Abstract
Objectives
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, also known as COVID-19 pandemic has caused an alarming situation worldwide. Since the first detection, in December 2019, there have been no effective drug therapy options for treating the SARS-CoV-2 pandemic. However, healthcare professionals are using chloroquine, hydroxychloroquine, remdesivir, convalescent plasma and some other options of treatments. This study aims to compare the biological, molecular, pharmacological, and clinical characteristics of these three treatment modalities for SARS-COV-2 infections, Chloroquine and Hydroxychloroquine, Convalescent Plasma, and Remdesivir.
Methods
A search was conducted in the “Institute of Science Information (ISI)-Web of Science, PubMed, EMBASE, ClinicalTrials.gov, Cochrane Library databases, Scopus, and Google Scholar” for peer reviewed, published studies and clinical trials through July 30, 2020. The search was based on keywords “COVID-19” SARS-COV-2, chloroquine, hydroxychloroquine, convalescent plasma, remdesivir and treatment modalities.
Results
As of July 30, 2020, a total of 36,640 relevant documents were published. From them 672 peer reviewed, published articles, and clinical trials were screened. We selected 17 relevant published original articles and clinical trials: 05 for chloroquine and/or hydroxychloroquine with total sample size (n = 220), 05 for Remdesivir (n = 1,781), and 07 for Convalescent Plasma therapy (n = 398), with a combined total sample size (n = 2,399). Based on the available data, convalescent plasma therapy showed clinical advantages in SARS-COV-2 patients.
Conclusions
All three treatment modalities have both favorable and unfavorable characteristics, but none showed clear evidence of benefit for early outpatient disease or prophylaxis. Based on the current available data, convalescent plasma therapy appears to show clinical advantages for inpatient use. In the future, ongoing large sample size randomized controlled clinical trials may further clarify the comparative efficacy and safety of these three treatment classes, to conclusively determine whom to treat with which drug and when to treat them.
Keywords: COVID-19, Hydroxychloroquine, Chloroquine, Remdesivir, Convalescent Plasma
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, also known as the COVID-19 pandemic has wreaked havoc globally. As of July 30, 2020, over 216 countries had been affected, and the virus had infected 17,106,007 people with a fatality rate of 668,910 (3.91%) (World Health Organization, 2020). Human infections with SARS-CoV-2 have raised great public health and socioeconomic concern all around the world (Meo et al., 2020a, Meo et al., 2020b). The COVID-19 pandemic-related healthcare crisis is partly due to the absence of well-established therapeutic tools to limit the spread of this pandemic.
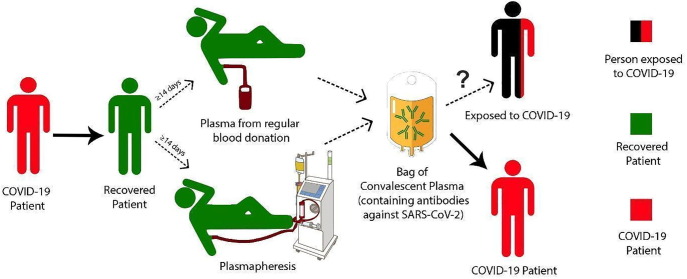
Chloroquine and hydroxychloroquine are well known for their antimalarial efficacy, and hydroxychloroquine is also widely used for rheumatoid arthritis and lupus. Recently these two 4-aminoquinolines have been recommended to treat SARS-CoV-2 infections and other coronaviruses because they can inhibit viral entry (Devaux et al., 2020). Meanwhile, immediate passive immunization through convalescent plasma transfusion acts as a natural source of pathogen-specific antibodies that can counteract viral infection (Fig. 1 ). The broad-spectrum class of viral RNA-dependent RNA polymerases (RdRp) inhibitors (including remdesivir) is used to treat medically important viral diseases. Remdesivir has recently been advanced to clinical trials for SARS-CoV-2 infections with some promising features (Shannon et al., 2020).
Fig. 1.
Convalescent plasma donation and transfusion cycle for treatment of SARS-CoV-2 patients.
These three treatment modalities have a mixed history of success in various cultural and economic contexts. Since the first detection of SARS-CoV-2 in December 2019, there is still no effective drug therapy available for this infection. The present study aims to compare the biological, pharmacological, and clinical characteristics of three treatment modalities: 1) chloroquine and hydroxychloroquine, 2) convalescent plasma, and 3) remdesivir for the COVID-19 Pandemic. This study looks to identify the best option among these three treatments for SARS-CoV-2 infected patients in various stages of infection.
2. Materials and methods
We searched clinical trials and original studies available as of July 30, 2020 that were written in English or contained an abstract written in English about the biological, pharmacological and clinical characteristics of chloroquine or hydroxychloroquine, convalescent plasma, and remdesivir for treating SARS-CoV-2 infection.
The data was obtained from the World Health Organization (2020) and articles published in the Institute of Scientific Information (ISI) Web of Knowledge, Thomson Reuter journals, PubMed, Medline, and clinical trial registries. The studies were explored through the search term “COVID-19” “SARS-COV-2” and/or keywords or key phrases including: 1) antiviral drugs, 2) chloroquine, 3) hydroxychloroquine, 4) convalescent plasma, and 5) remdesivir.
