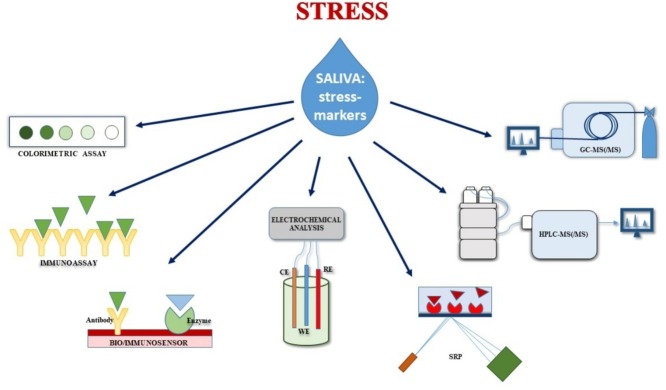
Graphical abstract
Abbreviations: 17−OHP, (17α-hydroxyprogesterone); AA, (alpha-amylase); AED, (androstenedione); AIE, (Aggregation-induced Emission); AIM, (Angular Interrogation Mode); APCI, (Atmospheric-Pressure Chemical Ionization); AuNPs, (Gold NanoParticles); CL, (ChemiLuminescence assay); CLIA, (ChemiLuminescence ImmunoAssay); c-Mab, (Monoclonal Antibody); CNF-SPE, (Carbon NanoFiber Electrodes); CNT, (multiwall Carbon NanoTubes); CV, (Cyclic Voltammetry); DAD, (Diode Array Detection); DHEA, (dehydroepiandrosterone); DHEA-S, (dehydroepiandrosterone-sulfate); DLLME, (Dispersive Liquid-Liquid MicroExtraction); DPV, (Differential Pulse Voltammetry); ECLIA, (ElectroChemiLuminescence ImmunoAssay); ESI, (Electrospray Ionization); FIA, (Flow Injection Analysis); FO, (Fiber Optic); FOSPR, (Fiber Optic Surface Plasmon Resonance); HMP, (2-Hydrazino-1-MethylPyridine); HPLC, (High Performance Liquid Chromatography); HRV-SCort, (Heart Rate Variability combined with Salivary Cortisol); IA, (ImmunoAssay); iCIEF, (Imaging detection Capillary Isoelectric Focusing); IIM, (Intensity Interrogation Mode); IL-DLLME, (Ionic Liquid Dispersive Liquid-Liquid MicroExtraction); LC, (Liquid Chromatography); LFIA, (Lateral Flow ImmunoAssay); LLE, (Liquid-Liquid Extraction); LMR, (Lossy Mode Resonance); LSPR, (Localized Surface Plasmon Resonance); MALDI-MS, (Matrix-Assisted Laser Desorption Ionization-Mass Spectrometry); MD, (MaltoDextrin); MEPS, (MicroExtraction by Packed Sorbent); MHPG, (3-Methoxy-4-hydroxyphenylglycol); MIP, (Molecularly Imprinted Polymer); MS/MS, (tandem mass spectrometry); n.c., (not calculated); n.s., (not specified); NU, (Novo Unit); PEG, (polyethylene glycol); PIT, (Photochemical Immobilization Technique); POC, (Point Of Care); PPAuNPs, (Polyaniline Protected gold Nanoparticles); PPY, (Polypyrrole); QCM, (Quartz-Crystal Microbalance); rGO, (reduced Graphene Oxide); sAA, (salivary alpha-amylase); SPCE, (Screen-Printed Carbon Electrodes); SPE, (Solid-Phase Extraction); SPME, (Solid-Phase MicroExtraction); SPR, (Surface Plasmon Resonance); SPRS, (Surface Plasmon Resonance Spectroscopy); SWV, (Square Wave Voltammetry); TR-IFMA, (Time-Resolved Immuno-Fluorometric Assay); UHPLC, (Ultra-High-Performance Liquid Chromatography); UV, (UltraViolet detector); Vis, (Visible detector); XLC, (eXtraction Liquid Chromatography)
Keywords: Biomarker, Immunoassay, Mass spectrometry, Point of care device, Chromatography, Psychological stress
Abstract
Stress and stress-related diseases are leading to drastic consequences in private and professional life. Therefore, the need for stress prevention strategies is of personal and economic interest. Especially during the recent period related to covid-19 outbreak and lock-down, an ongoing discussion of increasing stress etiology is reported. Biomarker analysis may help to assist diagnosis and classification of stress-related diseases and therefore support therapeutical decisions. Due to its non-invasive sampling, the analysis of saliva has become highly attractive compared to the detection methods in other specimen. This review article summarizes the status of research, innovative approaches, and trends. Scientific literature published since 2011 was excerpted with concentration on the detection of up to seven promising marker substances. Most often reported cortisol represents the currently best evaluated stress marker, while norepinephrine (noradrenaline) or its metabolite 3-methoxy-4-hydroxyphenylglycol is also a quite commonly considered stress marker. Other complementary stress marker candidates are testosterone, dehydroepiandrosterone (DHEA) and its sulfonated analogue DHEA-S, alpha-amylase, secretory immunoglobulin A, and chromogranin A. Several working groups are researching in the field of stress marker detection to develop reliable, fast, and affordable methods. Analytical methods reported mainly focused on immunological and electrochemical as well as chromatographic methods hyphenated to mass spectrometric detection to yield the required detection limits.
1. Introduction
According to the endocrinologist Hans Selye, stress is the non-specific response of the body to any demand. While generally connected with adaptations of the body by the activation of the sympathetic nervous system, psychological stress in psychology is often separated as positive and negative stress. Positive stress (eustress) helps to improve performance and motivation (either mental or physical), whereas distress is considered as excessive amounts of stress, which may lead to health risks. In public language, stress is most often connected with distress. Thus, in psychology, stress is mainly related to emotional strain and pressure.
Prolonged emotional pressure of distressing periods, or chronic stress, may lead to a broad spectrum of physical and psychological diseases [[1], [2], [3], [4], [5]]. Whether an experience is perceived or not as distressing, strongly depends on individual aspects (e.g., environment, socioeconomic stability, passed personal history, or mental health) and is physiologically hard to estimate. Several factors like persisting liability, ongoing anxiety, desperateness, or a lack of prospects are tightening this experience. Hence, there is an increased risk for people who are working in fields with ongoing emotional pressure, like physicians, caregivers, nurses, social workers or teachers, etc. [[6], [7], [8], [9], [10]]. Especially during nowadays coronavirus pandemic, an ongoing discussion of increasing stress etiology in health caregivers, but also in the general public is reported [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. In public perception, increasing stress load leads to symptoms summarized as burnout. Chronic stress and burnout also influence how people will physiologically react, cope, and adapt to a future acute stress event. Thus, the diagnosis of chronic stress is becoming more and more important in health protection [22]. Methods often rely on psychological scales achieved by questionnaires, that strongly depend on the individual patient [23]. Additionally, the physiological response to mental and physical stress involves various compounds that may, therefore, be used as biomarkers in biological signal-based methods.
The most commonly considered biomarker for stress determination in humans is cortisol. This well-examined hormone is part of the stress response, and its concentration varies in acute and chronic stressful situations. In addition, there are a lot of other biomarkers with a strong correlation to the perceived stress load. Their detection methods are as multifarious as their amount. Many research groups are working in the field of stress marker detection. Therefore, the number of reported detection methods is increasing too. Most of the manuscripts published in the scientific literature are dealing with the detection of a few stress markers in different matrices. Due to its non-invasive and stress-free sampling, the analysis of saliva has become highly attractive compared to the detection methods in blood, liquor, or hair. Therefore, the aim of this review is to give an overview of state-of-the-art analysis, to summarize the different detection approaches, and to provide a lookout for prospective methods.
2. Background
Stress, caused by exceptional circumstances, is leading to a dysregulation of biological homeostasis. Thereby, these dysfunctions are concerning all hormonal, neurohumoral, and physiological axes. The measurement of marker substance levels of these axes can be used for estimating the stress response level of an individual. The interactions of these substances and the physiology of the axes will be briefly introduced in the following.
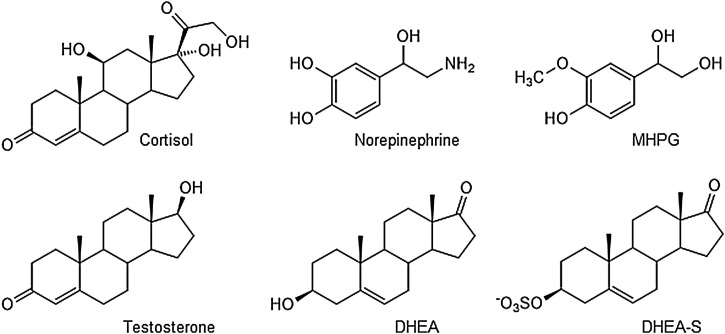
The immediate stress reaction, or the fight-or-flight response, involves the rapid activation of the autonomic nervous system (adrenal medulla, ANS), which leads to an increased concentration of epinephrine and norepinephrine (chemical structure in Fig. 1 ) in blood. Only limited research focused on the detection of the parent compounds themselves as they display limited chemical stability and are rapidly metabolized to yield 3-methoxylated metabolites. Therefore, the level of their metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG, Fig. 1) is also associated with a stress response. Its reference value from 12.85 ng/mL was determined by Reuster et al. [24], while Okumura et al. reported a method able to quantify norepinephrine at about 0.17 pmol/mL (i.e., 30 pg/mL) and epinephrine at about 0.1 pmol/mL (20 pg/mL) in saliva [25]. Furthermore, they mentioned a stress-related increase of the salivary neurotransmitter dopamine in some cases, most likely related to strong fear or anxiety. As increasing catecholamine levels are also associated with an induction of an elevated metabolic rate, metabolomics may also be considered an interesting future approach. However, at present, no publications related to metabolomics investigations on human saliva in psychological stress were retrieved within our database search.
Fig. 1.
Chemical structures of the small molecule stress-related biomarkers cortisol, norepinephrine, 3-methoxy-4-hydroxyphenylglycol (MHPG), testosterone, dehydroepiandrosterone (DHEA), and DHEA sulfate (DHEA-S).
As hypothesized, the hypothalamus-pituitary-adrenal (HPA) axis responds deferred. Limbic and hypothalamic structures are coordinating the emotional, cognitive, neuroendocrine, and autonomic inputs. On its activation, corticotropin-releasing-hormone (CRH) is excreted. This leads to a release of adrenocorticotropic hormone (ACTH) in the pituitary, which triggers the release of glucocorticoids in the adrenal gland. While ACTH is reported to increase as a stress-related response by Ramos et al., others found ACTH levels varying with age, sex and type of stress [[26], [27], [28]]. Salivary ACTH levels are only rarely used in stress marker research.
Triggered by the HPA signaling the most important stress-related glucocorticoid is cortisol. The normal ranges of cortisol in blood and saliva for healthy individuals are around 30−160 ng/mL (blood) and 1–1.6 ng/mL (saliva), respectively [29]. It is crucial to notice, though, that cortisol concentration is influenced by several factors such as sex, age, population, circumstances, and circadian rhythm [30]. In the case of an acute stress situation, the cortisol level is increasing and reaches the concentration peak around 20−30 min after the stressor event. If the stressor is still ongoing over a long-term period, cortisol levels are decreasing. If stress becomes chronic, the response of the HPA axis is attenuated. Therefore, cortisol (Fig. 1) is also considered as a marker of long-term response in humans.
Reflexively, a high cortisol concentration influences the testosterone (Fig. 1) level in blood, which is reported to decrease from its normal range (0.35 nmol/L [31]). The pre-stages of testosterone are dehydroepiandrosterone (DHEA) and its sulfated form DHEA-S (Fig. 1). Concomitant with an altered level of cortisol, the level of DHEA and DHEA-S change as well [32]. This means that its average concentration in saliva (0.2–2.7 ng/mL) also depends strongly on age, sex, and daytime [33]. While the acute stage of stress, the level of DHEA (-S) increases, while its level decreases in a long-term situation.
In parallel with the HPA axis, further paths are activated: gonadal axis, adipose axis, and immune system. Thereby, several other biological marker-substances are associated with acute and chronic stress. Thus, in addition to epinephrine, norepinephrine, cortisol, and testosterone, there are further proteins and enzymes correlated with stress.
