Abstract
Background & Aims
The SARS-CoV-2 pandemic had a sudden, dramatic impact on healthcare. In Italy, since the beginning of the pandemic, colorectal cancer (CRC) screening programs have been forcefully suspended. We aimed to evaluate whether screening procedure delays can affect the outcomes of CRC screening.
Methods
We built a procedural model considering delays in the time to colonoscopy and estimating the effect on mortality due to up-stage migration of patients. The number of expected CRC cases was computed by using the data of the Italian screened population. Estimates of the effects of delay to colonoscopy on CRC stage, and of stage on mortality were assessed by a meta-analytic approach.
Results
With a delay of 0-3 months, 74% of CRC is expected to be stage I-II, while with a delay of 4-6 months there would be a 2%-increase for stage I-II and a concomitant decrease for stage III-IV (P = .068). Compared to baseline (0-3 months), moderate (7-12 months) and long (> 12 months) delays would lead to a significant increase in advanced CRC (from 26% to 29% and 33%, respectively; P = .008 and P < .001, respectively). We estimated a significant increase in the total number of deaths (+12.0%) when moving from a 0-3-months to a >12-month delay (P = .005), and a significant change in mortality distribution by stage when comparing the baseline with the >12-months (P < .001).
Conclusions
Screening delays beyond 4-6 months would significantly increase advanced CRC cases, and also mortality if lasting beyond 12 months. Our data highlight the need to reorganize efforts against high-impact diseases such as CRC, considering possible future waves of SARS-CoV-2 or other pandemics.
Keywords: SARS-CoV-2, Colorectal Cancer Screening, Colonoscopy, Colon Cancer, Fecal Immunochemical Test
Abbreviations used in this paper: CRC, colorectal cancer; DS, delay stage; FIT, fecal immunochemical test; SARS-CoV-2, severe acute respiratory syndrome–associated coronavirus 2; SM, stage mortality
What You Need to Know.
Background
Because of the SARS-CoV-2 pandemic, colorectal cancer screening programs have been suspended in many countries.
Findings
Delays beyond 6 months would progressively lead to increased disease mortality rates through the shift to more advanced stages detected at screening. Results were obtained by applying meta-analytic estimates to the Italian population.
Implications for patient care
Our data highlight the need to avoid breaking the workflow of colorectal cancer screening beyond 12 months in the light of possible future waves of SARS-CoV-2 or other pandemics.
The severe acute respiratory syndrome–associated corona virus 2 (SARS-CoV-2) pandemic era affected the healthcare organizations/systems in every country, irrespective of differences in political governance, economic resources, or type of healthcare reimbursement. Mounting pressure for emergency admissions and intensive care support resulted in a surge of access to hospitals, prompting the downscaling of almost all clinical activities to face the unexpected spread of the disease and its life-threatening complications.1 In Europe, the enormous diversion of medical resources toward SARS-CoV-2–dedicated wards dominated the clinical scenarios,2 with almost all planned public healthcare activities, including cancer screening, being suspended. As of now, in Italy it is difficult to discern the excess of deaths registered in the early spring according to their relationship with SARS-CoV-2 or with high-impact illnesses that remained unrecognized and possibly untreated.
Regarding cancer incidence and mortality, colorectal cancer (CRC) represents a major burden worldwide3 but can be counteracted by screening strategies. Currently in the United States, the implementation of an opportunistic approach by screening colonoscopy has contributed to a gradual reduction of CRC incidence,4 whereas European countries mostly rely on programmatic screening by biannual fecal immunochemical test (FIT).5 The adoption of FIT reduces CRC mortality mainly through the detection of early stage tumors, which leads to a down-staging process.6 To meet their benchmark reduction of CRC burden, FIT-based screening programs rely on a multilayer system involving public health authorities, kit distribution facilities, FIT measuring labs, and ultimately hospitals/centers where FIT+ subjects undergo colonoscopy. Since the beginning of the pandemic, this entire system has been put on hold. On March 8 and 11, 2020, the Italian government issued decrees that greatly reduced gathering and interpersonal contacts, which added to the above-mentioned pressures on the healthcare system, further restraining cancer screening.7 Heterogeneous decisions involved the management of tests at the first level (by interrupting active calls) as well as at the second level (by placing on hold endoscopic examination for FIT+ subjects), with some coordinating units opting to shut down both levels.
Recent data highlighted the detrimental effects on mortality of delaying diagnosis in symptomatic patients with CRC in the United Kingdom8 because of SARS-Cov-2 pandemic. However, screening delays might have even more dramatic effects than delayed diagnosis, once the down-staging effect is reversed by the delays. Accordingly, we sought to assess the impact of the pandemic on programmatic CRC screening by building a meta-analytic procedural model that considers time delays in the access to colonoscopy and estimates the effect on mortality because of the consequent up-stage migration of patients over time.
Methods
Study Design
We followed a stepwise rationale in building the procedural model, which was based on the following relationships: (1) the stage at the time of diagnosis affects CRC mortality; and (2) the stage at diagnosis largely depends on an early detection of malignant and premalignant lesions, which is strictly related to and improved by timeliness of screening programs. Considering the delay in the screening procedures imposed by SARS-CoV-2 pandemic, we explored the relationships of delay stage (DS) and stage mortality (SM) under the constraints of different delay spans, with the aim of providing an estimate of the effect of pandemic on CRC mortality. The evaluation of the 2 relationships DS and SM was based on literature evidence that was obtained by performing 2 separate meta-analyses.
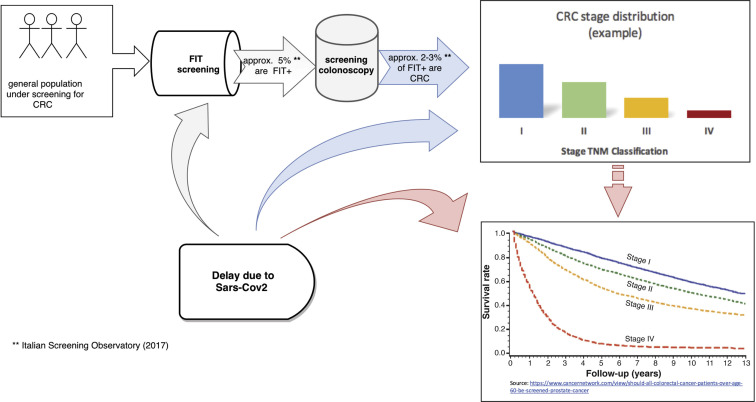
Eventually the chain effect of the screening delay due to Sars-Cov-2 pandemic was quantified in terms of the number of deaths expected to occur in a general, asymptomatic population that would have otherwise adhered to and undergone programmatic screening (Figure 1 ).
