Abstract
Glucose-regulated protein (GRP)-78, the key regulator of endoplasmic reticulum (ER) stress, is associated with endometrial cancer (EC) development and progression. However, its role in the continuum from complex atypical hyperplasia (CAH) to EC is unknown and the focus of this study.
Methods.
252 formalin-fixed, paraffin-embedded endometrial biopsies from patients with CAH diagnosed between 2003 and 2011 were evaluated for GRP78 expression by immunohistochemistry. Expression was also evaluated in subsequent biopsies from those patients treated with progestins. Differences in GRP78 expression were assessed using standard statistical methods.
Results.
GRP78 expression was undetectable in 45(18%) patients with CAH, while 120(48%) CAH cases showed moderate/strong expression. Among women who ultimately underwent hysterectomy for CAH (n = 134), 54(40%) had occult EC while 57(43%) had persistent CAH. Those with occult EC upon hysterectomy had significantly stronger GRP78 expression than those who did not have occult EC (p = 0.007). Greater GRP78 expression within CAH remained independently associated with the presence of an occult EC (p = 0.017). Thirty-four of 54 (63%) patients with occult EC had moderate/strong GRP78 expression compared to 36 of 80 (45%) patients with persistent CAH, benign or non-atypical hyperplastic endometrium. In those treated with progestins, samples with persistent CAH and EC were more likely to have high levels of GRP78 expression in the initial biopsies than those who responded (p = 0.014).
Conclusions.
Increased GRP78 expression in untreated CAH correlates with the presence of an occult EC. In addition, CAH specimens with greater GRP78 expression may identify patients who are less likely to respond to progestin therapy.
Keywords: Endometrial hyperplasia, Occult endometrioid adenocarcinoma, GRP78, Immunohistochemistry, Response to therapy
1. Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy in the developed world and the second most common in the developing world [1]. Endometrial hyperplasia is a known precursor lesion to endometrial carcinoma, and the presence of atypia in an endometrial biopsy (EMB) increases the riskup to 9-fold [2]. In addition, there is a 43% risk of concurrent EC in those diagnosed with atypical hyperplasia [3]. This high risk supports hysterectomy as the standard treatment for most women with complex atypical hyperplasia (CAH). However, some women have not completed childbearing at the time of diagnosis or have medical comorbidities that preclude them from being acceptable surgical candidates. Generally, these patients are offered close monitoring while on treatment with progestins. Physicians are challenged to assess an individual’s risk of progression to carcinoma, and histology alone inadequately predicts clinical outcome [4,5].
Few alternative methods exist to determine which patients are more likely to progress to cancer than others. Although clinical risk factors and potential biomarkers for progression have been examined, no reliable marker or markers have been identified to help with risk assessment and clinical management [6,7]. Glucose-regulated protein (GRP)-78, a key marker of endoplasmic reticulum (ER) stress, is overexpressed in many types of cancer including endometrial cancer [8,9]. GRP78 overexpression is associated with activation of the unfolded protein response (UPR) as well as tumor growth [10]. ER stress and GRP78 overexpression in both EC cells and even neighboring adipocytes have been implicated as possible mechanisms for EC development, as demonstrated in in vitro and in vivo models [11,12]. Although some studies have been published on the overexpression of GRP78 in endometrial cancer and its association with possible therapeutic resistance, no studies have evaluated the expression of GRP78 in precancerous endometrial lesions [13]. Furthermore, no studies have explored the role of GPR78 as a biological predictor of complex atypical hyperplasia progressing to endometrial carcinoma.
Although progestin therapy elicits reversal of endometrial hyperplasia to benign endometrium in the majority of cases [14], no predictors exist to prospectively identify those who are less likely to respond to treatment [15]. Examined in the setting of endometrial cancer, GRP78 has been shown to contribute to resistance to cytotoxic agents [16]. In addition, we have recently shown its importance in endometrial cancer development in an in vivo transgenic model [17]. Nevertheless, while GRP78 appears to play a role in EC development, whether or not changing levels of GRP78 might also predict progestin-induced regression of EC or its precursor lesion, complex atypical hyperplasia, is unknown.
The aim of this study was to evaluate the dynamic expression of GRP78 expression in endometrial disorders including CAH, EC and endometrium treated with progestin therapy. We hypothesize that ER stress is a factor in the progression of CAH to endometrial carcinoma.
