Abstract
Hydroxychloroquine (HCQ) has been implicated in antiviral activity in vitro against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, there is still controversy about whether HCQ should be used for coronavirus disease 2019 (COVID-19) patients due to the conflicting results in different clinical trials. To systematically assess the benefits and harms of HCQ for the treatment of COVID-19. Data sources were systematically searched from Pubmed, Biorxiv, ChiCTR, Clinicalrials.gov, and the Cochrane library of RCTs for studies published from inception to June 1, 2020, to obtain any possible inclusion. This meta-analysis of inclusion criteria was directed on the basis of the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P). Pooled studies by the title and abstract were screened and removed in the light of meta-analysis by two reviewers. Seven studies involving 851 participants with COVID-19 were eligible for analysis. There was no significant difference in RT-PCR negative conversion between HCQ group and standard treatment (ST) group (RR = 1.11, 95% CI = 0.77–1.59, P = 0.591). The rate of exacerbated pneumonia on chest CT in HCQ group was lower than that in ST group (RR = 0.44, 95% CI = 0.20–0.94, P = 0.035). There was no statistical difference in progressed illness between the HCQ group and the ST group (RR = 0.66, 95% CI = 0.18–2.43, P = 0.530). Death (RR = 1.92, 95% CI = 1.26–2.93, P = 0.003) was distinctly different in HCQ group compared with ST group in the treatment of COVID-19. Our meta-analysis demonstrated that there was no robust evidence to support prescribing HCQ as a treatment for COVID-19.
Keywords: Hydroxychloroquine, COVID-19, Meta-analysis
Introduction
Since December 2019, an outbreak of pneumonia caused by SARS-CoV-2 in Wuhan has spread rapidly throughout China and even around the world. On February 11, 2020, the disease caused by SARS-CoV-2 was officially named as coronavirus disease (COVID-19) by the WHO. In the past few months, there have been more than 7 million confirmed cases and 406 thousand deaths all over the world, posing unprecedented challenges to healthcare systems and amounting to a huge economic burden worldwide. However, there are no vaccines or specific antivirals available for SARS-CoV-2 infection.
Hydroxychloroquine (HCQ), employed initially for the treatment of malaria, has rapidly gained worldwide attention for its in vitro antiviral effect on SARS-CoV-2 (Liu et al. 2020), and its efficacy may be attributed to different mechanisms. HCQ was reported to reduce glycosylation of angiotensin-converting enzyme (ACE) II receptors and thus interfering with the binding of SARS-CoV-2 to the ACE2 receptor (Liu et al. 2020). Moreover, HCQ is weakly alkaline and increases pH of endosomes and lysosomes, leading to defects in protein degradation, endocytosis, and exocytosis, which are necessary for viral infection, replication, and propagation (Al-Bari 2015). The immunomodulatory and anti-inflammatory effects of HCQ can enhance its antiviral activity. HCQ can inhibit antigen processing and presentation to T cells, which reduces T cell activation and differentiation and expression of inflammatory cytokine (Zhou et al. 2020). Furthermore, new research has proposed that HCQ may prevent SARS-CoV-2 infection by inhibiting its binding with ganglioside (Fantini et al. 2020). Based on evidence from in vitro experiments on the suppression of activity of SARS-CoV-2, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for emergency use of oral formulations of chloroquine phosphate (CQ) and HCQ for the treatment of COVID-19 on March 29, 2020.