Figure 1.
Illustrative description of the rationale: SARS-Cov-2 effects on screening programs and consequently on the CRC stage distribution and survival rates. CRC, colorectal cancer; FIT, fecal immunochemical test; SARS-Cov-2, severe acute respiratory distress syndrome–associated coronavirus 2.
Search Strategy and Study Selection
To pursue the main aim of the study, we conducted a systematic literature search through the PubMed and Scopus databases in a 2-step process. For the first literature review on DS, we built a pipeline of articles providing the distribution of CRC stage by delay strata (outcome of interest: percentage/proportion of CRC by stage and delay), identified by the time for access to colonoscopy after a positive FIT. For the second step concerning SM, the outcome of interest was the 5-year (age-adjusted) survival rates stratified by stage of CRC patients. Specifically, we applied the following search strategies:
-
(1)
for the DS meta-analysis, the search string was “(delay OR time to colonoscopy) AND (fecal immunochemical test OR screening) AND (colon OR colorectal AND cancer) AND (stage OR tnm)”;
-
(2)
for the SM meta-analysis, the string was “(colon cancer OR colorectal cancer) AND (stage OR tnm) AND (mortality OR survival)”.
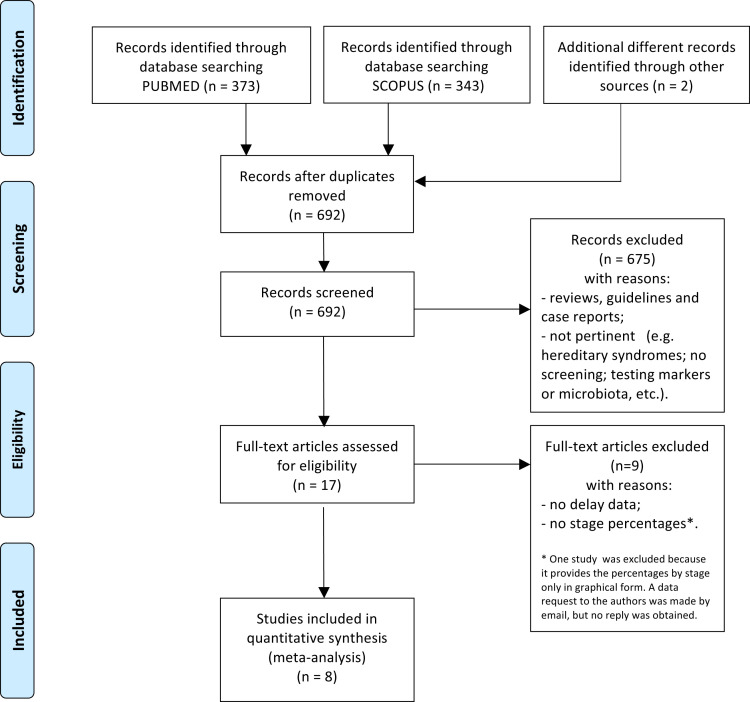
In addition, the following filters were applied for both strings and databases: Journal article; Publication date from January 1, 2010 to May 19, 2020; Humans; English. The “sort by: Best Match Filters” approach was used for all the PubMed queries. Moreover, for the second string, the search was limited to [Title/Abstract] fields in both databases and limited to Subject area “Medicine” and “Multidisciplinarity” in Scopus database. We performed these systematic literature reviews according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and flow diagrams.9 Records were first screened by checking titles and abstracts; subsequently, relevant eligible articles were assessed by reading the full texts. For both the DS and SM meta-analyses, 2 of the authors (LR, LL) carried out the search independently. Any disagreements were discussed and resolved with the involvement of a third researcher. All the screened articles and corresponding decisions were recorded in an Excel (Microsoft Corp, Redmond, WA) spreadsheet. The detailed search sequences are shown in Supplementary Figures 1 and 2 (PROSPERO registration number CRD42020186832).
Supplementary Figure 1.
Flowchart for the delay stage meta-analysis.
Supplementary Figure 2.
Flowchart for the stage mortality meta-analysis.
Data Extraction and Assessment of the Quality of the Included Studies
From the DS search we selected 8 articles (Supplementary Figure 1)10, 11, 12, 13, 14, 15, 16, 17 and extracted the following variables (Supplementary Table 1): year of publication, country, delay (in months), stage, number of CRC cases by stage and delay, and total number of CRC cases.
Because delay categories were not homogeneous across the included studies (with some articles using monthly delay intervals and others adopting longer time lags), we adopted 4 categories for delay times (ie, 0–3 months, 4–6 months, 7–12 months, and >12 months) because these intervals covered most waiting times across the selected studies. The studies also differed as to the reported stage categories; some used TNM classification (ie, stage I, II, III, IV), and others reported pooled stages (ie, “advanced” for stages III and IV). To allow comprehensive data capture for the analysis, we adopted the early vs advanced stage classification by aggregating data of stages I and II (early stage) and data of stages III and IV (advanced stage).
For the SM search, 10 articles were selected (Supplementary Figure 2),18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and the following variables were extracted (Supplementary Table 2): year of publication, country, stage, 5-year age-standardized survival rate by stage, and total number of CRC cases. Also, for this analysis we adopted the stage classification of early vs advanced tumors.
The Newcastle-Ottawa Scale for cohort studies (score range 0–9) was used to assess the quality of the included studies.28 Studies with score greater than 5 were considered of high quality (Supplementary Table 3).
Statistical Analysis
We used the data gathered from the DS literature search to calculate the meta-analytic pooled estimate of the proportion of CRC cases by stage that are detected at programmatic screening. Considering the SM relationship, the same approach was adopted to obtain a pooled estimate of the 5-year survival/mortality rate, stratified by stage. For all the estimates, 95% confidence intervals were computed. Because of the heterogeneity in patient populations and methods of measurement of stage and survival among selected articles, we applied a random-effects meta-analysis. To evaluate homogeneity of findings among studies, the I2 index (defined as the percentage of total variability due to the heterogeneity across studies) was computed. Proportions/percentages were compared through test for binomial proportions.
