Abstract
OBJECTIVE.
The purpose of this study was to assess the accuracy of portal vein pulsatility for noninvasive diagnosis of high-risk nonalcoholic fatty liver disease (NAFLD).
MATERIALS AND METHODS.
This retrospective study included patients with biopsy-proven diagnosis of NAFLD who underwent duplex Doppler ultrasound assessment of the main portal vein within 1 year of liver biopsy (January 2014 to February 2018). Doppler ultrasound images were reviewed. The spectral waveform was used to measure the maximum (Vmax) and minimum (Vmin) velocity of blood in the portal veins. Venous pulsatility index (VPI) defined as (Vmax – Vmin) / Vmax was calculated. ROC curve analysis was used to calculate AUC as a measure of accuracy to determine the value of this index for diagnosis of high-risk NAFLD and compared with that of the following four clinical decision aids: NAFLD fibrosis score (FS), fibrosis-4 index (FIB-4), BARD score (body mass index, aspartate aminotransferase [AST]–to–alanine aminotransferase ratio, diabetes mellitus), and AST-to-platelet ratio index (APRI). The value of adding VPI to these indexes was also investigated.
RESULTS.
Of 123 study subjects, 33 (26.8%) had high-risk NAFLD and were found to have a lower VPI than the other 90 subjects (0.19 vs 0.32; p < 0.001). VPI, NAFLD FS, FIB-4, and APRI had statistically significant diagnostic values for high-risk NAFLD. VPI had the highest optimism-corrected AUC (VPI, 0.84 [95% CI, 0.77–0.91]; NAFLD FS, 0.74 [95% CI, 0.63–0.83]; FIB-4, 0.81 [95% CI, 0.72–0.89]; APRI, 0.73 [95% CI, 0.61–0.82]). Addition of VPI to any of the four scoring systems significantly improved the diagnostic value of the score for high-risk NAFLD.
CONCLUSION.
VPI may be an accurate noninvasive biomarker for diagnosis of high-risk NAFLD.
Keywords: biomarker, duplex Doppler ultrasound, high-risk nonalcoholic fatty liver disease, portal venous pulsatility index
Nonalcoholic fatty liver disease (NAFLD), which is characterized by liver parenchymal fat deposition (hepatic steatosis) in the absence of excessive alcohol consumption, is the most common liver disease in the world and is often asymptomatic [1]. NAFLD affects approximately one-third of adults in the United States and has been found to be associated with other components of metabolic syndrome, such as obesity, dyslipidemia, and diabetes [2].
Among all of the pathologic features evaluated in patients with NAFLD, liver fibrosis is the best predictor of outcome [3, 4]. NAFLD can be categorized into the following five fibrosis stages based on the system developed by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) [5]: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, bridging septa between central and portal veins; and F4, cirrhosis [6].
Stage F2 or greater liver fibrosis is associated with a marked increase in long-term liver-specific and all-cause mortality [3, 7]. For this reason, the term “high-risk NAFLD” has emerged to describe the condition of these patients. Detection of high-risk NAFLD is critical in the management of NAFLD, because it defines the patient population most likely to benefit from interventions and participate in research. Although liver biopsy is the traditional reference standard for liver fibrosis staging, it is expensive, invasive, and subject to sampling and interpreter variability [8]. Consequently, numerous noninvasive liver fibrosis staging tools have been studied, including imaging methods, serum biomarkers, and combined clinical-laboratory decision aids, such as the NAFLD fibrosis score (FS), fibrosis-4 index (FIB-4), BARD score (body mass index [BMI; weight in kilograms divided by the square of height in meters], aspartate aminotransferase [AST]-to-alanine aminotransferase ratio, diabetes mellitus), and AST to platelet ratio index (APRI) [9, 10]. These tools are widely used in clinical practice, but limitations remain.
The current study was focused on an ultrasound fibrosis biomarker: portal vein pulsatility, which is an imaging biomarker measured by duplex Doppler assessment of the portal vein and quantified as the venous pulsatility index (VPI). VPI is calculated as (Vmax – Vmin) / Vmax, where Vmax is the maximum and Vmin is the minimum pulsed-wave Doppler ultrasound–estimated velocity of blood in the portal vein. Assessment of portal vein pulsatility is quantitative, noninvasive, and rapid and can be performed with routine ultrasound scanners that are available at the point of care. Although a few studies have investigated the distribution of VPI in patients with NAFLD [11–14], the accuracy of this method for identifying high-risk NAFLD is not known. Accordingly, we evaluated the value of VPI for diagnosing high-risk NAFLD in a sample of patients with biopsy-proven NAFLD and assessed whether VPI adds diagnostic value to that of the existing NAFLD FS, FIB-4, BARD score, and APRI.
Materials and Methods
Study Population
This single-center retrospective observational study received institutional review board approval, and the requirement for obtaining written consent was waived. Data management throughout the study met HIPAA requirements. Electronic medical records were searched to identify adults who had undergone liver biopsy between January 2014 and February 2018, had a biopsy-proven diagnosis of NAFLD, and had undergone pulsed-wave Doppler ultrasound assessment of the main portal vein within 1 year before or after the liver biopsy. No additional inclusion or exclusion criteria were considered for the study population.