Recently, there was still conflict on the efficacy and safety of HCQ for the treatment of COVID-19. Chen et al. evaluated the efficacy of HCQ in the treatment of 62 patients diagnosed with COVID-19. All participants were randomly divided into two groups, and HCQ group was assigned to receive an additional 5-day HCQ (400 mg/day) treatment. Compared with control group, the body temperature recovery time and the cough remission time of the HCQ group were significantly shortened, and more patients had improved pneumonia (80.6% vs. 54.8%) (Chen et al. 2020). Moreover, an uncontrolled non-comparative observational study demonstrated that COVID-19 patients treated with a combination of HCQ and azithromycin (AZ) had a significantly rapid fall of nasopharyngeal viral load, and more patients rapidly discharged from ICU with a mean stay for 5 days (Gautret et al. 2020b). However, emerging data and published literature have raised new questions on whether HCQ’s benefits outweigh the risks. Geleris et al. (Geleris et al. 2020) conducted an observational trial which found that HCQ made no difference to intubation or death of COVID-19 patients. Subsequently, the biggest multinational registry analysis of 96,032 patients with COVID-19 described that the use of HCQ is independently associated with decreased in-hospital survival and increased risk of ventricular arrhythmia (Mehra et al. 2020b). Unfortunately, this registry analysis based on the Surgisphere data has been retracted, because the integrity and reliability of the Surgisphere database cannot be guaranteed (Mehra et al. 2020c). Therefore, we performed the present meta-analysis to evaluate the efficacy and safety of HCQ in the treatment of COVID-19 patients.
Methods
Search strategy and study selection
Two reviewers performed a systematic search in Pubmed, Biorxiv, ChiCTR, Clinicalrials.gov, and the Cochrane library of RCTs. The following keywords were searched in combination, i.e., “COVID-19” or “novel coronavirus pneumonia,” “hydroxychloroquine” or “HCQ.” The articles published date from inception to June 1, 2020, to obtain any possible inclusion. Studies in any language were selected. A total of 506 studies were identified.
Study inclusion and exclusion criteria
This meta-analysis of inclusion criteria was directed on the basis of the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P). Pooled studies by the title and abstract were screened and removed in the light of meta-analysis by two reviewers. Afterwards, the rest of the articles were removed by browsing their full text according to the following inclusion criteria and exclusion criteria: (a) the subjects were diagnosed as COVID-19 by RT-PCR testing; (b) the study type included random controlled trial or observational study; (c) the subjects were treated at least by standard treatment (ST group) and hydroxychloroquine plus standard treatment (HCQ group); (d) endpoints included RT-PCR negative conversion, exacerbated pneumonia on chest CT, progressed illness, and death. The outcome of “exacerbated pneumonia on chest CT” referred to a change of lung lesion enlargement on chest radiology. The outcome of “progressed illness” was meant to a disease progression to a worse stage, like mild to moderate or moderate to severe. Exclusion criteria were as follows: (a) systematic review and meta-analysis; (b) letter to the editor or animal experiment; (c) incomplete data.
Data extraction and methodological quality assessment
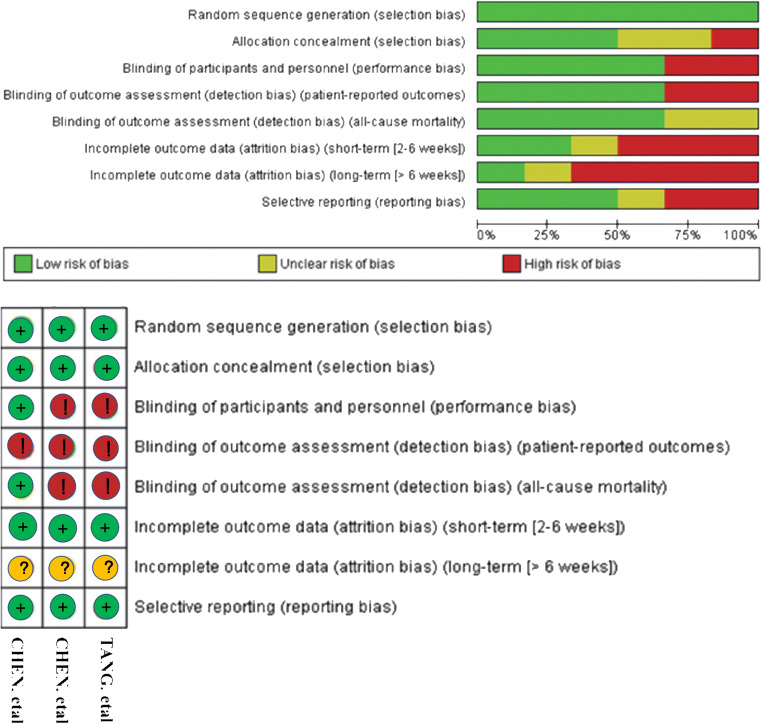
We initially viewed the title and abstract of the articles, and then read the full text if the article was relevant. Articles were included in this analysis if they met the above criteria. The standard EXCEL forms were used to extract relevant data including study, year, country, language, sample size, treatment, and outcomes of HCQ group and ST group respectively by two reviewers. The quality of included studies was independently appraised by two reviewers, with disagreements resolved by consensus. RCTs were evaluated by the Cochrane Evaluation Manual’s bias risk assessment tool, and the bias risk assessment of observational studies was conducted using the NOS scales.