To assess the burden on mortality caused by delayed screening procedures because of the SARS-CoV-2 pandemic, we applied the pooled estimates obtained from the DS-SM meta-analyses to the annual expected number of CRC cases in Italy. To obtain the latter, we first computed the screening program target population (ie, the population invited to undergo FIT) by using the Italian population data (ISTAT; data available on http://demo.istat.it/pop2019/index.html; last available data referring to January 1, 2019) stratified by age classes (50–69 years). Finally, using the data from the National Screening Observatory (https://www.osservatorionazionalescreening.it/content/lo-screening-colorettale; Report 2018, containing data referring to 2017, was the most updated information at the time of this writing) regarding the percentage of invited persons who performed the test, the percentage of positive screening tests, and the CRC detection rate, we estimated the annual numbers of participants, of positive screening tests, and of incident CRC cases.
Statistical analyses were performed by using R: a Language and Environment for Statistical Computing, version 3.6.3 (R Core Team, 2019; R Foundation for Statistical Computing) and its metafor package.29 The level of statistical significance was set at P < .05.
Results
Pooled Estimates From the Delay Stage and Stage Mortality Meta-analyses
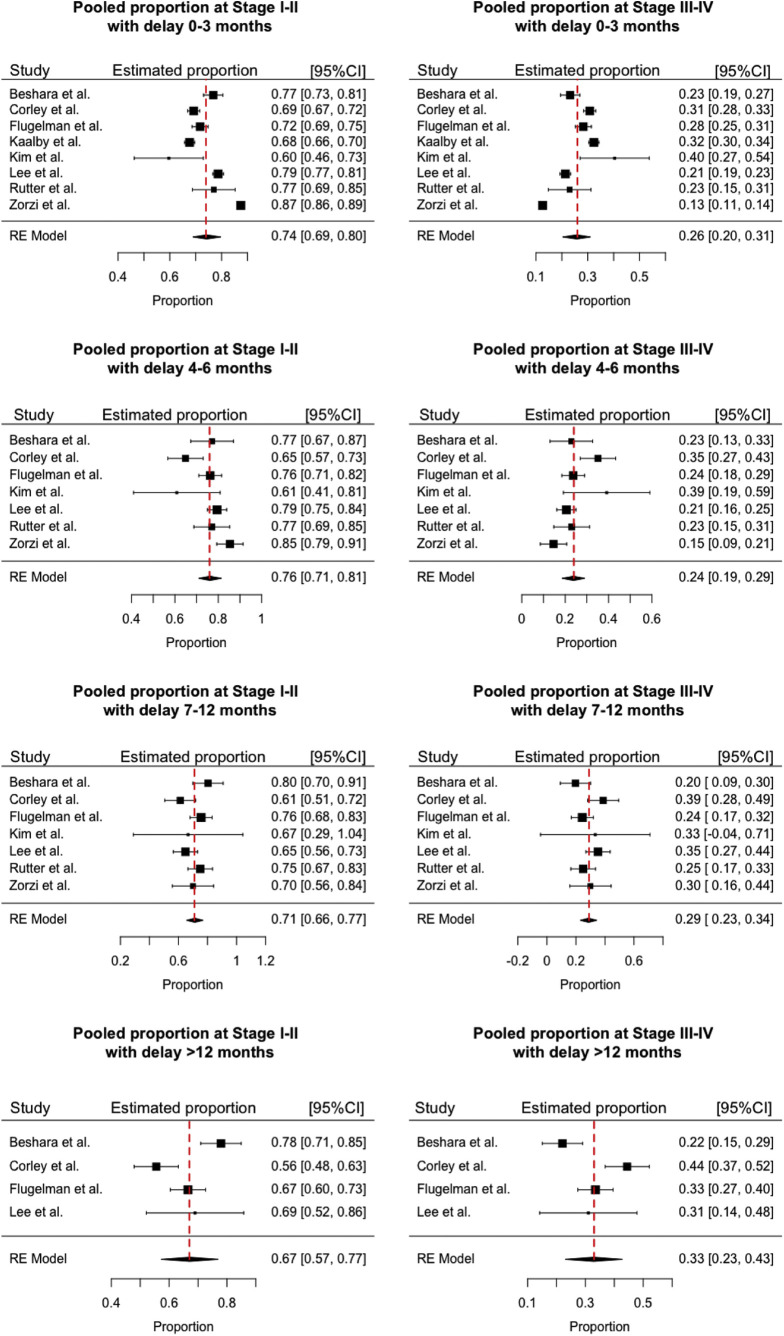
The pooled estimates of stage distribution of screen-detected CRC according to pre–SARS-CoV-2 era data are reported in Supplementary Figure 3. With a brief, already present delay of 0–3 months, 74% of the CRC is expected to be stage I–II, and a delay of 4–6 months will lead to a nonsignificant increase in stage I–II prevalence (from 74% to 76%) and to a concomitant decrease of stage III–IV (from 26% to 24%) prevalence (P = .068) (Table 1 ). Conversely, compared with the reference 0–3 months, our analysis estimated a significant increase in advanced cancers detected at screening for a moderate 7- to 12-month delay (from 26% to 29%; P = .008), which progressively worsened after a 12-month delay (up to 33%; P < .001). These results indicate a shift in the distribution of screen-detected CRC by stage at diagnosis, marked by a significant rise in the proportion of advanced cases with a delay beyond 6 months.
Supplementary Figure 3.
Proportion of colorectal cancer by stage (I–II and III–IV) at different delays (0–3 months, 4–6 months, 7–12 months, >12 months). Pooled estimates by delay stage meta-analysis. I2 index: 97% (0–3 months), 72.4% (4–6 months), 48.2% (7–12 months), 82.6% (>12 months). CI, confidence interval.
Table 1.
