Significance
Proteasome inhibitors are a highly effective treatment for multiple myeloma, a cancer of plasma cells. These studies were undertaken to understand why myeloma cells are exceptionally sensitive to proteasome inhibition. One proposed explanation is that myeloma proteasomes must continually degrade the large amounts of misfolded immunoglobins these cells produce. If so, myeloma cells should also be unusually sensitive to heat shock, which increases substrate load on 26S proteasomes. Unlike other cells studied, myeloma cell lines undergo extensive apoptosis at 43 °C, and, thus, are highly susceptible to proteotoxic stress. Also, shifting to only 39 °C markedly enhanced their sensitivity to proteasome inhibitors. Thus, mild hyperthermia (e.g. 39 °C) may enhance the efficacy of proteasome inhibitors in the treatment of myeloma.
Keywords: multiple myeloma, heat shock, Noxa, ubiquitin-proteasome system, protein misfolding
Abstract
Proteasome inhibitors, such as bortezomib (BTZ), are highly effective and widely used treatments for multiple myeloma. One proposed reason for myeloma cells’ exceptional sensitivity to proteasome inhibition is that they produce and continually degrade unusually large amounts of abnormal immunoglobulins. We, therefore, hypothesized that, heat shock may also be especially toxic to myeloma cells by causing protein unfolding, increasing further the substrate load on proteasomes, and, thus, putting further stress on their capacity for protein homeostasis. After a shift from 37 to 43 °C, all four myeloma lines studied underwent extensive apoptosis in 4 h, unlike 13 nonmyeloma cell lines, even though the myeloma cells induced heat-shock proteins and increased protein degradation similar to other cells. Furthermore, two myeloma lines resistant to proteasome inhibitors were also more resistant to 43 °C. Shifting myeloma cells to 43, 41, or 39 °C (which was not cytotoxic) dramatically increased their killing by proteasome inhibitors and inhibitors of ubiquitination or p97/VCP. Combining increased temperature with BTZ increased the accumulation of misfolded proteins and substrate load on the 26S proteasome. The apoptosis seen at 43 °C and at 39 °C with BTZ was mediated by caspase-9 and was linked to an accumulation of the proapoptotic Bcl-2-family member Noxa. Thus, myeloma cells are exceptionally sensitive to increased temperatures, which greatly increase substrate load on the ubiquitin-proteasome system and eventually activate the intrinsic apoptotic pathway. Consequently, for myeloma, mild hyperthermia may be a beneficial approach to enhance the therapeutic efficacy of proteasome inhibitors.
Multiple myeloma, a cancer of the immunoglobulin-producing plasma cells, is the second most frequent hematological malignancy. Myeloma cells are exceptionally sensitive to killing by inhibitors of the proteasome (1–3). Consequently, these agents (i.e., BTZ, carfilzomib, or ixazomib) are used worldwide to treat myeloma and have dramatically extended the lifespan of patients (1–4). The 26S proteasome is the primary site of protein degradation in mammalian cells and is especially important for the rapid elimination of critical regulatory proteins and misfolded potentially toxic proteins. Several mechanisms have been proposed for the exceptional sensitivity of myeloma cells to proteasome inhibition (1, 2, 4–6). One explanation is that proteasomes are required for production and activation of the transcription factor NFκB, which, in addition to its antiapoptotic actions in all cells, is particularly important in myeloma cells for production of key growth factors (e.g., IL-6) (1, 2, 4). Another explanation for their special sensitivity is that myeloma cells have an unusually high substrate load on their proteasomes because they continually synthesize very large amounts of abnormal immunoglobulins (1, 2, 7, 8). Although both heavy and light chains are secreted in large amounts and contribute to disease pathogenesis, a large fraction of these abnormal proteins is also degraded by the endoplasmic reticulum (ER)-associated degradation pathway (8, 9) in which misfolded secretory proteins are ubiquitinated, extracted from the ER by the p97/VCP complex, and degraded by cytosolic proteasomes (10). Consequently, substrate load on this quality-control pathway is especially high in myeloma cells and in normal plasma cells (1, 8, 11).
By preventing the clearance of such abnormal proteins, proteasome inhibitors cause misfolded proteins to accumulate and, thus, trigger the cytosolic heat-shock response, the ER stress response (i.e., the unfolded protein response [UPR], refs. 12, 13), and eventually apoptosis (2). Accordingly, the sensitivity of myeloma cells to proteasome inhibitors seems to correlate with the amount of proteins being degraded (7, 8, 14). Susceptibility to these inhibitors increases during plasma cell differentiation as cells produce more immunoglobulins (8, 15), and blocking protein synthesis in cells (16) reduces killing by proteasome inhibitors, presumably by decreasing the load of ubiquitinated substrates on proteasomes (11).
This “substrate load” hypothesis (7) predicts that other treatments that increase protein unfolding, such as heat shock (e.g., a shift from 37 to 43 °C), should also be particularly toxic to myeloma cells because it should further increase the demand on the ubiquitin proteasome system (UPS) and the cell’s sensitivity to proteasome inhibitors. Cells adapt to increased temperatures by increasing their rates of protein degradation (17, 18) and by inducing heat-shock proteins (HSPs). This transcriptional response increases the production of two key components of the cells’ proteostasis network (19): molecular chaperones, which reduce protein unfolding and aggregation (20–22), and ubiquitin and certain ubiquitination enzymes, which enhance the degradation of damaged proteins (18, 23). As a consequence, mammalian cells can survive high temperatures (e.g., 43 °C) for extended periods. It is noteworthy that the induction of heat-shock proteins is triggered by the accumulation of misfolded proteins in the cytosol as occurs with increased temperature or after proteasome inhibition (12, 21, 24, 25).
We have tested the prediction that myeloma cells, unlike other cells, cannot withstand heat shock. We demonstrate here that myeloma cells are exceptionally sensitive to heat shock and that increasing temperatures by increasing substrate load on the cell’s proteostasis network potentiates the killing by proteasome inhibitors and other agents that reduce protein degradation (26, 27). These observations indicate that the buildup of misfolded proteins in the cytosol as occurs with increased temperatures or proteasome inhibition is critical in activating apoptosis and suggest that raising body temperatures, even to only 39 °C, is a rational approach to further enhance the killing of myeloma cells by proteasome inhibitors.
Results
Myeloma Cells Are Exceptionally Sensitive to Heat Shock.
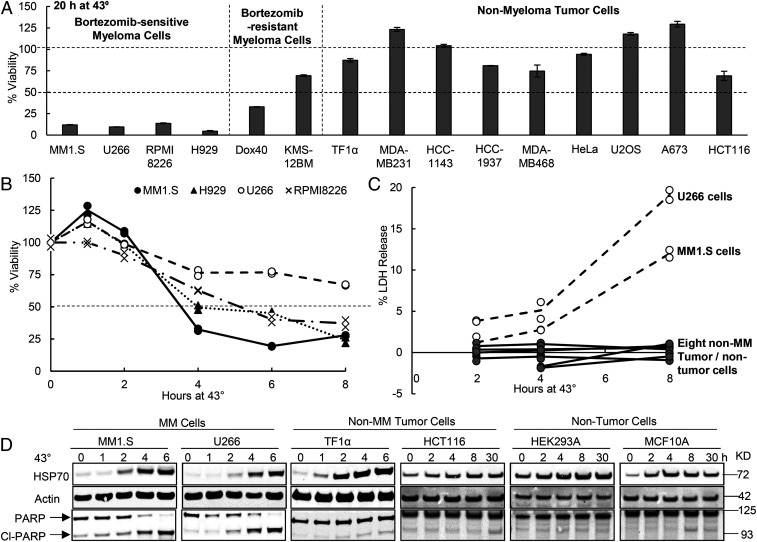
To test whether myeloma cells are less able to withstand heat shock than other cells, we shifted four myeloma lines and nine nonmyeloma lines from 37 to 43 °C and measured their viability by the MTS assay which measures mitochondrial reduced nicotinamide adenine dinucleotide production. By 20 h at 43 °C, the four myeloma lines tested (MM1.S, H929, RPM18226, and U266) showed a dramatic loss of viability (by roughly 90%), but all nine nonmyeloma tumor cells (Fig. 1A) analyzed showed little or no cell death. Loss of myeloma cell viability was evident even upon exposure to 43 °C for only 4 h when these myeloma cells had decreased in viability by 20–50% (Fig. 1B). When cell death at 43 °C was monitored for two myeloma lines MM1.S and U266 by following leakage of the cytoplasmic enzyme lactic dehydrogenase (LDH), they leaked 12–20% of their total LDH by 8 h, while the eight nonmyeloma cells (tumor or nontumor) leaked <2% (Fig. 1C). Furthermore, upon incubation of these myeloma lines at 43 °C for 4 h, there was clear evidence of apoptosis as shown by cleavage of most of the cellular poly (ADP ribose) polymerase (PARP) (Fig. 1D). By contrast, none of the four nonmyeloma lines (tumor or nontumor) studied showed an increase in PARP cleavage at 43 °C (Fig. 1D).