We searched chloroquine and/or hydroxychloroquine as the representative therapy for the viral entry inhibitor treatment modality. Convalescent plasma represented the passive immunity treatment modality. Finally, remdesivir represented the RdRp inhibitor treatment modality.
Two co-authors carefully reviewed all the documents; each article was selected based on its title and abstract. Moreover, two more co-authors further confirmed the selection of these articles. We excluded brief communications, letters to the editor, review articles, systematic reviews, case studies, articles published without peer review, and articles only available on pre-print websites. Moreover, we also excluded articles that were published but later retracted from the journal(s). Our selected articles had to denote a specific treatment and assess whether there was an improvement based on standardized factors that we included in our selection. After the relevant studies were identified, we compared eight biological and pharmacological features for the three modalities, including 1) origin, 2) drug delivery route, 3) metabolites, 4) half-life, 5) half maximal effective drug concentration (EC50) values, 6) absorption, 7) pathway for excretion, and 8) mechanism of action (Table 1 ). We also compared three clinical characteristics for the three modalities, including 1) contraindications, 2) side effects, and 3) safety precautions. Based on the current available data, findings were transferred on a sheet and were tabulated and analyzed.
Table 1.
Comparison of biological and pharmacological characteristics of chloroquine/hydroxychloroquine, remdesivir and convalescent plasma therapy for treatment of SARS-CoV-2 patients.
| Pharmacological Characteristics | Chloroquine and Hydroxychloroquine | Convalescent Plasma Therapy | Remdesivir |
|---|---|---|---|
| Origin | Cinchona bark derived | Human derived | Synthetic |
| Drug Delivery Route | Oral (Pastick et al., 2020) | Intravenous transfusion | Intravenous infusion |
| Metabolism | Chloroquine: metabolized in the liver into N-desethylchloroquine. (Projean et al., 2003). Hydroxychloroquine: metabolized in the liver into desethyl chloroquine (Lim et al., 2009). |
N/A because this is not a drug | Intracellularly metabolized into an analog of adenosine triphosphate (Jorgensen et al., 2020) |
| Half-life | Chloroquine: 20–60 days Hydroxychloroquine: 22.4 days (Pastick et al., 2020) |
IgG: 21 days IgM: 10 days (Rosado et al., 2020) |
Nucleotide triphosphate metabolite: 20 h |
| EC50 Value | Chloroquine: 23.90 μM Hydroxychloroquine: 6.14 μM (Sanders et al., 2020) |
N/A because this is not a drug | 0.77 μM (Sanders et al., 2020) |
| Absorption | Chloroquine: Rapid absorption (89%) by gastrointestinal tract hydroxychloroquine: Rapid absorption 74% by gastrointestinal tract. (Browning, 2014, Pastick et al., 2020). | N/A because this product is administered intravenously | N/A because this drug is administered intravenously |
| Pathway for excretion | Chloroquine: 50–60% excreted in urine, 10–20% as metabolite. Hydroxychloroquine: 50–60% excreted in the urine, 8–10% for chloroquine and 15–24% for hydroxychloroquine eliminated through the feces. 5% is sloughed off in skin, 2% excretion in breast milk (Browning, 2014, McCarthy and Price, 2015) |
Proteins in plasma are thought to be broken down in the liver and recycled for use in other proteins and tissue (Kelley and Roberts, 1956) | Renal and hepatic. Low renal excretion, 49%excreted in urine GS‐as 441,524 in urine And small percentage in excreted in feces (Singh et al., 2020, Jorgensen et al., 2020) |
| Mechanism of action | Increases endosomal pH and interferes with glycosylation of SARS-CoV-2 cell surface receptors to prevent virus binding to target cells (Pastick et al., 2020). Reduces mitogen-activated protein kinase activation, inhibiting virus replication, virion assembly, and budding (Devaux et al., 2020). | Provides immediate short-term immunization through antibodies contained in plasma that leads to viral neutralization. Suppression of viremia and other mechanisms like antibody-dependent cellular cytotoxicity (ADCC) may also play a role. | Metabolizes in active form. Interferes with action of RdRp, which is required for viral replication. Remdesivir acts as a disrupting nucleotide analog to stop replicative activity of RdRp. (Eastman et al., 2020) |
Ethical approval: For this study the data and related information were obtained from the publicly available websites, World Health Organization, Institute of Scientific Information (ISI) Web of Knowledge, PubMed, Medline, EMBASE, ClinicalTrials.gov, Cochrane Library databases, Scopus, and Google Scholar, hence ethical approval was not required.
3. Results
As of July 30, 2020, 36,640 published documents were initially identified. Of these, we screened 672 peer reviewed published articles and clinical trials. Finally, we identified 17 relevant published original articles and clinical trials: 05 for chloroquine and/or hydroxychloroquine, 07 for convalescent plasma therapy, and 05 for remdesivir.
For chloroquine and hydroxychloroquine, the 05 clinical trials contained sample sizes of n = 30, n = 62, n = 36, n = 11, and n = 81 (total n = 220) (Table 2 ). For convalescent plasma therapy for SARS-COV-2 infections, the 07 clinical trials contained sample contained with sample sizes of n = 103, n = 25, n = 5, n = 20, n = 46, n = 10n = 189 (total n = 398) (Table 3 ). For remdesivir, the 05 clinical trials contained sample sizes of n = 53, n = 397, n = 1,059, n = 237, n = 35 (total n = 1,781) (Table 4 ). The combined sample size for all of these studies was n = 2,389.
