Abstract
Background
The traditional Chinese Medicine (TCM) herbal formula Lian Hua Qing Wen (LHQW) improves the results of COVID-19 treatment. Three very recent studies analyzed with network pharmacology some working mechanisms of LHQW. However, we used more techniques and also included Angiotensin converting enzyme 2 (ACE2) (a SARS-CoV receptor, possibly the viral entry point in alveolar lung cells) and the immune system, as cytokine storm is essential in the late phase.
Purpose
Extensive detailed Network Pharmacology analysis of the LHQW- treatment mechanism in COVID-19.
Methods
TCM-herb-meridian and protein interaction network (PIN) of LHQW, based on LHQW herbs meridian information and the protein-protein interaction (PPI) information of the LHQW-component targets. Hub and topological property analyses to obtain crucial targets and construct the crucial LHQW-PIN. Functional modules determination using MCODE, GO and KEGG pathway analysis of biological processes and pathway enrichment. Intersection calculations between the LHQW-proteins and ACE2 co-expression-proteins.
Results
LHQW herbs have relationships to Stomach-, Heart-, Liver- and Spleen-systems, but most (10 of the 13 herbs) to the Lung system, indicating specific effects in lung diseases. The crucial LHQW PIN has the scale-free property, contains 2,480 targets, 160,266 PPIs and thirty functional modules. Six modules are enriched in leukocyte-mediated immunity, the interferon-gamma-mediated signaling pathway, immune response regulating signaling pathway, interleukin 23 mediated signaling pathway and Fc gamma receptor-mediated phagocytosis (GO analysis). These 6 are also enriched in cancer, immune system-, and viral infection diseases (KEGG). LHQW shared 189 proteins with ACE2 co-expression proteins.
Conclusions
Detailed network analysis shows, that LHQW herbal TCM treatment modulates the inflammatory process, exerts antiviral effects and repairs lung injury. Moreover, it also relieves the “cytokine storm” and improves ACE2-expression-disorder-caused symptoms. These innovative findings give a rational pharmacological basis and support for treating COVID-19 and possibly other diseases with LHQW.
Keywords: Traditional Chinese Medicine, Lian Hua Qing Wen (LHQW), COVID-19, Network pharmacology
Abbreviations: ACE2, angiotensin I converting enzyme 2; ACEI, angiotensin-converting enzyme inhibitors; AKT1, AKT Serine/Threonine Kinase 1; ARB, angiotensin II receptor blocker; COVID-19, coronavirus disease 2019; FYN, FYN Proto-Oncogene; GO, Gene Ontology; HMGB1, high mobility group box 1; IFNγ, interferon gamma; JAK1, Janus Kinase 1; KEGG, Kyoto Encyclopedia of Genes and Genomes; LHQW, Lian-Hua-Qing-Wen; MCODE, Molecular Complex Detection; MERS, Middle East respiratory syndromes; PCNA, proliferating cell nuclear antigen; PIK3CG, Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Gamma; PIN, protein interaction network; PLCG1, phospholipase C gamma 1; PPI, protein-protein interaction; SARS, severe acute respiratory syndrome; SARSCoV2, severe acute respiratory syndrome coronavirus 2; Src, Family Tyrosine Kinase; TCM, Traditional Chinese medicine; TNF, tumor necrosis factor; VAV1, Vav Guanine Nucleotide Exchange Factor 1; VCAM1, vascular cell adhesion molecule 1; WHO, World Health Organization
Graphical abstract
Introduction
In December 2019, an outbreak of the novel coronavirus pneumonia, named “COVID-19” (Coronavirus Disease 2019) by the World Health Organization (WHO), started in Wuhan, China. The disease was caused by severe acute respiratory syndrome coronavirus 2 (SARSCoV2). Coronavirus 2 belongs to the same family of viruses responsible for the severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndromes (MERS). These had caused severe epidemics in 2002 and 2012 (Huang et al., 2020). COVID-19 rapidly triggered a global health emergency. By September 7, 2020, the numbers of Coronavirus cases and deaths worldwide was reported by Johns Hopkins University Worldometer to 27,312,084 and 893,455. The measures taken in China (isolation, test, treatment and trace) were originally regarded in the western world as draconic. However, from March 20, 2020 (within 2,5 months after the start of the epidemic in Wuhan) the tide had turned. The WHO Director-General called this “an amazing achievement” (News, 2020). This is certainly true in view of the proportionally dramatically much higher number of cases (per capita of the population) in Italy, Spain, other countries in the European Union and the USA, where government measures to contain the disease were taken much slower and (certainly initially) followed less strictly by part of the public.
Common symptoms in hospitalized patients with COVID-19 include fever (98%), cough (76%) and myalgia or fatigue (44%). Severe complications include acute respiratory distress syndrome (ARDS), RNAemia, acute cardiac injury and multiple organ failure (Huang et al., 2020). Diffuse multiorgan microvascular thrombosis were also seen in an autopsy review of COVID-19 non-survivors (Campbell and Kahwash, 2020). Patients aged 30-79 years accounted for 87% of all cases (Wang et al., 2020d). Persons with underlying cardiovascular, lung and metabolic diseases, older patients (especially over 80) and men have a higher risk of getting COVID-19 and a higher mortality rate.
There is overwhelming evidence that COVID-19 results in innate and adaptive immune cell activation in the infected host (Li et al., 2020; Lin et al., 2020). A rapid and well-coordinated innate immune response may exert important antiviral effector functions. However, exaggerated immune responses may lead to Cytokine Storm. This refers to a very acute over-protective immune response with massive release of interleukins and tumor necrosis factor alpha (Filippou and Karagiannis, 2020). Cytokine storm syndrome includes ARDS, shock and multiorgan failure (Mehta et al., 2020; Pedersen and Ho, 2020). In fact, many patients with COVID-19 suffer from the cytokine storm syndrome (Chen et al., 2020), which is associated with multiorgan failure and high lethality (Mehta et al., 2020; Neurath, 2020). Effectively suppressing the cytokine storm is an important way to prevent the deterioration of patients with COVID-19 and save the patients' lives (Ye et al., 2020).
As there is currently no vaccine or specific treatment, symptomatic and antiviral therapies have mainly been used for COVID-19. Early supportive treatments, including nutrient supplements (vitamin C), oxygen therapy, Chinese herbal medicine and antibacterial therapy have been helpful for patients with mild symptoms at the early stage of infection. For critically ill patients, high-flow oxygen therapy, extracorporeal membrane oxygenation, glucocorticoid therapy, and administration of convalescent plasma have been used (Wang et al., 2020d). Antiviral treatments with lopinavir/ ritonavir, ribavirin, interferon-α, chloroquine phosphate, arbidol and their combinations have been suggested (Lim et al., 2020; Team, 2020; Wang et al., 2020c). The US Food and Drug Administratiom site authorized remdesivir for COVID-19 treatment. However, there are currently no other effective specific antiviral drugs or drug combinations for COVID-19 and a vaccine worldwide available for any person is still many months away. Thus, other medicines for the treatment of COVID-19, supported by high-level evidence, are urgently needed.
In 2002, in the fight against severe acute respiratory syndrome (caused by SARS-CoV), Traditional Chinese medicine (TCM) treatment has been proven to be very successful (Gao et al., 2020a). It has also got an important role in the prevention and cure of COVID-19 (Gao et al., 2020a). The TCM formula Lian Hua Qing Wen (LHQW) was recommended by the National Health Commission of China as a therapeutic drug in the guidelines for diagnosis and treatment of COVID-19 (the seventh edition, 03/04/2020) (NHCPRC, 2020). According to TCM, LHQW exerts Qingwen Jiedu efficiency, which roughly can be translated as antipyretic and antitoxic effects (for more details, see Chinese Pharmacopoeia 2020 edition (Beijing Tongrentang Co., Ltd, Beijing, China)). LHQW is commonly used for both the prevention and treatment of viral influenza in China, and consists of 13 TCM herbs including Forsythiae Fructus (Lian Qiao), Lonicerae Japonicae Flos (Jin Yin Hua), Ephedrae Herba (Ma Huang), Armeniacae Semen Amarum (Ku Xing Ren), Gypsum Fibrosum (Shi Gao), Isatidis Radix (Ban Lan Gen), Dryopteridis Crassirhizomatis Rhizoma (Mian Ma Guan Zhong), Houttuyniae Herba (Yu Xing Cao), Pogostemonis Herba (Guang Huo Xiang), Rhei Radix et Rhizoma (Da Huang), Rhodiolae Crenulatae Radix et Rhizoma (Hong Jing Tian), menthol (Bo He Nao), and Glycyrrhizae Radix et Rhizoma (Gan Cao). In clinical studies, LHQW significantly improves fever, cough, and fatigue in COVID-19 patients and other suspected cases, and reduces the duration and proportion of cases progressing to a more severe state (Cheng et al., 2020a; Yao et al., 2020). A very recent prospective randomized multicenter trial of patients treated with LHQW showed a higher recovery rate in the LHQW treatment than the control group (92% vs. 82%), shorter median time to symptom recovery (median: 7 vs. 10 days), time to recovery of fever (2 vs. 3 days), fatigue (3 vs. 6 days) and coughing (7 vs. 10 days). Moreover, also the rate of improvement in chest computed tomographic manifestations and clinical cure were higher in the treatment group. No serious adverse events were reported (Hu et al., 2020).
