Abstract
Background:
Tumor buds are associated with lympho-vascular invasion and lymph node metastases leading to the assumption that they are involved in the early metastatic process. Hence, it would be important to know if tumor buds can be targeted with the most widely used targeted therapies in breast cancer (BC) and if changes in hormone and Her2 status occur. The aim of this study was to answer these questions by determining whether hormone receptor (HR) and Her2 status are expressed in the tumor buds of a large cohort of BCs.
Design:
We constructed a tumor bud next-generation tissue microarray (ngTMA) consisting of n = 199 BCs of non-special type. Generally, two 1 mm punches were taken from the tumor bud areas in the periphery (PTB) and within the tumor center (ITB). HR and Her2 status was assessed using immunohistochemistry and fluorescence in situ hybridization, respectively. HR status was positive if ≥1% of tumor bud cells were positive. Her2 status was considered positive if bud cells showed strong complete membranous Her2 over-expression or Her2 amplification.
Results:
Most tumor buds were positive for estrogen (ER) (PTB: 86%; ITB: 88.3) and progesterone receptor (PgR) (PTB: 72%; ITB: 72.8%) and Her2 was positive in: PTB 11.5% and ITB 11%. A difference between the main tumor mass and tumor buds (PTB and ITB) was seen for PgR in 3.5% of cases (n = 7). No differences were seen for ER and Her2 between tumor buds and main tumor mass.
Conclusion:
Most tumor buds (96.5%) share the same HR and Her2 expression profile of the main tumor mass, implying that tumor buds relay on the same pathways as the main tumor mass and might be equally responsive to targeted therapies.
Keywords: Tumor budding, Breast cancer, Hormone receptors, Her2
1. Introduction
Tumor buds are small cell clusters or single tumors cells, detaching from the main tumor mass. This phenomenon can be seen within the tumor (intra-tumoral budding), or at the tumor periphery (peripheral tumor budding) [22,27,19]. Tumor budding is best characterized in colon cancer [6,19,31] but it is increasingly recognized and described in other tumor types such as e.g. breast, pancreatic-, esophagus-, larynx- and other cancers [16–18,22–25,27]. In breast cancer (BC) and other tumor types, high numbers of tumor buds are associated with lympho-vascular invasion (LVI) and/or lymph node metastasis [16,18,22,27]. Additionally, high numbers of tumor buds are associated with shorter overall and cancer-specific survival in BC [8,18] and this has been described in other tumor types as well [6,15]. The association of vascular invasion and tumor budding led to the assumption that tumor buds are involved in the early metastatic process by undergoing epithelial-mesenchymal transition (EMT) [8,19]. It is well-known that tumor cells undergoing EMT are more invasive and prone to metastasize that can lead to worse overall survival in cancer patients [1,10,23].
Inhibiting tumor cells involved in the early metastatic process would be of great clinical value since metastatic disease remains the major cause of cancer deaths with around 30% of BC patients developing metastasis [4,20]. This indicates that a better understanding of the metastatic process is needed in order to develop novel targeted approaches for highly aggressive and invasive cancer to improve patient outcomes. If tumor buds are involved in the early metastatic process, then it would be advantageous to determine whether and how they can be uniquely targeted. Tumor buds are known to have characteristics of EMT and in breast cancer, estrogen and Her2 overexpression was shown to be involved in EMT [11,13]. However, little is known regarding the expression of markers commonly targeted in breast cancer such as the estrogen and progesterone receptors and Her2 status in tumor buds.
Hence, the aim of this study was to examine the immunophenotypic profile of tumor buds to determine if they are targetable with the most widely used targeted therapies in BC, such as anti-hormonal and anti-Her2 therapy. In the current study, we report the estrogen, progesterone and Her2 receptor status of a large number of tumor buds in BC of non-special type (NST) using a next-generation tissue microarray (ngTMA).
2. Material and methods
Patients:
We selected 199 NST BCs out of our previously described cohort of 356 therapy naïve, unilateral BCs diagnosed in female patients that underwent surgery between 2005 and 2011 at the Inselspital Bern, Switzerland [7]. T category was available for all BCs and N category was available for n = 181 (91%). Tumor grading, estrogen-, progesterone-, and Her2 receptor status (ER, PgR and Her2) and the molecular subtypes according to the St. Gallen 2013 criteria from the main tumor mass was available from our previous studies [7,22]. The median age at diagnosis was 64 years (range: 33–98 years) and clinical information regarding chemotherapy was available for 100 (50.2%) cases; for anti-hormone therapy for n = 102 (51.3%), for anti-her2 therapy in n = 95 (47.7%); and for radiation therapy for n = 140 (70.9%) cases. The clinic-pathological characteristics are shown in Table 1. The study was approved by the ethical commission of the University of Bern (Registration 200/2014).
