Significance
A large body of work highlights disparities in survival rates across Black and White newborns during childbirth. We posit that these differences may be ameliorated by racial concordance between the physician and newborn patient. Findings suggest that when Black newborns are cared for by Black physicians, the mortality penalty they suffer, as compared with White infants, is halved. Strikingly, these effects appear to manifest more strongly in more complicated cases, and when hospitals deliver more Black newborns. No such concordance effect is found among birthing mothers.
Keywords: racial bias, birthing outcomes, concordance, mortality, health care
Abstract
Recent work has emphasized the benefits of patient–physician concordance on clinical care outcomes for underrepresented minorities, arguing it can ameliorate outgroup biases, boost communication, and increase trust. We explore concordance in a setting where racial disparities are particularly severe: childbirth. In the United States, Black newborns die at three times the rate of White newborns. Results examining 1.8 million hospital births in the state of Florida between 1992 and 2015 suggest that newborn–physician racial concordance is associated with a significant improvement in mortality for Black infants. Results further suggest that these benefits manifest during more challenging births and in hospitals that deliver more Black babies. We find no significant improvement in maternal mortality when birthing mothers share race with their physician.
The relationship between a decision maker’s ascriptive characteristics and advocates who do or do not share those characteristics has long been a source of intense scrutiny by scholars across a wide range of disciplines. Researchers in sociology have noted the benefits of female leadership for young women working at firms (1, 2). Management scholars note increased leniency in enforcing regulatory compliance when inspectors and their targets share similar backgrounds (3). Economists have shown that academic performance is higher when students share race with teachers (4). In addition, legal scholars have found higher incarceration rates among defendants paired with judges of a different race (5).
However, despite the prevalence of these findings, little evidence on the effect of gender and racial concordance in medicine existed until recently. Although received work indicates concordance can increase rapport between the patient and the physician for minorities (6–9), its effect on clinical care outcomes is only beginning to coalesce (10, 11). Researchers have, for example, found that gender concordance increases the willingness of women to participate in preventative screenings (12), and racial concordance with one’s physician can increase health care utilization among underresourced communities (11, 13). Nonetheless, limited empirical evidence exists that these communication and care benefits translate into material health benefits (14, 15).
In this work, we investigate the potential for patient–physician racial concordance to ameliorate the disparities experienced by a particularly vulnerable group—Black newborns. As both scholars (16) and the popular press (17) have noted, Black newborns face starkly worse clinical outcomes than White newborns in the United States. Black newborns are more than twice as likely to die in their first year as White newborns [1,090 vs. 490 deaths per 100,000 births, respectively (18)]. The reasons behind these disparities range from increased rates of eclampsia and preeclampsia during pregnancy (19), to preterm delivery (20), to social determinants like socioeconomic inequality and racial bias (21–23). In fact, mortality among Black infants outstrips medical inequalities in many other health domains (24, 25). New evidence can inform approaches to address this pressing social issue. Furthermore, to the extent that newborns cannot verbally communicate with their physician, we are able to observe the effects of concordance without trust or communication issues affecting the patient–physician relationship. Inasmuch as prior research has struggled to disentangle the mechanisms behind concordance’s effect (10, 26), the setting allows us to explore concordance in the absence of one invoked mechanism—communication. Thus, if concordance effects manifest, we are able to rule out communication as the exclusive mechanism.
Research posits that racial concordance between a newborn and their physician may mitigate disparities for at least two reasons. First, research suggests concordance is not only salient for adults. Indeed, a growing body of literature explores the question of whether actors exhibit different levels of bias toward both children and adults. Wolf et al. (27), for example, examine whether adults’ spontaneous racial bias toward children differs from their spontaneous racial bias toward adults, finding that people have significantly greater favorability toward their in-group. Strikingly, this bias was exhibited equally toward adults and children. It is therefore possible that such an effect might manifest exclusively as a function of spontaneous bias. At the same time, extant research indicates that mortality across White and Black newborns is starkly different (28), suggesting Black newborns may have different needs and be more medically challenging to treat due to social risk factors and cumulative racial and socioeconomic disadvantages of Black pregnant women (29). To the extent that physicians of a social outgroup are more likely to be aware of the challenges and issues that arise when treating their group (10, 30, 31), it stands to reason that these physicians may be more equipped to treat patients with complex needs.
Results indicate four key findings. First, Black infants experience inferior health outcomes regardless of who is treating them. However, clinical penalties for Black newborns treated by Black physicians are halved compared with the penalties Black newborns experience when cared for by White physicians. Second, these benefits accrue more sharply in more medically complicated cases, insofar as the performance disparity across White and Black physicians increases as the number of newborn comorbidities rises. Third, these effects are more pronounced at hospitals that deliver more Black newborns. Finally, we observe no effect of concordance on mortality for birthing mothers, suggesting communication is not the exclusive mechanism by which concordance benefits will manifest.