Statistical analysis
The analyses were carried out according to recommendations from the Cochrane Collaboration using Stata SE. The main statistical process included heterogeneity test, meta-analysis, funnel plot analysis, and Egger test. Heterogeneity among studies was evaluated according to I2. If I2 < 50%, a fixed effects model with Mantel-Haenszel (M-H) method was utilized to calculate RR and 95% CI. If I2 > 50%, a random effects model with Der Simonian-Laired (D + L) method was used. Potential publication biases were explored using visual inspection of Egger’s weighted regression test if the quantity of articles available was more than ten.
Results
Search results and study characteristics
The total of 506 papers were identified by online search. According to the title, abstract, and full text, 7 studies, 3 RCTs, 2 POSs, and 2 retrospective studies evaluating differences in between HCQ group versus ST group were included (Fig. 1). Eight hundred fifty-one patients were included in this meta-analysis (441 in the HCQ group vs. 410 in the ST group). Five of the 7 studies were published in English, one in Chinese and one in French. The sample size and the outcomes of studies are presented in Table 1.
Fig. 1.
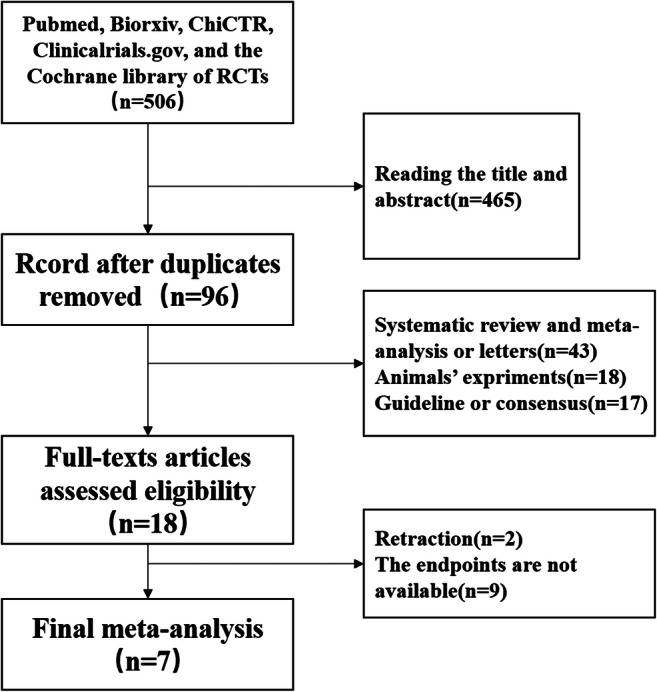
The flow of study selection process
Table 1.
