Abstract
IMPORTANCE
Hospitalizations for worsening heart failure (WHF) represent an enormous public health and financial burden, with physicians, health systems, and payers placing increasing emphasis on hospitalization prevention. In addition, maximizing time out of the hospital is an important patient-centered outcome. In this review, we discuss the concept of outpatient WHF, highlight the rationale and data for the outpatient treatment of WHF as an alternative to hospitalization, and examine opportunities and strategies for developing outpatient “interceptive” therapies for treatment of worsening symptoms and prevention of hospitalization.
OBSERVATIONS
Worsening heart failure has traditionally been synonymous with an episode of in-hospital care for worsening symptoms. While WHF often leads to hospitalization, many patients experience WHF in the outpatient setting and carry a similarly poor prognosis. These findings support WHF as a distinct condition, independent of location of care. For those that are hospitalized, most patients have an uncomplicated clinical course, with diuretics as the only intravenous therapy. Although complicated scenarios exist, it is conceivable that improved tools for outpatient management of clinical congestion would allow a greater proportion of hospitalized patients to receive comparable care outside the hospital. Most patients with WHF have a gradual onset of congestive signs and symptoms, offering a potential window in which effective therapy may abort continued worsening and obviate the need for hospitalization. To date, outpatient WHF has received minimal attention in randomized clinical trials, but this high-risk group possesses key features that favor effective clinical trial investigation.
CONCLUSIONS AND RELEVANCE
As the public health and economic burdens of heart failure continue to grow, recognizing the entity of outpatient WHF is critical. Efforts to reduce heart failure hospitalization should include developing effective therapies and care strategies for outpatient WHF. The outpatient WHF population represents a major opportunity for therapeutic advancements that could fundamentally change heart failure care delivery.
For most patients with heart failure (HF), the clinical course is characterized by periods of clinical stability of variable duration punctuated by episodes of worsening symptoms that often require hospitalization. Each subsequent hospitalization is associated with further increased risk of death and worsening quality of life.1–3 However, while hospitalization is consistently marked by high postdischarge event rates, the nature and time course of clinical deterioration that precedes hospitalization is highly variable. A minority of patients may experience relatively rapid and severe deterioration (eg, arrhythmia, “flash” pulmonary edema, ischemia) and require immediate attention in the emergency department (ED).4 By contrast, most may have worsening symptoms over a more prolonged period and have at least some contact with outpatient clinicians before hospitalization.5 This extended time course of clinical worsening offers a potential window of opportunity for intervention before hospitalization that has not previously been a focus for HF therapeutics.
Given the enormous public health and financial burden of HF, there has been increasing emphasis placed on preventing recurrent HF hospitalization among clinicians, health systems, and payers. While data suggest that some early readmissions may be preventable, most interventions aimed to reduce readmissions have been ineffective.6–8 From a patient perspective, maximizing “home time” (ie, time alive and out of a health care institution) and minimizing presentations to the ED and hospital are important patient-centered outcomes.9,10 Accordingly, in an era of increasing focus on patient-centered care, as well as continued reimbursement and practice improvement pressure, increased consideration for the management of worsening HF (WHF) as an outpatient is warranted. Indeed, the possibility of safe and effective outpatient WHF management as an alternative to hospitalization for even a segment of this population could yield significant cost savings and improvements in patient quality of life. Using this framework, we discuss the concept of outpatient WHF, defined as the deterioration of HF signs and symptoms in a patient with chronic HF after a period of clinical stability that requires escalation of therapy without an urgent need for hospitalization or ED presentation. In this review, we describe outpatient WHF in the context of prior randomized clinical trials and available data, review the rationale and data for the outpatient treatment of WHF as an alternative to hospitalization, and examine opportunities and strategies for developing outpatient “interceptive” therapies for the treatment of worsening symptoms and prevention of hospitalization. For purposes of discussion, we did not consider treatment in the ED or observation unit as outpatient care, as this has been discussed previously11,12 and is not the focus of this review. Although such episodes may not meet formal inpatient criteria for purposes of billing, they use hospital resources, staff members, and infrastructure and may be indistinguishable from hospital admission from the patient’s perspective (eg, extended time or an overnight stay in the hospital and away from home).