These positive clinical findings require investigating the underlying therapeutic mechanism of action of LHQW against COVID-19, as this can provide theoretical support for its wider application against the SARSCoV2 infection and possibly other diseases. With the rapid progress in bioinformatics, systems biology, and polypharmacology, the network-based approach to drug discovery is considered promising for cost-effective drug development. The Network Pharmacology concept was first proposed based on systems biology (Li et al., 2014). Since Network Pharmacology can provide a full or partial understanding of the principles of network theory and systems biology, it has been considered the next paradigm in drug discovery (Hopkins, 2008). Network Pharmacology has been used to study amongst others the “compound-proteins/ genes-disease” pathways, which are capable of describing complexities among the biological systems, drugs, and diseases from a network perspective, sharing a similar holistic philosophy to TCM (Zhang et al., 2019).
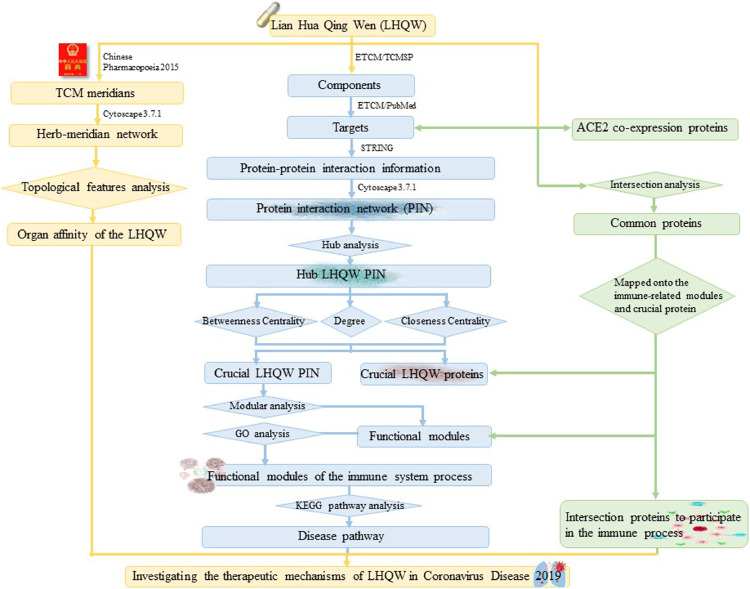
Proteins as vital macromolecules rarely act alone. Protein interaction networks (PIN) are biologically particularly important. They play a vital role in a variety of human cellular processes by protein-protein interactions (PPIs), also known as function module. The analysis of PIN modules is beneficial for understanding the complex network structure and biological activities in the cell. It´s application on studying the effects of TCM herbal treatment, provides an efficient way to understand the TCM targets interactions. These in turn highlight the mechanisms of action (Zhao et al., 2015). Therefore, we applied PIN module analysis integrating the hub and topological analysis to investigate LHQW complex network and targets action mechanisms. As well, to explain by TCM meridian theory, which specific organ system LHQW has selective therapeutic effects on, we constructed the LHQW meridian network. Moreover, we performed intersection analysis between LHQW proteins and ACE2 co-expression proteins, to explore the effect of improvement on ACE2-expression-disorder-caused symptoms. We thus systematically investigated the therapeutic mechanisms of LHQW in COVID-19 from three different dimensions based on Network Pharmacology. As to our knowledge, this has not been done before for the therapeutic mechanisms of LHQW. The schematic diagram of the systematic strategies for investigating the therapeutic mechanisms of LHQW in COVID-19 is shown in Fig. 1 .
Fig. 1.
A schematic diagram of the systematic strategies for investigating the therapeutic mechanisms of Lian Hua Qing Wen in Coronavirus Disease 2019 (COVID-19).
Three other studies have very recently been published on Network Pharmacology analysis of LHQW in COVID-19. Supplementary Data Table S1 compares the methods used in these and our study. Clearly the current study used many more techniques than the three previous studies.
Materials and methods
Data preparation
Collection of LHQW meridian, components, and targets
The information on the TCM meridians of herbs in LHQW was collected from the Chinese Pharmacopoeia 2020 edition (Beijing Tongrentang Co., Ltd, Beijing, China). The components of all the herbs in LHQW, except Dryopteridis Crassirhizomatis Rhizoma, were collected from the Encyclopedia of Traditional Chinese Medicine database (ETCM) (Xu et al., 2019a) (http://www.nrc.ac.cn:9090/ETCM/index.php/Home/Index/) using the herbs´ Chinese phonetic alphabet. The components of Dryopteridis Crassirhizomatis Rhizoma were collected from the Traditional Chinese Medicine Systems Pharmacology Database (Ru et al., 2014) (http://tcmspw.com/tcmsp.php). Then, the components were imported to the ETCM database to obtain the targets (except calcium sulfate of Gypsum Fibrosum, which was obtained from PubMed retrieval). Targets with a confidence score of > 0.8 were selected as the targets of LHQW. Regarding this threshold, different databases have different confidence score standards. The range of confidence score is 0-1. In general, the higher the confidence score, the more accurate it is. However, the higher the confidence score, the less data is obtained. We therefore used 0.8, as this is according to the original research. The same strategy is followed in the values mentioned below.
Collection of protein-protein interaction (PPI) data
The protein-protein interaction (PPI) data were imported from the STRING (Search Tool for Recurring Instances of Neighboring Genes) database (Szklarczyk et al., 2019) (https://string-db.org/). In STRING databases, there are three confidence score thresholds used, 0.4, 0.7 and 0.9. We selected PPI data with a confidence score of > 0.9 to construct the protein interaction network (PIN), which satisfied the accuracy and quantity of PPI.
Collection of ACE2 co-expression proteins
Angiotensin converting enzyme 2 (ACE2) is an enzyme attached to the outer surface (cell membranes) of cells in the lungs, arteries, heart, kidney, and intestines (Donoghue et al., 2000; Hamming et al., 2004). ACE2 binds to the receptor-binding motif (RBM) in the receptor-binding domain (RBD) of SARS-CoV and functions as a receptor for SARS-CoV (Li et al., 2005). It also serves as the entry point into cells for some coronaviruses (Kuba et al., 2005). ACE2 has been linked to SARS-CoV-2 infection (Chan et al., 2020). The SARS-CoV receptor interacts with the spike protein and mediates SARS-CoV-2 infection of the type II alveolar cells of the lungs (Chan et al., 2020). The proteins that were co-expressed with ACE2 in the colonic epithelial cells were obtained from the study by Wang et al (Wang et al., 2020b).
Network construction
Based on the PPI data obtained from the STRING database, the identified targets of LHQW were used to construct the LHQW-related network. Then, in agreement with previous studies (Li et al., 2007; Zhang et al., 2013), the nodes with more than 2-fold of the median degree of all nodes were extracted from the LHQW PIN to construct the hub LHQW PIN. Next, the nodes, their topological features including “Degree”, “Betweenness Centrality” and “Closeness Centrality” greater than the median values of all nodes, were extracted from the hub LHQW PIN to construct the crucial LHQW PIN. “Degree”, “Betweenness Centrality”, and “Closeness Centrality” can measure a protein's topological importance in the network. The larger a protein's degree/ betweenness centrality/ closeness centrality is, the more important the protein is in the PPI network (Valente and Fujimoto, 2010; Zhang et al., 2013). The values of “Degree”, “Betweenness Centrality” and “Closeness Centrality” were calculated through network analysis. Next, we applied Cytoscape 3.7.1 (Graphical Networks, Gaithersburg, MD, U.S.A.) to visualize the network.
