Abstract
Background
Black patients have higher rates of hospitalization for acute heart failure (AHF) than other race/ethnic groups. We sought to determine if diuretic efficiency is associated with racial differences in risk for rehospitalization after AHF.
Methods
A post hoc analysis was performed on 721 subjects (age 68 ± 13 yrs, 22% Black) enrolled in 3 AHF clinical trials: ROSE-AHF, DOSE-AHF, and CARRESS-HF. Repeated-measures ANOVA was used to test for a race × time effect on measures of decongestion. Diuretic efficiency was calculated as net fluid balance per total furosemide equivalents. In a subset of subjects, Cox regression was used to examine the association between race and rehospitalization according to plasma renin activity (PRA).
Results
Compared to non-Black patients, Black patients were younger and more likely to have nonischemic HF. During the first 72–96 hours there was greater fluid loss (P=0.001), decrease in NT-pro BNP (P=0.002), and lower levels of PRA (P<0.0001) in Black patients. Diuretic efficiency was higher in Black than non-Black patients (403 [IQR 221–795] vs 325 [IQR 154–698]; P=0.014). However, adjustment for baseline PRA attenuated the association between Black race and diuretic efficiency. Over a median follow-up of 68 (IQR 56–177) days, there was an increased risk of all-cause and HF-specific rehospitalization in non-Black patients with increasing levels of PRA, while the risk of rehospitalization was relatively constant across levels of PRA in Black patients.
Conclusions
Higher diuretic efficiency in Black patients with AHF may be related to racial differences in activity of the renin-angiotensin-aldosterone system.
Keywords: Acute heart failure, racial disparities, diuretic efficiency, plasma renin activity
INTRODUCTION
Heart failure (HF) is the leading cause of cardiovascular hospitalization and the fifth leading cause of hospitalization overall in the United States.1 Moreover, important disparities exist in the likelihood of HF hospitalization based on race/ethnicity. The rate of HF hospitalization for Black men and women is nearly two and half-fold higher when compared with Whites, with costs that are significantly higher in the first year after HF hospitalization.2, 3 While the relative rate of HF hospitalization has improved for other race/ethnic minorities, the disparity in HF hospitalization between Black and White patients has not decreased during the last decade.2 Although access to care and socioeconomic status contribute to racial differences in HF outcomes, biologic mechanisms that might contribute to differential risk for volume overload and/or diuretic response remain underexplored.
Multiple metrics of diuretic responsiveness during acute heart failure (AHF) have been shown to provide incremental prognostic information beyond that provided by traditional markers including changes in weight or fluid balance.4–6 Diuretic efficiency, defined as the net fluid output per milligram of loop diuretic administered during a hospitalization for AHF, is associated with worse long-term outcomes.7 Patients with low diuretic efficiency have a higher risk for death.5, 7 Moreover, although prior analyses suggest that patients with higher baseline activity of the renin-angiotensin-aldosterone system (RAAS) require higher outpatient loop diuretic doses, the loop diuretic dose administered during AHF is not associated with greater RAAS activation.8 Prior studies have documented a distinct volume-overloaded, hypertensive phenotype whereby increased sodium and volume retention does not appear to be directly secondary to increased RAAS activity, and is characterized by low renin and aldosterone levels.9 Moreover, the presence of low renin hypertension, which is thought to be more common in Black patients, may predict response to diuretics.10 Given known racial differences in biomarkers of RAAS activity, we pooled data from 3 randomized trials of AHF management to determine: 1) whether metrics of diuretic responsiveness differ by race/ethnicity, and 2) whether racial differences in RAAS activity influence diuretic responsiveness and/or risk for rehospitalization.
METHODS
Data source
A post-hoc analysis was performed on subjects enrolled in 3 prospective, multicenter, randomized clinical trials conducted by the National Heart, Lung, and Blood Institute (NHLBI) Heart Failure Network: Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF), Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF), and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF). The design and results of these trials have been reported previously.11–13 All studies were approved by the institutional review board at participating sites, and all study participants provided written informed consent. The data is publicly available and can be requested via the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
ROSE-AHF enrolled 360 subjects at 26 sites across North America from 2010 to 2013.11 Participants were hospitalized with AHF based on ≥1 symptom and 1 sign of HF regardless of left ventricular ejection fraction (LVEF), with evidence of renal dysfunction (estimated glomerular filtration rate [eGFR] of 15–60 mL/min/1.73 m2). Subjects were randomized in an open 1:1 allocation ratio to a dopamine or a nesiritide strategy within 24 hours of admission. Within each strategy, Subjects were randomized in a double blind 2:1 ratio to active therapy or placebo, resulting in an overall 1:1:1 division of dopamine:nesiritide:placebo among groups.
DOSE-AHF enrolled 308 subjects at 26 sites across North America from 2008 to 2009.12 Participants were hospitalized with AHF based on ≥1 symptom and 1 sign of HF regardless of LVEF. A 2X2 factorial design was used to randomize subjects to a strategy of high- versus low-dose furosemide therapy and continuous versus intermittent bolus dose administration within 24 hours of admission. Subjects were required to have a baseline diuretic requirement of ≥80 mg oral furosemide equivalents daily, and those with serum creatinine >3.0 mg/dL were excluded.
CARRESS-HF enrolled 188 subjects at 22 sites across North America from 2008 to 2012.13 Participants were hospitalized with AHF based on ≥2 signs of HF regardless of LVEF, and had persistent congestion with worsened renal function within 12 weeks earlier or 10 days after AHF admission. Subjects were randomized in a 1:1 ratio to ultrafiltration or a stepped pharmacologic therapy strategy utilizing diuretics titrated to maintain a urine output of 3 to 5 L/d. Subjects with a serum creatinine >3.5 mg/dL were excluded.
Study Population
Baseline data was combined from subjects enrolled in ROSE-AHF, DOSE-AHF, and the stepped pharmacologic therapy arm of CARRESS-HF (N=94). Subjects with missing data on urine output (N=16) or those with net fluid gain during the hospitalization (N=25) were excluded, leaving 721 subjects for the final analysis.
Metrics of diuretic responsiveness
Diuretic efficiency was estimated as net fluid balance per 40 mg of furosemide equivalents administered during the AHF hospitalization.7 Furosemide equivalents were defined based on the following conversions: 80 mg oral furosemide = 40 mg intravenous (IV) furosemide = 20 mg oral / IV torsemide = 1 mg oral / IV bumetanide.14 Diuretic efficiency was dichotomized into high versus low based on the median value.