Fig. 1.
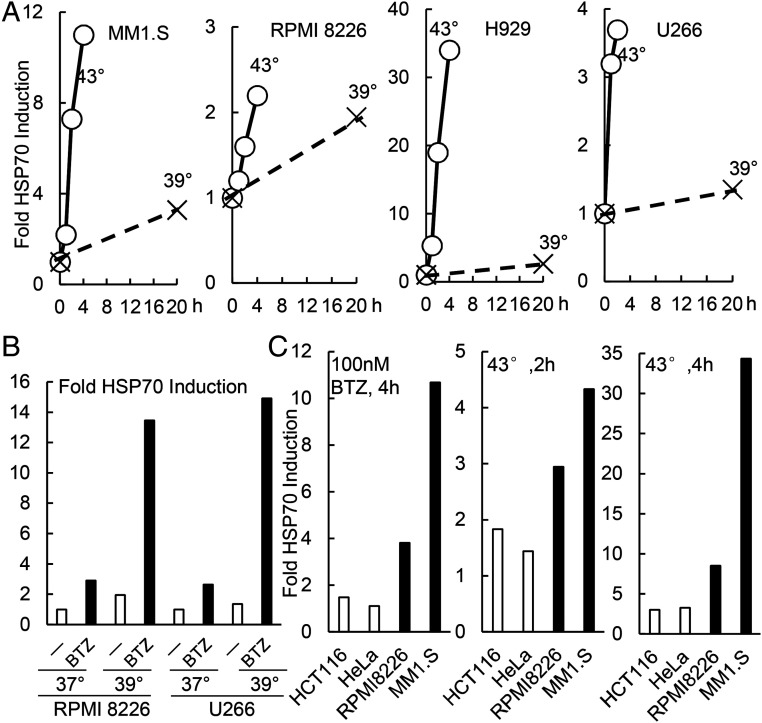
Myeloma cells are much more susceptible to killing by exposure to 43 °C than other cell lines. (A) Myeloma cells are exceptionally sensitive to 43 °C. Cells growing at 37 °C were switched to 43 °C for 20 h. Cell viability was measured by the MTS assay and normalized to the viability of cells kept at 37 °C for 20 h. Error bars represent SDs (n = 3). (B) At 43 °C, myeloma lines showed decreased viability within 4 h. Myeloma lines were switched to 43 °C, and cell viability was measured by the MTS assay. Data shown are from two biological replicates which yielded similar results. (C) In 8 h at 43 °C, myeloma cell lines (U266 and MM1.S) showed leakage of LDH, a measure of cell death, unlike eight nonmyeloma tumor or nontumor cell lines (HCT116, HAP1, HEK293A, M17, MCF10A, MDA-MB-231, HCC1143, and HCC1937). Data shown are from two biological replicates. (D) Although after exposure to 43 °C, myeloma and nonmyeloma (tumor or nontumor) cells showed a large accumulation of HSP70 (i.e., the heat-shock response), only myeloma cells cleaved PARP, an indicator of apoptosis. Protein levels were analyzed by Western blot.
To learn whether the susceptibility of myeloma cells to heat shock is linked to their susceptibility to proteasome inhibition, we also studied the viability at 43 °C of two myeloma lines that are relatively resistant to BTZ, Dox40, and KMS-12BM (SI Appendix, Fig. S1). The mechanisms for their BTZ resistance are not completely understood and may be related to an overexpression of multidrug resistance pump (reported for Dox40 cells, ref. 28). Intriguingly, both these cells also showed much greater resistance to 43 °C (which cannot be affected by drug secretion) than the four BTZ-sensitive myeloma lines, although they were not as resistant as the nine nonmyeloma cell lines studied (Fig. 1A). Thus, all of the myeloma lines tested are more susceptible to killing at 43 °C than the 13 nonmyeloma lines (Fig. 1 A and C), and the sensitivity of different myeloma lines to heat shock correlates with their sensitivity to proteasome inhibition. This correlation implies that heat shock and proteasome inhibition kill myeloma cells by a common mechanism, presumably by causing an accumulation of misfolded proteins.
Myeloma and Nonmyeloma Cells Induce HSP70 and Activate Protein Degradation Similarly.
Cells adapt to increased temperatures by inducing heat-shock genes (22). Therefore, one possible explanation for the unusual susceptibility of myeloma cells to 43 °C could be an inability to induce heat-shock proteins (e.g., HSP70) (20–22). Even though MM1.S and U266 cells were rapidly killed upon shifting to 43 °C, they markedly induced the expression of HSP70 within 2–4 h in a similar fashion to four nonmyeloma cells studied (Fig. 1D). The UPR, which is induced by protein misfolding in the ER, is activated in myeloma and plasma cells and was reported to be stimulated by heat shock (12, 29). However, exposure of three myeloma lines (H929, U266, and RPMI8226) to 43 °C for 6 h did not increase markers of the UPR, such as eIF2α phosphorylation or the accumulation of BiP or XBP1s (SI Appendix, Fig. S2A). A mediator of UPR-associated apoptosis is the c-Jun N-terminal kinase (JNK) (30). Although exposure of H929, U266, and RPMI8226 lines to 43 °C enhanced the phosphorylation of c-Jun (SI Appendix, Fig. S2A), inhibiting JNK with 10 µM SP600125 did not reduce the extensive killing of U266 or RPMI8226 cells upon exposure to 43 °C for 20 h or the more limited cell death at 41 °C (SI Appendix, Fig. S2B). Thus, in contrast to the increase in cytosolic misfolded proteins at 43 °C, which caused HSP induction, this increase in temperature did not seem to cause further proteotoxic stress in the ER of myeloma cells where the levels of misfolded immunoglobins are already high at 37 °C. The finding that the UPR is not activated clearly indicates that the proteotoxic stress resulting from thermal damage to cytosolic or nuclear proteins is the important cause of myeloma cell death.