Network analysis
Topological analysis
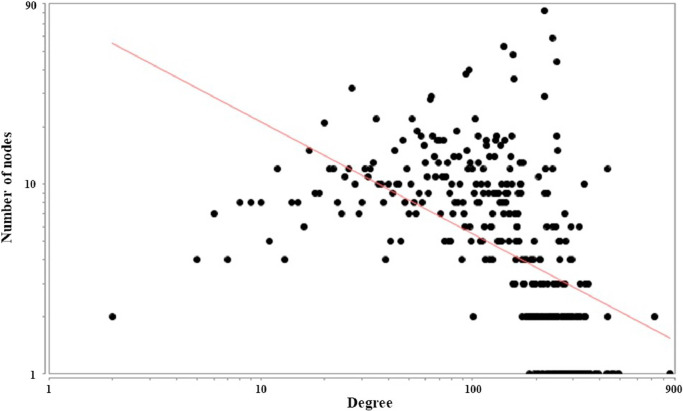
If biological networks’ degree distribution follows a power law distribution P (k) ~ k-γ(γ < 3) (Barabasi and Albert, 1999), it has scale-free topology that possesses fragility and robustness which allow networks to have a fault tolerant behavior (Manke et al., 2005). The crucial LHQW PIN scale-free property wasanalyzed based on the degree distribution by Network Analyzer (Assenov et al., 2008) in Cytoscape.
The identification of the main active components of LHQW
The components regulating the proteins whose “Degree”, “Betweenness Centrality”, and “Closeness Centrality” are greater than the median values of all nodes in the crucial LHQW PIN were selected as the main active components of LHQW.
Modular analysis
We used the Molecular Complex Detection (MCODE) algorithm (Bader and Hogue, 2003) to explore the functional modules in the crucial LHQW PIN. MCODE is based on vertex weighting by local neighborhood density and outward traversal from a locally dense seed protein to isolate the dense regions according to the given parameters. The algorithm has the advantage over other graph clustering methods of having a directed mode. This allows fine-tuning of the clusters of interest without considering the rest of the network and allows examination of cluster interconnectivity, which is relevant for protein networks.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis
We used the STRING database for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. STRING also implements well-known classification systems, such as GO and KEGG for enrichment analysis. The GO project (Ashburner et al., 2000) is a collection of well-defined biological terms spanning biological processes, molecular functions, and cellular components, and can facilitate biologically meaningful annotation of gene products. KEGG (Kanehisa et al., 2017) is an integrated database resource for biological interpretation of completely sequenced genomes by pathway mapping, and is the procedure to map genes in the genome to manually created pathway maps. It has been developed into a more comprehensive knowledge base for assisting biological interpretations of large-scale molecular datasets.
Results
Information on components and targets of LHQW
A total of 525 components and 624 targets were obtained through database retrieval after removing the duplicate values. The number of compounds contained in the herbs of LHQW and the corresponding putative targets are shown in Table 1 .
Table 1.
Number of compounds contained in the herbs of LHQW and corresponding putative targets.
| Herb | Chinese name | Number of compounds | Number of targets |
|---|---|---|---|
| Forsythiae Fructus | Lian Qiao | 49 | 238 |
| Lonicerae Japonicae Flos | Jin Yin Hua | 47 | 187 |
| Ephedrae Herba | Ma Huang | 28 | 114 |
| Armeniacae Semen Amarum | Ku Xing Ren | 64 | 237 |
| Gypsum Fibrosum | Shi Gao | 1 | 19 |
| Isatidis Radix | Ban Lan Gen | 25 | 311 |
| Dryopteridis Crassirhizomatis Rhizoma | Mian Ma Guan Zhong | 30 | 76 |
| Houttuyniae Herba | Yu Xing Cao | 34 | 242 |
| Pogostemonis Herba | Guang Huo Xiang | 59 | 270 |
| Rhei Radix et Rhizoma | Da Huang | 88 | 147 |
| Rhodiolae Crenulatae Radix et Rhizoma | Hong Jing Tian | 7 | 108 |
| Menthol | Bo He Nao | 1 | 4 |
| Glycyrrhizae Radix et Rhizoma | Gan Cao | 131 | 243 |
Based on the ETCM, the corresponding components of the obtained protein are drug-likeness. Because the proteins with a confidence score > 0.8 were used to construct the LHQW PIN. In ETCM, the potential targets for herbal ingredients using MedChem Studio, an efficient drug similarity search tool to identify known drugs with high structural similarity (Tanimoto score > 0.8) to herbal ingredients. The therapeutic targets in the DrugBank database of known drugs are considered as the candidate targets of the herbal ingredients with Tanimoto Scores > 0.8 to the known drugs (Xu et al., 2019b).
Meridian information of herbs in LHQW
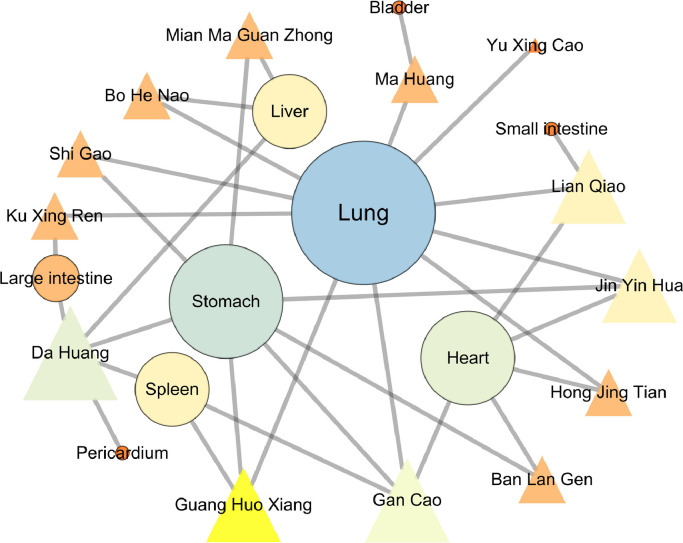
We used the meridian information of the herbs to construct the herb-meridian network, as shown in Fig. 2 . Organ affinity of the LHQW herbs is highest for the lung (10), followed by the stomach (7), heart (5), spleen (3), liver (3), large intestine (2), bladder (1), small intestine (1) and pericardium (1). Thus, LHQW may have specifically strong effects in the treatment of lung diseases.
Fig. 2.
The herb-meridian network of LHQW. The triangle and circular nodes represent the herbs and meridians, respectively. The node size is proportional to the node degree. The lung meridian with the largest degree is 10, followed by the stomach, heart, spleen and liver meridians in the network, with degree values of 7, 5, 3, and 3 respectively.
PIN construction of crucial LHQW proteins
The LHQW PIN was constructed using the PPI information of the LHQW targets. The LHQW PIN consisted of 6,438 nodes and 267,063 edges (Supplementary Data Figure S1A). Then, the PIN of the hub LHQW proteins was constructed including 3,391 nodes and 212,278 edges (Supplementary Data Figure S1B) using the PPI information of the hub proteins. Next, the median values of three topological features including “Degree,” “Betweenness Centrality,” and “Closeness Centrality” were calculated to identify crucial LHQW proteins. The median values of “Degree,” “Betweenness Centrality,” and “Closeness Centrality” of the hub LHQW PIN were 175, 0.00014396 and 0.35658099, respectively. The hub LHQW proteins with “Degree” > 175, “Betweenness Centrality” > 0.00014396, and “Closeness Centrality” > 0.35658099 were crucial LHQW proteins. As a result, 2,480 proteins were identified as such crucial LHQW proteins. Among them, 43 proteins are directly bound with LHQW. The network of crucial LHQW proteins consisted of 2,480 nodes and 160,266 edges (Supplementary Data Figure S1C).
Topological analysis
The degree distribution of the crucial LHQW PIN follows a power law distribution and the equation is Y = 85.453 x−0.590. As shown in Fig. 3 , the crucial LHQW PIN has the property of scale-free.
Fig. 3.
Degree distribution of the crucial LHQW PIN
The identification of the main active components of LHQW
223 of 525 components are identified as the main active components. The information of the main active components of LHQW is shown in Supplementary Data Table S2.