Biomarkers of neurohormonal activation
RAAS activation was measured by plasma renin activity (PRA) and aldosterone levels. Plasma samples were collected from subjects enrolled in the DOSE-AHF and the CARRESS-HF studies at baseline (N=356), at 72 h for subjects in DOSE-AHF or 96 h for subjects in CARRESS-HF (N=310), and analyzed at a central core laboratory. PRA was measured with a GammaCoat PRA iodine-125 radio-immunoassay kit (Diasorin, Still-water, Minnesota); expected values range from 0.85 to 16.34 ng/ml/h, and the interassay coefficient of variation provided by the manufacturer is <10%. Aldosterone was measured by radioimmunoassay (Diasorin); expected values range from 7.5 to 150 pg/ml, and the interassay coefficient of variation provided by the manufacturer is <5.3%.
Clinical covariates and outcomes
Baseline characteristics were recorded at randomization for all patients. Physical signs and symptoms of congestion, serum chemistries, total fluid intake, total urine output, weight, and cardiac biomarkers were collected at baseline, 24, 48, and 72 hours (ROSE-AHF, DOSE-AHF, CARRESS-HF), and 96 hours (CARRESS-HF). The primary clinical outcomes were time to all-cause and HF-specific rehospitalization.
Statistical analysis
Data are presented as mean ± standard deviation (SD), median (interquartile range [IQR]), or N (%) of patients, as appropriate. Biomarkers and diuretic efficiency were log-transformed prior to use as predictor variables in parametric tests. Analyses were performed according to self-reported race, Black (N=160) vs non-Black (White N=534, other N=27). Baseline characteristics were compared between Black and non-Black patients using the Student t-test for normally distributed continuous variables, the Wilcoxon rank-sum test for non-normally distributed continuous variables, and the χ2 test for categorical variables. Repeated measures were analyzed using analysis of variance (ANOVA) with Greenhouse-Geisser correction for normally distributed continuous variables (change in weight, creatinine, net fluid loss, blood pressure), or the Friedman test for non-normally distributed continuous variables (NT-pro BNP, PRA, aldosterone). To determine which baseline variables were associated with diuretic efficiency, generalized linear regression models were fitted with a gamma distribution and log link. A sensitivity analysis was also performed to determine baseline variables associated with diuretic efficiency after excluding those subjects who were on sequential nephron blockade (N=86) as outpatient therapy. Cox proportional hazards regression was used to examine the association of diuretic efficiency with the risk of all-cause and HF-specific rehospitalization for all subjects. The scaled Schoenfeld residuals were used to assess the proportional hazards assumption.
As an exploratory analysis, we examined the association between subjects’ baseline and 72- to 96-hour PRA level and time to rehospitalization in separate models. Only PRA was used in survival models since prior analyses demonstrated a marginal change in aldosterone levels with decongestion during AHF.8, 15 Cox proportional hazards regression was used to examine the association between PRA and risk of rehospitalization, with a race by PRA interaction term added to the main effect terms to assess any effect modification based on race. The relative hazard of all-cause and HF-specific rehospitalization was computed from the fully adjusted model for observed values of PRA while holding the values of covariates at their referent values and plotted (on the y-axis) against PRA (on the x-axis, modeled using restricted cubic splines with 3 knots to account for nonlinearity of effects) by race. Cox regression was also used to examine time to rehospitalization with PRA dichotomized into high versus low based on the median value separately for Black vs. non-Black patients, using the 4 combinations of race and PRA above or below the median. All Cox models were adjusted for age, sex, race, EF, HF etiology (ischemic vs nonischemic), hypertension, systolic blood pressure, body mass index (BMI), eGFR, study, and baseline therapy with angiotensin converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB) and aldosterone antagonists.
A p value of 0.05 was considered statistically significant. Data were analyzed using SAS v9.4 (SAS Institute Inc., Cary, NC) and R statistical software (version 3.5.1).
RESULTS
Baseline characteristics of the analytic cohort are described in Table 1. Black patients were younger with lower EF, and were more likely to have nonischemic HF, and be on ACEi, ARB, hydralazine, and nitrate therapy. As baseline outpatient therapy, the median dose of loop diuretic was similar between groups, however non-Black patients were more likely to be on sequential nephron blockade with a thiazide-type and a loop diuretic. In the prior 12 months, Black patients had more hospitalizations. At the time of randomization, Black patients were more likely to have high grade orthopnea and an S3 gallop, while non-Black patients were more likely to have severe peripheral edema on exam (Table 2). Even after 72 to 96 hours of diuresis, orthopnea remained more common in Black subjects.
Table 1.
Baseline characteristics of the analytic cohort.
| Non-Black N=561 | Black N=160 | P-Value | |
|---|---|---|---|
| Age, years | 70 ± 12 | 59 ± 13 | <0.0001 |
| Male | 418 (75) | 110 (69) | 0.1 |
| Study | 0.4 | ||
| • ROSE-AHF | 273 (80) | 69 (20) | |
| • DOSE-AHF | 220 (77) | 67 (23) | |
| • CARRESS-HF | 68 (74) | 24 (26) | |
| Hypertension | 452 (81) | 142 (89) | 0.017 |
| Diabetes mellitus | 319 (57) | 83 (52) | 0.2 |
| Nonischemic HF etiology | 269 (48) | 123 (77) | <0.0001 |
| Ejection Fraction, % | 38 ± 17 | 31 ± 16 | <0.0001 |
| Heart Failure with Preserved Ejection Fraction | 184 (33) | 33 (21) | 0.003 |
| Years since HF diagnosis | 6.5 ± 6.6 | 4.6 ± 4.1 | <0.0001 |
| NYHA Class | 0.9 | ||
| • 1–2 | 20 (4) | 6 (4) | |
| • 3 | 343 (65.0 | 95 (66) | |
| • 4 | 165 (31) | 43 (30) | |
| Baseline medical therapy | |||
| • ACEi/ARB | 293 (53) | 104 (65) | 0.009 |
| • Beta-blocker | 451 (82) | 141 (88) | 0.07 |
| • Aldosterone receptor antagonist | 148 (27) | 54 (34) | 0.09 |
| • Hydralazine | 66 (12) | 61 (38) | <0.0001 |
| • Nitrates | 134 (24) | 68 (43) | <0.0001 |
| • Digoxin | 136 (25) | 47 (29) | 0.2 |
| • Loop diuretic dose (mg furosemide equivalents) | 120 (80, 160) | 80 (80, 160) | 0.1 |
| • Sequential nephron blockade | 74 (13) | 12 (8) | 0.049 |
| Body mass index, kg/m2 | 33 ± 9 | 35 ± 11 | 0.017 |
| Systolic blood pressure, mm Hg | 117 ± 18 | 122 ± 20 | 0.005 |
| Diastolic blood pressure, mm Hg | 65 ± 12 | 74 ± 14 | <0.0001 |
| # CV hospitalizations in prior 12 months | 1.7 ± 1.6 | 2.2 ± 2.1 | 0.006 |
| # HF hospitalizations in prior 12 months | 1.3 ± 1.4 | 1.9 ± 1.8 | <0.0001 |
| Serum chemistries | |||
| • Sodium, mg/dL | 138.2 ± 3.8 | 137.5 ± 4.1 | 0.035 |
| • Total bilirubin, mg/dL | 1.1 ± 0.7 | 1.2 ± 0.8 | 0.3 |
| • Blood urea nitrogen, mg/dL | 39 (27, 56) | 29( 19, 44) | <0.0001 |
| • Creatinine, mg/dL | 1.7 ± 0.6 | 1.7 ± 0.6 | 0.7 |
| • eGFR, mL/min/1.72m2 | 46 ± 19 | 58 ± 26 | 0.7 |
| • NT-pro BNP (pg/mL) | 4,627 (2,273 – 9,833) | 4,349 (2,369 – 9,709) | 0.9 |
| Days from randomization to discharge | 6 (4, 9) | 6 (4, 8) | 0.5 |
Values are mean ± standard deviation, median (interquartile range), or N(%).
