Abstract
Objective
To review published economic evaluations of antiviral treatment for pandemics and outbreaks of respiratory illnesses.
Methods
We conducted a systematic review to identify economic evaluations of antiviral treatment for pandemics and outbreaks of respiratory illnesses, including coronavirus disease 2019 (COVID-19). We searched Medline (EBSCOhost), EMBASE (Ovid), EconLit (Ovid), National Health Service Economic Evaluation Database (Ovid), and Health Technology Assessment (Ovid). The search was last rerun on July 5, 2020. Citation tracking and reference checking were used. Only full economic evaluations published as peer-reviewed articles in the last 10 years were included. Studies were quality assessed using the National Institute for Health and Care Excellence economic evaluation checklist.
Results
Overall, 782 records were identified, of which 14 studies met the inclusion criteria. The studies were mostly conducted in high-income countries. All were model-based. Seven (50%) were cost-utility analyses, 4 (28.6%) were cost-effectiveness analyses, 2 (14.3%) were cost-consequences analyses, and 1 (7.1%) was a cost-benefit analysis. Strategies including antiviral treatment were found to be either cost-saving or cost-effective, at the study-specific willingness-to-pay thresholds. Empirical treatment was more cost-effective than test-guided treatment for young adults but less so for older adults.
Conclusions
Antiviral treatment for managing pandemics and outbreaks of respiratory illnesses that have very high case fatality rate, similar to COVID-19 pandemic, are likely to be cost-effective either as a standalone intervention or part of a multifaceted strategy. Investing in the development of such curative treatments and promptly evaluating their cost-effectiveness, relative to other strategies in use at the time of their introduction should be the focus going forward to inform resource allocation decisions particularly in low- and middle-income countries.
Keywords: pandemics, outbreaks, influenza, coronavirus, COVID-19, economic evaluation, systematic review, health technology assessment
Introduction
On December 31, 2019, a pneumonia of unknown cause detected in Wuhan, China was reported to the World Health Organization (WHO).1 The cause for this pneumonia has subsequently been identified as the new coronavirus severe acute respiratory syndrome coronavirus 2. This viral infection has spread across the globe, leading the WHO to declare it a pandemic. Just over 6 months later, the number of cases surpassed 10 million and the global death toll was reported to be over 500 000.2
The lack of both a protective vaccine and an effective treatment for this new coronavirus disease 2019 (COVID-19), coupled with the high rate of transmission, have made this a global challenge of unparalleled magnitude. Worldwide, governments had to implement 1 or more of a range of drastic control strategies from travel restrictions, school closures, social distancing, and self-isolation to city, regional, and country lockdowns, with their considerable economic consequences.3 The primary aim of these control measures is to flatten the pandemic spread curve to ensure that the pressure on their limited healthcare resources can be managed until a vaccine or an effective treatment is available.4
Lessons learned from previous pandemics and outbreaks like severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and swine flu (H1N1), however, show that pharmacological treatments for pandemics and outbreaks of respiratory illnesses can vary in effectiveness and, more importantly, cost-effectiveness depending on various factors.5 , 6 Assessing the cost-effectiveness of potential treatments for managing the current pandemic will be crucial to avoid wasting valuable time and resources and optimize their use prior to widespread provision.
A key mitigation strategy is the use of potentially curative treatments, such as antivirals. A number of these are currently being studied by the WHO, in its SOLIDARITY clinical trial for COVID-19 treatments and other trials, eg, remdesivir and interferon beta-1a.7 Drugs that are already approved or under investigation for other indications and could be repurposed for use in COVID-19 are of particular interest, as the use of repurposed drugs cuts down the development time and costs required to bring an effective treatment to the market.8 Economic evaluations of the use of these antivirals during previous pandemics and outbreaks can, thus, offer an important insight into their likely cost-effectiveness for treating COVID-19. They can also inform cost-effectiveness analysis of other treatment classes currently being assessed in clinical trials, by understanding the pandemic spread dynamics, its cost drivers, and the influential parameters driving cost-effectiveness. This will be valuable information for health technology assessment (HTA) organizations that will eventually appraise these treatments.
Hence, the aim of this study was to identify, critically appraise, and review published economic evaluations of antiviral treatment for the management of pandemics and outbreaks of respiratory illnesses, to provide an evidence base that could be used to inform economic evaluations and HTA of COVID-19 treatments as they become available. We also aimed to provide recommendations for constructing models for evaluating the cost-effectiveness of antiviral treatments in this context.
Methods
Data Sources
We conducted a targeted systematic review of the literature to identify full economic evaluations of antivirals as a treatment in pandemics and outbreaks of respiratory illnesses (MERS, SARS, H1N1, and COVID-19). The following databases were searched, from earliest available date: Medline (EBSCOhost), EMBASE (Ovid), EconLit (Ovid), National Health Service Economic Evaluation Database (Ovid), and Health Technology Assessment (Ovid). The searches were last run on July 5, 2020. Citation tracking of the key articles identified was undertaken as well as reference checking.
Search Strategies
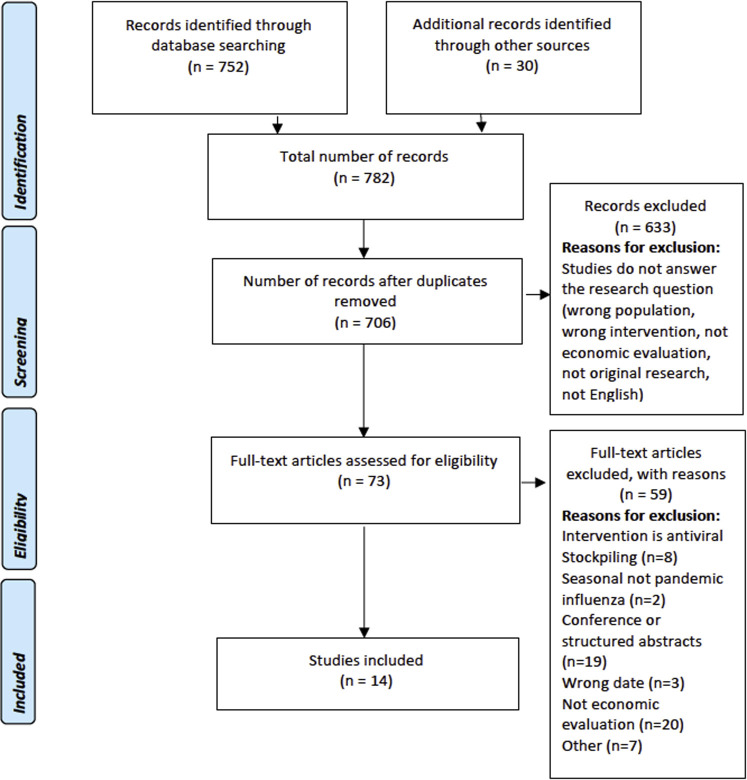
The search strategies used combined search terms relating to antivirals (“antiviral∗”, “anti viral∗”, “antivirus”), as a class, with terms relating to pandemics and outbreaks of respiratory viruses and their related complications (“Influenza,” “Flu,” “MERS,” “SARS,” “H1N1,” “COVID,” “CORONA,” “pneumonia”). These were combined with database-specific filters, used in the National Institute for Health and Care Excellence (NICE) clinical guideline development, to identify economic evaluation studies in the general databases Medline and EMBASE. Searches were limited to return articles published in English. The search Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram is illustrated in Figure 1 . Search strategies for Medline and EMBASE databases are provided in Appendix 1 (see Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.07.002).
