Abstract
Healthy aging is associated with a multitude of structural changes in the brain. These physical age-related changes are accompanied by increased variability in neural activity of all kinds, and this increased variability, collectively referred to as “neural noise,” is argued to contribute to age-related cognitive decline. In this study, we examine the relationship between two particular types of neural noise in aging. We recorded scalp EEG from younger (20–30 years) and older (60–70 years) adults performing a spatial visual discrimination task. First, we used the 1/f-like exponent of the EEG power spectrum, a putative marker of neural noise, to assess baseline shifts toward a noisier state in aging. Next, we examined age-related decreases in the trial-by-trial consistency of visual stimulus processing. Finally, we examined to what extent these two age-related noise markers are related, hypothesizing that greater baseline noise would increase the variability of stimulus-evoked responses. We found that visual cortical baseline noise was higher in older adults, and the consistency of older adults’ oscillatory alpha (8–12 Hz) phase responses to visual targets was also lower than that of younger adults. Crucially, older adults with the highest levels of baseline noise also had the least consistent alpha phase responses, while younger adults with more consistent phase responses achieved better behavioral performance. These results establish a link between tonic neural noise and stimulus-associated neural variability in aging. Moreover, they suggest that tonic age-related increases in baseline noise might diminish sensory processing and, as a result, subsequent cognitive performance.
Introduction
Neural activity fluctuates over time even in the absence of external stimuli (Faisal et al., 2008; Dinstein et al., 2015). Such variability is seen at all measurement scales, from single-unit spiking to the fMRI BOLD signal, and is present both at rest and across repeated trials. These kinds of variability are collectively referred to as “noise” and are variously linked to numerous neurological disorders as well as decreased cognitive performance. Defining “noise” is particularly complicated in neuroscience (Faisal et al., 2008), especially given that even pre-stimulus variability in neuronal activity is likely functional (Hartmann et al., 2015); however here we build off recent studies that operationalize a specific aspect of noise using a signal processing definition based on the shape of the power spectrum of the encephalographic (EEG) signal (Voytek et al., 2015; Voytek & Knight 2015). By definition, white noise is a purely stochastic signal with a “flat” power spectrum (equal power at all frequencies), whereas neural activity is 1/f-like in that power decreases exponentially as a function of frequency (Miller et al., 2009; He, 2014). The rate of this spectral power decrease—the exponent of the 1/f-like power spectrum in log-log space—indexes the statistical structure of neural activity in that less negative or flatter exponent indicates decorrelated, more-white-noise-like signals (Voytek et al., 2015; Voytek & Knight 2015; Gao, 2015). In addition, the spectral exponent has also been shown to index the excitation-inhibition (EI) balance of the underlying neural population (Gao et al., 2017). In this EI framing, a flatter exponent suggests a shift away from inhibition and toward “noisier” or more stochastic excitatory spiking. While the neural basis of the 1/f-like signal is debated, many theories relate to “noisiness” directly or indirectly; these include the EI balance theory above, as well as others, such as its relationship in EEG to signal entropy (Waschke, Wostmann, Obleser, 2017), or to signal complexity in invasive human EEG (Sheehan et al., 2018) and to the fMRI BOLD signal (Garrett et al., 2018).
In support of the neural noise hypothesis of aging, which proposes that healthy aging leads to diminished signal-to-noise in neural communication (Cremer and Zeef, 1987, Voytek & Knight, 2015), recent studies have shown age-related increases in EEG 1/f-like noise and how these changes relate to alterations in cognitive performance (Voytek et al., 2015, Waschke et al., 2017; McNair et al., 2018). Potentially reflecting increased EI balance, these increases in baseline noise would be consistent with reported reduced inhibitory signaling in aging (Hickmott and Dinse, 2013). Additionally, the consistency of activity evoked by identical or near-identical stimuli, which is already highly variable across trials (Scholvinck et al., 2015), has also been found to be reduced in older adults. For instance, the amplitude and latency of various visual event-related potential (ERP) components can, depending on electrode locations and stimulus paradigms, be more variable in older adults (Kugler et al., 1993; Lorenzo-Lopez et al., 2007). Similarly, decreases in the consistency of stimulus-evoked oscillatory alpha (roughly 8–12 Hz) phase responses, measured using intertrial phase coherence (ITC), have also been reported (Tran et al., 2016, though see Sander et al., 2012, and Wiegand and Sander, 2019). Given concomitant age-related increases in baseline noise and response variability, it is possible that more prominent baseline noise might have a direct effect on the consistency with which older adults respond to and process external stimuli.
Here, we investigate the relationship between these two kinds of neural noise—tonic baseline noise and trial-by-trial response variability—as well as their association with age-related cognitive decline. To do so, we recorded EEG from younger (20–30 years) and older (60–70 years) adults performing a spatial visual discrimination task (Rolle et al., 2015, Rolle et al., 2017, Voytek et al., 2017). To assess baseline noise, the spectral exponent of pre-trial visual posterior neural activity was used (hereafter referred to as “spectral exponent”). To estimate response variability, we measured the increase in oscillatory alpha ITC evoked by visual target presentation. We hypothesized that older adults would have higher levels of baseline noise and higher response variability than would younger adults, and that older adults with higher levels of baseline noise would also have higher variability in stimulus-evoked responses.
Materials and Methods
Behavioral task
All participants gave informed consent in accordance with protocols approved by the UCSF Committee on Human Research in the Human Research Protection Program. Healthy right-handed younger (20–30 years old) and older (60–70 years old) adults with normal or corrected-to-normal vision participated in a previously described visual discrimination task (Rolle et al., 2015, Rolle et al., 2017, Voytek et al., 2017). In this task, a modified Posner attentional cueing task, we parametrically manipulated the amount of spatial information provided by a pre-target visual cue, in doing so instructing participants to focus or divide their attention across narrow or broad areas of visual space (Figure 1A). Each trial consisted of spatial cue presentation (100 ms), a preparatory period (1500–2000 ms, random, uniformly distributed, central fixation cross on-screen), and simultaneous visual target and non-target presentation (50 ms). This was followed by a 1250 ms inter-trial interval.
Figure 1.