Table 2.
Signs and symptoms of volume overload by racial group.
| Baseline | 72–96 Hours | |||||
|---|---|---|---|---|---|---|
| Non-Black N=561 | Black N=160 | P-Value | Non-Black N=507 | Black N=143 | P-Value | |
| Jugular venous distension | 0.07 | 0.7 | ||||
| • <8 cm | 32 (6) | 8 (5) | 160 (34) | 38 (30) | ||
| • 8–12 cm | 129 (24) | 48 (33) | 184 (39) | 55 (43) | ||
| • 13–16 cm | 173 (32) | 50 (34) | 83 (18) | 24 (19) | ||
| • >16 cm | 207 (38) | 41 (28) | 45 (9) | 10 (8 | ||
| Orthopnea | <0.0001 | 0.018 | ||||
| • None | 60 (11) | 7 (5) | 137 (85) | 25 (19) | ||
| • 1 pillow | 92 (17) | 6 (5) | 129 (28) | 28 (21) | ||
| • 2 pillow | 210 (39) | 54 (36) | 135 (29) | 52 (40) | ||
| • 3+ pillows | 173 (32) | 85 (56) | 66 (14) | 26 (20) | ||
| Peripheral edema | 0.035 | 0.5 | ||||
| • None to trace | 121 (22) | 47 (30) | 316 (62) | 89 (62) | ||
| • Mild to moderate | 226 (40) | 67 (42) | 136 (27) | 34 (24) | ||
| • Severe | 213 (38) | 45 (28) | 55 (11) | 20 (14) | ||
| Rales present | 320 (57.6) | 79 (50.0) | 0.3 | * | * | * |
| S3 gallop present | 112 (20.4) | 44 (28.2) | 0.039 | * | * | * |
| Ascites present | 166 (31.5) | 40 (29.4) | 0.6 | * | * | * |
Data not recorded on the follow-up case report forms.
Metrics of diuretic responsiveness
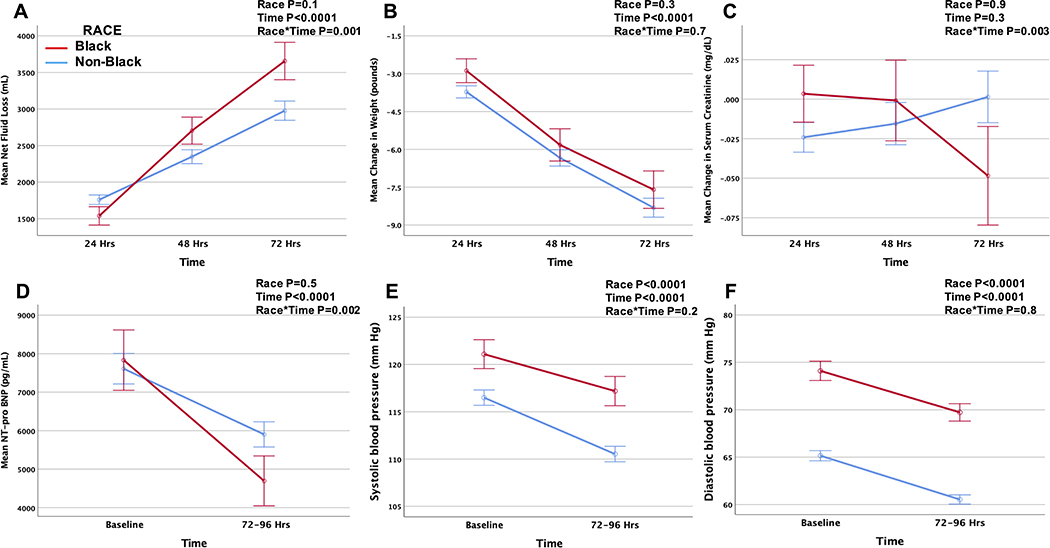
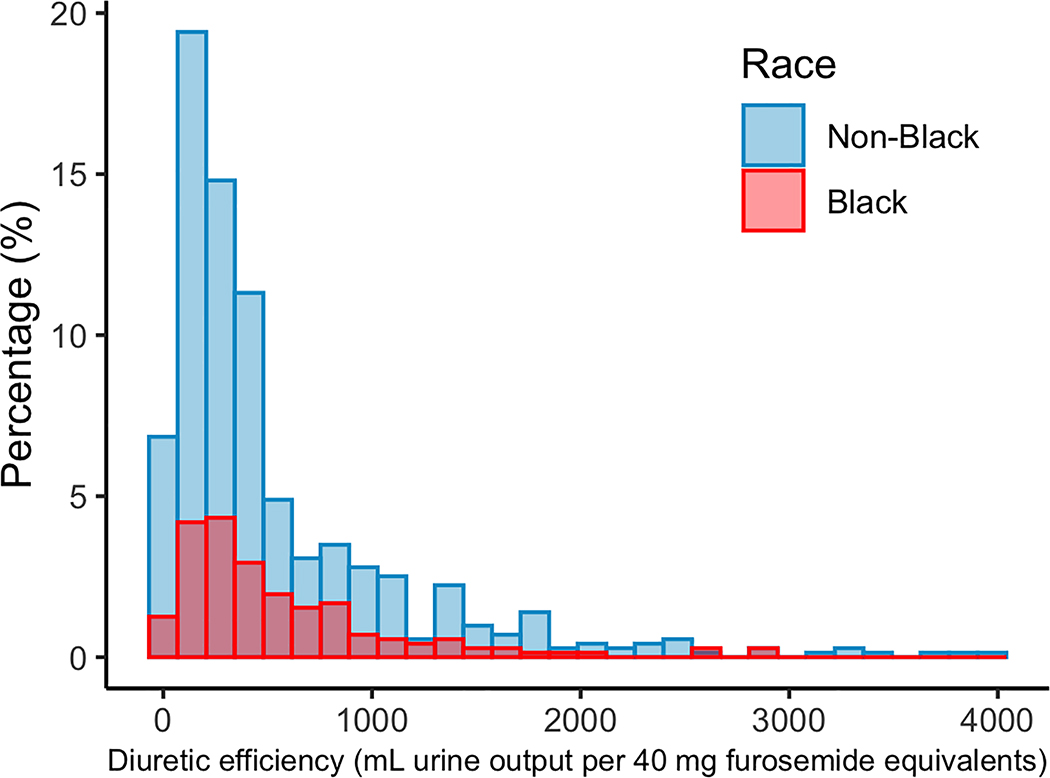
During the 72 to 96 hours after randomization, there was a greater net fluid loss, decrease in NT-pro BNP, and improvement in serum creatinine in Black compared to non-Black patients, while change in weight and blood pressure was comparable (Figure 1). The total furosemide equivalents administered during the hospitalization was lower in Black patients despite a comparable net fluid balance (Table 3), such that overall diuretic efficiency was higher in Black compared to non-Black patients (Figure 2). Similarly, Black patients were more likely than non-Black patients to be classified as having high diuretic efficiency (57% vs. 48%, P=0.046). In a model adjusting for study, age, sex, race, hypertension, blood pressure, outpatient diuretic therapy, and baseline eGFR and BUN, Black race remained predictive of higher diuretic efficiency (ß =0.201, P=0.037). In a sensitivity analysis excluding those subjects who were on sequential nephron blockade as outpatient therapy, black race remained associated with higher diuretic efficiency (ß =0.219, P=0.031).