Figure 1.
Review flowchart.
Inclusion and Exclusion Criteria
The records identified were assessed for inclusion by a single reviewer against the following criteria:
-
•
Peer-reviewed, published full economic evaluation studies or comparative cost analysis
-
•
Published in the last 10 years (2010 onward)
-
•
Assessed the cost-effectiveness of antivirals as an intervention for pandemics and outbreaks of influenza-like viruses in any country and for any age
The main exclusion criteria were as follows:
-
•
Studies assessing antivirals for other viral illnesses (eg, hepatitis C, HIV, etc)
-
•
Studies assessing antiviral stockpiling as a mitigation strategy
-
•
Studies solely assessing antivirals as prophylactic interventions
-
•
Studies assessing antivirals as treatment for seasonal rather than pandemic influenza viruses
The initial inclusion decision was made by the first author (D.D.) based on the titles and abstracts, while final inclusion was decided based on the full-text article. All excluded records were examined by a second reviewer (K.S.) to ensure the quality of the the inclusion/exclusion decisions. Any disagreements were resolved through discussions among the reviewers.
Quality Assessment
The methodological quality of the included studies was assessed using the methodological limitations section of the NICE economic evaluation checklist (see Appendix 2 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.07.002), with each study being assessed as having minor limitations, potentially serious limitations, or serious limitations.9 Those with serious limitations were excluded from the review.
Data Extraction
Data extraction tables were designed to record the details of the included studies’ characteristics and findings. The data extracted covered country and setting, currency, cost year, population, interventions, type of economic evaluation, analysis approach, time horizon, analysis perspective, cost categories, and health outcomes. A separate table reporting the findings of the included studies recorded the incremental cost-effectiveness ratio (ICER) (where relevant), sensitivity analyses, as well as the authors’ conclusions regarding the cost-effectiveness of strategies including antivirals. Where results were reported for several scenarios, results related to the most severe scenario were extracted. Data extraction was undertaken by a single reviewer (D.D.) with a random sample checked by a second reviewer (K.S.) for accuracy.
Results
Overall, 782 records were identified through database searching, reference checking, and citation tracking. After deduplication, the titles and abstracts of the remaining records were assessed. Of these, 73 articles were shortlisted for inclusion and their full text assessed. This resulted in 14 studies that were included in this review (see Fig. 1 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart).10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 The included studies were rated as having minor (2/14, 14.3%) or potentially serious (12/14, 85.7%) limitations. The characteristics of the included studies are summarized in Table 1 .
Table 1.
Study characteristics.
| Study | Country and Currency | Population | Intervention(s) and Comparator | Type of Evaluation | Analysis Approach | Perspective | Time Horizon | Cost Categories | Cost Year | Discounting | Health Outcome(s) | Source of Antiviral Efficacy Data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al 201022 | United States US dollars |
Adult patients presenting to the clinic or emergency room with influenza-like illness symptoms. 2 separate cohorts: younger adults (ages 20 to 64 years) and older adults (ages 65 to 85 years) Under both seasonal (not presented here) and pandemic influenza scenarios |
7 strategies of testing and treating: (1) using clinical judgment alone to guide antiviral use, (2) using PCR to determine whether to initiate antivirals, (3) using a rapid (point-of-care) test to determine antiviral use, (4) using combination of a point-of-care test and clinical judgment, (5) using clinical judgment and confirming the diagnosis with PCR testing, (6) treating all with antivirals, and (7) not treating anyone with antivirals (comparator) Antiviral regimen: 75 mg of oseltamivir twice a day for 5 days |
CUA | Monte Carlo decision analytic computer simulation | Societal Third-party payer |
Lifetime | Medications Hospitalization Clinic visits Staff time Tests Adverse events |
2009 | Costs: 3% Outcomes: 3% |
Primary: QALYs Other: Mortality Hospitalization Side effects |
Published systematic reviews and meta-analysis |
| Lugner et al 201016 | The Netherlands Euros |
Population of The Netherlands in 2007 High-risk groups include immunocompromised individuals, people with chronic respiratory diseases, and all people older than 65 years in nursing homes. |
No antiviral treatment Antiviral treatment within the first 48 hours of symptoms Antiviral used: oseltamivir |
CEA | Static (decision tree) and dynamic (SEIR [Susceptible-Exposed-Infectious-Removed]) models | Societal | NR | Over the counter drugs Visits to general practitioner (GP) Antibiotic prescriptions owing to influenza-related complication Hospitalizations Therapeutic intervention with AV drugs Productivity loss |
2005 | Costs: Not discounted Health outcomes: 1.5% |
Primary outcome: Life years Other outcomes: hospitalization |
Published literature |
| Perlroth et al 201019 |
United States US dollars |
Demographically typical US community under pandemic influenza conditions | 48 possible combinations of 4 social distancing strategies (child social distancing, adult social distancing, school closures, and household quarantine) and 2 antiviral medication treatments (antiviral treatment and antiviral household prophylaxis) and a “do nothing” strategy Antiviral treatment: A strategy in which patients with diagnosed cases (80% of symptomatic individuals) are given an antiviral within 48 hours of symptom onset at a probability of 30%, 60%, or 90%, depending on the compliance scenario, for 5 days. Antiviral used: oseltamivir (zanamivir in sensitivity analysis) |
CUA | Networked individual-level computational model | Societal | NR | Outpatient visits-Hospitalization Antiviral medication-Dispensing costs Daily wages Lost school days |
2009 | Costs: 3% Outcomes: 3% |
Primary outcome: QALYs Other outcomes: clinical cases averted, deaths averted |
Published literature |
| Andradóttir et al 201123 | Canada US dollars |
A typical midsized North American city, (Hamilton, Canada) under pandemic influenza conditions with R0 value of 1.4 | Strategies representing various combinations of vaccination, antiviral treatment and household prophylaxis, school closure, and general social distancing compared to no intervention | CCA | Stochastic, individual-level simulation model of influenza spread within a structured population | NR | 180 days | Vaccinations Antiviral use Illness-related absence from work Outpatient visits Prescription and over-the-counter drugs Hospitalization Lost earnings due to death |
2008 | NR | Illness attack rate | NR |
| Halder et al 201112 | Australia US dollars |
Community in the southwest of Western Australia (Albany) with a population of approximately 30 000 simulated baseline epidemic had an effective R0 of 1.2 and an illness attack rate of 13% | Wide range of interventions including school closure, antiviral treatment and prophylaxis, workplace non-attendance (a 50% reduction in workplace attendance), and community contact reduction (a 50% reduction in community contact) both individually and in combination, making 6 different clusters (from cluster A to cluster F) Cluster A: captures the single baseline (unmitigated) scenario, cluster B: groups ISC scenarios, cluster C: groups solely antiviral drug–based scenarios, cluster D: groups combined antiviral and limited duration school closure scenarios, cluster E: combines social distancing strategies such as workplace non-attendance (WP) and community contact reduction (CCR) with school closure and antiviral drug strategies, cluster F: groups certain combinations of school closure and social distancing strategies |
CEA | Individual-based disease simulation model | Societal | Lifetime | Direct healthcare costs: GP visit Hospitalization ICU admission Pharmaceutical costs (eg, costs related to antiviral drugs) Productivity loss |
2010 | Costs: 3% Outcomes: 3% |
Attack rate reduction, cases averted | Local data collected during H1N1 2009 pandemic |