To examine these questions, we leverage data from the State of Florida’s Agency for Healthcare Administration (AHCA). These data, which have been used extensively in public health and economics (32–34), provide a census of patients admitted to Florida hospitals between 1992 and 2015. We do not extend prior to 1992 because information on patient race is unavailable. We end our investigation in quarter 3 of 2015 because the AHCA switches comorbidity coding from ICD-9 to ICD-10. This allows us to maintain consistent measurement during the sample. These data grant us access to detailed information about both the mother and newborn, including the following: race, comorbidities, outcomes, the hospital where they are treated, and more. We also receive access to information about the attending physician in charge of the patient’s care, e.g., name, specialty certifications, and date of licensure. Physician race is not coded by the data and is captured from publicly searchable pictures of the physician. Newborn arrival is directly coded by the AHCA and is defined as “a baby born within the facility or the initial admission of an extramural birth infant to an acute care facility within 24 h of birth.” To ease interpretation, all newborns not White or Black, and all physicians not coded as White or Black, are dropped from the sample, isolating our examination to strictly White and Black patients and physicians. A discussion of this process is in SI Appendix. Summary statistics are in SI Appendix, Table S1A and a correlation matrix is in SI Appendix, Table S1B.
We first consider model free evidence from the SI Appendix, Table S1A. Consistent with extant research, we see a large mortality penalty for Black newborns (21, 24). In the sample, the raw mortality rate is 289 per 100,000 births among the 1.35 million White newborns and is 784 per 100,000 births among the 0.46 million Black newborns. Applying the sample mortality rate to the current number of Black newborns born in the United States implies ∼4,400 Black newborn deaths annually. If these newborns experienced the same mortality rate as White newborns, this number would fall by roughly 2,800 deaths annually. We also note differences across the newborn patient pools in SI Appendix, Table S1A. Black physicians, for example, appear more likely to treat underresourced patients, i.e., those on Medicaid, and specifically more underresourced White patients. Black physicians are also more likely to be female. Rates of board certification in pediatrics are broadly similar across groups, as are rates of cesarean sections. Furthermore, Black physicians care for newborns with slightly higher comorbidity count. It is also worth comparing the included sample to the omitted sample. As can be seen, omitted patients are similar in terms of mortality, physician gender distribution, length of stay, cesarean rates, and comorbidity counts. However, the omitted patients are less likely to be treated by a pediatrician, and there are differences in insurance provider, which does raise the possibility of selection. Finally, we consider caseload. Conservatively, because newborn care is not the only responsibility a pediatrician may have, we observe that Black pediatricians have a slightly higher caseload (83 patients per year vs. 67 patients per year), but the SDs are large, and rates are trending up. These differences underscore the need to rigorously investigate how physician–patient racial concordance affects disparities in mortality. To do so, we estimate the following:
| [1] |
where y is a dichotomous variable indicating whether or not infant-i born under the care of physician-j in quarter-t expires. y equals zero if the infant survives and 100 if it infant expires. indicates infant race (1 for Black, 0 for White). indicates physician race (1 for Black, 0 for White). expresses the interaction between infant patient and physician race. ijt is the idiosyncratic error term.
Our primary interest is whether the Black–White newborn mortality risk differs depending on physician race. This is captured by . The estimator is an ordinary least squares (OLS) to avoid interpretation issues associated with nonlinear estimators like logit regression (35). Huber–White SEs, clustered on the physician, are applied to avoid the heteroscedasticity problems OLS creates. We first estimate the pooled regression without controls. We subsequently include controls for insurance provider (e.g., Medicaid, self-pay) and for the 65 most-prevalent comorbidities [to account for newborn-specific heterogeneity (SI Appendix, Table S2)]; quarter-year fixed effects; hospital fixed effects; hospital-year fixed effects; and physician fixed effects. Note that the inclusion of physician fixed effects does cause , physician race, to be dropped from the model as it is time invariant. Hospital-year fixed effects are included in deference to the concern that the effects might change over time, and across location. Finally, we split the sample by physician race to allow the controls to enter through physician race.
