Abstract
Purpose:
Radium 223 dichloride (radium-223) is an alpha particle–emitting bone-directed therapy that prolongs overall survival in men with bone-predominant metastatic castration-resistant prostate cancer (mCRPC). Docetaxel is an antimicrotubule cytotoxic agent that improves survival in mCRPC. We investigated whether combining these potentially cross-sensitising agents to dually target tumour and bone would be safe and effective.
Patients and methods:
Phase 1 was a dose escalation study to define a recommended phase 2 dose (RP2D) of docetaxel and radium-223. In phase 2a, patients were randomised 2:1 to the recommended combination regimen or docetaxel at a dose of 75 mg/m2 every 3 weeks (q3w). Patients with bone-predominant mCRPC were eligible. End-points were safety, efficacy and treatment-related changes in serum and imaging biomarkers.
Results:
Twenty patients were enrolled in phase 1; 53 patients were randomised in phase 2a: 36 to combination treatment and 17 to docetaxel alone. The RP2D for the combination was radium-223 55 kBq/kg every six weeks × 5 doses, plus docetaxel 60 mg/m2 q3w × 10 doses. Febrile neutropenia was dose limiting. A higher rate of febrile neutropenia was seen in the docetaxel monotherapy arm (15% vs 0%); the safety profile of the treatment groups was otherwise similar. The combination arm had more durable suppression of prostate-specific antigen (median time to progression, 6.6 vs 4.8 months, respectively), alkaline phosphatase (9 vs 7 months) and osteoblastic bone deposition markers.
Conclusions:
Radium-223 in combination with docetaxel at the RP2D was well tolerated. Exploratory efficacy data suggested enhanced antitumour activity for the combination relative to docetaxel alone. Comparative studies with end-points of clinical benefit are warranted. ClinicalTrials.gov number: NCT01106352.
Keywords: Castration-resistant prostate cancer, Radium 223 dichloride, Docetaxel, Combination treatment
1. Introduction
Prostate cancer is bone-tropic, rendering it particularly susceptible to treatments that target bone formation and osteoblastic activity. The cancer-induced abnormal bone metabolism that places patients at risk of death and morbidity can also be leveraged to deliver life-prolonging therapy.
Radium 223 dichloride (radium-223), a calcium mimetic alpha particle–emitting radiopharmaceutical, targets hydroxyapatite. It selectively accumulates in areas of increased bone turnover that surround metastatic lesions, where it emits four high-energy, short-range (<100 μm) alpha particles with resulting minimal radiation effects on the adjacent bone marrow [1,2]. In preclinical models, it reduces abnormal bone production, tumour burden and dysregulated bone deposition [3,4]. Clinically, radium-223—given at a dose of 55 kBq/kg every 4 weeks for 6 doses—prolongs life and the time to first symptomatic skeletal event in patients with bone-predominant metastatic castration-resistant prostate cancer (mCRPC) and no known visceral metastases [5].
Docetaxel is a chemotherapeutic agent that interferes with microtubule dynamics and has a radiosensitising effect [6]. Docetaxel given at a dose of 75 mg/m2 every 3 weeks (q3w) in combination with prednisone prolongs life in patients with mCRPC [7].
We hypothesised that combining bone-targeted alpha radiation therapy with chemotherapy in patients with mCRPC might be an effective treatment approach, predicated on the concepts of multicompartment targeting and possible cross-sensitisation in bone lesions [8]. We conducted a phase 1/2a study to investigate this combination.
2. Patients and methods
2.1. Patients
Eligible patients had progressive mCRPC with ≥2 bone metastases, testosterone ≤50 ng/dL, Karnofsky Performance Status of ≥70%, life expectancy of ≥6 months and adequate organ functionality (white blood cell count ≥3 × 109/L, with an absolute neutrophil count ≥1.5 × 109/L, a platelet count ≥100 × 109/L and haemoglobin ≥10.0 g/dL; total bilirubin level ≤ upper limit of normal (ULN) and aspartate aminotransferase and alanine aminotransferase concentrations ≤1.5 × ULN; creatinine ≤1.5 × ULN and albumin >30 g/L). The patients-needed to have had two consecutive prostate-specific antigen (PSA) increases at least one week apart, with a minimum value of 2 ng/mL at screening, or two or more new bone lesions when analysed by bone scintigraphy. Those patients on a first-generation androgen inhibitor needed to progress through a 4-week withdrawal. The exclusion criteria included the following: visceral metastases, defined as >2 lung metastases and/or liver metastases that were ≥2 cm in size, symptomatic nodal disease and malignant lymphadenopathy >3 cm in short-axis diameter. Patients should not have received >10 previous docetaxel doses or previous treatment with a bone-seeking radiopharmaceutical.
