Significance
Plant functional traits are central instruments in developing understanding and predicting biodiversity patterns and ecosystems processes. Snow is an important ecological factor in cold climates, but its contribution to the evolution of functionality of tundra vegetation is poorly known and insufficiently addressed in the research. We show here that snow has a fundamental effect in mediating climate change impacts on functional composition and diversity of Arctic tundra vegetation. As a whole, Arctic landscapes may lose spatial heterogeneity because plant communities will be functionally more alike, although the local functional diversity may increase. Our results highlight that future snow conditions and their fine-scale variability should be acknowledged in the next generation of Arctic vegetation−ecosystem models.
Keywords: remote sensing, species distribution modeling, microclimate, winter ecology, alpine mountain
Abstract
The Arctic is one of the least human-impacted parts of the world, but, in turn, tundra biome is facing the most rapid climate change on Earth. These perturbations may cause major reshuffling of Arctic species compositions and functional trait profiles and diversity, thereby affecting ecosystem processes of the whole tundra region. Earlier research has detected important drivers of the change in plant functional traits under warming climate, but studies on one key factor, snow cover, are almost totally lacking. Here we integrate plot-scale vegetation data with detailed climate and snow information using machine learning methods to model the responsiveness of tundra communities to different scenarios of warming and snow cover duration. Our results show that decreasing snow cover, together with warming temperatures, can substantially modify biotic communities and their trait compositions, with future plant communities projected to be occupied by taller plants with larger leaves and faster resource acquisition strategies. As another finding, we show that, while the local functional diversity may increase, simultaneous biotic homogenization across tundra communities is likely to occur. The manifestation of climate warming on tundra vegetation is highly dependent on the evolution of snow conditions. Given this, realistic assessments of future ecosystem functioning require acknowledging the role of snow in tundra vegetation models.
Anthropogenic climate change is causing major changes in the physical environment, which, in turn, may fundamentally alter biodiversity and species compositions across the planet (1). Species differ in their size, resource use, and biochemical pathways, that is, in their functional traits (2–4). Hence, turnover in species assemblages may lead to major shifts in the functional composition of biological communities and, consequently, cause large-scale alterations in ecosystems processes (5, 6). Taxonomic approaches, such as species-level models, offer only limited understanding of the importance of climate warming-induced changes in functioning of ecosystems (7, 8). In contrast, trait-based ecology and the accelerated availability of trait data have revolutionized the field of ecosystem science (9, 10), allowing developing novel contributions for the climate change impact assessments. Plant functional traits and functional diversity (FD) can thus provide essential information for researchers and climate-smart conservation planning about the processes and stability of ecosystems otherwise challenging to quantify (6).
Functional traits, particularly in plants, may provide critical understanding of the resource use in biotic communities and their impacts on the ecosystems (2, 4). In practice, functional traits are measurable properties of plant size, structure, and biogeochemistry (6) that are intrinsically related to the key functions of ecosystems such as productivity or cycling of water, carbon, and nutrients (5, 11). FD, in turn, defines the range, variability, and evenness of functional traits in a community, describing one important component of biodiversity (2, 3, 12). However, in contrast to its taxonomic equivalents, it involves understanding of communities based on what organisms do, rather than on their evolutionary history (13). Indeed, ecosystem processes are often more consistently linked with FD than with pure species count (2), and experimental and analytical evidence shows that FD can provide a mechanistic link between organisms and ecosystems (13). The main mechanism for how FD can affect the ecosystem processes is the “niche complementarity effect”: Higher FD diversity allows a greater range of functional traits in a given community, leading to more efficient and diverse resource use in spatiotemporally varying environments (2), thereby potentially increasing the stability and resilience of ecosystems (14, 15).
Arctic communities provide one key environment to study the consequences of altering functionality of species communities in the face of climate change (16). This is because direct human impacts on the environment are smaller than in other parts of the globe, but, at the same time, the Arctic is warming from 2 to 3 times faster than the global average (17). Arctic soils are a major storage of carbon, and the whole Arctic system may witness important feedbacks emerging from changes in vegetation that could reinforce global climate warming (18, 19). Indeed, there are a few comprehensive studies showing the importance of growing season-related factors in driving functional composition of Arctic and alpine vegetation (5, 20–22). However, at the same time, it remains largely unknown how the changes in one key environmental driver in the Arctic, snow, will affect the functional composition and diversity of the Arctic ecosystems. This is a significant shortcoming, as snow is a fundamental mediator of climate conditions at local scales and has long been considered as one of the most important environmental filters for Arctic and alpine plants (23, 24). Another shortcoming is that earlier studies have hitherto focused on explaining plot-scale patterns in functional properties and paid limited attention to how trait composition and diversity might change across landscapes (2, 22, 25).
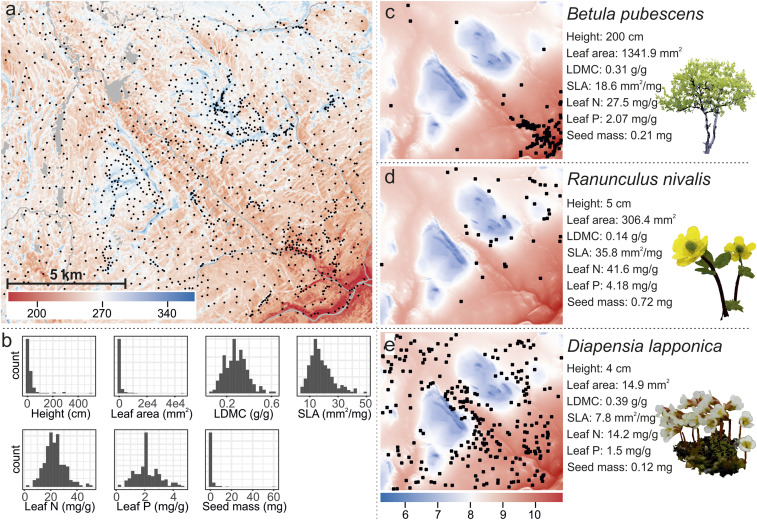
Here, to improve the understanding of the sensitivity of the functions in the Arctic ecosystems to the changes in both snow cover duration (SCD) and temperature, we used multidecadal fine-scale information of snow cover dynamics linked with summer temperature data and plot-scale cover values of 135 vascular plant species across an extensively studied tundra landscape (195 km2; 5,300 plots within 1,325 study sites; Material and Methods and Fig. 1). We examined the role of changing temperatures and snow conditions on community weighted means (CWMs) of seven widely applied functional traits and FD.