Prevalence (and Corresponding Expected Number of CRCs) of Early and Advanced Stages for CRCs Detected at Delayed Screening, According to Increasing Time Delays to Access to Colonoscopy (Estimates by DS Meta-analysis)
| Diagnostic delay (mo) | Stage at diagnosis | Stage prevalence | 95% Confidence intervala | Expected CRCsb | P valuec |
|---|---|---|---|---|---|
| 0–3 | I–II | 0.74 | (0.69–0.80) | 2356 | Reference |
| III–IV | 0.26 | (0.20–0.31) | 828 | ||
| 4–6 | I–II | 0.76 | (0.71–0.81) | 2420 | .068 |
| III–IV | 0.24 | (0.19–0.29) | 764 | ||
| 7–12 | I–II | 0.71 | (0.66–0.77) | 2261 | .008 |
| III–IV | 0.29 | (0.23–0.34) | 923 | ||
| >12 | I–II | 0.67 | (0.57–0.77) | 2133 | <.001 |
| III–IV | 0.33 | (0.23–0.43) | 1051 |
CRC, colorectal cancer; DS, delayed stage.
Lower and upper limit of 95% confidence interval.
Total number of cases is always equal to 3184 for each delay scenario.
P values refer to comparison of binomial proportions by stage of expected number of CRCs at 0–3 months vs higher delays on total number of CRC cases (3184), eg, .068 is the P value of the hypothesis test for comparing 2420/3184 vs 2356/3184.
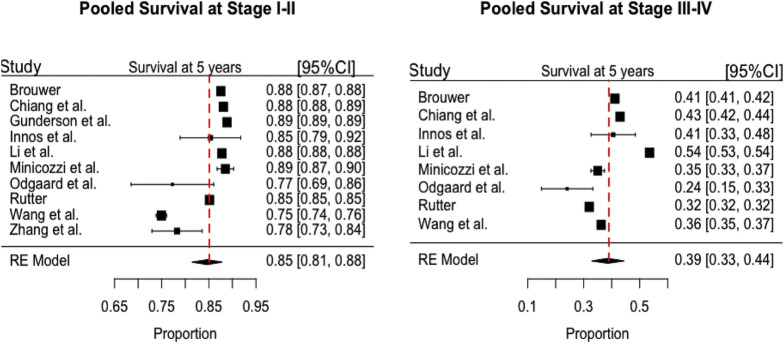
Next, we evaluated the survival of subjects supposed to undergo programmatic screening according to pooled survival rates (at 5 years) estimated by SM meta-analysis (Supplementary Figure 4, Table 2 ). This analysis revealed a survival rate of 0.85 (95% confidence interval, 0.81–0.88) for stage I–II diagnoses and 0.39 (95% confidence interval, 0.33–0.44) for stage III–IV.
Supplementary Figure 4.
Survival rates at 5 years by stage (I–II and III–IV). Pooled estimates by stage mortality meta-analysis. I2 index: 99% (stage I–II), 99% (stage III–IV). CI, confidence interval.
Table 2.
Five-Year Survival Rates of Patients With Colorectal Cancer Detected at Screening by the Stage at Diagnosis
| Stage at diagnosis | Survival rate at 5 y | 95% Confidence intervala |
|---|---|---|
| I–II | 0.85 | (0.81–0.88) |
| III–IV | 0.39 | (0.33–0.44) |
NOTE. Pooled estimates by stage mortality meta-analysis.
Lower and upper limits of the 95% confidence interval.
Estimate of Screening Participants and Number of Colorectal Cancers in Italy: An Illustrative Example of the Delay Effect on Stage and Mortality
To quantify the expected number of deaths resulting from the funnel effect on the otherwise ongoing screening, we focused on the Italian population and determined the number of individuals targeted by the screening program (in the 50- to 69-year old group equaling 834,4721 individuals). We estimated a total of 6,439,047 invitations for screening according to Vicentini et al.30 Furthermore, on the basis of 2017 data from the most recent National Screening Observatory report, we estimated 3184 CRC cases for the whole year, corresponding to 2.28% subjects with a positive FIT (Supplementary Table 4). The number of CRC cases was used to quantify the expected number of deaths.
By applying the proportions of the estimates in the pre–SARS-CoV-2 era (Table 1) to 3184 incident CRC cases, we derived the expected number of cases, stratified by stage occurring in the scenario overrun by delays. These estimates, combined with the mortality rates (derived from Table 2), allowed calculating the expected number of deaths at 5 years for each delay-stage scenario, which are reported in Table 3 . A significant increase in the total number of deaths (+12%) was estimated when moving from a 0- to 3-month (reference delay) to a >12-month delay (P = .005). We did not detect significant changes in the number of deaths with respect to baseline for delays <12 months.
Table 3.
Expected Number of Deaths at 5 Years for Colorectal Cancer Detected at Delayed Screening According to Diagnostic Delays and Stage at Diagnosis
| Diagnostic delay (mo) | Stage at diagnosis | Expected deathsa | Relative change (%) | P valueb | All stages |
||
|---|---|---|---|---|---|---|---|
| Expected deathsa | Relative change (%) | P valueb | |||||
| 0–3 | I–II | 353 | Reference | — | 858 | Reference | |
| III–IV | 505 | ||||||
| 4–6 | I–II | 363 | 2.8 | .294 | 829 | –3.4 | .427 |
| III–IV | 466 | –7.7 | |||||
| 7–12 | I–II | 339 | –4.0 | .139 | 902 | 5.1 | .228 |
| III–IV | 563 | 11.5 | |||||
| >12 | I–II | 320 | –9.3 | <.001 | 961 | 12.0 | .005 |
| III–IV | 641 | 26.9 | |||||
For example, 353 is given by 2356 (Table 1) multiplied by mortality rate (1–0.85) derived from Table 2. Sum of 353 and 505 (equal to 858) represents the expected total number of deaths at 0–3 months in the target population of 3184 colorectal cancer cases.
P values refer to comparison of proportions by stage of expected number of deaths at 0–3 months vs higher delays on total number of deaths, eg, 0.294 is the P value of the hypothesis test for comparing 363/829 vs 353/858. Test for binomial proportions is also used for comparing the proportion of deaths with respect to total number of colorectal cancer cases (3184), eg, 0.427 is the P value of the comparison of 829/3184 vs 858/3184.