2.2. Study design
This two-part phase 1/phase 2a study, conducted at eight centres, seven in the United States and one in France, aimed to establish a recommended phase 2 dose (RP2D) of radium-223 in combination with docetaxel and to investigate safety and exploratory efficacy end-points at the RP2D.
In phase 1, between 9 and 18 patients were to be enrolled and treated according to a 3 + 3 design. The dose escalation scheme is shown in Fig. 1A. Dose-limiting toxicity (DLT) was assessed during the 6-week period after the first radium-223 injection. DLT was defined as absolute neutrophil count <0.5 × 109/L for >7 days without fever despite granulocyte-colony stimulating factor support, grade ≥3 febrile neutropenia (after a protocol amendment), platelet count <25 × 109/L for >7 days, grade ≥3 diarrhoea despite optimal medical management, grade ≥4 vomiting or constipation.
Fig. 1.
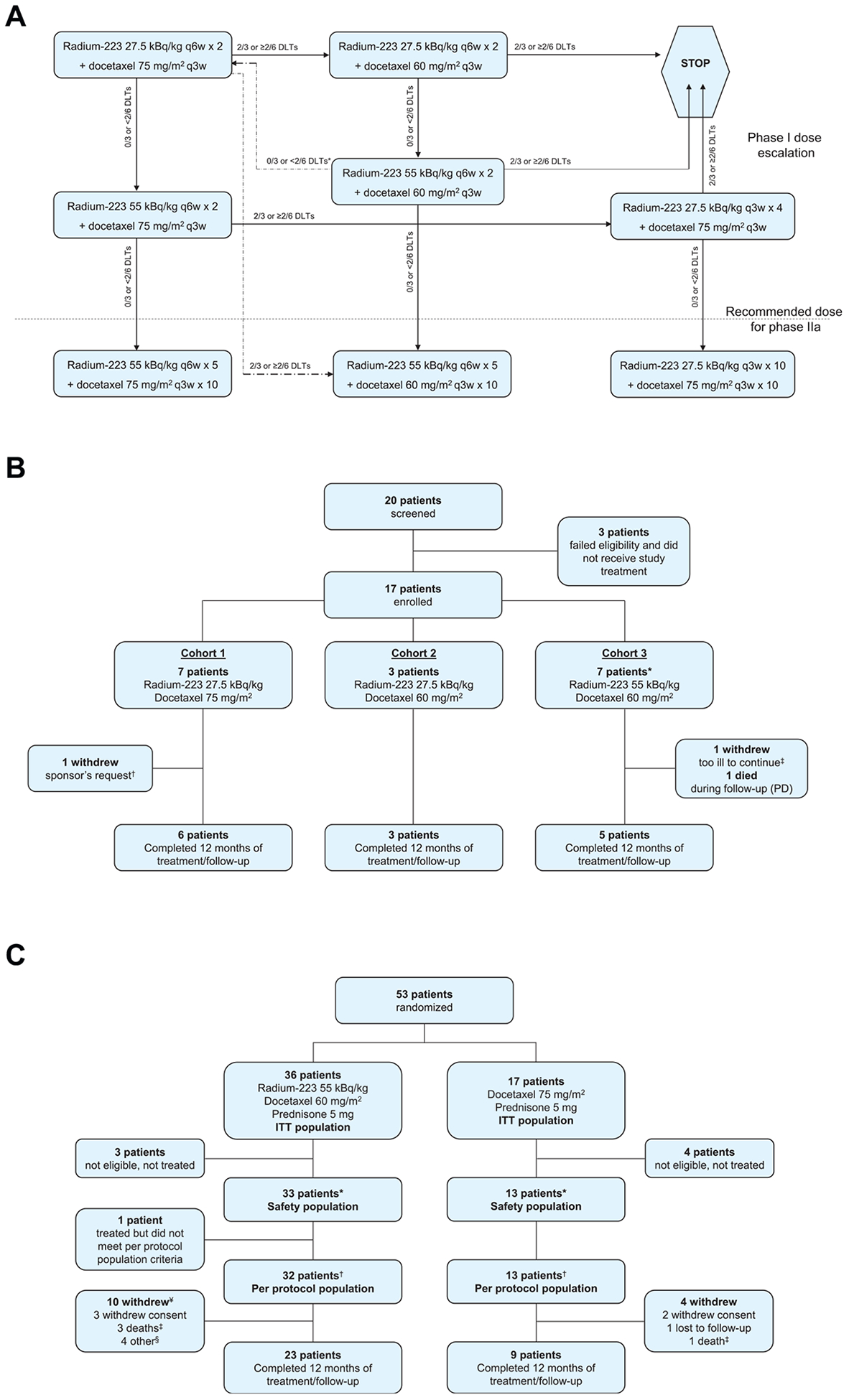
Study profile. (A) Dose escalation scheme.*A return to the very first dose cohort could be considered in the event of 0/3 or <2/6 DLTs at 55 kBq/kg radium-223 + 60 mg/m2 docetaxel q3w. If then 2/3 or ≥2/6 DLTs occurred at docetaxel 75 mg/m2, the chosen regimen for the phase 2a cohort was to be radium-223 50 kBq/kg × 5 + docetaxel 60 mg/m2 q3w × 10. (B) Phase 1 dose escalation cohorts. *One patient was replaced, unable to receive both combined doses of radium-223 and docetaxel because of docetaxel hypersensitivity. †Withdrew before receiving both doses of radium-223 to receive another treatment deemed necessary by the study sponsor. ‡Withdrew after receiving both doses of radium-223, too ill to attend the 12-month follow-up visit. (C) Phase 2a safety and efficacy cohort.*25 patients in the combination arm received all planned radium-223 doses, 20 patients in the combination arm and 5 patients in the docetaxel arm received all planned docetaxel doses; the dose for 4 patients in the docetaxel arm was stepped down to 60 mg/m2. The study was completed through 12 months of follow-up from the start of treatment with 23 (70%) patients in the combination arm and 9 (69%) in the docetaxel arm. †Received at least 40% of drug dose, no protocol violation. ¥Including the one patient who was excluded from the per protocol population. †All deaths occurred during follow-up and were due to disease progression. §3 patients entered hospice, and 1 had disease progression. PD, progressive disease; DLT, dose-limiting toxicity; ITT, intention to treat; q3w, every 3 weeks; q6w, every 6 weeks.
Radium-223 was started at a dose of 27.5 kBq/kg (according to the National Institute of Standards and Technology [NIST] 2016 update [9]), every six weeks (q6w), and could be escalated to 55 kBq/kg (according to NIST 2016 update [9]). The starting dose of docetaxel was 75 mg/m2 q3w, with a planned reduction to 60 mg/m2 in the event of DLT. We prioritised achieving full-dose radium-223 over full-dose chemotherapy in the dose escalation scheme, given that there are survival data using docetaxel as part of combination therapy at its step-down dose but no survival data using a lower dose of radium-223 [10]. Radium-223 