Abstract
The simultaneous maturation of multiple digital and telecommunications technologies in 2020 has created an unprecedented opportunity for ophthalmology to adapt to new models of care using tele-health supported by digital innovations. These digital innovations include artificial intelligence (AI), 5th generation (5G) telecommunication networks and the Internet of Things (IoT), creating an inter-dependent ecosystem offering opportunities to develop new models of eye care addressing the challenges of COVID-19 and beyond. Ophthalmology has thrived in some of these areas partly due to its many image-based investigations. Tele-health and AI provide synchronous solutions to challenges facing ophthalmologists and healthcare providers worldwide. This article reviews how countries across the world have utilised these digital innovations to tackle diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, glaucoma, refractive error correction, cataract and other anterior segment disorders. The review summarises the digital strategies that countries are developing and discusses technologies that may increasingly enter the clinical workflow and processes of ophthalmologists. Furthermore as countries around the world have initiated a series of escalating containment and mitigation measures during the COVID-19 pandemic, the delivery of eye care services globally has been significantly impacted. As ophthalmic services adapt and form a “new normal”, the rapid adoption of some of telehealth and digital innovation during the pandemic is also discussed. Finally, challenges for validation and clinical implementation are considered, as well as recommendations on future directions.
Keywords: Telemedicine, Tele-ophthalmology, Tele-screening, Diabetic retinopathy screening, Artificial intelligence, Deep learning, Digital transformation, Digital innovations, COVID-19, Digital technology
1. Introduction
2020 marked the synchronous maturation of several key digital innovations in information and communications technology, which advanced at an unprecedented rate this new century. Every sector and industry, including healthcare, has been impacted by digital transformation. Digital innovations including the further consolidation of tele-health, the development of 5th generation wireless networks (5G), artificial intelligence (AI) approaches such as machine learning (ML) and deep learning (DL), and the Internet of Things (IoT), as well as digital security capabilities such as blockchain, have created an extraordinary ecosystem for new opportunities in healthcare and other industries (Ting et al., 2020). These developments could potentially address some of the most urgent challenges facing health service providers and policy makers, including universal, equitable, sustainable healthcare coverage to a growing, ageing population. They can fundamentally change screening, diagnosis and monitoring of diseases, enable more accurate profiling of disease progression and further refine and/or personalise treatments.
Against this backdrop, 2020 has also been dominated by an unprecedented global crisis: the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since its emergence in Wuhan, China in late 2019 (Parrish et al., 2020), within months, on March 11, 2020, the World Health Organization (WHO) has announced COVID-19 was a “pandemic” (World Health Organization, 2020). With the non-linear rapid disease expansion, COVID-19 has caused widespread healthcare, socio-political and economic impact (Kuo et al., 2020; Siegel, 2020; Berlinger, 2020). Countries and healthcare systems around the world have been forced to rapidly adapt to tele-health and digital innovations to mitigate the impact of the risk of virus transmission to what is widely regarded as the “new normal”.
This article summarises digital technologies that may be applied in ophthalmology with attention to how they are apply to tele-health. A review of different tele-health models and the use of AI that are applicable to the delivery of ophthalmic services and more specifically how it is already incorporated in the management of diabetic retinopathy, retinopathy of prematurity, glaucoma, age-related macular degeneration, refractive error correction and prediction, anterior segment diseases and cataract is presented. The variation of global practices in teleophthalmology implementation and adoption, and potential challenges for implementation teleophthalmology and AI is discussed. Finally, this review proposes how ophthalmology may adapt to the “new normal” using tele-health and digital innovations considering the COVID-19 pandemic.
2. World Health Organization (WHO) guidelines for digital health
In 2019, WHO started developing a framework for the adoption of digital innovations and technology in healthcare. The WHO recommendations on digital interventions in healthcare promotes assessment on the basis of ‘benefits, harms, acceptability, feasibility, resource use and equity considerations’, and views these tools as still very much that – tools – in the journey to achieving universal health coverage and sustainability (World Health Organisation, 2019).
There are several digital interventions that have been prioritised for review by the WHO. Of relevance to this discussion are: the use of client-to-provider telemedicine to complement health service delivery; the use of provider-to-provider telemedicine; targeted customised health information transmission; health worker decision making support; digitised health information tracking; and education. In all these scenarios, the review highlights the need for monitoring of patient safety, privacy, traceability, accountability and security, with plans in place to address any breaches. Processes for these have been innate within the pharmaceutical and other medical devices industries, and new technological entrants to this traditional sector should consider these during development of the services. There will also be ethical conundrums that have yet to be articulated and debated. The engaged clinician should seek to be involved in the development of these new advances to closely align any innovations to solve unmet clinical needs. Simultaneously, clinicians should examine if any innovation complies with quality, ethical, and sustainable healthcare, as legislation invariably lags behind such momentous leaps in innovation.
3. Digital technology
3.1. Telemedicine
Telemedicine enable clinicians to evaluate their patients remotely. This can be desirable for several reasons. First, telemedicine can facilitate more efficient and equitable distribution of limited healthcare resources. This allows delivery of care to distant areas where there is a shortage of doctors and other professionals, reduces travel and the associated carbon footprints, and connects patients with rare diseases to speciality care and address the transport challenges some patients face. Waiting times could be reduced through increased capacity and access to care for both chronic and acute disease patient. In the acute setting, patients could receive immediate specialist input even if one is not available locally.
Second, amid the COVID-19 pandemic and in mitigating infection risk in the healthcare setting, real-time telemedicine has been rapidly incorporated into routine care delivery. The patient population telemedicine aims to serve is no-longer focused on targeting remote regions. Instead it is rapidly becoming a new standard of care. It enables triaging prior to patients’ arrival into hospital to avoid unnecessary visits and exposure risks and has been adopted by multiple centres across the world (Hollander and Carr, 2020; Ting et al., 2020; Wickham et al., 2020; Bourdon et al., 2020).
Third, video-consultations in combination with innovative service design already exist that further limits patient journeys and clinic visits whilst maximising the quality of the telemedicine consultation. In Scotland, optometric practices have been set up strategically across some regions to provide primary eye care services (NHS Scotland, 2020). Smart phones attached to slit-lamps enable ocular biomicroscopic videography, empowering ophthalmologists to view the patient's examination features in real-time without the patient attending. Also, simplification of image sharing of data such as OCT scans can be achieved by screen sharing, which has long been a challenge both within ophthalmology and in radiology due to the variety of available formats and software.
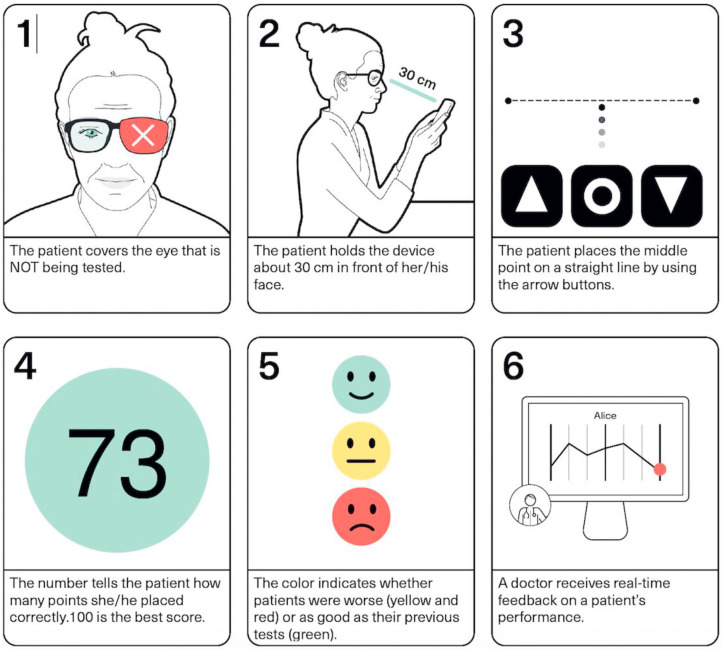
A movement away from traditional clinic visits might be further aided by the use of home devices used in the monitoring of visual acuity, visual fields, and intraocular pressure (Ittoop et al., 2016; Anderson et al., 2017; Amirsolaimani et al., 2017; Ciuffreda and Rosenfield, 2015; Wisse et al., 2019), though the more complicated devices such as tonometers may be prohibitively expensive.
Effective tele-screening programmes require multiple components. First, there should be a reliable, cost-effective and operator-friendly data gathering system. A preferred goal is to achieve longitudinal consistency of data format to facilitate comparisons. The device itself should be simple, with mechanisms in place to facilitate data transmission to the IoT. Ideal designs should involve networks where multiple, simpler devices can communicate with a central station. System updates would involve the central stations to enable streamlined logistics and cost efficiency, particularly if the network has widely dispersed simpler devices.
Second, the data must be processed and enabled to identify the disease of interest. The most frequently adopted model at present is the use of trained persons to read the collected images, as in diabetes tele-retinal screening programmes. Whilst larger numbers can be screened this way in comparison to direct clinician reviews, it remains a costly and resource intensive process involving highly trained graders. While DL is starting to be incorporated to this process, the potential benefits from this adaptation are unknown. Regulatory bodies recognise the potential of AI in healthcare, and the FDA has approved the use of an AI algorithm for the diagnosis of DR in the primary care setting (Abramoff et al., 2018).
Finally, the outcome must be conveyed in a timely manner to the patient and the healthcare provider to facilitate appropriate medical management. This communication again could involve a clinician consultation, but most normal outcomes may be communicated in an automated manner such as via a smart phone app or text message.
Beyond simply replicating current services albeit remotely, the collection, storage and transmission of offer the potential of combining telemedicine with AI. When used prospectively with longitudinal data, vast swathes of new knowledge such as disease progression and real-world, real-time incidence calculation could be harnessed. If well adopted, the data collected would enter the realms of big data, and far exceed the capabilities of data capture that most individual studies are able to achieve. Moreover, this could grow into a consistent source of longitudinal data which would be valuable in the development of disease progression forecasting capabilities, incorporating AI.
3.2. 5th Generation (5G) telecommunications
5G wireless communications was designed to meet the challenges of serving large-scale complex network connections. These networks have extremely low latency, higher capacity, and improve the speed of data transmission through the use of higher frequency millimetre waves compared to existing networks (Simko and Mattsson, 2019). Latency in 5G transmission can be less than 1 ms of delay compared to about 70 milliseconds on the 4G network, and give significant improvement to the users’ perception of the service (Samsung, 2015). Download speeds on 5G networks can be increased 20 fold from the current 1 gigabit per second on 4G (Nordrum, Clark, and staff. 2017). And all this magnitude increase in function whilst simultaneously reducing energy consumption by the connected devices (Agiwal et al., 2016). 5G networks will deliver an end-to-end latency of less than 5 milli-seconds and over-the-air latency of less than 1 ms - which is one-tenth of the 4G network latency (Samsung, 2015).
5G utilises small cells, which are miniature base stations that have low power requirements. However, because 5G transmits at higher frequencies, signal attenuation becomes a greater challenge, and these base stations need to be placed closer than 4G base stations (every 250 m or so) (National Academies of Sciences et al. 2019). To ensure consistent signal transmission, base stations will need to be densely populated. Despite the base stations being smaller in size, the increased infrastructure needs of a 5G network with these cells will not be practical in sparsely populated rural regions. Thus whilst telemedicine has been traditionally regarded as being able to contribute to healthcare delivery to these areas in a meaningful way, it may in fact continue to exclude those who already struggle to access physical care.
In addition to being able to support increasing bandwidth demands from users and patients, 5G enables Ultra-High-Definition (UHD) multimedia streaming with enhanced user experience. The high-resolution images can be more easily transferred. Better quality and reliable video-consultations with improved patient experience may contribute to forging better physician-patient relationship. Real-time slitlamp examinations streamed in high-definition has the potential to become common place. With imperceptible latency, the clinician could control a slit-lamp remotely whilst looking at a mobile device displaying the eye being examined remotely. The immersive experience promised by 5G can also be used to augment the learning experience, particularly the visually-based tasks such as surgery.
Despite these great expectations, 5G will not be the panacea for all connectivity challenges. The reported speeds assume that every network is using 5G, but not surprisingly the implementation of 5G will be gradual as new cells are built and installed. This incremental adoption of expensive infrastructure means that the network will need to remain compatible with legacy networks, and with other operators who may be implementing at a different speed (Rashid, 2020).
In being compatible, and with the networks essentially being a patchwork of wireless connections incorporating various generations, the same vulnerabilities found in older generation networks will remain. Well-knowns flaws of the data packet transmission protocol that is used across the different generations of networks, the General Packet Radio Service (GPRS) Tunneling Protocol (GTP), include not validating users’ physical location permitting attackers to spoof locations and allowing attackers to impersonate other users or use false credentials, so the impersonated subscriber is charged for costs incurred. Attackers can block all connections stemming from a single node so legitimate subscribers cannot access a connection in the given geographical region, in a denial-of-service attack (Rashid, 2020). The most basic requirements of connectivity in healthcare are security and reliability, and despite the impressive numbers 5G promises, it may be still some time before these two basic tenets are consistently achieved.
3.2.1. 5G and the COVID-19 pandemic
The lockdown orders across the world has brought a sudden strain on existing cellular networks. As countries responded, work, education, healthcare, and most other human interactions were suddenly pushed onto the virtual arena. The pandemic has shown that telemedicine is not only reserved for the remote and underserved. In fact, telemedicine can routinely serve the wider population if it can be shown to be safe, efficient, and inclusive, with measures to ensure security, robustness and capacity, particularly in densely populated regions with massive competing demands for bandwidth.
Though few examples currently exist, 5G telemedicine has already been implemented. In China, the successful utilisation of a 5G telemedicine network was reported in Sichuan province (Hong et al., 2020). The newly established China Telecom 5G Dual Gigabit system covered all 208 designated COVID-19 hospitals in the province, with a single hospital as the central node. Real-time video telemedicine service allowed multidisciplinary management of COVID-19 patients with simultaneous review of CT imaging by experts remotely. 5G contributed to the quality of video transmission and the accessibility of experts are reported to have contributed to the lower case fatality ratio in Sichuan compared to Hubei and the global average. Additionally, the authors report remote control of CT equipment by experts at the central hospital, overcoming shortages of qualified technicians and ensuring quality images.
3.2.2. 6G
6G research and development has already been launched, with anticipated launch in the next decade (Samsung, 2020). Both humans and machines will use 6G which will allow for truly immersive extended reality (XR) and high-fidelity mobile hologram which could have enormous implications for healthcare. 6G will address the issues of limited computational power of mobile devices through flexible integration of entities within the networks. Additionally it is set to address much of the security and privacy challenges associated with increasing data collection and sharing.
3.3. The Internet of Things (IoT)
Over the last decade the number of mobile devices has surpassed the global population figure (Simko and Mattsson, 2019). Thus, there is simultaneously increasing interconnection between devices and machines, maintaining connections without deliberate human intervention. This network is referred to as the Internet of Things, to differentiate it from the traditional internet which connects people. It is the network of physical objects embedded with sensors and the ability to transmit and process data, communicating with other machines or humans, frequently in an automated fashion. The current networks serve to connect individuals, but as individuals begins to wear health monitoring devices such as smart watches, live in smart homes with connected fridges and heating systems, wireless metering, mobile payments and commute in smart cities in driverless cars, the capacity needed on the networks increases exponentially. 5G is designed to support this ubiquitous connectivity that will truly enable IoT, virtually connecting every aspect of human lives. Connected devices are predicted reach around 500 billion, that is around 59 times the then projected human population, by 2030 when mass commercialization of 6G is anticipated (Samsung, 2020).
This connectivity can change healthcare services. When a patient enters a clinic, their arrival can automatically be registered from their personal devices, and their clinical journey once in hospital can be streamlined to minimise wait times. For instance, directing the patient first for an OCT scan if there is a long wait for visual fields. New clinical data including images such as OCTs will be automatically uploaded into the patient's EHR, and integration with automation may trigger alerts or make new diagnosis. The patient's drug histories will be current, drug interaction warnings issued, and new prescriptions could be dispensed locally or delivered to the patient instead of waiting in queue. Healthcare records from different providers could be integrated to form an update summary so all clinicians will have an overview of the patient's most recent healthcare interactions. Lifestyle tracker data may be integrated into healthcare data, such as activity levels and diabetic retinopathy screening. Individual surgeon's preferences can be stored on the IoT cloud, so phacoemulsification settings would automatically adjust for surgeons operating at different sites. Workflow efficiency in clinics and operating rooms can improved, and there is potential for reducing errors such as intra-ocular lens related errors, with increased automation. Lens stock can also be updated automatically, reducing administrative burden and surgeries being cancelled due to lack of stock, particularly for premium lenses. Increased automation can potentially reduce healthcare errors by moving away from less effective human orientated processes such as training, policies and checklists. The WHO checklists could be superseded by IoT linking the patient's mobile device with the operating room, automated delivery of the chosen intraocular lens, and other connections that minimise human intervention.
With the IoT, vast volumes of data are being generated. Big data can be used for monitoring, but potentially, combined with big data processing and AI, data output can enable prediction and optimization of existing functions. The transmission of this data and fundamentally what is enabling the potential of the IoT is a massive shift in communications technology and 5G networks. Additionally, advances in edge processing, that is processing of the data at the place where each device is located, allows for reduced latency, and less dependence on network bandwidth and availability, and potentially enhanced security.
3.4. Artificial intelligence, machine learning and deep learning
The concept of Artificial Intelligence (AI) was first discussed in 1956 (McCarthy et al., 2006), referring to technology used to mimic human behaviour. Since then, the field has made remarkable strides in development. As a subfield of AI, Machine Learning (ML) was conceptualised by Arthur Samuel in 1959 (Samuel, 2000). He emphasised the importance for systems to learn from experience automatically instead of being programmed. In the 1980s, ML demonstrated great potential in computer foresight and predictive analytics, including clinical practice and machine translation (Bengio et al., 2013). Deep Learning (DL), a subfield of ML, has ushered in new breakthroughs in information technology. DL may study underlying features in data from multiple processing layers using neural networks, similar to the human brain (LeCun et al., 2015). Since the 2010s, DL has garnered immense attention in many fields, especially in image recognition and speech recognition (Schmidhuber, 2015). In medical practice, DL is effective in image-centric specialties, proving itself by detecting pulmonary tuberculosis from chest radiographs and malignant melanoma from digital skin photographs (Lakhani and Sundaram, 2017; Esteva et al., 2017).
Conventional diagnostic methods for ophthalmic diseases depend on the clinical assessment and, increasingly, image-capturing devices of various modalities. This process is time-consuming and costly, but also makes ophthalmology one of the specialities particularly well-suited to DL techniques and its real-world application. The application of DL to ophthalmic images, such as digital fundus photographs and visual fields, has been reported to achieve the automated screening and diagnosis of common vision-threatening diseases, including diabetic retinopathy (DR) (Abramoff et al., 2016; Gulshan et al., 2016; Raumviboonsuk et al., 2019; Ting et al., 2017), glaucoma (Liu et al., 2019; Li et al., 2018; Masumoto et al., 2018a; Asaoka et al., 2016), age-related macular degeneration (AMD) (Grassmann et al., 2018; Burlina et al., 2017) and retinopathy of prematurity (ROP) (Brown et al., 2018) with high accuracy. As such, DL may prove to be a valuable and viable adjunct to the existing diagnostic processes, and there may be a role for it to serve as an alternative to ophthalmologists and trained human image graders.
Recently, new DL algorithms were adopted for use on optical coherence tomography (OCT) images (Medeiros et al., 2019; Schlegl et al., 2018; Kapoor et al., 2019), which may increase the sensitivity of detection at the early stage of disorders, especially in AMD and DR with the detection of diabetic macular oedema (Bogunovic et al., 2017). The integration of DL into ophthalmology practice is expected to revolutionise the current disease management process, improve early detection and there are hopes that it will ultimately improve outcomes (Balyen and Peto, 2019; Tan et al., 2019, Tan et al., 2019), although the cost-effectiveness of these systems remain unclear (Xie et al., 2020).
With the potential of AI and DL to make inroads in ophthalmic delivery services, it is incumbent upon the clinician to critically assess how these innovations work and when they might be safely implemented into clinical practice.
3.5. Home monitoring devices, augmented and virtual reality
5G, and in time 6G, will support virtual reality (VR) where a simulated presence is generated by computer graphics and allows users to interact with the simulated elements in a seemingly real way.
Augmented reality, where computer-aided information is generated and graphically augmented to the display real-time, can also have broad implications for healthcare. Counselling patients and pre-operative consent can likely be enhanced with augmented reality, and non-clinical functions in hospitals such as navigation, in particular for visually-impaired patients.
The current landscape in terms of use of VR and AR in ophthalmology is nascent. VR creates a digital experience where the user environment is immersive. In the VR environment, the user usually wears a wrap-around headset that limits peripheral vision. AR blends digital information with real-world environmental data, enabling users to interact with digital images and view the actual physical surroundings simultaneously. AR integrates virtual objects into a real-world space, whereas VR usually blocks out information from the actual environment and transports users into a virtual simulated world (Pietro et al., 2018).
Within the past decade, VR devices such as IrisVision™, and NuEyes™ have been used to aid patients with visual impairment (Deemer et al., 2018). IrisVision™ VR headset holds a smartphone that records a patient's surroundings and displays the image in the peripheral vision and can also magnify the image. NuEyes™ used a VR immersive system to magnify images but is no longer in production. The main limitation of VR for patient use is the occlusive and digitally immersive nature of the headsets. The user cannot visualize the peripheral environment well and thus precludes safe use while ambulating or moving while wearing a VR headset.
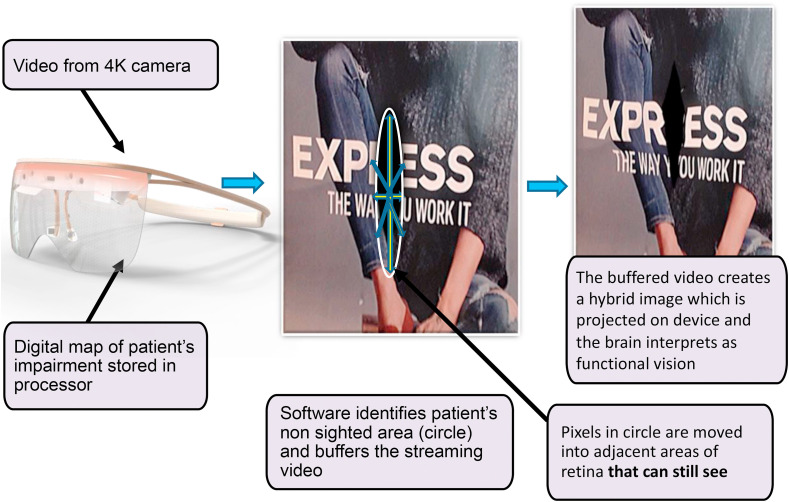
The advantage of AR over VR for purposes for visual rehabilitation is significant, as AR allows patients to maintain peripheral vision and interact in the real-world environment with digital enhancement. The Oculenz™ AR headset enables patients to view the image normally blocked within the scotoma of each eye to be visualized by adjacent functional retina, using image remapping strategy. Studies have shown that remapping may be helpful to improve vision, especially in reading (Gupta et al., 2018). Oculenz™ AR platform ( Fig. 1 ) has embedded 4 K cameras and algorithms that delineate the scotoma in each eye and remaps the previously missing image onto healthy neighboring retina. The mapping algorithm customizes the image placement as the disease changes. In order for remapping to work effectively, the display image needs to be stabilized on the adjacent retina regardless of gaze direction (Deemer et al., 2018). Oculenz is able to maintain alignment of the eye gaze and the projected image with patented eye tracking technology. While still in development, early patient trials show this device can improve 4–5 lines on Snellen chart after one use without magnification. The final version of the headset is slated to be available in 2021.
Fig. 1.
Customized image displacement onto functional retina by the Oculenz Augmented Reality Headset for patients with visual impairment from macular degeneration.
A few home monitoring systems have been developed for ophthalmic applications, some of which are described in Table 3 . Currently ForeseeHome™ and PsyPad™ are non-VR/AR platforms that can monitor patients with AMD (Chew et al., 2016; Adams et al., 2018). A novel method for AMD monitoring is to use AR technology. A key feature of the Oculenz platform is in-home monitoring of the scotoma or visual defect. Its AI algorithm tracks scotoma progression. If a change in the scotoma is detected, AI quantifies this change and alerts the physician's office. The ability to remotely and continuously monitor a patient's disease with precision is important. Patients with macular disease often cannot detect subtle, progressive changes in their vision. Hence, by the time a patient recognizes a change, it may be too late for a physician to intervene to preserve vision.
Table 3.
Digital home vision monitoring devices.
| Device | Type of device | Type of test | Function | Clinical application |
|---|---|---|---|---|
|
ForeseeHome Notal Vision, Inc. |
Desktop device | Preferential hyperacuity perimetry (PHP) central 14 degrees of field | Self-testing for AMD (Loewenstein et al., 2003) | FDA approved, covered by Medicare |
|
myVisiontrack® Genentech |
Mobile device | Shape discrimination hyperacuity (SDH) test | Self-testing for AMD (Kaiser et al., 2013) | FDA approved for AMD and DME monitoring |
| Alleye, Oculare Medical Inc. | Mobile device | Hyperacuity: central 12 degrees of field | Self-testing for AMD: discriminates between dry and wet AMD (Schmid et al. 2018, 2019) | FDA approved and CE-marked for vision monitoring in AMD |
Recently AR technology has emerged in ophthalmic surgery applications. Namely, AR headsets have been developed to improve ergonomics and enhance visualization in the operating room. Historically ophthalmic surgeons operated viewing through oculars of the operating microscope, creating surgeon movement restriction during surgery and spine problems due to ergonomic constraints of extended viewing at a microscope. Alcon (Ngenuity™) and Zeiss (Artevo™) have created digital head up displays for ophthalmic surgery to address these issues (Eckardt and Paulo, 2016; Palacios et al., 2020). These digital viewing systems allow the surgeon more comfortably while operating and wearing 3D glasses to view surgery on a large monitor positioned beside the patient's surgical bed. Instead of viewing a large monitor with 3D glasses to the side of the surgeon which is the currently configuration in digital heads up ophthalmic surgery, two new AR systems project operative digital images directly in front of the surgeon. Beyeonics™ has developed a platform that allows for digital information from the microscope to be projected onto a tethered headset worn by the surgeon. Limitations of that headset include being not wireless and heavy (1.6 lbs./730 g). ORlenz™ AR headset was developed to improve the ergonomics and visualization issues in ophthalmic surgery. It differs from Beyeonics™ Clarity headset in that it is wireless, lighter, and is higher resolution at 60 pixels per degree. Both systems use an AR platform to enhance visualization and reduce occupational injuries by not requiring direct microscope viewing. Both headsets are undergoing development and are not yet available to use outside of clinical trials.
3.6. Digital innovation and transformation
The intense focus on the capabilities that new technologies offer, can lead one to underestimate the challenges inherent in the actual digital transformation of ophthalmology. While other industries are rapid to embrace new technology, healthcare is notably slower. There is a real risk that high hopes for the new technologies described elsewhere in this paper will flounder upon the reality of healthcare systems that remain digitally immature. Some barriers to innovation in healthcare are perfectly legitimate, for example the real risk that sub-optimal deployment of a digital technology could lead to patient harm. Other barriers are entirely artificial, and foremost among these are the perverse incentives created by billing and tariff systems. In the UK, for example, there has only recently been a move to correct the imbalance between poorly reimbursed remote consultations and well reimbursed face-to-face consultations (Brennan et al., 2018).
When a technology has successfully navigated the ethical, financial, regulatory, and safety barriers to implementation in healthcare, the rate of attrition remains high. In order to be scalable beyond local pilots, the technology must either fit in seamlessly with existing clinical practice, or it must be sufficiently compelling to cause clinical practice to change (as we have seen with OCT platforms in ophthalmology). The failure of the UK's National Programme for IT is a case study for this phenomenon (Robertson et al., 2011). Where local adoption has been successful, innovations can be slow to spread through a fragmented system, with funding for spread of innovation often a small fraction of the research and development budget (Collins, 2018).
A partial solution to these challenges has been the creation of innovation units embedded in hospitals and academic medical centres (e.g. Cleveland Clinic Innovations and the Digital Clinical Lab at Moorfields Eye Hospital). These units can help to develop digital technologies that improve healthcare delivery in the real world, rather than developing solutions that can't easily be incorporated into routine practice. While innovation units can earmark resources, a major enabler is their ability to bring together multi-disciplinary teams that allow the development of useful solutions. These include, among others, engineers, developers, behavioural scientists, intellectual property specialists, and clinicians. The development of local capabilities to drive digital innovation mirrors the acceptance that national initiatives, such as EMR deployment, can be more successful when driven from “bottom up” process whereby local solutions are integrated in a modular fashion (Aanestad and Jensen, 2011).
A key enabler to this modular approach to innovation is the adoption of shared interoperability standards. Without these standards, we run the risk of creating a complex ecosystem of technologies that are incapable of communicating with each other. Ophthalmology is particularly retrograde on this, with most devices using vendor-specific file formats. Vendor-neutral approaches will improve the ability of AI algorithms, for example, to work on a common data substrate. These standards have long been suggested, but we are now beginning to see concerted effort towards their adoption, for example SMART-on-FHIR, a standards-based interoperable apps platform for EHR (Mandel et al., 2016) and SNOMED CT, a structured clinical vocabulary for use in EHR (Bodenreider et al., 2018).