WHF as an Entity Outside the Hospital and ED
While sometimes used interchangeably, WHF should be differentiated from the term acute HF in that it excludes “de novo” patients and requires a chronic HF diagnosis.13 Nonetheless, both terms have. traditionally been synonymous with an episode of hospital-based care for worsening symptoms.14 Thisoverlap between WHF and hospitalized HF contributed to widespread appreciation of hospitalization as a sentinel event in the natural history of the condition. Indeed, observational studies found subsequent mortality rates for patients hospitalized for HF to be 3-fold higher than for patients who were never hospitalized, with risk increasing further with each admission.1,15 These findings, in combination with biomarker data, led to a hypothesis that hospitalized HF represented a distinct pathophysiological process analogous to acute myocardial infarction, a period of irreversible end-organ injury that left patients with a permanently heightened risk of adverse outcomes.16,17
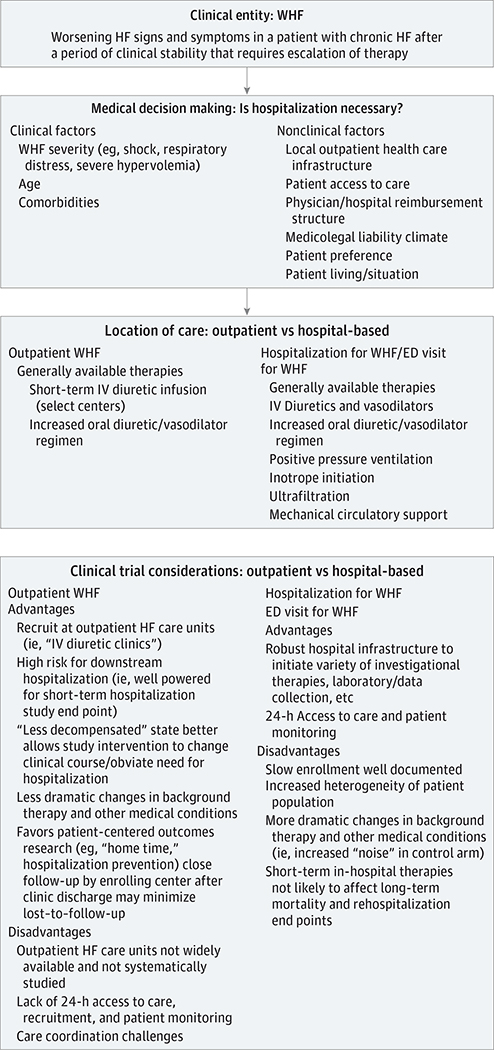
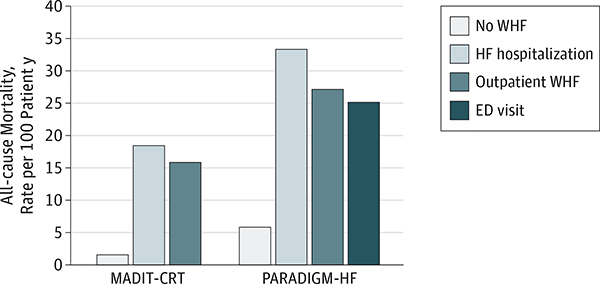
However, emerging data suggest that the concept of WHF and the clinical event of hospitalization should be disentangled (Figure 1; Box).14,18–20 While WHF often leads to hospitalization, patients may experience WHF in the outpatient setting, and hospitalization or ED presentation may be avoided with adequately adjusted therapy. Reconciling how a location of care (ie, the hospital) could be consistently linked to a biologic process is problematic. Although cardiac troponin may be detectable in as many as 90% of patients hospitalized for HF (without suspicion of having acute coronary syndrome), troponin elevation is not unique to hospitalized patients or confined to the duration of the hospital stay, with data supporting a high prevalence of persistent or new troponin elevations after hospital discharge despite clinical stability.21,22 Although hospitalization may identify patients with WHF who are at high risk, patients with WHF who are not hospitalized carry a similarly poor prognosis (Figure 2).18,19 Rather, the decision for patient hospitalization is inherently subjective, influenced by many nonclinical factors, including patient preferences, access to care, global and regional practice patterns, and health system reimbursement and liability incentives. For these reasons, it is unsurprising that despite numerous attempts with statistical modeling and machine learning, our ability to predict HF hospitalization remains poor.23–25 For purposes of reflecting patient risk of mortality and HF progression, we suggest the recognition of WHF as the key event in the natural history of HF, independent of where care is delivered.14 Although the epidemiology of outpatient WHF in routine clinical practice remains unknown and future work is needed to clarify practical means of identifying these episodes for systematic study, limiting attention to only patients hospitalized for WHF significantly underestimates the true WHF burden. Hospitalization, on the other hand, may be better viewed as a health care resource and treatment strategy.
Figure 1. Conceptual Framework for Worsening Heart Failure (WHF).
Worsening heart failure may be managed in both the outpatient and inpatient settings, as determined by many factors. Studying the inpatient vs outpatient WHF populations in randomized clinical trials presents specific advantages and disadvantages for successful clinical trial execution. ED indicates emergency department; HF, heart failure; IV, intravenous.
Box. Summary Points.
Worsening HF is common and defined as worsening HF signs and symptoms in a patient with chronic HF after a period of clinical stability that require escalation of therapy.
Worsening HF is a clinical entity independent of the location of care. Worsening HF can be managed as an inpatient, in the ED, or as an outpatient. The choice of location of care is inherently subjective and subject to multiple potential clinical and nonclinical factors.
There are no strong evidence-based guideline recommendations or therapies for the management of WHF other than optimization of chronic HF therapy.
Outpatient WHF is under-recognized as an area of clinical need, and these patients are not traditionally enrolled in WHF clinical trials.
Outpatient management of WHF is a viable alternative for a large proportion of the WHF population. Most patients with WHF have a relatively slow onset and progression of symptoms, providing a “window” during which successful outpatient intervention may restore baseline clinical status without the need for hospitalization or an ED visit.
- Current outpatient management approaches for WHF empirically center on:
- Escalation of oral diuretic and/or vasodilator regimens.
- Referral to an outpatient HF care unit for intravenous diuretic therapy.