Table 1.
Patient characteristics of the whole cohort (n = 199).
| Feature | Frequency n (%) | |
|---|---|---|
| Age at diagnosis (years) | Median (min, max) | 64 (33, 98) |
| Tumor size (centimeter) | Mean (min, max) | 2.4 (0.6, 8.4) |
| ER | positive | 165 (82.9) |
| negative | 34 (17.1) | |
| PgR | positive | 142 (71.4) |
| negative | 57 (28.6) | |
| Her2 | negative | 177 (88.9) |
| positive | 21 (10.6) | |
| No data | 1 (0.5) | |
| Nottingham Grad | G1 | 21 (10.6) |
| G2 | 97 (48.7) | |
| G3 | 81 (40.7) | |
| LVI | Yes | 82 (41.2) |
| No | 104 (52.3) | |
| No data | 13 (6.5) | |
| VI | Yes | 21 (10.6) |
| No | 161 (80.9) | |
| No data | 17 (8.5) | |
| Pn1 | Yes | 26 (13.1) |
| No | 148 (74.3) | |
| No data | 25 (12.6) | |
| pT | T1 | 92 (46.2) |
| T2 | 93 (46.8) | |
| T3 | 7 (3.5) | |
| T4 | 7 (3.5) | |
| pN | N0 | 84 (42.3) |
| N1mi | 11 (5.5) | |
| N1 | 60 (30.2) | |
| N2 | 17 (8.5) | |
| N3 | 9 (4.5) | |
| No data | 18 (9.0) | |
| Recurrence | Yes | 12 (6.0) |
| No data | 187 (94) | |
| Molecular subtypes (St. Gallen 2013) | Luminal A | 106 (53.3) |
| Luminal B (her2 negative) | 44 (22.1) | |
| Luminal B (her2 positive) | 14 (7.0) | |
| Her2 non-luminal | 7 (3.5) | |
| Triple negative | 26 (13.1) | |
| no data | 2 (1.0) | |
| Anti-hormonal therapy | Yes | 42 (21.1) |
| No | 60 (30.2) | |
| No data | 97 (48.7) | |
| Chemotherapy | Yes | 37 (18.6) |
| No | 63 (31.7) | |
| No data | 99 (49.7) | |
| Anti-her2 therapy | Yes | 5 (2.5) |
| No | 90 (45.2) | |
| No data | 104 (52.3) | |
| Radiation therapy | Yes | 100 (50.3) |
| No | 40 (20.1) | |
| no data | 59 (29.6) |
Next-generation tissue microarray (ngTMA) of tumor buds: The ngTMA was constructed as previously described (3DHistech, Budapest, Hungary) [33]. In brief, pathologists reviewed breast cancer cases using H&E slides [7]. The decision, what block should be use for the peripheral and intra-tumoral bud ngTMA was made by one pathologist (CT). The H&E slides where then scanned and uploaded to the digital platform to perform annotation on the computer screen. Whenever feasible, two areas from peripheral and central tumor buds were punched. We successfully made two 1 mm punches in 199 and 193 of PTB and ITB cases, respectively.
Definition and assessment of tumor buds: We used our previous definition of tumor buds: One isolated tumor cell or a small tumor cell clusters of up to 5 tumor cells [22]. The slides of the tumor bud ngTMA were stained with ER, PgR and Her2 using the same antibodies and conditions as in our previous study [22]. Briefly, any nuclear ER and PgR staining, regardless of intensity, was considered as positive. The cases were then dichotomized into negative and positive cases according to the cut-off of ≥ 1% [9]. Her2 status was evaluated according to ASCO/CAP guidelines 2013 [30]. ER and PgR positive tumor cells were estimated in the tumor buds and a positive rate of ≥ 1% positive tumor cells was regarded as a positive hormone (HR) status. For HR status any intensity of nuclear staining was regarded as positive and the cases were dichotomized into negative and positive cases according to the cut-off of ≥ 1%. A strong, complete membranous staining for Her2, or a Her2 amplification was considered Her2 positive. Difference in ER and PgR status was defined according to the dichotomized result obtained for the main tumor and the tumor buds or according to the defined positive expression of Her2 status.