The study characteristics
| Study | Year | Language | Country | Type | Blind | N | Age | Gender (male/female) | Patient characteristics | N of HCQ | N of ST | HCQ treatment | Outcomes | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. | 2020 | English | China | RCT | Double-blinded | 62 | 44.7 | 33/29 | Moderate | 31 | 31 | 400 mg/day × 5 days | Exacerbated pneumonia on chest CT, progressed illness | - | |
| Chen et al. | 2020 | Chinese | China | RCT | Open label | 30 | 48.6 | 21/9 | Moderate | 15 | 15 | 400 mg/day × 5 days | RT-PCR negatively, exacerbated pneumonia on chest CT, and progressed illness | - | |
| Tang et al. | 2020 | English | China | RCT | Open label | 150 | 46.1 | 82/68 | Moderate | 70 | 80 | Load dosage 1200 mg , 800 mg × 2–3 weeks | RT-PCR negatively, progressed illness | - | |
| Magagnoli et al. | 2020 | English | America | Retro | – | 368 | 68 | – | Mild/moderate | 210 | 158 | HCQ/HCQ + AZ | Death | 7 | |
| Gautret et al. | 2020 | English | France | POS | – | 30 | 52.5 | – | Mild | 14 | 16 | 600 mg/day × 10 days | RT-PCR negatively | 7 | |
| Barbosa et al. | 2020 | French | America | Retro | – | 38 | 62.7 | – | Moderate | 17 | 21 | Load dosage 800 mg, 200–400 mg/day × 3–4 days | Death | 6 | |
| Mahevas et al. | 2020 | English | France | POS | – | 173 | 60 | – | Pneumonia requiringO2 | 84 | 89 | 600 mg/day × 2 days | Death | 7 |
The outcomes of RT-PCR negative conversion and exacerbated pneumonia on chest CT
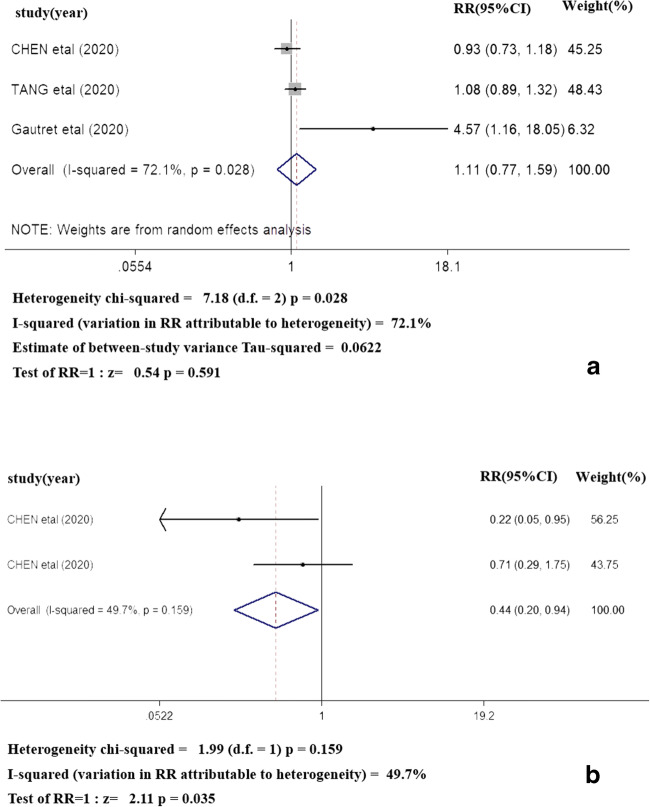
Three of 7 studies reported the outcome of RT-PCR negative conversion between HCQ group and ST group, and heterogeneity is extremely high (I2 = 72.1%, P = 0.028), which use random effect model. In brief, RT-PCR negative conversion was not significantly different in HCQ group compared with ST group in treatment of COVID-19 (RR = 1.11, 95% CI = 0.77–1.59, P = 0.591). Two papers reported the outcome of exacerbated pneumonia on chest CT and heterogeneity is extremely high (I2 = 49.7%, P = 0.159), which use fixed effect model. The rate of exacerbated pneumonia on chest CT in HCQ group was lower than that in ST group (RR = 0.44, 95% CI = 0.20–0.94, P = 0.035). The detailed outcomes of RT-PCR negative conversion and exacerbated pneumonia on chest CT are lined in Fig. 2.
Fig. 2.