- The outpatient WHF population is highly conducive to study in randomized clinical trials. Specific advantages include:
- Outpatient HF care units/“diuretic clinics” are becoming more common, routinely capture extensive patient data, and often spend several hours with patients at each visit. These clinics are an increasingly viable setting for clinical trial recruitment.
- Outpatients are generally more stable and less prone to changes in background therapy, thus improving “signal-to-noise” ratios for a clinical trial “control” group.
- The inherently high risk of WHF progression despite outpatient treatment favors even smaller studies having adequate power to assess downstream effects on a HF hospitalization, “days alive and out of the hospital,” or “home time” trial end point.
- Favors patient-centered outcomes research (eg, “home time,” hospitalization prevention).
There is an urgent unmet need for developing effective and evidence-based therapy for outpatient WHF. Agents that reduce congestion and have high bioavailability may offer the most promise. The development of subcutaneous drug delivery approaches offers the possibility of effective decongestion in the home without the need for intravenous access and/or hospitalization.
Abbreviations: ED, emergency department; HF, heart failure; WHF, worsening heart failure.
Figure 2. Heart Failure (HF) Randomized Clinical Trials Describing All-cause Mortality Rates for Patients With Worsening Heart Failure (WHF) Categorized by the Location of Care.
Worsening HF is associated with a high subsequent risk of death, irrespective of treatment as an outpatient, inpatient, or in the emergency department (ED). The outpatient WHF definition in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) was signs and/or symptoms consistent with HF and outpatient treatment with intravenous decongestive therapy. The outpatient WHF definition in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial was 1 or more of the following without associated hospitalization or ED visit: (1) sustained increase in diuretic dose for 1 month, (2) intravenous treatment for HF, and (3) the addition of a new drug for treating WHF. Data from Skali et al18 and Okumura et al.19
Treatment of Outpatient WHF as an Alternative to Hospitalization
Signs and symptoms of congestion (eg, dyspnea, orthopnea, and peripheral edema) are the most common reasons for HF hospitalization and are the focus of initial therapy.26 While systematic and validated tools for guiding decisions regarding need for hospitalization do not exist, clinical experience finds hospitalization unquestionably necessary for many patients with WHF, such as those with cardiogenic shock, respiratory failure, and unstable arrhythmia. However, the value and necessity of hospitalization can be debated for a significant proportion of patients with WHF and is likely influenced by many “nonbiologic” but important factors, such as local health care resources and patient preferences. For these patients, the reason for hospitalization may most closely relate to a lack of readily accessible outpatient therapies that can quickly improve symptoms rather than true safety concerns. Nonetheless, to consider alternatives to hospitalization and the potential “opportunity cost” of outpatient WHF management, one must reflect on the state of contemporary in-hospital HF treatment.
Unfortunately, despite decades of randomized clinical trials, the treatment of acute HF is largely unchanged from the 1970s and there remain no class I, level of evidence A guideline recommendations for patients hospitalized with HF.27,28 The current treatment for patients is primarily symptomatic and remains heavily centered on the empiric use of diuretics to relieve clinical congestion. United States registry data highlight that more than 90% of patients with HF receive intravenous (IV) diuretics during hospitalization, with minimal use of any other IV therapy.29,30 For many patients, an escalated diuretic regimen may represent the only added in-hospital treatment. No formal evidence-based or guideline recommended discharge criteria exist. Most patients have a relatively rapid and robust symptomatic response to standard diuretic-based therapy, with as many as 76% reporting dyspnea improvement within 6 hours of presentation.31 Although more complicated scenarios do exist, this relatively crude and simple in-hospital care plan supports the hypothesis that a proportion of patients with WHF could safely and effectively receive comparable care outside the hospital.
Outpatient WHF as an Opportunity for Intervention
For most patients who are eventually hospitalized for WHF, the term acute HF may be a misnomer. For these patients, the onset of signs and symptoms is not sudden, but rather follows a gradual onset over days to weeks that eventually prompts urgent treatment. For example, of 3580 patients in the EuroHeart Failure Survey II,4 2327 (65%) presented with decompensated HF (ie, a gradual onset of symptoms) as opposed to more sudden presentations, such as acute pulmonary edema, cardiogenic shock, or hypertensive HF. Similarly, data from ambulatory hemodynamic monitoring show that gradual increases in filling pressures tend to occur weeks before the eventual hospitalization.5 Moreover, clinical experience suggests that many patients with HF contact outpatient clinicians with worsening symptoms without feeling compelled to present directly to the ED.