Fig. 1.
SCD (in days) and the 1,325 study sites across the study area in northern Norway (A). The distributions of the species-level trait values of the 135 study species for seven studied functional traits (B). Summer temperatures (degrees Celsius) on maps and occurrences and median trait values for three contrasting example species common in the area (C–E).
We used an ensemble of machine learning models to reconstruct the species communities in current climate at 30-m spatial resolution by utilizing fine scale topoclimatic data and SCD derived from 135 Landsat satellite images. We related species communities primarily to the SCD and summer temperature variables but included also other key environmental factors (e.g., topographic soil wetness) in the models to take their potential confounding impacts into account (26). Then, we used these models to project the species compositions in future climatic conditions by simulating 36 different scenarios of summer warming and decrease in SCD (temperatures: no change and representative concentration pathway [RCP] scenarios 2.6, 4.5, and 8.5 for years 2040–2069; SCD: no change and eight descending steps from 5% down to 40% decrease). We extracted 65,706 species-level trait measurements from three databases (10, 27, 28) and calculated species-specific median values for seven traits, namely plant vegetative height, leaf area, leaf dry matter content (LDMC), specific leaf area (SLA), leaf nitrogen content (LeafN), leaf phosphorus content (LeafP) and seed mass. CWM trait values and several FD indices were calculated for the modeled plant communities.
Results and Discussion
The ensemble models were able to capture the variation in CWMs of the seven traits rather rigorously. Cross-validated R2 value between observed and modeled CWMs was highest for height (0.553) and seed mass (0.485) followed by leaf area (0.465), LeafN (0.464), SLA (0.452), and LeafP (0.447), while the R2 for LDMC was the lowest (0.386). Model residuals showed slight spatial autocorrelation (SI Appendix, Figs. S1–S7) only within the shortest distances for some traits (height, leaf area, SLA, and LDMC), whereas, for others, the spatial effect was negligible (LeafP, LeafN, and seed mass).
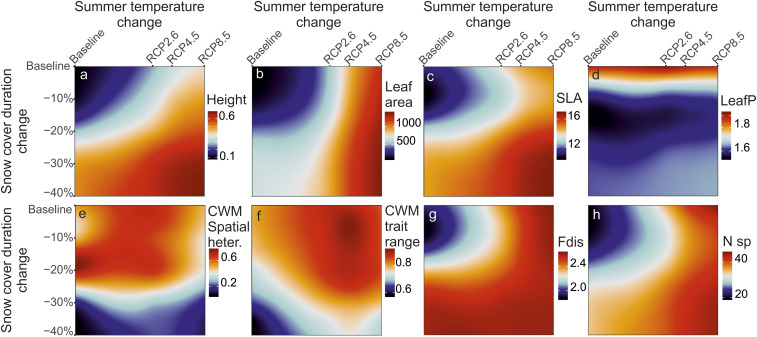
The CWM traits projected over the study area under the 36 different temperature and snow scenarios showed that most of the CWM traits were highly sensitive to changes in both summer temperature and SCD (Fig. 2 A–D and SI Appendix, Figs. S15, S23, and S24), but some were clearly more driven by snow conditions (LeafN, LeafP, and seed mass) and others by temperatures (leaf area). Simulated warmer future climate with longer snow-free season led to taller plants with bigger leaves and faster resource acquisition strategies (e.g., high SLA or nitrogen content; Fig. 3 and SI Appendix, Figs. S8–S14 and S16–S22). The only clear exception was that LeafP decreased rapidly when shorter SCD was simulated (Fig. 2D).
Fig. 2.
The evolution of CWM traits, variability, and FD. The evolution of CWM of four traits under modeled scenarios in summer temperatures and SCD (values between 36 modeled scenarios interpolated in the trait surfaces) (A–D). Color indicates the average values calculated over the study area for each of the scenario. The CWM traits shown are plant height (A), leaf area (B), SLA (C), and LeafP (D). (E–H) The evolution of community-level heterogeneity of the CWM traits calculated as a SD within a 100-m-radius moving window; values of the seven studied traits stacked, normalized, and averaged (E; heter., heterogeneity), the overall range of CWM values within the whole study area (F; values of the seven studied traits stacked, normalized, and averaged), average FD (G; Fdis, functional dispersal), and average species richness (H; N sp, i.e., pure species count). Current condition in upper left corner, and the extreme scenario in lower right. Only areas above 400 m a.s.l. are considered in calculations.
