Abstract
Background
Breast cancer is prevalent and has high cure rates. The resultant increase in numbers of breast cancer survivors (BCS) may overwhelm the current oncology workforce in years to come. We postulate that primary care physicians (PCPs) could play an expanded role in comanaging survivors, provided they are given the appropriate tools and training to do so.
Objective
To explore the perspectives of PCPs towards managing BCS in a community-based shared-care programme with oncologists.
Methods
Eleven focus groups and six in-depth interviews were conducted with seventy PCPs recruited by purposive sampling. All sessions were audio-recorded, transcribed verbatim and coded by three independent investigators. Thematic data analysis was performed and the coding process facilitated by NVivo 12.
Results
Majority of PCPs reported currently limited roles in managing acute and non-cancer issues, optimizing comorbidities and preventive care. PCPs aspired to expand their role to include cancer surveillance, risk assessment and addressing unmet psychosocial needs. PCPs preferred to harmonize cancer survivorship management of their primary care patients who are also BCS, with defined role distinct from oncologists. Training to understand the care protocol, enhancement of communication skills, confidence and trust were deemed necessary. PCPs proposed selection criteria of BCS and adequacy of their medical information; increased consultation time; contact details and timely access to oncologists (if needed) in the shared-care programme.
Conclusions
PCPs were willing to share the care of BCS with oncologists but recommended role definition, training, clinical protocol, resources and access to oncologist’s consultation to optimize the programme implementation.
Keywords: breast, cancer survivors, chronic disease, continuity of care, medical comorbidity, primary care
Key Messages.
PCPs acknowledge discrepancy in their current and aspirational role in cancer.
They are willing to expand their role provided they receive support and training.
Shared-care empowers PCP and patient, while ensuring sustainable health care cost.
PCPs recommend role delineation, training, guidelines and care coordination.
Successful implementation hinges on patient’s conviction in their PCPs’ value.
Introduction
According to Globocan estimates, the worldwide incidence of cancer is predicted to increase from 18.1 million in 2018 to 29.5 million by 2040 (1). The increase in incidence, coupled with improved cure rates has resulted in the burgeoning population of cancer survivors (2,3). This increasing trend may overwhelm the current oncology workforce if survivorship care remains specialist-centric, as is the case in many countries today.
Singapore is a high-income country situated in Asia and is also facing rising cancer incidence where the estimated lifetime risk for developing cancer is one in every four to five people (4). Breast cancer is the most common cancer among Singaporean women, and many survivors are expected to have long-term survival. National Cancer Centre Singapore (NCCS) is a tertiary centre that treats two-thirds of patients in the public sector, with the oncologist-led model being the predominant model of survivorship care. Patients may choose subsidized care within the public health care network or opt to pay out-of-pocket or through insurance claims for private health care.
Previous studies have looked at different cancer survivorship models of care, including oncologist-led, oncology nurse-navigator-led, specialized multidisciplinary clinics or shared-care models (5–7). In our proposed shared-care model involving primary care physicians (PCPs), an oncologist assumes responsibility for cancer-related care with the PCP focussing on primary care. This model adopts a risk-stratified approach where low-risk breast cancer survivors (BCS) are managed by alternating visits with oncologists and PCPs (8). Expanding PCPs’ role in survivorship care must go beyond relieving oncologists to allow them to focus on active cancer treatment. Indeed, there is a recent paradigm shift of focus from ‘fighting the battle against cancer’ to ‘living with cancer’ as a chronic disease (9).
Current oncologist-centric follow-up care is associated with unmet physical and psychosocial needs (10). BCS grapple with a multitude of health needs which range from postsurgical changes, cancer-related fatigue, psychological concerns, menopausal symptoms, long-term effects of cancer treatment, in addition to cancer surveillance. PCPs are best placed to address unmet needs in the psychosocial domains, optimize comorbidities, ensure adherence to lifestyle modification and preventive care (11).