Figure 1.
Net fluid loss (A), and change in weight (B), serum creatinine (C), NT-pro BNP (D), systolic blood pressure (E) and diastolic blood pressure (F) during the first 72 hours after randomization by racial group.
Table 3.
Measures of volume status, diuretic responsiveness, and RAAS biomarkers by racial group.
| Non-Black N=561 | Black N=160 | P-Value | |
|---|---|---|---|
| Total diuretic dose (mg)* | |||
| • Furosemide (N=690) | 460 (239, 840) | 360 (180, 695) | 0.018 |
| • Torsemide (N=199) | 80 (40, 200) | 80 (40, 180) | 0.9 |
| • Bumetanide (N=69) | 7 (2, 19) | 13 (4, 25) | 0.5 |
| Total furosemide equivalents‡ (mg) | 550 (280, 960) | 440 (220, 776) | 0.021 |
| Total urine output‡ (mL) | 9,100 (7,000, 12,300) | 9,100 (6,500, – 12,400) | 0.7 |
| Net fluid balance‡ (mL) | 4,700 (2,700, 6,900) | 4,900 (2,700, 7,800) | 0.4 |
| Change in weight‡ (pounds) | −9.5 (−15.6, −5.1) | −8.1 (−15.2, −2.9) | 0.1 |
| Diuretic Efficiency‡ | 324.6 (153.8, 698.3) | 403.0 (221.4, 795.2) | 0.014 |
| Baseline PRA (ng/mL/hr) | 5.9 (1.2, 20.7) | 1.7 (0.4, 6.2) | <0.0001 |
| Baseline aldosterone (pg/mL) | 228.4 (118.2, 390.3) | 172.2 (83.1, 309.3) | 0.005 |
Values are median (interquartile range), or N (%).
Values are for intravenous equivalents.
Values for measures of decongestion reflect totals during the course of the AHF hospitalization.
Figure 2.
Distribution of diuretic efficiency by racial group.
Clinical outcomes according to diuretic efficiency
During a median follow up of 68 (interquartile range [IQR] 56, 177) days, 251 (35%) subjects were rehospitalized. Each doubling of diuretic efficiency was associated with a 12% lower risk of all-cause (adjusted HR 0.88, 95% CI 0.82 – 0.95; P=0.001) and HF-specific (adjusted HR 0.88, 95% CI 0.79 – 0.98; P=0.015) rehospitalization.
Measures of RAAS activation in subjects enrolled in DOSE-AHF and CARRESS-HF
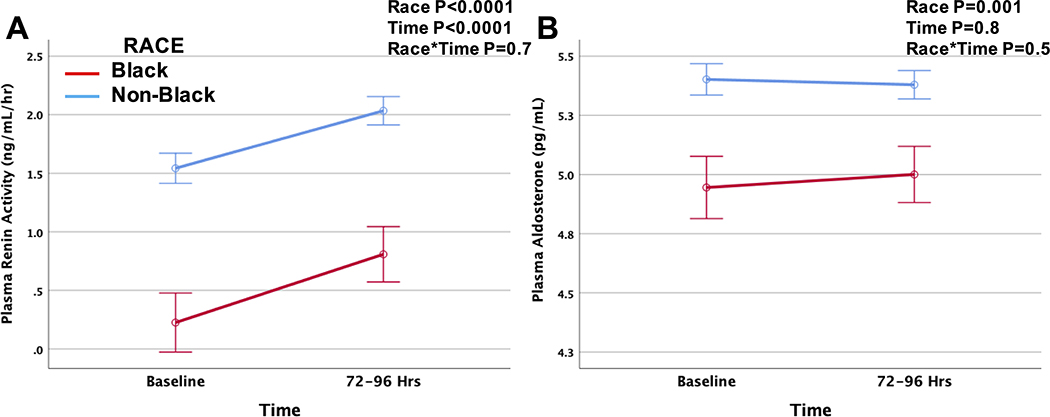
Baseline clinical characteristics were similar between the AHF trials (Supplemental Table 1). At baseline, PRA and aldosterone levels were lower in Black as compared to non-Black patients (Table 3). During the first 72 to 96 hours after randomization, PRA levels rose with decongestion in all subjects while aldosterone levels remained stable (Figure 3). However, levels of PRA and aldosterone remained lower in Black patients throughout the first 72 to 96 hours. Lower baseline PRA (ß=−0.108, P=0.003) and aldosterone (ß =−0.301, P<0.0001) levels were univariately associated with higher diuretic efficiency. There was no association between change in PRA and diuretic efficiency. In subjects with baseline PRA measurements, Black race remained predictive of higher diuretic efficiency (adjusted ß=0.353, P=0.025) even after adjustment for study, age, sex, race, hypertension, blood pressure, baseline eGFR, BUN, and outpatient medical therapy (diuretics, ACE/ARB and aldosterone antagonist use). The addition of baseline PRA to the multivariable model attenuated the association between Black race and diuretic efficiency (ß =0.337, P=0.037), and PRA was no longer associated with diuretic efficiency (ß=−0.016, P=0.6).
Figure 3.
Change in serum PRA (A) and aldosterone (B) levels during the first 72 to 96 hours after randomization by racial group. Biomarkers are log-transformed for analysis.
