Significance
Macroautophagy is essential for the maintenance of cellular homeostasis and physiology in mammals, and relies on vesicle fusion between the autophagosome and the lysosome, forming the autolysosome to degrade unwanted cytosolic contents for recycling. The membrane fusion between the autophagosome and lysosome requires ATG8 family proteins and autophagy-related SNARE proteins including Syntaxin17, VAMP8, and SNAP29, but with poorly understood mechanisms. In this study, through systemic biochemical and structural characterizations, we reveal three different states of the key autophagosomal SNARE protein Syntaxin17 and provide mechanistic insights into the autoinhibited state of Syntaxin17 as well as its interactions with ATG8 family proteins, SNAP29 and VAMP8. Our findings are valuable for further understanding the functions of Syntaxin17 in the autophagosome–lysosome fusion process.
Keywords: autophagy, SNARE, Syntaxin17, GABARAP, autophagosome–lysosome fusion
Abstract
Syntaxin17, a key autophagosomal N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) protein, can associate with ATG8 family proteins SNAP29 and VAMP8 to facilitate the membrane fusion process between the double-membraned autophagosome and single-membraned lysosome in mammalian macroautophagy. However, the inherent properties of Syntaxin17 and the mechanistic basis underlying the interactions of Syntaxin17 with its binding proteins remain largely unknown. Here, using biochemical, NMR, and structural approaches, we systemically characterized Syntaxin17 as well as its interactions with ATG8 family proteins, SNAP29 and VAMP8. We discovered that Syntaxin17 alone adopts an autoinhibited conformation mediated by a direct interaction between its Habc domain and the Qa-SNARE motif. In addition, we revealed that the Qa-SNARE region of Syntaxin17 contains one LC3-interacting region (LIR) motif, which preferentially binds to GABARAP subfamily members. Importantly, the GABARAP binding of Syntaxin17 can release its autoinhibited state. The determined crystal structure of the Syntaxin17 LIR–GABARAP complex not only provides mechanistic insights into the interaction between Syntaxin17 and GABARAP but also reveals an unconventional LIR motif with a C-terminally extended 310 helix for selectively binding to ATG8 family proteins. Finally, we also elucidated structural arrangements of the autophagic Syntaxin17–SNAP29–VAMP8 SNARE core complex, and uncovered its conserved biochemical and structural characteristics common to all other SNAREs. In all, our findings reveal three distinct states of Syntaxin17, and provide mechanistic insights into the Syntaxin17-mediated autophagosome–lysosome fusion process.
Macroautophagy (hereafter referred to as autophagy) relies on the double-membraned vesicle called the autophagosome to fuse with the lysosome, forming the autolysosome for degradation of enclosed cytoplasmic materials in eukaryotes (1–5). Through autophagy, eukaryotic cells can recycle macromolecular constituents, such as bulk protein aggregates, glycogen, dysfunctional organelles, and invading pathogens, to maintain cellular homeostasis and/or adapt to multiple cellular stresses (1, 2). Thereby, autophagy plays critical roles in numerous physiological processes, such as energy metabolism, immune response, embryogenesis, and aging (6–8). Dysfunctions of autophagy are associated with many human diseases, including cancer, immune disorders, and neurodegenerative diseases (8–11). During the autophagy pathway, the formation of the autolysosome represents one of the essential steps for ultimate autophagic degradation, and depends on the tight coordination of autophagic vesicle fusions (1, 4–6, 12). So far, many proteins have been identified as being involved in these processes in mammals, including autophagic N-ethylmaleimide–sensitive (NSF) factor attachment protein receptor (SNARE) proteins (13, 14), relevant tethering factors such as the HOPS complex, ATG14, and EPG5 (15–18), ATG8 family proteins (19–21), and related regulatory proteins including Rab7, RILP, TECPR1, PLEKHM1, BRUCE, and Pacer (22–27). However, the detailed molecular mechanisms underlying the cooperation of these proteins to promote the formation of the autolysosome are still not well-understood.
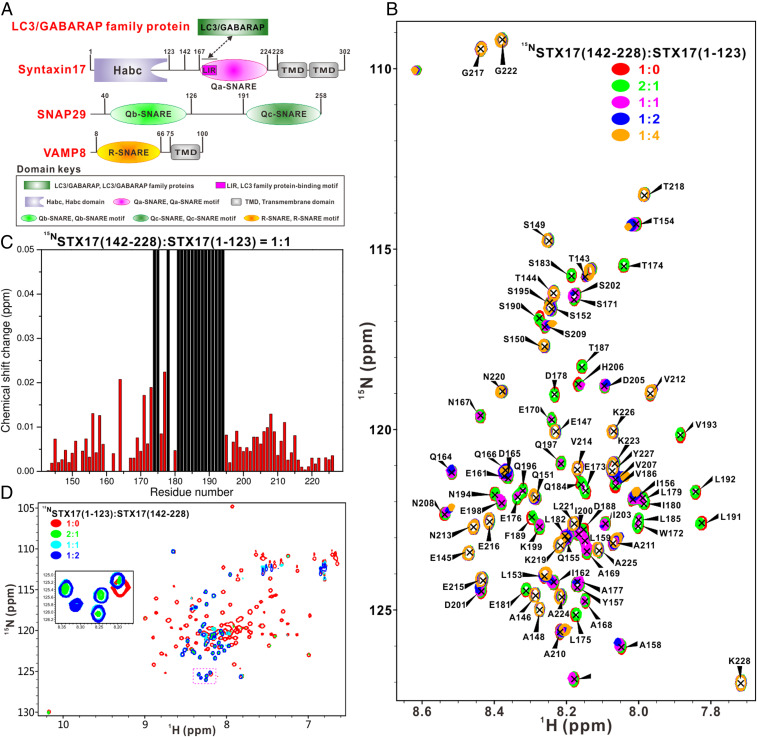
Cellular membrane fusion processes are known to be mediated by SNARE proteins, which can assemble into a membrane-bridging four-helix bundle (composed of Qa-, Qb-, Qc-, and R-SNAREs) to provide the mechanical thrust for effectively driving membrane fusion (28). The fusion event between the double-membraned autophagosome and the single-membraned lysosome during autophagy in mammals is reported to be mediated by two autophagy-specific SNARE complexes, the Syntaxin17 (hereafter called STX17)–SNAP29–VAMP8 SNARE complex and the recently discovered YKT6–SNAP29–Syntaxin7 SNARE complex (13, 14). As the key player for promoting autophagosome–lysosome fusion, the STX17-containing SNARE complex is composed of the autophagosomal SNARE STX17 (Qa-SNARE), the cytosolic SNARE SNAP29 (Qbc-SNAREs), and the lysosomal SNARE VAMP8 (R-SNARE) (Fig. 1A). Structurally, SNAP29 mainly contains Qb- and Qc-SNARE motifs, while VAMP8 is composed of an R-SNARE motif followed by a transmembrane domain (Fig. 1A). As a Qa-type SNARE protein, STX17 contains an N-terminal Habc domain, a Qa-SNARE motif followed by two unique tandem transmembrane domains (Fig. 1A). The two transmembrane domains of STX17 are demonstrated to form a hairpin structure and are required for the localization of STX17 on the autophagosome (13). The Qa-SNARE motif of STX17 can coassemble with SNAP29 Qb-SNARE, Qc-SNARE motifs, and the R-SNARE motif of VAMP8, forming the SNARE core complex (13), and the structure of this core SNARE complex was determined in a previous study from Zhong’s group (16). However, the biochemical properties of this autophagic SNARE complex and the detailed molecular basis underpinning the regulation of this autophagic SNARE complex formation are still largely unknown. Intriguingly, in addition to its canonical role in assembling the SNARE core complex, the Qa-SNARE motif of STX17 is also implicated in interactions with many other autophagy-related proteins, such as ATG14 (16), TBK1 (29), and ATG8 family proteins (30). In particular, a recent study showed that STX17 can directly interact with ATG8 family proteins and IRGM, an autophagy-related small GTPase, through its Qa-SNARE motif region and two transmembrane domains, respectively (30). Importantly, these interactions of STX17 with ATG8 family proteins and IRGM are essential for the efficient recruitment of STX17 to the autophagosome (30). However, due to the lack of detailed structural characterizations, the molecular mechanisms governing the interactions of STX17 with these proteins remain elusive.