4. Digital innovations for eye diseases
4.1. Diabetic retinopathy
Diabetes is one of the biggest healthcare challenges in the world (Wong and Sabanayagam, 2019). The global prevalence in 2019 was estimated to be 9.3% (463 million people), and is anticipated to rise to 10.9% (700 million) by 2045 (Saeedi et al., 2019). Diabetic retinopathy (DR) accounts for 4.8% of global blindness, and the overall prevalence of DR in type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) using pooled data from the United States, Australia, Europe and Asia is 34.6%, with 7% of patients harbouring vision threatening DR (VTDR) (Yau et al., 2012).
Annual fundoscopy for patients with diabetes mellitus is a key strategy by the WHO in the prevention of sight loss (World Health Organisation, 2000), a message echoed by the International Council of Ophthalmology (2017). Practices vary internationally both in terms of the technique for fundoscopy, including direct ophthalmoscopy, slit-lamp biomicroscopy facilitated posterior segment exam with hand held lenses, dilated or undilated retinal photographs, tele-retinal screening and video recording, and in terms of the screener, including general physicians, optometrists, trained technicians, and ophthalmologists (Ting et al., 2016).
If screening programmes were instituted by countries across the world using fundus photography, there would be close to one billion images generated on an annual basis based on a global prevalence of diabetes of nearly half a billion people (Saeedi et al., 2019). Traditional reading of these images by trained personnel is neither sustainable nor an efficient use of expertise. In short, technology is essential to facilitate capture, storage and interpretation of nearly a billion retinal photographs per year. And with longitudinal data to better inform prognostication, there is potential for patients to be safely risk stratified with tailored screening frequency, even modality, and have access to their personalised projected disease progression.
4.1.1. Tele-screening in DR
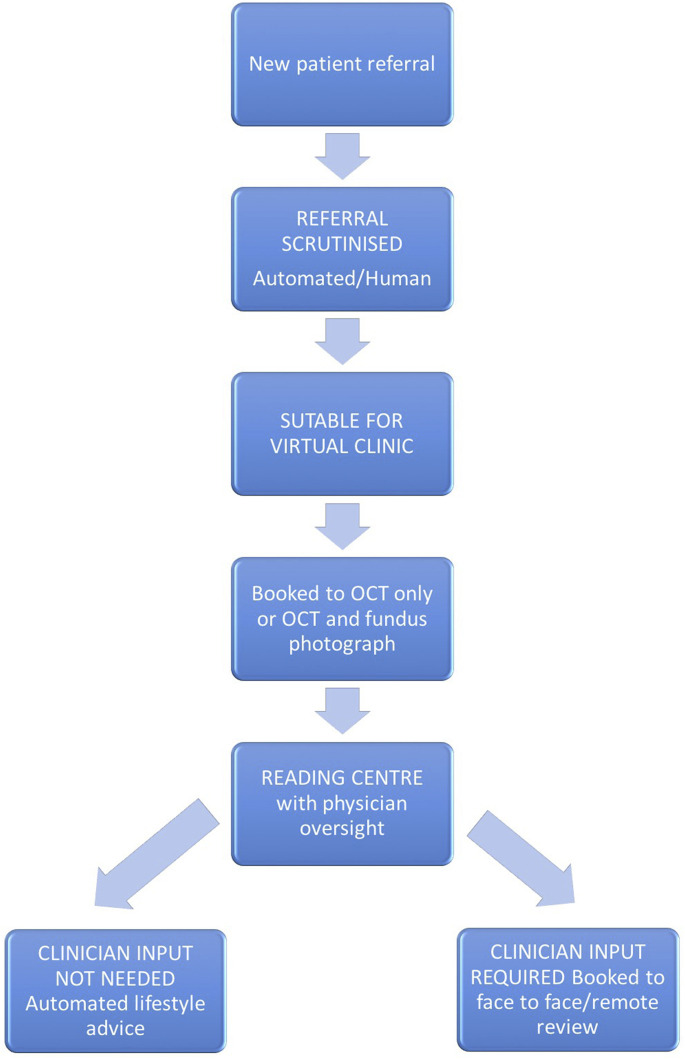
DR screening programmes based on telemedicine with digital fundus photography by specially trained graders has been well established in some developed nations like the UK and Singapore (Scotland et al., 2010; Nguyen et al., 2016). Tele-screening addresses many of the present geographic challenges and the unequal distribution of services across many areas, thus helping to increase screening coverage (Salongcay and Silva, 2018). Tele-screening facilitates task-sharing and task-shifting between clinicians and health professionals, e.g., trained graders instead of ophthalmologists reading retinal images, and offers a fast, accurate and cost-effective solution to DR screening in all resource settings. Fig. 2 provides an example of semi-automated remote triage workflow for medical retina. The availability of trained retinal graders is a major limitation in many countries, and a potential solution to this would be an AI-based DR screening algorithms, including ones that use fundus on phone (FOP) retinal imaging. Comprehensive practical guidance on telemedicine in diabetic retinopathy has been recently updated by the American Telemedicine Association Ocular Telehealth Special Interest Group (Horton et al., 2020). Table 1 provides a summary of DR screening across a number of countries and their adoption of AI and tele-screening.
Fig. 2.
Example of semi-automated remote triage workflow for medical retina.
Table 1.
Countries, their national screening strategies and the adoption of tele-screening and artificial intelligence in diabetic retinopathy screening.
| Countries | Screening sites | Number of screening sites | Retinal photographer | Width of views | Number of Fields | Graders of retinal images | Turnover time of grading |
|---|---|---|---|---|---|---|---|
| Australia (Atkinson-Briggs et al. 2019; Moynihan and Turner 2017) | Optometry and ophthalmology clinics, primary care | 17 (Kimberley DR screening) | Nurses and other health workers | 30° (Kimberley) | 1 (KDEHC) | Retinal specialists | variable |
| Zambia (Lewis et al. 2018) | Mobile van screening (single province) | 1 van, 5 sites | Trained technicians | 45 ° × 40 ° | 2 | Nurses and non-medical trained graders | |
| South Africa (Khan et al. 2013) | Community centres with mobile camera transported between sites (pilot) | Trained technicians | Medical officer with ophthalmic experience | ||||
| Tanzania (Cleland et al. 2016) | Mobile screening (single region) (pilot) | Covers 18 health facilities | Trained technicians | 45° | 2 | Ophthalmology residents | 2 weeks |
| China (Jia et al. 2019) | Trained technicians | 45° | 2 | Professional graders, ophthalmologists | |||
| Singapore (Nguyen et al. 2016) | Primary care facilities/polyclinics, hospitals, optometrists | 20 | Nurses | 45° | 2 | trained and accredited grading technicians | <1hr |
| England, United Kingdom (Scanlon 2017) | Fixed or mobile, optometry practices, eye clinics, hospitals | 1500 graders, 62 digital screening providers | Grader/clinical lead | 45° | 2 | Non-clinical technicians/optometrists/nurses, all supported by an ophthalmologist, usually retinal specialist | <3 weeks |
| United States (Tozer et al. 2015) | Primary care clinics, endocrinology clinics | 88 fixed, 10 portable sites across 25 states (JVN) | Certified technician | 45° | 3 | Automated diagnosis with ophthalmologist validation | <4 weeks |
In England, the National Health Service Diabetic Eye Screening Programme (DESP) has an established and effective universal programme which achieves substantial uptake. Since its inception in 2003 and reaching coverage of the entire English population by 2008, the DESP adoption rate includes 82% of the 2 million eligible population (Public Health England, 2016). With over 60 screening centres, dilated mydriatic retinal photographs are taken by trained technicians, and graded by trained graders, who may be non-clinicians, nurses, or optometrists, and overseen by a consultant ophthalmologist. Partially as a result of this successfully implemented programme, DR is no longer the leading cause of certifiable blindness in the working population in England (Scanlon, 2008, 2017).
In Singapore, about one third of patients with diabetes have DR and 17% have sight-threatening DR (Khoo et al., 1990). In 2010, the Singapore Integrated DR Program (SiDRP) (Nguyen et al., 2016), a national-level, telemedicine-based DR screening program, was developed and screened up to 200,000 people with diabetes. Retinal photographs taken from the 18 primary care facilities are transmitted via a telemedicine platform and assessed by trained graders. The clinics receive the reports on the same day, and 90% within 1 h. Comparing telemedicine screening to the existing physician-assessed model, the saving in terms of direct costs was SGD 144/person (EUR 94.20). Extrapolating this to the SiDRP population, this translates to a lifetime saving of ~SGD 29 million (Nguyen et al., 2016). Work is underway to integrate a DL system for referable DR, VTDR, and related eye diseases (Ting et al., 2017) within SiDRP (Bellemo et al., 2019), potentially becoming the first such autonomous reading system to be integrated into a national DR screening program.
In US, the two largest DR screening programmes are the Joslin Vision Network (JVN) and the Department of Veteran Affairs model (VA). JVN offers a simple process, where patients are able to undergo screening at their primary care physician or endocrinologist's office. The image is graded at a centralised reading centre along with a limited clinical data such as blood pressure and blood glucose as per the Joslin Diabetes Eye Health Care Model, and a recommended treatment plan is sent to the referring centers (Aiello et al., 1998). JVN demonstrates the ability to diagnose DR severity in a non-ophthalmic setting, which may be more effective at identifying early treatable disease and preventing visual loss, potentially at a more competitive cost (Cavallerano et al., 2005). The VA model is attractive since the entire VA system utilises same electronic health record (EHR), allowing for ease of data sharing. Similarly, Kaiser Permanente, a large Health Maintenance Organization (HMO), adopts the same EHR within the system, enabling telescreening to occur at the level of primary care office, and grading performed at a reading centre.
For-profit companies aimed at DR screening, such as Intelligent Retinal Imaging Systems, IDx, and Eyenuk, are increasing, especially since IDx invented IDx-DR, the first and only Food and Drug Administration (FDA)-approved AI system for the autonomous detection of DR. Historically, low and inconsistent reimbursement for telemedicine along with high up-front cost of camera purchase have been some of the barriers to the uptake of teleophthalmology adoption. As a result, most of teleophthalmology in the US was limited previously to research or provided as a community service (DeNomie et al., 2019). However, COVID-19 has prompted increased reimbursement for telemedicine service by Medicare and other insurance companies and increased utilisation of teleophthalmology by eye care specialists, including store-and-forward method, telephone and/or video conferencing, and a hybrid model. This may be only a temporary phenomenon to accommodate the patient volume while practising social distancing during the pandemic, but in some specialties including ophthalmology, implementation of tele-health may have lasting change on delivery of care in the US.
India has over 65 million diabetics with DR prevalence estimated to be around 18% (Rema et al., 2005). With much of the population in rural areas, smartphone-based imaging devices such as Remidio fundus on phone (FOP) camera have been used in teleophthalmology screening, showing the comparable ability with conventional mydriatic fundus cameras. In 2017, 16,226 individuals with diabetes were screened using Remidio FOP camera for DR with 7% of the individuals were suggested for further evaluation and treatment (Rajalakshmi et al., 2015).
China has the world's largest population of adults with diabetes with a prevalence estimated to be around 10%–11% of the population (Gwatidzo and Stewart Williams, 2017; Yang et al., 2010; Wang et al., 2017), creating a high burden of DR (Song et al., 2018). As the population ages and the prevalence of DM increases, DR is becoming one of the most common blinding disorders in China (Jonas et al., 2017). Hence, efficient DR screening strategies have been explored and implemented, in line with the national Healthy China 2030 strategy, to support the “prevention first” principle and early screening for chronic diseases (Chen et al., 2019).
The large-scale telemedicine-enabled program of Lifeline Express (LEX) has carried out free DR screening nationwide at 29 DR screening centres across China (Wong et al., 2018). In addition to the acquisition of fundus images in mobile vans or primary care institutions, smartphones are also used to provide electronic medical reports of fundus images via WeChat, the most popular messenger app in China. Between April 2014 and December 2016, 34,506 patients with diabetes underwent screening and 27.2% (9396) were reported to have DR (Wong et al., 2018).
4.1.2. Automated DR screening for telemedicine
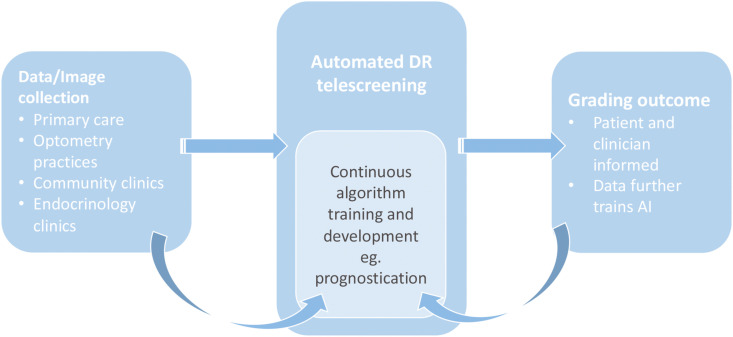
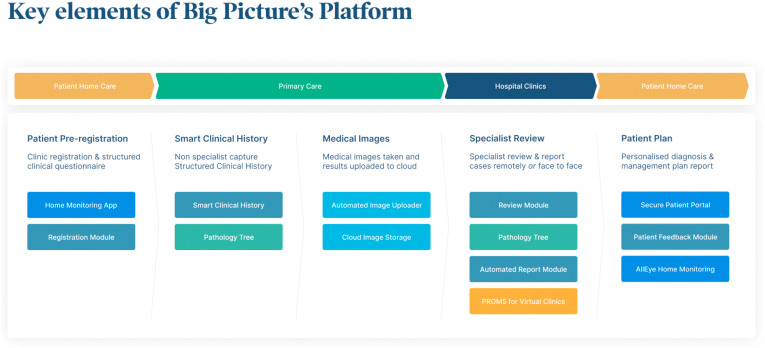
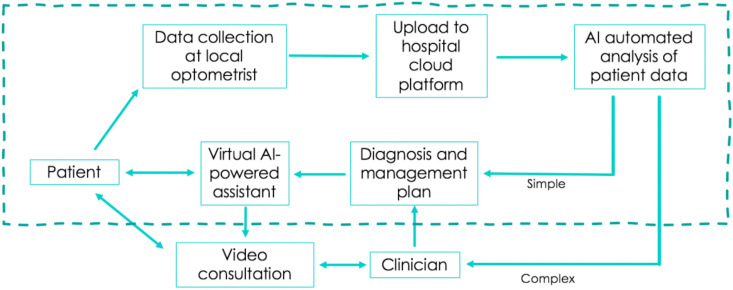
The adoption of DL in telescreening for DR makes it possible for non-eye health professionals to perform DR screening and make recommendations without the help of ophthalmologists (Cheung et al., 2019; Balyen and Peto, 2019; Schmidt-Erfurth et al., 2018a, Schmidt-Erfurth et al., 2018b). Fig. 3 illustrates how AI can be integrated into DR screening programmes, whilst also utilising the data generated during the screening process to aide in the further development of existing and new algorithms. Fig. 4 demonstrates the electronic systems that are already in place to streamline the management of a patient's journey, with virtual integration of each step of their journey from registration to EHR to management of images. Myriad DL programmes are being developed for DR diagnosis, with several models evolving into clinical adoption. Table 2 provides a summary of all the artificial intelligence systems with the respective training datasets and diagnostic performance for different retinal diseases using fundus photographs.
Fig. 3.
The role of AI in supporting tele-screening in DR, and the reciprocal contribution by tele-screening processes in improving AI algorithm performance and development of new capabilities.
Fig. 4.
Streamlined patient journey with a single platform. Courtesy of Big Picture Eye Health.
Table 2.
The summary of the artificial intelligence systems with the respective training datasets and diagnostic performance for different retinal diseases using fundus photographs.
| AI systems | Year | Disease | Imaging modality | Race | Clinical Validation | Independent testing datasets (retinal images) | AUC | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic Retinopathy | ||||||||||
| Abramoff et al. (Abramoff et al. 2016) | 2016 | Referable DR (worse than any DR) | Fundus photo | White | Messidor-2 | 874 | 0.98 | 96.80% | 87.00% | |
| Gulshan et al. (Gulshan et al. 2016) | 2016 | Referable DR | Fundus photo | White | EyePACS-1 | 9963 | 0.991 | 97.50% | 93.40% | |
| White | Messidor-2 | 1748 | 0.94 | 96.10% | 93.90% | |||||
| Gargeya and Leng (Gargeya and Leng 2017) | 2017 | Referable DR | Fundus photo | White | Messidor-2 | – | 0.99 | – | – | |
| White | E-Ophtha | – | 0.96 | – | – | |||||
| Ting et al. (Ting et al. 2017) | 2017 | Referable DR | Fundus photo | Asians (Chinese, Malays, Indians and others) | SiDRP 14-15 | 35,948 | 0.94 | 90.50% | 91.60% | |
| Chinese | Guangdong | 15,798 | 0.949 | 98.70% | 81.60% | |||||
| Malay | SIMES | 3052 | 0.889 | 97.10% | 82% | |||||
| Indians | SINDI | 4512 | 0.917 | 99.30% | 73.30% | |||||
| Chinese | SCES | 1936 | 0.919 | 100% | 76.30% | |||||
| Chinese | BES | 1052 | 0.929 | 94.40% | 88.50% | |||||
| African | AFEDS | 1968 | 0.98 | 98.80% | 86.50% | |||||
| White | RVEEH | 2302 | 0.983 | 98.90% | 92.20% | |||||
| Hispanics | Mexican | 1172 | 0.95 | 91.80% | 84.80% | |||||
| Chinese | CUHK | 1254 | 0.948 | 99.30% | 83.10% | |||||
| Chinese | HKU | 7706 | 0.964 | 100% | 81.30% | |||||
| Krause et a l(Krause et al. 2018) | 2018 | Referable DR | Fundus photo | White | EyePACS-2* | – | 0.986 | 97.10% | 92.3%* | |
| Abramoff et al. (Abràmoff et al. 2018) | 2018 | Referable DR (worse than any DR) | Fundus photo | White | FDA Pivotal Trial | 892 | – | 87.20% | 90.70% | |
| Li et al. (Li et al. 2018) | 2018 | Referable DR | Fundus photo | Chinese | ZhongShan | 8000 | 0.989 | 97.00% | 91.40% | |
| NIEHS | 7181 | 0.955 | 92.50% | 98.50% | ||||||
| SIMES | 15,679 | |||||||||
| AusDiab | 12,341 | |||||||||
| Ruamviboonsuk et a l(Ruamviboonsuk et al. 2019) | 2019 | Referable DR | Fundus photo | Thai | Thailand Diabetes Registry | 25,326 | 0.987 | 96.8% | 95.6% | |
| Gulshan et al. (Gulshan et al. 2019) | 2019 | Referable DR | Fundus photo | Indian | Sankara | 3779 | 0.980 | 92.1% | 95.2% | |
| Aravind | 1983 | 0.963 | 88.9% | 92.2% | ||||||
| Glaucoma | ||||||||||
| Li et al. (Li et al. 2018) | 2018 | CDR³0.7 and glaucomatous changes | Fundus photo | Chinese | LabelMe | 8000 | 0.986 | 95.60% | 92.00% | |
| Ting et al. (Ting et al. 2017) | 2017 | CDR³0.8 and glaucomatous changes | Fundus photo | Chinese, Malay, Indian and others | SiDRP 14-15 | 71,896 | 0.942 | 96.40% | 87.20% | |
| Shibata et a l(Shibata et al. 2018) | 2018 | Glaucoma | Fundus photo | Japanese | Matsue Red Cross Hospital | 110 | 0.965 | NR | NR | |
|
Masumoto et al. (Masumoto et al. 2018b) |
2018 |
Glaucoma |
Wide-field fundus photo |
Japanese |
Tsukazaki Hospital |
282 |
0.872 |
81.30% |
80.20% |
|
| AMD | ||||||||||
| Burlina et al. (Burlina et al. 2017) | 2017 | Referable AMD | Fundus photo | White | AREDS 1 | 26,764 images (AREDS 2) | 0.94–0.96 | 71.00–88.40% | 91.40–94.10% | |
| Ting et a l(Ting et al. 2017) | 2017 | Referable AMD | Fundus photo | Chinese, Malay, Indian and others | SiDRP 14-15 | 71,896 | 0.931 | 93.20% | 88.70% | |
|
Grassmann et al. (Grassmann et al. 2018) |
2018 |
Any AMD |
Fundus photo |
White |
AREDS 1 |
33,886 |
100% (Late Stage AMD) |
96.5% (Late Stage AMD) |
||
| ROP | ||||||||||
| Brown et al. (Brown et al. 2018) | 2018 | ROP | Retcam photo | White | i-ROP | 100 | 93.0% (plus) | 94% (plus) | ||
| 100% (pre-plus/worse) | 94% (pre-plus/worse) | |||||||||
CDR cup-disc ratio, AUC area under the curve, U unknow
Prior to the DL era, the iGradingM algorithm could perform ‘disease/no disease’ grading for DR, with a very high detection rate of 97.3% for referable DR (Philip et al., 2007), and with a sensitivity of 97.4–99.1% and specificity of 98.3–99.3% (Goatman et al., 2011). Subsequent studies suggest that iGrading (version 1.1 by Medalytix), as well as other commercial automated grading systems including Retmarker (version 0.8.2. 2014/02/10 by Retmarker Ltd, formerly Critical-Health) (Tufail et al., 2016) are comparable to that of trained graders. Further study based on retinal images from 20,258 patients in routine annual DR screening showed 85% sensitivity by Retmarker and 94% by EyeArt, indicating the potential to replace one or more steps of current DR screening programmes (Tufail et al., 2017).
Since 2016, many groups have published on the application of DL for DR screening (Table 2) (Gulshan et al., 2016; Ting et al., 2017; Gargeya and Leng, 2017). In April 2018, the DL algorithm developed by Abramoff et al., called IDx-DR, received the first approval from the FDA for detecting more-than-mild DR in adults who have DM using DL without clinician-assisted interpretation. The software was tested in a pre-registered US FDA prospective clinical trial in 10 primary practice sites throughout the USA. Abramoff et al., (2018) reported the first DL-enhanced algorithm for referable DR and VTDR, with a sensitivity of 87.2% and a specificity of 90.7% in detection of referable DR (worse than mild DR) with a gradability rate of 96.1%. Multiple other DL systems have been developed with high sensitivity and specificity for DR screening, and these are summarized in Table 2.
More recently, Li et al. developed a DL system to automatically detect the most common sign of DR, retinal haemorrhages, based on 16,827 ultra-widefield fundus (UWF) images (11,339 individuals) from the Chinese Medical Alliance for Artificial Intelligence (CMAAI) (Li et al., 2020). With both sensitivities and specificities over 96% in various settings, this system has significant potential to detect more DR patients, given that the retina view scope of UWF images is five times larger than that of tradition fundus images (Nagiel et al., 2016).
It has been shown that AI could potentially grade DR for epidemiology studies and clinical trials (Ting et al., 2019). With the continued improvement AI diagnostic performances in various specialties, AI could potentially reduce the need of professional graders in reading centers with clinicians adopting a supervisory role.
4.2. Retinopathy of prematurity
Retinopathy of prematurity (ROP) is a vasoproliferative disease of the premature retina which can progress to tractional retinal detachment, that can result in complete visual loss. Every year, more than 30,000 children lose their sight from ROP worldwide, and the prevalence is still increasing (Gilbert, 2007; Hellstrom et al., 2013). Despite being a leading cause of childhood blindness globally, visual loss is mostly preventable with timely treatment (Cryotherapy for Retinopathy of Prematurity Cooperative, 2001; Early Treatment For Retinopathy Of Prematurity Cooperative, 2003). Numerous clinical studies had shown that timely ablation of the peripheral avascular retina using laser photocoagulation or cryotherapy reduced unfavorable structural and visual outcomes significantly (McNamara et al., 1991; Hunter and Repka, 1993; Cryotherapy for Retinopathy of Prematurity Cooperative 2001; Laser ROP Study Group, 1994). In recent years, anti-VEGF treatment has also extended its application to ROP, showing significant structural and visual improvement. Therefore, regular screening for early detection and timely delivery of treatment are essential for visual preservation in at-risk infants.
4.2.1. Tele-screening of ROP
Screening systems differ across the world, reflecting not only the different healthcare systems, but also the region-specific distribution of ROP risk. A worldwide survey showed a region-specific distribution of ROP risk, with Eastern Europe (37.4%) and Latin America (23.9%) at the highest ranks (Gilbert, 2008, 2007; Hellstrom et al., 2013). The imbalance between increased survival of preterm babies due to advanced neonatal care, the paucity of sophisticated titratable oxygen delivery, and the lack ROP monitoring from experienced persons are key contributory factors. A similar situation also occurred in India and China, where the highest numbers of preterm babies are born (Chen and Li, 2006; Howson et al., 2013; Dutta et al., 2016).
Once patients have been diagnosed, there remains variation in the management of ROP due to the subjective nature of the diagnosis (Chiang et al., 2007; Wallace et al., 2008). The key retinal biomarker for treatment is “plus disease”, defined as venous dilatation and arteriolar tortuosity within the posterior retinal vessels. Another relevant feature of ROP is pre-plus disease, defined as vascular abnormality less than plus disease but more than normal, and this requires close observation (International Committee for the Classification of Retinopathy of 2005).
Over the past decade, wide-field digital imaging (WFDI) systems have been modified to evaluate pediatric retina patients. This has enhanced the ability for children with ROP to be screened through telemedicine methods. These imaging systems also allow for documentation of retinal findings and have the potential to improve diagnostic accuracy. In 2000, a store-and-forward telemedicine system using the WFDI system was successfully trialed, with trained nurses capturing WFDI and sending the images to experienced ophthalmologists (Schwartz et al., 2000). Since then, telemedicine for ROP has been vigorously evaluated for its diagnostic accuracy and reliability. The diagnostic accuracy of any ROP from multiple studies showed favorable results with sensitivity from 0.46 to 0.86 and specificity from 0.86 to 1.00 (Chiang et al. 2006, 2007; Dhaliwal et al., 2009; Roth et al., 2001; Yen et al., 2000).
Telemedicine has been shown to be useful in screening for ROP while being fast, cost-effective and having minimal impact on systemic status with several clinical studies reporting favorable long-term results (Brady, D'Amico, and Campbell, 2020). Additionally it is superior to indirect ophthalmoscopy in terms of objective documentation of serial retinal images to inform identify disease progression, and facilitates second opinions, education, and research (Chiang et al., 2012; Isaac et al., 2018; Richter et al., 2009; Shah et al., 2018; Brady, D'Amico, and Campbell, 2020).
Recently, smartphone-based fundus imaging (SBFI) has been introduced for screening purposes of ROP and showed competitive outcomes when compared with conventional contact fundus imaging (Goyal et al., 2019; Patel et al., 2019; Wintergerst et al., 2019). Novel imaging devices in combination with AI technology, as seen with the work by the Imaging and Informatics in Retinopathy of Prematurity (i-ROP) Research Consortium, could facilitate cost-effective telemedicine-based ROP screening in low-resource settings. The need for exacting protocols on image acquisition and image interpretation has been highlighted by many experts as a key priority (Abdul Aziz, Isaac, and Tehrani 2014).
4.2.2. Global ROP screening programs
While the US is a developed country with advanced medical services, it still has challenges with having enough healthcare providers to screen and treat children at risk for developing ROP. Only 11% of ophthalmologists in the United States were able to perform ROP screening using binocular indirect ophthalmoscopy, and even less (6%) are able to perform laser photocoagulation for ROP (Trese, 2008; Kemper et al., 2008). Therefore, a number of groups in the US are actively participating in telemedicine for ROP screening. In 2012, the American Academy of Ophthalmology (AAO) published an Ophthalmic Technology Assessment (OTA) on the use of WFDI for ROP screening, with a favorable performance of WDFI by reviewing 450 cases screened with telemedicine. In 2015, the American Academy of Pediatrics and the AAO released a joint systematic review of telemedicine for ROP screening for consensus. The guidelines recommend serial binocular indirect ophthalmoscopy (BIO) examinations but allowed digital imaging with at least 1 ROP examination before treatment or discharging infants from further ROP monitoring. Currently, different programs are provided depending on the hospital's situation. An analysis of survey responses from the medical directors of 847 level III NICUs reported 21% of the NICUs used retinal imaging devices for ROP screening.
The Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP) is a well-established ROP telemedicine program initiated by Dr Darius Moshfeghi with the goal of identifying at-risk infants for ROP throughout the San Francisco Bay Area (Murakami et al., 2010). The SUNDROP was started in 2005 to overcome a shortage of ROP experts and first provide screening at a level-2 neonatal intensive care unit (NICU) which then expanded to 6 NICUs in the area. The SUNDROP study reported 6-year results of ROP screening from 6 NICUs, which involved 26,970 images from 1216 eyes showing that 3.6% of examined premature infants required treatment. The retinal images were taken by trained nurses and uploaded for remote evaluation by an experienced ophthalmologist. The study showed 100% sensitivity, 99.8% specificity, 93.8% positive predictive value, and 100% negative predictive value for the detection of treatment-warranted ROP (Fijalkowski et al., 2014).
The Imaging & Informatics in Retinopathy of Prematurity (i-ROP) Research Consortium led by the Casey Eye Institute of the Oregon Health & Science University (OHSU) is an international group of 12 academic centers who are working together to develop better methods for diagnosing, understanding, and treating ROP through computer-based image analysis, genetic analysis, and biomedical informatics analysis. They have raised questions about the subjective nature of the definition of “plus disease” or “pre-plus disease” and the role of individual clinical judgment in cases that are not precisely covered by previously published treatment guidelines (Gupta et al., 2016). To address these challenges in ROP diagnosis, they developed a computer-based image analysis system that demonstrated 95% diagnostic accuracy, which was comparable with that of 11 expert clinicians (79–99%) (Campbell et al., 2016). Based on their findings, they proposed a continuous severity score for vascular abnormalities by ranking disease severity to enhance inter-expert agreement (Kalpathy-Cramer et al., 2016).
