Abstract
The immune system plays an important role in controlling cancer growth. However, cancers evolve to evade immune detection. Immune tolerance and active immune suppression results in unchecked cancer growth and progression. A major contributor to immune tolerance is the tumor physiologic microenvironment, which includes hypoxia, hypoglucosis, lactosis, and reduced pH. Preclinical and human studies suggest that exercise elicits mobilization of leukocytes into circulation (also known as “exercise-induced leukocytosis”), especially cytotoxic T cells and natural killer cells. However, the tumor physiologic microenvironment presents a significant barrier for these cells to enter the tumor and, once there, properly function. We hypothesize that the effect of exercise on the immune system’s ability to control cancer growth is linked to how exercise affects the tumor physiologic microenvironment. Normalization of the microenvironment by exercise may promote more efficient innate and adaptive immunity within the tumor. This review summarizes the current literature supporting this hypothesis.
Introduction
Although widely used to manage chronic diseases (1, 2), incorporating exercise into cancer therapy is relatively new. Epidemiologic studies have shown that aerobic exercise reduces cancer incidence and progression after diagnosis, in a variety of malignancies (3–6). Clinically recommended exercise levels (e.g., 150 minutes of moderate exercise per week) are associated with up to 40% risk reduction for developing breast and colon cancers (4, 7, 8). Part of these effects may be related to how exercise affects antitumor immune function (9–11).
Preclinical studies have revealed how exercise changes the physiologic tumor microenvironment. For example, exercise reduces tumor hypoxia and improves vascular maturity and perfusion (12–15). It is well established that tumor hypoxia contributes to tumor progression, radioresistance, and chemoresistance. Improved perfusion and reduced hypoxia in the tumor microenvironment could improve drug delivery, enhance tumor response to chemotherapy, and lead to better prognosis (12, 16–18).
Current FDA-approved immune-based therapies for cancer are, in large part, designed to reverse tumor immune escape and tumor-induced immune suppression (for example, immune checkpoint blockade; ref. 19). Despite significant success with immune-based therapies, a substantial proportion of patients do not respond (20). Thus, there is a rationale for adjunct strategies to improve clinical benefit associated with immunotherapy (21, 22).
Figure 1 summarizes our hypothesis that exercise-induced normalization of tumor microvasculature, hypoxia, and metabolism promotes competent cytotoxic immune cell infiltration into tumors.
Figure 1.
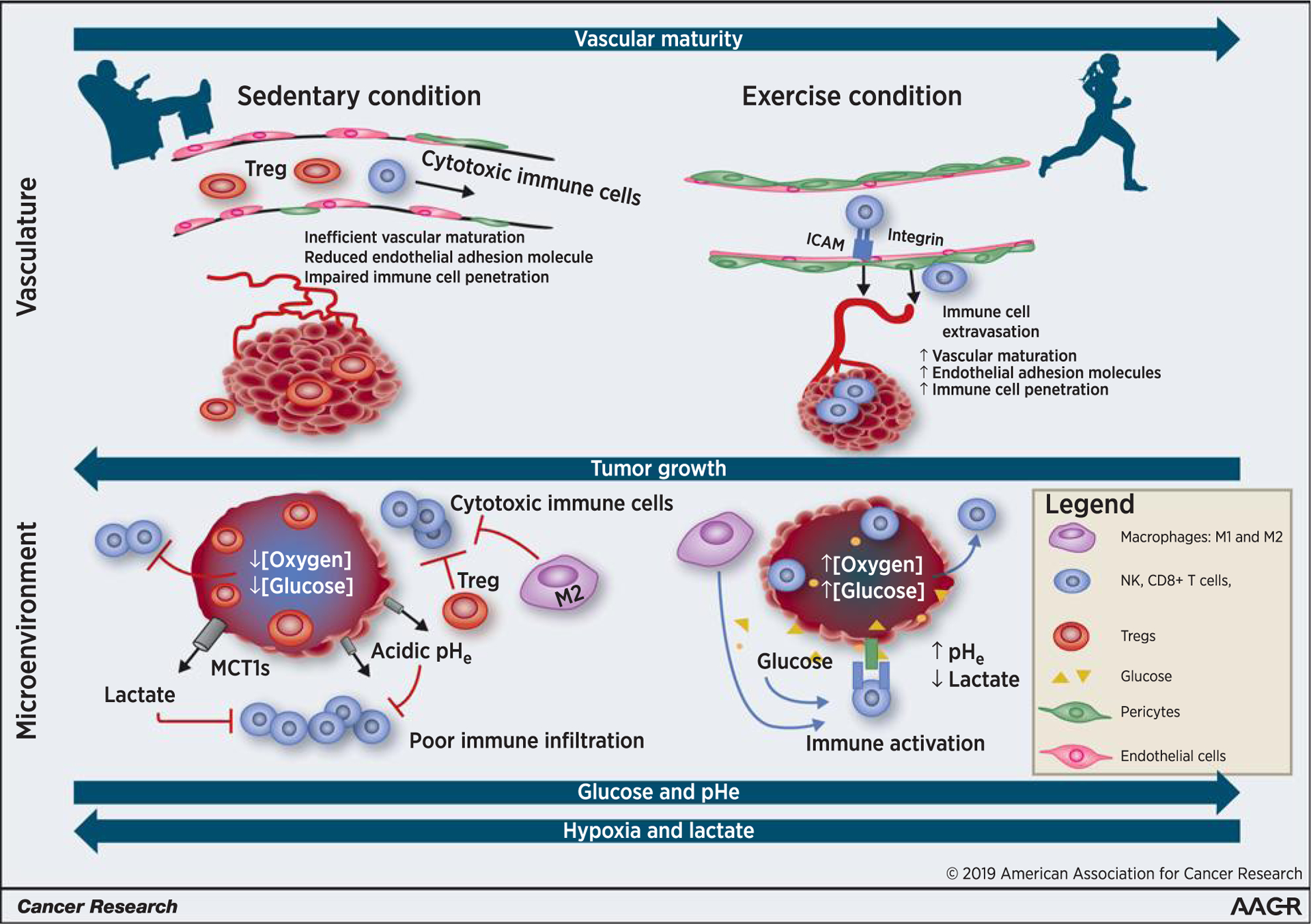
Exercise primes the tumor toward a more aerobic, less glycolytic physiologic microenvironment. The schematic demonstrates the large-scale vascular (top) and microscale physiologic microenvironment (bottom) changes within the tumor of an exercised versus sedentary individual. It is not drawn to scale. Exercise, in normalizing tumor vasculature, increases endothelial adhesion molecule expression, promotes the extravasation of cytotoxic immune cell (NK cells, CD8+ T cells, and type 1 macrophages) and infiltration of these cells into the tumor. Conversely, under the sedentary condition, tumors manifest a hypoxic, aberrantly vascularized, and highly glycolytic tumor. The two tumor cross-sections on the bottom represent the physiologic microenvironment of a sedentary versus exercised individual. High lactate, low pHe, and hypoglucotic environment within the sedentary tumor promote immune-suppressive Tregs but inhibit the function of tumor-infiltrating cytotoxic immune cells. The immune cells that are in the sedentary tumor microenvironment are metabolically outcompeted by the highly proliferative cancer cells. Exercise, by normalizing tumor vasculature, yields a better perfused tumor, with improved energy substrate (glucose availability), improved oxygen concentrations, and decreased glycolytic lactate production. The net effect of this is an enhanced metabolic and immunologic environment, one that results in more potent immune activation and more effective tumor cell cytolysis.
