Abstract
An outbreak of SARS-CoV-2 in a skilled nursing facility (SNF) can be devastating for residents and staff. Difficulty identifying asymptomatic and presymptomatic cases and lack of vaccination or treatment options make management challenging. We created, implemented, and now present a guide to rapidly deploy point-prevalence testing and 3-tiered cohorting in an SNF to mitigate an outbreak. We outline key challenges to SNF cohorting.
Keywords: Cohorting, COVID-19, SARS-CoV-2, long-term care, point prevalence, asymptomatic carriers
Problem/Significance
Post-acute and long-term care skilled nursing facilities (SNFs) are known to be high-risk environments for outbreaks including multidrug-resistant organisms (MDRO) and respiratory viruses.1 , 2 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to multiple outbreaks in SNFs.3, 4, 5, 6 In the absence of a vaccine or treatment, the primary resources available to SNFs to control the spread of SARS-CoV-2, have included appropriate personal protective equipment (PPE), hand hygiene, and cohorting of residents. Outbreak investigations of SARS-CoV-2 at other SNFs demonstrated that presymptomatic as well as symptomatic spread likely contributed to rapid propagation of outbreaks and that use of only symptom-based testing and cohorting was insufficient.3, 4, 5, 6 To account for the risk of presymptomatic and asymptomatic spread, we propose a 3-tier cohorting approach to control transmission. This differs from MDROs where only 2-tier cohorts are required or influenza that has additional mitigation strategies.7, 8, 9, 10 We coupled the efficacy of prevalence testing in an SNF11 with the development of 3 resident cohorts: positive (red), negative-cleared (green), and negative-exposed (yellow). In this article, we demonstrate that this strategy can be used to reduce horizontal transmission in an SNF.
Innovation
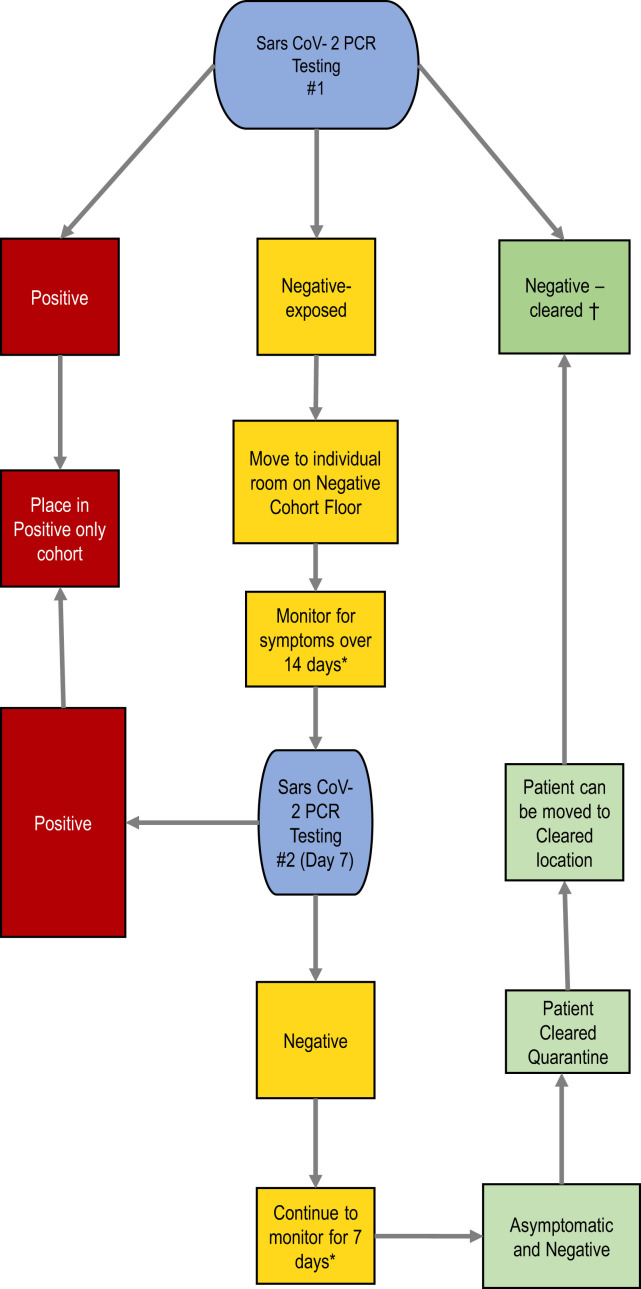
This report summarizes our experience implementing facility-wide point-prevalence testing and 3-tiered cohorting during an SNF outbreak of SARS-CoV-2. Our approach leveraged a partnership between an SNF and an academic medical center with microbiological capabilities, infection-control specialists, and geriatricians to implement mass point-prevalence testing twice at the SNF. We used a stepwise guide to direct the 3-tier cohorting based on the results of point-prevalence testing administered at the onset of the outbreak and individual exposure risk (Figure 1 ). The strategy resulted in 3 separate cohort groups: positive (red), negative-cleared (green), and negative-exposed (yellow). This protocol can be used at SNFs experiencing an outbreak.
