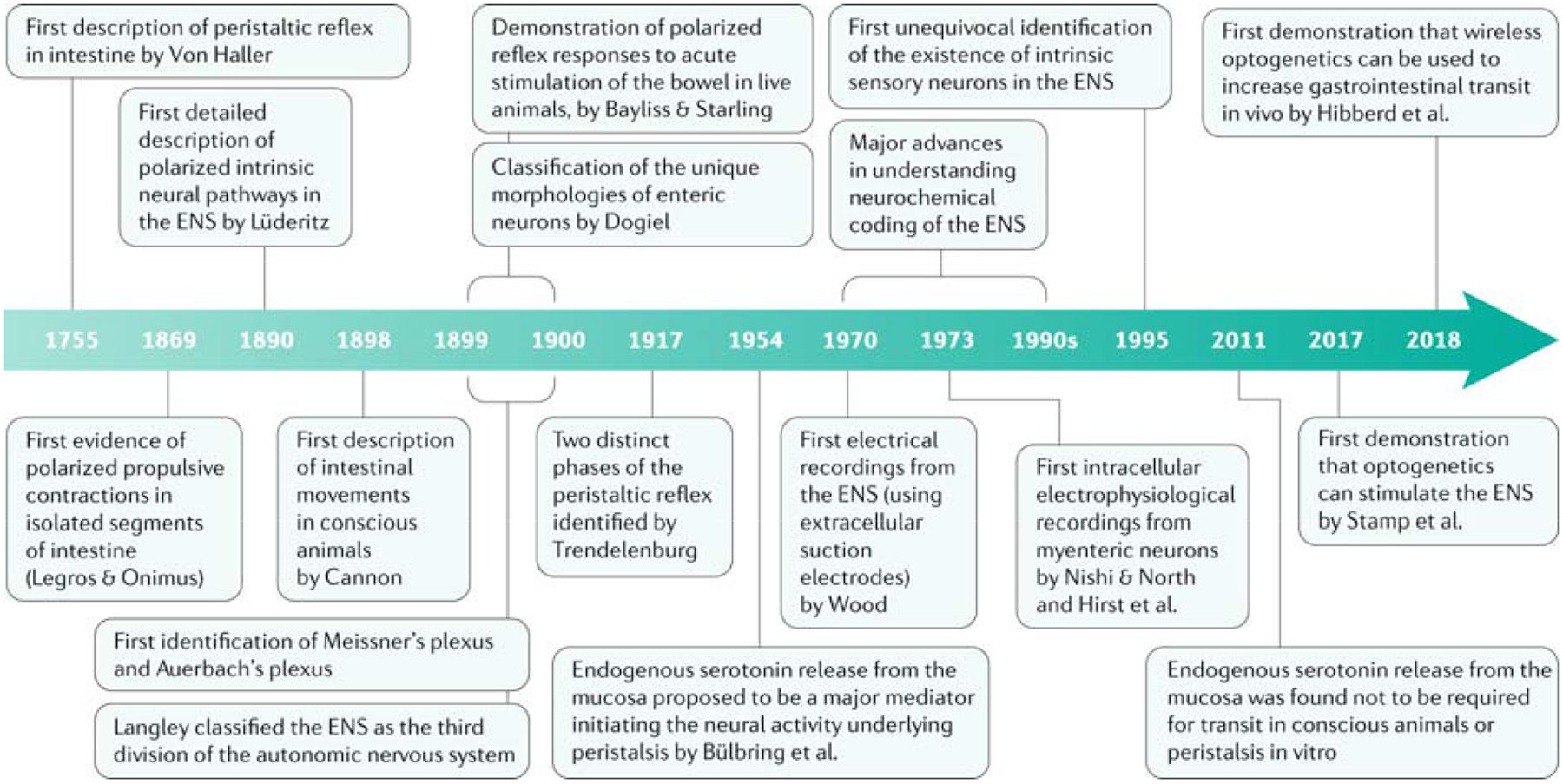
The figure shows the timeline of the major advances made in our understanding of the enteric nervous system (ENS) and the identification of motility reflexes. The first evidence of a reflex response in an isolated segment of the gut was provided in 1755 by Von Haller1. It was not until 1995 that the first unequivocal evidence that the ENS contained its own population of sensory neurons was presented2. For Cannon, Legros & Onimus and Langley see refs181,182,183
Fig. 2: The major extrinsic neural pathways between the ENS and spinal cord and brain.
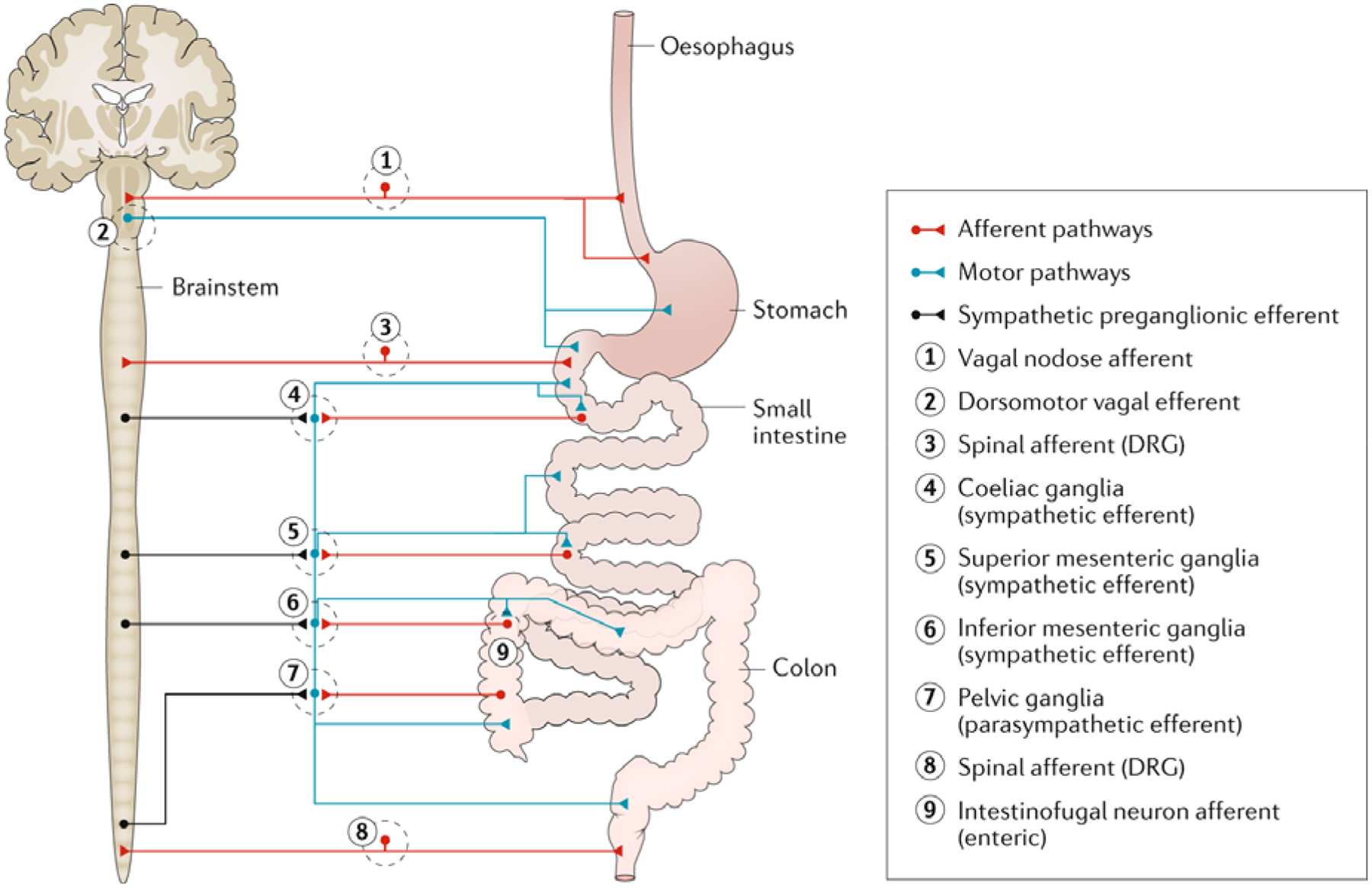
The major extrinsic motor pathways between the enteric nervous system (ENS) and spinal cord and brain are shown in blue. These motor pathways constitute the parasympathetic (vagal motor) and sympathetic nervous systems (arising from the thoracolumbar spinal cord and synapsing on the coeliac, superior mesenteric ganglia and inferior mesenteric ganglia). In the gut, the sympathetic nerves are inhibitory, whereas the parasympathetic nerves are excitatory. In the upper gut (oesophagus and stomach), the major extrinsic sensory nerves arise from the vagus nerve, whereas in the lower gut (colon), the influence of the vagus is reduced and the major extrinsic sensory nerves to the colon arise from spinal afferent nerves, whose cell bodies lie in dorsal root ganglia (DRG).
The ENS consists of many thousands of discrete small ganglia that retain neural continuity with each other forming two distinct ganglionated neuronal plexuses, called the myenteric and submucosal plexus14. Unlike skeletal muscle which is only innervated by excitatory neurons20, smooth muscle cells of the gastrointestinal tract are densely innervated by both excitatory and inhibitory motor neurons21–24. Within each plexus lies a heterogeneous population of individual neurons with distinct neurochemical coding, projections and functional roles14,25,26. Ganglia within the submucosal and myenteric plexuses are connected to neighboring ganglia via internodal strands that carry axons over substantial distances (even up to 13 cm) to facilitate the rapid conduction of neuronal signals along the bowel27,28. There is considerable redundancy in the ENS. Loss of large numbers of enteric neurons does not necessarily lead to loss of motility or function. Mice that lose at least half their ENS can still generate rhythmic propagating neurogenic colonic motor complexes and live a normal life span29. The evolutionary process that has led to the development of the ENS has not been restricted to vertebrates. Simple invertebrates in the cnidarian phylum with radial symmetry, such as Hydra, evolved an intrinsic nervous system without any central ganglia (that is, a CNS)30 that is clearly capable of generating propulsive peristaltic-like movements31 and expelling waste.
In general, the role of the myenteric plexus is to coordinate muscle movements underlying propulsion of content, while the submucosal plexus is broadly involved in secretion and absorption14. It is presumed that the myenteric plexus fulfills a similar role in invertebrates. The importance of the ENS for life is best exemplified in vertebrates that are born with genetic mutations that lead to complete loss of enteric ganglia in the terminal colorectum32,33. A variety of animal species with a complete loss of enteric neurons over a substantial length of colorectum lack neurogenic motility patterns and have improper expulsion of colonic contents. This defect usually leads to the development of megacolon and animals commonly die shortly after birth32,5.
There has been long-term interest in generating enteric neurons in aganglionic regions of bowel of a variety of mammals born with Hirschsprung disease32,34, or in mammals that develop enteropathies associated with a deficiency in number of enteric neurons (for example, Chagas disease). In the past few years, studies have demonstrated that transplanted neural progenitor-containing neurospheres from mouse and human can integrate into the aganglionic mouse colon both in vivo35–37 and ex vivo38,39. However, because of the extreme phenotype of the aganglionic mouse model used, the vast majority of the offspring died within the first 1–2 months of birth (independent of the neurosphere treatment), making it difficult to determine the potential benefits of the treatment. It has now been demonstrated that ENS progenitors from human pluripotent stem (PS) cells can be efficiently derived and isolated leading to their differentiation into functional enteric neurons40. This research showed that ENS precursors derived in vitro display targeted migration in the developing embryo of chicks and colonization of the large intestine of adult rodents40. Exciting in vivo studies have demonstrated that enteric neural stem cells can be successfully transplanted and integrated in vivo to restore nitrergic neurons in nitric oxide deficient mouse colon41. This study demonstrated that transplantation of enteric neural stem cells in vivo leads to a recovery of neuronal nitric oxide synthase positive enteric neurons and a restoration of motility. These effects were associated with the development of elaborate networks of transplanted cells41. This finding was a major advance because it demonstrated for the first time that enteric neural stem cell transplantation can lead to an improvement in colonic function.