Modulation of the Tumor Microenvironment by Exercise
Immune cell trafficking into tumors
Aberrant tumor vasculature downregulates endothelial adhesion molecules, which impairs leukocyte entry into tumors (23, 24). The normalization of tumor vasculature increases endothelial cell adhesion molecule expression, facilitating leukocyte entry into tumor parenchyma (25, 26). In murine tumor models, the combination of anti-PD-L1 and vascular endothelial growth factor (VEGF) blockade increased the density of high endothelial venules and caused a 3- to 10-fold increase in tumor-infiltrating T and B lymphocytes (26). Vascular normalization of tumors also occurs following exercise in murine models of cancer (12, 16). Therefore, exercise-induced normalization of tumor vasculature may increase endothelial adhesion molecule expression and promote immune cell infiltration into tumors.
The effect of exercise on immunity toward infection
Many recent reviews have highlighted the role of exercise in maintaining a healthy immune system and controlling infections and chronic inflammation-associated disease (11), including cancer (9, 10, 27, 28). Moderate exercise in mice (20–30 minutes/day of treadmill running) increased the survival rate by 2-fold following influenza infection compared with inactivity. In contrast, intense exercise (2.5 hours/day of treadmill running) increased morbidity (29). Epidemiologic human studies support moderate exercise as more beneficial to immune function than intense exercise (30). Women who walked briskly for 45 minutes 5 days/week had reduced duration of upper respiratory tract infection (URTI) symptoms compared with sedentary counterparts (5.1 days vs. 10.8; ref. 31). However, marathon runners who averaged >96 km/week had 2-fold higher odds of URTI compared with runners who averaged 32 km/week (32). Results such as those led to an “open-window” hypothesis: following vigorous exercise, an individual is transiently immunosuppressed (33). A recent review by Campbell and Turner argues that acute/vigorous exercise is not immunosuppressive; in the long run, exercise improves immune cell function, and exercise-induced immune cell redistribution is beneficial (27). This hypothesis is coined the “acute stress” or “exercise immune-enhancement” (27, 34).
Studies that examined the effect of exercise on immune cell phenotype and function in preclinical and clinical settings are listed in Tables 1A and 1B.
Table 1A.
Preclinical publications reporting immunologic effects in tumor-bearing mice associated with exercise
| Article | Model | Exercise modality | Tumor growth effects of exercise | Immune effects of exercise |
|---|---|---|---|---|
| Innate immune response adaptation to mice subjected to administration of DMBA and physical activity | BALB/c with DMBA induced mammary tumor | Swim training 5 days/week for 8 weeks in both no tumor and mammary tumor mice | Not addressed | ↑IL12-producing macrophages |
| ↑IFNy | ||||
| ↑M1 cytokines | ||||
| ↑TNF-a | ||||
| Abdallah et al. 2014 | ↓TGF-B | |||
| PMID: 24520305 | ||||
| Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice | Young (6 mo) and ages (22 mo) BALB/cByJ mice, nontumor bearing | Treadmill running sessions for 16 weeks | Not addressed | ↑Macrophage cytolytic activity |
| ↑NO− production by macrophages among young mice with exercise | ||||
| Lu et al. 1999 | ||||
| PMID: 9950928 | ||||
| Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner | PyMT transgenic mouse with mammary carcinoma | Voluntary wheel running starting at 42 days of age, until tumor becomes fulminant | ↓Tumor volume with exercise in first 4 weeks by 50% | ↓CCL22 |
| Goh et al. 2013 | ||||
| PMID: 24312199 | ||||
| Voluntary running suppresses tumor growth through epinephrine and IL6-dependent NK cell mobilization and redistribution | C57BL/6 mice with B16F10 tumors and Lewis Lung carcinoma mice | Voluntary wheel running before, after, or during tumor challenge | ↓B16F10 tumor growth by 50%−60% with 4 weeks of exercise prior to inoculation | ↑NK tumor infiltration |
| ↑NK cell killing against YAC-1 and B16 cells | ||||
| ↓Lung metastases in B16F1O tumor-bearing mice | ↑IL1a and iNOS | |||
| ↑IL6 expression | ||||
| Pedersen et al. 2016 | ↑IL6 receptor + NK cell subset | |||
| PMID: 26895752 | ||||
| Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise | BALB/c mice with orthotopic mammary tumors ± cyclophosphamide treatment | Voluntary wheel running before and during tumor growth | ↓Tumor volume with exercise alone, and greatest decrease with exercise + cyclophosphamide | ↓PDGF-R expression |
| ↑CD31 colocalization with desmin | ||||
| ↑VEGF expression | ||||
| n = 11–12 per group | ||||
| Betof et al. 2015 | ||||
| PMID: 25780062 | ||||
| Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise | Copenhagen rats with R-3327 MatLyLu prostate tumor | Treadmill exercise, for 5 min/day for 5 days at a 10° incline and a speed of 15 meters/min | Not assessed | ↑Tumor blood flow by 200% during exercise |
| ↓Maximal vasoconstriction in response to epinephrine | ||||
| n = 12 vehicle controls | ||||
| McCullough et al. | n = 42 tumor-bearing mice | ↓Tumor hypoxia by 2-fold | ||
| PMID: 24627275 |
Table 1B.