Fig. 1.
Algorithm for testing and cohorting.∗If patient develops symptoms, retest with nasopharyngeal (NP) PCR swab. †During ongoing outbreak, 14-day quarantine recommended before negative-cleared unit. A downloadable PDF of this form is available at www.sciencedirect.com.
Implementation
This study was granted institutional review board and quality improvement approval. Our university-employed geriatricians provide clinical care for an urban SNF with >200 beds. The SNF is a mix of post-acute and long-term care. It has 4 floors, including 1 memory-care unit floor.
Testing
We instituted point-prevalence testing of all residents at the SNF in mid-April 2020.
Test labels were preprinted and ordering of test kits was completed in partnership with the academic medical center laboratory. SNF residents were classified as “unexposed” or “exposed.” We defined “exposed” as per current Centers for Disease Control and Prevention guidance.12 At the time, given uncertainty of exposure during an ongoing outbreak we initially assumed all residents would have had interaction that met criteria. For the initial point-prevalence testing, all residents were assumed to be exposed given the ongoing facility and community outbreak. A team of 10 university medical center nurses, combined with SNF staff who were trained in rapid sample collection by nasal swab, completed sampling on 120 residents in 2 hours. Samples were delivered to the university laboratory via courier. Testing of samples was performed with SARS-CoV-2 nucleic acid detection 21 reverse-transcriptase polymerase chain reaction (RT-PCR) (Roche, Basel, Switzerland). A second sampling was performed 1 week later by the staff at the SNF on those residents who were negative after initial point prevalence. Residents were then subsequently re-cohorted (Figure 1). In addition to point-prevalence evaluation, residents who developed symptoms between point-prevalence testing were retested and cohorted as appropriate.
Cohorting
Based on the initial point-prevalence results, 2 cohorts were established. Two floors were designated COVID-19–positive floors (red) where sharing rooms was permitted. One floor was designated COVID-19 negative-exposed, and residents were placed in their own room given ongoing community transmission, potential false negative result, and risk of conversion. The memory floor was separated into 2 units by storm doors dividing positive (red) and negative-exposed (yellow) cohorts, as off-floor relocation was believed to cause more harm than benefit given the care needs of residents with dementia. At the end of 14 days and following the second point-prevalence at 1 week, the third cohort was established. Residents with 2 negative tests or a negative initial test plus 14 symptom-free days were moved to the negative-cleared (green) location.
In addition to the resident relocation strategy, we implemented broad infection-control strategies; the optimal recommendations are outlined in Table 1 . In some cases, the infection-control strategies were cohort-specific (eg, individual rooms). In other cases, the infection-control strategies were universally applied. For example, all residents in the facility were placed on droplet precautions (surgical facemask) and contact precautions (gown and gloves). Staff were assigned to the same unit as best possible during all shifts.
Table 1.
Categories of Resident Cohorting
| Category | Description | Infection Control |
|---|---|---|
| Positive (Red) |
|
|
| Negative- exposed (yellow) |
|
|
| Negative-cleared (green) |
|
|
CDC, Centers for Disease Control and Prevention.
Evaluation
Our initial point-prevalence testing (n = 120) identified 43 negative-exposed residents and 77 positive residents. Of those residents who were negative, repeat point-prevalence testing performed 1 week later revealed 12 residents had converted to SARS-CoV-2 positive. Ten (83%) of the 12 residents who converted after repeat point-prevalence testing were on the memory-care unit. There were only 2 residents from the negative-exposed cohort (yellow) floor that converted from negative to positive on the repeat prevalence testing. The 29 remaining negative-exposed on repeat testing were then considered negative-cleared (green).