There has been some controversy regarding the life span and turnover rates of enteric neurons. The controversy arises because original studies in mice showed that neurogenesis continues postnatally through to about P21, at which point neurogenesis declines42. However, a very different concept was proposed in 2017 by Kulkarni and colleagues where the rate of enteric neuronal turnover and neurogenesis in adult mice occurs at a rapid rate, whereby >85% of myenteric neurons in the adult mouse small intestine are less than 2 weeks of age43. If this is true, this rate of neuronal turnover would require substantial cell death and a concern with this concept is that apoptosis has been shown to occur infrequently44, if at all in the developing ENS of mice44,45. The study by Kulkarni and colleagues proposed that despite ongoing neuronal cell loss (due to apoptosis), the substantial turnover and neurogenesis of adult enteric neurons means that the total number of myenteric neurons remains constant in adult mouse small intestine,43. This was suggested to occur because neurons formed from dividing precursor cells located within myenteric ganglia that express nestin and p75NTR43. This study suggested there was a loss of approximately 4–5% of myenteric neurons per day, or about 30% of myenteric neurons every 7 days43. If the majority of neurons are turning over at such a high rate, it is unclear how retrograde neuronal tracing studies can visualize labeled enteric neurons many days after application of tracers to autonomic ganglia46. It is also unclear how synaptic connections between neurons and neurotransmission can persist if nerve cell bodies are turning over so rapidly47. Future studies will be required to provide a better understanding of the rates of neuronal turn over and whether similar turnover rates occur throughout the full length of gastrointestinal tract.
Just like the heart, the gut contains non-neuronal pacemaker cells which generate rhythmic electrical rhythmicity in the smooth muscle cells. These electrical oscillations in the smooth muscle are called slow waves. The pacemaker cells of the GI-tract that generate slow waves have been identified as Interstitial Cells of Cajal (ICC)48 Slow waves cause phasic contractions of the gut without any requirement of activity from enteric neurons49. It is accepted that the ENS is required for propulsion along the gut. However, according to evidence in experimental models, pacemaker-type ICC (at the level of the myenteric plexus) and the electrical rhythmicity these cells generate is not required for normal gastrointestinal function, at least in the small intestine, where they can be selectively ablated50,51. By contrast, mice that fail to develop an ENS, but still develop ICC in the aganglionic smooth muscle region die52. These mice are unable to generate sufficient propulsion or polarized contractions that could propel content and animals die soon after birth53. This research showed that while ICC at the level of the myenteric plexus (referred to as Interstitial Cells of Cajal – Myenteric, termed “ICCMY”)were important for slow-wave-mediated peristalsis49, these pacemaker cells and the rhythmic electrical depolarisations they generate were unable to take over the critical role of the ENS. Interestingly, mutant mice that lack pacemaker-type ICC at the level of the myenteric plexus and consequently lack electrical slow waves in the small intestine live a full life span with minor, or no obvious gastrointestinal deficits50,51 and still generate propagating neurogenic contractions54. Evidence exists that another population of ICC lying adjacent to the smooth muscle cells (termed ICC-IM) are important for neurotransmission55,56, although this is still controversial and some remain unconvinced57–59 as excitatory60 and inhibitory61 neurotransmission persists in animals without ICC-IM.
With the rapid increase in interest in sensory communication within the gut and between the gut and brain axis, our understanding of sensory innervation of the gastrointestinal tract has been the focus of much attention. Since the gastrointestinal tract is the only internal organ that has evolved with its own sensory neurons there is intense interest in understanding how the mechanisms of activation of intrinsic sensory neurons differs from extrinsic sensory nerve endings in the gastrointestinal tract (Fig.3). Also, understanding the relative contribution of intrinsic versus extrinsic sensory nerves to the generation of chemosensory and mechanosensory reflex responses is of supreme relevance to the development of future therapeutic interventions to modify gastrointestinal function.
Fig. 3: Different types of intrinsic sensory neurons and extrinsic sensory nerve endings in the enteric nervous system.
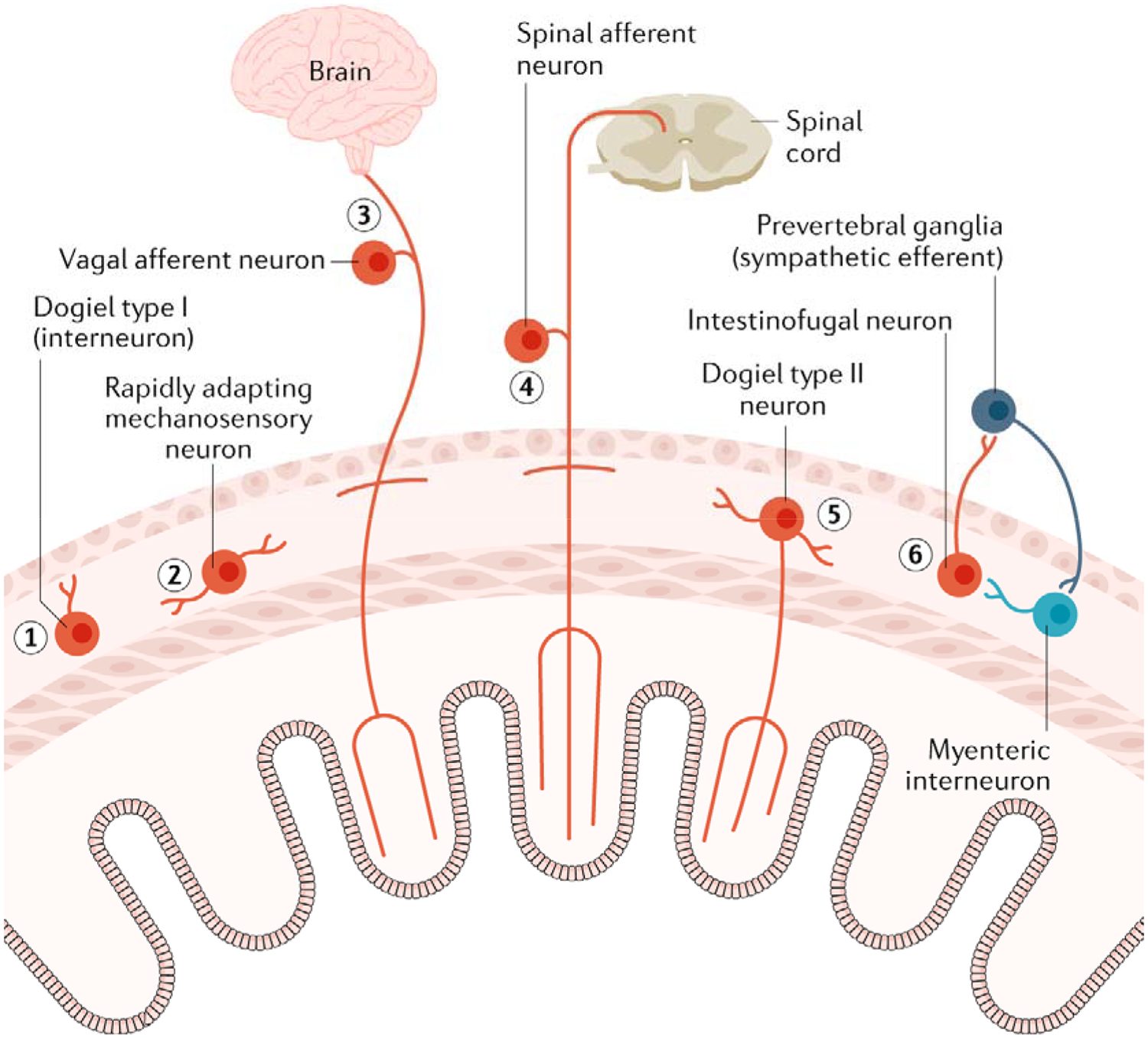
A range of intrinsic sensory neurons and extrinsic sensory nerve endings are known to exist in the enteric nervous system (ENS). (1) Dogiel type I neurons represents a class of myenteric interneuron in the colon that have been identified as being largely length sensitive and tension insensitive. (2) At least two classes of cholinergic and nitrergic myenteric neurons in the myenteric plexus have been demonstrated to be rapidly adapting myenteric excitatory neurons. (3) Extrinsic vagal afferent nerve endings innervate largely the upper gut and behave predominantly as slowly adapting tension receptors. (4) Spinal afferent nerve endings provide a very rich sensory innervation to the lower gut (distal colon) and are potently activated by stretch and increases in muscle tension. (5) Dogiel type II neurons in the myenteric plexus are both chemosensory and mechanosensitive and receive fast and slow synaptic inputs from other enteric neurons. (6) Intestinofugal neurons are usually thought of as second-order neurons but have been shown to be directly mechanosensitive and respond to direct mechanical compression stimuli.
Intrinsic sensory neurons
Mechanotransduction
It was first demonstrated in the mid-1700s that the gastrointestinal tract is unique, in that it can respond to sensory stimuli in vitro1. Other visceral organs such as the bladder, uterus and lungs rely on sensory stimuli being conveyed to the CNS, via spinal or vagal afferents to elicit motor reflexes62.
There have been important advances in our understanding of the intrinsic sensory innervation of gastrointestinal tract. That there exists a population of sensory neurons intrinsic to the gut wall had been suspected for many years. In fact, in the mid-1700s, Von Haller seems to have been the first to identify that the isolated gut could retain an ability to respond to sensory stimuli, despite losing connectivity with the spinal cord. He stated that if he touched the intestines using a knife or corrosives, the intestine responded as if it were still connected to the spinal cord and brain1. This was the first documented evidence that the gut could respond to stimuli without any apparent neural pathways between the gut and central nervous system.1.However, it was not until the first intracellular electrophysiological recordings were made from enteric neurons that evidence emerged for a class of sensory neuron actually within the ENS63,64. It was not until the mid-1990s that compelling evidence was presented that some enteric neurons in guinea-pig small intestine exhibited sensory properties3. These studies initially revealed that neurons with Dogiel type II morphology were indeed responsive to chemical2,14 and mechanical stimuli65. Dogiel type II neurons have distinct characteristics consisting of large cell somas and multipolar processes that ramify extensively within the myenteric plexus66 and have projections both into the mucosa (Table 1)2,14,67 and circumferentially, or aborally along the intestine27,68. The notion that neurons with Dogiel type II morphology could function as mechanosensory neurons was also supported by the work of Mao and colleagues in the mouse small intestine69. They showed during patch recordings from Dogiel type II nerve cell bodies that were immersed in a low Ca2+ solution (to block all synaptic transmission), that these neurons generated bursts of action potentials in response to mechanical compression of neighboring nerve fibre tracts. Their findings provided additional evidence that mechanosensation might be a ubiquitous property of Dogiel type II neurons in the ENS of different species.
Table 1.