2. Methods
2.1. Study population and design
After approval by the University of Southern California Institutional Review Board (IRB), all unique cases of newly diagnosed CAH between 2003 and 2011 at the Los Angeles County + USC Medical Center were identified from the pathology database (CoPath), and clinicopathologic- and outcome data were obtained from medical records. An expert gynecologic pathologist (PMF) confirmed all diagnoses of CAH from endometrial biopsies and hysterectomies in this study. All pretreatment specimens were histologically confirmed to have no evidence of treatment or progestin-effect. For the patients who proceeded with medical management rather than hysterectomy, we retrieved the first post-treatment biopsy after at least 3 months of progestin therapy. Progestin therapy included the levonorgestrel intrauterine device and oral progestins such as megestrol and medroxyprogesterone.
2.2. Immunohistochemistry
Archival endometrial samples were evaluated for GRP78 as previously described [11,12]. Briefly, freshly cut formalin-fixed, paraffin-embedded samples were deparaffinized and rehydrated. Antigen retrieval was accomplished with BD Retrievagen A (BD Pharmingen, Carpenteria, CA) according to manufacturer instructions. GRP78 was detected with rabbit anti-GRP78 (1:100, Santa Cruz Biotechnologies, Santa Cruz, CA) followed by the goat anti-rabbit antibody (VECTASTAIN® avidin-biotin complex (ABC) kit, Vector Laboratories, Burlingame, CA). Visualization was achieved with 3,3′-diaminobenzidine (DAB).
The study pathologist (PMF), blinded to clinical and treatment data, scored all specimens as negative, weak, moderate and strong. Negative controls absent of primary antibody were used as a reference. Overexpression of GRP78 was defined as moderate/strong intensity [12]. Internal consistency was evaluated by re-presenting a random subset of previously scored slides to the same pathologist, blinded to the original score.
2.3. Statistical analysis
Differences in GRP78 expression levels between patients with different disease characteristics were assessed using t-tests, analysis of variance, or linear regression analyses. All reported p values were 2-sided, and a p < 0.05 was considered statistically significant. Statistical analyses were performed using STATA software (version 11.0; StataCorp LP College Station, TX).
3. Results
3.1. Population characteristics and GRP78 expression
For the purpose of this study, we analyzed 252 cases of CAH. The patient characteristics are described in Table 1. The median age of this cohort was 38 years old. The majority of the patients were Hispanic (79%) and pre-menopausal (58%). Of the 158 patients with data on parity, 42% were nulligravid, with an additional 9% of the patients being nulliparous. In addition, the majority of patients were obese. Of the 156 women with weight data, 82% had a BMI greater than or equal to 30 kg/m2 and 43% had a BMI greater than or equal to 40 kg/m2. (See Table 2.)
Table 1:
Population demographics of complex atypical hyperplasia patients (N = 252).
| No. (%) | |
|---|---|
| Age (yrs) (median, range) | 38(18–65) |
| < 40 | 140 (56%) |
| ≥40 | 112 (44%) |
| Race | |
| Hispanic | 200 (79%) |
| White | 19(8%) |
| Asian | 14(6%) |
| Black | 13 (5%) |
| Other | 6 (2%) |
| Parity | |
| Nulligravid | 67(42%) |
| Nulliparous | 14(9%) |
| Multiparous | 77(49%) |
| Missing | 94 |
| Menopausal | |
| No | 146 (81%) |
| Yes | 34(19%) |
| Missing | 72 |
| BMI (kg/m2) (median, range) | (37.8, 22.7–74.1) |
| < 30 | 28(18%) |
| 30- < 35 | 34 (22%) |
| 35- < 40 | 27 (17%) |
| ≥40 | 67 (43%) |
| Missing | 96 |
| Diabetes | |
| Yes | 33 (21%) |
| No | 124 (79%) |
| Missing | 95 |
Table 2:
Summary of treatment and GRP78 overexpression.
