Abstract
In recent years, regenerative medicine is gaining momentum and is giving hopes for restoring function of diseased, damaged, and aged tissues and organs and nanotechnology is serving as a catalyst. In the ophthalmology field, various types of allogenic and autologous stem cells have been investigated to treat some ocular diseases due to age-related macular degeneration, glaucoma, retinitis pigmentosa, diabetic retinopathy, and corneal and lens traumas. Nanomaterials have been utilized directly as nanoscaffolds for these stem cells to promote their adhesion, proliferation and differentiation or indirectly as vectors for various genes, tissue growth factors, cytokines and immunosuppressants to facilitate cell reprogramming or ocular tissue regeneration. In this review, we reviewed various nanomaterials used for retina, cornea, and lens regenerations, and discussed the current status and future perspectives of nanotechnology in tracking cells in the eye and personalized regenerative ophthalmology. The purpose of this review is to provide comprehensive and timely insights on the emerging field of nanotechnology for ocular tissue engineering and regeneration.
Keywords: ocular regeneration, nanoscaffolds, electrospun nanofibers, self-assembled peptides, nanotopography, stem cells, cell reprogramming, immunomodulators
1. Introduction
Regenerative medicine is an emerging, highly interdisciplinary field that provides innovative solutions to repair, regrow, or replace cells, tissues, or organs that are damaged or lost due to trauma, chronic diseases, congenital defects or aging, and not able to undergo self-repair [1–4]. Regenerative medicine started with successful kidney transplantation in 1954, with subsequent successful transplantation of liver, pancreas, and heart in the 1960s [5, 6]. Following the introduction of cyclosporine in 1978, successful single-, double- and heart-lung, and living-donor lung and liver lung transplants were developed [5, 6]. Over the last several decades, regenerative medicine has advanced to regenerate tissues, organs, and cells using engineering approaches, which involve scaffold design, cell engineering, and chemical, physical, and biological stimuli [7]. Engineered skin (TransCyte, 1997; Apligraf, 1998; Dermagraft, 2001), bone (INFUSE, 2002), and connective tissues (MACI, 2016) for the repairs/replacements of skin due to ulcers, wounds, and burns, and damaged bones and knee cartilages, respectively, were approved by U.S. Food and Drug Administration (FDA) [6–9]. Engineered vascular grafts for heart bypass surgery and cardiovascular disease treatment are currently under clinical trials [10, 11]. In the ophthalmology field, increasing efforts have also been undertaken over the past 10 years to use engineering approaches to regenerate lost or damaged eye tissues to treat vision loss and blindness caused by various ocular degenerative diseases, traumas, or infections [12]. The degenerative ocular diseases include cataract, glaucoma, age-related macular degeneration (AMD), diabetic retinopathy [13, 14], and some inherent genetic retinal defects like Leber congenital amaurosis, retinitis pigmentosa, X-linked retinoschisis, and Stargardt’s disease [15, 16]. Currently, there is no proven therapy for the treatments of these retinal, corneal, and lens degenerative diseases. The existing managements for these diseases include 1) laser surgery and vitrectomy, but they are destructive and fail to address the underlying biology of these diseases [13, 17]; 2) photodynamic therapy, but it may result in retinal and vitreous haemorrhages and retinal pigment epithelial cell (RPE) tearing [18]; 3) angiostatic steroids, but they require frequent intravitreal injections and have many side effects after long-term use [19]; 4) antivascular endothelial growth factor agents, but they do not address the role of inflammation in the pathogenesis and progression of the disease and there are still 60–65% of AMD patients who are refractory to the anti-VEGF therapy [18]; 5) gene therapy such as Luxturna, the first gene therapy approved by the FDA in December 2018 for the treatment of inherited retinal dystrophies, but it has side effects including eye redness, cataract, retinal tear and increased intraocular pressure and also substantial challenges in production, clinical study design, long-term safety studies and commercialization [20]; and 6) tissue transplantation, but it has issues of donor shortage, high number of allogeneic tissue rejections, and rampant post-operative complications, such as infection. With recent advances in exogenous delivery of living allogenic or autologous cells, particularly stem cells that have plasticity and capacity for self-renewal and reprogramming of endogenous cells [2, 21–23], cell therapy has become an intriguing potential therapy that overcomes/avoids the limitations of the methods mentioned above to regenerate ocular tissues for the treatment of diseases of the retina, cornea, and lens [24].
Different types of stem cells including embryonic stem cells (ESCs), limbal stem cells (LSCs), mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) have been investigated toward replacement of lost retinal ganglion cells and photoreceptors in various retinal degenerative diseases and transplantation of patient-specific cornea or lens [25, 26]. ESCs are derived from embryos that develop from fertilized eggs [25]. LSCs reside in the basal epithelial layer of the limbus within a cellular environment and are quiescent but have capability to proliferate upon activation [27]. MSCs exists abundantly in the body and can be obtained from various tissues including the bone marrow, eye (ciliary pigment epithelial cells and marginal zone, iris, corneal endothelial cells, conjunctival epithelial cells, hair follicle and dental pulp [21, 25]. iPSCs are derived from somatic cells such as skin fibroblasts, blood cells and adipose cells by introduction of exogenous transcription factors including cellular myelocytomatosis oncogene (c-Myc), Kruppel-like factor 4 (KLF4), sex determining region Y-box 2 (SOX2), octamer-binding transcription factor 4 (OCT4) or genes that express these factors [28, 29]. They have advantages of propagating infinitely and maintaining capability of differentiation into many different cell types without the ethical concerns related to ESCs [28]. Some of ESCs, LSCs, MSCs and iPSCs have been advanced to clinical trials to treat retinal and corneal degenerative diseases (https://clinicaltrials.gov/). These clinical trials include phase I/II clinical trials of intravitreal injection of autologous bone marrow-isolated stem/progenitor cells to treat retinitis pigmentosa, AMD, and Stargardt’s disease (NCT03772938, NCT01560715, NCT02709876); subretinal transplantation of human central nervous system stem cells for the treatment of AMD (NCT01632527), and subretinal (NCT02464436) and intravitreal (NCT02320812) administrations of human retinal progenitor cells for the treatment of retinitis pigmentosa. There are also few phase I/II clinical trials taking place currently in different parts of the world including Brazil (NCT02903576), China (NCT02749734, NCT03046407, NCT03944239), United Kingdom (NCT03167203, NCT01469832) and United States (NCT01345006, NCT01344993, NCT02590692) aimed at investigating the therapeutic potentials of subretinal transplanted hESC-derived RPE cells for the treatments of dry and wet AMDs and Stargardt’s disease. Likewise, ex vivo cultured LSCs are under Phase I/II clinical trials to reverse superficial corneal pathological conditions associated with scars, ulcers and burns (NCT02948023, NCT03295292) or LSC deficiency that leads to conjunctivalization, progressive opacification, chronic ulceration and neovascularization of the cornea with pain and loss of vision (NCT02577861, NCT00736307, NCT03549299, NCT02318485, NCT01562002). The cultured LSCs has even advanced into clinical practice to treat LSC deficiency [24]. However, despite the successes mentioned above, cell therapy approaches are still at their early stage to regenerate eye tissues/organ. Effective methods need to be developed for cell transplantation, adhesion, proliferation, and differentiation in order to regenerate functional eye tissues/organ.
During the past 15 years, considerable efforts have been made to exploit the advancements in nanotechnology to speed up stem cell research and development [30]. For example, magnetic nanoparticles have been utilized to isolate and sort stem cells [31]. Many inorganic nanoparticles including nanodiamonds, iron oxide nanoparticles, quantum dots, and upconversion nanoparticles have been applied for molecular imaging and tracing of stem cells [32]. Different nanocarriers including carbon nanotubes and magnetic nanoparticles have been used to deliver genes or drugs into stem cells [30, 33]. In particular, biomaterials have been designed into nanofibrous scaffolds and nano-topographical surfaces for controllable regulation of migration, proliferation, and differentiation of stem cells [30, 32]. Nanoscaffolds can mimic the 3-dimensional extracellular microenvironment better than those made of conventional matrix: 1) their unique high surface area to volume ratio can provide higher density of epitopes for cell adhesion and differentiation [34], and 2) their nanostructures can render better porosity, mechanical properties, conductivity, bacterial resistance, and stimuli responsive for cell growth and differentiation [23, 35]. Nanoscaffolds have been formed by using electrospinning, self-assembly, phase-separation, or lithography methods [36, 37]. In electrospinning, a high voltage is applied to produce charged fibers from polymer solutions with diameters in nanometer scale [38, 39]. Self-assembled nanoscaffolds are formed from amphiphilic peptides that contain alternating hydrophobic amino acid residues such as alanine, valine, leucine, isoleucine, and phenylalanine, and hydrophilic residues of positively charged amino acids including lysine, arginine, histidine, and negatively charged amino acids including aspartic acids and glutamic acids [40, 41]. Depending on the distribution of the ionic amino acids, the peptides can be classified as modulus I, II, III or IV, each containing charged amino acids in the order of +-+-…, ++--++--…, +++---+++---…, or ++++----++++----…, respectively. The moduli can also be mixed to obtain mixed-modulus-self-assembled nanofibers. The orientation of the charge can be designed in reverse order to provide an entirely different supramolecular arrangement, with distinct molecular behavior [40]. Although the mechanism of the assembly is not yet fully understood, the amphiphilic peptides spontaneously assemble into different type of nanostructures such as nanofibers and nanotapes in millimolar salt concentration under physiological pH [41, 42]. Phase-separation is a long-established method that is used for fabrication of porous fibrous membranes or sponges by inducing separation of a polymer solution into polymer-poor (low polymer concentration) and polymer-rich (high polymer concentration) phases. In formation of nanoscaffolds, the phase separation is usually induced thermally to form nanofibrous foams that are similar in size to natural scaffold present in the extracellular matrix [36]. By using lithography technique, various nanotopographies including nanowells, nanopillars and nano-grooves and ridges have been formed and used as nanoscaffolds [43, 44]. The nanoscaffolds obtained by the methods mentioned above have been investigated as scaffolds for regeneration of bone [45, 46], neuronal [47], ocular [48, 49], cardiovascular [50], dental [51], and cartilage [52] tissues. In this article, we comprehensively review various nanoscaffolds including electrospun nanofibers, self-assembled peptides and nanotopographies (Fig. 1) used for cornea, lens, and retina regenerations. Furthermore, we summarize nanomaterials as carrier for gene and immunomodulators to reprogram cells and restore healthy immune system, respectively, for ocular tissue regeneration. We also discuss current perspectives in nanotechnology for tracking cells in the eye and personalized regenerative ophthalmology. The focus of this review is a novel concept of nanotechnology for ocular regeneration. The traditional concept of nanotechnology for ocular drug delivery [53], nanomaterials that act as regenerative antioxidants or mainly used for prevention of ocular tissue degeneration [54, 55] are out of the scope of this review.
Fig. 1.
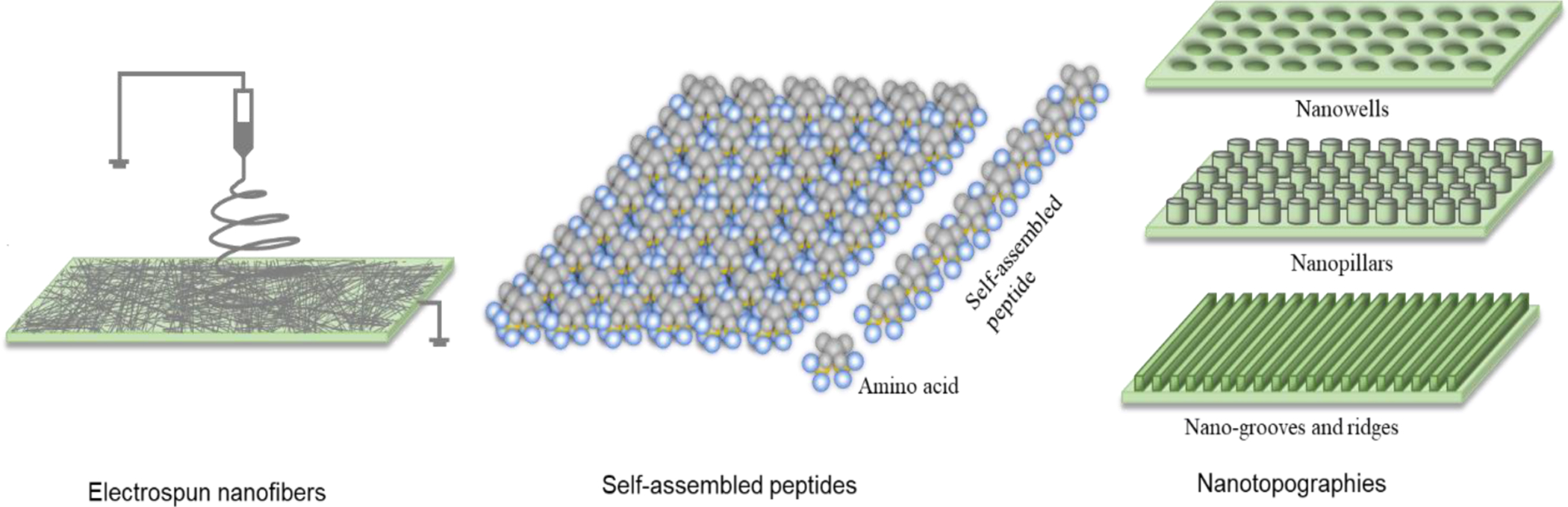
Schematic representations of nanoscaffolds including electrospun nanofibers, self-assembled peptides and nanotopographies used for ocular regeneration.
2. Nanoscaffolds for ocular tissue regeneration
2.1. Nanoscaffolds for retinal tissue regeneration
The retina is a highly organized complex sensory tissue that translates the incoming light into electrical signals, which is then further converted into meaningful images by the central nervous system [56]. It is made up of seven distinct layers formed from seven different types of cells including RPE cells, photoreceptor cells (rods and cones), interneurons (horizontal cells, bipolar cells and amacrine cells) and ganglion cells [56, 57]. The RPE cells provide nutritional support for the neural retina and phagocytose outer segments of the photoreceptors on a diurnal basis so that new outer segments are continuously added towards the proximal end of the cells [58, 59]. They also replenish the visual pigment opsin-bound 11-cis retinal by back isomerization of trans-retinal, which is formed from 11-cis retinal during the visual process, and maintain the ionic homeostasis in the subretinal space and cytokine balance in the retina/RPE/choroid [59]. The rod cells (low light sensors) and cone cells (daytime color vision) convert the optical image into an electrical signal, which will be sent to the brain via the optic nerve [56]. The three interneurons, namely horizontal, bipolar, and amacrine cells, provide lateral interactions within the retinal circuit and process the photoreceptor signals [13, 60]. The ganglion cells receive signals from the bipolar cells and send the signals to the brain for further processing [61]. Like other neuronal cells, the photoreceptors and other retinal neuronal cells in mammals are nondividing and any acquired or inherited degeneration of these neuronal cells may result in retinal diseases including AMD, glaucoma, retinitis pigmentosa, and diabetic retinopathy, that can cause visual impairment and complete blindness [13]. Specifically, AMD presents as two forms, dry and wet, that are characterized by the accumulation of insoluble drusen in the adjacent Bruch’s membrane and choroidal neovascularization that leads to hemorrhage and fluid leakage due to the degeneration and rapid loss of the RPE cells and associated cone photoreceptors located in the macula and fovea regions, respectively [62]. Glaucoma is characterized by increased intraocular pressure (IOP) and subsequent progressive retinal ganglion cells death and optic nerve atrophy [22, 63]. Retinitis pigmentosa is characterized by initial rod cell degeneration in the periphery of the retina, followed by the degeneration of cone cells as the disease progresses, leading to complete physical blindness [60]. Diabetic retinopathy (DR) is characterized by neurovascular degeneration including increased vascular permeability, retinal neovascularization, activation of glia cells [14, 64–70], loss of pericytes [68, 71–80], increased apoptosis of ganglion and amacrine cells [78, 80–82], and development of axonal dystrophy and synaptic degeneration [80, 83–85]. Currently, barring the diagnostic anti-angiogenic therapy, there is no effective treatment option for these retinal diseases [86]. Recent advances in tissue engineering and nanotechnology lead to novel approaches using nanoscaffolds to grow different cells including fetal and adult RPE cells, PC12 cells [87], 3T3 cells [87], definitive neural stem cells [88], ganglion cells [89], and retinal ganglion cells (RGC) [90] to regenerate retinal tissues for the treatments of these retinal diseases [91–94].
Different electrospun nanofibers (Table 1) and self-assembled nanofibers (Table 2) have been investigated as nanoscaffolds. Electrospun nanoscaffolds can be prepared using natural or synthetic polymers or combinations of the two (Table 1) [95] and most of the nanoscaffolds investigated promoted healthy cell growth, leading to release of important RPE characterizing genes such as zona occludin-1 (ZO-1), RPE65, and pigment epithelium-derived factor [92, 96] and expression of photoreceptors and retinal neuronal genes in conjunctiva-derived MSCs [97]. For example, Warnke et al. (2013) grew human primary RPE cells on PLGA and collagen nanofibrillar membranes, PLGA films and cover glass. The RPE cells formed in vivo-like monolayer of hexa/polygonal cells with long, sheet-like microvilli on the nanofibrillar membranes, while the cells on the films and cover glass were flatter and spread out with less than 10% of clearly visible microvilli (Fig. 2) [91]. The common natural polymers used for the preparation of electrospun nanoscaffolds in retinal tissue regeneration include gelatin [98], chitosan [99], hyaluronic acid [100], fibrin [101], laminin [49], and collagen [102]. Whereas, commonly used synthetic polymers are poly(lactic-co-glycolic acid) (PLGA) [91], poly(L-lactic acid) (PLA) [49, 103], poly caprolactone (PCL) [104], poly(L-lactide-co-ε-caprolactone) [91] and polyimide [105]. Generally, natural polymers are more suitable for promoting cell attachment and biological activity than synthetic polymers because of their chemistry but have less mechanical strength and shorter half-life than synthetic polymers [89]. Contrarily, it is easier to design and control the biodegradation, mechanical, and transport properties of synthetic nanoscaffolds than the ones made of natural polymers to obtain extracellular-mimicking matrix for cell growth and differentiation [101]. Besides their chemical composition, nanoscaffolds also have advantages of guiding cells to grow in a specific direction along their alignment to obtain tissues with native tissue-mimicking microarchitecture [106]. For example, Kador et al. (2016) fabricated radially electrospun nanoscaffolds to mimic radially oriented nerve fibers, and found about 72% of the axons grew along the nanoscaffolds compared to a 30% growth on unaligned scaffolds [107]. However, aligned nanoscaffolds may not be necessarily good for cell proliferation and differentiation. For example, Jahani et al. (2010) showed that MSCs cultured on randomly aligned nanofiber scaffolds proliferated better as compared to those cultured on aligned nanofibers [88]. In another study, Nadri et al. (2013) found that conjunctiva derived MSCs cultured on aligned PLA nanofiber scaffolds expressed higher amounts of photoreceptor and retinal neuronal genes but had denser tissue matrix and smooth surface morphology that discouraged the differentiation of the MSCs into retinal neuronal cells than those cultured on unaligned scaffolds [97]. Besides the alignment, the diameter of nanofibers also plays a significant role in cell growth. For example, Liu et al. (2014) showed that nanoscaffolds made of nanofibers with diameter of around 200 nm, regardless of the polymer used, better mimicked the extracellular matrix of the Bruch’s membrane and promoted adhesion of human fetal RPE cells than those with dimeter around 1000 nm [108]. Porosity of nanoscaffolds is another important factor affecting cell growth. Generally, high porosity provides better interconnectivity to transfer nutrients to RPEs, and thus enhances the proliferation of the cells [94, 108].