Clinical outcomes according to PRA and race
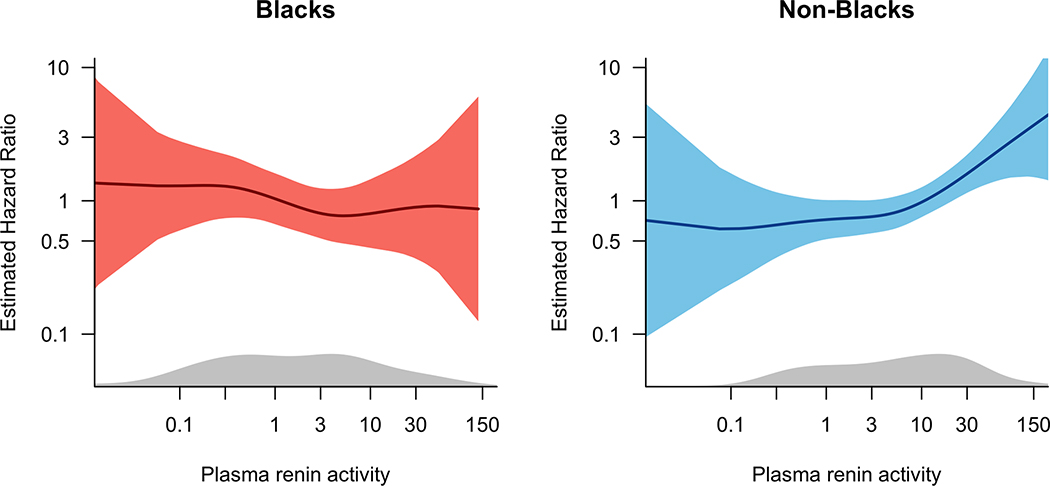
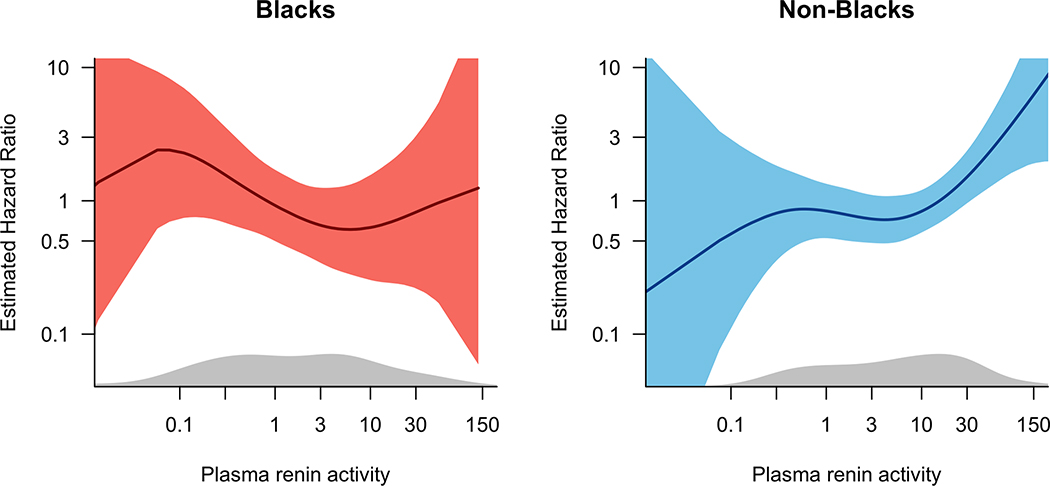
Baseline characteristics of subjects according to race and PRA categories are presented in Supplemental Table 2. An exploratory analysis was performed to examine the risk of rehospitalization by racial group in those subjects with measured PRA levels at baseline (N=356, p=0.037 for race*PRA statistical interaction) and at 72 to 96 hours (N=310). In race-stratified analyses, the risk of all-cause and HF-specific rehospitalization increased in non-Black subjects with increasing levels of PRA, while the risk of rehospitalization was relatively constant across levels of PRA in Black subjects (Figure 4). Similarly, with PRA dichotomized at the race-specific median, the risk of all-cause and HF-specific rehospitalization was higher for Black patients, with similar point estimates for the hazard ratio regardless of PRA levels (Table 4).
Figure 4.
Plot of hazard of all-cause and HF-specific rehospitalization according to racial group and baseline PRA.
Table 4.
Association of race and PRA categories with hazard of rehospitalization (Cox regression models).
| N | Events | HR (95% CI) | P Value | |
|---|---|---|---|---|
| All-cause hospitalization | ||||
| Baseline PRA (N=356) | ||||
| • Non-Black, low PRA | 136 | 41 | REFERENCE | |
| • Non-Black, high PRA | 136 | 49 | 1.43 (0.90 – 2.28) | 0.1 |
| • Black, low PRA | 40 | 19 | 3.14 (1.68 – 5.87) | <0.001 |
| • Black, high PRA | 40 | 20 | 2.08 (1.13 – 3.83) | 0.019 |
| 72- to 96-hr PRA (N=310) | ||||
| • Non-Black, low PRA | 87 | 18 | REFERENCE | |
| • Non-Black, high PRA | 88 | 37 | 2.55 (1.55 – 4.20) | <0.001 |
| • Black, low PRA | 24 | 10 | 4.51 (2.25 – 9.04) | <0.001 |
| • Black, high PRA | 25 | 13 | 3.96 (2.02 – 7.75) | <0.001 |
| HF hospitalization | ||||
| Baseline PRA (N=356) | ||||
| • Non-Black, low PRA | 136 | 24 | REFERENCE | |
| • Non-Black, high PRA | 136 | 25 | 1.14 (0.62 – 2.12) | 0.7 |
| • Black, low PRA | 40 | 9 | 2.30 (0.98– 5.41) | 0.057 |
| • Black, high PRA | 40 | 10 | 1.58 (0.68 – 3.64) | 0.3 |
| 72- to 96-hr PRA (N=310) | ||||
| • Non-Black, low PRA | 87 | 8 | REFERENCE | |
| • Non-Black, high PRA | 88 | 19 | 2.00 (1.04 – 3.83) | 0.037 |
| • Black, low PRA | 24 | 4 | 2.62 (0.95 – 7.23) | 0.06 |
| • Black, high PRA | 25 | 6 | 2.74 (1.09 – 6.94) | 0.033 |
Risk of rehospitalization estimated for subjects enrolled in DOSE-AHF and CARRESS-HF who had measured biomarkers of RAAS activation. PRA was analyzed at baseline for subjects enrolled in DOSE-AHF and CARRESS-HF, and at 72 hr for subjects in DOSE-AHF or 96 hr for subjects in CARRESS-HF. Subjects are classified as high vs. low PRA based on race-specific median values. Models are adjusted for study, age, sex, history of hypertension, blood pressure, HF etiology, ejection fraction, BMI, eGFR, and baseline ACE-I/ARB and aldosterone antagonist use.