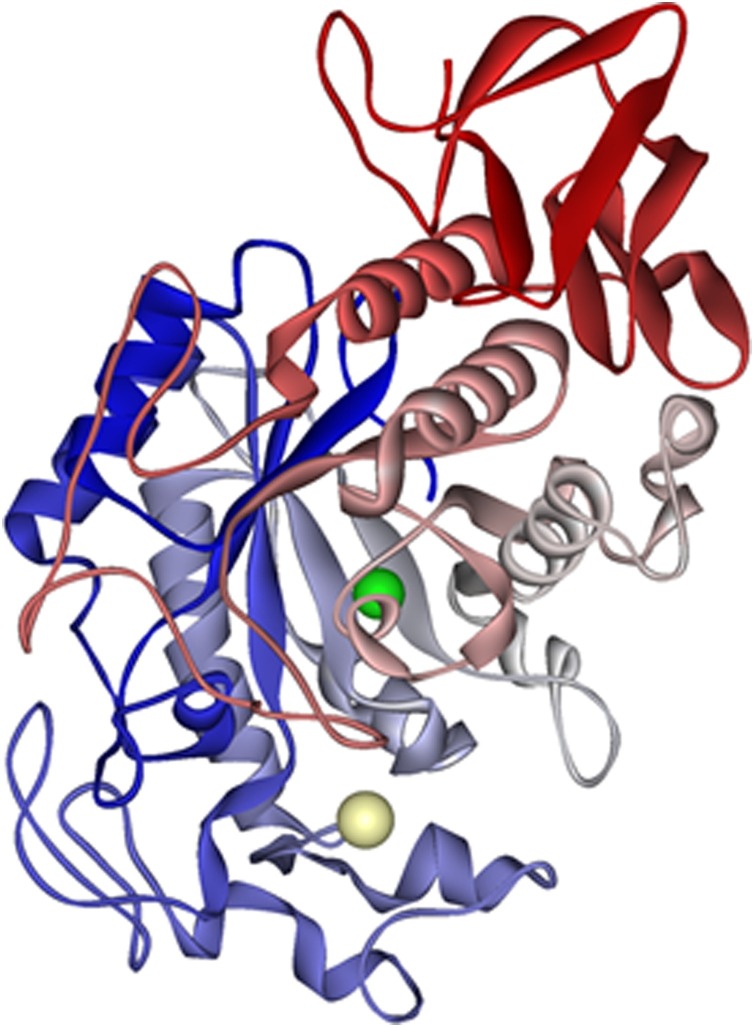
The increase in catecholamines leads to a greater need for energy. Alpha-amylase (AA, Fig. 2 ) is an enzyme, which also occurs in saliva (salivary AA (sAA)) with a normal range between 90–250 U/mL [29]. Its task is to split carbohydrates into digestible oligosaccharides, which are very important during a fight-or-flight response situation. The increase of AA activity is, therefore, also associated with acute stress.
Fig. 2.
Structure of alpha amylase, based on Ramasubbu et al. [157].
Another stress-related protein is the secretory immunoglobulin A (sIgA), which is secreted by B cells from the immune system. It plays a significant role in mucosal defense against pathogens and occurs in healthy individuals and normal conditions in a range of ca. 60.3 ± 3.46 μg/mL in saliva [34]. B cells have a high density of β2-adrenergic receptors. In case of a stress reaction, the increased level of epinephrine and cortisol works along this pathway.
Another stress response marker is chromogranin A (CgA, Fig. 3 ), which is part of the pathway of the autonomic nervous system. It is the precursor to several functional peptides like vasostatin-1, vasostatin-2, pancreastatin, catestatin, or parastatin. These peptides negatively modulate the neuroendocrine function of releasing cells, like cells of the adrenal medulla, which releases adrenaline to the blood. In this way, the chromogranin A level is associated with stress.
Fig. 3.
Chromogranin A: amino acid sequence of the recognized domain, used by Escribano et al. [142].
In some papers, serotonin is considered as a stress biomarker, but mainly in blood or urine, where the concentration is higher [29]. Moreover, Egri et al. found out that in children the salivary serotonin levels do not correlate well with plasma levels and Leung et al. highlighted that they do not correlate with central serotonin turnover either, making serotonin a non-suitable salivary biomarker for evaluation of serotonergic system functioning [35,36].
3. Methods
Based upon two publications from Danhof-Pont et al. [37] and Yamaguchi et al. [38], we have searched the databases of Pubmed and ISI Web of Science for articles published since 2011, which are dealing with stress marker detection in saliva. According to the described stress markers (x), we have worked with the Boolean search terms (x) AND detection AND saliva. The received articles were then excerpted given their relation to stress research, developed or used method, the achieved detection limits, and feasibility.
4. Assays
Well established and innovative analytical techniques are both used for biomarker determination. Initially, radioimmunoassays (RIAs) were very common, but other immunoassays (IA) were developed and optimized later to avoid the use of radioactive reagents. Currently, the enzyme-linked immunosorbent assay (ELISA) and immune-based biosensors are of more frequent use [[39], [40], [41], [42]].
In general, IA are susceptible to cross-reactivity dependent on the selectivity of antibodies used therein. Molecularly imprinted polymers (MIPs) are recently studied as alternatives and used in assays to overcome the economic downsides of antibody-based assays [43]. Similarly, bio- or chemiluminescent assays may serve as alternatives [[44], [45], [46], [47], [48], [49]].
Major advantages of IA include their effective usability in the clinical routine with several commercially available kits. However, classical IAs represent single analyte assays only. Furthermore, cross-reactivities with structurally related compounds may confound the analytical results, especially if differences in concentration between the analyte and cross-reacting agent are very high.
In addition to classical immunoassays, immunological methods and electrochemical analysis are often combined in stress marker analyses [[50], [51], [52], [53], [54], [55]]. Bio- and immunosensors are widespread thanks to their versatility, easy use, sensitivity, and short analysis time [45,46,[50], [51], [52], [53], [54], [55], [56]]. Furthermore, due to their possibility of miniaturization, they offer an excellent opportunity for developing point of care (POC) devices.
The majority of steroidal stress biomarkers in saliva are still analyzed with IAs because they are technically easy to use, rapid, and relatively cheap. There are, though, some downside not always negligible. IAs are based on the chemical binding reaction between antibodies and a specific analyte, but sometimes the assay selectivity can be mined by the antibodies cross-reactivity with structurally similar compounds. As reported in literature, this is the case of cortisol and cortisone in saliva [[57], [58], [59]]. Similarly, in plasma sample analyses cross reactivities for DHEA-S and testosterone were reported [60,61]. Often overlooked high differences in the concentration levels (even in order of magnitude) may require cross-reactivities far below 1 % which are often stated as "no cross reactivity" in IA kits. Furthermore, cross-reactivity is not the only variable that can influence the reliability of an IA. Since they might be designed differently, also the resulting interaction antibodies-analytes might change, and it is often not possible to compare different IAs results [57]. Therefore, some conversion tables have been developed to obtain comparable factor scores between results obtained with the most common IAs [57]. Buttler et al. [62] demonstrated high imprecision of some kits for dehydroepiandrosterone-sulfate by comparison of different IA kits and isotope-dilution-LC–MS/MS.
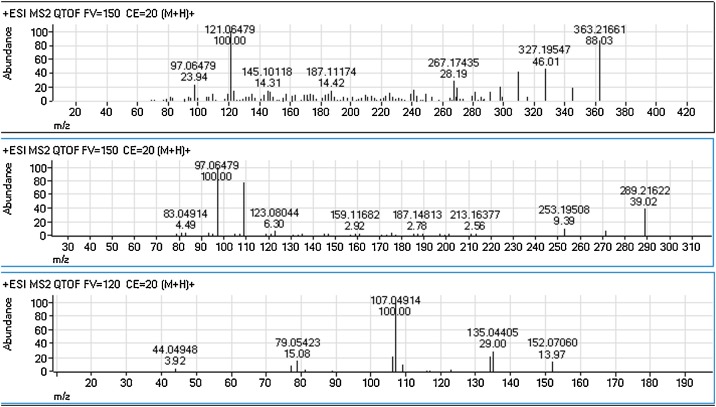
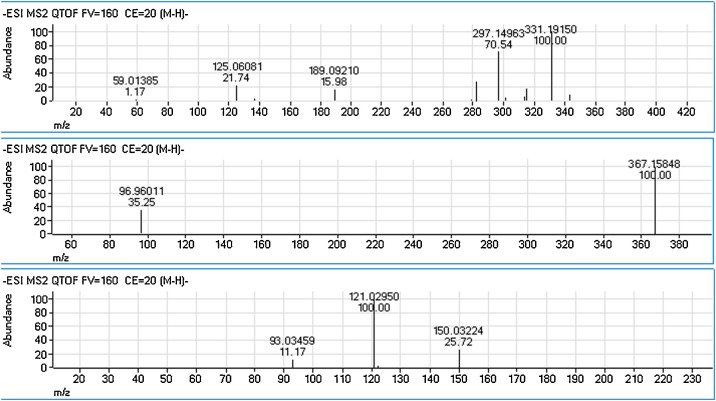
Thus, several authors instead use liquid chromatography (LC) or gas chromatography (GC) mostly coupled with mass spectrometry (MS). These hyphenation methods also allow for multi-analyte design, which cannot be achieved in classical IA analyses. Well-established in steroid profiling, GC–MS combines high selectivity due to its very high power of chromatographic separation and additional mass selective separation with its potential for trace level analysis. Fujimaru et al. and Yamada et al. utilized GC–MS for the determination of the (nor-)epinephrine metabolite MHPG [9,63]. To extend the scope of analytes, we have tested a GC–MS method for the simultaneous determination of stress related small molecule biomarkers. First experiments had shown that one could combine cortisol, testosterone, DHEA, and MHPG. These substances were well detectable as trimethylsilyl (TMS) derivatives by GC–MS. Currently, method validation is in progress. However, the analysis of sulfoconjugates is rather complicated by GC–MS. Thus, HPLC based methods were also tested for combined analysis of the above-mentioned small molecule stress biomarkers. In bioanalysis, HPLC is often coupled with (tandem) mass spectrometry (LC–MS(/MS)), which leads to higher selectivity and sensitivity and concomitantly a much lower limit of detection (LOD). It offers a fast, reliable, highly selective, and robust determination of a wide variety of biomarkers for use in multi-analyte designs [[64], [65], [66]]. The product ion spectra of cortisol, testosterone, and norepinephrine, DHEA-S, and MHPG are displayed in Fig. 4 (positive ionization) and Fig. 5 (negative ionization). The use of HPLC based methods may also help to overcome the lack of suitability for protein-based biomarkers in GC-based methods. However, a comprehensive detection of small molecules like catecholamines and steroids together with larger proteins may remain challenging, especially if sample pre-treatment needs to be incorporated in the method. Before running an HPLC-MS analysis of a salivary sample, either a solid-phase extraction (SPE) or a liquid-liquid extraction (LLE) is traditionally performed. In the last years, attempts have been made to reduce the consumption of organic solvents in the sample preparation. Therefore, microextraction techniques, ionic liquids (IL), or a combination of the two (IL-DLLME) [64,[67], [68], [69], [70]] have been introduced.
Fig. 4.
Product ion spectra (LC-QTOF-MS) of cortisol (upper), testosterone (middle), norepinephrine (lower), all positive ionization (ESI+), collision energy CE = 20V.
Fig. 5.
Product ion spectra (LC-QTOF-MS) of cortisol (upper), dehydroepiandrosterone sulfate (DHEA-S, middle) and 3-methoxy-4-hydroxyphenylglycol (MHPG, lower), all negative ionization (ESI-), collision energy CE = 20 V.
More specific details of analytical methods are given in the individual sections of specific biomarkers.
5. Saliva sampling and storage for stress marker analyses
Saliva represents a highly attractive specimen in bioanalysis due to its non-invasive sampling. It has the undeniable advantage of being easy to get, even from newborns and elderly, non-invasive, and non-stressful, and is therefore frequently used in stress-related research. The sampling procedure, though, requires some precautions to obtain accurate and reliable results. For several analytes concentrations in saliva are highly correlated to serum concentrations [71,72]. As shown in this review, there are different collection methods resulting in data that are not always interchangeable: comparing analytical results with different procedures of sampling might lead to incorrect deductions. Sampling is most often performed using easy collection devices. Standard protocols are developed and reported for many fields of analysis and adaptation to field conditions was achieved already many years ago [73].
However, several potentially confounding factors need to be considered to obtain significant and reliable data. Lipson and Ellison reported no influence of the selection of the type of sampling tubes (glass or polystyrene) on salivary steroid concentrations [73].
For all the biomarkers considered, some factors can influence the analysis. It is, therefore, recommended not to eat, smoke, drink, or brush the teeth for at least one hour before the collection, and to avoid blood contamination while collecting (i.e., by scratching the gum) [[74], [75], [76], [77]].