Ultrasound Imaging
Ultrasound studies during the designated period were performed as part of routine clinical care by licensed medical sonographers using a Logiq E9 (GE Healthcare) ultrasound scanner. According to the standard protocol, pulsed-wave Doppler ultrasound assessment of the portal vein in all patients was performed after the patient had fasted for 4 hours. For imaging, the patient was in a supine position and performed a breath-hold at the end of normal expiration. Ultrasound imaging data were retrieved through the PACS. A postdoctoral research fellow blinded to the histopathologic diagnosis retrospectively reviewed each ultrasound examination and used the spectral waveform images to measure the maximum (Vmax) and minimum (Vmin) estimated portal venous velocity. These measurements were then used to calculate VPI, defined as (Vmax – Vmin) / Vmax (Fig. 1).
Fig. 1—
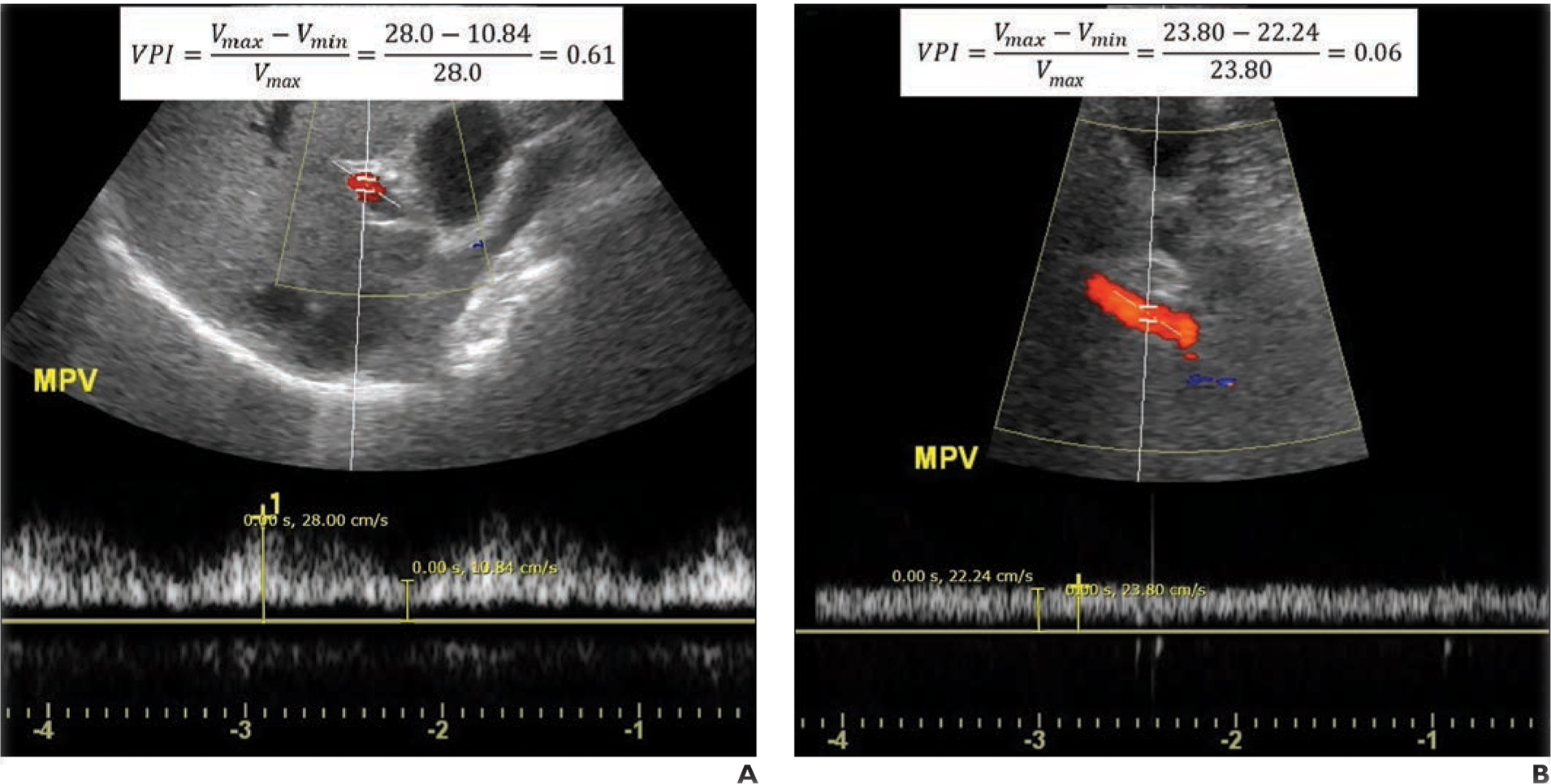
Calculation of portal venous pulsatility index (VPI).
A, 47-year-old man with nonalcoholic fatty liver disease (NAFLD) with fibrosis stage of F0. B-mode sonographic image at level of main portal vein (MPV) with superimposed color Doppler and spectral Doppler ROIs shows how maximum (Vmax) and minimum (Vmin) velocity are calculated from spectral waveform. Calculated VPI of 0.61 is elevated and would be unlikely to reflect NAFLD.
B, 59-year-old man with NAFLD with fibrosis stage of F4. Duplex ultrasound image shows spectral Doppler waveform measured in MPV has minimal temporal variation. Low calculated VPI of 0.06 corresponds to high-risk NAFLD.