Table 1.
Electronspun nanofiber-based scaffolds investigated for retinal tissue regeneration.
| Source | Material | Fiber Diameter and Orientation | Type of Cells Supported | Summary of Findings | Ref. |
|---|---|---|---|---|---|
| Natural | Crosslinked gelatin/chitosan mixture. | Ratio 70/30; 185 nm Ratio 50/50; 170 nm |
Human RPE cells | The scaffolds significantly improved cell growth compared to tissue culture plaster. The degradation rate was slower for the 70/30 nanoscaffolds until day 21, but on day 35, they degraded about 10% more. In addition, higher expression of RPE65 and cytokeratin were obtained in cells grown on the 70/30 nanoscaffolds. | [88] |
| Natural/ Synthetic | Bovine collage type 1, PLGA (85:15) | Collagen: 299 nm, PLGA: 331 nm | Primary human RPE cells | Unlike cells grown on a cover glass, cells implanted on an ultrathin PLGA or collagen nanofibrous membrane had well-formed, long and sheet-like microvilli morphology that was almost identical to those in native retina tissue. However, there was no significant difference in gene expression, proliferation, attachment, and phagocytosis of cells grown on the two surfaces. | [85] |
| Synthetic | Polyamide | 180 nm; random | Fetal and adult RPE cells | Compared to glass surface, the scaffolds enhanced the proliferation of fetal and adult RPE cells 1.25- and 4-fold, respectively. | [87] |
| Synthetic | PLA | 500–800 nm; aligned and random | Conjunctiva derived MSCs | Stem cells grown on aligned nanofiber scaffolds expressed higher amounts of photoreceptor and retinal neuronal genes compared with those grown on unaligned nanoscaffolds. However, stem cells cultured on unaligned nanoscaffolds expressed rhodopsin 2-fold more than those cultured on aligned scaffolds favoring their differentiation into photoreceptors. | [91] |
| Synthetic | Poly(DL-lactide) | 640 nm | Porcine RPE cells | RPE cells grown on nanoscaffolds supported by a poly(4-dioxanone) frame had polygonal morphology and high transepithelial electrical resistance (TEER) value, and expressed ZO-1 and RPE65 proteins after 1 month of growth. The frame helped to retain the cell membrane in flat shape and allowed better handling for transplanting the cell membrane to the subretinal space of enucleated porcine eyes ex vivo. | [90] |
| Synthetic | Hydrolyzed PCL (hydrolyzed for better biocompatibility, wettability and cell adhesion) | 186 nm | Human RPE cells | Increased proliferation of stretched and hexagonal cells and RPE65 expression was achieved. | [103] |
| Synthetic | polyethylene terephthalate, poly(L-lactide-co-ε-caprolactone) | 175 nm and 993 nm | Human fetal RPE cells | Cell density increased by around 30% in the 175 nm fiber and ZO-1 appeared more uniform. The cell membrane did not cause obvious inflammation to Chinchilla and New Zealand rabbits after subretinal transplantation and remained in place for 2 weeks. | [102] |
| Synthetic | Polypyrrole -Graphene + PLGA | 150 nm; aligned | Retinal ganglion cells | Upon electrical stimulation, neurite length increased by 137% and cells remained viable compared with 50% viability without electrical stimulation after 10 days of encapsulations in the nanoscaffolds. | [105, 106] |
| Synthetic | Polyimide | 26 nm | Human ESC-derived RPEs | When nanoscaffolds were transplanted in Royal College of Surgeons rats, the rats showed no signs of inflammation. However, when cells implanted along with nanoscaffolds, there was mononuclear cell infiltration that destroyed the outer nuclear layer in the eyes. | [99, 107] |
| Synthetic | Amine(1,6-hexanediamine) functionalized 2-methoxy-5-(2-ethylhexyloxy)- 1,4-phenylenevinylene:PCL | 526–630 nm; aligned and random | PC12 cell 3T3 cell |
Amine functionalization provided better cell adhesion due to the electrical interaction between the positively charged amine and the negatively charged cell surface. External electrical stimulation had more dominant effect on neurite formation than the nanofiber orientation. Upon electrical stimulation, the proliferation of the cells grown in the nanofibers was 1.5 folds faster than that of cells grown on tissue culture polystyrene. The nanoscaffolds were stable for 45 days. | [81] |
| Hybrid | Crosslinked gelatin (crosslinked via carbodiimide cross-linker in ethanol/water solvent) | 119–202 nm | ARPE-19 | The nanofiber cross-linking density, thermal and biological stability, and size was controlled by controlling the ratio of ethanol and water used as solvent. ARPE-19 cells grew well on the nanofibers, regardless of the ethanol amount used. No sign of inflammation on New Zealand rabbits after implantation was observed. | [108] |
| Hybrid | PLA, laminin | 70 nm; random |
Human RPEs | Cells grown on the hybrid nanoscaffolds expressed 1.5– 2 folds higher ZO-1 and slightly higher other proteins compared to cells grown on non-hybrid nanofibrous membranes made of PCL, PLGA, or poly(L-lactide-coD,L-lactide). The scaffolds showed no sign of inflammation in rdy rats. | [109] |
| Hybrid | Wild antheraea pernyi silk fibroin /PCL/gelatin | 253 nm | ARPE-19 | The hybrid nanoscaffolds increased cell proliferation rate and expression of pigment epithelium-derived factor by around 1.5 and 1.2 fold, respectively, more than tissue culture polystyrene. The scaffolds caused no sign of inflammation to Chinchilla rabbits after implantation. | [86] |
| Hybrid | Gelatin, poly(ethylene oxide); loaded with nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) loaded gelatin nanoparticles | 236 – 256 nm | Definitive neural stem cell | The nanoscaffolds sustained the NGF and BDGF release for more than two months. When the growth factor containing nanoparticles were included in the nanoscaffolds, cells with longer axon diameter, thicker myelin sheath, larger myelinated fiber, and more than 2 fold of (area ratio %) of myelin basic protein were obtained after 14 days compared to the cells cultured in same nanoscaffolds without the nanoparticles. | [82] |
| Hybrid | PGS coated with a hybrid PCL and laminin nanofiber | 199 nm | Photoreceptor layer obtained from an embryonic retina tissue | The nanofiber mesh enhanced the adherence of a photoreceptor layer obtained from an embryonic retina tissue with PGS membrane to enhance transplantation of the isolated layer. | [83] |
Table 2.
Self-assembled peptide amphiphile-based nanoscaffolds investigated for retinal tissue regeneration
| Peptide | Fiber diameter | Cell type | Summary of findings | Ref. |
|---|---|---|---|---|
| (RADA)16 | 10–15 nm | RGC | Increasing the peptide concentration from 0.05% to 1% decreased the porosity and resulted in decreased RGC cell numbers, RGC neurite extension, and astrocyte numbers by about 40%, 65%, and 75%, respectively. | [110] |
| Fmoc-FFKK, Fmoc-FKFK, |
Fmoc-FKFK – 5 nm Fmoc-FFKK – 8 nm |
Primary murine neuron | The Fmoc-FFKK and Fmoc-FKFK scaffolds maintained 100% and 75% cell viability, respectively, for over 40 days. In addition, cells grown on both scaffolds showed higher electrical activity compared to neurons grown on poly-D-lysine. | [113] |
| (RADA)16-I | 10 nm | The scaffolds promoted closing of neural tissue gaps in Syrian hamsters with transected optic tract lesion, leading to axon regrowth with no inflammatory response after injection in the brain. 75% of the hamsters injected with the self-assembled peptide nanofiber scaffold regained vision after 90 days, while saline-injected controls did not. | [112] | |
| Kr5 (K-(SL)6-K-G-PRKLYDY) | 13 nm | Human umbilical vein endothelial cell; Stromal cell |
The nanofibrous hydrogel was not toxic to the stromal cells and it had concentration dependent antiangiogenic effect in human umbilical vein endothelial cell. After subcutaneous injection in rats, the host cells infiltrated into the hydrogel to form a highly vascularized structure. |
[114] |
Fig. 2.
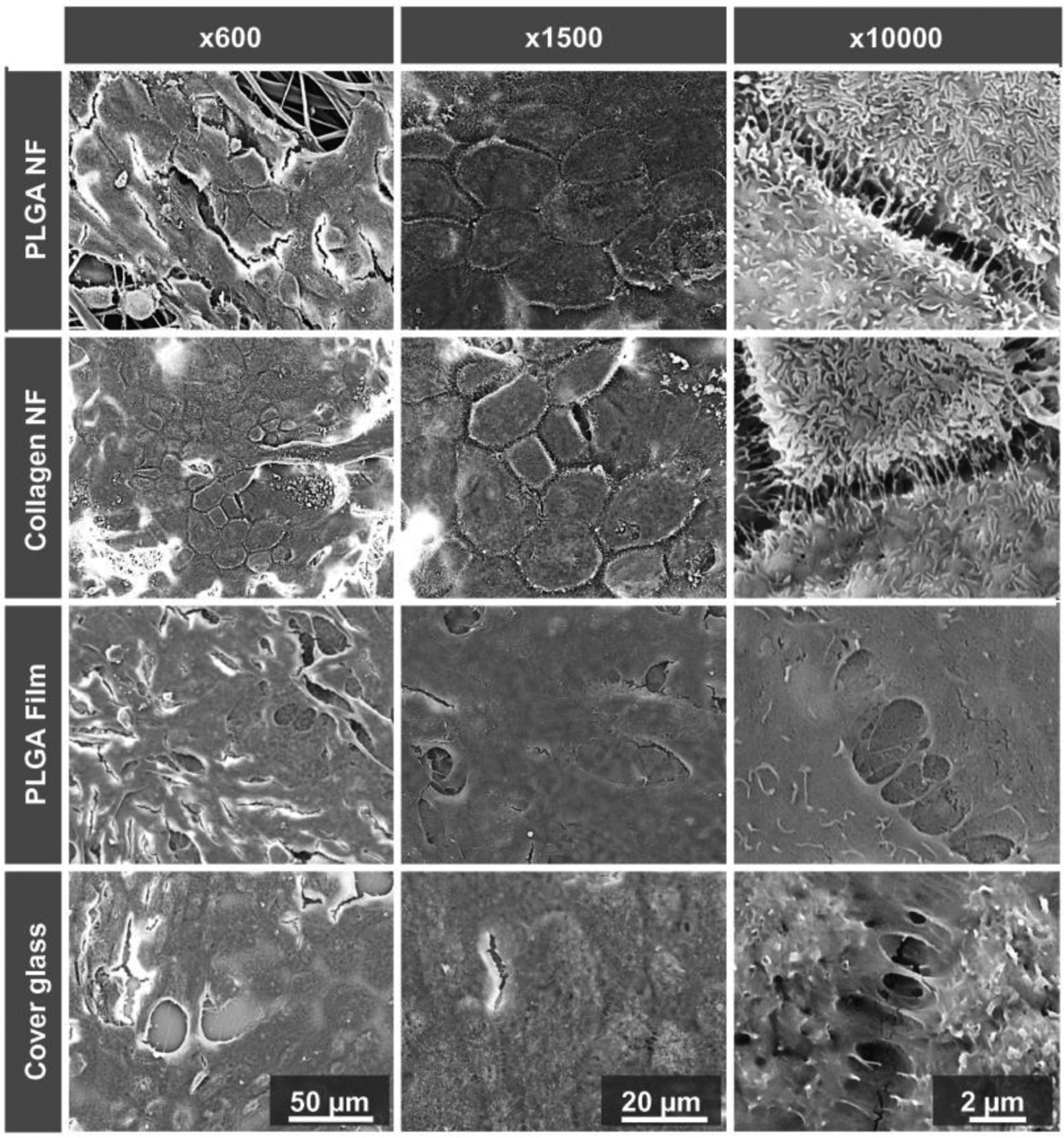
SEM images of RPE cells on PLGA and collagen nanofibrillar membranes, PLGA films and cover glass after 11 days of incubation. The RPE cells formed in vivo-like monolayer of hexa/polygonal cells (shown on x600 and x1500) with long, sheet-like microvilli (shown on x10000) on the PLGA and collagen nanofibrous membranes. Figure is adapted with permission from reference [91].
To further improve cell growth in nanoscaffolds, surface modification, electrical responsive property and controlled drug release have been added to nanoscaffolds [87, 89, 109, 110]. When a biodegradable poly(glycerol sebacate) (PGS) membrane was coated with electrospun nanofiber meshes composed of PCL and laminin, the adhesion of a photoreceptor layer isolated from embryonic retinal tissue to the membrane was increased by the nanofiber meshes, and the transplantation of the photoreceptor layer became easier to be handled [89]. When the surface of PCL-based nanoscaffolds was treated with alkaline hydrolysis, the wettability of the nanoscaffolds was improved so that human RPE cells could adhere to the scaffold surface better for 45 days of healthy growth in the nanoscaffolds [109]. When electrically conductive polypyrrole and graphene were added into nanoscaffolds, under electrical stimulation the neurite length of the retinal ganglion cells grown in the nanoscaffolds was increased by 137% compared with that of the cells grown in control nanoscaffolds without electrical stimulus responsiveness. In addition, electrical stimulation of the neuronal cells promoted the growth of the cells on both random and aligned nanoscaffolds, rendering the alignment unnecessary [87]. When nerve growth factor (NGF) and brain-derived growth factor (BDGF)-loaded gelatin nanoparticles were incorporated into multi-channeled scaffolds composed of nanofibers, NGF was released immediately from the nanofibers to aid the initial stage of axon regeneration and BDGF was sustained released from the gelatin nanoparticles to promote axon growth at the late stage of myelination [88].
The unique molecular recognition property and supramolecular architectures in the size of 5–200 nm of self-assembled peptide nanofiber scaffolds (SAPNS) makes SAPNS attractive as novel 3D scaffolds for retinal tissue regeneration. The common amino acid sequences used for the formation of SAPNS toward retinal tissue regeneration include RADA [111–113], FFkk, FkFk [114], SL-Kr5 (K-(SL)6-K-G-PRKLYDY) [115], and K2(SL)6K2 [115, 116]. SAPNS were used to promote proliferation of endogenous neurons to close neural gaps for functional return of vision [113] and as efficient neuronal storage media [114]. The activity of SAPNS is dependent on the concentration of the peptide used. For example, in a study when the concentration of the (RARA)16 peptide was increased from 0.05% to 1%, the porosity of SAPNS decreased and resulted in reduced adhesion and growth of retinal ganglion cells [111]. The SAPNS can also be in hydrogel form. For example, Moore et al. (2018) used K2(SL)6K2 sequence to form nanofibrous multidomain peptide hydrogels that were capable of provoking inflammatory response, allowing infiltration of host cells, which would then release growth factors and cytokines that would diffuse out of the hydrogels, degrading, initiating vascularization, and bringing innervation [116].
2.2. Nanoscaffolds for corneal tissue regeneration
Corneal diseases are the major causes of blindness worldwide and a wide range of infectious and inflammatory conditions contribute to corneal vision loss and blindness [117]. From superficial to deep layers, the cornea is made of corneal epithelial, stroma and endothelial layers, with the epithelial and stroma layers separated by the Bowman’s membrane and the stroma and endothelial layers separated by the Descemet’s membrane [118]. The corneal epithelium is a non-keratinized stratified squamous epithelium that supports corneal structure and function. It has a finite life span of 7 to 10 days and is continuously renewed by corneal epithelial stem cells located in the basal layer of limbus [119]. During homeostasis or injury, the limbal epithelial stem cells regenerate transient-amplifying cells that migrate centripetally from the limbus into the corneal basal layer, and then proliferate and differentiate into corneal epithelial cells to replace the lost ones [120]. The basement membrane is a transparent film made of highly ordered collagen fibers. If injured, it may form a scar that may cause vision loss [121]. The stroma forms the thickest layer of the cornea and primarily contains water and highly organized collagen fibers. These fibers have a unique shape, arrangement and spacing to render the cornea transparent [122]. The Descemet’s membrane is a thin self-repairing strong film made of collagen fibers that are different from those of the stroma [123]. The endothelium is the innermost layer of the cornea and its primary function is to pump any excess fluid out of the stroma to keep it thin and transparent. Unlike the epithelial cells, once the endothelium is destroyed by disease or trauma, it cannot be repaired or replaced by the body [124]. Corneal transplantation is the mainstay treatment for corneal vision loss. It is usually performed by partial or full replacement of the damaged or diseased cornea. However, due to the shortage of cornea donors as well as the risks of infection and tissue rejection, different electrospun (Table 3), self-assembled amphiphilic peptide nanofibers (Table 4) and nanotopographies (Table 5) have been investigated to support growth of different types of corneal cells including corneal fibroblasts [125], keratocytes [126], epithelial [127] and endothelial cells [128] for corneal epithelium, stroma, and endothelium regeneration or full corneal tissue replacement [49, 129].
Table 3.