Table 2.
Clinical trial outcomes of viral entry inhibitor drugs (chloroquine/hydroxychloroquine) for SARS-CoV-2 patients.
| Author (s) and year of study | Type of study | Dosage of Viral Entry Inhibitor | Study Outcomes |
|---|---|---|---|
| Chen et al., 2020a | Randomized controlled trial. Sample size: 30 | 400 mg hydroxychloroquine per day for five days | On day seven, throat swabs were negative for 13 (86.7%) cases in the hydroxychloroquine group and 14 (93.3%) cases in control group. |
| Chen et al., 2020b | Randomized controlled trial. Sample size: 62 | 400 mg hydroxychloroquine per day for five days | Temperature and cough remission times were shortened in the hydroxychloroquine group. Pneumonia improved in the hydroxychloroquine group (80.6%) compared to the control (54.8%). |
| Gautret et al., 2020 | Single arm, controlled trial. Sample size: 36 |
200 mg hydroxychloroquine three times per day for ten days | Hydroxychloroquine treatment was supplemented with azithromycin in six patients, treatment was significantly effective for clearing the viral load. |
| Molina et al., 2020 | Uncontrolled Clinical trial. Sample size: 11 |
600 mg hydroxychloroquine per day for 10 days. 500 mg azithromycin on day one, then 250 mg azithromycin per day for four days | No evidence of viral clearance in severe COVID-19 patients as shown by PCR assays. In one patient, treatment was halted because of prolonged QT interval. |
| Borba et al., 2020 | Double-masked, randomized clinical trial. Sample size: 81 |
High dose group: 600 mg chloroquine twice per day for 10 days Low dose group: 450 mg chloroquine twice on day one then once per day for the following four days All patients also received azithromycin and most received oseltamivir. |
No apparent benefit of chloroquine was found, seven patient of high-dose group developed prolonged QT interval compared to four of low-dose group. Five of the high-dose group had a history of heart disease compared to zero of the low-dose group. |
Table 3.
Clinical trial outcomes of convalescent plasma for SARS-CoV-2 patients.
| Author (s) and year of study | Type of study | Dosage of convalescent plasma | Study outcomes |
|---|---|---|---|
| Li et al., 2020 | Randomized clinical trial. Sample size: 103 | 4–13 mL convalescent plasma/kg of body weight; Transfusion rate: 100 mL per hour. | The treatment was associated with a higher negative conversion rate of viral PCR at 72 h (87.2% vs 37.5% of the control group. But it did not result in a statistically significant to clinical improvement within 28 days (51.9% of the CP group vs 43.1% in control group. |
| Salazar et al., 2020 | Uncontrolled trial. Sample size: 25 |
One dose of 300 mL convalescent plasma. | On day seven of transfusion, 9 patients improved, 13 patients had no change, 3 patients deteriorated, and one patient died. No adverse events found within 24 h of transfusion. |
| Shen et al., 2020 | Uncontrolled trial. Sample size: 05 | 2 consecutive transfusions of 200–250 mL convalescent plasma. All patients also received antiviral agents and methylprednisolone. | In four of the five patients, body temperatures normalized, SOFA scores decreased and PAO2/FIO2 increased. 12 days after transfusion, viral loads cleared, patients improved one week after transfusion. |
| Duan et al., 2020 | Cohort non-randomized, controlled trial. Sample size: 20 | One dose of 200 mL convalescent plasma. | Clinical symptoms, CT scan and laboratory parameters improved three days after convalescent plasma therapy. No adverse events observed. In a control group of ten patients, seven stable, and three died. |
| Cesare Perotti et al., 2020 | Multicenter study sample size 46 | Each patient received two units and one patient received three units. For first infusion had a titer of 1:160 or 1:320; one patient received 1:80. At the second infusion titers were 1:80, 1:160, 1:320. The third infusion had a titer of 1:320 | Convalescent plasma therapy reduced mortality from 15% expected to 6% observed. |
| Olivares-Gazca et al., 2020 | Clinical trial, sample size 10 | As per standard procedure | 7 patients on chest x-ray, 6 patients on CT scans showed improvement of the lung injury. Decreases in C-reactive protein and D-dimer levels. Three of five patients on mechanical ventilation support were extubated and two patients died. |
| Abolghasemi et al., 2020 | Multicenter study sample size 189; 115 received plasma therapy and 74 were in control group | First time, 500 cc plasma was infused, if no response, after 24 h another unit of plasma was also administered. | Length of hospital stay was significantly lower (9.54 days) for the convalescent plasma therapy group compared to the control group (12.88 days). 7% of patients in convalescent plasma group required intubation while 20% required intubation in the control group. |
Table 4.