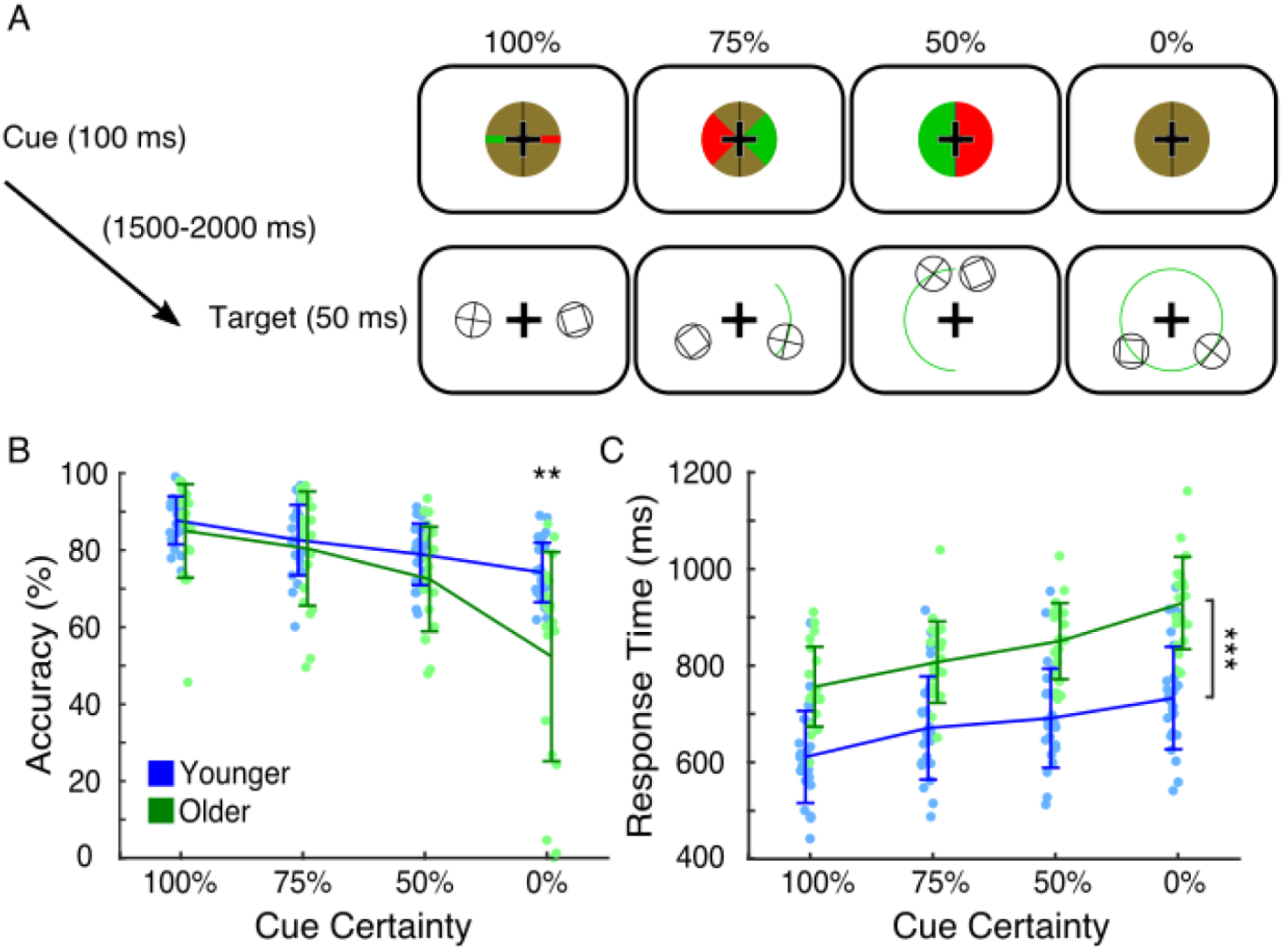
Spatial attention task and behavioral performance results. (A) Trial structure and visual stimuli presented. Cues presented at the beginning of each trial contained varying levels of spatial information about upcoming targets (plus signs enclosed in circles), indicating with green wedges of varying sizes the hemifield and potential location of targets. Targets were always presented at 4.5° central eccentricity and simultaneously presented with non-target stimuli of matched visual properties in the opposite visual hemifield, mirrored across the meridian. In the 100% certainty condition, targets appeared exactly to the left or right of center. In the 75% certainty condition, targets appeared anywhere in a 90° arc in the indicated hemifield. In the 50% certainty condition, targets appeared anywhere in a 180° arc in the indicated hemifield. In the 0% certainty condition, green wedges were not supplied, and targets appeared anywhere in the full 4.5° central eccentricity circle. Potential target locations are indicated here with green arcs (not actually shown to participants). (B) Younger (blue) and older (green) adults’ accuracy across cue conditions (participants plotted individually, error bars of standard deviation). Older adults had lower accuracy than younger adults only in the 0% certainty condition (** p < 0.01). (C) Younger (blue) and older (green) adults’ response times across cue conditions (participants plotted individually, error bars of standard deviation). Older adults had slower response times than younger adults in all cue conditions (*** p < 0.001 for each).
The spatial cue consisted of a green- and red-checkered circle surrounding the fixation cross, color luminances being matched. The circle was bisected along the vertical meridian with a black line. For the 100% certainty condition, the checkerboard was broken along the horizontal meridian by a solid red line in one hemifield and a solid green line in the other hemifield. These lines were the same vertical width as the arms of the fixation cross, and they extended the entire radius of the cue circle. In this condition, the hemifield of the green line was perfectly informative of the upcoming target location, which would appear 4.5° away from center exactly on the horizontal meridian in the green-line hemifield. For the 75% certainty condition, the cue instead had green and red 90° wedges centered along the horizontal meridian. In this condition, the hemifield of the green wedge was still perfectly informative of the target hemifield, but the target could appear anywhere along a 90° arc, also centered across the horizontal meridian, at 4.5° central eccentricity. For the 50% certainty condition, the two hemifields of the cue were either completely green or completely red, indicating that the upcoming target would appear anywhere along the 180° semicircle (a whole hemifield) at 4.5° central eccentricity. For the 0% certainty condition, no lines or wedges were presented, indicating that the upcoming target would appear anywhere around the 360° circle at 4.5° central eccentricity. Cue condition (100%, 75%, 50%, or 0% cue certainty) and target hemifield were randomized on a trial-by-trial basis.
The visual target consisted of a plus sign enclosed by a circle. Participants were tasked to indicate, via manual button press with their dominant hand, whether the plus was exactly vertical and horizontal (index finger) or slightly rotated (middle finger). A non-target stimulus was simultaneously presented in the opposite hemifield, mirrored across the vertical meridian. This non-target stimulus was a box enclosed by a circle, its basic visual components (two horizontal and two vertical bars enclosed in a circle) thus similar to that of targets.
Prior to the main experiment, each participant underwent individual psychophysical thresholding to normalize accuracy across participants. The thresholding procedure was a two-down, one-up staircase converging on ~70% accuracy (Leek, 2001). In the thresholding task, participants were only presented with 50% certainty cues and were initially shown either a vertical “+” or a 45°-rotated “X”. With every correct trial, the “X” rotated 1.5° closer toward vertical, and with every incorrect response, it rotated 3.0° away from vertical. Once behavioral asymptote was reached, the average angle across the final 10 trials was used as the final angle for the main experiment.
Data acquisition
EEG data was collected using a BioSemi ActiveTwo 64-channel DC amplifier with 24-bit resolution and was sampled at 1024 Hz. In addition to 64 scalp electrodes, both horizontal (HEOG) and vertical (VEOG) electrooculograms were recorded at both external canthi and with a left-inferior eye electrode, respectively. Data was referenced offline to the average potential of two mastoid electrodes. To ensure exact timing relative to stimulus presentation, event onset times were based on timing information provided by a photodiode attached to the stimulus presentation monitor.
Data processing
Data was preprocessed and analyzed in MATLAB® (R2017a, Natick, MA) and Python with custom scripts using the EEGLAB MATLAB toolbox (Delorme and Makeig, 2004), the CircStats MATLAB toolbox (Berens, 2009), and the FOOOF Python module (Haller and Donoghue et al., 2018). Continuous EEG data was resampled from 1024 Hz to 256 Hz. Data was highpass filtered above 1 Hz using a one-way, Hamming-windowed sinc finite impulse response (FIR) filter (−6 dB cutoff frequency 0.5 Hz, 1 Hz transition band) and lowpass filtered below 50 Hz using a one-way, Hamming-windowed sinc FIR filter (−6dB cutoff frequency 56.25 Hz, 12.5 Hz transition band). Bad channels (no more than 4 per participant) were spherically interpolated. Of those with interpolated channels, there was an average of 2.23 channels (3.5% of channels) per participant.