India has the highest number of premature births in the world, with more than 3.5 million premature infants born each year (Vinekar et al., 2019). There are a number of successful ROP telemedicine screening programs in India, such as the Karnataka Internet Assisted Diagnosis for Retinopathy of Prematurity (KIDROP), and the Retinopathy of Prematurity Eradication-Save Our Sight (ROPE-SOS) program through Aravind Eye Hospital (AEH), Coimbatore (Valikodath et al., 2018). These programs and the Indian ROP society have made critical improvements in educating families, pediatricians and other healthcare providers on the importance of ROP management. As a result, the number of infants screened has increased significantly throughout the country and sustainable ROP screening and treatment programs have been established.
In Chile, Retcam and telemedicine-based ROP screening has been established with an expert review guideline under government support (Ossandon et al., 2018). At least five images were captured in each eye; one image demonstrating the posterior pole and the other four of each of the fundus quadrants. Images were stored and transmitted via a secured inter-hospital virtual private network to a central reading center, where they were analyzed using the RetCam review station software by two independent ROP experts. Results were sent by secured email to the clinician on the same day. They used telemedicine in all screening and evaluations. Clinical examination using BIO was done only before providing treatment to confirm that treatment is required. The agreement rate was reported to be 98% between imaging and clinical judgment of cases requiring treatment.
Argentina scaled up services for ROP significantly in a short time due to the efforts of dedicated professionals, the Ministry of Health, a national ROP committee, international cooperation, and external funding (Hariharan et al., 2018). The ROP Argentina Group, an advisory body for the National Board of Maternity, Childhood, and Adolescence has coordinated the national program for the prevention of blindness in childhood by ROP since 2010. The telemedicine-based ROP screening program started with 14 facilities and reached 98 facilities from all over the country in 2016. A total of 227,138 births, which accounted for 29.4% of all births and 51.3% of births in public sector facilities were evaluated using telemedicine. It was encouraging that when the incidence of severe ROP and unusual cases were found to be high at specific facilities, changes to modify oxygen management to mitigate ROP took place (Alda et al., 2018). A direct comparison from multiple facilities and dissemination of the results would facilitate the improvement of medical care.
Many of the specialists who were utilising telemedicine for ROP screening prior to COVID-19 expressed the benefit of having this system in place during the pandemic, since it allowed them to screen these infants while limiting the number of people examining, thus reducing the potential viral exposure to this vulnerable population.
4.2.3. AI in ROP
The paucity of experienced ROP specialists necessitates the application of AI. In 2020, the first AI system for ROP, which was developed by the imaging and informatics for ROP (i-ROP) consortium, received breakthrough status by the FDA. This DL algorithm (DeepROP) has been incorporated into a system termed “i-ROP DL”. This system is a DL based diagnostic algorithm explicitly developed for the detection of plus disease (Brown et al., 2018) or diagnostic categories of ROP (Redd et al., 2018) from WFDI. The i-ROP consortium evaluated the accuracy and sensitivity of telemedicine grading of dilated fundus imaging versus binocular indirect ophthalmoscopy by comparing it with a consensus reference standard diagnosis (Biten et al., 2018). I-ROP DL has been shown to have very high accuracy for detecting plus disease from wide-angle posterior pole retinal images and with robust sensitivities and specificities for detecting both plus and pre-plus disease, it may even perform better than expert human examiners in detecting plus disease (Brown et al., 2018).
4.3. Glaucoma
Glaucoma is characterised by structural changes in the optic nerve head (ONH), variably raised intraocular pressure (IOP), retinal ganglion cell death, loss of visual field (VF) and eventual vision loss (Weinreb et al., 2014) and is the main cause of irreversible blindness, affecting ~64.3 million patients aged from 40 to 80 years worldwide (Stevens et al., 2013; Bourne et al., 2013; Tham et al., 2014). This number is expected to increase to 112 million by 2040 (Tham et al., 2014). However, most cases of chronic glaucoma may be asymptomatic early on, which increases the difficulty in diagnosis. When patients seek medical advice due to poor visual acuity related to glaucoma, the disease is often in its late stages. Care costs increase 4-fold when late disease is managed (Lee et al., 2006) leading to significant financial burden in most countries. Although most irreversible loss of vision can be prevented by timely diagnosis and treatment (Tatham et al., 2014; Tatham et al., 2015), unlike other eye diseases, one major challenge is to identify the large number of undiagnosed patients. The limited numbers of screening programs is a reflection that the disease does not fulfil all the criteria for effective population screening, particularly in early stage disease where an unacceptably high false positive detection rate exists (Samples, 2010).
Unlike other diseases such as DR or ROP, glaucoma is diagnosed according to consensus findings from intraocular pressure (IOP) measurements, fundus photographs, VF exams and OCT, rather than by detecting specific ocular biomarkers (Jonas et al., 2017). While fundus photographs are a mainstay in glaucoma diagnosis because they allow for an assessment of the optic cup to disc ratio (CDR), neuroretinal rim integrity, peripapillary atrophy and retinal nerve fibre layer (RNFL) defects (Haleem et al., 2013), early signs are not easily recognized. The inability to establish quantitative ONH parameters for the detection of early disease relates to the fact that optic nerves come in different sizes and shapes, while the number of axons coursing through the ONH is thought to be relatively constant. Similarly, independent of glaucoma, the distribution of RNFL thickness will vary considerably depending on the refractive status of the eye. Thus, detection of early disease requires experienced ophthalmologists curtailing the cost-effectiveness in glaucoma screening (Fleming et al., 2005; Moyer et al., 2013; Miller et al., 2017; Pizzi et al., 2018).
Another commonly used standard for glaucoma assessment is VF testing. VF testing represents a read out of the entire visual system from the pre-corneal tear film to the occipital lobes. Given the highly organized topographic structure of the visual system, particularly the optic nerve territory, it is possible to use VF test findings to infer whether glaucomatous optic nerve damage is present. There are various platforms to measure VFs in clinical practice, such as the Humphrey Visual Field Analyzer and Oculus Field Analyzer. The subjectivity and variability of the procedure contribute to the unreliability of the results (Russell et al., 2012; Wu and Medeiros, 2018), and it may be difficult for non-specialized ophthalmologists to decipher a VF report. Furthermore, since generating quantitative information about the RNFL from fundus inspection is particularly challenging, OCT imaging has become a critical modality used in the evaluation of structural damage of ONH and parapapillary RNFL thickness, which is associated with the diagnosis and rate of glaucoma progression. Numerous platforms for automated assessment of the RNFL and ONH are also available but OCT reports can also be challenging to decipher. Many OCT artefacts that can influence OCT interpretation and much of the collected data could be re-organized in ways to make them useful in glaucoma management.
Considering that widespread screening for glaucoma is costly and time-consuming, and the accuracy of diagnosis is limited according to the experience of ophthalmologists (Haleem et al., 2013), advanced tools to make better use of information are mandated to ensure the effective detection of suspicious findings.
4.3.1. Tele-glaucoma and virtual clinic
One currently available advanced technology to address glaucoma screening is telemedicine, which may effectively detect glaucomatous changes in patients, especially from fundus photographs (Arora et al., 2014). Through a combination of fundus photography, IOP measurements and VF screening, teleophthalmology can increase the sensitivity of glaucoma screenings in community or primary healthcare settings and provide healthcare access to patients in resource-depleted areas (Kumar et al. 2006, 2007; Maa et al., 2014), and recent guidance on the adoption of telemedicine in glaucoma has been developed (Gan et al., 2020).
Virtual clinics are increasingly adopted in the UK to facilitate remote glaucoma management. Virtual clinics use electronic patient records collected by technicians and consultants in community clinics or from a mobile clinic facility, and then delivers feedback on the decisions made by ophthalmologists for patients being examined remotely (Wright and Diamond, 2015). As the largest tele-glaucoma study to date, this program reported an 87% level of agreement on disease stratification status between optometrists and specialists. In the UK, around 50% of the Hospital Eye Service units are using glaucoma virtual clinics (Court and Austin, 2015; Kotecha et al., 2015), and their rate efficiency, patient safety and acceptability have been shown to be at least equivalent to that of standard care (Gunn et al., 2018; Clarke et al., 2017). While virtual clinics may have limitations in detecting unstable diseases, they may serve important functions for more slowly progressive diseases (Clarke et al., 2017). Glaucoma is typically slowly progressive and as such, telemedicine strategies may facilitate periodic monitoring, timely referral and screening (Sreelatha and Ramesh, 2016).
In the past 20 years, a large number of pilot teleophthalmology programs were carried out around the world, demonstrating the feasibility in the detection and management of glaucoma (Labiris et al., 2003; Owsley et al., 2015; Rathi et al., 2017; Zhao et al., 2017). For example, the Wills Eye Glaucoma Research Center designed a 5-year telemedicine screening program, the Philadelphia Telemedicine Glaucoma Detection and Follow-up Study (Hark et al., 2017). This program illustrated how to improve access to eye care and reduce glaucoma-related vision loss in high-risk populations (Waisbourd et al., 2016; Hark et al., 2016). Another telemedicine glaucoma program in Northern Canada at University of Alberta relied on real-time consultations with glaucoma specialists via VoIP (Voice-over Internet Protocol) in primary eye care clinics (Kassam et al., 2013). Overall, several studies reported that about half of the examined patients had favorable attitudes towards such programs (Gagnon et al., 2004; Valikodath et al., 2017; Rhodes et al., 2019), with positive implications for further improvement.
In China, where more than 90% of glaucoma may be undiagnosed (Song et al., 2011; Liang et al., 2011), telemedicine-based public health care delivery in ophthalmology has been adopted since 2012 (Xu et al., 2012). This population-based public health care project was designed to screen all elderly people (age 55–85 years) of the rural areas. Based on fundus images, 1606 of 37,281 (4.31%) participants were found to have glaucoma. Moreover, a novel teleophthalmology system was developed and centered at Zhongshan Ophthalmic Center, linking with 10 rural hospitals in Guangdong province (Xiao et al., 2017). This integrated system combines colour fundus imaging, cloud-based web application and tablet applications for providing glaucoma and DR grading, comprehensive eye examination, eye disease diagnosis and treatment. In addition, the system automatically sends mobile messages to patients reminding them about upcoming visits, which can improve their medical compliance. Moreover, from a quality control perspective, educational modules within the system train image graders and rural doctors regarding fundus image grading on glaucoma and DR.
4.3.2. AI in glaucoma
AI has fostered new breakthroughs in automated screening for glaucoma, including supervised and unsupervised ML. For glaucomatous colour fundus photo detection, early methods for glaucoma classification focused on segmentation of the optic disc and cup based on the combination of feature extraction techniques and supervised or unsupervised ML (Almazroa et al., 2015), with AUC ranging from 0.792 to 0.887 (Singh et al., 2016; Chakrabarty et al., 2016; Issac et al., 2015; Annan et al., 2016).
Recent DL technologies with predictive learning features that worked directly from the globally labelled images as glaucomatous or not based on clinical consensus reported an AUC ranging from 0.942 to 0.986, avoiding errors in localization and segmentation (Li et al., 2018; Ting et al., 2017). Furthermore, Liu et al., (2019) investigated a DL system and assessed its generalisability in various data sets, reporting similar high sensitivity (82.2%–96.1%) and specificity (70.4%–97.1%). All these studies used monoscopic, two-dimensional colour images and it is unclear whether stereoscopic images may increase the accuracy of diagnosis with DL methods. This is an example where the unexplainable ‘black box’ phenomenon of AI can offer exciting new insights into diseases and disease processes, identifying features that humans have not yet been able to. Li et al. developed a DL system for detecting glaucomatous optic neuropathy based on 48,166 colour fundus photographs, with an AUC of 0.986, sensitivity of 95.6% and specificity of 92.0% (Li et al., 2018). Zheng et al., (2020) developed a DL model for automated detection of glaucoma based on spectral domain OCT images with an AUC of 0.99. Finally, one cannot overstate the innovative machine-to-machine learning approach of Medieros et al. whereby a convoluted neural network (CNN) was trained to learn the average RNFL thickness as determined from OCT platforms from fundus photographs35. Table 4 includes AI systems with their respective training datasets and diagnostic performance for optic disc pathology using OCT. The widespread availability of such an algorithm could extend the utility of fundus images acquired in non-ophthalmic centres.
Table 4.
Artificial intelligence systems with their respective training datasets and diagnostic performance for macula and optic disc pathology using OCT.
| AI systems | Year | Disease | Imaging modality | Race | Clinical Validation | Independent testing datasets (retinal images) | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|
| Macula OCT | |||||||||
| Lee et al. (Lee et al. 2017) | 2017 | Exudative AMD | Spectralis OCT | White | Clinic-based | 20,613 | 0.928 | 84.60% | 91.50% |
| Treder et al. (Treder, Lauermann, and Eter 2018) | 2018 | Exudative AMD | Spectralis OCT | White | Clinic-based | 100 | NR | 92% | 96% |
| De Fauw et al. (De Fauw et al. 2018) | 2018 | Urgent, semi-urgent, routine, and observation only | Topcon OCT | White | Clinic-based | 997 | 0.992 (urgent referral) | Accuracy: 94.5% | |
| Spectralis OCT | White | 116 | 0.999 (urgent referral) | Accuracy: 96.6% | |||||
|
Schmidt-Erfurth et al. (Schmidt-Erfurth et al. 2018) |
2018 |
AMD (Prediction of visual acuity) |
Spectralis OCT |
White |
Harbor Clinical trial |
614 |
Accuracy: R2 = 0.7 |
||
| Optic nerve OCT | |||||||||
| Ran et al. (Ran et al. 2019) | 2019 | Glaucoma optic neuropathy (GON) | Cirrus OCT | Hong Kong Eye Hospital | 976 (3D) | 0.969 | 89% | 96% | |
| 976 (2D) | 0.921 | 85% | 85% | ||||||
| Prince of Wales | 546 | 0.893 (3D) | 79% | 84% | |||||
| 0.770 (2D) | 72% | 75% | |||||||
| Tuen Mun Eye Center | 267 | 0.897 (3D) | 90% | 79% | |||||
| 0.752 (2D) | 78% | 64% | |||||||
| Byers Eye Institute | 1231 | 0.917 (3D) | 78% | 86% | |||||
| 0.888 (2D) | 84% | 66% | |||||||
| Medeiros et al. (Medeiros et al. 2019) | 2019 | Glaucomatous optic neuropathy (GON) | Optic disc photographs and SD-OCT | White | Duke Glaucoma Repository | 6564 | 0.944 | 76% | 95% |
| 90% | 80% | ||||||||
Compared to optic disc images, VF data are characterised by low dimensionality and high noise, and such datasets could be refined using unsupervised ML algorithms. The two most reported unsupervised algorithms are clustering and component analysis (Hilton et al., 1996; Yousefi et al. 2016, 2018). In 1994, Goldbaum et al. created a two-layer neural network to detect glaucomatous eyes via visual fields and attained a sensitivity of 65% and specificity of 72% (Goldbaum et al., 1994). Further studies similarly demonstrated that ML may perform comparably or better than human experts in the mean deviation, pattern standard deviation and glaucoma hemifield test (Goldbaum et al., 2002; Chan et al., 2002). A back-propagation neural network reported to successfully detect visual field progression with an AUC of 0.92 (Lin et al., 2003). Further advances using algorithms based on CNN showed higher sensitivity and specificity than traditional ML methods. Asaoka et al. developed a neural network to detect pre-perimetric glaucoma with an AUC of 0.92 (Asaoka et al., 2016), and Li et al. achieved an accuracy of 87% in the discovery of glaucomatous visual fields, outperforming ophthalmologists and traditional algorithms (Li et al., 2018).
Extensive studies have been done to detect the progression of the VF. The Bayesian independent component analysis mixture model (Sample et al., 2005) was used in a “change detection using an optimization framework” (Yousefi et al., 2015), and recently, the Gaussian mixture model and expectation (GEM) (Yousefi et al., 2018) showed a significant decrease in the required time to detect the progression in participants, giving a high sensitivity and specificity. Using an alternative form of unsupervised learned termed archetypal analysis, Wang et al., (2019) reported that functional progression could be detected with an accuracy of 0.77, higher than reference standards agreed by three separate glaucoma specialists.
Early studies based on ML control using time-domain OCT showed an accuracy no worse than non-AI analytic methods (Burgansky-Eliash et al., 2005; Huang et al., 2007). The latest OCT technologies, SD-OCT and swept source-OCT, when combined with DL are reported to be more sensitive in the detection of early glaucoma than standard of care (Muhammad et al., 2017; Kermany et al., 2018; Devalla et al., 2018), with a high AUC up to 0.937. Recently, a study depicted a model that detects the RNFL thickness from OCT with an AUC of 0.944 (Medeiros et al., 2019).
Moreover, the mixture of functional and structural outcomes by ML controls was developed. Initially, Brigatti L et al. (Brigatti et al., 1996) combined VF with fundus photography, which had an accuracy of 88% in early detection, better than either of the data that were analyzed separately. Recently, more parameters have been introduced, including OCT RNFL thickness, standard automated perimetry and confocal scanning laser ophthalmoscopy imaging (Bowd et al., 2012), marking improvements in glaucoma detection. Christopher et al. (Christopher et al., 2020) published a DL technique predicting the VF loss from SD OCT scans, showing the potential to lessen the amount of VF testing required.
However, several limitations exist and further studies are required in this area. It can be difficult for DL, and humans, to classify glaucoma in the eyes with less severe disease manifestations or multiple comorbid eye conditions, especially high myopia (Li et al., 2018; Masumoto et al., 2018a), which requires a larger image database. Furthermore, in order to develop a more dependable screening method, other clinical parameters, including IOP, central corneal thickness and glaucoma genetic informativity biomarkers (Craig et al., 2020) should be integrated. Finally, the application and validation of these advanced methods in a real-world screening setting need additional investigations to bolster its support.
4.4. Age-related macular degeneration
Age-related macular degeneration (AMD) is a leading cause of visual loss and legal blindness of elderly persons in the developed world (Friedman et al., 2004). The 15-year cumulative incidence in the United States was 14.3% for early AMD and 3.1% for late AMD (Klein et al., 2007), and it is increasing as the aged population grows. Early AMD is generally asymptomatic; a majority of patients with AMD are unaware of their diagnosis (Gibson, 2012). The AAO recommends routine screening of patients aged 65 years or older every 1–2 years. The development of anti-VEGF treatments at monthly or bi-monthly intervals revolutionized the treatment of wet AMD by improving visual outcomes significantly (Brown et al., 2006; Rosenfeld et al., 2006). However, added clinical burden to individual and health care systems, economic and logistical burdens of frequent intravitreal injections have strained healthcare resources. Although several efforts have made to reduce the clinical burden by modifying injection protocols, such as ‘treat and extend’ (Gupta et al., 2010; Regillo et al., 2008), the number of patient visits by AMD patients continue to increase due to the growth of the aged population and the chronic and relapsing nature of the disease (Day et al., 2011).
The most intractable problem of treating AMD is the frequent and time-consuming appointments requiring review, evaluation and possible subsequent intravitreal injection. Since AMD treatment is determined mainly from the VA and OCT findings, telemedicine could be as useful as face-to-face office consultation. A meta-analysis in 2018 suggested that teleophthalmology for AMD is as effective as face-to-face examination, and potentially increases patient participation in screening (Kawaguchi et al., 2018). Also, a simulation study showed that telemonitoring of high risk AMD patients is cost-effective compared with scheduled examinations alone ($35 663 per quality-adjusted life-year gained) (Wittenborn et al., 2017).
In 2015, the first prospective randomised study to assess the efficacy of telemedicine for both in the initial screening and recurrence monitoring of neovascular AMD was reported in Canada (Li et al., 2015). Best corrected visual acuity, IOP, color fundus photography, and macula OCT were incorporated in a “store and forward” telemedicine model. Those in the telemedicine arm attended a local ophthalmologist who performed the screening, and the data was stored on a database, which was then reviewed electronically by a retina specialist. In those referred for initial screening of neovascular AMD, there was no statistically significant difference in patient waiting times to further diagnostic tests and to treatment. There was also no significant difference in patient satisfaction except for parking issues. In those monitored for recurrence, there was no significant difference in the visual outcome between groups (20/184.8 vs. 20/180.7, p = 0.99).
This “store and forward” model still utilises an ophthalmologist as the initial screener. While a technician can be for initial data acquisition used for screening, telemedicine can be applied further so that initial screening and subsequent monitoring can be remote, out of the clinical setting and into the home.
4.4.1. Home monitoring for AMD
Home monitoring and self-care have taken centre stage in modern medicine. Remote in-home monitoring is currently practiced to monitor acute and chronic diseases such as body temperature to assess an upper respiratory infection, blood pressure for hypertension (Noah et al., 2018), peak-flow lung capacities for chronic obstructive pulmonary disease (Kaptein et al., 2014) and asthma (Gibson et al., 2003; Kaptein et al., 2014), and blood glucose for diabetes (Vas et al., 2017). Several large programmes in recent decades have demonstrated clear effects in the timely detection of a worsening disease status, prompting targeted treatment (Vas et al., 2017; Gibson et al., 2003).
There is clearly a need for home monitoring in conditions such as AMD and diabetic macular oedema (DMO) to identify when there is visual decline and allow for timely management. Moreover, patients who are under stable monitoring may be able to alert their ophthalmologists when they need to be seen, rather than relying on a generic timeline.
The Amsler grid developed by Marc Amsler, a Swiss Ophthalmologist, has become synonymous with home monitoring in ophthalmology (Amsler, 1947). It assesses between 12 and 15 degrees of field served by the macula and is typically used to identify and monitor macular dysfunction (metamorphopsia) from conditions such as AMD, epiretinal membrane, or cystoid macular oedema secondary to conditions like DR, retinal vein occlusion, and uveitis (Kalinowska et al., 2018; Okamoto et al., 2012; Xu et al., 2018). Although digitised versions of the Amsler grid are now available, currently test results are neither recorded nor linked to other clinical parameters such as VA or OCT retinal scans.
Recent years have seen the introduction of several digital strategies to replace the Amsler Grid with initial promising results emerging from preferential hyperacuity perimetry (PHP) in patient self-testing for AMD (Loewenstein et al., 2003). The ForeseeHome™ (Notal Vision, Inc.) is a standalone device which is approved by the FDA and covered by Medicare. The test is a series of dotted lines that appear on the device screen, and patients use the provided mouse to click where a bump appears. Testing of each eye takes 3 min. The system runs on a standalone desktop device with unidirectional flow of information to the company's data centre via a wireless connection when prescribed by the patient's physician. The HOME study, a randomised trial of a home monitoring system designed for early detection of conversion of intermediate to neovascular AMD, demonstrated a smaller loss of vision in the device arm compared to standard care. This study was terminated early by the Data and Safety Monitoring Committee for efficacy (Group et al. 2014). A subsequent study showed that the home device arm had a higher neovascular AMD-detection rate than standard care group (relative risk = 16.0 [95% CI: 8.8–29.3]), resulting in less visual loss from baseline when compared with standard care group (−3 letters vs. −11.5 letters, p = 0.03) (Chew et al., 2016). Notal Vision is developing a cloud-based OCT platform to monitor neovascular AMD. In 2018, the FDA granted breakthrough device designation to Notal Home OCT that uses AI and a ML algorithm to detect retinal fluid changes at home.
The global rise of mobile health (mHealth) industry has led to the natural evolution of home monitoring using mobile or tablet devices. The International Telecommunication Union reports that over 5 billion wireless subscribers exist currently with over 70% of them residing in low- and middle-income countries. Smartphone-based Peek Acuity distance visual acuity tests, conducted in the Nakuru Eye Disease Cohort in central Kenya showed accurate and repeatable measurements compared to gold-standard 5-letter-per-line retro-illuminated logMAR charts (Bastawrous et al., 2015). Peek Acuity follows the standard ETDRS chart design with a tumbling E design. The patient indicates the direction of the arms of the E, and the tester swipe the screen accordingly. Single optotypes within a box are used to limit confusion simulating crowding. Standardized images/settings to counting fingers, hand movements and light perception are available. Peek Acuity Pro is a CE registered class 1 medical device.
For hyperacuity tests, there are currently two FDA-approved medical software applications, myVisiontrack™ and Alleye™ on mobile devices available for vision testing. myVisiontrack™ (mVT) uses a shape discrimination hyperacuity test. A feasibility pilot study on 160 patients with neovascular AMD receiving treatment demonstrated that they were willing and able to comply with daily self-testing (Kaiser et al., 2013). The mVT application was the first United States FDA-approved application (it received clearance on 3/24/13) for monitoring of AMD and DME using a smartphone (Micheletti et al., 2016). The patients touch the shape that looks different to track changes in vision. Based on its strength that is using a smartphone as the primary device, 84.7% on average patients (64% of those patients were older than 75 years) complied with a daily test, and 98.9% complied with weekly test (Kaiser et al., 2013).
The Alleye™ application (Fig. 5 ), which similarly tests hyperacuity, but examines a larger area of the macula (12° compared to 3 degrees of field) has demonstrated its ability to detect neovascular AMD and discriminately classify between dry and wet disease (Schmid et al., 2018, Schmid et al., 2019). To date, there are no longitudinal studies that demonstrate the clinical utility of mHealth based home monitoring applications. Table 5 summarises the aforementioned home monitoring systems.
Fig. 5.
Illustration demonstrating how the Alleye™ application works. Courtesy of Alleye.
Table 5.
Novel techniques in refractive error.
| Publication | Device or system | Year | Country | Number of subjects | Comparison with manifest refraction |
|---|---|---|---|---|---|
| Gaiser H et al. (Gaiser et al. 2013) | A cell phone based refracting device (NETRA-G) | 2013 | England | 27 | Mean absolute error of 0.31 ± 0.37D |
| Ciuffreda KJ et al. (Ciuffreda and Rosenfield 2015) | A handheld, smartphone-based autorefractor (SVOne) | 2015 | U.S. | 50 | No significant difference |
| Wisse RPL et al. (Wisse et al. 2019) | Online Refractive Evaluation Trial | 2019 | Netherlands | 100 | Intraclass correlation coefficient of 0.92 |
| Tan TE et al. (Tan et al. 2019) | A deep learning system based on fundus photographs | 2019 | Singapore | 15,876 | Mean absolute error of 1.20D |
| Varadarajan AV et al. (Varadarajan et al. 2018) | A deep learning algorithm based on fundus photographs | 2018 | England | 226,870 | Mean absolute error of 0.56D |
Further clinical validation is required for mHealth based home monitoring tests in ophthalmology. The promise of which has implications for the management of other chronic eye diseases, such as DR and glaucoma, will allow greater patient autonomy and may improve their commitment to ongoing treatment which have been demonstrated in other diseases (Fischer et al., 2012). In addition, home monitoring such as home OCT may allow more individualized treatment interval, which may prove to be important during pandemic as well as in the future to limit in-person visit only when needed.
4.4.2. Established tele-screening and monitoring in AMD
The use of telemedicine for AMD in the United States has centered on AMD screening and remote-monitoring systems with some utilising artificial intelligence applications but as yet there are no large-scale programs for either screening or monitoring of AMD (Brady and Garg, 2020). There are unique challenges to the screening and monitoring of AMD with lack of consensus on the suitability of the disease for population screening, and the need for OCTs rather than simple fundus photographs as used in DR screening and AI algorithms (Brady and Garg, 2020). The Mayo Clinic established a telemedicine model for the treatment of patients with neovascular AMD who require intravitreal injection. The Mayo Clinic Health System includes local ophthalmologists with integrated EMR and imaging systems. Patients were treated with an initial course of 3–4 monthly intravitreal injections, with the interval between injections gradually extended up to every 12 weeks. Patients were given the option to follow-up locally with their home ophthalmologist for the management of their wet AMD under the direction of the Mayo Clinic retina specialist. Using OCT data and the local ophthalmologist's eye examination (clinical visit record including VA, IOP, and dilated fundus examination) performed at a local site, the Mayo Clinic retinal specialist made their recommendation for adjunctive anti-VEGF injections. The anti-VEGF injections were performed on the day of the examination or within 1 week at the local clinic. Data from 83 eyes of 59 patients with wet AMD who were followed up using an e-consultation system demonstrated that 68.5% of intravitreal injections were performed with the local ophthalmologist, and only 2.5% of the e-consultations recommended a return to the Mayo clinic for an in-person examination (Starr et al., 2019).
In the UK, non-doctor led virtual AMD clinics have been piloted and adopted since 2012 to address the increased needs for follow-up appointments of the patient with AMD (Tsaousis et al., 2016). The virtual clinic is capable of performing visual acuity tests and OCT scans and offers up to two consecutive follow-up visits. Accordingly, the virtual clinics accounted for approximately 40% of AMD service appointments. With the introduction of the virtual clinics, patients were followed up with a mean of 5.3 weeks compared to 6.9 weeks in the period of conventional clinics. The percentage of patients with mean VA improvement >15 letters was higher in patients monitored in the virtual visit compared with conventional group: 6.9% pre-virtual clinics compared to 23.1% with the virtual clinics, although p-values were not reported. While the number of appointments increased with virtual clinics, less time was needed during the virtual appointments. Although the numbers of injections were comparable, there was significant reduction in the time spent at each appointment from 71 min to 47 min (p < 0.001). This virtual service model can present benefits over the current system given potential improved patient visual and efficiency outcomes.