Clinical publications examining associations between exercise and immune function in patients with cancer
| Article | Subjects | Exercise modality | Tumor growth effects of exercise | Immune effects of exercise |
|---|---|---|---|---|
| Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors | Postmenopausal breast cancer survivors sedentary group | Cycle ergometry sessions 3 times per week for 15 weeks | Not addressed | ↑Natural killer cell cytotoxic activity |
| n = 28 exercise group | ||||
| n = 25 | ||||
| Fairey et al. 1985 | ||||
| PMID: 15772062 | ||||
| Effect of aerobic training on the host systemic milieu in patients with solid tumors: an exploratory correlative study | Mixed solid tumor patients | Aerobic cycle ergometry sessions 3 times per week at 55%−100% VO2 peak for 12 weeks | Not addressed | ↑VO2 peak |
| ↓Macrophage Inflammatory Protein 1 | ||||
| sedentary group = 21 | ↓VEGF | |||
| No change in TNF-a | ||||
| exercise group = 23 | ↑CD8+ and CD4+ T cells | |||
| No change in NK cells | ||||
| Glass et al. 2015 | ||||
| PMID: 25584487 | ||||
| Exercise and lymphocyte activation following chemotherapy for breast cancer | Breast cancer patients recovering from chemotherapy | Treadmill or outdoor running or walking sessions for 6 month | Not addressed | ↑Percentage of CD4+ CD69+ T cells (activated T lymphocytes) in blood |
| No change in IFNy | ||||
| sedentary group = 21 | No change in IL6 | |||
| Hutnick et al. 2005 | exercise group = 28 | |||
| PMID: 16286849 | ||||
| Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy | Women with newly diagnosed, histologically confirmed unresected stage IIB-IIIC breast adenocarcinoma | Three supervised aerobic cycle ergometry sessions per week, 20 to 45 min/session (duration), at 55% to 100% of VO2 peak for 12 weeks | Not addressed | ↑VO2 Peak |
| ↑CEP surface markers of VEGFR-2 | ||||
| ↑PLGF (proangiogenic factor) | ||||
| ↑IL8 | ||||
| ↓Expression of PAK4, FYB, and TNFRSF10D (converges on NF-kB pathway) in tumors | ||||
| Jones et al. 2013 | Chemo alone: n = 10 | |||
| PMID: 23842792 | Chemo + exercise: n = 10 |
Exercise and the innate immune system
Exercise studies reported increased natural killer (NK) cell and macrophage reactivity against tumors. NK cells directly kill tumor cells via a perforin-dependent mechanism (35, 36). Subsets of NK cells are also involved in cross-talk with dendritic cells (37). This cross-talk can enhance antigen presentation and downstream effector cell responses (38–41). Pedersen and colleagues demonstrated that voluntary wheel running halved tumor incidence compared with sedentary controls in mice with diethylnitroasmine-induced liver tumors (42). Further, exercise prior to melanoma implantation in mice exhibited a 6-fold increase in NK cell infiltration into primary tumors, reduced tumor growth rate (by 60%), and halved the number of lung metastases compared with sedentary controls (42). Activity of adrenergic receptors on immune cells was key to the stimulation of NK cell activity; beta-blockers inhibited the activation of NK cells associated with exercise (42).
Tumor-associated macrophages (TAM) also contribute to innate antitumor immunity (43). Antitumor/M1 macrophages secrete proinflammatory cytokines (i.e., IFNγ and IL12), which support NK cell activation and Th1 T-cell immunity. However, in advanced cancer, TAMs differentiate to a protumor/M2 phenotype and secrete immunosuppressive cytokines such as IL10 (44).
In murine mammary carcinoma models, swimming promoted antitumor/M1 polarization of peritoneal macrophages following lipopolysaccharide (LPS) stimulation, whereas similarly treated macrophages in sedentary controls maintained a protumor/M2 phenotype (44). Additionally, chronic treadmill training increased tumor cell cytolysis by peritoneal macrophages by 50% (45). These data suggest that exercise-mediated shifts in macrophage polarization may increase antitumor function of TAMs.
Exercise and the adaptive immune system
In humans, large increases in the number of circulating low-, medium-, and high-differentiated T cells occur during or shortly after exercise. Exercise intensity increases the relative distribution of low-, medium-, and high-differentiated T cells with main effects seen in medium-differentiated CD4+ T cells and low- and medium-differentiated CD8+ T cells (46). These cells exhibit higher cytokine production and enhanced response to cytomegalovirus. Upon exercise cessation, circulating lymphocyte and NK cell numbers rapidly decline, even to levels below baseline, suggesting that they quickly enter back into tissues (47).
In post-chemotherapy patients with breast cancer, 12 weeks of supervised exercise did not change the mean circulating numbers of CD3+, CD4+, CD8+, B or NK cells, but increased the percentage of CD4+/CD9+cells (CD9 is a marker of T-cell activation) by 50% compared with controls who did not exercise. In comparison, the percentage of CD9+ lymphocytes declined in the control group. In vitro response to lymphocyte mitogens in the exercising group was increased, compared with controls (48). These results suggest that immune competency is increased by exercise in post chemotherapy breast cancer patients who exercise. It is not known whether this change in immune competency extends to tumor immunity, however.
To our knowledge, the role of exercise on regulatory T cells (Treg) has not been examined clinically; results in murine models have been mixed. In the MMTV-PyMT transgenic mouse mammary carcinoma, 10 weeks of voluntary wheel running decreased tumor size and caused a 4-fold reduction in CCL22 expression. CCL22 is an M2 macrophage-associated chemokine responsible for recruiting Tregs (49). There was no difference in numbers of M1 or M2 macrophages between groups, suggesting that the reduction in CCL22 is related to functional, rather than quantitative, change in M2 macrophages. A second study reported that physical activity decreased the percentage of splenic Tregs in mammary carcinoma-bearing mice (50). However, others have suggested that exercise increases Tregs (51). To date, the impact of exercise on Treg function and distribution has not been systemically evaluated. Understanding the relationship between exercise and Treg levels with various exercise conditions could be used to increase effector T-cell/Treg ratio; the ratio of effector T cells to Tregs is associated with cancer therapy response (52–54).
Humoral Immunity and Exercise
B cells, like T and NK cells, are mobilized by exercise. Short bouts of cycling in healthy human subjects increased circulating levels of multiple B-cell subsets, with the largest proportionate increase in immature B cells. Immature B cells may redistribute to peripheral tissues for maturation and antigen detection (55). In elderly patients, 10 months of aerobic exercise, compared with flexibility and balance training, increased antibody responses to influenza vaccine (56). However, it is not known whether such effects influence tumor immunity.
Exercise-induced modulation of the tumor physiologic microenvironment and its role in immune response
To our knowledge, there are no studies that directly link exercise-induced changes in the tumor physiologic microenvironment with changes in tumor immune response. However, the literature highlighted below supports the hypothesis that modulation of the physiologic microenvironment by exercise can improve antitumor immunity.
We discuss four features: hypoxia, glucose concentration, lactate concentration, and extracellular pH (pHe). Although discussed separately, these physiologic conditions often occur simultaneously in space and time. Future exercise intervention studies should consider simultaneous measurement of these four features coincident with the evaluation of immune function.
Tumor hypoxia and innate and adaptive immune function
Most tissues possess physiologic oxygen tension above 20 mmHg (57). Whereas oxygen delivery matches metabolic demand in normal tissue, oxygen demands overpower limited supply in tumors (58). The imbalance between supply and demand leads to intratumoral hypoxia (pO2 < 10 mmHg; refs. 58, 59). Hypoxia is prevalent in many solid cancers and contributes to chemoresistance, radioresistance, and reduced survival (6, 60, 61).
Hypoxia inhibits macrophage and NK cell activities (62, 63). Hypoxic conditions reduce the expression of NK cell surface receptor NKG2D, as well as its ligand in vitro (64, 65). Hypoxia impairs NK cytolysis of cancer cells of both hematopoietic and solid tumor origin (66). Hypoxia post-translationally upregulates the HIF1α subunit of the transcription factor, hypoxia inducible factor-1 (HIF1), in nearly all mammalian cells, including tumor, endothelial, and stromal cells (67). Upregulation of HIF1 induces the production of VEGF, granulocyte-stimulating factors, and IL8. This in turn recruits myeloid-derived suppressor cells (MDSC) and TAMs to tumor sites (68–70). High levels of HIF1 promote myeloid cell differentiation into immunosuppressive protumor/M2 TAMs and MDSCs (71, 72).
Hypoxia also inhibits adaptive immune cell function. Hypoxia interferes with immune plasticity and disrupts the balance between effector T cells and Tregs (25, 73). Further, T-cell motility is reduced in hypoxia (74). In murine models of colorectal carcinoma, hypoxia reduced differentiation of CD4+ T cells by 20% to 40%, while enhancing the number and function of Tregs (75). Hypoxic effector T cells exhibit decreased IFNγ and IL2 production (62).
Although there are many studies investigating T-cell function in the context of the hypoxic tumor microenvironment, few have examined B-cell function under hypoxic conditions. Studies indicate that hypoxia and oxygen gradients vary in lymphoid tissues, which could impact B-cell function (76–79). Germinal centers in lymph nodes and spleen are important for B-cell maturation. Germinal centers are hypoxic with high levels of HIF1α. Thus, B cells encounter varying conditions of hypoxia as they migrate to and from lymphoid organs into circulation. It is plausible that hypoxia controls B-cell migration, differentiation/function, and activation in response to antigens as well as tolerance (76–79). Lee and colleagues identified B cells as key immune cells in pancreatic cancer progression (80). In a mouse model of pancreatic ductal adenocarcinoma (PDAC), they demonstrate the importance of hypoxia and stabilization of HIF1α. They show that pancreas-specific Hif1a deletion promotes PDAC initiation with a concurrent increase of B cells in the pancreas, whereas B-cell depletion suppresses pancreatic cancer progression (80).
Key mechanisms underlying hypoxic immunosuppressive effects include (i) signaling through adenosine receptors (A2A adenosine receptors), (ii) desensitization of chemokine receptors, (iii) downregulation of major histocompatibility complex (MHC) class I molecules on tumor cells (81, 82), and (v) recruitment of immunosuppressive cells into the tumor microenvironment. Hypoxia-induced inhibitory effects may be reversible, however. Supplemental oxygen breathing revives effector immune responses. Housing tumor-bearing mice at hyperoxic conditions (60% O2) reversed hypoxia-driven adenosinergic action, enhanced tumor infiltration by CD8+ T cells by 3-fold, and shifted cytokine production toward immunostimulatory cytokines (IFNγ; ref. 81).