Modular and enrichment analysis
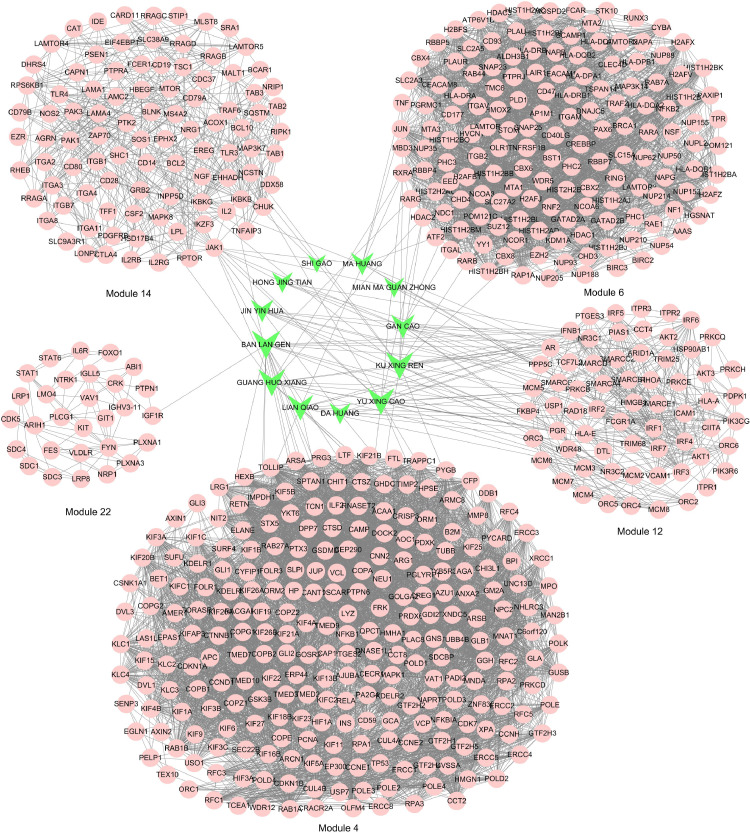
Functional modules of the crucial LHQW PIN were explored by MCODE. A total of 30 modules were identified with a score of > 3. GO enrichment analysis was then performed to investigate the biological processes of 30 functional modules. The detail information on the GO enrichment analysis of the 30 modules is shown in Supplementary Data Table S3. There were 6 functional modules that were closely related to the immune system process with an FDR (false discovery rate) of < 1⁎10−6. These were leukocyte mediated immunity (module 4 and 6), interferon gamma mediated signaling pathway (module 12), immune response regulating signaling pathway (module 14), interleukin 23 mediated signaling pathway (module 19), and Fc gamma receptor-mediated phagocytosis (module 22). Furthermore, 5 modules, except module 19, contained the protein bound with LHQW (Fig. 4 ).
Fig. 4.
Functional modules of the immune system process that contained the proteins bound with LHQW. The pink nodes represent the module proteins and the green nodes represent the herbs in LHQW; the node size is proportional to the node degree.
Module 4, 6, 12, and 14 are implemented in the immune system process, mainly including immune-related proteins, for example, HMGB1 (high mobility group box 1), PCNA (proliferating cell nuclear antigen), TNF (tumor necrosis factor), AKT1 (AKT Serine/Threonine Kinase 1), VCAM1 (vascular cell adhesion molecule 1), PIK3CG (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Gamma), and JAK1 (Janus Kinase 1). These proteins are regulated by oleanolic acid, pseudoephedrine, luteolin, kaempferol, sitosterol, and quercetin.
Oleanolic acid, a triterpenoid, is known for its anti-inflammatory and anti-cancer properties. HMGB1, as a late mediator of inflammation, up-regulates pro-inflammatory cytokines in several inflammatory diseases. Oleanolic acid could down-regulate the cell surface expression of the receptor of HMGB1, thereby inhibiting HMGB1-dependent pro-inflammatory responses by inhibiting activation of nuclear factor-κB (NF-κB) and production of tumor necrosis factor-α (TNF-α) by HMGB1 (Yang et al., 2012). PCNA, a nuclear scaffolding protein pivotal in DNA synthesis, controls neutrophil survival through its cytosolic association with procaspases. Targeting PCNA in inflammatory neutrophils holded promise as a multifaceted anti-inflammatory strategy (Ohayon et al., 2019). Oleanolic acid could downregulate the expression of PCNA to inhibit prostate cell proliferation (Cheon et al., 2020). Pseudoephedrine, a stereoisomer of ephedrine, is commonly used as a nasal decongestant in combination with other anti-inflammatory drugs for the symptomatic treatment of some common pathologies such as common cold. PSE has been found to inhibit interleukin-2 and TNF alpha-gene transcription in stimulated Jurkat cells (Fiebich et al., 2012). Luteolin is the main active pharmacological components of chrysanthemum indicum var. albescens methanol extract which exerts an anti-inflammatory effect by targeting AKT1 and AKT2 in the NF-κB signaling pathway in macrophage-mediated inflammatory responses (Yang et al., 2017). Kaempferol could inhibite AKT1-mediated phosphorylation of Smad3 at Thr179 residue (Jo et al., 2015). Beta-sitosterol significantly inhibited the TNF alpha-induced expression of VCAM1, which also played a key role in various inflammatory diseases (Gupta et al., 2010). Quercetin showed potent binding affinities to PIK3CG, and exerted a potent protective effect against LPS/CS-induced airway inflammation through inhibition of ERK, PI3K/Akt, and PKCα pathways (Ren et al., 2019). JAK1 is a member of Janus Kinases (JAK) which are intracellular tyrosine kinases linked to intracellular domains of many cytokine receptors. Oral JAK inhibitors have been developed as anti-cytokine therapy in rheumatoid arthritis (Choy, 2019). Luteolin promoted IFN-β-induced Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway activation by enhancing the phosphorylation of JAK1, Tyk2, and STAT1/2, thereby promoting STAT1 accumulation in the nucleus and endogenous IFN-α-regulated gene expression.
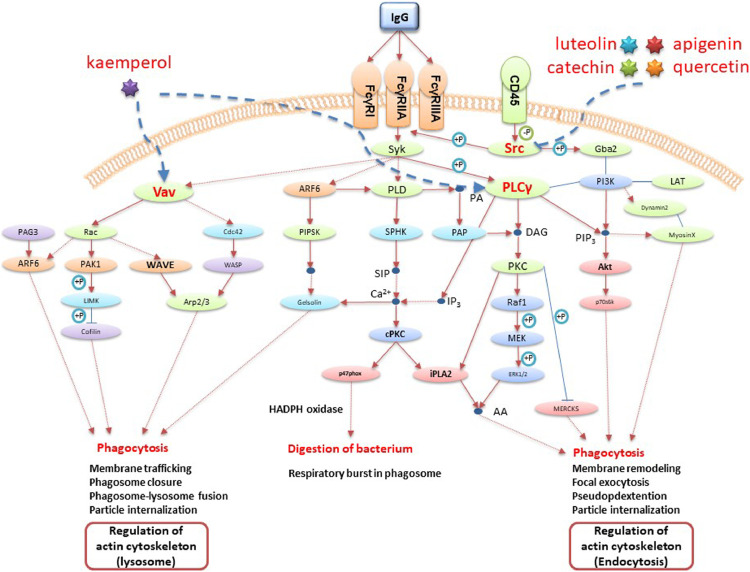
Module 22 is involved in Fc gamma receptor-mediated phagocytosis, and includes FYN (FYN Proto-Oncogene, Src Family Tyrosine Kinase) regulated by apigenin, catechin, quercetin and luteolin et al; VAV1 (Vav Guanine Nucleotide Exchange Factor 1) and PLCG1 (phospholipase C gamma 1) regulated by kaemperol et al. (Fig. 5 ). Fc gamma receptors (FcγRs) are critical regulators of inflammation, recognize IgG and so elicit a variety of effector functions including phagocytosis (Kusner et al., 1999). Phagocytosis plays a key role in host defense mechanisms by the uptake and destruction of infectious pathogens. One of the most striking features of FcγR-mediated phagocytosis is the dynamic and rapid organization of the actin cytoskeleton to form the phagocytic cup upon receptor activation by opsonized particle binding (Chimini and Chavrier, 2000).
Fig. 5.
Illustration of the Fc gamma receptor-mediated phagocytosis regulated by the LHQW main active components
FYN belongs to Src family kinases. Src family kinases are redundantly expressed in macrophages and are implicated in signaling by FcγRs, participating in actin polymerization in phagocytic cups and particle internalization (Majeed et al., 2001). Apigenin, catechin and quercetin, as flavonoids, exert anti-inflammatory effect. One key flavonoid inhibitory mechanism is blocking kinase activity through interactions with Src family kinases (Wright et al., 2015). Luteolin could inhibite nuclear translocation of FYN (Yang et al., 2018). VAV1 is a member of Vav family of guanine nucleotide exchange factors which are specifically required for FcγR-induced Rac activation through the exchange of GDP for GTP (Hall et al., 2006). PLCG1 (also known as PLCγ) plays an important role in the regulation of intracellular signaling cascades and actin reorganization, which mediates the production of the second messenger molecules diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (Dittmar et al., 2002). Kaempferol has been found to suppress the specific tyrosine phosphorylation of key components (Syk, VAV1, and PLCγ2) of collagen receptor signaling pathways, and also attenuate downstream responses, including cytosolic calcium elevation, P-selectin surface exposure, and integrin-αIIbβ3 activation (Wang et al., 2015).
