Abstract
Background
Great efforts by the scientific community are rapidly expanding the evidence on the clinical interplay between Covid-19 and inflammatory bowel disease (IBD).
Aims
We performed a systematic review of the literature on published Covid-19 cases occurring in patients with IBD.
Methods
PubMed Central/Medline and Embase were systemically searched for records up to May 31, 2020.
Results
13 cohort studies and 5 single case reports were included in the qualitative synthesis. A cumulative number of approximately 800 patients with IBD and Covid-19 were identified. The case fatality rate ranged from 0% to 20.0%. Overall, immunomodulators and biologics were not associated with higher risk of Covid-19 or with negative outcomes, while the use of systemic corticosteroids was related to worse prognosis in some studies.
Conclusions
This systematic review highlighted two main points that may help clinicians dealing with IBD in reassuring their patients: (1) patients with IBD do not seem to be at higher risk of being infected by SARS-COV-2 than the general population; (2) in case of Covid-19, treatment with immunomodulators or biologics is not associated with worse prognosis, while systemic steroids are suspected to be potentially detrimental, even if more data are needed to confirm this point.
Keywords: Biologics, Covid-19, Immunomodulators, Sars-CoV-2
1. Introduction
At the end of 2019, China reported several cases of severe pneumonia of unknown cause that would subsequently be identified as attributable to the novel Severe Acute Respiratory Syndrome-coronavirus-2 (SARS-CoV-2) [1]. Such disease was later called coronavirus disease 2019 (Covid-19) [2]. Considering the global transmission of Covid-19, the disease has been defined as a pandemic by the World Health Organization on March 11, 2020 [3]. As of May 31, 2020, there had been 5934,936 confirmed cases of Covid-19 globally with 367,166 deaths [4]. At the time of manuscript drafting, USA and Brazil are experiencing the most worrisome consequences of the pandemic, with a rapid growth of cases, deaths and burden for the health systems. As one might expect, such a large-scale event can only generate a huge impact at every level. In the healthcare setting, the possible impact of Covid-19 in patients with chronic diseases - including inflammatory bowel disease (IBD) – should be considered. Indeed, both patients and physicians are currently focused on two main issues. First, are patients with IBD at increased risk for Covid-19? Second, could the immunomodulators or biologics used for treatment of IBD increase the risk of developing severe forms of Covid-19? Actually, the interplay between Covid-19 and IBD is currently poorly known, given the short time from the viral disease outbreak. Anyway, great efforts by the scientific community are rapidly expanding the evidence on this topic.
In an attempt to provide the current evidence on the clinical interplay between Covid-19 and IBD – even in this rapidly evolving current scenario, where novel data emerge every day – we performed a systematic review of the literature on Covid-19 cases occurring in patients with IBD. Current evidence was resumed in order to support gastroenterologists dealing with IBD in this times dominated by Covid-19 pandemic.
2. Materials and methods
Information Sources, Search Strategies and Eligibility Criteria
The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statements were followed [5]. Primary sources of the reviewed studies, including English sources only, were PubMed Central/Medline and Embase, which were searched systemically for records published up to May 31, 2020. Searches included combinations of the following medical subject headings (MeSH): “Covid-19”, “Covid19”, “Pandemic”, “Sars-Cov-2”, with one or more of the following: “inflammatory bowel disease”, “Crohn's disease”, “Crohn disease”, “Crohn”, “Ulcerative colitis”. The terms were combined using the set operator AND. Database searches were supplemented with literature searches of reference lists from potentially eligible articles by both reviewers to find additional studies.
Papers selected for the analysis included retrospective or prospective studies reporting clinical data on patients with IBD and infection with Sars-Cov-2. There were no restrictions in the number of patients described by each study, so that also case reports were included. Studies reported solely as abstracts and narrative reviews were not included in the qualitative synthesis.