Baseline noise and response variability were assessed using pre-trial and target-evoked EEG activity, respectively. For baseline noise, epochs containing the 500 ms of activity prior to spatial cue onset were extracted (−500 to 0 ms relative to cue onset). Cue-locked epochs were rejected if the amplitude of any EEG channel exceeded ± 125 μV, any frontal EEG channel exceeded ± 75 μV, either HEOG channel exceeded ± 40 μV, or the difference between HEOG channels exceeded ± 40 μV. Response variability was assessed using the 1000 ms of activity centered around target onset (−500 to 500 ms relative to target onset). A similar epoch rejection procedure was used, though in the case of target-locked epochs, both pre-cue and pre-target baselines (−100 to 0 ms) were used to detect artifacts. Trials were rejected independently between cue- and target-locked epochs.
Due to low trial numbers after artifact rejection, 6 and 9 younger and older adults, respectively, were excluded from further analysis for each having fewer than thirty total (irrespective of cue condition) clean target-locked epochs from correctly answered trials. For clean cue-locked epochs from correctly answered trials, there was no difference in total epoch number between younger and older adults (p = 0.27; Younger: mean trial number 230, minimum 71, maximum 496; Older: mean 206, minimum 135, maximum 274). On average, 19.9% and 18.4% of cue-locked epochs from correctly answered trials were rejected in younger and older adults, respectively (Younger: minimum 0.95%, maximum 77.6%; Older: minimum 2.6%, maximum 43.6%). For clean target-locked epochs from correctly answered trials, there was no difference in total epoch number between groups (p = 0.34; Younger: mean trial number 151, minimum 32, maximum 519; Older: mean 123, minimum 32, maximum 253), nor was there a difference in epoch number between groups in any cue condition (100%: p = 0.49; 75%: p = 0.56; 50%: p = 0.38; 0%: p = 0.11). On average, 48.4% and 51.2% of target-locked epochs from correctly answered trials were rejected in younger and older adults, respectively (Younger: minimum 0%, maximum 87.8%; Older: minimum 3.9%, maximum 89.5%).
Data analysis
Baseline noise was estimated using the spectral exponent of the EEG power spectrum (Voytek et al., 2015; Gao et al., 2017). Specifically, each clean cue-locked epoch per channel was tapered with a Hanning window and zero-padded to one second in length. Using this zero-padded tapered trial, a power spectrum was constructed using the absolute value squared of the complex-numbered output of the Discrete Fourier Transform. This power spectrum was then decomposed using the FOOOF algorithm, which separates power spectra into aperiodic, 1/f-like components and zero or more oscillatory peaks whose power exceeds that of aperiodic activity by some threshold. With FOOOF, we extracted the aperiodic component over the 2-to-20-Hz frequency range of each power spectrum (aperiodic_mode = ‘fixed’, peak_width_limits = [2, inf], peak_threshold = 1, and default settings otherwise). The negative of the corresponding aperiodic exponent was used to index pre-cue baseline noise.
Response variability was quantified using trial-by-trial alpha phase consistency, or alpha intertrial phase coherence (ITC). To generate time-frequency ITC plots, clean target-locked epochs padded with an extra second of data per side were bandpass filtered with one-way, Hamming-windowed sinc FIR filters with a 4 Hz passband and center frequencies ranging from 4 to 50 Hz in 2-Hz steps. To calculate individualized alpha-band ITC, clean target-locked epochs padded with an extra second of data per side were bandpass filtered with a one-way, Hamming-windowed sinc FIR filter (2 Hz transition band) with a 4 Hz passband centered on each participant’s peak alpha frequency (PAF). Each participant’s PAF was estimated using power spectra constructed using the activity of channel Oz during target-locked epochs (−500 to 500 ms) and as the average across trials of the frequency of maximum power between 7 and 14 Hz. This frequency was determined using oscillatory peaks extracted with FOOOF (aperiodic_mode = ‘fixed’, peak_width_limits = [2, inf], peak_threshold = 1, and default settings otherwise). Estimates of PAF did not differ between younger and older adults (p = 0.18), and across all participants, PAF estimates did not differ between usage of peak_threshold 1 and 2 (p = 75) or between peak_threshold 1 and 3 (p = 0.79). The angle of the Hilbert transform of the bandpass filtered data was used to extract alpha analytic phase. The phase time series consists of cosine phase values of (π, π] radians, with π radians corresponding to the trough and zero radians to the peak of the oscillation. This method yields results equivalent to sliding-window fast Fourier transform and wavelet approaches (Bruns, 2004). The mean vector length of each timepoint’s phase distribution across trials was calculated. This mean vector length represents the degree of ITC, with ITC of unity reflecting a single adopted phase across trials and a value of zero reflecting uniformly distributed phases across trials.
Statistical analyses
Correctly answered trials from all cue conditions were pooled together for EEG analyses. To normalize electrode locations, channel recordings were swapped right to left across the midline as though targets were always presented in the right visual hemifield, making left- and right-hemisphere channels contralateral and ipsilateral to all targets, respectively. All analyses were performed on data from EEG channels O1/2, PO3/4, and PO7/8, with channels O1, PO3, and PO7 considered contralateral to targets. For baseline noise estimates, spectral exponent values were averaged across contralateral and ipsilateral channels separately. For response variability estimates, and because of ITC values being sensitive to differences in the number of data points used, we implemented a random resampling procedure to control for differences in epoch number between participants. Per participant, we randomly selected 30 target-locked epochs without replacement, and using these epochs, we constructed ITC time series per channel of interest. These time series were baselined using the −500 to 0 ms window prior to target onset and averaged across contralateral and ipsilateral channels separately. For time-frequency ITC plots, this procedure was repeated 500 times per participant, and the medians of ITC distribution were used for subsequent analysis. For alpha ITC analyses, after peak ITC in the 0 to 500 ms after target onset was determined, this procedure was repeated 2000 times per participant, and the median of this distribution was used for subsequent analysis.
Multiple-factor statistical analyses were assessed via ANOVAs, with age as a between-group factor and hemisphere as a within-group factor. Where sphericity assumptions were violated, degrees of freedom were adjusted using Greenhouse-Geisser corrections. Because ITC values prior to baselining are bound between 0 and 1, peak ITC values were log10-transformed prior to ANOVA analysis, and any single-factor comparisons or correlations involving ITC were analyzed using non-parametric tests. Ultimately, 25 out of 31 younger adults and 20 out of 29 older adults were included in the final analysis. One older participant was excluded only from behavioral response time analyses due to a lack of correct trials in the 0% certainty condition.
Results
Older adults had lower accuracy and longer response times
We compared younger and older adults’ accuracy levels in a visual discrimination task in which pre-target spatial cues had varying levels of information about the locations of upcoming targets (Figure 1A; Younger: n = 25; Older: n = 20). Accuracy showed main effects of age and cue information as well as an interaction between the two (age: F1,43 = 8.52, p= 0.0056, η2 = 0.091; cue: F3,129 = 38.01, ε = 0.47, pGG < 10−8, η2 = 0.30; age*cue: F3,129 = 8.14, ε = 0.47, pGG = 0.0024, η2 = 0.085). Post-hoc analysis indicated that younger and older adults did not differ in accuracy in the 100% or 75% certainty conditions (p = 0.36; p = 0.57), but older adults trended towards showing modest accuracy deficits in the 50% condition (p = 0.071) and had significant deficits in the 0% condition (Figure 1B; 74% vs 52%, t21.48 = 3.48, p = 0.0022, Cohen’s d = 1.15).