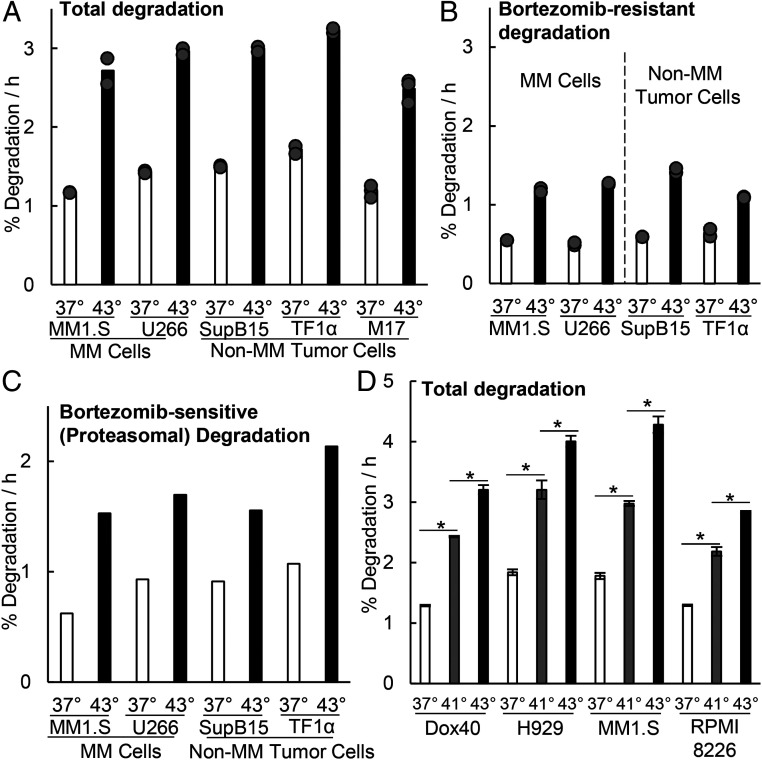
Another key adaptive response to heat shock is increased intracellular proteolysis, which enhances the clearance of unfolded proteins. Upon transfer from 37 to 43 °C, mammalian cells show an almost immediate twofold increase in the overall breakdown of endogenous cytosolic proteins by the UPS (18). Also, among the many proteins induced upon heat shock are two Ub-conjugating enzymes Ube2d2/Ubc4 and Ube2d1/Ubc5 (23), and the poly-Ub gene UBC (11), which together help cells maintain high levels of degradation by the UPS (17). To test if myeloma cells are also capable of similar large increases in proteolysis, long-lived cell proteins were radiolabeled for 20 h with 3H-phenylalanine and then chased for 2 h to allow clearance of short-lived proteins. Upon shift to 43 °C, after the chase, the myeloma lines MM1.S and U266 just like three nonmyeloma lines, rapidly doubled their overall rate of degradation of cell proteins that are normally long lived at 37 °C (Fig. 2A). This increased proteolysis primarily reflects degradation by proteasomes (i.e., the fraction that can be inhibited by 1 µM BTZ) (Fig. 2C), which composed about 60% of the total proteolysis at both 37 and 43 °C (Fig. 2 B and C), although the remaining degradation, primarily autophagy (31), also increased about twofold (Fig. 2B). Surprisingly, at both temperatures, these degradation rates did not differ between the myeloma and the nonmyeloma lines.
Fig. 2.
Exposure of myeloma cells to 43 °C increases degradation of long-lived cellular proteins by the proteasome. After switching to 43 °C, the myeloma and nonmyeloma cells tested all increased their overall rates of protein degradation and proteasome-mediated degradation by about twofold. (A and B) Long-lived cell proteins (the bulk of cell proteins) were labeled with 3H-phenylalanine at 37 °C, and after a 2 h chase to allow clearance of short-lived proteins, (A) their degradation rates were measured for 2 h (31, 62) when cells were exposed to 43 °C or kept at 37 °C, and (B) the BTZ-resistant degradation represents the rates of proteolysis measured in these cells in the presence of 1 µM BTZ and reflects primarily lysosomal degradation (31, 62). Data shown are from three biological replicates for M17 cells and two for other cells. (C) Proteasomal degradation (i.e., BTZ-sensitive) rates also increased about twofold at 43 °C. These rates were calculated by subtracting the average of the BTZ-resistant degradation rate (B) from the average total degradation rate (A) as described previously (31, 62). The BTZ-inhibited fraction composed about 60% of the proteolysis at both 37 and 43 °C. (D) At 41 °C, overall rates of protein degradation also increased but by less than at 43 °C. Degradation rates were measured in four myeloma lines exposed for 2 h to 41 or 43 °C or maintained at 37 °C. Error bars are SDs (n = 4). *P < 0.05.
Although 43 °C is commonly used to induce the heat-shock response, any increase in temperature should promote the unfolding of some cell proteins and enhance protein degradation and substrate load on the proteasome. In fact, when myeloma or nonmyeloma cells were exposed to 41 °C, overall protein degradation also increased, although to a lesser extent than at 43 °C (Fig. 2D). Thus, the myeloma cells’ exceptional sensitivity to high temperatures cannot be explained by a failure to induce heat-shock proteins or to enhance degradation. Presumably these adaptations, although adequate in most cells, in the myeloma cells are insufficient to handle the additional proteotoxic stress resulting from heat shock.
Increased Temperatures Markedly Enhance the Sensitivity of Myeloma Cells to Proteasome Inhibitors.
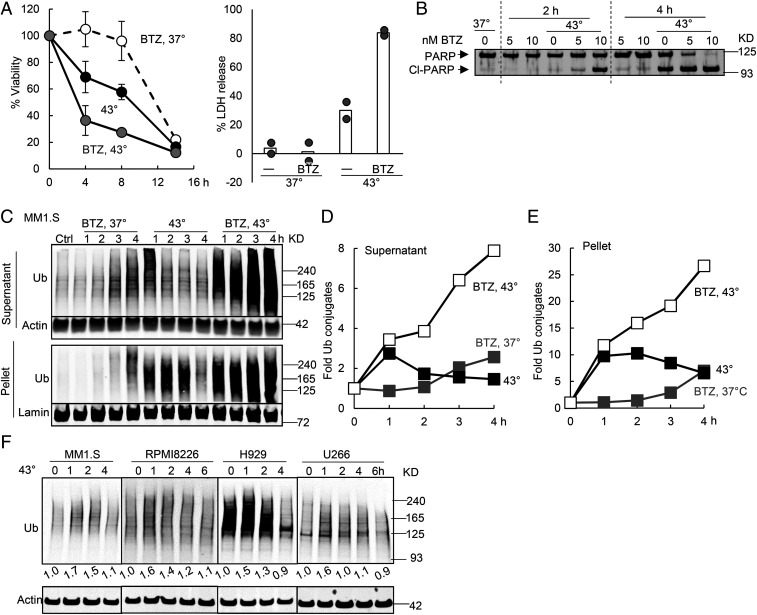
This unusual susceptibility of myeloma cells to heat shock and proteasome inhibitors strongly suggests an inability to handle the increased substrate load on the UPS. If so, increased temperatures and proteasome inhibition should have additive effects in causing cell death. By itself, shifting myeloma cells to 43 °C causes a greater and faster loss of viability than exposure to BTZ at 37 °C. For example, exposure of MM1.S cells to 43 °C for 4 h decreased the number of viable cells by about 1/3 (Fig. 3 A, Left) and caused marked cleavage of PARP (Fig. 3B) and by 8 h appreciable release of LDH (Fig. 3 A, Right). By contrast, treatment of these cells with 10 nM BTZ at 37 °C did not cause appreciable loss of viability until 14 h and no leakage of LDH at 8 h (Fig. 3A). However, exposure to this concentration of BTZ at 43 °C caused in 2 h marked PARP cleavage (Fig. 3B), in 4 h a 60% loss of viability (Fig. 3A), and by 8 h, nearly 80% LDH had leaked (Fig. 3A). Thus, the increases in temperature and proteasome inhibition have synergistic effects in killing myeloma cells. Apparently, at 43 °C, myeloma cells generate large quantities of misfolded cytosolic proteins that are quickly degraded by the UPS, but the presence of BTZ prevents their clearance (Fig. 3 C–E), leading to their rapid buildup to toxic levels (see below).
Fig. 3.