Clinical trial outcomes of an RdRp inhibitor drug (remdesivir) for SARS-CoV-2 patients.
| Author (s) and year of study | Types of study | Dosage of RdRp Inhibitor | Study Outcomes |
|---|---|---|---|
| Grein et al., 2020 | Uncontrolled, non-randomized clinical trial. Sample size: 53 |
40 patients: 200 mg of remdesivir intravenously on day one, then 100 mg of remdesivir once per day for 9 days 10 patients: 5–9 days of treatment 3 patients: < 5 treatments |
Improvement in oxygen-support in 36 of 53 patients, and 17 of 30 patients receiving mechanical ventilation were extubated. Eight patients showed worsening symptoms and seven of the patients passed away after treatment. 32 patients experienced side effects. |
| Goldman et al., 2020 | Randomized clinical trial. Sample size: 397 |
200 patients: 5 day treatment. 197 patients: 10 day treatment. Day 1: 200 mg remdesivir. Following days: 100 mg remdesivir once daily. |
No significant difference between five-day and ten-day courses of remdesivir; without control group magnitude of benefit could not be assessed. |
| Beigel et al., 2020 | Double blind, randomized clinical trial. Sample size: 1059 | Intravenous remdesivir. Day 1: 200 mg, following days 100 mg per day for nine days. |
Patients who received treatment with remdesivir had a shortened recovery time. |
| Wang et al., 2020 | Double blind, randomized Clinical trial. Sample size: 237 | Intravenous remdesivir. Day 1: 200 mg remdesivir. Days 2–10: 100 mg remdesivir once per day. | Remdesivir was not associated with statistically significant clinical benefits. |
| Antinori et al., 2020 | Double blind, randomized Clinical trial. Sample size: 35 | 10-days course of remdesivir | 22 patients completed the 10-day courser, while 13 discontinued, because of adverse events. On day 28, 14 patients were discharged, and one was died. In ICU, 6 were discharged, 8 patients died, three stayed on mechanical ventilation and one improved. The adverse events were hypertransaminasemia and acute kidney injury. |
3.1. Biological, molecular and pharmacological characteristics
Regarding biological, molecular and pharmacological characteristics, we compared two viral entry inhibitors in the 4-aminoquinoline family (hydroxychloroquine and chloroquine) against immediate passive immunization and RdRp-inhibition to assess which mechanism of action could be more useful for treating SARS-CoV-2 infections. The 4-aminoquinolines were originally derived from cinchona bark. Hydroxychloroquine and chloroquine are now synthetically produced (Permin et al., 2016). Convalescent plasma is purified from human plasma. Remdesivir is a synthetic nucleotide prodrug that is metabolized to an active nucleoside analogue. Regarding the drug delivery routes, both remdesivir and convalescent therapy are administered intravenously, while the two 4-aminoquinolines are administered orally. (Projean et al., 2003). Convalescent plasma therapy confers passive immunity by providing antibodies, and is active upon administration. Remdesivir is a prodrug, and is metabolized intracellularly into its active form.
Comparing the half-lives of the representative examples from the three modalities, hydroxychloroquine and chloroquine have the longest half-lives, followed by convalescent plasma, and then by remdesivir (Pastick et al., 2020, Rosado et al., 2020). The long half-lives of the 4-quinolines means that an accidental overdose of one of these drugs can cause prolonged toxicity. Considering EC50 values, only the 4-aminoquinolines and remdesivir can be compared, since convalescent plasma therapy is not a drug. For 4-aminoquinolines, hydroxychloroquine may be the safer option compared to chloroquine, because of its lower EC50 value. Although convalescent plasma therapy does not have an EC50 value since it is not a drug, it requires a minimum antibody titer of 1:160. When comparing the absorption pathway, the 4-aminoquinolines are rapidly absorbed by the gastrointestinal tract, allowing these drugs to be administered in tablet form (Browning, 2014) rather than the intravenous infusion route necessary for convalescent plasma therapy and remdesivir. Regarding, the pathways for excretion, the 4-aminoquinolines are excreted by both the kidneys and liver. In the cases of renal failure or hepatic failure the doses should be decreased. Immunoglobulins in convalescent plasma therapy are recycled and excreted (Duan et al., 2020). The remdesivir has almost complete clearance upon first pass metabolism in the liver. The antiviral mechanisms, hydroxychloroquine/chloroquine prevent viral binding to cell surface receptors (Devaux et al., 2020). Convalescent plasma confers immunity to the patient through the transfusion of a recovered patient’s antibodies rather than affecting the patient’s cells directly (Gasparyan et al., 2020). Remdesivir prevents replication of intracellular virus particles by inhibiting RdRp (Eastman et al., 2020).
3.2. Clinical trials based findings
A comparison of three clinically important characteristics for a representative example of each treatment is presented in Table 5 . These three features include: 1) contraindications, 2) side effects, and 3) safety precautions. Regarding contraindications, convalescent plasma therapy, compared to the 4-aminoquinolines and remdesivir, has the fewest contraindications. In addition, the side effects of convalescent plasma therapy, compared to the 4-aminoquinolines and remdesivir, tend to be the least debilitating. The potential worst side effects for remdesivir and 4-aminoquinolines, compared to those of convalescent plasma, tend to be more severe, because 4-aminoquinolines may prolong the QTc interval, which can lead to serious arrhythmias (Borba et al., 2020, Giudicessi et al., 2020, Molina et al., 2020), and remdesivir may cause liver failure (Antinori et al., 2020). (Table 5) A concern for convalescent plasma treatment is transfusion-transmitted infection, but through pathogen inactivation measures, this risk can be eliminated (Gajic et al., 2007). Among the seven reviewed convalescent plasma studies, only two patients (both from the Li et al. 2020 study) were reported to have adverse reactions to convalescent plasma, and these resolved within a few hours.
Table 5.
