Abstract
Background
Laparoscopic cholecystectomy is a safe ambulatory procedure in appropriately selected patients; however, day case rates remain low. The objective of this systematic review and meta-analysis was to identify interventions which are effective in reducing the length of stay (LOS) or improving the day case rate for elective laparoscopic cholecystectomy.
Methods
Comparative English-language studies describing perioperative interventions applicable to elective laparoscopic cholecystectomy in adult patients and their impact on LOS or day case rate were included.
Results
Quantitative data were available for meta-analysis from 80 studies of 10,615 patients. There were an additional 17 studies included for systematic review. The included studies evaluated 14 peri-operative interventions. Implementation of a formal day case care pathway was associated with a significantly shorter LOS (MD = 24.9 h, 95% CI, 18.7–31.2, p < 0.001) and an improved day case rate (OR = 3.5; 95% CI, 1.5–8.1, p = 0.005). Use of non-steroidal anti-inflammatories, dexamethasone and prophylactic antibiotics were associated with smaller reductions in LOS.
Conclusion
Care pathway implementation demonstrated a significant impact on LOS and day case rates. A limited effect was noted for smaller independent interventions. In order to achieve optimal day case targets, a greater understanding of the effective elements of a care pathway and local barriers to implementation is required.
Introduction
Laparoscopic cholecystectomy (LC) is the standard of care in the management of symptomatic gallstone disease.1 , 2 Since its introduction, the length of stay (LOS) associated with LC has steadily reduced and it is now widely accepted as an appropriate and safe ambulatory procedure in carefully selected patients.2, 3, 4, 5 Implementation of standard clinical pathways for LC have been reported to successfully reduce LOS and increase day case success.6, 7, 8, 9, 10, 11, 12 Day case laparoscopic cholecystectomy (DCLC) is associated with cost-savings3 , 13 , 14 without increasing the risk of adverse events or readmissions.3 , 15 , 16 It has a high rate of patient satisfaction17 , 18 and is comparable with inpatient stay with respect to quality of life, return to work and normal activity.16 Additionally, in the current climate every effort should be made to reduce the LOS associated with elective surgery in order to minimise potential exposure to COVID-19.
Despite this, a recent study from the United Kingdom (UK) and Ireland report a DCLC rate of only 49%.19 While this has improved significantly from 6.4% in the UK in 2005,20 it remains well below the 75% target, and DCLC rates are highly variable between hospitals and health systems.21 Patient-related factors often pose barriers to universal DCLC implementation. - DCLC is less likely to be successful in older patients, male patients, those with higher ASA scores, previous acute gallstone-related admissions, and preoperative endoscopic intervention19; therefore, proper patient selection is important. The most frequently cited modifiable reasons for failed discharge where DCLC was intended are uncontrolled pain, nausea, drain insertion, urinary retention, late return from theatre, and patient wishes or expectations.3 , 9 , 12 , 14 , 22 , 23 In addition to patient selection criteria, the other necessary components of a DCLC patient pathway, from an institutional and a technical surgical perspective, are not well defined in the literature.
The objective of this meta-analysis was to identify perioperative interventions which reduce the LOS or increase the day case rate associated with elective laparoscopic cholecystectomy in adult patients. A previous systematic review focused on interventions to facilitate ambulatory laparoscopic cholecystectomy,24 however no meta-analysis has been performed on this subject.
Materials and methods
Search strategy
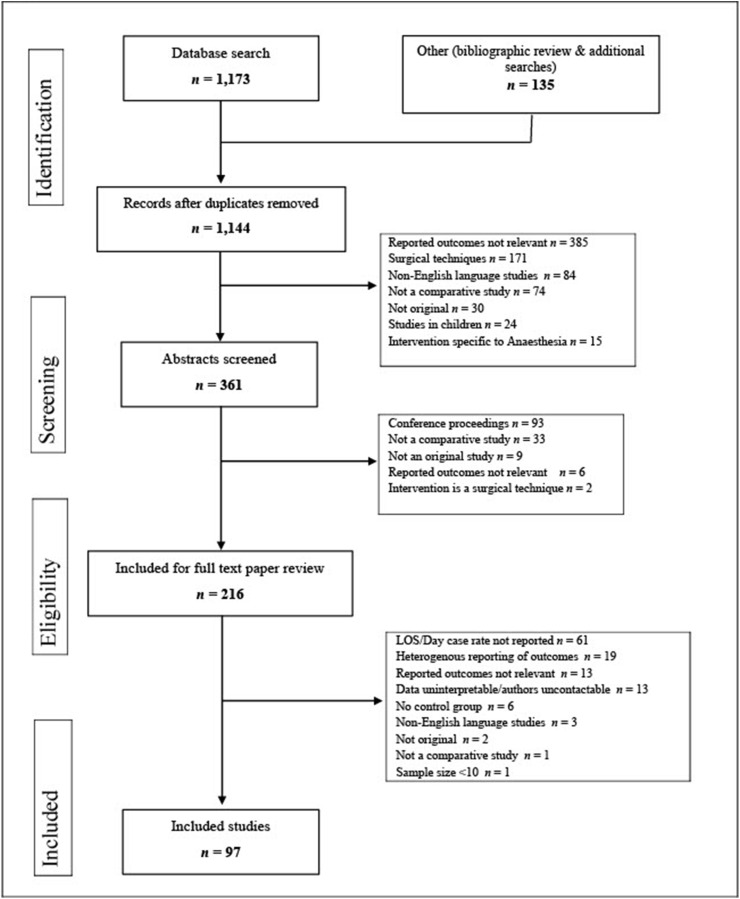
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.25 PubMed, PubMed Central, Medline, Embase, and Cochrane databases were searched using a Boolean search algorithm for articles published up to January 2019. Original comparable studies were included if they examined the effects of any clinical intervention during adult inpatients' trajectories for elective LC (Fig. 1 ). Exclusion criteria were papers where data were unavailable or uninterpretable and authors were uncontactable, and papers in languages other than English. Ethical approval and written consent were not required for this systematic review and meta-analysis.
Figure 1.
Study inclusion and exclusion criteria. ∗Refers to interventions which are delivered specifically by the Anaesthesiologist, e.g. administration of parenteral or intrathecal anaesthesia, pressor management intraoperatively, etc
An initial search of the above databases was used to identify the interventions to be included and subsequent additional searches for each intervention using Boolean algorithms were carried out on Pubmed. All search terms used are available as a Supplementary file (appendix I). The initial search was designed to be as broad as possible to identify interventions published in relation to elective LC. The citations from the initial search were reviewed to create a list of interventions which are relevant to common clinical practice in laparoscopic cholecystectomy and may be modified by surgical teams. The interventions included appear in Fig. 1. Subsequent searches were conducted using Boolean search terms related to each intervention separately. A manual search of reference lists and published review papers were also conducted to ensure optimal identification of relevant publications. All search results were compiled in a reference manager database (Endnote, Version X7, Thompson Reuters, New York, NY). Duplicates were removed automatically and then by hand.
Data extraction
Two independent reviewers (J.R. and E.O'C.) applied the inclusion and exclusion criteria (Fig. 1) to retrieve citations, the abstracts were reviewed, and full-text articles were selected. Reviewers extracted data from the full-text articles and applied exclusion criteria; discrepancies were agreed by consensus. For each study, data on baseline characteristics (journal, year published, country, study period, total number of patients, sex, study methodology, and definition of perioperative intervention) were extracted. Where drug classes were used and grouped together for meta-analysis, the specific dosages and drugs used in each study are available in the Supplementary file.
Authors were contacted if data were not available or interpretable. Where median and range were presented, the methods described by Hozo and colleagues26 , 27 were followed to derive mean and standard deviation (SD). Where means were presented without SD, but p values were available, the average of the two SDs was imputed.28 Study methodological quality and risk of bias were assessed by applying the MINORS criteria for observational studies29 and the Jadad score for randomized controlled trials (RCTs).30 This information is available in the Supplementary tables provided in the appendix.