was administered every other chemotherapy dose rather than monthly to optimise the likelihood of patient acceptance and compliance by having only one day of treatment per cycle, at a dosing interval known to have favourable clinical effects [11]. The number of doses was capped at five in an abundance of caution to protect long-term marrow integrity in the event of enhanced toxicity that would not be detected by blood count assessments during treatment. In all cohorts, docetaxel was to be administered every 3 weeks and was to be continued in the absence of progressive disease or unacceptable toxicity. Docetaxel and radium-223 were administered on the same day, with docetaxel administered first, followed by radium-223 as soon as practically feasible. Prednisone 5 mg was given orally twice daily, continuously. Dexamethasone premedication was given before docetaxel dosing as per each institution’s practice. Growth factor support was allowed only as secondary prophylaxis.
In phase 2a, using a schedule generated by an independent statistician, patients were randomly assigned centrally 2:1, using a block randomisation scheme (block size of three), via an interactive voice response system, to combination therapy or docetaxel alone, respectively. A preplanned early stopping rule applied in the event of significant toxicity in the combination arm. The treatment period was a maximum of 30 weeks (10 doses of docetaxel), followed by 22 weeks of follow-up.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation E6, Good Clinical Practice. The protocol and all amendments were approved by the independent ethics committee/institutional review boards at each site, and written informed consent was obtained from the patients before any assessments were performed.
2.3. Assessments
Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities version 13.0. Severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. The safety assessment period for AEs was from the start of study treatment to 6 weeks after the end of study treatment (8 weeks for serious AEs [SAEs]). Data on marrow sequelae and any second malignancies were collected up to 12 months after the start of study treatment. Exploratory efficacy assessments included on-treatment changes in bone alkaline phosphatase (bALP), total ALP (tALP), uri-nary C-telopeptide of type 1 (uCTX-1), N-terminal propeptide of procollagen type 1 (P1NP), pyridinoline cross-linked carboxyterminal telopeptide (ICTP), PSA and circulating tumour cells (CTCs).
2.4. Statistical considerations
The primary objectives were to establish a recommended dose of radium-223 combined with docetaxel and to investigate safety and explore efficacy at this dose level. The safety population included all patients who received treatment. To examine the antitumour effect of treatment in this exploratory study, the efficacy population comprised patients who received ≥40% (2 infusions) of the specified number of radium-223 doses (combination arm) or docetaxel doses (docetaxel arm) and had no major protocol violations (per protocol population). No formal statistical testing was planned.