Furthermore, we computed the relative percentage changes in expected deaths by stage (Table 3). At 4–6 months, we observed 2.8% increase in mortality for stage I–II cancers and 7.7% decrease for stage III–IV. These changes correspond to 2% shift in the early vs advanced stage distribution reported in Table 1. However, mortality by stage at 4–6 months as well as at 7–12 months was not significantly different from the baseline scenario (P = .294 and P = .139, respectively). Conversely, we observed a significant change by comparing the baseline with the >12-month mortality distribution by stage (P < .001).
Discussion
We estimated the effects of the lockdown because of the SARS-CoV-2 pandemic on programmatic CRC screening and their impact on disease burden by a rigorous, meta-analytical model. Our analysis indicates that delays up to 4–6 months do not significantly reduce the performance of screening, whereas a lockdown sustained for longer time frames would negatively affect mortality rates. This should be ascribed to the progressive shift of screen-detected cancers toward advanced stages, a change becoming significant in the 7- to 12-month delay interval. In usual circumstances, only a minor fraction of FIT-positive subjects would receive a delayed colonoscopy (ranging from 0.8% to 6.2% after 6 months).11 , 17 The effects of the pandemic may modify previously reassuring estimates in a time-dependent way, because a fraction of neoplastic lesions detected by FIT may progress from months 6 to 12,17 increasing overall CRC deaths by more than one tenth, including an excess of 1 out of 4 advanced cases.
At 6 months, the model envisioned a nonsignificant, transitory additional 2% diagnostic rate of stage I–II tumors, coupled with 2% reduction in stage III–IV tumors, in keeping with data used in the DS meta-analysis,11 , 17 in which some studies reported reduced risks of detecting advanced stages. We do not have a direct explanation for these changes, which might be interpreted as “waiting time paradox” at the first delay interval. Of interest, diagnostic delays up to 6 months have been associated with earlier stages across symptomatic patients, in keeping with a similar paradox31 , 32 that needs to be investigated in further research. Our analysis indicates that the backlog sustained beyond 6 months would unequivocally ensue in a significant excess of advanced stages detected through screening and thus in up-staging rather than down-staging. Although such an increase of advanced stages in the 7- to 12-month interval of delay will not affect the mortality rate at 5 years, it will nevertheless increase disease burden and human and economic costs.
The results presented in this study were obtained by applying the meta-analytic estimates to the Italian screened population. However, the figures obtained from the DS-MS meta-analyses could be easily applied to other CRC target screening populations. Moreover, the proposed procedural model could be recommended also for other cancers amenable to screening programs.
The main limitation of our study, which is based on meta-analytic results rather than on direct data, is the small number of primary studies included in the 2 meta-analyses. Although this could affect the robustness of the findings, the adopted random-effect meta-analysis models mitigate this drawback. Importantly, our meta-analytic approach represents a strength, because it generated a prompt quantification of the results of the delay on the outcome of CRC screening that may help planning timely public health decisions.
In addition, it is worth noting that our quantification on the Italian screening population should be considered as an illustrative example of the consequence of the screening delay on CRC stage shift and mortality. Once the observed data on CRC stage and mortality are gathered after the pandemic, it will be very informative to make a comparison with our results.
Finally, from the studies included in our meta-analysis, we could not unequivocally extrapolate the reasons explaining the delays in colonoscopy after a positive FIT test. Only Flugelman et al12 reviewing the data from the 304 patients (17% of the cohort) who deferred follow-up beyond 1 year found that the main reason (in 90% of them) was the lack of adherence to positive fecal test follow-up guidelines.
The appearance of an urgent, unexpected, and unmet medical need is bringing collateral, unestimated impact on fields subject to constant improvement in the last decades. Steering resources to counteract the SARS-CoV-2 pandemic creates a bottleneck in other medical areas, queuing scheduled procedures and second level exams. In Italy, programmatic screenings have been suspended since mid-March according to government transitory regulation plan. Concerns raised by institutional players did not result in an objective evaluation of the situation ahead.
In the United States, the Centers for Medicare and Medicaid Services issued guidance that colonoscopies for CRC screening had to be delayed from mid-March, raising the concern that delay of CRC screening for 23 million U.S. adults would lead to a delayed diagnosis and increased mortality.33 The need to resume key healthcare activities, including oncologic screening programs, is not counterbalanced by strategic plans considering the backlog. Indeed, in Italy, these stops will break a chain of about 6,000,000 yearly invitations for FIT, so that just a 2-month delay accounts for the lack of approximately 1,000,000 notices, with a parallel decrease in the 50% rate of pick-up, and a consequent loss of the estimated 5% positive tests among responders. Unfortunately, because of the highly unlikely scenario of full resumption of screening activities in the short-term, we could envision that such backlog would lead to a long-lasting carryover with significant negative consequences on the epidemiology of the disease.
The incoming challenge is to look forward to the demand for medical activities in the post–SARS-CoV-2 era, not only with respect to acute disorders but also for chronic and multifactorial diseases such as cancer, for which preventive attitudes and screening programs play a major role in reducing disease burden and mortality. In this perspective, designing new, proactive plans requires the proper evaluation of the scenarios ahead to counteract stops and delays imposed by the SARS-Cov-2 pandemic. This is of outmost importance in the view of possible future waves of SARS-CoV-2 infections.34
In conclusion, our study shows that CRC screening delays beyond 6 months would result in a significantly higher number of more advanced CRC cases; correspondingly, delays beyond 12 months would increase disease mortality. Thus, we believe that alternative strategies should envision future lockdowns and social distancing, rethinking the paths of distribution and analysis of the tests, and the possibility of managing screening-only, SARS-CoV-2–free, dedicated facilities.
CRediT Authorship Contributions
Luigi Ricciardiello, MD (Conceptualization: Lead; Data curation: Equal; Funding acquisition: Lead; Supervision: Lead; Writing – original draft: Lead)
Clarissa Ferrari, PhD (Data curation: Lead; Formal analysis: Lead; Methodology: Lead)
Michela Cameletti, PhD (Data curation: Lead; Formal analysis: Lead; Methodology: Lead)
Federica Gaiani, MD (Data curation: Equal)
Francesco Buttitta, MD (Data curation: Equal)
Franco Bazzoli, MD (Writing – review & editing: Equal)
Gian Luigi De’ Angelis, MD (Writing – review & editing: Equal)
Alberto Malesci, MD (Writing – review & editing: Equal)
Luigi Laghi, MD, PhD (Conceptualization: Lead; Data curation: Equal; Funding acquisition: Lead; Supervision: Lead; Writing – original draft: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by The AIRC Foundation for Cancer Research in Italy (AIRC) under investigator Grant N. 21723 (to LR) and Investigator Grant N. 22234 (to LL).