Histopathologic Evaluation
Pathology reports archived in the medical records were reviewed, and information on fibrosis staging of the liver specimens was extracted. These slides had been reviewed by several board-certified general pathologists blinded to the VPI values. For all pathology assessments, the length of each specimen in millimeters and the number of portal tracts visualized were recorded. Visualization of a minimum of three portal triads and a minimum 1-cm biopsy sample was considered adequate for histologic examination. Subjects’ conditions were classified as high-risk NAFLD if the biopsy NASH CRN fibrosis stage [5] was F2 or greater and as low-risk NAFLD otherwise.
Additional Data Collection
Additional clinical data extracted from the patients’ medical records included demographics (age, sex), medical history (history of diabetes), anthropometric and body composition indexes (weight, height, BMI), and laboratory findings (platelet count and serum levels of albumin, alanine aminotransferase [ALT], and AST). Using each patient’s demographic, anthropometric, and laboratory data, we calculated NAFLD FS [15], FIB-4 [16], BARD score [17], and APRI [18] using the following formulas. NAFLD FS was calculated as −1.675 + 0.037 × age in years + 0.094 × BMI + 1.13 × impaired fasting glucose or diabetes (1, present; 0, absent) × AST-to-ALT ratio −0.013 × platelet count in 109/L × albumin in g/dL. FIB-4 was calculated as (age in years × AST in U/L) / (platelet count in 109/L × [ALT in U/L]1/2). BARD score was calculated as AST-to-ALT ratio ≥ 0.8, −2 points; BMI ≥ 28, −1 point; presence of diabetes, −1 point. APRI was calculated as 100 × (AST / upper normal limit for AST) / platelet count in 109/L.
Statistical Analysis
Demographic, anthropometric, and laboratory summaries were calculated for the entire patient cohort. Categoric variables were summarized as frequencies and percentages, and continuous variables were summarized as mean and SD. Risk scores (NAFLD FS, FIB-4, BARD, APRI, and VPI) were summarized by low- versus high-risk NAFLD as median and 25th and 75th percentiles and compared by Wilcoxon test.
A series of logistic regression models were constructed to quantify the relations between the log-it-transformed low- and high-risk NAFLD values and each predictor and to evaluate the predictive utility of the risk score in differentiating low- from high-risk NAFLD. Multivariable logistic regression models were also constructed to determine whether VPI improves discriminatory ability when used in conjunction with other risk scores. For each model, we assessed the linearity assumption associated with each risk score by modeling the risk score as a linear term and modeling it in a flexible manner using restricted cubic splines. The use of splines did not significantly improve our model fit, as assessed by ANOVA, and thus we present the more parsimonious model that uses linear terms only. Odds ratios, 95% CIs, and Wald p were computed to summarize each model.
Optimism-corrected ROC AUCs and their bootstrapped 95% CIs were computed for each predictor and model to quantify model discriminatory utility. We acknowledge that we used the same dataset to develop and evaluate our models [19]. We computed pairwise comparisons of optimism-corrected AUC values using the bootstrapped distribution of these corrected AUC values. The average difference and the bootstrapped 95% CIs were defined as the median and the 2.5 and 97.5 percentiles of the distribution of differences in optimism-corrected AUC values. The corresponding p was derived from these CIs by use of the methods of Altman and Bland [20]. All analyses were performed with R software (version 3.4.3, R Project) and the R regression modeling strategies (rms, R version 5.1–1) and bootstrap (boot S-Plus, R version 1.3–11) functions [21].
Results
Medical records of 337 patients with liver biopsy evidence of fatty liver were reviewed; 164 of these patients had a history of alcohol consumption. Among the 173 patients with a diagnosis of NAFLD, 28 did not undergo pulsed-wave Doppler ultrasound assessment of the main portal vein within 1 year before or after the liver biopsy, and the spectral waveform images of 22 patients were not technically usable for VPI calculation. After the exclusions, 123 patients (69 woman, 54 men; age range, 22–78 years; mean age, 50.3 years) were included in the study. According to the NASH CRN scoring system, 50 (40.7%) patients had F0 disease; 40 (32.5%), F1; 11 (8.9%), F2; 18 (14.6%), F3; and four (3.3%), F4 disease. Overall, 33 (26.8%) patients were found to have fibrosis stages of F2 and higher and were classified as having high-risk disease. The other 90 (73.2%) patients had low-risk NAFLD. Table 1 presents the demographic characteristics and medical history of the subjects, their anthropometric and body composition indexes, and their laboratory findings.
TABLE 1:
Demographic, Anthropometric, and Laboratory Summaries of the Sample
| Variable | Total (n = 123) | Low-Risk NAFLD (n = 90) | High-Risk NAFLD (n = 33) |
|---|---|---|---|
| Age (y) | 50.3 ± 12.2 | 47.7 ± 11.7 | 57.3 ± 10.7 |
| Sex | |||
| Women | 69 (56) | 52 (58) | 17 (52) |
| Men | 54 (44) | 38 (42) | 16 (48) |
| Diabetes | 35 (28) | 25 (28) | 10 (30) |
| Weight (kg) | 90.1 ± 18.6 | 88.6 ± 17.5 | 94.2 ± 21.2 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Body mass indexa | 31.9 ± 5.5 | 31.5 ± 5.3 | 33.1 ± 6.1 |
| Platelet count (× 109/L) | 236.6 ± 78.2 | 247.8 ± 76.2 | 205.9 ± 76.3 |
| Albumin (g/dL) | 4.5 ± 0.4 | 4.6 ± 0.4 | 4.5 ± 0.4 |
| AST (U/L) | 50.7 ± 43.9 | 47.7 ± 47.0 | 58.9 ± 33.4 |
| ALT (U/L) | 66.1 ± 47.9 | 64.9 ± 49.1 | 69.3 ± 45.1 |
Note—Quantitative variables are summarized as mean ± SD, and qualitative variables are summarized as frequency with percentages in parentheses. NAFLD = nonalcoholic fatty liver disease, AST = aspartate aminotransferase, ALT = alanine aminotransferase.