Inhibiting the proteasome during the exposure of myeloma cells to 43 °C caused synergistic buildup of ubiquitinated proteins and apoptosis. (A) MM1.S cells were incubated at 37 or 43 °C with or without 10 nM BTZ. Exposure to BTZ at 43 °C caused synergistic killing when cell viability was measured by the MTS assay (Left, n = 3, error bars are SDs) or LDH release (Right, n = 2, both data points for two typical experiments are shown). (B) Exposure of MM1.S cells to 10 nM BTZ at 43 °C caused PARP cleavage by 2 h, but exposure to BTZ or 43 °C alone for 2 h did not. (C–E) Exposure of MM1.S cells to 43 °C caused a rapid accumulation of ubiquitinated proteins (detected by K48 linkage-specific antibody) especially in the 10,000 × g pellet. Their levels then decreased in both the supernatant and the pellet due to proteasomal degradation since BTZ prevented this decrease. Cells were exposed to 43 °C with or without 10 nM BTZ. Cell proteins were fractionated by centrifugation at 10,000 × g for 10 min in the presence of 1% Triton X-100 and analyzed by Western blotting (C). The amount of ubiquitinated proteins in C was quantified in the supernatant after normalization to actin (D) or in the pellet after normalization to lamin (E). (F) When four myeloma lines were shifted to 43 °C, there was a rapid accumulation of ubiquitin conjugates (detected with P4D1 antiubiquitin antibody) in 1 h, followed by a decrease.
After the shift of MM1.S cells to 43 °C for only 1 h, their lysates contained threefold higher levels of ubiquitinated proteins in the supernatant (after 10,000 × g centrifugation, containing 95% cellular proteins) and 10-fold more in the 10,000 × g pellet (containing 5% cellular proteins) (Fig. 3 C–E). This rapid increase in ubiquitinated proteins in both fractions after the switch to 43 °C must reflect the sudden appearance and ubiquitination of thermally damaged proteins. The buildup of ubiquitinated proteins in insoluble form indicates that these proteins form aggregates as also occurs upon proteasome inhibition (32). This increase in ubiquitin conjugates was much faster and larger than that seen after exposure to proteasome inhibitors at 37 °C. However, after 1 h at 43 °C, the amount of ubiquitin conjugates decreased rapidly in the soluble fraction and gradually in the pellet (Fig. 3 C–E). A rapid buildup of ubiquitinated proteins followed by a decrease was also observed in other myeloma lines RPMI8226, H929, and U266 (Fig. 3F). This decrease in ubiquitin conjugates by 2–3 h at 43 °C strongly suggests that the cells’ capacity to degrade the thermally damaged proteins increased with time, perhaps due to the induction of components of the UPS (18, 23). Accordingly, the addition of BTZ completely blocked this decrease and caused ubiquitin conjugate levels to continue to rise (Fig. 3 C–E).
In the nonmyeloma cell lines HCT116 and MCF10A, at 43 °C, the levels of ubiquitin conjugates remained low (for, at least, 30 h), as the cells grew. However, in the myeloma lines MM1.S and U266 (SI Appendix, Fig. S3), ubiquitinated proteins accumulated again in both supernatant and pellet by 8 h when there was widespread apoptosis (Fig. 1B). Thus, myeloma cells appear less able to handle the increased proteotoxic stress, presumably due to their greater load of misfolded immunoglobins. As expected, treating these cells at 43 °C with BTZ, which cause a further buildup of the damaged proteins, led to an even more rapid loss of viability (Fig. 3A).
At 39 or 41 °C Myeloma Cells also Showed Much Greater Sensitivity to Inhibitors of Proteasomes, Ubiquitination, and p97/VCP.
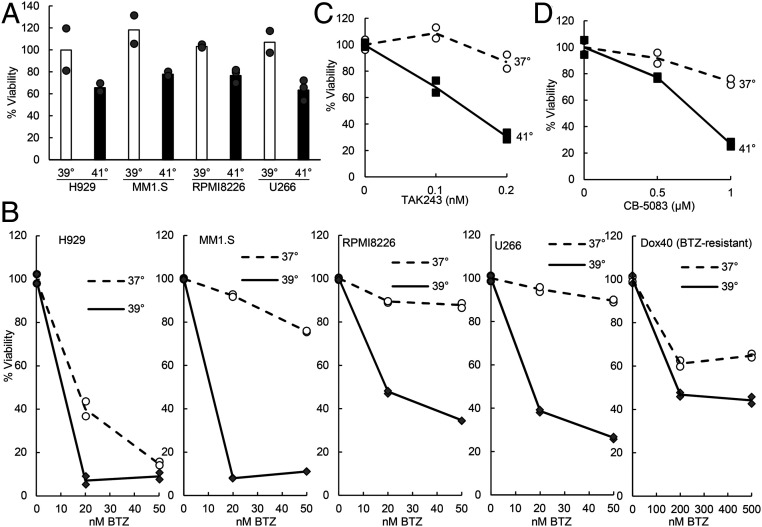
Unlike the extensive cell death seen at 43 °C, shifting four myeloma lines to 41 °C caused a modest (20–40%) decrease in viability by 20 h (Fig. 4A), and at 39 °C, no cell death was observed by this time. We, therefore, tested whether exposure to these temperatures might also increase the sensitivity of the myeloma cells to proteasome inhibition. At both 39 °C (Fig. 4B) and 41 °C (SI Appendix, Fig. S4A), the four myeloma lines tested were all much more sensitive to killing by 20 or 50 nM BTZ than they were at 37 °C. At 41 °C, MM1.S cells were also much more susceptible to killing by another proteasome inhibitor carfilzomib (SI Appendix, Fig. S4B). The sensitivity of even the BTZ-resistant myeloma line Dox40 to BTZ also increased at 39 °C (Fig. 4B), although as expected, killing them at 39 °C still required much higher doses of BTZ than for the typical myeloma lines. For the seven nonmyeloma lines tested, no similar synergy between 39 or 41 °C and BTZ in causing cell death was observed (SI Appendix, Fig. S4C). Because at 39 °C, only myeloma cells showed a large increase in killing by low concentrations of BTZ (20 nM), this temperature should enhance further the selective elimination of myeloma cells by proteasome inhibitors.
Fig. 4.
Exposure to 39 or 41 °C increases the sensitivity of myeloma cells to inhibitors of the proteasome, the ubiquitin activating enzyme (E1), and the p97/VCP ATPase. (A) Although shifting four myeloma lines to 39 °C for 20 h did not cause any loss of viability by the MTS assay, exposure to 41 °C caused a clear decrease in viable cells that was less than that seen at 43 °C (Fig. 1). Data shown are from two to three biological replicates. (B) At 39 °C, all myeloma lines were more sensitive to BTZ than at 37 °C, but this difference was smaller for the BTZ-resistant Dox40 line, which required much higher concentrations of BTZ (28). These myeloma lines were exposed for 20 h to 39 °C with the indicated concentrations of BTZ. Data shown are from two biological replicates. (C) At 41 °C, MM1.S cells were much more sensitive to the inhibitor of the ubiquitin activating enzyme TAK243 than at 37 °C. Cells were treated for 8 h. Data shown are from two biological replicates. (D) The p97 inhibitor CB-5083 also caused a greater loss of viability of MM1.S cells at 41 °C than at 37 °C, data points from two biological replicates are shown. Cells were treated for 8 h. In B–D, at each temperature, viabilities of cells without inhibitor treatment were all set as 100%.
These synergistic effects of increased temperatures and proteasome inhibition strongly suggest that myeloma cells are killed by increased substrate load on the UPS. Degradation by the 26S proteasome generally requires substrate ubiquitination, and the degradation of many ubiquitinated proteins requires their extraction from larger structures (e.g., from the ER or protein aggregates) by the p97/VCP ATPase (33). Because of their abilities to block protein degradation by the UPS, inhibitors of ubiquitin activation (e.g., TAK243, ref. 27) and of p97 (e.g., CB-5083, ref. 34) have been developed as possible treatments for cancers. We, therefore, tested if increased temperature also enhanced the sensitivity of MM1.S cells to these agents. At 41 °C, treatment with TAK243 or CB-5083 for 8 h enhanced the killing by these agents above the levels seen at 37 °C (Fig. 4 C and D). Thus, even a mild increase in temperature (to 39 or 41 °C) enhanced the sensitivity of myeloma cells not only to proteasome inhibitors, but also to inhibitors of ubiquitination or p97, which should also cause a further buildup of thermally damaged proteins by inhibiting their degradation.