Statistics:
We used the Pearson Chi-Square test to calculate significant correlations between categorical variables. A p-value of < 0.05 was considered statistically significant. Analyses were carried out using the IBM SPSS Statistics 22.0 (IBM, Armonk, NY, USA).
3. Results
3.1. Peripheral tumor buds (PTB)
Informative ER, PgR and Her2 results were available for 172 (86.9%), 168 (84.8%), and 156 (78.8%) cases. ER status was positive in 148 (86%) cases. Her2 status was positive in 18 (11.5%). PgR was positive in 121 (72%). No difference in ER or Her2 status was seen between main tumor mass and tumor buds. However there was a difference in receptor status between main tumor mass and buds for PgR in 6 (3.6%; 6/168) cases. All cases showed a positive PgR status in the main tumor mass but were negative in PTBs. Comparing the differences of PgR status with the molecular subtypes of the main tumor mass differences in 2 (2%) of luminal A, and 4 luminal B (Her2-negative) (10%) were observed. No differences in other molecular subtypes were seen.
3.2. Intra-tumoral buds (ITB)
Informative ER, PgR and Her2 results were available for 179 (92.7%), 169 (87.6%), and 173 (89.6%) cases. As for PTB, no difference in ER or Her2 status was seen between main tumor mass and tumor buds. ER status was positive in 158 (88.3%) cases. PgR status was positive in 123 (72.8%) cases, and Her2 status was positive in 19 (11%) cases. A difference in receptor status between main tumor mass and buds was seen again only for PgR in 2 (1.2%: 2/169) cases. The two cases were positive for PgR status in the main tumor mass but negative in ITB. Comparing the differences of PgR status with the molecular subtypes of the main tumor mass differences in 1 luminal B (Her2-negative) (2%) and in 1 luminal B (Her2-positive) (7%) was seen. No differences in other molecular subtypes were seen. The summary of cases that different PgR status is given in Table 2. Examples of positive HR and Her2 status and difference of PgR status are shown in Fig. 1.
Table 2.
Breast cancers with differences of PgR expression.
| ID | Main tumor mass | PTB | ITB | Molecular subtype |
|---|---|---|---|---|
| 10 | PgR positive | No PgR expression | PgR positive | luminal A |
| 21 | PgR positive | No PgR expression | No PgR expression | luminal B (her2−) |
| 43 | PgR positive | No PgR expression | PgR positive | luminal B (her2−) |
| 54 | PgR positive | No PgR expression | No data available | luminal B (her2−) |
| 248 | PgR positive | No PgR expression | PgR positive | luminal A |
| 181 | PgR positive | No PgR expression | PgR positive | Luminal B (her2−) |
| 142 | PgR positive | PgR positive | No PgR expression | luminal B (her2+) |
Color code: red=postive, green=no expression. (For interpretation of the references to colour in this Table note, the reader is referred to the web version of this article.)
Fig. 1.
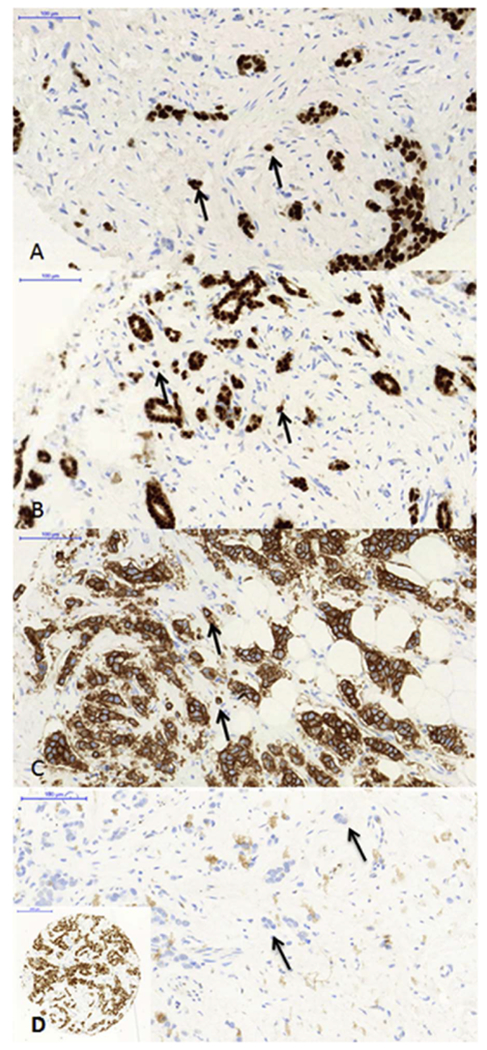
Examples of hormone and Her2 expression in tumor buds and BC with difference in PgR status.