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.09.008.
Supplementary Material
Supplementary Table 1.
Articles Included in the Delay-Stage Meta-analysis
| Article | Year | Country | Age (y) | Delay (mo) | Stage | n CRC | n total CRC |
|---|---|---|---|---|---|---|---|
| Beshara et al | 2019 | Israel | 50–74 | 0–3 | 1–2 | 377 | 753 |
| Beshara et al | 2019 | Israel | 50–74 | 0–3 | 3- | 114 | |
| Beshara et al | 2019 | Israel | 50–74 | 4–6 | 1–2 | 54 | |
| Beshara et al | 2019 | Israel | 50–74 | 4–6 | 3–4 | 16 | |
| Beshara et al | 2019 | Israel | 50–74 | 7–12 | 1–2 | 45 | |
| Beshara et al | 2019 | Israel | 50–74 | 7–12 | 3–4 | 11 | |
| Beshara et al | 2019 | Israel | 50–74 | >12 | 1–2 | 106 | |
| Beshara et al | 2019 | Israel | 50–74 | >12 | 3–4 | 30 | |
| Corley et al | 2017 | USA | 50–70 | 0–3 | 1–2 | 1017 | 1834 |
| Corley et al | 2017 | USA | 50–70 | 0–3 | 3–4 | 452 | |
| Corley et al | 2017 | USA | 50–70 | 4–6 | 1–2 | 85 | |
| Corley et al | 2017 | USA | 50–70 | 4–6 | 3–4 | 46 | |
| Corley et al | 2017 | USA | 50–70 | 7–12 | 1–2 | 50 | |
| Corley et al | 2017 | USA | 50–70 | 7–12 | 3–4 | 31 | |
| Corley et al | 2017 | USA | 50–70 | >12 | 1–2 | 81 | |
| Corley et al | 2017 | USA | 50–70 | >12 | 3–4 | 72 | |
| Flugelman et al | 2019 | Israel | 50+ | 0–3 | 1–2 | 583 | 1419 |
| Flugelman et al | 2019 | Israel | 50+ | 0–3 | 3–4 | 230 | |
| Flugelman et al | 2019 | Israel | 50+ | 4–6 | 1–2 | 193 | |
| Flugelman et al | 2019 | Israel | 50+ | 4–6 | 3–4 | 60 | |
| Flugelman et al | 2019 | Israel | 50+ | 7–12 | 1–2 | 93 | |
| Flugelman et al | 2019 | Israel | 50+ | 7–12 | 3–4 | 30 | |
| Flugelman et al | 2019 | Israel | 50+ | >12 | 1–2 | 153 | |
| Flugelman et al | 2019 | Israel | 50+ | >12 | 3–4 | 77 | |
| Kaalby et al | 2019 | Denmark | 50+ | 0–3 | 1–2 | 1498 | 3639 |
| Kaalby et al | 2019 | Denmark | 50+ | 0–3 | 3–4 | 716 | |
| Kaalby et al | 2019 | Denmark | 50+ | NA | 1–2 | 1099 | |
| Kaalby et al | 2019 | Denmark | 50+ | NA | 3–4 | 326 | |
| Kim et al | 2019 | Korea | 50+ | 0–3 | 1–2 | 31 | 81 |
| Kim et al | 2019 | Korea | 50+ | 0–3 | 3–4 | 21 | |
| Kim et al | 2019 | Korea | 50+ | 4–6 | 1–2 | 14 | |
| Kim et al | 2019 | Korea | 50+ | 4–6 | 3–4 | 9 | |
| Kim et al | 2019 | Korea | 50+ | 7–12 | 1–2 | 4 | |
| Kim et al | 2019 | Korea | 50+ | 7–12 | 3–4 | 2 | |
| Lee et al | 2019 | Taiwan | 50–69 | 0–3 | 1–2 | 1202 | 2003 |
| Lee et al | 2019 | Taiwan | 50–69 | 0–3 | 3–4 | 326 | |
| Lee et al | 2019 | Taiwan | 50–69 | 4–6 | 1–2 | 255 | |
| Lee et al | 2019 | Taiwan | 50–69 | 4–6 | 3–4 | 66 | |
| Lee et al | 2019 | Taiwan | 50–69 | 7–12 | 1–2 | 81 | |
| Lee et al | 2019 | Taiwan | 50–69 | 7–12 | 3–4 | 44 | |
| Lee et al | 2019 | Taiwan | 50–69 | >12 | 1–2 | 20 | |
| Lee et al | 2019 | Taiwan | 50–69 | >12 | 3–4 | 9 | |
| Rutter et ala | 2018 | USA | 50–75 | 0–3 | 1–2 | 77 | 300 |
| Rutter et ala | 2018 | USA | 50–75 | 0–3 | 3–4 | 23 | |
| Rutter et ala | 2018 | USA | 50–75 | 4–6 | 1–2 | 77 | |
| Rutter et ala | 2018 | USA | 50–75 | 4–6 | 3–4 | 23 | |
| Rutter et ala | 2018 | USA | 50–75 | 7–12 | 1–2 | 75 | |
| Rutter et ala | 2018 | USA | 50–75 | 7–12 | 3–4 | 25 | |
| Zorzi et al | 2020 | Italy | 50–69 | 0–3 | 1–2 | 2457 | 2981 |
| Zorzi et al | 2020 | Italy | 50–69 | 0–3 | 3–4 | 354 | |
| Zorzi et al | 2020 | Italy | 50–69 | 4–6 | 1–2 | 111 | |
| Zorzi et al | 2020 | Italy | 50–69 | 4–6 | 3–4 | 19 | |
| Zorzi et al | 2020 | Italy | 50–69 | 7–12 | 1–2 | 28 | |
| Zorzi et al | 2020 | Italy | 50–69 | 7–12 | 3–4 | 12 |
NOTE. n CRC represents the number of CRC cases for each delay and stage; n total CRC is the total number of CRC cases.
Data from the MIcrosimulation SCcreening ANalysis-ColoRectal Cancer (MISCAN-colon) microsimulation model were used.