Fig. 1.
| [1] |
ATG8 family proteins are small ubiquitin-like proteins, and include six orthologs in mammals known as MAP1LC3A (LC3A), MAP1LC3B (LC3B), MAP1LC3C (LC3C), GABARAP, GABARAPL1, and GABARAPL2 (31–33). They can be further classified into two subfamilies, the LC3 subfamily and the GABARAP subfamily (31, 32). ATG8s are decorated on the membrane of the phagophore by conjugating with a phosphatidylethanolamine (PE) lipid catalyzed by the E3-like ATG5–ATG12–ATG16L1 complex during the action of autophagy (4, 5, 32–34). The PE-conjugated ATG8s are present both on the inner and outer membranes of the emerging closed autophagosome before fusion with the lysosome (4, 5). They are demonstrated to play crucial roles in autophagosome biogenesis, autophagic cargo engulfment, autophagic vesicle transport, and fusion of the autophagosome with the lysosome or endosome by associating with relevant proteins that contain a short motif called the LC3-interacting region (LIR) in mammals (19, 31–33, 35–39). The canonical LIR motif contains a consensus core sequence ΘXXΓ (where Θ represents an aromatic Trp, Tyr, or Phe residue; Γ represents a bulky hydrophobic Leu, Ile, or Val residue; and X represents any amino acid residue) (31, 38, 40). Additional negatively charged serine/threonine phosphorylation sites and/or acidic residues preceding the LIR core sequence are also routinely found in typical LIR motifs, which are proven to regulate the interactions of LIR-containing proteins with ATG8 family members (31, 38, 40, 41). Intriguingly, our previous study together with other groups’ reports revealed that some LIR motifs, such as that of FYCO1, ankyrin-G, and ankyrin-B, also include C-terminal extensions that can participate in the interaction with ATG8 family orthologs following the core ΘXXΓ sequence (42–44). In particular, these C-terminal extensions not only can facilitate strong binding to ATG8s but also endow LIR-containing proteins with binding selectivity to different ATG8 orthologs (42–44). Notably, previous functional studies well-demonstrated that the GABARAP subfamily is preferentially involved in the autophagosome–lysosome fusion process and the LC3s are unable to replace GABARAPs for the fusion between the autophagosome and lysosome (19, 45), suggesting that some proteins involved in the autophagosome–lysosome fusion process may also contain C-terminal extensions for selectively binding to the GABARAP subfamily. Interestingly, STX17 was recently reported to contain two putative LIR motifs within its Qa-SNARE motif region, and can bind to ATG8 family proteins, especially LC3B and GABARAP (30). However, how the two putative LIR motifs of STX17 interact with ATG8 family proteins is still elusive.
In this study, we biochemically and structurally characterized the key autophagosomal SNARE STX17 as well as its interactions with the ATG8 family proteins, SNAP29 and VAMP8, and uncovered three different states of STX17. Specifically, we discovered that the isolated STX17 adopts an autoinhibited “closed” conformation, in which the N-terminal half of the STX17 Qa-SNARE motif occupies the Habc domain of STX17. In addition, we revealed that STX17 only contains one LIR motif, which preferentially binds to GABARAP subfamily members. We determined the high-resolution structure of the STX17 LIR–GABARAP complex, and uncovered the molecular mechanism underpinning the interaction between STX17 and GABARAP. Notably, the determined STX17 LIR–GABARAP complex structure also highlights the importance of the C-terminal extension following the LIR core motif for some LIR-containing proteins to selectively interact with ATG8 family proteins. Finally, we also investigated the biochemical and structural features of the autophagic STX17–SNAP29–VAMP8 SNARE core complex, and elucidated characteristic biochemical properties and structural arrangements of this autophagic SNARE complex.
Results
The STX17 SNARE Motif Can Interact with the N-Terminal Habc Domain of STX17 and Alter Its Conformation.
To gain molecular insights into the function of STX17 in the autophagosome–lysosome fusion process, we first conducted a detailed sequence alignment analysis of the cytoplasmic region of STX17 and found that the N-terminal Habc region (residues 1 to 123) and the Qa-SNARE motif (residues 167 to 224) of STX17 are highly conserved during evolution (SI Appendix, Fig. S1A), in line with their known functions of interacting with other proteins (13, 17). Then, we purified a uniformly 15N-labeled STX17(142–228) fragment that includes the entire Qa-SNARE motif (residues 167 to 224), and acquired its 1H-15N heteronuclear single-quantum coherence (HSQC) spectrum (SI Appendix, Fig. S2A). The small dispersion of the NMR peaks in the 1H dimension of the 1H-15N HSQC spectrum together with the determined secondary structure of this STX17 Qa-SNARE region based on the 13Cα and 13Cβ chemical shift values of each residue after backbone chemical shift assignments indicated that the isolated STX17 Qa-SNARE motif is basically unstructured (SI Appendix, Fig. S2). Interestingly, titration of the 15N-labeled STX17(142–228) with the unlabeled STX17(1–123) protein showed that a selected set of peaks in the 1H-15N HSQC spectrum of STX17(142–228) underwent significant dose-dependent peak broadening or chemical shift changes (Fig. 1B), indicating that the STX17 Qa-SNARE region can specifically interact with the N-terminal Habc domain of STX17. Further plotting of the peak broadening and amide backbone chemical shift changes as a function of residue number revealed that the significant perturbations are mainly rich in the N-terminal part of the STX17 Qa-SNARE motif (residues 174 to 194) (Fig. 1C), suggesting that this region is the major binding site for interacting with the STX17 Habc domain.
In contrast to that of STX17(142–228), the 1H-15N HSQC spectrum of STX17(1–123) is well-dispersed, indicating that this region constitutes an independently well-folded domain (Fig. 1D). Further titration of 15N-labeled STX17(1–123) with unlabeled STX17(142–228) showed that the majority of peaks in the 1H-15N HSQC spectrum underwent significant dose-dependent peak broadening (Fig. 1D), confirming the existence of a direct interaction between the Habc domain and Qa-SNARE motif of STX17. Strikingly, in the presence of STX17(142–228), a set of peaks appeared in the 1H-15N HSQC spectrum of STX17(1–123) (Fig. 1D). Based on this observed NMR phenomenon and a series of NMR titration experiments using different truncation mutants of the STX17 Qa-SNARE motif, we further confirmed that the N-terminal region of the STX17 Qa-SNARE motif is responsible for the interaction with STX17(1–123) (SI Appendix, Fig. S3). Unfortunately, due to the serious concentration-dependent peak broadening that was likely induced by nonspecific self-associations (SI Appendix, Fig. S4A), we were unable to achieve the backbone assignments for STX17(1–123). However, in the presence of a saturated amount of STX17(142–228) protein, we were able to finish the backbone assignments for the remaining NMR peaks (SI Appendix, Fig. S4B). Interestingly, we found that the newly appeared NMR peaks arose from residues 94 to 121, which are located in the predicted extreme C-terminal α-helix of the Habc domain (SI Appendix, Fig. S1A), but were demonstrated to be unstructured in the presence of STX17(142–228) based on our NMR analysis (SI Appendix, Fig. S4C). Taken together, all these data clearly demonstrated that the Qa-SNARE motif of STX17 can directly bind to the STX17 Habc domain and alter its conformation by partially unfolding its extreme C-terminal α-helix. Thus, the cytoplasmic region of STX17 may adopt an autoinhibited closed conformation imposed by an intramolecular interaction between its Habc domain and the Qa-SNARE motif. Notably, the STX17 Qa-SNARE motif only showed a negligible weak interaction with the full-length SNAP29 or the VAMP8 R-SNARE motif (residues 8 to 66) proteins based on our NMR titration results (SI Appendix, Fig. S5). Importantly, further NMR analyses revealed that neither the full-length SNAP29 nor the VAMP8 R-SNARE motif alone was able to release the autoinhibited state of STX17 (SI Appendix, Fig. S6). Consistent with these NMR-based analyses (SI Appendix, Figs. S5 and S6), further analytical gel filtration chromatography-based assays revealed that full-length SNAP29 cannot obviously interact with VAMP8(1–75) that includes the entire cytoplasmic region of VAMP8 nor with STX17(1–228) (SI Appendix, Fig. S7 A and B), and there is an extremely weak interaction between STX17(1–228) and VAMP8(1–75) (SI Appendix, Fig. S7C). As expected, when mixing these three proteins together, we can readily detect a ternary SNARE complex containing SNAP29 full-length, VAMP8(1–75), and STX17(1–228) (SI Appendix, Fig. S7 D and E). Therefore, unlike the neuronal SNARE complex, the formation of a binary STX17–SNAP29 (Qa/Qbc) t-SNARE complex during the autophagic SNARE complex assembly process is unfeasible in vitro.
The STX17 SNARE Region Contains One LIR Motif That Can Selectively Bind to Mammalian ATG8 Orthologs.