Telemedicine screening of AMD with a handheld portable nonmydriatic fundus camera is a newly described technique that can offer low-cost and effective screening, especially in the place with personnel shortages and limited photographic equipment (Jin et al., 2017). Combined with a WIFI transmission system, the images from portable fundus camera are transmitted from referral centres to the image reading board that is comprised of retinal specialists. After analyses, the diagnoses and comments are transmitted back to the referral centres. In addition, employment of AI with fundus images is another efficient method to screen for AMD. Keel et al., (2019) analyzed 56,113 retinal images to develop a DL system for neovascular AMD detection and validated this system using an additional 86,162 images. The DL system showed robust performance for AMD detection, with an AUC, sensitivity and specificity of 0.995, 96.7%, and 96.4%, respectively. Table 4 includes a summary of AI systems with their respective training datasets and diagnostic performance for macula pathology using OCT.
4.5. Myopia
Refractive error is a key public health concern with more than 650 million people suffering from insufficient or no refractive correction globally (Global Burden of Disease Study, 2015) with the incidence of myopia increasing and poised to escalate further with urbanization and higher literacy rates (Pan et al., 2012). According to the WHO, uncorrected refractive errors account for 43% of all visual impairments (Snellen Acuity <6/18-3/60) in 2010, causing 250 billion US dollars in loss of productivity (Pascolini and Mariotti, 2012; Smith et al., 2009; Fricke et al., 2012). Adding to this, the optometrist to population ratio is 1:10,000 in high-income countries and 1:600,000 in low and middle-income countries (Di Stefano, 2001).
To evaluate refractive error, traditional visual acuity examination is time-consuming, and requires the availability of equipment, and examiners skilled in the art of prescribing spectacles. The procedure is also challenging for people with difficulty in expressing themselves, such as young children, the elderly, and patients with verbal communication disabilities. Moreover, the equipment for prescribing, notably the lenses required, is costly (Amirsolaimani et al., 2017). With the development of more automated approaches, automatic refractive error measurements become more widely used in large scale screening. However, limitations still exist like the need of costly investments for its equipment as well as the hiring of experienced examiners. Consequently, economic implications due to incorrect dispensing remain high even in developed countries (Vitale et al., 2006). Providing good quality refraction services acceptable to the general population is greatly needed.
While myopia alone increases the risk posterior segment complications, these risks are notably increased in pathologic myopia (PM) when potentially blinding posterior segment pathological changes appear as a result of the globe elongation (Grossniklaus and Green, 1992). The diagnosis of PM, defined as peripapillary atrophy, myopic maculopathy, peripheral retinal breaks, generally requires a complete examination that includes assessment of the visual acuity and visual field and color fundus photograph acquisition tasks that are labor intensive and skill-dependent. Although some PM manifestations are not treatable, myopic choroidal neovascularization occur in 11% of PM patients and is a complication that can benefit from early identification and treatment (Hayashi et al., 2010). Thus, there is clearly a need for a sustainable method of monitoring PM eyes to reduce blinding complications, especially given that many PM patients are young or middle aged.
4.5.1. Tele-myopia
The focus of tele-myopia has been on to prediction of refractive error from easily obtainable and consistent methods proven in other disease; namely, using the acquisition of fundus photographs. To be able to accurate define refractive error to enable a prescription that is acceptable to the patient would be a significant leap forward in solving the burden of refractive error.
Several advanced techniques that assess refractive error accurately have been developed, and Table 5 provides a summary of some novel techniques in refractive error assessment. DL algorithms have made it possible to predict refractive error accurately using fundus photographs, a feat that has proved impossible for ophthalmologists to perform, where only the spherical component can be predicted (Varadarajan et al., 2018). Algorithms are also able to detect PM from fundus photographs (Tan et al., 2009). The combination of fundus images and demographic or clinical data may further improve prediction accuracy (Cheng et al., 2012; Zhang et al., 2013). Recently, Lin et al., (2018) developed a model to predict the onset of high myopia at specific time points among school-aged teenagers, with an area under the AUC ranged from 0.801 to 0.837 in 8 years.
However, limitations are still present in these novel technologies in the assessment of refractive error. Visual impairment caused by other eye diseases, such as cataracts, glaucoma and retinal diseases cannot be detected accurately, and the measure of refractive error is consequently unreliable. Additionally, subjective factors such as patient fatigue impact output. Further studies may be required to improve the test-retest reproducibility as well as the consistency.
The Massachusetts Institute of Technology developed the Near Eye Tool for Refractive Assessment (NETRA), which may provide an interactive and inexpensive screening method to estimate the refractive error using mobile phones (Gaiser et al., 2013). This portable device demonstrated comparable accuracy with marketed autorefractors and costed only $30 for production, providing a reliable and cost-effective tool for refractive error screening, especially in areas without trained optometrists (Bastawrous A et al., 2012). However, its accuracy requires further study encompassing a broader range of spectacle prescriptions. Another smartphone-based autorefractor is SVOne, a portable Hartmann-Shack wavefront aberrometer developed by Smart Vision Labs in New York (Ciuffreda and Rosenfield, 2015). It is reported to have the potential to replace the standard optometric examination with comparable performance and features retinoscopy, subjective refraction, and two commercially available autorefractors. The Mayo Clinic ophthalmology department developed the Jaeb Visual Acuity Screener (JVAS) (Yamada et al., 2015), a computerized visual acuity screening program for children, which has a sensitivity ranging from 88% to 91% and specificity from 73% to 86%. This program provides an effective method for school systems to rapidly identify refractive error amongst children.
In Amsterdam, in order to increase access to refractive error screening, the Dutch company Easee BV developed an algorithm for a web-based tool for refractive error measurement using a smartphone and a standard computer screen (Wisse et al., 2019). This technology demonstrated excellent correlation compared to the manifest refraction (gold standard) in 200 eyes, with an intraclass correlation coefficient (ICC) of 0.92.
Based on 687,063 longitudinal EMRs (129,242 individuals) in 8 ophthalmic centres between January 2005 and December 2015, researchers from Zhongshan Ophthalmic Centre in China have identified myopia development rules, and built an AI model to predict the onset of myopia and its progression for children and teenagers, providing a scientific basis for intervention to prevent myopia progression (Lin et al., 2018). With respect to the prediction of high myopia development by age 18 years, the model provided clinically acceptable accuracy over 3 years (the AUC ranged from 0.940 to 0.985), 5 years (the AUC ranged from 0.856 to 0.901), and even 8 years (the AUC ranged from 0.801 to 0.837), by predictors including age at examination, spherical equivalent (SE), and annual progression rate. In addition, Li et al. 2019, 2020 developed DL systems with high accuracy (over 96%) in detecting common comorbidities of high myopia, such as lattice degeneration, retinal breaks and retinal detachment, based on UWF images, which could further improve visual prognosis of myopia patients via timely medical intervention.
In India in 2014, a tele-based (virtually monitored) visual acuity examination with good performance was to be applied into a tele-eyecare system by the School of Allied Health Sciences in Manipal University and the Department of Ophthalmology in Kasturba Medical College (Sreelatha et al., 2014). Afterward, the Department of Teleophthalmology of the Medical Research Foundation carried out a pilot study, providing virtual tele-health eye care consultations for patients in low-resource areas (John et al., 2015). Instead of using traditional face-to-face video consultations, they employed real-time imaging for ophthalmologists to access, permitting virtual visits and instant sharing of fundus photographs, which yielded a 71% diagnosis rate of refractive error.
4.6. Anterior segment diseases
The health and integrity of cornea and lens are critical for normal vision. Despite considerable advancement in surgical technology, intraocular lens design, and biometry calculation for cataract surgery (Liu et al., 2017; Ting et al., 2017; Brandsdorfer and Kang, 2018) cataract remains the leading cause of reversible blindness in the world, affecting approximately 12.6 million people globally (Flaxman et al., 2017). On the other hand, corneal opacity represents the 5th leading cause of blindness, with 1.3 million people being affected (Flaxman et al., 2017). Whilst not being well captured in most epidemiological studies, unilateral corneal blindness – primarily caused by corneal ulceration and trauma – is estimated to have affected 23 million people globally (Oliva et al., 2012). Furthermore, cataract and corneal opacity-related blindness is significantly more prevalent in under-resourced developing countries, adding another dimension to the challenges in tackling these global burdens (Flaxman et al., 2017). In this section, we review the evidence in the literature in relation to how tele-health and AI could be or have been deployed to a variety of anterior segment conditions, particularly for cataract and corneal diseases, in different countries.
4.6.1. Cataract screening and integration with AI
Inadequate service provision for cataract screening remains a major barrier to tackling the rising burden of cataract-related blindness and visual impairment. Early detection and timely management of cataract are essential for improving patient's life quality and reducing healthcare burdens (Limwattananon et al., 2018). In 2008–2009, an ophthalmic mass screening programme, known as the Beijing Eye Public Health Care Project, was established to screen all elderly patients (55–85 years old) residing in the rural regions surrounding Beijing (Xu et al., 2012). With a systematic set-up of the programme and incorporation of a tele-health approach (where visual acuity data, anterior segment photographs and fundus photographs were electronically transferred from the community to the reading centre of Beijing Institute of Ophthalmology and evaluated within 24 h), the programme was able to screen more than 500,000 people for cataract, DR, glaucoma and other major ocular diseases. More importantly, the programme demonstrated superior cost-effectiveness where it only costed around 0.50 USD to screen one patient (Xu et al., 2012).
In the Beijing Eye Public Health Care Project if visual acuity was less than 0.30, individuals were referred to primary care centres where ocular photographs were taken. Then the photographs were transmitted electronically to a reading centre where the causes for visual impairment were diagnosed. Among 37,281 individuals, 19,163 were diagnosed with cataract, and were recommended to visit local ophthalmologists. Recently, a universal AI platform and multilevel collaborative pattern displayed robust performance for cataracts detection in three-step tasks: (1) capture mode recognition (AUC: 99.3%–99.7%), (2) cataracts diagnoses (normal lens, cataracts or postoperative eye with AUCs of 99.8%, 99.9% and 99.9% using mydriatic-slit lamp mode and AUCs >99% using other capture modes) and (3) identification of referable cataracts (AUCs >91% in all tests) (Wu et al., 2019) Even for detection of rare cataract variants like congenital cataracts, the AI agent shows robust performance, which has the potential to serve as a complementary screening approach, especially in undeveloped and remote regions (Wu et al., 2019).
In addition, the workflow of tele-cataract screening measured by ophthalmologist-to-population service ratio, can be further enhanced with integration of AI technology (Ting et al., 2019; Ting et al., 2020). Wu et al. presented a three-tiered, collaborative tele-medicine platform for cataract screening in China, starting from primary home-based self-monitoring, followed by secondary community-based assessment and tertiary referral to hospital specialty services for visually significant cataract. The self-monitoring was performed at home using a mobile phone by the patient and a website-based reference platform for cataract diagnosis. In cases of suspected cataract, slit-lamp photographs along with medical history were obtained during the community-based assessment and uploaded to a website-based cloud platform for confirmation of diagnosis and further management. Furthermore, when incorporated with a universal AI technology, the workflow was improved 10-fold.
4.6.2. Cornea tele-diagnosis
Infectious keratitis represents the leading cause of corneal blindness globally, particularly in developing countries (Ung et al., 2019). To address this issue, Maamari et al., (2014) developed a novel tele-health platform using mobile phones to detect and diagnose corneal abrasions and ulcers in Chiang Mai, Thailand. A smartphone attachment, consisting of a +25-dioptre lens and white and blue light-emitting diode (LED) light sources, was specially designed to acquire white-light and fluorescein images, respectively. When compared to the on-site ophthalmologist (based on slit-lamp assessment), the diagnostic performance of detecting a corneal ulcer by the off-site ophthalmologists (based on photographic assessment only) was excellent (83–89% sensitivity and 91–97% specificity) (Maamari et al., 2014). Similarly, Woodward et al. (Woodward et al., 2017) piloted a study evaluating the reliability and accuracy of a tele-health approach (using two portable cameras – iTorch 5S and Nidek VersaCam) in detecting a variety of corneal diseases, including corneal abrasions, ulcers, scars, and pterygia. The sensitivity and specificity to detect corneal pathologies ranged from 54 to 75% and 82–98%, respectively, with corneal scars having the lowest accuracy score. The findings suggested that the quality and resolution of the obtained anterior segment images were not yet adequate for tele-health applications, highlighting further need for refinement (Woodward et al., 2017).
Dry eye disease (DED) is another prevalent ocular surface disease that is estimated to affect around 5–50% of the population (Stapleton et al., 2017). Patients affected by this chronic disease often require multiple follow-up visits for monitoring and treatment. To reduce the workload of the healthcare system and the number of physical visits by the patients, Amparo and Dana, (2018) evaluated the feasibility of remote assessment and monitoring of DED using electronic versions of validated questionnaires such as Ocular Surface Disease Index (OSDI) and Symptom Assessment in Dry Eye (SANDE) questionnaires. Patients were found to be sufficiently motivated to report their symptoms at least once a month with a good correlation between the two dry eye questionnaires (r = 0.67), underscoring the potential utility of a tele-health approach for monitoring DED (Amparo and Dana, 2018). In a similar vein, Inomata et al. (Inomata et al., 2019) reported using a smartphone app, DryEyeRhythm, to determine the characteristics and risk factors of diagnosed and undiagnosed symptomatic DED, allowing for earlier detection and intervention.
Furthermore, Alabi et al., (2019) have investigated the potential utility of tele-health consultation in evaluating the suitability of donor corneal tissue for transplantation. With high quality digital images obtained from the slit-lamp, OCT and/or specular microscopy, the quality of donor corneal tissues could be reliably assessed remotely (Alabi et al., 2019).
4.7. Acute care services
Acute care ophthalmology presents a challenge due to the sheer volume of patient visits and the limited exposure to ophthalmology during training amongst non-ophthalmic providers. There are approximately 2 million admissions to the emergency department each year for ophthalmic conditions in the US (Rathi et al., 2017) and over 5 million visits to general practitioners a year in the UK were for ophthalmic conditions (The Royal College of Ophthalmologists, 2015). Diagnosis via telemedicine presents different challenges in comparison to screening. Screening is repetitive and elective, and the process can be planned with clarity for the input, processing and outputs. For diagnosis, on the other hand, a telemedicine diagnostic service must consider a much wider variety of conditions and include more abnormal conditions. In addition, it is more challenging to streamline and process input data in manner that achieves high diagnostic accuracy. Achieving such accuracy requires highly trained personnel.
The Scottish Government Health Department invested £6.6 million in 2010 to fund the Eyecare Integration Project (Scotland) with an aim to establish an integrated electronic referral system between community optometry services and hospital eye services in replacement of the traditional postal referral service (Khan et al., 2015). This programme was first initiated and piloted at Fife, Scotland, in 2005–2007 to assess the feasibility, safety and cost-effectiveness of the electronic referral system for ophthalmic diseases. The study demonstrated excellent (97%) clinical agreement between the clinical and e-diagnosis, high (97%) patient satisfaction, and 37% reduction of unnecessary referral to the hospital eye services. Moreover, the referrals (with digital images if necessary) were processed within 24 h, enabling a timely triage and management of any urgent and sight-threatening diseases. When this programme went live throughout southeast Scotland, the referral-to-consultation waiting time was reduced from 14 weeks to 4 weeks. The foundation of this integration project enabled the safe delivery of eye care services during the COVID-19 pandemic with many primary and urgent eye care services enabling non-hospital patient care (NHS Scotland, 2020).
A cloud-based referral system in the UK has demonstrated that more than half of referrals for possible retinal pathologies to hospital eye services from optometrists could be avoided with a consultant ophthalmologist reviewing fundus photographs of the referred patients (Kern et al., 2019), similar to the pathway shown in Fig. 6 . Although there are still many factors to be addressed such as safety, economic benefit, patient satisfaction, and outcomes for those patients who were not referred, there are notable advantages such as timely patient triage, enhanced provider correspondence and education. This system enabled the referring doctor to be able to receive the patient outcome via the platform, allowing each case to be an educational opportunity.
Fig. 6.
Smart healthcare telemedicine service. Courtesy of Mr Peter Thomas.
The dash box refers to automated pathway, which could proceed without an ophthalmologist reviewing the case and images. Example of ‘simple’ case: dry AMD diagnosed and recorded but no clinical action required and clinician oversight not required. Example of ‘complex case: macular hole potentially suitable for surgery, with clinician alerted and further clinical decision to be made.
The safety of remote triage in emergency ophthalmology still needs to be demonstrated. One early study showed that of 500 patients who were triaged remotely in an emergency unit, 1% had delayed treatment due to misdiagnosis (Bourdon et al., 2020). Prior to widespread adoption of tele-triage, the potential for harm needs to be more accurately characterised as well as mechanisms put in place to mitigate the shortcomings of remote reviews. Since in the absence the visual clues for diagnosis are limited for patients calling from home, there should be a lower threshold for in person review for those unable to give a clear history such as children, patients with cognitive impairment and learning disabilities, and where language barriers exist. Certain symptoms and signs should always warrant in-person review.
5. Applications in post-COVID-19 “new normal”
5.1. COVID-19 pandemic outbreak
In March 2020, Ferguson et al. modelled two fundamental strategies in the control of community spread of SARS-CoV-2; mitigation versus suppression (Ferguson et al., 2020). Mitigation focusses on reducing social contacts aiming to slow but not interrupting transmission so as not to overwhelm health systems. Suppression strategies involve more aggressive social distancing measures with testing and isolation of cases in an effort to stop transmission. With the mitigation approach, the study found that 8 of 10 people may still be affected, resulting in 510,000 deaths in the UK and 2.2 million deaths in the US by the end of the pandemic. The study suggested infected cases could be significantly decreased with a suppression strategy (“lockdown”), which involved closing schools/universities, case isolation, household quarantine and social distancing.
As country after country began imposing “lockdown” measures, including quarantines and travel bans in an unprecedented scale (Parmet and Sinha, 2020). China placed Wuhan under strict quarantine on January 23, 2020, and as Wan et al. concluded in their paper, “The end of cordon sanitaire in Wuhan: the role of non-pharmaceutical interventions”, it was these measures that allowed Wuhan to lift restrictions on April 8, 2020. In the absence of vaccine and proven specific treatments, the authors propose that the experience and results achieved by Wuhan could serve as a good reference for leaders and policy-makers around the world in formulating strategies and policies in fighting against COVID-19 (Wan et al., 2020).
Health care systems have been implementing strategies to maximise capacity for patients falling ill with COVID-19 with the principles of suppression in mind. In ophthalmology and many other specialties, non-urgent appointments and routine/elective surgeries were cancelled. In an effort to curtail the numbers of patients presenting to healthcare settings while still providing essential service to patients, hospitals and clinics were forced to rapidly upscale telemedicine services (Saleem et al., 2020; Sim et al., 2020; Wickham et al., 2020). As in other specialties, telemedicine was employed to follow-up routine patients, and to triage and manage new patients presenting to ophthalmology departments. Telephone consultations alone could suffice for some patients, but the addition of video features allows the clinician additional information to more appropriately triage a patient. Live video information can be particularly useful in specialties such as oculoplastics (Kang et al., 2020) and strabismus, but also in external eye diseases where corneal infiltrates may be observed. Furthermore, telemedicine allows for non-verbal communication and aids in fostering physician-patient engagement. Effective triage not only keeps many patients out of the hospital but can also shorten the patient's journey once they arrive in hospital. A patient with classic symptoms of a retinal detachment may bypass the emergency department and be referred directly to a vitreoretinal surgeon.
The rapid introduction of telemedicine and teleophthalmology during the pandemic has moved beyond the traditional model of connecting specialists with patients from remote and underserved regions. Instead it has the potential to become the new standard of care, in particular for triaging patients prior to their hospital attendance. The new telemedicine systems replacing routine care needs evaluation to ensure patient safety.
Governments such as the China and the US have taken steps to facilitate the rapid upscaling of these services, with the Chinese national health insurance agency covering virtual consultation fees, and the US Centres for Medicare and Medicaid Services (CMS) implementing temporary waivers to enable flexibility within the healthcare system (Webster, 2020). The manifold surge in uptake reported by CMS is staggering: nearly 1.7 million beneficiaries receiving telehealth services in the last week of April 2020 compared to around 13,000 beneficiaries a week prior to the pandemic (Verma, 2020). Of the 9 million beneficiaries who used a telehealth service three months from mid March 2020, 30% were conducted over the telephone suggesting there is still significant work to be done in terms of telecommunications network, healthcare facilities and clinicians adopting new applications, and consideration of patient factors.
As countries consider the model of eyecare in the post-COVID-19 “new normal”, there are several key considerations (Table 6 ). First, services must allow for sustainable social distancing measures for protection of patients, staff and the public. Second, those at high risk of serious morbidity and mortality with COVID-19 should be facilitated to isolate wherever possible with access to services at home. Third, plans must be in place for the management of patients who develop eye conditions concurrently with COVID-19. Fourth, contingency to manage the ‘surge’ of patients who have had deferred appointments or presented late as a result of “lockdown”. Fifth, services should have the agility to expand and shut down to essential provisions responsively in preparation for future peaks of COVID-19, and indeed other future pandemics. Finally, there should be measures in place to continually assess the outcomes of these services to ensure quality of care.
Table 6.
Considerations and new models of care in post-COVID-19 “new normal”.
| Consideration | New models of care | Digital innovations considerations |
|---|---|---|
| Social distancing | Reduce clinic visit number and duration | Teleconsultations/5G |
| Utilize pre-hospital forward triage via telemedicine | Remote vision testing | |
| Minimum in-clinic touch points, assessments, tests, consults, pharmacy | IoT for registration and appointment logistics | |
| Re-evaluate clinic space norms | AI-assisted triage | |
| Prioritize care (urgent, semi-urgent, routine, ‘unnecessary’) | Use of AR/VR for remote consultations | |
| Protecting health care workers | Remote working through telemedicine | Staff up-skilling/re-training |
| Split teams | Restructuring of human resources | |
| Education and meetings held remotely | ||
| Treating COVID-19 patients with eye diseases | Forward triage to enable planning of in-person examination | AI-assisted triage |
| Early involvement of final clinical decision maker | ||
| Post lockdown surge management | Increasing role of non-ophthalmologists | Teleconsultations/5G |
| Increasing workforce capacity through working from home | Remote EHR access | |
| Preparation for future peaks and pandemics | Contingency planning | Data security |
| Robust, reliable and secure telemedicine provision for entire patient journey | Network integrity | |
| Train more staff to utilize telemedicine platforms | Actively reduce digital exclusion |
The COVID-19 pandemic has come at a time when many technologies and the necessary infrastructure are mature and already established. Much can be achieved with simple and universally available technologies such as telephones, messaging, and video-calling, albeit via safer and secure applications. Subsequently, more sophisticated eye examinations via telemedicine can occur. This pandemic has significantly altered the landscape of health care delivery and may have permanent implications. Time is still needed to establish the safety telemedicine on a massive scale, but the paradigm shift in acceptability to both patients and doctors will be profound. Aside from the technical and infrastructural challenges, there are concerns over how patients will respond to such a shift in healthcare delivery, and if the loss of rapport gained from physical interaction will cause harm. Clinicians are also discovering that face-to-face healthcare delivery in the post-COVID era has also changed. Face masks and social distancing result in loss some of the non-verbal communication, impede the delivery of empathy. Though there is physical distancing over a video-consultation, patients are able to see their doctor, and both are able to see the facial expressions of the other. Acceptance in both patients and physicians is on the increase (Pappot et al., 2020; Hao, 2020). Even when teleophthalmology services have been rapidly adopted during the pandemic, feedback from a prospective study of 66 patients in an oculoplastics service reported 62% preferred the video consultations to face-to-face, and in this group ranging from 18 years to 88 years (mean 50.7 years), 92% would recommend video consultation to others (Kang et al., 2020). Teleophthalmology user platform design can and should be designed to improve acceptability and accessibility for all potential users without excluding those with minimal digital literacy. Thus studies into patient attitude should take care to compare tele-consultations with in-person visits in the current and future state, rather than the past.
5.2. New models of care
It would not be possible to provide care at pre-COVID-19 levels whilst practicing social distancing and maintaining a safe environment for patients and staff alike. New models of care are being and need to continue to be rapidly upscaled to enable safe delivery of care until an effective vaccine or treatment is found for COVID-19.
The overriding principle of safe care in the COVID-19 in ophthalmic practice is minimizing exposure: mainly by reducing the number and duration of in-person clinic visits. Assessments, tests, consultations and even pharmacy and interventions need to be minimised to those essential for safe care. The integration of teleophthalmology will be fundamental and can be utilised at multiple points of a patient's eye care journey. Telemedicine can be and already is being adopted for large screening programmes, most notably and successfully for DR (Scanlon, 2017; Nguyen et al., 2016; Cavallerano et al., 2005). Teleophthalmology can be upscaled to provide broader coverage, and promote onward referral of patients from screening to hospital eye services remotely with patients only seen in person if necessary, such as to provide treatment.
Teleophthalmology and in particular the use of video consultations facilitates forward triaging (Wickham et al., 2020), and routine clinic assessments particularly in subspecialties where visual clues are more readily discernible without need for magnification and close examination, such as oculoplastics (Kang et al., 2020) and strabismus. Fig. 7 provides an example of semi-automated remote triage workflow for emergency ophthalmology.
Fig. 7.
Example of semi-automated remote triage workflow for emergency ophthalmology.
Non-ophthalmologist health care workers including optometrists, nurses and technicians should be trained in multiple skills if possible so that a single person may perform several tasks such as assessment of visual acuity and intraocular pressure, instead of patients moving through a number of different clinical staff each performing a specific task. This improves efficiency and limits exposure risk. Furthermore, integrating second opinion services to primary care and optometry practices may enable more appropriate referral into specialized eye units.
These measures protect patients and health care workers and contribute to the larger public health measures. Telemedicine also enable ophthalmologists in isolation to continue to contribute in clinical work and lessen the impact of key staff shortages.
5.3. Opportunities for digital technology
This current climate provides the perfect ecosystem to reassess care delivery and to adopt the synergistic and complementary digital technologies discussed above, incorporating teleophthalmology and AI utilising and facilitated by 5G networks, IoT and Big Data analysis. There is widespread media interest and raising of public awareness of the role telemedicine has already started to play in risk mitigation during the pandemic.
The emergency department may be a good candidate for widespread introduction of virtual triage prior to attending in person. The patient benefits as they may discover they do not need to attend in person, and can be treated with medicines prescribed remotely. If they do need to attend, their in hospital journal may be much more efficiently managed, being seen directly by the specialists if appropriate. Additionally, with the maturation of chatbots, much of the patient counselling can be done seamlessly from the video consultation.
The healthcare providers too reap the benefits of reduced in person attendance, costs associated with additional time and space utilisation, as well as use of personal protective equipment at a time where sustainability must also always be considered. Staff who are able to work from home can contribute, facilitating efficient use of human resources. Reduced attendances also reduces the general workforce risk of COVID-19, avoiding the highly undesirable scenario of transmission between clinicians and patients.
Safety of such systems, the remote triaging and automated counselling need to be evaluated, and until then, clinicians need to oversee each consultation as is standard process prior to the pandemic.
The figure below demonstrates how a virtual video-based triaging system, with semi-automated features such as registration and counselling, might work. When patients register, there can be early algorithmic assessment of their presenting complaint. Symptoms such as flashing lights and floaters, new binocular double vision and new anisocoria will invariably require in-person examination, and as such can be directed early to a physical appointment without the patient waiting for a full virtual assessment first. Patients who do not necessarily require clinician input, such as mild dry eyes or chalazia, or followup patients who have seen resolution of their symptoms, for example treated pre-septal cellulitis or contact-lens related keratitis, can be directed to a chatbot or video for discussion. The remaining patients will be connected to a clinician when can proceed with a full history and basic examination which may involve visual acuity assessment using web-based tools. For conditions that may be managed remotely, such as early pre-septal cellulitis, mild recurrent anterior uveitis or indeed early non-vision involving contact lens associated keratitis, medication can be prescribed and sent to the patient via a dedicated delivery service or local phamarcy. If necessary, plans can be made for the patient to attend in person for review.
Finally, the disparity in the coverage of reimbursement among different health care insurance policies and different regions that have deterred the uptake of tele-health intervention is changing. Teleconsultations are now offered by providers in the UK NHS (Wickham et al., 2020), and covered by the Chinese national health insurance scheme and by Medicare and Medicaid in the US (Webster, 2020). These changes are a product of national health strategies and this new permissive regulatory environment and funding along with patient and physician acceptance, will allow the digital transformation of ophthalmology services.
Digital transformation through the adoption of teleophthalmology and AI is more than simply buying new software and hardware, and the next section explores some of the key challenges to be overcome.
6. Challenges for clinical implementations
6.1. Validation of digital innovation
Real-world validation has proven to be challenging. The size and heterogenous nature of the digital health sector with its constant and rapid evolution has created a complex environment for physicians, healthcare providers, patients and regulatory bodies in assess these tools to address unmet clinical needs (Mathews et al., 2019). There is a need for a rigorous and transparent validation framework, which has some flexibility in being applied to a broad range of technological innovations. One proposed framework suggests evaluation based on technical and clinical considerations, usability, and cost (Mathews et al., 2019).
Technical evaluation is the most obvious, and is the first step to validation. This is the fundamental aspect of the technology, and should address if the technology performs its purported function, its accuracy and robustness. For example, does a video consultation platform enable patients to register to a virtual waiting room and be connected to the appropriate clinicians in a safe and effective manner, with due consideration for data protection.
Clinical validation approaches should reflect those that are well established in clinical research, but can be tailored for digital technologies. Such studies are still uncommon and may be at least in part due to the lack of clinical experts simultaneously engaged with technological advances (Hatef et al., 2018) The cost of prospective clinical trials as a comparison to existing gold standards may be off-putting for some in the technology sector who seek rapid product cycles and returns.
Usability, and also accessibility, and the intended user of the technology must be assessed. Clinicians may need new skills in order to effectively use the tools. The effectiveness of their use by patients unsupervised should be assessed, as well as consideration of those who face barriers in adopting the technologies.
Cost, and cost effectiveness, as well as the longer term costs should be estimated. Costs may be obvious, such as purchasing the rights to an algorithm, or hidden, such as increased referrals seen through telemedicine screening services. Implications for all stakeholders needs to be considered, from the patient to clinician, to funding bodies as well as the state.
Regulatory bodies attempt to provide guidance for users and payers. AI is considered as a medical device, and regulation for its use falls to organisations such as the US Food and Drug Administration (FDA) and CE marking, a certification mark that indicates compliance with health, safety, and environmental protection standards for products sold within the European Economic Area (EEA). Approvals for technologies that change at such a rapid pace will no doubt continue to provide challenges to regulators. As approvals become increasingly standardized for different digital modalities, and the regulators gain experience in assessing their suitability for clinical use, the efficiency and effectiveness of the assessments should improve. With the algorithms not being a physical product, and that the algorithms may indeed be ‘live’ and be continually improved, there should be mechanisms in place to allow updates to be submitted for reapproval so that upgrades need not go through the full approval process. Increased requirement by regulators for developers and companies may gradually influence greater rigors in all aspects of validation to help create clinically meaningful and cost-effective technologies that are likely to be adopted by users. Some specific challenges to validation, as well as implementation, in telemedicine and AI are discussed below.
6.2. Challenges to clinical deployment of telemedicine in ophthalmology
With the ageing population, rising disease prevalence and expanding treatment options, drastic and innovative tackling measures are necessary to curb the mounting pressure on ophthalmic services (Buchan et al., 2019). This pressure has been further intensified by the pandemic. According to a UK-wide study conducted prior to the pandemic, patients incurred significant sight loss (3 or more Snellen-line vision loss in at least one eye) due to delayed hospital-initiated follow-up (Foot and MacEwen, 2017). In addition, studies have shown that delay in hospital review during COVID-19 pandemic lockdown could result in significant psychological impact on people with visual impairment (Ting et al., 2020). The excess ophthalmic morbidity as a result of deferred eye treatment during the pandemic is yet to be evaluated. The introduction of tele-health and AI present an attractive solution to increase service capacity in ophthalmology. Their introduction into a complex system at such a scale, and potentially impacting all patient journeys in the future requires urgent and careful consideration of a number of factors (Greenhalgh et al., 2020; Keesara et al., 2020).
The costs of tele-health ophthalmic equipment and additional personnel training, potential barrier to new technology adoption amongst physicians and patients, and heterogeneity in the insurance policy and medico-legal regulations are key challenges for clinical implementation of teleophthalmology (Rathi et al., 2017). Common ophthalmic imaging equipment such as slit-lamp microscopy, fundus camera, and optical coherence tomography all pose high cost. Additional costs are incurred for training personnel and technicians to acquire sufficiently high-quality images for clinical use. To overcome these barriers, various research groups have developed programs to acquiring high-quality anterior and posterior segment images, instead of using conventional ophthalmic imaging (Ludwig et al., 2016; Johnson et al., 2015; Goyal et al., 2019; Hogarty et al., 2019). However, noteworthy is that these devices will need to gain validation and FDA or equivalent regulatory agency approval before they can be introduced into clinical practice. Digital accessibility, the affordability and availability of both smartphones with necessary capabilities such as high image resolution and sufficient internet bandwidth may limit the number of patients who are able to receive telemedicine services.
Technical concerns exist that still require ongoing improvement, including ensuring security and reliability, contingency, and improved audio and video quality. Although successful examples of screening DR, ROP, AMD, glaucoma and cataract using digital slit-lamp and fundus images have been reported and implemented in some countries, use of tele-health in anterior segment diseases requires further evaluation and clinical validation. For instance, the sensitivity of detecting corneal pathologies, particularly corneal scars, using portable cameras (with 5.0 megapixels) was not adequate for tele-health application (Woodward et al., 2017). In addition, teleophthalmology evaluation may fail to detect subtle signs such as corneal oedema and anterior chamber inflammation (cells and flare) that may be only detectable with high-resolution, high-contrast and dynamic assessment (Threlkeld et al., 1999; Smith et al., 2003), particularly when the high-quality images/videos are required to be compressed before being transferred electronically. These issues highlight the importance of disease selection in when establishing a tele-health programme.
The use of a universal patient co-managed EHR system could help streamline the process of data acquisition, analysis and transfer among different institutions at different levels of care, though this aim has yet to be actualised in the real-world setting. Common perceived barriers among physicians for integrating EHR within their clinical practice include time taken to implement, cost, absence of computer skill, workflow disruption and security and privacy concerns (Ajami and Bagheri-Tadi, 2013). Some of these issues might be potentially overcome with education and training of the end-users and provision of financial incentives by the government for meaningful use of EHR system (Patel et al., 2013).
After validating the technological and clinical performance, cost-effectiveness represents the next hurdle to be overcome before the implementation of a specific tele-health programme. A notable example was reported in the UK where a large randomised controlled trial in England evaluating the cost-effectiveness of tele-health intervention for long-term conditions (including heart failure, chronic obstructive pulmonary disease, and diabetes) demonstrated no additional benefit when compared to standard care (Henderson et al., 2013). That said, tele-ophthalmology intervention, particularly for DR screening, has proven to be a cost-effective approach and is already being implemented in many countries, including the US, UK, and Singapore, at nationwide levels (Kirkizlar et al., 2013; Scanlon, 2017; Nguyen et al., 2016). Further cost-effectiveness analysis of tele-health intervention for other ophthalmic conditions will be required before integrating them with traditional care.
Implementation requires leadership at all levels. National and professional regulatory bodies should widely engage in discussions to develop best protocols based on learnings post successful and failed programs, offer support to local institutions and individuals, and highlight areas requiring further research. Regional clinical champions with a compelling narrative and sound knowledge of existing workflows should lead implementation, ensuring new platforms and processes are developed to provide a seamless journey from start to finish, including the delivery of prescriptions. Strategies should be embedded to examine the successes and failures of new processes to enable a cycle of ongoing reflection and improvement.
Patient receptivity and satisfaction is another potential barriers for the adoption of tele-health technology, but this is already changing in light of the pandemic (Pappot et al., 2020). Wildenbos et al. (Wildenbos et al., 2018) conducted a systematic review evaluating the ageing barriers on tele-health and reported four major deterring domains on the usability of tele-health among the elderly population (>50 years old), including cognition, motivation, physical ability and perception. Recent studies have highlighted a better patients’ satisfaction towards tele-medicine approach compared to traditional face-to-face consultation (Zahlmann et al., 2002; Khan et al., 2015), suggesting a positive change in the culture and perception among patients.
Orbis International uses a free online ophthalmic telemedicine program partnering doctors in developing countries with expert mentors internationally (Prakalapakorn et al., 2012). In a survey of this offering, they reported e-consultations were well received by users, with 96% of 107 users wishing to continue its use, and 94% of the 78 not using the system wishing to do so. Whilst success in terms of patient and physician satisfaction has been demonstrated with this ‘store-and-forward’ format for individuals, there are real challenges to their scalability. Logistical challenges of such a program include the lack of medical information consistency (especially in cases which require external expert input are usually of a higher level of complexity), combining medical data acquired in different formats, and ensuring secure, timely, and uncorrupted data transmission. Additional challenges include language barrier, interpretability of handwritten notes, and interconversion between analogue and digital images. Often the manpower involved in data capture and transmission limits service capabilities, especially in an acute setting.
From a medicolegal perspective, physician-patient interaction in tele-health is currently considered the same as face-to-face consultation. Though physicians are concerned about missing a diagnosis or finding (due to inadequate medical information or suboptimal image quality), the digital images used could serve as a powerful objective evidence of the consultation. Another noteworthy aspect is that laws governing physician-patient interactions are disparate across states and countries. Having an overarching regulation of telemedicine would expedite the introduction and implementation of telemedicine in routine healthcare service (American Academy of Ophthalmology AAO Telemedicine Task Force, 2018). There is emerging correlation between telemedicine and over-prescribing and the highlights the need for ongoing review of new services and realworld outcomes to better inform the delicate and changing balance between accessibility and quality of care (Hoffman, 2020).
Regulation of telemedicine is also evolving. The Centres of Medicare and Medicaid Services (CMS) broadened provision of telehealth services as part of the emergency response to the COVID-19 pandemic to enable provision of care whilst limited community spread of the virus (Centres for Medicare and Medicad Services 2020). Under a new waiver, Medicare can pay for much broader range of telehealth services compared to quite limited provisions previously. However, how this will continue, and the impact of changing regulation and funding streams in this post-COVID era remains to be seen.
6.3. Challenges in clinical deployment of AI
AI has remained largely constrained to the research domain with few examples of real-world adoption in ophthalmology and healthcare more generally. There are many contributing factors for this. Whilst there is enormous interest and increasingly robust evidence for the role of AI in DR screening, there are still several caveats that need to be considered.
DL algorithm uses the “black box” approach where clinical features that confirm a diagnosis are not apparent. To underscore the reasons prompting a specific diagnosis by algorithms would be highly beneficial as it allows for clinicians to understand assess if the correct features were identified, and to offer new insight into diseases not previously known. This lack of explainability is a hurdle both for clinician and patient trust. It is challenging when there is disagreement between the algorithm and the patient and root cause analysis stops short. It is not possible to know if there is an inherent error in the algorithm that might be corrected. Processes need to be in place such disagreements, such as an independent third party of a multi-disciplinary team meeting as would occur where there is clinical uncertainty.
There needs to be recognition though, that AI may be proven to be more accurate than a physician, and detect features humans cannot, as demonstrated by an algorithm being able to identify sex from fundus photographs (Poplin et al., 2018). Thus it becomes harder to adjudicate between the clinician and AI, when the adjudicator will invariably be another clinician, in particular if the AI decision making process is unexplainable. In these cases, it may become unethical not to use AI, even though we do not fully understand how they work. It is unlikely though, that an individual algorithm will be able to replace the holistic role of a physician, and increasingly the role of the physician could evolve the use of AI for specific tasks, and digest the various outputs to collectively to manage the patient.
Education on the use and appraisal of AI systems should be incorporated into medical school programs, and clinicians already in practice will need training to facilitate its adoption when the technology reaches maturation for clinical practice. Technically able staff who would not form part of existing human resources will need to be recruited, and work with clinicians to champion adoption. In cases of poor image quality, automated processes may be able to enhance those images and enable their reading by the algorithm. However, those with residual artefacts will remain ungradable and require referral to a clinician.
Early AI algorithms were tested on images collected in the clinical trials setting with strict inclusion and exclusion criteria (Burlina et al., 2017). The real-world validation of diagnostic performance and therapeutic decision-making of AI algorithms still need to be tested with large-scale unfiltered clinical data.
There are still other important issues to explore like patients’ acceptance and confidence in AI; the reproducibility, reliability, and usability of AI; and medico-legal challenges before DL algorithms can be deployed in the clinical workflow of diabetic eye disease screening programs (Cheung et al., 2019). DR diagnostic and screening algorithms vary greatly, and before any adoption in clinical practice, due consideration must be given to the training datasets and validation, and the intended use, in particular, the degree of autonomy the algorithms were designed to have (Abramoff et al., 2020).
The legal ramifications of adopting AI still needs to be clarified. The American Medical Association (AMA) advocates that in case of system failure or misdiagnosis from autonomous AI systems, that the developers must take liability and maintain their own medical liability insurance with their users (American Medical Association, 2019). However there is still no clear regulation around this, particularly when AI is assistive, and the clinician makes the final diagnosis and management plan with input from the algorithms.
AI has not yet matured to reach a stage of being able to diagnose and manage patients without human input. At present, where AI is in clinical and experimental use, it is in an assistive role, with clinician oversight and ultimate responsibility. This distinction between assistive and autonomous functions has clear ramifications for ultimate responsibility.
Data integrity, protection and cyber security will need to be continually addressed and enhanced. Broadly speaking, there are two types of data in AI which fall under different regulatory requirements: training data and testing data. Training data used in the development of algorithm should always follow the standard Institutional Review Boards (IRB) ethics approval protocol to de-identify and anonymise the data. The data used from patients thereafter for testing should follow the country specific regulation on medical data, such as Health Insurance Portability and Accountability Act (HIPAA) in the US and General Data Protection Regulation (GDPR) in the European Union. The transfer of data between countries is heavily regulated and often challenging. For example, GDPR prohibits the transfer of personal data to countries outside the European Economic Area (EEA) with certain exemptions apply, such as appropriate safeguards or the country in question has been deemed by the European Commission that the adequate standard of protection has been met.
There are concerns of the implications of other data being inadvertently captured, since a fundus photograph may be rich in data that humans cannot interpret, and could reveal different characteristics or information such as gender, cancer, or cardiovascular disease. However, for such data to be interpreted, it needs to be analyzed specifically by an algorithm for that purpose, and this ultimately remains a responsibility for AI service providers to comply with all local data and cybersecurity regulations.
6.4. Equity of access
Telemedicine and AI offer an opportunity to provide a limited and valuable resource – that of the physician's time and skillset – to a wider population in a more accessible way. It can potentially reduce health inequities, a fundamental principle of medical ethics, but there must be careful consideration of the design of services and algorithms, as well as their implementation in order to achieve this.
Such a sudden move to virtual care as necessitated by the pandemic has given little time to address the challenges to those who are excluded, and indeed identify those patients in the first instance. There needs to be urgent work in this arena, recognising that the causes are multi-faceted, relating to demographics, individual skill, current health status, access to infrastructure as well as training and support (Levin-Zamir and Bertschi, 2018). There needs to be recognition that the accessibility of virtual healthcare systems is fluid, and that patients may encounter different challenges at different times. For example, a technology savvy patient with good dexterity and high-speed internet connection may find it hard to navigate a webpage if they have blurred vision. Similarly, with some guidance and support, patients with limited computer literacy might be able to successfully access online platforms with simple and intuitively-designed user interfaces.
There is emerging data on patient demographics in the use of teleconsultations during the pandemic. Over 9 million Medicare beneficiaries received telehealth services in three months from Mid-March. Interestingly 22% of the beneficiaries used telehealth services in rural areas in contrast to 30% of those from urban areas (Verma, 2020). The Centre of Medicare and Medicaid (CMS) reports that beneficiaries are receiving telemedicine services across age-groups (34% below 65 years, and 29% above 85 year), with no significant differences by race or ethnicity (Verma, 2020).
Discrimination by AI – AI can be built into EHR, and when patients are booked, it can predict the likelihood of a patient not attending, and compensate for that with an overbooking. However, if algorithm is wrong and patient shows up, then less time is available with potential poor quality of care. Patients who frequently fail to show for appointments often have more complex medical and psychosocial needs, and can potentially come to more harm, thus propagating health inequities. The algorithm utilises personal characteristics such as ethnicity, socio-economic status, religion, body mass index, and may further marginalise those who are already vulnerable. Even removing personal characteristics modelling has demonstrated that the potential for discrimination could not be removed (Murray et al., 2020). Of course reducing wasted appointments increases efficiency, but may inadvertently potentiate the challenges for the vulnerable. The ‘black box’ nature of AI obscure the reason behind labelling a patient as high risk for not attending which makes it difficult to remove the underlying obstacles. However those at risk of missing appointments as identified by AI does not need to translate to discrimination. Rather it can be used to identify and support vulnerable patients who struggle for various reasons to attend. If EHR reminded clinicians that a patient may miss their follow-up, measures can be taken such as offering a virtual appointment, engage in suitable local community support services which offer support with transport and other social services.
Whilst AI might appear to be objective, biases can be inherent in the algorithms (Gianfrancesco et al., 2018; Rajkomar et al., 2018). Inherent to ML is that the algorithm learns from historic data and those under-represented in these data sets may suffer from inaccurate diagnoses, and this to a larger extent is why validation using real-world data is important. The design of these algorithms and their introduction into clinical practice should incorporate the principles of equity, so that the output does no harm. Proactive steps can be taken at each step of the data collection, training and evaluation stages, such as broad stakeholder engagement, ensuring data represents the protected groups and that such data is identifiable to guard against cohort bias, and formulate systems to continually evaluate key metrics across different groups (Rajkomar et al., 2018). Excessive reliance on algorithms without thoughtful ongoing assessment could see existing inequities merely reflected and even exacerbated (Gianfrancesco et al., 2018).
7. Future research and recommendation on digital innovations
With the rapid advancement in digital technology, including EHR, smartphone and 4G/5G technologies, tele-health is likely to pave the way for assessment and management in the field of ophthalmology. In order for a comprehensive and robust teleophthalmology platform to thrive, a well-planned eye care delivery system must exist that considers the resources that are available in specific regions.
In 2018, the AAO Telemedicine Task Force published an information statement regarding the development and implementation of teleophthalmology, including validation of a teleophthalmology programme against a reference standard, requirement and standards of data acquisition and communication devices, competency and qualification of involved personnel, quality assurance, and data protection (American Academy of Ophthalmology AAO Telemedicine Task Force, 2018). In principle, it is recommended that a tele-health programme should be implemented and integrated with evidence-based clinical practices where traditional process of care is already established (American Academy of Ophthalmology AAO Telemedicine Task Force, 2018). Successful teleophthalmology examples such as screening and monitoring of DR, AMD, ROP and glaucoma have already been reported in several countries (Labiris et al., 2018; Scanlon, 2017; Kirkizlar et al., 2013).
Cost and manpower remain the foremost challenges in establishing a successful teleophthalmology programme. Training of allied health professionals, including nurses, optometrists and technicians, by the ophthalmologists would help to share the overall workload of teleophthalmology, allowing the ophthalmologists to manage more complex cases. This model has already been adopted in several resource-rich countries for delivering a range of eye care service, including DR, glaucoma and cataract (Scanlon, 2017; Kotecha et al., 2017; Azuara-Blanco et al., 2007; Kirkwood et al., 2006).
The pyramidal 5-tier model of eye care delivery developed by L V Prasad Eye Institute (LVPEI) may serve as a sustainable and cost-effective framework for integrating tele-health technology in providing eye care and mass screening in underserved rural areas (Rao et al., 2012). This model is operated by a diverse cohort of eye care personnel, ranging from local volunteers, optometrists, technicians, ophthalmologists, allied health professionals involved in visual rehabilitation, eye banking, health advocacy workers and researchers. A similar model was also reported in China using a 3-tier eye care delivery service for screening and stratifying the severity of cataract (Wu et al., 2019). However, these models could only be successfully delivered and implemented if all personnel are fully trained and accredited by ophthalmologists or relevant accrediting agency for performing the specific given task. The replicability of these frameworks may also vary from country to country due to cultural differences.
Understanding the prevalence of the common ocular diseases at a national public health level, country-specific, is paramount as it helps policymakers and relevant stakeholders to maximise the cost-effectiveness of the tele-medicine programmes by targeting highly prevalent diseases. In addition, common diseases that are dependent on image-based diagnosis with universally agreed-upon, evidence-based classifications (e.g. DR, AMD, glaucoma and cataract) should also be prioritised in the set-up of teleophthalmology programmes. The data derived from tele-health may also be harnessed to generate big data research and to offer more diverse information such as patient journey education and disease progression forecasting (McCall, 2020). Aspiring to health equality and protection of vulnerable groups should be a key consideration in every stage of digital innovation and implementation.
The existing digital technologies are predominantly focussed on diagnosis. AI of the future can increasingly play a role in the guidance of treatment, such as prediction of how likely patients are to respond to treatments such as intra-vitreal injections in wet AMD or DMO. Increasing use of AI in the prediction of refractive outcomes following cataract surgery can help refine lens selection. For children requiring patching or those requiring accommodation exercises, digital solutions may be able to help adherence to treatments, with gamification and introduction of incentives for compliance, although debate will exist around if such use of technology is desirable for children.
Recently, ML associating perimetric cone sensitivities to local OCT in patients with retinitis pigmentosa was applied to predict visual function in Lebers congenital amaurosis (LCA) (Sumaroka et al., 2019). Though the training dataset was small, cone vision improvement potential in some LCA was shown to be predictable. This may permit individual prediction of likely response to treatments and influence selection to clinical trials so that those with maximal potential gains are selected.
Increasingly, isolated algorithms will integrate data from across modalities, and across disciplines. The utilisation of multi-modal imaging is important for specific diagnosis (for e.g., determination of the neovascular AMD subtype, diagnosis of glaucoma and etc). Multi-modal machine learning can be used to evaluate whether the predictive or diagnostic power of the AI algorithms will increase with the addition of more imaging modalities. Additionally, data from history, and other metrics such as blood pressure HbA1c can be used to increase the predictive power of the algorithms, and data collected from other specialities such as endocrinology and rheumatology could contribute.
Multi-modal inputs may be help improve the diagnostic and predictive power of AI systems, and move closer to simulating the decision-making process of a clinician, but deployment of such multi-modal algorithms in the real-world setting can be difficult. If the AI has been trained using the ground truth generated by a multi-modal imaging and additional biomarkers but during clinical use only a limited data is collected, then that algorithm may not be applicable. Therefore a balance needs to be achieved between what is practical for routine clinical use versus a complex algorithm that incorporates multiple inputs.
AI may also play a role in interpreting genetic diseases, such as those with variable expressivity and phenotypes. DL has been applied in genomics but still remains in its infancy. There have been studies that have shown some success with various -omics data, including prediction of expression (Chen et al., 2016), prediction of drug response in cell lines (Sakellaropoulos et al., 2019), prediction of tissue-of-origin and cancer type (Sakellaropoulos et al., 2019), and prediction of DNA function from sequence alone (Quang and Xie, 2016). DL models are still in its infancy for study of genomics and none has been validated in clinical practice. Multiple generic challenges exist, such as the lack of explainable AI, balanced datasets representing both disease and healthy states, and integration of heterogeneous data, which is akin to some of the challenges presented by multi-modal algorithms discussed above (Koumakis, 2020).
Medical schools and medical training programmes also need to adapt and incorporate understanding of digital innovations into training. Clinicians should learn to interpret studies on areas such as AI or DL algorithms (Ting et al., 2019) to know if and when such technologies would be suitable for their practice. Medical students should also learn to conduct remote consultations, be that video or telephone based only. Without the patient being physically present, the focus of consultations changes somewhat with the importance of excluding pathologies that require in person assessment rather than simply managing the presenting complaint. Nuanced changes to communication strategies need also to be developed adapted for virtual consultations, and clinicians need to develop at least some basic understanding of the technical aspect of each platform to enable simple trouble-shooting for new users. Finally patient attitudes need to be studied whilst recognising these will evolve, as any reaction to something novel. Education driven by evidence and not politics or other motivations, communicated effectively to reach a wide audience will be crucial in influencing patients to make their own considered decisions.
8. Conclusions
Myriad innovations have created a milieu ripe for telemedicine in ophthalmology to thrive and COVID-19 has hastened the development and embracement of these digital technologies. The growing AI and telecommunications technologies can potentially transform the delivery of the data-rich and image-dependent specialty of ophthalmology globally. 5G, IoT and AI are starting to be introduced into ophthalmology, but the potential for reliably linked machines such as OCTs and fundus cameras and algorithms changing ophthalmic service delivery is significant, and is likely to become more prevalent as the 5G network coverages grows, enabling a more mature IoT. These technologies may be able to make key contributions towards the provision of quality, sustainable eye care to all patients, and experiences from the pandemic has revealed the utility of telemedicine even in well-resourced and densely populated. Challenges associated with implementation of these technologies remain, including validation, patient acceptance, and education and training of end-users on these technologies. Physicians must continue to adapt to the changing models of care delivery, and collaborate with broader teams involving technology experts and data scientists to achieve universal quality and sustainable ophthalmic services.
Financial disclosure
Drs T Wong and D Ting are the patent holder for a deep learning system for retinal diseases. Dr L Pasquale is a consultant for Verily. Dr L Lam is a consultant for Ocutrx. Dr D Sim is the global medical lead for Big Picture Medical.
CRediT authorship contribution statement
Ji-Peng Olivia Li: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, revision, Project administration. Hanruo Liu: Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Darren S.J. Ting: Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, revision. Sohee Jeon: Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. R.V. Paul Chan: Data curation, Writing - original draft, Writing - review & editing, Supervision. Judy E. Kim: Data curation, Writing - original draft, Writing - review & editing, Supervision. Dawn A. Sim: Data curation, Writing - original draft, Writing - review & editing, Visualization. Peter B.M. Thomas: Data curation, Writing - original draft, Writing - review & editing, Visualization. Haotian Lin: Data curation, Writing - original draft, Writing - review & editing. Youxin Chen: Data curation, Writing - review & editing. Taiji Sakomoto: Data curation, Writing - original draft, Writing - review & editing, Supervision. Anat Loewenstein: Data curation, Writing - original draft, Writing - review & editing, Supervision. Dennis S.C. Lam: Data curation, Writing - original draft, Writing - review & editing, Supervision. Louis R. Pasquale: Data curation, Writing - original draft, Writing - review & editing, Supervision. Tien Y. Wong: Conceptualization, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision. Linda A. Lam: Conceptualization, Data curation, Writing - original draft, Writing - review & editing, Visualization. Daniel S.W. Ting: Conceptualization, Data curation, Writing - original draft, Writing - review & editing, Visualization, revision, Supervision.