We also examined differences in response time between younger and older adults. As with accuracy, response time showed an interaction between age and cue information in addition to separate main effects of each (age: F1,42 = 36.60, p < 10−6, η2 = 0.41; cue: F3,126 = 68.09, ε = 0.43, pGG < 10−17, η2 = 0.23, age*cue: F3,126 = 3.52, ε = 0.43, pGG = 0.035, η2 = 0.016). Post-hoc analysis indicated that older adults had longer response times in all cue conditions (Figure 1C; 100%: 584 ms vs. 728 ms, t41.04 = −5.78, p < 10−6, Cohen’s d = −1.73; 75%: 643 ms vs. 779 ms, t40.26 = −4.64, p < 10−4, Cohen’s d = −1.40; 50%: 664 ms vs. 817 ms, t41.09 = −5.40, p < 10−5, Cohen’s d = −1.61; 0%: 703 ms vs. 900 ms, t37.78 = −6.17, p < 10−6, Cohen’s d = −1.89).
Baseline noise and response variability were elevated in older adults
We estimated pre-trial baseline noise levels using the spectral exponent of pre-cue visual posterior EEG activity. To do so, we pooled trials from all cue conditions and examined average spectral exponent contralateral and ipsilateral to attended target locations. Baseline spectral exponent showed a main effect of age (Figure 2; age: −1.03 vs. −0.81, F1,43 = 12.54, p < 10−3, η2 = 0.22), but no main effect of hemisphere or interaction between age and hemisphere (hemisphere: F1,43 < 1.0, p = 0.42; age*hemisphere: F1,43 = 3.35, p = 0.074). These results indicate that older adults had higher levels of pre-trial baseline noise than did younger adults.
Figure 2.
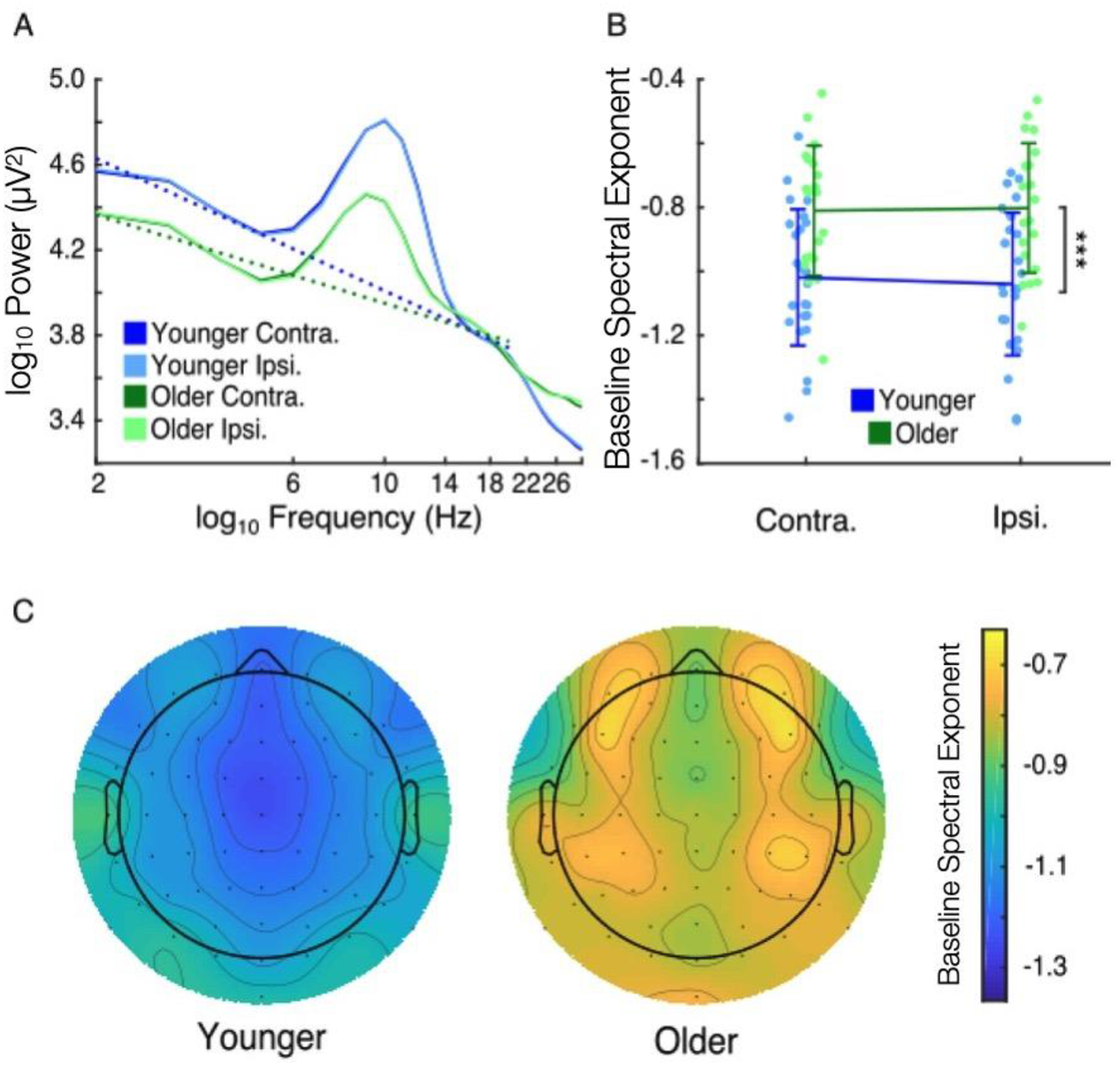
Baseline noise, characterized using the spectral exponent of pre-trial visual EEG activity, was higher in older adults. (A) Grand-average log-log-space power spectra of younger (blue) and older adults (green) both contralateral (darker) and ipsilateral (lighter) to eventual target presentation. Dotted lines indicate estimated spectral exponents. (B) Contralateral and ipsilateral spectral exponent values of younger (blue) and older (green) adults (participants plotted individually, error bars of standard deviation). Older adults had higher spectral exponent values (main effect of age, *** p < 0.001), suggesting higher levels of pre-trial baseline noise. (C) Grand-average topographies of baseline spectral exponent in younger (left) and older (right) adults.
Both younger and older adults showed increases in low-frequency intertrial phase coherence (ITC) in response to visual target presentation (Figure 3A). Based on this as well as previous work (Tran et al., 2016), we used target-evoked alpha-band ITC to estimate trial-by-trial response variability (Figure 3B). Peak ITC after target onset showed a main effect of age, with older adults having lower evoked ITC than younger adults (Figures 3C and 3D; 0.29 vs 0.23, F1,43 = 4.11, p = 0.049, η2 = 0.079). There was no main effect of hemisphere or interaction between age and hemisphere (hemisphere: F1,43 < 1.0, p = 0.52; age*hemisphere: F1,43 < 1.0, p = 0.36). Thus, older adults had higher variability in their target-evoked alpha phase responses than did younger adults.
Figure 3.