Electrospun nanofiber scaffolds investigated for corneal tissue regeneration
| Source | Materials | Fiber Diameter | Cell Type | Summary of Findings | Ref. |
|---|---|---|---|---|---|
| Natural | Collagen type I | 30–50 nm | Rabbit corneal fibroblasts | In the aligned scaffold more than 50% downregulation of α-smooth muscle actin (α-SMA) was obtained compared to the unaligned scaffold and tissue culture plate. In addition, in case of aligned scaffolds, the rabbit corneal fibroblasts elongated along the fiber alignment, but no cell elongation was observed on unaligned fibers and tissue culture plate. | [124] |
| Natural | Collagen type I | 137 ± 49 nm | Rabbit corneal fibroblast | Expression of α-smooth muscle actin (α-SMA) was significantly lower in aligned fiber scaffolds than unaligned scaffolds and tissue culture plate. In addition, in the aligned scaffold the light scattering was minimal that resulted in improved optical properties. | [146] |
| Hybrid | Polyvinyl acetate/ collagen type I | 200–800 nm | Human corneal keratocytes (HKs) and human corneal epithelial cells (HCECs) | The mechanical properties, such as strength, stiffness, and elasticity, of the hybrid scaffolds was about 30x higher than the pure collagen scaffolds. Making aligned scaffolds further improved the mechanical and optical properties of the hybrid scaffolds 2 fold for HKs to grow orderly along the alignment. | [139] |
| Hybrid | PLGA/ collagen type I | 500–2000 nm | HKs and HCECs | The tensile strength and light transmittance of the hybrid scaffolds were 3.41 MPa and 63%, respectively. The HKs maintained their natural phenotype and the HCECs formed multilayered epithelium similar to the natural epithelium when cultured for 2 weeks. | [125] |
| Hybrid | Collagen type I /Chondroitin Sulfate (crosslinked via carbodiimide cross-linker) | Collagen: 400–500 nm Hybrid: 300–400 nm |
HCECs | The tensile strength of the crosslinked nanoscaffolds was about 4x higher than the non-crosslinked nanoscaffolds and the crosslinking decreased scaffold degradation by 70%−80%. However, the viability of HCECs was 40–50% lower in the cross-linked nanoscaffolds than in the non-cross-linked nanoscaffolds. | [126] |
| Hybrid | PCL-PGS and PCL-chitosan | PCL-PGS: 300–500 nm; PCL-chitosan: 100–200 nm |
HKs and HCECs | Elongated HKs and HCECs grew on the aligned fibers, which had a nearly 100% light transmittance. But the HCECs metabolic activity was about 40% higher in the PCL-PGS nanoscaffolds than the PCL- chitosan nanoscaffolds. | [130] |
| Hybrid | PCL-PGS | 150–500 nm | Human corneal endothelial cells and Human conjunctival epithelial cells |
In a wet state, the aligned scaffolds had nearly 100% light transmittance. In addition, in scaffolds with higher PGS content, delayed but normal cell growth, higher cell orientation (~50%) and natural HCEC morphology were obtained. | [127] |
| Synthetic | PLA | Aligned: 696 nm | Bovine corneal keratocytes | The PLA scaffolds supported the growth of the cells and maintained the cell morphology. They had slower degradation rate, but only 80% light transmittance, compared with collagen scaffolds. | [144] |
| Synthetic | PCL | 100 nm | Human corneal epithelial stem cells | Similar viability of the stem cells grown in the PCL nanofibrous scaffolds and a human amniotic membrane (clinically standard substrate for corneal surface repair) was obtained over 15 days, with no sign of differentiation. | [140] |
| Hybrid | Silk fibroin -poly(L-lactic acid-co-ε-caprolactone SF:P(LLA-CL) |
100–650 nm | HCECs | Formation of monolayer cells with expression of tight junction protein ZO-1 and functional genes (e.g. Na+K+-ATPase, solute carrier family-4 member-4 (SLC4A4), voltage-dependent anion channel 3, voltage-sensitive 3 chloride channel, aquaporin-1), was observed. | [145] |
| Hybrid | PCL-Keratin (50:50) | 144 nm | Human mesenchymal stem cell (hMSCs) | Addition of keratin into PCL nanoscaffolds improved cell adhesion by 20%, aided cytoplasmic extension and resulted in elongated morphology. The hybrid nanoscaffolds made the cells to grow longer than 7 days, which could not be achieved by the PCL nanoscaffolds. | [134] |
| Hybrid | PLGA (50:50)/Collagen Type I; 3-layered nanoscaffolds (PLGA-Collagen-PLGA) were crosslinked by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) or glutaraldehyde. | Aligned: 200–300 nm Unaligned:500–1000 nm |
Human endometrial stem cells | Both crosslinked scaffolds had improved physical and mechanical properties, wettability, and cell viability. However, the EDC crosslinked nanoscaffolds exhibited around 52%, 127%, 1.3%, and 23% higher Young’s modulus, tensile strength, strain% at break, and cell viability, respectively, and around 5% lower degradation after 84 days than the glutaraldehyde crosslinked nanoscaffolds. | [158] |
Table 4.
Self-assembled peptide nanoscaffolds and nanotapes investigated for corneal tissue regeneration
| Peptide Amphiphile | Peptide Amphiphile Structure | Cell Type | Summary Findings | Ref. |
|---|---|---|---|---|
| C12-VVAGKYIGSR; obtained from laminin | Nanofiber: 20–30 nm | HCKs | HCKs proliferated on the nanofibers similarly as on collagen surface. Injection of the scaffolds to damaged New Zealand white rabbit corneas induced keratocyte migration and collagen I synthesis, which was indicative of corneal stroma regeneration. | [148] |
| C16–KTTKS; obtained from human collagen type I | Nanotape | HCKs | HCKs grew about 400% slower but produced about 200% more collagen on the nanotape than in basal medium after 3 days of incubation. | [159] |
| C16-YEALRVANEVTLN; obtained from lumican | Nanotape | HCKs | The self-assembled nanotape increased collagen production up to 1.25 times with increase in the concentration of the aggregated amphiphilic peptide from 0.00125 wt% to 0.0025 wt% in 21 days, compared to the scaffolds made of the monomeric peptide. | [149] |
| C16-TPGPQGIAGQ-RGDS/C16-ETTES | Peptide amphiphile coated on a topography | HCKs and HCECs | Formed corneal stromal tissue that was capable of supporting corneal epithelium. There was also no evidence of corneal haze or contracture after 9 months of peptide amphiphile film implantation in New Zealand white rabbit corneal stroma. | [153, 154, 160] |
| RFL4FR; synthetic origin | Nanotape | HCKs | Cells grown on the nanotape made of low concentration of RFL4FR had comparable morphology and viability to those cultured on tissue culture plate. But higher concentration of the peptide amphiphile led to lower cell adhesion. | [152] |
| C16-G3-RGDS/C16-ETTES; obtained from fibronectin | Nanotape C16-ETTES: 19.6 ±1.3 nm C16-G3-RGDS: 19.7±4.4 nm |
HCKs | Formed highly-organized 3D stromal tissue via deposition of collagen fibrils with diameter comparable to that found in stromal lamellae of the native human cornea. 5x more immobile cells and 2.4x thicker tissue were formed by HCKs grown on the peptide amphiphile coated surface than on the uncoated polystyrene surface. | [150] |
Table 5.
Nanotopographies investigated for corneal tissue regeneration.
| Topography | Material used | Size | Cell type | Summary of findings | Ref. |
|---|---|---|---|---|---|
| Nanopillar | Tissue culture polystyrene coated with a mixture of fibronectin and collagen | 250 and 1000 nm pillars | HCECs | The pillar patterns increased cell proliferation 2–3 folds depending on the seeding density. The tight junction protein ZO-1 production also increased 1.5 times in the 1000 nm pillars than in the unpatterned tissue culture polystyrene. The cells grown on the 250 nm pillars assumed polygonal shape and maintained the shape upon detachment from the patterned surface and reattachment onto other surfaces. | [40] |
| Nanopillar | Physically and chemically crosslinked gelatin methacrylate hydrogel fabricated by nano-imprinted polyethylene terephthalate | 1000 nm pillar with 6 μm spacing | HCECs | Significantly higher expression of functional markers such as Na+/K+-ATPase and a 2-fold increase in ZO-1protein were obtained in cells grown on the nanopillars than on unpatterned films. | [155] |
| Groove and ridge topography | RGD peptide functionalized poly(ethylene glycol) diacrylate hydrogel fabricated with polydimethylsiloxane | 400 nm pitch groove with 200–400 nm depth | HCEC | 50% faster wound healing and 2x laminin-332 expression were obtained compared with cells grown on a flat surface. | [156] |
| Nanopillar and nano well | Nano-patterned polydimethylsiloxane coated with bovine fibronectin and bovine collagen-І as well as laminin and chondroitin sulfate | 250 nm pillar and 1000 nm well and pillar | HCEC | Cells grown on the 1000 nm pillars coated with fibronectin-collagen had more regular morphology and expressed 1.2 times higher Na+/K+-ATPase and 2 times more ZO-1 compared with cells grown on the 250 nm fibronectin-collagen-coated pillars. However, on the laminin-chondroitin sulfate coated surfaces the 250 nm pillars exhibited higher ZO-1 expression than the 1000 nm pillars. | [157] |
| Nanopillar and nanowell | Nano-patterned polydimethylsiloxane substrates coated with poly(methylmethacrylate) | 250 and 1000 nm pillars and wells | Bovine corneal endothelial cells | Significantly higher expression of Na+/K+-ATPase and ZO-1 activity were obtained on the 250 nm pillars compared with the nanowell and unpatterned substrates. | [41] |
ZO-1, a tight junction protein used as a marker for assessing proper functioning of corneal endothelial cells; Laminin-332, an extracellular matrix protein used as a marker to evaluate the migratory status of cells upon epithelial wound healing; Na+/K+-ATPase, a marker for assessing proper functioning of corneal endothelial cells.
The electrospun nanofibers investigated for corneal tissue regeneration are made of various natural, synthetic and hybrid materials, similar to those discussed for retinal tissue regeneration under Section 2.1. The commonly investigated natural polymers/proteins are chitosan [130, 131], gelatin [132], collagen [133], keratin [134, 135] and silk fibrin [136, 137]. The commonly studied synthetic polymers are poly(ethylene glycol) [138], PLGA [126, 139], poly(vinyl acetate) [140], PGS [131], and PCL [128, 131, 141]. Scaffolds intended for corneal tissue regeneration should have appropriate porosity, mechanical and optical properties [142, 143]. Generally, scaffolds of natural polymer origin better mimic the natural environment for corneal cell proliferation and differentiation, but they have weaker mechanical strength and degrade relatively faster compared to scaffolds made of synthetic polymers [54, 127, 141]. For example, Aslan et al. (2018) compared cell proliferation on aligned PLA and collagen type I nanoscaffolds and found that the light transmittance of collagen nanoscaffolds was close to the native cornea, but only 80% light transmittance could be achieved with PLA nanoscaffolds. However, the rate of scaffold degradation in the collagen nanoscaffolds was faster than that in the PLA nanoscaffolds [144]. To obtain good light transmittance and degradation properties of nanoscaffolds for corneal cell proliferation, mixtures of different natural and synthetic polymers have been used for the fabrication of nanoscaffolds. These mixture systems include collagen with PLGA [126], polyvinyl acetate with collagen type I [140], collagen type I with chondroitin sulfate [127], PCL with PGS [128], and silk fibroin with poly(L-lactic acid-co-ε-caprolactone) [145]. Stafiej et al. (2017) investigated the growth of human corneal endothelial cells (HCECs) and human corneal keratocytes (HCKs) in aligned PCL-PGS and PCL-chitosan based nanoscaffolds. Even though both the scaffolds supported the growth and elongation of the both cells, the metabolic activity of the HCECs grown in PCL-PGS nanoscaffolds was approximately 40% higher than that in the PCL-chitosan scaffolds while the metabolic activity of the HCKs on the both scaffolds nearly was pretty much the same [131]. Arabpour et al. (2019) compared PCL based nanoscaffolds to PCL-keratin based nanoscaffolds and observed continued cell growth in PCL-keratin based nanoscaffolds after day 7, while it halted in PCL based nanoscaffolds [135]. Besides the chemical composition of polymers mentioned above, the physical properties including size, alignment and density of nanoscaffolds also play important roles for corneal cell growth. For example, rabbit corneal fibroblasts grown on aligned collagen nanofiber scaffolds had lower α-smooth muscle actin expression and better cell elongation and light transmittance than those grown on unaligned nanoscaffolds and tissue culture dishes (Fig. 3.) [125, 146]. In another study, rabbit corneal cells grown on radially aligned nanoscaffolds showed a 1.2 fold increase in both gene expression and proliferation compared with those grown on unaligned nanoscaffolds [147]. Compared to microfiber scaffolds, nanofiber scaffolds cause less inflammatory responses, because they mimic mechanical properties of the native cornea [128]. Although still debatable, Wu et al. (2018) showed that once the fiber size reached to the nano-range, the diameter of the nanofibers had no significant effect on corneal cell proliferation, activity, and growth pattern [140].
Fig. 3.
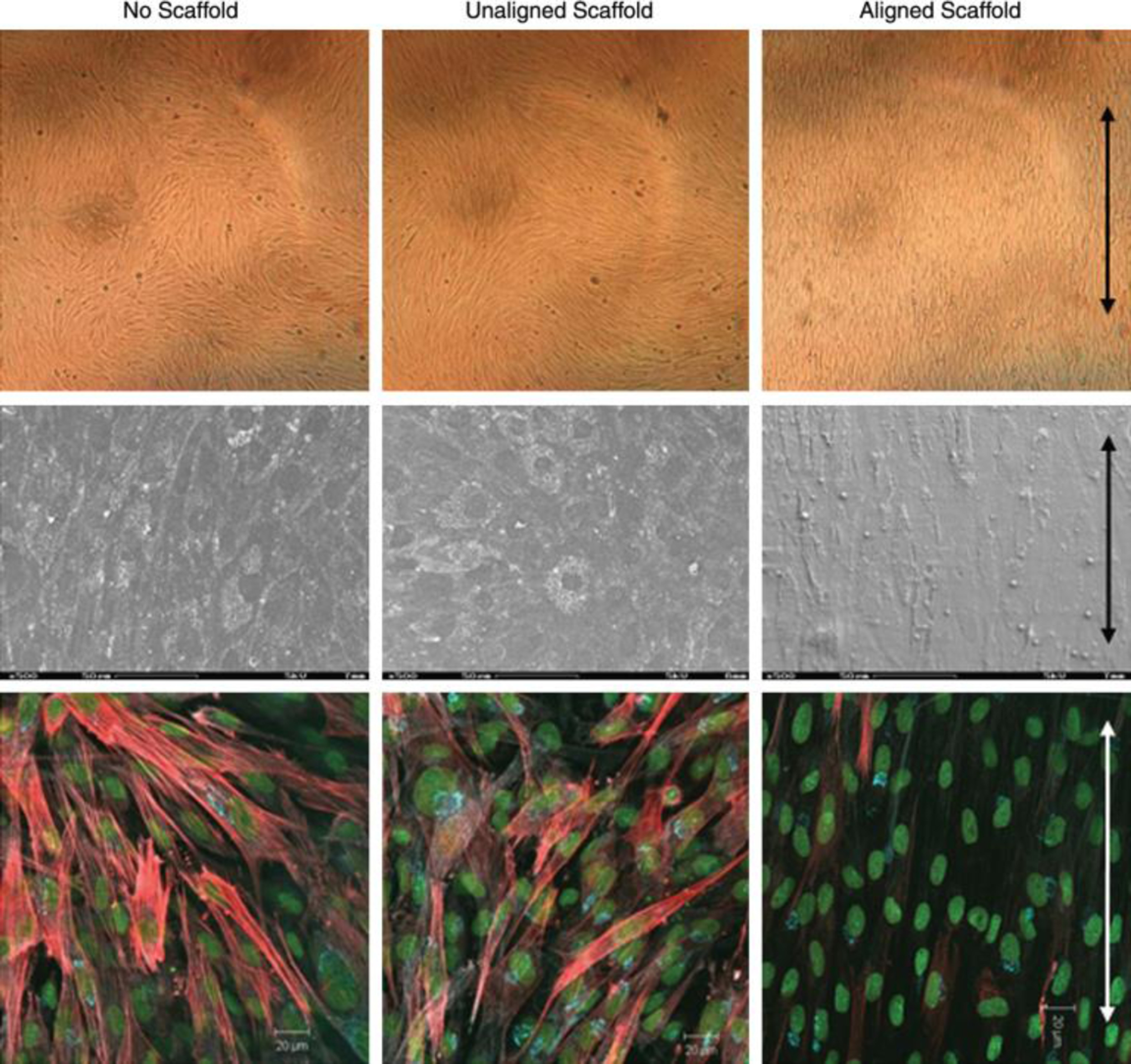
Rabbit corneal fibroblasts grown on aligned fibers expressed less α-SMA expression than those grown on unaligned fibers and tissue culture dishes. Top row: Cells cultured on culture dish, unaligned and aligned electrospun fibrous scaffolds. Middle row: SEM images of the morphology of the cells. Bottom row: IF images of cells. Cell nuclei are labeled with CYTOX green; α-SMA is labeled with rhodamine (red). Arrows indicate the direction of fiber alignment. Figure is adapted with permission from reference [125].
Peptide amphiphiles (PA) with sequences VVAGKYIGSR from laminin [148], KTTKS from collagen type I [149], YEALRVANEVTLN from lumican [150], RGDS from fibronectin [151–154], and synthetically made RFL4FR [155] were reported to self-assemble into nanofibers. After these self-assembling peptides were injected into rabbit’s cornea or used as nanotapes on curved templates, slates, and slides, enhanced keratocyte adhesion, migration and growth and collagen production were observed, suggesting the potential application of these self-assembling peptides for corneal tissue regeneration [148, 151–153, 155]. By using nanotopography technology, patterned nanoscaffolds can be obtained to provide physical and biological cues similar to the natural basement membrane. The patterned nanoscaffolds are commonly prepared using molds of patterned surfaces that are made of different materials including tissue culture polystyrene [43], polyethylene terephthalate [157], and polydimethylsiloxane [158] coated by different polymers such as collagen type I, fibronectin [43], laminin, chondroitin sulfate [159], thin hydrogels [157, 158], and poly(methylmethacrylate) [44]. Both the surface feature of the molds and the types of the coating materials play important roles for corneal cell growth. For example, Muhammad et al. (2015) compared the growth of human corneal endothelial cells on 250 and 1000 nm pillars and 1000 nm wells made of tissue culture polystyrene with fibronectin-collagen coating. Although the cell density and expression of ZO-1 was highest on the 1000 nm pillars, desired hexagonal morphology of HCECs was obtained on the 250 nm pillars [43]. In another study, the authors reported that the best corneal endothelial cell proliferation was found on 1000 nm pillars with fibronectin-collagen coated surface, and but on 250 nm pillars with laminin-chondroitin sulfate coated surface [159].