2.1. Searches and data collection process
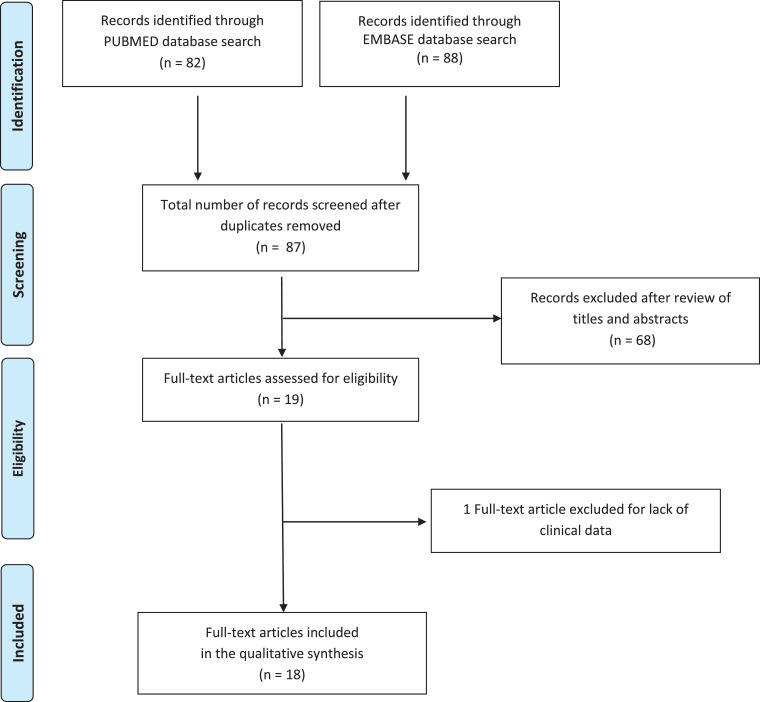
Among the 87 records that were identified through electronic search after duplicates removal, both reviewers independently evaluated the titles and abstracts, removed 68 studies that did not meet the inclusion criteria, and selected 19 potentially relevant reports that were identified and retrieved for detailed evaluation. Among these, 18 papers [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23] were included in the qualitative synthesis (Fig. 1 ).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the search process. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For each study, the reviewers independently extracted the data of interest, and entered them into a structured database using a Microsoft Excel spreadsheet. In particular, the data of interest included the name of the first author, the country where the study was conducted, the period of observation/reporting, the number of IBD patients who developed Covid-19, the number of IBD patients with Covid-19 older than 60 years, the case fatality rate, and the modality of diagnosis or exclusion of Covid-19. Furthermore, data on association between IBD treatments and severity of Covid-19 were also collected. Discrepancies among reviewers about qualitative and quantitative data collection were infrequent (overall interobserver variation < 10%), and were resolved through discussion until consensus was reached.
3. Results
3.1. Characteristics of the studies included in the qualitative synthesis
Among the 18 studies included in the qualitative synthesis, 13 were cohort studies, and 5 were single case reports. The main characteristics and finding of the 13 cohort studies are shown in Table 1 . Notably, 2 of them came from China, 2 from Italy, 1 from Italy and France, 2 from Spain, 4 from the U.S.A., and 2 collected data from all parts of the world (one pediatric setting, the other one covered both pediatric and adult patients). A cumulative number of approximately 800 patients with IBD and Covid-19 were identified. A precise number cannot be provided, as a certain degree of overlap of patients between the different reports and databases may be hypothesized. The case fatality rate ranged from 0% to 20.0%. Table 2 reports data on comparisons of case fatality rate of Covid-19 between patients with IBD and the general population (4 studies).
Table 1.
Cohort studies included in the systematic review reporting data on occurrence of Covid-19 in patients with inflammatory bowel disease.