The Cochrane Evaluation Manual’s bias risk assessment of 3 RCTs
The outcomes of progressed illness and death
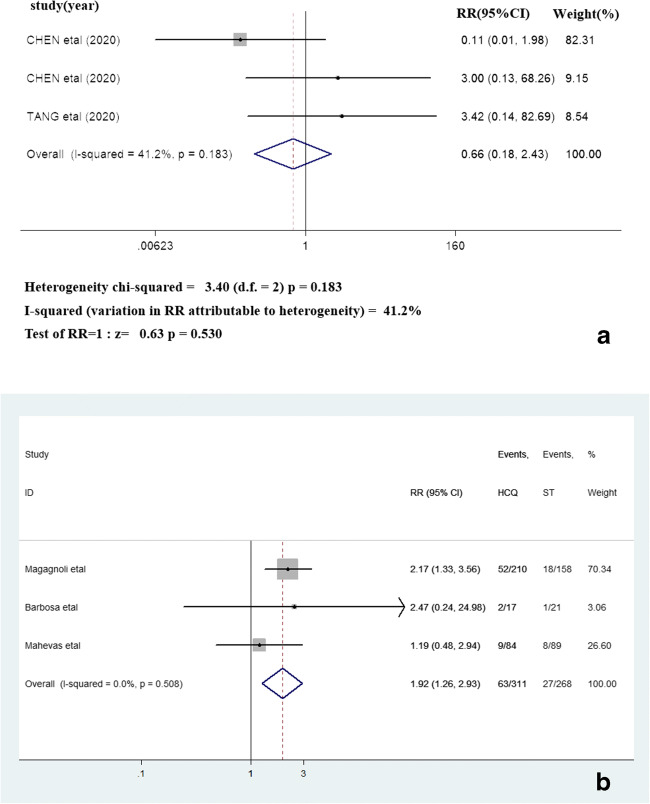
Three papers reported progressed illness, and heterogeneity is extremely high (I2 = 41.2%, P = 0.183), which use fixed effect model. Three of 9 papers reported the outcome of death, and heterogeneity is extremely high (I2 = 0.0%, P < 0.001), which use fixed effect model. And there was no statistical difference in progressed illness between the HCQ group and ST group (RR = 0.66, 95% CI = 0.18–2.43, P = 0.530). Death (RR = 1.92, 95% CI = 1.26–2.93, P = 0.003) was distinctly significant different in HCQ group compared with ST group in treatment of COVID-19. The detailed outcomes of progressed illness and death are lined in Fig. 3.
Fig. 3.
The outcomes of RT-PCR negative conversion (2A) and exacerbated pneumonia on chest CT (2B)
Discussion
HCQ, as an aminoquinoline, has been extensively used for the prevention and therapy of malaria and rheumatic diseases (Martinez et al. 2020). In the context of the COVID-19 pandemic and absence of effective drugs, HCQ has been approved for the treatment of hospitalized patients with COVID-19 on the grounds of anti-inflammatory and antiviral effects. HCQ has received worldwide attention as a potential treatment for COVID-19 due to positive results from some small clinical trials, which showed the association between HCQ and outcomes such as symptom resolution and viral clearance (Gautret et al. 2020a; Gautret et al. 2020b). However, Rosenberg et al. (Rosenberg et al. 2020) found that the in-hospital mortality was not significantly associated with HCQ, and cardiac arrest was significantly more likely in patients receiving HCQ combined with AZ, compared with patients receiving neither drug in logistic model. In the light of these conflicting results, it is necessary to evaluate the benefits and harms of HCQ use in the treatment of COVID-19 patients (Fig. 4).
Fig. 4.
The outcomes of progressed illness (3A) and death (3B)
In our current study, we found the exacerbated pneumonia on chest CT in HCQ group was improved, but the illness progression and PCR negative conversion were not changed. Although some small studies demonstrated that HCQ was associated with the shortened recovery time, many factors contributed to conflicting results, such as the sample sizes, the absence of randomized control and placebo control group, the single-center design, low methodological quality, the dissimilar baseline characteristics, and unmeasured confounding and bias. Hernandez et al. (Hernandez et al. 2020) indicated that evidence was conflicting and insufficient with regard to the effect of HCQ on all-cause mortality, disease progression, symptom resolution, and viral load. In addition, we found that the mortality of HCQ group was higher than that of ST group. Recent meta-analysis assessing the efficacy of HCQ for COVID-19 therapy found no significant difference or inconclusive effects in mortality between HCQ group and supportive care arm. However, HCQ + AZ group had significantly higher mortality than supportive care group (Patel et al. 2020). In our study, all patients taking HCQ were included, thus the effect of HCQ combined with AZ on mortality cannot be ruled out. Moreover, the different dosages, duration of follow-up, and baseline differences are likely explanations for the considerable variability seen in these outcomes.