Weight in kilograms divided by the square of height in meters.
The distribution of VPI measurements across different stages of fibrosis is presented in Figure 2. Risk scores by NAFLD category are presented in Table 2. Median risk scores for NAFLD FS, FIB-4, BARD score, and APRI tended to be higher among those with high-risk NAFLD (p < 0.001; BARD, p = 0.053). The median VPI score was significantly lower among patients in the group with high-risk disease (p < 0.001).
Fig. 2—
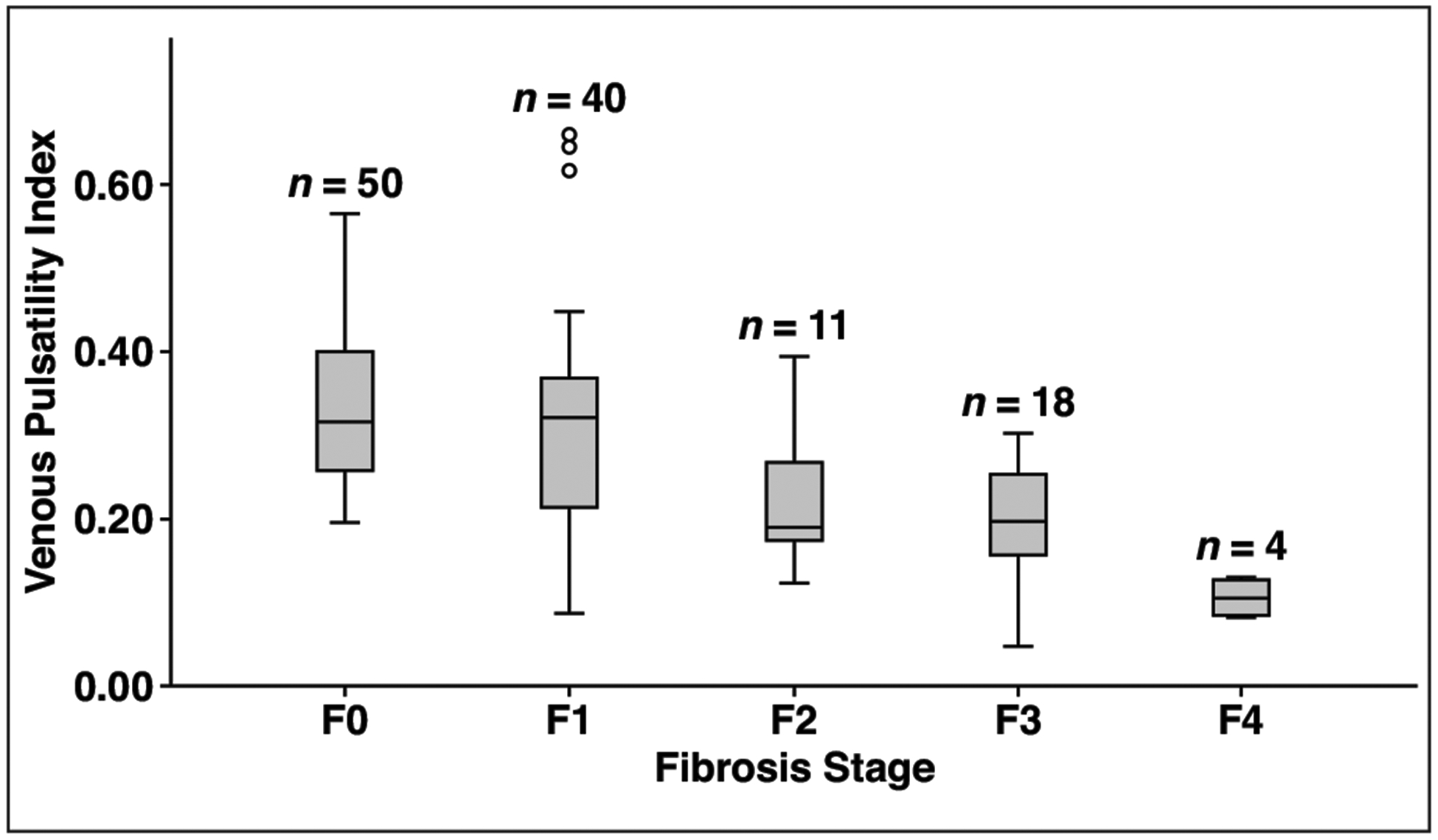
Box plot shows that higher fibrosis stages are associated with lower venous pulsatility index (VPI). In particular, VPI in high-risk nonalcoholic fatty liver disease (NAFLD) (≥ F2) appears less than VPI in low-risk NAFLD (F0–F1). Upper and lower limits of whiskers are defined as highest and lowest values within 1.5 interquartile range of upper (75th percentile) and lower (25th percentile) limits of box. Circles denote outliers.