A: ER positive breast cancer and positive tumor buds.
B: PgR positive breast cancer and positive tumor buds.
C: Her2 positive breast cancer with strong complete membrane her2 positivity in tumor buds.
D: Breast cancer (ID10) with a positive PgR status of the main tumor mass (inlet) and negative PgR status in PTB.
A-D: Black arrows are pointing towards tumor buds.
3.3. Significant associations of HR status and PTB
ER positive PTB were significantly (p < 0.05) associated with the following characteristics of the main tumor: ER positivity, PgR positivity low proliferation (< 20%), low tumor grade, and luminal molecular subtypes.
PgR positive PTB were significantly (p < 0.05) associated with the following characteristics of the main tumor: ER positivity, PgR positivity low proliferation (< 20%), low tumor grade, vascular invasion (V1), and luminal molecular subtypes.
3.4. Significant associations of HR status and ITB
ER positive ITB were significantly (p < 0.05) associated with the following characteristics of the main tumor: ER positivity, PgR positivity, negative Her2 status, low proliferation (< 20%), low tumor grade tumors, and luminal molecular subtypes.
PgR positive PTB were significantly (p < 0.05) associated with the following characteristics of the main tumor: ER positivity, PgR positivity, low proliferation (< 20%), low tumor grade, vascular invasion (V1), and luminal molecular subtypes.
4. Discussion
Targeting BC tumor buds, a tumor cell population regarded as highly migratory and invasive [8] could be crucial in interfering with tumor progression. Tumor buds were shown to have an EMT phenotype e.g. with vimentin, or ß-catenin expression and loss of e-cadherin [6,18]. In breast cancer (BC), it was reported that ER and Her2 play a role in EMT. In experiments with BC cell lines, it was shown that ER disrupts tight-junctions [13] and Her2 overexpression in breast epithelial cell lines with stem cell properties leading to EMT by loss of epithelial markers [11,13]. According to this data, we hypothesized that hormone receptor (HR) and Her2 status might change in tumor buds, which may be important in targeting tumor buds with anti-hormonal or anti-Her2 therapies. However, hormone and Her2 status are highly conserved, and ITB and PTB shared in the majority of cases the HR and Her2 status of the main tumor mass. These results indicate that BC tumor buds seem to rely on the same pathways as the cohesive tumor cells. This assumption, that main tumor mass and tumor buds can share the same profiles, is supported by a recent observation made in colon cancer, which revealed that the driver mutations in the main tumor mass and in tumor buds remained unchanged [3]. According to these results, we argue that tumor buds are equally responsive to anti-hormonal and anti-Her2 therapy as the main tumor mass.
We observed only different results for PgR status, whereas ER and Her2 showed consistent expression between tumor buds and the main tumor mass. Differences in PgR receptor could have different reasons. HR status can be heterogeneous within the tumor and all BCs with different results were heterogeneous for PgR expression and positivity might not have been captured in the tumor bud ngTMA. Although working with TMAs has been regarded as a weakness, TMAs are an excellent high throughput screening tool for protein expression [2,26], and ngTMAs have been shown to be suitable for characterizing tumor buds [33]. However, an underlying biological background of PgR status change cannot be excluded. In vivo experiments in breast cancer cell lines showed that progesterone can lead to inhibition of EMT relevant proteins such as e.g. e-cadherin due to binding with progesterone receptor alpha [34] and in endometrial cancer, it was suggested that the Wnt/beta-catenin pathway is inhibited by progesterone in the presence of progesterone receptor [29]. Hence, loss of PgR would favor EMT and EMT is reported to be a characteristic of tumor buds [18,19]. Loss of PgR expression was reported to be an independent factor for low survival rates in breast cancer [21]. A large cohort with long-term followup and survival data is needed to determine if loss of PgR expression in tumor buds represents a change in tumor biology towards a more aggressive tumor phenotype.
