Abstract
Diffuse leptomeningeal glioneuronal tumors (DLGNTs) are newly recognized as an entity in the 2016 revision of the WHO Classification of tumors of the central nervous system. They typically present as diffuse leptomeningeal infiltrates along the neuraxis with focal and superficial involvement of the parenchyma. Here, we report a DLGNT with unusual radiological and histological features. A 13-year-old girl presented with scoliosis and back pain. Magnetic resonance imaging demonstrated a syrinx from C2 to T11 and an intramedullary mass from T6 to T9–10. No leptomeningeal involvement was recognized. Histological examination of the gross total resection specimen revealed a low-grade neuroepithelial neoplasm predominantly infiltrating the spinal cord and only focally involving the leptomeninges. Chromosome microarray identified co-deletion of the short arm of chromosome 1 and the long arm of chromosome 19 as well as fusion of the KIAA1549 and BRAF genes. Nextgeneration sequencing demonstrated wild-type alleles at the mutational hotspots of IDH1 (R132) and IDH2 (R140 and R172). In contrast to most reported DLGNTs, the tumor described in this manuscript was characterized by a predominant parenchymal component and only minor leptomeningeal involvement both radiographically and histologically. Our case, therefore, expands the spectrum of radiological and histopathological features of this new entity. It also highlights the critical role of molecular genetic testing in establishing the diagnosis of DLGNT in unusual cases.
Keywords: Diffuse leptomeningeal glioneuronal tumors (DLGNT), BRAF, KIAA1549-BRAF fusion, 1p/19q co-deletion, Scoliosis, Syrinx
Introduction
Diffuse leptomeningeal glioneuronal tumors (DLGNTs) are newly established as a diagnostic entity in the 2016 WHO classification of tumors of the central nervous system [1]. They are characterized by diffuse leptomeningeal growth in the brain and spinal cord, and the neoplastic cells frequently show histological and immunohistochemical features of both glial and neuronal differentiation. Typically, these tumors are composed of oligodendroglioma-like cells in the leptomeninges and a relatively minor intraparenchymal component [1, 2]. The typical radiographic findings are predominant diffuse leptomeningeal enhancement and minor solid parenchymal involvement [2–4]. Molecular genetic testing frequently reveals fusion of the KIAA1549 and serine/threonine-protein kinase B-raf (BRAF) genes (KIAA1549-BRAF fusion) [4], as well as deletion of the short arm of chromosome 1(1p) and/or the long arm of chromosome 19 (19q) [2, 4]. Although most DLGNTs are histologically low-grade, high-grade tumors have also been reported [2, 5, 6].
Here, we present a case of a 13-year-old girl who presented with radiographic findings and histological features not typical for DLGNT. The patient had a history of “idiopathic” scoliosis with back and neck pain as well as tingling and numbness in the arms. Imaging studies demonstrated an intramedullary mass with an associated syrinx and hematomyelia. A gross total resection of the mass was performed. The submitted specimens showed an infiltrating neuroepithelial neoplasm with a predominant parenchymal component and focal leptomeningeal involvement. Molecular genetic testing revealed KIAA1549-BRAF fusion, chr1p/19q co-deletion, and the absence of IDH1 and IDH2 mutations.