DISCUSSION
Among subjects enrolled in multicenter trials examining decongestive strategies in AHF, we found that loop diuretic efficiency is associated with risk for rehospitalization, and we identified important differences in diuretic responsiveness according to racial group. Compared to non-Blacks, Black patients had a greater net fluid loss and decrease in NT-pro BNP for a given diuretic dose, indicative of higher diuretic efficiency with loop diuretics among Black compared to non-Black patients. Moreover, we found that PRA and aldosterone levels were lower in Black patients throughout the AHF episode, and that the lower levels of PRA observed in Black patients were associated with a higher risk of all-cause and HF-specific rehospitalization.
Multiple studies have documented higher rates of hospitalization and readmission among Black patients with HF as compared to other race-ethnic groups. Using the National Inpatient Sample, Ziaeian et al. demonstrated that the age-adjusted rate of HF hospitalization is more than twice as high for Black men and women as compared to Whites, with no significant change in the disparity between 2002 and 2013.2 By comparison, although Hispanic men and women had HF hospitalization rates that were 32% and 55% greater than Whites in 2002, the difference had narrowed to only 4% and 8% greater than Whites by 2013. Moreover, health care costs in the year after an index admission for HF are higher among Black patients than other race/ethnic groups.3
Activation of the RAAS plays a fundamental role in HF pathophysiology, with RAAS blockade forming the foundation of HF therapy.16–18 Cells of the renal juxtaglomerular apparatus up- or down-regulate their secretion of renin in response to detection of decreases or increases in total body sodium-volume content. It is well established that impaired cardiac output and the sensation of arterial underfilling caused by decreased activation of mechanoreceptors in the vascular tree leads to persistent RAAS activation, causing adverse cardiac remodeling and fluid retention with signs and symptoms of congestion.17, 18 Indeed, prior analyses have confirmed that PRA and plasma aldosterone levels are higher in patients with more advanced HF.8 Moreover, decongestion during AHF leads to increased PRA 8, 15 likely due to volume contraction with diuresis, which we have confirmed in the current analysis. Although the precise association of RAAS biomarkers with net fluid loss during AHF remains unclear 8, our study findings are consistent with prior data that shows that patients with worse diuretic efficiency are at higher risk for poor clinical outcomes 5, 7.
Our analysis adds to the literature by examining whether clinical outcomes differ between racial groups according to biomarkers of RAAS activity. We identified that levels of PRA and aldosterone are lower in Black patients with AHF. Both Black and non-Black patients with high PRA had increased risk of hospitalizations compared to non-Black patients with low PRA, consistent with prior observations that levels of RAAS biomarkers are elevated with severe HF. However, our findings are novel in that we also identified a high risk for rehospitalization in Black subjects with low PRA.
One factor that could contribute to the higher rates of HF hospitalization among Black patients is a higher degree of salt-sensitivity that increases the propensity towards volume retention. The term “salt-sensitivity” has been used to describe persons who display acute fluctuations in blood pressure with changes in sodium intake, and prior data suggest that ~50% of all hypertensives and as many as 75% of non-Hispanic Black hypertensives are “salt-sensitive”.19 It is well documented that there are important racial differences in patterns of RAAS activation.20, 21 Prior studies have shown that after similar states of sodium intake and volume loading were achieved in Blacks and Whites, Black subjects had less urinary sodium excretion and had a greater suppression of PRA.22 The greater sodium reabsorption in Black patients could be due to mechanisms unrelated to RAAS activity, including reduced potassium intake, decreased urinary kallikrein excretion, upregulation of epithelial sodium channel activity, impaired natriuretic peptide production, and APOL1 gene nephropathy risk variants.19,23–25 Alternatively, lower levels of RAAS biomarkers in Black patients could reflect an appropriate suppressive response to excess sodium intake and resultant volume overload.
Although it is not entirely clear why Black patients are more likely to retain sodium, elegant studies have demonstrated that low-renin, essential hypertensive patients have a better response to diuretics than normal-renin hypertensive patients, perhaps as a reflection of the greater degree of volume overload.10 In our analysis, we demonstrate similar findings, whereby Black patients had a greater decrease in NT-proBNP levels and fluid loss in the first 72 hours after randomization, and a better response to diuretics manifest as higher diuretic efficiency. Fluid loss occurred to a greater degree than weight loss, consistent with prior studies that have shown a poor correlation between net fluid loss and weight decrease.26 Greater fluid loss in Black patients may also reflect non-recorded fluid intakeas a surrogate for increased thirst and/or non-adherence, both of which are associated with a poor prognosis.27, 28 Moreover, serum creatinine decreased in Black patients but increased in non-Black patients, indicating a trend towards improved renal function. In prior analyses of the DOSE-AHF trial, improved renal function was associated with worse clinical outcomes.29 Brisco et al. noted that patients in DOSE-AHF with improved renal function had more severe signs and symptoms of congestion on admission, but were less likely to be “congestion free” at 72 hours, potentially indicative of suboptimal decongestion.
There are multiple clinical implications to our findings, as identifying novel ways to reduce HF hospitalizations is a key priority for the medical community. Since the introduction of the Hospital Readmissions Reduction Program, hospital systems have incurred substantial penalties for higher than expected rates of readmissions for HF.30 Moreover, hospitals that serve higher proportions of race/ethnic minority patients often incur higher penalties.31, 32 Black patients are more likely to reside in neighborhoods with limited access to low sodium, healthy foods 33, 34, which has been shown to increase the risk for all-cause and HF hospitalizations.34 Lower levels of RAAS biomarkers may reflect volume overload associated with excess dietary sodium intake due to food insecurity. Moreover, prior studies have shown that Black patients with HF are less likely to be treated by a cardiologist, which may also influence clinical outcomes.35 Black patients enrolled in these AHF trials had less aggressive outpatient diuretic regimens, and were noted by the investigators to have a different pattern of signs and symptoms of AHF including a higher likelihood of having severe orthopnea and S3 gallop, but lower likelihood of having severe lower extremity edema. These subtle differences in the clinical signs and symptoms of AHF by racial group, which have also been noted in prior studies36, 37, may make the recognition of AHF or persistent congestion more difficult in Black patients, leading to a higher risk for readmission after discharge. Additional studies are needed using novel techniques in diverse cohorts that examine both the interstitial and intravascular compartments as they pertain to sodium and fluid homeostasis in AHF.38
There are limitations of this analysis that are worth noting. Although values of all diuretics were converted to equivalent milligrams of furosemide, known differences in the bioavailability of loop diuretics may affect diuretic response. Moreover, our analysis was unable to account for the use of non-loop diuretics (i.e. thiazide or thiazide-like diuretics), or to account for evolution of other clinical variables during decongestion, including hematocrit. Although biomarkers of RAAS activation were only available for subjects enrolled in DOSE-AHF and CARRESS-HF, the Supplemental Table suggests that clinical characteristics were relatively similar between subjects enrolled in all three trials. Moreover, race/ethnicity was equally distributed among the different treatment groups in each trial 11–13, so we do not expect race-related effects of these treatments leading to a lower/higher diuretic efficiency. There is also no data on subjects’ dietary habits, to determine if low renin and/or aldosterone levels may reflect excess dietary sodium intake. Still, prior experimental analyses have shown that Black patients display persistently lower PRA values than non-Black patients in response to both high and low sodium diets 39, 40, as well as after furosemide infusion 10. Finally, although we adjusted for use of HF GDMT in our multivariable models, PRA is ideally measured and interpreted without the confounding effects of MRA, beta-blockers, ACEi, ARBs, and diuretics.
In conclusion, this analysis confirms that Black patients enrolled in clinical trials examining decongestive strategies in AHF have better diuretic efficiency than non-Black patients. Indeed, the in Black patients in our exploratory analysis demonstrated lower levels of PRA and aldosterone, highly suggestive of a relative expansion of extracellular fluid volume and/or sodium balance in Black relative to non-Black patients. Moreover, the risk of rehospitalization was higher for Black patients regardless of PRA levels. More research is needed to determine if racial differences in sodium and volume expansion in chronic and acute HF, in part due to differential responsiveness of the RAAS or other biologic pathways, contributes to racial disparities in the overall risk for HFH.
Supplementary Material
WHAT IS NEW?
Black patients enrolled in acute heart failure (AHF) trials have better diuretic efficiency (greater net fluid loss for a given loop diuretic dose) than non-Black patients.
Black patients were on less aggressive outpatient diuretic therapy prior to hospitalization for AHF, and were more likely to have high-grade orthopnea as the primary symptom of congestion.
In the setting of AHF, Black patients display lower levels of the neurohormones renin and aldosterone. Moreover, the association of Black race with diuretic efficiency is related to plasma renin levels, suggesting that racial differences in activity of the renin-angiotensin-aldosterone system (RAAS) may influence diuretic efficiency.
WHAT ARE THE CLINICAL IMPLICATIONS?
Black patients have higher rates of hospitalization for AHF than other race/ethnic groups. Moreover, the disparity in rates of hospitalization between Black and non-Black patients has not decreased in the past decade, such that more research is needed to determine what factors are driving the high rates of morbidity in this population.
Racial differences in volume expansion secondary to non-RAAS mechanisms may influence the higher risk for hospitalization in Black subjects. Additional studies are needed to examine racial differences in sodium and fluid homeostasis in subjects with acute and chronic HF.
ACKNOWLEDGMENTS
Data were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) and any analysis of these data does not necessarily reflect the opinions or views of NHLBI.
SOURCES OF FUNDING
Dr. Morris has received research grants from NHLBI (NIH K23 HL124287 and R03 HL146874) and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program). Dr. Felker has received research grants from NHLBI (NIH U-10 HL110312) and American Heart Association. Dr. Testani has received research grants from NHLBI (NIH R01 HL128973, R01 139629, and R01 HL148354). Dr. Tang has received research grants from NIDDK (R01 DK106000) and NHLBI (HL 126827).
DISCLOSURES
Drs. Morris, Nayak, Ko, D’Souza, Redfield and Tang have nothing to disclose. Dr. Felker has received research grants from Amgen, Merck, Cytokinetics, and Roche Diagnostics; he has acted as a consultant on unrelated projects for Novartis, Amgen, BMS, Medtronic, Cardionomic, Relypsa, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, Rocket Pharma, and SC Pharma. Dr. Testani has received grants and personal fees on unrelated projects from Sequana Medical, BMS, 3ive labs, Boehringer Ingelheim, Sanofi, FIRE1, as well as personal fees from Astra Zeneca, Novartis, Cardionomic, Bayer, MagentaMed, Renalguard, W.L. Gore, Windtree therapeutics, and grants from Otsuka and Abbott. Dr. Butler has served as a paid consultant or advisor on unrelated projects for Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, G3, Innolife, Janssen, Medtronic, Merck, Novartis, Relypsa, Stealth Peptide, SC Pharma, Vifor, and ZS Pharma.
ABBREVIATIONS
- ACEi
angiotensin converting enzyme inhibitor
- AHF
acute heart failure
- ARB
angiotensin receptor blocker
- HF
heart failure
- LVEF
left ventricular ejection fraction
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
REFERENCES
- 1.McDermott KW, Elixhauser A and Sun R. Trends in Hospital Inpatient Stays in the United States, 2005–2014 HCUP Statistical Brief #225. June 2017. Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.pdf. [Google Scholar]
- 2.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH and Fonarow GC. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circulation: Cardiovascular quality and outcomes. 2017;10:e003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziaeian B, Heidenreich PA, Xu H, DeVore AD, Matsouaka RA, Hernandez AF, Bhatt DL, Yancy CW and Fonarow GC. Medicare Expenditures by Race/Ethnicity After Hospitalization for Heart Failure With Preserved Ejection Fraction. JACC: Heart Failure. 2018;6:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP and Kimmel SE. Clinical Characteristics and Outcomes of Patients With Improvement in Renal Function During the Treatment of Decompensated Heart Failure. Journal of Cardiac Failure. 2011;17:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiernan MS, Stevens SR, Tang WHW, Butler J, Anstrom KJ, Birati EY, Grodin JL, Gupta D, Margulies KB, LaRue S, Dávila-Román VG, Hernandez AF and de las Fuentes L. Determinants of Diuretic Responsiveness and Associated Outcomes During Acute Heart Failure Hospitalization: An Analysis From the NHLBI Heart Failure Network Clinical Trials. Journal of Cardiac Failure. 2018;24:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valente MAE, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC and Hillege HL. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. European Heart Journal. 2014;35:1284–1293. [DOI] [PubMed] [Google Scholar]
- 7.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR and Tang WHW. Loop Diuretic Efficiency: A Metric of Diuretic Responsiveness With Prognostic Importance in Acute Decompensated Heart Failure. Circulation: Heart Failure. 2014;7:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, Khazanie P, Redfield MM, Stevenson LW, O’Connor CM, Goldsmith SR, Bart BA, Anstrom KJ, Hernandez AF, Braunwald E and Felker GM. Decongestion Strategies and Renin-Angiotensin-Aldosterone System Activation in Acute Heart Failure. JACC: Heart Failure. 2015;3:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratt JH, Rebhun JF, Zhou L, Ambrosius WT, Newman SA, Gomez-Sanchez CE and Mayes DF. Levels of mineralocorticoids in whites and blacks. Hypertension. 1999;34:315–319. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan NM, Kem DC, Holland OB, Kramer NJ, Higgins J and Gomez-Sanchez C. The Intravenous Furosemide Test: A Simple Way to Evaluate Renin Responsiveness. Annals of Internal Medicine. 1976;84:639–645. [DOI] [PubMed] [Google Scholar]