We previously conducted a pilot focus group study on private PCPs’ perspectives on community-based cancer survivorship care in Singapore and found that majority of private PCPs encounter very few cancer survivors in their practice (12). They reported barriers like patients’ lack of confidence in primary care, financial constraints, limited time and lack of empanelment.
The current study aims to explore the perspectives of PCPs (both private and public) towards managing BCS in a community-based shared-care programme with oncologists. We limited the discussion to the context of patients defined by oncologists as having low-risk breast cancer, i.e. cancers with a lower risk of recurrence or death, after taking into consideration tumour characteristics, prognostic and predictive biomarkers, gene expression profiling and patient-related factors (13).
Methods
Participants and setting
This study analysed focus groups discussions (FGDs) and in-depth interviews (IDIs) data examining PCPs’ perspectives of a shared-care survivorship concept for BCS. Purposive sampling (14) was employed to capture the views of PCPs from a broad range of experience, which included private PCPs and public PCPs from SingHealth, National Healthcare Group, National University Polyclinics in Singapore from June 2018 to November 2018. Participants included must be actively practising family medicine and be at least 3 years postgraduation. PCPs who practice in non-family medicine fields or non-community areas (e.g. emergency departments, secondary or tertiary care hospitals) were excluded from this study.
The key facilitator, R.W.Y.F. encountered some participants through training programmes organized by the College of Family Physicians Singapore. Participants from SingHealth Polyclinics were recruited by J.H.M.Q., the site principal investigator. Key stakeholders and institutional leaders from clinical services, education and research domains were invited to IDIs to delve deeper into personal perceptions. Eighty PCPs were approached by phone or email, three did not respond and seven did not participate due to scheduling difficulties. This accounted for a response rate of 88% (70 PCPs).
Data collection
Eleven FGDs and six IDIs lasting between 30 and 80 minutes were facilitated by R.W.Y.F. or A.C., assisted by another coinvestigator. Written informed consent was taken before the interviews, which were conducted in English within private meeting rooms at NCCS or SingHealth Polyclinics. Confidentiality and anonymity were maintained by assigning each participant with a serial number and deidentifying transcripts. Participants completed an anonymized demographic survey before the session.
We utilized a semistructured topic guide adopting a constructivist paradigm, to explore the participants’ perspectives on a broad range of ideas (Table 1). The interview guide was pilot tested with a focus group comprising of five PCPs, which resulted in minor revisions. Each participant was reimbursed with Singapore Dollar 30 (British Pound 18) for attendance. All sessions were audio-recorded, transcribed verbatim and audited by an independent coinvestigator (L.L.S.), for accuracy in transcription. The interviews continued until data saturation when no new themes emerged (15).
Table 1.
Guiding questions used in FGDs and IDIs held in 2018
| Themes | Questions |
|---|---|
| Background survey on current practice | Can you share with us some of your experience(s) with cancer survivors? |
| Discuss the perceived barriers of the proposed shared-care model | What are some of the barrier(s) that you can foresee with this shared-care model—patient related, physician related and health care system related? |
| Gather feedback on the Survivorship Care Plan (SCP) to facilitate communications planning | What information should be included in the SCP? |
| Explore some of the motivations for participation in the shared-care model | What are some of your motivation(s) to participate in this shared-care model? |
| Relationship with stakeholders | Who do you think are or should be stakeholders in this shared-care model, and possible barrier(s) that affect communication and seamless coordination and transition of care? |
| Community resources | Who are the community resources available and whom we can engage/ refer for effective shared-care? |
Data analysis
Thematic data analysis (16) was performed with investigators’ paying close attention to the data. To maximize reliability, the transcripts were coded by three independent investigators (Y.K., G.Y.L.W., D.Z.W.N.), with two independent analysts per transcript. The analysts familiarized with the initial transcripts independently and developed a codebook which was applied to the remaining transcripts. The codes were analysed and collated into themes, which were reviewed and defined. Coding comparison queries were run to identify differences between independent coders and minor differences were reconciled upon discussion. The coding process was facilitated by the QSR NVivo 12. Member checking was conducted and participants were invited to comment on a summary of the study findings. Demographic information collected were summarized using descriptive statistics.
