Abstract
Although fibrosis is a common pathological feature of most end-stage organ diseases, its pathogenesis remains unclear. There is growing evidence that mitochondrial dysfunction contributes to the development and progression of fibrosis. The heart, liver, kidney and lung are highly oxygen-consuming organs that are sensitive to mitochondrial dysfunction. Moreover, the fibrotic process of skin and islet is closely related to mitochondrial dysfunction as well. This review summarized emerging mechanisms related to mitochondrial dysfunction in different fibrotic organs and tissues above. First, it highlighted the important elucidation of mitochondria morphological changes, mitochondrial membrane potential and structural damage, mitochondrial DNA (mtDNA) damage and reactive oxidative species (ROS) production, etc. Second, it introduced the abnormality of mitophagy and mitochondrial transfer also contributed to the fibrotic process. Therefore, with gaining the increasing knowledge of mitochondrial structure, function, and origin, we could kindle a new era for the diagnostic and therapeutic strategies of many fibrotic diseases based on mitochondrial dysfunction.
Subject terms: Mitochondria, Metabolic disorders
Facts
Fibrosis is the major pathophysiologic basis and ultimate pathway for most parenchymatous organ injury.
Mitochondria play a central role in energy metabolism and even decide the cellular fate.
Mitochondrial dysfunction could induce fibrotic diseases.
Targeting mitochondria may help alleviate fibrosis.
Open questions
What are the mechanisms of mitochondrial dysfunction in fibrosis?
How are mitochondria involved in the development of fibrosis?
How to prevent or slow down fibrosis through targeting mitochondria?
Introduction
The fibrotic disease is a major health problem worldwide. As the common pathological pathway to organ injury and failure, fibrosis usually represents an unsatisfactory prognosis1. It is the major cause of death in the world and causes substantial medical and economic burdens1,2.
As the power house of cells, mitochondrion maintains the basic functions of every single cell in our body, including energy metabolism, cell differentiation modulation, signaling transduction and apoptosis3,4. Reactive oxygen species (ROS) are byproducts of normal metabolism. The functional mitochondrion has the ability to control the balance of ROS biogenesis and scavenging. However, severe redox stress events will lead to the disruption of homeostasis. Excessive ROS will destroy the normal structure and function of mitochondria and release from mitochondria via mitochondrial permeability transition pore (mPTP) opening mechanism5. When mitochondrial dysfunction occurs, the normal cellular biological processes are disrupted, and the oxygen homeostasis is destroyed in tissue.
In the process of fibrosis, the injured tissue shows the characteristics of oxidative stress, hypoxia, and inflammation6,7. In this damaging microenvironment, mitochondrial dysfunction usually occurs, which is closely related to the development of fibrotic diseases8. This review highlighted the emerging mechanisms related to mitochondrial dysfunction in different fibrotic organs and tissues, including mitochondria morphological changes, mitochondrial membrane potential damage, mitochondrial DNA (mtDNA) damage, ROS production, mitophagy abnormality, mitochondrial transfer, etc. Furthermore, we summarized the therapeutic strategies targeting mitochondria, aiming to provide new clinical therapy for the combined effect of mitochondria.
Mechanism of fibrotic process
Fibrosis is the terminal development of chronic inflammation in many organs1. When tissue damage is severe or repeated beyond the regeneration ability of surrounding parenchymal cells, normal tissue repair becomes excessive, interstitial fibrous connective tissue will repair a large number of proliferation, resulting in pathological changes of fibrosis6. It is generally believed that activated fibroblasts act as the key cells that ultimately lead to fibrosis. Activated fibroblasts (i.e. myofibroblasts) express α-smooth muscle actin (α-SMA), increase the expression of fibro-collagen (type I, III, V, and VI) and other extracellular matrix (ECM) macromolecules, and inhibit ECM degrading enzymes9,10. Moreover, transforming growth factor-β (TGF-β), a common anti-inflammatory cytokine mainly produced by macrophages, plays a critical role in the fibrosis development11, which is an effective inducer of myofibroblasts, and stimulates the expression of key genes in fibrosis through several downstream pathways, especially Smad signaling11–13.
Oxidative stress and hypoxia are pretty relevant to fibrosis6,7. In fibroblasts, hypoxia could increase ROS production in mitochondria14, in which ROS affects the synthesis, secretion, and degradation of ECM. And there is a strong correlation between TGF-β signal transduction and ROS15,16.
Mitochondrial dysfunction
Normal functions of mitochondrion
Mitochondrion is the power house of cells. As a semi-autonomous organelle, mitochondrion maintains the basic cellular function, including adenosine-triphosphate (ATP) production, ROS biogenesis and scavenging, cell differentiation modulation, signaling transduction, and apoptosis3,4. Mitochondrial inner membrane contains enzymes involving electron transport chain (ETC) and ATP production, and electrochemical gradients across the inner membrane drive the process of oxidative phosphorylation (OXPHOS)17–23. The energy of most cells in the body is produced by mitochondria through tricarboxylic acid (TCA) cycle and ETC. ETC consists of five subunit enzyme complexes located in mitochondrial inner membrane, including complexes I, II, III, IV, and V24.
Mitochondrial dysfunction
Mitochondrial dysfunction refers to the damage of mitochondrial structure, respiratory chain defects, biogenic dysfunction, gene damage, reduction of mitochondria number and changes in oxidative protein activity in cells and tissues. ROS is a byproduct of oxygen metabolism, and mitochondria have been found to serve as the main source of ROS in mammals. The imbalance between ROS production and removal results in cumulative ROS contacting with mitochondria and cellular components, leading to oxidative damage to mitochondrial proteins, DNA, and lipids25,26. The mPTP located in the mitochondrial inner membrane could be open under the conditions of increasing ROS. Mitochondrial permeability transition can induce mitochondrial depolarization and swelling, decrease of ETC activity and release of apoptotic factors27–29. In addition, mtDNA lacking histone protection is highly sensitive to ROS and prone to be damaged and mutated under oxidative stress, resulting in respiratory chain defects and decrease of mitochondrial biogenesis30,31.
Mechanisms of mitochondrial self-repair
Meanwhile, mitochondria have multiple mechanisms of self-repair and renewal. Enzymatic defense systems play an antioxidant role such as superoxide dismutase (SOD), catalase (CAT). Mitochondrial biogenesis maintains the number and size of mitochondria. Several transcription factors regulate mitochondrial biogenesis32,33. Peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α) interacts with many transcription factors/proteins to promote mitochondrial biogenesis and OXPHOS via acting as a transcription co-activator for nuclear receptors34,35.
Furthermore, mitochondrial dynamics is a process in which mitochondria form a network through dynamic balance of fission and fusion. Mitophagy can remove dysfunctional mitochondria by fusion with lysosomes36,37, thereby controlling the number of mitochondria and maintaining energy metabolism stability38,39. ROS can induce mitophagy by activating phosphatase and tensin homology deleted on chromosome 10 (PTEN) induced putative kinase 1 (PINK1)/Parkin pathway40. As a ubiquitin kinase, cellular prion protein (PrPc) binds PINK1, enters the mitochondrial inner membrane and is degraded under normal physiological conditions. Under oxidative stress, PINK1 recognizes and aggregates on the surface of damaged mitochondrial extracorporeal membrane, activates phosphorylation, and recruits Parkin translocation. Ubiquitous mitochondria are encapsulated to form mitophagosome, which are fused with lysosomes and reduced by hydrolases41,42.
Mitochondrial dysfunction in solid organ fibrosis
Mitochondrial dysfunction in cardiac fibrosis
Cardiac fibrosis with ECM deposition can lead to impaired cardiac function and potential heart injury43. ROS could directly regulate the production of interstitial ECM by modulating both expression and metabolism of matrix protein44. Importantly, the majority of ROS in cardiac fibrosis comes from mitochondria45. Therefore, treatment strategies targeting mitochondria are critical. For example, Dai et al. confirmed that the overexpression of antioxidant enzyme CAT targeted to mitochondria, but not wild-type peroxisomal CAT, alleviated mitochondrial oxidative damage, cardiac fibrosis and hypertrophy46. Mitoquinone (MitoQ), a mitochondrial-targeted antioxidant, could inhibit fibrosis in pressure overloaded hearts via targeting mitochondrial ROS-mediated signaling TGF-β1, NADPH oxidase 4 (NOX4), and Nrf2 pathway47. Bendavia and alogliptin could improve mitochondrial dysfunction, relieved cardiac fibrosis by improving mitochondrial biogenesis48,49.