Whetzel et al. and Shirtcliff et al. compared cotton swab (Salivette) and passive droll collection methods [33,78]. No significant differences in the two approaches were detected in case of cortisol and DHEA-S. Similar findings for cortisol were obtained by Gallagher et al., and Hodgson and Granger in the comparison of passive droll and Salivette swab collection [79,80]. The comparison of passive droll collection with two commercial kits, OraSure and Oracol reported by Nurkka et al. also resulted in non-significant influences for sIgA [81]. In contrast, Shirtcliff et al. reported significantly different concentrations for testosterone and sIgA when comparing Salivette and passive droll collection. The authors hypothesized that this effect might be caused by a non-specific binding or cross-linking reaction of the antibody used in the immunoassay, or that an interfering substance is filtered out by the cotton [78]. Lipson and Ellison found a high correlation of salivary steroids (progesterone, androstenedione, testosterone and cortisol) in non-stimulated collection and stimulated collection utilizing gum chewing (with or without sugar additives), candy, or lemon juice [73]. However, significantly different concentrations compared to unstimulated collection were determined for cortisol (lemon juice, lower concentrations), testosterone and androstenedione (sugarless gum, lower concentrations), as well as for progesterone (stimulation by sugared gum, higher concentrations). Additionally, interindividual variances in the slope of the correlation equation were found for progesterone concentrations comparing unstimulated and sugarless gum stimulated collection (higher or lower concentrations depending on the individuals) [73].
For evaluation of sAA activity Beltzer et al., and Rohleder et al. highlighted that the salivary flow rate has a non-critical impact [82,83]. However, it is observed that the activity of sAA strongly depends on the sampling area of the mouth [82,84]. Thus, differences in the sampling devices impact the results even if they do not result in different flows of saliva. Robles et al. hypothesized that parotid and submandibular glands produce a higher level of sAA in comparison with sublingual glands [84]. On the analysis of MHPG and CgA, there are only a few studies. To our knowledge, only Higashi et al. studied the MHPG concentration with (stimulated) and without (unstimulated) gum-mastication, resulting in a significant decrement in MHPG amount caused by salivary stimulation [85].
Additionally, the correct time for sampling is crucial for later result interpretation. Analyses of biomarkers have different time windows in case of chronic or acute stress. For example, a specific stress event causes an increase of cortisol after 15−20 min from the exposure; then, the level begins to decrease even if the stressor persists for some time, therefore, taking a sample of saliva after this window will not be useful [86]. Especially in monitoring of chronic stress a longitudinal evaluation is often performed and needs careful experimental design. Most of the biomarkers taken into consideration in this review are secreted with a trend, or circadian cycle, regulated mainly by day/night alternation and their amounts in saliva change within 24 h [33,[86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]]. The concentration of cortisol, for instance, starts rising in the morning and reaches its peak within 40 min after awakening. Then, the level begins to decrease until the minimum, or nadir, between 10 pm and 2 a.m. Normally, cortisol has a stable 24 h profile, overall unaffected by acute changes in habits or behavior. Intense training, lack of sleep, or fasting disrupt only momentarily the circadian cycle that will quickly return to homeostasis. In the case of chronic stress, instead, the level of cortisol remains high during the 24 h resulting in an attenuated circadian cycle and a blunted response to future stressors [[86], [87], [88], [89], [90],96].
It is also critical to consider the medical history of the subject examined because some diseases can cause an alteration of the basal level of the biomarker. The Cushing's and the Chronic fatigue syndromes, i.e., create a blunted cortisol wake/sleep variation similar to the one due to chronic stress [86,87,96].
Another aspect to consider is the storage of the salivary samples prior to the analytical determination.
The steroids are reported as quite stable biomarkers in saliva. Clements and Parker demonstrated that saliva samples for the analysis of cortisol resist at least five days with temperature variations between 15 °C and 38 °C [97]. Moreover, cortisol concentrations are stable at 5 °C for up to 3 months or at -20 °C and−80 °C for at least one year. The only variation registered was after one month of storage at room temperature [77]. Durdiaková et al. studied the stability of testosterone at room temperature, at 4 °C, at −20 °C, and at −80 °C. In each of these cases, no significant variations were found within at least one month [98]. To our knowledge, there are no stability studies for DHEA-S and MHPG in saliva.
In contrast, sample storage for the analysis of the protein biomarkers required more attention. Bellagambi et al. studied the stability of sAA for short and long term storage. The enzymatic activity remained stable for at least 8 h at room temperature (analysis at ten different time points) and after four weeks at −80 °C. The activity showed a decrease of about 15 % at 4 °C, always after four weeks [99]. In IgA analyses, snap-freezing with glycerol in liquid nitrogen resulted in significantly higher concentrations in comparison to those detected after storage for 4–8 hours at 4 °C with and without the addition of protease enzyme inhibitors before freezing at −70 °C [81]. In contrast, Ng et al. found stable salivary IgA concentrations for up to 3 months at 30 °C [100]. Escribano et al. evaluated the stability of CgA in porcine saliva specimen in different storage conditions. They proved that there is not a significant reduction in concentrations up to one year at −20 °C or−80 °C, and up to seven freezing/thawing cycles [101].
6. Stress related biomarkers
As relevant salivary stress markers, Yamaguchi et al. focused on cortisol, DHEA-S, testosterone, sAA, CgA, and sIgA [38]. These compounds were also examined by Danhof-Pont et al., who reported further 32 candidates for biomarkers in case of burnout [37]. These biomarkers concerned the HPA axis, the steroid hormones, the autonomic nervous system (ANS), the immune system, different metabolic processes, and the antioxidant defense system.
These findings were confirmed by our literature search. Since 2011, a significant number of publications reported the detection of cortisol in saliva (more than 300), but not all of them had a direct relation to stress research. Other well-examined biomarkers in saliva are testosterone (ca. 70 publications), chromogranin A (11 publications), salivary alpha-amylase (ca. 100 publications), and secretory immunoglobulin A (50 publications). Other interesting candidates were the sulfated precursor of testosterone DHEA-S (10 publications) and the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) (2 publications). Not all of these papers are related to stress biomarker research, thus, a supplementary selection has been made.
In the following sections, we itemize the most relevant biomarkers and their analytical methods for determination.
6.1. Cortisol
Cortisol (chemical structure in Fig. 1) is the most commonly utilized stress biomarker. It is reported to show a strong correlation between serum and salivary concentrations [102]. This correlation allows the detection of active cortisol in saliva, with a non-invasive, stress-free sampling.
Since 1979, when Kabra et al. reported the first HPLC method using UV detection for the detection of cortisol in serum/plasma [103], the variety of approaches has drastically increased.
Nowadays, cortisol determination is often performed by LC–MS/MS to provide the desired selectivity and sensitivity. Further chromatographic methods utilize high performance thin layer chromatography (HPTLC) and eXtraction Liquid Chromatography (XLC). GC methods are not reported for the detection of cortisol in saliva within the monitored time frame. However, from its analysis in other biological matrices, it is known that GC separation requires the derivatization of cortisol, which may hamper its quantitation [104].
Miller et al. refer to LC–MS as a well-established reference method [105]. Thus, they used it to compare the suitability of diff ;erent commercial IA. Their findings revealed nonlinear relations between IA and LC–MS/MS methods, which they discussed in terms of IA cross-reactivity with saliva matrix components. In contrast, Kaushik et al. referred to immunoassays as the gold standard in cortisol detection because these methods are simple, rapid, economical, sensitive, robust, and reproducible [106]. Radioactive labeled IAs (RIAs) are no longer preferable because of the risk of the radioactively labeled assays, and the trend shifted towards fluorescence labeling. Nevertheless, the analyte still needs to be pre-treated.
Modern label-free detection methods like surface plasmon resonance spectroscopy (SPRS) are developed to avoid the preparation step. Tahara et al. described an SPR method for detecting cortisol [107]. The sensor surface was modified with carboxylated polyethyleneglycol and cortisol 3-(O-carboxymethyl) oxime as a cortisol analog. This indirect competitive setting worked with anti-cortisol antibodies, which first bind to free cortisol in the sample. Afterwards, the remaining unbound antibodies were captured on the sensor surface. The received response of the layer thickness was inversely proportional to the concentration of free cortisol in the sample. The developed method yielded a limit of detection (LOD) of 38 pg/mL.
The use of fiber-optic (FO) based SPR sensors allowed to reach the lowest LODs ever registered. In 2017, Usha et al. reported a fiber optic salivary cortisol sensor, using lossy mode resonance (LMR) with molecular imprinting of ZnO nanocomposites and polypyrrole (PPY) [108]. Very recently (March 2020), Pandey et al. described a fiber-optic plasmonic sensor for cortisol quantitation. They realized two FO sensors based on plasmonic gratings: one made of SiO2 and a thin Ag layer, and the other of SiC/Ag [109]. They operated in Angular Interrogation Mode (AIM) and Intensity Interrogation Mode (IIM) to determine the LODs. In AIM, assuming 0.001° as the smallest angular shift detectable, they calculated a 9.9 pg/mL LOD for the SiO2/Ag sensor probe and 9.8 pg/mL for SiC/Ag. With IIM they obtained even lower limits, i.e., 22.6 fg/mL for SiO2/Ag and 68.17 fg/mL for SiC/Ag, which is by far below the concentration range that is expected for salivary cortisol.
Electrochemical (EC) methods are usually easy, fast, and fewer solvent consuming. There are different typologies of EC analysis; the most common are cyclic voltammetry (CV), differential pulse voltammetry (DPV), square wave voltammetry (SWV), amperometry, and potentiometry. Furthermore, electrochemical methods allow the realization of devices for POC use. There are some publications collected in this review (Table 1 ) that combine the advantages of the electrochemical sensors with the high selectivity of the immune assays.
Table 1.
Overview of different cortisol detection methods and their LOD. Reference values of salivary cortisol are reported in the range of 1–1.6 ng/mL [29].
| Method/Analytical technique | Ref. | Saliva collection | LOD | Comment |
|---|---|---|---|---|
| Bioluminescence | [44] | n.s. | 360 pg/mL | |
| Chemiresistor Immunosensor | [45] [46] |
Artificial saliva n.s. |
1 pg/mL 10 pg/mL |
Single-walled, carbon nanotube-based chemiresistive transducer c-Mab covalently immobilized on rGO |
| CL | [47] | Artificial saliva | 80 pg/mL | |
| CLIA | [110] [111] |
Sponge Artificial saliva |
160 pg/mL 0.47 pg/mL |
In pigs, SPE |
| Electrochemical biosensor (immunosensor and IA) | [50] [51] [ 52] [ 53] [ 54] [55] |
Salivette® PBS solution Artificial saliva n.s. Salivette® Passive droll |
10 pg/mL 0.36 pg/mL 1.03 pg/mL 0.4 pg/mL 1.7 ng/mL 1 ng/mL |
CV PPAuNPs on Au electrode, CV + DPV POC, 35 min analysis POC Disposable immune-sensor, CV + SWV Fluid control: vertical + lateral flow (no pre-treatment) |
| ELISA | [39] [40] [41] |
n.s. Alpco Diagnostics salivettes Oral swab |
28 pg/mL 1 ng/mL 1.02 ng/mL |
Cortisone detection LOD (saliva) is cited from kit supplier Biomimetic ELISA: MIP, colorimetric detection |
| ELISA: indirect assay ELISA: direct competitive assay |
[42] | n.s. | 0.5 ng/mL 1.2 ng/mL |
Cortisol-ovalbumin conjugated Cortisol-alkaline phosphatase-conjugated |
| IL-DLLME + LC-UV/Vis | [70] | Salivette® tubes | 160 pg/mL | Cortisone detection |
| LFIA | [112] [113] [10] |
Artificial saliva Salivette® Salivette® |
0.5 ng/mL 0.3 ng/mL 0.4 ng/mL |
Naked eye, AuNPs as signal labeling with Ag enhancement system, POC CL imaging system, POC CL biosensor, astronauts |
| LC-MS/MS | [114] [66] |
Spitting directly into a tube Salivette® |
100 pg/mL 10 pg/mL |
ESI, pre-treatment: LLE, simultaneous detection of melatonin and testosterone SPE, quadrupole linear ion trap, simultaneous quantitation of 17-OHP, aldosterone, AED, cortisol, cortisone, DHEAS, estradiol, progesterone, and testosterone |
| LMR-MIP | [108] | Artificial saliva | 25.9 fg/mL | Fiber optic sensor |
| MEPS-HPLC-DAD | [69] | Disposable plastic pipette | 1.5 ng/mL | Simultaneous cortisone and corticosterone detection |
| SPME + LC-MS/MS | [68] [64] |
Salisoft® tubes Salisoft® tubes |
1.1 pg/mL 0.9 pg/mL |
Simultaneous Testost. and DHEA detection Simultaneous DHEA detection |
| SPR (FOSPR) | [109] | Salivette® | AIM: 9,9 pg/mL (SiO2); 9.8 pg/mL (SiC) IIM: 226 fg/mL (SiO2); 6817 fg/mL (SiC) |
Plasmonic grating fiber optic-based SPR sensor (SiO2 and SiC) |
| SPR (Indirect competitive IA + SPR) | [107] | Oral swab | 38 pg/mL | compared with di ELISAs |
| Turbulent Flow chromatography + LC-MS/MS | [115] | Salivette® | 1.4 pg/mL | Simultaneous cortisone and melatonin detection |
| XLC-MS/MS | [116] | Salivette® | 72 pg/mL | Simultaneous cortisone detection |
| ECLIA | [49] | Salivette® | n.c. | Simultaneous detection of Cortisol, DHEA-S, IgA |
Several additional methods are published, diff ;erent in sample pre-treatment and detection. To resume the results, Table 1 gives an overview.