Statistical analysis
Data were analysed using RevMan software (Review Manager, version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Statistical expertise was available to the authors. Studies were included for meta-analysis if three or more existed which reported the LOS or day case rate for the same comparator. Mean LOS was compared between studies using the mean difference calculated using the inverse variance method in a fixed effects model. Mean length of stay was measured in hours for the purpose of analysis. Day case rate was compared between studies using the odds ratio calculated using the Mantel-Haenszel technique in a fixed effect model. An objective measure of heterogeneity was obtained by calculation of the I2 statistic from the Cochran Q test; an I2 value greater than 50% was taken to denote significant heterogeneity between studies. Where statistical heterogeneity existed in the analysis a random effects model was employed. Forest plots were included in the text where statistical significance was achieved; all forest plots for non-significant meta-analyses are included in the Supplementary file.
Results
Literature search
The literature search revealed 1173 publications, and a further 135 were identified from hand searches and bibliographic sources (Fig. 2 ). Following exclusion of duplicates and abstract review, 216 studies were subject to full text review. Quantitative data were available for meta-analysis from 80 studies. There were an additional 17 studies included for systematic review. Details for all 97 included studies have been provided in a Supplementary file. The included studies evaluated 14 peri-operative interventions. In the 80 studies suitable for meta-analysis, the mean LOS for 10,615 patients was 61.2 h, with a 41.1% day case rate where reported. The LOS and day case rates of perioperative interventions are summarised in Table 1 . The forest plots for non-significant meta-analyses have been provided in the Supplementary file.
Figure 2.
PRISMA diagram showing selection of articles for review
Table 1.
Meta-analyses of perioperative interventions to reduce the length of stay (LOS) or increase the day case rate in patients undergoing laparoscopic cholecystectomy
| Intervention | Outcome | Studies (n) | Participants (n) | Effect (95% CI)a | p value |
|---|---|---|---|---|---|
| Care pathway | LOS | 5 | 962 | 24.9 (18.7–31.2) | <0.001b |
| Care pathway | Day case rate | 6 | 2321 | OR 3.5 (1.5–8.1) | 0.005b |
| Carbohydrate supplement | LOS | 5 | 307 | 0.0 (−2.7 to 2.8) | 0.97 |
| Reduced pressure pneumoperitoneum | LOS | 6 | 1001 | 4.2 (−0.4 to 8.9) | 0.08 |
| Preoperative non-steroidal anti-inflammatory drugs | LOS | 6 | 447 | 1.7 (1.0–2.4) | <0.001b |
| Antiemetic | |||||
| Dexamethasone | LOS | 8 | 1053 | 1.4 (0.2–2.6) | 0.02b |
| Ondansetron | LOS | 6 | 395 | 0.8 (−0.1 to 1.7) | 0.08 |
| Antibiotics | LOS | 12 | 3410 | 0.6 (0.1–1.2) | 0.02b |
| Local anaesthesia (LA) | |||||
| Incisional LA | LOS | 4 | 382 | 9.4 (−5.0 to 23.8) | 0.2 |
| Intraperitoneal LA | LOS | 8 | 784 | 1.19 (−0.6 to 3.0) | 0.2 |
| Intraperitoneal LA | Day case rate | 4 | 308 | OR 1.8 (0.9–3.6) | 0.11 |
| Combined incisional and intraperitoneal LA | LOS | 5 | 360 | 2.7 (−0.1 to 5.5) | 0.06 |
| Drain insertion | LOS | 8 | 1629 | 11.97 (−1.5 to 25.5) | 0.08 |
Units of effect size for length of stay are reported as hours. Effect size for day case rate is reported as odds ratios (OR).
Indicates statistical significance.
Care pathway implementation
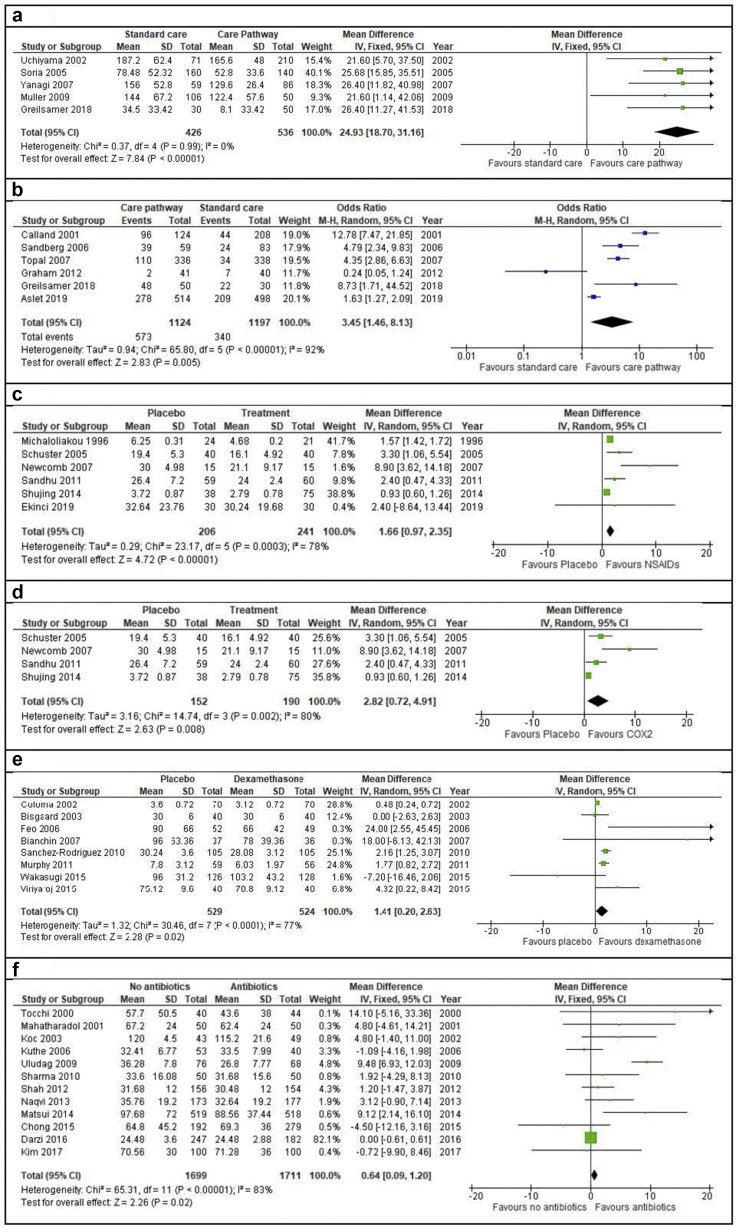
Implementation of a dedicated care pathway for patients undergoing elective LC was examined in 10 non-randomized studies.6, 7, 8, 9, 10, 11, 12 , 14 , 31 , 32 Implementation of a care pathway was associated with a significantly shorter mean LOS (MD 24.9 h; 95% CI, 18.7–31.2; p < 0.001; Fig. 3 a) and an improved day case rate (OR, 3.5; 95% CI, 1.5–8.1; p = 0.005; Fig. 3b).
Figure 3.
a – Meta-analysis of mean length of stay for dedicated care pathway implementation for laparoscopic cholecystectomy versus standard care pathway implementation. b – Meta-analysis of day case rate for dedicated care pathway implementation for LC versus standard care pathway implementation. c – Meta-analysis of mean length of stay in patients who received preoperative non-steroidal anti-inflammatory drugs (NSAIDs) during laparoscopic cholecystectomy versus those who received no NSAIDs or a placebo. d – Subgroup analysis of mean length of stay for those who received COX2 inhibitors versus no COX2 inhibitors or placebo. e – Mean length of stay for preoperative dexamethasone versus no dexamethasone or placebo. f – Meta-analysis of mean length of stay for patients who received prophylactic antibiotics during laparoscopic cholecystectomy versus those who received no antibiotics
Preoperative carbohydrate loading
The delivery of preoperative carbohydrate loading in the form of supplement drinks was examined in seven RCTs33, 34, 35, 36, 37, 38, 39 and no difference was found in either mean LOS (p = 0.970) or day case rate between those receiving carbohydrate loading and those fasting or receiving a placebo.