Comparison of two major types of enteric neurons.
| Characteristic | Enteric neuron type | |
|---|---|---|
| S neurons | AH neurons | |
| Exhibit prominent Fast EPSPs | Yes | Less common |
| Exhibit slow EPSPs | Yes | Common |
| Projections into the mucosa | Rare or never from the myenteric plexus | Yes, common from the myenteric plexus |
| Mechanosensory properties | Yes | Yes |
| Chemosensory properties | None known | Yes |
| Morphology | Dogiel type 1 | Dogiel type 2 |
| Functional classes | Intrinsic sensory neurons, interneurons and motor neurons | Intrinsic sensory neurons and possibly some interneurons |
Table shows the major differences in the characteristics of Myenteric S neurons and AH neurons in the myenteric plexus. EPSP, excitatory postsynaptic potential.
While it had been thought that Dogiel type II neurons were the only intrinsic sensory neuron in the ENS, research has shown that other populations of enteric neurons with Dogiel type I morphologies can also have mechanosensory properties (Table 1)70–72. Dogiel type I neurons were originally classified as having small to medium sized cell bodies with short, broad, flat dendrites and a single axon73. Later studies identified Dogiel type 1 neurons as predominantly being interneurons or motor neurons14. In mouse ileum, neuronal imaging of the ENS has revealed 22% of myenteric neurons are rapidly-adapting mechanosensitive myenteric neurons (RAMENs)while in the colon they represent 15% of myenteric neurons74. In guinea-pig intestine, 45% of cultured myenteric neurons responded with mechanosensory properties74–76. RAMENs have now been shown to be prevalent in the ENS of a variety of species and these neurons encode dynamic changes in force. This was demonstrated when action potential firing frequency was found to be proportional to the deformation applied to the ganglion in which the cell soma was located74. These neurons were confirmed in the small intestine of mice74, gastric corpus of guinea-pigs76 and human colon75. Notably, strain forces activated all classes of mechanosensitive enteric neurons, whereas shear stress was less effective77. On the basis of these findings, there is sound reasoning that neurons with either Dogiel type I or Type II properties can not only respond to sensory stimuli as primary afferents, but also receive synaptic inputs from other neurons. Indeed, intracellular electrophysiological studies have revealed that a population of Dogiel type I neurons (interneurons) in the myenteric plexus of guinea-pig colon are mechanically sensitive and also receive fast synaptic inputs70. Curiously, there are major differences in the mechanisms of mechanotransduction of Dogiel type II neurons in the guinea-pig small intestine, compared with Dogiel type I neurons in the large intestine of the same species. For example, in the guinea-pig distal colon, Dogiel type I neurons are largely stretch-sensitive, which continue to fire action potentials when smooth muscle tension is reduced or abolished (that is when the muscles are paralysed)70. By contrast, in the small intestine of the guinea-pig, Dogiel type II neurons are sensitive to changes in muscle tension and when the muscles are paralysed, these neurons are markedly less responsive to changes in circumferential length than Dogiel type I neurons65,78. Why the mechanisms of mechanotransduction are so different between the small bowel and colon are not clear, but could be related to the different composition of the luminal contents and the fact that largely liquid is propelled in the small intestine, whereas largely solids are propelled in the distal colon. One of the major unresolved mysteries of Dogiel type II neurons is whether rapid release of substances (like serotonin) from enteroendocrine cells can dynamically activate the terminals of these neurons that project into the mucosa67. There has been an assumption that endogenous serotonin is released from enterochromaffin (EC) cells following mucosal distortion and that this can directly activate the terminals of mucosally-projecting Dogiel type II neurons terminals. Despite it being well accepted that exogenous 5-HT can activate the terminals of Dogiel type II neurons directly79, there is no direct evidence that endogenous 5-HT released from EC cells can rapidly activate their terminals directly (Fig.4). These neurons do form a unique sensory circuit, at least in guinea-pig intestine, that involves preferentially activating ascending excitatory interneurons and excitatory motor neurons80.
Fig. 4: Possible mechanisms underlying the activation of two major classes of intrinsic sensory neuron in the ENS.
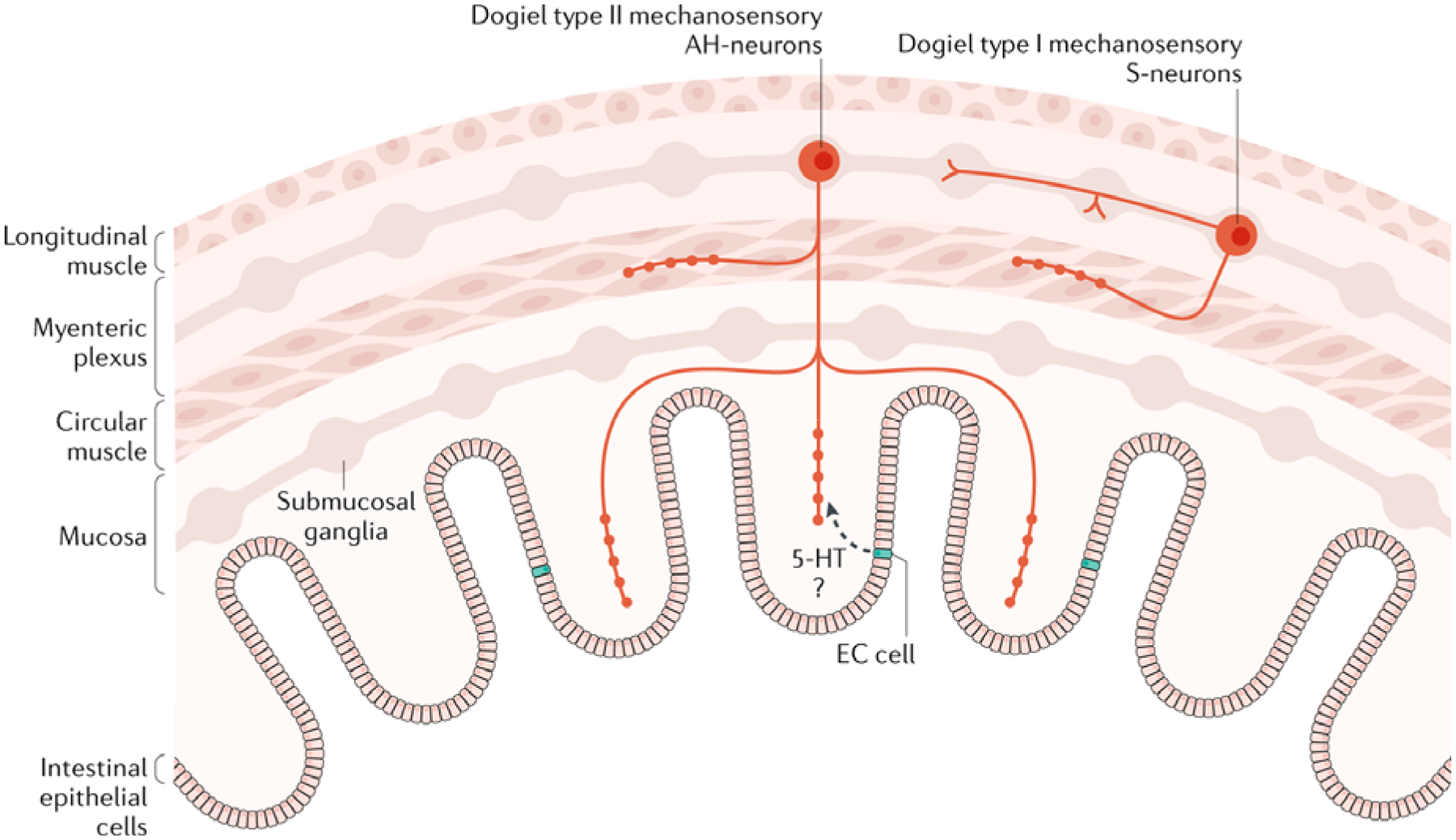
Dogiel type I neurons are mechanosensory and have been functionally identified as interneurons. These neurons also have fine nerve projections into the circular muscle, which could facilitate stretch sensitivity and connectivity with the circular muscle layer. Dogiel type II neurons have projections into the mucosa. They also have been shown to have fine varicose projections into the circular muscle layers. The functional role of enterochromaffin (EC) cells in the physiological activation of the nerve endings of these intrinsic sensory neurons is unknown but serotonin (5-hydroxytryptamine; 5-HT) could have a role.
Our understanding of the mechanisms underlying mechanotransduction of intestinofugal neurons in the ENS has progressed, although their precise functional role in the body remains somewhat mysterious. Intestinofugal neurons are neurons with cell bodies in the gut wall, typically in the myenteric plexus, which have axon projections out of the gut wall to sympathetic neurons in prevertebral ganglia (Fig.2&3)81,82. When activated by mechanical stimulation of the gut, these neurons give off fast synaptic potentials82–85 and sometimes slow86 synaptic potentials in sympathetic neurons. The current belief is that when intestinofugal neurons are activated in the ENS, they probably function to cause increased sympathetic inhibition of gut motility, via noradrenergic neurons in prevertebral ganglia. To support this theory, there is clear experimental evidence that when one segment of colon is distended, extrinsic sympathetic reflex pathways can inhibit neighboring isolated segments of colon in an organ bath87.
In contrast to what is known about intrinsic sensory neurons in the ENS, which directly respond to chemical or mechanical stimuli without any synaptic transmission28,70, intestinofugal neurons in the distal colon81–83,85 and proximal colon84 are largely second order neurons that are activated indirectly (Fig.3), by fast synaptic inputs from other enteric neurons that do respond directly to sensory stimuli81–85. However, in both regions of bowel, a population of intestinofugal neurons seem to be directly responsive to mechanical stimuli, since blockade of synaptic transmission in the colon does not prevent distension-activated firing of these neurons83,84. It is known that intestinofugal neurons, at least in the distal colon of laboratory animals, are potently activated by changes in circumferential stretch, but do not respond to longitudinal stretch88. Curiously, this finding is in direct contrast to the mechanotransduction of spinal afferent endings in the colon (whose cell bodies lie outside the gut wall, in dorsal root ganglion (DRG)), which are potently activated by both circumferential and longitudinal stretch89. At present, what is known about mechanosensation of intestinofugal neurons in the colon88,90 is similar to our current knowledge of intrinsic sensory neurons in the colon, in so much as is they are both largely insensitive to changes in muscle tension71,88. That is, paralysis of smooth muscle contraction and reducing muscle tone or tension in the colon of laboratory animals has no detectable effect on stretch-activated firing of either class of enteric neuron70,82,88. In addition, intestinofugal neurons actually decrease their firing during muscle contraction90,91. Miller and Szurszewski suggested that intestinofugal neurons respond primarily to changes in length, rather than as tension receptors; and function to monitor changes in intracolonic volume91. This hypothesis was based on their findings that clearly demonstrated that intestinofugal neurons increased their firing with increases in circumferential length; and decrease firing with contraction, that is when intraluminal volume decreases. Again, this aspect is vastly different from spinal afferent nerve endings that innervate the same region of colon, which show increased firing of action potentials with increases in muscle tension (under either isotonic92 or isometric conditions92–94. The ionic mechanisms underlying the transduction of mechanical and chemical stimuli of intrinsic sensory neurons in the ENS are not well understood. It is known that mechanotransduction of spinal afferent endings is very rapid involving direct mechano-gated channels and does not require exocytotic release of transmitters, since mechanotransduction persists in zero calcium solution93,95, and is not blocked by conventional stretch-activated ion channel or transient receptor potential (TRP) channel blockers95. This aspect suggests that stretch-activated ion channels involve direct physical mechanotransduction. Whether the Piezo ion channel family are involved, as they are for direct mechanotransduction of somatic afferents in skin96 awaits further study. Intestinofugal neurons can also be directly mechanically-sensitive and respond to Von Frey hair probing (Fig.3)97, confirming early suggestions that a population of these neurons can indeed be directly mechanosensitive83,84. The level to which these neurons participate in dynamic control of gastrointestinal motility remains unclear in vivo.