- 11.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WHW, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Dávila-Román VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Renal Dysfunction: The ROSE Acute Heart Failure Randomized Trial. JAMA. 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E and O’Connor CM. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. New England Journal of Medicine. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM and Braunwald E. Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome. New England Journal of Medicine. 2012;367:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley LF, Carter DM, Matta L, Cheng JW, Stevens C, Belenkiy RM, Burpee LJ, Young MA, Weiffenbach CS, Smallwood JA, Stevenson LW and Desai AS. Intravenous Diuretic Therapy for the Management of Heart Failure and Volume Overload in a Multidisciplinary Outpatient Unit. JACC: Heart Failure. 2016;4:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Nijst P, Verbrugge FH, Martens P, Bertrand PB, Dupont M, Francis GS, Tang WW and Mullens W. Plasma renin activity in patients with heart failure and reduced ejection fraction on optimal medical therapy. J Renin Angiotensin Aldosterone Syst. 2017;18:1470320317729919–1470320317729919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givertz MM. Manipulation of the Renin-Angiotensin System. Circulation. 2001;104:e14–e18. [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E Heart Failure. JACC: Heart Failure. 2013;1:1–20. [DOI] [PubMed] [Google Scholar]
- 18.Schrier RW and Abraham WT. Hormones and Hemodynamics in Heart Failure. New England Journal of Medicine. 1999;341:577–585. [DOI] [PubMed] [Google Scholar]
- 19.Richardson SI, Freedman BI, Ellison DH and Rodriguez CJ. Salt sensitivity: a review with a focus on non-Hispanic blacks and Hispanics. Journal of the American Society of Hypertension : JASH. 2013;7:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt J, Rebhun J, Zhou L, Ambrosius W, Newman S, Gomez-Sanchez C and Mayes D. Levels of mineralocorticoids in whites and blacks. Hypertension. 1999;34:315–319. [DOI] [PubMed] [Google Scholar]
- 21.Pratt JH, Jones JJ, Miller JZ, Wagner MA and Fineberg NS. Racial Differences in Aldosterone Excretion and Plasma Aldosterone Concentrations in Children. New England Journal of Medicine. 1989;321:1152–1157. [DOI] [PubMed] [Google Scholar]
- 22.Luft FC, Grim CE, Fineberg N and Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation. 1979;59:643–650. [DOI] [PubMed] [Google Scholar]
- 23.Nesbitt S and Victor RG. Pathogenesis of Hypertension in African Americans. Congestive Heart Failure. 2004;10:24–29. [DOI] [PubMed] [Google Scholar]
- 24.Pratt JH, Ambrosius Walter T, Agarwal R, Eckert George J and Newman S. Racial Difference in the Activity of the Amiloride-Sensitive Epithelial Sodium Channel. Hypertension. 2002;40:903–908. [DOI] [PubMed] [Google Scholar]
- 25.Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND and MacGregor GA. Association of hypertension with T594M mutation in beta subunit of epithelial sodium channels in black people resident in London. The Lancet. 1998;351:1388–1392. [DOI] [PubMed] [Google Scholar]
- 26.Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, Coca SG and Tang WHW. Substantial Discrepancy Between Fluid and Weight Loss During Acute Decompensated Heart Failure Treatment. Am J Med. 2015;128:776–83.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldréus N, van der Wal MHL, Hahn RG, van Veldhuisen DJ and Jaarsma T. Thirst Trajectory and Factors Associated With Persistent Thirst in Patients With Heart Failure. Journal of Cardiac Failure. 2014;20:689–695. [DOI] [PubMed] [Google Scholar]
- 28.Waldréus N, Hahn RG and Jaarsma T. Thirst in heart failure: a systematic literature review. European Journal of Heart Failure. 2013;15:141–149. [DOI] [PubMed] [Google Scholar]
- 29.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WHW and Testani JM. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. Journal of Cardiac Failure. 2016;22:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy CP, Vaduganathan M, Patel KV, Lalani HS, Ayers C, Bhatt DL, Januzzi JL, Jr., de Lemos JA, Yancy C, Fonarow GC and Pandey A. Association of the New Peer Group–Stratified Method With the Reclassification of Penalty Status in the Hospital Readmission Reduction Program. JAMA Network Open. 2019;2:e192987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joynt KE, Orav EJ and Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaskin D, Hossein Z, Vazin R, Love D and Steinwachs D. Racial and Ethnic Composition of Hospitals’ Service Areas and the Likelihood of Being Penalized for Excess Readmissions by the Medicare Program. Medical Care. 2018;56:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon C, Purciel-Hill M, Ghai NR, Kaufman L, Graham R and Van Wye G. Measuring food deserts in New York City’s low-income neighborhoods. Health & Place. 2011;17:696–700. [DOI] [PubMed] [Google Scholar]
- 34.Morris AA, McAllister P, Grant A, Geng S, Kelli HM, Kalogeropoulos A, Quyyumi A and Butler J. Relation of Living in a “Food Desert” to Recurrent Hospitalizations in Patients With Heart Failure. American Journal of Cardiology. 2019;123:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breathett K, Liu WG, Allen LA, Daugherty SL, Blair IV, Jones J, Grunwald GK, Moss M, Kiser TH, Burnham E, Vandivier RW, Clark BJ, Lewis EF, Mazimba S, Battaglia C, Ho PM and Peterson PN. African Americans Are Less Likely to Receive Care by a Cardiologist During an Intensive Care Unit Admission for Heart Failure. JACC: Heart Failure. 2018;6:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels LB, Bhalla V, Clopton P, Hollander JE, Guss D, McCullough PA, Nowak R, Green G, Saltzberg M, Ellison SR, Bhalla MA, Jesse R and Maisel A. B-Type Natriuretic Peptide (BNP) Levels and Ethnic Disparities in Perceived Severity of Heart Failure: Results From the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT) Multicenter Study of BNP Levels and Emergency Department Decision Making in Patients Presenting With Shortness of Breath. Journal of Cardiac Failure. 2006;12:281–285. [DOI] [PubMed] [Google Scholar]
- 37.Kamath SA, Drazner MH, Wynne J, Fonarow GC and Yancy CW. Characteristics and Outcomes in African American Patients With Decompensated Heart Failure. Archives of Internal Medicine. 2008;168:1152–1158. [DOI] [PubMed] [Google Scholar]
- 38.Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WHW and Mullens W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. Journal of the American College of Cardiology. 2015;65:378–388. [DOI] [PubMed] [Google Scholar]
- 39.He FJ, Markandu ND, Sagnella GA and MacGregor GA. Importance of the Renin System in Determining Blood Pressure Fall With Salt Restriction in Black and White Hypertensives. Hypertension. 1998;32:820–824. [DOI] [PubMed] [Google Scholar]
- 40.Luft F, Grim C, Higgins JJ and Weinberger M. Differences in response to sodium administration in normotensive white and black subjects. The Journal of Laboratory and Clinical Medicine. 1977;90:555–562. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.