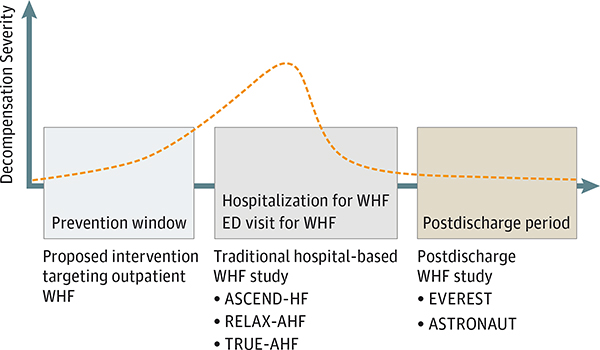
Given the often observed interval between the onset of worsening symptoms and eventual HF hospitalization, a compelling but not yet adequately tested strategy would be to intervene earlier in the process of HF decompensation to decongest the patient and obviate the need for hospitalization. Considering the persistent challenges in successful drug development in hospitalized WHF populations, such a strategy of targeting outpatient WHF in the “prevention window” upstream of hospitalization may offer key benefits for study in randomized clinical trials (Figure 3). This window would not encompass periods of clinical stability, as targeted with conventional and experimental telemonitoring strategies, but rather would apply to the time when patient-reported symptoms or objective measures first begin to worsen. As a potentially less severe manifestation of WHF, early decompensation during the “prevention window” may be more amenable to successful intervention and diminish the time needed for the patient to return to their clinical baseline.
Figure 3. Schematic Representation of the Time Course of Decompensation.
This representation highlights traditional randomized clinical trial enrollment and treatment strategies compared with the proposed approach of introducing the study intervention upstream of hospitalization in the outpatient worsening heart failure (WHF) setting. The randomized clinical trials listed are representative examples of each strategy (ie, lists are not all-inclusive). The orange dashed line represents the severity of decompensation. ED indicates emergency department.
Current Tools and Approaches for Outpatient WHF Management
Diagnosis
The traditional diagnosis of WHF, irrespective of the care setting, has been reactive and generally dependent on patients manifesting overt signs and symptoms of hypervolemia. Targeting a more active approach, a wealth of prior and ongoing research has been conducted with implantable cardiac monitors, including incorporation within implantable cardioverter-defibrillator systems.32,33 For current clinical practice, the regulatory approval of implantable hemodynamic monitoring with the CardioMEMS HF system (Abbott Laboratories) may have particular relevance for outpatient WHF diagnosis.34 Compelling evidence across randomized clinical trial and “real-world” experiences support a robust reduction in HF hospitalization with use of the device, with the mechanism of benefit likely mediated by an increased number of targeted changes to diuretic and vasodilator therapy.34–36 Although uptake has been slow, the cost-effectiveness debated, and the relative safety and durability in routine practice less well-defined, available data support the device as a potential strategy to characterize the leading edge of the “prevention window” and identify outpatient patients with WHF who stand to benefit from targeted intervention.37,38
Treatment
Currently, outpatient treatment of WHF is generally limited to 2 primary strategies, including (1) referral to an outpatient HF care unit for intravenous diuretic therapy (less common) or (2) prescription of an augmented oral diuretic or vasodilator regimen (more common). Despite a lack of rigorous data, clinical experience would suggest that outpatient initiation of IV inotropes for WHF is rare. Likewise, while patients who are progressing from stage C to stage D endstage HF may undergo outpatient evaluations for ventricular assist devices or transplantation, this subset comprises a small minority of the general HF population.39
Recognizing that diuresis is the primary intervention during the inpatient stay and that most patients quickly respond to therapy (ie, within few hours), many centers have developed specialized multidisciplinary outpatient clinics in efforts to facilitate the early treatment of worsening congestion and decrease hospitalizations.40 Outpatients who are experiencing WHF symptoms can obtain same-day appointments or walk-in visits with treatment teams composed of physicians, nurse practitioners, nurses, and social workers.40 This team then provides an evaluation via clinical and laboratory diagnostics and is equipped to initiate appropriately targeted therapies, including IV diuretics, electrolyte repletion, and titration of evidence-based medications. Few centers have published their experiences with such outpatient WHF treatment facilities.40–44
Duke University Hospital initiated a same-day access clinic in 2012 after observing that more than 50% of HF admissions admitted from the ED were relatively low-risk, with the primary treatment need limited to decongestion.41 In the first 3 years of operation, the clinic handled more than 3000 patient visits, leading to a concurrent 10% reduction in 30-day readmissions and an avoidance of financial penalties for excess readmission.41 While broad uptake and systematic study of the outpatient HF care unit as a management strategy remains to be seen, results from select centers have been encouraging.40 Nonetheless, it is clear that the target patient population for such an approach exists at every medical practice, regardless of whether such specialized care pathways are already in place. Limited data suggest that the population seen in such clinics may mirror a particularly high-risk hospitalized WHF population. Specifically, patients typically have New York Heart Association class III to IV symptoms; significant elevation in natriuretic peptide levels; a high prevalence of comorbidities, including chronic kidney disease; and a high maintenance dose of loop diuretic.41–43
Referral to an outpatient HF care unit may not be possible or deemed medically necessary in all situations. The more conventional approach to outpatient WHF management involves an intensification of oral medical therapy to target worsening congestion. Despite clinical experience suggesting that this situation is extremely common, to our knowledge, there are no rigorous data that characterize the frequency of this clinical situation, the typical patient profile, or the resultant outcomes in routine practice. Common clinical approaches include a temporary empiric increase in daily oral loop diuretic dose or the addition of a second diuretic, such as metolazone or spironolactone. For patients with uncontrolled hypertension, escalated vasodilator regimens may be prescribed. These medication changes can occur following either an in-person clinic assessment or patient telephone calls and are frequently triaged by HF nurses and support staff. Depending on the patient’s response to therapy and clinical judgement, follow-up phone calls, clinic visits, laboratory assessments, and/or hospital admissions are prescribed.