| First author [ref] | Country | Period of observation or reporting | No. of IBD patients with diagnosis of Covid-19 | No. of patients with Covid-19 > 60 yrs | Case fatality rate | Modality of Covid-19 cases identification | Association between IBD treatments and severity of Covid-19 |
|---|---|---|---|---|---|---|---|
| Mao R [6] | China (IBD Elite Union, which incorporates the seven largest IBD referral centres in China) | December 2019 – March 8, 2020 | 0/20,000 IBD patients | - | - | NA | - |
| An P [7] | China (Renmin Hospital of Wuhan University) | January 3 - March 30, 2020 | 0/318 IBD patients | - | - | Communication by phone or e-mail; furthermore, 29 patients underwent chest CT scans and virological testing. | - |
| Norsa L [8] | Italy (Province of Bergamo) | February 19 - March 23, 2020 | 0/522 IBD patients | - | - | Communication by the patients by phone or e-mail | No case despite 22% of patients were treated with IS and 16% with biologics |
| Allocca M [9] | France (Nancy University Hospital) and Italy (Humanitas, Milan) | NA | 15/6000 IBD patients (0.0025%) | 1/15 | 0/15 | Tele-medicine and infusion center visits. Diagnosis of suspected cases with virological testing | No deaths despite 14/15 patients were treated with IS and/or biologics |
| Bezzio C [10] | Italy | 11 - 29 March 2020 | 79 | NA | 6/79 (7.6%) | 49 patients: virological testing; 30 patients: clinical and radiological diagnosis | Therapy with biologics and IS did not associate with worse COVID-19 prognosis. A trend toward worse prognosis was found for corticosteroids use. |
| Rodríguez-Lago I [11] | Spain (Basque country) | February 27 - April 8, 2020 | 40 | NA | 2/40 (5.0%) | Virological testing | Low case fatality rate despite 28% of patients were under IS and 18% under biologics |
| Taxonera C [12] | Spain (Hospital Clinico San Carlos, Madrid) | April 8, 2020 | 12/1912 IBD patients (0.0063%) | 3/12 | 2/12 (16.7%) | Virological testing | No increased risk of COVID-19 and associated mortality compared with the general population despite 7 patients were on treatment with IS and/or biologics |
| Gubatan J [13] | USA (Northern California) | March 04 - April 14, 2020 | 5/168 IBD patients (3.0%) | 4/5 | 1/5 (20.0%) | Virological testing | - |
| Hajifathalian K [14] | USA (New York) | March 4 - April 9, 2020 | 17/1059 total patients with Covid-19 | NA | NA | Virological testing | - |
| Lukin DJ [15] | USA (New York) | NA | Retrospective cohort: 60 Longitudinal cohort: 29/119 IBD patients (24.4% - 9 confirmed, 20 highly suspected cases) | NA | 0/17 | Confirmed cases: virological testing; highly suspected cases: fever >37.8 and >1 new symptom including cough, sore throat, dyspnea, anosmia, diarrhea, with a known close contact with COVID-19. | Baseline corticosteroid use was higher among Covid-19 patients with IBD compared with those with IBD without Covid-19, while no difference was noted about biologic or immunomodulator use |
| Khan N [16] | USA (Veterans’ Affairs Healthcare System) | January 1 - May 15, 2020 | 36/37,857 IBD patients (0.0009%) | NA | NA | Virological testing | Thiopurines and anti-TNFs were not associated with a significant increased risk of Covid-19 |
| Turner D [17] | Global (pediatric setting) | March 26, 2020 | 8 | - | 0/8 | Diagnosis confirmed with virological testing in 6/8 cases | All cases had mild infection without needing hospitalization despite treatment with IS and/or biologics |
| Brenner EJ [18] | Global (pediatric and adult setting) | NA* | 525 | 101/525 | 16/525 (3.0%) | Virological testing | Risk factors for severe Covid-19 included systemic corticosteroids and 5-aminosalicylate use, while anti-TNFs were not associated with severe Covid-19. |
Date of acceptance of the article: May 8, 2020
Abbreviations: IBD: Inflammatory Bowel Diseases; IS: immunosuppressants.
Table 2.
Studies reporting comparisons of case fatality rate of Covid-19 between patients with inflammatory bowel disease and the general population.