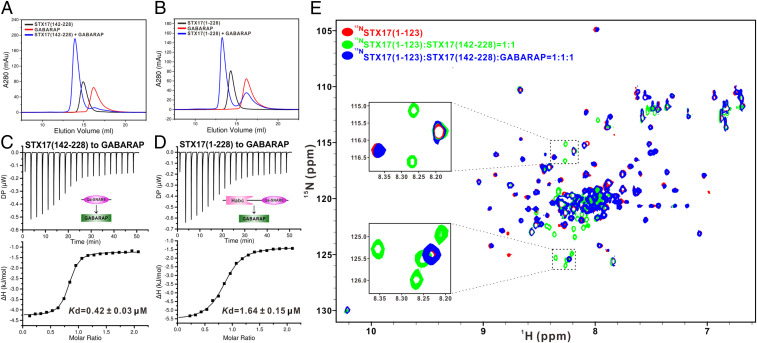
Since mammalian ATG8 family proteins, especially LC3B and GABARAP, were reported to interact with the STX17 Qa-SNARE region (30), we wondered whether these mammalian ATG8 orthologs might regulate the closed conformation of STX17. To test this hypothesis, we purified two STX17 fragments, STX17(142–228) and STX17(1–228), that include the entire cytoplasmic region of STX17 and should adopt an autoinhibited state, and investigated their interactions with the six mammalian ATG8 homologs. Using analytical gel filtration chromatography-based analyses, we found both STX17(142–228) and STX17(1–228) can directly interact with all of the six mammalian ATG8 orthologs (Fig. 2 A and B and SI Appendix, Figs. S8 and S9). Further quantitative analyses of the interactions of these two STX17 fragments with different ATG8 homologs using isothermal titration calorimetry (ITC) measurements revealed that STX17 can selectively bind to six mammalian ATG8 orthologs with distinct binding affinities (Fig. 2 C and D and SI Appendix, Figs. S10–S12). In particular, STX17 preferentially binds to GABARAP subfamily members (GABARAP, GABARAPL1, and GABARAPL2) rather than LC3 subfamily members (LC3A, LC3B, and LC3C) (Fig. 2 C and D and SI Appendix, Figs. S10 and S11). Notably, the STX17(1–228) fragment displays a relatively weaker binding affinity toward ATG8 family members than that of STX17(142–228) based on our ITC analyses (Fig. 2 C and D and SI Appendix, Figs. S10 and S11), suggesting that the STX17 N-terminal Habc region somehow interferes with the interactions between the STX17 SNARE region and ATG8 orthologs, consistent with our aforementioned observation that the N-terminal Habc domain of STX17 can directly interact with the STX17 SNARE motif (Fig. 1 B–D). Importantly, additional NMR characterizations uncovered that in contrast to SNAP29 and VAMP8 (SI Appendix, Fig. S6), GABARAP can compete against the N-terminal Habc domain for binding to the SNARE motif of STX17, thereby easily relieving the autoinhibited state of STX17 (Fig. 2E).
Fig. 2.
GABARAP can directly bind to STX17 and relieve the autoinhibited conformation of STX17. (A and B) Analytical gel filtration chromatography analyses of the interactions between GABARAP and STX17(142–228) (A) or STX17(1–228) (B). (C and D) ITC-based measurements of the binding affinities of GABARAP with STX17(142–228) (C) or STX17(1–228) (D). (E) Superposition plot of the 1H-15N HSQC spectra of STX17(1–123) (red), STX17(1–123) titrated with STX17(142–228) at a molar ratio of 1:1 (green), and STX17(1–123) saturated with STX17(142–228) followed by adding GABARAP at a molar ratio of 1:1:1 (blue). For clarity, the Insets show enlarged views of two selected regions of the overlaid 1H-15N HSQC spectra. DP, differential power measured by the ITC machine.
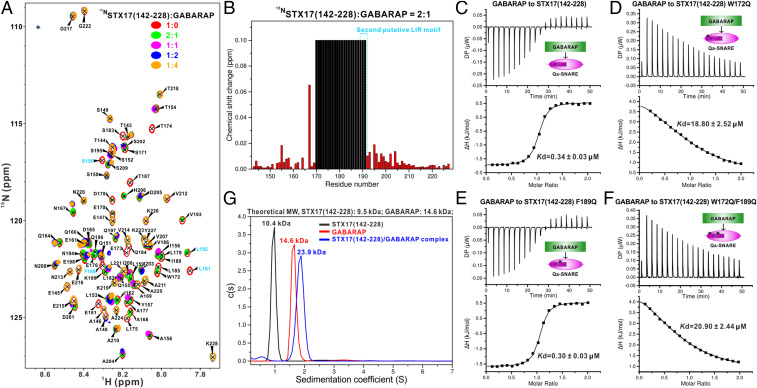
Then, we sought to understand how the STX17 SNARE motif recognizes the mammalian ATG8 orthologs. We chose GABARAP and LC3A as two representatives of the GABARAP subfamily and the LC3 subfamily, respectively, and carefully characterized their interactions with STX17(142–228). NMR-based analyses showed that both GABARAP and LC3A can interact with STX17(142–228) (Fig. 3A and SI Appendix, Fig. S13A), and the major binding sites on STX17 are located within the N-terminal part (residues 170 to 191) of the STX17 Qa-SNARE motif (Fig. 3B and SI Appendix, Fig. S13B). Notably, the binding sites of the STX17 SNARE motif for interacting with mammalian ATG8 orthologs and the STX17 Habc domain are highly overlapped (Figs. 1 B and C and 3 A and B and SI Appendix, Fig. S13 A and B), therefore mechanistically explaining why mammalian ATG8 orthologs can compete with the N-terminal Habc domain for binding to the SNARE motif of STX17. Interestingly, detailed sequence analysis showed that the STX17 SNARE region contains two putative LIR motifs, “WETL” (residues 172 to 175) and “FSLL” (residues 189 to 192), although the critical aromatic Phe residue in the second putative LIR motif is not strictly conserved in mammals (SI Appendix, Fig. S1A). Further NMR-based analyses revealed that the NMR resonances of the first putative LIR motif show much more significant changes than those of the second putative LIR region when titrated with GABARAP or LC3A (Fig. 3 A and B and SI Appendix, Fig. S13 A and B), suggesting that only the first putative LIR motif may directly participate in the interaction with GABARAP or LC3A. To further test whether these two putative LIR motifs are directly involved in the GABARAP or LC3A binding, we mutated the two crucial aromatic residues (W172 and F189) within the two putative LIR motifs of STX17(142–228) and constructed three mutants, STX17(142–228) W172Q, STX17(142–228) F189Q, and the STX17(142–228) W172Q/F189Q double mutant. Then, we used these three mutants together with the wild-type STX17(142–228) and quantitatively compared their interactions with GABARAP and LC3A using ITC-based assays (Fig. 3 C–F and SI Appendix, Fig. S13 C–F). The obtained ITC results showed that the W172Q mutation dramatically reduces and totally abolishes the interaction of STX17(142–228) with GABARAP and LC3A, respectively (Fig. 3 C and D and SI Appendix, Fig. S13 C and D), while the F189Q mutation does not affect the binding of STX17(142–228) to GABARAP and LC3A (Fig. 3 C and E and SI Appendix, Fig. S13 C and E), confirming that only the first putative LIR motif of STX17 is directly involved in the interactions with GABARAP and LC3A (Fig. 3 A and B and SI Appendix, Fig. S13 A and B). Surprisingly, the STX17(142–228) W172Q and W172Q/F189Q mutants still displayed some residual binding abilities to GABARAP but not LC3A (Fig. 3 D and F and SI Appendix, Fig. S13 D and F), and they can weakly bind to GABARAP with similar Kd values, ∼19 and ∼21 μM, respectively (Fig. 3 D and F), implying that, except for the canonical hydrophobic LIR core sequence, there are additional structural features that may contribute to the interaction of STX17 with GABARAP but not LC3A. Finally, using analytical ultracentrifugation analyses, we further elucidated that STX17(142–228) and GABARAP both form monomers in solution and, importantly, they can interact with each other to form a 1:1 stoichiometric complex (Fig. 3G), consistent with our notion that the Qa-SNARE region of STX17 only contains one LIR motif.
Fig. 3.
STX17 Qa-SNARE region contains one LIR motif that is required for interaction with GABARAP. (A) Superposition plot of the assigned 1H-15N HSQC spectra of STX17(142–228) titrated with increasing molar ratios of unlabeled GABARAP proteins. In this representation, the four core residues (F189, S190, L191, L192) of the second putative LIR motif of STX17 are further highlighted and colored in sky blue. (B) Plot of backbone amide chemical shift differences and peak broadening as a function of the residue number of STX17(142–228) between the wild type and the protein titrated with GABARAP at a molar ratio of 2:1. In this representation, the residues with disappeared NMR peaks due to peak broadening are shown in black, and the combined 1H and 15N chemical shift changes are defined as shown in Eq. 1. In addition, the corresponding four core residues (residues 189 to 192) of the second putative LIR motif of STX17 are also indicated. (C–F) ITC-based measurements showing the binding affinities of GABARAP with STX17(142–228) (C), STX17(142–228) W172Q mutant (D), STX17(142–228) F189Q mutant (E), and STX17(142–228) W172Q/F189Q double mutant (F). (G) Overlay plot of the sedimentation velocity data of STX17(142–228) (black), GABARAP (red), and STX17(142–228)–GABARAP complex (blue). These results demonstrate that STX17(142–228) and GABARAP form monomers and interact with each other to form a 1:1 stoichiometric complex.
The Overall Structure of the STX17 LIR Motif in Complex with GABARAP.
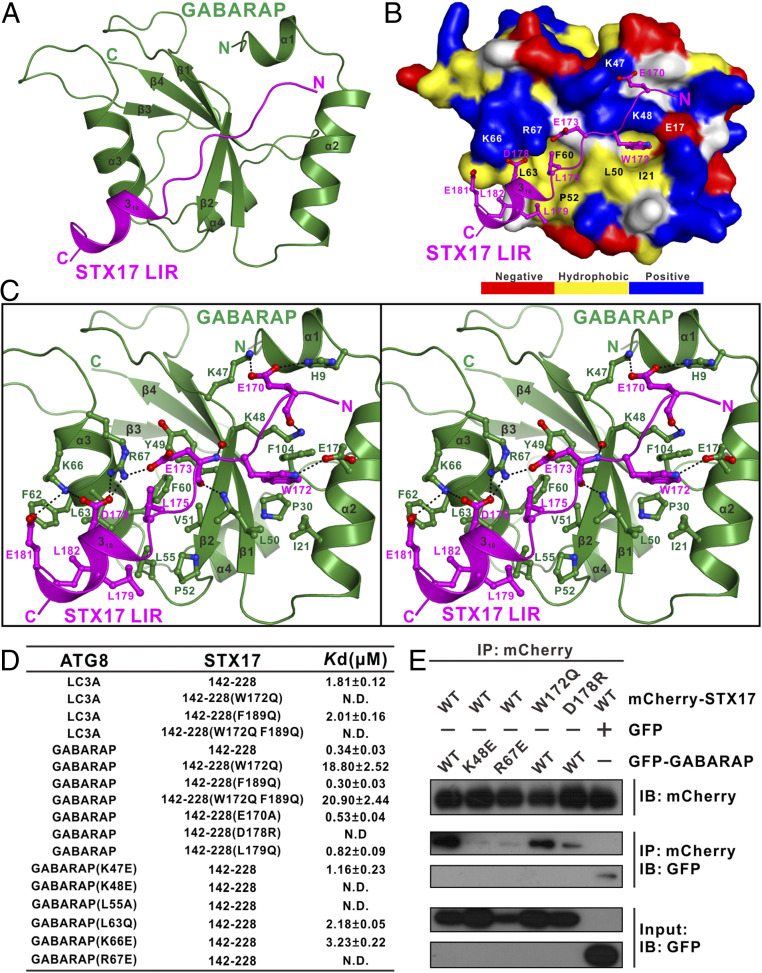
To further elucidate the molecular mechanism governing the interaction between the STX17 LIR motif and mammalian ATG8 proteins, we sought to determine their complex structures. Initially, we purified the STX17(142–228)–GABARAP complex to conduct a crystal screening but, unfortunately, we only obtained crystals with poor diffractions. Given that the STX17(142–228) fragment is basically unstructured, we further narrowed down the N- and C-terminal boundaries of STX17 and chose a STX17(167–188) fragment, which includes the entire proven LIR motif but lacks the second putative LIR sequences (SI Appendix, Fig. S1A). Fortunately, using the purified STX17(167–188)–GABARAP complex, we obtained good crystals that diffracted to 2.0-Å resolution. The crystal structure of the STX17 LIR–GABARAP complex was determined using the molecular replacement method (SI Appendix, Table S1). In the final refined structural model, each asymmetric unit contains four STX17 LIR–GABARAP complex molecules, and each STX17 LIR–GABARAP complex has a 1:1 stoichiometry (Fig. 4A), in line with our biochemical result (Fig. 3G). As expected, the GABARAP molecule in the complex structure adopts a typical ATG8 homolog protein architecture consisting of two N-terminal α-helices followed by a ubiquitin-like core that is assembled by a four-stranded β-sheet together with two α-helices (Fig. 4A). In the complex structure, the GABARAP-bound STX17 LIR motif is mainly composed of two parts: an N-terminal extended structure formed by the canonical LIR core containing the signature ΦXXΨ (WETL) sequences and an additional C-terminal 310-helix extension (Fig. 4 A and B and SI Appendix, Fig. S1A), in agreement with our aforementioned ITC results (Fig. 3 D and F). The entire STX17 LIR motif packs extensively with a solvent-exposed elongated groove mainly formed by the α1-, α2-, and α3-helices together with the β2-strand of GABARAP, burying a total surface area of ∼778 Å2 (Fig. 4 A and B and SI Appendix, Fig. S14A). Intriguingly, structural comparisons of the STX17 LIR–GABARAP complex with currently known complexes of ATG8s bound with unconventional LIR motifs that contain C-terminal extensions, such as the FYCO1 LIR–LC3A complex (Protein Data Bank [PDB] ID code 5CX3) (42), AnkB LIR–GABARAP complex (PDB ID code 5YIR), and AnkG LIR–GABARAPL1 complex (PDB ID code 5YIP) (43), revealed that the overall interaction modes of the extended LIR motifs from STX17, FYCO1, and AnkB/G toward ATG8 family proteins are very similar but, strikingly, only the STX17 LIR includes a C-terminal extension with a 310 helix (SI Appendix, Fig. S14B).
Fig. 4.
Structural analyses of the STX17 LIR–GABARAP complex. (A) Ribbon diagram showing the overall structure of the STX17 LIR–GABARAP complex. In this drawing, GABARAP is shown in forest green, and the STX17 LIR motif is in magenta. (B) The combined surface representation and ribbon-stick model showing the molecular interface of GABARAP in the STX17 LIR–GABARAP complex. In this representation, GABARAP is shown in the surface model and STX17 LIR is in the ribbon-stick model. The hydrophobic amino acid residues of GABARAP in the surface model are drawn in yellow, the positively charged residues are in blue, the negatively charged residues are in red, and the uncharged polar residues are in gray. (C) Stereoview of the ribbon-stick representation showing the detailed interactions between GABARAP and STX17 LIR. In this drawing, the side chains of the key residues are shown in stick-ball mode, and the hydrogen bonds involved in the binding are shown as dotted lines. (D) The measured binding affinities between various forms of GABARAP–LC3A and the STX17 Qa-SNARE motif or their mutants by ITC-based analyses. (E) Mutagenesis-based co-IP assays confirming the interactions between GABARAP and STX17 observed in the determined STX17 LIR–GABARAP complex structure. IB, immunoblotting.
The Molecular Interface of the STX17 LIR–GABARAP Complex.
Detailed structural analysis of the binding interface of the STX17 LIR–GABARAP complex revealed that the binding between STX17 LIR and GABARAP is mediated by extensive hydrophobic contacts and polar interactions (Fig. 4 B and C and SI Appendix, Fig. S14C). In particular, the aromatic side chain of W172 of STX17 LIR occupies a hydrophobic pocket of GABARAP mainly assembled by the hydrophobic side chains of I21, P30, L50, and F104 as well as the aliphatic side chain of K48 and, meanwhile, the side-chain group of STX17 W172 also forms a hydrogen bond with the side chain of E17 located at the α2-helix of GABARAP (Fig. 4 B and C). In parallel, the hydrophobic side chains of L175, L179, and L182 from STX17 LIR pack against a hydrophobic patch that is situated at the β2/α3-groove and formed by the side chains of the Y49, V51, P52, L55, F60, F62, and L63 residues of GABARAP (Fig. 4 B and C). Moreover, the backbone oxygen of STX17 E170 forms a strong hydrogen bond with the side chain of the K48 residue located at the β2-strand of GABARAP, and the backbone oxygen and amide group of STX17 E173 form two backbone hydrogen bonds with the K48 and L50 residues of GABARAP (Fig. 4C). In addition, the STX17 LIR–GABARAP complex is further stabilized by two charge–charge interaction networks, one of which is located at the N-terminal region of GABARAP and is assembled by the negatively charged STX17 E170 residue coupled with the positively charged H9 and K47 residues of GABARAP, while the other is formed between the negatively charged E173, D178, and E181 residues of STX17 LIR and positively charged K66 and R67 residues of GABARAP (Fig. 4 B and C and SI Appendix, Fig. S14C). Notably, all these key interface residues of STX17 and GABARAP are highly conserved during evolution (SI Appendix, Fig. S1). Using ITC and coimmunoprecipitation (co-IP) analyses, we further verified the interactions observed in the structure of the STX17 LIR–GABARAP complex. Consistent with our structural data, the ITC results showed that individual point mutations of the key interface residues either from STX17 or GABARAP, such as the E170A, W172Q, D178R, and L179Q mutations of STX17(142–228) or the K47E, K48E, L55A, L63Q, K66E, and R67E mutations of GABARAP, all largely reduce or completely disrupt the interaction between STX17(142–228) and GABARAP (Fig. 4D and SI Appendix, Fig. S15). Importantly, consistent with our in vitro ITC results, further co-IP experiments revealed that point mutations of key interface residues including the K48E and R67E mutations of GABARAP and the W172Q and D178R mutations of STX17 all significantly attenuate or essentially abolish the interaction between full-length STX17 and GABARAP in cotransfected cells (Fig. 4E). Notably, further detailed structure-based sequence alignment and structural comparison analyses showed that several key binding-interface residues are quite different among the six mammalian ATG8 orthologs (SI Appendix, Fig. S16). For instance, the residues corresponding to the bulky hydrophobic L55 and F62 in GABARAP, which are critical for the hydrophobic interaction with the C-terminal 310 helix (SI Appendix, Fig. S16B), are a relatively smaller Val residue in LC3A and LC3B and a polar Lys or Ser residue in LC3s, respectively (SI Appendix, Fig. S16A); the residue corresponding to the positively charged K47 in GABARAP is a neutral Thr residue in LC3s, and the residue corresponding to the positively charged R66 in GABARAP is a neutral Ser residue in LC3C (SI Appendix, Fig. S16A). Consistent with these sequence- and structure-based analyses, further ITC-based assays demonstrated that the GABARAP K47T/L55V/F62K triple mutant has a comparable binding affinity for STX17(142–228) to that of LC3A and, conversely, the V58L/K65F double mutant of LC3A has a much increased binding ability for STX17(142–228) (SI Appendix, Fig. S17). In all, the identification of these nonconserved interface residues among different mammalian ATG8 orthologs not only provided a mechanistic explanation for the selective binding of STX17 LIR toward different mammalian ATG8 orthologs but also rationalized our aforementioned biochemical results that the STX17(142–228) W172Q and W172Q/F189Q mutants can weakly bind to GABARAP but not to LC3A that lacks the crucial bulky hydrophobic L55 and F62 residues for interacting with the C-terminal 310-helix extension of STX17 (Fig. 3 D and F and SI Appendix, Fig. S13 D and F).
The Biochemical and Structural Properties of the STX17–SNAP29–VAMP8 SNARE Complex.
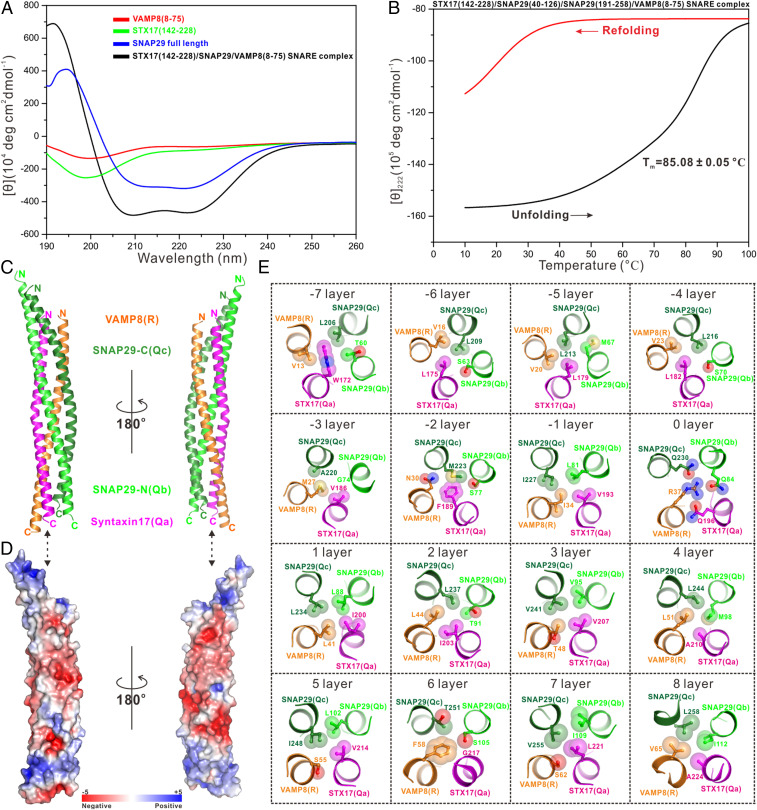
Given that GABARAP can occupy the N-terminal part of the STX17 SNARE motif and form a stable complex with STX17, next we wanted to know whether GABARAP may affect the assembly of the STX17–SNAP29–VAMP8 SNARE complex. Using analytical gel filtration chromatography-based analyses, we showed that the Qb- and Qc-SNARE motifs of SNAP29 alone are unable to interact with or disturb the GABARAP–STX17 complex (SI Appendix, Fig. S18A); however, in the presence of an additional VAMP8 R-SNARE motif, a stable SNARE complex containing the STX17 Qa-SNARE motif, the VAMP8 R-SNARE motif, as well as the Qb- and Qc-SNARE motifs of SNAP29, was readily formed (SI Appendix, Fig. S18 B and C), indicating that SNAP29 and VAMP8 together can easily compete with GABARAP for binding to STX17 to assemble the STX17–SNAP29–VAMP8 SNARE complex. Since the membrane fusion between the double-membraned autophagosome and single-membraned lysosome is morphologically distinct from the conventional fusion event between two single-membraned vesicles, we wondered whether this autophagic STX17–SNAP29–VAMP8 SNARE complex has striking biochemical properties. To test this hypothesis, we first purified this autophagic SNARE complex, which includes the SNARE motifs of STX17 and VAMP8 together with their short neck regions, and the Qb- and Qc-SNARE motifs of SNAP29, and then used circular dichroism (CD) spectroscopy to examine its secondary structure features and thermal stability. In contrast to that of the isolated SNARE regions of STX17 and VAMP8 as well as the full-length SNAP29, the SNARE complex showed significant characteristic α-helical content, as indicated by its CD spectrum (Fig. 5A). It is noteworthy that the isolated SNARE region of STX17 is intrinsically disordered based on the CD analysis (Fig. 5A), consistent with the aforementioned NMR results (SI Appendix, Fig. S2). Interestingly, further CD-based analysis revealed that with increasing temperature, this autophagic SNARE complex undergoes unfolding with a melting temperature (Tm) of ∼85 °C (Fig. 5B), which is a little higher than that of the late endosomal SNARE complex (Tm 78 °C) but slightly lower than its early endosomal (Tm 87 °C) and neuronal (Tm 90 °C) counterparts (46–48). Subsequent reduction of the temperature of the sample led to the initiation of refolding at a much lower temperature of ∼51 °C and further cooling to 10 °C resulted in a partial refolding of the original α-helical content (Fig. 5B), showing the characteristic unfolding–refolding hysteresis of a typical SNARE core complex (28, 49).
Fig. 5.
Biochemical and structural characterizations of the STX17–SNAP29–VAMP8 SNARE complex. (A) Overlay plot of the CD spectra of the STX17–SNAP29–VAMP8 SNARE complex and related individual autophagic SNARE proteins. (B) Thermal unfolding and refolding analyses of the STX17–SNAP29–VAMP8 SNARE complex monitored by CD spectroscopy at 222 nm. (C) Ribbon diagram showing the overall structure of the STX17–SNAP29–VAMP8 SNARE complex. (D) Surface charge potential representation (contoured at ±5 kT/eV; blue/red) of the STX17–SNAP29–VAMP8 SNARE complex with the same orientation as in C, revealing two highly negatively charged patches. (E) Detailed interior interactions of the autophagic STX17–SNAP29–VAMP8 SNARE complex formed by 16 layers of interacting amino acid side chains.
We also solved the crystal structure of this autophagic SNARE core complex (SI Appendix, Table S1). As expected, this autophagic SNARE complex forms a four-helix bundle with all four helices aligned in parallel, and STX17 and VAMP8 each contribute one helix while SNAP29 contributes two helices to the bundle (Fig. 5C and SI Appendix, Fig. S19). Notably, a similar overall architecture was also observed in a previously reported structure of the STX17–SNAP29–VAMP8 SNARE complex with slightly different protein boundaries (16). Interestingly, the electrostatic potential surface of this autophagic SNARE complex showed two highly negatively charged patches (Fig. 5D), which are on opposite sides of the surface and are implicated in the binding to the NSF-mediated SNARE-disassembly machinery (28, 50). Similar to other known SNARE complexes, the interior of this autophagic SNARE complex is formed by 16 layers of interacting amino acid side chains that are mostly hydrophobic, and the hydrophilic 0 layer is formed by three Gln residues (Q196 of STX17 and Q84 and Q230 of SNAP29) and one Arg residue (R37 of VAMP8) (Fig. 5E and SI Appendix, Fig. S19). Detailed structural analyses of the other 15 layers revealed that the −fifth, −first, first, fourth, and eighth layers are all formed by four hydrophobic side chains, whereas the −seventh, −sixth, −fourth, second, third, fifth, and seventh layers are all composed of three hydrophobic side chains and one polar side chain of a Ser or Thr residue, of which the hydroxyl group throughout points to the outside of the layer (Fig. 5E). Notably, the arrangements of the side chains in the −third, −second, and sixth layers are highly asymmetric (Fig. 5E). Next, we sought to use the glutathione S-transferase (GST)–fusion protein pull-down assay to verify the contributions of different intact layers to the assembly of the autophagic SNARE complex. We individually disrupted the intact −seventh, −fifth, 0, fourth, and seventh layers of the SNARE complex by mutation of key residues involved in the layer formation either from STX17 or SNAP29. The W172Q (−seventh layer), L179Q (−fifth layer), and Q196L (0 layer) mutations of STX17 as well as the L213Q (−fifth layer) and Q230L (0 layer) mutations of SNAP29 essentially abolished the SNARE core complex formation, whereas the A210Q (fourth layer) and L221Q (seventh layer) mutations of STX17 only partially weakened the core complex formation (Fig. 5E and SI Appendix, Fig. S20), suggesting that the N-terminal and central layers are much more important than the C-terminal layers during the assembly of the autophagic SNARE complex, in keeping with the zipper-like mode for canonical SNARE complex assembly (28, 51). In addition, the four-helix bundle structure of this autophagic SNARE complex is further stabilized by extensive surface interactions between different helices (SI Appendix, Figs. S17 and S21).
Discussion
In this study, we uncovered that the STX17 Qa-SNARE motif can directly interact with its N-terminal Habc domain, suggesting that, in isolation, STX17 adopts a closed conformation. Considering the poor quality of the 1H-15N HSQC spectrum of the STX17 Habc region in the presence of the STX17 SNARE motif (Fig. 1D and SI Appendix, Fig. S3), we sought to use X-ray crystallography to solve the autoinhibited structure of STX17 Habc in complex with the Qa-SNARE motif. Unfortunately, after numerous trials, we failed to obtain good crystals for structure determination, presumably due to the dynamic nature of this unstable complex, as indicated by our NMR analyses (Fig. 1 B and D). Hence, further studies are required to elucidate the detailed molecular mechanism underlying the interaction between the Habc domain and the Qa-SNARE motif of STX17.
Strikingly, a similar intramolecular interaction between the N-terminal Habc domain and the C-terminal Qa-SNARE motif was observed in Syntaxin1, a neuronal Qa-SNARE protein involved in synaptic membrane fusion (52). However, unlike that of Syntaxin1, the binding of the STX17 SNARE motif to its Habc domain can induce a partial unfolding of the extreme C-terminal α-helix of the STX17 Habc domain (Fig. 1D and SI Appendix, Fig. S4C). Importantly, previous studies of Syntaxin1 had well-demonstrated that the closed conformation of Syntaxin1 is essential for its interaction with Munc18-1, a major regulator of synaptic vesicle fusion, and represents a critical intermediate for neuronal exocytosis (52). Similarly, STX17 was reported to interact with the HOPS tethering complex through the Munc18-like subunit Vps33 for mediating autophagosome–lysosome fusion (15, 17, 53). However, whether HOPS binds to a closed or an open conformation of STX17 during the autophagosome–lysosome fusion process and what the potential in vivo function is of the conformational change of STX17 Habc induced by the Qa-SNARE motif remain to be elucidated.
Our study showed that SNAP29 alone is unable to open the closed conformation of STX17 to form a stable STX17–SNAP29 (Qa/Qbc) t-SNARE complex in vitro (SI Appendix, Figs. S6A and S7B). However, mammalian ATG8 orthologs, such as GABARAP, could easily release the autoinhibited conformation of STX17 by competitively binding to the N-terminal region of the STX17 SNARE motif and form a stable binary complex with STX17. Since previous relevant functional studies have well-demonstrated that the inactivation of all six mammalian ATG8 orthologs or the mammalian ATG8 conjugation machinery significantly attenuates the recruitment of STX17 to the autophagosome as well as the fusion between the autophagosome and lysosome (19, 20), our work may provide direct structural evidence for the essential function of mammalian ATG8s in mediating the autophagosome–lysosome fusion process. However, given that STX17 is exclusively recruited to the external membrane of the autophagosome instead of the phagophore that is also decorated with mammalian ATG8s (13, 20), the sole interactions between STX17 and mammalian ATG8s are likely insufficient to accomplish the recruitment of STX17 to the emerging autophagosome rather than the phagophore. Interestingly, a recent study showed that the autophagy-related small GTPase IRGM in its active form can directly associate with STX17 by binding to its two transmembrane domains (30), which was proven to be essential for the translocation of STX17 to autophagic membranes (13). Unfortunately, we were unable to obtain soluble IRGM proteins either from Escherichia coli or insect cells, thereby preventing detailed biochemical and structural characterizations. Additional work is required to elucidate the detailed mechanism governing the temporal and spatial regulation of the interaction of STX17 with mammalian ATG8s as well as the recruitment of STX17 to the autophagosome.
Furthermore, our systemic biochemical and structural characterizations revealed that the key binding-interface residues of STX17 involved in GABARAP binding and the STX17–SNAP29–VAMP8 SNARE complex assembly are heavily overlapped (Figs. 4C and 5E and SI Appendix, Figs. S1A and S19B), such as the W172 and L175 residues of STX17. Therefore, due to the potential steric exclusion, once the STX17–SNAP29–VAMP8 SNARE complex is formed, GABARAP and likely other ATG8 orthologs are unable to associate with this autophagic SNARE complex (SI Appendix, Fig. S18 B and C). Interestingly, based on careful structural analyses (Fig. 4C and SI Appendix, Fig. S22A), we rationally designed and obtained a STX17 D178R point mutation, which disrupted the interaction of STX17 with GABARAP without affecting the assembly and the stability of the STX17–SNAP29–VAMP8 SNARE complex (SI Appendix, Figs. S15B and S22B). Therefore, this mutation may potentially be useful for the future functional study of STX17 as well as the dissection of different roles mediated by the STX17–ATG8 ortholog interaction and this STX17-containing SNARE complex for autophagosome–lysosome fusion.
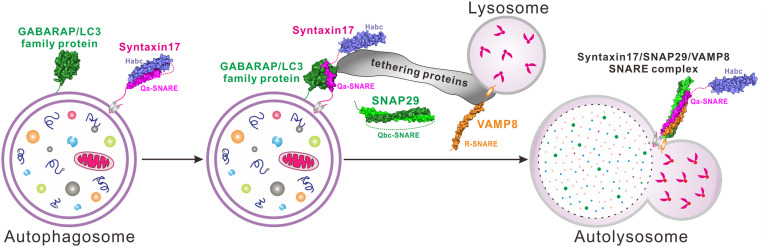
In summary, we proposed a model depicting three different states of STX17 in cooperation with mammalian ATG8s as well as the autophagy-related SNARE proteins SNAP29 and VAMP8 during the autophagosome–lysosome fusion process (Fig. 6). In this model, STX17 alone is in an autoinhibited closed state and, particularly, its Habc region somehow packs with the N-terminal part of its Qa-SNARE motif to stabilize the intrinsically disordered SNARE motif of STX17, thereby preventing its unnecessary degradation or interactions with other proteins on the autophagosome before assembling the STX17–SNAP29–VAMP8 SNARE complex (Fig. 6). However, in the presence of GABARAP/LC3 family proteins, the mammalian ATG8 ortholog can competitively bind to the N-terminal part of the STX17 Qa-SNARE motif that contains an extended LIR motif and abolish the autoinhibited conformation of STX17 (Fig. 6), thereby releasing the N-terminal Habc domain of STX17 and inducing its conformational rearrangement. Then, relevant tethering factors, such as the HOPS complex, ATG14, and EPG5, are recruited, which in turn work with STX17 and promote the further recruitment of cytosolic Qbc-SNARE SNAP29 and bring the VAMP8-residing lysosome into close proximity to the autophagosome (Fig. 6). Finally, mediated by their respective SNARE motifs, STX17, SNAP29, and VAMP8 assemble into the autophagic SNARE complex to clamp the membranes together and initiate the fusion between the autophagosome and lysosome, eventually leading to the formation of the autolysosome and the subsequent degradation of the enclosed materials (Fig. 6).