Randomized Clinical Trial Considerations for Outpatient WHF
Despite the urgent need for evidence-based care strategies and interventions, the outpatient WHF population has received minimal attention within randomized clinical trials. Phase III WHF trials of investigational therapies completed to date have exclusively enrolled hospitalized patients (Table).45–53 Only recently have some larger phase II studies targeted the broader WHF population, irrespective of inpatient or outpatient treatment.54,55 Nevertheless, the high-risk outpatient WHF group possesses several key features that favor effective randomized clinical trial investigation. For example, the outpatient WHF population may be less prone to changes in background therapy, hemodynamics, and symptoms as compared with hospitalized patients, allowing for a more stable control group against whom to test novel therapies (ie, improved “signal-to-noise” ratio). In addition, to facilitate an effective study, it is important to consider potential clinical trial end points, patient recruitment settings, and the types of therapies that may be promising.
Table.
Examples of Prior Clinical Trials of Worsening Heart Failure
| Clinical Trial | Study Drug | Key Results |
|---|---|---|
| Inpatient Enrollment; Short-term In-hospital Intravenous Study Therapy | ||
| OPTIME45 | Milrinone | Neutral for primary end point of cumulative d of hospitalization for CV cause within 60 d did not differ by treatment arm. Hypotension requiring intervention and new atrial arrhythmias were more frequent with milrinone. |
| VERITAS46 | Tezosentan | Neutral for coprimary end points of dyspnea relief over 24 h and the incidence of death or worsening heart failure at 7 d. |
| PROTECT47 | Rolofylline | Neutral for the primary end point of treatment success, treatment failure, or no change in the patient’s clinical condition; defined according to survival, heart failure status, and changes in renal function. |
| ASCEND-HF48 | Nesiritide | Neutral for the coprimary end points of change in dyspnea at 6 h and 24 h and the composite end point of rehospitalization for heart failure or death within 30 d. |
| REVIVE-I and II49 | Levosimendan | Levosimendan resulted in more frequent hypotension and cardiac arrhythmias during the infusion period and a numerically higher risk of death. |
| RELAX-AHF50 | Serelaxin | Positive for 1 of the 2 dyspnea end points. Patients who were treated with serelaxin had fewer deaths at 180 d. Topline results of the confirmatory RELAX-AHF2 trial reported as neutral for the coprimary end points of 180-d CV mortality and worsening heart failure through day 5. |
| TRUE-AHF51 | Ularitide | Neutral for coprimary end points of CV mortality over the duration of the trial follow-up and hierarchical clinical composite measuring the change in symptoms, presence of persistent or worsening in-hospital heart failure, and death during the first 48 h. |
| Inpatient Enrollment; Oral Study Therapy Initiated In-hospital and Continued as Outpatient | ||
| EVEREST52 | Tolvaptan | Neutral for the coprimary end points of all-cause mortality and CV death or hospitalization for heart failure. |
| ASTRONAUT53 | Aliskiren | Neutral for the primary end point of CV death or heart failure rehospitalization at 6 mo. |
| Inpatient or Outpatient Enrollment; Oral Study Therapy Continued as Outpatient | ||
| SOCRATES-REDUCED54 | Vericiguat | Neutral for the primary end point of change in log-transformed NT-proBNP from baseline to 12 weeks. |
| SOCRATES-PRESERVED55 | Vericiguat | Neutral for the coprimary end points of change in log-transformed NT-proBNP and left atrial volume from baseline to 12 weeks |
Abbreviations: CV, cardiovascular; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Randomized Clinical Trial End Point Considerations
Continued symptom progression despite escalating outpatient therapy is common in the outpatient WHF population. Thus, while hospitalization is imminent for many patients, even a modestly sized clinical trial could offer ample study power for detecting short-term (eg, 30-day) differences in a hospitalization, “days alive and out of the hospital,” or a “home time” trial end point. In addition, akin to traditional inpatient HF clinical trials, longer-term time-to-event mortality and hospitalization end points would be possible and may be required for regulatory approval.
Recruitment Considerations
The growing number of outpatient HF care units are increasing the promise and practicality of these clinics as prime clinical trial recruitment tools. Currently, enrollment from outpatient HF units is not traditionally done, with most HF clinical trials enrolling stable outpatients during routine follow-up or patients with WHF during hospitalization. Thus, patient recruitment in the outpatient HF care unit environment would not face significant competition from other studies. Moreover, patients in these outpatient units are typically monitored closely for several hours while therapies are administered, presumably allowing adequate time for clinical trial personnel to engage patients, perform screening, gather consent, perform randomization, and collect baseline data. As standard practice, HF care units perform a medical history, physical examination, and a careful medication reconciliation at each visit, offering the possibility to integrate data already routinely collected into an electronic case report form for streamlined trial conduct. In addition, by design, all patients who attend outpatient HF care units are followed closely by the enrolling center after clinic discharge, which may minimize problems with loss-to-follow-up and withdrawn consent compared with conventional inpatient WHF clinical trial populations.