| Characteristic | N | % |
|---|---|---|
| Treatment type | ||
| Hysterectomy | 134 | 53 |
| Progestin therapy (LNG-IUS, provera, megestrol) | 118 | 47 |
| Post-hysterectomy diagnosis | ||
| Malignancy/EC | 54 | 40 |
| CAH | 57 | 43 |
| Non-atypical hyperplasia/benign | 23 | 17 |
| GRP78 overexpression in preoperative CAH biopsy | ||
| Overexpressed GRP78/Total EC | 34/54 | 63 |
| Overexpressed GRP78/Total CAH | 25/57 | 44 |
| Overexpressed GRP78/Total non-atypical + benign | 11/23 | 48 |
| GRP78 overexpression in pre-progestin CAH biopsy | ||
| Overexpressed GRP78/Total EC | 5/6 | 83 |
| Overexpressed GRP78/Total CAH | 15/30 | 50 |
| Overexpressed GRP78/Total non-atypical + benign | 7/25 | 20 |
One hundred and twenty (48%) pretreatment CAH biopsies displayed GRP78 overexpression, while 87(34%) showed weak GRP78 expression and 45(18%) were negative for GRP78 expression [Fig. 1]. The incidence of GRP78 overexpression in CAH was similar regardless of age, 45% among those <40 years of age and 51% among those ≥ 40 years of age (p = 0.96) [Fig. 2A]. In addition, no significant difference in endometrial GRP78 expression was noted among BMI categories (<30 vs. 30–<35 vs. 35–<40 vs. ≥40, 46%, 47%, 52%, 48%, p = 0.86) [Fig. 2B]. GRP78 overexpression in the endometrium was not associated with pregnancy history (43% among nulligravid vs. 29% among nulliparous vs. 57% multiparous, p = 0.33) [Fig. 2C].
Fig. 1.
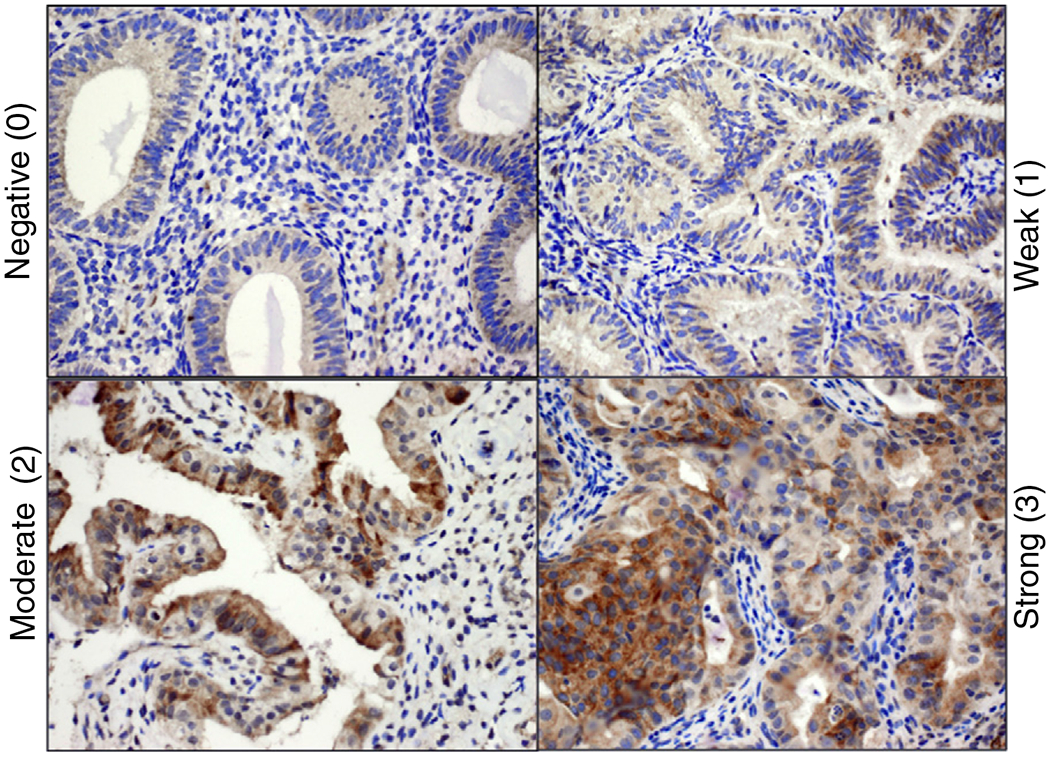
GRP78 expression in complex atypical hyperplasia. Intensity is characterized as negative (0), weak (1), moderate (2), and strong (3).
Fig. 2.