6.2. Testosterone
The detection of testosterone (chemical structure in Fig. 1) in saliva is described in ca. 70 publications since 2011, mainly in correlation with doping or physical stress in sport. Escribano et al. presented an automated chemiluminescent immunoassay for detecting salivary testosterone in pigs. The reported method utilized a commercial kit with a solid-phase, competitive chemiluminescent enzyme immunoassay [48]. Furthermore, Gosetti et al. summarized the detection of steroids with ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) in different human matrices, including saliva [117]. This review only refers to the detection of 17-hydroxyprogesterone in saliva; the analysis of testosterone is mainly described in other matrices, like hair, blood, or urine.
Other publications are dealing with testosterone detection in saliva: Jensen et al., Sobhi et al., Alzahrani et al., and Gomez-Gomez et al. used HPLC-MS/MS [114,[118], [119], [120]]. All four research groups used electrospray ionization source in positive mode. Out of these four papers, Sobhi et al. detected testosterone alone in human saliva while the others reported multi-compound analyses.
Immunoassays are well established and accepted in the scientific community, but they require the use of natural receptors or antibodies that are often very expensive. To overcome this problem, molecularly imprinted polymers (MIPs) are gaining more attention as an advantageous alternative. Kellens et al. used a microfluidic system combined with an in situ photo-polymerization on functionalized diamond substrates to obtain a controlled and consistent distribution of micro-patterned MIPs on the sensor substrate. So, they realized a selective sensor platform for testosterone [43].
There are some additional methods published, diff ;erent in sample pre-treatment and detection. To resume the results, Table 2 gives an overview.
Table 2.
Overview of different testosterone detection methods and their LOD.
| Method/Analytical technique | Ref. | Saliva collection method | LOD | Comment | |
|---|---|---|---|---|---|
| Biosensor | SPR LSPR |
[56] | PBS solution | 0.05 ng/mL | Competitive inhibition assay with AuNPs, POC |
| CLIA | [48] | Oral sponge | 0.16 ng/mL | In pigs, automates CLIA | |
| ECLIA | [49] | Salivette® | n.c. | Simultaneous detection of Cortisol, DHEA-S, IgA | |
| Impedance immunosensor | [121] | n.s. | 3.9 ng/mL | AuNPs Modified Electrode | |
| LC-MS/MS | [114] [118] [ 119] [ 120] [66] |
Spit dir. into a tube Spit dir. into a tube Spit dir. into a tube Salivette® Salivette® |
3 pg/mL 43.2 pg/mL (LLOQ) > 0.3 ng/mL 4 pg/mL 4.9 pg/mL |
LLE, Simultaneous detection of melatonin and cortisol DLLME, positive mode Simultaneous detection of 15 other steroids Derivatization with HMP, simultaneous detection of DHEA SPE, quadrupole linear ion trap, simultaneous quantitation of 17-OHP, aldosterone, AED, cortisol, cortisone, DHEA-S, estradiol, progesterone, and testosterone |
|
| MALDI-MS | [114,122] | Spitting directly into a tube | 1.7 μg/mL | Simultaneous detection of progesterone, cortisone, hydrocortisone. | |
| MIP-sensor | [43,122] | n.s. | 0.14 ng/mL | on Functionalized Diamond-Coated Substrates | |
| Multimode sensors | [43,123] | n.s. (rinsed mouth) |
STOCH. MODE CNT: 3.4 pg/mL Graphite: 0.12 pg/mL Graphene: 0.7 fg/mL Fullerene: 49.0 pg/mL DPV MODE CNT: 3.8 pg/mL Graphite: 11.9 ng/mL Graphene: 19.2 ng/mL Fullerene: 4.2 ng/mL |
Carbon based sensors and CNT modified with MD. DPV and stochastic mode | |
Abbreviations: 17−OHP (17α-hydroxyprogesterone), AED (Androstenedione), AuNPs (Gold NanoParticles), CLIA (ChemiLuminescence ImmunoAssay), CNT (multiwall Carbon NanoTubes), DLLME (Dispersive Liquid-Liquid MicroExtraction); ECLIA (ElectroChemiLuminescence ImmunoAssay), HMP (2-Hydrazino-1-MethylPyridine), LC (Liquid Chromatography), LLE (liquid-liquid extraction), LSPR (Localized Surface Plasmon Resonance), MALDI-MS (Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry), MD (MaltoDextrin), MIP (Molecularly Imprinted Polymer), MS/MS (tandem mass spectrometry), n.c. (not calculated), n.s. (not specified), SPR (Surface Plasmon Resonance), UHPLC-MS/MS (Ultra-High-Performance Liquid Chromatography-tandem Mass Spectrometry).
6.3. Dehydroepiandrosterone-sulfate (DHEA-S)
Dehydroepiandrosterone-sulfate (DHEA-S, chemical structure in Fig. 1) appears to be an interesting stress marker, albeit its detection is not very deeply examined. Yamaguchi et al. pointed out that DHEA-S has an excellent serum-saliva correlation (0.86, Salimetrics assay) because it enters saliva via ultrafiltration through the tight junctions between the acinar cells [38]. Since 2011, only a few published works dealt with the analysis of this steroid in saliva.
Various methods for salivary DHEA-S are reported, either utilizing IA or HPLC-based methods. Additionally, non-sulfated salivary DHEA is discussed as stress related biomarker as well. Kits are commercially available and have been assessed for saliva analysis [33,124]. However, cross-reactivity evaluations of commercially available IA kits hardly consider the expected differences in concentration ranges between DHEA and DHEA-S. Francavilla et al. analyzed DHEA-S in saliva to evaluate the correlation between serum and salivary concentrations using an electrochemiluminescence immunoassay [49]. Due to their selectivity, LC–MS/MS based methods are considered more appropriate for the determination of salivary DHEA and DHEA-S. Two studies reported a protocol for simultaneous measurement of cortisol and DHEA-S using LC–MS/MS on a triple quadrupole (QqQ) instrument [64,65]. Yasuhara et al. proposed a combination of solid-phase microextraction (SPME) with electrospray (ESI) MS/MS. After examining several on-line in-tube columns and diff ;erent parameters, they optimized the detection and achieved a LOD of 13 pg/mL for DHEA-S. Cao et al., instead, using a liquid-liquid extraction obtained a LOD of 0.03 ng/mL for DHEA-S. LC–MS/MS based methods for the determination of non-sulfated DHEA in a multi-analyte setting are reported by Kataoka et al. [68] also utilizing on-line in-tube SPME.
Gaudl et al. used an on-line solid-phase extraction (SPE) with LC quadrupole linear ion trap mass spectrometry for the simultaneous quantitation of 17α-hydroxyprogesterone, aldosterone, androstenedione, cortisol, cortisone, dehydroepiandrosterone-sulfate (DHEA-S), estradiol, progesterone, and testosterone [66]. They obtained a LOD of 814 pmol/L for DHEA-S.
6.4. 3-Methoxy-4-hydroxyphenylglycol (MHPG)
The detection of MHPG as a metabolite of norepinephrine is described in a couple of studies. The chemical structures of both are given in Fig. 1. Fujimaru et al. tested 39 nurses to examine the relationship between cortisol, MHPG, secretory immunoglobulin A, and stress [9]. Yamada et al. instead, proposed MHPG as a biological marker for anxiety disorders. They analyzed 196 healthy volunteers and 42 outpatients with anxiety disorders [63].
In both studies, MHPG was detected using GC–MS. The reached LOD for MHPG was calculated only by Fujimaru et al. and was 0.55 ng/mL, with intra and interassay coefficients of variation (CVs) of 3.95 % and 5.70 %, respectively. Reference values of normal salivary MHPG concentration were reported as 12.85 ng/mL by Reuster et al. [24].
Its precursors, norepinephrine and epinephrine, show limited chemical stability and are therefore hardly covered directly in biomarker studies. Okomura et al. report their analysis after stabilization by adding reduced glutathione /ethylene glycol tetraacetic acid (EGTA) based on a method reported by Schwab et al. [25,125].
6.5. Salivary alpha-amylase (sAA)
The detection of alpha-amylase is well investigated (structure in Fig. 2). There are already commercial easy-to-use, quick tests, and POC devices available. The most common detection method for this marker is by measuring its activity. Several different assay methods and unit definitions make it almost impossible to compare reported activities. As one example, one NU (Novo Unit) is defined as the amount of the enzyme that breaks down 0.00526 g of starch per hour. Alternatively, its activity is given as unit that liberates 1.0 mg of maltose from starch in 3 min at pH 6.9 at 20 °C [126].
Ohtomo et al. developed a flow injection spectrophotometric analysis for analyzing alpha-amylase activity in saliva [127]. The principle of this method was the degradation of a starch-iodine complex by alpha-amylase activity. The reached LOD was 60 NU/mL. Another spectrophotometric method is described by Fuentes et al. [128] for the determination of AA in porcine saliva. The LOD was 11.65 IU/L. Unfortunately, they did not share any information on their calculations, so that both LODs are even harder to compare.
Fuentes-Rubio et al. analytically and clinically validated a time-resolved immunofluorometric assay (TR-IFMA) for the quantitation of sAA in sheep [129] and horses [130]. They also compared the results obtained for the equine saliva with a commercial enzymatic assay. In both cases, the assay is based on an indirect, non-competitive sandwich method. As capture reagent, they used the anti-alpha-amylase polyclonal antibody and as a detector, the Eu3+-chelate labeled anti-alpha-amylase polyclonal antibody.
Alternatively, electrochemistry is often used also to determine sAA. Indeed, electrochemical techniques allow the realization of simple, not expensive and portable assays suitable for point-of-care testing. For example, Garcia et al. proposed an amperometric method with screen-printed carbon electrodes. They detected indirectly sAA thanks to a sequence of two chemical reactions. The first reaction is the hydrolysis of starch to maltose by alpha-amylase, followed by the conversion of [Fe(CN)6]3− into [Fe(CN)6]4− due to the reducing sugar produced in the first step [131]. Many of the optimized devices can be used directly on the POC. Zhang et al. realized a smartphone-based potentiometric biosensor for testing sAA, a kit ready to use. The saliva sample, obtained by passive drooling on the sensing chip with preloaded reagents, starts the conversion of Fe(CN)6 3− to Fe(CN)6 4-. The potentiometric sensor sends the measure to the smartphone, and an application quantifies sAA concentration through a calibration curve [132].
Another device for in situ testing was developed by Della Ventura et al. [133]. They functionalized the gold surface of the electrodes of a quartz-crystal microbalance (QCM) with antibodies by the photochemical immobilization technique (PIT). This simple method immobilizes and activates the antibodies with UV radiation.
Lastly, Tsyrulneva et al. developed a simple, colorimetric method with a paper membrane strip that also can be used without a qualified analyst or expensive instrument [131]. They exploit the colored byproduct formed during the cleavage of the alpha-bond of 2-chloro-4-nitrophenyl-α-D-maltotrioside by sAA, to obtain a semi-quantitative analysis. They combined the assay with a complementary RGB (red, green, blue components) analysis, to make the method quantitative and calculated a LOD of 11 μg/mL. They demonstrated that other salivary components do not cause relevant interferences. In their review paper, Yamaguchi et al. reported a hand-held biosensor for sAA suitable for POC testing [38]. As no LOD is reported, this article is only listed here for completeness and not included in Table 3 .
Table 3.
Overview of different sAA detection methods and their LOD.
| Method/Analytical technique | Ref. | Saliva collection method | LOD | Comment |
|---|---|---|---|---|
| AIE fluorescent probe | [134] | n.s. | 0.007 U/mL | based on the tetraphenylethylene motif and g-cyclodextrin. |
| Amperometry | [131] | n.s. | 1.1 U/mL | SPCE, indirect determination, POC |
| Colorimetric Assay | [135] [136] |
n.s. n.s. |
11 μg/mL 94,988 U/L (neat) 189 U/L (1:500) |
paper membrane, POC Phadebas® paper, not with mixed body fluids, H2O insoluble polymers bound to blue dye molecules |
| Colorimetric biosensor | [137] | Collector pad | 10 U/mL | Test strip, POC |
| FIA | [127] | n.s. | 60 NU/mL | Spectrophotometric analysis, no pre-treatment |
| iCIEF (concentration measurement) | [138] | Spitting directly into a tube | n.c. | POC, Sample preparation: ultrafiltration, gel-filtration, and starch affinity interaction |
| Immunosensor | [133] | Plastic collection tube under the tongue | 77 U/L | Influence of saliva viscosity, QCM, PIT |
| Potentiometric biosensor | [132] | Passive drooling | 0.12 U/mL | Smartphone based system, POC |
| Spectrophotometry | [128] | Oral sponge | 11.65 IU/L | In pigs |
| TR-IFMA | [129] [130] |
Oral sponge Oral sponge |
0.09 ng/mL 0.097 ng/mL |
In sheep In horses |
Abbreviations: AIE (Aggregation-induced Emission), FIA (Flow Injection Analysis), iCIEF (Imaging detection Capillary Isoelectric Focusing), n.c. (not calculated), n.s. (not specified), PIT (Photochemical Immobilization Technique), POC (Point Of Care), QCM (Quartz-Crystal Microbalance), SPCE (screen-printed carbon electrodes), TR-IFMA (Time-Resolved Immuno-Fluorometric Assay).