Exercise modulates tumor hypoxia
Several studies show that exercise reduces tumor hypoxia. In an orthotopic rat prostate cancer model, acute exercise increased tumor blood flow 2-fold, thereby increasing O2 delivery to tumors. Tumor hypoxic fraction was reduced by up to 15% (14, 16). Treadmill running increased microvessel density, promoted vessel maturity, and reduced hypoxic tumor fraction compared with sedentary controls (12,13,16). Chronic voluntary wheel running increased microvessel density by 50%, tripled the area of pericyte-covered vasculature, and halved hypoxic fraction (12) in mice bearing the 4T1 tumor. Schadler and colleagues demonstrated that increased vascular maturity associated with exercise was related to activation of calcineurin-NFAT_TSP-1 signaling induced by increased intravascular shear stress (16). In theory, reduced tumor hypoxia should destabilize HIF1α expression. Interestingly, however, Jones and colleagues showed increased HIF1α in the MDA-MB-231 xenograft with exercise, despite decreases in hypoxia (13). These findings suggest that exercise alters intratumoral HIF1α expression in ways independent of the improved oxygenation level. The fact that HIF1 levels can be upregulated in some tumors, regardless of the oxygenation status, leads to a cautionary note about the downstream effects of exercise on tumor growth. More studies are required to understand these implications.
Exercise and glucose deprivation
The high rate of glucose consumption in tumors, and deficiencies in glucose delivery by dysfunctional tumor vasculature, can lead to tumor subregions with near-zero glucose concentrations, even in nonnecrotic regions (83). Viable tumor cells residing in hypoglucotic (low glucose concentration) regions likely rely on other substrates to maintain viability, such as glutamine or fatty acids (84). Glucose availability is essential for effective immune function, because activated immune cells rely on glycolysis to produce precursors for cell division (85). Thus, one might expect that immune function would be inhibited in hypoglucotic tumor subregions. The effects of exercise on glucose metabolism in immune cells are understudied, however.
Glass and colleagues reported on the effects of daily treadmill exercise in three claudin-low murine tumor models (86). “Claudin-low” represents breast tumors that express genomic markers of dedifferentiation (87). Exercise inhibited growth compared with sedentary controls in one tumor line but stimulated growth in a second one. The tumor line that showed an accelerated growth rate with exercise had upregulated HIF1 levels. HIF1 is a master transcriptional regulator of glycolysis (88). Glycolysis is associated with accelerated tumor growth, because it generates precursors necessary for cell division (88). Corroborating the HIF1 result, metabolomic analysis revealed that glycolysis was upregulated by exercise in the growth-accelerated tumor. Exercise slowed tumor growth in three of six colorectal cancer patient-derived xenograft (PDX) tumors, whereas it exerted no effect on tumor growth in three others (89). The growth-inhibited lines showed metabolomic changes consistent with reduced mitochondrial metabolism. It is unknown whether exercise affects the nature of glucose consumption in tumor-associated immune cells and, if so, how that might affect the immune function.
Lactate metabolism
Tumor cells exist in an acidic microenvironment as a result of either anaerobic or aerobic glycolysis. In hypoxic conditions, cells must use glycolysis to generate energy (90). Aerobic glycolysis produces precursors for DNA and lipid synthesis (91). Lactic acid is the end-product of glycolysis, expelled from cells via monocarboxylic acid transporters (MCT; ref. 92). Acidity is created mainly by lactic acid via glycolysis (93). Although lactate was traditionally thought to be a glycolytic waste product, it is now established that lactate can be consumed by aerobic tumor cells; alanine and glutamate are primary catabolites (94). Consumption of lactate by aerobic tumor cells reserves glucose for hypoxic tumor cells deeper within the tumor (90). The sharing of energy substrates between aerobic and hypoxic tumor cells is a key mechanism for hypoxic tumor cell survival (90). It is not known whether immune cells can catabolize lactate.
Lactate, acidosis, pH, and immune response
In contrast to normal tissues, which possess an extracellular pH (pHe) of about 7.5, the median pHe in solid tumors is 6.8–7.0 (95, 96). Elevated lactate and the associated low tumor pHe impair lymphocyte cytotoxicity, chemotaxis, cellular respiration, and proliferation (97). At pHe < 6.5, in vitro random leukocyte motility is greatly decreased (98). At pHe < 6.7, lymphocyte and NK cell cytotoxic activities against leukemia target cells are approximately halved (99,100). Similarly, NK cells cultured with 10 to 15 mmol/L lactate, a physiologic tumor lactate range (101, 102), exhibit 5-fold reduction in target cell cytotoxicity (103).
Immunosuppression in acidic environments may partially be a consequence of compensatory shifts in T-cell metabolism (104), as activated T cells are particularly reliant on glycolysis (105). High lactate generated by tumor cells (106) disturbs the gradient that drives T-cell lactate efflux, because MCT1 facilitates lactate transport out of cells in a manner that is dependent upon the concentration gradient across the cell wall (107). Increased intracellular lactate suppresses T-cell metabolism and function (105).
Exercise and tumor lactate/acidity
Aerobic exercise normalizes the immunosuppressive, acidic tumor microenvironment. Treadmill running in mice with mammary carcinomas reduced tumor and circulating lactate concentrations by approximately 17% compared with sedentary counterparts (108). In sarcoma bearing rats (109), treadmill running decreased glucose conversion to lactate by approximately 50% in peripheral macrophages and lymphocytes. The reduction in tumor lactate production was accompanied by a 2-fold increase in peripheral macrophage phagocytic activity and a 75% increase in peripheral lymphocyte proliferation (measured in vitro). Although changes in the profile of peripheral immune cells in response to exercise do not necessarily reflect changes in the tumor, the study, nevertheless, demonstrates that reducing lactate exposure potentiates the immune function.
Future Directions and Conclusion
In this review, we explore the hypothesis that exercise modulates the tumor physiologic microenvironment and, consequently, influences immune function and activity. The current literature supports the concept that well-oxygenated, less acidic environments (i) improve the function of T cells and NK cells; (ii) promote antitumor activity in TAMs; and (iii) reduce expression of some immune checkpoints. Additional preclinical and clinical trials of exercise should be conducted in which immune function is studied in the context of the tumor physiologic microenvironment, with particular attention paid to hypoxia, glucose concentration, lactate concentration, and extracellular pH (pHe).
