Abstract
Objectives
The symptoms of Coronavirus disease 2019 (COVID-19) vary among patients. The aim of this study was to investigate the clinical manifestation and disease duration in young versus elderly patients.
Methods
We retrospectively analyzed 187 patients (87 elderly and 100 young patients) with confirmed COVID-19. The clinical characteristics and chest computed tomography (CT) extent as defined by a score were compared between the two groups.
Results
The numbers of asymptomatic cases and severe cases were significantly higher in the elderly group (elderly group vs. young group; asymptomatic cases, 31 [35.6%] vs. 10 [10%], p < 0.0001; severe cases, 25 [28.7%] vs. 8 [8.0%], p = 0.0002). The proportion of asymptomatic patients and severe patients increased across the 10-year age groups. There was no significant difference in the total CT score and number of abnormal cases. A significant positive correlation between the disease duration and patient age was observed in asymptomatic patients (ρ = 0.4570, 95% CI 0.1198–0.6491, p = 0.0034).
Conclusions
Although the extent of lung involvement did not have a significant difference between the young and elderly patients, elderly patients were more likely to have severe clinical manifestations. Elderly patients were also more likely to be asymptomatic and a source of COVID-19 viral shedding.
Keywords: COVID-19, Young patients, Elderly patients, Clinical characteristics, Asymptomatic patients
1. Introduction
Coronavirus disease 2019 (COVID-19), which leads to severe acute respiratory distress syndrome (ARDS), was first reported in Wuhan, Hubei Province, China in December 2019 [[1], [2], [3]]. It spread rapidly to other countries, and the number of affected patients is increasing. The World Health Organization declared a pandemic on March 11, 2020. There were 3,767,744 confirmed COVID-19 cases and 259,593 related deaths in the world by May 8, 2020 [4].
The symptoms of COVID-19 varied among the patients. Patients with minor symptoms presented with a headache, fever, fatigue, and/or cough, and those symptoms improved without any specific treatment. Severe cases presented with severe dyspnea or hypoxia and needed intensive care. An older age is reported to be associated with the disease severity and a high mortality [2]. On the other hand, some COVID-19 patients had no symptoms, even though they had radiological abnormalities [5]. These asymptomatic carriers could result in person-to-person transmission and are considered a source of the COVID-19 infection [[6], [7], [8], [9]]. Although Liu et al. reported the clinical features of COVID-19 in elderly patients [10], the manifestation of asymptomatic cases or the disease duration are not well understood. The aim of this study was to investigate the clinical manifestation and disease duration of elderly patients, including both symptomatic and asymptomatic cases.
2. Methods
2.1. Study population
We retrospectively analyzed 192 patients with confirmed COVID-19 who were admitted to our hospital from February 11 to April 30, 2020. All patients were diagnosed with COVID-19 infections by a viral reverse transcription polymerase chain reaction (RT-PCR). The RT-PCR using a pharyngeal swab was performed when the patients had clinical symptoms or close contact with a known COVID-19 patient. In the symptomatic patients, an initial RT-PCR from a pharyngeal source was performed 2 days after the resolution of the clinical symptoms. In the asymptomatic patients, the RT-PCR was performed 2–5 days after admission. The RT-PCR was repeatedly performed every 1–2 days. Discharge criteria included two consecutive negative RT-PCR results.
The definition of asymptomatic cases was as follows: COVID-19 was confirmed by the RT-PCR, but carriers did not have any clinical symptoms (some despite radiological abnormalities). Severe cases were defined as those with hypoxia requiring oxygen therapy (due to the pneumonia). Antiviral therapy or corticosteroids were administered for severe cases at the discretion of the clinical attending physician. Our hospital treated all COVID-19 patients in the general wards; therefore, no cases were treated in the intensive care unit.
Patients younger than 18 years old were excluded from this analysis. A total of 187 patients were enrolled in the study and divided into an elderly group (≧60 years old) and young group (<60 years old).
2.2. Data collection
For each COVID patient, the laboratory results and chest computed tomography (CT) studies were evaluated within the 48 h after the first admission. Our clinical data were collected retrospectively from the institutional medical records by two physicians (H.M. and H.O.). The following data were extracted: clinical information, clinical symptoms, and laboratory findings. The disease duration was defined as that from the symptom onset to the first RT-PCR negative result in symptomatic patients and from a RT-PCR positive test to the first RT-PCR negative test in asymptomatic patients.