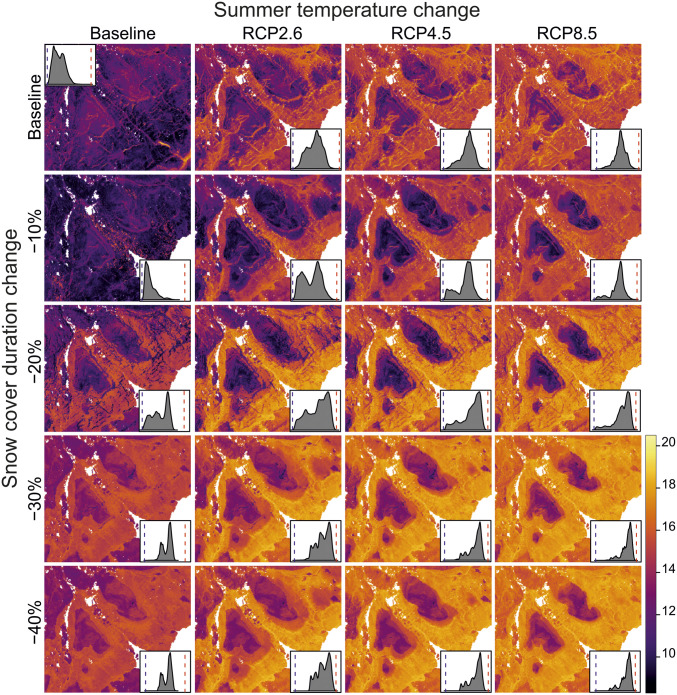
Fig. 3.
CWM SLA predicted over the study area in current climate and under 19 different scenarios combining specific change in summer temperatures or SCD for years 2049–2070. Insets show the distribution of CWM SLA for each of the scenarios in the study area. Dashed lines show minimum and maximum CWM SLA values under each of the scenarios. Areas below 400 m a.s.l. were excluded from the scenario maps to avoid prediction to nonanalog climate space and extrapolation outside the model calibration data. Other white areas in maps represent major water bodies. SLA was chosen as an example trait, due to its commonness in ecological literature (for similar figures of other traits, see SI Appendix, Figs. S8–S14).
We calculated community-level heterogeneity in the projected CWM traits as an SD of CWMs with a moving window of 100-m radius. These calculations showed that the high spatial variability in CWM traits between communities was highly dependent on late-lying snow patches (Fig. 2E; see also maps in Fig. 3). The overall range of the CWM traits within the whole study area (the difference between the minimum and maximum of the projected CWM values for each of the scenarios and traits) showed a similar pattern, but the largest shrinkage of range occurred in the scenario with maximum shortening of SCD and no warming (Fig. 2F). The CWM trait range increased in scenarios with summer warming but only with small concurrent changes in SCD.
Both shorter SCD and warmer summer temperatures increased local FD (calculated as multidimensional dispersion of the functional trait values in each community) but decreased functional evenness in the communities (Fig. 2G and SI Appendix, Figs. S25–S32). The increase in FD largely coincided with the increase in species count in local communities, although this increase was higher in the scenarios with the maximum rate of warming than in the scenarios with the greatest decrease in SCD (Fig. 2H).
Our previous study with the same dataset as used here suggested that declining SCD may cause a high rate of regional extinctions among snow specialist species (29). According to our models here, 15 species are projected to lose more than 95% of their distributional area under the most severe combination of climate and SCD scenarios (RCP8.5 and −40% SCD). The trait profiles of these 15 species threatened by regional extinction differed from the other species. These species were significantly shorter (5 cm on average vs. 25 cm; Wilcoxon two-tailed rank sum test, P < 0.001) and had smaller leaves (67 mm2 vs. 685 mm2; P < 0.001) and seed mass (0.28 mg vs. 0.93 mg; P = 0.013) and marginally lower SLA (15.4 mm2/mg vs. 18.3 mm2/mg; P = 0.069) and higher LDMC (0.33 g/g vs. 0.28 g/g; P = 0.10). Leaf nitrogen (21.0 mg/g vs. 23.4 mg/g; P = 0.42) and phosphorus (2.3 mg/g vs. 2.2 mg/g; P = 0.68) contents showed no detectable difference in trait values between the groups.
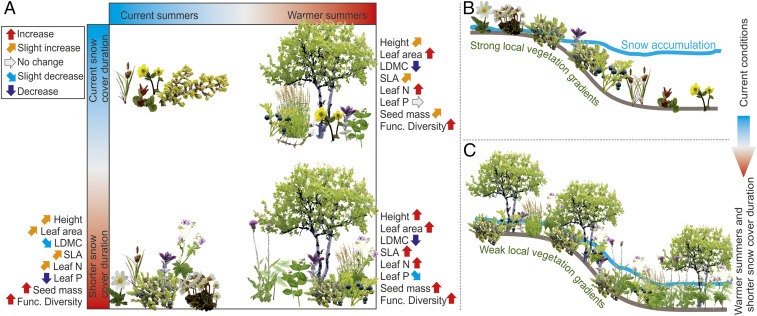
Our models display clear local-scale effects of temperature on functional composition and diversity of tundra vegetation (Fig. 4). Nevertheless, we found also clear deviations from these trends triggered by heterogeneous SCD, highlighting the importance of snow for functional properties of vegetation in cold climate ecosystems. In the forthcoming decades, increasing temperatures and shorter SCD have the potential to jointly drive the tundra toward strong directional changes in plant traits, altering the functionality of the whole ecosystem. Importantly, depending on the magnitude of the environmental change and trait in question, changes in these two environmental factors will either buffer or reinforce each other’s impacts on ecosystem processes.
Fig. 4.
A conceptual summary of the main results. (A) Increasing temperatures and/or shorter SCD will have directional effects on CWM of the most important plant functional (Func.) traits, but the magnitude of the effects is highly dependent on which one of the climatic factors is changing (A). Under current climate, topography generates uneven snow accumulation and high functional turnover across the local SCD gradients (B). Shorter SCD and warmer summer temperatures will increase the local functional richness but reduce the functional variability between communities, that is, subtract the importance of local topographic gradients associated with snow accumulation and persistence (C).