Pneumoperitoneum pressure
The effect of reduced pneumoperitoneum pressure was examined in six RCTs40, 41, 42, 43, 44, 45 and no difference was found in the mean LOS between patients undergoing surgery with a pressure of ≤10 mmHg compared to those undergoing surgery with pressure of 10–15 mmHg (p = 0.080).
Preoperative non-steroidal anti-inflammatory drugs (NSAIDs)
The effect of preoperative NSAIDs was examined in six RCTs.46, 47, 48, 49, 50, 51 There was a significant reduction in mean LOS in patients receiving NSAIDs compared to placebo (MD 1.7 h; 95% CI, 1.0–2.4; p < 0.001; Fig. 3c). A subgroup analysis of studies specifically involving COX2 inhibitors also demonstrated a significant difference in mean LOS (MD 2.8 h; 95% CI, 0.7–4.9; p = 0.008; Fig. 3d).47, 48, 49, 50
Preoperative anti-emetics
The effect of preoperative dexamethasone was examined in eight RCTs,52, 53, 54, 55, 56, 57, 58, 59 with a mean reduction in LOS by 1.4 h noted amongst patients receiving dexamethasone compared with placebo (95%, CI 0.2–2.6; p = 0.020; Fig. 3e). No difference in LOS was noted in six RCTs60, 61, 62, 63, 64, 65 comparing preoperative ondansetron with placebo (p = 0.080).
Prophylactic intra-operative antibiotics
The effect of prophylactic antibiotics was examined in 11 RCTs and one comparative study.66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 Patients who received antibiotics before skin incision had a marginally shorter hospital stay, by 0.6 h, than those who received no antibiotics (p = 0.020, Fig. 3f).
Local/regional anaesthesia
The effect of incisional local anaesthesia (LA) was examined in four RCTs,78, 79, 80, 81 which did not lead to a reduction in LOS compared with placebo (p = 0.200). The effect of intraperitoneal LA was examined in 12 studies,81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92 which demonstrated no change in LOS (p = 0.200) or day case rates (p = 0.110). A total of five studies81 , 93, 94, 95, 96 were found examining the effect of combined intraperitoneal and incisional LA and this likewise did not show a significant reduction (p = 0.060). Three studies97, 98, 99 examined the effect of intraoperative transversus abdominis plane (TAP) blocks versus no TAP blocks and systematic review found no difference in LOS or day case rate.
Prophylactic drain insertion
A total of eight studies100, 101, 102, 103, 104, 105, 106, 107 were found which reported on the effects of routine prophylactic drain insertion and found no significant difference in mean LOS between those receiving drainage and those with no drain insertion (p = 0.080). Two RCTs revealed that drain insertion significantly increased the likelihood of admissions in excess of 48 h.108 , 109 These studies were not suitable for meta-analysis as there were not enough studies reporting this LOS outcome (>/<48 h) in relation to drain insertion.
Preoperative education
Three studies110, 111, 112 were identified examining the effect of intensive versus standard preoperative education and none found differences in LOS or day case rate. This intervention was not amenable to meta-analysis as there were not enough studies for inclusion in either LOS or day case rate.
Perioperative fluid management
One RCT113 found a significant improvement in day case rate in patients who received a liberal intraoperative intravenous fluid regimen, however, a further four RCTs114, 115, 116, 117 found no significant difference in mean LOS. None of these studies were amenable to meta-analysis due to heterogenous reporting of LOS outcomes.
Warmed pneumoperitoneum
Mean LOS was reported in two RCTs comparing warmed, humidified pneumoperitoneum with standard pneumoperitoneum118 , 119 and no significant difference in mean LOS was noted in either study. This intervention was not amenable to meta-analysis as it did not meet the number of studies required for inclusion.
Country and time period
Mean LOS was noted to be significantly longer in Japanese studies than the rest of the world (130.17 vs 44.22 h; p = 0.001). There was no difference in mean LOS between studies published before or after 2010 (52.11 vs 50.47 h; p = 0.850). A table of studies by country and year has been provided in the supplementary results file.
Discussion
This review was designed to examine the evidence base for clinical interventions that have the potential to reduce LOS following LC. DCLC is associated with cost-savings3 , 13 , 14 and a high rate of patient satisfaction.17 , 18 In addition to this, shorter hospital stays lead to reduced healthcare associated infections120 and are likely to lead to reduce waiting list times due to better utilisation of resources. The 80 studies included in this meta-analysis, related to 10,615 LC patients who had a mean LOS of 61.2 h and a 41.1% day case rate. A total of 14 interventions were examined, of which only four were found to influence length of stay or likelihood of successful management as a day case: implementation of a dedicated care pathway, preoperative antibiotics, preoperative NSAIDs, and the antiemetic dexamethasone. Each of these demonstrated a statistically significant reduction in mean LOS. The largest effect size was seen with care pathway implementation, which led to a mean reduction in LOS of 24.9 h and a day case rate of 51.0%, compared with 27.9% for those treated prior to pathway implementation. The majority of the remaining interventions, used in isolation, did not lead to a reduction in LOS. Even among the three individual interventions with a significant difference, the change was of limited clinical importance, reducing LOS by 0.6–2.8 h. The reduction in LOS associated with the delivery of preoperative antibiotics is likely spurious. This is supported by the fact that antibiotics were only found to reduce LOS by 0.6 h, which is minimal.
A number of factors were not independently associated with reduced LOS in this meta-analysis. The provision of preoperative education, which played a role in several of the care pathways7, 8, 9, 10 , 31 and which is generally considered mandatory to achieve same day discharge20 did not lead to a reduced LOS in the studies included. Unfortunately, only three studies reported LOS outcomes with respect to this intervention, each of insufficient power to detect a difference.110 , 112 Equally unexpectedly, the insertion of prophylactic drainage was not associated with increased LOS. Avoidance of unnecessary drain insertion is generally recommended5 and the authors hypothesise that the similar LOS observed among drained and undrained patients relates to study protocols designed to compare endpoints such as pain, development of collections, and drain outputs, at standard timepoints.
LOS is a challenging outcome measure, subject to both incentives and perverse incentives that may be financial or organisational.121 The definition of a day case varies between departments, institutions and health systems ranging from just a few hours to 23.122 Accuracy of LOS reporting is difficult to assess or internally validate. The authors assume for this meta-analysis that in each system the same problems arise in a constant way, such that the measure of mean difference between groups then most accurately represents a valid unit, which varies by the intervention studied. The current study excluded papers which did not report LOS, resulting in exclusion of a large amount of good quality studies evaluating specific interventions. A further potential weakness is that many included studies were underpowered to identify changes in LOS, as it was often a secondary outcome. Interestingly, many studies had longer than average postoperative stays, possibly suggesting that investigators evaluating a new intervention exercised caution in discharging trial subjects or kept patients in hospital to capture data at specific timepoints. Japanese studies were found to have a significantly longer overall LOS than other countries, however the difference in LOS seen with each intervention should not have changed despite this finding. For example, regarding the care pathway intervention, despite the fact that patients involved in studies from Japan had longer overall LOS, there was still a significant reduction seen between those who were involved in a care pathway and those who were not. The available literature was heterogenous in terms of reporting of outcomes and interventions delivered. A further challenge is the potential publication bias where studies with negative results could be under-reported.