Increased Temperatures and BTZ Synergistically Cause HSP70 Induction, Which Reflects Levels of Misfolded Cytosolic Proteins.
Presumably, combining increased temperatures with BTZ (or TAK243 or CB-5083) causes much greater killing of myeloma cells because these treatments together increase substrate load on the cell’s proteostasis network. To test this idea, we measured the induction of the heat-shock protein HSP70 (25, 35–37), whose transcription by HSF1 occurs in response to the accumulation of unfolded proteins in the cytosol and nucleus (25, 35–37). As noted above, exposure to 43 °C caused a dramatic induction of HSP70 in MM1.S, RPMI8226, H929, and U266 cells (Fig. 5A). At 39 °C, HSP70 expression also increased in these cells but to a much less extent than at 43 °C (Fig. 5A). Proteasome inhibitors also induce the heat-shock response (12), and at 37 °C, treating two myeloma lines with 10 nM BTZ for 20 h caused a two to threefold increase in HSP70 levels (Fig. 5B). However, at 39 °C, BTZ caused a 13–15-fold accumulation of HSP70 by 20 h in RPMI8226 and U266 cells (Fig. 5B). Thus, although 39 °C or BTZ alone leads to a small accumulation of damaged proteins and little or no cell killing, together these treatments synergize to cause much greater protein misfolding, eventually reaching levels that induce apoptosis. Also, greater HSP70 accumulation was observed in the myeloma cells exposed to BTZ and other increased temperatures (e.g., when U266 cells were exposed to 41 °C and BTZ, SI Appendix, Fig. S5). By comparison, nonmyeloma cells accumulated much less HSP70 in response to these proteotoxic treatments (Fig. 5C), strongly suggesting less substrate load on their proteostasis network. For example, two myeloma, but not two nonmyeloma cells, increased HSP70 by more than twofold upon exposure to 43 °C for 2 h or to 100 nM BTZ for 4 h (Fig. 5C).
Fig. 5.
At 39 °C, unlike 43 °C, myeloma cells show very little HSP70 expression, but when treated with BTZ, there was a marked induction of HSP70. (A) Four myeloma lines cells show much less induction of HSP70 upon a shift to 39 °C than to 43 °C. Myeloma cells were switched to 43 or 39 °C, and the fold increase in HSP70 was expressed relative to levels in cells maintained at 37 °C as measured by Western blotting and normalized to actin. (B) Exposure to BTZ at 39 °C caused synergistic induction of HSP70. Two myeloma lines were incubated for 20 h at 37 or 39 °C with or without 10 nM BTZ present, and the amount of HSP70 was measured. (C) Two myeloma lines (MM1.S and RPMI8226) and two nonmyeloma lines (HCT116 and HeLa) were exposed for 4 h to 100 nM BTZ or for 2–4 h to 43 °C. Cell lysates were analyzed by Western blotting, and the levels of HSP70 (normalized to actin) were shown. There are much higher fold increases of HSP70 levels in myeloma cells than in nonmyeloma cells. Data shown in this figure are from a typical experiment.
At 43 °C, Myeloma Cells Activate the Intrinsic Apoptotic Pathway and Accumulate the Proapoptotic Bcl-2 Family Member Noxa.
The loss of myeloma cell viability at 43 °C appears to result from apoptosis because these cells showed extensive cleavage of PARP (Fig. 1D). To further examine the importance of apoptosis or another programmed cell death pathway, necroptosis (38), we incubated myeloma cells at 43 °C with the pan-caspase inhibitor Z-VAD-FMK to block apoptosis or with either the RIPK1 inhibitor necrostatin-1 or the MLKL inhibitor necrosulfonamide to prevent necroptosis. Treatment with Z-VAD-FMK, but not necrostatin-1 or necrosulfonamide, prevented the loss of viability of MM1.S, U266, and RPMI8226 cells at 43 °C (Fig. 6A and SI Appendix, Fig. S6A). Also Z-VAD-FMK (Fig. 6B and SI Appendix, Fig. S6B), unlike the inhibitors of necroptosis (Fig. 6B) prevented the loss of plasma membrane integrity at 43 °C as shown by the leakage of LDH by 8 h (Fig. 6B) and of radiolabeled cellular proteins in 3 to 4 h (SI Appendix, Fig. S6B). Furthermore, upon exposure of U266 cells to 43 °C, there is no phosphorylation of RIPK1 at Ser166 (SI Appendix, Fig. S6C), which occurs when RIPK1 is activated to trigger necroptosis (39), and there is rapid precipitation of RIPK1 into insoluble inclusions (SI Appendix, Fig. S6C).
Fig. 6.
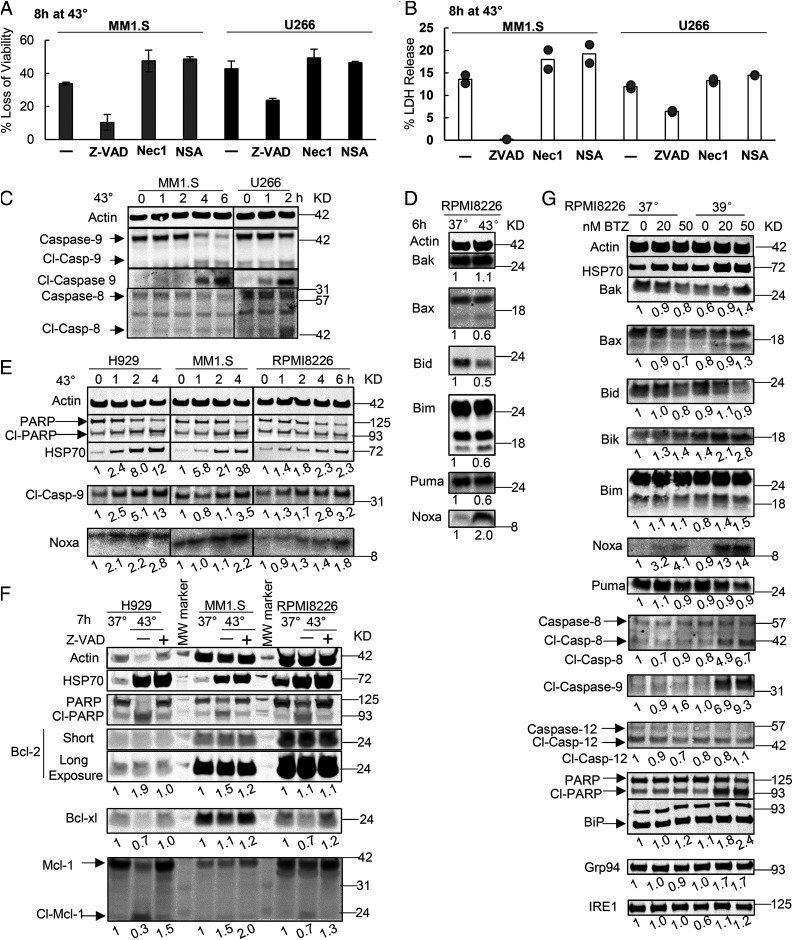
Upon shifting to 43 °C, myeloma cells activate the intrinsic apoptotic pathway and cause the accumulation of Noxa (A and B) Killing of the two myeloma lines at 43 °C was suppressed by an inhibitor of apoptosis but not inhibitors of necroptosis. MM1.S or U266 cells were exposed for 8 h to 43 °C and 40 µM Z-VAD-FMK to prevent apoptosis or 20 µM necrostatin-1 (Nec-1) or necrosulfonamide (NSA) to block necroptosis. Viability was measured by (A) the MTS assay (n = 3, error bars are SDs) and (B) cell death by LDH release assay (results from two biological replicates are shown). (C) Exposure of two myeloma lines to 43 °C triggered cleavage of caspase-9 but not caspase-8. Cl-caspase represents the cleaved form. (D) Shifting RPMI8226 cells to 43 °C caused in 6 h a twofold accumulation of Noxa but not other proapoptotic Bcl-2 proteins. (E) Exposing three myeloma lines to 43 °C led to cleavage of PARP and caspase-9 and Noxa accumulation. (F) Exposing myeloma lines to 43 °C did not affect the levels of Bcl-2 but caused caspase-dependent cleavage of Bcl-xl and Mcl-1, which was blocked by Z-VAD-FMK. Thus, their cleavage occurs during apoptosis and does not cause it. (G) RPMI8226 cells were treated with increasing concentrations of BTZ for 20 h at 37 or 39 °C. Exposure to both 39 °C and BTZ caused cleavage of caspase-8 and caspase-9 and a marked accumulation of Noxa but not of other proapoptotic Bcl-2 proteins or ER chaperones (BiP or Grp94). Also shown below each blot are the fold changes in different Bcl-2 proteins or cleaved caspases after normalization to actin.
Apoptosis can be activated either by the intrinsic pathway, which results from damage to the mitochondrial outer membrane, or the extrinsic pathway, which is activated through membrane receptors (40). To determine which pathway causes apoptosis of myeloma cells at 43 °C, we tested which initiator caspases are activated. Shifting MM1.S or U266 cells to 43 °C triggered the cleavage and activation of caspase-9, which mediates the intrinsic pathway, but not caspase-8, which mediates the extrinsic pathway (Fig. 6C). When the intrinsic pathway is activated, the proapoptotic Bcl-2 proteins accumulate in the cytosol and overwhelm the antiapoptotic Bcl-2 family members (41). To determine if the levels of any proapoptotic Bcl-2 protein might increase at 43 °C, we measured the levels of BH1-3 proteins Bak and Bax as well as BH3-only proteins Bid, Bim, Bik, Bad, Puma, and Noxa. Incubation of RPMI8226, H929, and MM1.S cells at 43 °C for 4 or 6 h caused a large increase in the levels of Noxa (Fig. 6 D and E) but not in Bak, Bax, Bid, Bim, Bik, or Puma (Fig. 6D). Exposure to 43 °C also did not alter the level of the main prosurvival Bcl-2 protein Bcl2, although there was caspase-dependent cleavage of two other prosurvival Bcl-2 proteins Bcl-xl and Mcl-1 (Fig. 6F). Since Z-VAD-FMK prevented these decreases in Bcl-xl and Mcl-1 (Fig. 6F), their cleavage resulted from the apoptosis and did not cause it. Therefore, the only consistent change in the Bcl-2 family of proteins at 43 °C was the accumulation of Noxa, which has been reported to rise and trigger apoptosis upon proteasome inhibition at 37 °C (42, 43).
The selective killing of myeloma cells by BTZ at 39 or 41 °C was also due to apoptosis because treatment with Z-VAD-FMK prevented their killing upon exposure to both 41 °C and BTZ for 20 h (SI Appendix, Fig. S4A). Also, incubating the myeloma line RPMI8226 cells with BTZ at 39 °C caused cleavage of PARP and caspase-9 (which increased nearly 10-fold), although there was also an increase of cleaved caspase-8 (nearly a fivefold increase). The combined treatment with both 39 °C and BTZ caused small increases in BiP and Grp94, but there was no increase in IRE1 and no cleavage of the UPR-associated caspase-12 (44) (Fig. 6G). Therefore, these conditions caused only a mild UPR, which did not seem to trigger apoptosis. The exposure of myeloma cells to both 39 °C and BTZ also caused a robust increase in Noxa (>10 fold) and a mild increase in Bik (twofold) and Bim (50%) without any change in Bak, Bax, Bid, and Puma (Fig. 6G) as was found at 43 °C. Thus, Noxa accumulation correlates with and probably causes the activation of the intrinsic apoptotic pathway in myeloma cells at 43 °C and by BTZ at 39 °C.
Discussion
The Susceptibility of Myeloma Cells to Proteotoxic Stress.
These various observations demonstrate that myeloma cells, presumably due to their continual expression of large amounts of misfolded immunoglobins, have much less capacity to withstand heat shock than the 13 other cell lines studied. Even at 41 °C, the myeloma lines showed significant cell death. Consequently, the increased demand from the elevated temperatures on their proteostasis network rapidly triggers apoptosis. Interestingly, the exposure to 43 °C caused much faster death of the myeloma cells than treatment with BTZ at 37 °C presumably because 43 °C causes immediate damage to many cell proteins unlike proteasome inhibition, which causes only a gradual accumulation of misfolded proteins (Fig. 3C).
Not surprisingly, promoting protein unfolding in myeloma cell lines with increased temperatures (even by only 2 °C) and inhibiting degradation with BTZ synergistically triggered PARP cleavage and apoptosis. Although heat shock and BTZ were also reported to have additive toxicities in certain nonmyeloma cells (45–47), the synergistic killing was much more pronounced for the five myeloma lines (Fig. 4B) than for the seven nonmyeloma lines studied here (SI Appendix, Fig. S4C). At 41 °C, the myeloma cells were also more sensitive to inhibitors of ubiquitin activation (UAE1) and of p97/VCP. Both these agents block the breakdown of cell proteins, but unlike proteasome inhibitors, they actually decrease substrate load on the proteasome. While inhibition of proteasomes or p97 increases levels of ubiquitinated proteins, the UAE1 inhibitor drastically reduces their levels. Because cell death occurred similarly with these treatments as with BTZ, the cause of the apoptosis must be the increased load of nondegraded proteins on the cell’s proteostasis network and not simply the load of ubiquitinated substrates on the proteasome.
The extent of HSP70 induction provides a useful measure of the levels of unfolded proteins in cells under conditions that cause protein damage (25, 37). As expected, those treatments that led to rapid death of myeloma cells (i.e., exposure to 43 or to 39 °C plus BTZ) caused a dramatic induction of HSP70, while treatments that caused much less apoptosis (i.e., exposure to 39 °C alone or to BTZ at 37 °C) caused a much smaller increase in HSP70. It is also noteworthy that upon shifting to 43 °C, the myeloma lines showed a similar ability to induce HSP70 as the nonmyeloma lines, although in myeloma cells, this protective response does not prevent apoptosis. A distinct pool of unfolded newly synthesized proteins in the ER triggers the UPR, which, if persistent, can trigger apoptosis. Because proteasome inhibition, which causes further accumulation of abnormal immunoglobins (48), the UPR was proposed to cause the resulting apoptosis of myeloma cells. However, when myeloma cells are exposed to 43 °C or BTZ at 39 °C, the classic markers of the UPR (Fig. 6G and SI Appendix, Fig. S2A) (such as levels of Bip or phopho-eIF2α) did not appreciably increase. Although JNK kinase, which can mediate UPR-induced apoptosis, increased, blocking its activity did not reduce cell death. Thus, ER stress is unlikely to cause myeloma cell apoptosis under these conditions.
In mammalian cells at 37 °C, proteasomes catalyze the rapid degradation of short-lived proteins and about 70–80% of the much more abundant long-lived cell proteins (31), and they play a similar role in their accelerated degradation at 43 °C (Fig. 2 B and C). Surprisingly, myeloma and nonmyeloma cells showed similar rates of degradation of these long-lived cell proteins at 37 °C and increased these rates similarly upon shift to 43 °C (Fig. 2 A–C). Overall proteolysis also increased at 41 °C, although less than at 43 °C, presumably because the extent of protein unfolding depends on the increase in temperature. The shift to 43 °C, in addition to increasing overall proteolysis, caused a large accumulation of ubiquitinated proteins especially in the 10,000 × g pellet fraction which includes aggregates of damaged proteins. Although myeloma proteasomes were able to clear most of the large buildup of ubiquitinated proteins within 2 to 3 h, this action was clearly insufficient to alleviate the proteotoxic stress and prevent widespread apoptosis, apparently due to the greater load of misfolded proteins in the myeloma cells. Probably the clearest evidence that increased temperatures and proteasome inhibition cause apoptosis via a common proteotoxic mechanism was the observation that the two BTZ-resistant myeloma lines were also relatively resistant to heat shock. Thus, their BTZ resistance results not from changes in drug uptake, metabolism, or efficacy in proteasome inhibition, but from an increased capacity to withstand proteotoxic stress (e.g., the production of less misfolded proteins).