Exploratory efficacy end-points included time to PSA progression, time to bALP progression, time to tALP progression, time to first radiographic or clinical progression based on Response Evaluation Criteria in Solid Tumours (RECIST) [12] version 1.1 and Prostate Cancer Working Group 2 (PCWG2) [13] definitions and overall survival. Time-to-event end-points were measured from the first dose of study treatment. For this report, progression-free survival (PFS) events are defined as radiographic or clinical progression or death. Medians for time-to-event variables were estimated using the Kaplan–Meier method. Changes in biomarkers over time were computed as the area under the bone marker curve. Based on the Lehmann alternative power function for a two-sided 0.05-level test, the planned 42 patients were to be randomised. Assuming for a given marker that the odds were 3:1 that a patient in the combination group had a greater area under the bone turnover curve relative to a patient in the docetaxel group, the power of the test was 0.78. P values for exploratory efficacy end-points have not been corrected for multiplicity of testing and are provided for information only.
3. Results
3.1. Phase 1 dose escalation
Seventeen patients were treated in the phase 1 dose escalation cohort, including three with visceral disease; patient disposition and baseline characteristics are summarised in Fig. 1B and Supplementry Table 1. No DLTs occurred among the first three patients treated at full-dose chemotherapy and half-dose radium-223 (27.5 kBq/kg), but two developed febrile neutropenia, which was not then specified as a DLT. The cohort was expanded to six patients; no DLTs or additional febrile neutropenia events were seen. Owing to febrile neutropenia in two of six patients, the docetaxel dose was reduced to 60 mg/m2 in the second cohort, which also used radium-223 at a dose of 27.5 kBq/kg. No DLTs were seen in the first three patients enrolled in this cohort. Because it appeared that the docetaxel dose at 75 mg/m2 was accounting for the neutropenic fevers, the radium-223 dose was escalated to 55 kBq/kg in the third cohort, holding the docetaxel dose at 60 mg/m2. No DLTs were seen in the first three patients enrolled at this dose level. However, one patient developed grade 3 neutropenia and another developed grade 4 neutropenia, both without fever or infection. After reviewing the safety data, it was decided to add three more patients to this cohort. No DLTs occurred in these patients. The third cohort dose (55 kBq/kg radium q6w × 5 and 60 mg/m2 docetaxel q3w × 10) was consequently selected as the RP2D to be administered over 30 weeks. Haematological treatment-emergent AEs (TEAEs) occurring in phase 1 are shown in Supplementary Table 2.
3.2. Phase 2a cohort
3.2.1. Patients and treatment
Between December 19, 2012, and April 7, 2014, 53 patients were randomly assigned to receive combination therapy with docetaxel 60 mg/m2 and radium-223 55 kBq/kg q6w × 5 (n = 36) or docetaxel alone, at a standard dose of 75 mg/m2 q3w × 10 (n = 17, Fig. 1C, Supplementary Fig. 1); seven patients were found not to be eligible and were not treated. Baseline characteristics were similar between treatment groups (Table 1). Seven (15%) of 46 eligible patients had visceral metastases at baseline, five in the combination arm and two in the docetaxel arm.
Table 1.
Baseline characteristics (phase 2a cohort).
| Characteristic | Radium-223 + docetaxel N = 33 | Docetaxel N = 13 |
|---|---|---|
| Age, median (range), years | 68 (49–82) | 67 (55–82) |
| Weight, median (range), kg | 87 (61–120) | 78 (69–132) |
| Karnofsky Performance Status, median %, (range) | 90 (70–100) | 90 (70–100) |
| Albumin, median, g/L | 43.0 | 43.0 |
| Haemoglobin, median, g/L | 122.0 | 121.0 |
| PSA | ||
| >ULN, N (%) | 32 (97) | 13 (100) |
| Median (range), |μg/L | 99 (3–1000) | 43 (4–1042) |
| Total ALP | ||
| >ULN, N (%) | 20 (61) | 10 (77) |
| Median (range), U/L | 167 (62–1016) | 186 (74–472) |
| Bone ALP | ||
| >ULN, N (%) | 23 (70) | 11 (85) |
| Median (range), μg/L | 36 (10–331) | 47 (16–164) |
| LDH | ||
| >ULN, N (%) | 6 (18) | 2 (15) |
| Median (range), U/L | 191 (123–418) | 190 (124–328) |
| Patients with visceral metastatic lesions, N (%) | ||
| Any | 5 (15) | 2 (15) |
| Lung | 1 (3) | 1 (8) |
| Liver | 0 | 0 |
| Other | 4a (12) | lb (8) |
| Extent of disease (number of bone lesions), N (%) | ||
| 2–4 | 4 (12) | 0 |
| 5–9 | 7 (21) | 3 (23) |
| 10–20 | 9 (27) | 4 (31) |
| >20 | 13 (39) | 6 (46) |
| Time since initial diagnosis, median (range), months | 73 (7–292) | 45 (12–274) |
| Time since bone metastases, median (range), months | 23 (1–58) | 10 (0–92) |
| Prior anticancer therapies, N (%) | ||
| Hormonal therapies | ||
| Abiraterone + prednisone | 25 (76) | 8 (62) |
| Enzalutamide | 3 (9) | 5 (38) |
| Chemotherapy | ||
| Docetaxel | 2 (6) | 0 |
| Immunostimulants | ||
| Sipuleucel-T | 6 (18) | 4 (31) |
| Bone-modifying agents, N (%) | ||
| Bisphospnonates | 13 (39) | 5 (38) |
| Denosumab | 12 (36) | 3 (23) |
| Other, N (%) | ||
| Radiation | 24 (73) | 9 (69) |
ALP, alkaline phosphatase; LDH, lactate dehydrogenase; PSA, prostate-specific antigen; ULN, upper limit of normal.