Reflexivity
The main facilitator for both FGDs and IDIs was R.W.Y.F. who is an experienced female family medicine physician by training, caring for low-risk BCS. Another author (A.C.) who facilitated some FGDs is a male board-certified specialist pharmacist. Both have substantial experience in survivorship care at NCCS. The recordings, transcripts, coding, field and reflexive notes were maintained in organized secure archives, to establish a clear audit trail (17).
Results
Participant characteristics and practice setting
A total of 70 participants took part in eleven FGDs and 6 IDIs (Table 2). Majority (84.3%) of participants were Chinese and 58.6% aged between 30 and 39 years old. Two-thirds (68.6%) have 5–15 years of clinical practice experience, and 18.6% were highly experienced with >20 years’ experience. About 78.6% were public PCPs, and 92.9% were utilizing full or partial electronic records.
Table 2.
Participants’ demographics and characteristics (2018)
| Characteristic | n (%) |
|---|---|
| Demographic | |
| Gender | |
| Male | 34 (48.6%) |
| Female | 36 (51.4%) |
| Ethnicity | |
| Chinese | 59 (84.3%) |
| Indian | 7 (10.0%) |
| Others | 4(5.7%) |
| Practice experience (years) | |
| <5 | 4 (5.7%) |
| 5–10 | 30 (42.9%) |
| 11–15 | 18 (25.7%) |
| 16–20 | 5 (7.1%) |
| >20 | 13 (18.6%) |
| Age (years) | |
| 20–29 | 5 (7.1%) |
| 30–39 | 41 (58.6%) |
| 40–49 | 14 (20.0%) |
| 50–59 | 10 (14.3%) |
| Practice setting | |
| Current practice setting | |
| Public | 55 (78.6%) |
| Private general practitioner | 15 (21.4%) |
| Practice area | |
| North | 10 (14.3%) |
| South | 15 (21.4%) |
| East | 11 (15.7%) |
| West | 10 (14.3%) |
| Central | 24 (34.3%) |
| Types of medical records | |
| Paper records | 5 (7.1%) |
| Partial/ in transition | 5 (7.1%) |
| Full electronic records | 60 (85.8%) |
| Current experience with patients | |
| Average number of patients seen monthly | |
| <300 | 7 (10.0%) |
| 300–400 | 6 (8.6%) |
| 401–500 | 3 (4.3%) |
| 501–600 | 9 (12.9%) |
| >600 | 45 (64.3%) |
| Average amount of time spent with each patient (minutes) | |
| <5 | 1 (1.4%) |
| 5–10 | 44 (62.9%) |
| 11–15 | 21 (30.0%) |
| 16–20 | 3 (4.3%) |
| >20 | 1 (1.4%) |
| Average number of cancer survivors seen monthly | |
| <5 | 23 (32.9%) |
| 5–10 | 21 (30.0%) |
| 11–15 | 15 (21.4%) |
| 16–20 | 2 (2.9%) |
| >20 | 9 (12.9%) |
| Time spent caring for cancer survivors care on cancer-related issues (% of total consultation time spent in practice) | |
| <20 | 67 (95.7%) |
| 20–50 | 3 (4.3%) |
| >50 | 0 (0%) |
Approximately two-thirds (64.3%) of the participants experienced heavy patient loads, seeing more than 600 patients monthly. Majority of participants (62.9%) had short consultation times with only 5–10 minutes per patient visit.
The participants had a low level of engagement with cancer-related issues, with 62.9% seeing up to 10 BCS monthly. 95.7% of participants spent less than 20% of their total consultation time caring for cancer-related issues.
Two major themes emerged, which were roles of PCPs and recommendations for the shared-care model.