The findings of this paper can be used to improve outcomes associated with DCLC, but first it is necessary to establish which interventions are central to an effective day case pathway. This is difficult for a number of reasons. Firstly, the studies involving care pathways varied widely in their approach to elective surgery. A number of them specified admission prior to the day of surgery, retained patients for a number of days postoperatively, and referred to removal of routine drains. While the care pathway protocols may have reduced LOS, some of their elements or outcomes may have lost their relevance to contemporary surgery. The significant impact of care pathway implementation on shortening LOS in this meta-analysis may be attributed to the cumulative effect of multiple factors with smaller individual effects. For example, a number of factors are common to the studied care pathways including NSAID use, opiate minimisation, multimodal antiemetics, and combined skin and peritoneal local anaesthesia. It remains unclear whether the interventions themselves lead to the benefit observed when a care pathway is implemented, or whether the improved team dynamics and multi-disciplinary collaboration characteristic of care pathway implementation123 is responsible. Additionally, some of the pathway components were poorly described. Three papers did not describe the pathway sufficiently to be able to replicate it.8 , 14 , 31 Lastly, none of the studies included were randomised, and all involved study of outcomes pre- and post-implementation of care pathways. Non-randomized designs are subject to many potential biases including the Hawthorne effect, recall bias and publication bias. Such potential biases could influence the effect on LOS from clinical pathways.
Evaluation of the studied care pathways provides some insight into the methods for introduction, but very little information on the barriers that exist to their implementation. Many health systems have defined care pathways for day case laparoscopic cholecystectomy, including guidance provided locally by the Irish National Clinical Programme for Surgery.124 In spite of the availability of such a care pathway, Irish day case rates continue to fall below expected targets of 60%.125 In addition, even among hospitals of similar characteristics, utilisation of day case laparoscopic cholecystectomy is widely variable, with rates of 0%–95.8% reported across Irish hospitals in 2019.125 It is clear that a defined care pathway is necessary but not sufficient to effect change in LOS126; equal attention to factors relating to implementation is required. Attention to the context, planning and structures necessary for successful day case surgery implementation are also important but generally less emphasised by surgeons.127 Inclusion of implementation outcomes alongside intervention outcomes would greatly enhance the reproducibility of surgical literature. It is apparent that further research regarding care pathway components is unlikely to increase the effectiveness of care pathways. Rather, understanding methods of care pathway implementation is necessary to facilitate effective pathway use.
Implementing a care pathway which incorporates a range of perioperative interventions is more likely to lead to a significant reduction in LOS and increase in day case rate than any single intervention, although there has yet to be an RCT demonstrating this. Very few interventions in isolation have an effect on LOS after elective LC, and the effect size of such isolated interventions is small. Future studies should focus to a greater extent on the contextual and organisational factors associated with successful implementation of short-stay LC pathways instead of on the individual components of the care pathway.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
This paper is not based on a previous communication to a society or meeting.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hpb.2020.08.012.
Declarations of interest
None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Beckingham I., Royal College of surgeons of England. Association of Upper Gastrointestinal surgeons . Royal College of Surgeons of England; London: 2016. Commissioning guide: gallstone disease. [Google Scholar]
- 2.Mortimore G., NICE Internal Clinical Guidelines team . 2014. Gallstone disease: diagnosis and management. Clinical guideline [CG 188]https://www.nice.org.uk/guidance/cg188/chapter/1-Recommendations#managing-gallbladder-stones Available from: [Google Scholar]
- 3.Ahmad N.Z., Byrnes G., Naqvi S.A. A meta-analysis of ambulatory versus inpatient laparoscopic cholecystectomy. Surg Endosc. 2008;22:1928–1934. doi: 10.1007/s00464-008-9867-2. [DOI] [PubMed] [Google Scholar]
- 4.Tang H., Dong A., Yan L. Day surgery versus overnight stay laparoscopic cholecystectomy: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:556–561. doi: 10.1016/j.dld.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk A.H., de Reuver P.R., Besselink M.G., van Laarhoven K.J., Harrison E.M., Wigmore S.J., et al. Assessment of available evidence in the management of gallbladder and bile duct stones: a systematic review of international guidelines. HPB. 2017;19:297–309. doi: 10.1016/j.hpb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Uchiyama K., Takifuji K., Tani M., Onishi H., Yamaue H. Effectiveness of the clinical pathway to decrease length of stay and cost for laparoscopic surgery. Surg Endosc. 2002;16:1594–1597. doi: 10.1007/s00464-002-9018-0. [DOI] [PubMed] [Google Scholar]
- 7.Soria V., Pellicer E., Flores B., Carrasco M., Candel Maria F., Aguayo J.L. Evaluation of the clinical pathway for laparoscopic cholecystectomy. Am Surg. 2005;71:40–45. [PubMed] [Google Scholar]
- 8.Yanagi K., Sasajima K., Miyamoto M., Suzuki S., Yokoyama T., Maruyama H., et al. Evaluation of the clinical pathway for laparoscopic cholecystectomy and simulation of short-term hospitalization. J Nippon Med Sch. 2007;74:409–413. doi: 10.1272/jnms.74.409. [DOI] [PubMed] [Google Scholar]
- 9.Calland J.F., Tanaka K., Foley E., Bovbjerg V.E., Markey D.W., Blome S., et al. Outpatient laparoscopic cholecystectomy: patient outcomes after implementation of a clinical pathway. Ann Surg. 2001;233:704–715. doi: 10.1097/00000658-200105000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandberg W.S., Canty T., Sokal S.M., Daily B., Berger D.L. Financial and operational impact of a direct-from-PACU discharge pathway for laparoscopic cholecystectomy patients. Surgery. 2006;140:372–378. doi: 10.1016/j.surg.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Greilsamer T., Orion F., Denimal F., De Kerviler B., Jean M.H., Dimet J., et al. Increasing success in outpatient laparoscopic cholecystectomy by an optimal clinical pathway. ANZ J Surg. 2018 doi: 10.1111/ans.14297. [DOI] [PubMed] [Google Scholar]
- 12.Aslet M., Yates D., Wasawo S. Improving the day case rate for laparoscopic cholecystectomy via introduction of a dedicated clinical pathway. J Perioper Pract. 2019 doi: 10.1177/1750458919862701. 1750458919862701. [DOI] [PubMed] [Google Scholar]
- 13.Trevino C.M., Katchko K.M., Verhaalen A.L., Bruce M.L., Webb T.P. Cost effectiveness of a fast-track protocol for urgent laparoscopic cholecystectomies and appendectomies. World J Surg. 2016;40:856–862. doi: 10.1007/s00268-015-3266-3. [DOI] [PubMed] [Google Scholar]