| First Author [ref.] | Case fatality Rate IBD patients | Case fatality Rate General population | P | Comments |
|---|---|---|---|---|
| Allocca M [9] | 0% | 13–15% | Estimated case fatality rate among diagnosed cases of Covid-19 in the general population in Italy at the time of manuscript submission | |
| Bezzio C [10] | 7.6% | 13–15% | Estimated case fatality rate among diagnosed cases of Covid-19 in the general population in Italy at the time of manuscript submission | |
| Taxonera C [12] | 0.9 deaths per 1000 pts | 1 death per 1000 pts | 0.36 | Comparison of crude mortality rates between IBD patients and the general population of Madrid at the time of manuscript submission |
| Lukin DJ [15] | 0% | 5.9% | 0.22 | Direct comparison between cases (COVID-19 patients with IBD) and 1:2 age- and gender- matched controls (COVID-19 patients without IBD). |
3.2. Clinical data on Covid-19 in patients with IBD
Coherently with the initial diffusion of Sars-Cov-2, the first reports about Covid-19 in patients with IBD came from China. The IBD Elite Union, which incorporates the seven largest IBD centers in China with more than 20,000 IBD patients, reported no cases of Covid-19 dating March 8, 2020 [6]. Similar data were also obtained from Wuhan, the first zone of outbreak of the pandemic [7]. Three hundred and eighteen patients with IBD were registered in a prospective database at the Regional Medical IBD Center of China, Renmin Hospital of Wuhan University, between January 1, 2000, and December 8, 2019. Between December 8, 2019, and March 30, 2020, these patients were followed-up with intensive alerts, recommendations, and information messages provided with e-mails or phone calls, in order to improve prevention strategies against infection from Sars-CoV-2 and receive promptly diagnosis of Covid-19. As of March 30, none of these patients with IBD had confirmed or suspected Covid-19. The disease was also excluded in 29 patients by chest CT scans and virological testing.
Among the European countries, Italy – and especially the Northern Italy - was firstly assailed by the pandemic. Nonetheless, the first data on Covid-19 in IBD patients were quite reassuring. Norsa and colleagues reported no case of Covid-19 among their IBD cohort, which included 522 patients from the province of Bergamo – one of the area with the highest rate of Sars-CoV-2 infection in Lombardy [8]. Notably, applying a mathematical model, authors speculated that approximately 4% of the total population of the province should have been infected with Sars-CoV-2, so that 21 cases should be expected in their IBD cohort. However, case identification was not performed in all patients, as only those with severe symptoms and/or with established contact with infected patients received a nasopharyngeal swab at the times of the peak of the pandemic, leaving the asymptomatic subjects out of the count and, as a consequence, asymptomatic or mild cases could have been undiagnosed. Another study performed in a combined cohort of approximately 6000 IBD patients from France (Nancy University Hospital) and Italy (Humanitas, Milan) – both regions with high incidence of Covid-19 - reported only 15 cases of Covid-19, accounting for a cumulative incidence of 0.0025%, with no deaths despite all but one patients were treated with biological and/or immunosuppressive therapy [9]. Other relevant findings were obtained by a recent, prospective, observational cohort study initiated and supported by the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) [10]. All centres affiliated with IG-IBD were invited to participate in the study with an open call for participation. Between 11 and 29 March 2020, 79 patients with IBD and Covid-19 were identified. Thirty-six patients had Covid-19-related pneumonia (46%), 22 (28%) were hospitalised, and 6 (8%) died. Interestingly, while active IBD, older age and presence of comorbidities were found to be associated with a higher risk of Covid-19 pneumonia and death, therapy with biologics and immunosuppressants did not associate with worse outcomes, even if a trend towards aworse prognosis was reported for corticosteroids use.
Another European country which was gravely hit by the pandemic was Spain. In this regard, the first data – specifically from the Basque Country – were provided by Rodríguez-Lago and colleagues [11]. From February 27, 2020 to April 8, 2020, 40 patients with IBD and a positive test for SARS-CoV-2 from 5 Basque sites were reported. Notably, 28% and 18% of cases were under immunomodulators or biological therapy, respectively, none of these patients was admitted to intensive care unit, and only 2 died (an 86-year-old male and a 77-year-old male). Another study from the Hospital Clinico San Carlos of Madrid reported - through April 8, 2020 - 12 patients with Covid-19 out of 1918 IBD patients [12]. Interestingly, 7 patients (58%) were on treatment with immunomodulators and/or biologics, and 2 patients died. The authors calculated that the cumulative incidence of Covid-19 was 6.1 per 1000 IBD patients, and that IBD patients had a lower adjusted incidence ratio of Covid-19 (OR 0.74) with similar associated mortality ratio compared with the general population in Madrid.