| Lee et al 201118 | United States US dollars |
A hospital patient population unable to take oral antiviral treatment under seasonal and pandemic influenza scenarios | 4 strategies: Treatment without testing Treatment then testing Testing then treatment No treatment Testing for influenza was by polymerase chain reaction (PCR). Treatment was intravenous antiviral drugs for 5 days. For treatment then testing, patients started treatment and were given a PCR test. The results were received within 24 hours and treatment was discontinued, after the first dose, if the result was negative. For testing then treatment, treatment was started when the PCR results were received (within 24 hours) if the result was positive. Antiviral used: peramivir |
CUA | Decision tree model | Societal perspective Third-party payer perspective |
Lifetime | Antiviral costs Hospitalization Ventilation PCR testing Lost productivity (societal perspective) |
2009 | Costs: 3% Outcomes: 3% |
Primary outcome: QALYs |
published studies and reports by the Agency for Healthcare Research and Quality (AHRQ) |
| You et al 201210 | China US dollars |
Patients aged 18 years or above, had and signs compatible with influenza (eg, fever, cough), and required hospitalization because of signs of severe lower respiratory infection: hypoxemia, tachypnea, and/or pulmonary infiltrates on chest radiography | 4 management strategies: Immunofluorescence assay (IFA) PCR-guided oseltamivir treatment Empirical treatment plus PCR Empirical treatment alone Antiviral used: Oseltamivir, 75 mg twice daily therapy for 5 days |
CUA | Decision tree model | Healthcare provider | Lifetime | Hospital admission ICU Medication tests |
2011 | Costs: 3% Outcomes: 3% |
Primary outcome: QALYs Other outcomes: survival |
Published literature |
| Dugas et al 201315 | United States US dollars |
Adult patients presenting to the emergency department (ED) with symptoms of an acute respiratory infection, who met the Center for Disease Control and Prevention criteria for recommended antiviral treatment | 4 influenza testing and treatment strategies: Treat none Treat according to provider judgment Treat according to results of a PCR-based rapid diagnostic test Treat all Antiviral used: Oseltamivir (zanamivir in sensitivity analysis) |
CUA | Decision tree model | Societal perspective | Lifetime | Testing Antiviral treatment Hospital admission Primary care visits Complication treatments |
2011 | Costs: NR Outcomes: 3% |
Primary outcome: QALYs Other outcomes: mortality |
Published literature |
| Kelso et al 201317 | Australia US dollars |
A community in the southwest of Western Australia, the town of Albany with a population of approximately 30 000, to simulate the dynamics of an influenza pandemic |
School closure Antiviral drugs for treatment Antiviral drugs for prophylaxis Workplace non-attendance (workforce reduction) Community contact reduction These interventions were considered individually and in combination and social distancing interventions were considered for either continuous periods (that is, until the local epidemic effectively ceased) or periods of fixed duration (2 weeks or 8 weeks). |
CBA | Individual-based simulation mode | Societal perspective | Lifetime | GP visits Hospitalization ICU admission Antiviral treatment (including cost of stockpiling) Antiviral prophylaxis Productivity loss School closure |
2010 | Cost: 3% Outcomes: 3% |
Attack rate Hospitalization, ICU admission, mortality |
Local data collected during H1N1 2009 pandemic |
| Milne et al 201321 | Australia US dollars |
A real community in the southwest of Western Australia, the town of Albany with a population of approximately 30 000, to simulate the dynamics of an influenza pandemic |
School closure, antiviral drugs for treatment and prophylaxis, workplace non-attendance (workforce reduction), and community contact reduction These interventions were considered individually and in combination and social distancing interventions were considered for either continuous periods (that is, until the local epidemic effectively ceased) or periods of fixed duration (2 weeks or 8 weeks). |
CEA | Individual-based simulation mode | Societal perspective | Lifetime | GP visits Hospitalization ICU admission Antiviral treatment (including cost of stockpiling) Antiviral prophylaxis Productivity loss School closure |
2010 | Cost: 3% Outcomes: 3% |
Life years saved | Local data collected during H1N1 2009 pandemic |
| Kamal et al 201720 | United States US dollars |
US population of healthy adults, aged 18-64 years under 4 pandemic scenarios: (1) high transmissibility and high severity, (2) low transmissibility and low severity, (3) high transmissibility and low severity, and (4) low transmissibility and high severity. | Oseltamivir, 75 mg twice daily for 5 days Oseltamivir, 150 mg twice daily for 5 days No treatment |
CUA | Pharmacokinetic-pharmacodynamic/pharmacoeconomic modeling | Payer perspective and societal perspective | 1 year | Direct medical costs (medication and hospitalization) Direct nonmedical costs (transportation to and from hospital) |
2013 | NA | Primary outcome: QALYs |
Published literature. |
| Wu et al 201814 | United States US dollars |
US population of healthy adults, aged 18–64 years under 4 pandemic scenarios: (1) high transmissibility and high severity; (2) low transmissibility and low severity; (3) high transmissibility and low severity; and (4) low transmissibility and high severity. | Oseltamivir, 75 mg twice daily for 5 days Oseltamivir, 150 mg twice daily for 5 days No treatment |
CUA | Pharmacokinetic-pharmacodynamic/pharmacoeconomic modeling | Payer perspective and societal perspective | 1 year | Direct medical costs (medication and hospitalization) Direct nonmedical costs (transportation to and from hospital) |
2013 | NA | Primary outcome: QALYs |
Published literature. |
| Venkatesan et al 201911 | United Kingdom Pounds Sterling |
2009 UK population under pandemic scenario similar to 2009 H1N1 pandemic conditions in addition to 4 other hypothetical scenarios | Outpatient antiviral (neuraminidase inhibitors) treatment No treatment Antiviral used: Oseltamivir or zanamivir (data based on oseltamivir) |
CEA | Decision tree model | National Health Service (NHS) | < 1 year (1 pandemic episode) | Medication GP visits Phone consultations Hospitalization |
2017 | NA | Deaths hospitalizations | IPD meta-analysis |
| Beresniak et al 202013 | France Euro |
France general population under 6 pandemic scenarios: (1) scenario A (seasonal like), (2) scenario B (2009 pandemic like), (3) scenario C (community risk, low virulence), (4) scenario D (community risk, high virulence), (5) scenario E (high-risk groups), (6) scenario F (major event) | 18 potential interventions including (1) individual measures, (2) border control, (3) community infection control measures, (4) infection control using usual care circuit, (5) infection control using specific care circuit, (6) vaccination program of at risk population using specific healthcare facilities, (7) vaccination program of at-risk population using usual healthcare facilities, (8) vaccination program of health professionals using specific healthcare facilities, (9) vaccination program of health professionals using usual healthcare facilities, (10) vaccination of general population using specific healthcare facilities, (11) vaccination of general population using usual healthcare facilities, (12) antiviral preventive distribution, (13) antiviral curative distribution, (14) reduction of secondary infections, (15) pneumococcal vaccination, (16) development of new ICU capacity, (17) implementation of new equipment, and (18) screening intervention |
CCA | Monte simulation model | French healthcare system | 9 months (1 pandemic season) | The cost of the public health intervention The costs of the communication program Pharmaceutical interventions |
NR | NA | Outcome 1 was defined as ‘achieving mortality reduction >/= 40%’. Outcome 2 was defined as achieving disease incidence reduction >/= 30%, |
success probability input values were sampled from a uniform distribution between minimum and maximum probabilities |
CBA indicates cost-benefit analysis; CCA, cost-consequences analysis; CEA, cost-effectiveness analysis; CUA, cost-utility analysis; GP, general practitioner; ICU, intensive care unit; IPD, individual participant data; NR, not reported; NA, not applicable; PCR, polymerase chain reaction; QALY, quality-adjusted life-year.