Mechanism of Proteotoxic Cell Death of Myeloma Cells.
The death of myeloma cells at 43 °C is not because their proteins are inherently more susceptible to thermal denaturation, but because the accumulation of damaged proteins in such cells triggers programmed cell death through activation of the intrinsic apoptotic pathway and caspase-9. Similar proapoptotic changes were seen upon exposure of these cells to 39 °C plus a proteasome inhibitor. Accordingly, myeloma cell death at 41 or 43 °C was blocked by a pan-caspase inhibitor. The critical step in activating the intrinsic pathway is the permeabilization of the mitochondrial outer membrane, which is regulated by proapoptotic and antiapoptotic Bcl-2 family proteins. Upon exposure to 43 °C (or to 39 °C and BTZ) there was a marked buildup of only one proapoptotic Bcl-2 protein, the BH3-only protein Noxa. Thus, Noxa is likely to be the critical proapoptotic factor in myeloma cells under these conditions. Noxa has also been reported to induce apoptosis upon treatment of myeloma (42, 49), melanoma (43), mantle cell lymphoma (50), and triple-negative breast cells (51) with proteasome inhibitors, and treatment of esophageal cancer cells with bAP-15, a deubiquitinase inhibitor that also causes an accumulation of ubiquitinated proteins (52). Accumulation of Noxa can be triggered by ATF3 (53), although the major pathway leading to ATF3 activation, the UPR (54), was not activated at 43 °C. Noxa expression may also be activated by p53 (55) and c-myc (52), which build up after proteasome inhibition. Because Noxa itself is rapidly ubiquitinated and degraded (56), proteasome inhibitors may also promote its accumulation to cytotoxic levels. How misfolded proteins cause the buildup of Noxa is an important question for future study.
Hyperthermia as a Potential Therapeutic Modality for Multiple Myeloma.
There have been many studies of the use of hyperthermia in cancer treatment. Such attempts have generally used temperatures higher than 43 °C but have not proven successful because most cancer cells and their normal counterparts do not differ markedly in thermal tolerance (57). However, the present findings and a related recent study (29) raise the possibility that hyperthermia together with proteasome inhibitors may be useful in the treatment of myeloma. Although 41 and 43 °C are selectively toxic to myeloma cells, prolonged body temperatures of 43 °C can be life threatening. By contrast, body temperatures of 39 °C are seen with prolonged exercise, fevers, exposure to various drugs (58, 59), and cytokines (60). The present observations would predict that myeloma patients, when they are febrile, should be more responsive to proteasome inhibitors.
A variety of innovative techniques to raise temperatures of specific organs or the whole body have been developed recently. Although increased temperatures can be easily achieved for cancers in accessible areas (e.g., melanoma), myeloma cells grow in bone marrow throughout the body, and, thus, systemic hyperthermia would be necessary to be used therapeutically. After most of the present studies were completed, we learned of the work of Miki et al. (29), who used an innovative approach to raise bone marrow temperature in mice in a controlled manner (by marrow-directed nanomagnets and NMR) and could promote the killing of myeloma cells. Although our findings about how increased proteotoxic stress causes apoptosis of myeloma cells does not support their proposed mechanism (i.e., that death at 43 °C induces UPR activation and Mcl-1 production), their intriguing observations and the present ones strongly suggest that mild hyperthermia in combination with inhibitors of proteostasis merits further investigation as a potential therapy for myeloma and, perhaps, also for other cancers shown to be sensitive to proteasome inhibitors or “addicted to” proteasome gene expression (51, 61).
Materials and Methods
Cell Lines and Growth Conditions.
HEK293A, HeLa, and U2OS cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, 10–013-CV). M17 cells were cultured in DMEM/F12 media (Mediatech, 10–092-CV). Triple-negative breast cancer cell lines MDA-MB-231, HCC-1143, HCC-1937, and MDA-MB-468 were provided by Joan Brugge (HMS) and cultured in DMEM/F12 media. MCF10A cells were cultured in DMEM/F12 media supplemented with 20 ng/mL epidermal growth factor (Peprotech, AF-100-15), 0.5 mg/mL hydrocortisone, 100 ng/mL cholera toxin (Sigma-Aldrich, C-8052), and 10 µg/mL insulin. HCT116 cells were cultured in McCoy’s 5A Media (ATCC, 30–2007). HAP1 cell lines were cultured in Gibco Iscove’s modified Dulbecco’s media (ThermoFisher Scientific, 12440061). A673 cells were cultured in l-15 media (ATCC, 30–2008). Myeloma cell lines MM1.S, U266, RPMI8226, H929, KMS-12BM, and Dox40 cells were provided by Drs. Teru Hideshima and Giada Bianchi (Dana Farber) and were cultured in RPMI1640 media (Mediatech, 10–040-CV). TF1α cells provided by Dr. Beili Zhu (Northeastern University) were cultured in RPMI1640 media. All media contain 10% fetal bovine serum (Sigma-Aldrich, F6178-100 mL) and 1% penicillin–streptomycin solution. All cells were maintained in a humidified incubator at 37 °C and 5% CO2 before shift to higher temperatures. Compounds used in cell treatments are described in SI Appendix, Materials and Methods.
Lysate Preparation, Fractionation, and Western Blotting.
To fractionate cell lysates, cells were lysed for 30 min in ice-cold 1% Triton X-100 buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 mM NaF, 1 mM ethylenediaminetetraacetic acid, 1 mM Na3VO4, 1 mM dithiothreitol, 1% Triton X-100, Roche protease inhibitor mixture tablet). Approximately 0.5 million cells were lysed in 100 µL buffer. For fractionation, at least, 1 million cells were used. After centrifugation 10,000 × g for 10 min, pellets were solubilized in 2% sodium dodecyl sulfate (SDS) (in 50 mM Tris-Cl pH 7.4) and sonicated. Alternatively, when not indicated, cells were directly lysed in the 2% SDS buffer and sonicated to prepare cell lysates. For lysates in Triton X-100 buffer, protein concentrations were quantified by the Bradford Assay (VWR, PI23238). For lysates in SDS buffer, protein concentrations were quantified by the bicinchoninic assay (VWR, PI23225). Primary antibodies for Western blots are described in SI Appendix, Materials and Methods and were detected by either enhanced chemiluminescence or the Odyssey CLx Infrared Imaging System. Quantification of signals by densitometry was performed using the Odyssey CLx Infrared Imaging System. Actin was used as the loading control for the lysate fraction soluble in 1% TX-100, and lamin was used as the loading control for the pellet fraction after centrifugation.
Cell Viability and Toxicity Assays.
CellTiter 96 aqueous nonradioactive cell proliferation (MTS) assay (Promega, G5421), which measures mitochondrial function, was used to measure cell viability. CytoTox 96 nonradioactive cytotoxicity assay (Promega, G1780) was used to measure the percentage of cells that lost membrane integrity and released LDH into the media. Detailed descriptions of these assays are presented in ref. 32 and SI Appendix, Materials and Methods.
Measurement of the Degradation of Long-Lived Cell Protein.
Cell proteins were incubated with 3H-phenylalanine for 20 h to label the bulk of cell proteins. After washing and resuspension in a large excess of nonradioactive phenylalanine, their degradation was measured by following the release of 3H-phenylalanine from proteins (31, 62). Detailed descriptions of these assays are presented in SI Appendix, Materials and Methods.
Statistics.
Unpaired Student’s t test of Microsoft Excel was used for statistical analysis throughout the entire paper. An asterisk (*) indicates that P < 0.05.