The first U.S. study that evaluated the prevalence of SARS-CoV-2 infection in IBD patients was performed in a Northern Carolina Cohort [13]. From March 4, 2020 to April 14, 2020, 14,235 individuals were tested for SARS-CoV-2 with 8.2% testing positive. Among the tested patients, the prevalence of IBD was 1.2% (168/14,235), and among these 168 IBD patients, the prevalence of COVID-19 was 3.0% (5/168). Interestingly, only age > 66 years was independently associated with increased risk of COVID-19 at multivariate logistic regression analysis. Four out of these 5 patients had a mild course, whereas one patient developed pneumonia and died. Another retrospective review conducted in New York between March 4, 2020 and April 9, 2020 recorded 17 patients with IBD out of 1059 total patients infected with Sars-CoV-2 [14]. A more recent study by Lukin and colleagues used the same source cohort of all Covid-19-positive patients at two New York hospitals of the previous study [15]. Eighty Covid-19 patients with IBD were matched for decade of age and gender in a 1:2 ratio to 160 Covid-19 patients without IBD. IBD cases more frequently presented with diarrhea, while negative outcomes (composite of death, intensive care unit admission, or intubation) were not significantly different between the two groups. In addition, in a separate longitudinal cohort of 119 active IBD patients, 24 were diagnosed with Covid-19 – a rate consistent with those estimated in the general population of New York. Compared with IBD patients without Covid-19, those with IBD and Covid-19 had a higher rate of clinically or endoscopically active disease, and higher baseline C-reactive protein values and fecal calprotectin. Furthermore, baseline corticosteroid use was higher among Covid-19 patients, while no overall differences were noted about biologic or immunomodulator use. About this latter point, Khan and colleagues performed a retrospective study to evaluate specifically the impact of anti-TNFs and thiopurines on the development of Covid-19 in a nationwide cohort of IBD patients extracted from the Veterans’ Affairs Healthcare System (VAHS) [16]. From January 1, 2020 to May 15, 2020, the authors identified 36 incident Covid-19 cases among 37,857 IBD patients. Interestingly, neither thiopurines nor anti-TNFs were associated with a significant increased risk of Covid-19.
About pediatric patients, several data have shown that Covid-19 has a mild course in children. It is therefore relevant to confirm this trend also in IBD pediatric patients, who generally have a more severe IBD course. In this regard, cumulative data obtained from an electronic reporting system circulated among the 102 centres affiliated with the Paediatric IBD Porto group of ESPGHAN recorded only 8 children with IBD and Covid-19 globally, all with mild infection without hospitalization despite treatment with immunomodulators and/or biologics [17].
Probably, the most relevant data on Covid-19 in IBD came from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) study. SECURE-IBD is an international web-based database (www.covidibd.org) endorsed by several national and international organizations, where physicians are encouraged to report all cases of virological-confirmed Covid-19 occurring in patients with IBD. The first published report [18] collected 525 IBD patients from 33 countries, even if the last report on the website (May 26, 2020) at the time of writing of this manuscript includes 1302 cases. Overall, the case fatality rate was low (3%), while 7% of cases experienced a composite outcome of intensive care unit admission, ventilator support, and/or death. Regarding risk factors for adverse outcomes, some of them were expected - older age and number of comorbidities – while other were related to IBD treatments, namely, the use of systemic corticosteroids and 5-ASA/sulfasalazine. Interestingly, the use of anti-TNFs was not an independent risk factor for more severe Covid-19, as well as the use of other biologics did not seem to be related to worse outcomes.
Finally, 5 case reports of patients with IBD and Covid-19 were published. Rosen and colleagues described a patient with acute severe ulcerative colitis during her first trimester of pregnancy who also had diagnosis of Covid-19 [19]. She was treated with cyclosporine and steroids for ulcerative colitis, and azithromycin and hydroxychloroquine for Covid-19. Unfortunately, the patient experienced a spontaneous abortion. Mazza S et al., described a case of Covid-19 pneumonia occurring in an 80-year-old female with acute severe ulcerative colitis [20]; the patient died despite treatment with systemic steroids plus non-invasive ventilation, and a combination of lopinavir/ritonavir and hydroxychloroquine. Another case report from Italy described a 30-year old male with Crohn's disease and Covid-19, whose pulmonary disease recovered quickly despite the patient was under treatment with adalimumab [21]. Another paper described a man in his late 60 s embarked on the Diamond Princess cruise ship who had been treated with infliximab and azathioprine for ulcerative colitis [22]. He was administered his last infusion of infliximab 4 days before boarding. On the cruise ship, he developed Covid-19, but after hospitalization no findings of pneumonia were observed by chest CT scanning, and his symptoms resolved within a few days without treatment. Another favorable course of Covid-19, with no need for hospitalization, was reported in a 33-year old woman with a 13-year history of ulcerative colitis on tofacitinib when infected with SARS-CoV-2 [23].