Generation of neurogenic motor patterns
There have been some important advances in our understanding of the intrinsic neuronal mechanisms and pathways that underlie distension-evoked peristalsis. Originally, in the 1950s, work from Edith Büllbring’s laboratory suggested that the release of 5-HT from the mucosa was important for distension-evoked peristalsis to occur, findings that were largely based on the observation that endogenous 5-HT was released when peristalsis occurred and that exogenous 5-HT could also elicit peristalsis98–100. Indeed, since then, others had also proposed that endogenous 5-HT release from the mucosa had an essential role in the generation of colonic peristalsis101,102 or colonic migrating motor complexes (CMMCs)103. Since 5-HT antagonists also could block these motor patterns in the colon of laboratory animals101,102, there seemed to be a compelling case that endogenous 5-HT was important for distension-evoked peristalsis to occur. This notion was contradicted in the past decade when a selective small molecule inhibitor of the enzyme tryptophan hydroxylase-1 (TPH-1) was given to conscious mice to selectively block synthesis of mucosal 5-HT104. This drug was found to have no effect on gastric emptying, colonic transit or overall gastrointestinal-transit times, raising doubts about earlier suggestions that mucosal 5-HT was an essential player in gastrointestinal motility104. In support of these findings, work by105 showed that in conscious mice genetic ablation of the gene Tph1 also had no changes in gastrointestinal transit in vivo105 and CMMCs still occurred15,106,107. Real-time amperometric recordings of 5-HT release have provided powerful insights into the role of mucosal 5-HT release. Studies showed that release of endogenous 5-HT that occurred at the same time as peristalsis or CMMCs was actually a consequence of the contraction underlying peristalsis108 and not the underlying cause of peristalsis or CMMCs109.
Although pharmacological or genetic blockage of mucosal 5-HT synthesis does not reduce gastrointestinal transit in vivo, studies have shown that inhibitors of serotonin reuptake cause a reduction in the threshold to elicit propulsive contractions in the small intestine of guinea-pigs110. It was concluded that background release of endogenous 5-HT from the mucosa alone is inadequate to modify the threshold for propulsive motor activity. However, if exogenous 5-HT was applied to the lumen, or there was increased release of endogenous 5-HT this was found to lower the threshold of propulsive contractions in the small bowel of guinea-pigs, which was mediated by 5-HT3 and 5-HT4 receptors110. Indeed, others had shown some time ago that mucosally-applied 5-HT reduces the threshold for peristalsis in the small intestine of guinea-pigs, that is likely mediated by the 5-HT3 receptor located on the mucosal, but not serosal surface of the bowel111. This aspect was determined when 5-HT3 antagonists affected peristaltic threshold when luminally applied, but not serosally-applied111. These findings supported earlier studies that showed distension-evoked peristalsis and the propulsion of natural fecal content did not cease when the mucosa was removed and all dynamic release of endogenous 5-HT from the mucosa was prevented112, leading to the conclusion that the mechanotransduction process underlying distension-evoked colonic peristalsis by natural fecal pellets required only the myenteric plexus and smooth muscle layers112,113. Similar results were obtained in the small intestine, where peristalsis was still shown to occur in intact isolated preparations where the mucosa and submucosa had been dissected away114. Taken together, these findings led to the inescapable conclusion that distension-evoked peristalsis involved an intrinsic neural reflex circuit in the myenteric plexus, whose stretch receptors must have been located outside the mucosa. The only remaining possibility was that the distension-evoked circuit lay in the myenteric plexus114. Further studies in the colon showed that the mechanosensory transduction process required for activation of distension-evoked ascending excitatory and descending inhibitory nerve pathways (at least in the colon) was critically dependent upon connectivity between the circular muscle and myenteric ganglia115;. Removal of the circular muscle prevented distension-evoked ascending excitatory and descending inhibitory pathways, but removal of the longitudinal muscle from the myenteric plexus did not reduce mechanotransduction115. In addition, removal of the circular muscle from the myenteric plexus abolished the vast majority of ongoing fast synaptic potentials in the ENS of the distal colon116, but removal of the longitudinal muscle did not70. One possibility is that intrinsic sensory neurons in the colon have fine nerve projections that lie in the circular muscle layer that are length-sensitive and these are destroyed when the circular layer is removed. Indeed, both Dogiel type II neurons70,117 and mechanosensitive Dogiel type I neurons have been shown to have fine processes in the circular muscle70.
Compelling evidence exists that populations of enteroendocrine cells in the gut are mechanosensitive and can release high quantities of 5-HT108,118,119. However, evidence from various laboratories has shown that this release of 5-HT is not required to initiate distension-evoked peristalsis, nor does it seem to be required for normal orderly transit of content in vivo17,104,105,113. There is no doubt that large quantities of 5-HT can be released into the blood stream in response to distension (much of which is taken up by platelets120). However, the final concentration of 5-HT that reaches the target effector cells is unclear. A major focus of future research will be to unravel the functional role of enteroendocrine cells in living animals. Indeed, there is evidence for major changes in expression of the serotonin reuptake transporter and increased expression of mucosal 5-HT in mammals with inflammatory bowel disease and it is possible that endogenous 5-HT is largely responsible for the changes in ENS excitability only in disease states121,122.
Direct recordings from isolated enterochromaffin (EC) cells revealed they are indeed electrically excitable119,123–125 and express major ion channels for the generation of action potentials108,118,119,124,125. This expression includes L-Type Ca2+ channels, which are essential for contraction or distension-evoked release of 5-HT108,118.
The identification of the mechanosensitive ion channels that underlie distension-evoked neurogenic motor patterns in the gut, such as peristalsis, is of major interest, in particular the Piezo family of mechano-gated ion channels and their role in stretch-evoked reflexes in visceral organs such as the gut126,127. A study published in 2018 has shown that in guinea-pig intestine, the mechanosensitive ion channel Piezo 1 is expressed in enteric nitrergic and some cholinergic enteric neurons, whereas Piezo 2 was rarely expressed in any enteric neurons128. We wait with excitement to learn if Piezo channels are involved in the stretch-activation of peristalsis. Interestingly, (EC cells have been shown to express the mechanosensitive ion channel Piezo 2, which is located in close apposition to vesicles containing serotonin119. These studies also showed that mechanical sensitivity of a subset of isolated EC cells relies on Piezo 2 ion channels125. This finding could be important because this channel has emerged as a major player in mechanotransduction in Merkel cells in skin to modulate the conversion of touch into itch sensation96. Also, studies suggest that some population of enteroendocrine cells also make synaptic-like connections with vagal afferent endings129, very much like the Merkel cell-neurite complex in the skin. It was concluded from these studies that synaptically released glutamate is used by an epithelial sensor cell in the gut to rapidly transduce luminal stimuli to the CNS129.
There are major differences in mechanotransduction processes that convert mechanical distension of the small intestine and colon into neurogenic motor patterns. In the small intestine, the mechanisms underlying distension-evoked peristalsis require muscle tone or tension in the smooth muscles130. This is supported by direct recordings from intrinsic sensory neurons in the small intestine that display substantially reduced stretch-sensitivity following muscle paralysis78 (discussed earlier). By contrast, in the colon, paralysis of tone or tension in the smooth muscle has little or no effect on distension-evoked peristaltic reflex pathways131,132, nor the propagation of peristalsis133. This finding means that the sensory neurons underlying the activation of colonic peristalsis are primarily length-sensitive, rather than muscle tension or tone-sensitive. The precise ionic transduction process by which mechanical stretch activates enteric neurons in the colon to elicit peristalsis or CMMCs is not fully understood.
ENS circuits and motility patterns
Chemotransduction
Although endogenous 5-HT release from the mucosa is not required for normal gastrointestinal motility or transit in healthy bowel, there is evidence that endogenous 5-HT release from the mucosa can modulate ENS activity and gastrointestinal motor patterns, via particular chemosensory stimulants applied to the lumen134. For example, in the isolated small intestine, studies have suggested that endogenous 5-HT modulates activity in intrinsic neural circuits that underlies nutrient-induced segmental contractions134. Segmental contrations are different from classic peristaltic contractions in that segmental contractions have been long proposed to involve myogenic electrical rhythmicity in the smooth muscle and consist of135. In contrast, peristalsis is critically dependent upon the ENS and cannot occur when the ENS activity is absent. Interestingly, studies have shown that segmental contractions that are induced by nutrients, at least in guinea-pig small intestine, can occur when slow waves and electrical rhythmicity are not recorded136. This finding raises serious doubts about whether myogenic rhythmicity in the smooth muscle is in fact a prerequisite for segmentation to occur136. Evidence supporting the notion that serotonin and CCK released from the mucosa may be involved in segmentation was provided when nutrient induced segmentation (induced by decanoic acid) was found to be depressed when the 5-HT receptor antagonists (granisetron and SB-207266) and CCK antagonist, devazepide were applied to the lumen. It was proposed that decanoic acid activated a novel intrinsic pathway in which segmentation involves 5-HT3 and 5-HT4 receptors and CCK-1 and CCK-2 receptors134.
Potent enterotoxins, such as cholera toxin, can also potently modify gastrointestinal motility by stimulating secretomotor neurons137, leading to the release of 5-HT from enteroendocrine cells137,138. Most commonly, cholera toxin causes diarrhoea by increasing secretion of water into the intestinal lumen139. Studies have shown that cholera toxin administered into the lumen of the jejunum ex vivo can have opposing effects on distinct motor patterns. For example, in organ bath experiments using isolated segments of guinea-pig small intestine, cholera has been reported to enhance propulsive contractile activity, in the same preparation it can also suppress nutrient-induced140. It was also proposed that cholera toxin is able to stimulate more than one intrinsic neural pathway in the ENS and that the segmenting pattern and propulsive motor patterns are differentially regulated. Support for the notion that ENS activity can be modulated by agents such as cholera exposed to the lumen comes from direct intracellular recordings that have shown increased excitability of myenteric Dogiel type II neurons141, which was proposed to occur via a mechanism that involved NK3 receptors but not 5-HT3 receptors141. A particularly interesting finding was reported when cholera toxin was infused into the colon and effects on motility compared not only between male and female mice, but also between the stages of oestrus cycle in female mice. It was found that cholera toxin immediately and reversibly inhibited CMMCs in female mice that were in the oestrus stage of cycle, but not in female mice in pro-oestrus, or in male mice. The inhibitory effects of cholera toxin on CMMC frequency seemed to be mediated by the 5-HT3 receptor and EC cells, since these effects of cholera toxin were absent from female mice in which the enzyme TPH1 was genetically ablated. These investigators showed that the number of EC cells that contain 5-HT was approximately 30% higher in female mice during the oestrus stage than with female mice in the pro-oestrus stage, or in male mice15. Taken together, while mutation of the gene Tph1 that synthesizes mucosal 5-HT does not lead to changes in gastrointestinal transit in vivo in mice, there is still evidence that chemosensory activation of the mucosa can lead to changes in ENS circuits via 5-HT receptors and possibly EC cells. In support of the notion that intrinsic sensory neurons can also respond to chemosensory stimuli, direct recordings from Dogiel type II neurons have shown that these neurons do respond to electrical stimuli of the mucosa142 or chemical stimulation of the mucosa with 5-HT or acetate79.