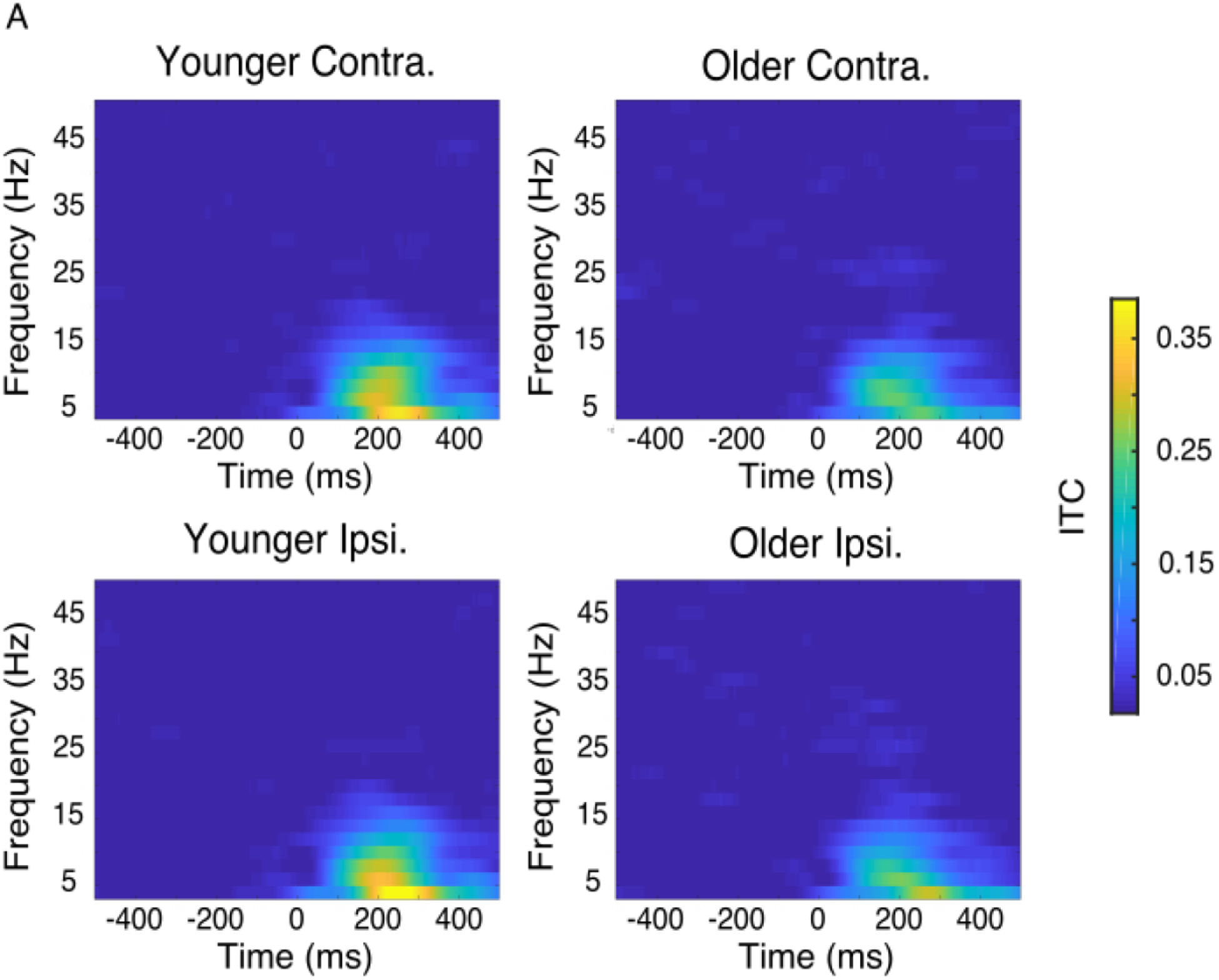
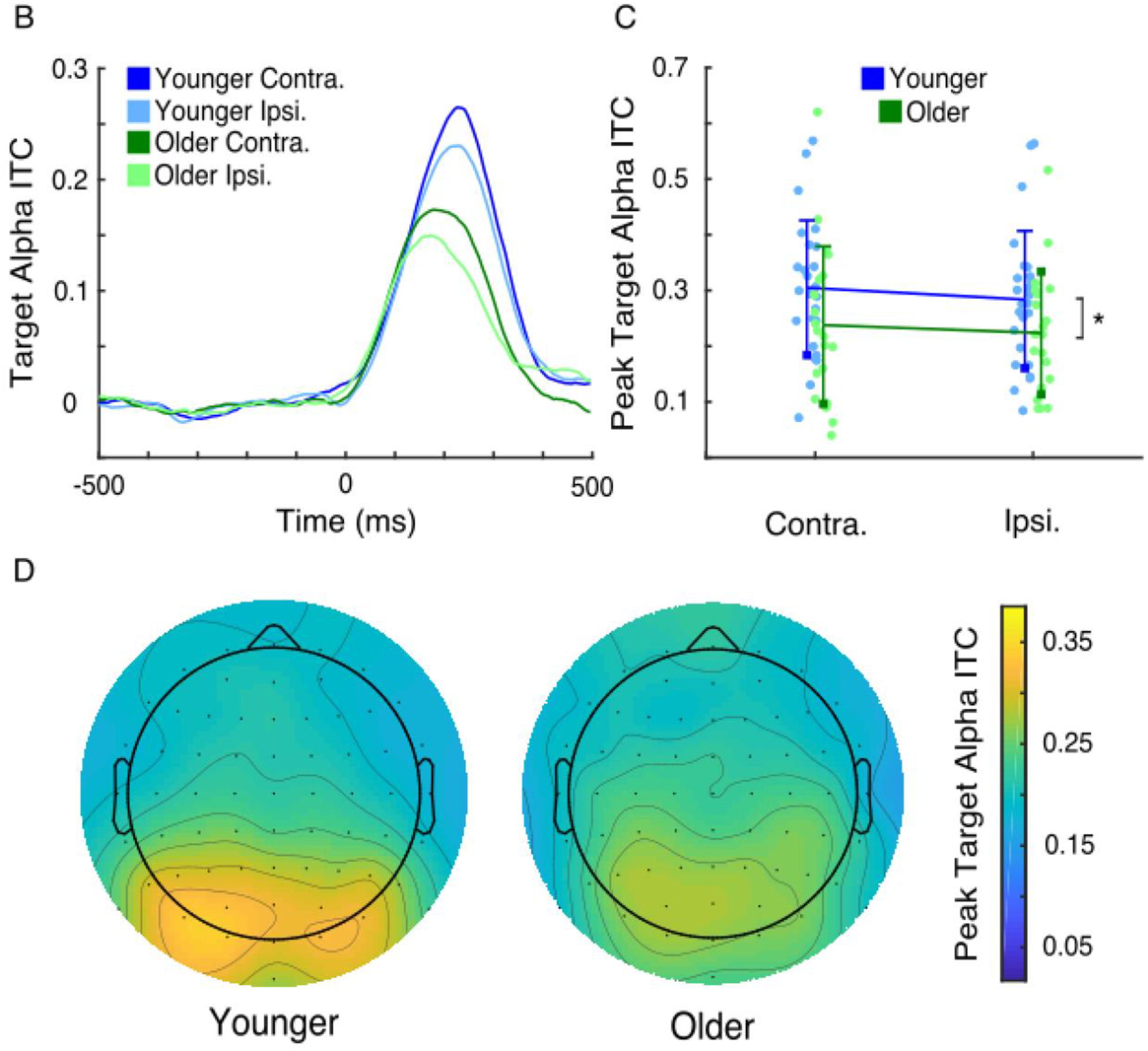
Trial-by-trial response variability, characterized using the consistency of target-evoked alpha phase responses (alpha intertrial coherence, or ITC), was elevated in older adults. (A) Grand-average time-frequency plots for target-evoked ITC in younger (left) and older (right) adults both contralateral (upper) and ipsilateral (lower) to target presentation. (B) Grand-average time courses of baselined target-evoked alpha ITC of younger (blue) and older adults (green) both contralateral (darker) and ipsilateral (lighter) to target presentation. (C) Contralateral and ipsilateral peak alpha ITC values of younger (blue) and older (green) adults (participants plotted individually, error bars of standard deviation). Older adults had lower peak alpha ITC values (main effect of age, * p < 0.05), indicating higher levels of response variability. (D) Grand-average topographies of peak target-evoked alpha ITC in younger (left) and older (right) adults.