References
- Centres for Medicare & Medicad Services . 2020. MEDICARE TELEMEDICINE HEALTH CARE PROVIDER FACT SHEET.https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet [Google Scholar]
- Aanestad M., Jensen T.B. Building nation-wide information infrastructures in healthcare through modular implementation strategies. J. Strat. Inf. Syst. 2011;20:161–175. [Google Scholar]
- Abdul Aziz A.A., Isaac M., Tehrani N.N. Using telemedicine to screen for retinopathy of prematurity. CMAJ (Can. Med. Assoc. J.) 2014;186:1012–1014. doi: 10.1503/cmaj.131357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M.D., Lou Y.Y., Erginay A., Clarida W., Amelon R., Folk J.C., Niemeijer M. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig. Ophthalmol. Vis. Sci. 2016;57:5200–5206. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- Abramoff M.D., Lavin P.T., Birch M., Shah N., Folk J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abràmoff Michael D., Lavin Philip T., Birch Michele, Shah Nilay, James C Folk. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M.D., Leng T., Ting D.S.W., Rhee K., Horton M.B., Brady C.J., Chiang M.F. Automated and computer-assisted detection, classification, and diagnosis of diabetic retinopathy. Telemed. J. e Health. 2020;26:544–550. doi: 10.1089/tmj.2020.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M., Ho C.Y.D., Baglin E., Sharangan P., Wu Z., Lawson D.J., Luu C.D., Turpin A., McKendrick A.M., Guymer R.H. Home monitoring of retinal sensitivity on a tablet device in intermediate age-related macular degeneration. Transl. Vis. Sci. Technol. 2018;7:32. doi: 10.1167/tvst.7.5.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agiwal M., Roy A.S., Saxena N. Next generation 5G wireless networks: a comprehensive survey. IEEE Commun. Surv. Tutorials. 2016;19:1617–1655. [Google Scholar]
- Aiello L.M., Bursell S.E., Cavallerano J., Gardner W.K., Strong J. Joslin vision network validation study: pilot image stabilization phase. J. Am. Optom. Assoc. 1998;69:699–710. [PubMed] [Google Scholar]
- Ajami S., Bagheri-Tadi T. Barriers for adopting electronic health records (EHRs) by physicians. Acta Inf. Med. 2013;21:129–134. doi: 10.5455/aim.2013.21.129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi R.O., Ansin A., Clover J., Wilkins J., Rao N.K., Terry M.A., Tran K.D., Sales C.S. Novel use of telemedicine for corneal tissue evaluation in eye banking: establishing a standardized approach for the remote evaluation of donor corneas for transplantation. Cornea. 2019;38:509–514. doi: 10.1097/ICO.0000000000001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alda E., Lomuto C.C., Benitez A.M., Bouzas L., Brussa M., Cattaino A., Dinerstein N.A., Erpen N., Galina L., Mansilla C., Marinaro S., Quiroga A., Saidman G., Sanchez C., Sepulveda T., Visintin P. Results of the national program for the prevention of blindness in childhood by retinopathy of prematurity in Argentina (2004-2016) Arch. Argent. Pediatr. 2018;116:386–393. doi: 10.5546/aap.2018.eng.386. [DOI] [PubMed] [Google Scholar]
- Almazroa A., Burman R., Raahemifar K., Lakshminarayanan V. Optic disc and optic cup segmentation methodologies for glaucoma image detection: a survey. J. Ophthalmol. 2015;2015:180972. doi: 10.1155/2015/180972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academcy Ophthalmology (AAO) Telemedicine Task Force . American Academy of Ophthalmology Clinical Statements; 2018. Telemedicine for ophthalmology information statement.https://www.aao.org/clinical-statement/telemedicine-ophthalmology-information-statement [Google Scholar]
- American Medical Association . 2019. Augmented intelligence in health care. Policy.https://www.ama-assn.org/system/files/2019-08/ai-2018-board-policy-summary.pdf [Google Scholar]
- Amirsolaimani B., Peyman G., Schwiegerling J., Bablumyan A., Peyghambarian N. A new low-cost, compact, auto-phoropter for refractive assessment in developing countries. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amparo F., Dana R. Web-based longitudinal remote assessment of dry eye symptoms. Ocul. Surf. 2018;16:249–253. doi: 10.1016/j.jtos.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Amsler M. L'Examen qualitatif de la fonction maculaire. Ophthalmologica. 1947;114:248–261. [Google Scholar]
- Anderson A.J., Bedggood P.A., Kong Y.X.G., Martin K.R., Vingrys A.J. Can home monitoring allow earlier detection of rapid visual field progression in glaucoma? Ophthalmology. 2017;124:1735–1742. doi: 10.1016/j.ophtha.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Annan Li, Cheng Jun, Wong Damon Wing Kee, Jiang Liu. Integrating holistic and local deep features for glaucoma classification. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016:1328–1331. doi: 10.1109/EMBC.2016.7590952. 2016. [DOI] [PubMed] [Google Scholar]
- Arora S., Rudnisky C.J., Damji K.F. Improved access and cycle time with an ''in-house'' patient-centered teleglaucoma program versus traditional in-person assessment. Telemed. J. e Health. 2014;20:439–445. doi: 10.1089/tmj.2013.0241. [DOI] [PubMed] [Google Scholar]
- Asaoka R., Murata H., Iwase A., Araie M. Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology. 2016;123:1974–1980. doi: 10.1016/j.ophtha.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Atkinson-Briggs S., Jenkins A., Keech A., Ryan C., Brazionis L., Retinopathy Centre of Research Excellence in Diabetic Integrating diabetic retinopathy screening within diabetes education services in Australia's diabetes and indigenous primary care clinics. Intern. Med. J. 2019;49:797–800. doi: 10.1111/imj.14309. [DOI] [PubMed] [Google Scholar]
- Azuara-Blanco A., Burr J., Thomas R., Maclennan G., McPherson S. The accuracy of accredited glaucoma optometrists in the diagnosis and treatment recommendation for glaucoma. Br. J. Ophthalmol. 2007;91:1639–1643. doi: 10.1136/bjo.2007.119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balyen L., Peto T. Promising artificial intelligence-machine learning-deep learning algorithms in ophthalmology. Asia. Pac. J. Ophthalmol. 2019;8:264–272. doi: 10.22608/APO.2018479. [DOI] [PubMed] [Google Scholar]
- Bastawrous A., Leak C., Howard F., Kumar V. Validation of near eye tool for refractive assessment (NETRA) ― pilot study. J. Mob. Technol. Med. 2012;1(3):6–16. [Google Scholar]
- Bastawrous A., Rono H.K., Livingstone I.A., Weiss H.A., Jordan S., Kuper H., Burton M.J. Development and validation of a smartphone-based visual acuity test (Peek acuity) for clinical practice and community-based fieldwork. JAMA Ophthalmol. 2015;133:930–937. doi: 10.1001/jamaophthalmol.2015.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemo V., Lim G., Rim T.H., Tan G.S.W., Cheung C.Y., Sadda S., He M.G., Tufail A., Lee M.L., Hsu W., Ting D.S.W. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr. Diabetes Rep. 2019;19:72. doi: 10.1007/s11892-019-1189-3. [DOI] [PubMed] [Google Scholar]
- Bengio Y., Courville A., Vincent P. Representation learning: a review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 2013;35:1798–1828. doi: 10.1109/TPAMI.2013.50. [DOI] [PubMed] [Google Scholar]
- Berlinger Joshua. CNN; 2020. WHO Warns Governments 'this Is Not a Drill' as Coronavirus Infections Near 100,000 Worldwide. [Google Scholar]
- Biten H., Redd T.K., Moleta C., Campbell J.P., Ostmo S., Jonas K., Chan R.V.P., Chiang M.F. Diagnostic accuracy of ophthalmoscopy vs telemedicine in examinations for retinopathy of prematurity. JAMA Ophthalmol. 2018;136:498–504. doi: 10.1001/jamaophthalmol.2018.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenreider O., Cornet R., Vreeman D.J. Recent developments in clinical terminologies - SNOMED CT, LOINC, and RxNorm. IMIA Yearb. Med. Inf. 2018;27:129–139. doi: 10.1055/s-0038-1667077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic H., Montuoro A., Baratsits M., Karantonis M.G., Waldstein S.M., Schlanitz F., Schmidt-Erfurth U. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest. Ophthalmol. Vis. Sci. 2017;58:BIO141–B150. doi: 10.1167/iovs.17-21789. [DOI] [PubMed] [Google Scholar]
- Bourdon H., Jaillant R., Ballino A., El Kaim P., Debillon L., Bodin S., N’Kosi L. Teleconsultation in primary ophthalmic emergencies during the COVID-19 lockdown in Paris: experience with 500 patients in March and April 2020. J. Fr. Ophtalmol. 2020;43(7):577–585. doi: 10.1016/j.jfo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne R.R., Stevens G.A., White R.A., Smith J.L., Flaxman S.R., Price H., Jonas J.B., Keeffe J., Leasher J., Naidoo K., Pesudovs K., Resnikoff S., Taylor H.R., Group Vision Loss Expert Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet. Glob. Health. 2013;1:e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- Bowd C., Lee I., Goldbaum M.H., Balasubramanian M., Medeiros F.A., Zangwill L.M., Girkin C.A., Liebmann J.M., Weinreb R.N. Predicting glaucomatous progression in glaucoma suspect eyes using relevance vector machine classifiers for combined structural and functional measurements. Investig. Ophthalmol. Vis. Sci. 2012;53:2382–2389. doi: 10.1167/iovs.11-7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady C.J., Garg S. Telemedicine for age-related macular degeneration. Telemed. J. e Health. 2020;26:565–568. doi: 10.1089/tmj.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady C.J., D'Amico S., Campbell J.P. Telemedicine for retinopathy of prematurity. Telemed. J. e Health. 2020;26:556–564. doi: 10.1089/tmj.2020.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsdorfer A., Kang J.J. Improving accuracy for intraocular lens selection in cataract surgery. Curr. Opin. Ophthalmol. 2018;29:323–327. doi: 10.1097/ICU.0000000000000493. [DOI] [PubMed] [Google Scholar]
- Brennan S., Serle J., Clover B. 2018. 'Tariff Set for Sweeping Change from Next Year.https://www.hsj.co.uk/finance-and-efficiency/tariff-set-for-sweeping-change-from-next-year/7023543.article [Google Scholar]
- Brigatti L., Hoffman D., Caprioli J. Neural networks to identify glaucoma with structural and functional measurements. Am. J. Ophthalmol. 1996;121:511–521. doi: 10.1016/s0002-9394(14)75425-x. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Kaiser P.K., Michels M., Soubrane G., Heier J.S., Kim R.Y., Sy J.P., Schneider S., Anchor Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Brown J.M., Campbell J.P., Beers A., Chang K., Ostmo S., Chan R.V.P., Dy J., Erdogmus D., Ioannidis S., Kalpathy-Cramer J., Chiang M.F., Imaging, and consortium informatics in retinopathy of prematurity research automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–810. doi: 10.1001/jamaophthalmol.2018.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J.C., Norman P., Shickle D., Cassels-Brown A., MacEwen C. Failing to plan and planning to fail. Can we predict the future growth of demand on UK Eye Care Services? Eye. 2019;33:1029–1031. doi: 10.1038/s41433-019-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgansky-Eliash Z., Wollstein G., Chu T., Ramsey J.D., Glymour C., Noecker R.J., Ishikawa H., Schuman J.S. Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest. Ophthalmol. Vis. Sci. 2005;46:4147–4152. doi: 10.1167/iovs.05-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlina P.M., Joshi N., Pekala M., Pacheco K.D., Freund D.E., Bressler N.M. Automated grading of age-related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. 2017;135:1170–1176. doi: 10.1001/jamaophthalmol.2017.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.P., Ataer-Cansizoglu E., Bolon-Canedo V., Bozkurt A., Erdogmus D., Kalpathy-Cramer J., Patel S.N., Reynolds J.D., Horowitz J., Hutcheson K., Shapiro M., Repka M.X., Ferrone P., Drenser K., Martinez-Castellanos M.A., Ostmo S., Jonas K., Chan R.V., Chiang M.F., Imaging, Research Consortium Informatics R.O.P. Expert diagnosis of plus disease in retinopathy of prematurity from computer-based image analysis. JAMA Ophthalmol. 2016;134:651–657. doi: 10.1001/jamaophthalmol.2016.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallerano A.A., Cavallerano J.D., Katalinic P., Blake B., Rynne M., Conlin P.R., Hock K., Tolson A.M., Aiello L.P., Aiello L.M., Team Joslin Vision Network Research A telemedicine program for diabetic retinopathy in a veterans Affairs medical center--the Joslin vision network eye health care model. Am. J. Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Chakrabarty L., Joshi G.D., Chakravarty A., Raman G.V., Krishnadas S.R., Sivaswamy J. Automated detection of glaucoma from topographic features of the optic nerve head in color fundus photographs. J. Glaucoma. 2016;25:590–597. doi: 10.1097/IJG.0000000000000354. [DOI] [PubMed] [Google Scholar]
- Chan K., Lee T.W., Sample P.A., Goldbaum M.H., Weinreb R.N., Sejnowski T.J. Comparison of machine learning and traditional classifiers in glaucoma diagnosis. IEEE Trans. Biomed. Eng. 2002;49:963–974. doi: 10.1109/TBME.2002.802012. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li X. Characteristics of severe retinopathy of prematurity patients in China: a repeat of the first epidemic? Br. J. Ophthalmol. 2006;90:268–271. doi: 10.1136/bjo.2005.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li Y., Narayan R., Subramanian A., Xie X. Gene expression inference with deep learning. Bioinformatics. 2016;32:1832–1839. doi: 10.1093/bioinformatics/btw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Li F., Harmer P. Healthy China 2030: moving from blueprint to action with a new focus on public health. Lancet Public Health. 2019;4:e447. doi: 10.1016/S2468-2667(19)30160-4. [DOI] [PubMed] [Google Scholar]
- Cheng J., Tao D., Liu J., Wong D.W., Tan N.M., Wong T.Y., Saw S.M. Peripapillary atrophy detection by sparse biologically inspired feature manifold. IEEE Trans. Med. Imag. 2012;31:2355–2365. doi: 10.1109/TMI.2012.2218118. [DOI] [PubMed] [Google Scholar]
- Cheung C.Y., Tang F., Ting D.S.W., Tan G.S.W., Wong T.Y. 2019. Artificial Intelligence in Diabetic Eye Disease Screening', Asia Pac J Ophthalmol (Phila) [DOI] [PubMed] [Google Scholar]
- Group Areds Home Study Research, Chew E.Y., Clemons T.E., Bressler S.B., Elman M.J., Danis R.P., Domalpally A., Heier J.S., Kim J.E., Garfinkel R. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E.Y., Clemons T.E., Harrington M., Bressler S.B., Elman M.J., Kim J.E., Garfinkel R., Heier J.S., Brucker A., Boyer D., Areds Home Study Research Group Effectiveness OF different monitoring modalities IN the detection OF neovascular age-related macular degeneration: the home study, report number 3. Retina. 2016;36:1542–1547. doi: 10.1097/IAE.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.F., Keenan J.D., Starren J., Du Y.E., Schiff W.M., Barile G.R., Li J., Johnson R.A., Hess D.J., Flynn J.T. Accuracy and reliability of remote retinopathy of prematurity diagnosis. Arch. Ophthalmol. 2006;124:322–327. doi: 10.1001/archopht.124.3.322. [DOI] [PubMed] [Google Scholar]
- Chiang M.F., Jiang L., Gelman R., Du Y.E., Flynn J.T. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch. Ophthalmol. 2007;125:875–880. doi: 10.1001/archopht.125.7.875. [DOI] [PubMed] [Google Scholar]
- Chiang M.F., Melia M., Buffenn A.N., Lambert S.R., Recchia F.M., Simpson J.L., Yang M.B. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:1272–1280. doi: 10.1016/j.ophtha.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher M., Bowd C., Belghith A., Goldbaum M.H., Weinreb R.N., Fazio M.A., Girkin C.A., Liebmann J.M., Zangwill L.M. Deep learning approaches predict glaucomatous visual field damage from OCT optic nerve head en face images and retinal nerve fiber layer thickness maps. Ophthalmology. 2020;127:346–356. doi: 10.1016/j.ophtha.2019.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K.J., Rosenfield M. Evaluation of the SVOne: a handheld, smartphone-based autorefractor. Optom. Vis. Sci. 2015;92:1133–1139. doi: 10.1097/OPX.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J., Puertas R., Kotecha A., Foster P.J., Barton K. Virtual clinics in glaucoma care: face-to-face versus remote decision-making. Br. J. Ophthalmol. 2017;101:892–895. doi: 10.1136/bjophthalmol-2016-308993. [DOI] [PubMed] [Google Scholar]
- Cleland C.R., Burton M.J., Hall C., Hall A., Courtright P., Makupa W.U., Philippin H. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop. Med. Int. Health. 2016;21:417–426. doi: 10.1111/tmi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. 2018. 'Adoption and Spread of Innovation in the NHS.https://www.kingsfund.org.uk/publications/innovation-nhs [Google Scholar]
- Court J.H., Austin M.W. Virtual glaucoma clinics: patient acceptance and quality of patient education compared to standard clinics. Clin. Ophthalmol. 2015;9:745–749. doi: 10.2147/OPTH.S75000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J.E., Han X., Qassim A., Hassall M., Cooke Bailey J.N., Kinzy T.G., Khawaja A.P., An J., Marshall H., Gharahkhani P., Igo R.P., Jr., Graham S.L., Healey P.R., Ong J.S., Zhou T., Siggs O., Law M.H., Souzeau E., Ridge B., Hysi P.G., Burdon K.P., Mills R.A., Landers J., Ruddle J.B., Agar A., Galanopoulos A., White A.J.R., Willoughby C.E., Andrew N.H., Best S., Vincent A.L., Goldberg I., Radford-Smith G., Martin N.G., Montgomery G.W., Vitart V., Hoehn R., Wojciechowski R., Jonas J.B., Aung T., Pasquale L.R., Cree A.J., Sivaprasad S., Vallabh N.A., consortium Neighborhood, Biobank Eye U.K., Vision Consortium, Viswanathan A.C., Pasutto F., Haines J.L., Klaver C.C.W., van Duijn C.M., Casson R.J., Foster P.J., Khaw P.T., Hammond C.J., Mackey D.A., Mitchell P., Lotery A.J., Wiggs J.L., Hewitt A.W., MacGregor S. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet. 2020;52:160–166. doi: 10.1038/s41588-019-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryotherapy for Retinopathy of Prematurity Cooperative, Group Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch. Ophthalmol. 2001;119:1110–1118. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- Day S., Acquah K., Lee P.P., Mruthyunjaya P., Sloan F.A. Medicare costs for neovascular age-related macular degeneration, 1994-2007. Am. J. Ophthalmol. 2011;152:1014–1020. doi: 10.1016/j.ajo.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fauw, Jeffrey Joseph R Ledsam, Romera-Paredes Bernardino, Nikolov Stanislav, Tomasev Nenad, Blackwell Sam, Askham Harry, Glorot Xavier, O'Donoghue Brendan, Visentin Daniel. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat. Med. 2018;24:1342. doi: 10.1038/s41591-018-0107-6. [DOI] [PubMed] [Google Scholar]
- Deemer A.D., Bradley C.K., Ross N.C., Natale D.M., Itthipanichpong R., Werblin F.S., Massof R.W. Low vision enhancement with head-mounted video display systems: are we there yet? Optom. Vis. Sci. 2018;95:694–703. doi: 10.1097/OPX.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNomie M., Medic V., Castro A., Vazquez M.B., Rodriguez B., Kim J. Lessons learned from a community-academic project using telemedicine for eye screening among urban Latinos. Prog. Community Health Partnersh. 2019;13:183–189. doi: 10.1353/cpr.2019.0018. [DOI] [PubMed] [Google Scholar]
- Devalla S.K., Chin K.S., Mari J.M., Tun T.A., Strouthidis N.G., Aung T., Thiery A.H., Girard M.J.A. A deep learning approach to digitally stain optical coherence tomography images of the optic nerve head. Invest. Ophthalmol. Vis. Sci. 2018;59:63–74. doi: 10.1167/iovs.17-22617. [DOI] [PubMed] [Google Scholar]
- Dhaliwal C., Wright E., Graham C., McIntosh N., Fleck B.W. Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. Br. J. Ophthalmol. 2009;93:355–359. doi: 10.1136/bjo.2008.148908. [DOI] [PubMed] [Google Scholar]
- Di Stefano A.F. VISION 2020: the right to sight. A global initiative for the elimination of avoidable blindness. Optometry. 2001;72:619–622. [PubMed] [Google Scholar]
- Dutta S., Raghuveer T., Vinekar A., Dogra M.R. Can we stop the current epidemic of blindness from retinopathy of prematurity? Indian Pediatr. 2016;53(Suppl. 2):S80–S84. [PubMed] [Google Scholar]
- Early Treatment For Retinopathy Of Prematurity Cooperative, Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- Eckardt C., Paulo E.B. HEADS-UP surgery for vitreoretinal procedures: an experimental and clinical study. Retina. 2016;36:137–147. doi: 10.1097/IAE.0000000000000689. [DOI] [PubMed] [Google Scholar]
- Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks (vol 542, pg 115, 2017) Nature. 2017;546 doi: 10.1038/nature21056. 686-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Laydon D., Nedjati-Gilani G., Imai N., Ainslie K., Baguelin M., et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. 2020. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf URL: [Accessed on March 23, 2020]'. [DOI] [PMC free article] [PubMed]
- Fijalkowski N., Zheng L.L., Henderson M.T., Wang S.K., Wallenstein M.B., Leng T., Moshfeghi D.M. Stanford university network for diagnosis of retinopathy of prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surg. Lasers Imaging Retina. 2014;45:106–113. doi: 10.3928/23258160-20140122-01. [DOI] [PubMed] [Google Scholar]
- Fischer M.J., Scharloo M., Abbink J., van 't Hul A., van Ranst D., Rudolphus A., Weinman J., Rabe K.F., Kaptein A.A. Concerns about exercise are related to walk test results in pulmonary rehabilitation for patients with COPD. Int. J. Behav. Med. 2012;19:39–47. doi: 10.1007/s12529-010-9130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Silvester A., Stevens G.A., Tahhan N., Wong T.Y., Taylor H.R. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet. Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- Fleming C., Whitlock E.P., Beil T., Smit B., Harris R.P. Screening for primary open-angle, glaucoma in the primary care setting: an update for the US Preventive Services Task Force. Ann. Fam. Med. 2005;3:167–170. doi: 10.1370/afm.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foot B., MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye. 2017;31:771–775. doi: 10.1038/eye.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke T.R., Holden B.A., Wilson D.A., Schlenther G., Naidoo K.S., Resnikoff S., Frick K.D. Global cost of correcting vision impairment from uncorrected refractive error. Bull. World Health Organ. 2012;90:728–738. doi: 10.2471/BLT.12.104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D.S., O'Colmain B.J., Munoz B., Tomany S.C., McCarty C., de Jong P.T., Nemesure B., Mitchell P., Kempen J., Group Eye Diseases Prevalence Research Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gagnon M.P., Cloutier A., Fortin J.P. Quebec population and telehealth: a survey on knowledge and perceptions. Telemed. J. e Health. 2004;10:3–12. doi: 10.1089/153056204773644526. [DOI] [PubMed] [Google Scholar]
- Gaiser H., Moore B., Pamplona V., Solaka N., Schafran D., Merrill D., Sharpe N., Geringer J., Raskar R. Comparison of a novel cell phone-based refraction technique (Netra-G) with subjective refraction. Investig. Ophthalmol. Vis. Sci. 2013;54 [Google Scholar]
- Gan K., Liu Y., Stagg B., Rathi S., Pasquale L.R., Damji K. Telemedicine for glaucoma: guidelines and recommendations. Telemed. J. e Health. 2020;26:551–555. doi: 10.1089/tmj.2020.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargeya Rishab, Leng Theodore. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124:962–969. doi: 10.1016/j.ophtha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Gianfrancesco M.A., Tamang S., Yazdany J., Schmajuk G. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern. Med. 2018;178:1544–1547. doi: 10.1001/jamainternmed.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.M. Diabetic retinopathy and age-related macular degeneration in the U.S. Am. J. Prev. Med. 2012;43:48–54. doi: 10.1016/j.amepre.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Gibson P.G., Powell H., Coughlan J., Wilson A.J., Abramson M., Haywood P., Bauman A., Hensley M.J., Walters E.H. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst. Rev. 2003:CD001117. doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Changing challenges in the control of blindness in children. Eye. 2007;21:1338–1343. doi: 10.1038/sj.eye.6702841. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study, Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goatman K., Charnley A., Webster L., Nussey S. Assessment of automated disease detection in diabetic retinopathy screening using two-field photography. PloS One. 2011;6 doi: 10.1371/journal.pone.0027524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbaum M.H., Sample P.A., White H., Colt B., Raphaelian P., Fechtner R.D., Weinreb R.N. Interpretation of automated perimetry for glaucoma by neural network. Invest. Ophthalmol. Vis. Sci. 1994;35:3362–3373. [PubMed] [Google Scholar]
- Goldbaum M.H., Sample P.A., Chan K., Williams J., Lee T.W., Blumenthal E., Girkin C.A., Zangwill L.M., Bowd C., Sejnowski T., Weinreb R.N. Comparing machine learning classifiers for diagnosing glaucoma from standard automated perimetry. Invest. Ophthalmol. Vis. Sci. 2002;43:162–169. [PubMed] [Google Scholar]
- Goyal A., Gopalakrishnan M., Anantharaman G., Chandrashekharan D.P., Thachil T., Sharma A. Smartphone guided wide-field imaging for retinopathy of prematurity in neonatal intensive care unit - a Smart ROP (SROP) initiative. Indian J. Ophthalmol. 2019;67:840–845. doi: 10.4103/ijo.IJO_1177_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann F., Mengelkamp J., Brandl C., Harsch S., Zimmermann M.E., Linkohr B., Peters A., Heid I.M., Palm C., Weber B.H.F. A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology. 2018;125:1410–1420. doi: 10.1016/j.ophtha.2018.02.037. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T., Wherton J., Shaw S., Morrison C. Video consultations for covid-19. BMJ. 2020;368:m998. doi: 10.1136/bmj.m998. [DOI] [PubMed] [Google Scholar]
- Grossniklaus H.E., Green W.R. Pathologic findings in pathologic myopia. Retina. 1992;12:127–133. doi: 10.1097/00006982-199212020-00009. [DOI] [PubMed] [Google Scholar]
- Gulshan V., Peng L., Coram M., Stumpe M.C., Wu D., Narayanaswamy A., Venugopalan S., Widner K., Madams T., Cuadros J., Kim R., Raman R., Nelson P.C., Mega J.L., Webster D.R. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. J. Am. Med. Assoc. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- Gulshan V., Rajan R.P., Widner K., Wu D., Wubbels P., Rhodes T., Whitehouse K., Coram M., Corrado G., Ramasamy K., Raman R., Peng L., Webster D.R. Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019;137(9):987–993. doi: 10.1001/jamaophthalmol.2019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn P.J.G., Marks J.R., Au L., Waterman H., Spry P.G.D., Harper R.A. Acceptability and use of glaucoma virtual clinics in the UK: a national survey of clinical leads. BMJ Open Ophthalmol. 2018;3 doi: 10.1136/bmjophth-2017-000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta O.P., Shienbaum G., Patel A.H., Fecarotta C., Kaiser R.S., Regillo C.D. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–2140. doi: 10.1016/j.ophtha.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Gupta M.P., Chan R.V.P., Anzures R., Ostmo S., Jonas K., Chiang M.F., Imaging, Research Consortium Informatics R.O.P. Practice patterns in retinopathy of prematurity treatment for disease milder than recommended by guidelines. Am. J. Ophthalmol. 2016;163:1–10. doi: 10.1016/j.ajo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Mesik J., Engel S.A., Smith R., Schatza M., Calabrese A., van Kuijk F.J., Erdman A.G., Legge G.E. Beneficial effects of spatial remapping for reading with simulated central field loss. Invest. Ophthalmol. Vis. Sci. 2018;59:1105–1112. doi: 10.1167/iovs.16-21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwatidzo S.D., Stewart Williams J. Diabetes mellitus medication use and catastrophic healthcare expenditure among adults aged 50+ years in China and India: results from the WHO study on global AGEing and adult health (SAGE) BMC Geriatr. 2017;17:14. doi: 10.1186/s12877-016-0408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem M.S., Han L.X., van Hemert J., Li B.H. Automatic extraction of retinal features from colour retinal images for glaucoma diagnosis: a review. Comput. Med. Imag. Graph. 2013;37:581–596. doi: 10.1016/j.compmedimag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Hao K. 2020. Doctors Are Using AI to Triage Covid-19 Paitents. The Tools May Be Here to Stay.https://www.technologyreview.com/2020/04/23/1000410/ai-triage-covid-19-patients-health-care/ Accessed May 25. [Google Scholar]
- Hariharan L., Gilbert C.E., Quinn G.E., Barg F.K., Lomuto C., Quiroga A., McLeod-Omawale J., Zin A., Ortiz Z., Alda E., Bouzas L., Brussa M., Cattaino A., Dinerstein A., Erpen N., Fandino A., Galina L., Manzitti J., Marinaro S., Sepulveda T., Visintin P., Silva J.C., Magluta C., Benitez A. Reducing blindness from retinopathy of prematurity (ROP) in Argentina through collaboration, advocacy and policy implementation. Health Pol. Plann. 2018;33:654–665. doi: 10.1093/heapol/czy004. [DOI] [PubMed] [Google Scholar]
- Hark L., Waisbourd M., Myers J.S., Henderer J., Crews J.E., Saaddine J.B., Molineaux J., Johnson D., Sembhi H., Stratford S., Suleiman A., Pizzi L., Spaeth G.L., Katz L.J. Improving access to eye care among persons at high-risk of glaucoma in Philadelphia - design and methodology: the Philadelphia glaucoma detection and treatment project. Ophthalmic Epidemiol. 2016;23:122–130. doi: 10.3109/09286586.2015.1099683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark L.A., Katz L.J., Myers J.S., Waisbourd M., Johnson D., Pizzi L.T., Leiby B.E., Fudemberg S.J., Mantravadi A.V., Henderer J.D., Zhan T., Molineaux J., Doyle V., Divers M., Burns C., Murchison A.P., Reber S., Resende A., Bui T.D.V., Lee J., Crews J.E., Saaddine J.B., Lee P.P., Pasquale L.R., Haller J.A. Philadelphia telemedicine glaucoma detection and follow-up study: methods and screening results. Am. J. Ophthalmol. 2017;181:114–124. doi: 10.1016/j.ajo.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Hatef E., Sharfstein J.M., Labrique A.B. Innovation and entrepreneurship: harnessing the public health skill set in a new era of health reforms and investment. J. Publ. Health Manag. Pract. 2018;24:99–101. doi: 10.1097/PHH.0000000000000665. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Ohno-Matsui K., Shimada N., Moriyama M., Kojima A., Hayashi W., Yasuzumi K., Nagaoka N., Saka N., Yoshida T., Tokoro T., Mochizuki M. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–1611. doi: 10.1016/j.ophtha.2009.11.003. 611 e1-4. [DOI] [PubMed] [Google Scholar]