Comparing our modeling results with previous experimental and gradient studies that have related plant functional traits to snow and temperature conditions is challenging for several reasons: 1) Tundra experiments have been usually rather short term and measured the trait variation mainly within species (intraspecific trait variation [ITV]); 2) studies considering whole communities and multiple traits are rare; 3) experiments that have simulated both the advancement of snow melt and warming of summer temperatures are largely missing, and the majority of the studies have delayed the snow melt instead (30); 4) in many cases, the vegetation responses triggered by experimental treatments were driven by single species, which makes generalizations difficult (31, 32); and 5) studies are very heterogeneous in terms of duration, gradient lengths, treatment magnitudes, ambient snow and temperature conditions, and overall methodologies. However, despite this variability, commonly reported results largely concur with our findings, including the increase of plant height and leaf sizes as a response to shorter snow season and higher summer temperatures (24, 33–35), and a higher leaf nutrient concentration and SLA in late melting sites or under snow-adding treatments (24, 36, 37). In this study, we examined the indirect effects of changing environmental conditions on CWM traits (i.e., species turnover), but intraspecific trait responses can also be important, especially at shorter time scales (24). However, both turnover and ITV seem to shift the community trait compositions in the same direction; for example, both warmer conditions and shorter SCD lead to taller individuals within species as well as directional community turnover toward taller species (20).
Our study area is compact, but it contains wide environmental gradients and range of habitat types representative of large parts of the tundra biome. Even if the species pool somewhat varies between regions, similar vegetation shifts along the snow accumulation gradients have been reported all across the tundra biome (23, 37). Thus, we consider our results applicable for a wide range of tundra areas where topographic heterogeneity allows uneven snow accumulation and duration. In flat and dry regions, snow may still be an important filter for species, but applying our results to such areas should be done by carefully considering the ambient temperature and snow conditions of the target region. Furthermore, Arctic regions where changing snow and thermal conditions will trigger drastic changes in permafrost and the currently waterlogged soils may react very differently compared to our Low Arctic system (38, 39). In essence, snow conditions are projected to change all over the tundra biome, but the magnitude may vary. Thus, the different future projections simulated here can be relevant for different regions. Furthermore, it should be noted that the current limited knowledge makes it difficult to assess how snow conditions at different topographic positions will respond to climatic changes. Thus, our approach to simulate similar percentage change in SCD across the snow accumulation and elevational gradients is a useful but nevertheless simplified approximation of the potential changes.
The importance of snow in controlling functional trait composition of tundra vegetation may rise from several mechanisms. Firstly, SCD limits the length of the growing season, but it is also tightly linked to the thickness of the snowpack and consequently its insulating capacity. Thus, snow has strong local control on both summer and winter thermal conditions and incoming solar energy (32, 40, 41). Secondly, snow conditions are also directly or indirectly linked to many environmental factors other than energy and temperatures. These include, for example, soil moisture, wind desiccation, ice crystal abrasion, soil forming processes, and nutrient mineralization (32, 41, 42). All these mechanisms may select plant species according to their life forms, size, structure, and biochemistry and thus act as strong local filters, producing a wide range of functionally different plant communities along the snow gradients. The fine-scale snow accumulation is largely controlled by local topography in tundra, but topography is also driving soil moisture and water flow, which are not linked to snow and meltwaters. However, we controlled the soil moisture effects emerging from local topography by including a topographic wetness index in our models. This enabled us to dissect the unique impacts of SCD on species traits, not blurred by the potentially confounding effect of the local topography.
It is noteworthy, that according to our models, climate change will increase the average FD in tundra but decrease the variability between communities. This outcome is based on the fact that if the late-lying snowbed environments are lost, the communities will be more diverse as such but functionally more alike; that is, there will be increasing similarity in their functional trait profiles. Therefore, the ecosystem resilience and stability may increase locally but decrease at the scale of the whole ecosystem, due to these conflicting trends (2, 14). Our results show that the loss of snowbed environments will wipe out snow specialist species that differ from the other species, especially in their size-related traits (short species with small leaves and seeds), whereas, for leaf economics, there are no clear trends. Thus, especially in the case of size-related traits, species extinctions may also play an important role in the landscape-level functionality, not only compositional turnover caused by colonizing species (29, 34).
Recent coarse-scale investigation (43) found relatively weak trait–environment relationships of plot-scale plant communities at global scale but large within-region variability, indicating high relevance of local conditions. Our results support those findings: Even though the wide temperature gradient in our study area had a clear effect on functional composition, the local snow conditions were notably important determinants of how the effects of climate change are manifested in tundra vegetation. This suggests that snow conditions represent local-scale agents whose importance is masked out in coarse-scale analyses. Heterogeneous SCD is a characteristic element of Arctic and alpine landscapes. It is evident that this environmental variability enables relatively high taxonomic diversity, as found in earlier studies (25, 29, 42), but also high FD and variation between communities as highlighted here.
An Arctic-wide study explored relationships between temperature, moisture, and largely the same functional traits as investigated here (20). It concluded that the temperature−trait relationships are strongly dependent on local soil moisture conditions, and that, especially, traits related to leaf economics had weaker links to summer temperatures than expected. We propose that snow conditions have a role similar to soil moisture and mediate the effects of other environmental factors on trait compositions. We also showed that the traits that did not show such clear relationships with temperature at Arctic-wide scale had, here, clear links to snow conditions, an environmental factor that is not typically considered in studies investigating trait−environment relationships in cold ecosystems.
The importance of snow has been demonstrated for plant distributions, biodiversity patterns, and functions of tundra ecosystems (24, 29, 44), but at least two bottlenecks exist, obstructing putting this knowledge into action and routine integration of fine-scale snow information into ecosystem models of Arctic tundra. Firstly, availability of snow information at ecologically relevant spatiotemporal scales is rather poor. Here we provided a procedure applicable to any region to construct such information, but, for successful applications across large areas, closer cooperation between remote sensing specialists and ecologists is needed.