TABLE 2:
Clinical Risk Scores and Venous Pulsatility Index Values Stratified by Low- and High-Risk Nonalcoholic Fatty Liver Disease (NAFLD) Pathologic Diagnoses
| Scoring System | Total (n = 123) | Low-Risk NAFLD (n = 90) | High-Risk NAFLD (n = 33) | pa |
|---|---|---|---|---|
| Venous pulsatility index | 0.28 (0.21, 0.36) | 0.32 (0.24, 0.37) | 0.19 (0.14, 0.24) | < 0.001 |
| NAFLD fibrosis score | −1.85 (−1.70, −0.67) | −2.22 (−2.05, −1.08) | −0.69 (−1.73, −0.14) | < 0.001 |
| FIB-4 | 1.35 (0.82, 1.82) | 1.11 (0.76, 1.64) | 2.09 (1.46, 2.91) | < 0.001 |
| BARD score | 2.00 (1.00, 3.00) | 2.00 (1.00, 3.00) | 3.00 (1.00, 3.00) | 0.053 |
| APRI | 0.48 (0.32, 0.70) | 0.42 (0.28, 0.60) | 0.68 (0.42, 1.02) | < 0.001 |
Note—Values are medians with 25th and 75th percentiles in parentheses. FIB-4 = fibrosis-4 index, BARD = body mass index (weight in kilograms divided by the square of height in meters), aspartate aminotransferase (AST)–to–alanine aminotransferase ratio, diabetes mellitus; APRI = AST-to-platelet ratio index.
Comparison of high- and low-risk NAFLD. Computed by Wilcoxon test.
Results from the univariate logistic regression models (Table 3) coincided with the summaries presented in Table 2. For example, the odds ratios associated with NAFLD FS, FIB-4, BARD, and APRI were greater than 1, which indicates that each unit increase in risk score is associated with increased risk of high-risk NAFLD. Similarly, the odds ratio associated with a 0.1-unit increase in VPI was 0.85 (95% CI, 0.80–0.91) and thus associated with a 15% reduction in the risk of high-risk NAFLD. Similar patterns were observed when existing risk scores and VPI were combined. Interestingly, the effect of VPI on high-risk NAFLD remained constant (≈ 0.85) across models, indicating that this metric may be characterizing an attribute of high-risk NAFLD not accounted for by the four evaluated risk scores. Optimism-corrected AUC values associated with each univariate model ranged from 0.61 for BARD (95% CI, 0.46–0.72) to 0.81 for FIB-4 (95% CI, 0.72–0.89) for clinical risk models; the corrected AUC for VPI was 0.84 (95% CI, 0.77–0.91). After adding VPI to each risk score, we observed increases in all optimism-corrected AUC values (e.g., BARD, 0.86; 95% CI, 0.78–0.92) (Table 3 and Fig. 3).
TABLE 3:
Logistic Regression Analysis Quantifying Diagnostic Performance of Risk Scores for Predicting High-Risk Nonalcoholic Fatty Liver Disease (NAFLD)
| Model | Odds Ratioa | P | Optimism-Corrected ROC AUC |
|---|---|---|---|
| Univariate | |||
| VPI | 0.85 (0.80–0.91) | < 0.001 | 0.84 (0.77–0.91) |
| NAFLD-FS | 1.81 (1.33–2.48) | < 0.001 | 0.74 (0.63–0.83) |
| FIB-4 | 4.82 (2.47–9.42) | < 0.001 | 0.81 (0.72–0.89) |
| BARD score | 1.39 (0.98–1.96) | 0.063 | 0.61 (0.46–0.72) |
| APRI | 3.71 (1.32–10.46) | 0.013 | 0.73 (0.61–0.82) |
| Multivariable | |||
| VPI + NAFLD FS | 0.89 (0.81–0.95) | ||
| VPI | 0.84 (0.78–0.91) | < 0.001 | |
| NAFLD FS | 1.90 (1.30–2.79) | 0.001 | |
| VPI + FIB-4 | 0.90 (0.83–0.96) | ||
| VPI | 0.86 (0.79–0.93) | < 0.001 | |
| FIB-4 | 4.13 (1.99–8.58) | < 0.001 | |
| VPI + BARD score | 0.86 (0.78–0.92) | ||
| VPI | 0.84 (0.78–0.90) | < 0.001 | |
| BARD score | 1.57 (1.03–2.38) | 0.034 | |
| VPI + APRI | 0.85 (0.77–0.92) | ||
| VPI | 0.86 (0.80–0.92) | < 0.001 | |
| APRI | 2.13 (0.79–5.72) | 0.134 |
Note—Values in parentheses are 95% CIs. VPI = venous pulsatility index, FS = fibrosis score, FIB-4 = fibrosis-4 index, BARD = body mass index (weight in kilograms divided by the square of height in meters), aspartate aminotransferase (AST)–to–alanine aminotransferase ratio, diabetes mellitus; APRI = AST-to-platelet ratio index.
Associated with a 1-unit change in either NAFLD-FS, FIB-4, BARD score, or APRI and a 0.1-unit change in VPI.