Furthermore, mitochondria in cardiac fibrosis often show diverse dysfunctional forms. The mtDNA lacking histone protection is highly sensitive to ROS. The deletion of mtDNA induced by angiotensin II was reported in cardiac fibrosis of hypertensive cardiomyopathy. Primary damage to mtDNA induced by zidovudine or homozygous mutation of mitochondrial polymerase γ, could also improve cardiac fibrosis46. Valli et al. showed that age-dependent cardiac fibrosis was closely associated with mitochondrial dysfunction due to PGC-1β deficiency, a transcriptional regulator of mitochondrial genes50.
Nucleotide-binding domain and leucine-rich repeat containing PYD-3 (NLRP3) is a pattern recognition receptor, and it usually responds to inflammation in the form of a multiprotein platform (NLRP3 inflammasome)51. Recent studies displayed that NLR family, pyrin domain containing 3 (NLRP3) regulated mitochondrial ROS production in human cardiac fibroblasts. NLRP3 localized to mitochondria regulated myofibroblast differentiation and Smad signal transduction by inducing ROS. Notably, this mechanism is independent of inflammasome. This study indicates the new role of mitochondrial NLRP3 protein involved in fibrosis in non-professional immune cells52.
Mitochondrial membrane potential and membrane structural damage are also important characteristics in cardiac fibrosis. It is reported that alogliptin alleviated interstitial fibrosis in diabetic rabbits by reducing the production of mitochondrial ROS, preventing the mitochondrial membrane depolarization, and improving the swelling of mitochondria49. Similarly, melatonin and ephedrine-4 could alleviate oxidative stress and cardiac fibrosis through maintaining the integrity of mitochondrial membrane and preventing the release of cytochrome C53.
Additionally, the dysfunction of oxidative respiratory chain and metabolic-related enzymes is also emerging as the pivotal mechanism of cardiac fibrosis. Kennedy et al. identified that the deficiency of nuclear-encoded mitochondrial inorganic pyrophosphatase (PPA2) due to biallelic missense mutations was associated with cardiac fibrosis by using whole-exome sequencing, of which mechanism is related to suppress the activity of respiratory chain complex I and IV, and decreased the expression of citrate synthase in fibroblasts54. According to the study by Fraccarollo et al. the activation of nitric oxide (NO)/heme-independent soluble guanylate cyclase (sGC) provided protection through increased expression of uncoupling protein 3 (UCP3) and manganese SOD (MnSOD) genes against mitochondrial SOD production and progressive fibrotic remodeling. Ultimately, this process inhibited human cardiac fibroblast differentiation and ECM accumulation55. And one of the mitochondrial sirtuins, Sirt4, accelerated Ang II-induced pathological cardiac hypertrophy via suppressing MnSOD activity in cardiomyocytes from transgenic mice56. Among them, UCP3 had an effect on ETC, which could decrease protonmotive force and attenuate ROS production through mild uncoupling57. And the deletion of MnSOD would have adverse effects on mitochondrial ETC, TCA cycle, mtDNA stability, and iron metabolism58.
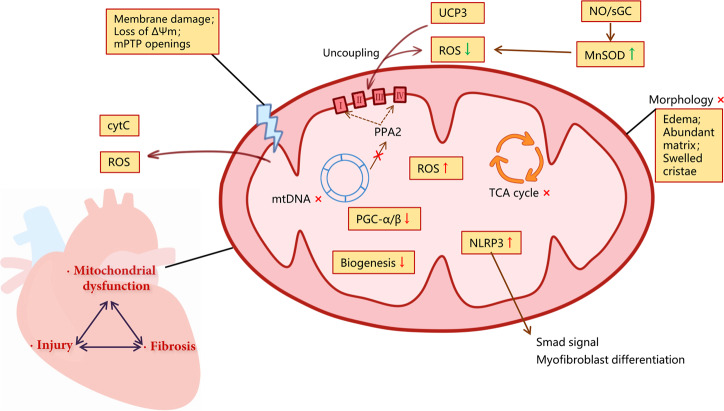
We summarized the details of every mechanism above in Fig. 1.
Fig. 1. Mitochondrial dysfunction in cardiac fibrosis.
Cardiac fibrosis, heart injury, and mitochondrial dysfunction are mutually causal, and the mechanisms overlap. Mitochondrial dysfunction is accompanied by morphological changes, mitochondrial membrane potential, and structural damage, and mtROS production. Excessive mtROS will destroy the normal structure and function of mitochondria, which further leads to the disorder of mitochondrial metabolic function. The release of risk factors like ROS and CytC from mitochondria further aggravates injury and inflammation. Meanwhile, transcriptional regulator deficiency and inhibited mitochondrial biogenesis pathways limit the self-repair function. NLRP3 which was localized to mitochondria regulates myofibroblast differentiation and Smad signal transduction by inducing ROS. As one of the protective pathways, UCP3 and NO/sGC can reduce ROS by mild decoupling and upregulating MnSOD.
Mitochondrial dysfunction in pulmonary fibrosis
Pulmonary fibrosis, a pathological change in the development of various and age-related end-stage lung diseases, generally is featured by not only activation and proliferation of fibroblast accompanied by tissue damage and inflammation, but also increased deposition of mesenchymal collagen59. The vast majority of patients with pulmonary fibrosis have an unknown cause (idiopathic), defined as idiopathic interstitial pneumonia (IIP) or idiopathic pulmonary fibrosis (IPF)60. Recently, there is growing evidence that mitochondrial dysfunction may contribute to the pathogenesis of IPF. We have sketched Fig. 2 to summarize the key information of every mechanism below.
Fig. 2. Mitochondrial dysfunction in pulmonary fibrosis.
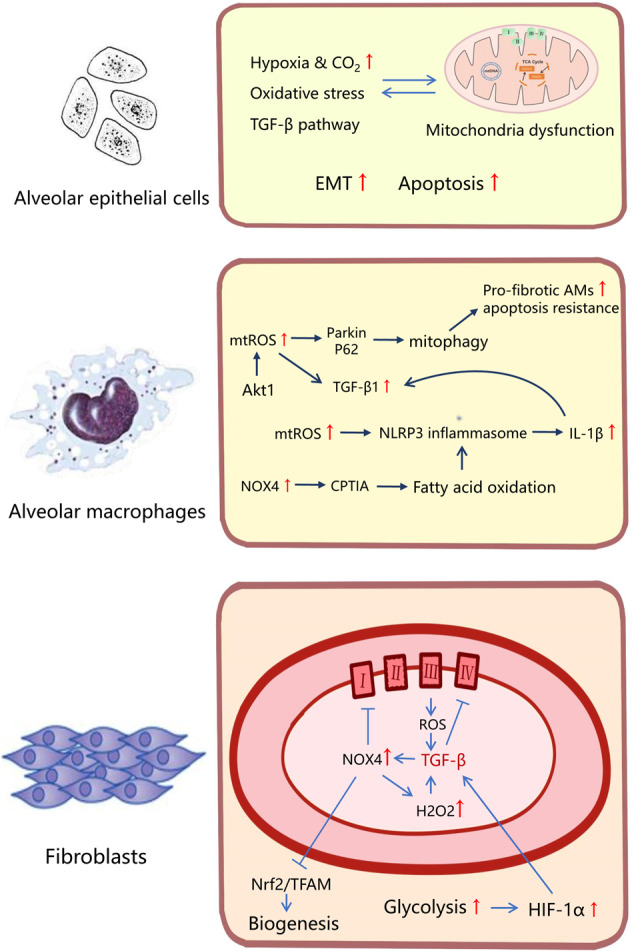
The mitochondrial dysfunction of different cells shows different characteristics in pulmonary fibrosis. The mitochondrial abnormalities and mitochondria-mediated apoptosis in AECs could conduce to pulmonary fibrosis in a critical way. HIF, high level of mtROS and endogenous TGF-β1 signaling interact with apoptosis and EMT. In AMs, Akt1-mediated mtROS could cause mitophagy, which contributed to the apoptotic resistance of pro-fibrotic AMs. As a risk factor in fibrosis, TGF-β1 was activated in response to ROS and NLRP3 inflammasome, which could also induce mitochondrial dysfunction in AMs. The deficiency of NOX4 reduced the mitochondrial fatty acid oxidation, which could inhibit NLRP3 inflammasome activation. ROS produced by complex III were required for TGF-β to induce gene expression in human lung fibroblasts. In turn, TGF-β could also increase the ROS level through the mechanism like inhibition of complex IV. Moreover, generation of H2O2 dependent on NOX4 was demanded for myofibroblast differentiation induced by TGF-β. Furthermore, the metabolic reprogramming in myofibroblast shows a augmented glycolysis, which contributed to pulmonary fibrosis via promoting the stabilization of HIF-1α.
The mitochondrial abnormalities and mitochondria-mediated apoptosis in alveolar epithelial cells (AECs) could conduce to pulmonary fibrosis in a critical way. Mitochondrial ROS, always with an increased level, mediated by a variety of mechanisms. Hypoxia and high CO2 level can decrease oxygen consumption and ATP production in AECs and impair cell proliferation through mitochondrial ROS61,62. Furthermore, EMT of AECs was induced by hypoxia through hypoxia inducible factor (HIF), high level of mitochondrial ROS, and endogenous TGF-β1 signaling61. It was reported that oxidative stress induced preferential mtDNA damage in a variety of AECs. Sirtuin (silent mating type information regulation 2 homolog) 3 (SIRT3) deficiency could improve lung fibrosis by augmenting apoptosis and mtDNA damage in AECs63. As a key enzyme for base excision repair with the function of alleviating pulmonary fibrosis, 8-oxoguanine DNA glycosylase (Ogg1) associated with aconitase-2 (Aco-2) could prevent mtDNA damage, p53 mitochondrial translocation, and intrinsic apoptosis in AECs64. Furthermore, thyroid hormone could increase biogenesis via activating PGC-1α and promote mitophagy via PINK1 in mice, which helped suppress mitochondria-mediated apoptosis and reversing bleomycin-induced mitochondrial abnormalities in AECs65.
Compared with the normal lung fibroblasts, there had been shown that not only a decrease of mitochondrial mass and morphologic alteration, but also the low level of oxygen consumption rate and ATP production in the ones of pulmonary fibrosis66. Excessive TGF-β pathway could result in robust profibrotic gene expression in fibroblasts, leading to fibrosis. It was confirmed that ROS produced by complex III were required for TGF-β to induce gene expression in primary normal human lung fibroblasts67. TGF-β could also directly or indirectly increase the ROS level through various mechanisms, such as inhibition of complex IV and activation of NADPH oxidase61. Moreover, generation of hydrogen peroxide (H2O2) dependent on NOX4 was demanded for myofibroblast differentiation, which was induced by TGF-β168. Also, lysocardiolipin acyltransferase (LYCAT) could protect against pulmonary fibrosis through negatively modulating TGF-β-induced lung fibroblast differentiation via the decline of NOX-dependent H2O2 generation and mitochondrial superoxide69. In addition, augmented glycolysis contributed to pulmonary fibrosis via promoting the stabilization of HIF-1α in myofibroblast, which could increase the expression of TGF-β1 and regulate the glycolytic enzymes70. In lung fibroblasts, NOX4 inhibited mitochondrial bioenergetics and biogenesis through decreasing induction and activation of endogenous nuclear factor (erythroid-derived-2)-like-2 factor (Nrf2) and mitochondrial transcription factor A (TFAM) or directly inhibiting complex I of ETC, whereas inactivation of TORC1/PGC-1 axis could repress mitochondrial biogenesis and bioenergetics via downgrading the expression of Nrf1 and TFAM71,72. Metformin could attenuate lung fibrosis development through NOX4 inhibition. Activation of AMPK mediated by metformin inhibits NOX4 expression induced by TGF-β73. It was reported that AMPK activity was lower in fibrotic regions. AMPK-deficient fibroblasts reduced basal oxygen consumption, diminished mitochondrial reserve capacity and maximal respiration74. In a bleomycin model, metformin could reverse the established lung fibrosis. Metformin could active AMPK reprogramming metabolism of IPF fibroblasts via diminishing mTOR activation and promoting autophagy, as well as downregulating homeostasis levels of ECM proteins. AMPK activation also upregulated mitochondrial biogenesis. Furthermore, it restored myofibroblast sensitivity to intrinsic apoptosis, particularly induced by antimycin A, a mitochondrial inhibitor of ETC complex II74.
Importantly, there was evidence of vital role of alveolar macrophages (AMs) in the process of pulmonary fibrosis. Dioscin could alleviate crystalline silica-induced excessive mitochondrial ROS release, AMs apoptosis, and mitochondrial dysfunction, such as MMP depolarization and low ATP production. Dioscin promoted AMs autophagy, decreasing production of inflammatory factors in vivo and in vitro, thereby reducing collagen deposition and inflammatory infiltration75. In AMs, Akt1-mediated mitochondrial ROS could cause mitophagy, which contributed to the apoptotic resistance of pro-fibrotic AMs, expression of TGF-β1 and activation of myofibroblasts76. TGF-β1 could induce mitochondrial dysfunction in AMs, such as suppression of the OXPHOS, low level of mitochondrial ATP production, and MMP depolarization77. Notably, the ROS production in AMs induced NLRP3 inflammasome activation, which was related to mitochondrial dysfunction78. And in the mouse model of NLRP3-mediated Streptococcus pneumoniae infection, the deficiency of NOX4 reduced the mitochondrial fatty acid oxidation, which could inhibit LRP3 inflammasome activation and improved survival5. The mechanisms of mitochondria involved in NLRP3 inflammasome activation further suggested that mitochondria may play a critical role in chronic inflammation.
Mitochondrial dysfunction in renal fibrosis
Renal fibrosis is the formation of scars in the parenchyma, which is the commonly accepted to serve as ultimate pathway for almost all chronic and progressive nephropathy1. Like other organs, decreased expression of Gα-binding protein (GABP), PGC-1α, and PPAR-α indicated a decrease of mitochondrial biogenesis in fibrosis79. Additionally, the integrity of mitochondrial morphology and structure was often destroyed in renal fibrosis80. It was reported that melatonin prevented mitochondrial edema, cristae dilatation and maintained the integrity of mitochondrial membrane, thereby alleviating renal fibrosis8. TNF receptor-associated protein 1 (TRAP1) could inhibit the fibrosis-related proteins expression in renal tubular epithelial cells (TECs) and tubulointerstitial fibrosis by alleviating mitochondrial vacuolation, swelling, matrix density reduction and mitochondrial cristae rupture, and increasing the number of mtDNA copies81.
Moreover, the changes in mitochondria permeability could lead to the release of cytochrome C and other substances, which mediated apoptosis82. The oxidative damage of cardiolipin-sensitized mitochondria to calcium, induced mitochondrial permeability transition and destroyed the permeability barrier of IMM, which caused the collapse of mitochondrial potential, the decoupling of OXPHOS and apoptosis, and the release of cytochrome C to cytosol83,84. A cell-permeable peptide Bendavia (SS-31) targeted the inner mitochondrial membrane and binded to cardiolipin, which could protect mitochondria in medullary TECs83. Bendavia improved oxidative stress and tubulointerstitial fibrosis and restored renal vascular endothelial function in vivo and in vitro84. The anti-fibrotic mechanism was to reduce oxidative damage of mitochondrial cardiolipin.
According to the study of Zhang et al.85 after renal ischemia reperfusion injury (IRI), ROS production and mtDNA damage increased, which could lead to EMT and further renal fibrosis. Postconditioning (POC) therapy can reduce renal fibrosis by protecting mitochondria from oxidative stress-induced mtDNA damage. Furthermore, in renal fibrosis and renal injury induced by AAI, decrease of mtDNA-encoded cytochrome C oxidase subunit 1 (COX-1) and nuclear DNA (nDNA)-encoded nicotinamide adenine dinucleotide dehydrogenase (ubiquinone)-1b subcomplex 8 (NDUFb8) suggested that mtDNA and nDNA were both victims. However, with the progression of aristolochic acid nephropathy (AAN) in renal cortex, NDUFb8 level restored, while COX-1 level maintained low80. Similarly, the activity of respiratory complex 1, which is partly encoded by mtDNA, was more significantly impaired than that of respiratory complex II, completely encoded by nDNA86. These results suggested that mtDNA damage would be more severe than nDNA damage on fibrosis.
In terms of oxidative respiratory chain and metabolic enzymes, cardiolipin peroxidation disrupts respiratory chain complex and inhibits mitochondrial respiratory compounds83,84. As mentioned before, COX-I and NDUFb8 defects also played critical roles in respiratory chain damage80. Moreover, Ang-II induced renal injury by alleviating mitochondrial dysfunction, which was related to the decrease of ATP synthase activity, 1,25-dihydroxyvitamin D3 alleviated this situation87.
NLRP3 could be considered as another important risk factor. It is reported that activated NLRP3 inflammasome is involved in kidney injury process via mitochondrial dysfunction in TECs and macrophages. NLRP3 caused mitochondrial dysfunction, increased ROS and finally led to fibrosis88,89. However, studies also showed that NLRP3 promoted the TGF-β/Smad signaling pathway in TECs independent of the inflammasome90. In renal tubular cells, NLRP3 transferred from the cytosol to the mitochondria and targeted to mitochondrial antiviral signal protein (MAVS) during hypoxia, which played a critical role in mitochondrial ROS accumulation and disfunction91. Therefore, the deletion of NLRP3 is emerging as a potential therapeutic target, which can attenuate fibrosis via protecting the damaged mitochondrial function.
During the treatment of chronic kidney disease (CKD) based on mesenchymal stem cells (MSCs), MSCs in patients with CKD experienced accelerated aging and suppressed efficacy. It is reported that melatonin enhances the role of MSCs in CKD treatment and alleviates fibrosis, which improves mitochondrial function through high expression of PrPc. High expression of PrPc can increase the activity of complexes I and IV, thereby enhancing OXPHOS of mitochondria. Moreover, PINK1 could promote mitochondrial dynamics and metabolism92.
Mitochondrial dysfunction in hepatic fibrosis
Hepatic fibrosis is the major pathophysiologic basis and final common pathway of various chronic hepatic diseases, such as alcoholic liver disease, viral infection and non-alcoholic steatohepatitis (NASH)93. In the process of liver injury94, HSCs transform from static physiological state to fibrotic phenotype95,96. This transformation is induced by inflammatory mediators, ROS and apoptotic bodies arising from dying hepatocytes and activated HSCs. Growing evidence supported that the hepatic fibrosis via HSCs activation was associated with mitochondrial dysfunction.
According to the chemiosmotic theory, mitochondrial electron transfer is accompanied by proton flux and coupled by redox proton pump mediated by mitochondrial complexes (CI, CIII, and CIV). Mitochondrial uncouplers can make the energy generated by electron transfer in the respiratory chain not be used for the phosphorylation of ADP, but can only be emitted in the form of heat. A recent study suggested that mitochondrial uncouplers could inhibit HSCs activation via reducing ATP and ROS level97.
Augmenter of liver regeneration (ALR) is a hepatocyte survival factor induced by mitochondrial dysfunction/damage and cell death upon inhibition of its synthesis. Ai et al. found that inhibition of ALR expression aggravated hepatic fibrosis, probably through enhancing mitochondrial fusion and HSCs migration. In HSCs, ALR could induce the mitochondrial Ca2+ influx increase, which attributed to the HSCs migration. ALR transfection retarded HSCs migration and suppressed F-actin assembly, while promoting mitochondrial fission and diminishing ATP synthesis98. ALR gene therapy, which has been shown to improve the hepatic fibrosis effectively, could inhibit the ATP loss, reduce intrahepatic ROS level, enhance the activity of ATPase, and decrease expression of TGF-β1, PDGF, and α-SMA99.
NLRP3 inflammasome induces caspase 1-dependent release of proinflammatory cytokines IL-1 β and IL-18, which induce cell death under inflammatory and stress conditions. As one of many important NLRP3 inflammasome activators, ROS have been reported to promoting the chronic liver disease, including hepatic fibrosis. Especially in HSCs, the up regulation of NOX4 expression, which is a producer of ROS, has been found to be related to the activation of NLRP3 inflammatory and the increase of collagen production. Didymin can notably ameliorate chronic hepatic injury and collagen deposition, with inhibition of HSCs proliferation and induction of apoptosis, and it also significantly causes mitochondrial membrane depolarization, usually accompanied by cytochrome C release in HSCs. Didymin can improve the hepatic fibrosis mainly by inhibition of ERK/MAPK and PI3K/Akt pathways through increasing Raf kinase inhibitor protein (RKIP) expression in HSCs100. p66Shc, a redox enzyme that regulates mitochondrial ROS generation, contributes to hepatic fibrosis, whereas its inhibition can ameliorate the liver fibrosis through restraining the activation of HSCs via down-regulating mitochondrial ROS production and NLRP3 expression101.
For mitochondrial homeostasis and normal ATP level, it is critical to keep the normal ETC and normal activity of enzyme related to respiratory chain. Poly (ADP-ribose) polymerase (PARP) is a key mediator of liver fibrosis, and its inhibition or genetic deletion can protect against hepatic fibrosis via ameliorating the abnormal ETC and improving the activation of complex I and IV102. Enzyme activity related to respiratory also plays a very important role in the entire unit. Nabanita et al. found that melatonin could ameliorate hepatic fibrosis via restoring the enzymatic activities associated with respiratory chain, decreasing mitochondrial ROS production and inhibition of HSCs activation103. As we already know, the activation of HSCs around hepatic sinusoids is the main source of liver fibrosis in any etiology. So promoting HSCs apoptosis is a strategy worth considering. Chen et al. found that dihydroartemisinin prevented liver fibrosis through promoting HSCs apoptosis via down-regulating the PI3K/Akt pathway. Dihydroartemisinin could induce HSCs apoptosis via promoting loss of mitochondrial transmembrane potential (MTP) in HSCs, transfer of cytochrome C from mitochondria to cytoplasm, and the decreased ratio of anti-apoptotic BCL-2 to pro-apoptotic Bax.
Mitochondrial autophagy is a specific selection process, which is precisely regulated by various factors such as PINK1, Parkin, and so on. It is an important regulatory mechanism for cells to clear damaged mitochondria and maintain their homeostasis. Qiu et al. found that PM2.5 induced liver fibrosis through triggering mitophagy mediated by ROS. PM2.5 could induce mitophagy through up-regulating PINK1/Parkin signal pathway via increased ROS, and thus activate HSCs104. Additionally, melatonin could protect against liver fibrosis via upregulating mitophagy and mitochondrial biogenesis in mice105. In addition, NLRP3 inflammasome activated by NOX4-independent ROS could induce pro-inflammatory factors, including IL-1β, which increased chronic liver inflammation and promoted activation of HSCs. Cai et al. found that angiotensin-(1–7) improved hepatic fibrosis via modulating the NLRP3 inflammasome through redox balance regulation including upregulation of GSH, Nrf2, antioxidant response element (ARE), and down-regulation of hydrogen peroxide, NOX4106. In hepatic cells, the overexpression of BCL-2, which is the anti-apoptotic protein with the function of inhibiting hepatic cells apoptosis, can delay fibrosis progression via maintaining the normal ROS level107.
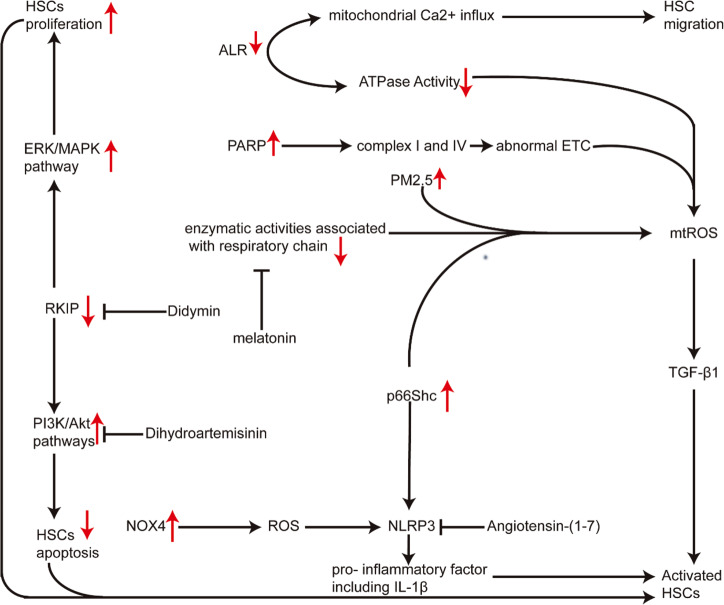
Figure 3 shows the keynotes of all the mechanisms above.