Comparison of clinical characteristics between representative examples of viral entry inhibitors, immediate passive immunization, and an RdRp inhibitor for the treatment of SARS-CoV-2 infected patients.
| Clinical Characteristics | Chloroquine and Hydroxychloroquine | Convalescent Plasma Therapy | Remdesivir |
|---|---|---|---|
| Contra- indications | Coronary artery disease, myocardial infarction, arrhythmias, eye disease, and/or glucose-6-phosphate dehydrogenase (G6PD) deficiency (Blignaut et al., 2019, Braga et al., 2015, Devine et al., 2017, Giudicessi et al., 2020). | IgE antibodies against IgA in blood products or a history of severe reactions to plasma (Braga et al., 2015). | Abnormal liver function or decreased estimated glomerular filtration rate (Antinori et al., 2020; (Jorgensen et al., 2020) |
| Side effects | Nausea, vomiting, diarrhea, skin rash, arrhythmias, ventricular tachycardia, blurred vision, paresthesia, and/or insomnia (Blignaut et al., 2019, Braga et al., 2015, Devine et al., 2017, Giudicessi et al., 2020). | Allergic reactions, circulatory overload, or acute lung injury (Gajic et al., 2007, Li et al., 2020). | Nausea, vomiting, abnormal liver function (Antinori et al., 2020) |
| Safety precautions | Monitor plasma levels of K+, Mg2+, & Ca2 + . Check the QTc interval, and watch for any concurrent medications that may prolong QTc; if yes, then monitor QTc during therapy. Check G6PD status. (Devine et al., 2017, Giudicessi et al., 2020) | Consenting donors should have anti-SARS-CoV-2 antibodies. (Duan et al., 2020) | Check the status of liver function and kidney function (Antinori et al., 2020). |
4. Discussion
The present study compares the biological, molecular, pharmacological and clinical characteristics of 1) chloroquine and hydroxychloroquine, 2) convalescent plasma, and 3) remdesivir for treating SARS-COV-2 infections. We found that each treatment modality has both favorable and unfavorable characteristics, and may be more effective at one stage of the disease than another. Based on the current available findings, convalescent plasma therapy appeared to show clinical advantages, but none of these three treatment modalities showed clear evidence of benefit for outpatient disease or prophylaxis.
4.1. Chloroquine and hydroxychloroquine
Hydroxychloroquine and chloroquine inhibit SARS-COV-2 binding to the ACE-2 receptor. These drugs can be engulfed into endosomes and lysosomes, which will lead to increased pH in these cellular compartments, which in turn impedes membrane fusion. Finally, hydroxychloroquine and chloroquine decrease cytokine release, which could help mitigate the cytokine storm that can occur in SARS-CoV-2-infected patients (Pastick et al., 2020). Hydroxychloroquine has been postulated to be less toxic than chloroquine (Zhou et al., 2020). Chloroquine promotes the cytosolic uptake of zinc, which has an antiviral effect by disrupting RdRP activity (Skalny et al., 2020). Azithromycin has also been proposed as an adjunct to hydroxychloroquine/chloroquine therapy, but it has not been rigorously tested (Gautret et al., 2020). Regarding cardiac risks of 4-aminoquinolines, studies have found prolongation of QTc interval. In a study conducted by (Molina et al. 2020), one out of eleven patients had to terminate treatment with hydroxychloroquine early because of the QTc risk. In a study by Borba et al. (2020, 11) out of 81 patients were diagnosed with a prolonged QTc interval following treatment with hydroxychloroquine (Table 2).
A recent study by (Mazzanti et al., 2020) did not demonstrate a significant difference in QTc intervals between hydroxychloroquine versus hydroxychloroquine/azithromycin and/or lopinavir/ritonavir combination therapies. The authors concluded the effect of hydroxychloroquine on QTc interval prolongation would be reduced by the short course of treatment used for SARS-CoV-2 patients, since hydroxychloroquine therapy does not reach a steady state until after 180 days of therapy. In a study by (Maraj et al., 2020), 23% of 91 patients who were treated with unspecified doses of hydroxychloroquine combined with azithromycin experienced prolonged QTc intervals.
4.2. Convalescent plasma
Convalescent plasma (CP) therapy, a classic adaptive immunotherapy, has been applied to the prevention and treatment of many infectious diseases for over a century. Convalescent plasma is being considered as a viable treatment for SARS-CoV-2 infections because it can provide short-term, immediate immune protection. Previously, it was discovered that neutralizing antibodies (NAbs) were able to bind spike-receptor binding proteins on the surface of SARS-Cov and Middle East Respiratory Syndrome (MERS) viruses. The antibodies in CP bind to the SARS-Cov-2 viruses, inhibiting them from binding to cells (Rojas et al., 2020).
Convalescent plasma, compared to remdesivir and hydroxychloroquine or chloroquine, has demonstrated the least severe side effects. In seven studies of convalescent plasma for SARS-CoV-2 infections, six of them showed improvement in patient conditions after treatment (Abolghasemi et al., 2020, Cesare Perotti et al., 2020, Duan et al., 2020, Olivares-Gazca et al., 2020, Salazar et al., 2020, Shen et al., 2020), and one study showed no improved outcomes (Li et al., 2020).