More work is required to establish how physiologic effects of exercise modulate the tumor microenvironment. Corroborative measurements of circulating and intratumoral immune cells may help to clarify the link between tumor microenvironment changes and changes in immune function/activity. Additionally, metabolic profiling of immune cells isolated from tumors of exercising and sedentary subjects would shed light on the metabolic adaptations in an exercise-primed tumor microenvironment. In order to be interpretable, preclinical and clinical studies of exercise require carefully defined and controlled exercise regimens. Preclinical models of exercise in tumor-bearing animals can shed light on underlying mechanisms and help to optimize combinations of exercise with other therapeutic approaches.
Rigorous studies that elucidate the link between exercise and immune cell function in the tumor microenvironment and in the periphery will also serve as a guide of how to implement exercise in the context of immunotherapies that harness the immune system against cancer. Successful immunotherapy relies upon the ability of innate and adaptive immune cells, such as macrophages, NK cells and T cells, to infiltrate the tumor parenchyma and eliminate tumor cells. The factors in the tumor physiologic microenvironment that inhibit the penetration and function of host immune cells will likely also interfere with immunotherapies. To elaborate further, two examples are provided below:
CAR T cells are engineered to express chimeric antigen receptors (CAR) that specifically target and eliminate tumor cells, independent of the MHC (110). Because MHC expression is downregulated by hypoxia (81, 82), CAR T cells have an advantage over host T cells that rely on the MHC-based recognition of tumor cells in the hypoxic tumor microenvironment. However, T-cell (including CAR T-cell) trafficking to the tumor via the vasculature and penetration into the tumor bed are inhibited by hypoxia. In other words, the T cell has to reach tumor cells before it can kill them. Thus, the tumor physiologic microenvironment, and not the MHC, is the gatekeeper for effective T-cell infiltration into the tumor bed. As discussed above, high lactate concentrations and low pH can interfere with the ability of T cells to kill tumor cells. These effects are not reliant on MHC expression. It is important to note that CARs have been effective in treating certain types of lymphomas (111, 112). The physiologic microenvironment of lymphomas or blood cancers may be more permissive toward an effective immune function. For example, lymphomas are only mildly hypoxic (113). However, results regarding lactate levels are mixed. Elevated lactate levels are not common in CNS lymphomas (114). Further, there is some evidence that lymphomas are more reliant on oxidative phosphorylation than glycolysis (115), which would reduce lactate concentrations. However, elevated lactate concentrations and lowered pHe have been observed in preclinical lymphoma models (116, 117). Compared with normals, blood lactate concentrations are relatively high in dogs with non-Hodgkin lymphoma (118). These data suggest that lactate levels may be relatively normal in some lymphomas, but not all. If lymphomas are relatively oxic, then the physiologic microenvironment would be permissive to enhanced CAR T-cell function; however, lactate levels may counterbalance the positive effects of normoxia. The effects of elevated lactate on the CAR T-cell immune function are relatively unexplored in lymphomas. Further, the role that exercise may play in the lymphoma physiologic microenvironment is not defined.
Immune-checkpoint inhibitors are antibodies that bind immune receptors to inhibit T-cell function. T-cell function is inhibited when PD-1 on T cells interacts with its ligand, PD-L1, on tumor cells or myeloid immune cells. PD-L1 is regulated by HIF1, so in situations where exercise reduces hypoxia and its dependent transcription factor, HIF1, one would expert decreased PD-L1 expression (119–121). Reduced PD-L1 would reduce PD-L1/PD-1 interaction and improve T-cell function within the tumor. It is unknown whether T-cell function would be improved in conditions where hypoxia is lowered but lactate levels remain the same. Thus, correction of hypoxia by exercise may be insufficient to restore immune cell function if lactate concentrations remain elevated as a result of aerobic glycolysis.
It is clear that additional studies are required to resolve the effects of exercise on the physiologic microenvironment. Parallel studies examining the function of innate and adaptive immunity in the microenvironment, as influenced by exercise, are necessary to decipher the full potential of this therapy.
Acknowledgments
This work was partially funded by the Medical Research Fellowship from the Howard Hughes Foundation (X. Zhang) and by the Conquer Cancer Foundation (ABW).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 2000;342:454–60. [DOI] [PubMed] [Google Scholar]
- 3.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol 2012;2:2775–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol 2010;28:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gammon MD, Schoenberg JB, Britton JA, Kelsey JL, Coates RJ, Brogan D, et al. Recreational physical activity and breast cancer risk among women under age 45 years. Am J Epidemiol 1998;147:273–80. [DOI] [PubMed] [Google Scholar]
- 6.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 2016;76:4032–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer 1996;73:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenreich CM. Physical activity and breast cancer risk: the effect of menopausal status. Exerc Sport Sci Rev 2004;32:180–4. [DOI] [PubMed] [Google Scholar]
- 9.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer 2017;17: 620–32. [DOI] [PubMed] [Google Scholar]
- 10.Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc Sport Sci Rev 2017;45:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idorn M, Straten PT. Exercise and cancer: from “healthy” to “therapeutic”? Cancer Immunol Immunother 2017;66:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol 2010;108:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst 2014;106:dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol 2012;113:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016;7:65429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Iskra B, Kleinerman E, Alvarez-Florez C, Andrews T, Shaw A, et al. Aerobic exercise during early murine doxorubidn exposure mitigates cardiac toxicity. J Pediatr Hematol Oncol 2018;40:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kathleen A Ashcraft KRC, Zessin AS, Boss M-K, Zhang X, Rickard AG, Betof AS, et al. Physical activity increases tumor response to radiation and reduces spontaneous metastasis. Manuscript in preparation 2018. [Google Scholar]