Secondly, snow has complex relationships with global climate patterns. The local snow conditions are a result of tangled interactions between temperature, precipitation, wind, radiation, and local topography (45). This complexity exists also in our mountainous study area, as SCD has surprisingly low correlation with temperatures (rs = −0.45). In recent decades, northern Fennoscandia has experienced a slight increase in winter precipitation and maximum snow depth but a significant decrease in spring snow depth and SCD (29, 46, 47). This indicates that gains in winter snowfall are not enough to counterbalance the advanced snow melt caused by higher spring and summer temperatures (48). However, rough projections of the future snow conditions present huge variation between Arctic regions and demonstrate high uncertainty. Here we simulated a wide range of SCD scenarios, but it is difficult to evaluate which one is the most probable for the study area and is even more problematic for other Arctic regions. Given the high importance of snow conditions for the future of tundra vegetation, these uncertainties in snow projections represent an important open question to be tackled by future research and methodological development in climate change impact modeling.
Finally, we acknowledge that we have used correlative models in our data analyses that cannot easily account for population dynamics, functional plasticity, dispersal limitation, species interactions, and time lags in responses (49). We also recognize that the most drastic climate and snow scenarios used in our study include an increased risk for making extrapolations to novel environmental conditions (outside the range of the data used in model fitting), which produces some uncertainties in those projections. Another important point is that the traits vary also within species, and this ITV can be important along the environmental gradient, especially in the shorter term. However, we consider that there are multiple aspects in our study design and methodology that reduce the potential shortcomings related to these questions: 1) The study area is rather compact, and, thus, no severe dispersal limitation is likely to occur; 2) our study area consists of the maximum environmental gradients found in northern Fennoscandia; and, 3) although ITV can be important, the ITV seems to follow the same patterns as the trait variation between species, and it should not change our conclusions but rather make them stronger.
Conclusions
Previous studies have shown the importance of snow in modulating microclimate, altering species distributions, extinction rates, and species richness (7, 29, 40, 42, 44). Here we demonstrate that the same holds for plant functional composition and diversity in Arctic tundra. SCD is strongly linked to functional traits, and the local manifestation of climate warming effects on tundra vegetation is dependent on the evolution of the snow conditions. Consequently, realistic assessments of the future Arctic vegetation patterns and ecosystem functioning require acknowledging the role of snow in tundra vegetation models and robust projections of the future snow conditions at fine spatial scales.
Materials and Methods
Study Area.
The study area is located in northern Norway and consists of 195 km2 of mountainous tundra (70°0′N, 26°14′E). The altitude spans from 120 m above sea level (m a.s.l.) to 1064 m a.s.l. (Mt. Rastigaisa is the highest peak). Therefore, the area contains large environmental gradients, especially in temperatures and snow conditions (Fig. 1). The lowest valleys are forested (mainly Betula pubescens subsp. chzerepanovii), and the tree line reaches the altitudes of 250 m a.s.l. to 350 m a.s.l., depending on slope aspect. The tundra above is mostly dominated by dwarf shrubs (e.g., Empetrum nigrum subsp. hermaphroditum and Betula nana subsp. nana) (50). Different snowbeds, wetlands, and meadows are inhabited more by forbs and graminoids, but these habitats are relatively small and restricted to topographically sheltered locations and along mountain creeks (44). The area is grazed by reindeer mainly in winter and by lemmings and voles that have distinctive population dynamics with rather rarely occurring peak years (51). The wide environmental gradients and contrasting plant communities within the relatively compact study area with minimal direct human impact serve as a suitable modeling environment for testing hypotheses of the potential effects of climate changes on natural vegetation patterns (52).
Vegetation Data.
The plant community data consist of 5,300 1-m2 vegetation plots clustered within 1,325 study sites. In each site, four plots were situated 5 m from the center of the study site toward the four principal compass directions. All vascular plants from all plots were identified at species level (with a few exceptions, such as Taraxacum spp.), and their percentage cover values were estimated. In this study, we used site-level data with species cover values averaged over the four plots within each site. Nomenclature and species identification follow the species lists by the Finnish Biodiversity Information Facility (https://laji.fi). The data were collected in summers 2014–2018.
Trait Data.
Trait observations for seven widely used plant functional traits were downloaded from three international databases: Tundra Trait Team (TTT) (10), TRY Plant Trait Database (TRY) (9, 27), and the Botanical Information and Ecological Network (BIEN) (28). The seven traits were plant vegetative height, SLA (leaf area per leaf dry mass), LDMC, leaf area, LeafN, LeafP and seed dry mass. The total number of available trait observations for the study species was 65,746. We calculated a median trait value per species per trait (median instead of mean to handle possible outliers in the heterogeneous original data), preferably by using the TTT data, because the TTT dataset is collected from ecosystems similar to the study system here. If TTT contained less than five trait observations per trait for a given species, we also used data from TRY and BIEN. For species that lack species-level trait observations, we calculated genus or family-level values. For cryptogams that do not have seeds but spores instead, we used the minimum seed mass found among the other study species (0.001 mg) to reflect their dispersal capability. The species-specific medians covered 96.2 to 99.4% of the cover weighted community data, and thus, the genus- and family-level trait values had only a trivial effect on the CWM traits.
TRY trait observations correspond to TRY trait ID numbers 3106 (height), 47 (LDMC), 3108, 3109, 3110, 3111, 3112, 3113, and 3114 (leaf area; for species with compound leaves only, 3108, 3110, 3112 and 3114), 14 (leaf N), 15 (leaf P), 26 (seed mass), and 3115, 3116, and 3117 (SLA).
FD is a rather complex concept of biodiversity and is dependent on several choices made by the researcher (13). During recent decades, there have been suggested multiple methodologically contrasting indices that may also give contrasting results (53). Therefore, we calculated six indices that describe the aspects of FD and treat the trait data differently. Prior to the FD calculations, we log-transformed the species-specific median values for height, leaf area, and seed mass, because these three traits had few very large values possibly fully overriding the effects of other traits and species in calculating FD. After this, we also standardized all seven traits. We used the species abundances as weights in the FD calculations (except in calculating FDgp, a dendrogram-based functional diversity index). See Table 1 for a summary of the calculated FD indices.
Table 1.