4. Discussion
This systematic review aimed at summarizing the current evidence on the prevalence and impact of Covid-19 – the global health crisis of our time - in patients with IBD. Even if the evidence on this topic is rapidly evolving, and despite the limitation related to the eligibility to English-only studies, we believe that this cumulative analysis of clinical data was able to give relevant indications to clinicians dealing with IBD.
Regarding the possibility that patients with IBD may have an increased risk for Covid-19, we know that SARS-CoV-2 is detectable in stools [24], but there is no evidence that the content of ACE2 (i.e. the viral receptor) in the ileum and colon - that is increased by inflammation of IBD [25] - may favor entry of the virus within the intestinal cells (a theoretical transmission by an extra-respiratory route). This could be due to the fact that a soluble form of ACE2 – also increased in patients with IBD [26] - might prevent the binding of the viral receptor to the full-length intestinal ACE2. So, patients with IBD should have no increased risk of developing SARS-CoV-2 infection, as deeply discussed in a recently published paper [27]. Translating these concepts of basic science into clinical data, our review identified a cumulative number of approximately 800 patients with IBD and Covid-19. A precise number cannot be provided, as overlap of patients between the different studies or databases may be hypothesized. Anyway, this number is relatively small even taking into account an unavoidable certain degree of under-reporting. For example, considering the very high prevalence of SARS-COV-2 infection in Italy and USA, we would expect higher prevalence or incidence than those reported by several studies. Overall, these findings confirm that patients with IBD are not at higher risk of being infected by SARS-COV-2 than the general population.
The second hot question regards the theoretical risk of developing severe forms of Covid-19 in patients with IBD on immunomodulators or biologics. Indeed, several drugs used for the treatment of IBD can promote the occurrence of infections due to the interference of these compounds on the mechanisms of signaling of the immune system [28]. Data arising from our review are reassuring. Overall, the case-fatality rate among the studies was not higher than that reported in the general population. Furthermore, the negative outcomes were associated to older age, presence of comorbidities, and male sex – all risk factors which had already been found in the general population [29] – but not with immunosuppressants or biologics. Of note, the overall effect estimate of anti-TNFs in the SECURE-IBD was 0.9 (even if not significant): this finding may suggest that patients with IBD on immunomodulatory treatments – particularly those who directly interfere with cytokines action and production - may be even protected against the severe forms of Covid-19 [30], as they could interfere with key points of the “cytokine storm” syndrome, the pathophysiological mechanism driving the severe forms of Covid-19 [31]. Obviously, these latter considerations need confirmation in large-scale clinical studies. Conversely, an association with worse outcomes was found for the use of systemic steroids, which was associated with negative outcomes in the SECURE-IBD, in the study by Lukin and colleagues, and in the IG-IBD study (even if only with a trend towards statistical significance). However, it should be noted that the effects of continuing systemic steroids or stopping them after Covid-19 diagnosis in IBD patients are still unknown, so more data are needed to clarify this issue. Similarly, the association between worse outcomes and use of 5-ASA/sulfasalazine highlighted by SECURE-IBD is currently unclear and deserves further confirmation.
In conclusion, this systematic review highlighted two main points that may help clinicians dealing with IBD in reassuring their patients: (1) patients with IBD do not seem to be at higher risk of being infected by SARS-COV-2 than the general population; (2) in case of Covid-19, treatment with immunomodulators or biologics is not associated with worse prognosis, while systemic steroids are suspected to be potentially detrimental, even if more data are needed to confirm this issue. This latter point should be kept in mind for the management of patients with IBD, particularly in case of the hypothesized second peak of the pandemic that may occur in the next autumn.
Funding
None.