Fig. 3—
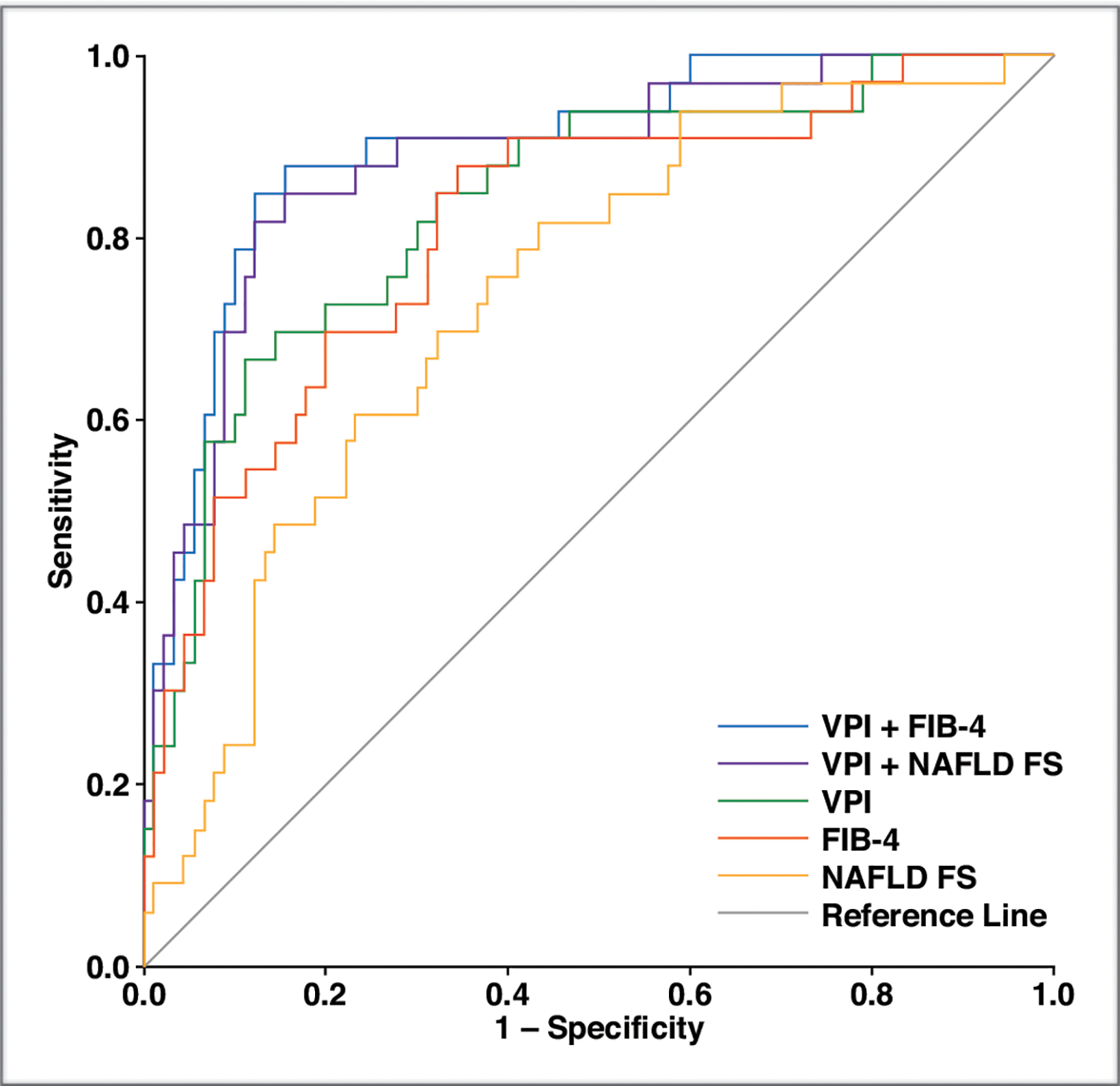
Graph shows ROC curves of univariable models of venous pulsatility index (VPI), fibrosis-4 index (FIB-4), and nonalcoholic fatty liver disease fibrosis score (NAFLD FS) and multivariable models of VPI + NAFLD FS and VPI + FIB-4 for diagnosis of high-risk NAFLD.
Differences in the optimism-corrected AUC values between each risk score and VPI and the incremental effect of VPI are presented in Table 4. The VPI optimism-corrected AUC value was significantly greater than the corrected AUC obtained with the BARD scoring system but not the corrected AUCs obtained with NAFLD FS, APRI, and FIB-4. The addition of VPI to every clinical risk model resulted in a statistically significant increase in optimism-corrected AUC. Conversely, when added to VPI, the only risk model that resulted in a statistically significant increase in corrected AUC was FIB-4.
TABLE 4:
Risk Score Comparisons and Estimates of the Incremental Effect of Venous Pulsatility Index (VPI)
| Comparison or Estimate | Difference in Optimism-Corrected ROC AUCa | P |
|---|---|---|
| Risk score comparison | ||
| NAFLD FS vs VPI | −0.10 (−0.24 to 0.02) | 0.106 |
| FIB-4 vs VPI | −0.03 (−0.16 to 0.09) | 0.645 |
| BARD score vs VPI | −0.24 (−0.42 to −0.08) | 0.005 |
| APRI vs VPI | −0.12 (−0.26 to 0.01) | 0.081 |
| Incremental effect of VPI | ||
| VPI + NAFLD FS vs VPI | 0.04 (0.00–0.09) | 0.088 |
| VPI + NAFLD FS vs NAFLD FS | 0.14 (0.06–0.26) | 0.002 |
| VPI + FIB-4 vs VPI | 0.06 (0.01–0.12) | 0.040 |
| VPI + FIB-4 vs FIB-4 | 0.09 (0.02–0.18) | 0.031 |
| VPI + BARD score vs VPI | 0.01 (−0.01 to 0.05) | 0.493 |
| VPI + BARD score vs BARD score | 0.25 (0.12–0.42) | 0.001 |
| VPI + APRI vs VPI | 0.01 (−0.01 to −0.06) | 0.570 |
| VPI + APRI vs APRI | 0.13 (0.02–0.27) | 0.028 |
Note—Values in parentheses are 95% CIs. NAFLD FS = nonalcoholic fatty liver disease fibrosis score, FIB-4 = fibrosis-4 index, BARD = body mass index (weight in kilograms divided by the square of height in meters), aspartate aminotransferase (AST)–to–alanine aminotransferase ratio, diabetes mellitus.