These data provide strong theoretical support for the use of LHQW in the treatment of COVID-19 through regulating the immune process and Fc gamma receptor-mediated phagocytosis by main active components.
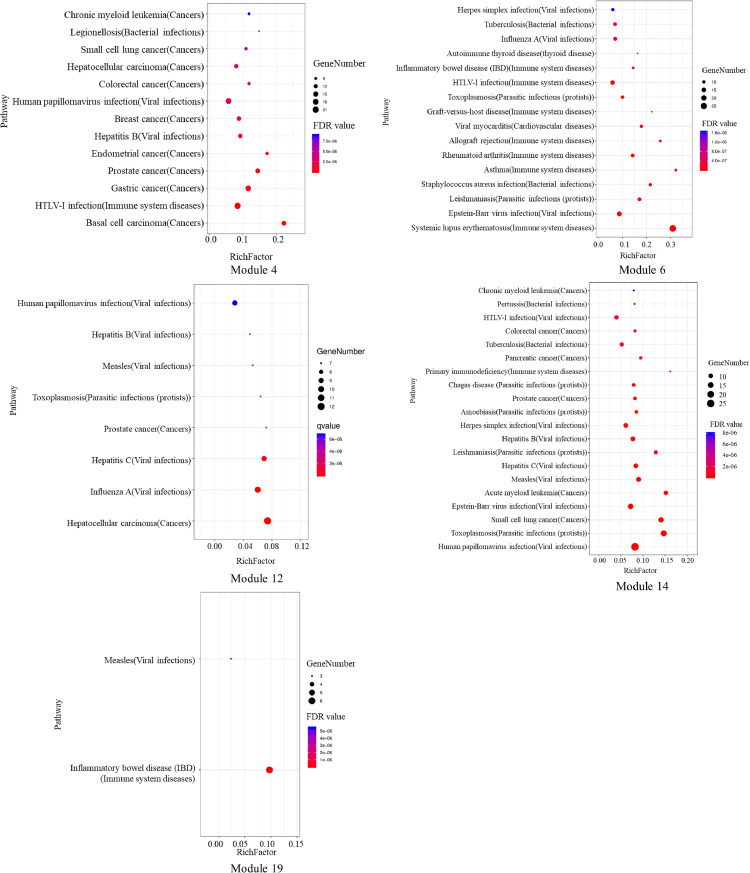
As COVID-19 is related to "cytokine storm", it is important that 6 functional modules are significantly related to the immune system process. These modules were therefore used for the KEGG pathway analysis. Then, the pathway terms with FDR of < 1⁎10−6 were mapped to the KEGG human diseases to obtain the disease pathway (Fig. 6 ). Among them, the module 22 did not obtain the corresponding disease. The identified disease pathways were mainly involved in cancer, immune system diseases, and viral infections, and were associated with the inflammatory process. For example, small cell lung cancer has been suggested to be strongly associated with inflammation according to clinical and epidemiologic studies (Cho et al., 2011) and HTLV-I infection is an immune system disease. Studies on the interactions between the inflammatory gene polymorphisms and HTLV-I infection for total death and incidence of cancer showed an increased hazard ratio for total death in the Amami islands region in Japan, after adjustment for various factors with gene polymorphisms (Kairupan et al., 2017). Infection with influenza A virus causes significant cell death within the upper and lower respiratory tracts and lung parenchyma. In severe infections, high levels of cell death can exacerbate inflammation and compromise the integrity of the epithelial cell barrier, leading to respiratory failure (Atkin-Smith et al., 2018). This indicated that LHQW as far as immune modules are concerned, could play an antiviral effect and repair lung injury by intervening at several phases of the inflammatory process.
Fig. 6.
The KEGG disease pathway of immune related modules in crucial LHQW PIN. There are only 5, not 6 figures, as module 22 did not obtain the corresponding disease.
Intersection analysis between LHQW proteins and ACE2 co-expression proteins
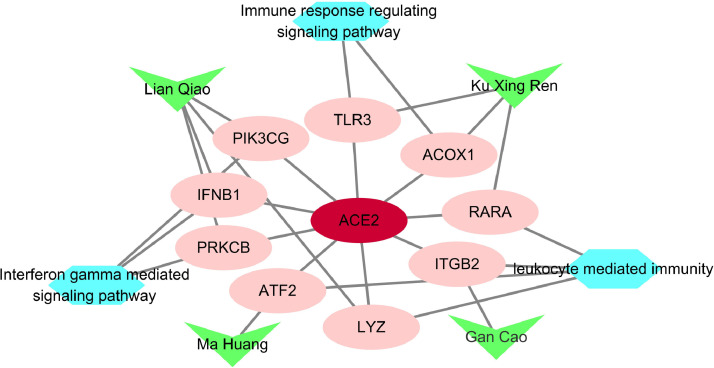
A total of 5556 proteins co-expressed with ACE2 were obtained. Intersection calculation between the LHQW proteins and ACE2 co-expression proteins was performed to investigate the moderation effect of LHQW on ACE2 co-expression proteins. As a result, 189 common proteins were obtained and mapped onto the 6 immune-related modules. 9 proteins were contained each in module 4, 6, 12, and 14, and could be regulated by the herbs in LHQW including Forsythiae Fructus (Lian Qiao), Ephedrae Herba (Ma Huang), Armeniacae Semen Amarum (Ku Xing Ren), and Glycyrrhizae Radix et Rhizoma (Gan Cao) (Fig. 7 ). Among them, ITGB2 was the crucial protein of hub LHQW PIN.
Fig. 7.
Intersection proteins between the LHQW proteins and ACE2 co-expression proteins by herbs to participate in the immune process. Ellipse nodes represent the intersection proteins, hexagon nodes represent the immune process, and triangle nodes represent the herbs in LHQW.
Discussion
LHQW has therapeutic effects on COVID-19. It improves the recovery rate, shortens the time to symptom recovery and improves the recovery of chest radiologic abnormalities (Hu et al., 2020). In light of the efficacy and safety profiles, LHQW should be recommended for COVID-19 treatment. It is therefore very important to investigate the pharmacological mechanisms of LHQW. Three very recent previous studies in Chinese non-Pubmed journals, have performed network pharmacology analysis of the LHQW mechanisms. However, the current study used many more techniques. Moreover, to explain by TCM meridian theory, which specific organ systems LHQW has selective therapeutic effects on, we constructed the LHQW meridian network. This new aspect is lacking in the three previous studies. Also new in our study is that we performed intersection analysis between LHQW proteins and ACE2 co-expression proteins, to explore the effect of improvement on ACE2-expression-disorder-caused symptoms. As ACE2 protein is the entry port for corona virus, this new aspect is of the utmost importance. We thus systematically investigated the therapeutic mechanisms of LHQW in COVID-19 from three different dimensions based on Network Pharmacology. The current study thus has a much greater depth than the previously published 3 articles. Finally, all these 3 articles were written in Chinese and published in journals which are not on PubMed. This would make them inaccessible to 75% of the world´s scientific population.
Traditional Chinese Medicine is a holistic medicine based on the observation of curative effects through many centuries of empirical clinical practice. Its effectiveness depends on multiple ingredients, targets and pathways. It is too complex to be analyzed by traditional experimental statistical methods which are based on the paradigm of “one gene, one drug, one disease” (Wermuth, 2004). Therefore, we elucidated the mechanism of action of LHQW on COVID-19 by applying Network Pharmacology. This method can identify the multi-component and multi-target agents involved by analyzing various networks of the complex and multi-level interactions (Li et al., 2014; Tao et al., 2013).
We constructed the LHQW PIN based on the whole protein-protein interactions obtained from the STRING database. Detection of protein interactions are limited by techniques development and proteomics studies. The 624 proteins may in fact be a small part of all the real targets of LHQW, but this is as much as the current status of knowledge is. Only using the protein interactions among the 624 targets to construct the PIN may be part of the whole LHQW pharmacological spectrum. Therefore, to make a construction which is closer to the whole network of LHQW PIN, we expanded the interaction proteins of the 624 proteins, as follows. Suppose that proteins A and B belong to the 624 targets of LHQW and C does not. If A interacts with C and C interacts B, C is also in our constructed LHQW PIN. This explains why the number of nodes of LHQW PIN was larger than the number of targets.