There are several additional methods published, diff ;erent in sample pre-treatment and detection. An overview is given in Table 3.
6.6. Secretory immunoglobulin A (sIgA)
Not many publications were retrieved, which are dealing with the detection of secretory immunoglobulin A in saliva, and only a few in the perspective of stress-related research work.
Wang et al. developed a detection device based upon gold nanoparticles (AuNPs) conjugated with antigen-binding fragment (Fab) fragments against sIgA [139]. These prepared AuNPs were added to the sample, mixed with fluorescent-labeled sIgA, which competed with free sIgA in the sample. The measured fluorescent signal in the solution was, therefore, proportional to the concentration of free sIgA. Wang et al. reached a LOD of 50 ng/mL.
In contrast to the following work, the influence of cross-reactivity by the use of Fab fragments is unknown and not examined. Kvietkauskaite and his colleagues used a sandwich ELISA for investigating stress markers in soldiers [6]. They determined a LOD of 0.4 ng/mL for sIgA. Theoretical cross-reactivity was precluded by the authors.
Electrochemical methods and immunoassays have also been used to detect sIgA. Rizwan et al. developed an electrochemical immunosensor with gold nanoparticle (AuNP) and polyethylene glycol (PEG) nanocomposite with immobilized Anti-sIgA monoclonal antibody (c-Mab) deposited on modified carbon nanofiber electrodes (CNF-SPE) [140]. With this electrochemical immunosensor, they obtained an extremely low LOD (500 fg/mL).
Another electrochemical immunosensor was developed by Lim et al. [141]. They covalently immobilized sIgA to magnetic beads and then incubated with biotin-conjugated secondary antibody and with streptavidin-hydrogen peroxidase. Finally, they immobilized the obtained magnetic beads on a single-walled carbon nanotube working electrode for amperometric measurements. This immunosensor reaches a LOD of 5 pg/mL and has a linear range of 5 pg/mL-10 ng/mL.
According to our database search, the most recent paper about sIgA in salivary matrix is published by Francavilla et al. They used a pre-validated immunoturbidimetric assay (Roche Diagnostics S.p.a, Monza, Italy) to quantitate and then evaluate the correlation between serum and salivary concentrations of IgA and steroid hormones, such as cortisol and testosterone [49].
6.7. Chromogranin A (CgA)
Chromogranin A is associated with an increase in liberated catecholamines. Its role as a stress marker is not often reported: only seven publications were found and only two of them report the validation of their method.
Escribano et al. developed and validated a time-resolved immunofluorometric assay for detecting salivary CgA in pigs [142]. Here, they used polyclonal antibodies against CgA (Catestatin) and combined them with the commercially available kit (DELFIA, Perkin Elmer). The relevant amino acid sequence used for detection in this assay is displayed in Fig. 3. This kit utilizes the lanthanide Europium, which is liberated after antibody-reaction. The concentration of liberated Eu is proportional to the concentration of CgA. The reached LOD was 4.27 ng/mL. This method was also used by Casal et al. in their study about the effects of an environmental enrichment and herbal supplement on physiological stress indicators in pigs [143].
Two more studies on the stress biomarkers in porcine saliva were carried out. The first by Huang et al. analyzed CgA with western blot [144]. The other, by Tecles et al., reports the development and validation of a time-resolved immunofluorometric assay (TR-IFMA). They obtained a 4.27 ng/mL LOD and a CV < 10 % for intra and inter-assay [145].
CgA was tested as a salivary stress biomarker also in humans. Rai et al. tested a commercially available ELISA. However, the sensitivity of the kit was not in the focus of the work and thus not reported [146]. Abekura et al. used a commercial ELISA kit as well. They studied the correlation between sleep bruxism and psychological stress by combining objective (CgA) and subjective (ten-division visual analog scale) parameters [147]. In 2019 Lihala et al. correlated CgA concentrations and personally perceived stress levels before and after non-surgical periodontal therapy [148].
It is essential to consider when to collect a sample of saliva to quantitate CgA. Its concentration is not constant during the day. Salivary CgA has higher levels during the night, reaches the highest peak just after awakening, then decreases rapidly, and remains low through the day [94].
7. Conclusions and future perspectives
With ongoing discussions on psychological (dis-)stress, reliable and objective diagnostic tools are highly desired to categorize the stress level. An event of acute stress has a disrupting effect on the physiological homeostasis and it causes, for example, a momentary increase in sAA or cortisol levels. If the distressing event is prolonged or repeated over-time, the ability of hormonal, neurohumoral, and physiological axes might result blunted and not adequate. Therefore, chronic stress not only leads to a feeling of exhaustion, mental distance from society and work, loss of productivity, but also to secondary psychological and physical diseases.
Thus, research on stress-related biomarkers is of persevering interest. In view of the fact that stress and its consequences are very multifaceted, harmless, comfortable, and economical detection devices are of increasing interest.
Classical clinical assays like IA offer easy analytical approaches for single analytes that may be designed for POC testing. Provided a sufficient sensitivity, economic efficiency is becoming more critical. Therefore, test methods, which are lower priced and easy to use, are of major interest, especially in research fields like stress prophylaxis and personalized medicine. To prevent risks related to stressful jobs or other conditions, the necessity of fast, sufficiently sensitive, and portable devices is of primary importance. This goal appears suitable for electrochemical developments. Dependent on the selectivity of the antibody utilized cross-reactivity may result in the overestimation of analytes when compared with MS data, particularly at lower concentrations. When comparing the data obtained with MS and IAs, there is often no linear correlation between the results [57,149,150]. To overcome this issue and produce more reliable kits, IAs manufacturers are nowadays often using MS based techniques to validate and calibrate their kits more accurately [59].
To supplement clinical testing and confirmation of the POC device results exact and highly sensitive detection methods are developed meanwhile. In this field, mainly sophisticated methods based on chromatography coupled to mass spectrometric detection appear to be the methods of choice. While many detection methods reported in literature are designed for detecting only one or two markers simultaneously, LC–MS/MS or GC–MS(/MS) based methods offer the possibility for multi-analyte methods. After successful optimization, they may cover several stress-related biomarkers in one single analytical method. This allows for an efficient simultaneous measurement and helps to reliably assess possible stress-related risks in a multifactorial design. As one example, Gaudl et al. report the simultaneous determination of 17−OHP, aldosterone, AED, cortisol, cortisone, DHEAS, estradiol, progesterone, and testosterone in saliva by one LC–MS/MS method [66].
The capability of LC—MS/MS based methods to cover multiple analytes is most persuasively demonstrated in metabolomics studies. Only recently, metabolomics investigations are also reported for stress-related conditions. For further reading on this topic, we would like to refer to the review paper of Mellon et al. [151]. Complementary, Nathalie Michels recently reviewed multi-omics approaches in the context of psychological stress [152]. Using gene expression analysis Le-Niculescu et al. discovered and validated NUB1, APOL3, MAD1L1, and NKTR as predictive biomarkers in addition to FKBP5, DDX6, B2M, LAIR1, and RTN4 [153]. Additionally, Dean et al. recently reported the use of multi-omics for the identification of biomarkers for the diagnosis of post-traumatic stress disorders [154]. They finally reported a set of 28 markers, that yielded 81 % accuracy. Using three out of these markers, namely gamma glutamyl tyrosine, insulin, and the methylation marker cg01208318, they still achieved 60 % accuracy in the validation group. Unfortunately, the read-out for most of the multi-analyte methods is still challenging and multivariate statistics are often required. However, the discovery of further suitable biomarkers may be achieved by these designs. Their suitability needs further validation. Furthermore, their recovery in saliva needs to be evaluated in future studies as well.
Further innovative approaches may off ;er attractive solutions for developing cost and sensitivity efficient devices. Martin et al. recently showed that the use of aptamers is leading to promising results in the detection of small molecules, especially in terms of selectivity [155]. They did not report the LOD of the methods but opened a new perspective for combining the detection of small and large molecule marker substances [156]. This approach shows another potential future trend.
Funding
Financial support of the study from the State of Berlin (Elsa-Neumann scholarship of Ginevra Giacomello) was received.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The State of Berlin, Germany, is acknowledged for granting the Elsa-Neumann PhD scholarship of Ginevra Giacomello. The authors thank Bernhard Wuest, Agilent Technologies Inc. for his assistance in mass spectrometry.
References
- 1.Cozma S., Dima-Cozma L., Ghiciuc C., Pasquali V., Saponaro A., Patacchioli F. Salivary cortisol and α-amylase: subclinical indicators of stress as cardiometabolic risk. Braz. J. Med. Biol. Res. 2017;50 doi: 10.1590/1414-431X20165577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhama K., Latheef S.K., Dadar M., Samad H.A., Munjal A., Khandia R., Karthik K., Tiwari R., Yatoo M.I., Chakraborty S. Molecular signatures of biomarkers with a special reference to stress and related Diseases/Disorders: diagnostic, prognostic and therapeutic values-current progress and futuristic vision. Front. Mol. Biosci. 2019;6:91. doi: 10.3389/fmolb.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell G., Lightman S. The human stress response. Nat. Rev. Endocrinol. 2019;15:525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 4.Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 5.Baron K.G., Reid K.J. Circadian misalignment and health. Int. Rev. Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvietkauskaite R., Vaicaitiene R., Mauricas M. The change in the amount of immunoglobulins as a response to stress experienced by soldiers on a peacekeeping mission. Int. Arch. Occup. Environ. Health. 2014;87:615–622. doi: 10.1007/s00420-013-0899-0. [DOI] [PubMed] [Google Scholar]
- 7.Akinola M., Mendes W.B. Stress-induced cortisol facilitates threat-related decision making among police officers. Behav. Neurosci. 2012;126:167–174. doi: 10.1037/a0026657. [DOI] [PubMed] [Google Scholar]
- 8.De Andrés-García S., Cano-López I., Moya-Albiol L., González-Bono E. Negative affect, perceived health, and endocrine and immunological levels in caregivers of offspring with schizophrenia. Psicothema. 2016;28:377–382. doi: 10.7334/psicothema2015.76. [DOI] [PubMed] [Google Scholar]
- 9.Fujimaru C., Okamura H., Kawasaki M., Kakuma T., Yoshii C., Matsuishi T. Self-perceived work-related stress and its relation to salivary IgA, cortisol and 3-methoxy-4-hydroxyphenyl glycol levels among neonatal intensive care nurses. Stress Health. 2012;28:171–174. doi: 10.1002/smi.1414. [DOI] [PubMed] [Google Scholar]
- 10.Zangheri M., Mirasoli M., Guardigli M., Di Nardo F., Anfossi L., Baggiani C., Simoni P., Benassai M., Roda A. Chemiluminescence-based biosensor for monitoring astronauts’ health status during space missions: results from the International Space Station. Biosens. Bioelectron. 2019;129:260–268. doi: 10.1016/j.bios.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Shuai L., Yu H., Wang Z., Qiu M., Lu L., Cao X., Xia W., Wang Y., Chen R. Acute stress, behavioural symptoms and mood states among school-aged children with attention-deficit/hyperactivity disorder during the COVID-19 outbreak. Asian J. Psychiatr. 2020;51 doi: 10.1016/j.ajp.2020.102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umucu E., Lee B. Examining the impact of COVID-19 on stress and coping strategies in individuals with disabilities and chronic conditions. Rehabil. Psychol. 2020 doi: 10.1037/rep0000328. [DOI] [PubMed] [Google Scholar]