Roles of PCPs
Current role
The current role of PCPs is mainly limited to acute, non-cancer-related issues. Areas PCPs have done well include optimal care for comorbidities, health promotion and preventive care. Their existing relationship and rapport with BCS can impact behavioural change and address psychosocial issues like anxiety, depression and mood disorders.
(PCPs) have a bit of advantage in swaying them (BCS) to improve their disease…we are definitely more trained in terms of psychological issues…Because it happens with chronic illness also. FGD#41, public
Aspirational role
PCPs aspired to expand their role to include cancer surveillance, risk assessment and addressing unmet psychosocial needs in the cancer domain. PCPs preferred to harmonize cancer survivorship management of their primary care patients who are also BCS. PCPs indicated the need to have a distinct role for PCPs in this shared-care model.
we (PCPs can) keep a lookout if they (BCS) develop some acute complaints, for example, chronic cough or back pain, we will be a bit more proactive in doing further investigations to rule out any cancer recurrence. FGD#3, public
PCPs hope to address psychological barriers of BCS for transition and reintegration back into society; either to return to work or their previous social role.
Constraints and motivations behind the role
PCPs encounter constraints in this aspirational role and are unclear of the demarcation of their responsibility in this shared-care model. They are concerned about the increased workload imposed on their busy practice.
Despite this, PCPs believe in the value of shared-care in providing accessible care for an increasing population of cancer survivors. Shared-care promotes patient autonomy, supports empowerment and encourages ownership. Furthermore, it shifts the focus from disease-specific to patient-centric care and encourages specificity in the physician–patient relationship.
So, I think it’s actually good, I mean, it’s IDEAL if the patient has ONE primary care physician to follow up with, providing this patient with continuous and holistic care, and you are actually bridging the gap between tertiary care and (the) transition back to primary care. FGD#2, public
From the physicians’ perspective, shared-care allows PCPs to provide comprehensive care and opportunity to widen their skill set and enhance their capabilities. To the health care system, shared-care promotes right-siting of patients to the community, alleviating strain on tertiary care.
Recommendations for shared-care
Patient selection and trust
PCPs should select BCS from their current pool of primary care patients whom they are already seeing for chronic conditions. The existing therapeutic doctor–patient relationship can be maintained, and BCS’ trust and confidence in their PCPs could be strengthened to manage both cancer and non-cancer-related issues throughout their journey.
they (BCS) came from us in the first place, …perhaps we (PCPs) are the ones who pick up a breast lump, we refer them, or maybe if it’s one of our chronic patients, she’s known to us, …these types of patients will be the better ones to decant back, because the trust is already built, and they are familiar with us. FGD#51, public
Another enabler is for oncologists to stratify and select BCS with a low risk for cancer recurrence and stabilized health issues. Patients selected should be mutually agreed on by oncologists and PCPs.
I(PCP) reiterate the point that active issue(s) should be resolved because it’s very difficult for us primary care to manage the active issues at one time. (For) minor adjustments, we will make, once there’s variation. So, the general principle (here) is (a) minor issue, maintenance issue, and (whether) we can build a relationship, then we can refer back, get back into the system in case there are problems. IDI#1, public
Equipping PCPs with skills and knowledge, demarcation of roles and responsibilities
PCPs recognized their limitations in knowledge and experience in cancer care, with the rapid advancements in oncology. They called attention to their lack of training and confidence, thus recommending clinical attachments at NCCS to observe specialists’ clinical and communication skills.
Training doesn’t need to be very deep, like, extensive. It just means that you need the means (to handle) common everyday problems that occur in the context of cancer survivorship. FGD#16, private
The exposure would enable PCPs to appreciate the multiple needs of survivors and their management strategies. The design of the training programme should identify and target PCPs’ knowledge gaps and deliver sufficient information without overloading PCPs.
Mutually agreed demarcation of roles and responsibilities is critical. Oncologists should manage recurrence and long-term side effects of cancer treatment, while PCPs focus on health maintenance, preventive care and identifying red flags suggestive of recurrence for prompt referral to the oncologists.
Enhance infrastructure support
To address the barrier of limited consultation time, public PCPs recommended care of BCS to be initiated at Family-Physician Clinics. These clinics are run by public PCPs with postgraduate family medicine training, which focusses on delivering patient-centric care and allows extended consultation time for managing patients with complex care. PCPs reiterated the need for sustainable financial resources and timely communication. A defined survivorship care plan should be incorporated into the shared electronic medical record (EMR) with updated practice guidelines, dynamic care templates and workflow prompts.
a whole programme, not just the training…, but the information flow, the support, the resourcing, the referring-back mechanism…, good follow-up programme, and obviously there must be resources and capability to manage that. IDI#1, public
a digitalised form and there can be constant updates…, two-way communication where what we wrote can be seen by the oncologists and the oncologists’ (notes) can be seen by us. FGD#62, private
Care coordinators, proficient in accessing community resources, are essential to facilitate proper handover and ensure smooth transition of care.
Discussion
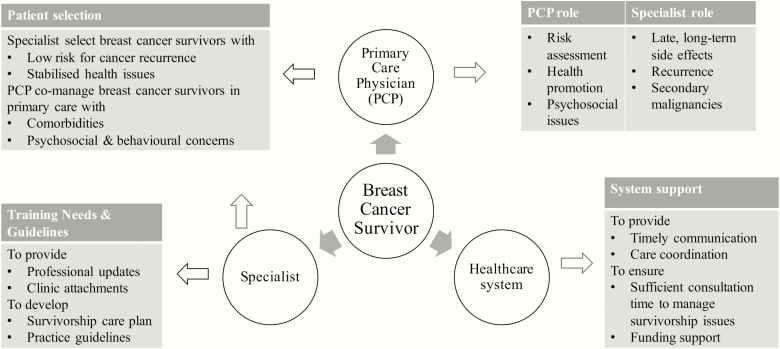
Key findings in our study include PCPs’ willingness to expand their current role to include cancer surveillance, risk assessment and addressing unmet psychosocial needs in the cancer domain. This discrepancy between current and aspirational/desired role was also reported by family physicians in Canada, which they felt was hindered by patient-based, system and professional barriers (18). We proposed to use a patient-centric care approach to help us conceptualize the recommendations of the participants, which were derived from the themes of the study (Fig. 1). A similar strategy was described by studies to incorporate risk stratification, clarity of roles, timely communication, care coordination and clear routes for access back to specialist care (19,20). A multifaceted training programme, evidence-based guidelines for common survivorship issues and updated care plans are essential components of high-quality survivorship care and are gradually being adopted into routine services in Australia (21).
Figure 1.
Roles and recommendations derived from study themes for shared-care in BCS.
The added value of this larger study provided opportunities to explore and understand the different challenges and needs between private and public settings. The recommendations can be implemented on a national level (e.g. policy change to enhance financial reimbursement to private PCPs and develop an accessible/integrated training programme) or at different practice settings (e.g. team-based care in public polyclinics and linking up private PCPs into primary care networks to share resources).
Private PCPs, who are self-funded proposed for policymakers to recognize cancer as a chronic disease to allow BCS to leverage on the current chronic disease care support system (22). This will lower out-of-pocket cost for survivorship care. In contrast, public PCPs receive direct funding from the government to deliver subsidized primary care supported by multidisciplinary primary health care professionals. As a result of the health care financing policy in Singapore, the bulk of chronic disease management is handled by public PCPs. As BCS age, many of them develop chronic medical conditions. The polyclinics are thus ideal sites for the holistic management of BCS (Table 3).
Table 3.