The CT findings were reviewed by two radiologists with 5 and 11 years of experience (W.M. and Y.S.) blinded to the clinical information, and the final decision was reached by consensus. For all the patients, axial CT images were evaluated semi-quantitatively using a scoring system for the COVID-19 findings based on the previous studies [11,12]. Each lung was divided into six zones without regard to the anatomical lobe; for the cranial-caudal dimension, three zones were defined as upper (above the carina), middle (below the carina and above the inferior pulmonary vein), and lower (below the inferior pulmonary vein). For the medial-lateral dimension, each outer third of the axial slice was defined as peripheral, and the middle third area was defined as central. Therefore, a total of 12 lung zones were presented in each patient. Each zone was graded according to a visual assessment of the involvement as follows: 0 (0%), 1 (1–5%), 2 (6–25%), 3 (25–50%), 4 (51–75%), and 5 (75–100%). The final score was summed over all 12 zones and ranged from 0 (no involvement) to 60 (maximum involvement).
The clinical characteristics and clinical manifestations were compared between the two groups. The correlation between the patient age and disease duration was also analyzed.
2.3. Statistical analysis
Statistical analyses were performed using JMP® Pro software, version 11.2 (SAS Institute). A Shapiro-Wilk test was performed to assess the data distribution. The continuous variables were represented by median values with interquartile ranges and compared using a Mann-Whitney test for non-parametric data. The categorical data were compared using a chi-square test. A Spearman's correlation test was performed to assess the relationship between the age and number of admission days or disease duration. The Cochran-Armitage test was used for a trend analysis. A value of P <0.05 indicated statistical significance.
3. Results
3.1. Clinical manifestations of the two groups
A total of 187 patients were enrolled in our study, 87 elderly patients and 100 young patients. Table 1 shows the clinical characteristics of the two groups. The median age of the elderly group was 72 years (IQR; 67–76 years), and the median age of the young group was 41 years (IQR; 35–48 years). The patents in the elderly group were more often female and more likely to have hypertension, diabetes, and cardiovascular disease.
Table 1.
Clinical manifestations of the two groups.
| Elderly group (n = 87) | Young group (n = 100) | p | |
|---|---|---|---|
| Clinical parameters | |||
| Age, years | 72 (67–76) | 41 (35–47.8) | <0.0001∗ |
| Gender, male, n (%) | 44 (50.1) | 65 (65.0) | 0.0460∗ |
| Contact episode, n (%) | 35 (40.2) | 47 (47.0) | 0.3520 |
| BMI, kg/m2 | 22.3 (19.5–25.6) | 23.0 (21.2–25.5) | 0.1729 |
| Current smoker, n (%) | 5 (5.8) | 24 (24.0) | 0.0006∗ |
| Past Medical History | |||
| Hypertension | 32 (36.8) | 6 (6.0) | <0.0001∗ |
| Diabetes | 10 (11.5) | 1 (1.0) | 0.0023∗ |
| Respiratory disease | 10 (11.5) | 10 (10.0) | 0.7416 |