- 13.Fagiolini A., Cuomo A., Frank E. COVID-19 diary from a psychiatry department in Italy. J. Clin. Psychiatry. 2020;81 doi: 10.4088/JCP.20com13357. [DOI] [PubMed] [Google Scholar]
- 14.Torales J., O’Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry. 2020 doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar R.P. COVID-19 and mental health: a review of the existing literature. Asian J. Psychiatr. 2020;52 doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bo H.X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T., Wu X., Xiang Y.T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2020:1–2. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horesh D., Brown A.D. Traumatic stress in the age of COVID-19: a call to close critical gaps and adapt to new realities. Psychol. Trauma. 2020;12:331–335. doi: 10.1037/tra0000592. [DOI] [PubMed] [Google Scholar]
- 18.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burtscher J., Burtscher M., Millet G.P. (Indoor) isolation, stress and physical inactivity: vicious circles accelerated by Covid-19? Scand. J. Med. Sci. Sports. 2020 doi: 10.1111/sms.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuja K.H., Aqeel M., Jaffar A., Ahmed A. COVID-19 pandemic and impending global mental health implications. Psychiatr. Danub. 2020;32:32–35. doi: 10.24869/psyd.2020.32. [DOI] [PubMed] [Google Scholar]
- 21.Freeman M.P. COVID-19 from a psychiatry perspective: meeting the challenges. J. Clin. Psychiatry. 2020;81 doi: 10.4088/JCP.20ed13358. [DOI] [PubMed] [Google Scholar]
- 22.Bruffaerts R., Vilagut G., Demyttenaere K., Alonso J., Alhamzawi A., Andrade L.H., Benjet C., Bromet E., Bunting B., de Girolamo G., Florescu S., Gureje O., Haro J.M., He Y., Hinkov H., Hu C., Karam E.G., Lepine J.P., Levinson D., Matschinger H., Nakane Y., Ormel J., Posada-Villa J., Scott K.M., Varghese M., Williams D.R., Xavier M., Kessler R.C. Role of common mental and physical disorders in partial disability around the world. Br. J. Psychiatry. 2012;200:454–461. doi: 10.1192/bjp.bp.111.097519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H., Jia J., Guo Q., Xue Y., Li Q., Huang J., Cai L., Feng L. User-level psychological stress detection from social media using deep neural network. Proceedings of the 22nd ACM International Conference on Multimedia, Association for Computing Machinery; Orlando, Florida, USA; 2014. pp. 507–516. [Google Scholar]
- 24.Reuster T., Buechler J., Winiecki P., Oehler J. Influence of reboxetine on salivary MHPG concentration and cognitive symptoms among patients with alcohol-related Korsakoff’s syndrome. Neuropsychopharmacology. 2003;28:974–978. doi: 10.1038/sj.npp.1300118. [DOI] [PubMed] [Google Scholar]
- 25.Okumura T., Nakajima Y., Matsuoka M., Takamatsu T. Study of salivary catecholamines using fully automated column-switching high-performance liquid chromatography. J. Chromatogr. B. 1997;694:305–316. doi: 10.1016/s0378-4347(97)00106-0. [DOI] [PubMed] [Google Scholar]
- 26.Brodish A., Odio M. Age-dependent effects of chronic stress on ACTH and corticosterone responses to an acute novel stress. Neuroendocrinology. 1989;49:496–501. doi: 10.1159/000125158. [DOI] [PubMed] [Google Scholar]
- 27.Stagl M., Bozsik M., Karow C., Wertz D., Kloehn I., Pillai S., Gasser P.J., Gilmartin M.R., Evans J.A. Chronic stress alters adrenal clock function in a sexually dimorphic manner. J. Mol. Endocrinol. 2018;60:55–69. doi: 10.1530/JME-17-0146. [DOI] [PubMed] [Google Scholar]
- 28.Ramos A.T., Tufik S., Troncone L.R. Control of stress-induced ACTH secretion by vasopressin and CRH: additional evidence. Neuropsychobiology. 2016;73:184–190. doi: 10.1159/000445480. [DOI] [PubMed] [Google Scholar]
- 29.Steckl A.J., Ray P. Stress biomarkers in biological fluids and their point-of-Use detection. ACS Sens. 2018;3:2025–2044. doi: 10.1021/acssensors.8b00726. [DOI] [PubMed] [Google Scholar]
- 30.Mert M., Tanakol R., Karpuzoglu H., Abbasoglu S., Yarman S., Boztepe H., Alagol F. Spectral effect: each population must have its own normal midnight salivary cortisol reference values determined. Arch. Med. Sci. 2013;9:872–876. doi: 10.5114/aoms.2013.38681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Sanchez V., Moreno-Perez O., Garcia de Guadiana L., Sanchez-Pellicer P., Alfayate R., Mauri M., Sanchez-Paya J., Pico A. Reference ranges for serum and salivary testosterone in young men of Mediterranean region. Endocrinol. Nutr. 2015;62:4–10. doi: 10.1016/j.endonu.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Jiang X., Zhong W., An H., Fu M., Chen Y., Zhang Z., Xiao Z. Attenuated DHEA and DHEA-S response to acute psychosocial stress in individuals with depressive disorders. J. Affect. Disord. 2017;215:118–124. doi: 10.1016/j.jad.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Whetzel C.A., Klein L.C. Measuring DHEA-S in saliva: time of day differences and positive correlations between two different types of collection methods. BMC Res. Notes. 2010;3:204. doi: 10.1186/1756-0500-3-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Amelio R.P., Le Moli S., Seminara R., Aiuti F. Serum and salivary IgA levels in normal subjects: comparison between tonsillectomized and non-tonsillectomized subjects. Int. Arch. Allergy Immunol. 1982;68:256–259. doi: 10.1159/000233108. [DOI] [PubMed] [Google Scholar]
- 35.Egri C., Dunbar M., Horvath G.A. Correlation between salivary, platelet and central serotonin levels in children. Canadian J. Neurol. Sci. 2020;47:214–218. doi: 10.1017/cjn.2019.334. [DOI] [PubMed] [Google Scholar]
- 36.Leung J., Selvage C., Bosdet T., Branov J., Rosen-Heath A., Bishop C., Sirrs S., Horvath G. Salivary serotonin does not correlate with central serotonin turnover in adult phenylketonuria (PKU) patients. Mol. Genet. Metab. Rep. 2018;15:100–105. doi: 10.1016/j.ymgmr.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danhof-Pont M.B., van Veen T., Zitman F.G. Biomarkers in burnout: a systematic review. J. Psychosom. Res. 2011;70:505–524. doi: 10.1016/j.jpsychores.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi M., Shetty V. Salivary sensors for quantification of stress response biomarker. Electrochemistry. 2011;79:442–446. [Google Scholar]
- 39.Al-Dujaili E.A., Baghdadi H.H., Howie F., Mason J.I. Validation and application of a highly specific and sensitive ELISA for the estimation of cortisone in saliva, urine and in vitro cell-culture media by using a novel antibody. Steroids. 2012;77:703–709. doi: 10.1016/j.steroids.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Russell E., Koren G., Rieder M., Van Uum S.H. The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther. Drug Monit. 2014;36:30–34. doi: 10.1097/FTD.0b013e31829daa0a. [DOI] [PubMed] [Google Scholar]
- 41.Spano G., Cavalera S., Di Nardo F., Giovannoli C., Anfossi L., Baggiani C. Development of a biomimetic enzyme-linked immunosorbent assay based on a molecularly imprinted polymer for the detection of cortisol in human saliva. Anal. Methods. 2019;11:2320–2326. [Google Scholar]
- 42.Sesay A.M., Micheli L., Tervo P., Palleschi G., Virtanen V. Development of a competitive immunoassay for the determination of cortisol in human saliva. Anal. Biochem. 2013;434:308–314. doi: 10.1016/j.ab.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Kellens E., Bove H., Vandenryt T., Lambrichts J., Dekens J., Drijkoningen S., D’Haen J., Ceuninck W., Thoelen R., Junkers T., Haenen K., Ethirajan A. Micro-patterned molecularly imprinted polymer structures on functionalized diamond-coated substrates for testosterone detection. Biosens. Bioelectron. 2018;118:58–65. doi: 10.1016/j.bios.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.B., Takenaka Y., Torimura M. A bioluminescent probe for salivary cortisol. Bioconjug. Chem. 2011;22:1835–1841. doi: 10.1021/bc200220k. [DOI] [PubMed] [Google Scholar]
- 45.Tlili C., Myung N.V., Shetty V., Mulchandani A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011;26:4382–4386. doi: 10.1016/j.bios.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y.H., Lee K., Jung H., Kang H.K., Jo J., Park I.K., Lee H.H. Direct immune-detection of cortisol by chemiresistor graphene oxide sensor. Biosens. Bioelectron. 2017;98:473–477. doi: 10.1016/j.bios.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Pires N.M., Dong T. Measurement of salivary cortisol by a chemiluminescent organic-based immunosensor. Biomed. Mater. Eng. 2014;24:15–20. doi: 10.3233/BME-130778. [DOI] [PubMed] [Google Scholar]
- 48.Escribano D., Fuentes-Rubio M., Ceron J.J. Salivary testosterone measurements in growing pigs: validation of an automated chemiluminescent immunoassay and its possible use as an acute stress marker. Res. Vet. Sci. 2014;97:20–25. doi: 10.1016/j.rvsc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Francavilla V.C., Vitale F., Ciaccio M., Bongiovanni T., Marotta C., Caldarella R., Todaro L., Zarcone M., Muratore R., Bellia C., Francavilla G., Mazzucco W. Use of saliva in alternative to serum sampling to monitor biomarkers modifications in professional soccer players. Front. Physiol. 2018;9:1828. doi: 10.3389/fphys.2018.01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasha S.K., Kaushik A., Vasudev A., Snipes S.A., Bhansali S. Electrochemical immunosensing of saliva cortisol. J. Electrochem. Soc. 2014;161:B3077–B3082. [Google Scholar]
- 51.Arya S.K., Dey A., Bhansali S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens. Bioelectron. 2011;28:166–173. doi: 10.1016/j.bios.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Abdulsattar J.O., Greenway G.M., Wadhawan J.D. Electrochemical immunoassay for the detection of stress biomarkers. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vabbina P.K., Kaushik A., Pokhrel N., Bhansali S., Pala N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015;63:124–130. doi: 10.1016/j.bios.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 54.Kamarainen S., Maki M., Tolonen T., Palleschi G., Virtanen V., Micheli L., Sesay A.M. Disposable electrochemical immunosensor for cortisol determination in human saliva. Talanta. 2018;188:50–57. doi: 10.1016/j.talanta.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi M., Matsuda Y., Sasaki S., Sasaki M., Kadoma Y., Imai Y., Niwa D., Shetty V. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosens. Bioelectron. 2013;41:186–191. doi: 10.1016/j.bios.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yockell-Lelievre H., Bukar N., McKeating K.S., Arnaud M., Cosin P., Guo Y., Dupret-Carruel J., Mougin B., Masson J.F. Plasmonic sensors for the competitive detection of testosterone. Analyst. 2015;140:5105–5111. doi: 10.1039/c5an00694e. [DOI] [PubMed] [Google Scholar]
- 57.Miller R., Plessow F., Rauh M., Groschl M., Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013;38:50–57. doi: 10.1016/j.psyneuen.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Inder W.J., Dimeski G., Russell A. Measurement of salivary cortisol in 2012 - laboratory techniques and clinical indications. Clin. Endocrinol. (Oxf) 2012;77:645–651. doi: 10.1111/j.1365-2265.2012.04508.x. [DOI] [PubMed] [Google Scholar]
- 59.Taylor T., West D.J., Howatson G., Jones C., Bracken R.M., Love T.D., Cook C.J., Swift E., Baker J.S., Kilduff L.P. The impact of neuromuscular electrical stimulation on recovery after intensive, muscle damaging, maximal speed training in professional team sports players. J. Sci. Med. Sport. 2015;18:328–332. doi: 10.1016/j.jsams.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Middle J.G. Dehydroepiandrostenedione sulphate interferes in many direct immunoassays for testosterone. Ann. Clin. Biochem. 2007;44:173–177. doi: 10.1258/000456307780118082. [DOI] [PubMed] [Google Scholar]
- 61.Rosner W., Auchus R.J., Azziz R., Sluss P.M., Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J. Clin. Endocrinol. Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 62.Buttler R.M., Kruit A., Blankenstein M.A., Heijboer A.C. Measurement of dehydroepiandrosterone sulphate (DHEAS): a comparison of Isotope-Dilution Liquid Chromatography Tandem Mass Spectrometry (ID-LC-MS/MS) and seven currently available immunoassays. Clin. Chim. Acta. 2013;424:22–26. doi: 10.1016/j.cca.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 63.Yamada S., Yamauchi K., Yajima J., Hisadomi S., Maeda H., Toyomasu K., Tanaka M. Saliva level of free 3-methoxy-4-hydroxyphenylglycol (MHPG) as a biological index of anxiety disorders. Psychiatry Res. 2000;93:217–223. doi: 10.1016/s0165-1781(00)00118-9. [DOI] [PubMed] [Google Scholar]