Supplementary Material
Acknowledgments
We are grateful to Dhweeja Dasarathy for valuable assistance in performing certain experiments, Amelia Gould for assistance in preparation of this paper, and Teru Hideshima, Giada Bianchi, and Benjamin Ebert (Dana Farber) for kindly providing myeloma and other blood cell lines. Z.S. was a Novartis Fellow of the Life Sciences Research Foundation and a fellow of the Leukemia and Lymphoma Society. Our research was supported by Grants from the National Institute of General Medical Sciences (GM051923-18), Project ALS, and Genentech.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001323117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Kisselev A. F., van der Linden W. A., Overkleeft H. S., Proteasome inhibitors: An expanding army attacking a unique target. Chem. Biol. 19, 99–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson P. G., Mitsiades C., Hideshima T., Anderson K. C., Bortezomib: Proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med. 57, 33–47 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Goldberg A. L., Development of proteasome inhibitors as research tools and cancer drugs. J. Cell Biol. 199, 583–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah J. J., Orlowski R. Z., Proteasome inhibitors in the treatment of multiple myeloma. Leukemia 23, 1964–1979 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan D., Hideshima T., Anderson K. C., Proteasome inhibition in multiple myeloma: Therapeutic implication. Annu. Rev. Pharmacol. Toxicol. 45, 465–476 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Hideshima T., Anderson K. C., Biologic impact of proteasome inhibition in multiple myeloma cells–From the aspects of preclinical studies. Semin. Hematol. 49, 223–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi G. et al., The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood 113, 3040–3049 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Meister S. et al., Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 67, 1783–1792 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Ruggiano A., Foresti O., Carvalho P., Quality control: ER-associated degradation: Protein quality control and beyond. J. Cell Biol. 204, 869–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Z., Brodsky J. L., Protein quality control in the secretory pathway. J. Cell Biol. 218, 3171–3187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi M., Crinelli R., Arbore V., Magnani M., Induction of ubiquitin C (UBC) gene transcription is mediated by HSF1: Role of proteotoxic and oxidative stress. FEBS Open Bio 8, 1471–1485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush K. T., Goldberg A. L., Nigam S. K., Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J. Biol. Chem. 272, 9086–9092 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Walter P., Ron D., The unfolded protein response: From stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Cenci S. et al., Pivotal Advance: Protein synthesis modulates responsiveness of differentiating and malignant plasma cells to proteasome inhibitors. J. Leukoc. Biol. 92, 921–931 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Cenci S. et al., Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J. 25, 1104–1113 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fels D. R. et al., Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 68, 9323–9330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicherla B., Goldberg A. L., Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J. Cell Biol. 182, 663–673 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parag H. A., Raboy B., Kulka R. G., Effect of heat shock on protein degradation in mammalian cells: Involvement of the ubiquitin system. EMBO J. 6, 55–61 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balch W. E., Morimoto R. I., Dillin A., Kelly J. W., Adapting proteostasis for disease intervention. Science 319, 916–919 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Balchin D., Hayer-Hartl M., Hartl F. U., In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Saibil H., Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindquist S., The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191 (1986). [DOI] [PubMed] [Google Scholar]
- 23.Seufert W., Jentsch S., Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 9, 543–550 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff S. A., Goldberg A. L., Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell 41, 587–595 (1985). [DOI] [PubMed] [Google Scholar]
- 25.Ananthan J., Goldberg A. L., Voellmy R., Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232, 522–524 (1986). [DOI] [PubMed] [Google Scholar]
- 26.Zhuang J. et al., Ubiquitin-activating enzyme inhibition induces an unfolded protein response and overcomes drug resistance in myeloma. Blood 133, 1572–1584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyer M. L. et al., A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Watts G. S. et al., cDNA microarray analysis of multidrug resistance: doxorubicin selection produces multiple defects in apoptosis signaling pathways. J. Pharmacol. Exp. Ther. 299, 434–441 (2001). [PubMed] [Google Scholar]
- 29.Miki H. et al., Effective impairment of myeloma cells and their progenitors by hyperthermia. Oncotarget 9, 10307–10316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R., Emi M., Tanabe K., Murakami S., Role of the unfolded protein response in cell death. Apoptosis 11, 5–13 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Zhao J., Zhai B., Gygi S. P., Goldberg A. L., mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl. Acad. Sci. U.S.A. 112, 15790–15797 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sha Z., Schnell H. M., Ruoff K., Goldberg A., Rapid induction of p62 and GABARAPL1 upon proteasome inhibition promotes survival before autophagy activation. J. Cell Biol. 217, 1757–1776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dantuma N. P., Hoppe T., Growing sphere of influence: Cdc48/p97 orchestrates ubiquitin-dependent extraction from chromatin. Trends Cell Biol. 22, 483–491 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Le Moigne R. et al., The p97 inhibitor CB-5083 is a unique disrupter of protein homeostasis in models of multiple myeloma. Mol. Cancer Ther. 16, 2375–2386 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Zou J., Guo Y., Guettouche T., Smith D. F., Voellmy R., Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Neef D. W., Jaeger A. M., Thiele D. J., Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 10, 930–944 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anckar J., Sistonen L., Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 80, 1089–1115 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Christofferson D. E., Yuan J., Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263–268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degterev A. et al., Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hengartner M. O., The biochemistry of apoptosis. Nature 407, 770–776 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Wang C., Youle R. J., The role of mitochondria in apoptosis*. Annu. Rev. Genet. 43, 95–118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin J. Z. et al., Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 65, 6282–6293 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Fernández Y. et al., Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: Therapeutic implications. Cancer Res. 65, 6294–6304 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Morishima N., Nakanishi K., Takenouchi H., Shibata T., Yasuhiko Y., An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Aguilar L. E., Tumurbaatar B., Ghavaminejad A., Park C. H., Kim C. S., Functionalized non-vascular nitinol stent via electropolymerized polydopamine thin film coating loaded with bortezomib adjunct to hyperthermia therapy. Sci. Rep. 7, 9432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neznanov N., Komarov A. P., Neznanova L., Stanhope-Baker P., Gudkov A. V., Proteotoxic stress targeted therapy (PSTT): Induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget 2, 209–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saliev T. et al., Hyperthermia enhances bortezomib-induced apoptosis in human white blood cancer cells. J. Therm. Biol. 67, 9–14 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Lee A. H., Iwakoshi N. N., Anderson K. C., Glimcher L. H., Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. U.S.A. 100, 9946–9951 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Bougie P. et al., Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 67, 5418–5424 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Pérez-Galán P. et al., The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 107, 257–264 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Petrocca F. et al., A genome-wide siRNA screen identifies proteasome addiction as a vulnerability of basal-like triple-negative breast cancer cells. Cancer Cell 24, 182–196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sha B. et al., Deubiquitylatinase inhibitor b-AP15 induces c-Myc-Noxa-mediated apoptosis in esophageal squamous cell carcinoma. Apoptosis 24, 826–836 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Wang Q. et al., ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 106, 2200–2205 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohini M., Haritha Menon A., Selvamurugan N., Role of activating transcription factor 3 and its interacting proteins under physiological and pathological conditions. Int. J. Biol. Macromol. 120, 310–317 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Oda E. et al., Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Fennell D. A., Chacko A., Mutti L., BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene 27, 1189–1197 (2008). [DOI] [PubMed] [Google Scholar]
- 57.van der Zee J., Heating the patient: A promising approach? Ann. Oncol. 13, 1173–1184 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Musselman M. E., Saely S., Diagnosis and treatment of drug-induced hyperthermia. Am. J. Health Syst. Pharm. 70, 34–42 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Walter E., Carraretto M., Drug-induced hyperthermia in critical care. J. Intensive Care Soc. 16, 306–311 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson Rowsey P., Thermoregulation: Cytokines involved in fever and exercise. Annu. Rev. Nurs. Res. 31, 19–46 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Tsvetkov P. et al., Oncogenic addiction to high 26S proteasome level. Cell Death Dis. 9, 773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sha Z., Zhao J., Goldberg A. L., Measuring the overall rate of protein breakdown in cells and the contributions of the ubiquitin-proteasome and autophagy-lysosomal pathways. Methods Mol. Biol. 1844, 261–276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.