Supplementary Table 2.
Articles Included in the Stage-Mortality Meta-analysis
| Article | Year | Country | Age (y) | Stage | n SURV | n CRC |
|---|---|---|---|---|---|---|
| Brouwer et al | 2018 | Netherlands | 0+ | 1–2 | 28,714 | 32,802 |
| Brouwer et al | 2018 | Netherlands | 0+ | 3–4 | 13,426 | 32,633 |
| Chiang et al | 2016 | Taiwan | 15+ | 1–2 | 13,465 | 15,286 |
| Chiang et al | 2016 | Taiwan | 15+ | 3–4 | 8001 | 18,613 |
| Gunderson et al | 2010 | USA | 0+ | 1–2 | 64,258 | 72,307 |
| Gunderson et al | 2010 | USA | 0+ | 3–4 | NA | NA |
| Innos et al | 2018 | Estonia | 15+ | 1–2 | 99 | 116 |
| Innos et al | 2018 | Estonia | 15+ | 3–4 | 60 | 148 |
| Li et al | 2018 | China | 0+ | 1–2 | 117,167 | 133,483 |
| Li et al | 2018 | China | 0+ | 3–4 | 55,199 | 103,135 |
| Minicozzi et al | 2013 | Italy | 15+ | 1–2 | 1124 | 1270 |
| Minicozzi et al | 2013 | Italy | 15+ | 3–4 | 520 | 1485 |
| Odgaard et al | 2018 | Greenland | 28–92 | 1–2 | 68 | 88 |
| Odgaard et al | 2018 | Greenland | 28–92 | 3–4 | 20 | 83 |
| Rutter et al | 2013 | USA | 20+ | 1–2 | 103,971 | 122,114 |
| Rutter et al | 2013 | USA | 20+ | 3–4 | 35,752 | 111,764 |
| Wang et al | 2019 | USA | 3–129 | 1–2 | 4418 | 5895 |
| Wang et al | 2019 | USA | 3–129 | 3–4 | 2492 | 6875 |
| Zhang et al | 2014 | China | 30–93 | 1–2 | 180 | 230 |
| Zhang et al | 2014 | China | 30–93 | 3–4 | NA | NA |
NOTE. n SURV and n CRC represent the number of people who survived and of colorectal cancer cases for each stage, respectively.
Supplementary Table 3.
Quality Index for the Studies Included in the DS and SM Meta-analysis Computed by using the Newcastle-Ottawa Scale
| Newcastle-Ottawa scale domains |
Total | |||
|---|---|---|---|---|
| Selection | Comparability | Outcome | ||
| DS meta-analysis | ||||
| Beshara et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Corley et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Flugelman et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Kaalby et al | ||||
| Items | 1A, 2C, 3A, 4A | 1AB | 1B, 2B, 3A | |
| Point | 3 | 2 | 2 | 7 |
| Kim et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 2 | 2 | 3 | 7 |
| Lee et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Rutter et al | ||||
| Items | 1C, 2C, 3A, 4A | 1B, 2A, 3A | ||
| Point | 2 | 0 | 3 | 5 |
| Zorzi et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| SM meta-analysis | ||||
| Brouwer et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Chiang et al | ||||
| Items | 1B, 2A, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 4 | 2 | 3 | 9 |
| Gunderson et al | ||||
| Items | 1C, 2C, 3A, 4A | 1A | 1B,2A,3C | |
| Point | 2 | 1 | 2 | 5 |
| Innos et al | ||||
| Items | 1C, 2C, 3A, 4A | 1A | 1B, 2A, 3A | |
| Point | 2 | 1 | 3 | 6 |
| Li et al | ||||
| Items | 1A, 2A, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 4 | 2 | 3 | 9 |
| Minicozzi et al | ||||
| Items | 1B, 2C, 3A, 4A | 1A | 1B, 2A, 3A | |
| Point | 3 | 1 | 3 | 7 |
| Odgaard et al | ||||
| Items | 1A, 2C, 3A, 4A | 1A | 1B, 2A, 3A | |
| Point | 3 | 1 | 3 | 7 |
| Rutter et al | ||||
| Items | 1A, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
| Wang et al | ||||
| Items | 1A, 2A, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 4 | 2 | 3 | 9 |
| Zhang et al | ||||
| Items | 1B, 2C, 3A, 4A | 1AB | 1B, 2A, 3A | |
| Point | 3 | 2 | 3 | 8 |
NOTE. Scale range is 0–9.
DS, delay stage; SM, stage mortality.
Supplementary Table 4.
Details Regarding Computation of the Screening Target Population and Expected Number of CRC Cases in Italy
| Italy macro-region | 50- to 69-year-old Italian total population | Target population | N invitations | N participants | N FIT+ | CRC |
|---|---|---|---|---|---|---|
| North | 7,727,209 | 3,863,605 | 3,670,424 | 1,908,621 | 89,705 | 1909 |
| Center | 3,342,758 | 1,671,379 | 1,504,241 | 526,484 | 27,904 | 790 |
| South-Islands | 5,619,474 | 2,809,737 | 1,264,382 | 303,452 | 21,849 | 486 |
| Total | 16,689,441 | 8,344,721 | 6,439,047 | 2,738,557 | 139,457 | 3184 |
NOTE. The Italian population data stratified by age are retrieved from the Italian National Statistics Institute (ISTAT) website (http://demo.istat.it/pop2019/index.html; last available data referring to January 1, 2019). In particular, we consider 3 macro-regions (North, Center, and South-Islands) and the 50 to 69 age class that is the target population of the screening program. Considering that the screening program is biennial, we compute the screening target population by halving the 50 to 69 age total population. As reported in Vicentini et al,30 there are differences in the Italian macro-regions in covering the target population (ie, sending invitations for screening), with 95% coverage in the North, 90% in the Center, and 45% in the South-Islands. Because of these percentages, we estimate a total of 6,439,047 sent invitations. The last report of the National Screening Observatory (https://www.osservatorionazionalescreening.it/content/lo-screening-colorettale; last available data referring to year 2017) provides information about the percentage of invited population that performed the FIT test (52% in the North, 35% in the Center, and 24% in the South-Islands) and the percentage of positive tests (4.7% in the North, 5.3% in the Center, and 7.2% in the South-Islands). We thus obtain a total of 2,738,557 participants and 139,457 positive tests. Moreover, because of a CRC detection rate (provided by the National Screening Observatory) equal to 1% (North), 1.5% (Center), and 1.6% (South-Islands), we estimate to have 3184 CRC cases in the whole year, corresponding to 2.28% of the estimated number of FIT+.