- 64.Yasuhara R., Ehara K., Saito K., Kataoka H. Automated analysis of salivary stress-related steroid hormones by online in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Methods. 2012;4:3625–3630. [Google Scholar]
- 65.Cao Z.T., Wemm S.E., Han L., Spink D.C., Wulfert E. Noninvasive determination of human cortisol and dehydroepiandrosterone sulfate using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019;411:1203–1210. doi: 10.1007/s00216-018-1549-x. [DOI] [PubMed] [Google Scholar]
- 66.Gaudl A., Kratzsch J., Bae Y.J., Kiess W., Thiery J., Ceglarek U. Liquid chromatography quadrupole linear ion trap mass spectrometry for quantitative steroid hormone analysis in plasma, urine, saliva and hair. J. Chromatogr. A. 2016;1464:64–71. doi: 10.1016/j.chroma.2016.07.087. [DOI] [PubMed] [Google Scholar]
- 67.Escudero L.B., Grijalba A.C., Martinis E.M., Wuilloud R.G. Bioanalytical separation and preconcentration using ionic liquids. Anal. Bioanal. Chem. 2013;405:7597–7613. doi: 10.1007/s00216-013-6950-x. [DOI] [PubMed] [Google Scholar]
- 68.Kataoka H., Ehara K., Yasuhara R., Saito K. Simultaneous determination of testosterone, cortisol, and dehydroepiandrosterone in saliva by stable isotope dilution on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013;405:331–340. doi: 10.1007/s00216-012-6479-4. [DOI] [PubMed] [Google Scholar]
- 69.Saracino M.A., Iacono C., Somaini L., Gerra G., Ghedini N., Raggi M.A. Multi-matrix assay of cortisol, cortisone and corticosterone using a combined MEPS-HPLC procedure. J. Pharm. Biomed. Anal. 2014;88:643–648. doi: 10.1016/j.jpba.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Abujaber F., Corps Ricardo A.I., Rios A., Guzman Bernardo F.J., Rodriguez Martin-Doimeadios R.C. Ionic liquid dispersive liquid-liquid microextraction combined with LC-UV-Vis for the fast and simultaneous determination of cortisone and cortisol in human saliva samples. J. Pharm. Biomed. Anal. 2019;165:141–146. doi: 10.1016/j.jpba.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Cadore E., Lhullier F., Brentano M., Silva E., Ambrosini M., Spinelli R., Silva R., Kruel L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports Sci. 2008;26:1067–1072. doi: 10.1080/02640410801919526. [DOI] [PubMed] [Google Scholar]
- 72.Nowak R., McMillen I., Redman J., Short R. The correlation between serum and salivary melatonin concentrations and urinary 6‐hydroxymelatonin sulphate excretion rates: two non‐invasive techniques for monitoring human circadian rhythmicity. Clin. Endocrinol. 1987;27:445–452. doi: 10.1111/j.1365-2265.1987.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 73.Lipson S.F., Ellison P.T. Development of protocols for the application of salivary steroid analysis to field conditions. Am. J. Hum. Biol. 1989;1:249–255. doi: 10.1002/ajhb.1310010304. [DOI] [PubMed] [Google Scholar]
- 74.Obayashi K. Salivary mental stress proteins. Clin. Chim. Acta. 2013;425:196–201. doi: 10.1016/j.cca.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 75.Hanneman S.K., Cox C.D., Green K.E., Kang D.H. Estimating intra- and inter-assay variability in salivary cortisol. Biol. Res. Nurs. 2011;13:243–250. doi: 10.1177/1099800411404061. [DOI] [PubMed] [Google Scholar]
- 76.Toda M., Morimoto K., Nagasawa S., Kitamura K. Effect of snack eating on sensitive salivary stress markers cortisol and chromogranin A. Environ. Health Prev. Med. 2004;9:27–29. doi: 10.1265/ehpm.9.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garde A.H., Persson R., Hansen A.M., Osterberg K., Orbaek P., Eek F., Karlson B. Effects of lifestyle factors on concentrations of salivary cortisol in healthy individuals. Scand. J. Clin. Lab. Invest. 2009;69:242–250. doi: 10.1080/00365510802483708. [DOI] [PubMed] [Google Scholar]
- 78.Shirtcliff E.A., Granger D.A., Schwartz E., Curran M.J. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 79.Hodgson N.A., Granger D.A. Collecting saliva and measuring salivary cortisol and alpha-amylase in frail community residing older adults via family caregivers. J. Vis. Exp. 2013 doi: 10.3791/50815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallagher P., Leitch M.M., Massey A.E., McAllister-Williams R.H., Young A.H. Assessing cortisol and dehydroepiandrosterone (DHEA) in saliva: effects of collection method. J. Psychopharmacol. 2006;20:643–649. doi: 10.1177/0269881106060585. [DOI] [PubMed] [Google Scholar]
- 81.Nurkka A., Obiero J., Kayhty H., Scott J.A. Effects of sample collection and storage methods on antipneumococcal immunoglobulin A in saliva. Clin. Diagn. Lab. Immunol. 2003;10:357–361. doi: 10.1128/CDLI.10.3.357-361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beltzer E.K., Fortunato C.K., Guaderrama M.M., Peckins M.K., Garramone B.M., Granger D.A. Salivary flow and alpha-amylase: collection technique, duration, and oral fluid type. Physiol. Behav. 2010;101:289–296. doi: 10.1016/j.physbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Rohleder N., Wolf J.M., Maldonado E.F., Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43:645–652. doi: 10.1111/j.1469-8986.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 84.Robles T.F., Sharma R., Harrell L., Elashoff D.A., Yamaguchi M., Shetty V. Saliva sampling method affects performance of a salivary alpha-amylase biosensor. Am. J. Hum. Biol. 2013;25:719–724. doi: 10.1002/ajhb.22438. [DOI] [PubMed] [Google Scholar]
- 85.Higashi T., Hijikuro M., Yamagata K., Ogawa S. Influence of saliva flow rate stimulated by gum-chewing on salivary concentrations of catecholamine metabolites. Clin. Chim. Acta. 2012;414:248–252. doi: 10.1016/j.cca.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 86.Oster H., Challet E., Ott V., Arvat E., de Kloet E.R., Dijk D.J., Lightman S., Vgontzas A., Van Cauter E. The functional and clinical significance of the 24-Hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017;38:3–45. doi: 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung S., Son G.H., Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim. Biophys. Acta. 2011;1812:581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 88.Collomp K., Baillot A., Forget H., Coquerel A., Rieth N., Vibarel-Rebot N. Altered diurnal pattern of steroid hormones in relation to various behaviors, external factors and pathologies: a review. Physiol. Behav. 2016;164:68–85. doi: 10.1016/j.physbeh.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 89.Guyon A., Balbo M., Morselli L.L., Tasali E., Leproult R., L’Hermite-Baleriaux M., Van Cauter E., Spiegel K. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J. Clin. Endocrinol. Metab. 2014;99:2861–2868. doi: 10.1210/jc.2013-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lam J.C.W., Shields G.S., Trainor B.C., Slavich G.M., Yonelinas A.P. Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress Health. 2019;35:15–26. doi: 10.1002/smi.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Born D.P., Faiss R., Willis S.J., Strahler J., Millet G.P., Holmberg H.C., Sperlich B. Circadian variation of salivary immunoglobin A, alpha-amylase activity and mood in response to repeated double-poling sprints in hypoxia. Eur. J. Appl. Physiol. 2016;116:1–10. doi: 10.1007/s00421-015-3236-3. [DOI] [PubMed] [Google Scholar]
- 92.Shirakawa S., Tsuchiya S., Tsutsumi Y., Kotorii T., Uchimura N., Sakamoto T., Yamada S. Time course of saliva and serum melatonin levels after ingestion of melatonin. Psychiatry Clin. Neurosci. 1998;52:266–267. doi: 10.1111/j.1440-1819.1998.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 93.Dabbs J.M., Jr. Salivary testosterone measurements: reliability across hours, days, and weeks. Physiol. Behav. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- 94.Den R., Toda M., Nagasawa S., Kitamura K., Morimoto K. Circadian rhythm of human salivary chromogranin A. Biomed. Res. 2007;28:57–60. doi: 10.2220/biomedres.28.57. [DOI] [PubMed] [Google Scholar]
- 95.Gupta S.K., Lindemulder E.A., Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J. Clin. Pharmacol. 2000;40:731–738. doi: 10.1177/00912700022009486. [DOI] [PubMed] [Google Scholar]
- 96.Copinschi G., Challet E. Endocrine rhythms, the sleep-wake cycle, and biological clocks. Endocrinology: Adult Pediatric. 2016:147–173. e149. [Google Scholar]
- 97.Clements A.D., Parker C.R. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 98.Durdiakova J., Fabryova H., Koborova I., Ostatnikova D., Celec P. The effects of saliva collection, handling and storage on salivary testosterone measurement. Steroids. 2013;78:1325–1331. doi: 10.1016/j.steroids.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 99.Bellagambi F., Degano I., Ghimenti S., Lomonaco T., Dini V., Romanelli M., Mastorci F., Gemignani A., Salvo P., Fuoco R. Determination of salivary α-amylase and cortisol in psoriatic subjects undergoing the Trier Social Stress Test. Microchem. J. 2018;136:177–184. [Google Scholar]
- 100.Ng V., Koh D., Fu Q., Chia S.E. Effects of storage time on stability of salivary immunoglobulin A and lysozyme. Clin. Chim. Acta. 2003;338:131–134. doi: 10.1016/j.cccn.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Escribano D., Gutierrez A.M., Fuentes-Rubio M., Ceron J.J. Saliva chromogranin A in growing pigs: a study of circadian patterns during daytime and stability under different storage conditions. Vet. J. 2014;199:355–359. doi: 10.1016/j.tvjl.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 102.VanBruggen M.D., Hackney A.C., McMurray R.G., Ondrak K.S. The relationship between serum and salivary cortisol levels in response to different intensities of exercise. Int. J. Sports Physiol. Perform. 2011;6:396–407. doi: 10.1123/ijspp.6.3.396. [DOI] [PubMed] [Google Scholar]
- 103.Kabra P.M.T., Marion L.J. Improved liquid-chromatographic method for determination os serum cortisol. Clinical Chemestry. 1979;25:1293–1296. L. L. [PubMed] [Google Scholar]
- 104.Teubel J., Wust B., Schipke C.G., Peters O., Parr M.K. Methods in endogenous steroid profiling - A comparison of gas chromatography mass spectrometry (GC-MS) with supercritical fluid chromatography tandem mass spectrometry (SFC-MS/MS) J. Chromatogr. A. 2018;1554:101–116. doi: 10.1016/j.chroma.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 105.Miller R., Plessow F., Kirschbaum C., Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom. Med. 2013;75:832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 106.Kaushik A., Vasudev A., Arya S.K., Pasha S.K., Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 107.Tahara Y., Huang Z., Kiritoshi T., Onodera T., Toko K. Development of indirect competitive immuno-assay method using SPR detection for rapid and highly sensitive measurement of salivary cortisol levels. Front. Bioeng. Biotechnol. 2014;2:15. doi: 10.3389/fbioe.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Usha S.P., Shrivastav A.M., Gupta B.D. A contemporary approach for design and characterization of fiber-optic-cortisol sensor tailoring LMR and ZnO/PPY molecularly imprinted film. Biosens. Bioelectron. 2017;87:178–186. doi: 10.1016/j.bios.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 109.Pandey A.K., Sharma A.K., Marques C. On the application of SiO2/SiC grating on Ag for high-performance Fiber optic plasmonic sensing of cortisol concentration. Materials (Basel) 2020;13 doi: 10.3390/ma13071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Escribano D., Fuentes-Rubio M., Ceron J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Invest. 2012;24:918–923. doi: 10.1177/1040638712455171. [DOI] [PubMed] [Google Scholar]
- 111.Abdulsattar J.O., Greenway G.M. A sensitive chemiluminescence based immunoassay for the detection of cortisol and cortisone as stress biomarkers. J. Anal. Sci. Technol. 2019;10:13. [Google Scholar]