Most studies were conducted in the United States (6/14, 42.9%)14 , 15 , 18, 19, 20 , 22 and Australia (3/14, 21.4%),12 , 17 , 21 in addition to 1 study each (1/14, 7.1%) conducted in the United Kingdom,11 France,13 The Netherlands,16 China,10 and Canada.23 Most used a societal perspective (10/14, 71.4%),12 , 14, 15, 16, 17, 18, 19, 20, 21, 22 either solely or in addition to a healthcare system or third-party payer perspective.
All the included studies focused on the H1N1 pandemic. Nine studies (9/14, 64.3%)10 , 11 , 14, 15, 16 , 18, 19, 20 , 22 reported the antiviral agent used, with the most commonly used regimen being 75 mg of oseltamivir given twice daily for 5 days (8/9, 88.9%). In 3 of these 8 studies, the antiviral zanamivir was used in a sensitivity analysis. Other agents used included peramivir (1/9, 11.1%).
Seven studies (50%) were cost-utility analyses,10 , 14 , 15 , 18, 19, 20 , 22 using quality-adjusted life-years (QALYs) as the main health outcome measure. Four studies (28.6%) were cost-effectiveness analyses,11 , 12 , 16 , 21 2 (14.3%) were cost-consequence analyses,13 , 23 and 1 (7.1%) was a cost-benefit analysis.17 The time horizon used ranged from 6 months to lifetime.
All the included studies used simulation models to assess the cost-effectiveness of the included interventions, with data on the effectiveness of the antivirals used based on published studies. The included studies reported that strategies including antiviral treatment, compared to either doing nothing or to strategies that do not include antivirals, were either cost saving or cost-effective at the study-specific willingness-to-pay thresholds. In the included cost-utility analyses, the ICER of the strategies including antivirals ranged from $68/QALY-gained to $39 674/QALY-gained from a societal perspective, compared to either doing nothing or to strategies that do not include antivirals.
In the studies that compared different testing strategies for guiding the initiation of antiviral treatment with either no treatment or treating all, the most cost-effective strategy was dependent on both the perspective and the target population, with empirical treatment or treatment based on clinical judgment emerging as the most likely to be cost-effective over a wide range of population characteristics with ICERs from $6246/QALY to $47 841/QALY.15 , 22
Sensitivity analyses undertaken showed that the main parameters that affect the results included antiviral effectiveness, antiviral cost, prevalence, viral basic reproduction number (R0), transmission rate, case fatality rate, and level of adherence to the mitigation strategies. The results of the included studies are summarized in Table 2 .
Table 2.
Results of included studies.
| Study | ICER/net benefit of antiviral based strategies (vs comparator) | Cost-effectiveness threshold (if applicable) | Sensitivity and scenario analysis | Author’s conclusion regarding antivirals |
|---|---|---|---|---|
| Lee et al 201022 | (Under pandemic influenza and 30% probability of influenza scenario) (A) Societal perspective: All adults: Clinical judgment dominant Younger adults (20-65 years): Clinical judgment, followed by PCR then PoC testing (all dominant) Older adults (65-85 years): PCR, then clinical judgment then PoC testing (all dominant) (B) Third-party payer perspective: All adults: Do nothing strategy (comparator), followed by clinical judgment ($47 841/QALY), and PoC testing ($202 124/QALY compared to clinical judgment) Younger adults (20-65 years): Clinical judgment ($30 098-$35 000) followed by PCR testing ($38 109-$46 432) Older adults (65-85 years): PCR testing dominated, followed by clinical judgment, and PoC testing ($287 530/QALY compared to clinical judgment) |
$50 000 per QALY gained | -Deterministic sensitivity analysis -Probabilistic sensitivity analysis -Scenario analyses explored the decision for higher-risk adults (ie, double the risk of hospitalization and mortality), older adults, and higher-risk older adults |
“When hospitalization risk and mortality were doubled, using clinical judgment (>/= 50% sensitive) to guide antiviral initiation emerged as the most cost-effective option with PCR testing being the closest competitor but only when at least 20% of cases were influenza. Among older adults (65+ years old), employing PCR to guide antiviral initiation emerged as the most cost-effective option with the closest competitor being clinical judgment when judgment sensitivity was at least 50%. Treating all patients with antivirals appeared to be cost-effective only in older adults.” |
| Lugner et al 201016 | Direct healthcare costs only: ICER: €1695 (static model) and €1637 (dynamic model) per life-year gained Societal perspective: intervention becomes cost-saving when including productivity loss |
NA | Deterministic sensitivity analysis | Therapeutic use of antiviral drugs is cost-effective compared with non-intervention, irrespective of which model approach is chosen. |
| Perlroth et al 201019 |
A strategy combining adult and child social distancing, school closure, antiviral treatment, and prophylaxis most cost-effective ICER: $31 300/QALY-gained All other strategies: dominated or extendedly dominated |
$100 000 per QALY gained | Deterministic and probabilistic sensitivity analysis | Multilayered mitigation strategies that include adult and child social distancing, use of antivirals, and school closure are cost-effective for a moderate to severe pandemic. If antivirals are not available or are not effective, a strategy of adult and child social distancing and school closure is most effective, resulting in a cost per QALY-gained of $40 800, relative to a strategy of adult and child social distancing. |
| Andradóttir et al 201123 | No intervention: The average overall illness attack rate is 34.1%, with an estimated total cost of $81.1 million. Antiviral use at low (10%) coverage alone: Overall attack rate of 31.3% with total cost $75.9 million School closure and social distancing alone: attack rate of 24.0%, with a total cost of $125.0 million Reactive low-efficacy vaccination of 35% of the population delivered in 3 batches combined with antivirals (10% coverage), and school closure and social distancing: overall average illness attack rate of 4.5% (total cost $32.2 million) if no delays occur in the initiation of vaccination, and 5.4% (total cost $36.8 million) if a 60-day delay occurs |
NA | None reported | Combining rolling, limited-duration, as-needed closures of individual schools and a practical social distancing policy with 35% reactive low-efficacy vaccination coverage and low-level (10%) antiviral use can reduce illness attack rates by 89% compared to no intervention, as well as total costs by 64%. |