We did not identify any difference, in particular, no change to positive hormone or Her2 status in tumor buds in triple negative BCs (TNBC). ER, PgR and Her2, up-or down regulation, might facilitate EMT and tumor budding but other pathways must play a role. TNBC can be positive for many EMT markers such as e.g. vimentin [5,12] and vimentin was shown to be expressed in tumor buds of BCs [18]. Hence, to elucidate underlying mechanisms of tumor budding in BC and BC subtypes, other pathways and markers need to be considered besides HR and Her2. Other factors such as processes within the tumor microenvironment should be included in this consideration. Several studies showed that the tumor stroma might play an important role in tumor budding formation and inflammatory cells can be associated with tumor buds [8,14,17,28,32], indicating that the complex phenomenon of tumor budding may involve all tumor compartments. At this point, we would argue that tumor buds are most efficiently targeted through their immuno profile. However, in the future, stroma and/or immune cell modulators may prove to be equally efficient.
In conclusion, for the first time, we identified that tumor buds generally reflect the targetable immuno profile of the main tumor mass. Therefore, we assume that tumor buds are equally responsive to anti-hormonal and anti-Her2 therapies. Different PgR status can occur in tumor buds but its significance is unclear and needs further investigation.
Acknowledgements
The ngTMA was constructed from tissues provided by the Tissue Bank Bern. We thank the Translational Research Unit for helping with construction of the BC tumor budding ngTMA (L. Schoeni, J. Galván) and performing immunohistochemistry (C. Hammer). Many thanks to M. Neuenschwander for performing fluorescence in-situ hybridization and M. Trippel and K. Pfaltz for helping with pathological review of BCs cases. Many thanks to A. Grogg for helping with the BC ngTMA from the main tumor mass and IHC evaluation.
Funding
This study was funded by the Claudia von Schilling Foundation for Breast Cancer Research, Germany.
Footnotes
Conflict of interest
None of the authors has a conflict of interest.
References
- [1].Bill R, Christofori G, The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 589 (2015) 1577–1587. [DOI] [PubMed] [Google Scholar]
- [2].Burandt E, Schreiber M, Stein A, Minner S, Clauditz TS, Bokenmeyer C, Jänicke F, Frisch M, Izbicki JR, Knecht R, Sauter G, Stahl PR, Continuous tissue microarray based identification of can with homogeneous target expression for successful targeted therapy in clinical routine practice, Genes Chromosomes Cancer 53 (2014) 228–239. [DOI] [PubMed] [Google Scholar]
- [3].Centeno I, Paasinen Sohns A, Flury M, Galván JA, Zahnd S, Koelzer VH, Sokol L, Dawson HE, Lugli A, Cathomas G, Zlobec I, DNA profiling of tumor buds in colorectal cancer indicates that they have the same mutation profile as the tumor from which they derive, Virchows Arch. 470 (2017) 341–346. [DOI] [PubMed] [Google Scholar]
- [4].Chambers AF, Groom AC, MacDonald IC, Dissemination and growth of cancer cells in metastatic sites, Nat. Rev. Cancer 2 (2002) 563–572. [DOI] [PubMed] [Google Scholar]
- [5].Cheung SY, Boey YJ, Koh VC, Thike AA, Lim JC, Igbal J, Tan PH, Role of epithelial-mesenchymal transition markers in triple-negative breast cancer, Breast Cancer Res. Treat. 152 (2015) 489–498. [DOI] [PubMed] [Google Scholar]
- [6].Dawson H, Lugli A, Molecular and pathogenetic aspects of tumor budding in colorectal cancer, Front. Med 2 (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grogg A, Trippel M, Pfaltz K, Ladrach C, Droeser RA, Cihoric N, Salhia B, Zweifel M, Tapia C, Androgen receptor status is highly conserved during tumor progression of breast cancer, BMC Cancer 15 (2015) 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gujam FJ, McMillan DC, Mohammed ZM, Edwards J, Going JJ, The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer, Br. J. Cancer 113 (2015) 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinica Oncology/College Of American Pathologists guideline recommendations for immuno-histochemical testing of estrogen and progesterone receptors in breast cancer, J. Clin. Oncol 6 (2010) 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S, EMT and tumor metastasis, Clin. Transla. Med 4 (6) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ingthorsson S, Andersen K, Hilmarsdottir B, Maelandsmo GM, Magnusson MK, Gudjonsson T, HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells is inhibited by coexpression of EGFR, Oncogene 35 (2016) 4244–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY, Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome, Hum. Pathol 46 (2015) 1267–1274. [DOI] [PubMed] [Google Scholar]