2.3. Nanoscaffolds for lens regeneration
Cataract is the most common form of lens disease that may cause blindness. Cataract surgery is the only treatment currently available for cataract disease, and involves invasive method to replace the natural lens with an artificial intraocular lens [160]. During the past several years, lens epithelial progenitor cells and pigment epithelial cells of the dorsal iris have shown promise in regeneration of the lens without surgery in rabbit, macaque and newt models as well as in human infants with congenital cataracts [161, 162]. Along the way, nanoscaffolds are also being developed for these cells for lens regeneration, where they are mostly injected into the lens capsule after removing its content. For example, Nibourg et al. (2016) used a low molecular weight supramolecular hydrogelator to form self-assembled nanofiber-based nanogels made of LMWG peptide (Fig. 4) to be used as extracellular environment for lens epithelial cell growth in an ex vivo porcine eye model. The nanofiber-based nanogels were filled into the capsules of porcine lenses after removing the lens content and the filled lenses were then extracted from the porcine eye and were cultured for three weeks. Lenses filled with hyaluronan were used as a control. Compared with the control, the nanogels helped the lens epithelial cells maintain their normal epithelial-like morphology and had less alpha smooth muscle actin (αSMA) expression (9.54 ± 11.29% vs. 30.08 ± 24.60%), which resulted in less capsular opacification. Further improvement up to 10 times in capsular opacification was observed after incorporating different extracellular matrix-derived peptides, such as IKVAV, YIGSR, RGDS, PHSRN, and DGEA [163]. Xi et al. (2013) fabricated polyurethane nanofibers by electrospinning method and used the nanofibers as scaffold substrates to grow human umbilical vein endothelial cells and human lens epithelial cells (SRA 01–04) [164]. They first investigated the effects of the alignments of nanofibers on the cell adhesion. They found that the attachment of human umblical vein endothelial cells on the aligned nanofibrous membranes were 4 and 2 times greater than planar and randomly aligned membranes, respectively. Furthermore, they compared cell migration on aligned membranes with fibers oriented perpendicular to the wound edges, anisotropic-latitude; aligned membranes with fibers oriented parallel to the wound edges, anisotropic-longitude; randomly aligned membranes; and planar membranes. They found that the area of SRA 01–04 on anisotropic-latitude nanofibrous membranes was 18.7, 8, and 2 times greater than that of the cells on anisotropic-longitude, random, and planar membranes, respectively. Furthermore, endothelial cells on aligned membranes had bipolar morphology, which was more favorable than polygonal morphology observed on randomly aligned membranes [164]. These studies provided an optimistic view to explore the potential of nanoscaffold-based lens regeneration approach and a new avenue of research with a goal to find better alternatives for the management of cataract and its subsequent complications. However, the use of nanofabricated scaffolds in ocular lens regeneration is an avenue yet to be well explored.
Fig. 4.
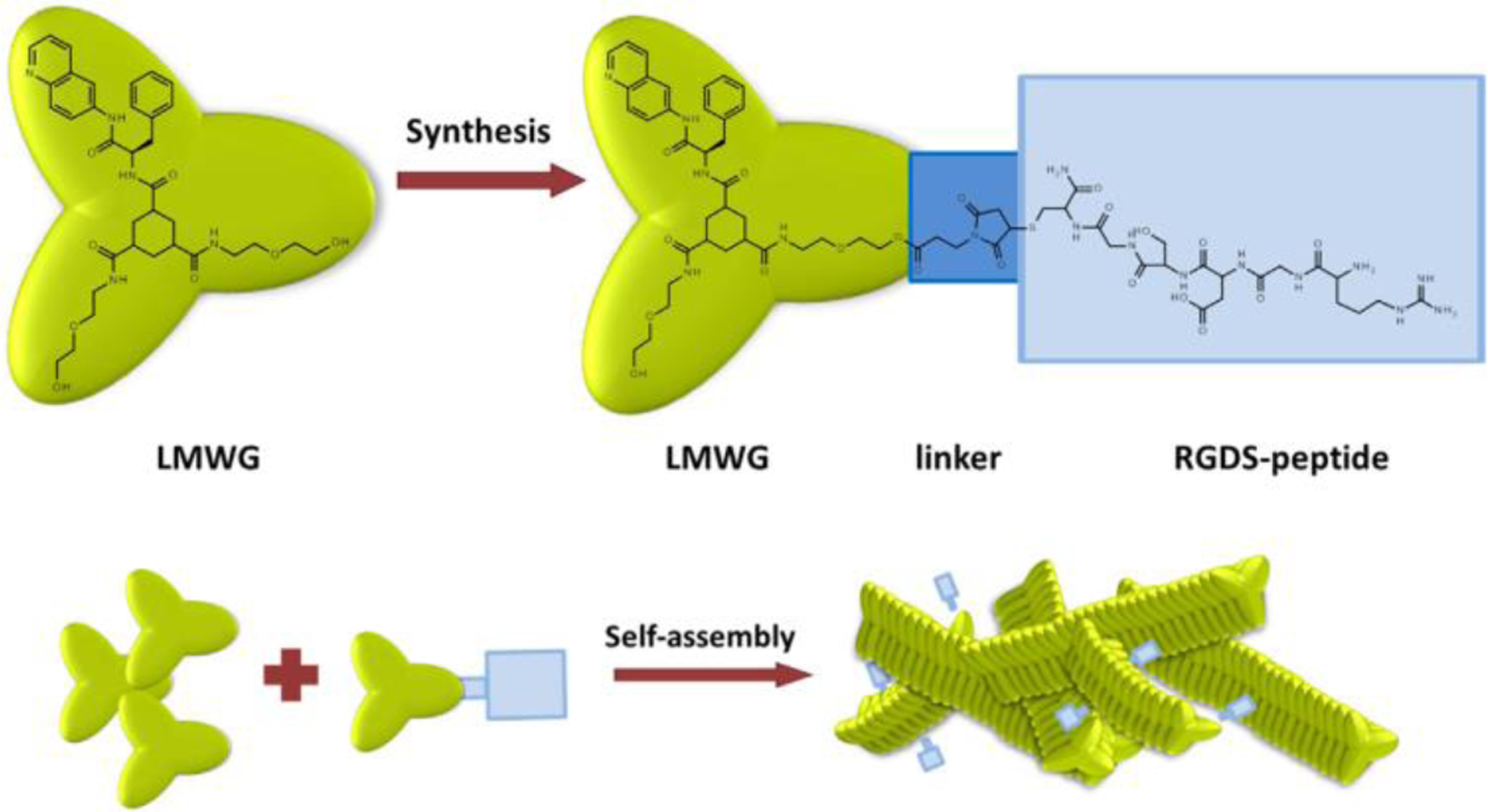
Schematic representation of the formation of self-assembled nanofiber-based nanogels made of LMWG molecule. LMWG molecule was conjugated with a RGSD peptide via a maleimide linker molecule and then formed into nanogels by self-assembly. Figure is adapted with permission from references [163].
3. Nanomaterials as gene delivery devices to reprogram cells for ocular regeneration
Stem-cell differentiation and cellular reprogramming can be fundamentally regulated through a process known as gene regulation [28, 165, 166]. Somatic cells and MSCs can be reprogrammed into iPSCs by introduction and ectopic expression of a combination of pluripotency genes including OCT3, OCT4, SOX2, KLF4, and c-Myc (Nanog and Lin14) [167–169]. Gene delivery can also promote differentiation of different stem cells or progenitor cells into specific cell lineages through up- or down-regulation of specific genes [28, 170]. Recently, mammalian Müller glial cells have been investigated as endogenous source of retinal progenitor cells. Zhao et al. (2013) reprogrammed mouse Müller glia into photoreceptors and retinal ganglion cells by downregulation of p53 genes [34, 171] that, upon incorporation into the host retina, expressed either retinal ganglion cell or photoreceptor markers such as Islet1 and Brn3 or rhodopsin and interphotoreceptor retinoid-binding protein (IRBP), respectively [171]. Similarly, Chen’s group (2016) transfected adult mouse Müller glial cells by a β-catenin gene loaded adeno-associated virus and enhanced the proliferation of Müller glial cells through activation of Wnt signaling [172]. The same research group later transfected the proliferated Müller glia cells with Otx2, Crx and Nrl expressing genes to differentiate them into rods [173]. Upon these two steps of de novo cell reprogramming and genesis of rod photoreceptors, they restored the visual responses in Gnat1rd17Gnat2cpfl3 double mutant mice, a model for congenital blindness. In another study, retinal stem cells located in the ciliary margin of the adult human eye were transfected by CHX10VP16, OTX2, and CRX genes-loaded lentivirus to differentiate them into all types of retinal cell types including photoreceptors in vitro [174]. Another alternative to cellular reprogramming using gene therapy is gene supplementation therapy that is based on the replacement of absent or abnormal genes that are responsible for the ocular diseases. It can be used to prevent the progression of some ocular degenerative diseases caused by mutations like retinitis pigmentosa, Leber congenital amaurosis, X-linked retinoschisis, and Stargardt’s disease [15, 16]. The retinal cells that are mostly affected by inherited retinal diseases are RPEs and photoreceptors and less so bipolar, amacrine, horizontal and ganglion cells [16]. For example, Leber congenital amaurosis is associated with mutations of RPE65 gene [175]. The RPE65 gene express a key enzyme that regulates the availability of photochemicals that absorbs light energy from light rays and converts the light energy into nerve impulses that travel to the visual cortex of the brain. In its absence, the toxic intermediates retinyl esters accumulate in the RPE, which eventually leads to RPE atrophy and subsequent death of cone and rod photoreceptors and blindness [56, 175]. Stargardt’s disease is associated with mutation of ATP-binding cassette subfamily a member 4 [16]. Therefore, repairing or replacement of these missing genes can prevent the progression of ocular degeneration. For example, genes have been successfully delivered into RPEs to restore genetic defects in autosomal-recessive retinitis pigmentosa in mice [176]. However, gene supplementation has its own limitations. For example, some neurodegenerative diseases like AMD are associated with multiple gene mutations and editing of multiple genes incurs additional risk such as unintended mutations [176]. Therefore, repopulating the affected tissue by cell-based therapy rather than gene supplementation therapy poses reduced risk [176] and in this review gene supplementation is discussed with less attention.
The anatomy of the eye allows direct deposition of therapeutic genes to the retina through intravitreal or subretinal injections [15]. However, efficient gene transfection, both in vitro and in vivo, has been challenging due to the poor permeability of genes into the cell and especially cell nucleus, degradation of genes in the cytoplasm by ubiquitous nucleases, and sequestration of genes by DNA-binding proteins and cytoskeletal elements in the cytoplasm [168, 177]. To improve the penetration of genes into the cell, holes were drawn in the cell plasma membrane for genes to enter into the cell using physical methods including direct microinjection and electroporation [178, 179]. However, the cells that can be performed by direct microinjection are only large cells such as oocytes and embryos and non-dividing cells such as neurons[180, 181]. Electrical stimulation from electroporation may also cause high rate of cell death [179]. To improve the transfection efficiency of genes, different viral and non-viral nanosized gene delivery vectors have been investigated [15]. Especially, viral vectors like retrovirus, lentivirus, adenovirus and adeno-associated virus possess high cellular transfection and gene expression capabilities and have been commonly used to reprogram cells [28]. They have even entered into different clinical trial stages to treat inherited retinal degenerative diseases, with adeno-associated viruses leading the way. However, despite the encouraging results, their progression is hampered by safety issues associated with their inherent immunogenicity, broad tissue tropism, and tumorigenicity due to insertional mutagenesis and uncontrolled gene expression [16, 28, 56, 170, 182]. In addition, the packaging capacities of viral vectors is limited. For instance, the packing capacity of the commonly used adeno-associated virus and lentivirus vectors is limited to approximately 5 and 9 kb, respectively [183]. Contrarily, the non-viral nano-particulate vectors such as lipoplexes, polyplexes, mesoporous silica nanoparticles, and dendrimers are relatively safer, cheaper, more reproducible, and small enough to enter cells by endocytosis. However, they have low gene transfection ability and may also have some degree of cytotoxicity and non-biodegradability issues [28, 184]. Therefore, the need of developing efficient, nontoxic, non-viral, gene nanocarriers with high gene transfection efficiency has long been of paramount importance [16, 185]. As a result, many types of non-viral nanocarriers (Fig. 5) have been investigated as vectors for gene delivery to target cells used in ocular regenerative medicine.
Fig. 5.
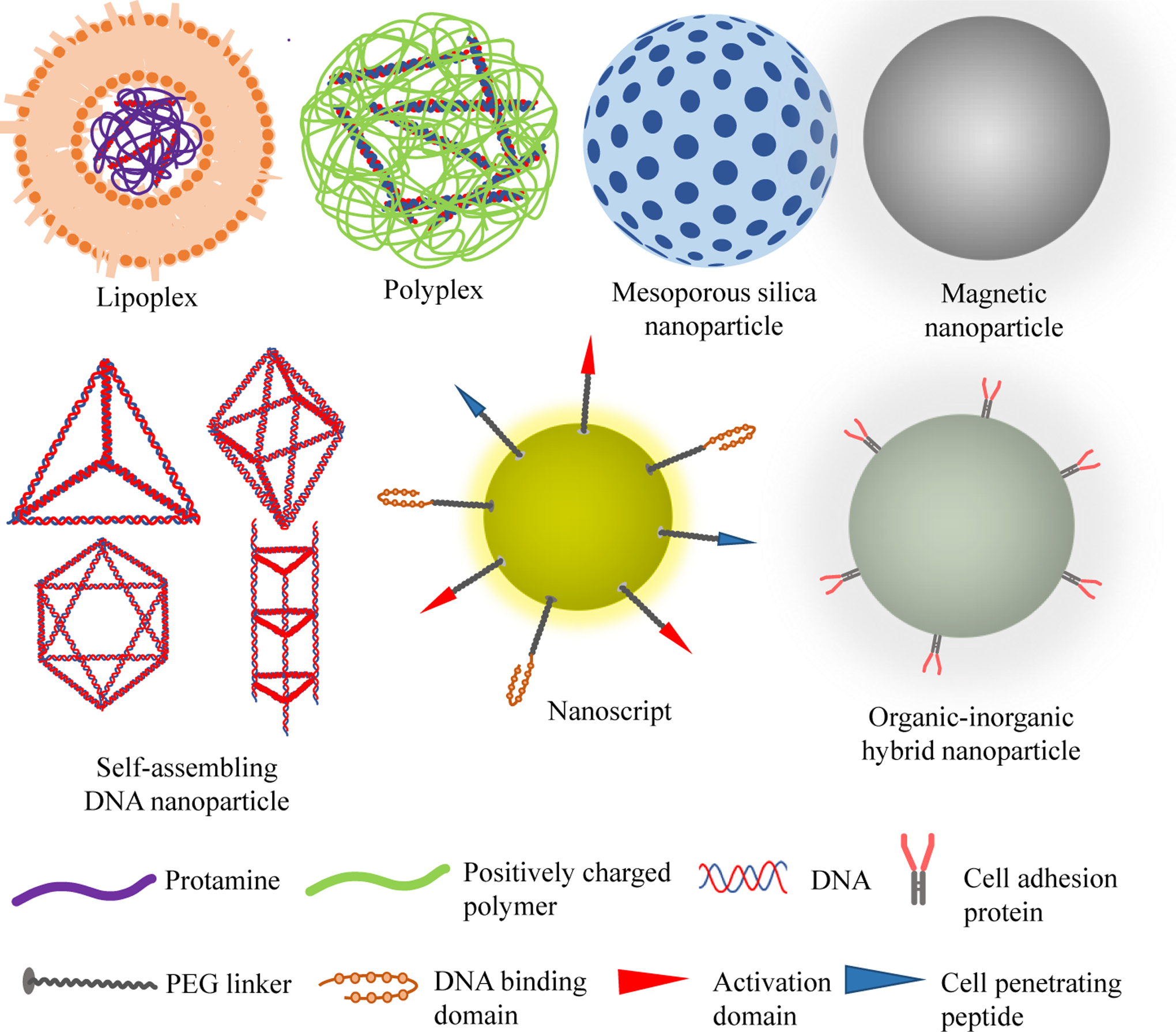
Schematic representation of different types of nanoparticles used as gene delivery carriers for ocular regeneration.
The first type of non-viral gene nanocarriers investigated for gene delivery for ocular tissue regeneration is liposome-protamine-DNA (LPD) complexes, also called lipoplexes [186]. LPD complexes contain a highly condensed DNA core surrounded by lipid bilayers, and they were initially prepared in two steps [187]. First, the negatively charged DNA was interacted electrostatically with the positively charged protamine – an arginine-rich protein isolated from the sperm of a mature fish – to form a condensed core. Then a cationic liposome, consisting of cationic lipids like DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) was added to the negatively charged protamine/DNA complexes to form LPD complexes. The LPD complexes were nanosized and prevented DNA from nuclease degradation [188]. Protamine also condensed DNA and presented sequences of six consecutive arginine residues, which enabled translocation of DNA from the cytoplasm to the nucleus of living cells [184]. As compared to plasmid DNA-loaded liposomes, which is without protamine, the LPD complexes better protected the plasmid DNA from enzymatic degradation and offered higher gene expression [186]. Inclusion of cholesterol as a helper lipid could further increase the in vivo transfection efficiency of the LPD nanocarriers with less need of the cationic lipids [188]. To improve the targeting capability of the LPD nanocarriers, various cell targeting peptides were enveloped inside the LPD nanocarriers [189]. For example, the group of Rajala (2014) developed an LPD nanocarrier that they described as an “artificial virus” for the delivery of RPE65 gene to the retina [56]. The nanocarrier was made by surface modification of the LPD complex by a nuclear localization signaling peptide (DKKKRKVDKKKRKVDKKKRKV) and a cell-penetrating transactivator of transcription peptide (YGRKKRRQRRR). Subretinal administration of the nanocarriers to 5-day old RPE65 knockout mice resulted in expression of the RPE65 in the test group but not in the control group. Similarly, electroretinography (ERG) measurements showed that the amplitudes of the scotopic b-wave (rod photoreceptor function indicator) and photonic b-wave (cone photoreceptor function indicator) of mice treated with RPE65 gene loaded nanocarriers increased by more than 100% as compared to mice treated with the control LPD. In a similar study, Wang et al. (2016) attached vitelli form macular dystrophy, mouse rhodopsin, red/green opsin, and thymocyte antigen as retinal cell-specific promoters and could specifically deliver genes to RPEs, rod cells, cone cells, and ganglion cell, respectively, in vivo [190].