Adrenal (2 patients), pleura, pancreas.
Adrenal.
3.2.2. Treatment exposure
The patients in the combination arm received a cumulative median of 1187 mg of docetaxel (range, 250–1520), versus 1270 mg (range, 643–1600) in the docetaxel monotherapy arm. The median number of docetaxel doses was 10 (range, 2–11) in the combination arm and 9 (range, 4–10) in the monotherapy arm. The median number of radium-223 doses in the combination arm was 5 (range, 1–5).
In the combination therapy arm, radium-223 and docetaxel administration was delayed in two patients because of TEAEs (cellulitis and osteoporosis), with docetaxel administration delayed in a further five patients (because of back pain, pain in extremity; oral abscess; pneumonia; toothache; diarrhoea, dehydration, pleural effusion, acute respiratory failure and pneumonia). There were three dose delays because of TEAEs in the docetaxel arm (hypotension; influenza-like illness, cough and melaena; cellulitis). In the combination arm, radium-223 and docetaxel were discontinued in 4 of 33 (12%) patients because of TEAEs (unilateral blindness; cerebrovascular accident; pneumonitis; asthenia and back pain), and docetaxel was discontinued in a further two (6%) patients (peripheral neuropathy; asthenia). In the docetaxel arm, 3 of 13 (23%) patients discontinued treatment because of TEAEs (febrile neutropenia; interstitial lung disease; peripheral neuropathy).
3.2.3. Safety
TEAE and TESAE incidence in the phase 2a safety population is summarised in Table 2 and Supplementary Table 3. Notably, there was less toxicity of any grade seen with combination therapy than docetaxel alone for neutropenia, febrile neutropenia, fatigue, dyspnoea, arthralgia and nausea. However, combination therapy was associated with more diarrhoea and back pain. The incidence of grade 3 or 4 TEAEs was low in both arms (Table 2), with the exception of neutropenia. Febrile neutropenia occurred in two patients (one grade 3 and one grade 4) in the docetaxel arm and none in the combination arm; growth factors were used to prevent or resolve neutropenia in four patients in the combination arm and two patients in the docetaxel arm. There were no TEAEs of thrombocytopenia reported in either arm during the treatment period, and median platelet laboratory values were similar for both treatment groups between baseline and day 8 (Supplementary Fig. 2). There were no grade 5 TEAEs. No fractures were observed.
Table 2.
TEAEs in the phase 2a treatment period (any grade and grade 3 or 4): safety population.
| TEAE | Any grade | Grade 3 or 4 | ||
|---|---|---|---|---|
| Radium-223 + Docetaxel N = 33 | Docetaxel N = 13 | Radium-223 + docetaxel TV = 33 | Docetaxel N = 13 | |
| Any | 33 (100) | 13 (100) | 16 (48) | 8 (62) |
| Haematologicala | ||||
| Neutropenia | 10 (30) | 5 (38) | 10 (30) | 5 (38) |
| Anaemia | 3 (9) | 1 (8) | 1 (3) | 0 |
| Leucopenia | 2 (6) | 2 (15) | 2 (6) | 2 (15) |
| Lymphopenia | 1 (3) | 0 | 1 (3) | 0 |
| Febrile neutropenia | 0 | 2 (15) | 0 | 2 (15) |
| Non-haematologicalb | ||||
| Fatigue | 17 (52) | 9 (69) | 0 | 0 |
| Nausea | 16 (48) | 8 (62) | 0 | 0 |
| Diarrhoea | 15 (45) | 5 (38) | 1 (3) | 0 |
| Back pain | 13 (39) | 4 (31) | 2 (6) | 0 |
| Alopecia | 12 (36) | 7 (54) | 0 | 0 |
| Peripheral oedema | 12 (36) | 5 (38) | 0 | 1 (8) |
| Constipation | 11 (33) | 5 (38) | 0 | 0 |
| Decreased appetite | 11 (33) | 4 (31) | 0 | 0 |
| Peripheral neuropathy | 10 (30) | 4 (31) | 0 | 0 |
| Dysgeusia | 7 (21) | 8 (62) | 0 | 0 |
| Arthralgia | 7 (21) | 6 (46) | 0 | 0 |
| Dyspnoea | 2 (6) | 5 (38) | 0 | 0 |
| Gastrointestinal reflux disease | 1 (3) | 4 (31) | 0 | 0 |
TEAEs, treatment-emergent adverse events.