FDA has recently revoked EUA for emergency use of CQ and HCQ to treat COVID-19 patients based on the ongoing analysis and emerging scientific data as following. The RECOVERY Trial enrolling over 11,000 patients in UK showed no evidence of benefit on outcomes of hospitalized COVID-19 patients treated with HCQ, such as 28-day mortality, hospital stay duration, and other outcomes (Torjesen 2020). A randomized trial assessing the efficacy of HCQ as postexposure prophylaxis (PEP) for SARS-CoV-2 infection among 821 asymptomatic participants revealed that HCQ failed to prevent COVID-19 when used as PEP within 4 days after high-risk or moderate-risk exposure, and appeared to be associated with more side effects (Boulware et al. 2020). This finding has been consistently replicated in the PEP CoV2 Study, which showed that HCQ could not prevent SARS-CoV-2 infection (ClinicalTrials.gov Identifier: NCT04304053, https://clinicaltrials.gov/ct2/show/NCT04304053). In general, these data convincingly rule out any meaningful clinical benefits of HCQ in both prevention and therapy for COVID-19 patients. Furthermore, there are ongoing reports of serious cardiac adverse events, such as QT prolongation, tip torsional ventricular tachycardia and cardiac arrest.
Wide use of HCQ will expose some patients to potentially fatal adverse reactions, including ventricular arrhythmias and cardiac arrest, especially when prescribed with AZ (Gerard et al. 2020). The combination of HCQ and AZ has been designed for the treatment of COVID-19 due to their synergistic antimicrobial properties (Nakornchai and Konthiang 2006). It is reported that patients receiving HCQ combined with AZ had an increased risk of cardiac arrest, and the most commonly reported adverse event was arrhythmia (Rosenberg et al. 2020). It has long been known that HCQ has a preponderance for causing cardiac rhythm abnormalities, because it can increase the electrical instability, which is characterized as QT interval prolongation. It has been reported that 1 to 18% of patients receiving HCQ had a severe increased corrected QT (QTc) interval (Bessiere et al. 2020; Ramireddy et al. 2020; Chorin et al. 2020), and the product labeling specifically highlights that QTc interval prolongation and torsade de pointes have been reported. Recent data from 1515 COVID-19 patients showed that approximately 10% of COVID-19 patients treated with CQ/HCQ developed QT prolongation (Jankelson et al. 2020). Moreover, patients treated with HCQ in combined with AZ had higher risk of QTc prolongation compared with use alone (Mercuro et al. 2020). In our study, the increased risk of death in patients treated with HCQ may be related to prolonged QT and cardiac arrest.
QTc interval prolongation is associated with blockade of the hERG potassium channel, which can potentially prolong ventricular repolarization and the duration of ventricular action potentials (Traebert et al. 2004). Early afterdepolarizations may trigger ventricular arrhythmias under some specific conditions (Giudicessi et al. 2020). Individuals with structural cardiovascular disease are more susceptible to arrhythmia. Growing evidence supported that patients with COVID-19 are more likely to suffer from cardiovascular injury, including acute myocardial injury, arrhythmia, and cardiac insufficiency (Shi et al. 2020; Guo et al. 2020). In addition, many COVID-19 patients have underling cardiovascular disease and are more prone to severe disease with worse clinical outcomes (Mehra et al. 2020a). The cardiovascular comorbidity and de-novo cardiovascular injury may explain the heightened vulnerability to arrhythmia among patients with COVID-19 who were treated with HCQ. Furthermore, it is reported that 2~50% COVID-19 patients suffered from diarrhea, which can cause hypokalemia and hypomagnesemia, leading to an increased risk of arrhythmia (D'Amico et al. 2020). Moreover, critically ill patients may have an altered metabolism of HCQ due to hepatic injury and renal insufficiency (Zhang et al. 2020; Rowland Yeo et al. 2020), which may increase the risk of adverse reactions.