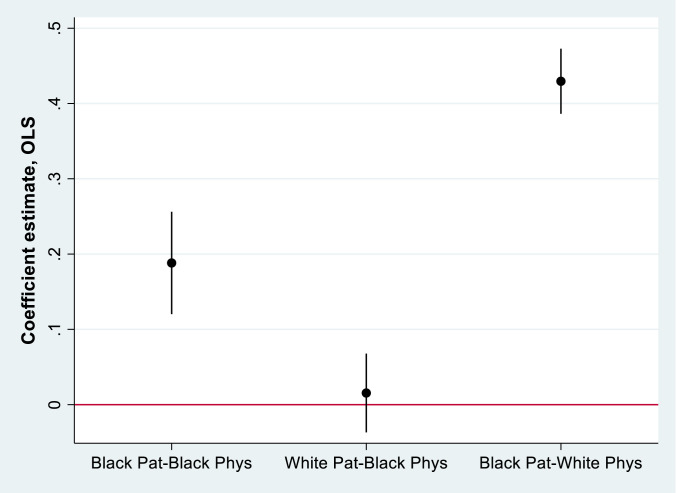
In the simple model absent controls, the Patient Black coefficient indicates that, under the care of White physicians, Black newborns experience triple the in-hospital mortality rate of White infants (column 1 of Table 1). Under the care of White physicians, the White newborn mortality rate is 290 per 100,000 births, as implied by the constant term (0.290). Black newborn mortality is estimated at 894 per 100,000 births (0.290 + 0.604). The Physician Black coefficient implies no significant difference in mortality among White newborns cared for by Black vs. White physicians (columns 1 to 5 of Table 1). In contrast, we observe a robust racial concordance benefit for Black newborns, as captured by the Physician Black * Patient Black interaction. Under the care of White physicians, Black newborns experience 430 more fatalities per 100,000 births than White newborns (column 4). Under the care of Black physicians, the mortality penalty for Black newborns is only 173 fatalities per 100,000 births above White newborns, a difference of 257 deaths per 100,000 births, and a 58% reduction in the racial mortality difference. Results of column 4 are graphed in Fig. 1 (to allow comparisons across race). Concordance appears to bring little benefit for White newborns but more than halves the penalty experienced by Black newborns. In the fully specified model, we add physician fixed effects to allow comparisons of Black and White infant mortality rates within physician (column 6). In these estimations, the mortality penalty for Black newborns is 39% lower under the care of Black physicians than White physicians. Attenuation of the concordance-coefficient as additional controls are added to the model indicates that these observables are correlated with both concordance and mortality outcomes. Thus, it is plausible that the models with fewer controls suffer from an omitted-variable bias. Results of the Oster (36) selection-on-unobservables diagnostic (psacalc) comparing models 1 and 6 equals 0.77, implying that selection on unobservables would have to be 77% as strong as selection on observables to reduce the estimated concordance effect to zero. As controls are added to the model, the diagnostic increases to 0.89 (column 2), 0.99 (column 3), and 1.55 (column 4), well above Oster’s suggested threshold of 1. This underscores the need for controls, which are chosen deliberately as strong predictors, and also indicates that caution regarding the persistence of omitted-variable bias is warranted. The disparity in outcomes persists in separate estimates of the performance of White and Black doctors (columns 7 and 8), showing a mortality penalty for Black newborns in both cases, but a penalty 53% smaller among those treated by Black physicians. Comparing the size of the estimates to prior research suggests the magnitude of the effect is plausible. Uninsured neonates, for example, experience 333 more fatalities per 100,000 births than insured neonates (729 fatalities per 100,000 for uninsured and 396 fatalities per 100,000 for insured) (37). Furthermore, Black newborns experience an additional 187 fatalities per 100,000 births due to low birth weight in general (38). However, both of these social factors are dwarfed by the increase of ∼18,000 deaths per 100,000 for newborns weighing less than 2,500 g, compared with newborns weighing more than 3,500 g (39).
Table 1.
Linear probability model estimates of the effect of racial concordance on survival of newborns
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Dependent variable | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death |
| Sample | Newborns | Newborns | Newborns | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs |
| Physician Black | −0.0103 (0.0652) | −0.00476 (0.0372) | −0.0309 (0.0399) | 0.0155 (0.0455) | 0.0438 (0.0430) | |||
| Patient Black | 0.604*** (0.0667) | 0.494*** (0.0445) | 0.506*** (0.0443) | 0.430*** (0.0385) | 0.425*** (0.0374) | 0.311*** (0.0321) | 0.318*** (0.0318) | 0.169*** (0.0381) |
| Physician Black * Patient Black | −0.494*** (0.0854) | −0.358*** (0.0670) | −0.331*** (0.0630) | −0.257*** (0.0606) | −0.236*** (0.0583) | −0.129** (0.0517) | ||
| Constant | 0.290*** (0.0320) | |||||||
| Insurance fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Comorbidity fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Time fixed effects (quarter) | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Hospital fixed effects | Yes | Yes | Yes | Yes | Yes | |||
| Hospital-year fixed effects | Yes | Yes | ||||||
| Physician fixed effects | Yes | Yes | Yes | |||||
| Observations | 1,812,979 | 1,812,979 | 1,812,979 | 1,812,972 | 1,812,938 | 1,811,979 | 1,583,958 | 228,052 |
| R-squared | 0.001 | 0.045 | 0.046 | 0.050 | 0.058 | 0.144 | 0.137 | 0.151 |
Mean of death, 0.4123. Robust SEs are clustered on the physician. BlackDocs, Black physicians; WhiteDocs, White physicians. ***P < 0.01, **P < 0.05, and *P < 0.1.
Fig. 1.
Effect of racial concordance on patient survival, disaggregated based on column 4 of Table 1. Patient White–Physician White serves as the baseline. Estimates displayed in the absence of the physician fixed effect to allow comparison across physician race. Includes controls, hospital fixed effect, and time fixed effects. The 95% CI is displayed.
The presence of such effects gives rise to auxiliary questions. Are there conditions under which concordance effects are more likely to manifest? Do these results extend to birthing mothers? With regard to conditions under which concordance effects are more likely to manifest, we approach the question in three ways. First, there may be differences across patients, with some cases being more complicated than others. Second, there may be differences across location, with some hospitals being more successful in caring for Black newborns. Finally, there may be differences in the training of physicians, with some physicians being more equipped to provide appropriate care to Black newborns.