Fig. 6.
Proposed cartoon model illustrating the three different states as well as the potential working mode of STX17 in cooperation with mammalian ATG8s, autophagic SNARE proteins SNAP29 and VAMP8, and relevant tethering factors for facilitating the membrane fusion process between the autophagosome and lysosome in macroautophagy.
Materials and Methods
Protein Expression and Purification.
Different DNA fragments encoding human STX17, SNAP29, VAMP8, and ATG8s and other related DNA fragments were amplified by PCR from the full-length human complementary DNA (cDNA). All these fragments were cloned into in-house modified versions of the pET32a vector for recombinant protein expression. For the coimmunoprecipitation assay, full-length STX17 and GABARAP DNA fragments were cloned into pmCherry-C1 and pEGFP-C1 vectors, respectively. All of the point mutations of STX17, SNAP29, GABARAP, and LC3A used in this study were created using the standard PCR-based mutagenesis method, further checked by PCR screen using 2× Taq Master Mix (Vazyme Biotech) enzyme and confirmed by DNA sequencing.
Recombinant proteins were expressed in BL21 (DE3) E. coli cells induced by 100 μM isopropyl β-d-1-thiogalactopyranoside at 16 °C. The bacterial cell pellets were resuspended in binding buffer (50 mM Tris, pH 7.9, 500 mM NaCl, 5 mM imidazole), and then lysed by the FB-110XNANO homogenizer machine (Shanghai Litu Machinery Equipment Engineering). Then the lysate was centrifuged at 35,000 × g for 30 min to remove debris. His6-tagged proteins were purified using Ni2+-NTA agarose (GE Healthcare) affinity chromatography followed by size-exclusion chromatography (Superdex 75 or 200 column; GE Healthcare) in a buffer containing 20 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, and 1 mM dithiothreitol (DTT). Purities and molecular masses were verified by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) analysis.
To obtain the STX17 LIR–GABARAP complex proteins used for crystallization, GST-STX17(167–188) and His-GABARAP were first copurified by glutathione Sepharose 4B (GE Healthcare) affinity chromatography. Then, the N-terminal tags were cleaved by 3C protease, and the GST tag was further removed by glutathione Sepharose 4B affinity chromatography. Finally, the STX17(167–188)–GABARAP complex was further purified on a Superdex 75 size-exclusion column that was equilibrated with a column buffer containing 20 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, and 1 mM DTT. For the STX17–SNAP29–VAMP8 SNARE core complex proteins used for crystallization, Trx-STX17(142–228), MBP-SNAP29(40–126), GB1-SNAP29(191–258), and Trx-VAMP8(8–75) were first copurified by Ni2+-NTA agarose (GE Healthcare) affinity chromatography followed by size-exclusion chromatography (Superdex 200 column). Fractions containing the SNARE complex were collected and digested by 3C protease, loaded onto a MonoQ 10/10 ion-exchange column (GE Healthcare), and eluted with a linear NaCl gradient up to 500 mM to remove N-terminal tags. Finally, the autophagic STX17–SNAP29–VAMP8 SNARE complex was further purified using a Superdex 75 size-exclusion column equilibrated with a column buffer containing 20 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, 1 mM DTT, and 1 mM ethylenediaminetetraacetic acid (EDTA).
Uniformly 15N- or 15N/13C-labeled proteins were prepared by growing bacteria in M9 minimal medium using 15NH4Cl (Cambridge Isotope Laboratories; NLM-467) as the sole nitrogen source or 15NH4Cl and [13C6]glucose (Cambridge Isotope Laboratories; CLM-1396) as the sole nitrogen and carbon sources, respectively.
NMR Spectroscopy.
The stable isotope-labeled protein samples for NMR studies were concentrated to ∼0.1 mM for titration experiments and ∼0.6 mM for backbone resonance assignment experiments in 50 mM potassium phosphate buffer containing 50 mM NaCl (pH 6.5) and 1 mM DTT. NMR spectra were acquired at 25 °C on an Agilent 800-MHz spectrometer equipped with an actively z gradient-shielded triple-resonance cryogenic probe. Backbone resonance assignments of STX17(142–228) and the STX17 SNARE-bound Habc domain were achieved using a suite of heteronuclear correlation experiments including HNCO, HNCA, CA(CO)NH, HNCACB, and CBCA(CO)NH using a 15N/13C-labeled protein sample together with a three-dimensional 15N-separated NOESY (54).
Analytical Gel Filtration Chromatography.
Analytical gel filtration chromatography was carried out on an AKTA FPLC System (GE Healthcare). Protein samples were loaded onto a Superose 12 10/300 GL column (GE Healthcare) equilibrated with a buffer containing 20 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, and 1 mM DTT.
Isothermal Titration Calorimetry Assay.
ITC measurements were carried out on a MicroCal PEAQ-ITC calorimeter or an automated system (Malvern) at 25 °C. For each ITC experiment in this study, the protein samples in the cell and in the syringe were exchanged into the same buffer condition containing 20 mM Tris (pH 7.5), 100 mM NaCl, and 1 mM DTT using a HiPrep 26/10 desalting column. The titration processes were performed by injecting 40-μL aliquots of the syringe sample (∼500 μM) into the cell sample (∼50 μM) at time intervals of 2 min to ensure that the titration peak returned to baseline. In addition, relevant reference control experiments, in which the protein samples in the syringe are titrated into the control buffers, were also conducted. For each ITC dataset, the reference data would be subtracted from the raw data to obtain the net result for the final fitting analysis. The titration data were analyzed using the Malvern MicroCal PEAQ-ITC analysis program. The Kd error is the fitted error obtained from the data analysis software when using the one-site binding model to fit the ITC data.
Analytical Ultracentrifugation.
Sedimentation velocity experiments were performed on a Beckman XL-I analytical ultracentrifuge equipped with an eight-cell rotor at 42,000 rpm at 20 °C. The partial specific volume of different protein samples and the buffer density were calculated using the program SEDNTERP (http://www.rasmb.org/). The final sedimentation velocity data were analyzed and fitted to a continuous sedimentation coefficient distribution model using the program SEDFIT (55). The fitting results were further output to Origin 9.0 software and aligned with each other.
Protein Crystallization and Structural Elucidation.
Crystals of the STX17 LIR–GABARAP complex and autophagic STX17–SNAP29–VAMP8 SNARE complex were obtained using the sitting-drop vapor-diffusion method at 16 °C. Specifically, crystals of the STX17 LIR–GABARAP complex were formed about 1 wk after the freshly purified STX17 LIR–GABARAP complex (10 mg/mL in 20 mM Tris⋅HCl, pH 7.5, 100 mM NaCl, 1 mM DTT) was mixed with an equal volume of reservoir solution containing 0.12 M alcohols, 50% (vol/vol) precipitant Mix 4, and buffer system 3 at pH 8.5 from the Morpheus Screen Kit (Molecular Dimensions). Meanwhile, crystals of the STX17–SNAP29–VAMP8 SNARE complex (20 mg/mL in 20 mM Tris⋅HCl, pH 7.5, 100 mM NaCl, 1 mM DTT, 1 mM EDTA) were grown from buffer containing 2.5 M ammonium nitrate (pH 4.6) and 0.1 M sodium acetate trihydrate. Before the diffraction experiments, glycerol as appropriate was added as the cryoprotectant. X-ray datasets were collected at beamline BL17U1 or BL19U1 of the Shanghai Synchrotron Radiation Facility (SSRF) (56). The diffraction data were processed and scaled using HKL2000 (57).
The phase problem of the STX17 LIR–GABARAP complex was solved by the molecular replacement method using the modified structure of GABARAP (PDB ID code 5YIR) with PHASER (58). Meanwhile, the phase problems of the STX17–SNAP29–VAMP8 SNARE complex were solved by the molecular replacement method using the crystal structure of the endosomal SNARE core complex (PDB ID code 1GL2) as the search model. All initial structural models were rebuilt manually using Coot (59) and then refined using REFMAC (60) or PHENIX (61). The qualities of the final model were validated by MolProbity (62). The final refinement statistics of the solved structures in this study are listed in SI Appendix, Table S1. All of the structure figures were prepared using the program PyMOL (https://pymol.org/2/).
Coimmunoprecipitation Assay.
HEK293T cells transiently expressing proteins were harvested, washed with phosphate-buffered saline (PBS) buffer, and lysed for 1 h at 4 °C in lysis buffer containing 50 mM Tris⋅HCl (pH 7.8), 50 mM NaCl, 0.4% Nonidet P-40, 0.5 mM phenylmethanesulfonyl fluoride, and protease inhibitor mixture (AMRESCO). Lysates were centrifuged and then supernatants were incubated with the appropriate antibody pretreated with rProtein G agarose (Invitrogen) for 3 h with rotation at 4 °C. Precipitated proteins were washed with lysis buffer five times and then collected by brief centrifugation. Subsequently, the precipitated proteins were resolved by SDS/PAGE and detected by immunoblotting using a chemical luminescence-based detection method.