The second type of gene nanocarriers investigated for ocular regenerative medicine is polyplexes. Polyplexes are nanosized complexes formed by electrostatic interaction of cationic polymers with negatively charged DNA in aqueous solution, which leads to reversible linear to globule transition of DNA [22, 191]. At certain polycation to DNA ratios, DNA undergoes localized bending or distortion that facilitates the formation of nanoparticles with different shapes like rods, toroids and spheroids [191]. In addition, the polyplexes’ transfection efficiency increased with increasing their positive to negative charge ratios to a certain point and then decreasing at higher ratios [191]. The polyplexes were stable in nuclease-rich environment, and had relatively high gene transfection ability for both dividing and nondividing cells [22].
The third type of nanocarriers that have good gene delivery potential is mesoporous nanoparticles that contain pores with diameters between 2 and 50 nm. The porous structure and high surface area of mesoporous nanoparticles enable high gene loading/encapsulation and enhanced transfection [29, 182]. Change et al. (2017) differentiated iPSCs into dopaminergic neurons by co-delivering of nuclear receptor related-1 protein plasmid DNA and Rex1 siRNA using hexagonal mesoporous silica nanoparticles with dense positive charge, amine functional group and a size of 110±30 nm [169]. The DNA-siRNA-loaded mesoporous silica nanoparticles generated more robust and long-living neuronal cells than Lipofectamine™, a commercially available cationic liposome that is widely accepted as “gold-standard” for the safe delivery of exogenous DNA or RNA into cells[29, 192]. Cao et al. (2014) found that, based on fluorescent microscopic results, plasmid DNA encoding VEGF-loaded mesoporous iron oxide nanoparticles transfected MSCs better than free plasmid DNA [182].
Organic-inorganic hybrid nanocrystals are the fourth type of gene nanocarriers. Kutsuzawa et al. (2006) developed a plasmid DNA-loaded organic-inorganic hybrid nanocarriers for effective transfection of mouse ESC lines. The hybrid nanocarrier was formed by electrostatically embedding fibronectin and E-cadherin-Fc chimera on carbonate apatite nanocrystals. The fibronectin and E-cadherin-Fc chimera were used as cell adhesive molecules that bound with the transmembrane proteins fibronectin-specific integrins and E-cadherin, respectively, and synergistically enhanced the interaction of the nanocrystal with the cells. The hybrid nanocarrier enhanced gene transfection and transgene expression 20 and 3 times, as compared with the non-modified inorganic carbonate apatite nanocrystals and the commercially available Lipofectamine in the mouse embryonic cells, respectively, demonstrating the great potential of the hybrid nanocarriers for stem cell reprogramming [165].
The fifth type of nanocarriers investigated for ocular tissue regeneration is NanoScripts. NanoScripts are nanoparticles onto which specific small molecules are attached to mimic natural transcription factor proteins. The small molecules bind to specific portions of a genome in stem cells to regulate gene expression in the stem cells for regenerative medicine [166, 193]. For example, Patel et al. (2015) developed 45 nm Sox9-specific NanoScripts that repressed Sox9 expression in neural stem cells and initiated enhanced differentiation of the neural stem cells into neurons. The NanoScripts were designed by conjugation of Sox9-specific hairpin polyamide, a corepressor peptide with a sequence WRPW, and TAT sequence from the human immunodeficiency virus (HIV) virus, a membrane penetrating peptide, on magnetic nanoparticles via a PEG-based linker. The hairpin polyamide (PyPyPy-b-PyImPy-g-PyPyPy-b-PyImImb-Dp-NH2) comprised pyrrole (Py) and imidazole (Im) groups that bound to A–T and G–C base pairs on the DNA, respectively, and sterically hindered the attachment of enzymes such as RNA Polymerase II, to the binding site and in turn prevented gene transcription in neural stem cells. The short readily soluble WRPW peptide also induced gene repression by preventing the formation of basal transcriptional machinery at the binding site and initiating the Groucho family proteins, well-established corepressor factors that prevent the formation of the transcriptional basal complex. The membrane penetrating peptide assisted the NanoScripts cross the plasma and nuclear membranes [166].
The sixth and seventh types of gene nanocarriers that can potentially be used for ocular regeneration are self-assembling DNA and magnetic nanoparticles. Ma et al. (2018) showed that self-assembled DNA tetrahedronnanostructures (≈10 nm) enhanced the proliferation of neuroectodermal stem cells via activation of Wnt/β-catenin pathway in vitro. They also showed that the tetrahedron increased differentiation of neuroectodermal stem cells into neuron in serum free medium by inhibiting notch pathway [194]. Lee et al. (2011) transfected mouse embryonic fibroblast cells using paramagnetic nanoparticles loaded with the transcription factor genes OCT4, SOX2, KLF4 and c-Myc. The genes were incorporated in the magnetic nanoparticles via conjugation with cationic polymer polyethyleneimine coated on the magnetic nanoparticles. After the transfection, exogenous DNA-free iPSCs were obtained and the iPSCs showed similar properties as ESCs in terms of cell growth pattern, colony shape, differentiation potential and teratoma formation [195]. Similarly, Ruan et al. (2011) transfected 293T cells and human fibroblast cells with four transcription factor genes OCT4, SOX2, LIN28 and Nanog loaded in a magnetic nanocarrier coated with 5-generation polyamidoamine dendrimer. The magnetic nanocarrier produced 10 times higher OCT4, SOX2, LIN28 and Nanog-containing lentiviruses in 293T cells than Lipofectamine 2000, and reprogrammed human fibroblast cells into exogenous DNA-free ESC like iPSCs at 37 °C after 21 days of transfection [196].
4. Nanomaterials as immunomodulators in ocular regeneration
In ocular regenerative medicine, autologous, allogenic or bioengineered cells can be used [12]. Autologous cell therapy has the advantage of not being attacked by the immune system, but it has economic and regulatory issues. Contrarily, in allogenic cell therapy, one cell type can be used for all suitable patients, but it has an inherent problem of immune rejection [197]. Particularly, in ocular tissue transplantation, although photoreceptors are not expected to be immunogenic, glial cells and RPEs might cause immune rejection [22]. The immune rejection occurs mainly by the activation of alloreactive T cells and antigen presenting cells such as B lymphocytes, macrophages and dendritic cells. Acute rejection takes place when the T cells infiltrate into the allografts and stimulate inflammation and cytotoxicity. Complex interactions between the allografts and cytokines, CD4+ and CD8+ T cells and B cells result into chronic rejection and, eventually, graft loss. Specifically, recognition of the incompatibility of the donor cells by the recipient’s immune system via major histocompatibility complex (MHC) class I and II antigens causes recruitment of activated lymphocytes and initiation of immune effector mechanisms, leading to graft destruction [198].
Different cytokines from subsets of immune cells including innate lymphoid cells and Gamma Delta (γδ) T cells showed different effects on epithelial stratification and corneal restoration [199]. For example, Li et al. (2011) reported that during corneal abrasion, C-C chemokine receptor type 6 (CCR6) and Interleukin 17 (IL-17) positive γδ T cells infiltrated epithelium, causing chemokine (C-C motif) ligand 20 (CCL20) mRNA and protein expressions to increase 19 and 16 fold, respectively, 6 h after injury in wild type C57BL/6 mice [200]. They also found that corneal γδ T cells showed positive stains for the isoform of retinoic acid receptor-related orphan nuclear receptor gamma (RORγt), Interleukin 23 receptor (IL-23R), and Interleukin 22 (IL-22) in wild type C57BL/6 mice. After anti-CCL20 antibody was administered systemically or topically to wild type C57BL/6 mice, accumulation of γδ T cells was reduced by 50% along with a 60% reduction in stromal inflammation in terms of neutrophil response. Use of anti-IL-22 antibody decreased the peak epithelial cell division of the healing epithelium by 52.2% after 18 h of injury in wild type mice. Li et al. (2011) further reported that in γδ T cell-deficient mice, treatment with recombinant IL-22 promoted significant wound healing and increased peak epithelial cell division by 3 fold [200]. On the other hand, Zhang et al. (2017) found that cytokine IL-20 favoured corneal wound repair, evidenced by the following facts. IL-20 expression increased 3 fold after a 2 mm central epithelial abrasion in mice, and topical application of anti-IL-20 antibody slowed down the cornea healing process [201]. However, topical application of recombinant IL-20 in wounded wild-type, mutant and neutrophil-depleted mice reduced corneal inflammation by 42% as demonstrated by reductions in limbal vessel dilatation (arterioles 20%, venules 20%), platelet extravasation (74%), neutrophil recruitment (62.5%) and chemokine (C-X-C motif) ligand 1 (CXCL1) expression [201]. Gong et al. (2007) reported that increase of Th2-type cytokines IL-4 and IL-10 improved allograft survival in the rat cornea [202]. Ramesh et al. (2008) showed that manipulation of the oxidative pathway by activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) to be a promoter for a number of genes involved in cytokine and matrix metalloproteinases (MMP) expression, corneal inflammation could also be reduced [12].
To improve the immunosuppression efficiency of cytokines as well as other immunosuppressants such as Flt-23k (anti-VEGF intraceptor) [203], rapamycin [204], mycophenolic acid [205], tacrolimus [206] cyclosporin A [207], and dexamethasone [208] in the eye, a variety of nanomaterials have been investigated as carriers for the immunosuppressants. [209, 210]. PLGA nanoparticles with a size of 220 nm were used to load Flt-23k to decrease corneal opacification by 75% and improve graft survival by 20% after 2–5 and 8 weeks of operation, respectively, in a corneal graft mouse model compared with the Flt-23k alone control group [203]. Co-administration of the steroid triamcinolone with the Flt-23k-loaded nanoparticles further reduced the graft rejection rate from 80% to 8% [203]. Chitosan-coated, rapamycin-loaded PLA nanoparticles extended rabbit corneal allograft survival by about 3–4 days in comparison with 0.5% rapamycin suspension [204]. Weekly subconjunctival administration of 8% dexamethasone-loaded PLGA nanoparticles prevented corneal allograft rejection in rats for 9 weeks [208]. Mycophenolic acid-loaded PLGA nanoparticles prolonged allograft survival 50% compared with the daily dose of drug alone after intraperitoneal injection in mice (median allograft survival time 33 vs. 22 days), despite the fact that the drug dose in the nanoparticles was 1000‐fold lower than the dose of the free drug. The nanoparticles also helped mycophenolic acid to decrease antidonor CD4+ T‐cell IFN‐γ responses in the mouse spleen [205]. Niosomes derived from proniosomes containing poloxamer 188 and lecithin as surfactants and cholesterol as a stabilizer, were used to deliver tacrolimus to the cornea in a Sprague–Dawley (SD) rat xenotransplantation model, for prolonging the allograft survival [206]. The results showed that 0.1% tacrolimus-loaded niosomes delayed the rejection of corneal allograft and significantly prolonged the median allograft survival time (13.86 ± 0.80 days) as compared with 1% cyclosporine A (CsA) eye drops (10.57 ± 0.65 days), drug-free niosomes (6.25 ± 0.59 days), or untreated group (6.29 ± 0.42 days).
5. Summary and future perspectives
Regenerative ophthalmology is an exciting new field to regenerate compromised, lost or damaged ocular cells and tissues to treat vision loss and blindness caused by various ocular degenerative diseases, traumas, or infections. Recent advances in exogenous delivery of living allogenic or autologous cells, particularly stem cells make cell therapy an intriguing potential therapy to regenerate ocular tissues to preserve and restore vision [24]. However, cell therapy approaches are still at their early stage and face multiple major challenges to regenerate ocular tissues/organ. The challenges include requirement of chronic systemic immunosuppression that can have numerous side effects [211]; chronic graft-versus-host disease that can lead to dysfunction of the lacrimal gland and keratoconjunctivitis sicca, which diminish the patient’s quality of life [212]; and pro-inflammatory environment created by the diseased eye itself, including cytokines, chemokines, complement and nitric oxide that contribute to reactive gliosis and can also prevent regeneration [213]. Effective methods and biomaterials for cell transplantation, adhesion, proliferation, and differentiation and the addition of nanotechnology to ocular regenerative therapies shows promise for overcoming these challenges [30].
Different types of nanoscaffolds including electrospun nanofibers, self-assembled peptide nanofibers and nanotopographies have been designed and investigated for retinal, ocular and lens tissue regeneration. Generally, unlike traditional scaffolds, nanoscaffolds provide highly porous 3D frameworks that facilitate oxygen and nutrient transport and cellular waste removal and promote cellular attachment, proliferation and differentiation [35]. By modifying their content including surface coating and geometry including nanofiber length, diameter, arrangement and alignment, nanoscaffolds can be engineered to have appropriate chemical, physical, biological and mechanical properties mimicking the natural corneal and retinal microenvironments for ocular and stem cell growth and differentiation to regenerate ocular tissues. Usually, natural polymers including self-assembled peptides are more suitable for promoting cell attachment and biological activity by closely imitating the native extracellular matrix than synthetic polymers. However, they have less mechanical strength and shorter half-life than synthetic polymers. As a result, a combination of natural and synthetic polymers has been used to form transparent and biocompatible corneal nanoscaffolds and biomimetic retinal nanoscaffolds.
Non-viral gene nanocarriers, including LPD complexes, polyplexes, mesoporous nanoparticles, organic-inorganic hybrid nanocarriers, NanoScripts, self-assembled DNA nanostructures and magnetic nanoparticles, are emerging as promising tools for cell reprogramming for ocular tissue regeneration. They were utilized to deliver pluripotency genes including Oct3/OCT4, SOX2, KLF4 and c-Myc or OCT4, SOX2, Nanog and Lin14 that reprogram specialized cells into iPSCs. They have also been used to deliver genes that initiate or enhance differentiation of ESCs and iPSCs into specialized ocular cells including RPE cells and photoreceptors. In addition, nanocarriers have also been investigated as delivery devices for delivering specific genes including p53 gene, Otx2, Crx and Nrl expressing genes, or CHX10VP16, OTX2, and CRX to non-neuronal retina cells, mainly Müller glia cells and stem cells located in the ciliary margin of the eye, and reprograming them into photoreceptors and retinal ganglion cells. If this approach is successful, the impact will be significant, because it will allow utilization of intrinsic cells for retinal tissue regeneration and stop the progression of some hereditary ocular degenerative diseases. Therefore, continuous efforts should be devoted to optimize nanotechnology to reprogram somatic cells into iPSCs and stem cells that can be differentiated into specialized ocular cell lineages for successful ocular tissue regeneration.
During the past 10 years, nanotechnology has been used to control release of cytokines and immunosuppressants to prevent corneal tissue rejection and increase corneal graft survival rate. In the future, deeper studies of the effects of the size, shape, surface feature and elastic property of nanoparticles on the phagocytic clearance of the nanoparticles in the eye are expected. Combination of nanotechnology and immunoengineering to modulate both innate and adaptive immune systems to promote a pro-regenerative microenvironment at the defect/injury site will be critical for ocular healing and regeneration.
Successful ocular tissue engineering and regeneration research and application needs an appropriate method to track the proliferation and migration of transplanted cells in vivo. Different nanoparticles such as magnetic and gold nanoparticles and quantum dots have been used as cell-tagging agents for repeated or continuous tracking of cells in vivo in combination with magnetic resonance imaging (MRI), photoacoustic imaging, and optical imaging, respectively [214, 215]. In this process, nanomaterials that can be imaged are first incorporated into stem cells by endocytosis. Afterwards, the nanomaterials-containing stem cells are implanted at the target site and their migration and differentiation are monitored using the corresponding scanning device. However, the studies of nanomaterials as tagging agents for tracking cells in the eye are scarce and limited to the toxicity of superparamagnetic iron oxides and gold nanoparticles (SPIONs) to rabbit corneal endothelial cells [216] and MSCs [217–220], respectively, and tracking MSCs in the subretinal layer of the rat’s eye [218]. More development of nanotechnology for live cell imaging and tracking for disease diagnosis and tissue regeneration in the eye is expected in the future.
Personalized medicine includes tailoring of disease treatment to the individual person based on that person’s genetics or severity of disease to achieve better patient outcomes and is an emerging and growing field within ophthalmology [221, 222]. The use of nanotechnology can allow for personalization of therapeutics and optimization of drug dosages for a more precise and efficient treatment of ophthalmic diseases. To date, most studies have focused on the use of nanotechnology in the sustained release of drugs to treat ophthalmic conditions in a personalized manner [223]. In the future, nanotechnology is expected to be applied to personalize regenerative medicine using human stem cells from the same patient and delivering therapeutics to maintain a healthy environment for stem cells to grow and mature in the location of disease, such as using plasma rich in growth factors from the same patient [224].
In conclusion, nanotechnology has been revolutionizing tissue regeneration to restore function of diseased, damaged and aged tissues in the eye. However, the research in nanotechnology for regenerative ophthalmology is still at its early stage, and there are very limited number of in vivo experiments and to date, based on reports on clinicaltrial.gov, no clinical trial has been reported in this area. More preclinical animal studies including toxicology, pharmacokinetics, pharmacodynamics and bioeffect studies and appropriately designed clinical trials are expected to be conducted in the future. Before the clinical trials will be conducted, the physical, chemical, biological, and microbiological stability of nanomaterials in conjunction with stem cell therapy should be evaluated according to regulatory guidelines to ensure the safety, efficacy, and quality of the products. Cost-effective technology needs to be developed to scale up sterilized nanomaterial production for clinical translation. The ultimate goal is that nanotechnology-enabled regenerative therapy products will be developed to repair damaged ocular tissues based on individual patient’s conditions for personalized regenerative ophthalmology to improve, preserve and restore vision.
Acknowledgements
This work was financially supported by NIH R01EY023853.