A negative value in risk score comparison indicates superior diagnostic performance of VPI versus the given clinical risk prediction model.
To evaluate for sensitivity of our findings to the sonography-to-biopsy time interval, we performed a sensitivity analysis in a subgroup of subjects who underwent duplex Doppler ultrasound assessment within 6 months of liver biopsy (compared with the 1-year time frame defined in our inclusion criteria). A total of 93 subjects satisfied this interval restriction, and of these, 26 were classified as having high-risk NAFLD. Study results in this subset closely resembled those of the full cohort and are available on request.
Discussion
In the current study, we found that VPI is lower in patients with higher liver fibrosis stage. Furthermore, prediction based on VPI performs at a level similar to that reported for shear-wave elastography and serum biomarkers in multiple studies [22–25]. Moreover, the addition of VPI to all existing clinical prediction models resulted in statistically significant improvement in their diagnostic performance. This finding suggests that VPI measures a previously uncaptured sonographic sign of moderate or greater liver fibrosis in patients with NAFLD.
We used a 2-year patient inclusion window (1 year after and before liver biopsy) to improve statistical power. This decision was based on our a priori understanding that liver fibrosis severity would not be likely to change substantially over this time frame. This assumption is supported by a systematic review [26] that showed fibrosis progresses by one stage per 14.3 years in patients with NAFLD and by one stage per 7.1 years in patients with NASH. We confirmed the stability of our findings by performing a sensitivity analysis of a subgroup of imaging studies performed within 6 months of biopsy. The results were similar, affirming our confidence in our initial assumption.
Although various sonographic changes in the portal vein, such as reversal of flow or decrease in antegrade flow volume, have been previously described as possible indicators of liver disease [27], changes in portal vein pulsatility in these settings was first investigated in the 1990s [28–30]. Westra et al. [28] suggested that venous congestion of the hepatic parenchyma within the space confined by the liver capsule results in competition between inflow from the portal vein and hepatic artery during peak systole, which increases portal vein pulsatility. In 1995, Wachsberg et al. [29] found that this mechanism is not the sole contributing one because portal vein pulsatility can increase despite occlusion of hepatic arterial flow. They suggested that pulsatile portal vein inflow may be affected by transmission of pulsations from the adjacent inferior vena cava, the location of the sample volume relative to the inferior vena cava, or other factors that have not yet been determined.
In a comparison of patients with chronic liver disease and healthy subjects, Barakat [31] in 2002 found a significantly lower VPI in patients with chronic liver disease. The value was lower in patients with Child-Pugh class C disease than in those with Child-Pugh class A disease. The mechanism Barakat proposed for the observations was that the pathologic fibrotic changes in the liver decrease transmission of atrial pressure changes through the hepatic veins.
In 2008, Erdogmus et al. [13] found that VPI is significantly lower in patients with NAFLD than in healthy subjects. They attributed this alteration to the decreased compliance of liver vasculature due to fatty infiltration. Balci et al. [32] also reported similar results from the study of 105 patients with NAFLD and 35 healthy subjects. Three years later, their findings were confirmed by Solhjoo et al. [14], who compared the VPI of 31 patients with NAFLD with that of 31 healthy individuals. In a more recent study, Balasubramanian et al. [33] compared the VPI scores of 90 patients with NAFLD with those of 90 healthy control subjects and reached the same conclusion as prior investigators did. Results of other studies suggested that VPI increases in patients with cirrhosis [11, 12] owing to reversed portal venous flow, increased hepatic venular resistance, and arterioportal shunting caused by hepatic structural distortion. Further investigation is needed to better characterize the relations between VPI, steatosis, and liver fibrosis. In this regard, our study offers useful additional insight: our analyses show that VPI decreases with increasing fibrosis severity in patients with NAFLD and therefore may be a clinically useful predictor of liver fibrosis stage F2 or greater in these patients.
Our study had several limitations. First, the retrospective nature of the study may have led to unrecognized selection bias. Second, a single image reviewer measured VPI, which might have limited the generalizability of our findings to other reviewers. In some cases, it is difficult to precisely determine the best cycle to measure maximum and minimum velocity on the spectral waveform, and the exact points for measuring these two parameters are not always clearly defined on the available images. In the study the reviewer selected the highest and lowest points to determine maximum and minimum velocity, respectively. In clinical practice, it may be possible to mitigate this limitation by repeating image acquisition in real time. Third, a single model of ultrasound machine was used to acquire VPI, which may limit extrapolation to other ultrasound devices. Fourth, several sonographers acquired the images as part of routine clinical practice. This might have introduced variability, which could adversely affect the power of the study to detect associations between VPI and liver fibrosis stage. Conversely, that several technologists acquired the images suggests that our results may not depend on individual operator skill or bias. Fifth, histopathologic grading of liver fibrosis has known limitations in accuracy and variability. We used histopathologic grading because it continues to be the most widely accepted reference standard.