(Cesare Perotti et al., 2020) conducted a multicenter a study on 46 COVID-19 patients, mean age 63 years with bilateral infiltrates on chest X-ray in 36 patients. Forty three patients were alive and 3 patients (6.5%) died within 7 days. The PaO2/FiO2 (a biomarker of acute respiratory distress syndrome) (Fujishima 2014) improved by 112 units in survivors, chest radiogram severity decreased in 23%, ferritin and LDH decreased.
Similarly, (Olivares-Gazca et al., 2020) conducted a clinical trial on 10 patients who were treated with convalescent plasma. Over 8 days, the sequential organ failure assessment score dropped in all patients, body temperature, and ferritin levels decreased, chest X-rays improved in 7/10 cases, and computerized tomography scans of injured lungs improved in 6/10 patients.
(Abolghasemi et al., 2020) performed a multicenter non-randomized non-placebo-controlled study, with sample size 189; 115 received plasma therapy and 74 were in the control group. Following a 500 cc plasma infusion, if no response occurred, then after 24 h another unit of plasma was administrated. Survival was 98 of 115 (85.2%) for convalescent plasma patients and 56 of 74 (75.7%) for control patients (p = 0.09). The hospital stay duration was significantly lower (9.54 days) in the convalescent plasma group than in the control group (12.88 days, p = 0.002). This study provided evidence to support the efficacy of convalescent plasma therapy in SARS-COV-2 patients.
4.3. Remdesivir
In the five studies we reviewed that used remdesivir for SARS-CoV-2 treatment, we found varying results, including improvement (Antinori et al., 2020, Beigel et al., 2020, Grein et al., 2020), no change (Goldman et al., 2020, Wang et al., 2020), and worsening (Antinori et al., 2020, Grein et al., 2020). Remdesevir is designed to inhibit viral RdRp, an enzyme that is integral to viral RNA replication. Without viral RNA replication, the virus is unable to multiply and spread to the infected host’s other cells. Remdesivir is not a strong inhibitor of mammalian polymerases, and there is low risk for toxicity in human mitochondria. Remdesivir, a broad-spectrum antiviral drug, has been shown to counteract infections by other viruses, including coronaviruses like SARS-CoV and MERS (Brown et al., 2019).
(Antinori et al., 2020) conducted a prospective open-label clinical trial on remdesivir. They enrolled 35 patients with SARS-CoV-2 pneumonia, ages ≥ 18 years; 18 patients were in the intensive care unit, and 17 were in an infectious diseases unit. A 10-day course of remdesivir was completed by 22 patients (62.8%), and discontinued by 13 (37.1%). In the infectious diseases unit 14 (82.3%) patients were discharged, and one patient died (5.6%), whereas in the ICU 6 (33.3%) were discharged, and 8 (44.4%) patients died; the remaining patients were still hospitalized at study completion. The authors identified acute liver and kidney injury as adverse events. (Wang et al., 2020) reported a randomized, double-blind, placebo-controlled, multicenter trial in ten hospitals. They enrolled 237 patients, and randomly assigned 158 to Remdesivir, and 79 to placebo. Remdesivir use was not associated with a difference in time to clinical improvement than placebo. Adverse events were reported in 102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients.
In another randomized, open-label, clinical trial, (Goldman et al., 2020) studied 397 hospitalized SARS-CoV-2 patients, randomly assigned to receive intravenous remdesivir (200 patients for 5 days) and 197 for 10 days. All patients received 200 mg of remdesivir on day 1 and 100 mg once daily on subsequent trial days. The patients showed no significant difference in a clinical status scale between a 5-day course and a 10-day course of Remdesivir. The most common adverse events were nausea, worsening respiratory failure, elevated alanine aminotransferase level, and constipation. In addition, (Grein et al., 2020) performed an uncontrolled study of remdesivir treatment on 53 patients. During a median follow-up period of 18 days, 25 patients (47%) were discharged, and 7 patients (13%) died. In this cohort of patients, clinical improvement was observed in 36 of 53 patients (68%).
(Beigel et al., 2020) reported a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with SARS-COV-2 patients. A total of 1059 patients underwent randomization (538 assigned to remdesivir and 521 to placebo). Those who received remdesivir (200 mg on day 1, followed by 100 mg daily for 9 days) had a median recovery time of 11 days as compared with 15 days those who received placebo (rate ratio for recovery, 1.32; 95% CI, 1.12 to 1.55; P < 0.001). The estimates of mortality by 14 days were 7.1% with remdesivir and 11.9% with placebo (hazard ratio for death, 0.70; 95% CI, 0.47 to 1.04). Adverse events were reported for 114 (21.1%) remdesivir-treated patients and 141 (27.0%) placebo-treated patients.
5. Strengths and limitations
This is the first article to our knowledge that has compared the biological, pharmacological, and clinical features of chloroquine and/or hydroxychloroquine, remdesivir, and convalescent plasma as treatment modalities for SARS-CoV-2 patients. Another strength is that the clinical study data was gathered using reliable sources, including Web of Science, Pub-Med, Medline, EMBASE, and Scopus databases. A limitation of this study is the limited number of published randomized controlled trials for any of the three modalities, and the absence of direct comparison studies between these three modalities for treating SARS-CoV-2 infections. Moreover, the literature does not provide a clear indication as to when in the course of the disease each medication was initiated, which limits a conclusion about whether there is an optimal time (early or late) in the course of SARS-CoV-2 infection for each type of treatment to be administered.