- Hellstrom A., Smith L.E., Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C., Knapp M., Fernandez J.L., Beecham J., Hirani S.P., Cartwright M., Rixon L., Beynon M., Rogers A., Bower P., Doll H., Fitzpatrick R., Steventon A., Bardsley M., Hendy J., Newman S.P. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. Br. Med. J. 2013;346:f1035. doi: 10.1136/bmj.f1035. [DOI] [PubMed] [Google Scholar]
- Hilton S., Katz J., Zeger S. Classifying visual field data. Stat. Med. 1996;15:1349–1364. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1349::AID-SIM270>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hoffman L.C. Shedding light on telemedicine & online prescribing: the need to balance access to health care and quality of care. Am. J. Law Med. 2020;46:237–251. doi: 10.1177/0098858820933497. [DOI] [PubMed] [Google Scholar]
- Hogarty D.T., Hogarty J.P., Hewitt A.W. Smartphone use in ophthalmology: what is their place in clinical practice? Surv. Ophthalmol. 2019;65(2):250–262. doi: 10.1016/j.survophthal.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for covid-19. N. Engl. J. Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- Hong Z., Li N., Li D., Li J., Li B., Xiong W., Lu L., Li W., Zhou D. Telemedicine during the COVID-19 pandemic: experiences from western China. J. Med. Internet Res. 2020;22 doi: 10.2196/19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M.B., Brady C.J., Cavallerano J., Abramoff M., Barker G., Chiang M.F., Crockett C.H., Garg S., Karth P., Liu Y., Newman C.D., Rathi S., Sheth V., Silva P., Stebbins K., Zimmer-Galler I. third ed. vol. 26. 2020. pp. 495–543. (Practice Guidelines for Ocular Telehealth-Diabetic Retinopathy). Telemed. J. e Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson C.P., Kinney M.V., McDougall L., Lawn J.E., Group Born too soon Preterm Birth Action born too soon: preterm birth matters. Reprod. Health. 2013;10(Suppl. 1):S1. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.L., Chen H.Y., Lin J.C. Rule extraction for glaucoma detection with summary data from StratusOCT. Invest. Ophthalmol. Vis. Sci. 2007;48:244–250. doi: 10.1167/iovs.06-0320. [DOI] [PubMed] [Google Scholar]
- Hunter D.G., Repka M.X. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993;100:238–244. doi: 10.1016/s0161-6420(93)31664-7. [DOI] [PubMed] [Google Scholar]
- Inomata T., Iwagami M., Nakamura M., Shiang T., Yoshimura Y., Fujimoto K., Okumura Y., Eguchi A., Iwata N., Miura M., Hori S., Hiratsuka Y., Uchino M., Tsubota K., Dana R., Murakami A. Characteristics and risk factors associated with diagnosed and undiagnosed symptomatic dry eye using a smartphone application. JAMA Ophthalmol. 2019;138(1):58–68. doi: 10.1001/jamaophthalmol.2019.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee for the Classification of Retinopathy of, Prematurity The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- International Council of Ophthalmology . 2017. ICO guidelines for diabetic eye care. [Google Scholar]
- Isaac M., Isaranuwatchai W., Tehrani N. Cost analysis of remote telemedicine screening for retinopathy of prematurity. Can. J. Ophthalmol. 2018;53:162–167. doi: 10.1016/j.jcjo.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Issac A., Sarathi M.P., Dutta M.K. An adaptive threshold based image processing technique for improved glaucoma detection and classification. Comput. Methods Progr. Biomed. 2015;122:229–244. doi: 10.1016/j.cmpb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Ittoop S.M., SooHoo J.R., Seibold L.K., Mansouri K., Kahook M.Y. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv. Ther. 2016;33:1679–1690. doi: 10.1007/s12325-016-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Weng J., Zhu D., Ji L., Lu J., Zhou Z., Zou D., Guo L., Ji Q., Chen L., Chen L., Dou J., Guo X., Kuang H., Li L., Li Q., Li X., Liu J., Ran X., Shi L., Song G., Xiao X., Yang L., Zhao Z., Society Chinese Diabetes Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019;35:e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- Jin K., Lu H., Su Z., Cheng C., Ye J., Qian D. Telemedicine screening of retinal diseases with a handheld portable non-mydriatic fundus camera. BMC Ophthalmol. 2017;17:89. doi: 10.1186/s12886-017-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Premila M., Javed M., Vikas G., Wagholikar A. A pilot study to improve access to eye care services for patients in rural India by implementing community ophthalmology through innovative telehealth technology. Stud. Health Technol. Inf. 2015;214:139–145. [PubMed] [Google Scholar]
- Johnson K.A., Meyer J., Yazar S., Turner A.W. Real-time teleophthalmology in rural Western Australia. Aust. J. Rural Health. 2015;23:142–149. doi: 10.1111/ajr.12150. [DOI] [PubMed] [Google Scholar]
- Jonas J.B., Aung T., Bourne R.R., Bron A.M., Ritch R., Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- Jonas J.B., Wu S.L., Wang Y.X. Diabetic retinopathy in China: population-based studies and clinical and experimental investigations. Lancet. 2017;390 [Google Scholar]
- Kaiser P.K., Wang Y.Z., He Y.G., Weisberger A., Wolf S., Smith C.H. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: results of a pilot study. Retina. 2013;33:1863–1870. doi: 10.1097/IAE.0b013e3182899258. [DOI] [PubMed] [Google Scholar]
- Kalinowska A., Nowomiejska K., Brzozowska A., Maciejewski R., Rejdak R. Metamorphopsia score and central visual field outcomes in diabetic cystoid macular edema. BioMed Res. Int. 2018:4954532. doi: 10.1155/2018/4954532. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpathy-Cramer J., Campbell J.P., Erdogmus D., Tian P., Kedarisetti D., Moleta C., Reynolds J.D., Hutcheson K., Shapiro M.J., Repka M.X., Ferrone P., Drenser K., Horowitz J., Sonmez K., Swan R., Ostmo S., Jonas K.E., Chan R.V., Chiang M.F., Imaging, and Consortium Informatics in Retinopathy of Prematurity Research plus disease in retinopathy of prematurity: improving diagnosis by ranking disease severity and using quantitative image analysis. Ophthalmology. 2016;123:2345–2351. doi: 10.1016/j.ophtha.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Thomas P.B.M., Sim D.A., Parker R.T., Daniel C., Uddin J.M. Oculoplastic video-based telemedicine consultations: covid-19 and beyond. Eye. 2020;34(7):1193–1195. doi: 10.1038/s41433-020-0953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R., Whigham B.T., Al-Aswad L.A. Artificial intelligence and optical coherence tomography imaging. Asia. Pac. J. Ophthalmol. 2019;8:187–194. doi: 10.22608/APO.201904. [DOI] [PubMed] [Google Scholar]
- Kaptein A.A., Fischer M.J., Scharloo M. Self-management in patients with COPD: theoretical context, content, outcomes, and integration into clinical care. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:907–917. doi: 10.2147/COPD.S49622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam F., Sogbesan E., Boucher S., Rudnisky C.J., Prince W., Leinweber G., Pilipchuk T., Kogan S., Edwards M.C., Dorey M.W., Damji K.F. Collaborative care and teleglaucoma: a novel approach to delivering glaucoma services in Northern Alberta, Canada. Clin. Exp. Optom. 2013;96:577–580. doi: 10.1111/cxo.12065. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Sharafeldin N., Sundaram A., Campbell S., Tennant M., Rudnisky C., Weis E., Damji K.F. Tele-ophthalmology for age-related macular degeneration and diabetic retinopathy screening: a systematic review and meta-analysis. Telemed. J. e Health. 2018;24:301–308. doi: 10.1089/tmj.2017.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel S., Li Z., Scheetz J., Robman L., Phung J., Makeyeva G., Aung K., Liu C., Yan X., Meng W., Guymer R., Chang R., He M. Development and validation of a deep-learning algorithm for the detection of neovascular age-related macular degeneration from colour fundus photographs. Clin. Exp. Ophthalmol. 2019;47:1009–1018. doi: 10.1111/ceo.13575. [DOI] [PubMed] [Google Scholar]
- Keesara S., Jonas A., Schulman K. Covid-19 and health care’s digital revolution. N. Engl. J. Med. 2020;382(23):e82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- Kemper A.R., Freedman S.F., Wallace D.K. Retinopathy of prematurity care: patterns of care and workforce analysis. J aapos. 2008;12:344–348. doi: 10.1016/j.jaapos.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermany D.S., Goldbaum M., Cai W., Valentim C.C.S., Liang H., Baxter S.L., McKeown A., Yang G., Wu X., Yan F., Dong J., Prasadha M.K., Pei J., Ting M.Y.L., Zhu J., Li C., Hewett S., Dong J., Ziyar I., Shi A., Zhang R., Zheng L., Hou R., Shi W., Fu X., Duan Y., Huu V.A.N., Wen C., Zhang E.D., Zhang C.L., Li O., Wang X., Singer M.A., Sun X., Xu J., Tafreshi A., Lewis M.A., Xia H., Zhang K. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122–11231 e9. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Kern C., Fu D.J., Kortuem K., Huemer J., Barker D., Davis A., Balaskas K., Keane P.A., McKinnon T., Sim D.A. Implementation of a cloud-based referral platform in ophthalmology: making telemedicine services a reality in eye care. Br. J. Ophthalmol. 2019;104(3):312–317. doi: 10.1136/bjophthalmol-2019-314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T., Bertram M.Y., Jina R., Mash B., Levitt N., Hofman K. Preventing diabetes blindness: cost effectiveness of a screening programme using digital non-mydriatic fundus photography for diabetic retinopathy in a primary health care setting in South Africa. Diabetes Res. Clin. Pract. 2013;101:170–176. doi: 10.1016/j.diabres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Mustafa M.Z., Sanders R. Improving patient access to prevent sight loss: ophthalmic electronic referrals and communication (Scotland) Publ. Health. 2015;129:117–123. doi: 10.1016/j.puhe.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Khoo D.H., Tan K.T., Yeo K.T., Chew W., Yong V., Tan Y.T. Diabetic retinopathy--results of a two year screening programme in two medical units in Singapore. Ann. Acad. Med. Singapore. 1990;19:484–488. [PubMed] [Google Scholar]
- Kirkizlar E., Serban N., Sisson J.A., Swann J.L., Barnes C.S., Williams M.D. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604–2610. doi: 10.1016/j.ophtha.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Kirkwood B.J., Pesudovs K., Latimer P., Coster D.J. The efficacy of a nurse-led preoperative cataract assessment and postoperative care clinic. Med. J. Aust. 2006;184:278–281. doi: 10.5694/j.1326-5377.2006.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B.E., Knudtson M.D., Meuer S.M., Swift M., Gangnon R.E. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Kotecha A., Baldwin A., Brookes J., Foster P.J. Experiences with developing and implementing a virtual clinic for glaucoma care in an NHS setting. Clin. Ophthalmol. 2015;9:1915–1923. doi: 10.2147/OPTH.S92409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha A., Brookes J., Foster P.J. A technician-delivered 'virtual clinic' for triaging low-risk glaucoma referrals. Eye. 2017;31:899–905. doi: 10.1038/eye.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumakis L. Deep learning models in genomics; are we there yet? Comput. Struct. Biotechnol. J. 2020;18:1466–1473. doi: 10.1016/j.csbj.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause Jonathan, Gulshan Varun, Rahimy Ehsan, Karth Peter, Widner Kasumi, Corrado Greg S., Peng Lily, Webster Dale R. Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy. Ophthalmology. 2018;125:1264–1272. doi: 10.1016/j.ophtha.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Kumar S., Middlemiss C., Bulsara M., Guibilato A., Morgan W., Tay-Kearney M.L., Constable I.J., Yogesan K. Telemedicine-friendly, portable tonometers: an evaluation for intraocular pressure screening. Clin. Exp. Ophthalmol. 2006;34:666–670. doi: 10.1111/j.1442-9071.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Giubilato A., Morgan W., Jitskaia L., Barry C., Bulsara M., Constable I.J., Yogesan K. Glaucoma screening: analysis of conventional and telemedicine-friendly devices. Clin. Exp. Ophthalmol. 2007;35:237–243. doi: 10.1111/j.1442-9071.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- Kuo Lily, Boseley Sarah, Ian Sample, Tondo Lorenzo, Quinn Ben. The Guardian; 2020. Reporting on Coronavirus: 'fear Is Almost as Great a Threat as the Disease. [Google Scholar]
- Labiris G., Fanariotis M., Christoulakis C., Petounis A., Kitsos G., Aspiotis M., Psillas K. Tele-ophthalmology and conventional ophthalmology using in remote Greece a mobile medical unit. J. Telemed. Telecare. 2003;9:296–299. doi: 10.1258/135763303769211337. [DOI] [PubMed] [Google Scholar]
- Labiris G., Panagiotopoulou E.K., Kozobolis V.P. A systematic review of teleophthalmological studies in Europe. Int. J. Ophthalmol. 2018;11:314–325. doi: 10.18240/ijo.2018.02.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani P., Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284:574–582. doi: 10.1148/radiol.2017162326. [DOI] [PubMed] [Google Scholar]
- Laser ROP Study Group Laser therapy for retinopathy of prematurity. Laser ROP Study Group. Arch. Ophthalmol. 1994;112:154–156. doi: 10.1001/archopht.1994.01090140028007. [DOI] [PubMed] [Google Scholar]
- LeCun Y., Bengio Y., Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- Lee P.P., Walt J.G., Doyle J.J., Kotak S.V., Evans S.J., Budenz D.L., Chen P.P., Coleman A.L., Feldman R.M., Jampel H.D., Katz L.J., Mills R.P., Myers J.S., Noecker R.J., Piltz-Seymour J.R., Ritch R.R., Schacknow P.N., Serle J.B., Trick G.L. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch. Ophthalmol. 2006;124:12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- Lee Cecilia S., Baughman Doug M., Y Lee Aaron. Deep learning is effective for classifying normal versus age-related macular degeneration OCT images. Ophthalmology Retina. 2017;1:322–327. doi: 10.1016/j.oret.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Zamir D., Bertschi I. Media health literacy, eHealth literacy, and the role of the social environment in context. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15081643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A.D., Hogg R.E., Chandran M., Musonda L., North L., Chakravarthy U., Sivaprasad S., Menon G. Prevalence of diabetic retinopathy and visual impairment in patients with diabetes mellitus in Zambia through the implementation of a mobile diabetic retinopathy screening project in the Copperbelt province: a cross-sectional study. Eye. 2018;32:1201–1208. doi: 10.1038/s41433-018-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Powell A.M., Hooper P.L., Sheidow T.G. Prospective evaluation of teleophthalmology in screening and recurrence monitoring of neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2015;133:276–282. doi: 10.1001/jamaophthalmol.2014.5014. [DOI] [PubMed] [Google Scholar]
- Li F., Wang Z., Qu G.X., Song D.P., Yuan Y., Xu Y., Gao K., Luo G.W., Xiao Z.G., Lam D.S.C., Zhong H., Qiao Y., Zhang X.L. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med. Imag. 2018;18 doi: 10.1186/s12880-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., He Y., Keel S., Meng W., Chang R.T., He M. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology. 2018;125:1199–1206. doi: 10.1016/j.ophtha.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Li Z., Keel S., Liu C., He Y., Meng W., Scheetz J., Lee P.Y., Shaw J., Ting D., Wong T.Y., Taylor H., Chang R., He M. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care. 2018;41:2509–2516. doi: 10.2337/dc18-0147. [DOI] [PubMed] [Google Scholar]
- Li Z., Guo C., Nie D., Lin D., Zhu Y., Chen C., Zhang L., Xu F., Jin C., Zhang X., Xiao H., Zhang K., Zhao L., Yu S., Zhang G., Wang J., Lin H. A deep learning system for identifying lattice degeneration and retinal breaks using ultra-widefield fundus images. Ann. Transl. Med. 2019;7:618. doi: 10.21037/atm.2019.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Guo C., Nie D. Development and evaluation of a deep learning system for screening retinal hemorrhage based on ultra-widefield fundus images. Transl. Vis. Sci. Techn. 2020;9 doi: 10.1167/tvst.9.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Guo C., Nie D., Lin D., Zhu Y., Chen C., Wu X., Xu F., Jin C., Zhang X., Xiao H., Zhang K., Zhao L., Yan P., Lai W., Li J., Feng W., Li Y., Wei Ting D.S., Lin H. Deep learning for detecting retinal detachment and discerning macular status using ultra-widefield fundus images. Commun. Biol. 2020;3:15. doi: 10.1038/s42003-019-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.B., Friedman D.S., Zhou Q., Yang X., Sun L.P., Guo L.X., Tao Q.S., Chang D.S., Wang N.L., Group Handan Eye Study Prevalence of primary open angle glaucoma in a rural adult Chinese population: the Handan eye study. Invest. Ophthalmol. Vis. Sci. 2011;52:8250–8257. doi: 10.1167/iovs.11-7472. [DOI] [PubMed] [Google Scholar]
- Limwattananon C., Limwattananon S., Tungthong J., Sirikomon K. Association between a centrally reimbursed fee schedule policy and access to cataract surgery in the universal coverage scheme in Thailand. JAMA Ophthalmol. 2018;136:796–802. doi: 10.1001/jamaophthalmol.2018.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Hoffman D., Gaasterland D.E., Caprioli J. Neural networks to identify glaucomatous visual field progression. Am. J. Ophthalmol. 2003;135:49–54. doi: 10.1016/s0002-9394(02)01836-6. [DOI] [PubMed] [Google Scholar]
- Lin H.T., Long E.P., Ding X.H., Diao H.X., Chen Z.C., Liu R.Z., Huang J.L., Cai J.H., Xu S.J., Zhang X.Y., Wang D.N., Chen K.X., Yu T.Y., Wu D.X., Zhao X.T., Liu Z.Z., Wu X.H., Jiang Y.Z., Yang X., Cui D.M., Liu W.Y., Zheng Y.F., Luo L.X., Wang H.B., Chan C.C., Morgan I.G., He M.G., Liu Y.Z. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: a retrospective, multicentre machine learning study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.C., Wilkins M., Kim T., Malyugin B., Mehta J.S. Cataracts. Lancet. 2017;390:600–612. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- Liu H., Li L., Wormstone I.M., Qiao C., Zhang C., Liu P., Li S., Wang H., Mou D., Pang R., Yang D., Jiang L., Chen Y., Hu M., Xu Y., Kang H., Ji X., Chang R., Tham C., Cheung C., Ting D.S.W., Wong T.Y., Wang Z., Weinreb R.N., Xu M., Wang N. Development and validation of a deep learning system to detect glaucomatous optic neuropathy using fundus photographs. JAMA Ophthalmol. 2019;137(12):1353–1360. doi: 10.1001/jamaophthalmol.2019.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein A., Malach R., Goldstein M., Leibovitch I., Barak A., Baruch E., Alster Y., Rafaeli O., Avni I., Yassur Y. Replacing the Amsler grid: a new method for monitoring patients with age-related macular degeneration. Ophthalmology. 2003;110:966–970. doi: 10.1016/S0161-6420(03)00074-5. [DOI] [PubMed] [Google Scholar]
- Ludwig C.A., Murthy S.I., Pappuru R.R., Jais A., Myung D.J., Chang R.T. A novel smartphone ophthalmic imaging adapter: user feasibility studies in Hyderabad, India. Indian J. Ophthalmol. 2016;64:191–200. doi: 10.4103/0301-4738.181742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa A.Y., Evans C., DeLaune W.R., Patel P.S., Lynch M.G. A novel tele-eye protocol for ocular disease detection and access to eye care services. Telemed. J. e Health. 2014;20:318–323. doi: 10.1089/tmj.2013.0185. [DOI] [PubMed] [Google Scholar]
- Maamari R.N., Ausayakhun S., Margolis T.P., Fletcher D.A., Keenan J.D. Novel telemedicine device for diagnosis of corneal abrasions and ulcers in resource-poor settings. JAMA Ophthalmol. 2014;132:894–895. doi: 10.1001/jamaophthalmol.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J.C., Kreda D.A., Mandl K.D., Kohane I.S., Ramoni R.B. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J. Am. Med. Inf. Assoc. 2016;23:899–908. doi: 10.1093/jamia/ocv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Tabuchi H., Nakakura S., Ishitobi N., Miki M., Enno H. Deep-learning classifier with an ultrawide-field scanning laser ophthalmoscope detects glaucoma visual field severity. J. Glaucoma. 2018;27:647–652. doi: 10.1097/IJG.0000000000000988. [DOI] [PubMed] [Google Scholar]
- Masumoto Hiroki, Tabuchi Hitoshi, Nakakura Shunsuke, Ishitobi Naofumi, Miki Masayuki, Enno Hiroki. Deep-learning classifier with an ultrawide-field scanning laser ophthalmoscope detects glaucoma visual field severity. J. Glaucoma. 2018;27:647–652. doi: 10.1097/IJG.0000000000000988. [DOI] [PubMed] [Google Scholar]
- Mathews S.C., McShea M.J., Hanley C.L., Ravitz A., Labrique A.B., Cohen A.B. Digital health: a path to validation. NPJ Digit Med. 2019;2:38. doi: 10.1038/s41746-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall B. 15 ways Silicon Valley is harnessing Big Data for health. Nat. Med. 2020;26:7–10. doi: 10.1038/s41591-019-0708-8. [DOI] [PubMed] [Google Scholar]
- McCarthy J., Minsky M.L., Shannon C.E. A proposal for the Dartmouth summer research project on artificial intelligence - August 31, 1955. AI Mag. 2006;27:12–14. [Google Scholar]
- McNamara J.A., Tasman W., Brown G.C., Federman J.L. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98:576–580. doi: 10.1016/s0161-6420(91)32247-4. [DOI] [PubMed] [Google Scholar]
- Medeiros F.A., Jammal A.A., Thompson A.C. From machine to machine: an OCT-trained deep learning algorithm for objective quantification of glaucomatous damage in fundus photographs. Ophthalmology. 2019;126:513–521. doi: 10.1016/j.ophtha.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti J.M., Hendrick A.M., Khan F.N., Ziemer D.C., Pasquel F.J. Current and next generation portable screening devices for diabetic retinopathy. J. Diabetes Sci. Technol. 2016;10:295–300. doi: 10.1177/1932296816629158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.E., Thapa S., Robin A.L., Niziol L.M., Ramulu P.Y., Woodward M.A., Paudyal I., Pitha I., Kim T.N., Newman Casey P.A. Glaucoma screening in Nepal: cup-to-disc estimate with standard mydriatic fundus camera compared to portable nonmydriatic camera. Am. J. Ophthalmol. 2017;182:99–106. doi: 10.1016/j.ajo.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V.A., LeFevre M.L., Siu A.L., Peters J.J., Baumann L.C., Bibbins-Domingo K., Curry S.J., Ebell M., Flores G., Garcia F.A.R., Cantu A.G., Grossman D.C., Herzstein J., Nicholson W.K., Owens D.K., Phillips W.R., Pignone M.P., Wilt T., US Preventive Serv Task Force Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive services task Force recommendation statement. Ann. Intern. Med. 2013;159:342–348. doi: 10.7326/0003-4819-159-5-201309030-00008. [DOI] [PubMed] [Google Scholar]
- Moynihan V., Turner A. Coordination of diabetic retinopathy screening in the Kimberley region of Western Australia. Aust. J. Rural Health. 2017;25:110–115. doi: 10.1111/ajr.12290. [DOI] [PubMed] [Google Scholar]
- Muhammad H., Fuchs T.J., De Cuir N., De Moraes C.G., Blumberg D.M., Liebmann J.M., Ritch R., Hood D.C. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J. Glaucoma. 2017;26:1086–1094. doi: 10.1097/IJG.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Silva R.A., Jain A., Lad E.M., Gandhi J., Moshfeghi D.M. Stanford university network for diagnosis of retinopathy of prematurity (SUNDROP): 24-month experience with telemedicine screening. Acta Ophthalmol. 2010;88:317–322. doi: 10.1111/j.1755-3768.2009.01715.x. [DOI] [PubMed] [Google Scholar]
- Murray S.G., Wachter R.M., Cucina R.J. 2020. Discrimination by Artificial Intelligence in A Commercial Electronic Health Record—A Case Study.https://www.healthaffairs.org/do/10.1377/hblog20200128.626576/full/ [Google Scholar]
- Nagiel A., Lalane R.A., Sadda S.R., Schwartz S.D. ULTRA-WIDEFIELD fundus imaging: a review of clinical applications and future trends. Retina. 2016;36:660–678. doi: 10.1097/IAE.0000000000000937. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, Medicine, Policy, Affairs Global, Roundtable Government-University-Industry Research . In: The Transformational Impact of 5G: Proceedings of a Workshop-In Brief. Saunders J., editor. National Academies Press (US); 2019. The national Academies collection: reports funded by national institutes of health. Copyright 2019 By the National Academy of Sciences. All Rights Reserved.: Washington (DC)) [Google Scholar]
- Nguyen H.V., Tan G.S., Tapp R.J., Mital S., Ting D.S., Wong H.T., Tan C.S., Laude A., Tai E.S., Tan N.C., Finkelstein E.A., Wong T.Y., Lamoureux E.L. Cost-effectiveness of a national telemedicine diabetic retinopathy screening program in Singapore. Ophthalmology. 2016;123:2571–2580. doi: 10.1016/j.ophtha.2016.08.021. [DOI] [PubMed] [Google Scholar]
- NHS Scotland . 2020. 'Teleophthalmology Activation Guide.http://communityeyecare.scot.nhs.uk/telemedicine [Google Scholar]
- Noah B., Keller M.S., Mosadeghi S., Stein L., Johl S., Delshad S., Tashjian V.C., Lew D., Kwan J.T., Jusufagic A., Spiegel B.M.R. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018;1:20172. doi: 10.1038/s41746-017-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordrum A., Clark K., IEEE Spectrum staff . IEEE Spectrum; 2017. '5G Bytes: Small Cells Explained.https://spectrum.ieee.org/video/telecom/wireless/5g-bytes-small-cells-explained Accessed 20 February 2020. [Google Scholar]
- Okamoto F., Sugiura Y., Okamoto Y., Hiraoka T., Oshika T. Associations between metamorphopsia and foveal microstructure in patients with epiretinal membrane. Invest. Ophthalmol. Vis. Sci. 2012;53:6770–6775. doi: 10.1167/iovs.12-9683. [DOI] [PubMed] [Google Scholar]
- Oliva M.S., Schottman T., Gulati M. Turning the tide of corneal blindness. Indian J. Ophthalmol. 2012;60:423–427. doi: 10.4103/0301-4738.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossandon D., Zanolli M., Stevenson R., Agurto R., Ortiz P., Dotan G. A national telemedicine network for retinopathy of prematurity screening. J aapos. 2018;22:124–127. doi: 10.1016/j.jaapos.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Owsley C., Rhodes L.A., McGwin G., Jr., Mennemeyer S.T., Bregantini M., Patel N., Wiley D.M., LaRussa F., Box D., Saaddine J., Crews J.E., Girkin C.A. Eye Care Quality and Accessibility Improvement in the Community (EQUALITY) for adults at risk for glaucoma: study rationale and design. Int. J. Equity Health. 2015;14:135. doi: 10.1186/s12939-015-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R.M., Kayat K.V., Morel C., Conrath J., Matonti F., Morin B., Farah M.E., Devin F. Clinical study on the initial experiences of French vitreoretinal surgeons with heads-up surgery. Curr. Eye Res. 2020:1–8. doi: 10.1080/02713683.2020.1737136. [DOI] [PubMed] [Google Scholar]
- Pan C.W., Ramamurthy D., Saw S.M. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol. Optic. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- Pappot N., Taarnhoj G.A., Pappot H. Telemed J E Health; 2020. 'Telemedicine and E-Health Solutions for COVID-19: Patients' Perspective. [DOI] [PubMed] [Google Scholar]
- Parmet W.E., Sinha M.S. Covid-19 - the law and limits of quarantine. N. Engl. J. Med. 2020;382(15):e28. doi: 10.1056/NEJMp2004211. [DOI] [PubMed] [Google Scholar]
- Parrish R.K., 2nd, Stewart M.W., Duncan Powers S.L. Ophthalmologists are more than eye doctors-in memoriam Li Wenliang. Am. J. Ophthalmol. 2020;213:A1–A2. [Google Scholar]
- Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Patel V., Jamoom E., Hsiao C.J., Furukawa M.F., Buntin M. Variation in electronic health record adoption and readiness for meaningful use: 2008-2011. J. Gen. Intern. Med. 2013;28:957–964. doi: 10.1007/s11606-012-2324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T.P., Aaberg M.T., Paulus Y.M., Lieu P., Dedania V.S., Qian C.X., Besirli C.G., Margolis T., Fletcher D.A., Kim T.N. Smartphone-based fundus photography for screening of plus-disease retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2019;257:2579–2585. doi: 10.1007/s00417-019-04470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip S., Fleming A.D., Goatman K.A., Fonseca S., McNamee P., Scotland G.S., Prescott G.J., Sharp P.F., Olson J.A. The efficacy of automated "disease/no disease" grading for diabetic retinopathy in a systematic screening programme. Br. J. Ophthalmol. 2007;91:1512–1517. doi: 10.1136/bjo.2007.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietro C., Irene A., Mariano A.R. The past, present, and future of virtual and augmented reality researcb: a network and cluster analysis of the literature. Front. Psychol. 2018:1–20. doi: 10.3389/fpsyg.2018.02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi L.T., Waisbourd M., Hark L., Sembhi H., Lee P., Crews J.E., Saaddine J.B., Steele D., Katz L.J. Costs of a community-based glaucoma detection programme: analysis of the Philadelphia glaucoma detection and treatment project. Br. J. Ophthalmol. 2018;102:225–232. doi: 10.1136/bjophthalmol-2016-310078. [DOI] [PubMed] [Google Scholar]
- Poplin R., Varadarajan A.V., Blumer K., Liu Y., McConnell M.V., Corrado G.S., Peng L., Webster D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018;2:158–164. doi: 10.1038/s41551-018-0195-0. [DOI] [PubMed] [Google Scholar]
- Prakalapakorn S.G., Smallwood L.M., Helveston E.M. ORBIS telemedicine users. Ophthalmology. 2012;119:880–881. doi: 10.1016/j.ophtha.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Public Health England . 2016. NHS Diabetic Eye Screening Programme. Summary Statistics: England, 1 April 2015 to 31 March 2016. [Google Scholar]
- Quang D., Xie X. DanQ: a hybrid convolutional and recurrent deep neural network for quantifying the function of DNA sequences. Nucleic Acids Res. 2016;44:e107. doi: 10.1093/nar/gkw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi R., Arulmalar S., Usha M., Prathiba V., Kareemuddin K.S., Anjana R.M., Mohan V. Validation of smartphone based retinal photography for diabetic retinopathy screening. PloS One. 2015;10 doi: 10.1371/journal.pone.0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkomar A., Hardt M., Howell M.D., Corrado G., Chin M.H. Ensuring fairness in machine learning to advance health equity. Ann. Intern. Med. 2018;169:866–872. doi: 10.7326/M18-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran A.R., Shi J., Ngai A.K., Chan W.Y., Chan P.P., Young A.L., Yung H.W., Tham C.C., Cheung C.Y. Artificial intelligence deep learning algorithm for discriminating ungradable optical coherence tomography three-dimensional volumetric optic disc scans. Neurophotonics. 2019;6 doi: 10.1117/1.NPh.6.4.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G.N., Khanna R.C., Athota S.M., Rajshekar V., Rani P.K. Integrated model of primary and secondary eye care for underserved rural areas: the L V Prasad Eye Institute experience. Indian J. Ophthalmol. 2012;60:396–400. doi: 10.4103/0301-4738.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid F.Y. 2020. 5G Networks Will Inherit Their Predecessors' Security Issues.https://spectrum.ieee.org/tech-talk/telecom/security/5g-networks-will-juggle-legacy-security-issues-for-years [Google Scholar]