Neurotransmitters in the ENS
Despite the large number of different neurochemicals synthesized in the different classes of enteric neurons26, most neurochemicals shown to be expressed and synthesized in the ENS have not been demonstrated to behave as neurotransmitters. It is well established that the major excitatory neurotransmitter underlying enteric neuro-neuronal (a synaptic junction between two neurons) transmission is acetylcholine acting on nicotinic receptors on enteric neurons63,64. In most species, blockade of nicotinic transmission abolishes all fast synaptic neurotransmission17,143,144. In a smaller population of neurons, there is evidence that after nicotinic receptor blockade, adenosine triphosphate (ATP) acts on P2X receptors and serotonin acts on 5-HT3 receptors to elicit fast excitatory postsynaptic potentials145. Interestingly, while glutamate is a major fast excitatory neurotransmitter in the CNS146, evidence for glutamate as a neurotransmitter in the ENS is poor147. Indeed, glutamatergic receptors are expressed in some enteric neurons, but electrophysiological studies have found the evidence for glutamate as a neurotransmitter in the ENS is weak or absent148. Interestingly, a recent study using calcium imaging from myenteric neurons in mouse colon suggested that endogenous glutamate may mediate part of the slow EPSP, particularly in neurons that express calbindin149.
In addition to fast excitatory postsynaptic potential (EPSPs) in the ENS147, there is sound evidence for the existence of slow EPSPs in myenteric neurons. ATP has been shown to mediate slow EPSPs via P2Y1 receptors and there is also compelling evidence that tachykinins can mediate slow EPSPs in myenteric neurons via the NK1 receptor150–153. In the submucosal plexus, there is evidence that acetylcholine, ATP and 5-HT mediate fast synaptic transmission mediated by nicotinic, P2X and 5-HT3 receptors152. In addition, ATP can mediate an intermediate and slow EPSP in some submucosal neurons152 and 5-HT can also mediate some slow EPSPs in myenteric neurons via an action on the 5-HT7 receptor154.
Interestingly, in addition to a role for ATP as an excitatory enteric neuroneuronal transmitter in the ENS, ATP can mediate the fast inhibitory junction potential at the neuromuscular junction in gastrointestinal smooth muscle155. It has been argued that β-nicotinamide adenine dinucleotide (β-NAD) is the inhibitory neurotransmitter instead of ATP at neuromuscular junctions in gastrointestinal smooth muscle156,157. However, this stance has not been accepted by all groups and other explanations have been proposed158,159. For example, others have not been able to find evidence for a physiological role of β-NAD acting on receptors on the smooth muscle to cause inhibitory neurotransmission158. Also, others have demonstrated credible evidence that β-NAD can act prejunctionally by acting on adenosine A1 receptors to suppress the release of neurotransmitters at neuromuscular junctions; and this could account for reduced inhibitory neuromuscular transmission158. Hence, the notion that β-NAD is an inhibitory neurotransmitter is equivocal.
Compelling evidence indicates that vasoactive intestinal peptide (VIP) is a major inhibitory neurotransmitter released from secretomotor neurons160,161. Indeed, evidence suggests that 5-HT1A, SST1 and SST2 receptors mediate nonadrenergic inhibitory postsynaptic potentials (IPSPs) in the noncholinergic (VIP) secretomotor neurons162. GABAc receptors can also modulate synaptic transmission in the myenteric plexus, which was demonstrated when calcium transients elicited by brief trains of electrical stimuli were actually enhanced by antagonists of GABAc receptors in isolated mouse small intestine163. At present, there is no direct evidence that endogenous GABA is released following electrical stimulation of the ENS to elicit a synaptic potential in myenteric neurons. Interestingly, there is evidence that approximately one third of cell bodies in the myenteric plexus that are immunoreactive to GABA are also cholinergic. However, less than 5% of cholinergic neurons also contain GABA164.
ENS circuitry during neurogenic motility patterns
Although it is well accepted that activity from the ENS is required for the generation of neurogenic motility patterns in the small intestine165 and colon5,11,102,130,133,166–168, one of the great mysteries of the ENS has been how such a large population of individual neurons are temporally activated over large distances to cause the propulsion of content. Understanding how the ENS is physiologically activated as an intact neural network during healthy conditions is essential if we are to fully understand the aetiology of neurogenic disorders of the ENS. Important advances have been made in this area.
It has been found that there is a distinct clustering of clonally related enteric neurons in the ENS. This important observation led to the suggestion that in the small intestine the ENS develops from postmigratory neural crest-derived progenitors which form overlapping clonal units169. A major finding of this study was that it revealed that enteric neurons that are clonally-related generate synchronous activity following local stimulation of internodal strands. This suggests the presence of an underlying hard-wired circuit, such that large populations of enteric neurons can be synaptically activated at the same time (Fig.5). Consistent with this discovery, another study showed that during neurogenic CMMCs that propagated along the colon, the ENS generated a highly organized, rhythmic firing pattern that involved the simultaneous synaptic recruitment of many thousands of myenteric inhibitory and excitatory neurons at the same time170. This fundamental polarized neural circuit involved the convergence and synaptic recruitment of ascending and descending interneurons (Fig.5). This firing pattern occurred at ~2Hz that led to rhythmic depolarisations (EJPs) and hyperpolarisations (IJPs) in the smooth muscle at the same rate170. As the faecal content had propelled along the bowel, past the site of imaging, these neurons became temporally less correlated into a ‘desynchronized state’17,170. Another study using neuronal imaging and tracing from the large intestine have shown topographic differences in the enteric neuronal wiring patterns between the proximal and distal colon171. An ascending inhibitory myenteric pathway exists in the proximal colon, which was driven by nicotinic transmission. Interestingly, this phenomenon was not identified in the distal colon171. Taken together, there is sound evidence for local changes in the neuronal circuits along the full length of colon which may be related to the differences in motility requirements of these different regions.
Fig. 5: Major intrinsic neuronal circuits in the large intestine active during neurogenic motor patterns.
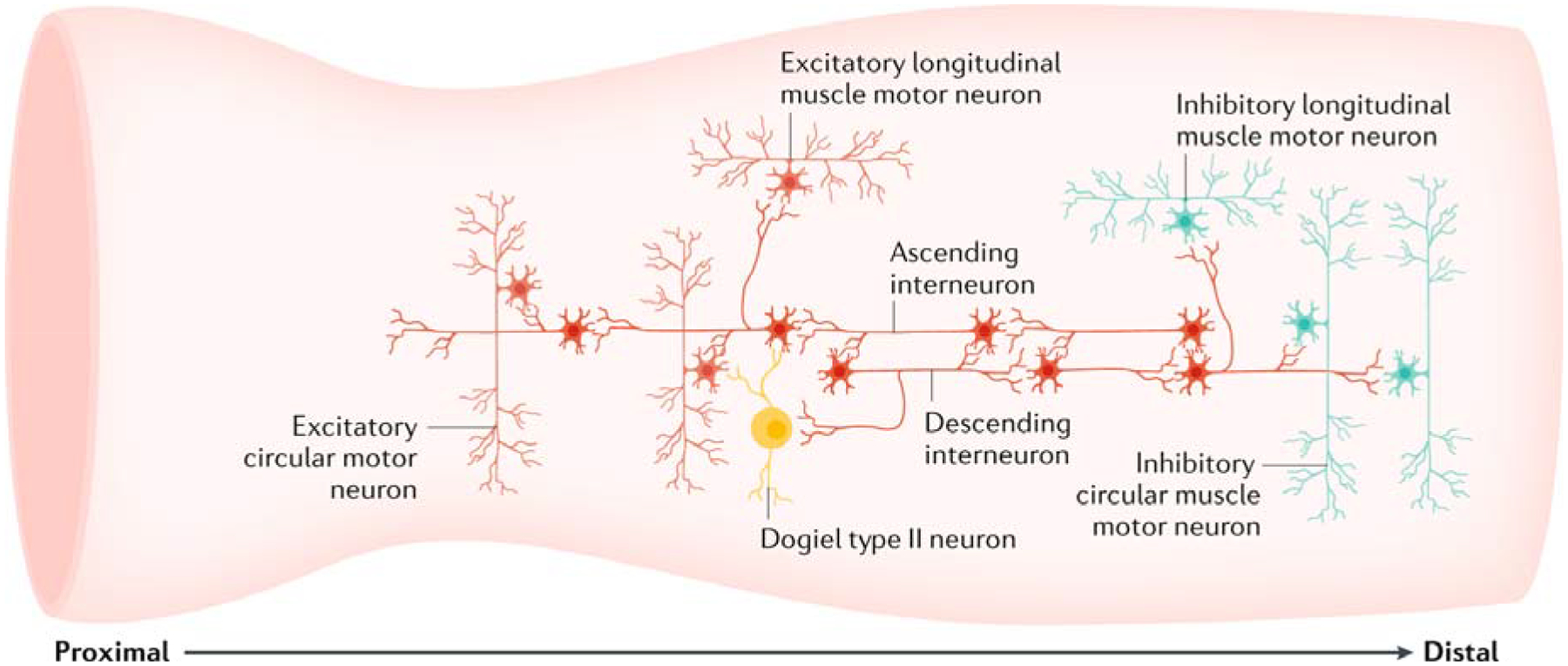
A fundamental pathway involves the synaptic convergence of ascending and descending interneurons, such that increased activation of ascending pathways leads to a corresponding increased activation of descending inhibitory pathways. Furthermore, common ascending interneurons simultaneously activate independent populations of excitatory motor neurons to the longitudinal muscle and circular muscle orally; at the same time, common descending interneurons simultaneously activate separate populations of inhibitory motor neurons to both the longitudinal muscle and circular muscle aborally. Thus, temporally coordinated firing of ascending and descending interneurons simultaneously activates cholinergic excitatory motor neurons and nitrergic inhibitory motor neurons. These pathways underlie major neurogenic motility patterns in the large intestine. In the colon, calbindin-immunoreactive Dogiel type II neurons project to the mucosa and receive nicotinic inputs from some populations of myenteric interneurons. The Dogiel type II neurons (in the colon) preferentially provide synaptic outputs to local cholinergic excitatory motor neurons and cholinergic ascending interneurons, and rarely or never to nitrergic neurons80.
Emerging technologies to study ENS neural circuits
Optogenetics was first developed about 15 years and is an exciting technique to control the excitability of particular cells of interest172,173. However, only in the past 5 years or so has there been a rapid increase in the use of this technique with high fidelity. The basic principle of optogenetics is that light-sensitive ion channels are genetically encoded into specific cells of interest. This means that it is possible to excite or inhibit cells (e.g. neurons) of interest using different wavelengths of light, provided the cells of interest express the excitatory or inhibitory light-sensitive ion channels. One of the most exciting benefits of using optogenetics is the ability to selectively stimulate or inhibit particular neurochemical classes of neurons. This was not possible with electrical stimulation of the ENS. Indeed, this benefit is highly relevant to studying the ENS, because one of the major obstacles to study ENS neural circuits is that the ENS contains a highly heterogeneous population of neurons that are difficult to be selectively targeted with conventional electrophysiological and imaging techniques. Advances in optogenetic techniques have provided unique tools for in vivo stimulation or inhibition of neuronal electrical activity, which is an efficient way to probe causal relationships between specific neuronal cells and behavior172,173. Optogenetic techniques have been extensively utilized in the CNS for a number of years174. But, only in the past 3 years has the field of optogenetics been demonstrated to control the excitability of the ENS17,34,175–177. To date, there have been two studies that have successfully demonstrated optogenetic technologies can potently control activity in the ENS34,175 and induce propagating neurogenic contractions in mice175.