- 14.Topal B., Peeters G., Verbert A., Penninckx F. Outpatient laparoscopic cholecystectomy: clinical pathway implementation is efficient and cost effective and increases hospital bed capacity. Surg Endosc. 2007;21:1142–1146. doi: 10.1007/s00464-006-9083-x. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan J., Gurusamy K.S., Davidson B.R. Day-surgery versus overnight stay surgery for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2013:Cd006798. doi: 10.1002/14651858.CD006798.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurusamy K., Junnarkar S., Farouk M., Davidson B.R. Meta-analysis of randomized controlled trials on the safety and effectiveness of day-case laparoscopic cholecystectomy. Br J Surg. 2008;95:161–168. doi: 10.1002/bjs.6105. [DOI] [PubMed] [Google Scholar]
- 17.Leeder P.C., Matthews T., Krzeminska K., Dehn T.C. Routine day-case laparoscopic cholecystectomy. Br J Surg. 2004;91:312–316. doi: 10.1002/bjs.4409. [DOI] [PubMed] [Google Scholar]
- 18.Rathore M.A., Andrabi S.I., Mansha M., Brown M.G. Day case laparoscopic cholecystectomy is safe and feasible: a case controlled study. Int J Surg. 2007;5:255–259. doi: 10.1016/j.ijsu.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 19.El-Sharkawy A.M., Tewari N., Vohra R.S. The cholecystectomy as A day case (CAAD) score: a validated score of preoperative Predictors of successful day-case cholecystectomy using the CholeS data set. World J Surg. 2019;43:1928–1934. doi: 10.1007/s00268-019-04981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHS . 2006. Delivering quality and value. Focus on: cholecystectomy.https://www.qualitasconsortium.com/index.cfm/reference-material/delivering-value-quality/focus-oncholecystectomy-commissioners-guide Available from: [Google Scholar]
- 21.Bailey C.R., Ahuja M., Bartholomew K., Bew S., Forbes L., Lipp A., et al. Guidelines for day-case surgery 2019: guidelines from the association of anaesthetists and the British Association of Day Surgery. Anaesthesia. 2019;74:778–792. doi: 10.1111/anae.14639. [DOI] [PubMed] [Google Scholar]
- 22.Solodkyy A. Re-audit of ‘true day case’ laparoscopic cholecystectomy in a high-volume specialist unit in a district general hospital. Surg Endosc Int Tech. 2017;31:S326. doi: 10.1007/s00464-017-5565-2. [DOI] [Google Scholar]
- 23.Al-Qahtani H.H., Alam M.K., Asalamah S., Akeely M., Ibrar M. Day-case laparoscopic cholecystectomy. Saudi Med J. 2015;36:46–51. doi: 10.15537/smj.2015.1.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn Y., Woods J., Connor S. A systematic review of interventions to facilitate ambulatory laparoscopic cholecystectomy. HPB. 2011;13:677–686. doi: 10.1111/j.1477-2574.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo Y.H., Lee K.F., Chin C.C., Huang W.S., Yeh C.H., Wang J.Y. Does body mass index impact the number of LNs harvested and influence long-term survival rate in patients with stage III colon cancer? Int J Colorectal Dis. 2012;27:1625–1635. doi: 10.1007/s00384-012-1496-5. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J.P., Deeks J.J. Cochrane Book Series; 2011. Selecting studies and collecting data. Cochrane Handbook for systematic reviews of interventions; pp. 151–185. [Google Scholar]
- 29.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 30.Clark H.D., Wells G.A., Huet C., McAlister F.A., Salmi L.R., Fergusson D., et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20:448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 31.Müller M.K., Dedes K.J., Dindo D., Steiner S., Hahnloser D., Clavien P.-A. Impact of clinical pathways in surgery. Langenbecks Arch Surg. 2009;394:31–39. doi: 10.1007/s00423-008-0352-0. [DOI] [PubMed] [Google Scholar]
- 32.Graham L., Neal C.P., Garcea G., Lloyd D.M., Robertson G.S., Sutton C.D. Evaluation of nurse-led discharge following laparoscopic surgery. J Eval Clin Pract. 2012;18:19–24. doi: 10.1111/j.1365-2753.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 33.Hausel J., Nygren J., Thorell A., Lagerkranser M., Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2005;92:415–421. doi: 10.1002/bjs.4901. [DOI] [PubMed] [Google Scholar]
- 34.Faria M.S., de Aguilar-Nascimento J.E., Pimenta O.S., Alvarenga L.C., Jr., Dock-Nascimento D.B., Slhessarenko N. Preoperative fasting of 2 hours minimizes insulin resistance and organic response to trauma after video-cholecystectomy: a randomized, controlled, clinical trial. World J Surg. 2009;33:1158–1164. doi: 10.1007/s00268-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 35.Pedziwiatr M., Pisarska M., Matlok M., Major P., Kisielewski M., Wierdak M., et al. Randomized clinical trial to compare the effects of preoperative oral carbohydrate loading versus placebo on insulin resistance and cortisol level after laparoscopic cholecystectomy. Pol Przegl Chir. 2015;87:402–408. doi: 10.1515/pjs-2015-0079. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.S., Song Y., Kim J.Y., Park J.S., Yoon D.S. Effects of preoperative oral carbohydrates on quality of recovery in laparoscopic cholecystectomy: a randomized, double blind, placebo-controlled trial. World J Surg. 2018;42:3150–3157. doi: 10.1007/s00268-018-4717-4. [DOI] [PubMed] [Google Scholar]
- 37.de Andrade Gagheggi Ravanini G., Portari Filho P.E., Abrantes Luna R., Almeida de Oliveira V. Organic inflammatory response to reduced preoperative fasting time, with a carbohydrate and protein enriched solution: a randomized trial. Nutr Hosp. 2015;32:953-7. doi: 10.3305/nh.2015.32.2.8944. [DOI] [PubMed] [Google Scholar]
- 38.Dock-Nascimento D.B., de Aguilar-Nascimento J.E., Magalhaes Faria M.S., Caporossi C., Slhessarenko N., Waitzberg D.L. Evaluation of the effects of a preoperative 2-hour fast with maltodextrine and glutamine on insulin resistance, acute-phase response, nitrogen balance, and serum glutathione after laparoscopic cholecystectomy: a controlled randomized trial. JPEN. 2012;36:43–52. doi: 10.1177/0148607111422719. [DOI] [PubMed] [Google Scholar]
- 39.Bisgaard T., Kristiansen V.B., Hjortso N.C., Jacobsen L.S., Rosenberg J., Kehlet H. Randomized clinical trial comparing an oral carbohydrate beverage with placebo before laparoscopic cholecystectomy. Br J Surg. 2004;91:151–158. doi: 10.1002/bjs.4412. [DOI] [PubMed] [Google Scholar]
- 40.Wallace D.H., Serpell M.G., Baxter J.N., O'Dwyer P.J. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg. 1997;84:455–458. [PubMed] [Google Scholar]
- 41.Sarli L., Costi R., Sansebastiano G., Trivelli M., Roncoroni L. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg. 2000;87:1161–1165. doi: 10.1046/j.1365-2168.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 42.Barczynski M., Herman R.M. A prospective randomized trial on comparison of low-pressure (LP) and standard-pressure (SP) pneumoperitoneum for laparoscopic cholecystectomy. Surg Endosc. 2003;17:533–538. doi: 10.1007/s00464-002-9121-2. [DOI] [PubMed] [Google Scholar]
- 43.Esmat M.E., Elsebae M.M., Nasr M.M., Elsebaie S.B. Combined low pressure pneumoperitoneum and intraperitoneal infusion of normal saline for reducing shoulder tip pain following laparoscopic cholecystectomy. World J Surg. 2006;30:1969–1973. doi: 10.1007/s00268-005-0752-z. [DOI] [PubMed] [Google Scholar]
- 44.Sandhu T., Yamada S., Ariyakachon V., Chakrabandhu T., Chongruksut W., Ko-iam W. Low-pressure pneumoperitoneum versus standard pneumoperitoneum in laparoscopic cholecystectomy, a prospective randomized clinical trial. Surg Endosc. 2009;23:1044–1047. doi: 10.1007/s00464-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 45.Joshipura V.P., Haribhakti S.P., Patel N.R., Naik R.P., Soni H.N., Patel B., et al. A prospective randomized, controlled study comparing low pressure versus high pressure pneumoperitoneum during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2009;19:234–240. doi: 10.1097/SLE.0b013e3181a97012. [DOI] [PubMed] [Google Scholar]
- 46.Michaloliakou C., Chung F., Sharma S. Preoperative multimodal analgesia facilitates recovery after ambulatory laparoscopic cholecystectomy. Anesth Analg. 1996;82:44–51. doi: 10.1097/00000539-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Schuster R., Stewart D., Schuster L., Greaney G., Waxman K. Preoperative oral rofecoxib and postoperative pain in patients after laparoscopic cholecystectomy: a prospective, randomized, double-blinded, placebo-controlled trial. Am Surg. 2005;71:827–829. [PubMed] [Google Scholar]
- 48.Newcomb W., Lincourt A., Hope W., Schmelzer T., Sing R., Kercher K., et al. Prospective, double-blinded, randomized, placebo-controlled comparison of local anesthetic and nonsteroidal anti-inflammatory drugs for postoperative pain management after laparoscopic surgery. Am Surg. 2007;73:618–624. discussion 24-5. [PubMed] [Google Scholar]
- 49.Sandhu T., Paiboonworachat S., Ko-iam W. Effects of preemptive analgesia in laparoscopic cholecystectomy: a double-blind randomized controlled trial. Surg Endosc. 2011;25:23–27. doi: 10.1007/s00464-010-1122-y. [DOI] [PubMed] [Google Scholar]
- 50.Shuying L., Xiao W., Peng L., Tao Z., Ziying L., Liang Z. Preoperative intravenous parecoxib reduces length of stay on ambulatory laparoscopic cholecystectomy. Int J Surg. 2014;12:464–468. doi: 10.1016/j.ijsu.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Ekinci M., Ciftci B., Celik E.C., Kose E.A., Karakaya M.A., Ozdenkaya Y. A randomized, placebo-controlled, double-blind study that evaluates efficacy of intravenous ibuprofen and acetaminophen for postoperative pain treatment following laparoscopic cholecystectomy surgery. J Gastrointest Surg. 2019 doi: 10.1007/s11605-019-04220-1. [DOI] [PubMed] [Google Scholar]
- 52.Coloma M., White P.F., Markowitz S.D., Whitten C.W., Macaluso A.R., Berrisford S.B., et al. Dexamethasone in combination with dolasetron for prophylaxis in the ambulatory setting: effect on outcome after laparoscopic cholecystectomy. Anesthesiology. 2002;96:1346–1350. doi: 10.1097/00000542-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Bisgaard T., Klarskov B., Kehlet H., Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:651–660. doi: 10.1097/01.sla.0000094390.82352.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feo C.V., Sortini D., Ragazzi R., De Palma M., Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93:295–299. doi: 10.1002/bjs.5252. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Rodriguez P.E., Fuentes-Orozco C., Gonzalez-Ojeda A. Effect of dexamethasone on postoperative symptoms in patients undergoing elective laparoscopic cholecystectomy: randomized clinical trial. World J Surg. 2010;34:895–900. doi: 10.1007/s00268-010-0457-9. [DOI] [PubMed] [Google Scholar]
- 56.Murphy G.S., Szokol J.W., Greenberg S.B., Avram M.J., Vender J.S., Nisman M., et al. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and postdischarge recovery outcomes. Anesthesiology. 2011;114:882–890. doi: 10.1097/ALN.0b013e3181ec642e. [DOI] [PubMed] [Google Scholar]
- 57.Wakasugi M., Tori M., Shimizu J., Kim Y.K., Noda T., Dono K., et al. Efficacy of preoperative dexamethasone for postoperative nausea and vomiting after laparoscopic cholecystectomy: a large-scale, multicenter, randomized, double-blind, placebo-controlled trial in Japan. J Hepatobiliary Pancreat Sci. 2015;22:802–809. doi: 10.1002/jhbp.285. [DOI] [PubMed] [Google Scholar]
- 58.Viriyaroj V., Boonsinsukh T., Rookkachart T., Yigsakmongkol N. The effects of single-dose preoperative intravenous dexamethasone on clinical outcome after laparoscopic cholecystectomy. J Med Assoc Thail. 2015;98(Suppl. 10):S112–S117. [PubMed] [Google Scholar]
- 59.Bianchin A., De Luca A., Caminiti A. Postoperative vomiting reduction after laparoscopic cholecystectomy with single dose of dexamethasone. Minerva Anestesiol. 2007;73:343–346. [PubMed] [Google Scholar]
- 60.Helmy S.A. Prophylactic anti-emetic efficacy of ondansetron in laparoscopic cholecystectomy under total intravenous anaesthesia. A randomised, double-blind comparison with droperidol, metoclopramide and placebo. Anaesthesia. 1999;54:266–271. doi: 10.1046/j.1365-2044.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz de Adana J., Tobalina Bonis R., Garcia Galan F., Hernandez Matias A., Fernandez Luengas D., Ortega Deballon P., et al. Antiemetic efficacy of ondansetron in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Rev Esp Enferm Dig. 1999;91:639–643. [PubMed] [Google Scholar]
- 62.Liberman M.A., Howe S., Lane M. Ondansetron versus placebo for prophylaxis of nausea and vomiting in patients undergoing ambulatory laparoscopic cholecystectomy. Am J Surg. 2000;179:60–62. doi: 10.1016/s0002-9610(99)00251-2. [DOI] [PubMed] [Google Scholar]
- 63.Elhakim M., Nafie M., Mahmoud K., Atef A. Dexamethasone 8 mg in combination with ondansetron 4 mg appears to be the optimal dose for the prevention of nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth. 2002;49:922–926. doi: 10.1007/bf03016875. [DOI] [PubMed] [Google Scholar]
- 64.Kaki A.M., Abd El-Hakeem E.E. Prophylaxis of postoperative nausea and vomiting with ondansetron, metoclopramide, or placebo in total intravenous anesthesia patients undergoing laparoscopic cholecystectomy. Saudi Med J. 2008;29:1408–1413. [PubMed] [Google Scholar]
- 65.Grover V.K., Mathew P.J., Hegde H. Efficacy of orally disintegrating ondansetron in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy: a randomised, double-blind placebo controlled study. Anaesthesia. 2009;64:595–600. doi: 10.1111/j.1365-2044.2008.05860.x. [DOI] [PubMed] [Google Scholar]
- 66.Tocchi A., Lepre L., Costa G., Liotta G., Mazzoni G., Maggiolini F. The need for antibiotic prophylaxis in elective laparoscopic cholecystectomy: a prospective randomized study. Arch Surg. 2000;135:67–70. doi: 10.1001/archsurg.135.1.67. discussion. [DOI] [PubMed] [Google Scholar]
- 67.Mahatharadol V. A reevaluation of antibiotic prophylaxis in laparoscopic cholecystectomy: a randomized controlled trial. J Med Assoc Thail. 2001;84:105–108. [PubMed] [Google Scholar]
- 68.Koc M., Zulfikaroglu B., Kece C., Ozalp N. A prospective randomized study of prophylactic antibiotics in elective laparoscopic cholecystectomy. Surg Endosc. 2003;17:1716–1718. doi: 10.1007/s00464-002-8866-y. [DOI] [PubMed] [Google Scholar]
- 69.Kuthe S.A., Kaman L., Verma G.R., Singh R. Evaluation of the role of prophylactic antibiotics in elective laparoscopic cholecystectomy: a prospective randomized trial. Trop Gastroenterol. 2006;27:54–57. [PubMed] [Google Scholar]
- 70.Uludag M., Yetkin G., Citgez B. The role of prophylactic antibiotics in elective laparoscopic cholecystectomy. JSLS. 2009;13:337–341. [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma N., Garg P.K., Hadke N.S., Choudhary D. Role of prophylactic antibiotics in laparoscopic cholecystectomy and risk factors for surgical site infection: a randomized controlled trial. Surg Infect. 2010;11:367–370. doi: 10.1089/sur.2008.084. [DOI] [PubMed] [Google Scholar]
- 72.Shah J.N., Maharjan S.B., Paudyal S. Routine use of antibiotic prophylaxis in low-risk laparoscopic cholecystectomy is unnecessary: a randomized clinical trial. Asian J Surg. 2012;35:136–139. doi: 10.1016/j.asjsur.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Naqvi M.A., Mehraj A., Ejaz R., Mian A. Role of prophylactic antibiotics in low risk elective laparoscopic cholecystectomy: is there a need? J Ayub Med Coll Abbottabad. 2013;25:172–174. [PubMed] [Google Scholar]
- 74.Matsui Y., Satoi S., Kaibori M., Toyokawa H., Yanagimoto H., Matsui K., et al. Antibiotic prophylaxis in laparoscopic cholecystectomy: a randomized controlled trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chong J.U., Lim J.H., Kim J.Y., Kim S.H., Kim K.S. The role of prophylactic antibiotics on surgical site infection in elective laparoscopic cholecystectomy. Korean J Hepatobiliary Pancreat Surg. 2015;19:188–193. doi: 10.14701/kjhbps.2015.19.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Darzi A.A., Nikmanesh A., Bagherian F. The effect of prophylactic antibiotics on post laparoscopic cholecystectomy infectious complications: a double-blinded clinical trial. Electron Physician. 2016;8:2308–2314. doi: 10.19082/2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H.J., Kang S.H., Roh Y.H., Kim M.C., Kim K.W. Are prophylactic antibiotics necessary in elective laparoscopic cholecystectomy, regardless of patient risk? Ann Surg Treat Res. 2017;93:76–81. doi: 10.4174/astr.2017.93.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlidis T.E., Atmatzidis K.S., Papaziogas B.T., Makris J.G., Lazaridis C.N., Papaziogas T.B. The effect of preincisional periportal infiltration with ropivacaine in pain relief after laparoscopic procedures: a prospective, randomized controlled trial. JSLS. 2003;7:305–310. [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y.Y., Yeh C.N., Lee H.L., Wang S.Y., Tsai C.Y., Lin C.C., et al. Local anesthesia with ropivacaine for patients undergoing laparoscopic cholecystectomy. World J Gastroenterol. 2009;15:2376–2380. doi: 10.3748/wjg.15.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin S., Hua J., Xu B., Yang T., He Z., Xu C., et al. Comparison of bupivacaine and parecoxib for postoperative pain relief after laparoscopic cholecystectomy: a randomized controlled trial. Int J Clin Exp Med. 2015;8:13824–13829. [PMC free article] [PubMed] [Google Scholar]
- 81.Cha S.M., Kang H., Baek C.W., Jung Y.H., Koo G.H., Kim B.G., et al. Peritrocal and intraperitoneal ropivacaine for laparoscopic cholecystectomy: a prospective, randomized, double-blind controlled trial. J Surg Res. 2012;175:251–258. doi: 10.1016/j.jss.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 82.Scheinin B., Kellokumpu I., Lindgren L., Haglund C., Rosenberg P.H. Effect of intraperitoneal bupivacaine on pain after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1995;39:195–198. doi: 10.1111/j.1399-6576.1995.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 83.Szem J.W., Hydo L., Barie P.S. A double-blinded evaluation of intraperitoneal bupivacaine vs saline for the reduction of postoperative pain and nausea after laparoscopic cholecystectomy. Surg Endosc. 1996;10:44–48. doi: 10.1007/s004649910011. [DOI] [PubMed] [Google Scholar]
- 84.Ahmed B.H., Ahmed A., Tan D., Awad Z.T., Al-Aali A.Y., Kilkenny J., 3rd, et al. Post-laparoscopic cholecystectomy pain: effects of intraperitoneal local anesthetics on pain control – a randomized prospective double-blinded placebo-controlled trial. Am Surg. 2008;74:201–209. [PubMed] [Google Scholar]
- 85.Zimmer P.W., McCann M.J., O'Brien M.M. Bupivacaine use in the Insuflow device during laparoscopic cholecystectomy: results of a prospective randomized double-blind controlled trial. Surg Endosc. 2010;24:1524–1527. doi: 10.1007/s00464-009-0804-9. [DOI] [PubMed] [Google Scholar]
- 86.Ingelmo P.M., Bucciero M., Somaini M., Sahillioglu E., Garbagnati A., Charton A., et al. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: a double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2013;110:800–806. doi: 10.1093/bja/aes495. [DOI] [PubMed] [Google Scholar]
- 87.Niknam F., Saxena A., Niles N., Budak U.U., Mekisic A. Does irrigation of the subdiaphragmatic region with ropivacaine reduce the incidence of right shoulder tip pain after laparoscopic cholecystectomy? A prospective randomized, double-blind, controlled study. Am Surg. 2014;80:E17–E18. [PubMed] [Google Scholar]
- 88.Ahmad A., Faridi S., Siddiqui F., Edhi M.M., Khan M. Effect of bupivacaine soaked gauze in postoperative pain relief in laparoscopic cholecystectomy: a prospective observational controlled trial in 120 patients. Patient Saf Surg. 2015;9:31. doi: 10.1186/s13037-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chundrigar T., Hedges A.R., Morris R., Stamatakis J.D. Intraperitoneal bupivacaine for effective pain relief after laparoscopic cholecystectomy. Ann R Coll Surg Engl. 1993;75:437–439. [PMC free article] [PubMed] [Google Scholar]
- 90.Paulson J., Mellinger J., Baguley W. The use of intraperitoneal bupivacaine to decrease the length of stay in elective laparoscopic cholecystectomy patients. Am Surg. 2003;69:275–278. discussion 8-9. [PubMed] [Google Scholar]
- 91.Roberts K.J., Gilmour J., Pande R., Nightingale P., Tan L.C., Khan S. Efficacy of intraperitoneal local anaesthetic techniques during laparoscopic cholecystectomy. Surg Endosc. 2011;25:3698–3705. doi: 10.1007/s00464-011-1757-3. [DOI] [PubMed] [Google Scholar]
- 92.Abet E., Orion F., Denimal F., Brau-Weber A.G., de Kerviler B., Jean M.H., et al. Interest of using ropivacaine for outpatient laparoscopic cholecystectomy: prospective randomized trial. World J Surg. 2017;41:687–692. doi: 10.1007/s00268-016-3797-2. [DOI] [PubMed] [Google Scholar]
- 93.Tsimoyiannis E.C., Glantzounis G., Lekkas E.T., Siakas P., Jabarin M., Tzourou H. Intraperitoneal normal saline and bupivacaine infusion for reduction of postoperative pain after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1998;8:416–420. [PubMed] [Google Scholar]
- 94.Hilvering B., Draaisma W.A., van der Bilt J.D., Valk R.M., Kofman K.E., Consten E.C. Randomized clinical trial of combined preincisional infiltration and intraperitoneal instillation of levobupivacaine for postoperative pain after laparoscopic cholecystectomy. Br J Surg. 2011;98:784–789. doi: 10.1002/bjs.7435. [DOI] [PubMed] [Google Scholar]
- 95.Yeh C.N., Tsai C.Y., Cheng C.T., Wang S.Y., Liu Y.Y., Chiang K.C., et al. Pain relief from combined wound and intraperitoneal local anesthesia for patients who undergo laparoscopic cholecystectomy. BMC Surg. 2014;14:28. doi: 10.1186/1471-2482-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Protic M., Veljkovic R., Bilchik A.J., Popovic A., Kresoja M., Nissan A., et al. Prospective randomized controlled trial comparing standard analgesia with combined intra-operative cystic plate and port-site local anesthesia for post-operative pain management in elective laparoscopic cholecystectomy. Surg Endosc. 2017;31:704–713. doi: 10.1007/s00464-016-5024-5. [DOI] [PubMed] [Google Scholar]
- 97.Tihan D., Totoz T., Tokocin M., Ercan G., Koc Calikoglu T., Vartanoglu T., et al. Efficacy of laparoscopic transversus abdominis plane block for elective laparoscopic cholecystectomy in elderly patients. Bosn J Basic Med Sci. 2016;16:139–144. doi: 10.17305/bjbms.2016.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siriwardana R.C., Kumarage S.K., Gunathilake B.M., Thilakarathne S.B., Wijesinghe J.S. Local infiltration versus laparoscopic-guided transverse abdominis plane block in laparoscopic cholecystectomy: double-blinded randomized control trial. Surg Endosc. 2019;33:179–183. doi: 10.1007/s00464-018-6291-0. [DOI] [PubMed] [Google Scholar]
- 99.Elamin G., Waters P.S., Hamid H., O'Keeffe H.M., Waldron R.M., Duggan M., et al. Efficacy of a laparoscopically delivered transversus abdominis plane block technique during elective laparoscopic cholecystectomy: a prospective, double-blind randomized trial. J Am Coll Surg. 2015;221:335–344. doi: 10.1016/j.jamcollsurg.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 100.Nomdedeu J., Salvador J., Piqueras R., Escrig J., Garcia R. The systematic use of drainage in laparoscopic cholecystectomy. A prospective study. Cir Esp. 1997;61:254–257. [Google Scholar]
- 101.Mrozowicz A., Rucinski P., Polkowski W. Routine drainage of the subhepatic area after laparoscopic cholecystectomy. Prospective, controlled study with random patient selection. Pol Przegl Chir. 2006;78:597–609. [Google Scholar]
- 102.Uchiyama K., Tani M., Kawai M., Terasawa H., Hama T., Yamaue H. Clinical significance of drainage tube insertion in laparoscopic cholecystectomy: a prospective randomized controlled trial. J Hepatobiliary Pancreat Surg. 2007;14:551–556. doi: 10.1007/s00534-007-1221-x. [DOI] [PubMed] [Google Scholar]
- 103.Tzovaras G., Liakou P., Fafoulakis F., Baloyiannis I., Zacharoulis D., Hatzitheofilou C. Is there a role for drain use in elective laparoscopic cholecystectomy? A controlled randomized trial. Am J Surg. 2009;197:759–763. doi: 10.1016/j.amjsurg.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Ishikawa K., Matsumata T., Kishihara F., Fukuyama Y., Masuda H., Kitano S. Laparoscopic cholecystectomy with and without abdominal prophylactic drainage. Dig Endosc. 2011;23:153–156. doi: 10.1111/j.1443-1661.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 105.Picchio M., De Angelis F., Zazza S., Di Filippo A., Mancini R., Pattaro G., et al. Drain after elective laparoscopic cholecystectomy. A randomized multicentre controlled trial. Surg Endosc. 2012;26:2817–2822. doi: 10.1007/s00464-012-2252-1. [DOI] [PubMed] [Google Scholar]
- 106.Gurer A., Dumlu E.G., Dikili E., Kiyak G., Ozlem N. Is a drain required after laparoscopic cholecystectomy? Eurasian J Med. 2013;45:181–184. doi: 10.5152/eajm.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma A., Mittal S. Role of routine subhepatic abdominal drain placement following uncomplicated laparoscopic cholecystectomy: a prospective randomised study. J Clin Diagn Res. 2016;10:Pc03–Pc05. doi: 10.7860/jcdr/2016/21142.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Georgiou C., Demetriou N., Pallaris T., Theodosopoulos T., Katsouyanni K., Polymeneas G. Is the routine use of drainage after elective laparoscopic cholecystectomy justified? A randomized trial. J Laparoendosc Adv Surg Tech A. 2011;21(2):119–123. doi: 10.1089/lap.2010.0003. [DOI] [PubMed] [Google Scholar]
- 109.Sharma A., Gupta S.N. Drainage versus no drainage after elective laparoscopic cholecystectomy. Kathmandu Univ Med J. 2016;14:69–72. [PubMed] [Google Scholar]
- 110.Subirana Magdaleno H., Caro Tarrago A., Olona Casas C., Diaz Padillo A., Franco Chacon M., Vadillo Bargallo J., et al. Evaluation of the impact of preoperative education in ambulatory laparoscopic cholecystectomy. A prospective, double-blind randomized trial. Cir Esp. 2018;96:88–95. doi: 10.1016/j.ciresp.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Blay N., Donoghue J. The effect of pre-admission education on domiciliary recovery following laparoscopic cholecystectomy. Aust J Adv Nurs. 2005;22:14–19. [PubMed] [Google Scholar]
- 112.de Aguilar-Nascimento J.E., Leal F.S., Dantas D.C., Anabuki N.T., de Souza A.M., Silva E.L.V.P., et al. Preoperative education in cholecystectomy in the context of a multimodal protocol of perioperative care: a randomized, controlled trial. World J Surg. 2014;38:357–362. doi: 10.1007/s00268-013-2255-7. [DOI] [PubMed] [Google Scholar]
- 113.Holte K., Klarskov B., Christensen D.S., Lund C., Nielsen K.G., Bie P., et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double-blind study. Ann Surg. 2004;240:892–899. doi: 10.1097/01.sla.0000143269.96649.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yao L., Wang Y., Du B., Song J., Ji F. Comparison of postoperative pain and residual gas between restrictive and liberal fluid therapy in patients undergoing laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2017;27:346–350. doi: 10.1097/sle.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 115.Belavic M., Sotosek Tokmadzic V., Brozovic Krijan A., Kvaternik I., Matijas K., Strikic N., et al. A restrictive dose of crystalloids in patients during laparoscopic cholecystectomy is safe and cost-effective: prospective, two-arm parallel, randomized controlled trial. Ther Clin Risk Manag. 2018;14:741–751. doi: 10.2147/tcrm.S160778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bonventre S., Inviati A., Di Paola V., Morreale P., Di Giovanni S., Di Carlo P., et al. Evaluating the efficacy of current treatments for reducing postoperative ileus: a randomized clinical trial in a single center. Minerva Chir. 2014;69:47–55. [PubMed] [Google Scholar]
- 117.Caliskan N., Bulut H., Konan A. The effect of warm water intake on bowel movements in the early postoperative stage of patients having undergone laparoscopic cholecystectomy: a randomized controlled trial. Gastroenterol Nurs. 2016;39:340–347. doi: 10.1097/sga.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 118.Farley D.R., Greenlee S.M., Larson D.R., Harrington J.R. Double-blind, prospective, randomized study of warmed, humidified carbon dioxide insufflation vs standard carbon dioxide for patients undergoing laparoscopic cholecystectomy. Arch Surg. 2004;139:739–743. doi: 10.1001/archsurg.139.7.739. discussion 43-4. [DOI] [PubMed] [Google Scholar]
- 119.Mouton W.G., Bessell J.R., Millard S.H., Baxter P.S., Maddern G.J. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Surg Endosc. 1999;13:106–108. doi: 10.1007/s004649900915. [DOI] [PubMed] [Google Scholar]
- 120.Wang L., Baser O., Wells P., Peacock W.F., Coleman C.I., Fermann G.J., et al. Benefit of early discharge among patients with low-risk pulmonary embolism. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185022. e0185022-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Appleby J. Day case surgery: a good news story for the NHS. BMJ. 2015;351:h4060. doi: 10.1136/bmj.h4060. [DOI] [PubMed] [Google Scholar]
- 122.Solodkyy A., Hakeem A.R., Oswald N., Di Franco F., Gergely S., Harris A.M. ‘True Day Case’ laparoscopic cholecystectomy in a high-volume specialist unit and review of factors contributing to unexpected overnight stay. Minim Invasive Surg. 2018;2018:1260358. doi: 10.1155/2018/1260358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith I., Cooke T., Jackson I., Fitzpatrick R. Rising to the challenges of achieving day surgery targets. Anaesthesia. 2006;61:1191–1199. doi: 10.1111/j.1365-2044.2006.04875.x. [DOI] [PubMed] [Google Scholar]
- 124.NCPS . Royal College of Surgeons in Ireland; 2014. National clinical programme in surgery: care pathway for the management of day case laparoscopic cholecystectomy.https://www.rcsi.com/surgery/-/media/feature/media/download-document/surgery/practice/ncp/surgery/publications/care-pathway-for-the-management-of-day-case-laparoscopic-cholecystectomy.pdf Available from: [Google Scholar]
- 125.Health service executive: management data report. Health Serv Exec. September 2019 https://www.hse.ie/eng/services/publications/performancereports/september-management-data-report.pdf [Internet] Available from: [Google Scholar]
- 126.Kehlet H. ERAS implementation-time to move forward. Ann Surg. 2018;267:998–999. doi: 10.1097/sla.0000000000002720. [DOI] [PubMed] [Google Scholar]
- 127.Hu Q.L., Liu J.Y., Hobson D.B., Cohen M.E., Hall B.L., Wick E.C., et al. Best practices in data use for achieving successful implementation of enhanced recovery pathway. J Am Coll Surg. 2019;229 doi: 10.1016/j.jamcollsurg.2019.08.1448. 626–632.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.