Comment
Our evaluation supports previous outbreak investigation studies that demonstrated asymptomatic individuals with positive tests.8 This may be even more relevant in the resident population in SNFs, which includes older residents who may exhibit more subtle signs of infection.
It is also noteworthy that 83% (10/12) of the SNF residents who converted to a positive test result on the second point-prevalence testing were on the memory-care floor. These findings suggest that the 3-tiered cohorting approach separating exposed from unexposed negative-test individuals is crucial to stop horizontal transmission among older adults with dementia. Only 2 residents on the exposed-negative floor converted to a positive test at the second point-prevalence testing; we anticipate more residents might have converted had they not been cohorted and placed in individual rooms. Individual rooms for all patients may not be possible at an SNF, and therefore prioritizing the isolation of exposed individuals is essential to mitigating and preventing spread of an outbreak. Memory-care units present additional inherent challenges, including the difficulties in instructing residents with dementia on appropriate PPE usage7 and minimization of mobilization outside of the personal room. Separating residents into smaller pods or increasing the staffing ratios on the memory unit may be potential strategies to decrease transmission when strict cohorting is not feasible.
Limitations to this innovation include that this protocol has only been piloted at a single institution to date. Although we were able to complete 2 point-prevalence testing days, we had rapid testing with 24-hour turn-around available making this feasible. The sustainability of and need for continued prevalence testing has not been evaluated. Finally, although residents were separated and placed on precautions, given PPE shortages, extended use of PPE was practiced and staff compliance to PPE was not evaluated. We did not perform universal testing of staff at the time of our point prevalence, but this may be an additional way to prevent asymptomatic transmission from staff. A cohorting approach that categorizes individuals based on testing results, as well as exposure risk, is essential to halting an ongoing outbreak. Additional strategies for specific populations, like memory-care residents, need to continue to be refined.
The pragmatic innovation described in this article may need to be modified for use by others; in addition, strong evidence does not yet exist regarding efficacy or effectiveness. Therefore, successful implementation and outcomes cannot be assured. When necessary, administrative and legal review conducted with due diligence may be appropriate before implementing a pragmatic innovation.
Acknowledgments
We thank the University of Chicago microbiology laboratory and testing team.
Footnotes
The authors declare no conflicts of interest.
This publication was made possible in part by Grant U1QHP28728 from the Health Resources and Services Administration, an operating division of the U.S. Department of Health and Human Services and Grant T1MHP39062.
References
- 1.Smith P.W., Bennett G., Bradley S. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control. 2008;36:504–535. doi: 10.1016/j.ajic.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Preparing for COVID-19 in Nursing Homes. https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care-strategies.html Available at:
- 3.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a long- term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimball A., Hatfield K.M., Arons M. Asymptomatic and presymptomatic SARS- CoV-2 infections in residents of a long-term care skilled nursing facility — King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M.C., Chaisson L.H., Borgetti S. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020 Jun 16 doi: 10.1093/cid/ciaa763. [Epub ahead of print] [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg S.A., Pu C.T., Thompson R.W. Asymptomatic spread of COVID-19 in 97 patients at a skilled nursing facility. J Am Med Dir Assoc. 2020;21:980–981. doi: 10.1016/j.jamda.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz-Price L.S., Quinn J.P. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2013;26:378–387. doi: 10.1097/01.qco.0000431853.71500.77. [DOI] [PubMed] [Google Scholar]
- 8.Spires S.S., Talbot H.K., Pope C.A., Talbot T.R. Paramyxovirus outbreak in a long-term care facility: The challenges of implementing infection control practices in a congregate setting. Infect Control Hosp Epidemiol. 2017;38:399–404. doi: 10.1017/ice.2016.316. [DOI] [PubMed] [Google Scholar]
- 9.Uršič T., Miksić N.G., Lusa L. Viral respiratory infections in a nursing home: A six-month prospective study. BMC Infect Dis. 2016;16:637. doi: 10.1186/s12879-016-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansbury L.E., Brown C.S., Nguyen-Van-Tam J.S. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11:356–366. doi: 10.1111/irv.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakaev I., Retalic T., Chen H. Universal testing-based response to COVID-19 outbreak by a long-term care and post-acute care facility. J Am Geriatr Soc. 2020;68:E38–E39. doi: 10.1111/jgs.16653. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Public health guidance for community-related exposure. www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html Available at: