Abstract
Background
Anion gap (AG) has been proved to be associated with prognosis of many cardiovascular diseases. This study is aimed at exploring the association of AG with inhospital all-cause mortality and adverse clinical outcomes in coronary care unit (CCU) patients.
Method
All data of this study was extracted from Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4) database. All patients were divided into four groups according to AG quartiles. Primary outcome was inhospital all-cause mortality. Lowess smoothing curve was drawn to describe the overall trend of inhospital mortality. Binary logistic regression analysis was performed to determine the independent effect of AG on inhospital mortality.
Result
A total of 3593 patients were enrolled in this study. In unadjusted model, as AG quartiles increased, inhospital mortality increased significantly, OR increased stepwise from quartile 2 (OR, 95% CI: 1.01, 0.74-1.38, P = 0.958) to quartile 4 (OR, 95% CI: 2.72, 2.08-3.55, P < 0.001). After adjusting for possible confounding variables, this association was attenuated, but still remained statistically significant (quartile 1 vs. quartile 4: OR, 95% CI: 1.02, 0.72-1.45 vs. 1.49, 1.07-2.09, P = 0.019). Moreover, CCU mortality (P < 0.001) and rate of acute kidney injury (P < 0.001) were proved to be higher in the highest AG quartiles. Length of CCU (P < 0.001) and hospital stay (P < 0.001) prolonged significantly in higher AG quartiles. Maximum sequential organ failure assessment score (SOFA) (P < 0.001) and simplified acute physiology score II (SAPSII) (P < 0.001) increased significantly as AG quartiles increased. Moderate predictive ability of AG on inhospital (AUC: 0.6291), CCU mortality (AUC: 0.6355), and acute kidney injury (AUC: 0.6096) was confirmed. The interactions were proved to be significant in hypercholesterolemia, congestive heart failure, chronic lung disease, respiratory failure, oral anticoagulants, Beta-blocks, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB), and vasopressin treatment subgroups.
Conclusion
AG was an independent risk factor of inhospital all-cause mortality and was associated with adverse clinical outcomes in CCU patients.
1. Introduction
Despite extraordinary progress in cardiovascular field in recent decades, cardiovascular diseases still remain the major cause of death all over the world, causing about 17.5 million deaths each year [1, 2]. Originating in 1962, coronary care unit (CCU) focuses on the treatment of patients with severe cardiovascular diseases, which greatly reduces the mortality rate of patients [3–7]. A cheap and readily available clinical indicator for assessing prognosis still makes sense for CCU patients.
As a traditional clinical indicator used to evaluate acid-base balance, anion gap (AG) has been used in clinical practice for more than 50 years [8, 9]. AG has been proved to be associated with prognosis of many cardiovascular diseases [10–14]. A meta-analysis proved that AG was strongly related to mortality in critically ill patients [15]. Moreover, AG was confirmed to be associated with higher blood pressure [16], insulin resistance [17], and cardiorespiratory fitness [18]. In general population, higher AG was also proved to be related to cardiovascular mortality [19]. On the basis of these evidence, we hypothesized that AG could influence the prognosis of CCU patients. The purpose of this study was to explore the relationship between AG and outcomes of CCU patients.
2. Method
2.1. Data Source
We retrieved all data from an openly available critical care database named Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4) [20], which included more than 60000 intensive care unit (ICU) stays and more than 50000 stays for adult patients. The data in MIMIC-III were collected from June 2001 to October 2012 in Beth Israel Deaconess Medical Center, including general information (patient demographics, birth and death, ICU admission, and discharge information), vital signs, laboratory data, the balance of body fluid, reports, medication, and nursing record. Protecting Human Research Participant exam was passed to gain access to MIMIC-III database, and our certificate number is 9027152. Structured Query Language (SQL) was used to extract all patient information from MIMIC-III database.
2.2. Study Population
All adult patients (≥18 years) admitted to CCU from MIMIC-III database were included. And only the first admission of each patient was included. Patients meeting the following criteria were excluded: (1) patients were under 18 years old, (2) length of CCU stay <2 days, (3) anion gap data missing, and (4) individual data missing ≥5%. A total of 3593 patients were included in this study (Figure 1).
Figure 1.
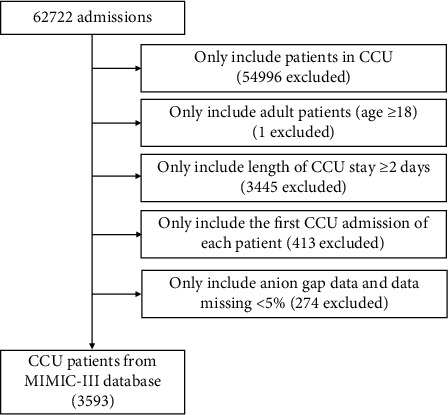
Flowchart of study population. CCU: coronary care unit.
2.3. Data Collection
All data used in this study was extracted using SQL from MIMIC-III database. Demographics, diagnoses of heart diseases, comorbidities and medical history, laboratory parameters, medication use, and survival data were collected. Demographic data included age, gender, and race. Diagnoses of heart diseases included coronary artery disease, acute myocardial infarction, third-degree atrioventricular block, atrial fibrillation, congestive heart failure, ventricular arrhythmias (ventricular tachycardia, ventricular flutter, and ventricular fibrillation), primary cardiomyopathy (hypertrophic obstructive cardiomyopathy and other primary cardiomyopathies), valve diseases (disorders of mitral, aortic, pulmonary, and tricuspid valve; rheumatic diseases of valves and congenital diseases of valves), endocarditis, and cardiogenic shock. Cardiogenic shock was identified by the presence of appropriate International Classification of Diseases, Ninth Versions (ICD-9) diagnosis and procedure code, which was adopted by the World Health Organization to code diagnoses, and previous study confirmed the validity of ICD-9 code in recording clinical conditions in dually coded database [21]. The ICD-9 code of cardiogenic shock used in this study was 78551. Comorbidities and medical history included hypertension, diabetes, chronic liver disease, hypercholesterolemia, chronic lung disease, chronic kidney disease, malignancy, autoimmune diseases, respiratory failure, prior myocardial infarction, and prior stroke. Medication use included antiplatelet, oral anticoagulant, Beta-blocks, ACEI, ARB, statin, and vasopressin. Laboratory parameters included AG, white blood cell, platelet, hemoglobin, creatinine, blood nitrogen urea, sodium, potassium, and glucose. All laboratory parameters were extracted within 24 hours after admission to CCU.
AG was calculated by the following formula: [AG = Na+(mmol/L) + K+(mmol/L)] − [Cl−(mmol/L) + HCO3−(mmol/L)], which was generally acknowledged [22]. And AG was recorded as initial AG and maximum AG, initial AG was the first test value after admission to CCU, and maximum AG was the maximum value during CCU stay.
2.4. Outcomes
The primary outcome was inhospital all-cause mortality, secondary outcomes included CCU all-cause mortality, acute kidney injury, maximum SOFA [23], maximum SAPSII [24], length of CCU, and hospital stay. Kidney Disease: Improving Global Outcomes (KDIGO) definition [25] was used for diagnosis of acute kidney injury.
Survival information was extracted from table named “patients” of MIMIC-III database. Data of length of CCU stay was extracted from table named “icustays” of MIMIC-III database. Data of length of hospital stay was extracted from table named “admissions” of MIMIC-III database. Acute kidney injury was confirmed based on KDIGO definition from table named “kdigo_creat” and “kdigo_uo” of MIMIC-III database. SOFA was extracted from table named “sofa” of MIMIC-III database. SAPSII was extracted from table named “sapsii” of MIMIC-III database.
2.5. Statistical Analysis
All the patients were stratified by AG quartiles. Continuous variables were summarized as mean ± standard deviation (SD) and median [interquartile range (IQR)]. Kruskal–Wallis test or one-way ANOVA analysis was used to test for difference. Categorical variables were summarized as number (percentage) and compared between groups using Chi-square test.
Binary logistic regression analysis was applied to identify the association between AG and inhospital all-cause mortality, and results were summarized as odds ratio (OR) with 95% confidence interval (CI). Covariates were incorporated into regression models based on statistical evidence and clinical judgment. Local weighted regression (Lowess) was applied to fit out curves in line with overall trend. Relative operating characteristic (ROC) curves were used to evaluate predictive ability of AG on inhospital all-cause mortality. All data processing and analysis were performed by Stata V.11.2. All tests were two sided, and P < 0.05 was considered statistically significant.
3. Result
3.1. Patient Characteristics
After screening step by step, a total of 3593 patients admitted to CCU were extracted from MIMIC-III database (Figure 1), most of whom were white and male. The baseline characteristics of patients stratified by AG quartiles are presented in Table 1. Initial AG and maximum AG of all patients were 15.0 ± 3.6 mmol/L and 17.7 ± 4.3 mmol/L, respectively. As AG quartiles increased, rates of ventricular arrhythmias, congestive heart failure, primary cardiomyopathy, cardiogenic shock, diabetes, respiratory failure, and chronic kidney diseases increased. But rates of coronary artery disease, hypertension, hypercholesterolemia, chronic lung diseases, and malignancy decreased as AG quartiles increased. Moreover, patients with higher AG had higher white blood cell, platelet, hemoglobin, glucose, creatinine, blood nitrogen urea, and potassium. Patients with higher AG also received less ACEI/ARB and statin treatment but more vasopressin treatment.
Table 1.
Characteristics of patients stratified by AG quartiles.
| Characteristics | Total (n = 3593) |
Quartiles of AG (mmol/L) | P value | |||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 868) AG < 13 |
Quartile 2 (n = 902) 13 ≤ AG < 15 |
Quartile 3 (n = 779) 15 ≤ AG < 17 |
Quartile 4 (n = 1044) 15 ≤ AG < 17 |
|||
| Age (years) | 69.2 ± 15.0 | 69.6 ± 14.4 | 68.7 ± 15.3 | 69.4 ± 15.0 | 69.0 ± 15.1 | 0.679 |
| Gender, n (%) | 0.986 | |||||
| Male | 2042 (56.8) | 497 (57.3) | 510 (56.5) | 440 (56.5) | 595 (57.0) | |
| Female | 1551 (43.2) | 371 (42.7) | 392 (43.5) | 339 (43.5) | 449 (43.0) | |
| Race, n (%) | 0.198 | |||||
| White | 2551 (71.0) | 640 (73.7) | 641 (71.1) | 554 (71.1) | 716 (68.6) | |
| Black | 263 (7.3) | 52 (6.0) | 66 (7.3) | 53 (6.8) | 92 (8.8) | |
| Other | 779 (21.7) | 176 (20.3) | 195 (21.6) | 172 (20.1) | 236 (22.6) | |
| Body mass index (kg/m2) | 28.2 ± 6.9 | 27.8 ± 6.7 | 28.3 ± 7.0 | 28.4 ± 6.8 | 28.3 ± 7.2 | 0.416 |
| Diagnoses of heart diseases, n (%) | ||||||
| Coronary artery disease | 1793 (49.9) | 405 (46.7) | 473 (52.4) | 418 (53.7) | 497 (47.6) | 0.006 |
| Acute myocardial infarction | 674 (18.8) | 143 (16.5) | 160 (17.7) | 161 (20.7) | 210 (20.1) | 0.082 |
| Atrial fibrillation | 1349 (37.6) | 319 (36.8) | 334 (37.0) | 304 (39.0) | 392 (37.6) | 0.786 |
| Ventricular arrhythmias | 206 (5.7) | 31 (3.6) | 39 (4.3) | 47 (6.0) | 89 (8.5) | <0.001 |
| Third-degree atrioventricular block | 153 (4.3) | 34 (3.9) | 36 (4.0) | 37 (4.8) | 46 (4.4) | 0.820 |
| Congestive heart failure | 1935 (53.9) | 421 (48.5) | 461 (51.1) | 415 (53.3) | 638 (61.1) | <0.001 |
| Primary cardiomyopathy | 294 (8.2) | 60 (6.9) | 62 (6.9) | 71 (9.1) | 101 (9.7) | 0.048 |
| Valve disease | 776 (21.6) | 165 (19.0) | 201 (22.3) | 173 (22.2) | 237 (22.7) | 0.203 |
| Endocarditis | 64 (1.8) | 18 (2.1) | 17 (1.9) | 13 (1.7) | 16 (1.5) | 0.824 |
| Cardiogenic shock | 471 (13.1) | 70 (8.1) | 92 (10.2) | 94 (12.1) | 215 (20.6) | <0.001 |
| Comorbidities and medical history, n (%) | ||||||
| Hypertension | 1456 (40.5) | 411 (47.4) | 409 (45.3) | 312 (40.1) | 324 (31.0) | <0.001 |
| Diabetes | 1205 (33.5) | 226 (26.0) | 287 (31.8) | 263 (33.8) | 429 (41.1) | <0.001 |
| Hypercholesterolemia | 1200 (33.4) | 313 (36.1) | 314 (34.8) | 271 (34.8) | 302 (28.9) | 0.003 |
| Chronic lung disease | 885 (24.6) | 244 (28.1) | 195 (21.6) | 198 (25.4) | 248 (23.8) | 0.013 |
| Respiratory failure | 1272 (35.4) | 279 (32.1) | 280 (31.0) | 264 (33.9) | 449 (43.0) | <0.001 |
| Chronic kidney disease | 752 (20.9) | 106 (12.2) | 165 (18.3) | 171 (22.0) | 310 (29.7) | <0.001 |
| Chronic liver disease | 125 (3.5) | 39 (4.5) | 22 (2.4) | 19 (2.4) | 45 (4.3) | 0.017 |
| Malignancy | 518 (14.4) | 125 (14.4) | 158 (17.5) | 105 (13.5) | 130 (12.5) | 0.013 |
| Autoimmune disease | 156 (4.3) | 40 (4.6) | 35 (3.9) | 34 (4.4) | 47 (4.5) | 0.879 |
| Prior myocardial infarction | 323 (9.0) | 77 (8.9) | 80 (8.9) | 83 (10.7) | 83 (8.0) | 0.256 |
| Prior stroke | 85 (2.4) | 19 (2.2) | 24 (2.7) | 12 (1.5) | 30 (2.9) | 0.270 |
| Laboratory parameters | ||||||
| AG (mmol/L) | 15.0 ± 3.6 | 10.9 ± 1.2 | 13.5 ± 0.5 | 15.5 ± 0.5 | 19.4 ± 2.6 | <0.001 |
| Maximum AG | 17.7 ± 4.3 | 14.7 ± 3.4 | 16.2 ± 2.9 | 17.7 ± 3.0 | 21.4 ± 4.1 | <0.001 |
| White blood cell (109/L) | 11.7 ± 5.6 | 10.0 ± 4.6 | 10.8 ± 4.8 | 11.7 ± 5.4 | 13.7 ± 6.3 | <0.001 |
| Platelet (109/L) | 236.8 ± 96.8 | 220.1 ± 91.3 | 234.8 ± 93.6 | 242.0 ± 95.1 | 248.4 ± 102.9 | <0.001 |
| Hemoglobin (g/dL) | 11.5 ± 2.0 | 11.2 ± 1.8 | 11.4 ± 1.9 | 11.6 ± 2.0 | 11.6 ± 2.1 | <0.001 |
| Glucose (mg/dL) | 154.6 ± 75.2 | 132.1 ± 52.0 | 141.5 ± 56.9 | 154.6 ± 69.0 | 184.6 ± 97.0 | <0.001 |
| Creatinine (mg/dL) | 1.58 ± 1.40 | 1.06 ± 0.63 | 1.24 ± 0.79 | 1.45 ± 0.95 | 2.41 ± 2.04 | <0.001 |
| Blood nitrogen urea (mg/dL) | 31.0 ± 21.6 | 22.6 ± 14.1 | 25.7 ± 16.2 | 31.3 ± 20.7 | 42.4 ± 26.2 | <0.001 |
| Sodium (mmol/L) | 138.0 ± 4.6 | 138.4 ± 4.3 | 138.3 ± 4.2 | 138.2 ± 4.2 | 137.2 ± 5.3 | <0.001 |
| Potassium (mmol/L) | 4.2 ± 0.8 | 4.0 ± 0.6 | 4.1 ± 0.7 | 4.2 ± 0.7 | 4.4 ± 0.9 | <0.001 |
| Medication use, n (%) | ||||||
| Antiplatelet | 2274 (63.3) | 556 (64.1) | 587 (65.9) | 480 (61.6) | 651 (62.4) | 0.425 |
| Oral anticoagulants | 1047 (29.1) | 259 (29.8) | 276 (30.6) | 232 (29.8) | 280 (26.8) | 0.260 |
| Beta-blocks | 2513 (69.9) | 624 (71.9) | 644 (71.4) | 533 (68.4) | 712 (68.2) | 0.184 |
| ACEI/ARB | 1882 (52.4) | 475 (54.7) | 495 (54.9) | 422 (54.2) | 490 (46.9) | 0.001 |
| Statin | 2095 (58.3) | 512 (59.0) | 552 (61.2) | 458 (58.8) | 573 (54.9) | 0.039 |
| Vasopressin | 234 (6.5) | 38 (4.4) | 46 (5.1) | 45 (5.8) | 105(10.1) | <0.001 |
Continuous variables were presented as mean ± SD. Categorical variables were presented as number (percentage). Abbreviation: AG: anion gap; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
3.2. Outcomes
The primary outcome was inhospital all-cause mortality. As shown in Table 2, the inhospital mortality of all patients in this study was 14.3%. As AG quartiles increased, inhospital mortality increased gradually (quartile 1 vs. quartile 4: 9.8% vs. 22.8%, P < 0.001); the same conclusion was drawn by Lowess smoothing curve shown in Figure 2. From unadjusted model comparing inhospital all-cause mortality among different AG groups, we observed that as AG quartiles increased, inhospital mortality increased significantly, OR increased stepwise from quartile 2 (OR, 95% CI: 1.01, 0.74-1.38, P = 0.958) to quartile 4 (OR, 95% CI: 2.72, 2.08-3.55, P < 0.001). After adjusting for more variables in model 3, this association was weakened, but still remained statistically significant (quartile 1 vs. quartile 4: OR, 95% CI: 1.02, 0.72-1.45 vs. 1.49, 1.07-2.09, P = 0.019) (Table 3).
Table 2.
Outcomes of patients stratified by AG quartiles.
| Outcomes | Total (n = 3593) |
Quartiles of AG (mmol/L) | P value | |||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 868) AG < 13 |
Quartile 2 (n = 902) 13 ≤ AG < 15 |
Quartile 3 (n = 779) 15 ≤ AG < 17 |
Quartile 4 (n = 1044) 15 ≤ AG < 17 |
|||
| Inhospital mortality, n (%) | 515 (14.3) | 85 (9.8) | 89 (9.9) | 103 (13.2) | 238 (22.8) | <0.001 |
| CCU mortality, n (%) | 399 (11.1) | 55 (6.3) | 73 (8.1) | 88 (11.3) | 183 (17.5) | <0.001 |
| Length of CCU stay (days) | 3.9 (2.8-6.6) | 3.5 (2.6-5.9) | 3.7 (2.8-5.9) | 3.9 (2.7-6.7) | 4.4 (3.0-7.3) | <0.001 |
| Length of hospital stay (days) | 8.3 (5.3-13.9) | 7.3 (4.8-12.5) | 7.8 (5.0-13.2) | 8.5 (5.3-13.7) | 9.7 (4.0-15.7) | <0.001 |
| Acute kidney injury, n (%) | 1924 (53.6) | 371 (42.7) | 434 (48.1) | 429 (55.1) | 690 (66.1) | <0.001 |
| Maximum SOFA | 4 (2-6) | 3 (1-5) | 3 (2-5) | 4 (2-6) | 5 (3-8) | <0.001 |
| Maximum SAPSII | 36 (28-46) | 33 (27-40) | 34 (26-43) | 36 (28-44) | 42 (33-52) | <0.001 |
Nonnormally distributed continuous variables were presented as median (IQR). Categorical variables were presented as number (percentage). Abbreviation: AG: anion gap; CCU: coronary care unit; SOFA: sequential organ failure assessment score; SAPS II: simplified acute physiology score II.
Figure 2.
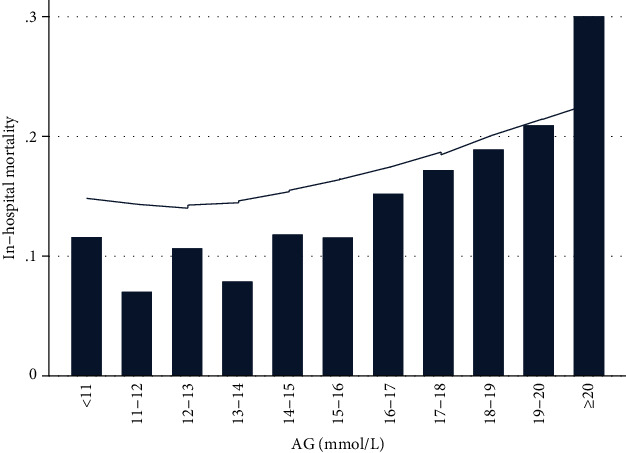
Association between anion gap and inhospital all-cause mortality presented through Lowess smoothing. Abbreviation: AG: anion gap.
Table 3.
The association between AG and inhospital all-cause mortality.
| AG (mmol) | |||
|---|---|---|---|
| OR (95% CI) | P value | P for trend | |
| Model 1 | <0.001 | ||
| Quartile 1: AG < 13 | Ref | ||
| Quartile 2: 13 ≤ AG < 15 | 1.01 (0.74-1.38) | 0.958 | |
| Quartile 3: 15 ≤ AG < 17 | 1.40 (1.03-1.90) | 0.029 | |
| Quartile 4: AG ≥ 17 | 2.72 (2.08-3.55) | <0.001 | |
| Continuous | 1.13 (1.10-1.16) | <0.001 | |
| Model 2 | <0.001 | ||
| Quartile 1: AG < 13 | Ref | ||
| Quartile 2: 13 ≤ AG < 15 | 1.02 (0.75-1.40) | 0.891 | |
| Quartile 3: 15 ≤ AG < 17 | 1.40 (1.03-1.91) | 0.031 | |
| Quartile 4: AG ≥ 17 | 2.78 (2.12-3.63) | <0.001 | |
| Continuous | 1.14 (1.11-1.16) | <0.001 | |
| Model 3 | |||
| Quartile 1: AG < 13 | Ref | <0.001 | |
| Quartile 2: 13 ≤ AG < 15 | 1.02 (0.72-1.45) | 0.897 | |
| Quartile 3: 15 ≤ AG < 17 | 1.22 (0.86-1.73) | 0.258 | |
| Quartile 4: AG ≥ 17 | 1.49 (1.07-2.09) | 0.019 | |
| Continuous | 1.06 (1.02-1.09) | 0.001 | |
Models were derived from binary logistic regression analysis. Model 1: unadjusted. Model 2: adjusted for age, gender, and race. Model 3: adjusted for age, gender, race, body mass index, coronary heart disease, acute myocardial infarction, atrial fibrillation, ventricular arrhythmias, third-degree atrioventricular block, congestive heart failure, primary cardiomyopathy, valve disease, endocarditis, cardiogenic shock, hypertension, diabetes, hypercholesterolemia, respiratory failure, chronic kidney disease, chronic liver disease, chronic lung disease, malignancy, autoimmune disease, prior myocardial infarction, prior stroke, oral anticoagulants, statin, vasopressin, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, antiplatelet, blood nitrogen urea, white blood cell, sodium, and creatinine. Abbreviation: AG: anion gap; OR: odds ratio; CI: confidence interval.
Secondary outcomes were CCU mortality, acute kidney injury, maximum SOFA, maximum SAPSII, length of CCU, and hospital stay. As shown in Table 2, CCU mortality of all patients was 11.1%, and as AG quartiles increased, the rate of CCU mortality increased stepwise from quartile 1 (6.3%) to quartile 4 (17.5%) (P < 0.001). Length of CCU stay (quartile 1 vs. quartile 4: 3.5 (2.6-5.9) vs. 4.4 (3.0-7.3), P < 0.001) and length of hospital staying (quartile 1 vs. quartile 4: 7.3 (4.8-12.5) vs. 9.7 (4.0-15.7), P < 0.001) increased significantly as AG increased. A total of 1924 patients were diagnosed with acute kidney injury based on KDIGO definition, and the incidence of acute kidney injury increased gradually from quartile 1 (42.7%) to quartile 4 (66.1%) (P < 0.001). Moreover, patients in highest AG quartile had highest maximum SAPSII (quartile 1 vs. quartile 4: 33 (27-40) vs. 42 (33-52), P < 0.001) and highest maximum SOFA (quartile 1 vs. quartile 4: 3 (1-5) vs. 5 (3-8), P < 0.001).
The relationship between inhospital mortality and AG quartiles in different subgroups is shown in Table 4. We did not observe significant interactions in most subgroups. Patients with chronic lung disease, congestive heart failure, and respiratory failure had lower risk of inhospital death. Moreover, patients who received oral anticoagulants, Beta-blocks, ACEI/ARB, and vasopressin treatment had lower risk of inhospital death too. But patients with hypercholesterolemia had higher risk of inhospital death.
Table 4.
Subgroup analysis of associations between inhospital all-cause mortality and AG (mmol/L).
| Subgroups | n | Quartile 1 AG < 13 |
Quartile 2 13 ≤ AG < 15 |
Quartile 3 15 ≤ AG < 17 |
Quartile 4 15 ≤ AG < 17 |
P
for interaction |
|---|---|---|---|---|---|---|
| Gender | 0.839 | |||||
| Male | 2042 | Ref | 1.21 (0.80-1.84) | 1.32 (0.86-2.01) | 2.98 (2.07-4.27) | |
| Female | 1551 | Ref | 0.79 (0.49-1.27) | 1.50 (0.96-2.33) | 2.43 (1.64-3.61) | |
| Age (years) | 0.968 | |||||
| <72 | 1832 | Ref | 1.08 (0.67-1.75) | 1.09 (0.66-1.80) | 2.82 (1.87-4.25) | |
| ≥72 | 1761 | Ref | 0.97 (0.64-1.47) | 1.62 (1.10-2.39) | 2.71 (1.90-3.85) | |
| Race | 0.244 | |||||
| White | 2551 | Ref | 1.07 (0.74-1.54) | 1.30 (0.91-1.87) | 2.57 (1.88-3.52) | |
| Black | 263 | Ref | 0.14 (0.16-1.28) | 0.77 (0.19-3.03) | 2.29 (0.80-6.57) | |
| Other | 779 | Ref | 1.13 (0.57-2.22) | 1.98 (1.05-3.73) | 3.41 (1.91-6.07) | |
| Body mass index (kg/m2) | 0.332 | |||||
| <27 | 1792 | Ref | 0.96 (0.64-1.46) | 1.49 (0.99-2.22) | 2.45 (1.72-3.48) | |
| ≥27 | 1801 | Ref | 1.08 (0.67-1.75) | 1.35 (0.84-2.17) | 3.14 (2.09-4.72) | |
| Coronary artery disease | 0.530 | |||||
| Yes | 1793 | Ref | 1.20 (0.73-1.97) | 1.68 (1.04-2.73) | 3.10 (2.00-4.81) | |
| No | 1800 | Ref | 0.94 (0.62-1.41) | 1.31 (0.88-1.95) | 2.55 (1.82-3.57) | |
| Acute myocardial infarction | 0.269 | |||||
| Yes | 674 | Ref | 1.15 (0.50-2.62) | 2.10 (0.99-4.46) | 3.65 (1.83-7.30) | |
| No | 2919 | Ref | 0.99 (0.70-1.39) | 1.29 (0.92-1.81) | 2.58 (1.93-3.45) | |
| Atrial fibrillation | 0.921 | |||||
| Yes | 1349 | Ref | 1.16 (0.72-1.86) | 1.40 (0.88-2.24) | 2.91 (1.93-4.40) | |
| No | 2244 | Ref | 0.90 (0.59-1.37) | 1.39 (0.93-1.08) | 2.59 (1.83-3.68) | |
| Ventricular arrhythmias | 0.065 | |||||
| Yes | 206 | Ref | 6.56 (0.76-56.55) | 9.17 (1.12-75.13) | 17.68 (2.30-135.7) | |
| No | 3387 | Ref | 0.94 (0.68-1.30) | 1.29 (0.94-1.76) | 2.45 (1.86-3.22) | |
| Third-degree atrioventricular block | 0.687 | |||||
| Yes | 153 | Ref | 0.30 (0.03-2.99) | 0.91 (0.17-4.86) | 2.51 (0.63-10.1) | |
| No | 3440 | Ref | 1.04 (0.76-1.42) | 1.43 (1.05-1.95) | 2.73 (2.08-3.58) | |
| Congestive heart failure | 0.013 | |||||
| Yes | 1935 | Ref | 0.84 (0.56-1.27) | 1.15 (0.77-1.71) | 1.97 (1.40-2.78) | |
| No | 1658 | Ref | 1.26 (0.77-2.05) | 1.78 (1.11-2.88) | 4.07 (2.66-6.23) | |
| Primary cardiomyopathy | 0.994 | |||||
| Yes | 294 | Ref | 0.97 (0.23-4.05) | 1.78 (0.51-6.22) | 2.64 (0.84-8.29) | |
| No | 3299 | Ref | 1.01 (0.73-1.39) | 1.39 (1.01-1.91) | 2.76 (2.10-3.64) | |
| Valve disease | 0.919 | |||||
| Yes | 776 | Ref | 0.75 (0.35-1.60) | 1.38 (0.69-2.78) | 2.34 (1.264.37) | |
| No | 2817 | Ref | 1.08 (0.77-1.53) | 1.42 (1.01-1.99) | 2.84 (2.12-3.82) | |
| Endocarditis | 0.617 | |||||
| Yes | 64 | Ref | 0.67 (0.10-4.58) | 0.91 (0.13-6.40) | 3.89 (0.80-18.97) | |
| No | 3529 | Ref | 1.02 (0.74-1.40) | 1.42 (1.04-1.94) | 2.71 (2.07-3.56) | |
| Cardiogenic shock | 0.352 | |||||
| Yes | 471 | Ref | 1.00 (0.48-2.09) | 1.43 (0.70-2.92) | 1.88 (1.01-3.51) | |
| No | 3122 | Ref | 0.97 (0.68-1.37) | 1.30 (0.92-1.83) | 2.55 (1.88-3.44) | |
| Hypertension | 0.126 | |||||
| Yes | 1456 | Ref | 1.01 (0.60-1.67) | 1.50 (0.90-2.48) | 3.44 (2.21-5.38) | |
| No | 2137 | Ref | 1.00 (0.67-1.48) | 1.30 (0.88-1.91) | 2.27 (1.62-3.17) | |
| Diabetes | 0.914 | |||||
| Yes | 1205 | Ref | 1.35 (0.73-2.49) | 1.72 (0.94-3.13) | 2.94 (1.72-5.02) | |
| No | 2388 | Ref | 0.91 (0.63-1.32) | 1.32 (0.93-1.90) | 2.79 (2.04-3.82) | |
| Hypercholesterolemia | 0.019 | |||||
| Yes | 1200 | Ref | 1.54 (0.80-2.95) | 2.22 (1.18-4.19) | 4.89 (2.75-8.69) | |
| No | 2393 | Ref | 0.88 (0.61-1.26) | 1.20 (0.84-1.71) | 2.17 (1.60-2.95) | |
| Chronic lung disease | <0.001 | |||||
| Yes | 885 | Ref | 0.94 (0.56-1.56) | 0.72 (0.42-1.22) | 1.55 (0.99-2.41) | |
| No | 2708 | Ref | 1.18 (0.78-1.77) | 2.04 (1.39-3.01) | 3.82 (2.70-5.42) | |
| Respiratory failure | 0.005 | |||||
| Yes | 1272 | Ref | 0.71 (0.46-1.09) | 1.14 (0.76-1.72) | 1.67 (1.18-2.38) | |
| No | 2321 | Ref | 1.62 (0.99-2.65) | 1.85 (1.12-3.04) | 4.26 (2.74-6.62) | |
| Chronic kidney disease | 0.813 | |||||
| Yes | 752 | Ref | 1.49 (0.65-3.40) | 1.35 (0.59-3.10) | 2.86 (1.37-5.97) | |
| No | 2841 | Ref | 0.93 (0.66-1.31) | 1.45 (1.04-2.01) | 2.78 (2.08-3.73) | |
| Chronic liver disease | 0.538 | |||||
| Yes | 125 | Ref | 1.22 (0.30-4.90) | 1.03 (0.23-4.66) | 2.23 (0.76-6.60) | |
| No | 3468 | Ref | 1.02 (0.74-1.40) | 1.44 (1.05-1.97) | 2.76 (2.10-3.63) | |
| Malignancy | 0.109 | |||||
| Yes | 518 | Ref | 0.97 (0.49-1.94) | 1.14 (0.55-2.39) | 1.82 (0.95-3.52) | |
| No | 3075 | Ref | 1.00 (0.70-1.42) | 1.47 (1.05-2.06) | 2.94 (2.19-3.95) | |
| Autoimmune disease | 0.680 | |||||
| Yes | 156 | Ref | 0.53 (0.12-2.30) | 1.21 (0.35-4.18) | 1.94 (0.65-5.77) | |
| No | 3437 | Ref | 1.04 (0.76-1.44) | 1.42 (1.04-1.94) | 2.78 (2.11-3.66) | |
| Prior myocardial infarction | 0.706 | |||||
| Yes | 323 | Ref | 1.43 (0.51-3.97) | 1.22 (0.43-3.44) | 2.77 (1.09-7.06) | |
| No | 3270 | Ref | 0.97 (0.70-1.35) | 1.43 (1.04-1.96) | 2.71 (2.06-3.58) | |
| Prior stroke | 0.413 | |||||
| Yes | 61 | Ref | - | 3.6 (0.29-44.82) | 3.6 (0.39-33.50) | |
| No | 3508 | Ref | 1.03 (0.75-1.41) | 1.38 (1.02-1.88) | 2.72 (2.08-3.55) | |
| Antiplatelet | 0.678 | |||||
| Yes | 2274 | Ref | 1.02 (0.69-1.53) | 1.61 (1.09-2.36) | 2.81 (2.00-3.96) | |
| No | 1319 | Ref | 0.99 (0.60-1.64) | 1.12 (0.68-1.84) | 2.57 (1.68-3.93) | |
| Oral anticoagulants | 0.011 | |||||
| Yes | 1047 | Ref | 0.99 (0.51-1.89) | 0.57 (0.26-1.25) | 1.57 (0.86-2.86) | |
| No | 2546 | Ref | 1.02 (0.71-1.46) | 1.69 (1.20-2.37) | 3.06 (2.26-4.13) | |
| Beta-blockers | 0.002 | |||||
| Yes | 2513 | Ref | 0.90 (0.61-1.31) | 1.24 (0.85-1.80) | 1.98 (1.43-2.76) | |
| No | 1080 | Ref | 1.28 (0.74-2.23) | 1.75 (1.03-2.98) | 4.58 (2.86-7.34) | |
| ACEI/ARB | <0.001 | |||||
| Yes | 1882 | Ref | 0.96 (0.58-1.59) | 1.17 (0.71-1.95) | 1.40 (0.87-2.24) | |
| No | 1711 | Ref | 1.04 (0.70-1.56) | 1.56 (1.06-2.31) | 3.43 (2.45-4.81) | |
| Statin | 0.846 | |||||
| Yes | 2095 | Ref | 1.02 (0.65-1.60) | 1.45 (0.93-2.24) | 2.62 (1.78-3.85) | |
| No | 1498 | Ref | 1.02 (0.66-1.59) | 1.37 (0.89-2.10) | 2.75 (1.90-3.98) | |
| Vasopressin | 0.042 | |||||
| Yes | 234 | Ref | 0.65 (0.27-1.56) | 0.89 (0.37-2.11) | 1.18 (0.56-2.47) | |
| No | 3359 | Ref | 1.05 (0.74-1.48) | 1.45 (1.04-2.04) | 2.78 (2.06-3.74) | |
| White blood cell (109/L) | 0.057 | |||||
| <10.5 | 1778 | Ref | 0.80 (0.52-1.23) | 1.22 (0.79-1.87) | 1.91 (1.29-2.84) | |
| ≥10.5 | 1815 | Ref | 1.25 (0.78-2.01) | 1.54 (0.98-2.43) | 3.10 (2.07-4.65) | |
| Platelet (109/L) | 0.170 | |||||
| <221 | 1793 | Ref | 1.10 (0.71-1.72) | 1.80 (1.18-2.75) | 3.30 (2.27-4.81) | |
| ≥221 | 1800 | Ref | 0.91 (0.58-1.41) | 1.07 (0.69-1.66) | 2.22 (1.52-3.24) | |
| Hemoglobin (g/dL) | 0.223 | |||||
| <11.4 | 1777 | Ref | 0.93 (0.62-1.39) | 1.24 (0.82-1.86) | 2.42 (1.70-3.43) | |
| ≥11.4 | 1816 | Ref | 1.16 (0.71-1.90) | 1.73 (1.09-2.76) | 3.31 (2.17-5.04) | |
| Glucose (mg/dL) | 0.828 | |||||
| <132 | 1773 | Ref | 1.12 (0.74-1.70) | 1.42 (0.93-2.16) | 2.88 (1.97-4.22) | |
| ≥132 | 1820 | Ref | 0.86 (0.53-1.39) | 1.32 (0.84-2.08) | 2.44 (1.65-3.62) | |
| Creatinine (mg/dL) | 0.177 | |||||
| <1.1 | 1537 | Ref | 0.99 (0.64-1.54) | 1.24 (0.78-1.97) | 2.20 (1.40-3.45) | |
| ≥1.1 | 2056 | Ref | 0.96 (0.61-1.52) | 1.37 (0.89-2.11) | 2.52 (1.72-3.68) | |
| Blood nitrogen urea (mg/dL) | 0.365 | |||||
| <24 | 1728 | Ref | 1.08 (0.68-1.73) | 1.34 (0.81-2.20) | 2.79 (1.77-4.39) | |
| ≥24 | 1865 | Ref | 0.83 (0.54-1.27) | 1.06 (0.71-1.27) | 1.76 (1.24-2.50) | |
| Sodium (mmol/L) | 0.123 | |||||
| <138 | 1456 | Ref | 1.16 (0.73-1.84) | 1.26 (0.79-2.00) | 2.28 (1.53-3.39) | |
| ≥138 | 2137 | Ref | 0.89 (0.58-1.37) | 1.50 (1.00-2.25) | 3.02 (2.10-4.33) | |
| Potassium (mmol/L) | 0.290 | |||||
| <4.1 | 1625 | Ref | 0.95 (0.60-1.50) | 1.70 (1.09-2.64) | 3.12 (2.09-4.65) | |
| ≥4.1 | 1968 | Ref | 1.04 (0.68-1.61) | 1.17 (0.77-1.79) | 2.39 (1.66-3.44) |
Binary logistic regression analysis was used, and results were presented as OR (odds ratio) and 95% CI (confidence interval). Abbreviation: AG: anion gap; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
As presented in Figure 3, moderate predictive ability of AG on inhospital (AUC, 95% CI: 0.6291, 0.6019-0.6564), CCU mortality (AUC, 95% CI: 0.6355, 0.6060-0.6650), and acute kidney injury (AUC, 95% CI: 0.6096, 0.5914-0.6278) was confirmed.
Figure 3.
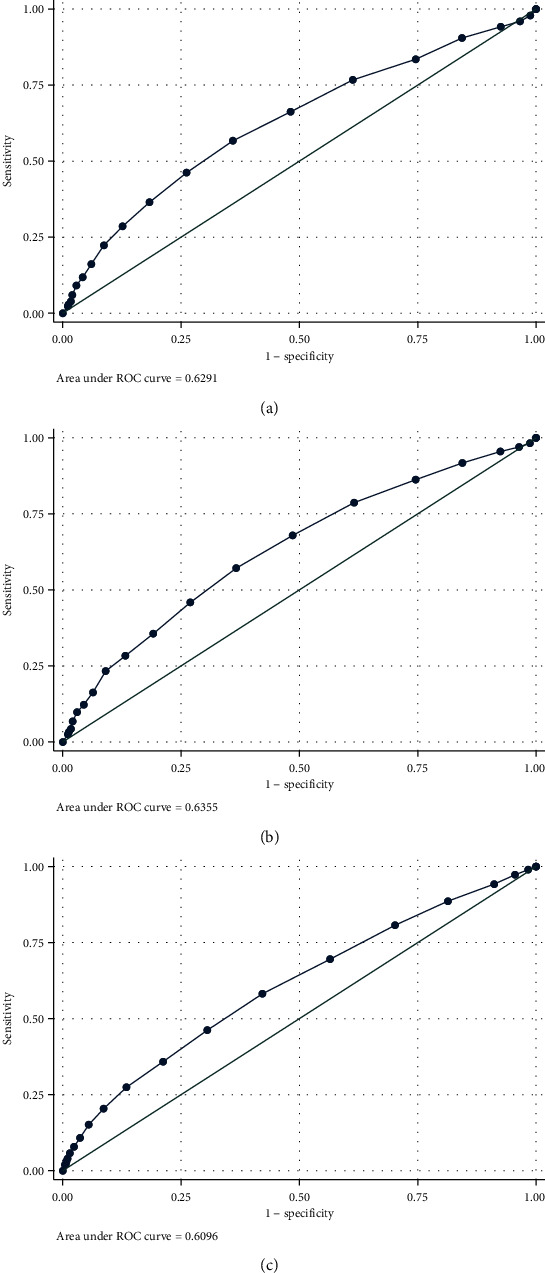
ROC curves of AG for prediction of inhospital all-cause mortality (a), CCU all-cause mortality (b), and acute kidney injury (c). Abbreviation: AG: anion gap; CCU: coronary care unit.
4. Discussion
This study explored the association of AG with inhospital mortality and other adverse outcomes of CCU patients. The main findings were as follows: (1) as AG quartiles increased, inhospital all-cause mortality increased significantly. (2) As AG quartiles increased, CCU mortality and the rate of acute kidney injury increased. (3) Patients with higher AG had higher maximum SOFA and maximum SAPSII. (4) Length of CCU and hospital stay prolonged significantly in higher AG quartiles. (5) Moderate predictive ability of AG on inhospital, CCU mortality, and acute kidney injury was confirmed. (6) The interactions were proved to be significant in hypercholesterolemia, congestive heart failure, chronic lung disease, respiratory failure, oral anticoagulants, Beta-blocks, ACEI/ARB, and vasopressin treatment subgroups.
Acid-base balance is very important for the maintenance of normal physiological function and cell metabolism [26]. As a common indicator to evaluate acid-base balance, AG is often used to define the types and causes of metabolic acidosis. Clinically, AG is usually calculated by the concentration of serum sodium, potassium, chloride, and bicarbonate [27], which is inexpensive and readily available.
AG has been proved to be associated with prognosis of many cardiovascular diseases [10–14]. Previous study which enrolled 18115 patients with coronary disease showed that higher AG was associated with worse cardiac function, more severe clinical symptoms, and acute myocardial infarction: for every unit increase in AG, the 30-day risk of all-cause death increased by 0.244 times [10]. In patients with myocardial infarction, increased AG was also associated with higher mortality and cardiogenic shock [12]. Another research enrolled 63 patients with cardiogenic shock following ST-segment elevation myocardial infarction (STEMI) came to a similar conclusion that higher AG was associated with higher mortality [13]. For patients with STEMI, AG was proved to be an independent risk factor for high inhospital mortality after percutaneous coronary intervention and could be used for risk stratification [14]. Moreover, a meta-analysis and another study revealed that AG may be a good choice to assess the prognosis of critically ill patients especially for those in areas with inadequate medical resources [15, 28]. Similarly, our data suggested that AG was associated with inhospital all-cause mortality of CCU patients independently, and maybe the adverse effects of increased AG on coronary artery disease and critically ill patients contribute to this result. Moreover, AG contributed to the diagnosis of acute kidney injury [8, 27]. Similarly, we found that as AG quartiles increased, incidence of acute kidney injury increased significantly. SOFA and SAPSII were good scoring system for predicting the prognosis of critically ill patients. In this study, we found that as AG increased, SOFA and SAPSII increased significantly, and this phenomenon may also explain higher inhospital mortality in patients with higher AG. Moreover, length of CCU and hospital stay prolonged significantly in higher AG quartiles, which will bring greater psychological, physical, and financial burden to patients, so more attention to AG in CCU patients may be needed.
In coronary artery disease, acute myocardial infarction, third-degree atrioventricular block, congestive heart failure, primary cardiomyopathy, valve disease, ventricular arrhythmias, endocarditis, cardiogenic shock, and atrial fibrillation subgroups, we can all come to the same conclusion that as AG increased, inhospital mortality increased. All above diseases almost covered most diseases of CCU. This result provided a strong support for us to use AG as a clinical indicator in CCU to predict prognosis. In hypercholesterolemia, congestive heart failure, chronic lung disease, respiratory failure, oral anticoagulants, Beta-blocks, ACEI/ARB, and vasopressin subgroups, the interactions were proved to be significant. Further research is needed to clarify the reasons.
5. Limitation
This study was a single retrospective study, and inevitable bias may affect the authenticity of the results. Moreover, the bulk of AG is largely determined by anions attached to circulation protein [29, 30], and as albumin decreases, so does AG [31, 32]. But due to the loss of albumin data, we did not include the albumin data in this study. Apart from the retrospective model, the main bias of this study was the lack of albumin values for a correct AG adjustment. In general, the more key variables a model contains, the more accurate its predictions will be. But constrained by public databases, a lot of information that may affect the model was not collected, like smoking and drinking alcohol. In addition to this, other important information was also not collected such as specific cause of death, cardiac function, and left ventricular ejection fraction. In order to verify the conclusion, prospective case-control study may be needed.
6. Conclusion
AG was an independent risk factor of inhospital all-cause mortality and was associated with adverse clinical outcomes in CCU patients. But more prospective case-control data are needed to confirm AG's role as a clinical indicator in CCU to predict prognosis.
Acknowledgments
Thanks to all co-authors for the contributions to data collection and processing.
Data Availability
All data used in this analysis were from an openly available critical care database named MIMIC-III. Protecting Human Research Participant exam was passed to gain access to MIMIC-III database, and our certificate number is 9027152.
Ethical Approval
Ethics approval of MIMIC-III database was obtained by the institutional review boards at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Geneva S., World Health Organization . Global Status Report on Noncommunicable Diseases 2010. World Health Organization; 2011. [Google Scholar]
- 2.Roth G. A., Abate D., Abate K. H., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fye W. B. Resuscitating a circulation abstract to celebrate the 50th anniversary of the coronary care unit concept. Circulation. 2011;124(17):1886–1893. doi: 10.1161/CIRCULATIONAHA.111.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julian D. G. The history of coronary care units. British Heart Journal. 1987;57(6):497–502. doi: 10.1136/hrt.57.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killip T., 3rd, Kimball J. T. Treatment of myocardial infarction in a coronary care unit. The American Journal of Cardiology. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 6.MacMillan R. L., Brown K. W. Comparison of the effects of treatment of acute myocardial infarction in a coronary unit and on a general medical ward. Canadian Medical Association Journal. 1971;105(10):1037–1040. [PMC free article] [PubMed] [Google Scholar]
- 7.Loughran J., Puthawala T., Sutton B. S., Brown L. E., Pronovost P. J., DeFilippis A. P. The cardiovascular intensive care unit—an evolving model for health care delivery. Journal of Intensive Care Medicine. 2016;32(2):116–123. doi: 10.1177/0885066615624664. [DOI] [PubMed] [Google Scholar]
- 8.Kraut J. A., Madias N. E. Serum anion gap: its uses and limitations in clinical medicine. Clinical journal of the American Society of Nephrology. 2006;2(1):162–174. doi: 10.2215/cjn.03020906. [DOI] [PubMed] [Google Scholar]
- 9.Kraut J. A., Nagami G. T. The serum anion gap in the evaluation of acid-base disorders: what are its limitations and can its effectiveness be improved? Clinical Journal of the American Society of Nephrology : CJASN. 2013;8(11):2018–2024. doi: 10.2215/CJN.04040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S. W., Zhou Y. J., Zhao Y. X., et al. The serum anion gap is associated with disease severity and all-cause mortality in coronary artery disease. Journal of geriatric cardiology : JGC. 2017;14(6):392–400. doi: 10.11909/j.issn.1671-5411.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q., Chen Q., Li L., et al. Serum anion gap on admission predicts intensive care unit mortality in patients with aortic aneurysm. Experimental and Therapeutic Medicine. 2018;16(3):1766–1777. doi: 10.3892/etm.2018.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahu A., Cooper H. A., Panza J. A. The initial anion gap is a predictor of mortality in acute myocardial infarction. Coronary Artery Disease. 2006;17(5):409–412. doi: 10.1097/00019501-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Attanà P., Lazzeri C., Chiostri M., Picariello C., Gensini G. F., Valente S. Strong-ion gap approach in patients with cardiogenic shock following ST-elevation myocardial infarction. Acute Cardiac Care. 2013;15(3):58–62. doi: 10.3109/17482941.2013.776691. [DOI] [PubMed] [Google Scholar]
- 14.Lazzeri C., Valente S., Chiostri M., Picariello C., Gensini G. F. Evaluation of acid-base balance in ST-elevation myocardial infarction in the early phase: a prognostic tool? Coronary Artery Disease. 2010;21(5):266–272. doi: 10.1097/MCA.0b013e32833b20c6. [DOI] [PubMed] [Google Scholar]
- 15.Glasmacher S. A., Stones W. Anion gap as a prognostic tool for risk stratification in critically ill patients - a systematic review and meta-analysis. BMC Anesthesiology. 2016;16(1):p. 68. doi: 10.1186/s12871-016-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor E. N., Forman J. P., Farwell W. R. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension. 2007;50(2):320–324. doi: 10.1161/HYPERTENSIONAHA.107.092643. [DOI] [PubMed] [Google Scholar]
- 17.Farwell W. R., Taylor E. N. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabetic Medicine : a Journal of the British Diabetic Association. 2008;25(7):798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 18.Abramowitz M. K., Hostetter T. H., Melamed M. L. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney International. 2012;81(10):1033–1042. doi: 10.1038/ki.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M., Jung S. J., Yoon S., Yun J. M., Yoon H. J. Association between the markers of metabolic acid load and higher all-cause and cardiovascular mortality in a general population with preserved renal function. Hypertension Research : official Journal of the Japanese Society of Hypertension. 2015;38(6):433–438. doi: 10.1038/hr.2015.23. [DOI] [PubMed] [Google Scholar]
- 20.Johnson A. E., Pollard T. J., Shen L., et al. MIMIC-III, a freely accessible critical care database. Scientific Data. 2016;3(1):p. 160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H., Li B., Duncan Saunders L., et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Services Research. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta A. N., Emmett J. B., Emmett M. GOLD MARK: an anion gap mnemonic for the 21st century. Lancet. 2008;372(9642):p. 892. doi: 10.1016/S0140-6736(08)61398-7. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J. L., Moreno R., Takala J., et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 24.Le Gall J. R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. Journal of the American Medical Association. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 25.Mehta R. L., Kellum J. A., Shah S. V., et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11(2):p. R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamm L. L., Nakhoul N., Hering-Smith K. S. Acid-base homeostasis. Clinical Journal of the American Society of Nephrology : CJASN. 2015;10(12):2232–2242. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Co I., Gunnerson K. Emergency Department Management of Acute Kidney Injury, Electrolyte Abnormalities, and Renal Replacement Therapy in the Critically Ill. Emergency Medicine Clinics of North America. 2019;37(3):459–471. doi: 10.1016/j.emc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Zheng C. M., Liu W. C., Zheng J. Q., et al. Metabolic acidosis and strong ion gap in critically ill patients with acute kidney injury. BioMed Research International. 2014;2014:8. doi: 10.1155/2014/819528.819528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corey H. E. The anion gap (AG): studies in the nephrotic syndrome and diabetic ketoacidosis (DKA) The Journal of Laboratory and Clinical Medicine. 2006;147(3):121–125. doi: 10.1016/j.lab.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Feldman M., Soni N., Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. The Journal of Laboratory and Clinical Medicine. 2005;146(6):317–320. doi: 10.1016/j.lab.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Figge J., Jabor A., Kazda A., Fencl V. Anion gap and hypoalbuminemia. Critical Care Medicine. 1998;26(11):1807–1810. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Carvounis C. P., Feinfeld D. A. A simple estimate of the effect of the serum albumin level on the anion gap. American Journal of Nephrology. 2000;20(5):369–372. doi: 10.1159/000013618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this analysis were from an openly available critical care database named MIMIC-III. Protecting Human Research Participant exam was passed to gain access to MIMIC-III database, and our certificate number is 9027152.