Case report
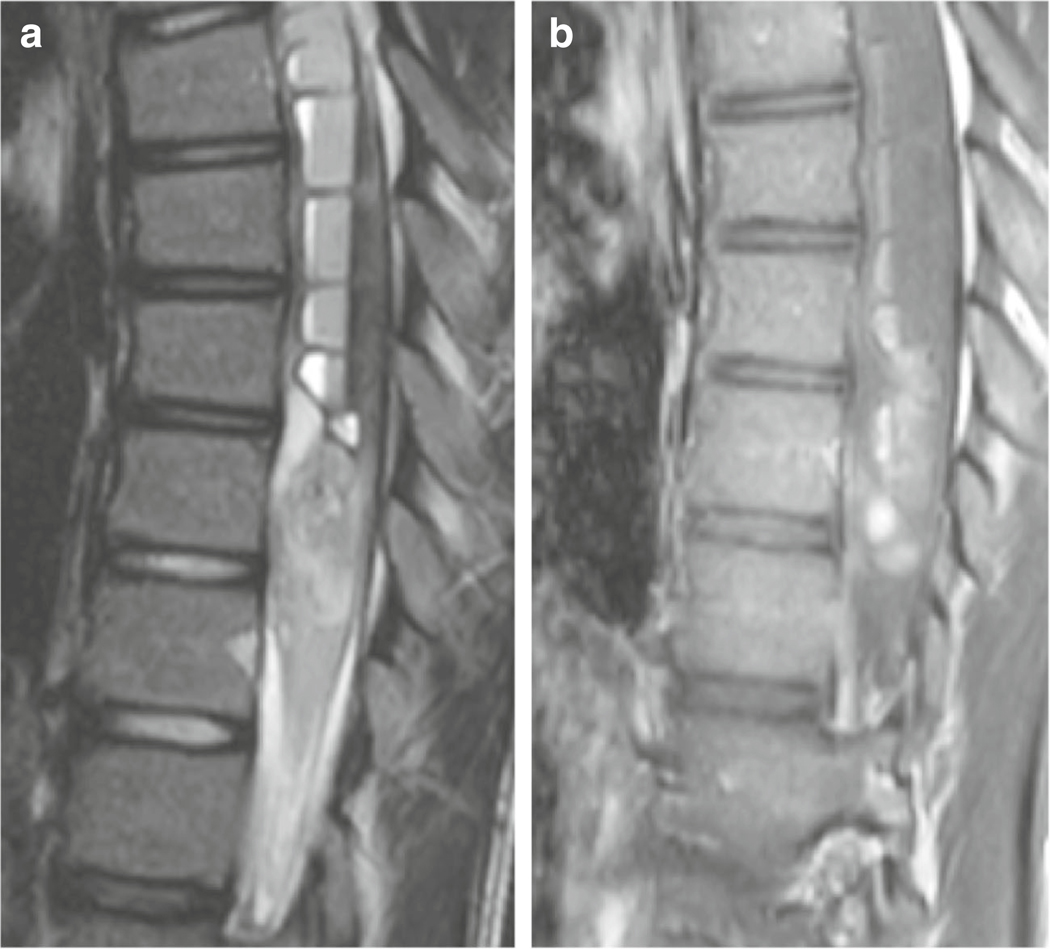
A 13-year-old girl presented to the Children’s Hospital Los Angeles with a history of scoliosis and recent upper thoracic and neck pain, numbness of the lower extremities, and bilateral hand weakness. Magnetic resonance imaging (MRI) demonstrated an S-shaped scoliotic curvature of the spine with dextroscoliosis in the thoracic and levoscoliosis in the lumbar region. MRI also revealed marked dilatation of the central canal, extending from C2 to T11, compatible with a syrinx. Within the syrinx, there was an associated blood-fluid level consistent with hemorrhage (Fig. 1a). Post-contrast images demonstrated intramedullary nodular enhancement, extending from T6 to T9–10 without leptomeningeal enhancement (Fig. 1b). The patient underwent laminectomy for a gross total resection. Intraoperatively, the spinal cord was noted to be markedly swollen and invaded by the tumor.
Fig. 1.
MR imaging features of the tumor. Sagittal T2-weighted (a) and T1-weighted (b) post-contrast images demonstrate an intramedullary mass with associated cord expansion and nodular enhancement. There is a large syrinx with a blood-fluid level consistent with haemorrhage
Histological examination revealed a neuroepithelial neoplasm diffusely infiltrating the spinal cord and focally involving the leptomeninges (Fig. 2). The infiltrating tumor cells were characterized by mild nuclear pleomorphism, oval or elongated nuclei, and scant cytoplasm (Fig. 2a, b). A small hemorrhagic area (Fig. 2c) in the parenchyma showed monomorphic cells with fine stippled chromatin and perinuclear haloes (Fig. 2d). Tumor cells in the leptomeninges formed microcysts or were loosely arranged (Fig. 2e, f). They had round or oval nuclei, fine dispersed chromatin, and scant cytoplasm. Occasional hyalinized small blood vessels were mixed with tumor cells (Fig. 2e). There were no neurocytic rosettes, mitotic figures, or foci of necrosis, and there was no microvascular proliferation.