The six FD indices used in the analyses
| Abbreviation | Description | R function and library | Ref. |
| FDis | Functional dispersion index | dbFD/FD | (54) |
| FDpg | Dendrogram-Based Functional Diversity index | FD_dendro/fundiv | (3) |
| FDw | Abundance weighted version of dendrogram-Based Functional Diversity index | FD_dendro/fundiv | (3) |
| FEve | Functional evenness index | dbFD/FD | (8) |
| FRic | Functional richness index | dbFD/FD | (8) |
| RaoQ | Rao's quadratic entropy index | dbFD/FD | (55) |
Environmental Data.
This study focuses mainly on the effects of changing summer temperatures (Tsummer), and SCD. Tsummer is an average free air temperature for June, July, and August. It is based on a gridded climate dataset introduced in Aalto et al. (56). Aalto et al. utilized the climate record of 942 climate stations in Sweden, Norway, and Finland, and a digital elevational model, to statistically model climate surfaces at 50-m resolution (30-y climate period: 1981–2010), resampled here to 10-m resolution. Tsummer is the only climatic variable used here, as all macroclimatic factors are highly correlated (r |>0.9|) in the compact study area. SCD is based on 135 cloud-free Landsat (Thematic Mapper 5, Enhanced Thematic Mapper Plus 7, and Operational Land Imager 8) images covering the whole study area from March to October in 1984–2016. We calculated a normalized difference snow index from the images and then binarized (snow/no snow) them. From the binarized imagery, we calculated average melting and snowfall days pixel by pixel using binomial regression. For a detailed description of the method, workflow, and validation of the SCD variable, see refs. 29, 44.
Arctic SCD is projected to decrease by 10 to 40% before 2050 (48). Because the range in SCD projections is that large, we simply reduced the observed pixel-based SCD by 5%, 10%, 15%, 20%, 25%, 30%, 35%, and 40% to test a wide range of possible snow trajectories. Thus, the absolute change is smaller in sites with short SCD and bigger in late-lying snowbeds. The Tsummer variable was projected for the period 2040–2069 forced by three RCP scenarios, 2.6, 4.5, and 8.5. The projected temperatures were averaged over 23 CMIP5 (Coupled Model Intercomparison Project phase 5) climate simulations (57). The nine SDC and four temperature scenarios (no-change scenario also included for both variables) resulted in 36 possible combinations which were all used in the vegetation projections over the study area.
Our target was to construct reasonable and realistic models for the cover of the study species. Therefore, we also included four other environmental predictors—in addition to Tsummer and SCD variables—known to be important for plants in tundra environments (26). These additional variables were potential annual incoming solar radiation (RAD), topographic wetness index (SAGA [System for Automated Geoscientific Analyses] wetness index algorithm; hereafter, TWI), surface soil quality (SOILQ), and soil edaphic conditions (EDAP).
RAD and TWI were derived from an ∼2-m-resolution digital elevation model (DEM). The DEM was constructed by mean merging and edge matching 10 individual surface model tiles produced by ArcticDEM program by the Polar Geospatial Center. They have applied stereo autocorrelation techniques to overlapping pairs of high-resolution Worldview satellite images to create three-dimensional models of the terrain surfaces. Stereographic methods are affected by high vegetation but are working well in tundra areas covered by low-stature plants.
The potential annual incoming solar radiation (in kilowatt hours per square meter per year) was calculated using the Potential Incoming Solar Radiation Tool from SAGA-GIS (System for Automated Geoscientific Analyses - Geographic Information System) Terrain Analysis toolbox and utilizing Sky View Factor analyses with 10-km radius from the same toolbox. The SAGA wetness index algorithm that was used to calculate the TWI variable is a modification of the traditional topographical wetness index. Prior to the TWI calculation, we preprocessed the DEM by filling possible sinks and then used it to calculate the specific catchment area and slope required by the TWI algorithm.
SOILQ represents the quality of the surface soil conditions with five classes: peat, fluvial sediments, glacial till, bolder field, and bare rock. SOILQ was digitized and interpreted from fine-scale satellite images (resolutions of 0.5 m to 1.4 m) and verified with field examinations. EDAP characterizes the nutritional status of the bedrock and was calculated as the downhill Euclidean distance to the shale belt (the only base-rich rock in the area) scaled from 0 to 100. Areas located on the shale belt were valued at 100, and areas uphill from shale belt or outside the area where the shale belt drains were set to 0. The geological bedrock data were gathered from the bedrock geology database maintained by Geological Survey of Norway (geo.ngu.no/kart/berggrunn/). Values of the predictors were extracted for the study points from rasters with the original resolutions, but, for spatial predictions, all predictors were resampled with bilinear interpolation to 30-m spatial resolution (as in Landsat satellite images used in constructing the SCD variable). SOILQ was an exception because it is a nominal variable, and therefore a maximum area aggregation was used instead of bilinear interpolation.
Modeling.
All data processing and statistical analyses were performed using statistical software R (58). The models were fitted with randomForest (59) and gbm (60) R libraries with the help of streamlined modeling functionalities of the caret library (61). All raster processing utilizes functions of the raster library (62).
We modeled the cover values of 135 species individually using two modeling methods, generalized boosted models (GBM) and random forests (RF) and their ensemble. Before the modeling, the absolute cover values were transformed to relative cover values, so that each species-specific cover value is a proportion of the total vegetation cover within each study site. The 135 species consist of all vascular plant taxa recorded in at least eight study sites. The modeling method specific modeling parameters were tuned by testing a range of parameter combinations (GBM: interaction depth = 3, 4, 5; n.trees = 500, 1,000, 1,500, 2,000, 2,500; shrinkage = 0.01, 0.005, 0.001; n.minobsinnode = 5, 10; RF: mtry = 2, 4, 6, 8, 10). The parameters leading to the best predictive performance were selected for each species separately.