CRC, colorectal cancer; FIT, fecal immunochemical test.
References
- 1.Hick J.L., Biddinger P.D. Novel coronavirus and old lessons: preparing the health system for the pandemic. N Engl J Med. 2020;382:e55. doi: 10.1056/NEJMp2005118. [DOI] [PubMed] [Google Scholar]
- 2.Gagliano A., Villani P.G., Cò F.M. COVID-19 epidemic in middle province of northern Italy: impact, logistics, and strategy in the first line hospital. Disaster Med Public Health Prep. 2020;14:372–376. doi: 10.1017/dmp.2020.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Pan J., Xin L., Ma Y.F. Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am J Gastroenterol. 2016;111:355–365. doi: 10.1038/ajg.2015.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senore C., Basu P., Anttila A. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2019;68:1232–1244. doi: 10.1136/gutjnl-2018-317293. [DOI] [PubMed] [Google Scholar]
- 6.Zorzi M., Dal Maso L., Francisci S. Trends of colorectal cancer incidence and mortality rates from 2003 to 2014 in Italy. Tumori. 2019;105:417–426. doi: 10.1177/0300891619838336. [DOI] [PubMed] [Google Scholar]
- 7.Lazzerini M., Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8:e641–e642. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sud A., Jones M., Broggio J. Quantifying and mitigating the impact of the COVID-19 pandemic on outcomes in colorectal cancer. medRxiv. 2020 [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336. [PMC free article] [PubMed] [Google Scholar]
- 10.Beshara A., Ahoroni M., Comanester D. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer. 2020;146:1532–1540. doi: 10.1002/ijc.32497. [DOI] [PubMed] [Google Scholar]
- 11.Corley D.A., Jensen C.D., Quinn V.P. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317:1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flugelman A.A., Stein N., Segol O. Delayed colonoscopy following a positive fecal test result and cancer mortality. JNCI Cancer Spectr. 2019;3:pkz024. doi: 10.1093/jncics/pkz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaalby L., Rasmussen M., Zimmermann-Nielsen E. Time to colonoscopy, cancer probability, and precursor lesions in the danish colorectal cancer screening program. Clin Epidemiol. 2019;11:659–667. doi: 10.2147/CLEP.S206873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim N.H., Lim J.W., Kim S. Association of time to colonoscopy after a positive fecal test result and fecal hemoglobin concentration with risk of advanced colorectal neoplasia. Dig Liver Dis. 2019;51:589–594. doi: 10.1016/j.dld.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y.C., Fann J.C.Y., Chiang T.H. Time to colonoscopy and risk of colorectal cancer in patients with positive results from fecal immunochemical tests. Clin Gastroenterol Hepatol. 2019;17:1332–1340.e3. doi: 10.1016/j.cgh.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Rutter C.M., Kim J.J., Meester R.G.S. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol Biomarkers Prev. 2018;27:158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zorzi M., Hassan C., Capodaglio G. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy. 2020;52:871–876. doi: 10.1055/a-1159-0644. [DOI] [PubMed] [Google Scholar]
- 18.Brouwer N.P.M., Bos A.C.R.K., Lemmens V.E.P.P. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758–2766. doi: 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang C.J., Lo W.C., Yang Y.W. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc. 2016;115:1076–1088. doi: 10.1016/j.jfma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Gunderson L.L., Jessup J.M., Sargent D.J. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Innos K., Reima H., Baburin A. Subsite- and stage-specific colorectal cancer trends in Estonia prior to implementation of screening. Cancer Epidemiol. 2018;52:112–119. doi: 10.1016/j.canep.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Feng Y., Dai W. Prognostic effect of tumor sidedness in colorectal cancer: a SEER-based analysis. Clin Colorectal Cancer. 2019;18:e104–e116. doi: 10.1016/j.clcc.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Minicozzi P., Kaleci S., Maffei S. Disease presentation, treatment and survival for Italian colorectal cancer patients: a EUROCARE high resolution study. Eur J Public Health. 2013;24:98–100. doi: 10.1093/eurpub/ckt056. [DOI] [PubMed] [Google Scholar]
- 24.Odgaard M., Lohse N., Petersen A.J. Oncological treatment and outcome of colorectal cancer in Greenland. Int J Circumpolar Health. 2018;77:1546069. doi: 10.1080/22423982.2018.1546069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutter C.M., Johnson E.A., Feuer E.J. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105:1806–1813. doi: 10.1093/jnci/djt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C.B., Shahjehan F., Merchea A. Impact of tumor location and variables associated with overall survival in patients with colorectal cancer: a mayo clinic colon and rectal cancer registry study. Frontiers in Oncology. 2019;9 doi: 10.3389/fonc.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M., Zhao Q.C., Liu Y.P. Prognostic analysis and comparison of colon cancer in Han and Hui patients. World J Gastroenterol. 2014;20:5082–5086. doi: 10.3748/wjg.v20.i17.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells G., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2012. http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from: Accessed May 31, 2020.
- 29.Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 30.Vicentini M., Zorzi M., Bovo E. Impact of screening programme using the faecal immunochemical test on stage of colorectal cancer: results from the IMPATTO study. Int J Cancer. 2019;145:110–121. doi: 10.1002/ijc.32089. [DOI] [PubMed] [Google Scholar]
- 31.Leiva A., Esteva M., Llobera J. Time to diagnosis and stage of symptomatic colorectal cancer determined by three different sources of information: a population based retrospective study. Cancer Epidemiol. 2017;47:48–55. doi: 10.1016/j.canep.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Murchie P., Raja E.A., Brewster D.H. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: effect on disease stage and survival. Br J Cancer. 2014;111:461–469. doi: 10.1038/bjc.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issaka R.B., Somsouk M. Colorectal cancer screening and prevention in the COVID-19 era. JAMA Health Forum. 2020;1 doi: 10.1001/jamahealthforum.2020.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kissler S.M., Tedijanto C., Goldstein E. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]