We first examine the degree to which increased medical complication affects the relationship. To execute these tests, we split the sample based on whether or not the newborn is diagnosed with at least one of the 65 comorbidities included in the set of controls. We then replicate the estimation of Eq. 1 on each subsample. Results are in Table 2. The estimated effect of concordance is statistically significant at conventional levels in the larger subsample of more complex cases (column 8) and similar but less precise for patients without comorbidities (column 3). Among cases with more than three comorbidities, the estimate is larger but less precise (SI Appendix, Table S3, column 8).
Table 2.
Linear probability model estimates of the effect of racial concordance on survival of newborns split by count of comorbidities
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Dependent variable | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death |
| Sample | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs |
| Restriction | 0 - Comorbid | 0 - Comorbid | 0 - Comorbid | 0 - Comorbid | 0 - Comorbid | 1 or more - Comorbid | 1 or more - Comorbid | 1 or more - Comorbid | 1 or more - Comorbid | 1 or more - Comorbid |
| Physician Black | −0.0348 (0.0722) | 0.0624 (0.0603) | 0.0110 (0.0521) | 0.0202 (0.0475) | ||||||
| Patient Black | 0.726*** (0.0601) | 0.731*** (0.0573) | 0.437*** (0.0421) | 0.433*** (0.0432) | 0.334*** (0.0916) | 0.301*** (0.0419) | 0.285*** (0.0408) | 0.234*** (0.0385) | 0.245*** (0.0379) | 0.103*** (0.0389) |
| Physician Black * Patient Black | −0.232* (0.132) | −0.262** (0.129) | −0.110 (0.0984) | −0.237*** (0.0578) | −0.202*** (0.0543) | −0.123** (0.0593) | ||||
| Insurance fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Comorbidity fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time fixed effects (quarter) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital-year fixed effects | Yes | Yes | Yes | Yes | ||||||
| Physician fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Observations | 547,394 | 547,325 | 546,516 | 492,726 | 53,852 | 1,265,576 | 1,265,545 | 1,264,762 | 1,090,665 | 174,123 |
| R-squared | 0.025 | 0.078 | 0.307 | 0.277 | 0.332 | 0.067 | 0.076 | 0.110 | 0.105 | 0.093 |
Mean of death, 0.4068 (columns 1–5)/0.4349 (columns 6–10). Robust SEs are clustered on the physician. BlackDocs, Black physicians; Comorbid, comorbidities; WhiteDocs, White physicians. ***P < 0.01, **P < 0.05, and *P < 0.1.
We next consider the institutional context in which newborn care is provided, splitting the sample at the median number of Black newborn cases per hospital-quarter (65 cases). We then replicate Eq. 1 for each subsample. Results are in Table 3. As can be seen, the benefits of concordance only manifest in hospital-quarters with a greater number of Black infants born (columns 1 to 5). However, as can be seen in columns 3 and 8, the mortality penalty for Black newborns treated by White physicians is 56% larger in hospitals managing a large number of Black newborns. This suggests, all else equal, that Black physicians are not performing better as the number of Black newborns increases (note the similarity in the coefficient size across columns 5 and 10). Instead, it appears that White physicians are underperforming (columns 4 and 9). To test whether this is related to the volume of newborns overall, we replicate the analysis splitting on the number of White newborns delivered in the hospital-quarter (median 235), and on the total number of newborns born in the hospital quarter (median 335). Results are in SI Appendix, Tables S4 and S5 and indicate that concordance benefits manifest for Black newborns regardless of the number of White or other children born within the hospital. In other words, the concordance benefit varies with the hospital’s level of experience caring for Black newborns, not with White newborns or newborns in general. In hospital-quarters with large numbers of Black newborns, those born under the care of White physicians experience especially high mortality penalties.
Table 3.
Linear probability model estimates of the effect of racial concordance on survival of newborns
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Dependent variable | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death |
| Sample | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs |
| Restriction | Above median | Above median | Above median | Above median | Above median | Below median | Below median | Below median | Below median | Below median |
| Physician Black | 0.0420 (0.0746) | 0.0926 (0.0740) | 0.0118 (0.0341) | 0.0137 (0.0280) | ||||||
| Patient Black | 0.482*** (0.0506) | 0.484*** (0.0510) | 0.350*** (0.0445) | 0.356*** (0.0431) | 0.147** (0.0569) | 0.351*** (0.0406) | 0.322*** (0.0330) | 0.228*** (0.0270) | 0.239*** (0.0306) | 0.170*** (0.0487) |
| Physician Black * Patient Black | −0.290*** (0.0778) | −0.297*** (0.0809) | −0.182** (0.0754) | −0.156** (0.0645) | −0.117** (0.0598) | −0.0568 (0.0521) | ||||
| Insurance fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Comorbidity fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time fixed effects (quarter) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital-year fixed effects | Yes | Yes | Yes | Yes | ||||||
| Physician fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Observations | 910,127 | 910,127 | 909,520 | 785,633 | 123,887 | 902,845 | 902,811 | 902,098 | 798,012 | 104,121 |
| R-squared | 0.059 | 0.062 | 0.139 | 0.133 | 0.169 | 0.040 | 0.059 | 0.178 | 0.171 | 0.124 |
Sample split by median number of Black newborn cases handled by hospital. Mean of death, 0.6111 (columns 1–5)/0.2198 (columns 6–10). Robust SEs are clustered on the physician. BlackDocs, Black physicians; WhiteDocs, White physicians. ***P < 0.01, **P < 0.05, and *P < 0.1.