| Cardiovascular disease | 9 (10.3) | 2 (2.0) | 0.0156∗ |
| Respiratory Rate, breaths per min | 18 (16–21) | 17 (16–18) | 0.1157 |
| Heart rate, beats per min | 84 (76–95) | 82 (75–89) | 0.1980 |
| Systolic blood pressure, mmHg | 138 (124–158) | 120 (110–132) | <0.0001∗ |
| Body temperature, °C | 36.8 (36.5–37.1) | 36.8 (36.5–37.3) | 0.8080 |
| Nationality | |||
| Asia | 64 (73.3) | 92 (92.0) | |
| Europe | 4 (4.7) | 4 (4.0) | |
| Oceania | 7 (8.1) | 1 (1.0) | |
| Northern America | 12 (14.0) | 1 (1.0) | |
| Southern America | 0 (0.0) | 2 (2.0) | |
| Clinical symptoms | |||
| Asymptomatic patients, n (%) | 31 (35.6) | 10 (10.0) | <0.0001∗ |
| Duration of symptoms prior to admission, days | 6.5 (4–11) | 7 (5–10) | 0.5676 |
| Severe cases, n (%) | 25 (28.7) | 8 (8.0) | 0.0002∗ |
| Fever (temperature >37.5 °C) | 41 (47.1) | 77 (77.0) | <0.0001∗ |
| Fatigue, n (%) | 18 (20.7) | 35 (35.0) | 0.0303∗ |
| Headache, n (%) | 10 (11.5) | 49 (49.0) | <0.0001∗ |
| Sore throat, n (%) | 10 (11.5) | 22 (22.0) | 0.0571 |
| Cough, n (%) | 28 (32.2) | 58 (58.0) | 0.0004∗ |
| Myalgia, n (%) | 0 (0.0) | 12 (12.0) | 0.0008∗ |
| Diarrhea, n (%) | 7 (8.1) | 22 (22.0) | 0.0086∗ |
| Nausea, n (%) | 5 (5.8) | 8 (8.0) | 0.5457 |
| Abdominal pain, n (%) | 3 (3.5) | 9 (9.0) | 0.1223 |
| Dyspnea, n (%) | 19 (21.8) | 9 (9.0) | 0.0141∗ |
| Laboratory Findings | |||
| WBC count, 104/μL | 5430 (4120–6580) | 4879 (3837–6325) | 0.2213 |
| Lymphocyte ratio, % | 24.4 (16.1–32.5) | 26.5 (21.6–36.5) | 0.0231∗ |
| Haemoglobin, g/dl | 14.0 (12.9–15.0) | 15.2 (14.1–15.9) | <0.0001∗ |
| Platelet count, 104/μL | 22.8 (17.8–27.1) | 21.3 (17.3–26.7) | 0.5087 |
| BUN, mg/dl | 15 (12–18) | 12 (10–15) | <0.0001∗ |
| Creatine kinase, mg/dl | 0.81 (0.65–0.97) | 0.76 (0.63–0.86) | 0.0839 |
| AST, IU/L | 26 (21–33) | 27 (21–36.8) | 0.4675 |
| ALT, IU/L | 21 (16–33) | 30 (18–50.5) | 0.0045∗ |
| LDH, IU/L | 200 (179–247) | 204.5 (167.3–256.5) | 0.6588 |
| Albumin, g/dL | 3.9 (3.6–4.2) | 4.3 (4.0–4.5) | <0.0001∗ |
| CRP, mg/dL | 0.87 (0.11–2.92) | 0.38 (0.13–1.92) | 0.1970 |
| PT-INR | 1.02 (0.98–1.05) | 1.0 (0.96–1.05) | 0.1688 |
∗ p < 0.05.
Although the number of asymptomatic cases was significantly higher in the elderly group (elderly group 31 (35.6%) than young group 10 (10%), p < 0.0001), the number of severe cases was significantly higher in the elderly group (elderly group 25 (28.7%) than young group 8 (8.0%), p = 0.0002). The younger group patients had a fever, fatigue, headache, cough, myalgia, and diarrhea with statistical significance. The most common symptom among the young group was a fever (elderly group 41 [47.1%] vs. young group 77 [77%], p < 0.0001).
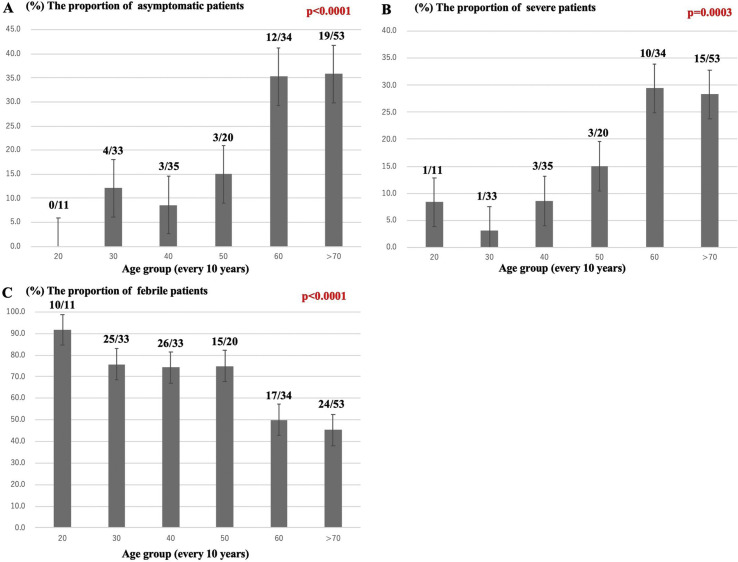
A trend analysis was performed to assess the proportion of clinical characteristics. One 18 year-old patient was included in the 20-year group. The proportions of asymptomatic patients and severe patients both increased across the age groups (Fig. 1 A and B). On the other hand, the proportion of patients with a fever decreased across the age groups (Fig. 1C).