One of the most exciting aspects of applying optogenetic technology to the ENS is the conspicuous advantage of being able to selectively stimulate or inhibit specific classes of neurons that lie only within the ENS. This can occur using cre/lox recombination technology which involves site specific recombination of light-sensitive ion channels (like Channelrhodopsin, ChR2) into specific neurochemical classes of enteric neurons17,175–177. This advance has been demonstrated using wireless optogenetic approaches whereby excitatory neurons were activated with micro-LEDs implanted onto the colon wall in vivo in mice, to cause an increased transit of colonic content in conscious freely-moving animals175,178.
There are some advantages and some disadvantages of using optogenetic tools to stimulate gastrointestinal transit in live animals (Box 1). The major advantages of using wireless optogenetics to stimulate gastrointestinal motility in vivo, as opposed to current orally-ingested agonists to stimulate the ENS (for example, 5-HT agonists) are that: focally applied light can be delivered instantaneously to target specific regions of gut, without requiring oral consumption of non-specific agonists that inadvertently stimulate other neural pathways in central or peripheral ganglia (Box 1). In in vitro experimentation using transgenic mouse models or Cre-inducible viruses, optogenetic approaches could provide a major advantage of being able to control ENS excitability via either excitatory or inhibitory light-sensitive ion channels, in specific neurochemical populations of neurons. This technical advance is a major step forward in the field. This approach would be impossible using conventional pharmacological agents, that do not act on only one class of neuron.
Box 1. Pros and cons of optogenetics to control the ENS.
Advantages
Allows selective expression of light-sensitive ion channels in specific classes of neurons
Light can instantaneously activate nerve pathways of interest
No oral ingestion of agonists required to selectively stimulate specific populations of neurons
Can identify the functional role of specific neural circuits
Disadvantages
Light delivery generates heat, which can alter neuronal function
Requires genetic modification of cells
Takes many weeks to express light-sensitive ion channels
Low yield of expression can occur using viruses
-
Not clear how long opsins can retain expression in vivo
ENS, enteric nervous system.
There are also some obvious downfalls using optogenetic techniques to control the ENS in live animals. Firstly, light-sensitive opsins must be expressed in the ENS. This step requires either the injection of viral vectors into the host animal, or the breeding of transgenic animals together. In either case, it involves genetically modifying specific cells of interest in the ENS. A second major disadvantage is that light is required to stimulate enteric neurons. Prolonged light stimulation can raise the temperature of the target organ, which has been shown to clearly alter function179.
A particularly exciting aspect of optogenetic technology is that it has now been demonstrated that it is possible to express sufficient levels of channelrhodopsin 2 (ChR2) in sensory neurons of non-transgenic vertebrates180, which was demonstrated using adeno-associated viruses that can be transported retrograde along nerve axons and effectively express ChR2 into nerve cell bodies. Conceptually, it could be possible to control gut motility and transit in conscious non-transgenic mammals using wireless optogenetics after injection of adeno-associated viruses into the gut wall. However, to do so would require overcoming the technical challenge of implanting wireless micro-LEDs onto the gut wall and ensuring that sufficient levels of ChR2 are expressed in the particular classes of neurons of interest. This approach would almost certainly have to involve surgery and the pros and cons would need to be carefully evaluated before any procedure(s) were undertaken.
Conclusions
This Review has discussed the current advances that have been made in the transduction of sensory stimuli into action potentials in intrinsic and extrinsic sensory neurons in the small and large intestine of vertebrates. There is substantial interest in improving our understanding the basis of gastrointestinal motility disorders and transit in a variety of conditions, such as slow-transit or idiopathic constipation, irritable bowel syndrome, or as occurs in inflammatory bowel disease. However, before we can hope to try to explain the causes of disordered neurogenic motility and transit in diseased or dysfunctional bowel, it is important that we first have a clear understanding of the fundamental control mechanisms underlying these neurogenic motor patterns in healthy bowel. This aspect has not yet been achieved. With the rapid improvements and advances in optogenetic techniques, it will be exciting to use these approaches to control the excitability of specific classes of neurons in the ENS to careful dissect their functional roles.
Key Points.
In vertebrates, the enteric nervous system (ENS) is critical for gastrointestinal function
There has been much progress in understanding the mechanisms by which mechanical or chemical stimulation of the gut is converted into neural activity with the ENS and propulsive motility.
Mechanosensory elements critical for distension-evoked colonic peristalsis have been identified to lie in the myenteric plexus and/or circular muscle of the gastrointestinal tract and do not require the mucosa or submucosal plexus.
Evidence suggests that substances released from cells in the mucosa (such as enteroendocrine cells) can modulate ENS activity, but release of mediators like serotonin are not required for distension-evoked peristalsis nor colonic migrating motor complexes.
Fundamental differences have been revealed in the mechanisms of activation of extrinsic spinal afferent nerves compared with intrinsic sensory nerves in the same region of bowel.
Recent refinements in optogenetic technologies can now permit stimulation of specific neurochemical classes of neurons in the ENS to elucidate function.
Acknowledgements
H.H. was supported by grants from the NIH, R01GM101218, R01DK103901 and R01AA027065, Washington University School of Medicine Digestive Disease Research Core Center (NIDDK P30 DK052574), The Center for the Study of Itch of Department of Anesthesiology at Washington University School of Medicine. N.J.S. is supported by NH&MRC of Australia, grants APP1156427 and APP1156416.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Von Haller A A Dissertation on the Sensible and Irritable Parts of Animals, by. Bulletin of the Institute of the History of Medicine, 651–699 (1755). [Google Scholar]
- 2.Kunze WA, Bornstein JC & Furness JB Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience 66, 1–4 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Furness JB, Johnson PJ, Pompolo S & Bornstein JC Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil 7, 89–96 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Trendelenburg P hysiologische und pharmakologischeUntersuchungen über die Dünndarmperistalti. Arch ExpPathol Pharmakol 81, 55–129 (1917). [Google Scholar]
- 5.Wood JD Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am J Dig Dis 18, 477–488 (1973). [DOI] [PubMed] [Google Scholar]
- 6.Costa M & Furness JB The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol 294, 47–60 (1976). [DOI] [PubMed] [Google Scholar]
- 7.Lüderitz C Experimentelle Untersuchungen uber die Entstehung der Dam-peristaltik. Virchows Arch. f. path. Anat 122, 1–28 (1890). [Google Scholar]
- 8.Lüderitz C Das motorische Verhalten des Magens bei Reizung seiner ausseren Flache. Arch. f. d. ges. Physiol 49, 158–174 (1891). [Google Scholar]
- 9.Bayliss WM & Starling EH The movements and innervation of the small intestine. J Physiol 24, 99–143 (1899). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartho L, Holzer P, Donnerer J & Lembeck F Evidence for the involvement of substance P in the atropine-resistant peristalsis of the guinea-pig ileum. Neurosci Lett 32, 69–74 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Smith TK & Robertson WJ Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J Physiol 506 (Pt 2), 563–577 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer NJ & Smith TK Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533, 787–799, doi:PHY_11847 [pii] (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonini M et al. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology 129, 1557–1566, doi: 10.1053/j.gastro.2005.08.005 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Furness JB The enteric nervous system. Blackwell Publishing, Oxford, U.K: (2006). [Google Scholar]
- 15.Balasuriya GK, Hill-Yardin EL, Gershon MD & Bornstein JC A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J Physiol 594, 4325–4338, doi: 10.1113/JP272071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer NJ, Dinning PG, Brookes SJ & Costa M Insights into the mechanisms underlying colonic motor patterns. J Physiol 594, 4099–4116, doi: 10.1113/JP271919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H & Spencer NJ in Physiology of the Gastrointestinal tract. 6th ed Vol. 1 (ed Said HM) Ch. 14, 629–669 (Elsevier/Academic Press, 2018). [Google Scholar]
- 18.Kuizenga MH et al. Neurally mediated propagating discrete clustered contractions superimposed on myogenic ripples in ex vivo segments of human ileum. Am J Physiol Gastrointest Liver Physiol 308, G1–G11, doi: 10.1152/ajpgi.00230.2014 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Spencer NJ et al. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am J Physiol Gastrointest Liver Physiol 302, G34–43, doi: 10.1152/ajpgi.00319.2011 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Weakly JN Similarites in synaptic efficacy along multiply innervated twich muscle fibers of the frog: a possible muscle-to-motoneuron interaction. Brain Res 158, 235–239 (1978). [DOI] [PubMed] [Google Scholar]
- 21.Bennett MR, Burnstock G & Holman ME Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol 182, 527–540 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulbring E & Tomita T Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol 189, 299–315 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furness JB Types of neurons in the enteric nervous system. J Auton Nerv Syst 81, 87–96 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Brookes SJ Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262, 58–70 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Costa M, Furness JB & Gibbins IL Chemical coding of enteric neurons. Prog Brain Res 68, 217–239 (1986). [DOI] [PubMed] [Google Scholar]
- 26.Costa M et al. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75, 949–967 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Brookes SJ, Song ZM, Ramsay GA & Costa M Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. J Neurosci 15, 4013–4022 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furness JB, Kunze WA, Bertrand PP, Clerc N & Bornstein JC Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54, 1–18, doi:S0301–0082(97)00051–8 [pii] (1998). [DOI] [PubMed] [Google Scholar]
- 29.Ro S, Hwang SJ, Muto M, Jewett WK & Spencer NJ Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am J Physiol Gastrointest Liver Physiol 290, G710–718, doi:00420.2005 [pii] 10.1152/ajpgi.00420.2005 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Burnett AL D. NA. The Nervous System of Hydra. I. Types, distribution and origin of nerve elements. J Exp Zool. 157, 217–226. (1964). [DOI] [PubMed] [Google Scholar]
- 31.Murillo-Rincon AP et al. Spontaneous body contractions are modulated by the microbiome of Hydra. Sci Rep 7, 15937, doi: 10.1038/s41598-017-16191-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obermayr F, Hotta R, Enomoto H & Young HM Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol 10, 43–57, doi: 10.1038/nrgastro.2012.234 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Young HM & McKeown SJ Motility: Hirschsprung disease--laying down a suitable path. Nat Rev Gastroenterol Hepatol 13, 7–8, doi: 10.1038/nrgastro.2015.213 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Stamp LA et al. Optogenetic Demonstration of Functional Innervation of Mouse Colon by Neurons Derived From Transplanted Neural Cells. Gastroenterology 152, 1407–1418, doi: 10.1053/j.gastro.2017.01.005 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Hotta R et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest 123, 1182–1191, doi: 10.1172/JCI65963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetz S et al. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One 9, e93605, doi: 10.1371/journal.pone.0093605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper JE et al. In Vivo Transplantation of Enteric Neural Crest Cells into Mouse Gut; Engraftment, Functional Integration and Long-Term Safety. PLoS One 11, e0147989, doi: 10.1371/journal.pone.0147989 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger M, Caldwell C, Barlow AJ, Burns AJ & Thapar N Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology 136, 2214–2225 e2211–2213, doi: 10.1053/j.gastro.2009.02.048 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa R et al. Migration and differentiation of transplanted enteric neural crest-derived cells in murine model of Hirschsprung’s disease. Cytotechnology 67, 661–670, doi: 10.1007/s10616-014-9754-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fattahi F et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105–109, doi: 10.1038/nature16951 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCann CJ et al. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat Commun 8, 15937, doi: 10.1038/ncomms15937 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham TD, Gershon MD & Rothman TP Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol 314, 789–798, doi: 10.1002/cne.903140411 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni S et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A 114, E3709–E3718, doi: 10.1073/pnas.1619406114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corpening JC, Cantrell VA, Deal KK & Southard-Smith EM A Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev Dyn 237, 1119–1132, doi: 10.1002/dvdy.21498 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianino S, Grider JR, Cresswell J, Enomoto H & Heuckeroth RO GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130, 2187–2198 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Furness JB, Kuramoto H & Messenger JP Morphological and chemical identification of neurons that project from the colon to the inferior mesenteric ganglia in the guinea-pig. J Auton Nerv Syst 31, 203–210, doi: 10.1016/0165-1838(90)90186-m (1990). [DOI] [PubMed] [Google Scholar]
- 47.Rao M & Gershon MD Neurogastroenterology: The dynamic cycle of life in the enteric nervous system. Nat Rev Gastroenterol Hepatol 14, 453–454, doi: 10.1038/nrgastro.2017.85 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Dickens EJ, Hirst GD & Tomita T Identification of rhythmically active cells in guinea-pig stomach. Journal of Physiology 514, 515–531 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A & Huizinga JD Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114, 724–736 (1998). [DOI] [PubMed] [Google Scholar]
- 50.Ward SM, Burns AJ, Torihashi S & Sanders KM Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480 (Pt 1), 91–97 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huizinga JD et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349, doi: 10.1038/373347a0 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Ward SM et al. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology 117, 584–594 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Gershon MD Lessons from genetically engineered animal models. II. Disorders of enteric neuronal development: insights from transgenic mice. Am J Physiol 277, G262–267 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Spencer NJ, Sanders KM & Smith TK Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol 553, 881–893, doi: 10.1113/jphysiol.2003.049700 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward SM et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20, 1393–1403 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein S et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun 4, 1630, doi: 10.1038/ncomms2626 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Goyal RK & Chaudhury A Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 298, G10–13, doi: 10.1152/ajpgi.00426.2009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goyal RK CrossTalk opposing view: Interstitial cells are not involved and physiologically important in neuromuscular transmission in the gut. J Physiol 594, 1511–1513, doi: 10.1113/JP271587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goyal RK Rebuttal from Raj K Goyal. J Physiol 594, 1517, doi: 10.1113/JP271972 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang RX, Wang XY, Chen D & Huizinga JD Role of interstitial cells of Cajal in the generation and modulation of motor activity induced by cholinergic neurotransmission in the stomach. Neurogastroenterol Motil 23, e356–371, doi: 10.1111/j.1365-2982.2011.01753.x (2011). [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Carmichael SA, Wang XY, Huizinga JD & Paterson WG Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol 298, G14–24, doi: 10.1152/ajpgi.00266.2009 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Driessen AK, Farrell MJ, Mazzone SB & McGovern AE Multiple neural circuits mediating airway sensations: Recent advances in the neurobiology of the urge-to-cough. Respir Physiol Neurobiol 226, 115–120, doi: 10.1016/j.resp.2015.09.017 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Nishi S & North RA Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol 231, 471–491 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirst GD, Holman ME & Spence I Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol 236, 303–326 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunze WA, Furness JB, Bertrand PP & Bornstein JC Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol 506 (Pt 3), 827–842 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bornstein JC, Furness JB & Kunze WA Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst 48, 1–15 (1994). [DOI] [PubMed] [Google Scholar]
- 67.Furness JB, Robbins HL, Xiao J, Stebbing MJ & Nurgali K Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317, 1–12, doi: 10.1007/s00441-004-0895-5 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Bornstein JC, Hendriks R, Furness JB & Trussell DC Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J Comp Neurol 314, 437–451, doi: 10.1002/cne.903140303 (1991). [DOI] [PubMed] [Google Scholar]
- 69.Mao Y, Wang B & Kunze W Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol 96, 998–1010, doi: 10.1152/jn.00204.2006 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Spencer NJ & Smith TK Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol 558, 577–596, doi: 10.1113/jphysiol.2004.063586 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith TK, Spencer NJ, Hennig GW & Dickson EJ Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil 19, 869–878, doi: 10.1111/j.1365-2982.2007.01019.x (2007). [DOI] [PubMed] [Google Scholar]
- 72.Mazzuoli-Weber G & Schemann M Mechanosensitivity in the enteric nervous system. Front Cell Neurosci 9, 408, doi: 10.3389/fncel.2015.00408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dogiel AS Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch Anat Physiol Leipzig Anat Abt Jg 1899, 130–158 (1899). [Google Scholar]
- 74.Mazzuoli G & Schemann M Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One 7, e39887, doi: 10.1371/journal.pone.0039887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kugler EM et al. Mechanical stress activates neurites and somata of myenteric neurons. Front Cell Neurosci 9, 342, doi: 10.3389/fncel.2015.00342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazzuoli-Weber G & Schemann M Mechanosensitive enteric neurons in the guinea pig gastric corpus. Front Cell Neurosci 9, 430, doi: 10.3389/fncel.2015.00430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kugler EM et al. Sensitivity to Strain and Shear Stress of Isolated Mechanosensitive Enteric Neurons. Neuroscience 372, 213–224, doi: 10.1016/j.neuroscience.2017.12.052 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Kunze WA, Clerc N, Bertrand PP & Furness JB Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J Physiol 517 (Pt 2), 547–561 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bertrand PP, Kunze WA, Bornstein JC, Furness JB & Smith ML Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol 273, G422–435 (1997). [DOI] [PubMed] [Google Scholar]
- 80.Smolilo DJ, Costa M, Hibberd TJ, Wattchow DA & Spencer NJ Morphological evidence for novel enteric neuronal circuitry in guinea pig distal colon. J Comp Neurol 526, 1662–1672, doi: 10.1002/cne.24436 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Crowcroft PJ, Holman ME & Szurszewski JH Excitatory input from the colon to the inferior mesenteric ganglion. J Physiol 208, 19P–20P (1970). [PubMed] [Google Scholar]
- 82.Miller SM & Szurszewski J Physiology of prevertebral ganglia. Physiology of the Gastrointestinal Tract 19, 795–877 (1994). [Google Scholar]
- 83.Bywater RA Activity following colonic distension in enteric sensory fibres projecting to the inferior mesenteric ganglion in the guinea pig. J Auton Nerv Syst 46, 19–26 (1994). [DOI] [PubMed] [Google Scholar]
- 84.Stebbing MJ & Bornstein JC Electrophysiological analysis of the convergence of peripheral inputs onto neurons of the coeliac ganglion in the guinea pig. J Auton Nerv Syst 46, 93–105 (1994). [DOI] [PubMed] [Google Scholar]
- 85.Miller SM & Szurszewski JH Colonic mechanosensory afferent input to neurons in the mouse superior mesenteric ganglion. Am J Physiol 272, G357–366 (1997). [DOI] [PubMed] [Google Scholar]
- 86.Jiang Z, Dun NJ & Karczmar AG Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science 217, 739–741 (1982). [DOI] [PubMed] [Google Scholar]
- 87.Kreulen DL & Szurszewski JH Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. J Physiol 295, 21–32, doi: 10.1113/jphysiol.1979.sp012952 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller SM & Szurszewski JH Circumferential, not longitudinal, colonic stretch increases synaptic input to mouse prevertebral ganglion neurons. Am J Physiol Gastrointest Liver Physiol 285, G1129–1138, doi: 10.1152/ajpgi.00292.2003 00292.2003 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 89.Lynn P, Zagorodnyuk V, Hennig G, Costa M & Brookes S Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. Journal of Physiology 564, 589–601 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hibberd TJ, Zagorodnyuk VP, Spencer NJ & Brookes SJ Viscerofugal neurons recorded from guinea-pig colonic nerves after organ culture. Neurogastroenterol Motil 24, 1041–e1548, doi: 10.1111/j.1365-2982.2012.01979.x (2012). [DOI] [PubMed] [Google Scholar]
- 91.Miller SM & Szurszewski JH Relationship between colonic motility and cholinergic mechanosensory afferent synaptic input to mouse superior mesenteric ganglion. Neurogastroenterol Motil 14, 339–348, doi:338 [pii] (2002). [DOI] [PubMed] [Google Scholar]
- 92.Lynn PA, Olsson C, Zagorodnyuk V, Costa M & Brookes SJH Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125, 786–794 (2003). [DOI] [PubMed] [Google Scholar]
- 93.Spencer NJ et al. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 153, 518–534, doi: 10.1016/j.neuroscience.2008.02.054 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zagorodnyuk VP, Kyloh M, Brookes SJ, Nicholas SJ & Spencer NJ Firing patterns and functional roles of different classes of spinal afferents in rectal nerves during colonic migrating motor complexes in mouse colon. Am J Physiol Gastrointest Liver Physiol 303, G404–411, doi: 10.1152/ajpgi.00047.2012 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Zagorodnyuk VP, Lynn P, Costa M & Brookes SJ Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol 289, G397–406, doi:00557.2004 [pii] 10.1152/ajpgi.00557.2004 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Feng J et al. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360, 530–533, doi: 10.1126/science.aar5703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hibberd TJ, Zagorodnyuk VP, Spencer NJ & Brookes SJ Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225, 118–129, doi: 10.1016/j.neuroscience.2012.08.040 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Büllbring E & Lin RCY The action of 5-hydroxytryptamine (5-HT) on peristalsis. J Physiol 138, 12P (1957). [Google Scholar]
- 99.Büllbring E & Lin RCY The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140, 381–407 (1958). [PMC free article] [PubMed] [Google Scholar]
- 100.Büllbring E, Lin RCY & Schofield G An investigation of the peristaltic reflex in relation to anatomical observations. Q. Journal. exp Physiol 43, 26–43 (1958). [DOI] [PubMed] [Google Scholar]
- 101.Jin JG, Foxx-Orenstein AE & Grider JR Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288, 93–97 (1999). [PubMed] [Google Scholar]
- 102.Kadowaki M, Wade PR & Gershon MD Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. American Journal of Physiology-Gastrointestinal and Liver Physiology 271, G849–857 (1996). [DOI] [PubMed] [Google Scholar]
- 103.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW & Smith TK Localized release of serotonin (5-Hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136, 1328–1338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yadav VK et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med 16, 308–312, doi:http://www.nature.com/nm/journal/v16/n3/suppinfo/nm.2098_S1.html (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31, 8998–9009, doi:31/24/8998 [pii] 10.1523/JNEUROSCI.6684-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heredia DJ et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591, 5939–5957, doi: 10.1113/jphysiol.2013.256230 jphysiol.2013.256230 [pii] (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vincent AD, Wang XY, Parsons SP, Khan WI & Huizinga JD Abnormal Absorptive Colonic Motor Activity in Germ Free Mice Is Rectified by Butyrate, an Effect Possibly Mediated by Mucosal Serotonin. Am J Physiol Gastrointest Liver Physiol, doi: 10.1152/ajpgi.00237.2017 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Bertrand PP Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol 577, 689–704, doi:jphysiol.2006.117804 [pii] 10.1113/jphysiol.2006.117804 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keating DJ & Spencer NJ Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138, 659–670 670 e651–652, doi: 10.1053/j.gastro.2009.09.020 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Gwynne RM, Clarke AJ, Furness JB & Bornstein JC Both exogenous 5-HT and endogenous 5-HT, released by fluoxetine, enhance distension evoked propulsion in guinea-pig ileum in vitro. Front Neurosci 8, 301, doi: 10.3389/fnins.2014.00301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tuladhar BR, Kaisar M & Naylor RJ Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br J Pharmacol 122, 1174–1178, doi: 10.1038/sj.bjp.0701503 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spencer NJ et al. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301, G519–527, doi: 10.1152/ajpgi.00101.2011 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Keating DJ & Spencer NJ What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol Res, doi: 10.1016/j.phrs.2018.06.017 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Tsuji S, Anglade P, Ozaki T, Sazi T & Yokoyama S Peristaltic movement evoked in intestinal tube devoid of mucosa and submucosa. Jpn J Physiol 42, 363–375 (1992). [DOI] [PubMed] [Google Scholar]
- 115.Spencer NJ, Dickson EJ, Hennig GW & Smith TK Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J Physiol 576, 519–531, doi: 10.1113/jphysiol.2006.109561 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lomax AE et al. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst 76, 45–61, doi:S0165183899000089 [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 117.Neunlist M, Michel K, Aube AC, Galmiche JP & Schemann M Projections of excitatory and inhibitory motor neurones to the circular and longitudinal muscle of the guinea pig colon. Cell Tissue Res 305, 325–330 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Bertrand PP Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil 16, 511–514, doi:NMO572 [pii] 10.1111/j.1365-2982.2004.00572.x (2004). [DOI] [PubMed] [Google Scholar]
- 119.Alcaino C et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A 115, E7632–E7641, doi: 10.1073/pnas.1804938115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baumgartner HR 5-Hydroxytryptamine uptake and release in relation to aggregation of rabbit platelets. J Physiol 201, 409–423, doi: 10.1113/jphysiol.1969.sp008763 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mawe GM & Hoffman JM Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10, 473–486, doi: 10.1038/nrgastro.2013.105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coates MD, Tekin I, Vrana KE & Mawe GM Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 46, 569–580, doi: 10.1111/apt.14226 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Raghupathi R et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. J Physiol 591, 5959–5975, doi: 10.1113/jphysiol.2013.259796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strege PR et al. Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release. Sci Rep 7, 15650, doi: 10.1038/s41598-017-15834-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang F et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 595, 79–91, doi: 10.1113/JP272718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu J, Lewis AH & Grandl J Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels. Trends Biochem Sci 42, 57–71, doi: 10.1016/j.tibs.2016.09.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alcaino C, Farrugia G & Beyder A Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr Top Membr 79, 219–244, doi: 10.1016/bs.ctm.2016.11.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mazzuoli-Weber G et al. Piezo proteins: incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res, doi: 10.1007/s00441-018-2926-7 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Kaelberer MM et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, doi: 10.1126/science.aat5236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spencer NJ, Smith CB & Smith TK Role of muscle tone in peristalsis in guinea-pig small intestine. J Physiol 530, 295–306 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spencer NJ, Hennig GW & Smith TK A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545, 629–648 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spencer NJ, Hennig GW & Smith TK Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 284, G231–241, doi: 10.1152/ajpgi.00291.2002 00291.2002 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 133.Costa M et al. New insights into neurogenic cyclic motor activity in the isolated guinea-pig colon. Neurogastroenterol Motil 29, 1–13, doi: 10.1111/nmo.13092 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Ellis M, Chambers JD, Gwynne RM & Bornstein JC Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 304, G749–761, doi: 10.1152/ajpgi.00358.2012 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Huizinga JD et al. The origin of segmentation motor activity in the intestine. Nat Commun 5, 3326, doi: 10.1038/ncomms4326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gwynne RM & Bornstein JC Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292, G1162–1172, doi:00441.2006 [pii] 10.1152/ajpgi.00441.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Farthing MJ Enterotoxins and the enteric nervous system--a fatal attraction. Int J Med Microbiol 290, 491–496, doi: 10.1016/S1438-4221(00)80073-9 (2000). [DOI] [PubMed] [Google Scholar]
- 138.Lundgren O 5-Hydroxytryptamine, enterotoxins, and intestinal fluid secretion. Gastroenterology 115, 1009–1012 (1998). [DOI] [PubMed] [Google Scholar]
- 139.Vanden Broeck D, Horvath C & De Wolf MJ Vibrio cholerae: cholera toxin. Int J Biochem Cell Biol 39, 1771–1775, doi: 10.1016/j.biocel.2007.07.005 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Fung C, Ellis M & Bornstein JC Luminal Cholera Toxin Alters Motility in Isolated Guinea-Pig Jejunum via a Pathway Independent of 5-HT(3) Receptors. Front Neurosci 4, 162, doi: 10.3389/fnins.2010.00162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koussoulas K, Gwynne RM, Foong JPP & Bornstein JC Cholera Toxin Induces Sustained Hyperexcitability in Myenteric, but Not Submucosal, AH Neurons in Guinea Pig Jejunum. Front Physiol 8, 254, doi: 10.3389/fphys.2017.00254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neunlist M, Dobreva G & Schemann M Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol (Lond) 517 (Pt 2), 533–546 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wood JD Cellular Neurophysiology of Enteric Neurons. Physiology of the Gastrointestinal tract In Handbook of Physiology, Vol. Chapter 21 629–669. (Elsevier, Inc., 2012). [Google Scholar]
- 144.Furness JB The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294, doi: 10.1038/nrgastro.2012.32 (2012). [DOI] [PubMed] [Google Scholar]
- 145.Galligan JJ Pharmacology of synaptic transmission in the enteric nervous system. Current Opinion in Pharmacology 2, 623–629 (2002). [DOI] [PubMed] [Google Scholar]
- 146.Zhou Y & Danbolt NC Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 121, 799–817, doi: 10.1007/s00702-014-1180-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wood JD in Handbook of Physiology Vol. 2 Physiology of the Gastrointestinal Tract. (ed Said Hamid M) Ch. 15, 361–272 (Academic Press, 2018). [Google Scholar]
- 148.Ren J, Hu HZ, Liu S, Xia Y & Wood JD Glutamate receptors in the enteric nervous system: ionotropic or metabotropic? Neurogastroenterol Motil 12, 257–264 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Swaminathan M, Hill-Yardin EL, Bornstein JC & Foong JPP Endogenous Glutamate Excites Myenteric Calbindin Neurons by Activating Group I Metabotropic Glutamate Receptors in the Mouse Colon. Front Neurosci 13, 426, doi: 10.3389/fnins.2019.00426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hu HZ et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550, 493–504, doi: 10.1113/jphysiol.2003.041731 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gwynne RM & Bornstein JC Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. Am J Physiol Gastrointest Liver Physiol 297, G179–186, doi: 10.1152/ajpgi.90700.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monro RL, Bertrand PP & Bornstein JC ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556, 571–584, doi: 10.1113/jphysiol.2004.060848 jphysiol.2004.060848 [pii] (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gwynne RM & Bornstein JC Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol 5, 1–17 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monro RL, Bornstein JC & Bertrand PP Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience 134, 975–986, doi:S0306–4522(05)00508–7 [pii] 10.1016/j.neuroscience.2005.05.006 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Crist JR, He XD & Goyal RK Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol 447, 119–131 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mutafova-Yambolieva VN et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A 104, 16359–16364, doi: 10.1073/pnas.0705510104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mutafova-Yambolieva VN & Sanders KM Appropriate experimental approach is critical for identifying neurotransmitter substances: application to enteric purinergic neurotransmission. Am J Physiol Gastrointest Liver Physiol 309, G608–609, doi: 10.1152/ajpgi.00225.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang GD et al. beta-Nicotinamide adenine dinucleotide acts at prejunctional adenosine A1 receptors to suppress inhibitory musculomotor neurotransmission in guinea pig colon and human jejunum. Am J Physiol Gastrointest Liver Physiol 308, G955–963, doi: 10.1152/ajpgi.00430.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wood JD Response to Mutafova-Yambolieva and Sanders. Am J Physiol Gastrointest Liver Physiol 309, G610–611, doi: 10.1152/ajpgi.00262.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bornstein JC, Costa M & Furness JB Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol 381, 465–482 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reed DE & Vanner SJ Converging and diverging cholinergic inputs from submucosal neurons amplify activity of secretomotor neurons in guinea-pig ileal submucosa. Neuroscience 107, 685–696 (2001). [DOI] [PubMed] [Google Scholar]
- 162.Foong JP, Parry LJ, Gwynne RM & Bornstein JC 5-HT(1A), SST(1), and SST(2) receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 298, G384–394, doi: 10.1152/ajpgi.00438.2009 ajpgi.00438.2009 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koussoulas K, Swaminathan M, Fung C, Bornstein JC & Foong JPP Neurally Released GABA Acts via GABAC Receptors to Modulate Ca(2+) Transients Evoked by Trains of Synaptic Inputs, but Not Responses Evoked by Single Stimuli, in Myenteric Neurons of Mouse Ileum. Front Physiol 9, 97, doi: 10.3389/fphys.2018.00097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sang Q & Young HM The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec 251, 185–199 (1998). [DOI] [PubMed] [Google Scholar]
- 165.Tonini M, Frigo G, Lecchini S, D’Angelo L & Crema A Hyoscine-resistant peristalsis in guinea-pig ileum. Eur J Pharmacol 71, 375–381 (1981). [DOI] [PubMed] [Google Scholar]
- 166.Tonini M, Costa M, Brookes SJ & Humphreys CM Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience 73, 287–297 (1996). [DOI] [PubMed] [Google Scholar]
- 167.Costa M et al. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. Am J Physiol Gastrointest Liver Physiol 305, G749–759, doi: 10.1152/ajpgi.00227.2013 ajpgi.00227.2013 [pii] (2013). [DOI] [PubMed] [Google Scholar]
- 168.Costa M et al. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea-pig colon. Neurogastroenterol Motil, doi: 10.1111/nmo.12646 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Lasrado R et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722–726, doi: 10.1126/science.aam7511 (2017). [DOI] [PubMed] [Google Scholar]
- 170.Spencer NJ et al. Identification of a Rhythmic Firing Pattern in the Enteric Nervous System That Generates Rhythmic Electrical Activity in Smooth Muscle. J Neurosci 38, 5507–5522, doi: 10.1523/JNEUROSCI.3489-17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li Z et al. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife 8, doi: 10.7554/eLife.42914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8, 1263–1268, doi: 10.1038/nn1525 (2005). [DOI] [PubMed] [Google Scholar]
- 173.Deisseroth K Optogenetics. Nat Methods 8, 26–29, doi: 10.1038/nmeth.f.324 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim CK, Adhikari A & Deisseroth K Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 18, 222–235, doi: 10.1038/nrn.2017.15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hibberd TJ et al. Optogenetic Induction of Colonic Motility in Mice. Gastroenterology 155, 514–528, doi: 10.1053/j.gastro.2018.05.029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boesmans W, Hao MM & Vanden Berghe P Optogenetic and chemogenetic techniques for neurogastroenterology. Nat Rev Gastroenterol Hepatol 15, 21–38, doi: 10.1038/nrgastro.2017.151 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Perez-Medina AL & Galligan JJ Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice. Am J Physiol Gastrointest Liver Physiol 317, G569–G579, doi: 10.1152/ajpgi.00089.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spencer NJ, Hibberd T, Feng J & Hu H Optogenetic control of the enteric nervous system and gastrointestinal transit. Expert Rev Gastroenterol Hepatol, 1–4, doi: 10.1080/17474124.2019.1581061 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Owen SF, Liu MH & Kreitzer AC Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 22, 1061–1065, doi: 10.1038/s41593-019-0422-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iyer SM et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32, 274–278, doi: 10.1038/nbt.2834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cannon WB The movements of the stomach studied by means of the Roetgen rays. Am. J. Physiol 1, 359–382 (1898). [Google Scholar]
- 182.Legros and Onimus. Recherches experimentales sur les mouvements de l’intestine. J. de l’Anat. et Physiol 37–66 (1869). [Google Scholar]
- 183.Langley JN in Textbook of Physiology (ed. Schaffer EA) 616–696 (Pentland, 1900). [Google Scholar]