Study Therapy Considerations
While the outpatient WHF population holds potential for studying many investigational HF therapies and care strategies, the burden of clinical congestion among these patients and the published data from outpatient HF care units suggest that therapies that target diuretic response (ie, defined as the change in weight per 40 mg of oral furosemide equivalent) may be especially promising. Indeed, the combination of high background rates of chronic kidney disease with generally high daily doses of maintenance diuretic supports diuretic resistance as a preeminent problem for these patients. Poor diuretic response is a common management challenge for clinicians and data consistently show strong negative prognostic implications.56–58 While rigorous evidence supporting best practices for treating diuretic resistance does not exist, traditional empiric approaches overlap with currently used treatments for outpatient WHF, including increasing the oral loop diuretic regimen, changing the oral loop diuretic (eg, from furosemide to torsemide), adding an adjunctive nonloop diuretic (eg, metolazone), and administering IV diuretics on an inpatient or outpatient basis. Among these strategies, given the potential for a significant reduction in bioavailability when patients enter the decompensated state, an escalation of oral diuretics is generally less effective than IV administration.59
Given the near ubiquitous use of IV loop diuretics in the treatment of WHF and diuretic resistance, research efforts have focused on developing compounds for subcutaneous (SC) delivery to improve bioavailability over standard oral medications.60,61 Such an approach could include an automated wearable patch and pump (similar in appearance to an insulin pump used for patients with diabetes) that would deliver the drug continuously, or a schedule of intermittent SC injections. The theoretical advantages of SC delivery could be enormous for patients with WHF who present to either outpatient diuretic clinics or the hospital. After an initial stabilization and standard IV diuretic therapy, patients could be discharged from either setting with a means of continued parenteral decongestion at home by using a highly bioavailable decongestive agent to further reduce symptom burden and risk of downstream HF hospitalization.
One such specific therapy in development is SC furosemide. Preliminary published data for this agent are encouraging, and randomized clinical trials are ongoing.61,62 One such study, the Subcutaneous Furosemide in Acute Decompensated Heart Failure trial (NCT03170219), is randomizing select hospitalized patients with WHF to standard inpatient care vs expedited hospital discharge with a wearable SC furosemide pump. Analogous to efforts with furosemide, similar work is ongoing to develop other SC peptides, such as B-type natriuretic peptide compounds. Although randomized clinical trial data have been inconsistent, IV natriuretic peptides may potentially offer decongestive benefits over standard care.48,63,64 Moreover, as compared with diuretics, these agents may carry the potential mechanistic advantage of more directly targeting the renal response to hypervolemia via augmentation of renal cyclic guanosine monophosphate and facilitation of a combined natriuresis and diuresis.65,66 To date, clinical work with SC B-type natriuretic peptide has been primarily limited to patients with stable symptomatic HF or asymptomatic patients with structural heart disease.65–67
Conclusions
Worsening HF is a distinct entity that can be managed in both the hospitalized and outpatient settings. Regardless of care location, WHF carries a poor prognosis and randomized clinical trials focused on intervening during HF hospitalization have failed to improve clinical outcomes. Most patients with WHF have a subacute presentation of worsening symptoms over days to weeks, creating a potential window for targeted outpatient interventions that aim to reduce congestion and the need for downstream hospitalization. Given the ubiquitous use of IV diuretics in contemporary in-hospital WHF management, the financial consequences of HF hospitalizations and readmissions, and the negative effects on patient quality of life, US hospital systems are increasingly developing outpatient IV diuretic clinics for treating WHF in the ambulatory setting. Likewise, ongoing research continues to explore therapies with the potential to shift the burden of WHF care outside of the hospital, with the development of investigational agents for SC delivery holding particular promise. As the public health and economic burden of WHF continues to grow, recognizing the specific entity of outpatient WHF is critical. At the present time, while effective and proven care strategies for outpatient WHF remain an unmet need, this high-risk population represents a major opportunity for therapeutic advancements that could fundamentally change HF care delivery.
Acknowledgments
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Greene receives support from grant 5T32HL069749-14 from the National Institutes of Health and a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis. Dr Felker has received research funding from Otsuka, Novartis, Roche Diagnostics, Amgen, Merck, the American Heart Association, and the National Heart, Lung, and Blood Institute and consulting fees from Novartis, Roche Diagnostics, Amgen, Trevena, Cytokinetics, Madeliene, Myokardia, Bristol-Myers Squibb, Stealth Biotherapeutics, and GlaxoSmithKline. Dr Mentz has received research support from the National Institutes of Health, Amgen, AstraZeneca, Bristol- Myers Squibb, GlaxoSmithKline, Gilead, Medtronic, Madeliene, Novartis, Otsuka, and ResMed; honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, and Thoratec/St Jude Medical; has served on an advisory board for Luitpold Pharmaceuticals and Boehringer Ingelheim; and has received a research grant from Merck.
Contributor Information
Stephen J. Greene, Duke Clinical Research Institute, Durham, North Carolina; Division of Cardiology, Duke University Medical Center, Durham, North Carolina.
Robert J. Mentz, Duke Clinical Research Institute, Durham, North Carolina; Division of Cardiology, Duke University Medical Center, Durham, North Carolina.
G. Michael Felker, Duke Clinical Research Institute, Durham, North Carolina; Division of Cardiology, Duke University Medical Center, Durham, North Carolina.