6. Conclusions
The mechanisms of action of the 4-aminoquinolines and remdesivir inhibit viral entry and replication, which would seem to be beneficial in earlier stages of viral infection. Convalescent plasma therapy strengthens the immune response to the virus, which would be beneficial for both early and late stage infections. The 4-aminoquinoline treatments might benefit from supplementation with zinc and/or azithromycin, however no efficacy results against COVID-19 with either of these combinations have been reported from well-controlled trials during the time period of our review. Immediate passive immunization in our series, although tested to only a limited extent, has generated more consistently positive outcomes in hospitalized patients than the two drug modalities. Only a limited supply of convalescent plasma is available, however, because this agent must be harvested from recovering COVID-19 patients, before their immunity dissipates. Large randomized controlled trials of all of these treatment modalities are currently underway and we expect them to provide valuable information for selecting therapy for SARS-CoV-2 infections in both early- and late-stage infection. Based on currently available data and clinical trials, convalescent plasma therapy appeared to show clinical advantages for inpatient use, but none of these three treatment modalities showed clear evidence of benefit for outpatient disease or prophylaxis. In the future the ongoing large sample size randomized controlled clinical trials may further clarify the comparative efficacy and safety of these three drugs, and elucidate a definitive approach as to whom to treat, and when to treat them.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Thankful to the “Researchers Supporting Project Number (RSP-2019/47), King Saud University, Riyadh, Saudi Arabia”.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abolghasemi H., Eshghi P., Cheraghali A.M., Imani Fooladi A.A., Bolouki Moghaddam F., Imanizadeh S., Moeini Maleki M., Ranjkesh M., Rezapour M., Bahramifar A., Einollahi B., Hosseini M.J., Jafari N.J., Nikpouraghdam M., Sadri N., Tazik M., Sali S., Okati S., Askari E., Tabarsi P., Aslani J., Sharifipour E., Jarahzadeh M.H., Khodakarim N., Salesi M., Jafari R., Shahverdi S. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus. Apheres. Sci. 2020:102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori S., Cossu M.V., Ridolfo A.L., Rech R., Bonazzetti C., Pagani G., Gubertini G., Coen M., Magni C., Castelli A., Borghi B., Colombo R., Giorgi R., Angeli E., Mileto D., Milazzo L., Vimercati S., Pellicciotta M., Corbellino M., Torre A., Rusconi S., Oreni L., Gismondo M.R., Giacomelli A., Meroni L., Rizzardini G., Galli M. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 2020;158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel, J.H., Tomashek, K.M., Dodd, L.E., Mehta, A.K., Zingman, B.S., Kalil, A.C., Hohmann, E., Chu, H.Y., Luetkemeyer, A., Kline, S., et al., 2020. Remdesivir for the Treatment of Covid-19 Preliminary Report. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2007764. [DOI] [PubMed]
- Browning, D.J., Pharmacology of Chloroquine and Hydroxychloroquine Hydroxychloroquine and Chloroquine Retinopathy. 2014; 4: 35-63.
- Blignaut M., Espach Y., vanVuuren M., Dhanabalan K., Huisamen B. Revisiting the Cardiotoxic Effect of Chloroquine. Cardiovasc. Drugs. Ther. 2019;33:1–11. doi: 10.1007/s10557-018-06847-9. [DOI] [PubMed] [Google Scholar]
- Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Jr, Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.-T., Monteiro W.M., Lacerda M.V.G. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- Braga C.B.e., Martins A.C., Cayotopa A.D.E., Klein W.W., Schlosser A.R., Silva A.F.d., Souza M.N.d., Andrade B.W.B., Filgueira-Júnior J.A., Pinto W.d.J., da Silva-Nunes M. Side Effects of Chloroquine and Primaquine and Symptom Reduction in Malaria Endemic Area (Mâncio Lima, Acre, Brazil) Interdiscip. Perspect. Infect. Dis. 2015;2015:1–7. doi: 10.1155/2015/346853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare Perotti, Fausto Baldanti, Raffaele Bruno, Claudia Del Fante, Elena Seminari, Salvatore Casari , Elena Percivalle, Claudia Glingani et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica, 2020; haematol.2020.261784. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed]
- Chen, J., Liu, D., Liu, L., Liu, P., Xu, Q., Xia, L., Ling, Y., Huang, D., Song, S., Zhang, D., Qian, Z., Li, T., Shen, Y., Lu, H. Zhejiang da xue bao. Yi xue ban. Journal of Zhejiang University. Medical sciences. 2020; 49 (2), 215-219. [DOI] [PMC free article] [PubMed]
- Chen, Z., Hu, J., Zhang, Z., Jiang, S., Han, S., Yan, D., Zhuang, R., Hu, B., Zhang, Z., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. CC-BY-NC-ND 4.0 International license. doi: https://doi.org/10.1101/2020.03.22.20040758.