Baseline noise was correlated with response variability in older adults
Given the observed age-related differences in baseline noise and response variability, we next investigated the relationship between these two measures. Specifically, we examined if, within groups, higher levels of baseline noise would be associated with increased variability in target-evoked alpha phase responses. In younger adults, no relationship between spectral exponent and peak alpha ITC was observed contralateral or ipsilateral to target presentation (Figure 4A; p = 0.88, p = 0.72). In older adults, on the other hand, participants with flatter spectral exponent also had lower levels of evoked alpha ITC, and this was the case both contralateral (Figure 4B; Spearman’s r = −0.45, p = 0.047) and ipsilateral to target presentation (Spearman’s r = −0.57, p = 0.0094). The correlation between spectral exponent and evoked alpha ITC was stronger for older versus younger adults ipsilaterally (Fisher’s z-transform = 1.78, p = 0.037) and trended similarly contralaterally (Fisher’s z-transform = 1.60, p = 0.055). Thus, older but not younger participants with more baseline noise also had higher variability in stimulus-evoked responses.
Figure 4.
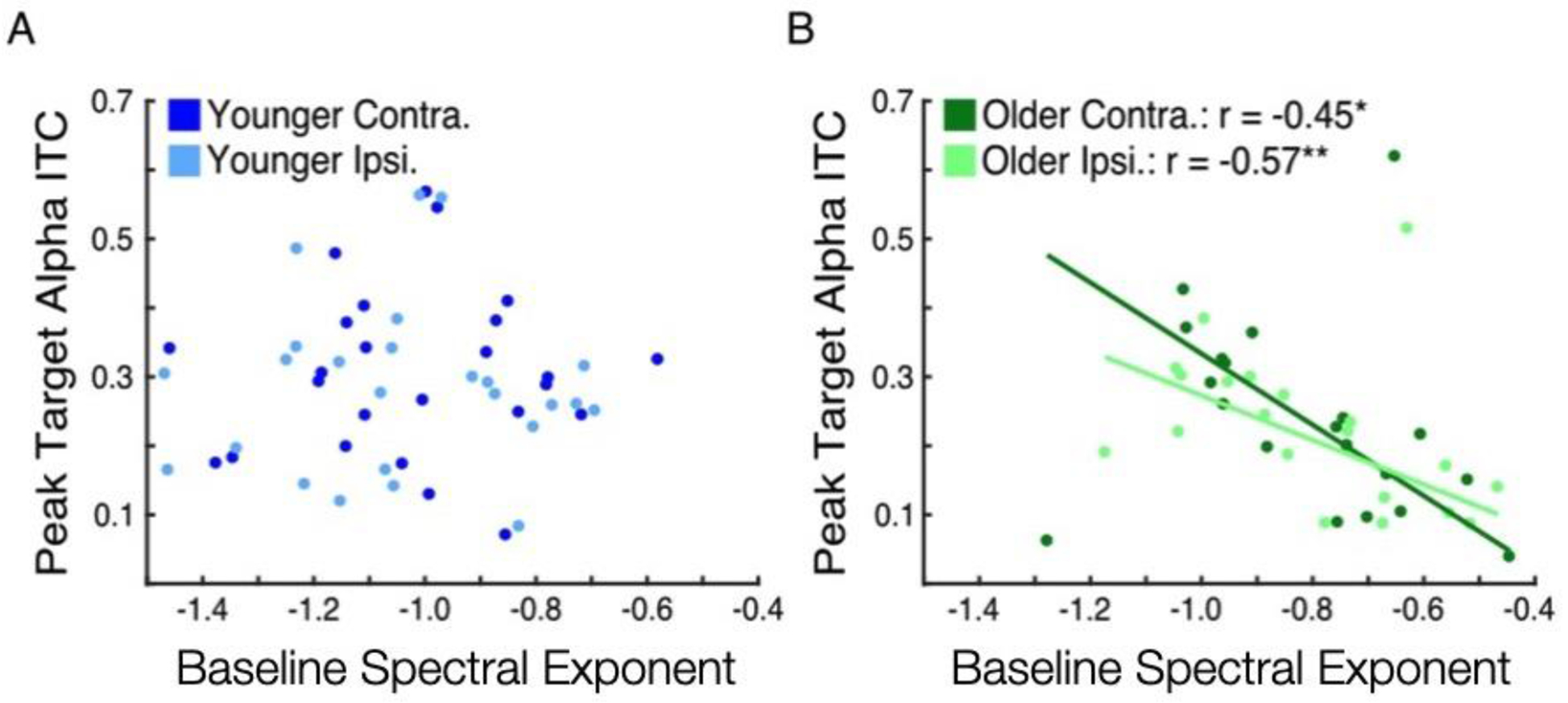
Baseline noise and response variability were correlated in older but not younger adults. (A) Peak target-evoked alpha ITC versus baseline spectral exponent in younger adults both contralateral (darker) and ipsilateral (lighter) to target presentation. No relationship was found. (B) Peak target-evoked alpha ITC versus baseline spectral exponent in older adults, with best-fit lines both contralateral (darker) and ipsilateral (lighter) to target presentation. In both hemispheres, increased baseline noise (less negative or flatter pre-trial spectral exponent) was associated with increased response variability (reduced peak target-evoked alpha ITC, * p < 0.05, ** p < 0.01).
Trial-by-trial response variability was correlated with accuracy in younger adults
We also examined whether levels of baseline noise or response variability were related to behavioral performance. For this investigation, accuracy across all trials (irrespective of cue condition) was used. Baseline spectral exponent contralateral to target presentation was not correlated with accuracy in younger or older adults (Figure 5A; Younger: p = 0.62; Older: p = 0.34). Regarding response variability, however, higher target-evoked contralateral alpha ITC was correlated with higher accuracy in younger adults (Figure 5B; Spearman’s r = 0.51, p = 0.0096). No such relationship was found across older adults (p = 0.51). Thus, contralateral alpha phase response consistency was related to behavioral performance in younger adults, though not in older adults.
Figure 5.