Extant research further suggests that highly specialized training can yield superior clinical care benefits. One particular form of training, specialty-based board certification, wherein physicians complete an additional 1- to 3-y fellowship has received considerable attention. Research suggests that such training increases understanding of the nuance of disease (40), increases information recall (41), and accelerates reaction to new information (42). We therefore replicate our estimations splitting the sample into physicians who are, and are not, board certified in pediatrics. Results are in Table 4. Two interesting findings are apparent. First, the absolute mortality penalty for Black newborns is smaller among both Black and White pediatricians, compared with nonpediatricians. Second, we see significant concordance benefits among both board-certified pediatricians and nonpediatricians (in both cases concordance diminishes the Black mortality penalty by roughly half). This suggests additional formal training may reduce the magnitude of the Black mortality penalty but does not appear to eliminate these differences. Results with neonatologists yield consistent results.
Table 4.
Linear probability model estimates of the effect of racial concordance on survival of newborns
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Dependent variable | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death |
| Sample | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs | Newborns | Newborns | Newborns | WhiteDocs | BlackDocs |
| Restriction | Peds | Peds | Peds | Peds | Peds | Non-peds | Non-peds | Non-peds | Non-peds | Non-peds |
| Physician Black | −0.000409 (0.0444) | 0.0273 (0.0409) | −0.144 (0.150) | −0.297* (0.178) | ||||||
| Patient Black | 0.312*** (0.0420) | 0.307*** (0.0408) | 0.260*** (0.0374) | 0.261*** (0.0373) | 0.127*** (0.0430) | 0.773*** (0.0921) | 0.776*** (0.0905) | 0.475*** (0.0647) | 0.511*** (0.0632) | 0.295*** (0.0755) |
| Physician Black * Patient Black | −0.205*** (0.0568) | −0.194*** (0.0508) | −0.134** (0.0552) | −0.503** (0.201) | −0.461** (0.194) | −0.249** (0.103) | ||||
| Insurance fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Comorbidity fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Time fixed effects (quarter) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hospital-year fixed effects | Yes | Yes | Yes | Yes | ||||||
| Physician fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Observations | 1,435,490 | 1,435,469 | 1,435,363 | 1,266,415 | 168,964 | 377,481 | 377,344 | 376,502 | 317,541 | 59,083 |
| R-squared | 0.055 | 0.061 | 0.079 | 0.078 | 0.040 | 0.051 | 0.080 | 0.255 | 0.233 | 0.265 |
Sample split by board certification in pediatrics. Mean of death 0.3059 (columns 1–5)/0.8312 (columns 6–10). Robust SEs are clustered on the physician. BlackDocs, Black physicians; WhiteDocs, White physicians; Peds, Pediatricians. ***P < 0.01, **P < 0.05, and *P < 0.1.
Finally, it is worth considering if the benefits of concordance extend to birthing mothers. Like newborns, Black birthing mothers in the United States suffer dramatically higher mortality than their White counterparts (17, 43). We replicate our estimations using the 2.1-mm birthing mothers in Florida over the same time period (1992 to 2015). It should be noted that the mother’s physician (i.e., the obstetrician) is almost always different from the newborn’s physician (i.e., the pediatrician). Immediately after birth, both mothers and newborns require care, newborns needing to establish things like Apgar scores or if meconium has been inhaled, while mothers need postpartum care in the form of stitches, placental expulsion, and so forth. This explains the differing sample sizes. Although data restrictions prevent us from linking an individual birthing mother to an individual newborn, the set of mothers studied here did give birth to the set of newborns studied above. Comorbidities are updated to be relevant to the maternal sample. Results are in Table 5. Consistent with prior work, we see a penalty for Black birthing mothers in general, although the base mortality rates are an order of magnitude lower than for infants. In the cross-tabs (column 1), among birthing mothers cared for by White physicians, Black mothers experience an additional 14 deaths per 100,000 births, tripling White mothers’ mortality rate of 7 per 100,000 births. There is no difference in mortality rates based on physician race. However, while the interaction of patient and physician race is directionally consistent with concordance benefits for Black mothers, the estimate is never significantly different from zero.
Table 5.