| Halder et al 201112 | Antiviral treatment: $1109/symptomatic case averted Antiviral treatment + household antiviral prophylaxis $701/symptomatic case averted Antiviral treatment + household antiviral prophylaxis + extended antiviral prophylaxis $641/symptomatic case averted ISC 2 weeks + antiviral treatment + household antiviral prophylaxis $632/symptomatic case averted ISC 2 weeks + antiviral treatment + household antiviral prophylaxis + extended antiviral prophylaxis $636/symptomatic case averted ISC 4 weeks + antiviral treatment + household antiviral prophylaxis $676/symptomatic case averted ISC 4 weeks + antiviral treatment + household antiviral prophylaxis + extended antiviral prophylaxis $640/symptomatic case averted ISC 8 weeks + antiviral treatment + household antiviral prophylaxis $777/symptomatic case averted ISC 8 weeks + antiviral treatment + household antiviral prophylaxis + extended antiviral prophylaxis $708/symptomatic case averted |
$1000/symptomatic case averted | Deterministic sensitivity analysis | The combination of antiviral drug strategies together with school closure strategies are found to be the most cost-effective in the cost per case averted, for a pandemic with H1N1 2009 characteristics. |
| Lee et al 201118 | Pandemic influenza, third-party payer perspective: Ventilated population, antiviral cost per day $100: Antiviral treatment then PCR: $39 674/QALY PCR then antiviral treatment: comparator Give no IV antiviral treatment Dominated Give IV antiviral treatment $68/QALY Nonventilated population, antiviral cost per day $100: Antiviral treatment then PCR: $39 674/QALY PCR then antiviral treatment: comparator Give no IV antiviral treatment Dominated Give IV antiviral treatment $797/QALY |
$50 000/QALY gained | Deterministic sensitivity analysis Probabilistic sensitivity analysis |
The authors concluded that intravenous antiviral treatment for hospitalized patients with influenza-like illness was cost-effective, especially with initial PCR testing to guide treatment |
| You et al 201210 | Empirical treatment alone: Comparator Immunofluorescence-assay (IFA): Dominated PCR-guided oseltamivir treatment: Dominated Empirical treatment plus PCR: Dominated |
$50 000/QALY gained | Deterministic sensitivity analysis Probabilistic sensitivity analyses |
During influenza epidemics, empirical antiviral treatment appears to be a cost-effective strategy in managing patients hospitalized with severe respiratory infection suspected of influenza, from the perspective of healthcare providers in Hong Kong. |
| Dugas et al 201315 | Treat according to provider judgment: Comparator Treat according to results of a PCR-based rapid diagnostic test: $1389/QALY Treat all: $6246/QALY Treat none: Dominated |
$50 000/QALY gained | Deterministic sensitivity analysis Probabilistic sensitivity analyses |
Overall, the most cost-effective method of influenza testing and treatment in high-risk ED patients depends on local influenza prevalence; however, with any active influenza, antiviral treatment of any kind is superior to no treatment. |
| Kelso et al 201317 | For high-severity pandemics: Continuous school closure, community contact reduction, antiviral treatment, and antiviral prophylaxis: Net benefit = $6996 per person compared to no intervention For low-severity pandemics: 8 weeks’ school closure combined with antiviral treatment and prophylaxis, costing $374 per person, which represents a net saving of $67 per person compared to no intervention |
NA | Deterministic sensitivity analysis | In the likely situation where the severity of an emerging pandemic is initially unknown (but is suspected to be greater than that of seasonal influenza), the results indicate that the most appropriate intervention strategy is to instigate school closure and community contact reduction, combined with antiviral drug treatment and household prophylaxis, as soon as transmission has been confirmed in the community. |
| Milne et al 201321 | Intervention strategies combining school closure with antiviral treatment and prophylaxis are the most cost-effective strategies, For a severe pandemic (CFR of 2.5%): ICER: $9000 per life-year saved For a low-severity pandemic (CFR of 0.1%): ICER: $58 k per life-year saved For a pandemic with very low severity similar to the 2009 pandemic (CFR of 0.03%): ICER: $155 k per life-year saved |
NA | Deterministic sensitivity analysis Probabilistic sensitivity analysis |
The most cost-effective strategies for mitigating an influenza pandemic involve combining sustained social distancing with the use of antiviral agents. For low-severity pandemics the most cost-effective strategies involve antiviral treatment, prophylaxis, and short durations of school closure. |
| Kamal et al 201720 | Societal perspective: High transmissibility and high severity Oseltamivir, 75 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs oseltamivir, 75 mg Cost-saving Payer perspective High transmissibility and high severity Oseltamivir, 75 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs oseltamivir, 75 mg Cost-saving |
$100 000/QALY-gained | Scenario analysis assuming different levels of antiviral uptake (25%, 50%, 80%) and different transmission levels (R0 = 1.9 and 2.7) | Oseltamivir reduced the median number of infected individuals, increased QALYs by deaths-averted, and was cost-saving under most pandemic scenarios. |
| Wu et al 201814 | Societal perspective: High transmissibility and high severity Oseltamivir, 75 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs oseltamivir, 75 mg Cost-saving Payer perspective High transmissibility and high severity Oseltamivir, 75 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs no treatment Cost-saving Oseltamivir, 150 mg vs oseltamivir, 75 mg Cost-saving |
$100 000/QALY gained | Deterministic sensitivity analysis Probabilistic sensitivity analysis Scenario analysis assuming different levels of antiviral uptake: 25%, 50%, 80% |
Oseltamivir reduced the median number of infected individuals, increased QALYs by deaths-averted, and was cost-saving under most pandemic scenarios. |
| Venkatesan et al 201911 | Overall population £7110 per hospitalization-averted High-risk population £2238 per hospitalization-averted Non–high-risk population £20 473 per hospitalization-averted |
NA | Deterministic sensitivity analysis Probabilistic sensitivity analysis |
Across pandemic scenarios, antiviral treatment can be cost-saving for population groups at high risk of influenza-related complications. |
| Beresniak et al 202013 | Antiviral curative distribution under: Scenario B (2009 pandemic influenza scenario) Costs: €9.19-€47.19 million Effectiveness:
|
NA | Probabilistic sensitivity analysis | Curative antiviral programs appeared more cost-effective than preventive distribution programs, whatever the pandemic scenario. Future preparedness strategies ought to be progressively enriched by cost-effectiveness considerations concerning a wide spectrum of relevant public health interventions. |
CFR indicates case fatality rate; ED, emergency department; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; ISC, individual school closure; NR, not reported; NA, not applicable; PCR, polymerase chain reaction; PoC, point of care; QALY, quality-adjusted life-year.