Data are number of patients (%).
Selected because of their relevance to radium-223 and chemotherapy.
Any grade occurring in ≥25% of patients in either treatment group.
3.2.4. Efficacy
PSA declines of >50% occurred in 61% of patients in the combination arm and 54% of patients in the docetaxel arm (Supplementary Fig. 3A). Plots of PSA level relative to baseline from week 4 to end of treatment show similar profiles for both arms, but PSA suppression was more pronounced with the combination arm (Supplementary Fig. 4A, Supplementary Table 4). A longer time to PSA progression was also observed with the combination arm (Fig. 2A; median, 6.6 vs 4.8 months).
Fig. 2.

KaplaneMeier plots for (A) time to PSA progression; (B) time to tALP progression; (C) time to bALP progression and (D) radiographic or clinical progression-free survival. *Per protocol population; intent-to-treat patients who received ≥40% of specified number of radium-223 injections or docetaxel, per dose escalation study results, and have no major protocol violations. †As per Prostate Cancer Working Group 2 (PCWG2). PSA progression for patients with an initial PSA decline from baseline is defined as a PSA increase ≥25% and ≥2 ng/mL above nadir, confirmed ≥3 weeks later; for those with no PSA decline from baseline, progression is defined as a PSA increase ≥25% and ≥2 ng/mL above baseline after 12 weeks. †tALP/bALP progression for patients with an initial decline in tALP/bALP from baseline was defined as a tALP/bALP increase ≥25% above the nadir, confirmed ≥3 weeks later; for patients with no tALP/bALP decline from baseline, progression was defined as a tALP/bALP increase ≥25% above the baseline after 12 weeks. ¥Time to radiographic or clinical progression is a composite end-point encompassing time to first radiographic or clinical progression or death. bALP, bone alkaline phosphatase; CI, confidence interval; PSA, prostate-specific antigen; tALP, total alkaline phosphatase.
The median PFS was 12.0 months in the combination arm and 9.3 months in the docetaxel arm (Fig. 2D). Twelve-month overall survival rates were similar (89% and 90%, respectively), although the high level of censoring precluded meaningful analysis. Disease progression based on RECIST and PCWG2 criteria is shown in Supplementary Table 5.
Changes in bone marker levels indicated a greater suppression of osteoblastic activity in the combination arm (Supplementary Fig. 3B, 3C, 4B, C, Supplementary Table 4). For both tALP and bALP, a longer median time to progression was observed for combination arm patients than docetaxel arm patients (9.0 vs 6.9 and 9.3 vs 7.4 months, respectively; Fig. 2B and C).
P1NP showed a decline pattern favouring the combination similar to that for bALP (Supplementary Fig. 3D, 4D). The weighted median area under the timeeactivity curve for P1NP was substantially smaller for the combination arm (25.0 v 46.2 μg*day/L), reflecting greater suppression of this marker (Supplementary Table 6).
Markers of osteoclastic activity, uCTX-1 and ICTP, showed similar patterns of decrease during treatment for combination arm and docetaxel arm patients (Supplementary Fig. 3E, 3F, Fig. 4E, F, Supplementary Table 6).
An antitumour treatment effect in both arms was suggested by the decrease in CTCs (Supplementary Table 7).
4. Discussion
To our knowledge, this trial is the first to explore the concept of dual targeting of osteoblastic bone and cancer cells using two concurrent agents, radium-223 and docetaxel, both of which prolong survival in patients with mCRPC. The concept of targeting bone and tumour is not novel. Prior studies have examined docetaxel in combination with bone-targeting agents that are not known to prolong survival, namely, strontium-89 and rhenium-188-hydroxyethylidine diphosphonate [14,15]. These studies only used one or two doses of the bone-seeking radiopharmaceutical, rather than as a repetitively dosed regimen integrated with chemotherapy. Neither of these studies yielded data sufficiently promising to warrant advancement to phase 3. This study, however, used only life-prolonging agents in a regimen in which patients were exposed to both agents throughout the treatment. Although the combination arm used the step-down dose of docetaxel commonly applied in clinical practice, the cumulative exposure to docetaxel in the two arms of the phase 2a cohort was similar, and the combination was associated with less neutropenia, fatigue, and certain gastrointestinal toxicities. Another factor that may have contributed to the safety profile of the combination is that we administered five doses of radium q6w, rather than six doses every four weeks. Combination therapy appeared to increase the proportion of patients with substantial declines in levels of PSA and bone formation biomarkers relative to docetaxel alone and appeared to delay time to progression of these markers.