Fig. 1.
Trend analysis of disease characteristics. The proportion of asymptomatic patients and severe patients increased across the 10-year age groups (A, B). On the other hand, the proportion of patients with a fever decreased across the 10-year age groups (C).
3.2. CT findings
A total of 185 patients underwent chest CT studies. Two patients with severe emphysema, one with severe interstitial pneumonia, one with non-tuberculous mycobacteria, and one with excessive motion artifact were excluded from the CT analysis. The remaining 180 CT studies were reviewed. Table 2 shows the CT findings of the symptomatic patients separated into the two groups. There was no significant difference in the total CT score and number of abnormal cases (elderly group 8.5 [2–16.3] vs. young group 5 [2–11], p = 0.0796) or in the number of abnormal cases, n% (elderly group 46 [85.2] vs. young group 71 [81.6], p = 0.5828). Table 3 shows the CT findings of the asymptomatic patients separated into the two groups. There was also no significant difference in the total CT score (elderly group 2.0 [0–5.0] vs. young group 1.0 [0–5.5], p = 0.9868) or the number of abnormal cases (elderly group 17 [58.6] vs. young group 7 [70.0], p = 0.5236).
Table 2.
CT findings of the two groups (symptomatic patients).
| Elderly group (n = 54) | Young group (n = 87) | p | ||
|---|---|---|---|---|
| Total CT score | 8.5 (2–16.3) | 5 (2–11) | 0.0796 | |
| The number of abnormal cases, n (%) | 46 (85.2) | 71 (81.6) | 0.5828 | |
| Right Lung | ||||
| Upper | Outer | 0 (0–2) | 0 (0–0) | 0.0032∗ |
| Inner | 0 (0–1) | 0 (0–0) | 0.0578 | |
| Middle | Outer | 2 (0–2) | 0 (0–2) | 0.0037∗ |
| Inner | 0 (0–1) | 0 (0–1) | 0.8509 | |
| Lower | Outer | 1.5 (0–2) | 2 (0–2) | 0.2104 |
| Inner | 0 (0–2) | 0 (0–1) | 0.3716 | |
| Left Lung | ||||
| Upper | Outer | 0 (0–2) | 0 (0–1) | 0.0645 |
| Inner | 0 (0–0.25) | 0 (0–0) | 0.0887 | |
| Middle | Outer | 1 (0–2) | 1 (0–2) | 0.2754 |
| Inner | 0 (0–1) | 0 (0–1) | 0.3134 | |
| Lower | Outer | 2 (0–2) | 2 (0–2) | 0.2400 |
| Inner | 0 (0–1) | 0 (0–1) | 0.8467 | |
∗ p < 0.05.
Table 3.
CT findings of the two groups (asymptomatic patients).
| Elderly group (n = 29) | Young group (n = 10) | p | ||
|---|---|---|---|---|
| Total CT score | 2.0 (0–5.0) | 1.0 (0–5.5) | 0.9868 | |
| The number of abnormal cases, n (%) | 17 (58.6) | 7 (70.0) | 0.5236 | |
| Right Lung | ||||
| Upper | Outer | 0 (0–0) | 0 (0–1.25) | 0.0708 |
| Inner | 0 (0–0) | 0 (0–0) | 0.7775 | |
| Middle | Outer | 0 (0–0.5) | 0 (0–1.25) | 0.6991 |
| Inner | 0 (0–0) | 0 (0–0.25) | 0.5533 | |
| Lower | Outer | 0 (0–1.5) | 0 (0–1.25) | 0.7670 |
| Inner | 0 (0–0.5) | 0 (0–0) | 0.4038 | |
| Left Lung | ||||
| Upper | Outer | 0 (0–0) | 0 (0–1.25) | 0.0708 |
| Inner | 0 (0–0) | 0 (0–0) | 0.4157 | |
| Middle | Outer | 0 (0–0) | 0 (0–1.25) | 0.4693 |
| Inner | 0 (0–0) | 0 (0–0.25) | 0.4980 | |
| Lower | Outer | 0 (0–2) | 0 (0–0.5) | 0.4525 |
| Inner | 0 (0–0) | 0 (0–0) | 0.3039 | |
3.3. Disease duration and days of admission in the two groups
Table 4 shows the disease duration and days of admission in the two groups. Two symptomatic elderly patients, whose disease onset was not recorded, and ten symptomatic young patients, who were discharged before the RT-PCR was confirmed negative, were excluded. One asymptomatic elderly patient, in whom the day of the positive RT-PCR result was not recorded, and one asymptomatic elderly patient, who underwent the RT-PCR on day 8 after admission, were also excluded. The days of admission in the symptomatic patients among the elderly group were significantly longer than that in the young group. The disease duration and days of admission in the asymptomatic patients in the elderly group were also significantly longer than that in the young group.