Our study shows that VPI may be a predictor of high-risk NAFLD and may improve the prognostic performance of widely used clinical prediction aids used for this purpose. Because VPI is routinely available at no or minimal cost additional to conventional diagnostic ultrasound, further investigation of the utility of VPI for the diagnosis of high-risk NAFLD is required. With further validation, VPI may become an important component of low-cost noninvasive multiparametric high-risk NAFLD diagnosis.
Footnotes
Based on a presentation at the Radiological Society of North America 2018 annual meeting, Chicago, IL.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84 [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61:1547–1554 [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 2011; 53:1874–1882 [DOI] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017; 377:2063–2072 [DOI] [PubMed] [Google Scholar]
- 7.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906 [DOI] [PubMed] [Google Scholar]
- 9.Gawrieh S, Chalasani N. NAFLD fibrosis score: is it ready for wider use in clinical practice and for clinical trials? Gastroenterology 2013; 145:717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaswala DH, Lai M, Afdhal NH. Fibrosis assessment in nonalcoholic fatty liver disease (NAFLD) in 2016. Dig Dis Sci 2016; 61:1356–1364 [DOI] [PubMed] [Google Scholar]
- 11.Iranpour P, Lall C, Houshyar R, et al. Altered Doppler flow patterns in cirrhosis patients: an overview. Ultrasonography 2016; 35:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNaughton DA, Abu-Yousef MM. Doppler US of the liver made simple. RadioGraphics 2011; 31:161–188 [DOI] [PubMed] [Google Scholar]
- 13.Erdogmus B, Tamer A, Buyukkaya R, et al. Portal vein hemodynamics in patients with non-alcoholic fatty liver disease. Tohoku J Exp Med 2008; 215:89–93 [DOI] [PubMed] [Google Scholar]
- 14.Solhjoo E, Mansour-Ghanaei F, Moulaei-Langorudi R, Joukar F. Comparison of portal vein Doppler indices and hepatic vein Doppler waveform in patients with nonalcoholic fatty liver disease with healthy control. Hepat Mon 2011; 11:740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854 [DOI] [PubMed] [Google Scholar]
- 16.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36 [DOI] [PubMed] [Google Scholar]
- 17.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008; 57:1441–1447 [DOI] [PubMed] [Google Scholar]
- 18.Kruger FC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J 2011; 101:477–480 [PubMed] [Google Scholar]
- 19.Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol 2014; 180:318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ 2011; 343:d2304. [DOI] [PubMed] [Google Scholar]
- 21.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge, UK: Cambridge University Press, 1997 [Google Scholar]
- 22.Samir AE, Dhyani M, Vij A, et al. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology 2014; 274:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall TJ, Milkowski A, Garra B, et al. RSNA/QIBA: shear wave speed as a biomarker for liver fibrosis staging In: 2013 IEEE International Ultrasonics Symposium (IUS). Piscataway, NJ: IEEE, 2013 [Google Scholar]
- 24.Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008; 47:455–460 [DOI] [PubMed] [Google Scholar]
- 25.Adams LA, Angulo PJ. Role of liver biopsy and serum markers of liver fibrosis in nonalcoholic fatty liver disease. Clin Liver Dis 2007; 11:25–35 [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13:643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohnishi K, Saito M, Nakayama T, et al. Portal venous hemodynamics in chronic liver disease: effects of posture change and exercise. Radiology 1985; 155:757–761 [DOI] [PubMed] [Google Scholar]
- 28.Westra SJ, Zaninovic AC, Vargas J, Hall TR, Boechat MI, Busuttil RW. The value of portal vein pulsatility on duplex sonograms as a sign of portal hypertension in children with liver disease. AJR 1995; 165:167–172 [DOI] [PubMed] [Google Scholar]
- 29.Wachsberg RH, Needleman L, Wilson DJ. Portal vein pulsatility in normal and cirrhotic adults without cardiac disease. J Clin Ultrasound 1995; 23:3–15 [DOI] [PubMed] [Google Scholar]
- 30.Gorka W, Gorka TS, Lewall DB. Doppler ultrasound evaluation of advanced portal vein pulsatility in patients with normal echocardiograms. Eur J Ultrasound 1998; 8:119–123 [DOI] [PubMed] [Google Scholar]
- 31.Barakat M Portal vein pulsatility and spectral width changes in patients with portal hypertension: relation to the severity of liver disease. Br J Radiol 2002; 75:417–421 [DOI] [PubMed] [Google Scholar]
- 32.Balci A, Karazincir S, Sumbas H, Oter Y, Egilmez E, Inandi T. Effects of diffuse fatty infiltration of the liver on portal vein flow hemodynamics. J Clin Ultrasound 2008; 36:134–140 [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian P, Boopathy V, Govindasamy E, Venkatesh BP. Assessment of portal venous and hepatic artery haemodynamic variation in nonalcoholic fatty liver disease (NAFLD) patients. J Clin Diagn Res 2016; 10:TC07–TC10 [DOI] [PMC free article] [PubMed] [Google Scholar]