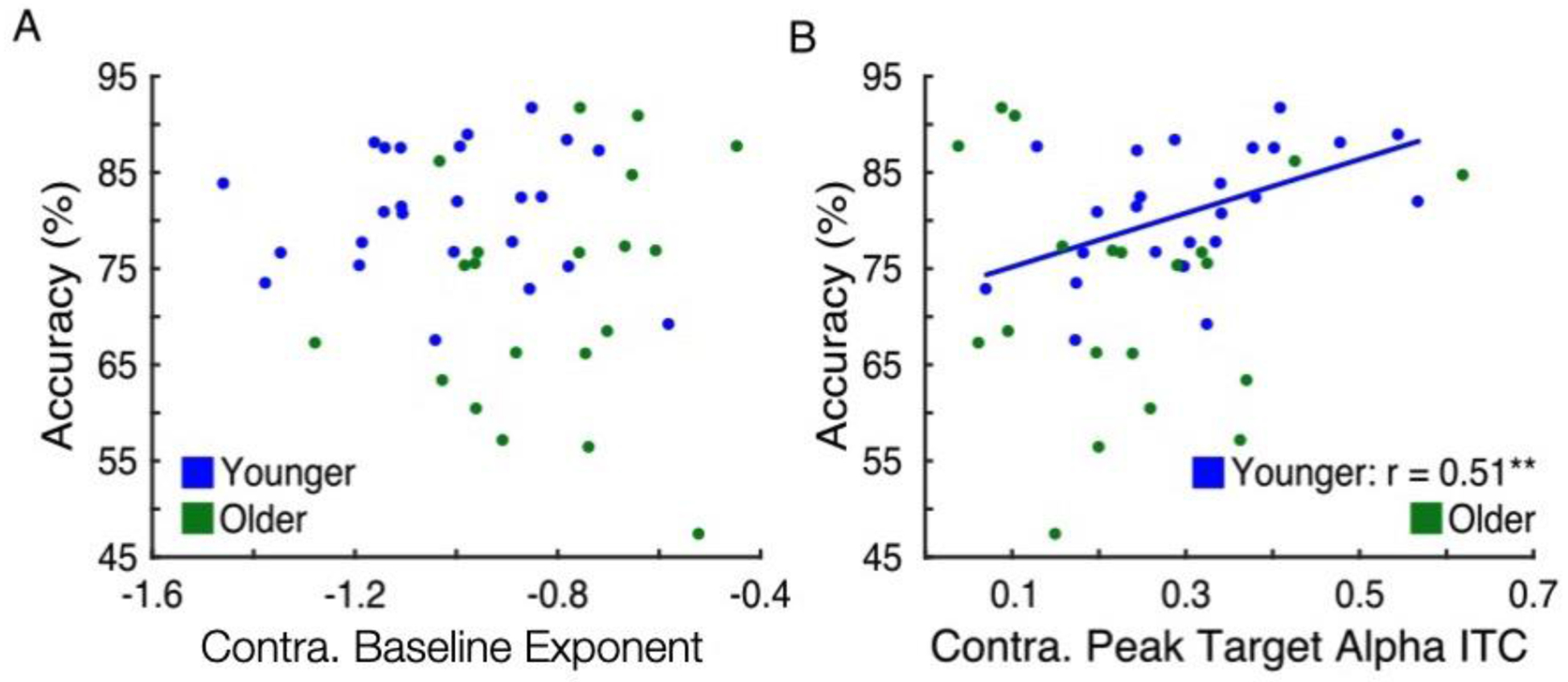
Baseline noise was not correlated with behavioral accuracy, but decreased response variability was associated with higher accuracy in younger but not older adults. (A) Accuracy across all cue conditions versus contralateral baseline spectral exponent in younger (blue) and older (green) adults. No relationship was found. (B) Accuracy versus contralateral peak target-evoked alpha ITC in younger (blue) and older (green) adults, with best-fit line for younger adults. In younger but not older adults, higher alpha ITC was associated with higher accuracy (** p < 0.01).
Discussion
In this study, we sought to examine the relationship between two types of neural noise—tonic baseline noise and trial-by-trial response variability—as well as how age-related changes in either relate to cognitive decline. We compared younger and older adults’ behavioral performance and visual cortical activity during a spatial visual discrimination task. Analysis of visual posterior EEG activity demonstrated that pre-trial baseline noise, characterized using the spectral exponent, was elevated in older adults relative to younger adults. In addition, we found that older adults also had greater inconsistency in their alpha phase responses to target presentation. In younger adults, alpha phase response consistency was correlated with higher accuracy, though not so in older adults. In older—but not younger—adults, higher baseline noise was associated with greater target-evoked response variability.
Concerning behavioral performance, older adults had longer response times relative to younger adults in each cue condition, but lower accuracy only when no spatial information was provided about upcoming targets. Comparable accuracy in task conditions where spatial information was provided indicates that both groups were using provided cue information to guide behavior. In the absence of spatial information, however, the briefness of target presentation and any difficulty in quickly distinguishing targets from non-targets (as in the 0% certainty condition, participants were unaware of the visual hemifield in which targets would eventually appear) may have contributed to lower accuracy in older adults.
As has been found in previous studies, pre-trial spectral exponent was flatter in older adults (Voytek et al., 2015; Waschke et al., 2017; McNair et al., 2018). More broadly, the spectral exponent of neural activity has been studied in the context of neural population spiking statistics (Voytek et al., 2015; Voytek and Knight, 2015; Gao, 2015), excitatory-inhibitory (EI) balance (Gao et al., 2017), cortical activation (Podvalny et al., 2015), and neural irregularity (Waschke et al., 2017). That the spectral exponent is flatter in older adults is thus consistent with previous findings of age-related increases in spontaneous, asynchronous baseline activity (Hong and Rebec, 2012) and could be indicative of age-related shifts in EI balance (Gao et al., 2017) and reductions in inhibitory signaling (Hickmott and Dinse, 2013). Several recent studies have also demonstrated the clinical importance of spectral exponent, including in deep brain stimulation treatment for depression (Veerakumar et al., 2019), as well as in pharmacological treatment for schizophrenia (Molina et al., 2020) or ADHD (Robertson et al., 2019).
Additionally, target-evoked alpha ITC was elevated in younger adults and correlated with behavioral performance in younger but not older adults. This relationship between target-evoked alpha phase response consistency and behavioral performance is consistent with previous findings (Klimesch et al., 2004; Hanslmayr et al., 2005; Werkle-Bergner et al., 2012; Yamagishi et al., 2008; Tran et al., 2016) and indicates that the degree to which cortical neural populations can precisely respond to external stimuli directly influences behavioral outcomes in younger adults. Age-related reductions in stimulus-evoked alpha phase consistency, as reported here and previously (Tran et al., 2016), suggest that such precision in sensory processing is reduced in older adults. Notably, the behavioral performance of older adults was also more variable, so it may be that the lower alpha ITC observed among older adults may be partially driven by this group-wise variability. At the same time, the lack of correlation between target-evoked alpha phase response consistency and accuracy across older adults in this study suggests that later processes are themselves altered as to account or compensate for these changes in early sensory processing.
Other studies, however, have reported no difference (Werkle-Bergner et al., 2012) or even elevated stimulus-evoked alpha ITC in older adults (Sander et al., 2012; Wiegand and Sander, 2019). These reported increases in alpha ITC are hypothesized to reflect older adults’ excessive neural entrainment to external stimuli, with such entrainment impeding subsequent processes necessary for successful behavioral outcomes. Contradictory results between these studies and the present study could be, and are likely due to, differences in the properties of stimuli being presented. For instance, here and in Tran et al., 2016, age-related changes in stimulus-evoked alpha phase responses were investigated for visual stimuli presented for only 50 ms durations. As such, these visual stimuli could have been insufficient to entrain older adults in the manner demonstrated in Sander et al., 2012, and Wiegand and Sander, 2019, which examined the alpha phase responses to visual stimuli of 100 ms duration and 85 dB tones, respectively. Moreover, that visual targets in this study were only 50 ms in duration, such duration being less than one alpha oscillation cycle in length, suggests that target presentation sometimes fell partially or even entirely during alpha phases non-optimal for sensory processing (Busch and VanRullen, 2010; Mathewson et al., 2009). If alpha phase effects are more pronounced in older adults–for instance, if older adults have larger differences in perceptual processing between optimal and non-optimal alpha phases–these changes could lead to more variable evoked responses to briefly presented stimuli. This might account for the inconsistency in target-evoked alpha phase responses we observed here in older adults.
As hypothesized, pre-trial spectral exponent was correlated with target-evoked alpha ITC in older adults, with flatter spectral exponent associated with reduced alpha phase response consistency. This result suggests that age-related changes in neural activity, such changes apparent even in the absence of external stimuli, might hamper older adults’ ability to consistently respond to and process upcoming stimuli. This might occur due to a shift towards a more excitatory state (Gao et al., 2017) wherein increased asynchrony in or rate of spiking activity (Voytek et al., 2015; Voytek & Knight, 2015) might reduce the consistency of visual stimulus processing. In addition, increased age-related noise in ongoing neural activity, if such noise persists through and after stimulus presentation, could dominate relatively weak or inconsistent responses to short-duration stimuli. Interestingly, a relationship between pre-trial spectral exponent and target-evoked alpha ITC was not present in younger adults. These results suggest that at the levels of baseline noise shown in younger adults, phase response consistency is largely unaffected by individual differences in ongoing baseline noise. In older adults, on the other hand, elevations in baseline noise levels might point to profound changes in ongoing neural activity that, if more greatly exacerbated, lead to greater effects on subsequent sensory processing.