- [13].Jiménez-Salazar JE, Posadas-Rodriquez P, Lazzarini-Lechuga RC, Luna-López A, Zentella-Dehesa A, Gomez-Quiroz LE, Königsberg M, Dominquez-Gómez G, Damián-Matsumura P, Membrane-initiated estradiol signaling of epithelial-mesenchymal transition-associated mechanisms through regulation of tight junctions in human breast cancer cells, Horm. Cancer 5 (2014) 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jing Y, Han Z, Zhang S, Liu Y, Wei L, Epithelial-mesenchymal Transition in tumor microenvironment, Cell Biosci. 1 (2011) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karamitopoulou E, Role of epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma: is tumor budding the missing link? Front. Oncol 3 (2013) 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A, Tumour budding is a strong and independent prognostic factor in pancreatic cancer, Eur. J. Cancer 49 (2013) 1032–1039. [DOI] [PubMed] [Google Scholar]
- [17].Kadota K, Yeh YC, Villena-Vargas J, Cherkassky L, Drill EN, Sima CS, Jones DR, Travis WD, Adusumilli PS, Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma, Chest 148 (2015) 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z, The prognostic value of tumor budding in invasive breast cancer, Pathol. Res. Pract 209 (2013) 269–275. [DOI] [PubMed] [Google Scholar]
- [19].Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, Zimaity HE, Fléjou J-F, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P, Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding concensus conference, Mod. Pathol (2017) 1–13. [DOI] [PubMed] [Google Scholar]
- [20].Mehlen P, Puisieux A, Metastasis: a question of life or death, Nat. Rev. Cancer 6 (2006) 449–458. [DOI] [PubMed] [Google Scholar]
- [21].Puride CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, Thompson AM, Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study, Br. J. Cancer 110 (2014) 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Salhia B, Trippel M, Pfaltz K, Cihoric N, Grogg A, Ladrach C, Zlobec I, Tapia C, High tumor budding stratifies breast cancer with metastatic properties, Breast Cancer Res. Treat. 150 (2015) 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Santamaria PG, Moreno-Bueno G, Portillo F, Cano A, EMT: present and future in clinical oncology, Mol. Oncol 11 (2017) 718–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sarioglu S, Acara C, Akman FC, Dag N, Ecevit C, Ikiz AO, Cetinayak OH, Ada E, for Dokuz Eylul Head and Neck Tumor Group (DEHNTG), Tumor budding as a prognostic marker in laryngeal carcinoma, Pathol. Res. Pract 206 (2010) 88–92. [DOI] [PubMed] [Google Scholar]
- [25].Seki M, Sano T, Yokoo S, Oyama T, Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth, Head Neck (Suppl. 1) (2016) E1582–E1590. [DOI] [PubMed] [Google Scholar]
- [26].Tapia C, Glatz K, Novotny H, Lugli A, Horde M, Seemayer AC, Tornillo L, Terracciano L, Spichting H, Mirlacher M, Simon R, Sauter G, Close association between HER-2 amplification and overexpression in human tumors of non-breast origin, Mod. Pathol 20 (2007) 192–198. [DOI] [PubMed] [Google Scholar]
- [27].Thies S, Guldener L, Slotta-Huspenina J, Zlobec I, Koelzer VH, Lugli A, Kroll D, Seiler CAA, Feith M, Langer R, Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas, Hum. Pathol 52 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [28].Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC, Histological categorization of fibrotic cancer stroma in advanced rectal cancer, Gut 53 (2004) 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doom HC, Ewing PC, Kim JJ, Grootegoed JA, Bruger CW, Fodde R, Blok LJ, Progesterone inhibition of Wnt/betacatenin signaling in normal endometrium and endometrial cancer, Clin. Cancer Res. 15 (2009) 5784–5793. [DOI] [PubMed] [Google Scholar]
- [30].Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G,Hayes DF, Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update, J. Clin. Oncol 31 (2013) 3997–4013. [DOI] [PubMed] [Google Scholar]
- [31].Zlobec I, Lugli A, Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget, Oncotarget 1 (2010) 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR, Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumor budding in colorectal cancer, J. Pathol 121 (2007) 260–268. [DOI] [PubMed] [Google Scholar]
- [33].Zlobec I, Suter G, Perren A, Lugli A, A next-generation tissue microarray (ngTMA) protocol for biomarker studies, J. Vis. Exp 91 (2014) 51893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zuo L, Lei W, You SY, Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells cia a membrane progesterone receptor mediated pathway, Breast Cancer Res. 12 (2010) R34. [DOI] [PMC free article] [PubMed] [Google Scholar]