Circular Dichroism Spectroscopy.
CD measurements were performed using a Chirascan instrument (Applied Photophysics). A Hellma quartz cuvette with a path length of 0.5 mm was used. CD spectra were obtained by measuring the purified wild type or mutants of the autophagic STX17–SNAP29–VAMP8 SNARE core complex at a concentration of 20 μM in a buffer containing 40 mM sodium phosphate buffer (pH 6.5). For the thermal melting experiment, the wavelength was set to 222 nm and the temperature from 10 to 105 °C. Then the unfolding measurement was started by gradually increasing the temperature to 105 °C at a rate of 30 °C/h. Subsequently, the temperature for refolding was again lowered to 10 °C at a rate of 30 °C/h.
GST Pull-Down Assay.
Direct interactions between different Syntaxin17, SNAP29, and VAMP8 proteins were assayed in PBS (pH 7.4). The Syntaxin17 fragment (residues 142 to 225/228) was tagged with GST, while the SNAP29 fragment (residues 40 to 126), SNAP29 fragment (residues 191 to 258), and VAMP8 fragment (residues 8 to 66/71) were tagged with MBP-His6, GB1-His6, and Trx-His6, respectively. Fifty micrograms of GST-tagged proteins and His-tagged proteins was mixed at a molar ratio of 1:2 in 1 mL of the assay buffer. The GST-STX17–SNAP29–VAMP8 complexes were pelleted by adding 30 µL fresh glutathione Sepharose 4B (GE Healthcare) beads. The pellets were washed six times with 1 mL of the assay buffer, and subsequently eluted with 60 µL of 30 mM glutathione buffer. Ten microliters of each eluted sample was separated by 12% SDS/PAGE and analyzed using Coomassie blue staining or Western blot.
Supplementary Material
Acknowledgments
We thank SSRF BL17U1 and BL19U1 for X-ray beamtime, Dr. Jianchao Li for help with X-ray diffraction data collection, and Prof. Jiahuai Han for the full-length Syntaxin17, SNAP29, and VAMP8 cDNA. This work was supported by grants from the National Key R&D Program of China (2016YFA0501903), National Natural Science Foundation of China (31470749, 21621002, 91753113, 21822705), Science and Technology Commission of Shanghai Municipality (17JC1405200), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the start-up fund from the State Key Laboratory of Bioorganic and Natural Products Chemistry and Chinese Academy of Sciences.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. Y.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006997117/-/DCSupplemental.
Data Availability.
The coordinates and structure factors of the STX17 LIR–GABARAP complex and the STX17–SNAP29–VAMP8 SNARE complex reported in this paper have been deposited in the Protein Data Bank (PDB ID codes 7BV4 and 7BV6, respectively).
References
- 1.Klionsky D. J., Emr S. D., Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z., Klionsky D. J., Eaten alive: A history of macroautophagy. Nat. Cell Biol. 12, 814–822 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb C. A., Yoshimori T., Tooze S. A., The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Feng Y., He D., Yao Z., Klionsky D. J., The machinery of macroautophagy. Cell Res. 24, 24–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y., Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Mizushima N., Autophagy: Process and function. Genes Dev. 21, 2861–2873 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Levine B., Mizushima N., Virgin H. W., Autophagy in immunity and inflammation. Nature 469, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B., Kroemer G., Biological functions of autophagy genes: A disease perspective. Cell 176, 11–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B., Kroemer G., Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nixon R. A., The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Dikic I., Elazar Z., Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Hurley J. H., Young L. N., Mechanisms of autophagy initiation. Annu. Rev. Biochem. 86, 225–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itakura E., Kishi-Itakura C., Mizushima N., The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Matsui T. et al., Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J. Cell Biol. 217, 2633–2645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang P. et al., The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol. Biol. Cell 25, 1327–1337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diao J. et al., ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 520, 563–566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takáts S. et al., Interaction of the HOPS complex with syntaxin 17 mediates autophagosome clearance in Drosophila. Mol. Biol. Cell 25, 1338–1354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z. et al., The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol. Cell 63, 781–795 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T. N. et al., Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 215, 857–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuboyama K. et al., The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 354, 1036–1041 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Nakatogawa H., Ichimura Y., Ohsumi Y., Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 130, 165–178 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez M. G., Munafó D. B., Berón W., Colombo M. I., Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 117, 2687–2697 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Wijdeven R. H. et al., Cholesterol and ORP1L-mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat. Commun. 7, 11808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D. et al., A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Mol. Cell 45, 629–641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEwan D. G. et al., PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 57, 39–54 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Ebner P. et al., The IAP family member BRUCE regulates autophagosome-lysosome fusion. Nat. Commun. 9, 599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng X. et al., Pacer mediates the function of class III PI3K and HOPS complexes in autophagosome maturation by engaging Stx17. Mol. Cell 65, 1029–1043.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Jahn R., Scheller R. H., SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Kumar S. et al., Phosphorylation of syntaxin 17 by TBK1 controls autophagy initiation. Dev. Cell 49, 130–144.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S. et al., Mechanism of Stx17 recruitment to autophagosomes via IRGM and mammalian Atg8 proteins. J. Cell Biol. 217, 997–1013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen T., Lamark T., Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80–103 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Shpilka T., Weidberg H., Pietrokovski S., Elazar Z., Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 12, 226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng J., Klionsky D. J., The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. “Protein modifications: Beyond the usual suspects” review series. EMBO Rep. 9, 859–864 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichimura Y. et al., A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Behrends C., Sowa M. E., Gygi S. P., Harper J. W., Network organization of the human autophagy system. Nature 466, 68–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolz A., Ernst A., Dikic I., Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495–501 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Pankiv S. et al., FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 188, 253–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkin V., Rogov V. V., A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76, 268–285 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Weidberg H. et al., LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 29, 1792–1802 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birgisdottir A. B., Lamark T., Johansen T., The LIR motif—Crucial for selective autophagy. J. Cell Sci. 126, 3237–3247 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Wild P. et al., Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng X. et al., Structural basis of FYCO1 and MAP1LC3A interaction reveals a novel binding mode for Atg8-family proteins. Autophagy 12, 1330–1339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J. et al., Potent and specific Atg8-targeting autophagy inhibitory peptides from giant ankyrins. Nat. Chem. Biol. 14, 778–787 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Olsvik H. L. et al., FYCO1 contains a C-terminally extended, LC3A/B-preferring LC3-interacting region (LIR) motif required for efficient maturation of autophagosomes during basal autophagy. J. Biol. Chem. 290, 29361–29374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaites L. P., Paulo J. A., Huttlin E. L., Harper J. W., Systematic analysis of human cells lacking ATG8 proteins uncovers roles for GABARAPs and the CCZ1/MON1 regulator C18orf8/RMC1 in macroautophagic and selective autophagic flux. Mol. Cell. Biol. 38, e00392-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwilling D. et al., Early endosomal SNAREs form a structurally conserved SNARE complex and fuse liposomes with multiple topologies. EMBO J. 26, 9–18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonin W. et al., A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19, 6453–6464 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ernst J. A., Brunger A. T., High resolution structure, stability, and synaptotagmin binding of a truncated neuronal SNARE complex. J. Biol. Chem. 278, 8630–8636 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Fasshauer D., Antonin W., Subramaniam V., Jahn R., SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat. Struct. Biol. 9, 144–151 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Zhao M. et al., Mechanistic insights into the recycling machine of the SNARE complex. Nature 518, 61–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y. et al., Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science 337, 1340–1343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dulubova I. et al., A conformational switch in syntaxin during exocytosis: Role of munc18. EMBO J. 18, 4372–4382 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graham S. C. et al., Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc. Natl. Acad. Sci. U.S.A. 110, 13345–13350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bax A., Grzesiek S., Methodological advances in protein NMR. Acc. Chem. Res. 26, 131–138 (1993). [Google Scholar]
- 55.Schuck P., Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z. et al., Automatic crystal centring procedure at the SSRF macromolecular crystallography beamline. J. Synchrotron Radiat. 23, 1323–1332 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Otwinowski Z., Minor W., Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Storoni L. C., McCoy A. J., Read R. J., Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Murshudov G. N., Vagin A. A., Dodson E. J., Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Adams P. D. et al., PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Davis I. W. et al., MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The coordinates and structure factors of the STX17 LIR–GABARAP complex and the STX17–SNAP29–VAMP8 SNARE complex reported in this paper have been deposited in the Protein Data Bank (PDB ID codes 7BV4 and 7BV6, respectively).