Disclosures
Fabio Salvatore Macaluso served as an advisory board member and/or received lecture grants from AbbVie, Biogen, MSD, Pfizer, Samsung Bioepis, and Takeda Pharmaceuticals. AAmbrogio Orlando served as an advisory board member for AbbVie, MSD, Janssen, Pfizer, Takeda Pharmaceuticals, and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer, and Takeda Pharmaceuticals.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Symptoms of coronavirus disease2019 (COVID–19). 2020 (https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html).
- 3.World Health Organization. Rolling updates on coronavirus disease (COVID-19). 2020 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen).
- 4.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report — 132. May 31, 2020. (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf?sfvrsn=d9c2eaef_2).
- 5.Stroup D.F., Berlin J.A., Morton S.C., et al. for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 6.Mao R., Liang J., Shen J., et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425‐427. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An P., Ji M., Ren H., et al. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5:525‐527. doi: 10.1016/S2468-1253(20)30121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norsa L., Indriolo A., Sansotta N., et al. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.062. S0016-5085(20)30445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allocca M., Fiorino G., Zallot C., et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the nancy and milan cohorts. Clin Gastroenterol Hepatol. 2020;18(9):2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezzio C., Saibeni S., Variola A., et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020 doi: 10.1136/gutjnl-2020-321411. gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Lago I., Ramírez de la Piscina P., Elorza A., et al. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain) Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.043. S0016-5085(20)30560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taxonera C., Sagastagoitia I., Alba C., et al. 2019 Novel Coronavirus Disease (COVID-19) in patients with Inflammatory Bowel Diseases. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15804. 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubatan J., Levitte S., Balabanis T., et al. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.009. S0016-5085(20)30601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajifathalian K., Krisko T., Mehta A., et al. Gastrointestinal and hepatic manifestations of 2019 Novel Coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.010. S0016-5085(20)30602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukin D.J., Kumar A., Hajifathalian K., et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan N., Patel D., Xie D., et al. Impact of Anti-TNF and Thiopurines medications on the development of COVID-19 in patients with inflammatory bowel disease: a Nationwide VA cohort study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner D., Huang Y., Martín-de-Carpi J., et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70:727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry Gastroenterology. 2020; doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed]
- 19.Rosen M.H., Axelrad J., Hudesman D., et al. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis. 2020 doi: 10.1093/ibd/izaa109. izaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazza S., Sorce A., Peyvandi F., et al. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69:1148–1149. doi: 10.1136/gutjnl-2020-321183. [DOI] [PubMed] [Google Scholar]
- 21.Tursi A., Angarano G., Monno L., et al. Covid-19 infection in Crohn's disease under treatment with adalimumab. Gut. 2020 doi: 10.1136/gutjnl-2020-321240. gutjnl-2020-321240. [DOI] [PubMed] [Google Scholar]
- 22.Kunisaki R., Tsukiji J., Kudo M. Potential inhibition of COVID-19-driven pneumonia by immunosuppressive therapy and anti-TNFα antibodies: a case report. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa105. jjaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs J., Clark-Snustad K., Lee S. Case report of a SARS-CoV-2 Infection in a patient with ulcerative colitis on tofacitinib. Inflamm Bowel Dis. 2020 doi: 10.1093/ibd/izaa093. izaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao F., Tang M., Zheng X., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg M., Royce S.G., Tikellis C., et al. Imbalance of the renin–angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2019 doi: 10.1136/gutjnl-2019-318512. [DOI] [PubMed] [Google Scholar]
- 26.Garg M., Burrell L.M., Velkoska E., et al. Upregulation of circulating components of the alternative renin–angiotensin system in inflammatory bowel disease: a pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16:559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- 27.Monteleone G., Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzola G., Macaluso F.S., Adamoli L., et al. Diagnostic and vaccine strategies to prevent infections in patients with inflammatory bowel disease. J Infect. 2017;74:433–441. doi: 10.1016/j.jinf.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Onder G., Rezza G., Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 30.Macaluso F.S., Orlando A. Could patients with inflammatory bowel disease treated with immunomodulators or biologics be at lower risk for severe forms of Covid-19? Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.026. S0016-5085(20)30619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C., Zhang X.R., Ju Z.Y., et al. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]