Main differences in health care structure between public and private PCPs in Singapore
| Public PCPs | Private PCPs | |
|---|---|---|
| Financing system | • Government subsidy for all eligible patients (citizens and permanent residents) and covers all medical conditions | • Fee-for-service for most patients |
| • Portable subsidies (Community Health Assist Scheme) for eligible patients. Maximum allocated for current approved chronic medical conditions is $540 Sing Dollar per year (302 British pound sterling) and selected dental services | ||
| Infrastructure | • Larger, multidoctor team-based care supported by nurses, allied health, pharmacists, on-site laboratory, radiology and sometimes dentists | • Solo practice or group clinic with a few doctors. Referral to private laboratories or radiological services as indicated |
| • Universal use of EMR and access to National Electronic Health Record (NEHR) | • Optional participation in Primary Care Networks | |
| • Optional use of EMR and access to National Electronic Health Record (NEHR) | ||
| Education and training | • Family medicine residency training | • Optional site for clinic attachment for family medicine training programmes |
| • Regular continuing medical education |
PCPs are well-positioned to provide holistic patient-centric care in view of their accessibility and broad-based training. Randomized trials have demonstrated similar recurrence detection rates, health outcomes (23) and better patient satisfaction when follow-up is done by PCPs (24). Survivors acknowledged the importance of having their PCPs involved in follow-up care (25). This is especially important for those with comorbidities affecting medical decision-making, health outcomes and quality of life (26). Cancer survivors are at increased risk for cardiovascular diseases, both as a result of cancer therapies and shared cardiovascular risk factors like obesity, inactivity, smoking and poor diet (27,28). Traditional cardiovascular risk prediction tools do not include cancer as a risk factor. Thus, it is imperative for PCPs to recognize the association and advocate risk reduction strategies, especially for older BCS (29).
Studies have shown that PCPs are more adept in providing psychosocial support and behavioural modification (30,31). Furthermore, PCPs have a long-standing relationship with their patient and family, which can promote and sustain behavioural changes like smoking cessation, exercise and weight maintenance. Participants in our study were confident in delivering health promotion and preventive care, which have been linked to improved outcomes (32).
PCPs in our study acknowledged their limited knowledge and lack of confidence in cancer care, a similar finding among PCPs overseas (33). In recent years, training in cancer survivorship for PCPs worldwide has undergone rapid progress; with online courses, resource toolkits and continuing medical education. American Society of Clinical Oncology has developed a core curriculum and competencies targeting health care practitioners for cancer survivorship education (34). In contrast, Singapore’s current education outreach lacks coordination and structure. PCPs in our study recommended the development of local clinical practice guidelines and adoption of a multifaceted education and training programme to cover prevention, early detection and survivorship.
Studies recognized the value of a positive working relationship between PCPs and specialists to support shared-care. This can be achieved by care coordinators engaging and keeping PCPs ‘in the loop’ (35,36). Most PCPs in our study have access and agreed that a centralized EMR could be a viable platform to support the routine usage of a web-based survivorship care plan to guide patient care and facilitate communication.
There are existing shared-care programmes for cancer survivors in Canada, Denmark, Australia, but not in Singapore. Overall, shared-care is highly acceptable, resulting in better patient compliance and satisfaction. In terms of effectiveness, shared-care has been shown to be similar to usual care (37,38). The current findings and recommendations of this study have implications for the integration of a shared-care model into routine primary care practice. Shared-care should be initiated early to establish a relationship among the various stakeholders. Once stable, BCS can be discharged completely back to their PCPs upon completion of endocrine therapy.
Several limitations of this study should be considered. Firstly, PCPs who participated may have a special interest in survivorship care and may not be representative of all PCPs. To minimize the impact of this limitation, we recruited a large number of participants from diverse practice settings and conducted FGDs/IDIs to provide a broad range of viewpoints. Secondly, we did not include BCS and specialists in this study, although we had described their perceptions and barriers in previous publications (39,40). Our next study will include different stakeholders to assess feasibility, adherence and cost-effectiveness of this shared-care model. Thirdly, although our results may not be generalizable to all PCPs or other cancer types, they can apply to PCPs who practise in a similar health care system caring for low-risk BCS.