Abbreviations:
- FDA
Food and Drug Administration
- AMD
age-related macular degeneration
- RPE
retinal pigment epithelial cell
- VEGF
vascular endothelial growth factor
- ESC
embryonic stem cell
- MSC
mesenchymal stem cell
- iPSC
induced pluripotent stem cell
- LSC
limbal stem cells
- KLF4
kruppel-like factor 4
- c-Myc
cellular myelocytomatosis oncogene
- OCT4
octamer-binding transcription factor 4
- SOX2
sex determining region Y-box 2
- hESC
human epithelial stem cell
- AFM
atomic force microscopy
- IOP
intra-ocular pressure
- DR
diabetic retinopathy
- RGC
retinal ganglion cell
- ZO-1
zona occludin-1
- PLGA
poly(lactic-co-glycolic acid)
- PLA
poly(L-lactic acid)
- PCL
poly caprolactone
- PGS
poly(glycerol sebacate)
- NGF
nerve growth factor
- BDGF
brain-derived growth factor
- TEER
transepithelial electrical resistance
- ARPE
adult retinal pigment epithelial cell
- SAPNS
self-assembled peptide nanofiber scaffolds
- HCECs
human corneal epithelial cell
- HCKs
human corneal keratocyte
- PA
peptide amphiphile
- α-SMA
α-smooth muscle actin
- SF:P(LLA-CL)
silk fibroin-poly(L-lactic acid-co-ε-caprolactone)
- SLC4A4
solute carrier family-4 member-4
- HMSC
human mesenchymal stem cell
- IRBP
interphotoreceptor retinoid-binding protein
- EDC
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide
- LPD
liposome-protamine-DNA complex
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- ERG
Electroretinography
- NLS
nuclear localization signaling peptide
- TAT
transactivator of transcription
- HIV
human immunodeficiency virus
- PEG
polyethylene glycol
- MHC
major histocompatibility complex
- CCR6
C-C chemokine receptor type 6
- IL-17
interleukin 17
- CCL20
chemokine (C-C motif) ligand 20
- RORγt
retinoic acid receptor-related orphan nuclear receptor gamma
- IL-23R
interleukin 23 receptor
- CXCL1
chemokine (C-X-C motif) ligand 1
- NF-Κb
nuclear factor kappa-light-chain-enhancer of activated B cells
- MMP
Matrix metalloproteinases
- SD
Sprague–Dawley
- CsA
cyclosporine A
- MRI
magnetic resonance imaging
- SPION
superparamagnetic iron oxide nanoparticle
References
- [1].Vacanti CA, The history of tissue engineering, Journal of Cellular and Molecular Medicine, 10 (2006) 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Daftarian N, Kiani S, Zahabi A, Regenerative therapy for retinal disorders, Journal of ophthalmic & vision research, 5 (2010) 250–264. [PMC free article] [PubMed] [Google Scholar]
- [3].Polykandriotis E, Popescu LM, Horch RE, Regenerative medicine: then and now--an update of recent history into future possibilities, Journal of cellular and molecular medicine, 14 (2010) 2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Niloy KK, Gulfam M, Compton K, Li D, Huang GTJ, Lowe TL, Methacrylated Hyaluronan Based Hydrogel Scaffolds with Tunable Mechanical and Biodegradable Properties Maintain Pluripotency Stemness in Human Dental Pulp Stem Cells, Regenerative Engineering and Translational Medicine, First Online: 10 July (2019). [Google Scholar]
- [5].Starzl TE, The early days of transplantation, JAMA, 272 (1994) 1705–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hunziker R, NIH FACT SHEET - Regenerative Medicine, National Institutes of Health, 2010, pp. 2. [Google Scholar]
- [7].Hoffman T, Khademhosseini A, Langer R, Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering, Tissue engineering. Part A, 25 (2019) 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marx V, Organs from the lab, Nature, 522 (2015) 373. [DOI] [PubMed] [Google Scholar]
- [9].Mao AS, Mooney DJ, Regenerative medicine: Current therapies and future directions, Proceedings of the National Academy of Sciences of the United States of America, 112 (2015) 14452–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pashneh-Tala S, MacNeil S, Claeyssens F, The Tissue-Engineered Vascular Graft-Past, Present, and Future, Tissue engineering. Part B, Reviews, 22 (2015) 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hunziker R, FACT SHEET - Regenerative Medicine, National Institutes of Health, 2010, pp. 2. [Google Scholar]
- [12].Ramaesh K, Stone N, Dhillon B, Therapeutic Strategies in Ocular Tissue Regeneration: The Role of Stem Cells, in: Santin M (Ed.) Strategies in Regenerative Medicine: Integrating Biology with Materials Design, Springer New York, New York, NY, 2009, pp. 1–25. [Google Scholar]
- [13].Karl MO, Reh TA, Regenerative medicine for retinal diseases: activating the endogenous repair mechanisms, Trends in molecular medicine, 16 (2010) 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Misra GR, Gardner TW, Lowe TL, Hydrogels for Ocular Posterior Segment Drug Delivery, in: Kompella UB, Edelhauser HF (Eds.) Drug Product Development for the Back of the Eye 2011, pp. 291–304. [Google Scholar]
- [15].Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF, Drug and gene delivery to the back of the eye: from bench to bedside, Investigative ophthalmology & visual science, 55 (2014) 2714–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ovando-Roche P, Georgiadis A, Smith AJ, Pearson RA, Ali RR, Harnessing the Potential of Human Pluripotent Stem Cells and Gene Editing for the Treatment of Retinal Degeneration, Current Stem Cell Reports, 3 (2017) 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS, Long-term effects of ranibizumab on diabetic retinopathy severity and progression, Archives of Ophthalmology, 130 (2012) 1145–1152. [DOI] [PubMed] [Google Scholar]
- [18].Ho CPS, Lai TYY, Current management strategy of polypoidal choroidal vasculopathy, Indian Journal of Ophthalmology, 66 (2018) 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sarao V, Veritti D, Boscia F, Lanzetta P, Intravitreal Steroids for the Treatment of Retinal Diseases, The Scientific World Journal, 2014 (2014) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gordon K, Del Medico A, Sander I, Kumar A, Hamad B, Gene therapies in ophthalmic disease, Nature reviews. Drug discovery, 18 (2019) 415–416. [DOI] [PubMed] [Google Scholar]
- [21].Ellis-Behnke R, Jonas JB, Redefining tissue engineering for nanomedicine in ophthalmology, Acta ophthalmologica, 89 (2011) e108–114. [DOI] [PubMed] [Google Scholar]
- [22].Zarbin MA, Montemagno C, Leary JF, Ritch R, Regenerative nanomedicine and the treatment of degenerative retinal diseases, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 4 (2012) 113–137. [DOI] [PubMed] [Google Scholar]
- [23].Mclaughlin S, Podrebarac J, Ruel M, Suuronen EJ, McNeill B, Alarcon EI, Nano-Engineered Biomaterials for Tissue Regeneration: What Has Been Achieved So Far?, Frontiers in Materials, 3 (2016). [Google Scholar]
- [24].Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL, Ali RR, Young M, Xie Y, Temple S, Regenerating Eye Tissues to Preserve and Restore Vision, Cell stem cell, 22 (2018) 834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nicoară SD, Șușman S, Tudoran O, Bărbos O, Cherecheș G, Aștilean S, Potara M, Sorițău O, Novel Strategies for the Improvement of Stem Cells’ Transplantation in Degenerative Retinal Diseases, Stem Cells International, 2016 (2016) 1236721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Y, Wang J, Luo Y, Chen S, Lewallen M, Xie T, Stem Cells and Ocular Tissue Regeneration, Asia-Pacific journal of ophthalmology (Philadelphia, Pa.), 2 (2013) 111–118. [DOI] [PubMed] [Google Scholar]
- [27].Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY, Limbal stem cells: identity, developmental origin, and therapeutic potential, Wiley Interdiscip. Rev. Dev. Biol, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu WH, Chang YL, Lo WL, Li HY, Hsiao CW, Peng CH, Chiou SH, Ma HI, Chen SJ, Human induced pluripotent stem cell and nanotechnology-based therapeutics, Cell transplantation, 24 (2015) 2185–2195. [DOI] [PubMed] [Google Scholar]
- [29].Long J, Kim H, Kim D, Lee JB, Kim D-H, A biomaterial approach to cell reprogramming and differentiation, Journal of materials chemistry. B, Materials for biology and medicine, 5 (2017) 2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Z, Ruan J, Cui D, Advances and prospect of nanotechnology in stem cells, Nanoscale Res Lett, 4 (2009) 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jing Y, Moore LR, Williams PS, Chalmers JJ, Farag SS, Bolwell B, Zborowski M, Blood progenitor cell separation from clinical leukapheresis product by magnetic nanoparticle binding and magnetophoresis, Biotechnology and Bioengineering, 96 (2007) 1139–1154. [DOI] [PubMed] [Google Scholar]
- [32].Zhou X, Yuan L, Wu C, Cheng c., Luo G, Deng J, Mao Z, Recent review of the effect of nanomaterials on stem cells, RSC Advances, 8 (2018) 17656–17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sahle FF, Gulfam M, Lowe TL, Design strategies for physical-stimuli responsive programmable nanotherapeutics, Drug Discovery Today, 23 (2018) 992–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zarbin MA, Montemagno C, Leary JF, Ritch R, Nanotechnology in ophthalmology, Canadian Journal of Ophthalmology / Journal Canadien d’Ophtalmologie, 45 (2010) 457–476. [DOI] [PubMed] [Google Scholar]
- [35].Jafari S, Ahmadian E, Fard JK, Yari Khosroushahi A, Biomacromolecule based nanoscaffolds for cell therapy, Journal of Drug Delivery Science and Technology, 37 (2017) 61–66. [Google Scholar]
- [36].Vasita R, Katti DS, Nanofibers and their applications in tissue engineering, International Journal of Nanomedicine, 1 (2006) 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu T, Li Y, Chen T, Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering, International Journal of Nanomedicine, 8 (2013) 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li W-J, Tuan RS, Fabrication and application of nanofibrous scaffolds in tissue engineering, Current protocols in cell biology, Chapter 25 (2009) Unit-25.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sarkar K, Gómez C, Zambrano S, Ramirez M, de Hoyos E, Vasquez H, Lozano K, Electrospinning to ForcespinningTM, 2010.
- [40].Zhang S, Discovery and design of self-assembling peptides, Interface focus, 7 (2017) 20170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Qiu F, Chen Y, Tang C, Zhao X, Amphiphilic peptides as novel nanomaterials: design, self-assembly and application, Int J Nanomedicine, 13 (2018) 5003–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Farkas E, Ryadnov M, Brunsveld L, Crain J, Heddle J, Amino Acids, Peptides and Proteins, Royal Society of Chemistry 2012. [Google Scholar]
- [43].Muhammad R, Peh GSL, Adnan K, Law JBK, Mehta JS, Yim EKF, Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells, Acta Biomaterialia, 19 (2015) 138–148. [DOI] [PubMed] [Google Scholar]
- [44].Teo BKK, Goh KJ, Ng ZJ, Koo S, Yim EKF, Functional reconstruction of corneal endothelium using nanotopography for tissue-engineering applications, Acta Biomaterialia, 8 (2012) 2941–2952. [DOI] [PubMed] [Google Scholar]
- [45].Smith IO, Liu XH, Smith LA, Ma PX, Nano-structured polymer scaffolds for tissue engineering and regenerative medicine, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 1 (2009) 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yi H, Ur Rehman F, Zhao C, Liu B, He N, Recent advances in nano scaffolds for bone repair, Bone Research, 4 (2016) 16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Koss KM, Unsworth LD, Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides, Acta Biomaterialia, 44 (2016) 2–15. [DOI] [PubMed] [Google Scholar]
- [48].Kamaleddin MA, Nano-ophthalmology: Applications and considerations, Nanomedicine, 13 (2017) 1459–1472. [DOI] [PubMed] [Google Scholar]
- [49].Karamichos D, Ocular Tissue Engineering: Current and Future Directions, Journal of Functional Biomaterials, 6 (2015) 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oh B, Lee CH, Nanofiber for cardiovascular tissue engineering, Expert opinion on drug delivery, 10 (2013) 1565–1582. [DOI] [PubMed] [Google Scholar]
- [51].Chieruzzi M, Pagano S, Moretti S, Pinna R, Milia E, Torre L, Eramo S, Nanomaterials for Tissue Engineering In Dentistry, Nanomaterials, 6 (2016) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].He X, Fu W, Feng B, Wang H, Liu Z, Yin M, Wang W, Zheng J, Electrospun collagen–poly(L-lactic acid-co-ε-caprolactone) membranes for cartilage tissue engineering, Regenerative Medicine, 8 (2013) 425–436. [DOI] [PubMed] [Google Scholar]
- [53].Janagam DR, Wu LF, Lowe TL, Nanoparticles for drug delivery to the anterior segment of the eye, Advanced Drug Delivery Reviews, 122 (2017) 31–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu H, Shabala L, Shabala S, Giraldo JP, Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention, Environmental Science: Nano, 5 (2018) 1567–1583. [Google Scholar]
- [55].Mitra RN, Merwin MJ, Han Z, Conley SM, Al-Ubaidi MR, Naash MI, Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration, Free Radic Biol Med, 75 (2014) 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rajala A, Wang Y, Zhu Y, Ranjo-Bishop M, Ma J-X, Mao C, Rajala RVS, Nanoparticle-Assisted Targeted Delivery of Eye-Specific Genes to Eyes Significantly Improves the Vision of Blind Mice In Vivo, Nano Letters, 14 (2014) 5257–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McLaughlin S, Podrebarac J, Ruel M, Suuronen EJ, McNeill B, Alarcon EI, Nano-Engineered Biomaterials for Tissue Regeneration: What Has Been Achieved So Far?, Frontiers in Materials, 3 (2016). [Google Scholar]
- [58].Zhang J-X, Wang N-L, Lu Q-J, Development of gene and stem cell therapy for ocular neurodegeneration, International Journal of Ophthalmology, 8 (2015) 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Khristov V, Jha BS, Rising A, Li Y, Qian H, Maminishkis A, Amaral J, Campos M, Bharti K, Induced Pluripotent Stem Cell-Derived Autologous Cell Therapy for Age-Related Macular Degeneration, in: Schwartz SD, Nagiel A, Lanza R (Eds.) Cellular Therapies for Retinal Disease: A Strategic Approach, Springer International Publishing, Cham, 2017, pp. 33–44. [Google Scholar]
- [60].Lamba DA, Karl MO, Reh TA, Strategies for retinal repair: cell replacement and regeneration, in: Verhaagen J, Hol EM, Huitenga I, Wijnholds J, Bergen AB, Boer GJ, Swaab DF (Eds.) Progress in Brain Research, Elsevier; 2009, pp. 23–31. [DOI] [PubMed] [Google Scholar]
- [61].Gao H, Zhang HL, Shou J, Chen L, Shen Y, Tang Q, Huang J, Zhu J, Towards retinal ganglion cell regeneration, Regenerative Medicine, 7 (2012) 865–875. [DOI] [PubMed] [Google Scholar]
- [62].Gregory KG Hageman S, Johnson Lincoln V., and Anderson Don, Age-Related Macular Degeneration (AMD), University of Utah Health Sciences Center; Copyright: (c) 2019 Webvision., Salt Lake City (UT), 1995. [PubMed] [Google Scholar]
- [63].Daliri K, Ljubimov AV, Hekmatimoghaddam S, Glaucoma, Stem Cells, and Gene Therapy: Where Are We Now?, International Journal of Stem Cells, 10 (2017) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hammes HP, Federoff HJ, Brownlee M, Nerve growth-factor prevents both neuroretinal programmed cell-death and capillary pathology in experimental diabetes, Molecular Medicine, 1 (1995) 527–534. [PMC free article] [PubMed] [Google Scholar]
- [65].Mertsch K, Hanisch UK, Kettenmann H, Schnitzer J, Characterization of microglial cells and their response to stimulation in an organotypic retinal culture system, Journal of Comparative Neurology, 431 (2001) 217–227. [PubMed] [Google Scholar]
- [66].Zeng HY, Green WR, Tso MOM, Microglial activation in human diabetic retinopathy, Archives of Ophthalmology, 126 (2008) 227–232. [DOI] [PubMed] [Google Scholar]
- [67].Poduslo JF, Curran GL, Berg CT, Macromolecular permeability across the blood-nerve and blood-brain barriers, Proceedings of the National Academy of Sciences of the United States of America, 91 (1994) 5705–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barber AJ, Antonetti DA, Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats, Investigative ophthalmology & visual science, 44 (2003) 5410–5416. [DOI] [PubMed] [Google Scholar]
- [69].Misra GP, Singh RSJ, Aleman TS, Jacobson SG, Gardner TW, Lowe TL, Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina, Biomaterials, 30 (2009) 6541–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Imai H, Misra GP, Wu LF, Janagam DR, Gardner TW, Lowe TL, Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic Rats, Investigative ophthalmology & visual science, 56 (2015) 7839–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW, Diabetic retinopathy: More than meets the eye, Surv. Ophthalmol, 47 (2002) S253–S262. [DOI] [PubMed] [Google Scholar]
- [72].Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, Grp JDRC, Diabetic retinopathy - Seeing beyond glucose-induced microvascular disease, Diabetes, 55 (2006) 2401–2411. [DOI] [PubMed] [Google Scholar]
- [73].Bearse MA, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S, A multifocal electroretinogram model predicting the development of diabetic retinopathy, Progress in Retinal and Eye Research, 25 (2006) 425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Barber AJ, Gardner TW, Abcouwer SF, The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy, Investigative ophthalmology & visual science, 52 (2011) 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD, Pharmacokinetics of Pars Plana Intravitreal Injections versus Microcannula Suprachoroidal Injections of Bevacizumab in a Porcine Model, Investigative ophthalmology & visual science, 52 (2011) 4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hernandez C, Simo R, Neuroprotection in Diabetic Retinopathy, Current Diabetes Reports, 12 (2012) 329–337. [DOI] [PubMed] [Google Scholar]