Discussion
This systematic review identified 14 full economic evaluations that assessed antivirals as interventions for treating pandemic influenza viruses. This body of evidence showed that antiviral treatment is a cost-effective strategy overall in managing pandemic influenza, both as a standalone intervention as well as when combined with other mitigation strategies such as vaccination, antiviral prophylaxis, social distancing, and school closures. Oseltamivir, 75 mg administered twice daily for 5 days, was the most commonly used regimen in the included studies.
Previously published systematic reviews have focused on assessing the cost-effectiveness of interventions for pandemic influenza viruses.5 , 6 They identified studies published up to 2014. Our systematic review is, to our knowledge, one of a few that addressed this topic. Nevertheless, our particular focus is on the cost-effectiveness of antiviral treatments compared to all other interventions. This makes our review the most relevant and up-to-date one for researchers working on developing antivirals for the treatment of COVID-19 and for HTA agencies that will eventually be tasked with providing guidance on the use of such agents.
The availability of a vaccine during the first wave of a pandemic is unlikely. Hence, the commonly proposed strategy, to use either alone or as part of a multifaceted strategy with other pandemic containment methods, is to treat all symptomatic individuals with currently available antiviral drugs. Perlroth et al examined 48 possible combinations of 6 main strategies including antiviral treatment.19 They reported that single interventions were less effective compared to strategies made up of multiple interventions, particularly for pandemics of high infection rates. The most cost-effective strategy was found to be one that combined adult and children social distancing with antiviral prophylaxis and antiviral treatment, with an ICER of $31 300/QALY gained. Similarly, Halder et al reported that the use of antiviral drugs as the sole strategy avoids some of the productivity losses that arise from other interventions owing to a reduced workforce for child care if school closure interventions are used as an alternative.12 The authors also reported that combining workforce reduction with school closure results in significant reduction in attack rates but these strategies, particularly when implemented for a long time, are unlikely to be cost-effective owing to their prohibitive productivity costs, particularly for pandemics of milder severity. These strategies, however, are suitable when there is no or limited access to antiviral drugs or if there is a significant risk of development of antiviral-resistant virus strains.
Although our review has focused on antivirals as a class, we identified the specific agents that were assessed for this indication. The studies included in our review have examined the following antivirals: oseltamivir, zanamivir, and peramivir. These agents have not been widely trialed for the current pandemic (COVID-19), and it is not clear whether they would be effective or not in this population. We did not identify, through our database searches, published economic evaluations of any of the other antiviral agents that have been included in the WHO Solidarity trial, the UK-based Randomised Evaluation of COVID-19 therapy (RECOVERY) trial, or other ongoing trials in COVID-19 (eg, remdesivir, lopinavir/ritonavir, interferon beta-1a, favipiravir).7 , 24 This is likely because we have focused our searches on respiratory illnesses; given the difference in the transmission dynamics of respiratory and blood-borne viruses, these antivirals were mainly developed and used in other disease areas (eg, HIV, Ebola, hepatitis). So, a review of economic evaluations of these antivirals for RNA viral diseases (HIV and Ebola) could be informative.
Nevertheless, the Institute for Clinical and Economic Review in the United States recently published its report “Alternative Pricing Models for Remdesivir and Other Potential Treatments for COVID-19,” on May 1, 2020, followed by its first update on June 24, 2020.25 , 26 This report includes an economic evaluation of remdesivir as a treatment option for hospitalized COVID-19 patients with lung involvement. It was intended to inform its pricing after the U.S. Food & Drug Administration issued an Emergency Use Authorization for it based on its pivotal trial (Adaptive COVID-19 Treatment Trial Part 1) preliminary results.27 , 28
The analysis compared remdesivir to usual care, in its base case, and to usual care with added dexamethasone, in a scenario analysis.25 , 26 The latter was done following the release of the RECOVERY trial results that showed a significant effect for dexamethasone on reducing mortality in hospitalized COVID-19 patients (age-adjusted rate ratio 0.83; 95% confidence interval [CI] 0.74-0.92; P < .001].25 , 26 , 29 The analysis was undertaken from the perspective of the healthcare sector using a decision analytic model. It applied a cost-effectiveness threshold of $50 000/QALY-gained to derive its main value-based price benchmark. Price benchmarks using 2 alternative threshold values ($100 000 and $150 000/QALY-gained) were also presented.
The model, however, assumes a mortality benefit for remdesivir, based on the point estimate from its pivotal trial, and explores the impact of an alternative assumption of no effect on mortality, owing to the lack of statistical significance in the trial results (for death, 0.70; 95% CI, 0.47-1.04).25, 26, 27 Given its low price (wholesale acquisition cost for a 10-day course of once-daily dexamethasone [6 mg tablet]: $14.87) and significant favorable effect on mortality, the addition of dexamethasone to usual care as a comparator led to a reduction in the Institute for Clinical and Economic Review’s estimate of remdesivir’s value-based price benchmark range by almost 50% (from $4580-$5080 to $2520-$2800 per course of treatment, assuming mortality benefit).26 An even lower price was estimated ($310 in the base case) if the assumed mortality benefit is removed.26
Subsequently, the marketing authorization, Gilead Sciences, announced the price for governments of developed countries to be $390 per vial.30 Gilead reported that most patients are expected to receive a 5-day treatment course, which showed equivalent efficacy to the 10-day course in trials.31 This means that a patient will be using 6 vials of remdesivir, which equates to $2340 per treatment course.30 Generic manufacturing has already started in India, Pakistan, and Egypt, under nonexclusive voluntary licensing agreements, with a price of Rs 4800 per vial reported in India, which is about 80% below its price in the United States.32
To our knowledge, the Institute for Clinical and Economic Review’s analysis is the only economic evaluation of any COVID-19 treatments in the public domain so far. It highlighted the substantial clinical evidence uncertainty that remains for remdesivir.26 The Institute for Clinical and Economic Review’s model, however, does not reflect the infection transmission dynamics and assumes that patients return to normal quality of life following discharge and ignores the impact of the long-term complications reported after recovery from COVID-19.33
It is widely accepted that antiviral treatment is more effective when administered as early as possible in the course of the illness. This has been seen in trials of remdesivir in COVID-19 patients as well, where better efficacy was observed in those who started treatment within 10 days of the onset of symptoms.34 So, earlier use of antivirals in the course of the illness as well as targeting those most likely to benefit from antiviral treatment can improve their cost-effectiveness. Using polymerase chain reaction or point-of-care testing to guide treatment decisions has been suggested as a potential way of doing the latter. According to Lee et al, in a pandemic scenario, prescribing antivirals to all symptomatic patients may be warranted for older adults (65-85 years) but not younger adults (20-64 years).22 Nevertheless, some older adults may be at much higher risk for complications. In this case, employing polymerase chain reaction to target antiviral use emerged as the most cost-effective option. Complications are also more likely in case of a more virulent virus strain. Lee at al also concluded that testing is consistently more cost-effective than doing nothing for older adults, while for healthy younger adults, particularly in the early days of a pandemic, doing nothing might be the optimal strategy.22 Studies that included adults of all ages without stratifying, however, concluded that in pandemic situations empirical antiviral treatment alone would dominate other test-and-treat strategies.10 This conclusion was sensitive to prevalence, with cost-effectiveness of empirical treatment increasing with prevalence, and the daily antiviral cost, with lower daily treatment cost associated with better cost-effectiveness of empirical treatment.10 The results were similarly valid in adults at high risk of complications owing to either comorbidities or age.15 These conclusions were consistent both from healthcare and societal perspectives and regardless of setting (inpatient or emergency department).10 , 15
The efficacy of antivirals in preventing transmission is not clear cut, with debates still ongoing. Hence, some researchers chose not to include this effect in their models,22 while others did.19 The main benefits of antiviral treatment in the included studies were reducing complications, reducing duration of illness, and reducing mortality. Data on these outcomes were generally based on observational studies and surveillance data collected during the 2009 H1N1 pandemic. This is expected to change in the case of COVID-19, where high-quality, large-scale clinical trials are under way and have started reporting their results, which can be used to inform economic models.7 , 24 Unfortunately, in most of the included studies, limited details were provided to allow accurate assessment of the level of certainty in the effectiveness evidence with some lacking reference to the publication or data source used. Adverse effects of antivirals were considered to be mild and were generally not included in the reviewed studies.
Costs in the included studies largely covered all the relevant categories; however, other categories may have to be considered for pandemics of a severity similar to COVID-19 including additional indirect macroeconomic impacts caused by disruption of trade and tourism, consumer demand and supply, and investor confidence.17 Overall, antiviral costs did not represent a high percentage of total costs in the included studies, where their costs were dwarfed by the cost of lost productivity, healthcare costs, and the cost owing to early mortality in studies adopting a societal perspective and/or comparing against other very costly strategies such as school closures and social distancing. Some studies included the cost of antiviral stockpiling, assuming a duration of 30 years between pandemics and a shelf life of 5 years.17 Models comparing different antivirals to each other or to other pharmacological treatments, though, are expected to be sensitive to differences in antiviral costs, as we have seen with the Institute for Clinical and Economic Review’s evaluation of remdesivir when dexamethasone was included.26
The cost categories included in these evaluations are dependent on the perspective used, which, for most of the studies, was a societal perspective. It is likely that a societal perspective would be the most relevant in such pandemic situations; however, some HTA organizations such as NICE specify using a healthcare payer perspective. Nevertheless, antiviral treatments appeared to be cost-effective regardless of the perspective used.14 , 18 , 20 , 22
Different modeling approaches have been used in the included studies. Dynamic models (eg, Susceptible-Exposed-Infectious-Removed type of models, which describes the transmission of the disease seemed to play an important role in exploring possible strategies to contain a pandemic outbreak of influenza through controlling transmission, compared to static (eg, decision tree and Markov) models.16 Hence, it would be recommended for economic models constructed to assess the cost-effectiveness of interventions for COVID-19 to be based on the dynamic disease transmission models. Modeling approaches that integrate pharmacology, epidemiology, and pharmacoeconomics also seem to be promising, because they can provide more accurate representation of the transmission dynamics combining these with data on the pharmaco-kinetics and dynamics of the drug. This may facilitate faster drug development within an adaptive licensing approach.14 , 20
Our review focused on economic evaluations of antiviral treatment for pandemics and outbreaks of respiratory illnesses of high severity and high infectivity, including the current COVID-19 pandemic. Nevertheless, the full scale of the current pandemic is yet to be determined. We focused on studies published in the last 10 years to ensure relevance to the current context. This may have resulted in missing studies that were conducted during the SARS outbreak, which would have been published in 2002-2004. We also did not identify studies of treatments that were trialed for SARs and MERs and have been suggested as potential treatments for COVID-19 (eg, hydroxychloroquine and chloroquine), likely for the same reason. However, the applicability of studies from 10 years back to the current healthcare practice environment would have been limited anyway. Nevertheless, this means that our conclusions are largely based on the use of antivirals in pandemics caused by influenza viruses. We believe, though, that these are likely to be generalizable to pandemics of respiratory illnesses caused by other viruses including the current coronavirus, severe acute respiratory syndrome coronavirus.
We only included studies published in English, which may have resulted in excluding relevant studies. Additionally, the studies included in our review were mainly conducted in high-income countries, so their results might not be generalizable to low- and middle-income countries (LMICs). Nevertheless, this is a limitation of the body of evidence in this area. Undertaking economic evaluations of pandemic mitigation strategies in LMICs during the COVID-19 pandemic must be high on research agendas to inform current as well as future pandemic mitigation efforts in these countries.
Although conclusions regarding the potential cost-effectiveness of antivirals and other potentially curative treatments for patients affected by COVID-19 can be drawn from this review, de-novo assessment of the cost-effectiveness of these agents should be undertaken taking into account the relevant influential factors identified in this review, including the pandemic case fatality rate; the virus transmission rate; the availability, acceptability, and feasibility of other interventions; as well as the demographics of the susceptible population.
Undertaking such economic evaluations alongside the ongoing clinical trials should be encouraged, because it would facilitate timely assessment and availability of the economic evidence required by HTA agencies for recommending these agents for wider use. Nevertheless, decision models will be essential to compare all available options and to extrapolate beyond the short follow-up of clinical trials. This is particularly important given the accumulating evidence that COVID-19 is a multisystem disease that can lead to long-term vascular, cardiovascular, neurologic, and mental-health complications.33
Based on the above, the following considerations are suggested when developing economic models and designing economic evaluations to assess the cost-effectiveness of antiviral treatments and other mitigation strategies for COVID-19:
-
•
Models should be individual-based dynamic disease transmission models that simulate the transmission of the infection and the changing level of susceptibility in the community with time.
-
•
Analysis should account for the probability of antimicrobial resistance developing.
-
•
Models should simulate the multi-morbid impact of COVID-19 and its potential long-term effects by using a lifetime time horizon rather than focusing on a single epidemic episode.
-
•
All relevant comparators, including combinations of antivirals with other strategies, should be included.
-
•
Undertake the analysis from both the societal and healthcare payer perspectives to inform potential cost-sharing arrangements, where benefits might be shared across different sectors, as well as pricing decisions where relevant.
-
•
Given the expected ongoing uncertainty around the characteristics of the current pandemic, a wide range of sensitivity and scenario analyses to assess the impact of parameter and structural uncertainty will be important. Key parameters to explore should include the viral basic reproduction number (R0), case fatality rate, and adherence to the different strategies compared.
The above is not intended to be an exhaustive list but rather a prompt for further discussions around the key considerations for economic modeling of mitigation strategies for pandemics of respiratory illnesses.
Conclusions
The use of antiviral treatments appears to be a cost-effective strategy for managing pandemics and outbreaks of respiratory illnesses that have a very high case fatality rate, similar to the current COVID-19 pandemic, either as standalone intervention or part of a multifaceted strategy.