- 112.Apilux A., Rengpipat S., Suwanjang W., Chailapakul O. Development of competitive lateral flow immunoassay coupled with silver enhancement for simple and sensitive salivary cortisol detection. Excli j. 2018;17:1198–1209. doi: 10.17179/excli2018-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zangheri M., Cevenini L., Anfossi L., Baggiani C., Simoni P., Di Nardo F., Roda A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015;64:63–68. doi: 10.1016/j.bios.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 114.Jensen M.A., Hansen A.M., Abrahamsson P., Norgaard A.W. Development and evaluation of a liquid chromatography tandem mass spectrometry method for simultaneous determination of salivary melatonin, cortisol and testosterone. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:2527–2532. doi: 10.1016/j.jchromb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 115.Fustinoni S., Polledri E., Mercadante R. High-throughput determination of cortisol, cortisone, and melatonin in oral fluid by on-line turbulent flow liquid chromatography interfaced with liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013;27:1450–1460. doi: 10.1002/rcm.6601. [DOI] [PubMed] [Google Scholar]
- 116.Jones R.L., Owen L.J., Adaway J.E., Keevil B.G. Simultaneous analysis of cortisol and cortisone in saliva using XLC-MS/MS for fully automated online solid phase extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;881-882:42–48. doi: 10.1016/j.jchromb.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 117.Gosetti F., Mazzucco E., Gennaro M.C., Marengo E. Ultra high performance liquid chromatography tandem mass spectrometry determination and profiling of prohibited steroids in human biological matrices. A review, J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2013;927:22–36. doi: 10.1016/j.jchromb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 118.Sobhi H.R., Henry H., Bruce S.J., Esrafili A., Rochat B. Simple measurement of testosterone in male saliva samples using dispersive liquid-Liquid microextraction followed by liquid chromatography-tandem mass spectrometry detection. J. Liq. Chromatogr. Relat. Technol. 2014;37:1278–1286. [Google Scholar]
- 119.Gomez-Gomez A., Miranda J., Feixas G., Arranz Betegon A., Crispi F., Gratacos E., Pozo O.J. Determination of the steroid profile in alternative matrices by liquid chromatography tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2020;197 doi: 10.1016/j.jsbmb.2019.105520. [DOI] [PubMed] [Google Scholar]
- 120.Alzahrani M.A., Alshuwaier G.O., Alialoud K.S., Gibson C., Khalaf A., Alhawiti A.S., Watson D.G. Development of a derivatization method for investigating testosterone and dehydroepiandrosterone using tandem mass spectrometry in saliva samples from young professional soccer players pre- and post-training. Sci. Pharm. 2019;87 [Google Scholar]
- 121.Sun Z.Z., An Y., Li H., Zhu H., Lu M.S. Electrochemical investigation of testosterone using a AuNPs modified electrode. Int. J. Electrochem. Sci. 2017;12:11224–11234. [Google Scholar]
- 122.Chen C.H., Bair M.J., Hsu C.W., Chiu T.C., Hu C.C. Analysis of steroid hormones in human saliva by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Methods. 2015;7:486–489. [Google Scholar]
- 123.Gugoasa L.A., Stefan-van Staden R.I., Calenic B., Legler J. Multimode sensors as new tools for molecular recognition of testosterone, dihydrotestosterone and estradiol in children’s saliva. J. Mol. Recognit. 2015;28:10–19. doi: 10.1002/jmr.2408. [DOI] [PubMed] [Google Scholar]
- 124.Dismukes A.R., Meyer V.J., Shirtcliff E.A., Theall K.P., Esteves K.C., Drury S.S. Diurnal and stress-reactive dehydroepiandrosterone levels and telomere length in youth. Endocr. Connect. 2016;5:107–114. doi: 10.1530/EC-16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwab K., Heubel G., Bartels H. Free epinephrine, norepinephrine and dopamine in saliva and plasma of healthy adults. European J. clin. Chem. Clin. Biochem. J. Forum European Clin. Chem. Societies. 1992:541–544. [PubMed] [Google Scholar]
- 126.Yoo Y.J., Hong J., Hatch R.T. Comparison of α‐amylase activities from different assay methods. Biotechnol. Bioeng. 1987;30:147–151. doi: 10.1002/bit.260300120. [DOI] [PubMed] [Google Scholar]
- 127.Ohtomo T., Igarashi S., Takagai Y. Flow injection spectrophotometric analysis of human salivary alpha-amylase activity using an enzyme degradation of starch-iodine complexes in flow channel and its application to human stress testing. Biol. Pharm. Bull. 2013;36:1857–1861. doi: 10.1248/bpb.b13-00214. [DOI] [PubMed] [Google Scholar]
- 128.Fuentes M., Tecles F., Gutierrez A., Otal J., Martinez-Subiela S., Ceron J.J. Validation of an automated method for salivary alpha-amylase measurements in pigs (Sus scrofa domesticus) and its application as a stress biomarker. J. Vet. Diagn. Invest. 2011;23:282–287. doi: 10.1177/104063871102300213. [DOI] [PubMed] [Google Scholar]
- 129.Fuentes-Rubio M., Fuentes F., Otal J., Quiles A., Hevia M.L. Validation of an assay for quantification of alpha-amylase in saliva of sheep. Can. J. Vet. Res. 2016;80:197–202. [PMC free article] [PubMed] [Google Scholar]
- 130.Fuentes-Rubio M., Fuentes F., Otal J., Quiles A., Tecles F., Ceron J.J., Hevia M.L. Measurements of salivary alpha-amylase in horse: comparison of 2 different assays. J. Veterinary Behavior-Clin. Appl. Res. 2015;10:122–127. [Google Scholar]
- 131.Garcia P.T., Guimaraes L.N., Dias A.A., Ulhoa C.J., Coltro W.K.T. Amperometric detection of salivary alpha-amylase on screen-printed carbon electrodes as a simple and inexpensive alternative for point-of-care testing. Sens. Actuators B-Chemical. 2018;258:342–348. [Google Scholar]
- 132.Zhang L., Yang W., Yang Y., Liu H., Gu Z. Smartphone-based point-of-care testing of salivary alpha-amylase for personal psychological measurement. Analyst. 2015;140:7399–7406. doi: 10.1039/c5an01664a. [DOI] [PubMed] [Google Scholar]
- 133.Della Ventura B., Sakac N., Funari R., Velotta R. Flexible immunosensor for the detection of salivary alpha-amylase in body fluids. Talanta. 2017;174:52–58. doi: 10.1016/j.talanta.2017.05.075. [DOI] [PubMed] [Google Scholar]
- 134.Shi J., Deng Q., Li Y., Chai Z., Wan C., Shangguan H., Li L., Tang B. An aggregation-induced emission probe based on host-guest inclusion composed of the tetraphenylethylene motif and gamma-cyclodextrin for the detection of alpha-amylase. Chem. Asian J. 2019;14:847–852. doi: 10.1002/asia.201801601. [DOI] [PubMed] [Google Scholar]
- 135.Tsyrulneva I., Alagappan P., Liedberg B. Colorimetric detection of salivary alpha-amylase using maltose as a noncompetitive inhibitor for polysaccharide cleavage. ACS Sens. 2019;4:865–873. doi: 10.1021/acssensors.8b01343. [DOI] [PubMed] [Google Scholar]
- 136.Wornes D.J., Speers S.J., Murakami J.A. The evaluation and validation of Phadebas((R)) paper as a presumptive screening tool for saliva on forensic exhibits. Forensic Sci. Int. 2018;288:81–88. doi: 10.1016/j.forsciint.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 137.Shetty V., Zigler C., Robles T.F., Elashoff D., Yamaguchi M. Developmental validation of a point-of-care, salivary alpha-amylase biosensor. Psychoneuroendocrinology. 2011;36:193–199. doi: 10.1016/j.psyneuen.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zarabadi A.S., Huang T., Mielke J.G. Capillary isoelectric focusing with whole column imaging detection (iCIEF): a new approach to the characterization and quantification of salivary alpha-amylase. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1053:65–71. doi: 10.1016/j.jchromb.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 139.Wang D., Zhu X., Zhao D., Cai Y., Zhao M. Single-step turn-on homogeneous fluorescent immunosensor for rapid, sensitive, and selective detection of proteins. Chem. Asian J. 2012;7:1546–1549. doi: 10.1002/asia.201200086. [DOI] [PubMed] [Google Scholar]
- 140.Rizwan M., Koh D., Booth M.A., Ahmed M.U. Combining a gold nanoparticle-polyethylene glycol nanocomposite and carbon nanofiber electrodes to develop a highly sensitive salivary secretory immunoglobulin A immunosensor. Sens. Actuators B-Chemical. 2018;255:557–563. [Google Scholar]
- 141.Lim S.A., Koh D., Ahmed M.U. Single wall carbon nanotube and magnetic bead based electrochemical immunosensor for sensitive detection of salivary secretory immuno-globulin a. Curr. Anal. Chem. 2018;14:399–405. [Google Scholar]
- 142.Escribano D., Soler L., Gutierrez A.M., Martinez-Subiela S., Ceron J.J. Measurement of chromogranin A in porcine saliva: validation of a time-resolved immunofluorometric assay and evaluation of its application as a marker of acute stress. Animal. 2013;7:640–647. doi: 10.1017/S1751731112002005. [DOI] [PubMed] [Google Scholar]
- 143.Casal N., Manteca X., Escribano D., Ceron J.J., Fabrega E. Effect of environmental enrichment and herbal compound supplementation on physiological stress indicators (chromogranin A, cortisol and tumour necrosis factor-alpha) in growing pigs. Animal. 2017;11:1228–1236. doi: 10.1017/S1751731116002561. [DOI] [PubMed] [Google Scholar]
- 144.Huang Y., Liu Z., Liu W., Yin C., Ci L., Zhao R., Yang X. Short communication: salivary haptoglobin and chromogranin A as non-invasive markers during restraint stress in pigs. Res. Vet. Sci. 2017;114:27–30. doi: 10.1016/j.rvsc.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 145.Tecles F., Escribano D., Martinez-Miro S., Hernandez F., Contreras M.D., Ceron J.J. Cholinesterase in porcine saliva: analytical characterization and behavior after experimental stress. Res. Vet. Sci. 2016;106:23–28. doi: 10.1016/j.rvsc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 146.Rai B., Kaur J. Salivary stress markers and psychological stress in simulated microgravity: 21 days in 6 degrees head-down tilt. J. Oral Sci. 2011;53:103–107. doi: 10.2334/josnusd.53.103. [DOI] [PubMed] [Google Scholar]
- 147.Abekura H., Tsuboi M., Okura T., Kagawa K., Sadamori S., Akagawa Y. Association between sleep bruxism and stress sensitivity in an experimental psychological stress task. Biomed. Res. 2011;32:395–399. doi: 10.2220/biomedres.32.395. [DOI] [PubMed] [Google Scholar]
- 148.Lihala R., Jayaram P., Chatterjee A., Joshi A. Effect of non-surgical periodontal therapy on stress and salivary Chromogranin-A levels: a clinico-biochemical study. Indian J. Dent. Res. 2019;30:213–218. doi: 10.4103/ijdr.IJDR_273_17. [DOI] [PubMed] [Google Scholar]
- 149.Wood P. Salivary steroid assays - research or routine? Ann. Clin. Biochem. 2009;46:183–196. doi: 10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- 150.El-Farhan N., Rees D.A., Evans C. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann. Clin. Biochem. 2017;54:308–322. doi: 10.1177/0004563216687335. [DOI] [PubMed] [Google Scholar]
- 151.Mellon S.H., Gautam A., Hammamieh R., Jett M., Wolkowitz O.M. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biol. Psychiatry. 2018;83:866–875. doi: 10.1016/j.biopsych.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 152.Michels N. Biological underpinnings from psychosocial stress towards appetite and obesity during youth: research implications towards metagenomics, epigenomics and metabolomics. Nutr. Res. Rev. 2019;32:282–293. doi: 10.1017/S0954422419000143. [DOI] [PubMed] [Google Scholar]
- 153.Le-Niculescu H., Roseberry K., Levey D.F., Rogers J., Kosary K., Prabha S., Jones T., Judd S., McCormick M.A., Wessel A.R., Williams A., Phalen P.L., Mamdani F., Sequeira A., Kurian S.M., Niculescu A.B. Towards precision medicine for stress disorders: diagnostic biomarkers and targeted drugs. Mol. Psychiatry. 2020;25:918–938. doi: 10.1038/s41380-019-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dean K.R., Hammamieh R., Mellon S.H., Abu-Amara D., Flory J.D., Guffanti G., Wang K., Daigle B.J., Jr., Gautam A., Lee I., Yang R., Almli L.M., Bersani F.S., Chakraborty N., Donohue D., Kerley K., Kim T.K., Laska E., Young Lee M., Lindqvist D., Lori A., Lu L., Misganaw B., Muhie S., Newman J., Price N.D., Qin S., Reus V.I., Siegel C., Somvanshi P.R., Thakur G.S., Zhou Y., Consortium P.S.B., Hood L., Ressler K.J., Wolkowitz O.M., Yehuda R., Jett M., Doyle F.J., 3rd, Marmar C. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol. Psychiatry. 2019:1–13. doi: 10.1038/s41380-019-0496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin J.A., Chavez J.L., Chushak Y., Chapleau R.R., Hagen J., Kelley-Loughnane N. Tunable stringency aptamer selection and gold nanoparticle assay for detection of cortisol. Anal. Bioanal. Chem. 2014;406:4637–4647. doi: 10.1007/s00216-014-7883-8. [DOI] [PubMed] [Google Scholar]
- 156.Sundaram P., Kurniawan H., Byrne M.E., Wower J. Therapeutic RNA aptamers in clinical trials. Eur. J. Pharm. Sci. 2013;48:259–271. doi: 10.1016/j.ejps.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 157.Ramasubbu N., Paloth V., Luo Y., Brayer G.D., Levine M.J. Structure of human salivary α-amylase at 1.6 Å resolution: implications for its role in the oral cavity. Acta Crystallogr. D Biol. Crystallogr. 1996;52:435–446. doi: 10.1107/S0907444995014119. [DOI] [PubMed] [Google Scholar]