- 19.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 21.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. [DOI] [PubMed] [Google Scholar]
- 23.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996;56: 1111–7. [PubMed] [Google Scholar]
- 24.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res 1992;52: 4265–8. [PubMed] [Google Scholar]
- 25.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstades and opportunities for innovative immunotherapy of cancer. Oncogene 2017;36:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Casado A, Martin-Ruiz A, Perez LM, Provendo M, Fiuza-Luces C, Luda A. Exercise and the hallmarks of cancer. Trends Cancer 2017;3: 423–41. [DOI] [PubMed] [Google Scholar]
- 29.Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun 2005;19:377–80. [DOI] [PubMed] [Google Scholar]
- 30.Nieman DC. Exercise, infection, and immunity. Int J Sports Med 1994;15 Suppl 3:S131–41. [DOI] [PubMed] [Google Scholar]
- 31.Nehlsen-Cannarella SL, Nieman DC, Balk-Lamberton AJ, Markoff PA, Chritton DB, Gusewitch G, et al. The effects of moderate exercise training on immune response. Med Sci Sports Exerc 1991;23:64–70. [PubMed] [Google Scholar]
- 32.Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness 1990;30:316–28. [PubMed] [Google Scholar]
- 33.Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol 2017;122:1077–87. [DOI] [PubMed] [Google Scholar]
- 34.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 2014;58:193–210. [DOI] [PubMed] [Google Scholar]
- 35.Pardo J, Balkow S, Anel A, Simon MM. Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control. Eur J Immunol 2002;32:2881–7. [DOI] [PubMed] [Google Scholar]
- 36.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T-cells and natural-killer-cells is greatly impaired in perforin deficient mice. Nature 1994;369:31–7. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176:1517–24. [DOI] [PubMed] [Google Scholar]
- 38.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood 2005;106:566–71. [DOI] [PubMed] [Google Scholar]
- 39.Ames E, Murphy WJ. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother 2014;63: 21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava RM, Lee SC, Andrade PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013;19:1858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK):dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res 2011;50:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab 2016;23:554–62. [DOI] [PubMed] [Google Scholar]
- 43.O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med 2012;209:1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdalla DR, Aleixo AA, Murta EF, Michelin MA. Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol Lett 2014;7:886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol 1999;276(2 Pt 2):R482–9. [DOI] [PubMed] [Google Scholar]
- 46.LaVoy EC, Hussain M, Reed J, Kunz H, Pistillo M, Bigley AB, et al. T-cell redeployment and intracellular cytokine expression following exercise: effects of exercise intensity and cytomegalovirus infection. Physiol Rep 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooney BV, Bigley AB, LaVoy EC, Laughlin M, Pedlar C, Simpson RJ. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: a detailed temporal analysis of leukocyte extravasation. Physiol Behav 2018;194:260–7. [DOI] [PubMed] [Google Scholar]
- 48.Hutnick NA, Williams NI, Kraemer WJ, Orsega-Smith E, Dixon RH, Bleznak AD, et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc 2005;37:1827–35. [DOI] [PubMed] [Google Scholar]
- 49.Goh J, Tsai J, Bammler TK, Farin FM, Endicott E, Ladiges WC. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS One 2013;8:e80123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdalla DR, Murta EF, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev 2013;22:251–8. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports 2012;22:643–52. [DOI] [PubMed] [Google Scholar]
- 52.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016;5: e1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JP, Riddell NE, Bums VE, Turner M, van Zanten J, Drayson MT, et al. Acute exercise mobilises CD8+T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun 2009;23:767–75. [DOI] [PubMed] [Google Scholar]
- 56.Woods JA, Keylock KT, Lowder T, Vieira VJ, Zelkovich W, Dumich S, et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J Am Geriatr Soc 2009;57:2183–91. [DOI] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 58.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008;8:425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res 2002;62:5381–5. [PubMed] [Google Scholar]
- 60.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638–48. [DOI] [PubMed] [Google Scholar]
- 61.Hypoxia Vaupel P. and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist 2008;13Suppl 3:21–6. [DOI] [PubMed] [Google Scholar]
- 62.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 2001; 167: 6140–9. [DOI] [PubMed] [Google Scholar]
- 63.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003;112:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada N, Yamanegi K, Ohyama H, Hata M, Nakasho K, Futani H, et al. Hypoxia downregulates the expression of cell surface MICA without increasing soluble MICA in osteosarcoma cells in a HIF-1 alpha-dependent manner. Int J Oncol 2012;41:2005–12. [DOI] [PubMed] [Google Scholar]
- 65.Noman MZ, Janji B, Berchem G, Chouaib S. miR-210 and hypoxic microvesicles: Two critical components of hypoxia involved in the regulation of killer cells function. Cancer Lett 2016;380:257–62. [DOI] [PubMed] [Google Scholar]
- 66.Fink T, Ebbesen P, Koppelhus U, Zachar V. Natural killer cell-mediated basal and interferon-enhanced cytotoxicity against liver cancer cells is significantly impaired under in vivo oxygen conditions. Scand J Immunol 2003;58:607–12. [DOI] [PubMed] [Google Scholar]
- 67.Semenza GL. Regulation of mammalian O-2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999;15:551–78. [DOI] [PubMed] [Google Scholar]
- 68.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008;13: 206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999;79:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL, Koh HY, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016;64: 797–813. [DOI] [PubMed] [Google Scholar]
- 71.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008;13:453–61. [DOI] [PubMed] [Google Scholar]
- 72.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207:2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437–47. [DOI] [PubMed] [Google Scholar]
- 74.Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol 2007;178:7747–55. [DOI] [PubMed] [Google Scholar]
- 75.Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol Biochem 2017;41: 1271–84. [DOI] [PubMed] [Google Scholar]
- 76.Piovan E, Tosello V, Indraccolo S, Masiero M, Persano L, Esposito G, et al. Differential regulation of hypoxia-induced CXCR4 triggering during B-cell development and lymphomagenesis. Cancer Res 2007;67: 8605–14. [DOI] [PubMed] [Google Scholar]