- [77].Whitmire W, Al-Gayyar MMH, Abdelsaid M, Yousufzai BK, El-Remessy AB, Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far, Molecular Vision, 17 (2011) 300–308. [PMC free article] [PubMed] [Google Scholar]
- [78].Mizutani M, Kern TS, Lorenzi M, Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy, Journal of Clinical Investigation, 97 (1996) 2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content - Vascular endothelial growth factor decreases occludin in retinal endothelial cells, Diabetes, 47 (1998) 1953–1959. [DOI] [PubMed] [Google Scholar]
- [80].Barber AJ, Antonetti DA, Gardner TW, Penn G State Retina Res, Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes, Investigative ophthalmology & visual science, 41 (2000) 3561–3568. [PubMed] [Google Scholar]
- [81].Caputo S, Dileo MAS, Falsini B, Ghirlanda G, Porciatti V, Minella A, Greco AV, Evidence for early impairment of macular function with pattern ERG in type-I diabetic-patients, Diabetes Care, 13 (1990) 412–418. [DOI] [PubMed] [Google Scholar]
- [82].Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW, Neural apoptosis in the retina during experimental and human diabetes - Early onset and effect of insulin, Journal of Clinical Investigation, 102 (1998) 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gastinger MJ, Barber AJ, Khin SA, McRill CS, Gardner TW, Marshak DW, Abnormal centrifugal axons in streptozotocin-diabetic rat retinas, Investigative ophthalmology & visual science, 42 (2001) 2679–2685. [PMC free article] [PubMed] [Google Scholar]
- [84].Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ, Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2(Akita) diabetic mice, Investigative ophthalmology & visual science, 49 (2008) 2635–2642. [DOI] [PubMed] [Google Scholar]
- [85].VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ, Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina, European Journal of Neuroscience, 28 (2008) 1–11. [DOI] [PubMed] [Google Scholar]
- [86].Pieramici DJ, Rabena MD, Anti-VEGF therapy: comparison of current and future agents, Eye (London, England), 22 (2008) 1330–1336. [DOI] [PubMed] [Google Scholar]
- [87].Borah R, Ingavle GC, Sandeman SR, Kumar A, Mikhalovsky SV, Amine-Functionalized Electrically Conductive Core–Sheath MEH-PPV:PCL Electrospun Nanofibers for Enhanced Cell–Biomaterial Interactions, ACS Biomaterials Science & Engineering, 4 (2018) 3327–3346. [DOI] [PubMed] [Google Scholar]
- [88].Chang YC, Chen MH, Liao SY, Wu HC, Kuan CH, Sun JS, Wang TW, Multichanneled Nerve Guidance Conduit with Spatial Gradients of Neurotrophic Factors and Oriented Nanotopography for Repairing the Peripheral Nervous System, ACS applied materials & interfaces, 9 (2017) 37623–37636. [DOI] [PubMed] [Google Scholar]
- [89].Pritchard CD, Arner KM, Neal RA, Neeley WL, Bojo P, Bachelder E, Holz J, Watson N, Botchwey EA, Langer RS, Ghosh FK, The use of surface modified poly(glycerol-co-sebacic acid) in retinal transplantation, Biomaterials, 31 (2010) 2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang T-C, Chuang J-H, Buddhakosai W, Wu W-J, Lee C-J, Chen W-S, Yang Y-P, Li M-C, Peng C-H, Chen S-J, Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold, International Journal of Molecular Sciences, 18 (2017) 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Warnke PH, Alamein M, Skabo S, Stephens S, Bourke R, Heiner P, Liu Q, Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers, Acta Biomater, 9 (2013) 9414–9422. [DOI] [PubMed] [Google Scholar]
- [92].Xiang P, Wu KC, Zhu Y, Xiang L, Li C, Chen DL, Chen F, Xu G, Wang A, Li M, Jin ZB, A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells, Biomaterials, 35 (2014) 9777–9788. [DOI] [PubMed] [Google Scholar]
- [93].Thieltges F, Stanzel BV, Liu Z, Holz FG, A nanofibrillar surface promotes superior growth characteristics in cultured human retinal pigment epithelium, Ophthalmic research, 46 (2011) 133–140. [DOI] [PubMed] [Google Scholar]
- [94].Noorani B, Tabandeh F, Yazdian F, Soheili Z-S, Shakibaie M, Rahmani S, Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells, International Journal of Polymeric Materials and Polymeric Biomaterials, 67 (2018) 754–763. [Google Scholar]
- [95].Hotaling NA, Khristov V, Wan Q, Sharma R, Jha BS, Lotfi M, Maminishkis A, Simon CG, Bharti K, Nanofiber Scaffold-Based Tissue-Engineered Retinal Pigment Epithelium to Treat Degenerative Eye Diseases, Journal of Ocular Pharmacology and Therapeutics, 32 (2016) 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Popelka S, Studenovska H, Abelova L, Ardan T, Studeny P, Stranak Z, Klima J, Dvorankova B, Kotek J, Hodan J, Rypacek F, A frame-supported ultrathin electrospun polymer membrane for transplantation of retinal pigment epithelial cells, Biomedical materials (Bristol, England), 10 (2015) 045022. [DOI] [PubMed] [Google Scholar]
- [97].Nadri S, Kazemi B, Eeslaminejad MB, Yazdani S, Soleimani M, High yield of cells committed to the photoreceptor-like cells from conjunctiva mesenchymal stem cells on nanofibrous scaffolds, Molecular Biology Reports, 40 (2013) 3883–3890. [DOI] [PubMed] [Google Scholar]
- [98].Rose JB, Pacelli S, Haj AJE, Dua HS, Hopkinson A, White LJ, Rose F, Gelatin-Based Materials in Ocular Tissue Engineering, Materials (Basel, Switzerland), 7 (2014) 3106–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sorkio AE, Vuorimaa-Laukkanen EP, Hakola HM, Liang H, Ujula TA, Valle-Delgado JJ, Österberg M, Yliperttula ML, Skottman H, Biomimetic collagen I and IV double layer Langmuir–Schaefer films as microenvironment for human pluripotent stem cell derived retinal pigment epithelial cells, Biomaterials, 51 (2015) 257–269. [DOI] [PubMed] [Google Scholar]
- [100].Hynes SR, Lavik EB, A tissue-engineered approach towards retinal repair: Scaffolds for cell transplantation to the subretinal space, Graefe’s Archive for Clinical and Experimental Ophthalmology, 248 (2010) 763–778. [DOI] [PubMed] [Google Scholar]
- [101].White CE, Olabisi RM, Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration, Journal of Tissue Engineering, 8 (2017) 2041731417720841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lu JT, Lee CJ, Bent SF, Fishman HA, Sabelman EE, Thin collagen film scaffolds for retinal epithelial cell culture, Biomaterials, 28 (2007) 1486–1494. [DOI] [PubMed] [Google Scholar]
- [103].Giordano GG, Thomson RC, Ishaug SL, Mikos AG, Cumber S, Garcia CA, Lahiri-Munir D, Retinal pigment epithelium cells cultured on synthetic biodegradable polymers, Journal of biomedical materials research, 34 (1997) 87–93. [DOI] [PubMed] [Google Scholar]
- [104].McHugh KJ, Tao SL, Saint-Geniez M, Porous poly(epsilon-caprolactone) scaffolds for retinal pigment epithelium transplantation, Investigative ophthalmology & visual science, 55 (2014) 1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ilmarinen T, Hiidenmaa H, Kööbi P, Nymark S, Sorkio A, Wang J-H, Stanzel BV, Thieltges F, Alajuuma P, Oksala O, Kataja M, Uusitalo H, Skottman H, Ultrathin Polyimide Membrane as Cell Carrier for Subretinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigment Epithelium, PLOS ONE, 10 (2015) e0143669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yang F, Murugan R, Wang S, Ramakrishna S, Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering, Biomaterials, 26 (2005) 2603–2610. [DOI] [PubMed] [Google Scholar]
- [107].Kador KE, Grogan SP, Dorthe EW, Venugopalan P, Malek MF, Goldberg JL, D’Lima DD, Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds, Tissue engineering. Part A, 22 (2016) 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Liu Z, Yu N, Holz FG, Yang F, Stanzel BV, Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography, Biomaterials, 35 (2014) 2837–2850. [DOI] [PubMed] [Google Scholar]
- [109].Shahmoradi S, Yazdian F, Tabandeh F, Soheili Z-S, Hatamian Zarami AS, Navaei-Nigjeh M, Controlled surface morphology and hydrophilicity of polycaprolactone toward human retinal pigment epithelium cells, Materials Science and Engineering: C, 73 (2017) 300–309. [DOI] [PubMed] [Google Scholar]
- [110].Anni S, P. JP, Kati J-U, B. JM, Heli S, G. AB, Surface Modified Biodegradable Electrospun Membranes as a Carrier for Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells, Tissue Engineering Part A, 21 (2015) 2301–2314. [DOI] [PubMed] [Google Scholar]
- [111].Ho D, Fitzgerald M, Bartlett CA, Zdyrko B, Luzinov IA, Dunlop SA, Swaminathan Iyer K, The effects of concentration-dependent morphology of self-assembling RADA16 nanoscaffolds on mixed retinal cultures, Nanoscale, 3 (2011) 907–910. [DOI] [PubMed] [Google Scholar]
- [112].Koss KM, Unsworth LD, Towards Developing Bioresponsive, Self-Assembled Peptide Materials: Dynamic Morphology and Fractal Nature of Nanostructured Matrices, Materials (Basel, Switzerland), 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ellis-Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, Schneider GE, Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision, Proc Natl Acad Sci U S A, 103 (2006) 5054–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Martin AD, Chua SW, Au CG, Stefen H, Przybyla M, Lin Y, Bertz J, Thordarson P, Fath T, Ke YD, Ittner LM, Peptide Nanofiber Substrates for Long-Term Culturing of Primary Neurons, ACS applied materials & interfaces, 10 (2018) 25127–25134. [DOI] [PubMed] [Google Scholar]
- [115].Nguyen PK, Sarkar B, Siddiqui Z, McGowan M, Iglesias-Montoro P, Rachapudi S, Kim S, Gao W, Lee EJ, Kumar VA, Self-Assembly of an Antiangiogenic Nanofibrous Peptide Hydrogel, ACS Applied Bio Materials, 1 (2018) 865–870. [DOI] [PubMed] [Google Scholar]
- [116].Moore AN, Lopez Silva TL, Carrejo NC, Origel Marmolejo CA, Li IC, Hartgerink JD, Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells, Biomaterials, 161 (2018) 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Griffith M, Polisetti N, Kuffova L, Gallar J, Forrester J, Vemuganti GK, Fuchsluger TA, Regenerative Approaches as Alternatives to Donor Allografting for Restoration of Corneal Function, The Ocular Surface, 10 (2012) 170–183. [DOI] [PubMed] [Google Scholar]
- [118].Obata H, Tsuru T, Corneal wound healing from the perspective of keratoplasty specimens with special reference to the function of the Bowman layer and Descemet membrane, Cornea, 26 (2007) S82–89. [DOI] [PubMed] [Google Scholar]
- [119].Sridhar MS, Anatomy of cornea and ocular surface, Indian journal of ophthalmology, 66 (2018) 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pillai RG, Stem cells for ocular tissue engineering and regeneration, Current topics in medicinal chemistry, 11 (2011) 1606–1620. [DOI] [PubMed] [Google Scholar]
- [121].Wilson SL, El Haj AJ, Yang Y, Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering, J Funct Biomater, 3 (2012) 642–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Meek KM, Knupp C, Corneal structure and transparency, Prog Retin Eye Res, 49 (2015) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Institute TNE, Facts About the Cornea and Corneal Disease, (2016).
- [124].Teichmann J, Valtink M, Nitschke M, Gramm S, Funk RH, Engelmann K, Werner C, Tissue engineering of the corneal endothelium: a review of carrier materials, J Funct Biomater, 4 (2013) 178–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wray LS, Orwin EJ, Recreating the microenvironment of the native cornea for tissue engineering applications, Tissue engineering. Part A, 15 (2009) 1463–1472. [DOI] [PubMed] [Google Scholar]
- [126].Kong B, Sun W, Chen G, Tang S, Li M, Shao Z, Mi S, Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat, Scientific Reports, 7 (2017) 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Akhshabi S, Biazar E, Singh V, Keshel SH, Geetha N, The effect of the carbodiimide cross-linker on the structural and biocompatibility properties of collagen-chondroitin sulfate electrospun mat, International journal of nanomedicine, 13 (2018) 4405–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Salehi S, Czugala M, Stafiej P, Fathi M, Bahners T, Gutmann JS, Singer BB, Fuchsluger TA, Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair, Acta Biomaterialia, 50 (2017) 370–380. [DOI] [PubMed] [Google Scholar]
- [129].Ghezzi CE, Rnjak-Kovacina J, Kaplan DL, Corneal Tissue Engineering: Recent Advances and Future Perspectives, Tissue Engineering. Part B, Reviews, 21 (2015) 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Liang Y, Liu W, Han B, Yang C, Ma Q, Zhao W, Rong M, Li H, Fabrication and characters of a corneal endothelial cells scaffold based on chitosan, Journal of materials science. Materials in medicine, 22 (2011) 175–183. [DOI] [PubMed] [Google Scholar]
- [131].Stafiej P, Kung F, Thieme D, Czugala M, Kruse FE, Schubert DW, Fuchsluger TA, Adhesion and metabolic activity of human corneal cells on PCL based nanofiber matrices, Materials science & engineering. C, Materials for biological applications, 71 (2017) 764–770. [DOI] [PubMed] [Google Scholar]
- [132].Parekh M, Ferrari S, Sheridan C, Kaye S, Ahmad S, Concise Review: An Update on the Culture of Human Corneal Endothelial Cells for Transplantation, Stem Cells Translational Medicine, 5 (2016) 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, Suuronen EJ, Liu Y, Brunette I, Griffith M, Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold, Biomaterials, 35 (2014) 2420–2427. [DOI] [PubMed] [Google Scholar]
- [134].Reichl S, Borrelli M, Geerling G, Keratin films for ocular surface reconstruction, Biomaterials, 32 (2011) 3375–3386. [DOI] [PubMed] [Google Scholar]
- [135].Cruz-Maya I, Guarino V, Almaguer-Flores A, Alvarez-Perez MA, Varesano A, Vineis C, Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization, Journal of biomedical materials research. Part A, (2019). [DOI] [PubMed] [Google Scholar]
- [136].Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M, Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium, The Lancet, 349 (1997) 990–993. [DOI] [PubMed] [Google Scholar]
- [137].Alaminos M, Del Carmen Sanchez-Quevedo M, Munoz-Avila JI, Serrano D, Medialdea S, Carreras I, Campos A, Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold, Investigative ophthalmology & visual science, 47 (2006) 3311–3317. [DOI] [PubMed] [Google Scholar]
- [138].Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Soderqvist M, Griffith M, A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study, Science translational medicine, 2 (2010) 46ra61. [DOI] [PubMed] [Google Scholar]
- [139].Hu X, Lui W, Cui L, Wang M, Cao Y, Tissue engineering of nearly transparent corneal stroma, Tissue engineering, 11 (2005) 1710–1717. [DOI] [PubMed] [Google Scholar]
- [140].Wu Z, Kong B, Liu R, Sun W, Mi S, Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold, Nanomaterials, 8 (2018) 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Baradaran-Rafii A, Biazar E, Heidari-keshel S, Cellular Response of Limbal Stem Cells on Polycaprolactone Nanofibrous Scaffolds for Ocular Epithelial Regeneration, Current eye research, 41 (2016) 326–333. [DOI] [PubMed] [Google Scholar]
- [142].Niu G, Choi J-S, Wang Z, Skardal A, Giegengack M, Soker S, Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation, Biomaterials, 35 (2014) 4005–4014. [DOI] [PubMed] [Google Scholar]
- [143].Torbet J, Malbouyres M, Builles N, Justin V, Roulet M, Damour O, Oldberg Å, Ruggiero F, Hulmes DJS, Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction, Biomaterials, 28 (2007) 4268–4276. [DOI] [PubMed] [Google Scholar]
- [144].Aslan B, Guler S, Tevlek A, Aydin HM, Evaluation of collagen foam, poly(l-lactic acid) nanofiber mesh, and decellularized matrices for corneal regeneration, Journal of biomedical materials research. Part B, Applied biomaterials, 106 (2018) 2157–2168. [DOI] [PubMed] [Google Scholar]
- [145].Chen J, Yan C, Zhu M, Yao Q, Shao C, Lu W, Wang J, Mo X, Gu P, Fu Y, Fan X, Electrospun nanofibrous SF/P(LLA-CL) membrane: a potential substratum for endothelial keratoplasty, International Journal of Nanomedicine, 10 (2015) 3337–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Phu D, Wray LS, Warren RV, Haskell RC, Orwin EJ, Effect of substrate composition and alignment on corneal cell phenotype, Tissue engineering. Part A, 17 (2011) 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kim JI, Kim JY, Park CH, Fabrication of transparent hemispherical 3D nanofibrous scaffolds with radially aligned patterns via a novel electrospinning method, Scientific Reports, 8 (2018) 3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Uzunalli G, Soran Z, Erkal TS, Dagdas YS, Dinc E, Hondur AM, Bilgihan K, Aydin B, Guler MO, Tekinay AB, Bioactive self-assembled peptide nanofibers for corneal stroma regeneration, Acta Biomater, 10 (2014) 1156–1166. [DOI] [PubMed] [Google Scholar]
- [149].Jones RR, Castelletto V, Connon CJ, Hamley IW, Collagen stimulating effect of peptide amphiphile C16-KTTKS on human fibroblasts, Molecular pharmaceutics, 10 (2013) 1063–1069. [DOI] [PubMed] [Google Scholar]
- [150].Walter MN, Dehsorkhi A, Hamley IW, Connon CJ, Supra-molecular assembly of a lumican-derived peptide amphiphile enhances its collagen-stimulating activity, Biomaterials science, 4 (2016) 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Gouveia RM, Koudouna E, Jester J, Figueiredo F, Connon CJ, Template Curvature Influences Cell Alignment to Create Improved Human Corneal Tissue Equivalents, Advanced Biosystems, 1 (2017) 1700135. [DOI] [PubMed] [Google Scholar]
- [152].Gouveia RM, Castelletto V, Alcock SG, Hamley IW, Connon CJ, Bioactive films produced from self-assembling peptide amphiphiles as versatile substrates for tuning cell adhesion and tissue architecture in serum-free conditions, Journal of Materials Chemistry B, 1 (2013) 6157–6169. [DOI] [PubMed] [Google Scholar]