Table 4.
Disease duration and admission days of the two groups.
| Symptomatic patients | Elderly group |
Young group |
p |
|---|---|---|---|
| n = 54 | n = 80 | ||
| Duration of disease, days | 16.5 (11.8–20) | 14 (12–18) | 0.1051 |
| Admission days, days |
12.5 (10–16) |
10 (7–13) |
0.0001∗ |
| Asymptomatic patients |
n = 29 |
n = 10 |
|
| Duration of disease, days | 10 (7.5–15.5) | 7.5 (7.0–10.0) | 0.0496∗ |
| Admission days, days | 11 (10–15) | 8.5 (6.8–10) | 0.0015∗ |
∗ p < 0.05.
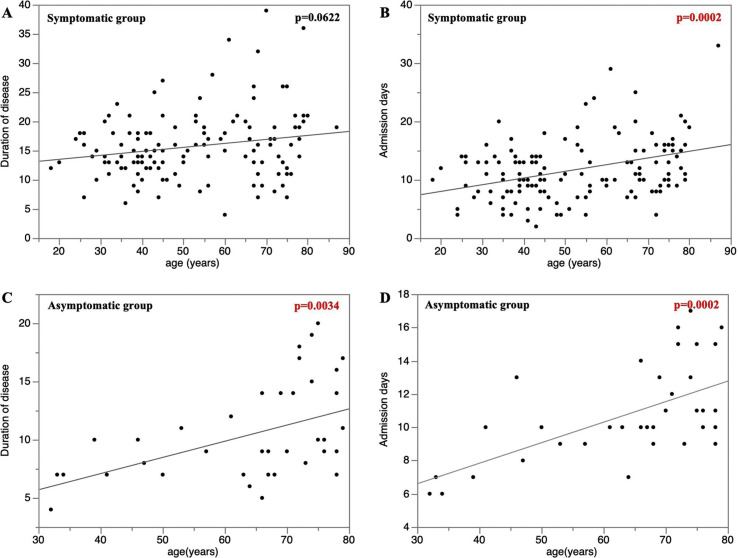
Fig. 2A and B shows the correlation between the duration of the disease, admission days, and patient age among the symptomatic groups. A significant positive correlation between the admission days and patient age was observed (Spearman's ρ = 0.3053, 95% CI 0.16–0.46, p = 0.0002). Fig. 2C and D shows a similar correlation for the asymptomatic groups. A significant positive correlation between the duration of the disease, days of admission, and patient age was observed (Spearman's correlation for the duration of the disease, ρ = 0.4570, 95% CI 0.1198–0.6491, p = 0.0034; Spearman's for admission days, ρ = 0.5583, 95% CI 0.2104–0.6998, p = 0.0002).
Fig. 2.
Correlation between the duration of the disease, days of admission, and the patient age. A and B shows the correlation between the duration of the disease, days of admission, and patient age among the symptomatic groups. A significant positive correlation between the days of admission and patient age was observed (Spearman's correlation,ρ = 0.3053, 95% CI 0.16–0.46, p = 0.0002). C and D shows that a significant positive correlation between the duration of the disease, days of admission, and patient age was observed in the asymptomatic groups (Spearman's correlation of the duration of the disease, ρ = 0.4570, 95% CI 0.1198–0.6491, p = 0.0034; Spearman's correlation for admission days, ρ = 0.5583, 95% CI 0.2104–0.6998, p = 0.0002).