It is limited that there may be substantial risk of bias in this meta-analysis because of the sample of articles rolled and some negative outcomes hidden by some researchers, even the suspended and stopped trial by WHO. The articles of RCTs may be qualitatively different, because of the blind method, but steerable in order to the population recruited from Chinese. The observational studies were evaluated by NOS, a scale used for the assessment of risk of bias, and in our work, the quality of 4 papers was middle to high. There may exist moderate risk of bias that was hard to avoid in the circumstance of the less quantity of trials available, whereas, in conclusion, our study showed the improved CT manifestations and increased risk of death in HCQ group. In addition, there was no significance on the progression to severe disease and PCR negative conversion when treated with HCQ. However, we just evaluated the relationship between the HCQ treatment and death without stratifying the reasons of death, and not measure QT intervals between two groups. Even with these limitations, this result argues against the widespread use of HCQ in COVID-19 patients and confirmation from better, properly powered, randomized controlled clinical trials is urgently needed.
Author contributions
Yanxiang Zang and Yue Li designed the study and analyzed data. Yanxiang Zang, Xuejie Han, and Meijiao He wrote the draft of the manuscript. Jing Shi checked the grammar. All authors contributed to the interpretation of data and critical revision of the manuscript, and had final approval of the submitted and published version. The authors declare that all data were generated in-house and that no paper mill was used.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no competing interest.
Ethical approval
This article is a meta-analysis, so it does not contain any studies with human participants performed by any of the authors.
Footnotes
Yanxiang Zang, Xuejie Han, and Meijiao He were co-first authors in this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessiere F, Roccia H, Deliniere A, Charriere R, Chevalier P, Argaud L, Cour M (2020) Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol [DOI] [PMC free article] [PubMed]
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B, Zhang Z (2020) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial - Renmin Hospital, Wuhan. medRxiv. 10.1101/2020.03.22.20040758
- Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park DS, Stefano C, Chinitz LA, Jankelson L (2020) QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm [DOI] [PMC free article] [PubMed]
- D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Eldin C, Finance J, Vieira VE, Tissot-Dupont HT, Honore S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard A, Romani S, Fresse A, Viard D, Parassol N, Granvuillemin A, Chouchana L, Rocher F, Drici MD, C. French Network of Pharmacovigilance (2020) “Off-label” use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: a survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapie [DOI] [PMC free article] [PubMed]
- Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol [DOI] [PMC free article] [PubMed]
- Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM (2020) Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. [DOI] [PubMed]
- Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC (2020) QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm [DOI] [PMC free article] [PubMed]
- Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GP, Zabaleta ME, Di Giulio C, Charris JE, Mijares MR (2020) The role of chloroquine and hydroxychloroquine in immune regulation and diseases. Curr Pharm Des. 26 [DOI] [PubMed]
- Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (2020b) RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet [DOI] [PMC free article] [PubMed] [Retracted]
- Mehra MR, Ruschitzka F, Patel AN. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS (2020) Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol [DOI] [PMC free article] [PubMed]
- Nakornchai S, Konthiang P. Activity of azithromycin or erythromycin in combination with antimalarial drugs against multidrug-resistant Plasmodium falciparum in vitro. Acta Trop. 2006;100:185–191. doi: 10.1016/j.actatropica.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Patel TK, Barvaliya M, Kevadiya BD, Patel PB, Bhalla HL (2020) Does adding of hydroxychloroquine to the standard care provide any benefit in reducing the mortality among COVID-19 patients?: a systematic review. J Neuroimmune Pharmacol [DOI] [PMC free article] [PubMed]
- Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, Cingolani E, Cheng S, Marban E, Albert CM, Chugh SS. Experience With hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9:e017144. doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg P, Kirkwood J, Muse A, DeHovitz J, Blog DS, Hutton B, Holtgrave DR, Zucker HA. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland Yeo K, Zhang M, Pan X, Ban Ke A, Jones HM, Wesche D, Almond LM (2020) Impact of disease on plasma and lung exposure of chloroquine, hydroxy-chloroquine and azithromycin: application of PBPK modelling. Clin Pharmacol Ther [DOI] [PMC free article] [PubMed]
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C (2020) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol [DOI] [PMC free article] [PubMed]
- Torjesen I. Covid-19: Hydroxychloroquine does not benefit hospitalised patients, UK trial finds. BMJ. 2020;369:m2263. doi: 10.1136/bmj.m2263. [DOI] [PubMed] [Google Scholar]
- Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]