The safety of this combination is increasingly clinically relevant. Patients generally receive abiraterone or enzalutamide as first-line therapy for mCRPC, with chemotherapy reserved for second-line or beyond. After abiraterone or after enzalutamide therapy, patients frequently manifest both bony disease and soft tissue disease [16,17] and remain sensitive to chemotherapy despite the presence of molecular changes that may render tumours resistant to further androgen receptor (AR)–directed therapy [18]. We therefore have an increasing clinical need for a regimen that is non-AR directed and delivers potent therapy both systemically to the cancer cells and also to the osteoblasts surrounding metastatic bone lesions. Radium-223 and docetaxel appear to fulfil these criteria well. This trial suggests that such an approach is safe, with patients followed up for 1 year without the emergence of long-term safety concerns. It is unknown whether the combination prolongs overall survival compared with radium-223 or docetaxel alone, thus warranting further investigation.
5. Conclusions
This study showed that radium-223 (55 kBq/kg q6w) plus docetaxel (60 mg/m2 q3w) was well tolerated and presented no greater safety concerns than docetaxel alone (75 mg/m2 q3w). Exploratory efficacy data suggested enhanced antitumour activity in the combination arm. Based on these results, the radium-223/docetaxel combination will be further explored in a phase 3 trial in patients with bone metastatic CRPC (NCT03574571).
Supplementary Material
Acknowledgements
This study was sponsored by Bayer. Medical writing services were provided by Dr. Jim Heighway of Cancer Communications and Consultancy Ltd, Knutsford, United Kingdom, and were funded by Bayer.
Role of the funding source
The study was designed by the funder in conjunction with the coordinating investigator (M.J.M.). The funder collected, analysed and interpreted the study data in collaboration with the authors and commissioned medical writing support for the drafting of the report. M.J.M. had the final responsibility for the decision to submit for publication.
Footnotes
Conflict of interest statement
M.J.M. discloses consultancy/advisory roles with Astellas Pharma, Bayer, Endocyte and Advanced Accelerator Applications and has received travel/accommodation expenses from Bayer and Endocyte, and his institution has received research funding from Bayer, Endocyte, Progenics and Sanofi; Y.L. discloses consultancy/advisory roles with Astellas Pharma, AstraZeneca, Janssen, Merck Sharp & Dohme, Pfizer, Roche, Seattle Genetics and Sanofi, and his institution has received research funding from Sanofi; C.J.S. declares stock ownership in relation to Leuchemix, consultancy/advisory roles with Astellas Pharma, AstraZeneca, Bayer, Genentech/Roche, Janssen Biotech, Pfizer and Sanofi and intellectual property interests in relation to Leuchemix and Exelixis, and his institution has received research funding from Astellas Pharma, Bayer, Janssen Biotech, Sanofi and Sotio; K.F. has received honoraria from Bayer and Sanofi, discloses consultancy/advisory roles with Amgen, Astellas Pharma, Bayer, Janssen and Sanofi and has received travel/accommodation expenses from Amgen; C.J.R. has received honoraria from Astellas Pharma, Bayer and Janssen Oncology, discloses consultancy/advisory roles with Bayer, Ferring and Millennium and has received research funding from BIND Biosciences, Karyopharm Therapeutics and Novartis; D.H.S. declares participation in speakers’ bureau for Bayer; E.S.A. has received honoraria from Astellas Pharma, AstraZeneca, Clovis Oncology, Dendreon, ESSA, Janssen Biotech, Medivation, Merck and Sanofi, discloses consultancy/advisory roles with Astellas Pharma, AstraZeneca, Clovis Oncology, Dendreon, ESSA, Janssen Biotech, Medivation, Merck and Sanofi, has an intellectual property interest in relation to Qiagen and has received travel/accommodation expenses from Dendreon, Medivation and Sanofi, and his institution has received research funding from Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca, Clovis Oncology, Constellation Pharmaceuticals, Dendreon, Exelixis, Genentech, Janssen Biotech, Johnson & Johnson, Merck, Millennium, Novartis, Sanofi and Tokai Pharmaceuticals; N.P-.T. has nothing to disclose; D.D. has received travel/accommodation expenses from Bayer Healthcare and General Electric, and her institution has received research funding from Bayer Healthcare; H.A.J. has received honoraria from Astellas Pharma, Bayer Healthcare and Ipsen and has an intellectual property interest in relation to Cambridge University Press, and her institution has received