Conclusion
Overall, we find that baseline noise and stimulus-evoked trial-by-trial response variability are both elevated during healthy aging, with increases in baseline noise associated with increased alpha phase response variability across older adults. Thus, age-related changes in sensory processing may be due in part to alterations in neural population activity that can be observed in ongoing, non-stimulus-evoked activity. These alterations may include age-related increases in neural asynchrony or EI balance, with such increases potentially affecting the capacity of older adults to reliably respond to upcoming stimuli. These findings point to the need for greater understanding of the ways in which age-related changes in ongoing neural activity or neural noise in general might affect neural mechanisms supporting sensory processing and cognition.
Acknowledgements
Tran is supported by the UC San Diego-NIH Institute for Neural Computation Training Program in Cognitive Neurosciences and a UC San Diego Achievement Rewards for College Scientists. Voytek is supported by a Sloan Research Fellowship (FG-2015-66057), the Whitehall Foundation (2017-12-73), the National Science Foundation under grant BCS-1736028, the NIH National Institute of General Medical Sciences grant R01GM134363-01, and a UC San Diego, Shiley-Marcos Alzheimer’s Disease Research Center (ADRC): Research Training in Alzheimer’s Disease grant.
Footnotes
The authors declare no competing financial interests.
References
- Berens P CircStat: A MATLABToolbox for Circular Statistics. J Stat. Soft 31, 1–21 (2009). [Google Scholar]
- Bruns A Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? Journal of Neuroscience Methods 137, 321–332 (2004). [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J & VanRullen R The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 29, 7869–7876 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer R & Zeef EJ What Kind of Noise Increases With Age? Journal of Gerontology 42, 515–518 (1987). [DOI] [PubMed] [Google Scholar]
- Delorme A & Makeig S EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods 134, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ & Behrmann M Neural variability: friend or foe? Trends in Cognitive Sciences 19, 322–328 (2015). [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LPJ & Wolpert DM Noise in the nervous system. Nat Rev Neurosci 9, 292–303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R Interpreting the electrophysiological power spectrum. Journal of Neurophysiology 115, 628–630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Peterson EJ & Voytek B Inferring synaptic excitation/inhibition balance from field potentials. NeuroImage 158, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, Gradye CL Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci Biobehav Rev 37(4), 610–624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M et al. Parameterizing neural power spectra. bioRxiv 299859 (2018). [Google Scholar]
- Hanslmayr S et al. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neuroscience Letters 375, 64–68 (2005). [DOI] [PubMed] [Google Scholar]
- Hartmann C, Lazar A, Nessler B, & Triesch J Where’s the Noise? Key Features of Spontaneous Activity and Neural Variability Arise through Learning in a Deterministic Network. PLoS Comput Biol 11(12) 292–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ Scale-free brain activity: past, present, and future. Trends in Cognitive Sciences 18, 480–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickmott P & Dinse H Effects of Aging on Properties of the Local Circuit in Rat Primary Somatosensory Cortex (S1) In Vitro. Cerebral Cortex 23, 2500–2513 (2013). [DOI] [PubMed] [Google Scholar]
- Hong SL & Rebec GV A new perspective on behavioral inconsistency and neural noise in aging: compensatory speeding of neural communication. Frontiers in Aging Neuroscience 4, 1–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W et al. Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Cognitive Brain Research 19, 302–316 (2004). [DOI] [PubMed] [Google Scholar]
- Kügler CF, Taghavy A & Platt D The event-related P300 potential analysis of cognitive human brain aging: a review. Gerontology 39, 280–303 (1993). [DOI] [PubMed] [Google Scholar]
- Leek MR Adaptive procedures in psychophysical research. Percept Psychophys 63, 1279–1292 (2001). [DOI] [PubMed] [Google Scholar]
- Lorenzo-López L, Amenedo E, Pazo-Álvarez P & Cadaveira F Visual target processing in high- and low-performing older subjects indexed by P3 component. Neurophysiologie Clinique/Clinical Neurophysiology 37, 53–61 (2007). [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM & Ro T To See or Not to See: Prestimulus Alpha Phase Predicts Visual Awareness. Journal of Neuroscience 29, 2725–2732 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair SW, Kayser SJ & Kayser C Consistent pre-stimulus influences on auditory perception across the lifespan. NeuroImage 186, 22–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG & Nijs den, M. Power-Law Scaling in the Brain Surface Electric Potential. PLoS Comput Biol 5, e1000609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JL et al. Memantine effects on EEG measures of putative excitatory/inhibitory balance in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvalny E et al. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114, 505–519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM, Furlong S, Voytek B, Donoghue T, Boettiger CA, Sheridan MA EEG Power Spectral Slope Differs by ADHD Status and Stimulant Medication Exposure in Early Childhood. J Neurophysiol 122(6), 2427–2437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle CE, Anguera JA, Skinner SN, Voytek B & Gazzaley A Enhancing Spatial Attention and Working Memory in Younger and Older Adults. Journal of Cognitive Neuroscience 29, 1483–1497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle CE, Voytek B & Gazzaley A Exploring the Potential of the iPad and Xbox Kinect for Cognitive Science Research. Games for Health Journal 4, 221–224 (2015). [DOI] [PubMed] [Google Scholar]
- Sander MC, Werkle-Bergner M & Lindenberger U Amplitude modulations and inter-trial phase stability of alpha-oscillations differentially reflect working memory constraints across the lifespan. NeuroImage 59, 646–654 (2012). [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD & Carandini M Cortical State Determines Global Variability and Correlations in Visual Cortex. Journal of Neuroscience 35, 170–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TC, Sreekumar V, Inati SK & Zaghloul KA Signal Complexity of Human Intracranial EEG Tracks Successful Associative-Memory Formation across Individuals. Journal of Neuroscience 38, 1744–1755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Hoffner NC, LaHue SC, Tseng L & Voytek B Alpha phase dynamics predict age-related visual working memory decline. NeuroImage 143, 196–203 (2016). [DOI] [PubMed] [Google Scholar]
- Veerakumar et al. , Field Potential 1/f Activity in the Subcallosal Cingulate Region as a Candidate Signal for Monitoring Deep Brain Stimulation for Treatment Resistant Depression. Journal of Neurophysiology 122(3), 1023–1035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B & Knight RT Dynamic Network Communication as a Unifying Neural Basis for Cognition, Development, Aging, and Disease. Biological Psychiatry 77, 1089–1097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B et al. Preparatory Encoding of the Fine Scale of Human Spatial Attention. Journal of Cognitive Neuroscience 29, 1302–1310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B et al. Age-Related Changes in 1/f Neural Electrophysiological Noise. Journal of Neuroscience 35, 13257–13265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke L, Tune S & Obleser J Local cortical desynchronization and pupil-linked arousal differentially shape brain states for optimal sensory performance. eLife 8, e51501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke L, Wöstmann M & Obleser J States and traits of neural irregularity in the age-varying human brain. Scientific Reports 7, 17381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkle-Bergner M, Freunberger R, Sander MC, Lindenberger U & Klimesch W Inter-individual performance differences in younger and older adults differentially relate to amplitude modulations and phase stability of oscillations controlling working memory contents. NeuroImage 60, 71–82 (2012). [DOI] [PubMed] [Google Scholar]
- Wiegand I & Sander MC Cue-related processing accounts for age differences in phasic alerting. Neurobiology of Aging 79, 93–100 (2019). [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Anderson SJ & Kawato M Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Research 1197, 115–122 (2008). [DOI] [PubMed] [Google Scholar]