- 77.Abbott RK, Thayer M, Labuda J, Silva M, Philbrook P, Cain DW, et al. Germinal center hypoxia potentiates immunoglobulin class switch recombination. J Immunol 2016;197:4014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen CD, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol 2017;18:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 2016;537:234-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, et al. HiF1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov 2016;6:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med 2015;7:277ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sethumadhavan S, Silva M, Philbrook P, Nguyen T, Hatfield SM, Ohta A, et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One 2017;12:e0187314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schroeder T, Yuan H, Viglianti BL, Peltz C, Asopa S, Vujaskovic Z, et al. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res 2005;65: 5163–71. [DOI] [PubMed] [Google Scholar]
- 84.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 2009;15:6479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouidhi S, Ben Ayed F, Benammar Elgaaied A. Targeting tumor metabolism: a new challenge to improve immunotherapy. Front Immunol 2018;9:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glass OK, Inman BA, Broadwater G, Courneya KS, Mackey JR, Goruk S, et al. Effect of aerobic training on the host systemic milieu in patients with solid tumours: an exploratory correlative study. Br J Cancer 2015;112: 825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J 2017;36:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu M, Sanderson SM, Zessin A, Ashcraft KA, Jones LW, Dewhirst MW, et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008;118:3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Icard P, Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta 2012;1826:423–33. [DOI] [PubMed] [Google Scholar]
- 92.Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One 2012;7:e33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillies RJ, Gatenby RA. Metabolism and its sequelae in cancer evolution and therapy. Cancer J 2015;21:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kennedy KM, Scarbrough PM, Ribeiro A, Richardson R, Yuan H, Sonveaux P, et al. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS One 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tannock IF, Rotin D. Add pH in tumors and its potential for therapeutic exploitation. Cancer Res 1989;49:4373–84. [PubMed] [Google Scholar]
- 96.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia 1995;11:211–6. [DOI] [PubMed] [Google Scholar]
- 97.Lardner A The effects of extracellular pH on immune function. J Leukoc Biol 2001;69:522–30. [PubMed] [Google Scholar]
- 98.Rotstein OD, Fiegel VD, Simmons RL, Knighton DR. The deleterious effect of reduced pH and hypoxia on neutrophil migration in vitro. J Surg Res 1988;45:298–303. [DOI] [PubMed] [Google Scholar]
- 99.Severin T, Muller B, Giese G, Uhl B, Wolf B, Hauschildt S, et al. pH-dependent LAK cell cytotoxicity. Tumour Biol 1994;15:304–10. [DOI] [PubMed] [Google Scholar]
- 100.Loeffler DA, Juneau PL, Heppner GH. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int J Cancer 1991;48: 895–9. [DOI] [PubMed] [Google Scholar]
- 101.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000;60:916–21. [PubMed] [Google Scholar]
- 102.Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51: 349–53. [DOI] [PubMed] [Google Scholar]
- 103.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol 2013;191:1486–95. [DOI] [PubMed] [Google Scholar]
- 104.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007;109:3812–9. [DOI] [PubMed] [Google Scholar]
- 105.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol 2004;172:4661–5. [DOI] [PubMed] [Google Scholar]
- 106.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999;343Pt 2:281–99. [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi M, Fujita I, Itagaki S, Hirano T, Iseki K. Transport mechanism for L-lactic acid in human myocytes using human prototypic embryonal rhabdomyosarcoma cell line (RD cells). Biol Pharm Bull 2005;28: 1197–201. [DOI] [PubMed] [Google Scholar]
- 108.Aveseh M, Nikooie R, Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J Physiol 2015;593: 2635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bacurau RF, Belmonte MA, Seelaender MC, Costa Rosa LF. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem Fund 2000;18:249–58. [DOI] [PubMed] [Google Scholar]
- 110.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 2016;16:566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown CE, Mackall CL. CAR T cell therapy: inroads to response and resistance. Nat Rev Immunol 2019;19:73–4. [DOI] [PubMed] [Google Scholar]
- 112.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Postema EJ, McEwan AJB, Riauka TA, Kumar P, Richmond DA, Abrams DN, et al. Initial results of hypoxia imaging using 1-alpha-D-(5-deoxy-5-(18)F -fluoroarabinofuranosyl)-2-nitroimidazole ((18)F-FAZA). Eur J Nucl Med Mol Imaging 2009;36:1565–73. [DOI] [PubMed] [Google Scholar]
- 114.Raizer JJ, Koutcher JA, Abrey LE, Panageas KS, DeAngelis LM, Lis E, et al. Proton magnetic resonance spectroscopy in immunocompetent patients with primary central nervous system lymphoma. J Neuro-Oncol 2005;71: 173–80. [DOI] [PubMed] [Google Scholar]
- 115.Gooptu M, Whitaker-Menezes D, Sprandio J, Domingo-Vidal M, Lin Z, Uppal G, et al. Mitochondrial and glycolytic metabolic compartmentalization in diffuse large B-cell lymphoma. Semin Oncol 2017;44: 204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Potzl J, Roser D, Bankel L, Homberg N, Geishauser A, Brenner CD, et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-gamma and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer 2017;140: 2125–33. [DOI] [PubMed] [Google Scholar]
- 117.Lee SC, Marzec M, Liu XB, Wehrli S, Kantekure K, Ragunath PN, et al. Decreased lactate concentration and glycolytic enzyme expression reflect inhibition of mTOR signal transduction pathway in B-cell lymphoma. NMR Biomed 2013;26:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McQuown B, Burgess KE, Heinze CR. Preliminary investigation of blood concentrations of insulin-like growth factor, insulin, lactate and -hydroxybutyrate in dogs with lymphoma as compared with matched controls. Vet Comp Oncol 2018;16:262–7. [DOI] [PubMed] [Google Scholar]
- 119.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1 alpha., and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dai XM, Pi GL, Yang SL, Chen GG, Liu LP, Dong HH. Association of PD-L1 and HIF-1 alpha coexpression with poor prognosis in hepatocellular carcinoma. Transl Oncol 2018;11:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014;74:665–74. [DOI] [PubMed] [Google Scholar]