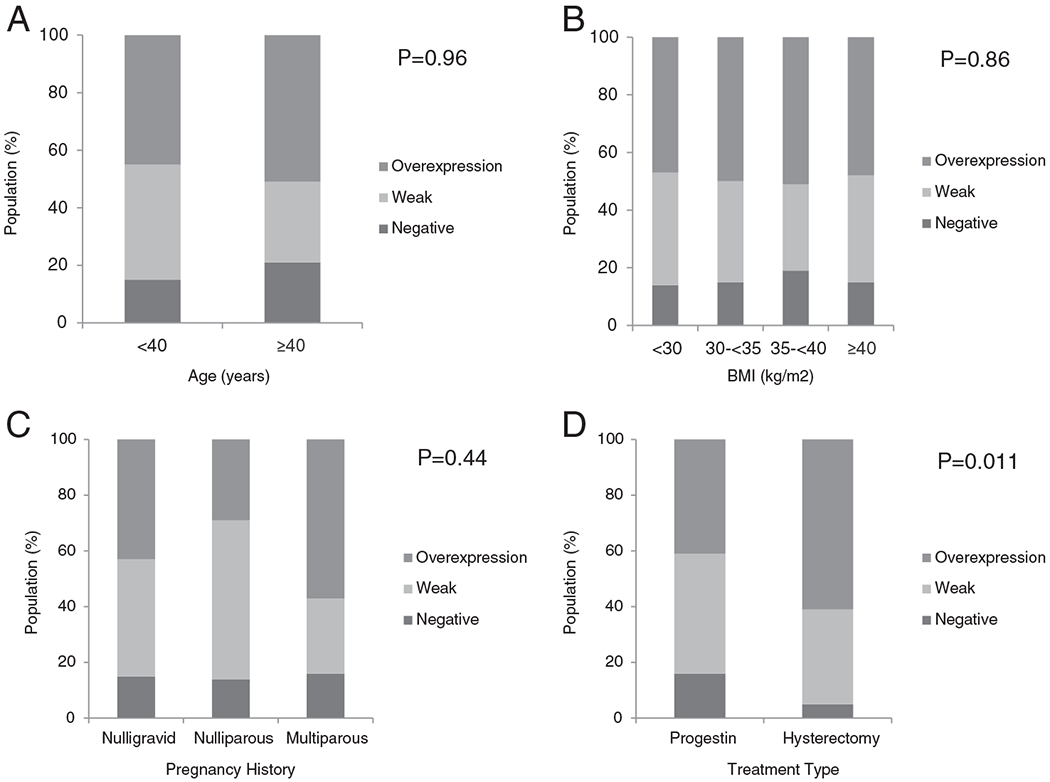
GRP78 expression in complex atypical hyperplasia as stratified by (A) by age (years), (B) body mass index (kg/m2), (C) pregnancy history, and (D) treatment modality.
3.2. GRP78 overexpression is associated with occult malignancy
134(53%) patients with newly diagnosed CAH ultimately underwent definitive hysterectomy. Among those who had a hysterectomy, 54(40%) had an occult malignancy. Of those who had cancer, 52(96%) were Grade 1 endometrioid histology and 51 (94%) had Stage 1 disease. To explore the role of GRP78 as a possible predictor of occult malignancy, we examined the relationship between GRP78 overexpression in the diagnostic biopsy and the presence of occult malignancy within the uterus at the time of hysterectomy. GRP78 overexpression within presurgical endometrial biopsies with CAH was identified in 34(63%) of those who had occult malignancy on subsequent hysterectomy [Fig. 2D]. Conversely, 70 patients who underwent hysterectomy were identified to have had GRP78 overexpression at the time of initial diagnosis. Among those patients, 34 (49%) had carcinoma seen at the time of hysterectomy and 36 (51%) did not. Sixty-four patients who underwent hysterectomy were identified to have had negative/weak GRP78 expression at initial diagnosis. Of these patients, 20(31%) had carcinoma and 44(69%) did not.
Of those without malignancy on the hysterectomy specimen, 57(43%) retained the original CAH diagnosis and 23( 17%) had hyperplasia without atypia or benign endometrium. Among patients without malignancy or CAH in their hysterectomy specimen, 11(48%) had GRP78 overexpression in their initial diagnostic endometrial biopsy.
Multivariate analysis was performed to evaluate the association of GRP78 overexpression and occult malignancy while control for age, BMI, gravity, and parity. Even after controlling for these clinical covariates, GRP78 overexpression within CAH remained an independent predictor of occult endometrial malignancy (p = 0.017).
3.3. Responsiveness to progestin therapy is less in those with GRP78 overexpression
Given that endometrial GRP78 overexpression was independently associated with occult uterine malignancy, we sought to determine if GRP78 overexpression might also predict a lower likelihood of CAH regression with progestin therapy. We identified 61 women (24%) within our cohort who had treatment-naïive CAH endometrial samples and a subsequent endometrial sampling after at least 3 months of progestin therapy. Among these patients undergoing medical management, 72% were <40 years old. The median time between the initial biopsy and the first biopsy after initiating progestin therapy was 4.1 months (range 1.1–29.2 months). Endometrial adenocarcinoma was diagnosed in 6 (10%) biopsies taken subsequent to initiating progestin therapy. Persistent CAH was present in 30 (50%) patients on progestin therapy. Twenty-five (41%) patients showed regression of hyperplasia in the first biopsy after initiating progestin therapy, and only 7 of the 25 patients (28%) with normal endometrium on the first biopsy after initiating progestin therapy demonstrated GRP78 overexpression in their initial EMB.
Among the 61 patients who received progestin therapy, 27 patients had overexpression of GRP78 at the initial diagnosis and 5(19%) of those patients had carcinoma on follow-up biopsy. Thirty-four patients had negative/weak GRP78 expression at initial diagnosis, 1(3%) had carcinoma of follow-up biopsy.
To determine whether pre-treatment endometrial GRP78 expression was associated with CAH regression, we evaluated GRP78 expression in the endometrium pre- and post-treatment. In the patients with benign or non-atypical pathology at the time of follow-up, GRP78 expression at initial diagnosis was significantly lower than those with persistent CAH. Moreover, patients who progressed to EC had greater GRP78 expression than those whose endometrial lesions regressed. Among those treated with progestins, we reviewed GRP78 expression in the initial biopsies. Greater GRP78 expression was seen in the initial biopsies of those who had persistent CAH or EC as compared with those without residual atypia in their follow-up biopsies [Fig. 3A; p = 0.014].
Fig. 3.
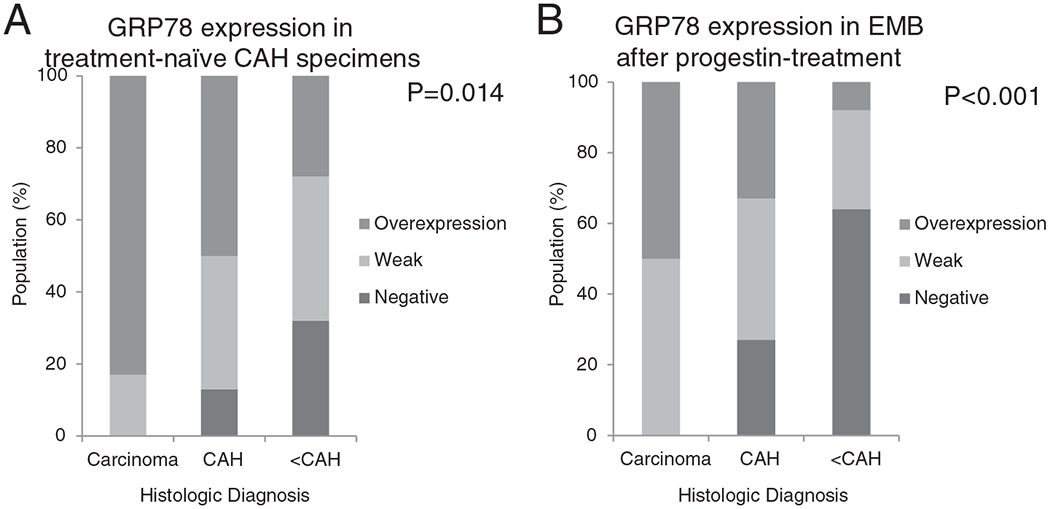
GRP78 expression in (A) treatment-naïve CAH EMB specimens show more GRP78 overexpression among endometrial lesions that progress to carcinoma or persist, and do not regress. (B) GRP78 overexpression is attenuated after progestin treatment with carcinoma and persistent CAH showing continued GRP78 overexpression compared with benign endometrial lesions and those without atypia.
When examining GRP78 expression in endometrium treated with progestins, the post-treatment biopsies showed less overall GRP78 expression as compared with pre-treatment biopsies. As depicted in Fig. 3B, 33% and 50% of those with persistent CAH and carcinoma, respectively, still showed GRP78 overexpression on post-treatment EMB despite progestin treatment [Figure 3B]. This would suggest that hyperplasia over expressing GRP78 may be less likely to regress during progestin therapy.
4. Discussion
In this study, GRP78 overexpression in CAH reflects the presence of an underlying, occult endometrial carcinoma. Overexpression of GRP78 has been identified in many aggressive cancer types including gastric, breast, liver, and prostate [8]. Endometrial carcinoma has been identified as another cancer that demonstrates overexpression of GRP78 [11,12,16]. Our study shows that GRP78, a marker of ER stress, is overexpressed in the endometrium of patients with CAH who have underlying carcinoma. Interestingly, the intensity of GRP78 staining was also observed to be attenuated after progestin-treatment, possibly reflecting diminished ER stress within the endometrium over the course of progestin treatment. Given the pathologic role of ER stress in EC and new transgenic in vivo data strongly implicating its important role in EC development [17], this study further implicates GRP78 and/or ER stress in the spectrum of precancerous endometrial hyperplasia to frank carcinoma.
In addition to cancer, ER stress and GRP78 overexpression have been shown to be associated with obesity [12]. The relationship between obesity and endometrial cancer has been primarily, but not entirely, attributed to estrogen excess [18]. Thus, other molecular processes likely contribute to this risk for cancer development. Given the prevalence of obesity among those diagnosed with endometrial hyperplasia, we hypothesized that this population may be subject to higher levels of ER stress, which may confer a greater likelihood of progression from a precancerous to cancerous state. Although our study revealed increased endometrial GRP78 expression among those with occult EC, BMI was not independently associated with GRP78 overexpression. Although there does not appear to be an independent association between BMI and GRP78 overexpression, information on the relationship between GRP78 expression and EC has implicated overexpression within individual adipocytes as opposed to the cancer cells themselves [12]. GRP78 expression in the adipocytes of women with precancerous lesions may be an interesting avenue of future study, potentially to better understand the role of adiposity in carcinogenesis.
Here, we found that responsiveness to progestin therapy appears to be relatively diminished in endometria overexpressing GRP78. Currently, there are few modalities available to clinicians to triage patients with CAH and manage those undergoing progestin therapy. Upson et al. found that high expression of PRB was predictive of resolution of hyperplasia if treated with oral progestins [15]. However, the mechanism of action of PRB remains unclear. Exploring several clinical and histologic risk factors, such as regression of hyperplasia at the first follow-up biopsy, Penner et al. proposed a risk assessment algorithm to predict progestin-response [6]. However, histological parameters to predict response were difficult to reliably incorporate into their model. Ultimately, the authors acknowledge that the availability of biological markers would be useful in identifying those at highest risk and those who would benefit most from progestin therapy [6]. Our findings suggest that GPR78, which has shown to have a biological role in EC development, may also be an indicator of progestin response, however, a limited sample size precludes extensive statistical analyses (e.g., positive- and negative-predictive value), and deeper interrogation of other molecules involved in ER stress are warranted to confirm these associations.
After diagnosing a precancerous lesion, physicians have the opportunity to recognize and intervene therapeutically, possibly preventing progression to cancer. A biochemical marker could be used as a tool for differentiating between those who will regress and those who will progress to cancer. To our knowledge, markers in precancerous endometrial lesions have not been well described and, furthermore, definitive information on marker expression during the course of treatment has not been elucidated. Given the high risk of concurrent endometrial cancer in the setting of CAH, hysterectomy is the standard treatment for women with a diagnosis of atypical hyperplasia, regardless of fertility desires. The addition of a biomarker would be a valuable tool in risk assessment and offer more information to patients when weighing treatment options. Specifically, if the patient choses to proceed with medical management as an initial treatment, the addition of a biochemical marker could help clinicians and patients as they pursue conservative management.
HIGHLIGHTS.
GRP78 is frequently detected in complex atypical hyperplasia (CAH).
High endometrial GRP78 expression correlated with malignancy at hysterectomy.
High GRP78 in CAH correlated with persistent hyperplasia despite progestin treatment.
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2015, CA Cancer J. Clin 65 (2015) 5–29. [DOI] [PubMed] [Google Scholar]
- [2].Kurman RJ, Kaminski PF, Norris HJ, The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients, Cancer 56 (1985) 403–412. [DOI] [PubMed] [Google Scholar]
- [3].Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ 2nd, Alberts D, Curtin J, Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a gynecologic oncology group study, Cancer 106 (2006) 812–819. [DOI] [PubMed] [Google Scholar]
- [4].Leitao MM Jr., Han G, Lee LX, Abu-Rustum NR, Brown CL, Chi DS, Sonoda Y, Levine DA, Gardner GJ, Jewell EE, Barakat RR, Soslow RA, Complex atypical hyperplasia of the uterus: characteristics and prediction of underlying carcinoma risk, Am. J. Obstet. Gynecol 203 (2010) 349 e1–6. [DOI] [PubMed] [Google Scholar]
- [5].Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, Gallup DG, Reproducibility of the diagnosis of atypical endometrial hyperplasia: a gynecologic oncology group study, Cancer 106 (2006) 804–811. [DOI] [PubMed] [Google Scholar]
- [6].Penner KR, Dorigo O, Aoyama C, Ostrzega N, Balzer BL, Rao J, Walsh CS, Cass I, Holschneider CH, Predictors of resolution of complex atypical hyperplasia or grade 1 endometrial adenocarcinoma in premenopausal women treated with progestin therapy, Gynecol. Oncol 124 (2012) 542–548. [DOI] [PubMed] [Google Scholar]
- [7].Allison KH, Tenpenny E, Reed SD, Swisher EM, Garica RL, Immunohistochemical markers in endometrial hyperplasia: is there a panel with promise? A review, Appl. Immunohistochem. Mol. Morphol. 16 (2008) 329–343. [DOI] [PubMed] [Google Scholar]
- [8].Luo B, Lee AS, The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies, Oncogene 32 (2013) 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li J, Lee AS, Stress induction of GRP78/BiP and its role in cancer, Curr. Mol. Med 6 (2006) 45–54. [DOI] [PubMed] [Google Scholar]
- [10].Luvsandagva B, Nakamura K, Kitahara Y, Aoki H, Murata T, Ikeda S, Minegishi T, GRP78 induced by estrogen plays a role in the chemosensitivity of endometrial cancer, Gynecol. Oncol 126 (2012) 132–139. [DOI] [PubMed] [Google Scholar]
- [11].Bifulco G, Miele C, Di Jeso B, Beguinot F, Nappi C, Di Carlo C, Capuozzo S, Terrazzano G, Insabato L, Ulianich L, Endoplasmic reticulum stress is activated in endometrial adenocarcinoma, Gynecol. Oncol 125 (2012) 220–225. [DOI] [PubMed] [Google Scholar]
- [12].Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, Yoo EJ, Dubeau L, Lee AS, Lin YG, The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival, Gynecol. Oncol 128 (2013) 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roller C, Maddalo D, The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment, Front. Pharmacol 4 (2013) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gunderson CC, Fader AN, Carson KA, Bristow RE, Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma:asystematicreview, Gynecol. Oncol 125 (2012) 477–482. [DOI] [PubMed] [Google Scholar]
- [15].Upson K, Allison KH, Reed SD, Jordan CD, Newton KM, Swisher EM, Doherty JA, Garcia RL, Biomarkers of progestin therapy resistance and endometrial hyperplasia progression, Am. J. Obstet. Gynecol 207 (2012) 36, e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gray MJ, Mhawech-Fauceglia P, Yoo E, Yang W, Wu E, Lee AS, Lin YG, AKT inhibition mitigates GRP78 (glucose-regulated protein) expression and contribution to chemoresistance in endometrial cancers, Int. J. Cancer 133 (2012) 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin YG, Shen J, Yoo E, Liu R, Yen HY, Mehta A, Rajaei A, Yang W, Mhawech-Fauceglia P, DeMayo FJ, Lydon J, Gill P, Lee AS, Targeting the glucose-regulated protein-78 abrogates Pten-null driven AKT activation and endometrioid tumorigenesis, Oncogene (2015) Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schmandt RE, Iglesias DA, Co NN, Lu KH, Understanding obesity and endometrial cancer risk: opportunities for prevention, Am. J. Obstet. Gynecol 205 (2011) 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]