Conclusions
PCPs in Singapore are willing to share the care of low-risk BCS with oncologists. Recommendations from PCPs included risk stratification, role definition, focussed training, viable care pathways, practice guidelines, timely communication and sustainable funding to equip them for this expanded role. However, the successful implementation of a shared-care programme must be centred around instilling in the BCS the belief that her PCP is a valued partner in her cancer journey.
Acknowledgements
Family Medicine Academic Clinical Program had no role in the development, conduct, analysis or reporting of the study. We acknowledge Ms Gladys Yan Lin Wong, Mr Daniel Zhi Wei Ng for their contribution in data analysis. Our heartfelt appreciation to all participants for their invaluable contribution to this study. The key results from this article have been presented as a poster at the Multinational Association of Supportive Care in Cancer Annual Meeting 2019 in San Francisco, and an oral presentation at the 7th Asia Pacific Primary Care Research Conference 2019 at Georgetown Penang.
Declaration
Funding: our research team would like to thank Family Medicine Academic Clinical Programme (FM ACP) Seed Grant for the financial and administrative support (FY17/P2/14-A58/01).
Ethical approval: ethical approval was obtained from the SingHealth Central Institutional Review Board (CIRB 2017-3107).
Conflict of interest: none.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271–89. [DOI] [PubMed] [Google Scholar]
- 3. Lim GH, Chow KY, Lee HP. Singapore cancer trends in the last decade. Singapore Med J 2012; 53: 3–9; quiz 10. [PubMed] [Google Scholar]
- 4. Singapore Cancer Registry Annual Registry Report: Trends in Cancer Incidence in Singapore 2010–2014 https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/cancer-trends-report-2010---2014_web.pdf?sfvrsn=0 (accessed on 18 September 2019).
- 5. Nekhlyudov L, O’malley DM, Hudson SV. Integrating primary care providers in the care of cancer survivors: gaps in evidence and future opportunities. Lancet Oncol 2017; 18: e30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaput G, Med CP, Sussman J. Integrating primary care providers through the seasons of survivorship. Curr Oncol 2019; 26: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol 2006; 24: 5117–24. [DOI] [PubMed] [Google Scholar]
- 8. Loh KW, Ng T, Choo SP et al. Cancer supportive and survivorship care in Singapore: current challenges and future outlook. J Glob Oncol 2018; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian 2010; 17: 47–50. [DOI] [PubMed] [Google Scholar]
- 10. Aaronson NK, Mattioli V, Minton O et al. Beyond treatment—psychosocial and behavioural issues in cancer survivorship research and practice. Eur J Cancer Suppl 2014; 12 (1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Runowicz CD, Leach CR, Henry NL et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin 2016; 66: 43–73. [DOI] [PubMed] [Google Scholar]
- 12. Chan A, Ngai GH, Chung WL et al. Practitioners’ perspectives on community-based breast cancer survivorship care in Singapore: a focus group study. Health Soc Care Community 2018; 26: 404–11. [DOI] [PubMed] [Google Scholar]
- 13. Strasser-Weippl K, Goss PE. Competing risks in low-risk breast cancer. American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology; Annual Meeting, 2013, pp. 32–9. [DOI] [PubMed] [Google Scholar]
- 14. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015; 42: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morse JM. The significance of saturation. Qual Health Res 1995; 5 (2):147–9. [Google Scholar]
- 16. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3 (2):77–101. [Google Scholar]
- 17. Bradshaw C, Atkinson S, Doody O. Employing a qualitative description approach in health care research. Glob Qual Nurs Res 2017; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Easley J, Miedema B, O’Brien MA et al. ; Canadian Team to Improve Community-Based Cancer Care Along the Continuum. The role of family physicians in cancer care: perspectives of primary and specialty care providers. Curr Oncol 2017; 24: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubin G, Berendsen A, Crawford SM et al. The expanding role of primary care in cancer control. Lancet Oncol 2015; 16: 1231–72. [DOI] [PubMed] [Google Scholar]
- 20. Hall SJ, Samuel LM, Murchie P. Toward shared care for people with cancer: developing the model with patients and GPs. Fam Pract 2011; 28: 554–64. [DOI] [PubMed] [Google Scholar]
- 21. Emery J. Cancer survivorship—the role of the GP. Aust Fam Physician 2014; 43: 521–5. [PubMed] [Google Scholar]
- 22. The Singaporean Health Care System. https://international.commonwealthfund.org/countries/singapore/ (accessed on 7 December 2019).