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19. Int. J Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine A., Parmiter M., Chu C.S., Bancone G., Nosten F., Price R.N., Lubell Y., Yeung S. Using G6PD tests to enable the safe treatment of Plasmodium vivax infections with primaquine on the Thailand-Myanmar border: a cost-effectiveness analysis. PLoS Negl. Trop. Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L.i., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L.i., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajic O., Rana R., Winters J.L., Yilmaz M., Mendez J.L., Rickman O.B., O'Byrne M.M., Evenson L.K., Malinchoc M., DeGoey S.R., et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am. J. Respir. Crit. Care Med. 2007;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparyan A.Y., Misra D.P., Yessirkepov M., Zimba O. Perspectives of immune therapy in coronavirus disease 2019. J. Korean Med. Sci. 2020;35(18) doi: 10.3346/jkms.2020.35.e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin. Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, J.D., Lye, D., Hui, D.S., Marks, K.M., Bruno, R., Montejano, R., Spinner, C. D., Galli, M., Ahn, M. Y., Nahass, R.G., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med., 2020; 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S.C.J., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre‐clinical data, and emerging clinical experience for COVID‐19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M.B., Roberts S. Metabolism of plasma proteins in vitro. J. Biol. Chem. 1956;222(2):555–564. [PubMed] [Google Scholar]
- Lim H.-S., Im J.-S., Cho J.-Y., Bae K.-S., Klein T.A., Yeom J.-S., Kim T.-S., Choi J.-S., Jang I.-J., Park J.-W. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. AAC. 2009;53(4):1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraj, I., Hummel, J.P., Taoutel, R., Chamoun, R., Workman, V., Li, C., Tran, L., DelVecchio, A., Howes, C., Akar, J.G. Incidence and Determinants of QT Interval Prolongation in COVID-19 Patients Treated with Hydroxychloroquine and Azithromycin. J. Cardiovasc Electrophysiol. 2020; 0.1111/jce.14594. [DOI] [PMC free article] [PubMed]
- Mazzanti A, Briani M, Kukavica D, Bulian F, Marelli S, Trancuccio A. Association of Hydroxychloroquine with QTc Interval in Patients with COVID-19. Circulation. 10.1161/CIRCULATIONAHA.120.048476. [DOI] [PubMed]
- McCarthy JS, Price RN. Antimalarial Drugs. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition), 2015; (1): 495-509.e5
- Meo, S.A., Alhowikan, A.M., Al-Khlaiwi, T., Meo, I.M., Halepoto, D.M., Iqbal, M., Usmani, A.M., Hajjar, W., Ahmed, N., 2020. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020; 24(4), 2012–2019. [DOI] [PubMed]
- Meo S.A., Al-Khlaiwi T., Usmani A.M., Meo A.S., Klonoff D.C., Hoang T.D. Biological and epidemiological trends in the prevalence and mortality due to outbreaks of novel coronavirus COVID-19. J. King Saud Univ. – Sci. 2020;32(4):2495–2499. doi: 10.1016/j.jksus.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Gazca J.C., Priesca-Marín J.M., Ojeda-Laguna M., Garces-Eisele J., Soto-Olvera S., Palacios-Alonso A., Izquierdo-Vega J., Chacon-Cano R., Arizpe-Bravo D., López-Trujillo M.A., Cantero-Fortiz Y., Fernandez- Lara D., Ruiz-Delgado G.J., Ruiz-Argüelles G.J. Infusion of convalescent plasma is associated with clinical improvement in critically Ill patients with COVID-19: a pilot study. RIC. 2020;72(3) doi: 10.24875/RIC.20000237. [DOI] [PubMed] [Google Scholar]
- Pastick K.A., Okafor E.C., Wang F., Lofgren S.M., Skipper C.P. Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open forum. Infect. Dis. 2020;7(4):ofaa130. doi: 10.1093/ofid/ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permin H., Norn S., Kruse E., Kruse P.R. On the history of Cinchona bark in the treatment of Malaria. Dansk medicinhistorisk arbog. 2016;44:9–30. [PubMed] [Google Scholar]
- Projean D., Baune B., Farinotti R., Flinois J.P., Beaune P., Taburet A.M., Ducharme J. In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation. Drug Metab. Dispos. Biol. Fate Chem. 2003;31(6):748–754. doi: 10.1124/dmd.31.6.748. [DOI] [PubMed] [Google Scholar]
- Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.-M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020;19(7):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado J., Cockram C., Merkling S.H., Demeret C., Meola A., Kerneis S., Terrier B. Serological signatures of SARS-CoV-2 infection: implications for antibody-based diagnostic. MedRxiv. 2020 doi: 10.1101/2020.05.07.20093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, E., Perez, K.K., Ashraf, M., Chen, J., Castillo, B., Christensen, P.A. et al. Treatment of COVID-19 Patients with Convalescent Plasma. Am. J. Pathol. 2020; S0002-9440(20)30257-1. [DOI] [PMC free article] [PubMed]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Shannon A., Le N.T., Selisko B., Eydoux C., Alvarez K., Guillemot J.C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Singh R., Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diab. Metab. Syndr.: Clin. Res. Rev. 2020;14(4):641–648. doi: 10.1016/j.dsx.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny, A.V., Rink, L., Ajsuvakova, O.P., Aschner, M., Gritsenko, V. A., Alekseenko, S. I. Zinc and respiratory tract infections: Perspectives for COVID‑19 (Review). International journal of molecular medicine. 2020; 46 (1): 17-26. [DOI] [PMC free article] [PubMed]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y.i., Luo G., Wang K.e., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y.i., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y.i., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization: Coronavirus, 2020. Available at: https://www.who.int/health-topics/coronavirus. Cited date: July 30, 2020.
- Zhou, D., Dai, S.M., Tong, Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020; 5 (7): 1667–1670. [DOI] [PMC free article] [PubMed]