The predictive performance of the models was tested with four-fold cross-validation repeated three times for each species. There the data are divided randomly into four subsets. Each subset is, in turn, removed from the data, and the model is fitted with the remaining data points and then used to predict to the withheld data. As this procedure is repeated three times, each data point is predicted exactly three times. As a final prediction, we took an average of these three predictions. The overall predictive performance at the community level was evaluated by examining how well the models were able to reconstruct the CWM of the seven traits. We used squared correlation (R2) between the observed and reconstructed CWM traits as the evaluation metric. The model performance was tested for GBM and RF separately and for ensemble modeling methods: simple mean and predictive performance (R2) weighted mean of the two models. The latter produced the highest predictive performance and was used to construct the spatial predictions across the study area and the range of future scenarios.
To analyze which species are likely to disappear from the study area, we fitted models similar to those we used to model the cover values, but with binary species data (presence/absence), and projected the species’ distributions under each temperature and SCD scenario (ensemble prediction: a species was considered as present if both modeling methods [GBM and RF] predicted occurrence). We checked which species were predicted to lose more than 95% of their current range within the study area and then tested whether the functional trait values of these species differed statistically from the traits of all other species, by using two-tailed Wilcoxon rank sum test.
Supplementary Material
Acknowledgments
We are grateful for research support provided by the Academy of Finland (Projects 286950 and 312559), Kone Foundation, Societas pro Fauna et Flora Fennica, the Doctoral Programme in Geosciences at the University of Helsinki, and “The protected area network in the changing climate (SUMI)” project funded by the Ministry of Environment, Finland. We thank all members of the BioGeoClimate Modelling Lab for assisting with the fieldwork.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001254117/-/DCSupplemental.
Data Availability.
All data used in this manuscript are publicly available. and are deposited in the Zenodo public data repository (https://doi.org/10.5281/zenodo.3956705) (63).
References
- 1.Blowes S. A. et al., The geography of biodiversity change in marine and terrestrial assemblages. Science 366, 339–345 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Diaz S., Cabido M., Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655 (2001). [Google Scholar]
- 3.Petchey O. L., Gaston K. J., Functional diversity (FD), species richness and community composition. Ecol. Lett. 5, 402–411 (2002). [Google Scholar]
- 4.Diaz S. et al., The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 15, 295–304 (2004). [Google Scholar]
- 5.Myers-Smith I. H., Thomas H. J. D., Bjorkman A. D., Plant traits inform predictions of tundra responses to global change. New Phytol. 221, 1742–1748 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Lavorel S., Garnier E., Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 16, 545–556 (2002). [Google Scholar]
- 7.Zhu L. K., Ives A. R., Zhang C., Guo Y. Y., Radeloff V. C., Climate change causes functionally colder winters for snow cover-dependent organisms. Nat. Clim. Chang. 9, 886–893 (2019). [Google Scholar]
- 8.Villéger S., Mason N. W. H., Mouillot D., New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Kattge J. et al., TRY—A global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011). [Google Scholar]
- 10.Bjorkman A. D. et al., Tundra Trait Team: A database of plant traits spanning the tundra biome. Glob. Ecol. Biogeogr. 27, 1402–1411 (2018). [Google Scholar]
- 11.Cornwell W. K. et al., Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Tilman D. et al., The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302 (1997). [Google Scholar]
- 13.Petchey O. L., Gaston K. J., Functional diversity: Back to basics and looking forward. Ecol. Lett. 9, 741–758 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Isbell F. et al., Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Loreau M. et al., Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 294, 804–808 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Callaghan T. V. et al., Biodiversity, distributions and adaptations of Arctic species in the context of environmental change. Ambio 33, 404–417 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Arctic Monitoring and Assessment Programme , Snow, Water, Ice and Permafrost in the Arctic (SWIPA) 2017 (Arctic Monitoring and Assessment Programme, 2017).
- 18.Lupascu M. et al., High Arctic wetting reduces permafrost carbon feedbacks to climate warming. Nat. Clim. Chang. 4, 51–55 (2014). [Google Scholar]
- 19.Pearson R. G. et al., Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Chang. 3, 673–677 (2013). [Google Scholar]
- 20.Bjorkman A. D. et al., Plant functional trait change across a warming tundra biome. Nature 562, 57–62 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Roos R. E. et al., Contrasting drivers of community-level trait variation for vascular plants, lichens and bryophytes across an elevational gradient. Funct. Ecol. 33, 2430–2446 (2019). [Google Scholar]
- 22.Thomas H. J. D. et al., Global plant trait relationships extend to the climatic extremes of the tundra biome. Nat. Commun. 11, 1351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billings W. D., Mooney H. A., Ecology of Arctic and alpine plants. Biol. Rev. Camb. Philos. Soc. 43, 481–529 (1968). [Google Scholar]
- 24.Happonen K. et al., Snow is an important control of plant community functional composition in oroarctic tundra. Oecologia 191, 601–608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart L., Simonsen C. E., Svenning J. C., Schmidt N. M., Pellissier L., Forecasted homogenization of high Arctic vegetation communities under climate change. J. Biogeogr. 45, 2576–2587 (2018). [Google Scholar]
- 26.Mod H. K., Scherrer D., Luoto M., Guisan A., What we use is not what we know: Environmental predictors in plant distribution models. J. Veg. Sci. 27, 1308–1322 (2016). [Google Scholar]