4. Discussion
To the best of our knowledge, this is the first report to compare the clinical manifestation and duration of the disease between elderly and young COVID-19 patients, which includes both symptomatic cases and asymptomatic cases. The main findings of this study were: (1) while there were many severe patients in the elderly group, elderly patients were more often asymptomatic and afebrile; (2) there was no significant difference in the total CT score and number of abnormal cases between the elderly and young groups; and (3) the duration of the disease had a significant positive correlation with the patient age in the asymptomatic group.
Although there were no significant differences in the CT score, the elderly patients had a higher incidence of severe and asymptomatic cases. Such cases with CT findings and a lack of clinical symptoms may be referred to as “silent pneumonia”. In addition, the serum LDH and CRP did not show any statistical difference between the two groups. This may indicate that lung damage from COVID-19 was similar between the two groups, despite the varying clinical manifestations. Differences in the elderly group's lung anatomy, including muscle atrophy, may account for the higher severity among the elderly, with reduced the airway clearance [13], lung reserve [14], and defense barrier function [15].
A prior study associated hypertension with the COVID-19 disease severity [16]. Higher blood pressure may be associated with the clinical manifestations and outcomes. A lower lymphocyte ratio in the elderly group may also be associated with the greater disease severity among the elderly group [17].
Another study reported a significant positive correlation between the age and peak viral load [18]. Our study showed that the duration of the disease had a significant positive correlation with the patient age in the asymptomatic group. The viral load in the asymptomatic patients was reported to be similar to that in the symptomatic patients, which suggested the transmission potential of asymptomatic patients [8]. Elderly patients are more likely to be asymptomatic with a higher viral load. As a consequence, elderly patients could contribute to the viral shedding of the COVID-19 infection. Special consideration should be given to this population for the purpose of infection control during this pandemic.
4.1. Study limitations
Our study had a few sources of potential selection bias. This study was a single center retrospective study with a relatively small sample size. Although our study included 33 severe cases, including four intubated patients, COVID-19 patients were triaged before admission, and severe cases were admitted preferentially to a different hospital. In addition, CT images were obtained and analyzed at an early stage of COVID-19. Presumably, later CT images would show the disease progression not captured in this study. Finally, we were unable to address the relationship between the mortality and patient age. Due to the limitation in the number of RT-PCR tests during the initial phase of the COVID-19 pandemic, the timing of the RT-PCR test for evaluating negative results was irregular among the patients.
5. Conclusions
Although there was no significant difference in the CT representation of the lung involvement between the young and elderly patients, the elderly patients were more likely to be severe cases. Furthermore, elderly patients were more likely to be asymptomatic and were a source of COVID-19 viral shedding.
Author's contributions
HM and HO, study conception and design; WM, KT, HS and YS, data collection and data analysis; YM, YT, SO, and MY, manuscript revision; KT, study supervision.
Funding source
This study did not receive any specific grant.
Ethical approval
This study was approved by the institutional review board of our institution (02-16).
Declaration of competing interest
All authors declared no conflict of interest associated with this study.
Acknowledgement
We thank the COVID-19 team from the Self Defense Forces Central Hospital, Tokyo. In addition, we appreciate the help in proofreading by Stephanie Anne Lee-Felker, M.D., Department of Radiological Sciences at the University of California, Los Angeles and Mr. John Martin.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO coronavirus disease (COVID-2019) dashboard. May 8 2020. https://covid19.who.int
- 5.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K., et al. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan. medRxiv. 2020 doi: 10.1016/S1473-3099(20)30482-5. https://www.medrxiv.org/content/10.1101/2020.03.18.20038125v2.full.pdf [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020 Feb 21 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 Mar 5;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020 May;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020 Mar 27 doi: 10.1016/j.jinf.2020.03.005. pii: S0163-4453(20)30116-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 Feb 13:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020 Mar 19;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020 Feb 27 doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo M.Z., Huang Y.G., Ma W.H., Xue Z.G., Zhang J.Q., Gong Y.H., et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J. 2020 Feb 27 doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., et al. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020 Feb 22;(2):12. doi: 10.3390/v12020244. pii: E244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19):a pooled analysis. Pol Arch Intern Med. 2020 Apr 30;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 17.Diagnosis and treatment protocol for novel coronavirus pneumonia. The general office of national Health commission office of state TCM administration printed and distributed on March 3, 2020.
- 18.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 May;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]