Linear probability model estimates of the effect of racial concordance on survival of birthing mothers
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Dependent variable | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death | 100 = Death |
| Sample | Birthing mothers | Birthing mothers | Birthing mothers | Birthing mothers | Birthing mothers | Birthing mothers | Birthing mothers | Birthing mothers |
| Physician Black | 0.000615 (0.00229) | −3.22e-05 (0.00237) | 0.000338 (0.00238) | −0.00290 (0.00270) | −0.00123 (0.00284) | |||
| Patient Black | 0.0145*** (0.00251) | 0.0118*** (0.00242) | 0.0118*** (0.00241) | 0.00886*** (0.00234) | 0.00805*** (0.00234) | 0.00480** (0.00223) | 0.00533** (0.00223) | 0.00141 (0.00399) |
| Physician Black * Patient Black | −0.00363 (0.00439) | −0.00307 (0.00442) | −0.00315 (0.00442) | −0.00401 (0.00469) | −0.00487 (0.00460) | −0.00224 (0.00422) | ||
| Constant | 0.00698*** (0.000749) | |||||||
| Insurance fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Comorbidity fixed effects | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Time fixed effects (quarter) | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Hospital fixed effects | Yes | Yes | Yes | Yes | Yes | |||
| Hospital-year fixed effects | Yes | Yes | ||||||
| Physician fixed effects | Yes | Yes | Yes | |||||
| Observations | 2,133,412 | 2,133,412 | 2,133,412 | 2,133,393 | 2,133,152 | 2,131,474 | 1,816,817 | 314,854 |
| R-squared | 0.000 | 0.000 | 0.000 | 0.001 | 0.009 | 0.098 | 0.111 | 0.011 |
Mean of death, 0.010655. Robust SEs are clustered on the physician. ***P < 0.01, **P < 0.05, and *P < 0.1.
This work is subject to limitations that offer fruitful directions for future research. First, we are unable to observe the mechanism that is driving the observed result, or the selection process of the physician. While most accounts, as well as our discussions with practicing pediatricians, suggest that newborns are assigned in a quasi-random format to the on-call pediatrician (the birth process itself being quasi-random due to timing), this is worth discussing. On the one hand, there may be selection on the part of patients, whereby the mothers of Black newborns are having difficulty accessing the optimal physician (or are choosing their pediatrician using an inefficient selection criterion). On the other hand, it is possible that training regarding the challenges faced by Black newborns is lacking (the prototypical patient being White). Robustness checks in the supplement suggest patient predicted mortality is not significantly correlated with physician race, nor is there heterogeneous physician availability based on practice and arrival times. Still, caution is warranted as there may be some inefficiency in the matching process.
Second, we are unable to observe the composition of the patient care team, i.e., residents, nursing staff, etc. Although the inclusion of hospital and hospital-year fixed effects should account for the effect of hospital level processes, and results in SI Appendix show the result is robust to the presence or absence of residents, future work is clearly needed to understand the role of the patient care team. Third, our sample only includes newborns admitted to the hospital, suggesting some selection effect as it eliminates home births. However, as out-of-hospital births account for only 1.6% of American births (44), this is of little concern. Fourth, there may be heterogeneous effects across mothers of varying socioeconomic status, which is correlated with race. Replication of the estimations across Medicaid and non-Medicaid patients (SI Appendix, Table S11) yields consistent concordance effects, inasmuch as the penalty is roughly halved in both samples. However, replication across Latino newborns yield no significant concordance effect (SI Appendix, Table S7). While this may be a function of the context, viz. Florida, it is worth exploring whether concordance exists across other ethnic minorities. Fifth, of the 9,992 physicians in the original sample, pictures could only be found for 8,045, and our analysis omits physicians missing a photo. Thus, the analysis yields consistent estimates only under an untestable, maintained missing-at-random assumption that unobservable influences are mean independent of missingness conditional on fully observed covariates (45, 46). Finally, we observe no evidence of physician performance improving as they treat more Black newborns (SI Appendix, Table S12). This is striking, as research has noted the importance of experience in quality improvement (42, 47).
Several important contributions stem from this work. Empirically, this study provides evidence that the Black–White newborn mortality gap is smaller when Black doctors provide care for Black newborns than when White doctors do—lending support to research that examines the importance of racial concordance in addressing health care disparities. Furthermore, this study demonstrates that gap reduction occurs in more medically complex cases and is isolated to newborn mortality rather than maternal mortality. For families giving birth to a Black baby, the desire to minimize risk and seek care from a Black physician would be understandable. However, the disproportionately White physician workforce makes this untenable because there are too few Black physicians to service the entire population. Moreover, it avoids the foundational concern of resolving the disparities in care offered by White physicians. Finally, it is important to note that physician performance varies widely among physicians of both races, suggesting that exclusively selecting on physician race is not an effective solution to mortality concerns.