REFERENCES
- 1.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266. [DOI] [PubMed] [Google Scholar]
- 2.Mills RM. The heart failure frequent flyer: an urban legend. Clin Cardiol. 2009;32(2):67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol. 2015;191:256–264. [DOI] [PubMed] [Google Scholar]
- 4.Nieminen MS, Brutsaert D, Dickstein K, et al. ; EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology. EuroHeart Failure Survey II (EHFSII): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27 (22):2725–2736. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14): 1433–1441. [DOI] [PubMed] [Google Scholar]
- 6.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012; 126(4):501–506. [DOI] [PubMed] [Google Scholar]
- 7.van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMA J 2011;183(7):E391–E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krumholz HM, Wang K, Lin Z, et al. Hospital-readmission risk–isolating hospital effects from patient effects. N Engl J Med. 2017;377(11): 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannah D, Lindholm B, Maisch L. Certain uncertainty: life after stroke from the patient’s perspective. Circ Cardiovasc Qual Outcomes. 2014;7 (6):968–969. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Liang L, Thomas L, et al. Assessment of home-time after acute ischemic stroke in Medicare beneficiaries. Stroke. 2016;47 (3):836–842. [DOI] [PubMed] [Google Scholar]
- 11.Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18(12):900–903. [DOI] [PubMed] [Google Scholar]
- 12.Collins SP, Pang PS, Fonarow GC, Yancy CW, Bonow RO, Gheorghiade M. Is hospital admission for heart failure really necessary? the role of the emergency department and observation unit in preventing hospitalization and rehospitalization. J Am Coll Cardiol. 2013;61(2):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene SJ, Hernandez AF, Dunning A, et al. Hospitalization for recently diagnosed versus worsening chronic heart failure: from the ASCEND-HF trial. J Am Coll Cardiol. 2017;69(25): 3029–3039. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Braunwald E, Gheorghiade M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA. 2014; 312(8):789–790. [DOI] [PubMed] [Google Scholar]
- 15.Solomon SD, Dobson J, Pocock S, et al. ; Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13): 1482–1487. [DOI] [PubMed] [Google Scholar]
- 16.Metra M, Cotter G, Davison BA, et al. ; RELAX-AHF Investigators. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–573. [DOI] [PubMed] [Google Scholar]
- 18.Skali H, Dwyer EM, Goldstein R, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014;16 (5):560–565. [DOI] [PubMed] [Google Scholar]
- 19.Okumura N, Jhund PS, Gong J, et al. ; PARADIGM-HF Investigators and Committees. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254–2262. [DOI] [PubMed] [Google Scholar]
- 20.Cook TD, Greene SJ, Kalogeropoulos AP, et al. Temporal changes in postdischarge mortality risk after hospitalization for heart failure (from the EVEREST Trial). Am J Cardiol. 2016;117(4):611–616. [DOI] [PubMed] [Google Scholar]
- 21.Felker GM, Mentz RJ, Teerlink JR, et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015;17(12):1262–1270. [DOI] [PubMed] [Google Scholar]
- 22.Greene SJ, Butler J, Fonarow GC, et al. ; ASTRONAUT Investigators and Coordinators. Pre-discharge and early post-discharge troponin elevation among patients hospitalized for heart failure with reduced ejection fraction: findings from the ASTRONAUT trial [published online October 17, 2017]. Eur J Heart Fail. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1(3):245–251. [DOI] [PubMed] [Google Scholar]
- 24.Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frizzell JD, Liang L, Schulte PJ, et al. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol. 2017;2(2):204–209. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. [DOI] [PubMed] [Google Scholar]
- 27.Ramírez A, Abelmann WH. Cardiac decompensation. N Engl J Med. 1974;290(9):499–501. [DOI] [PubMed] [Google Scholar]
- 28.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 29.Adams KF Jr, Fonarow GC, Emerman CL, et al. ; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–216. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–1133. [DOI] [PubMed] [Google Scholar]
- 31.Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31(7):832–841. [DOI] [PubMed] [Google Scholar]
- 32.Bourge RC, Abraham WT, Adamson PB, et al. ; COMPASS-HF Study Group. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51(11):1073–1079. [DOI] [PubMed] [Google Scholar]
- 33.Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. ; PARTNERS Study Investigators. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55(17):1803–1810. [DOI] [PubMed] [Google Scholar]
- 34.Abraham WT, Adamson PB, Bourge RC, et al. ; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011; 377(9766):658–666. [DOI] [PubMed] [Google Scholar]
- 35.Desai AS, Bhimaraj A, Bharmi R, et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real-world” clinical practice. J Am Coll Cardiol. 2017;69(19):2357–2365. [DOI] [PubMed] [Google Scholar]
- 36.Costanzo MR, Stevenson LW, Adamson PB, et al. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail. 2016;4(5):333–344. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu AT, Goldhaber-Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost-effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail. 2016;4(5):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaduganathan M, DeFilippis EM, Fonarow GC, Butler J, Mehra MR. Postmarketing adverse events related to the CardioMEMS HF system. JAMA Cardiol. 2017;2(11):1277–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122(6):585–596. [DOI] [PubMed] [Google Scholar]