Investing in and accelerating the development of new antivirals and other potentially curative treatments that specifically target COVID-19 is to be encouraged. Nevertheless, promptly evaluating their cost-effectiveness relative to other strategies in use at the time of their introduction should be a priority for researchers, decision makers, and healthcare systems, especially those in LMICs, to support evidence-informed resource allocation decisions. This is as countries carefully scale back the use of other economically and socially disruptive strategies and start counting the human and economic losses caused by COVID-19.
Footnotes
Author Contributions:Concept and design: Dawoud
Acquisition of data: Dawoud, Soliman
Analysis and interpretation of data: Dawoud, Soliman
Drafting of the manuscript: Dawoud, Soliman
Critical revision of the paper for important intellectual content: Dawoud, Soliman
Statistical analysis: Dawoud
Administrative, technical, or logisticsupport: Dawoud, Soliman
Conflict of Interest Disclosures: The authors reported no conflicts of interest.
Funding/Support: The authors received no financial support for this research.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.jval.2020.07.002.
Supplemental Material
References
- 1.World Health Organization Rolling updates on coronavirus disease (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3.Wikipedia Travel restrictions related to the 2019–20 coronavirus pandemic. https://en.wikipedia.org/wiki/Travel_restrictions_related_to_the_2019%E2%80%9320_coronavirus_pandemic
- 4.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini-Descomps H., Brender N., Maradan D. Value for money in H1N1 influenza: a systematic review of the cost-effectiveness of pandemic interventions. Value Health. 2017;20(6):819–827. doi: 10.1016/j.jval.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Velasco R.P., Praditsitthikorn N., Wichmann K., et al. Systematic review of economic evaluations of preparedness strategies and interventions against influenza pandemics. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization “Solidarity” clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- 8.Senanayake S.L. Drug repurposing strategies for COVID-19. Future Drug Discov. 2020 Mar 25 doi: 10.4155/fdd-2020-0010. [DOI] [Google Scholar]
- 9.National Institute for Health and Care Excellence Developing NICE guidelines: the manual. https://www.nice.org.uk/process/pmg20/chapter/introduction [PubMed]
- 10.You J.H.S., Chan E.S.K., Leung M.Y.K., Ip M., Lee N.L.S. A cost-effectiveness analysis of ‘test’ versus ‘treat’ patients hospitalized with suspected influenza in Hong Kong. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesan S., Carias C., Biggerstaff M., et al. Antiviral treatment for outpatient use during an influenza pandemic: a decision tree model of outcomes averted and cost-effectiveness. J Public Health (Oxf) 2019;41(2):379–390. doi: 10.1093/pubmed/fdy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder N., Kelso J.K., Milne G.J. Cost-effective strategies for mitigating a future influenza pandemic with H1N1 2009 characteristics. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beresniak A., Rizzo C., Oxford J., et al. Cost-effectiveness of public health interventions against human influenza pandemics in France: a methodological contribution from the FLURESP European Commission project. Eur J Public Health. 2020;30(1):43–49. doi: 10.1093/eurpub/ckz074. [DOI] [PubMed] [Google Scholar]
- 14.Wu D.B.C., Chaiyakunapruk N., Pratoomsoot C., et al. Cost-utility analysis of antiviral use under pandemic influenza using a novel approach – linking pharmacology, epidemiology and heath economics. Epidemiol Infect. 2018;146(4):496–507. doi: 10.1017/S0950268818000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dugas A.F., Coleman S., Gaydos C.A., Rothman R.E., Frick K.D. Cost-utility of rapid polymerase chain reaction-based influenza testing for high-risk emergency department patients. Ann Emerg Med. 2013;62(1):80–88. doi: 10.1016/j.annemergmed.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lugner A.K., Mylius S.D., Wallinga J. Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Econ. 2010;19(5):518–531. doi: 10.1002/hec.1485. [DOI] [PubMed] [Google Scholar]
- 17.Kelso J.K., Halder N., Postma M.J., Milne G.J. Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories. BMC Public Health. 2013;13:211. doi: 10.1186/1471-2458-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee B.Y., Tai J.H., Bailey R.R., et al. Economic model for emergency use authorization of intravenous peramivir. Am J Manag Care. 2011;17(1):e1–e9. [PMC free article] [PubMed] [Google Scholar]
- 19.Perlroth D.J., Glass R.J., Davey V.J., Cannon D., Garber A.M., Owens D.K. Health outcomes and costs of community mitigation strategies for an influenza pandemic in the United States. Clin Infect Dis. 2010;50(2):165–174. doi: 10.1086/649867. [DOI] [PubMed] [Google Scholar]
- 20.Kamal M.A., Smith P.F., Chaiyakunapruk N., et al. Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. Br J Clin Pharmacol. 2017;83(7):1580–1594. doi: 10.1111/bcp.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne G.J., Halder N., Kelso J.K. The cost effectiveness of pandemic influenza interventions: a pandemic severity based analysis. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B.Y., McGlone S.M., Bailey R.R., et al. To test or to treat? An analysis of influenza testing and antiviral treatment strategies using economic computer modeling. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andradottir S., Chiu W., Goldsman D., et al. Reactive strategies for containing developing outbreaks of pandemic influenza. BMC Public Health. 2011;11(suppl 1):S1. doi: 10.1186/1471-2458-11-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randomised Evaluation of Covid-19 Therapy (RECOVERY) https://www.recoverytrial.net/files/recovery-protocol-v7-0-2020-06-18.pdf
- 25.Institute for Clinical and Economic Review (ICER) Alternative pricing models for remdesivir and other potential treatments for COVID-19. https://icer-review.org/wp-content/uploads/2020/06/ICER-COVID_Initial_Abstract_05012020.pdf
- 26.Institute for Clinical and Economic Review (ICER) Alternative pricing models for remdesivir and other potential treatments for COVID-19. https://icer-review.org/wp-content/uploads/2020/06/ICER-COVID_Revised_Report_20200624.pdf
- 27.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 — preliminary report [e-pub ahead of print] N Engl J Med. 2020 May 22 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food & Drug Administration (FDA) Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
- 29.Horby P., Lim W.S., Emberson J., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. June 2020 2020.06.22.20137273. [Google Scholar]
- 30.Gilead Sciences An open letter from Daniel O’Day, Chairman & CEO, Gilead Sciences. Press release. https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman--ceo-gilead-sciences
- 31.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19 [e-pub ahead of print] N Engl J Med. 2020 May 27 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CNBC Mylan prices its generic remdesivir in India at $64 per 100 mg vial. July 6, 2020. https://www.cnbc.com/2020/07/06/mylan-prices-its-generic-remdesivir-in-india-at-64-per-100-mg-vial.html
- 33.Roberts C.M., Levi M., McKee M., Schilling R., Lim W.S., Grocott M.P.W. COVID-19: a complex multi-system disorder [e-pub ahead of print] Br J Anaesth. 2020 Jun 20 doi: 10.1016/j.bja.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.