These results underscore the need for research into drivers of differences between high- and low-performing physicians, and why Black physicians systemically outperform their colleagues when caring for Black newborns. Key open questions include the following: 1) whether physician race proxies for differences in physician practice behavior, 2) if so, which practices, and 3) what actions can be taken by policymakers, administrators, and physicians to ensure that all newborns receive optimal care. Furthermore, it serves as an important call to continue the diversification of the medical workforce (48). Prior work suggests stereotyping and implicit bias contribute to racial and ethnic disparities in health (49). Taken with this work, it gives warrant for hospitals and other care organizations to invest in efforts to reduce such biases and explore their connection to institutional racism (50, 51). Reducing racial disparities in newborn mortality will also require raising awareness among physicians, nurses, and hospital administrators about the prevalence of racial and ethnic disparities, their effects, furthering diversity initiatives, and revisiting organizational routines in low-performing hospitals (52). It is clear that patient–physician racial concordance provides benefits, particularly because of the inequities in clinical care outcomes experienced by Black patients. We hope this study provides a basis for additional work that advances our understanding of inequality, its origins, and how practitioners can work toward creating better and more-equitable birth outcomes.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913405117/-/DCSupplemental.
Data Availability.
Data and materials are available by limited use agreement from the Florida AHCA.
References
- 1.Cohen L. E., Broschak J. P., Haveman H. A., And then there were more? The effect of organizational sex composition on the hiring and promotion of managers. Am. Sociol. Rev. 63, 711–727 (1998). [Google Scholar]
- 2.Cohen P. N., Huffman M. L., Working for the woman? Female managers and the gender wage gap. Am. Sociol. Rev. 72, 681–704 (2007). [Google Scholar]
- 3.Gino F., Pierce L., Robin Hood under the hood: Wealth-based discrimination in illicit customer help. Organ. Sci. 21, 1176–1194 (2010). [Google Scholar]
- 4.Fairlie R. W., Hoffmann F., Oreopoulos P., A community college instructor like me: Race and ethnicity interactions in the classroom. Am. Econ. Rev. 104, 2567–2591 (2014). [Google Scholar]
- 5.Abrams D. S., Bertrand M., Mullainathan S., Do judges vary in their treatment of race? J. Legal Stud. 41, 347–383 (2012). [Google Scholar]
- 6.Gross R. et al., The association of gender concordance and primary care physicians’ perceptions of their patients. Women Health 48, 123–144 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Elliott A. M., Alexander S. C., Mescher C. A., Mohan D., Barnato A. E., Differences in physicians’ verbal and nonverbal communication with black and white patients at the end of life. J. Pain Symptom Manage. 51, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogo-Gupta L. J., Haunschild C., Altamirano J., Maldonado Y. A., Fassiotto M., Physician gender is associated with Press Ganey patient satisfaction scores in outpatient gynecology. Womens Health Issues 28, 281–285 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Street R. L. Jr., O’Malley K. J., Cooper L. A., Haidet P., Understanding concordance in patient-physician relationships: Personal and ethnic dimensions of shared identity. Ann. Fam. Med. 6, 198–205 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood B. N., Carnahan S., Huang L., Patient-physician gender concordance and increased mortality among female heart attack patients. Proc. Natl. Acad. Sci. U.S.A. 115, 8569–8574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsan M., Garrick O., Graziani G. C., Does Diversity Matter for Health? Experimental Evidence from Oakland, (National Bureau of Economic Research, 2018). [Google Scholar]
- 12.Malhotra J. et al., Impact of patient-provider race, ethnicity, and gender concordance on cancer screening: Findings from medical expenditure panel survey. Cancer Epidemiol. Biomarkers Prev. 26, 1804–1811 (2017). [DOI] [PubMed] [Google Scholar]
- 13.LaVeist T. A., Nuru-Jeter A., Jones K. E., The association of doctor-patient race concordance with health services utilization. J. Public Health Policy 24, 312–323 (2003). [PubMed] [Google Scholar]
- 14.Jerant A., Bertakis K. D., Fenton J. J., Tancredi D. J., Franks P., Patient-provider sex and race/ethnicity concordance: A national study of healthcare and outcomes. Med. Care 49, 1012–1020 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Krähenmann-Müller S. et al., Patient and physician gender concordance in preventive care in university primary care settings. Prev. Med. 67, 242–247 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Collins J. W. Jr., David R. J., Racial disparity in low birth weight and infant mortality. Clin. Perinatol. 36, 63–73 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Villarosa L., Why America’s black mothers and babies are in a life-or-death crisis. The New York Times Magazine, 11 April 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed 30 July 2020.
- 18.Foundation K. F., Number of births by race (2017). https://www.kff.org/other/state-indicator/births-by-raceethnicity/. Accessed 30 July 2020.
- 19.Fingar K., et al. , “Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014” (Statistical Brief 222, Agency for Healthcare Research and Quality, 2006). [PubMed]
- 20.Centers for Disease Control and Prevention , Infant mortality and low birth weight among black and white infants–United States, 1980–2000. MMWR Morb. Mortal. Wkly. Rep. 51, 589–592 (2002). [PubMed] [Google Scholar]
- 21.Brown Speights J. S. et al., State-level progress in reducing the Black–White infant mortality gap, United States, 1999–2013. Am. J. Public Health 107, 775–782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers B. D., Baer R. J., McLemore M. R., Jelliffe-Pawlowski L. L., Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality—California, 2011–2012. J. Urban Health 96, 159–170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace M., Crear-Perry J., Richardson L., Tarver M., Theall K., Separate and unequal: Structural racism and infant mortality in the US. Health Place 45, 140–144 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Love C., David R. J., Rankin K. M., Collins J. W. Jr., Exploring weathering: Effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Am. J. Epidemiol. 172, 127–134 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Schoendorf K. C., Hogue C. J., Kleinman J. C., Rowley D., Mortality among infants of black as compared with white college-educated parents. N. Engl. J. Med. 326, 1522–1526 (1992). [DOI] [PubMed] [Google Scholar]
- 26.Rogers H. L., Dumenci L., Epstein R. M., Siminoff L. A., Impact of patient gender and race and physician communication on colorectal cancer diagnostic visits in primary care. J. Womens Health (Larchmt.) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf L. J., Maio G. R., Karremans J. C., Leygue C., On implicit racial prejudice against infants. Group Process. Intergroup Relat. 20, 789–800 (2017). [Google Scholar]
- 28.Lu M. C., Halfon N., Racial and ethnic disparities in birth outcomes: A life-course perspective. Matern. Child Health J. 7, 13–30 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Geronimus A. T., The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn. Dis. 2, 207–221 (1992). [PubMed] [Google Scholar]
- 30.Tsugawa Y., Newhouse J. P., Zaslavsky A. M., Blumenthal D. M., Jena A. B., Physician age and outcomes in elderly patients in hospital in the US: Observational study. BMJ 357, j1797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke M. A., Fournier G. M., Prasad K., Physician social networks and geographical variation in medical care (Brookings, 2003). https://www.brookings.edu/research/physician-social-networks-and-geographic-variation-in-medical-care/. Accessed 30 July 2020.
- 32.Greenwood B. N., Agarwal R., Matching platforms and HIV incidence: An empirical investigation of race, gender, and socioeconomic status. Manage. Sci. 62, 2281–2303 (2016). [Google Scholar]
- 33.Burke M. A., Fournier G. M., Prasad K., The diffusion of a medical innovation: Is success in the stars? South. Econ. J. 73, 588–603 (2007). [Google Scholar]
- 34.Lu S. F., Rui H., Can we trust online physician ratings? Evidence from cardiac surgeons in Florida. Manage. Sci. 64, 2557–2573 (2017). [Google Scholar]
- 35.Hoetker G., The use of logit and probit models in strategic management research: Critical issues. Strateg. Manage. J. 28, 331–343 (2007). [Google Scholar]
- 36.Oster E., Unobservable selection and coefficient stability: Theory and evidence. J. Bus. Econ. Stat. 37, 187–204 (2017). [Google Scholar]
- 37.Morriss F. H., Jr., Increased risk of death among uninsured neonates. Health Serv. Res. 48, 1232–1255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenz J. M., Ananth C. V., Polin R. A., D’Alton M. E., Infant mortality in the United States. J. Perinatol. 36, 797–801 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Watkins W. J., Kotecha S. J., Kotecha S., All-cause mortality of low birthweight infants in infancy, childhood, and adolescence: Population study of england and wales. PLoS Med. 13, e1002018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan T. A. et al., The role of physician specialty board certification status in the quality movement. JAMA 292, 1038–1043 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Davis D. et al., Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA 282, 867–874 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Greenwood B. N., Agarwal R., Agarwal R., Gopal A., The role of individual and organizational expertise in the adoption of new practices. Organ. Sci. 30, 191–213 (2019). [Google Scholar]
- 43.MacDorman M. F., Declercq E., Thoma M. E., Trends in maternal mortality by socio-demographic characteristics and cause of death in 27 states and the District of Columbia. Obstet. Gynecol. 129, 811–818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDorman M. F., Mathews T. J., Declercq E., Trends in out-of-hospital births in the United States, 1990–2012. J. Midwifery Women’s Health 58, 494–501 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Rubin D. B., Inference and missing data. Biometrika 63, 581–592 (1976). [Google Scholar]
- 46.Schafer J. L., Graham J. W., Missing data: Our view of the state of the art. Psychol. Methods 7, 147–177 (2002). [PubMed] [Google Scholar]
- 47.Avdic D., Lundborg P., Vikström J., Estimating returns to hospital volume: Evidence from advanced cancer surgery. J. Health Econ. 63, 81–99 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Hardeman R. R., Medina E. M., Boyd R. W., Stolen breaths. N. Engl. J. Med. 383, 197–199 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Medicine Io., Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, (National Academies Press, Washington, DC, 2003). [PubMed] [Google Scholar]
- 50.Hardeman R. R., Medina E. M., Kozhimannil K. B., Structural racism and supporting black lives—the role of health professionals. N. Engl. J. Med. 375, 2113–2115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feagin J., Bennefield Z., Systemic racism and U.S. health care. Soc. Sci. Med. 103, 7–14 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Women CoHCfU, Committee Opinion: Racial and ethnic disparities in obstetrics and gynecology (American College of Obstetricians and Gynecologists, 2015). https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Racial-and-Ethnic-Disparities-in-Obstetrics-and-Gynecology. Accessed 30 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are available by limited use agreement from the Florida AHCA.