Fig. 2.
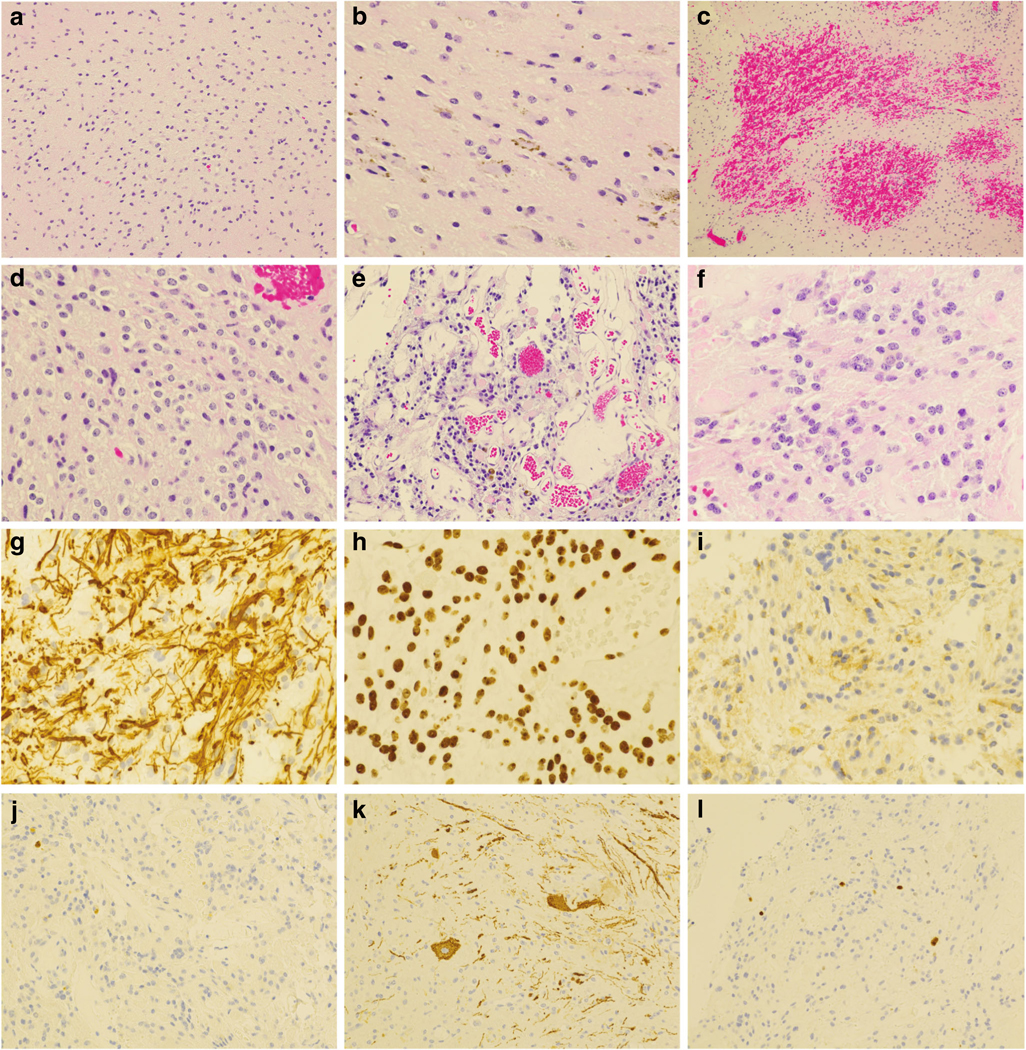
Histological features and immunohistochemical profile of the tumor. a, b H&E-stained sections demonstrate an infiltrating neuroepithelial neoplasm with low cellularity and mild nuclear pleomorphism. There are scattered hemosiderin pigments (b). c Acute hemorrhage within the tumor. d Intraparenchymal tumor cells with round nuclei and perinuclear haloes. e Tumor in the leptomeninges with microcysts. f Loosely arranged tumor cells in the leptomeninges. g–i The tumor cells are strongly immunopositive for GFAP (g) and Olig2 (h), and weakly immunoreactive for synaptophysin (i). j, k An immunostain for neurofilament protein (NF-L, Abcam antibody clone 2F11) highlights entrapped normal neurons and axons mixed with infiltrating tumor in the intraparenchymal component (j) but does not label any tumor cells (k). l Rare tumor cell nuclei have immunoreactivity for Ki67. Original magnification: × 100 for c; × 200 for a, e, j, and k; × 400 for b, d, f, g, h, and i
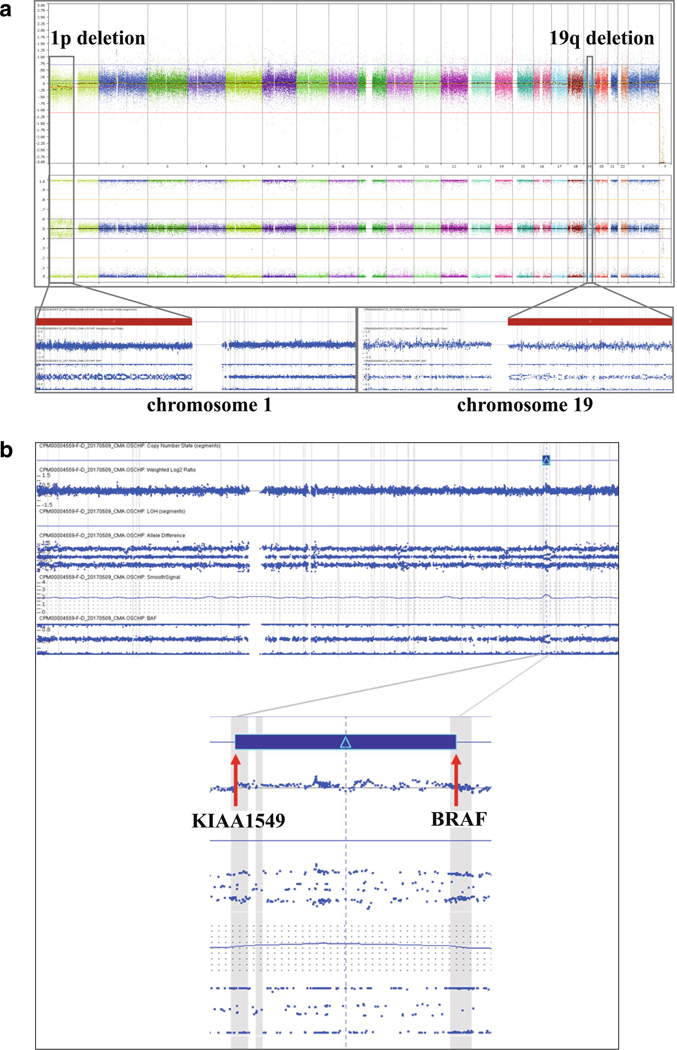
Immunohistochemical studies demonstrated that the tumor cells in the parenchyma of the cord and leptomeninges were strongly immunopositive for GFAP (Fig. 2g) and Olig2 (Fig. 2h). Occasional tumor cells in the leptomeninges were weakly immunoreactive for synaptophysin (Fig. 2i). An immunostain for low molecular weight neurofilament protein (NF-L, antibody 2F11 from Abcam) highlighted axons in the spinal cord as well as occasional intrinsic neurons (Fig. 2j); however, tumor cells in the spinal cord (Fig. 2j) and leptomeninges (Fig. 2k) were immunonegative. Rare tumor cells were immunopositive for Ki67, and the proliferation index was estimated at approximately 1–2% (Fig. 2l). Chromosomal microarray (CMA) (Affymetrix, Thermo-Fisher Scientific) demonstrated 1p/19q co-deletion (Fig. 3a) and a 1.9-Mb duplication in the 7q34 region in a subpopulation of the tumor cells, consistent with KIAA1549-BRAF fusion (Fig. 3b). Nextgeneration sequencing analysis did not find mutations in the hotspots of the IDH1 (R132) or IDH2 (R140 and R172) genes. Based on the histopathological features and genetic alterations, a final diagnosis of DLGNT was rendered.
Fig. 3.
Chromosomal microarray characterization of the tumor. a Chromosomal microarray analysis demonstrated loss of the short arm of chromosome 1 (1p) and loss of the long arm of chromosome 19 (19q). Top panel: whole genome view of the tumor (from left to right: chromosomes 1–22 followed by X and Y); middle panel: allele frequency plots; bottom panel: chromosome 1 (left) and chromosome 19 (right). b A 1.9-Mb copy number gain (duplication) is seen in the chromosome 7q34 region (top panel), with breakpoints in the KIAA1549 and BRAF genes. This duplication is predicted to result in a KIAA1549-BRAF fusion and subsequent constitutive activation of the BRAF kinase
Our patient had an uneventful post-operational recovery. It has been 18 months after her surgery. Repeated MRI demonstrated resolution of signal abnormalities. She has been followed by orthopedic surgeons and neurosurgeons for scoliosis. No chemo- or radiation therapy was given.
Discussion
Beck and Russel published the first case of what is now known as DLGNT in 1942 [7]. They used the term “oligodendrogliomatosis” to describe the characteristic cytological features of this entity. Subsequently, multiple cases of similar tumors were reported in pediatric [2, 8–14] and adult patients [15–17]. In most patients, tumors disseminated along the leptomeninges with a minor intraparenchymal involvement.
Our case is unusual with respect to both the radiographic appearance and histological features. Radiographically, there was no evidence of leptomeningeal enhancement. There was an ill-defined, solitary, enhancing intramedullary mass with associated hematohydromyelia. The most com mon MRI finding of DLGNTs is leptomeningeal enhancement involving the spinal cord and basilar cisterns, often in association with cystic T2 hyperintense lesions along the basilar cisterns and subarachnoid space [15, 16]. Our case is unusual due to the lack of leptomeningeal enhancement. Additionally, the presence of an intramedullary lesion in the spinal cord, although previously reported, is also unusual for DLGNT, especially in the absence of leptomeningeal enhancement [2].
Unlike most of the reported cases, the tumor samples submitted for histological evaluation demonstrated a predominantly infiltrating neuroepithelial tumor associated with hemorrhage. The involvement of the leptomeninges was focal. Although some tumor cells show features reminiscent of oligodendroglioma, they accounted for only a small portion of the samples. In the absence of the molecular genetic features, the radiological and histological features were most consistent with a diffuse astrocytoma. A final diagnosis of DLGNT was confirmed only after the demonstration of chr1p/19q co-deletion and KIAA1549-BRAF fusion in the absence of mutations in IDH1 and IDH2.
Several treatment options for DLGNT have been reported, including chemotherapy and radiation therapy depending on the clinical course of individual tumors [15, 18]. The presence of KIAA1549-BRAF fusion and subsequent alteration in the RAS/MEK/MAPK signaling pathway suggest MEK (or other MAPK pathways) inhibition is a potential therapeutic approach [4, 18].
In summary, we present a DLGNT with unusual radiographic and non-classic histological features. This case expands the radiological and histological spectrum of this new WHO entity. In addition, molecular genetic testing to evaluate KIAA1549-BRAF fusion, the status of the IDH1 and IDH2 genes and the status of chr1p/19q, is strongly recommended for establishing a diagnosis of DLGNT in unusual cases.
Acknowledgements
We would like to sincerely thank the patient and her family members for allowing us to report this case.
Footnotes
Compliance with ethical standards
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reifenberger G, Rodriguez F, Burger PC, Perry A, Capper D (2016) In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) Diffuse leptomeningeal glioneuronal tumour World Health Organization histological classification of tumours of the central nervous system, 4th edn International Agency for Research on Cancer, Lyon, pp 152–155 [Google Scholar]
- 2.Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D, Mosier S, Lin MT, Eberhart CG, Burger PC (2012) Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol 124(5):627–641 [DOI] [PubMed] [Google Scholar]
- 3.Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ (2017) 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics 37(7):2164–2180 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez FJ, Schniederjan MJ, Nicolaides T, Tihan T, Burger PC, Perry A (2015) High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN). Acta Neuropathol 129(4): 609–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler BA, Bookhout C, Jaikumar S, Hipps J, Lee YZ (2015) Disseminated oligodendroglial-like leptomeningeal tumor with anaplastic progression and presumed extraneural disease: case report. Clin Imaging 39(2):300–304 [DOI] [PubMed] [Google Scholar]
- 6.Schwetye KE, Kansagra AP, McEachern J, Schmidt RE, Gauvain K, Dahiya S (2017) Unusual high-grade features in pediatric diffuse leptomeningeal glioneuronal tumor: comparison with a typical low-grade example. Hum Pathol 70:105–112 [DOI] [PubMed] [Google Scholar]
- 7.Beck DJK, Russel DS (1942) Oligodendrogliomatosis of the cerebrospinal pathway. Brain 65:352–372 [Google Scholar]
- 8.Best PV (1963) Intracranial oligodendrogliomatosis. J Neurol Neurosurg Psychiatry 26:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wober G, Jellinger K (1976) Intramedullary oligodendroglioma with meningocerebral dissemination (author’s transl). Acta Neurochir 35(4):261–269 [DOI] [PubMed] [Google Scholar]
- 10.Armao DM, Stone J, Castillo M, Mitchell KM, Bouldin TW, Suzuki K (2000) Diffuse leptomeningeal oligodendrogliomatosis: radiologic/pathologic correlation. AJNR Am J Neuroradiol 21(6): 1122–1126 [PMC free article] [PubMed] [Google Scholar]
- 11.Ozkul A, Meteoglu I, Tataroglu C, Akyol A (2007) Primary diffuse leptomeningeal oligodendrogliomatosis causing sudden death. J Neuro-Oncol 81(1):75–79 [DOI] [PubMed] [Google Scholar]
- 12.Gardiman MP, Fassan M, Orvieto E, D’Avella D, Denaro L, Calderone M, Severino M, Scarsello G, Viscardi E, Perilongo G (2010) Diffuse leptomeningeal glioneuronal tumors: a new entity? Brain Pathol 20(2):361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardiman MP, Fassan M, Nozza P, Orvieto E, Garre ML, Milanaccio C, Severino M, Perilongo G, Giangaspero F (2012) Diffuse leptomeningeal glioneuronal tumours: clinicopathological follow-up. Pathologica 104(6):428–431 [PubMed] [Google Scholar]
- 14.Karlowee V, Kolakshyapati M, Amatya VJ, Takayasu T, Nosaka R, Sugiyama K, Kurisu K, Yamasaki F (2017) Diffuse leptomeningeal glioneuronal tumor (DLGNT) mimicking Whipple’s disease: a case report and literature review. Childs Nerv Syst 33(8):1411–1414 [DOI] [PubMed] [Google Scholar]
- 15.Pellerino A, Ruda R, Bertero L, Magistrello M, Franchino F, Cassoni P, Pasqualetti F, Pinessi L, Giangaspero F, Soffietti R (2018) Successful use of bevacizumab in an adult primary diffuse leptomeningeal glioneuronal tumor. J Neurosurg Sci 62(2):229–232 [DOI] [PubMed] [Google Scholar]
- 16.Fiaschi P, Badaloni F, Cagetti B, Bruzzone L, Marucci G, Dellacha A, Pavanello M, Ganci G, Padolecchia R, Valsania V (2018) Disseminated oligodendroglial-like leptomeningeal tumor in the adult: case report and review of the literature. World Neurosurg 114: 53–57 [DOI] [PubMed] [Google Scholar]
- 17.Cho HJ, Myung JK, Kim H, Park CK, Kim SK, Chung CK, Choi SH, Park SH (2015) Primary diffuse leptomeningeal glioneuronal tumors. Brain Tumor Pathol 32(1):49–55 [DOI] [PubMed] [Google Scholar]
- 18.Dodgshun AJ, SantaCruz N, Hwang J, Ramkissoon SH, Malkin H, Bergthold G, Manley P, Chi S, MacGregor D, Goumnerova L, Sullivan M, Ligon K, Beroukhim R, Herrington B, Kieran MW, Hansford JR, Bandopadhayay P (2016) Disseminated glioneuronal tumors occurring in childhood: treatment outcomes and BRAF alterations including V600E mutation. J Neuro-Oncol 128(2):293–302 [DOI] [PubMed] [Google Scholar]