Fig. 3. Mitochondrial dysfunction in hepatic fibrosis.
The proliferation and activation of HSCs is the central process during the development of HF. Inhibition of ALR expression aggravates liver fibrosis, probably via promoting HSC migration and mitochondrial fusion. The increased mitochondrial Ca2+ influx induced by ALR in HSCs attributes the HSC migration. The activation of PARP can aggravate hepatic fibrosis via deteriorating the abnormal ETC including the inhibition of complexes I and IV. p66Shc can contribute to hepatic fibrosis through the activation of HSCs via upregulating mtROS production and NLRP3 expression. Didymin can improve the hepatic fibrosis main by inhibition of ERK/MAPK and PI3K/Akt pathways via up-regulation of RKIP expression in HSCs. The NLRP3 inflammasome activated by NOX4-independent ROS can mediate activation of HSCs via inducing pro-inflammatory factor including IL-1β.
Mitochondrial dysfunction in tissues
Mitochondrial dysfunction in skin fibrosis
Radiation‐induced dermatitis can cause skin fibrosis, and radiation also damages mitochondria108. Radiation-induced subcutaneous fibrosis can also be associated with genetic variation of thioredoxin reductase 2 (Txnrd2), a mitochondrial enzyme involved in removal of ROS109. This reveals that the lost control of ROS clearance and production in mitochondria will lead to serious consequences. JP4-039 is a ROS scavenger with the significant affinity for mitochondrial inner membrane. Topical JP4-039 could prevent skin damage and fibrosis from radiation110.
Treatment that selectively induces apoptosis of myofibroblasts could reverse established fibrosis, like scleroderma111,112. In fact, the apoptosis induction targeting mitochondria has been gradually applied in the fibrosis treatment. It is reported that increasing of the mitochondrial priming could promote myofibroblast activation, which primed by proapoptotic BH3-only protein BIM. But meanwhile, the antiapoptotic protein BCL-XL sequestered BIM to ensure myofibroblast survival. The “BH3 mimetic” drug (ABT-263) can induce myofibroblasts apoptosis through inhibiting BCL-XL113.
Mitochondrial dysfunction in islet fibrosis
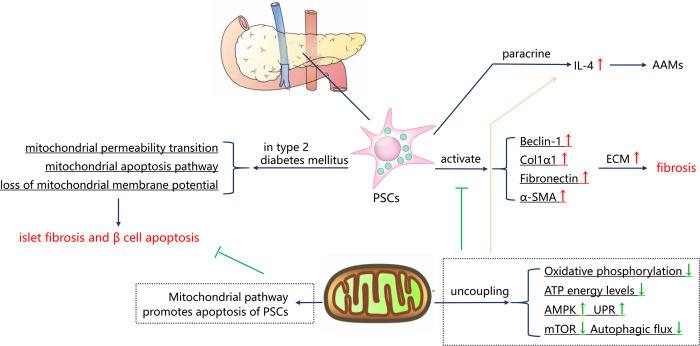
Activated pancreatic stellate cells (PSCs) regulates the remodeling of peripheral ECM and plays a paracrine role in adjacent cells. Activated PSCs mainly relies on OXPHOS of mitochondria rather than glycolysis to maintain ATP energy levels and sustained energy-dependent processes114,115. Rottlerin acts as an OXPHOS uncoupling agent of mitochondria, which can rapidly depolarize mitochondria, reduce mitochondrial mass, change dynamics, decrease ATP level, activate AMP-activated protein kinase (AMPK), unfolded protein response (UPR) signaling transduction, inhibit mTOR pathway, and block autophagic flux. Therefore, rottlerin reduces the expression of α-SMA and other ECM proteins in PSCs115. In addition, we have known that alternatively activated macrophages (AAMs) depend on IL-4 signal transduction, and PSCs are the source of IL-4116. Interestingly, the increased expression of IL-4 induced by rottlerin indicates that IL-4 is more easily expressed under low cell energy115. This suggests that metabolic reprogramming of PSCs may also play an immunomodulatory role in fibrotic microenvironment. Moreover, PSCs promote islet fibrosis and β cell apoptosis in type 2 diabetes mellitus. The mechanism is that PSCs cause mitochondrial dysfunction, including loss of mitochondrial membrane potential, mitochondrial permeability transition, and mitochondrial apoptosis117. Treatment that selectively induces PSCs apoptosis is a feasible strategy, like Tocotrienol can selectively induce the death of PSCs by targeting MTP118. An overview landscape of all the mechanisms above is shown in Fig. 4.
Fig. 4. Mitochondrial dysfunction in islet fibrosis.
Activated PSCs play a critical role in the remodeling of peripheral ECM, which mediates apoptosis and islet fibrosis by inducing mitochondrial dysfunction of islet cells. Selectively inducing PSCs apoptosis via mitochondrial pathway is a feasible strategy. Furthermore, activated PSCs mainly relies on oxidative phosphorylation of mitochondria to maintain ATP energy levels. The uncoupling of mitochondria decreases oxidative phosphorylation and ATP level to inhibit PSCs activation. But this low cell energy situation can promote the phenotype transformation of AAMs through IL-4 secretion.
Mitophagy and fibrotic disease
Mitophagy can remove damaged mitochondria and alleviate mitochondrial dysfunction in order to inhibit the development of fibrosis. The defects of mitophagy in fibrosis has been widely reported. In animal models of renal vascular hypertension, mitophagy was inhibited, such as Parkin, LC3-II, ATG5 protein deficiency, accompanied by renal fibrosis119. Inhibition of mitophagy activates platelet-derived growth factor receptor (PDGFR)/PI3K/AKT signaling pathway and increases ROS production, accompanied by enhanced differentiation and proliferation of myofibroblasts. Inhibitory mechanism of mitophagy involves the knockdown of PARK2 and the low expression of BECN1 induced by microRNA-1224–5p120–122. It is also reported that TGF-β induces the production of ROS and mitochondrial depolarization in pulmonary epithelial cells. However, TGF-β can stabilizes the key mitophagy initiating factor PINK1 on the surface of mitochondria, abrogates ROS, prevents cell death, which is necessary to limit fibrosis123. So, the appropriate mitophagy activation in fibrosis may be regarded as a cell self-protection mechanism.
Reducing mitochondria dysfunction by targeting mitophagy has the potential therapeutic value in fibrosis. Melatonin inhibits liver fibrosis by up-regulating PINK, increasing autophagy flux, and upregulating light chain-3 (LC3-II) degradation105. PINK1-mediated mitophagy reduces the accelerated aging of MSCs in patients with CKD and enhances the therapeutic effect of transplanted MSCs on renal fibrosis92. Interestingly, when mitophagy is excessive, it also mediates the initiation of apoptotic processes. BCL-B belongs to the BCL-2 protein family, inhibits autophagy by binding Parkin signal and inhibiting its phosphorylation. BCL-B knockdown activates mitophagy, promotes apoptosis of HSCs and prevents fibrosis124. However, self-renewal mediated by mitophagy is not always beneficial to alleviating fibrosis. Akt1 produces apoptotic resistance in IPF AMs by inducing increased ROS and mitophagy, and increasing the expression of TGF-β1 to promote pulmonary fibrosis76. PM2.5 activates PINK1/Parkin pathway by inducing excessive ROS to trigger mitophagy, which activates LX-2 cells and primary HSCs104.
Mitochondrial transfer and fibrosis
It is believed that mitochondrial transfer as a new mode of cell–cell communication can effectively replace defective mitochondria125. There are many different ways to transfer mitochondria, including microinjection, incubation with intact purified mitochondria, gap junction channel-mediated cell attachment, and direct transfer from donor cells such as MSCs125,126. Extensive studies have confirmed that mitochondrial transfer plays a protective role in diverse organs127,128. We have seen burgeoning interest in the relationship between mitochondrial metastasis and fibrosis. MSCs directly transfer mitochondria to receptor cells through spontaneously generated cytoplasmic bridges called tunnel nanotubes (TNT)129. Li et al. documented that intravenous injection of bone-marrow-derived MSCs (BM-MSCs) suppressed cigarette smoke (CS)-induced pulmonary fibrosis. However, it is notable that the treatment and mitochondrial transfer to co-cultured bronchial epithelial cells of induced pluripotential cell-derived MSCs (iPS-MSCs) were more effective130. In addition, renal scattered tubular cells (STC-like cells)-extracellular vesicles (EV) have capacity for repairing injured TECs and decreasing interstitial fibrosis, partly through transferring STC-like cells functional mitochondria131.
As mitochondrial transfer is relatively new research direction, there is still a gap to be filled in fibrotic field. The mechanism, function, and potential clinical application of mitochondrial transfer in fibrosis need and deserve further investigation.
Therapeutic strategies targeting mitochondria to alleviate fibrosis
In Table 1, we made a summary of therapies targeting mitochondrial dysfunction, which had been mentioned in each organ and tissue section above. These treatment ideas can be summarized as follows: (1) maintain the integrity of the mitochondrial membrane and prevent the release of pro-inflammatory or pro-apoptotic substances; (2) enhance mitochondrial self-repair ability, such as mitophagy, mitochondrial biogenesis; (3) reduce oxidative damage to mitochondrial structure, like mtDNA and cardiolipin; (4) inhibit oxidative stress through exogenous ROS scavenger; (5) restore mitochondrial function or increase the quantity of normal mitochondria through exogenous carriers like MSCs. We also found that melatonin and some small molecular peptides showed therapeutic effects combined with diverse mechanisms. Moreover, cell therapy gradually presents its amazing potential for repair and treatment.
Table 1.
Therapies targeting mitochondrial dysfunction to alleviate fibrosis.
| Organ/disease | Therapeutic strategies | Mechanism | Reference |
|---|---|---|---|
| Heart/ventricle diastolic dysfunction | Alogliptin (a dipeptidyl peptidase-4 inhibitor) | 1. Preventing the production of mitochondrial ROS and mitochondrial membrane depolarization; 2. Improving mitochondrial biogenesis by PGC-1α/NRF1/Tfam pathway. | 49 |
| Heart/cardiorenal syndrome | Melatonin and ephedrine-4 | Alleviating oxidative stress, maintaining the integrity of mitochondrial membrane and preventing the release of cytochrome C | 53 |
| Heart/heart failure | Mitoquinone (a mitochondrial-targeted antioxidant) | 1. Inhibiting TGF-β1 and NOX4 expression; 2. Preventing Nrf2 downregulation and activation of TGF-β1-mediated profibrogenic signaling in cardiac fibroblasts | 47 |
| Heart/hypertensive cardiomyopathy | Overexpress catalase targeted to mitochondria | Alleviating cardiac hypertrophy, fibrosis, and mitochondrial damage | 46 |
| Heart/renovascular hypertension | Bendavia (a mitochondrial targeted peptide) | Reducing oxidative stress through improving mitochondrial biogenesis | 48 |
| Lung | 8-oxoguanine DNA glycosylase (Ogg1) and aconitase-2 (Aco-2) | Preventing mtDNA damage, p53 mitochondrial translocation, and intrinsic apoptosis in alveolar epithelial cells | 64 |
| Lung | Thyroid hormone | Increasing biogenesis via activating PGC-1α and promote mitophagy via PINK1 | 65 |
| Lung | Lysocardiolipin acyltransferase (LYCAT) | Negatively modulating TGF-β-induced fibroblast differentiation via the decline of NOX-dependent H2O2 generation and mitochondrial superoxide | 69 |
| Lung | Metformin | 1. Activation of AMPK mediated by metformin inhibits NOX4 expression induced by TGF-β; 2. AMPK activation also upregulates mitochondrial biogenesis and restores myofibroblast sensitivity to intrinsic apoptosis; 3. AMPK activation reprograms metabolism of IPF fibroblasts via diminishing mTOR activation and promoting autophagy | 73 |
| Lung | BM-MSCs transplantation | MSCs directly transfer mitochondria to receptor cells through spontaneously generated cytoplasmic bridges called tunnel nanotubes | 130 |
| Renal | Melatonin | Maintaining the integrity of mitochondrial morphology and structure | 8 |
| Renal | TNF receptor-associated protein 1 (TRAP1) | 1. Maintaining the integrity of mitochondrial morphology and structure; 2. Increasing the number of mtDNA copies | 81 |
| Renal | Bendavia | Reducing oxidative damage of mitochondrial cardiolipin | 83,84 |
| Renal/renal ischemia reperfusion injury | Postconditioning therapy | Protecting mitochondria from oxidative stress-induced mtDNA damage | 85 |
| Renal | 1,25-dihydroxyvitamin D3 | Maintaining the ATP synthase activity | 87 |
| Renal | Deletion of NLRP3 | Alleviating oxidative stress and ROS production | 88–91 |
| Renal | Combined treatment of MSC and melatonin | 1. Melatonin enhanced the role of MSC in fibrosis treatment; 2. Melatonin improved MSC mitochondrial function and enhanced oxidative phosphorylation through high expression of PrPc; 3. PINK1-mediated mitophagy reduces the accelerated aging of MSCs in patients with CKD and enhances the therapeutic effect | 92 |
| Renal | STC-like cells-extracellular vesicles | Transferring STC-like cells functional mitochondria to repair injured TECs | 131 |
| Liver | Augmenter of liver regeneration (ALR) gene therapy | Improving the mitochondrial dysfunction, inhibiting oxidative stress, and suppressing activation of HSCs | 98,99 |
| Liver | Didymin | Inhibition of ERK/MAPK and PI3K/Akt pathways in HSCs | 100 |
| Liver | Melatonin | 1. Restoring the enzymatic activities associated with respiratory chain, decreasing mitochondrial ROS production and inhibition of HSCs activation; 2. Upregulating mitophagy and mitochondrial biogenesis in mice | 103 |
| Liver | Dihydroartemisinin | Promoting HSCs apoptosis via mitochondrial pathway and down-regulating PI3K/Akt | 105 |
| Liver | PARP inhibition | Ameliorating the abnormal ETC and improving the activation of complexes I and IV | 102 |
| Liver | Melatonin | Up-regulating PINK1 and down-regulating LC3-II/LC3 ratio to promote mitophagy | 105 |
| Liver | Deletion of BCL-B | Activates excessive mitophagy to promote apoptosis of HSCs | 124 |
| Skin/radiation-induced skin injury | JP4-039 (a mitochondrially targeted antioxidant) | 1. Acting as a ROS scavenger with the significant affinity for mitochondrial inner membrane; 2. Reducing apoptosis and preserving the skin’s antioxidant capacity | 110 |
| Skin/scleroderma | ABT-263 (a “BH3 mimetic” drug) | Inducing myofibroblasts mitochondrial apoptotic pathway through inhibiting BCL-XL | 113 |
| Islet | Rottlerin (an oxidative phosphorylation uncoupling agent) | Inhibiting PSCs activation by acting as an oxidative phosphorylation uncoupling agent | 115 |
| Islet | Tocotrienol | Selectively inducing the death of PSCs | 118 |
Concluding remarks
Mitochondrial dysfunction has long been thought to be closely related to the progression of fibrosis in many end-stage viscera diseases. Mitochondrial dysfunction can lead to changes in mitochondrial morphology, dynamics, metabolic pathways, mtDNA, increased oxidative stress and other harmful substances, and ultimately exacerbate the biogenesis and development of fibrosis. Additionally, the abnormality of mitophagy and mitochondrial transfer also played vital roles in the fibrotic process. Therefore, understanding the process and mechanism of mitochondrial dysfunction is of great therapeutic value for diseases characterized by fibrosis as their pathological feature. With our comprehending of mitochondrial structure, function, and origin in recent years, the diagnosis and treatment of many fibrosis diseases are expected to find a breakthrough in mitochondria.
Acknowledgements
This study was supported by grants of the National Natural Science Foundation of China (81700658) and the Hunan Provincial Natural Science Foundation-Outstanding Youth Foundation (2020JJ3058).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by Silvia von Karstedt
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Linyong Xu, Email: xybms@163.com.
Quan Zhuang, Email: zhuangquansteven@163.com.
References
- 1.Humphreys BD. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 2.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N. Engl. J. Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 3.Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle. 2017;8:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topf U, Wrobel L, Chacinska A. Chatty mitochondria: keeping balance in cellular protein homeostasis. Trends Cell Biol. 2016;26:577–586. doi: 10.1016/j.tcb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Moon, et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourji K, et al. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015;87:282–289. doi: 10.1016/j.freeradbiomed.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Prado NJ, et al. Antiarrhythmic effect linked to melatonin cardiorenal protection involves AT1 reduction and Hsp70-VDR increase. J. Pineal Res. 2018;65:e12513. doi: 10.1111/jpi.12513. [DOI] [PubMed] [Google Scholar]
- 9.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017;60:44–65. doi: 10.1016/j.preteyeres.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil H, et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang R, et al. The transcription factor GLI2 as a downstream mediator of transforming growth factor-beta-induced fibroblast activation in SSc. Ann. Rheum. Dis. 2017;76:756–764. doi: 10.1136/annrheumdis-2016-209698. [DOI] [PubMed] [Google Scholar]
- 13.Tomcik M, et al. Tribbles homologue 3 stimulates canonical TGF-beta signalling to regulate fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 2016;75:609–616. doi: 10.1136/annrheumdis-2014-206234. [DOI] [PubMed] [Google Scholar]
- 14.Grosche J, Meissner J, Eble JA. More than a syllable in fib-ROS-is: the role of ROS on the fibrotic extracellular matrix and on cellular contacts. Mol. Asp. Med. 2018;63:30–46. doi: 10.1016/j.mam.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Richter K, Konzack A, Pihlajaniemi T, Heljasvaara R, Kietzmann T. Redox-fibrosis: impact of TGFbeta1 on ROS generators, mediators and functional consequences. Redox Biol. 2015;6:344–352. doi: 10.1016/j.redox.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He T, et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J. Mol. Med. 2016;94:1359–1371. doi: 10.1007/s00109-016-1451-y. [DOI] [PubMed] [Google Scholar]
- 18.Hecker L, Cheng J, Thannickal VJ, Targeting NOX. enzymes in pulmonary fibrosis. Cell. Mol. Life Sci. 2012;69:2365–2371. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, et al. Profibrotic role for interleukin-4 in cardiac remodeling and dysfunction. Hypertension (Dallas, Tex.: 1979) 2015;66:582–589. doi: 10.1161/HYPERTENSIONAHA.115.05627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, et al. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J. Biol. Chem. 2017;292:14003–14015. doi: 10.1074/jbc.M117.802066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, et al. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017;91:375–386. doi: 10.1016/j.kint.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68-69:106–121. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology. 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 24.Guo, R., Gu, J., Zong, S., Wu, M. & Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 41, 10.1016/j.bj.2017.12.001 (2018). [DOI] [PMC free article] [PubMed]
- 25.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard M, Wright KJ, Ritchie D, Thomas MM, Hepple RT. Mitochondrial function in permeabilized cardiomyocytes is largely preserved in the senescent rat myocardium. PLoS ONE. 2012;7:e43003. doi: 10.1371/journal.pone.0043003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, et al. Uncoupling protein 3 mediates H(2)O(2) preconditioning-afforded cardioprotection through the inhibition of MPTP opening. Cardiovasc. Res. 2015;105:192–202. doi: 10.1093/cvr/cvu256. [DOI] [PubMed] [Google Scholar]
- 29.Ye F, et al. Cyclosporin A protects against Lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology. 2016;57:203–213. doi: 10.1016/j.neuro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Kaarniranta, K., Pawlowska, E., Szczepanska, J., Jablkowska, A. & Blasiak, J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int. J. Mol. Sci.20, 10.3390/ijms20102374 (2019). [DOI] [PMC free article] [PubMed]
- 31.Bouchez, C. & Devin, A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cells8, 10.3390/cells8040287 (2019). [DOI] [PMC free article] [PubMed]
- 32.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker RM, Corum D, Beeson CC, Schnellmann RG. Mitochondrial biogenesis as a pharmacological target: a new approach to acute and chronic diseases. Annu. Rev. Pharmacol. Toxicol. 2016;56:229–249. doi: 10.1146/annurev-pharmtox-010715-103155. [DOI] [PubMed] [Google Scholar]
- 34.Johri A, Chandra A, Flint Beal M. PGC-1alpha, mitochondrial dysfunction, and Huntington’s disease. Free Radic. Biol. Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsunemi T, La Spada AR. PGC-1alpha at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog. Neurobiol. 2012;97:142–151. doi: 10.1016/j.pneurobio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiyama T, et al. Essential roles of mitochondrial biogenesis regulator Nrf1 in retinal development and homeostasis. Mol. Neurodegener. 2018;13:56. doi: 10.1186/s13024-018-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Eisner V, Picard M, Hajnoczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018;20:755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sebastian D, Palacin M, Zorzano A. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol. Med. 2017;23:201–215. doi: 10.1016/j.molmed.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Kong H, et al. Morphine induces dysfunction of PINK1/Parkin-mediated mitophagy in spinal cord neurons implying involvement in antinociceptive tolerance. J. Mol. Cell Biol. 2019 doi: 10.1093/jmcb/mjz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 45.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai DF, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ. Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goh KY, et al. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. 2019;21:101100. doi: 10.1016/j.redox.2019.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eirin A, et al. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J. Hypertens. 2014;32:154–165. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, et al. Alogliptin prevents diastolic dysfunction and preserves left ventricular mitochondrial function in diabetic rabbits. Cardiovasc. Diabetol. 2018;17:160. doi: 10.1186/s12933-018-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valli H, et al. Age-dependent atrial arrhythmic phenotype secondary to mitochondrial dysfunction in Pgc-1beta deficient murine hearts. Mech. Ageing Dev. 2017;167:30–45. doi: 10.1016/j.mad.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mangan MSJ, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 52.Bracey NA, et al. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J. Biol. Chem. 2014;289:19571–19584. doi: 10.1074/jbc.M114.550624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chua S, et al. The cardioprotective effect of melatonin and exendin-4 treatment in a rat model of cardiorenal syndrome. J. Pineal Res. 2016;61:438–456. doi: 10.1111/jpi.12357. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy H, et al. Sudden cardiac death due to deficiency of the mitochondrial inorganic pyrophosphatase PPA2. Am. J. Hum. Genet. 2016;99:674–682. doi: 10.1016/j.ajhg.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraccarollo D, et al. Soluble guanylyl cyclase activation improves progressive cardiac remodeling and failure after myocardial infarction. Cardioprotection over ACE inhibition. Basic Res. Cardiol. 2014;109:421. doi: 10.1007/s00395-014-0421-1. [DOI] [PubMed] [Google Scholar]
- 56.Luo YX, et al. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2017;38:1389–1398. doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- 57.Toime LJ, Brand MD. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic. Biol. Med. 2010;49:606–611. doi: 10.1016/j.freeradbiomed.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holley AK, Bakthavatchalu V, Velez-Roman JM, St Clair DK. Manganese superoxide dismutase: guardian of the powerhouse. Int. J. Mol. Sci. 2011;12:7114–7162. doi: 10.3390/ijms12107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malsin ES, Kamp DW. The mitochondria in lung fibrosis: friend or foe? Transl. Res. 2018;202:1–23. doi: 10.1016/j.trsl.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Raghu, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, et al. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1120–L1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vohwinkel CU, et al. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011;286:37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jablonski, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017;31:2520–2532. doi: 10.1096/fj.201601077R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SJ, et al. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J. Biol. Chem. 2014;289:6165–6176. doi: 10.1074/jbc.M113.515130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu G, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alvarez D, et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L1164–L1173. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain M, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J. Biol. Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hecker L, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang LS, et al. Lysocardiolipin acyltransferase regulates TGF-beta mediated lung fibroblast differentiation. Free Radic. Biol. Med. 2017;112:162–173. doi: 10.1016/j.freeradbiomed.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 70.Xie N, et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernard K, et al. NADPH Oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J. Biol. Chem. 2017;292:3029–3038. doi: 10.1074/jbc.M116.752261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caporarello N, et al. PGC1alpha repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax. 2019;74:749–760. doi: 10.1136/thoraxjnl-2019-213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato N, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir. Res. 2016;17:107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rangarajan S, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du S, et al. Dioscin alleviates crystalline silica-induced pulmonary inflammation and fibrosis through promoting alveolar macrophage autophagy. Theranostics. 2019;9:1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, Carter AB. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. 2016;44:582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grunwell JR, et al. TGF-beta1 suppresses the Type I IFN response and induces mitochondrial dysfunction in alveolar macrophages. J. Immunol. 2018;200:2115–2128. doi: 10.4049/jimmunol.1701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stout-Delgado HW, et al. Age-dependent susceptibility to pulmonary fibrosis is associated with NLRP3 inflammasome activation. Am. J. Respir. Cell Mol. Biol. 2016;55:252–263. doi: 10.1165/rcmb.2015-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eirin A, et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension (Dallas, Tex.: 1979) 2012;60:1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 80.Liu X, et al. Possible role of mitochondrial injury in Caulis Aristolochia manshuriensis-induced chronic aristolochic acid nephropathy. Drug Chem. Toxicol. 2017;40:115–124. doi: 10.1080/01480545.2016.1188303. [DOI] [PubMed] [Google Scholar]
- 81.Chen JF, et al. TRAP1 ameliorates renal tubulointerstitial fibrosis in mice with unilateral ureteral obstruction by protecting renal tubular epithelial cell mitochondria. FASEB J. 2017;31:4503–4514. doi: 10.1096/fj.201700283R. [DOI] [PubMed] [Google Scholar]
- 82.Pozdzik AA, et al. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008;73:595–607. doi: 10.1038/sj.ki.5002714. [DOI] [PubMed] [Google Scholar]
- 83.Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Ren. Physiol. 2014;306:F970–F980. doi: 10.1152/ajprenal.00697.2013. [DOI] [PubMed] [Google Scholar]
- 84.Eirin A, et al. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc. Res. 2014;103:461–472. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang S, Tan X, Chen Y, Zhang X. Postconditioning protects renal fibrosis by attenuating oxidative stress-induced mitochondrial injury. Nephrol. Dial. Transplant. 2017;32:1628–1636. doi: 10.1093/ndt/gfw469. [DOI] [PubMed] [Google Scholar]
- 86.Jiang Z, et al. Possible role of mtDNA depletion and respiratory chain defects in aristolochic acid I-induced acute nephrotoxicity. Toxicol. Appl. Pharmacol. 2013;266:198–203. doi: 10.1016/j.taap.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Shen Q, Bi X, Ling L, Ding W. 1,25-Dihydroxyvitamin D3 attenuates angiotensin II-induced renal injury by inhibiting mitochondrial dysfunction and autophagy. Cell. Physiol. Biochem. 2018;51:1751–1762. doi: 10.1159/000495678. [DOI] [PubMed] [Google Scholar]
- 88.Wen Y, et al. NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget. 2016;7:54290–54302. doi: 10.18632/oncotarget.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu WH, et al. Compound K inhibits priming and mitochondria-associated activating signals of NLRP3 inflammasome in renal tubulointerstitial lesions. Nephrol. Dial. Transplant. 2019 doi: 10.1093/ndt/gfz073. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, et al. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J. Immunol. (Baltim., Md.: 1950) 2013;190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 91.Kim SM, et al. Inflammasome-independent role of NLRP3 Mediates mitochondrial regulation in renal injury. Front. Immunol. 2018;9:2563. doi: 10.3389/fimmu.2018.02563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han YS, et al. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrP(C)-dependent enhancement of the mitochondrial function. J. Pineal Res. 2019;66:e12535. doi: 10.1111/jpi.12535. [DOI] [PubMed] [Google Scholar]
- 93.Campana L, Iredale JP. Regression of liver fibrosis. Semin. Liver Dis. 2017;37:1–10. doi: 10.1055/s-0036-1597816. [DOI] [PubMed] [Google Scholar]
- 94.Mallat A, Lotersztajn S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013;305:C789–C799. doi: 10.1152/ajpcell.00230.2013. [DOI] [PubMed] [Google Scholar]
- 95.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 96.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 97.Guimaraes, et al. Mitochondrial uncouplers inhibit hepatic stellate cell activation. BMC Gastroenterol. 2012;12:68. doi: 10.1186/1471-230X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ai WL, et al. Deficiency in augmenter of liver regeneration accelerates liver fibrosis by promoting migration of hepatic stellate cell. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3780–3791. doi: 10.1016/j.bbadis.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 99.Song M, et al. Augmenter of liver regeneration (ALR) gene therapy attenuates CCl(4)-induced liver injury and fibrosis in rats. Biochem. Biophys. Res. Commun. 2011;415:152–156. doi: 10.1016/j.bbrc.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 100.Lin X, et al. Didymin alleviates hepatic fibrosis through inhibiting ERK and PI3K/Akt pathways via regulation of Raf kinase inhibitor protein. Cell. Physiol. Biochem. 2016;40:1422–1432. doi: 10.1159/000453194. [DOI] [PubMed] [Google Scholar]
- 101.Zhao Y, et al. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics. 2019;9:1510–1522. doi: 10.7150/thno.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukhopadhyay P, et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 2017;66:589–600. doi: 10.1016/j.jhep.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 103.Das, N. et al. Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res.62, 10.1111/jpi.12404 (2017). [DOI] [PubMed]
- 104.Qiu YN, et al. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 2019;167:178–187. doi: 10.1016/j.ecoenv.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 105.Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 2016;60:383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 106.Cai SM, et al. Angiotensin-(1-7) improves liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxid. Redox Signal. 2016;24:795–812. doi: 10.1089/ars.2015.6498. [DOI] [PubMed] [Google Scholar]
- 107.Mitchell C, et al. Protection against hepatocyte mitochondrial dysfunction delays fibrosis progression in mice. Am. J. Pathol. 2009;175:1929–1937. doi: 10.2353/ajpath.2009.090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sheng X, et al. Establishment and characterization of a radiation-induced dermatitis rat model. J. Cell. Mol. Med. 2019;23:3178–3189. doi: 10.1111/jcmm.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edvardsen H, et al. SNP in TXNRD2 associated with radiation-induced fibrosis: a study of genetic variation in reactive oxygen species metabolism and signaling. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:791–799. doi: 10.1016/j.ijrobp.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 110.Brand RM, et al. A topical mitochondria-targeted redox-cycling nitroxide mitigates oxidative stress-induced skin damage. J. Investig. Dermatol. 2017;137:576–586. doi: 10.1016/j.jid.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kisseleva T, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Troeger JS, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083.e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med.9, 10.1126/scitranslmed.aal3765 (2017). [DOI] [PMC free article] [PubMed]
- 114.Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front. Physiol. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Su HY, et al. The unfolded protein response plays a predominant homeostatic role in response to mitochondrial stress in pancreatic stellate cells. PLoS ONE. 2016;11:e0148999. doi: 10.1371/journal.pone.0148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xue J, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat. Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kikuta K, et al. Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem. Biophys. Res. Commun. 2013;433:292–297. doi: 10.1016/j.bbrc.2013.02.095. [DOI] [PubMed] [Google Scholar]
- 118.Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 2007;132:2518–2532. doi: 10.1053/j.gastro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 119.Fedorova LV, et al. Peroxisome proliferator-activated receptor delta agonist, HPP593, prevents renal necrosis under chronic ischemia. PLoS ONE. 2013;8:e64436. doi: 10.1371/journal.pone.0064436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurita Y, et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir. Res. 2017;18:114. doi: 10.1186/s12931-017-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kobayashi K, et al. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J. Immunol. (Baltim., Md.: 1950) 2016;197:504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 122.Wu, Q. et al. miR-1224-5p mediates mitochondrial damage to affect silica-induced pulmonary fibrosis by targeting BECN1. Int. J. Mol. Sci.18, 10.3390/ijms18112357 (2017). [DOI] [PMC free article] [PubMed]
- 123.Patel AS, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS ONE. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ding Q, et al. The role of the apoptosis-related protein BCL-B in the regulation of mitophagy in hepatic stellate cells during the regression of liver fibrosis. Exp. Mol. Med. 2019;51:6. doi: 10.1038/s12276-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinha, P., Islam, M. N., Bhattacharya, S. & Bhattacharya, J. Intercellular mitochondrial transfer: bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev.38, 10.1016/j.gde.2016.05.002 (2016). [DOI] [PMC free article] [PubMed]
- 126.Agrawal A, Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L103–L113. doi: 10.1152/ajplung.00320.2015. [DOI] [PubMed] [Google Scholar]
- 127.Berridge MV, Herst PM, Rowe MR, Schneider R, McConnell MJ. Mitochondrial transfer between cells: methodological constraints in cell culture and animal models. Anal. Biochem. 2018;552:75–80. doi: 10.1016/j.ab.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Jiang D, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7:e2467. doi: 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li X, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 2014;51:455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 131.Zou X, et al. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci. Rep. 2018;8:1263. doi: 10.1038/s41598-018-19750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]