TCM meridian theory is an important part of the TCM theory. “Meridian” refers to the location in the body of the drug action. Although TCM herbs (or its chemical components) circulate through the whole body and therefore reach all organs, each TCM herb typically has selective therapeutic effects on a specific organ system. In the herb-meridian network of LHQW, 10 of 13 herbs in LHQW have specific relationship affinity to the lung system, which therefore is the largest organ system degree node. This indicates that LHQW mainly acts on the lungs, but not only. Indeed, SARS-CoV-2 infection leads to the aggravation of the infection of the lung tissue in patients with severe viral pneumonia and respiratory illness. Radiologically, multiple bilateral lobular and subsegmental areas of consolidation or bilateral ground-glass opacities have been reported and respiratory tract insufficiency is critical in severely ill COVID-19 patients. The Network Pharmacology results therefore provide a theoretical basis for the therapeutic potential of LHQW in the treatment of COVID-19 caused by SARS-CoV-2. However, LHQW has also significant effects in the Heart, Stomach, Spleen and Liver TCM systems.
Another important aspect of COVID-19 is its association with “cytokine storm” (Huang et al., 2020). The release of mediators and chemokines by infected cells rapidly leads to a local accumulation of neutrophils at the site of the infection. COVID-19 non-survivors had higher levels of neutrophils than survivors (Wang et al., 2020a). In one study on the pathogenesis of SARS-CoV-2 infection, ICU patients had significantly higher levels of interleukin (IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-17), interferon gamma, fibroblast growth factor, and TNF than healthy controls (Gao et al., 2020b). Moreover, in severe cases, significantly higher serum levels of tumor necrosis factor (TNF), interleukin (IL)-2R, IL-6, IL-8 and IL-10 were detected as compared with milder cases (Qin et al., 2020). This suggests that the cytokine storm was associated with disease severity.
Considering the high levels of cytokines induced by SARS-CoV-2 infections, the treatment based on reducing the inflammation-induced lung injury could have benefit for infected patients. Studies that focus on the inhibition of IL-1β to reduce the cytokine storm have attracted most attention. Anakinra, an antagonist of IL-1β, can be used to treat the cytokine storm caused by infection. It significantly improved the 28-day survival rate of patients with severe sepsis (Shakoory et al., 2016) . IFN-λ may be an ideal treatment as IFN-λ primarily activates epithelial cells and reduces the mononuclear macrophage-mediated proinflammatory activity of IFN-αβ (Davidson et al., 2016). In addition, IFN-λ inhibits the recruitment of neutrophils to the sites of inflammation (Blazek et al., 2015). Also in persons with proven COVID-19, LHQW treatment can be very useful, as it not only will diminish the chance of progressing to severe COVID-19, but for those who experience a cytokine storm and still recover, the serious damage to lung tissue can have long-lasting chronic consequences. LHQW could perhaps diminish these long term side effects.
The GO enrichment results based on the LHQW PIN showed that 6 functional modules were closely related to the immune system process, including leukocyte mediated immunity, interferon gamma mediated signaling pathway, immune response regulating signaling pathway, interleukin 23 mediated signaling pathway, and Fc gamma receptor-mediated phagocytosis. Previous KEGG disease enrichment results showed that LHQW could intervene in small cell lung cancer, HILV-I infection, herpes simplex infection, human papillomavirus infection, hepatitis B, hepatitis C, and influenza A. It does so by promoting a regulated and protective immune response. Moreover, the levels of interleukin and IFNγ were observed to be higher in infected patients than in healthy people (Huang et al., 2020). Taken together, the results of the current study indicate that LHQW could modulate the inflammatory process to exert its therapeutic effects against COVID-19, and especially regulate interleukin 23 mediated signaling pathway and the IFNγ-mediated signaling pathway.
SARS-CoV-2 utilizes ACE2 to bind and gain entry to the host pneumocytes (Wan et al., 2020). ACE2 has been reported to be the receptor for COVID-19 (Chan et al., 2020). Patients previously using angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blocker (ARB) cardiologic therapy possibly have a higher risk for severe COVID-19 infection, as these therapies upregulate ACE2 expression, thereby facilitating entry of SARS-CoV-2 into the pneumocytes (Fang et al., 2020). Conversely, patients with COVID-19 may benefit from protective effect of acute lung injury of ACE2 (decreasing overall inflammation) (Cheng et al., 2020b; Rico-Mesa et al., 2020). Though the hypotheses on ACE2 vary, several cardiology associations (HFSA/ACC/AHA and ESC Hypertension Council) released an official statement and strongly recommend continuing treatment with ACEI/ARB in patients who were previously taking either class of medication (Rico-Mesa et al., 2020). Therefore, regulation of ACE2 may be beneficial for COVID-19 therapy.
The intersection results between the LHQW targets and ACE2 co-expression proteins showed that they shared 189 common proteins in all. Some of them participated in the immune-related biological processes that are regulated by LHQW. Moreover, integrin subunit beta 2 (ITGB2) was the crucial target. It plays an important role in the immune response and the diseases associated with ITGB2 including leukocyte adhesion deficiency and pertussis. ITGB2 is required for the negative regulation of WNT signaling in the lung to increase repair and regeneration after lung injury (Mukhametshina et al., 2013). These results show that LHQW has potential ameliorating effects on the symptoms caused by ACE2 expression disorder.
Yet, the other functional modules of LHQW may also be important. They are closely associated with the nucleic acid metabolic process and the cellular protein metabolic process. The virus has no ribosomes of its own, so it needs to use the ribosomes of the host cell to synthesize the protein shell of the virus, so as to complete its own replication (Zhao et al., 2020). The ribosome is the site of protein synthesis in the cell, where the process of translating mRNA into amino acids takes place. Therefore, the therapeutic mechanisms of LHQW may related to regulate the nucleic acid metabolic process and the cellular protein metabolic process. The process of nucleic acid metabolism and cell protein metabolism involve a very wide range of proteins. It would greatly exceed the goals of this article to analyze all these proteins as this would be a several person-years project.
LHQW has good therapeutic effects on COVID-19 (Cheng et al., 2020a; Yao et al., 2020). Three very recently published studies (Chinese language, in Chinese journals) have used Network Pharmacology to discuss its action mechanisms. Wang et al´s first study (published on 29 February 2020) found that 392 GO biological processes and 105 KEGG signal pathways are involved. These are mainly enriched in virus infection, inflammation and immunity. Wang et al´s study (published on 18 March 2020) showed that LHQW may improve the clinical symptoms of COVID-19 by regulating inflammatory signals and viruses. Ling et al's study (published on 30 March 2020) obtained many more entries: 1946 GO enrichment analysis entries which were mainly involved in T cell activation, viral receptors, and inflammatory responses. The number of KEGG pathways was also higher (166), including renin-angiotensin system, Toll-like receptor signaling pathway, JAK-STAT signaling pathway, T cell receptor signaling pathway, TNF signaling pathway and so on. We found 6 inflammatory functional modules enriched in three diseases. Also on technical methods, we have adopted more methods than the other 3 articles, by focusing on the results (for more details detail, see Supplementary Data Table S1).
Most importantly, our paper differed from these previous studies by focusing on the immune system process. We did so, as “cytokine storm” is crucial in the pathophysiology and especially the late stage of COVID-19. Therefore, we expanded the module analysis to include the inflammatory functional modules, to make the research results more specific and clear. We found that the 6 functional modules obtained are related to the immune system process, including leukocyte mediated immunity, interferon gamma mediated signaling pathway, immune response regulating signaling pathway, interleukin 23 mediated signaling pathway, and Fc gamma receptor-mediated phagocytosis, which are enriched in three diseases (cancer, immune system diseases and viral infection diseases). These findings are new.
The advantage of Network Pharmacology is that it can investigate very complex systems such as TCM herbal formulas containing multiple herbs in widely different diseases, in which each herb can have many different primary and secondary effects. Network Pharmacology connects herbs, components, targets and diseases through a network. It also mimics the way drugs act on human networks. This can never be done to the same extent by classical statistical methods. However, the method also has weak points. The information on active components and protein interactions is limited by separation techniques development and proteomics studies. Therefore, the results of Network Pharmacology is limited by the “state of the art” knowledge, known today in very large databases. This knowledge will expand enormously in the future. The results also correspond to the technology and research of the time. Moreover, our results were based on silicon computer (“dry”) experiment. Therefore, validation of the results by future “wet” experiments using cellular and molecular biological and organic chemistry methods, are needed to support the current findings. We plan to do the following experiments. First, to screen screening the quality of many commercially available single herbs. Herbs from different vendors worldwide can vary considerably. Secondly, to investigate the oral bioavailability in different persons, different genders and ages, by a pharmacokinetics study. It may well be, that certain patients did not get the optimal dosage, for their gender, age or other patient-specific characteristics.
Nevertheless, the current results provide an important basis for further studying the mechanism of LHQW in COVID-19 and targeted optimization of future studies.
Conclusions
The COVID-19 pandemic caused by SARS-CoV-2 has caused great global concerns. COVID-19 is associated with “cytokine storm”. LHQW, a TCM drug which acts by clearing heat and toxic materials according to TCM theory, has proven to be therapeutically useful in COVID-19 (Cheng et al., 2020a; Yao et al., 2020). Three very recent previous studies, published in Chinese, used Network Pharmacology to elucidate the biological mechanism of LHQW in COVID-19. We did the same, but used more in-depth techniques and included also ACE and cytokine storm, essential in the very early and late stages of Covid-19. A multidimensional analysis including meridian, GO biological process, KEGG disease pathway, and ACE2 co-expression intersection was used. Special emphasis was given on the “cytokine storm” immunological aspects, as this is crucial in the pathophysiology of COVID-19. The results show that LHQW has specific effects in the treatment of lung diseases as 10 of the 13 LHQW herbs have a strong outspoken lung meridian affinity relationship. This provides a theoretical basis for using LHQW in the treatment of COVID-19. Furthermore, the results from GO and KEGG enrichment show that LHQW modulates the inflammatory process and relieves the “cytokine storm”. Hence, it exerts its therapeutic effects against COVID-19 by including multiple processes via leukocyte mediated immunity, interferon gamma mediated signaling pathway, immune response regulating signaling pathway, interleukin 23 mediated signaling pathway, and Fc gamma receptor-mediated phagocytosis. Moreover, LHQW shared 189 common proteins with ACE2 co-expression proteins. ACE2 is an enzyme found in lung cells, is a receptor for SARS-CoV, serves as the entry point into cells for some coronaviruses and has been linked to SARS-CoV-2 infection. LHQW therefore also has potentially ameliorating effects on the symptoms caused by ACE2 expression disorder. This is a new finding.
The results give strong theoretical support to use LHQW in the treatment of COVID-19. Moreover, as described above, LHQW could also be useful in a broader range of clinical applications. They provide an important basis for further studying the mechanisms of LHQW in COVID-19 and targeted optimization of future validation studies.
Funding sources
This work was supported by the National Natural Science Foundation of China (81703825), the Natural Science Foundation Project of the Education Department of Sichuan Province (18ZB01869) and a grant from Dr. Med. Jan Baak Health Care Inc, Tananger, Norway.
Contribution of the authors
The table below summarizes the contribution from each author:
| Shichao Zheng | Jan P. Baak | Shuang Li | Wenke Kiao | Hong Ren | Huan Yang | Yanxiong Gan | Chuanbiao Wen | |
|---|---|---|---|---|---|---|---|---|
| Conceptualization | √ | √ | √ | |||||
| Data curation | √ | √ | √ | √ | √ | √ | ||
| Formal analysis | √ | √ | √ | |||||
| Investigation | √ | √ | √ | |||||
| Methodology | √ | √ | √ | √ | ||||
| Project administration | √ | √ | √ | |||||
| Resources | √ | √ | √ | √ | ||||
| Software | √ | √ | √ | |||||
| Validation | √ | √ | √ | |||||
| Visualization | √ | √ | √ | √ | √ | √ | ||
| Funding acquisition | √ | √ | √ | |||||
| Supervision | √ | √ | √ | |||||
| Writing - original draft | √ | √ | √ | |||||
| Writing review & editing | √ | √ | √ |
Declaration of Competing Interest
The authors declare that there is no financial or other conflict of interest regarding the publication of this paper.
Acknowledgements
We thank Dr. Helen van Oord, MSc, Melbourne, Victoria, Australia, and Dr. M. Smith. MSc, London, UK, for correcting the English.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2020.153336.
Appendix. Supplementary materials
References
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Harris M.A., Hill D.P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J.C., Richardson J.E., Ringwald M., Rubin G.M., Sherlock G., Gene Ontology C. Gene Ontology: tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenov Y., Ramírez F., Schelhorn S.E., Lengauer T., Albrecht M. Computing topological parameters of biological networks. Bioinformatics. 2008;24:282–284. doi: 10.1093/bioinformatics/btm554. [DOI] [PubMed] [Google Scholar]
- Atkin-Smith G.K., Duan M., Chen W., Poon I.K.H. The induction and consequences of Influenza A virus-induced cell death. Cell Death. Dis. 2018;9:1002–1012. doi: 10.1038/s41419-018-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader G.D., Hogue C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003;4:1–27. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi A.L., Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- Blazek K., Eames H.L., Weiss M., Byrne A.J., Perocheau D., Pease J.E., Doyle S., McCann F., Williams R.O., Udalova I.A. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J. Exp. Med. 2015;212:845–853. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020;141:1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infections. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Wang W., Li Y., Wu X., Zhou B., Song Q. Analysis of curative effect of 51 patients with novel coronavirus pneumonia treated with Chinese medicine Lianhua Qingwen: a multicentre retrospective study. Tianjin J. Tradit. Chin. Med. 2020;37:509–516. [Google Scholar]
- Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S.Y., Jin B.R., Kim H.J., An H.J. Oleanolic acid ameliorates benign prostatic hyperplasia by regulating PCNA-dependent cell cycle progression in vivo and in vitro. J. Nat. Prod. 2020;83:1183–1189. doi: 10.1021/acs.jnatprod.9b01210. [DOI] [PubMed] [Google Scholar]
- Chimini G., Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- Cho W.C.S., Kwan C.K., Yau S., So P.P.F., Poon P.C.M., Au J.S.K. The role of inflammation in the pathogenesis of lung cancer. Expert Opin. Ther. Targets. 2011;15:1127–1137. doi: 10.1517/14728222.2011.599801. [DOI] [PubMed] [Google Scholar]
- Choy E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953–962. doi: 10.1093/rheumatology/key339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., McCabe T.M., Crotta S., Gad H.H., Hessel E.M., Beinke S., Hartmann R., Wack A. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol. Med. 2016;8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T., Husemann A., Schewe Y., Nofer J.R., Niggemann B., Zänker K.S., Brandt B.H. Induction of cancer cell migration by epidermal growth factor is initiated by specific phosphorylation of tyrosine 1248 of c-erbB-2 receptor via EGFR. FASEB J. 2002;16:1823–1825. doi: 10.1096/fj.02-0096fje. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebich B.L., Collado J.A., Stratz C., Valina C., Hochholzer W., Muñoz E., Bellido L.M. Pseudoephedrine inhibits T-cell activation by targeting NF-κB, NFAT and AP-1 signaling pathways. Immunopharmacol. 2012;34:98–106. doi: 10.3109/08923973.2011.582118. [DOI] [PubMed] [Google Scholar]
- Filippou P.S., Karagiannis G.S. Cytokine storm during chemotherapy: a new companion diagnostic emerges? Oncotarget. 2020;11:213–215. doi: 10.18632/oncotarget.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Ma Y., Yang F., Zhang J., Yu C. ZHANG Boli:traditional Chinese medicine plays a role in the prevention and treatment on novel coronavirus pneumonia. Tianjin J. Tradit. Chin. Med. 2020;37:121–124. [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Balwani S., Kumar S., Aggarwal N., Rossi M., Paumier S., Caruso F., Bovicelli P., Saso L., DePass A.L., Prasad A.K., Parmar V.S., Ghosh B. Beta-sitosterol among other secondary metabolites of Piper galeatum shows inhibition of TNFalpha-induced cell adhesion molecule expression on human endothelial cells. Biochimie. 2010;92:1213–1221. doi: 10.1016/j.biochi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Hall A.B., Gakidis M.A., Glogauer M., Wilsbacher J.L., Gao S., Swat W., Brugge J.S. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and Safety of Lianhuaqingwen Capsules, a repurposed Chinese Herb, in Patients with Coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;153242 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E., Park S.J., Choi Y.S., Jeon W.K., Kim B.C. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia. 2015;17:525–537. doi: 10.1016/j.neo.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairupan T.S., Ibusuki R., Kheradmand M., Sagara Y., Mantjoro E.M., Nindita Y., Niimura H., Kuwabara K., Ogawa S., Tsumematsu-Nakahata N., Nerome Y., Owaki T., Matsushita T., Maenohara S., Yamaguchi K., Takezaki T. Interactions between inflammatory gene polymorphisms and HTLV-I infection for total death, incidence of cancer, and atherosclerosis-related diseases among the Japanese population. J. Epidemiol. 2017;27:420–427. doi: 10.1016/j.je.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S.A., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y.L., Deng W., Bao L.L., Zhang B.L., Liu G., Wang Z., Chappell M., Liu Y.X., Zheng D.X., Leibbrandt A., Wada T., Slutsky A.S., Liu D.P., Qin C.A., Jiang C.Y., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusner D.J., Hall C.F., Jackson S. Fc gamma receptor-mediated activation of phospholipase D regulates macrophage phagocytosis of IgG-opsonized particles. J. Immunol. 1999;162:2266–2274. [PubMed] [Google Scholar]
- Li F., Li W.H., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Fan T.-P., Jia W., Lu A., Zhang W. Network pharmacology in traditional Chinese medicine. Evid.-Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/138460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang Z.Q., Wu L.J., Zhang X.G., Li Y.D., Wang Y.Y. Understanding Zheng in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst. Biol. 2007;1:51–60. doi: 10.1049/iet-syb:20060032. [DOI] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.-W., Kang Y.M., Lee B., Park S.-J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes. Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Caveggion E., Lowell C.A., Berton G. Role of Src kinases and Syk in Fcgamma receptor-mediated phagocytosis and phagosome-lysosome fusion. J. Leukoc. Biol. 2001;70:801–811. [PubMed] [Google Scholar]
- Manke T., Demetrius L., Vingron M. Lethality and entropy of protein interaction networks. Genome informatics. Int. Conf. Genome Informat. 2005;16:159–163. [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhametshina R.T., Ruhs A., Singh I., Hasan D., Contreras A., Mehta A., Nikam V.S., Ahlbrecht K., Carraro G., Cabrera-Fuentes H.A., Jiang D., Voswinckel R., Seeger W., Bellusci S., Scharffetter-Kochanek K., Bagaeva T.V., Preissner K.T., Boettger T., Braun T., Krueger M., Barreto G. Quantitative proteome analysis of Alveolar Type-II cells reveals a connection of integrin receptor subunits Beta 2/6 and WNT Signaling. J. Proteome Res. 2013;12:5598–5608. doi: 10.1021/pr400573k. [DOI] [PubMed] [Google Scholar]
- Neurath M.F. Covid-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- News, U., 2020. COVID-19: WHO working on supply pipeline for protective equipment and testshttps://news.un.org/en/story/2020/03/1059792.