- [153].Gouveia RM, González-Andrades E, Cardona JC, González-Gallardo C, Ionescu AM, Garzon I, Alaminos M, González-Andrades M, Connon CJ, Controlling the 3D architecture of Self-Lifting Auto-generated Tissue Equivalents (SLATEs) for optimized corneal graft composition and stability, Biomaterials, 121 (2017) 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gouveia RM, Castelletto V, Hamley IW, Connon CJ, New Self-Assembling Multifunctional Templates for the Biofabrication and Controlled Self-Release of Cultured Tissue, Tissue engineering. Part A, 21 (2015) 1772–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].da Silva ER, Walter MN, Reza M, Castelletto V, Ruokolainen J, Connon CJ, Alves WA, Hamley IW, Self-Assembled Arginine-Capped Peptide Bolaamphiphile Nanosheets for Cell Culture and Controlled Wettability Surfaces, Biomacromolecules, 16 (2015) 3180–3190. [DOI] [PubMed] [Google Scholar]
- [156].Schmidt N, Mishra A, Lai GH, Wong GC, Arginine-rich cell-penetrating peptides, FEBS letters, 584 (2010) 1806–1813. [DOI] [PubMed] [Google Scholar]
- [157].Rizwan M, Peh GSL, Ang HP, Lwin NC, Adnan K, Mehta JS, Tan WS, Yim EKF, Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications, Biomaterials, 120 (2017) 139–154. [DOI] [PubMed] [Google Scholar]
- [158].Yanez-Soto B, Liliensiek SJ, Gasiorowski JZ, Murphy CJ, Nealey PF, The influence of substrate topography on the migration of corneal epithelial wound borders, Biomaterials, 34 (2013) 9244–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Koo S, Muhammad R, Peh GSL, Mehta JS, Yim EKF, Micro- and nanotopography with extracellular matrix coating modulate human corneal endothelial cell behavior, Acta Biomaterialia, 10 (2014) 1975–1984. [DOI] [PubMed] [Google Scholar]
- [160].Lee JO, Park H, Du J, Balakrishna A, Chen O, Sretavan D, Choo H, A microscale optical implant for continuous in vivo monitoring of intraocular pressure, Microsystems &Amp; Nanoengineering, 3 (2017) 17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RAJ, Xu Y, Chung C, Zhang Y, Lin D, Patel S, Wu F, Cai H, Hou J, Wen C, Jafari M, Liu X, Luo L, Zhu J, Qiu A, Hou R, Chen B, Chen J, Granet D, Heichel C, Shang F, Li X, Krawczyk M, Skowronska-Krawczyk D, Wang Y, Shi W, Chen D, Zhong Z, Zhong S, Zhang L, Chen S, Morrison SJ, Maas RL, Zhang K, Liu Y, Lens regeneration using endogenous stem cells with gain of visual function, Nature, 531 (2016) 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millán JL, Tsonis PA, Regenerative capacity in newts is not altered by repeated regeneration and ageing, Nature Communications, 2 (2011) 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Nibourg LM, Gelens E, de Jong MR, Kuijer R, van Kooten TG, Koopmans SA, Nanofiber-based hydrogels with extracellular matrix-based synthetic peptides for the prevention of capsular opacification, Experimental Eye Research, 143 (2016) 60–67. [DOI] [PubMed] [Google Scholar]
- [164].Xi Y, Dong H, Sun K, Liu H, Liu R, Qin Y, Hu Z, Zhao Y, Nie F, Wang S, Scab-Inspired Cytophilic Membrane of Anisotropic Nanofibers for Rapid Wound Healing, ACS applied materials & interfaces, 5 (2013) 4821–4826. [DOI] [PubMed] [Google Scholar]
- [165].Kutsuzawa K, Chowdhury EH, Nagaoka M, Maruyama K, Akiyama Y, Akaike T, Surface functionalization of inorganic nano-crystals with fibronectin and E-cadherin chimera synergistically accelerates trans-gene delivery into embryonic stem cells, Biochemical and Biophysical Research Communications, 350 (2006) 514–520. [DOI] [PubMed] [Google Scholar]
- [166].Patel S, Chueng STD, Yin PT, Dardir K, Song Z, Pasquale N, Kwan K, Sugiyama H, Lee KB, Induction of Stem-Cell-Derived Functional Neurons by NanoScript-Based Gene Repression, Angewandte Chemie - International Edition, 54 (2015) 11983–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Cai Y, Dai X, Zhang Q, Dai Z, Gene expression of OCT4, SOX2, KLF4 and MYC (OSKM) induced pluripotent stem cells: identification for potential mechanisms, Diagnostic Pathology, 10 (2015) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Pushp P, Kaur R, Lee HT, Gupta MK, Nanoparticles for Gene Delivery into Stem Cells and Embryos, Adv Polym Sci, 254 (2013) 51–85. [Google Scholar]
- [169].Chang J-H, Tsai P-H, Chen W, Chiou S-H, Mou C-Y, Dual delivery of siRNA and plasmid DNA using mesoporous silica nanoparticles to differentiate induced pluripotent stem cells into dopaminergic neurons, Journal of Materials Chemistry B, 5 (2017) 3012–3023. [DOI] [PubMed] [Google Scholar]
- [170].Gandra N, Wang D-D, Zhu Y, Mao C, Virus-Mimetic Cytoplasm-Cleavable Magnetic/Silica Nanoclusters for Enhanced Gene Delivery to Mesenchymal Stem Cells, Angewandte Chemie International Edition, 52 (2013) 11278–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zhao JJ, Ouyang H, Luo J, Patel S, Xue Y, Quach J, Sfeir N, Zhang M, Fu X, Ding S, Chen S, Zhang K, Induction of retinal progenitors and neurons from mammalian Muller glia under defined conditions, The Journal of biological chemistry, 289 (2014) 11945–11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yao K, Qiu S, Tian L, Snider WD, Flannery JG, Schaffer DV, Chen B, Wnt regulates proliferation and neurogenic potential of Müller glial cells through a Lin28/let-7 miRNA-dependent pathway in adult mammalian retina, Cell reports, 17 (2016) 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B, Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas, Nature, 560 (2018) 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Inoue T, Coles BLK, Dorval K, Bremner R, Bessho Y, Kageyama R, Hino S, Matsuoka M, Craft CM, McInnes RR, Tremblay F, Prusky GT, van der Kooy D, Maximizing Functional Photoreceptor Differentiation From Adult Human Retinal Stem Cells, Stem cells (Dayton, Ohio), 28 (2010) 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Koirala A, Conley SM, Makkia R, Liu Z, Cooper MJ, Sparrow JR, Naash MI, Persistence of non-viral vector mediated RPE65 expression: case for viability as a gene transfer therapy for RPE-based diseases, Journal of controlled release : official journal of the Controlled Release Society, 172 (2013) 10.1016/j.jconrel.2013.1008.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Tan YSE, Shi PJ, Choo CJ, Laude A, Yeong WY, Tissue engineering of retina and Bruch’s membrane: a review of cells, materials and processes, Br. J. Ophthalmol, 102 (2018) 1182–1187. [DOI] [PubMed] [Google Scholar]
- [177].Sahle FF, Lowe TL, Design Strategies for Programmable Oligonucleotide Nanotherapeutics, Drug Discovery Today, In Press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Selmeczi D, Hansen TS, Met O, Svane IM, Larsen NB, Efficient large volume electroporation of dendritic cells through micrometer scale manipulation of flow in a disposable polymer chip, Biomedical microdevices, 13 (2011) 383–392. [DOI] [PubMed] [Google Scholar]
- [179].Wang HY, Lu C, Microfluidic electroporation for delivery of small molecules and genes into cells using a common DC power supply, Biotechnol Bioeng, 100 (2008) 579–586. [DOI] [PubMed] [Google Scholar]
- [180].Lin TP, Microinjection of mouse eggs, Science (New York, N.Y.), 151 (1966) 333–337. [DOI] [PubMed] [Google Scholar]
- [181].Lu VB, Williams DJ, Won Y-J, Ikeda SR, Intranuclear microinjection of DNA into dissociated adult mammalian neurons, J Vis Exp, (2009) 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Cao B, Qiu P, Mao C, Mesoporous iron oxide nanoparticles prepared by polyacrylic acid etching and their application in gene delivery to mesenchymal stem cells, Microscopy research and technique, 76 (2013) 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Mitra RN, Zheng M, Han Z, Nanoparticle motivated gene delivery for ophthalmic application, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 8 (2016) 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Delgado D, del Pozo-Rodríguez A, Solinís MÁ, Rodríguez-Gascón A, Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: The importance of the entry pathway, European Journal of Pharmaceutics and Biopharmaceutics, 79 (2011) 495–502. [DOI] [PubMed] [Google Scholar]
- [185].Rakoczy EP, Narfström K, Gene therapy for eye as regenerative medicine? Lessons from RPE65 gene therapy for Leber’s Congenital Amaurosis, The International Journal of Biochemistry & Cell Biology, 56 (2014) 153–157. [DOI] [PubMed] [Google Scholar]
- [186].Li S, Huang L, In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes, Gene therapy, 4 (1997) 891–900. [DOI] [PubMed] [Google Scholar]
- [187].Wang Y, Rajala A, Rajala RVS, Lipid Nanoparticles for Ocular Gene Delivery, Journal of Functional Biomaterials, 6 (2015) 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Li S, Rizzo MA, Bhattacharya S, Huang L, Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery, Gene therapy, 5 (1998) 930–937. [DOI] [PubMed] [Google Scholar]
- [189].Ma K, Wang DD, Lin Y, Wang J, Petrenko V, Mao C, Synergetic Targeted Delivery of Sleeping-Beauty Transposon System to Mesenchymal Stem Cells Using LPD Nanoparticles Modified with a Phage-Displayed Targeting Peptide, Advanced functional materials, 23 (2013) 1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, Elisseeff JH, Design, clinical translation and immunological response of biomaterials in regenerative medicine, Nature Reviews Materials, 1 (2016) 16040. [Google Scholar]
- [191].Tiera MJ, Shi Q, Winnik FM, Fernandes JC, Polycation-based gene therapy: Current knowledge and new perspectives, Current Gene Therapy, 11 (2011) 288–306. [DOI] [PubMed] [Google Scholar]
- [192].Cardarelli F, Digiacomo L, Marchini C, Amici A, Salomone F, Fiume G, Rossetta A, Gratton E, Pozzi D, Caracciolo G, The intracellular trafficking mechanism of Lipofectamine-based transfection reagents and its implication for gene delivery, Scientific Reports, 6 (2016) 25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Patel S, Chueng S-TD, Yin PT, Dardir K, Song Z, Pasquale N, Kwan K, Sugiyama H, Lee K-B, Induction of Stem Cell-derived Functional Neurons via NanoScript-based Gene Repression, Angewandte Chemie (International ed. in English), 54 (2015) 11983–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ma W, Shao X, Zhao D, Li Q, Liu M, Zhou T, Xie X, Mao C, Zhang Y, Lin Y, Self-Assembled Tetrahedral DNA Nanostructures Promote Neural Stem Cell Proliferation and Neuronal Differentiation, ACS Applied Materials and Interfaces, 10 (2018) 7892–7900. [DOI] [PubMed] [Google Scholar]
- [195].Lee CH, Kim J-H, Lee HJ, Jeon K, Lim H, Choi H.y., Lee E-R, Park SH, Park J-Y, Hong S, Kim S, Cho S-G, The generation of iPS cells using non-viral magnetic nanoparticlebased transfection, Biomaterials, 32 (2011) 6683–6691. [DOI] [PubMed] [Google Scholar]
- [196].Ruan J, Shen J, Wang Z, Ji J, Song H, Wang K, Liu B, Li J, Cui D, Efficient preparation and labeling of human induced pluripotent stem cells by nanotechnology, International journal of nanomedicine, 6 (2011) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Dove A, Cell-based therapies go live, Nature biotechnology, 20 (2002) 339–343. [DOI] [PubMed] [Google Scholar]
- [198].Johnson HJ, Schonder KS, Solid-Organ Transplantation, in: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM (Eds.) Pharmacotherapy: A Pathophysiologic Approach, 10e, McGraw-Hill Education, New York, NY, 2017. [Google Scholar]
- [199].Chung L, Maestas DR Jr., Housseau F, Elisseeff JH, Key players in the immune response to biomaterial scaffolds for regenerative medicine, Adv Drug Deliv Rev, 114 (2017) 184–192. [DOI] [PubMed] [Google Scholar]
- [200].Li G, Yang P, Guo X, Huang N, Shen R, An in vitro evaluation of inflammation response of titanium functionalized with heparin/fibronectin complex, Cytokine, 56 (2011) 208–217. [DOI] [PubMed] [Google Scholar]
- [201].Zhang W, Magadi S, Li Z, Smith CW, Burns AR, IL-20 promotes epithelial healing of the injured mouse cornea, Exp Eye Res, 154 (2017) 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Gong N, Pleyer U, Vogt K, Anegon I, Flügel A, Volk H-D, Ritter T, Local Overexpression of Nerve Growth Factor in Rat Corneal Transplants Improves Allograft Survival, Investigative ophthalmology & visual science, 48 (2007) 1043–1052. [DOI] [PubMed] [Google Scholar]
- [203].Bi YL, Wu MF, Lu LX, Zhou Q, Du F, Sun XT, Tang SF, Xu GT, Functions of corneal endothelial cells do not change after uptake of superparamagnetic iron oxide nanoparticles, Molecular medicine reports, 7 (2013) 1767–1772. [DOI] [PubMed] [Google Scholar]
- [204].Yuan X-B, Yuan Y-B, Jiang W, Liu J, Tian E-J, Shun H-M, Huang D-H, Yuan X-Y, Li H, Sheng J, Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation, International Journal of Pharmaceutics, 349 (2008) 241–248. [DOI] [PubMed] [Google Scholar]
- [205].Shirali AC, Look M, Du W, Kassis E, Stout-Delgado HW, Fahmy TM, Goldstein DR, Nanoparticle Delivery of Mycophenolic Acid Upregulates PD-L1 on Dendritic Cells to Prolong Murine Allograft Survival, 11 (2011) 2582–2592. [DOI] [PubMed] [Google Scholar]
- [206].Li Q, Li Z, Zeng W, Ge S, Lu H, Wu C, Ge L, Liang D, Xu Y, Proniosome-derived niosomes for tacrolimus topical ocular delivery: in vitro cornea permeation, ocular irritation, and in vivo anti-allograft rejection, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 62 (2014) 115–123. [DOI] [PubMed] [Google Scholar]
- [207].Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R, Cyclosporine A delivery to the eye: A pharmaceutical challenge, European Journal of Pharmaceutics and Biopharmaceutics, 56 (2003) 307–318. [DOI] [PubMed] [Google Scholar]
- [208].Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang J-C, Tang L, Heflin T, Alwadani S, Eberhart CG, Stark WJ, Hanes J, Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats, Journal of Controlled Release, 201 (2015) 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Raveendran S, Rochani AK, Maekawa T, Kumar DS, Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers, Materials (Basel, Switzerland), 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Fischak C, Klaus R, Werkmeister RM, Hohenadl C, Prinz M, Schmetterer L, Garhöfer G, Effect of Topically Administered Chitosan-N-acetylcysteine on Corneal Wound Healing in a Rabbit Model, J Ophthalmol, 2017 (2017) 5192924–5192924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Wood EH, Tang PH, De la Huerta I, Korot E, Muscat S, Palanker DA, Williams GA, STEM CELL THERAPIES, GENE-BASED THERAPIES, OPTOGENETICS, AND RETINAL PROSTHETICS: Current State and Implications for the Future, Retina, 39 (2019) 820–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sivaraman KR, Jivrajka RV, Soin K, Bouchard CS, Movahedan A, Shorter E, Jain S, Jacobs DS, Djalilian AR, Superior Limbic Keratoconjunctivitis-like Inflammation in Patients with Chronic Graft-Versus-Host Disease, Ocul Surf, 14 (2016) 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Calkins DJ, Pekny M, Cooper ML, Benowitz L, The challenge of regenerative therapies for the optic nerve in glaucoma, Exp Eye Res, 157 (2017) 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA, Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells, Nature biotechnology, 19 (2001) 1141–1147. [DOI] [PubMed] [Google Scholar]
- [215].Accomasso L, Gallina C, Turinetto V, Giachino C, Stem Cell Tracking with Nanoparticles for Regenerative Medicine Purposes: An Overview, Stem cells international, 2016 (2016) 7920358–7920358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Zarbin MA, Arlow T, Ritch R, Regenerative nanomedicine for vision restoration, Mayo Clin Proc, 88 (2013) 1480–1490. [DOI] [PubMed] [Google Scholar]
- [217].E Connor E, Mwamuka J, Gole A, Murphy C, Wyatt M, Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity, 2005. [DOI] [PubMed]
- [218].Mok LP, Leow NS, Koh EA, Mohd Nizam HH, Ding LS, Luu C, Ruhaslizan R, Wong SH, Halim HW, Ng HM, Idrus BR, Chowdhury RS, Bastion MC, Subbiah KS, Higuchi A, Alarfaj AA, Then YK, Micro-Computed Tomography Detection of Gold Nanoparticle-Labelled Mesenchymal Stem Cells in the Rat Subretinal Layer, International Journal of Molecular Sciences, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Li J, Li JJ, Zhang J, Wang X, Kawazoe N, Chen G, Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells, Nanoscale, 8 (2016) 7992–8007. [DOI] [PubMed] [Google Scholar]
- [220].Ricles LM, Nam SY, Sokolov K, Emelianov SY, Suggs LJ, Function of mesenchymal stem cells following loading of gold nanotracers, Int J Nanomedicine, 6 (2011) 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Roybal CN, Velez G, Toral MA, Tsang SH, Bassuk AG, Mahajan VB, Personalized Proteomics in Proliferative Vitreoretinopathy Implicate Hematopoietic Cell Recruitment and mTOR as a Therapeutic Target, American journal of ophthalmology, 186 (2018) 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Velez G, Bassuk AG, Colgan D, Tsang SH, Mahajan VB, Therapeutic drug repositioning using personalized proteomics of liquid biopsies, JCI insight, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Ong FS, Kuo JZ, Wu WC, Cheng CY, Blackwell WL, Taylor BL, Grody WW, Rotter JI, Lai CC, Wong TY, Personalized Medicine in Ophthalmology: From Pharmacogenetic Biomarkers to Therapeutic and Dosage Optimization, Journal of personalized medicine, 3 (2013) 40–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Anitua E, Muruzabal F, de la Fuente M, Merayo J, Duran J, Orive G, Plasma Rich in Growth Factors for the Treatment of Ocular Surface Diseases, Current eye research, 41 (2016) 875–882. [DOI] [PubMed] [Google Scholar]