- 27.Kattge J. et al., TRY plant trait database–enhanced coverage and open access. Glob. Change Biol. 26, 119–188 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Maitner B. S. et al., The BIEN R package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol. Evol. 9, 373–379 (2018). [Google Scholar]
- 29.Niittynen P., Heikkinen R. K., Luoto M., Snow cover is a neglected driver of Arctic biodiversity loss. Nat. Clim. Chang. 8, 997–1001 (2018). [Google Scholar]
- 30.Wipf S., Rixen C., A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res. 29, 95–109 (2010). [Google Scholar]
- 31.Wahren C. H. A., Walker M. D., Bret-Harte M. S., Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob. Change Biol. 11, 537–552 (2005). [Google Scholar]
- 32.Leffler A. J., Klein E. S., Oberbauer S. F., Welker J. M., Coupled long-term summer warming and deeper snow alters species composition and stimulates gross primary productivity in tussock tundra. Oecologia 181, 287–297 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Komac B., Pladevall C., Penuelas J., Conesa J. V., Domenech M., Variations in functional diversity in snowbed plant communities determining snowbed continuity. Plant Ecol. 216, 1257–1274 (2015). [Google Scholar]
- 34.Pickering C., Green K., Barros A. A., Venn S., A resurvey of late-lying snowpatches reveals changes in both species and functional composition across snowmelt zones. Alp. Bot. 124, 93–103 (2014). [Google Scholar]
- 35.Venn S. E., Green K., Pickering C. M., Morgan J. W., Using plant functional traits to explain community composition across a strong environmental filter in Australian alpine snowpatches. Plant Ecol. 212, 1491–1499 (2011). [Google Scholar]
- 36.Semenchuk P. R. et al., Deeper snow alters soil nutrient availability and leaf nutrient status in high Arctic tundra. Biogeochemistry 124, 81–94 (2015). [Google Scholar]
- 37.Choler P., Consistent shifts in Alpine plant traits along a mesotopographical gradient. Arct. Antarct. Alp. Res. 37, 444–453 (2005). [Google Scholar]
- 38.Higgens R. A. F. et al., Changing lake dynamics indicate a drier Arctic in Western Greenland. J. Geophys. Res. Biogeosci. 124, 870–883 (2019). [Google Scholar]
- 39.Riordan B., Verbyla D., McGuire A. D., Shrinking ponds in subarctic Alaska based on 1950-2002 remotely sensed images. J. Geophys. Res. Biogeosci. 111, G04002 (2006). [Google Scholar]
- 40.Aalto J., Scherrer D., Lenoir J., Guisan A., Luoto M., Biogeophysical controls on soil-atmosphere thermal differences: Implications on warming Arctic ecosystems. Environ. Res. Lett. 13, 074003 (2018). [Google Scholar]
- 41.Webb E. E. et al., Increased wintertime CO2 loss as a result of sustained tundra warming. J. Geophys. Res. Biogeosci. 121, 249–265 (2016). [Google Scholar]
- 42.Carlson B. Z., Choler P., Renaud J., Dedieu J. P., Thuiller W., Modelling snow cover duration improves predictions of functional and taxonomic diversity for alpine plant communities. Ann. Bot. 116, 1023–1034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruelheide H. et al., Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2, 1906–1917 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Niittynen P., Luoto M., The importance of snow in species distribution models of arctic vegetation. Ecography 41, 1024–1037 (2018). [Google Scholar]
- 45.Liston G. E., Elder K., A distributed snow-evolution modeling system (SnowModel). J. Hydrometeorol. 7, 1259–1276 (2006). [Google Scholar]
- 46.Luomaranta A., Aalto J., Jylha K., Snow cover trends in Finland over 1961-2014 based on gridded snow depth observations. Int. J. Climatol. 39, 3147–3159 (2019). [Google Scholar]
- 47.Pulliainen J. et al., Patterns and trends of Northern Hemisphere snow mass from 1980 to 2018. Nature 581, 294–298 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Callaghan T. V. et al., The changing face of Arctic snow cover: A synthesis of observed and projected changes. Ambio 40, 17–31 (2011). [Google Scholar]
- 49.Guisan A., Thuiller W., Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Ryvarden L., The vascular plants of the Rastigaissa area (Finnmark, Northern Norway). Acta Boreal. 26, 1–56 (1969). [Google Scholar]
- 51.Tuomi M. et al., Herbivore effects on ecosystem process rates in a low-productive system. Ecosystems 22, 827–843 (2019). [Google Scholar]
- 52.Elsen P. R., Tingley M. W., Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 5, 772–776 (2015). [Google Scholar]
- 53.Schleuter D., Daufresne M., Massol F., Argillier C., A user’s guide to functional diversity indices. Ecol. Monogr. 80, 469–484 (2010). [Google Scholar]
- 54.Laliberté E., Legendre P., A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Botta-Dukat Z., Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 16, 533–540 (2005). [Google Scholar]
- 56.Aalto J., Riihimäki H., Meineri E., Hylander K., Luoto M., Revealing topoclimatic heterogeneity using meteorological station data. Int. J. Climatol. 37, 544–556 (2017). [Google Scholar]
- 57.Taylor K. E., Stouffer R. J., Meehl G. A., An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93, 485–498 (2012). [Google Scholar]
- 58.R Core Team , R: A Language and Environment for Statistical Computing, Version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria, 2019), https://www.R-project.org/. Accessed 3 January 2020.
- 59.Liaw A., Wiener M., Classification and regression by randomForest. R News 2, 18–22 (2002). [Google Scholar]
- 60.Greenwell B., Boehmke B., Cunningham J., GBM_Developers, gbm: Generalized Boosted Regression Models. R Package Version 2.1.5. https://cran.r-project.org/web/packages/gbm/index.html (2019).
- 61.Kuhn M., caret: Classification and Regression Training. R package version 6.0-85. https://cran.r-project.org/web/packages/caret/index.html (2020).
- 62.Hijmans R. J., raster: Geographic Data Analysis and Modeling. R package version 3.0-12. https://cran.r-project.org/web/packages/raster/index.html (2020).
- 63.Niittynen P., Luoto M., Data for “Decreasing snow cover alters functional composition and diversity of Arctic tundra.” Zenodo. 10.5281/zenodo.3956705. Deposited 22 July 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this manuscript are publicly available. and are deposited in the Zenodo public data repository (https://doi.org/10.5281/zenodo.3956705) (63).