research funding from GTx and Siemens Healthineers; H.V. discloses a consultancy role with MIM Software, outside the submitted work; O.P. is an employee of Bayer; C.L. is an employee of Bayer and owns company stock; J.A.C. discloses a consultancy/advisory role with Y-mAbs Therapeutics and Bayer and research support from Genentech, Regeneron, Gilead Pharmaceutical and Morphotek; C.S.H. has received honoraria from Genentech, discloses consultancy/advisory roles with Asana BioSciences, Astellas Pharma, Bayer, Blue Earth Diagnostics, Clovis Oncology, Dendreon, Endocyte, Ferring, Myriad Genetics, Orion Corporation and Tolmar Pharmaceuticals, has received travel/ accommodation expenses from Asana Biosciences, Astellas Pharma, Bayer, Blue Earth Diagnostics, Clovis Oncology, Dendreon, Endocyte, Ferring, Genentech, Menarini, Orion Pharma GmbH, Myriad Genetics and Pfizer and has an immediate family member who is employed by, has a leadership role in and holds stock in CTI BioPharma, and her institution has received research funding from Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, Dendreon, Emergent BioSolutions, Genentech, Medivation, Pfizer and Roche.
Data availability statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA ‘Principles for responsible clinical trial data sharing’. This pertains to scope, time point and process of data access.
As such, on request from qualified scientific and medical researchers, Bayer commits to sharing patient-level clinical trial data, study-level clinical trial data and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymised patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal.
Data access will be granted to anonymised patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2019.04.007.
References
- [1].Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006;12(20 Pt 2):6250s–7s. [DOI] [PubMed] [Google Scholar]
- [2].Henriksen G, Fisher DR, Roeske JC, Bruland OS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003;44(2): 252–9. [PubMed] [Google Scholar]
- [3].Suominen MI, Fagerlund KM, Rissanen JP, Konkol YM, Morko JP, Peng Z, et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone micro-environment in mouse models. Clin Cancer Res 2017;23(15): 4335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suominen MI, Rissanen JP, Kakonen R, Fagerlund KM, Alhoniemi E, Mumberg D, et al. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J Natl Cancer Inst 2013;105(12):908–16. [DOI] [PubMed] [Google Scholar]
- [5].Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213–23. [DOI] [PubMed] [Google Scholar]
- [6].Balcer-Kubiczek EK, Attarpour M, Jiang J, Kennedy AS, Suntharalingam M. Cytotoxicity of docetaxel (Taxotere) used as a single agent and in combination with radiation in human gastric, cervical and pancreatic cancer cells. Chemotherapy 2006;52(5): 231–40. [DOI] [PubMed] [Google Scholar]
- [7].Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351(15): 1502–12. [DOI] [PubMed] [Google Scholar]
- [8].Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Clin Pract Oncol 2007;4(3):172–80. [DOI] [PubMed] [Google Scholar]
- [9].Zimmerman BE, Bergeron DE, Cessna JT, Fitzgerald R, Pibida L. Revision of the NIST standard for (223)Ra: new measurements and review of 2008 data. J Res Natl Inst Stand Technol 2015;120:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351(15):1513–20. [DOI] [PubMed] [Google Scholar]
- [11].Parker CC, Pascoe S, Chodacki A, O’Sullivan JM, Germa JR, O’Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 2013;63(2):189–97. [DOI] [PubMed] [Google Scholar]
- [12].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2): 228–47. [DOI] [PubMed] [Google Scholar]
- [13].Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26(7):1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol 2016;2(4): 493–9. [DOI] [PubMed] [Google Scholar]
- [15].van Dodewaard-de Jong JM, de Klerk JMH, Bloemendal HJ, Oprea-Lager DE, Hoekstra OS, van den Berg HP, et al. A randomised, phase II study of repeated rhenium-188-HEDP combined with docetaxel and prednisone versus docetaxel and prednisone alone in castration-resistant prostate cancer (CRPC) metastatic to bone; the Taxium II trial. Eur J Nucl Med Mol Imaging 2017;44(8):1319–27. [DOI] [PubMed] [Google Scholar]
- [16].Maughan BL, Xhou XC, Suzman DL, Nadal R, Bassi S, Schweizer MT, et al. Optimal sequencing of docetaxel and abiraterone in men with metastatic castration-resistant prostate cancer. Prostate 2015;75(15):1814–20. [DOI] [PubMed] [Google Scholar]
- [17].Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol 2015;1(5):582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