- Rathi S., Tsui E., Mehta N., Zahid S., Schuman J.S. The current state of teleophthalmology in the United States. Ophthalmology. 2017;124:1729–1734. doi: 10.1016/j.ophtha.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raumviboonsuk P., Krause J., Chotcomwongse P., Sayres R., Raman R., Widner K., Campana B.J.L., Phene S., Hemarat K., Tadarati M., Silpa-Archa S., Limwattanayingyong J., Rao C., Kuruvilla O., Jung J., Tan J., Orprayoon S., Kangwanwongpaisan C., Sukumalpaiboon R., Luengchaichawang C., Fuangkaew J., Kongsap P., Chualinpha L., Saree S., Kawinpanitan S., Mitvongsa K., Lawanasakol S., Thepchatri C., Wongpichedchai L., Corrado G.S., Peng L., Webster D.R. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit Med. 2019;2:25. doi: 10.1038/s41746-019-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd T.K., Campbell J.P., Brown J.M., Kim S.J., Ostmo S., Chan R.V.P., Dy J., Erdogmus D., Ioannidis S., Kalpathy-Cramer J., Chiang M.F., Imaging, and Consortium Informatics in Retinopathy of Prematurity Research Evaluation of a deep learning image assessment system for detecting severe retinopathy of prematurity. Br. J. Ophthalmol. 2018;103:580–584. doi: 10.1136/bjophthalmol-2018-313156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regillo C.D., Brown D.M., Abraham P., Yue H., Ianchulev T., Schneider S., Shams N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am. J. Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Rema Mohan, Premkumar Sundaram, Anitha Balaji, Deepa Raj, Pradeepa Rajendra, Mohan Viswanathan. Prevalence of diabetic retinopathy in urban India: the Chennai urban rural epidemiology study (CURES) eye study, I. Investig. Ophthalmol. Vis. Sci. 2005;46:2328–2333. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- Rhodes L.A., Huisingh C.E., McGwin G., Girkin C.A., Owsley C. Glaucoma patient knowledge, perceptions, and predispositions for telemedicine. J. Glaucoma. 2019;28:481–486. doi: 10.1097/IJG.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G.M., Sun G., Lee T.C., Chan R.V., Flynn J.T., Starren J., Chiang M.F. Speed of telemedicine vs ophthalmoscopy for retinopathy of prematurity diagnosis. Am. J. Ophthalmol. 2009;148:136–142 e2. doi: 10.1016/j.ajo.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A., Bates D.W., Sheikh A. The rise and fall of England's National Programme for IT. J. R. Soc. Med. 2011;104:434–435. doi: 10.1258/jrsm.2011.11k039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y., Marina Study Group Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Roth D.B., Morales D., Feuer W.J., Hess D., Johnson R.A., Flynn J.T. Screening for retinopathy of prematurity employing the retcam 120: sensitivity and specificity. Arch. Ophthalmol. 2001;119:268–272. [PubMed] [Google Scholar]
- Ruamviboonsuk P., Krause J., Chotcomwongse P., Sayres R., Raman R., Widner K. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digital Medicine. 2019;2 doi: 10.1038/s41746-019-0099-8. (Article Number 25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R.A., Crabb D.P., Malik R., Garway-Heath D.F. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Investig. Ophthalmol. Vis. Sci. 2012;53:5985–5990. doi: 10.1167/iovs.12-10428. [DOI] [PubMed] [Google Scholar]
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., Shaw J.E., Bright D., Williams R., Diabetes Atlas Committee I.D.F. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- Sakellaropoulos T., Vougas K., Narang S., Koinis F., Kotsinas A., Polyzos A., Moss T.J., Piha-Paul S., Zhou H., Kardala E., Damianidou E., Alexopoulos L.G., Aifantis I., Townsend P.A., Panayiotidis M.I., Sfikakis P., Bartek J., Fitzgerald R.C., Thanos D., Mills Shaw K.R., Petty R., Tsirigos A., Gorgoulis V.G. A deep learning framework for predicting response to therapy in cancer. Cell Rep. 2019;29:3367–33673 e4. doi: 10.1016/j.celrep.2019.11.017. [DOI] [PubMed] [Google Scholar]
- Saleem S.M., Pasquale L.R., Sidoti P.A., Tsai J.C. Virtual ophthalmology: telemedicine in a covid-19 era. Am. J. Ophthalmol. 2020;216:237–242. doi: 10.1016/j.ajo.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salongcay R.P., Silva P.S. The role of teleophthalmology in the management of diabetic retinopathy. Asia. Pac. J. Ophthalmol. 2018;7:17–21. doi: 10.22608/APO.2017479. [DOI] [PubMed] [Google Scholar]
- Sample P.A., Boden C., Zhang Z., Pascual J., Lee T.W., Zangwill L.M., Weinreb R.N., Crowston J.G., Hoffmann E.M., Medeiros F.A., Sejnowski T., Goldbaum M. Unsupervised machine learning with independent component analysis to identify areas of progression in glaucomatous visual fields. Invest. Ophthalmol. Vis. Sci. 2005;46:3684–3692. doi: 10.1167/iovs.04-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples Schacknow. 2010. The Glaucoma Book: a Practical, Evidence-Based Approach to Patient Care. [Google Scholar]
- Samsung . Samsung Electronics CO., Ltd; 2015. 5G VIsion White Paper 1. [Google Scholar]
- Samsung . Samsung Research. 2020. 6G the next hyper-connected experience for all. [Google Scholar]
- Samuel A.L. Some studies in machine learning using the game of checkers (Reprinted from Journal of Research and Development, vol 3, 1959. IBM J. Res. Dev. 2000;44:207–226. [Google Scholar]
- Scanlon P.H. The English national screening programme for sight-threatening diabetic retinopathy. J. Med. Screen. 2008;15:1–4. doi: 10.1258/jms.2008.008015. [DOI] [PubMed] [Google Scholar]
- Scanlon P.H. The English national screening programme for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54:515–525. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegl T., Waldstein S.M., Bogunovic H., Endstrasser F., Sadeghipour A., Philip A.M., Podkowinski D., Gerendas B.S., Langs G., Schmidt-Erfurth U. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology. 2018;125:549–558. doi: 10.1016/j.ophtha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- Schmid M.K., Faes L., Bachmann L.M., Thiel M.A. Accuracy of a self-monitoring test for identification and monitoring of age-related macular degeneration: a diagnostic case-control study. Open Ophthalmol. J. 2018;12:19–28. doi: 10.2174/1874364101812010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M.K., Thiel M.A., Lienhard K., Schlingemann R.O., Faes L., Bachmann L.M. Reliability and diagnostic performance of a novel mobile app for hyperacuity self-monitoring in patients with age-related macular degeneration. Eye. 2019;33:1584–1589. doi: 10.1038/s41433-019-0455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhuber J. Deep learning in neural networks: an overview. Neural Network. 2015;61:85–117. doi: 10.1016/j.neunet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U., Bogunovic H., Sadeghipour A., Schlegl T., Langs G., Gerendas B.S., Osborne A., Waldstein S.M. Machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol. Retina. 2018;2:24–30. doi: 10.1016/j.oret.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U., Sadeghipour A., Gerendas B.S., Waldstein S.M., Bogunovic H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018;67:1–29. doi: 10.1016/j.preteyeres.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Schwartz S.D., Harrison S.A., Ferrone P.J., Trese M.T. Telemedical evaluation and management of retinopathy of prematurity using a fiberoptic digital fundus camera. Ophthalmology. 2000;107:25–28. doi: 10.1016/s0161-6420(99)00003-2. [DOI] [PubMed] [Google Scholar]
- Scotland G.S., McNamee P., Fleming A.D., Goatman K.A., Philip S., Prescott G.J., Sharp P.F., Williams G.J., Wykes W., Leese G.P., Olson J.A., Network Scottish Diabetic Retinopathy Clinical Research Costs and consequences of automated algorithms versus manual grading for the detection of referable diabetic retinopathy. Br. J. Ophthalmol. 2010;94:712–719. doi: 10.1136/bjo.2008.151126. [DOI] [PubMed] [Google Scholar]
- Shah P.K., Ramya A., Narendran V. Telemedicine for ROP. Asia Pac. J. Ophthalmol. (Phila) 2018;7:52–55. doi: 10.22608/APO.2017478. [DOI] [PubMed] [Google Scholar]
- Shibata Naoto, Tanito Masaki, Mitsuhashi Keita, Fujino Yuri, Matsuura Masato, Murata Hiroshi, Asaoka Ryo. Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Sci. Rep. 2018;8:14665. doi: 10.1038/s41598-018-33013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel Ethan. Forbes; 2020. 'Why 'Exponential Growth' Is So Scary for the COVID-19 Coronavirus. [Google Scholar]
- Sim D.A., Thomas P., Canning C. 2020. 'Tackling COVID-19 with Telemedicine.https://theophthalmologist.com/subspecialties/tackling-covid-19-with-telemedicine [Google Scholar]
- Simko M., Mattsson M.O. 5G wireless communication and health effects-A pragmatic review based on available studies regarding 6 to 100 GHz. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Dutta M.K., ParthaSarathi M., Uher V., Burget R. Image processing based automatic diagnosis of glaucoma using wavelet features of segmented optic disc from fundus image. Comput. Methods Progr. Biomed. 2016;124:108–120. doi: 10.1016/j.cmpb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Smith L.F., Bainbridge J., Burns J., Stevens J., Taylor P., Murdoch I. Evaluation of telemedicine for slit lamp examination of the eye following cataract surgery. Br. J. Ophthalmol. 2003;87:502–503. doi: 10.1136/bjo.87.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.S., Frick K.D., Holden B.A., Fricke T.R., Naidoo K.S. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull. World Health Organ. 2009;87:431–437. doi: 10.2471/BLT.08.055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Shan L., Cheng F., Fan P., Zhang L., Qu W., Zhang Q., Yuan H. Prevalence of glaucoma in a rural northern China adult population: a population-based survey in kailu county, inner Mongolia. Ophthalmology. 2011;118:1982–1988. doi: 10.1016/j.ophtha.2011.02.050. [DOI] [PubMed] [Google Scholar]
- Song P., Yu J., Chan K.Y., Theodoratou E., Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J. Glob. Health. 2018;8 doi: 10.7189/jogh.08.010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreelatha O.K., Ramesh S.V. Teleophthalmology: improving patient outcomes? Clin. Ophthalmol. 2016;10:285–295. doi: 10.2147/OPTH.S80487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreelatha O.K., Ramesh S.V., Jose J., Devassy M., Srinivasan K. Virtually controlled computerised visual acuity screening in a multilingual Indian population. Rural Rem. Health. 2014;14 [PubMed] [Google Scholar]
- Stapleton F., Alves M., Bunya V.Y., Jalbert I., Lekhanont K., Malet F., Na K.S., Schaumberg D., Uchino M., Vehof J., Viso E., Vitale S., Jones L. TFOS DEWS II epidemiology report. Ocul. Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Starr M.R., Barkmeier A.J., Engman S.J., Kitzmann A., Bakri S.J. Telemedicine in the management of exudative age-related macular degeneration within an integrated health care system. Am. J. Ophthalmol. 2019;208:206–210. doi: 10.1016/j.ajo.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Stevens G.A., White R.A., Flaxman S.R., Price H., Jonas J.B., Keeffe J., Leasher J., Naidoo K., Pesudovs K., Resnikoff S., Taylor H., Bourne R.R.A., Group Vision Loss Expert Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990-2010. Ophthalmology. 2013;120:2377–2384. doi: 10.1016/j.ophtha.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Sumaroka A., Garafalo A.V., Semenov E.P., Sheplock R., Krishnan A.K., Roman A.J., Jacobson S.G., Cideciyan A.V. Treatment potential for macular cone vision in leber congenital amaurosis due to CEP290 or NPHP5 mutations: predictions from artificial intelligence. Invest. Ophthalmol. Vis. Sci. 2019;60:2551–2562. doi: 10.1167/iovs.19-27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan N.M., Liu J., Wong D.K., Lim J.H., Zhang Z., Lu S., Li H., Saw S.M., Tong L., Wong T.Y. Automatic detection of pathological myopia using variational level set. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;2009:3609–3612. doi: 10.1109/IEMBS.2009.5333517. [DOI] [PubMed] [Google Scholar]
- Tan T.E., Ting D.S.W., Liu Y., Li S.H., Chen C., Nguyen Q., Wong C.W., Hoang Q.V., Lee S.Y., Wong E.Y.M., Yeo I.Y.S., Wong Y.L., Cheng C.Y., Saw S.M., Cheung G.C.M., Wong T.Y. Artificial intelligence using a deep learning system with transfer learning to predict refractive error and myopic macular degeneration from color fundus photographs. Investig. Ophthalmol. Vis. Sci. 2019;60 [Google Scholar]
- Tan Z., Scheetz J., He M. Artificial intelligence in ophthalmology: accuracy, challenges, and clinical application. Asia. Pac. J. Ophthalmol. 2019;8:197–199. doi: 10.22608/APO.2019122. [DOI] [PubMed] [Google Scholar]
- Tatham A.J., Weinreb R.N., Medeiros F.A. Strategies for improving early detection of glaucoma: the combined structure-function index. Clin. Ophthalmol. 2014;8:611–621. doi: 10.2147/OPTH.S44586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham A.J., Medeiros F.A., Zangwill L.M., Weinreb R.N. Strategies to improve early diagnosis in glaucoma. Prog. Brain Res. 2015;221:103–133. doi: 10.1016/bs.pbr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040 A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- The Royal College of Ophthalmologists . 2015. The way forward: emergency eye care. [Google Scholar]
- Threlkeld A.B., Fahd T., Camp M., Johnson M.H. Telemedical evaluation of ocular adnexa and anterior segment. Am. J. Ophthalmol. 1999;127:464–466. doi: 10.1016/s0002-9394(98)00355-9. [DOI] [PubMed] [Google Scholar]
- Ting D.S., Cheung G.C., Wong T.Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin. Exp. Ophthalmol. 2016;44:260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- Ting D.S.J., Rees J., Ng J.Y., Allen D., Steel D.H.W. Effect of high-vacuum setting on phacoemulsification efficiency. J. Cataract Refract. Surg. 2017;43:1135–1139. doi: 10.1016/j.jcrs.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Ting D.S.W., Cheung C.Y.L., Lim G., Tan G.S.W., Quang N.D., Gan A., Hamzah H., Garcia-Franco R., Yeo I.Y.S., Lee S.Y., Wong E.Y.M., Sabanayagam C., Baskaran M., Ibrahim F., Tan N.C., Finkelstein E.A., Lamoureux E.L., Wong I.Y., Bressler N.M., Sivaprasad S., Varma R., Jonas J.B., He M.G., Cheng C.Y., Cheung G.C.M., Aung T., Hsu W., Lee M.L., Wong T.Y. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. J. Am. Med. Assoc. 2017;318:2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D.S.J., Ang M., Mehta J.S., Ting D.S.W. Artificial intelligence-assisted telemedicine platform for cataract screening and management: a potential model of care for global eye health. Br. J. Ophthalmol. 2019;103:1537–1538. doi: 10.1136/bjophthalmol-2019-315025. [DOI] [PubMed] [Google Scholar]
- Ting D.S.W., Cheung C.Y., Nguyen Q., Sabanayagam C., Lim G., Lim Z.W., Tan G.S.W., Soh Y.Q., Schmetterer L., Wang Y.X., Jonas J.B., Varma R., Lee M.L., Hsu W., Lamoureux E., Cheng C.Y., Wong T.Y. Deep learning in estimating prevalence and systemic risk factors for diabetic retinopathy: a multi-ethnic study. NPJ Digit Med. 2019;2:24. doi: 10.1038/s41746-019-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D.S.W., Lee A.Y., Wong T.Y. An ophthalmologist's guide to deciphering studies in artificial intelligence. Ophthalmology. 2019;126:1475–1479. doi: 10.1016/j.ophtha.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D.S.J., Foo V.H., Yang L.W.Y., Sia J.T., Ang M., Lin H., Chodosh J., Mehta J.S., Ting D.S.W. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br. J. Ophthalmol. 2020;Online ahead of print doi: 10.1136/bjophthalmol-2019-315651. [DOI] [PubMed] [Google Scholar]
- Ting D.S.J., Krause S., Said D.G., Dua H.S. 2020. ’Psychosocial Impact of COVID-19 Pandemic Lockdown on People Living with Eye Diseases in the UK. Online ahead of print. Eye (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D.S., Lin H., Ruamviboonsuk P., Wong T.Y., Sim D. Artificial intelligence, the internet of things, and virtual clinics: ophthalmology at the digital translation forefront. Lancet Digital Health. 2020 doi: 10.1016/S2589-7500(19)30217-1. [DOI] [PubMed] [Google Scholar]
- Ting D.S.W., Carin L., Dzau V., Wong T.Y. Digital technology and COVID-19. Nat. Med. 2020;26:459–461. doi: 10.1038/s41591-020-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer K., Woodward M.A., Newman Casey P.A. Telemedicine and diabetic retinopathy: review of published screening programs. J. Endocrinol. Diabetes. 2015;2 doi: 10.15226/2374-6890/2/4/00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treder Maximilian, Lauermann Jost Lennart, Eter Nicole. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018;256:259–265. doi: 10.1007/s00417-017-3850-3. [DOI] [PubMed] [Google Scholar]
- Trese M.T. What is the real gold standard for ROP screening? Retina. 2008;28:S1–S2. doi: 10.1097/IAE.0b013e31816a5587. [DOI] [PubMed] [Google Scholar]
- Tsaousis K.T., Empeslidis T., Konidaris V.E., Kapoor B., Deane J. The concept of virtual clinics in monitoring patients with age-related macular degeneration. Acta Ophthalmol. 2016;94:e353–e355. doi: 10.1111/aos.12832. [DOI] [PubMed] [Google Scholar]
- Tufail A., Kapetanakis V.V., Salas-Vega S., Egan C., Rudisill C., Owen C.G., Lee A., Louw V., Anderson J., Liew G., Bolter L., Bailey C., Sadda S., Taylor P., Rudnicka A.R. An observational study to assess if automated diabetic retinopathy image assessment software can replace one or more steps of manual imaging grading and to determine their cost-effectiveness. Health Technol. Assess. 2016;20:1–72. doi: 10.3310/hta20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail A., Rudisill C., Egan C., Kapetanakis V.V., Salas-Vega S., Owen C.G., Lee A., Louw V., Anderson J., Liew G., Bolter L., Srinivas S., Nittala M., Sadda S., Taylor P., Rudnicka A.R. Automated diabetic retinopathy image assessment software: diagnostic accuracy and cost-effectiveness compared with human graders. Ophthalmology. 2017;124:343–351. doi: 10.1016/j.ophtha.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Ung L., Bispo P.J.M., Shanbhag S.S., Gilmore M.S., Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019;64:255–271. doi: 10.1016/j.survophthal.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valikodath N.G., Leveque T.K., Wang S.Y., Lee P.P., Newman Casey P.A., Hansen S.O., Woodward M.A. Patient Attitudes toward telemedicine for diabetic retinopathy. Telemed. J. e Health. 2017;23:205–212. doi: 10.1089/tmj.2016.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valikodath N., Shah P., Chan R.V.P., Campbell J.P., Chiang M.F., Bohm K., Chee R., Olson S., Beca F., Ramyadevi R., Murugan S., Venkatapathy N. Evaluation of birth weight and gestational age in infants with treatment requiring retinopathy of prematurity in ROPE-SOS trial. Invest. Ophthalmol. Vis. Sci. 2018;59:2749. [Google Scholar]
- Varadarajan A.V., Poplin R., Blumer K., Angermueller C., Ledsam J., Chopra R., Keane P.A., Corrado G.S., Peng L., Webster D.R. Deep learning for predicting refractive error from retinal fundus images. Investig. Ophthalmol. Vis. Sci. 2018;59:2861–2868. doi: 10.1167/iovs.18-23887. [DOI] [PubMed] [Google Scholar]
- Vas A., Devi E.S., Vidyasagar S., Acharya R., Rau N.R., George A., Jose T., Nayak B. Effectiveness of self-management programmes in diabetes management: a systematic review. Int. J. Nurs. Pract. 2017;23 doi: 10.1111/ijn.12571. [DOI] [PubMed] [Google Scholar]
- Verma S. 2020. Early Impact of CMS Expansion of Medicare Telehealth during COVID-19.https://www.healthaffairs.org/do/10.1377/hblog20200715.454789/full/ [Google Scholar]
- Vinekar A., Dogra M., Azad R.V., Gilbert C., Gopal L., Trese M. The changing scenario of retinopathy of prematurity in middle and low income countries: unique solutions for unique problems. Indian J. Ophthalmol. 2019;67:717–719. doi: 10.4103/ijo.IJO_496_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S., Cotch M.F., Sperduto R., Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999-2002. Ophthalmology. 2006;113:2163–2170. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Waisbourd M., Pruzan N.L., Johnson D., Ugorets A., Crews J.E., Saaddine J.B., Henderer J.D., Hark L.A., Katz L.J. The Philadelphia glaucoma detection and treatment project: detection rates and initial management. Ophthalmology. 2016;123:1667–1674. doi: 10.1016/j.ophtha.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.K., Quinn G.E., Freedman S.F., Chiang M.F. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J aapos. 2008;12:352–356. doi: 10.1016/j.jaapos.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan K.H., Huang S.S., Ko C.-N., Lam D.S.C. The end of cordon sanitaire in Wuhan: the role of non-pharmaceutical interventions. Publ. Health. 2020;185:6–7. doi: 10.1016/j.puhe.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q., Li Y., Zhao Z., Qin X., Jin D., Zhou M., Tang X., Hu Y., Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. J. Am. Med. Assoc. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Shen L.Q., Pasquale L.R., Petrakos P., Formica S., Boland M.V., Wellik S.R., De Moraes C.G., Myers J.S., Saeedi O., Wang H., Baniasadi N., Li D., Tichelaar J., Bex P.J., Elze T. An artificial intelligence approach to detect visual field progression in glaucoma based on spatial pattern analysis. Invest. Ophthalmol. Vis. Sci. 2019;60:365–375. doi: 10.1167/iovs.18-25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P. Virtual health care in the era of COVID-19. Lancet. 2020;395:1180–1181. doi: 10.1016/S0140-6736(20)30818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. J. Am. Med. Assoc. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham L., Hay G., Hamilton R., Wooding J., Tossounis H., da Cruz L., Siriwardena D., Strouthidis N. The impact of COVID policies on acute ophthalmology services-experiences from Moorfields Eye Hospital NHS Foundation Trust. Eye. 2020;34(7):1189–1192. doi: 10.1038/s41433-020-0957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenbos G.A., Peute L., Jaspers M. Aging barriers influencing mobile health usability for older adults: a literature based framework (MOLD-US) Int. J. Med. Inf. 2018;114:66–75. doi: 10.1016/j.ijmedinf.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Wintergerst M.W.M., Petrak M., Li J.Q., Larsen P.P., Berger M., Holz F.G., Finger R.P., Krohne T.U. Non-contact smartphone-based fundus imaging compared to conventional fundus imaging: a low-cost alternative for retinopathy of prematurity screening and documentation. Sci. Rep. 2019;9:19711. doi: 10.1038/s41598-019-56155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse R.P.L., Muijzer M.B., Cassano F., Godefrooij D.A., Prevoo Yfdm, Soeters N. Validation of an independent web-based tool for measuring visual acuity and refractive error (the manifest versus online refractive evaluation trial): prospective open-label noninferiority clinical trial. J. Med. Internet Res. 2019;21 doi: 10.2196/14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenborn J.S., Clemons T., Regillo C., Rayess N., Liffmann Kruger D., Rein D. Economic evaluation of a home-based age-related macular degeneration monitoring system. JAMA Ophthalmol. 2017;135:452–459. doi: 10.1001/jamaophthalmol.2017.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.Y., Sabanayagam C. The war on diabetic retinopathy: where are we now? Asia. Pac. J. Ophthalmol. 2019;8:448–456. doi: 10.1097/APO.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong I.Y.H., Ni M.Y., Wong I.O.L., Fong N., Leung G.M. Saving sight in China and beyond: the lifeline express model. BMJ Glob. Health. 2018;3 doi: 10.1136/bmjgh-2018-000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M.A., Musch D.C., Hood C.T., Greene J.B., Niziol L.M., Jeganathan V.S.E., Lee P.P. Teleophthalmic approach for detection of corneal diseases: accuracy and reliability. Cornea. 2017;36:1159–1165. doi: 10.1097/ICO.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . 2000. Programme for the prevention of blindness and deafness: global initiative for the elimination of avoidable blindness. [Google Scholar]
- World Health Organisation . 2019. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. [Google Scholar]
- Wright H.R., Diamond J.P. Service innovation in glaucoma management: using a web-based electronic patient record to facilitate virtual specialist supervision of a shared care glaucoma programme. Br. J. Ophthalmol. 2015;99:313–317. doi: 10.1136/bjophthalmol-2014-305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Medeiros F.A. Impact of different visual field testing paradigms on sample size requirements for glaucoma clinical trials. Sci. Rep. 2018;8:4889. doi: 10.1038/s41598-018-23220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Huang Y., Liu Z., Lai W., Long E., Zhang K., Jiang J., Lin D., Chen K., Yu T., Wu D., Li C., Chen Y., Zou M., Chen C., Zhu Y., Guo C., Zhang X., Wang R., Yang Y., Xiang Y., Chen L., Liu C., Xiong J., Ge Z., Wang D., Xu G., Du S., Xiao C., Wu J., Zhu K., Nie D., Xu F., Lv J., Chen W., Liu Y., Lin H. Universal artificial intelligence platform for collaborative management of cataracts. Br. J. Ophthalmol. 2019;103:1553–1560. doi: 10.1136/bjophthalmol-2019-314729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Vignarajan J., Chen T., Ye T., Xiao B., Congdon N., Kanagasingam Y. Content design and system implementation of a teleophthalmology system for eye disease diagnosis and treatment and its preliminary practice in Guangdong, China. Telemed. J. e Health. 2017;23:964–975. doi: 10.1089/tmj.2016.0266. [DOI] [PubMed] [Google Scholar]
- Xie Y., Nguyen Q.D., Hamzah H., Lim G., Bellemo V., Gunasekeran D.V., Yip M.Y.T., Lee X.Q., Hsu W., Lee M.L., Tan C.S., Wong H.T., Lamoureux E.L., Tan G.S.W., Wong T.Y., Finkelstein E.A., Ting D.S.W. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national program: a modelled economic analysis study. Lancet Digit. Health. 2020;2(5):E240–E249. doi: 10.1016/S2589-7500(20)30060-1. [DOI] [PubMed] [Google Scholar]
- Xu L., Jonas J.B., Cui T.T., You Q.S., Wang Y.X., Yang H., Li J.J., Wei W.B., Liang Q.F., Wang S., Yang X.H., Zhang L. Beijing eye public health care project. Ophthalmology. 2012;119:1167–1174. doi: 10.1016/j.ophtha.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Xu K., Gupta V., Bae S., Sharma S. Metamorphopsia and vision-related quality of life among patients with age-related macular degeneration. Can. J. Ophthalmol. 2018;53:168–172. doi: 10.1016/j.jcjo.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Yamada T., Hatt S.R., Leske D.A., Moke P.S., Parrucci N.L., Reese J.J., Ruben J.B., Holmes J.M. A new computer-based pediatric vision-screening test. J.f Aapos. 2015;19:157–162. doi: 10.1016/j.jaapos.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Lu J., Weng J., Jia W., Ji L., Xiao J., Shan Z., Liu J., Tian H., Ji Q., Zhu D., Ge J., Lin L., Chen L., Guo X., Zhao Z., Li Q., Zhou Z., Shan G., He J., Diabetes China national, and group metabolic disorders study prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E., Klein R., Krishnaiah S., Mayurasakorn K., O'Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y., Group Meta-Analysis for Eye Disease Study Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K.G., Hess D., Burke B., Johnson R.A., Feuer W.J., Flynn J.T. The optimum time to employ telephotoscreening to detect retinopathy of prematurity. Trans. Am. Ophthalmol. Soc. 2000;98:145–150. discussion 50-1. [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Goldbaum M.H., Varnousfaderani E.S., Belghith A., Jung T.P., Medeiros F.A., Zangwill L.M., Weinreb R.N., Liebmann J.M., Girkin C.A., Bowd C. Detecting glaucomatous change in visual fields: analysis with an optimization framework. J. Biomed. Inf. 2015;58:96–103. doi: 10.1016/j.jbi.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Balasubramanian M., Goldbaum M.H., Medeiros F.A., Zangwill L.M., Weinreb R.N., Liebmann J.M., Girkin C.A., Bowd C. Unsupervised Gaussian mixture-model with expectation maximization for detecting glaucomatous progression in standard automated perimetry visual fields. Transl. Vis. Sci. Technol. 2016;5 doi: 10.1167/tvst.5.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Kiwaki T., Zheng Y.H., Sugiura H., Asaoka R., Murata H., Lemij H., Yamanishi K. Detection of longitudinal visual field progression in glaucoma using machine learning. Am. J. Ophthalmol. 2018;193:71–79. doi: 10.1016/j.ajo.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Zahlmann G., Mertz M., Fabian E., Holle R., Kaatz H., Neubauer L., Strobl H., Walther H.D. Perioperative cataract OP management by means of teleconsultation. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240:17–20. doi: 10.1007/s00417-001-0396-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xu Y., Liu J., Wong D.W., Kwoh C.K., Saw S.M., Wong T.Y. Automatic diagnosis of pathological myopia from heterogeneous biomedical data. PloS One. 2013;8 doi: 10.1371/journal.pone.0065736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Guallar E., Gajwani P., Swenor B., Crews J., Saaddine J., Mudie L., Varadaraj V., Friedman D.S., ToP Glaucoma Study Group S. Optimizing glaucoma screening in high-risk population: design and 1-year findings of the screening to prevent (SToP) glaucoma study. Am. J. Ophthalmol. 2017;180:18–28. doi: 10.1016/j.ajo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Zheng C., Xie X., Huang L., Chen B., Yang J., Lu J., Qiao T., Fan Z., Zhang M. Detecting glaucoma based on spectral domain optical coherence tomography imaging of peripapillary retinal nerve fiber layer: a comparison study between hand-crafted features and deep learning model. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:577–585. doi: 10.1007/s00417-019-04543-4. [DOI] [PubMed] [Google Scholar]