- 23. Grunfeld E, Fitzpatrick R, Mant D et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract 1999; 49: 705–10. [PMC free article] [PubMed] [Google Scholar]
- 24. Grunfeld E, Levine MN, Julian JA et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol 2006; 24: 848–55. [DOI] [PubMed] [Google Scholar]
- 25. Hudson SV, Miller SM, Hemler J et al. Adult cancer survivors discuss follow-up in primary care: ‘not what I want, but maybe what I need’. Ann Fam Med 2012; 10: 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagel G, Wedding U, Röhrig B, Katenkamp D. The impact of comorbidity on the survival of postmenopausal women with breast cancer. J Cancer Res Clin Oncol 2004; 130: 664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strongman H, Gadd S, Matthews A et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019; 394: 1041–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blaes AH, Thavendiranathan P, Moslehi J. Cardiac toxicities in the Era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. Am Soc Clin Oncol Educ Book 2018; 38: 764–74. [DOI] [PubMed] [Google Scholar]
- 29. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 2011; 13: R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol 2012; 30: 2897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pascoe SW, Neal RD, Allgar VL, Selby PJ, Wright EP. Psychosocial care for cancer patients in primary care? Recognition of opportunities for cancer care. Fam Pract 2004; 21: 437–42. [DOI] [PubMed] [Google Scholar]
- 32. Ligibel JA, Basen-Engquist K, Bea JW. Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Educ Book 2019; 39: e22–33. [DOI] [PubMed] [Google Scholar]
- 33. McDonough AL, Rabin J, Horick N, et al. Practice, preferences, and practical tips from primary care physicians to improve the care of cancer survivors. J Oncol Pract 2019; 15: e600–6. [DOI] [PubMed] [Google Scholar]
- 34. Shapiro CL, Jacobsen PB, Henderson T et al. ReCAP: ASCO core curriculum for cancer survivorship education. J Oncol Pract 2016; 12: 145, e108–17. [DOI] [PubMed] [Google Scholar]
- 35. Lizama N, Johnson CE, Ghosh M, Garg N, Emery JD, Saunders C. Keeping primary care “in the loop”: general practitioners want better communication with specialists and hospitals when caring for people diagnosed with cancer. Asia Pac J Clin Oncol 2015; 11: 152–9. [DOI] [PubMed] [Google Scholar]
- 36. Haq R, Heus L, Baker NA et al. Designing a multifaceted survivorship care plan to meet the information and communication needs of breast cancer patients and their family physicians: results of a qualitative pilot study. BMC Med Inform Decis Mak 2013; 13: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Brettle A, Qiu L. The effectiveness of shared care in cancer survivors—a systematic review. Int J Integr Care 2018; 18: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adam R, Watson E. The role of primary care in supporting patients living with and beyond cancer. Curr Opin Support Palliat Care 2018; 12: 261–7. [DOI] [PubMed] [Google Scholar]
- 39. Chan A, Lum ZK, Ng T et al. Perceptions and barriers of survivorship care in Asia: perceptions from Asian Breast Cancer Survivors. J Glob Oncol 2017; 3: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ng T, Toh MR, Cheung YT, Chan A. Follow-up care practices and barriers to breast cancer survivorship: perspectives from Asian oncology practitioners. Support Care Cancer 2015; 23: 3193–200. [DOI] [PubMed] [Google Scholar]