- NHCPRC, 2020. National Health Commission of the Peaple's Republic of China: Notice on the issuance of COVID-19 protocol (trial seventh edition), http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. [DOI] [PMC free article] [PubMed]
- Ohayon D., De Chiara A., Dang P.M., Thieblemont N., Chatfield S., Marzaioli V., Burgener S.S., Mocek J., Candalh C., Pintard C., Tacnet-Delorme P., Renault G., Lagoutte I., Favier M., Walker F., Hurtado-Nedelec M., Desplancq D., Weiss E., Benarafa C., Housset D., Marie J.C., Frachet P., El-Benna J., Witko-Sarsat V. Cytosolic PCNA interacts with p47phox and controls NADPH oxidase NOX2 activation in neutrophils. J. Exp. Med. 2019;216:2669–2687. doi: 10.1084/jem.20180371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q.C., Li X.H., Li Q.Y., Yang H.L., Wang H.L., Zhang H., Zhao L., Jiang-Yong S.L., Meng X.L., Zhang Y., Shen X.F. Total flavonoids from sea buckthorn ameliorates lipopolysaccharide/cigarette smoke-induced airway inflammation. Phytother. Res. 2019;33:2102–2117. doi: 10.1002/ptr.6404. [DOI] [PubMed] [Google Scholar]
- Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr. Cardiol. Rep. 2020;22:31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., Jensen L.J., Mering C. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucl. Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W., Xu X., Wang X., Li B., Wang Y., Li Y., Yang L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013;145:1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- Team N.C.P.E.R.E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Valente T.W., Fujimoto K. Bridging: locating critical connectors in a network. Soc. Netw. 2010;32:212–220. doi: 10.1016/j.socnet.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Zhao, S., Liu, M., Zhao, Z., Xu, Y., Wang, P., Lin, M., Xu, Y., Huang, B., Zuo, X., Chen, Z., Bai, F., Cui, J., Lew, A., Zhao, J., Zhang, Y., Luo, H., Zhang, Y., 2020b. ACE2 expression by colonic epithelial cells is associated with viral infection, immunity and energy metabolism. medRxiv preprint.
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.B., Jang J.Y., Chae Y.H., Min J.H., Baek J.Y., Kim M., Park Y., Hwang G.S., Ryu J.S., Chang T.S. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic. Biol. Med. 2015;83:41–53. doi: 10.1016/j.freeradbiomed.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth C.G. Multitargeted drugs: the end of the 'one-target-one-disease' philosophy? Drug Discov. Today. 2004;9:826–827. doi: 10.1016/S1359-6446(04)03213-1. [DOI] [PubMed] [Google Scholar]
- Wright B., Watson K.A., McGuffin L.J., Lovegrove J.A., Gibbins J.M. GRID and docking analyses reveal a molecular basis for flavonoid inhibition of Src family kinase activity. J. Nutr. Biochem. 2015;26:1156–1165. doi: 10.1016/j.jnutbio.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Xu H.-Y., Zhang Y.-Q., Liu Z.-M., Chen T., Lv C.-Y., Tang S.-H., Zhang X.-B., Zhang W., Li Z.-Y., Zhou R.-R., Yang H.-J., Wang X.-J., Huang L.-Q. ETCM: an encyclopaedia of traditional Chinese medicine. Nucl. Acids Res. 2019;47:D976–D982. doi: 10.1093/nar/gky987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.Y., Zhang Y.Q., Liu Z.M., Chen T., Lv C.Y., Tang S.H., Zhang X.B., Zhang W., Li Z.Y., Zhou R.R., Yang H.J., Wang X.J., Huang L.Q. ETCM: an encyclopaedia of traditional Chinese medicine. Nucl. Acids Res. 2019;47:D976–d982. doi: 10.1093/nar/gky987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E.J., Lee W., Ku S.K., Song K.S., Bae J.S. Anti-inflammatory activities of oleanolic acid on HMGB1 activated HUVECs. Food Chem. Toxicol. 2012;50:1288–1294. doi: 10.1016/j.fct.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Yang J.T., Wang J., Zhou X.R., Xiao C., Lou Y.Y., Tang L.H., Zhang F.J., Qian L.B. Luteolin alleviates cardiac ischemia/reperfusion injury in the hypercholesterolemic rat via activating Akt/Nrf2 signaling. Naunyn-Schmiedeberg's Arch. Pharmacol. 2018;391:719–728. doi: 10.1007/s00210-018-1496-2. [DOI] [PubMed] [Google Scholar]
- Yang W.S., Kim D., Yi Y.S., Kim J.H., Jeong H.Y., Hwang K., Kim J.H., Park J., Cho J.Y. AKT-targeted anti-inflammatory activity of the methanol extract of Chrysanthemum indicum var. albescens. J. Ethnopharmacol. 2017;201:82–90. doi: 10.1016/j.jep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Yao K., Liu M., Li X., Huang J., Cai H. Retrospective clinical analysis on treatment of novel coronavirus-infected pneumonia with traditional chinese medicine Lianhua Qingwen. Chin. J. Exp. Tradit. Med. Formulae. 2020:1–7. [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhu X., Bai H., Ning K. Network pharmacology databases for traditional chinese medicine: review and assessment. Front. Pharmacol. 2019;10:123. doi: 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li Z., Yang M., Wang D., Yu L., Guo C., Guo X., Lin N. Identification of GRB2 and GAB1 coexpression as an unfavorable prognostic factor for hepatocellular carcinoma by a combination of expression profile and network analysis. PLoS One. 2013;8:e85170. doi: 10.1371/journal.pone.0085170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Guochun L., Yang Y., Shi L., Xu L., Yin L. A network pharmacology approach to determine active ingredients and rationality of herb combinations of Modified-Simiaowan for treatment of gout. J. Ethnopharmacol. 2015;168:1–16. doi: 10.1016/j.jep.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Zhao J., Tian S.S., Yang J., Liu J.F., D. Z.W. Investigating mechanism of Qing-Fei-Pai-Du-Tang for treatment of COVID-19 by network pharmacology. Chin. Tradit. Herbal Drugs. 2020;51:829–835. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.