- 40.Zsilinszka R, Mentz RJ, DeVore AD, Eapen ZJ, Pang PS, Hernandez AF. Acute heart failure: alternatives to hospitalization. JACC Heart Fail. 2017;5(5):329–336. [DOI] [PubMed] [Google Scholar]
- 41.DeVore AD, Allen LA, Eapen ZJ. Thinking outside the box: treating acute heart failure outside the hospital to improve care and reduce admissions. J Card Fail. 2015;21(8):667–673. [DOI] [PubMed] [Google Scholar]
- 42.Buckley LF, Carter DM, Matta L, et al. Intravenous diuretic therapy for the management of heart failure and volume overload in a multidisciplinary outpatient unit. JACC Heart Fail. 2016;4(1):1–8. [DOI] [PubMed] [Google Scholar]
- 43.Makadia S, Simmons T, Augustine S, et al. The diuresis clinic: a new paradigm for the treatment of mild decompensated heart failure. Am J Med. 2015;128(5):527–531. [DOI] [PubMed] [Google Scholar]
- 44.Hebert K, Dias A, Franco E, Tamariz L, Steen D, Arcement LM. Open access to an outpatient intravenous diuresis program in a systolic heart failure disease management program. Congest Heart Fail. 2011;17(6):309–313. [DOI] [PubMed] [Google Scholar]
- 45.Cuffe MS, Califf RM, Adams KF Jr, et al. ; Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287(12):1541–1547. [DOI] [PubMed] [Google Scholar]
- 46.McMurray JJ, Teerlink JR, Cotter G, et al. ; VERITAS Investigators. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298(17):2009–2019. [DOI] [PubMed] [Google Scholar]
- 47.Massie BM, O’Connor CM, Metra M, et al. ; PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363(15):1419–1428. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. [DOI] [PubMed] [Google Scholar]
- 49.Packer M, Colucci W, FisherL, et al. ; REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1(2):103–111. [DOI] [PubMed] [Google Scholar]
- 50.Teerlink JR, Cotter G, Davison BA, et al. ; Relaxin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. [DOI] [PubMed] [Google Scholar]
- 51.Packer M, Holcomb R, Abraham WT, et al. ; TRUE-AHF Investigators and Committees. Rationale for and design of the TRUE-AHF trial: the effects of ularitide on the short-term clinical course and long-term mortality of patients with acute heart failure. Eur J Heart Fail. 2017;19(5):673–681. [DOI] [PubMed] [Google Scholar]
- 52.Konstam MA, Gheorghiade M, Burnett JC Jr, et al. ; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. [DOI] [PubMed] [Google Scholar]
- 53.Gheorghiade M, Bohm M, Greene SJ, et al. ; ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309(11):1125–1135. [DOI] [PubMed] [Google Scholar]
- 54.Gheorghiade M, Greene SJ, Butler J, et al. ; SOCRATES-REDUCED Investigators and Coordinators. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED randomized trial. JAMA. 2015;314(21):2251–2262. [DOI] [PubMed] [Google Scholar]
- 55.Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the Soluble Guanylate Cyclase Stimulator in Heart Failure Patients with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017; 38(15):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valente MAE, Voors AA, Damman K, et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35(19):1284–1293. [DOI] [PubMed] [Google Scholar]
- 57.Voors AA, Davison BA, Teerlink JR, et al. ; RELAX-AHF Investigators. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail. 2014;16(11):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ter Maaten JM, Dunning AM, Valente MA, et al. Diuretic response in acute heart failure—an analysis from ASCEND-HF. Am Heart J. 2015;170(2):313–321. [DOI] [PubMed] [Google Scholar]
- 59.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102(3):314–318. [DOI] [PubMed] [Google Scholar]
- 60.Verma AK, da Silva JH, Kuhl DR. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother. 2004;38(4):544–549. [DOI] [PubMed] [Google Scholar]
- 61.Zacharias H, Raw J, Nunn A, Parsons S, Johnson M. Is there a role for subcutaneous furosemide in the community and hospice management of end-stage heart failure? Palliat Med. 2011;25(6): 658–663. [DOI] [PubMed] [Google Scholar]
- 62.Gilotra NA, Princewill O, Okwuosa IS, et al. Efficacy of intravenous furosemide versus a novel, pH-neutral furosemide formulation administered subcutaneously in outpatients with worsening heart failure [published online November 27, 2017]. JACC Heart Fail. doi: 10.1016/j.jchf.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 63.Packer M, O’Connor C, McMurray JJV, et al. ; TRUE-AHF Investigators. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376(20):1956–1964. [DOI] [PubMed] [Google Scholar]
- 64.Colucci WS, Elkayam U, Horton DP, et al. ; Nesiritide Study Group. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med. 2000;343(4):246–253. [DOI] [PubMed] [Google Scholar]
- 65.Vogel MW, Chen HH. Novel natriuretic peptides: new compounds and new approaches. Curr Heart Fail Rep. 2011;8(1):22–27. [DOI] [PubMed] [Google Scholar]
- 66.McKie PM,Schirger JA, Benike SL, et al. Chronic subcutaneous brain natriuretic peptide therapy in asymptomatic systolic heart failure. Eur J Heart Fail. 2016;18(4):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC Jr. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coil Cardiol. 2012;60(22):2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]