Abstract
The importance of patient selection for quality outcomes following radical cystectomy is critical. Clinical staging is one of the key elements necessary for patient selection, and staging relies on accurate preoperative imaging. Many imaging modalities are available and have been utilized for preoperative staging with published operating characteristics. In this update, we review recently published literature for advances in preoperative imaging prior to radical cystectomy.
Keywords: Bladder cancer, Clinical staging, Imaging, Radical cystectomy
Introduction
Radical cystectomy remains the gold standard treatment for muscle-invasive and non-muscle-invasive bladder cancers refractory to intravesical therapy. In order to optimize patient selection and clinical outcomes for patients undergoing radical cystectomy, accurate preoperative clinical staging and assessment is paramount. Clinical staging is performed by incorporating pathologic results from a transurethral resection, a bimanual examination to assess for local invasion and mobility, and cross-sectional radiologic studies. Preoperative imaging studies recommended by the National Comprehensive Cancer Network (NCCN) include chest imaging, imaging of the upper urinary tract, abdominal/pelvic CT or magnetic resonance imaging (MRI), and bone scan if alkaline phosphatase is elevated or if symptoms suggest bony metastasis [1].
For patients undergoing radical cystectomy, abdominal/pelvic imaging is most often performed with a contrast-enhanced CT scan including a delayed imaging phase to evaluate the upper urinary tract. While CT is readily available at nearly all institutions, it has been shown to have overall primary tumor staging accuracy of 35–55 % considering both under- and over-staging rates and 70–97 % for lymph nodes where operating characteristics are dramatically influenced by nodal size cutoff [2, 3]. Because of these low accuracy rates, other imaging modalities have been evaluated in an effort to increase preoperative staging accuracy. In this update, we reviewed the recently published literature on preoperative imaging modalities for patients undergoing radical cystectomy.
Ultrasound
Ultrasound is not often used in the USA for preoperative staging prior to radical cystectomy, and we did not identify any recently published studies utilizing this technique. One interesting study [4] published in 2012 evaluated the effectiveness of three-dimensional (3D) contrast-enhanced (CE) ultrasound (US) to differentiate between non-muscle- and muscle-invasive bladder tumors prior to transurethral resection. They also compared these results with 3D US and CE US. They found that 3D CE US was able to identify and differentiate all 16 muscle-invasive tumors (with 1 false positive) and had improved performance with a receiver operating curve of 0.976 compared to 3D US (0.881) and CE US (0.927). Additionally, the inter-reader agreement was better with 3D CE US with a kappa statistic of 0.914 compared with 0.717 and 0.794 for 3D and CE US, respectively. The promising results of this study certainly call for further evaluation of this technique.
Computed Tomography Scan
A recent study by Schmid et al. [5] evaluated if associations exist between preoperative bladder wall thickness and lymph node size on conventional CT with all-cause and bladder-cancer-specific mortalities. They found that an increase in bladder wall thickness of >150 % compared with unaffected bladder wall regions was associated with all-cause mortality (HR 1.68, 95 % CI 1.02–2.77, p = 0.043) and bladder-cancer-specific mortality (HR 2.00, 95 % CI 1.10–3.64, p = 0.027). They also showed that patients with lymph nodes sized 6–10 mm were associated with worse all-cause (HR 2.13; 95 % CI 1.32–3.46, p = 0.018) and bladder-cancer-specific survival (HR 2.77 95 % CI 1.48–5.17, p = 0.002) than patients with nodes ≤5 mm. While retrospective in design, this study is one of the few studies to show an association between preoperative imaging findings and the overall and cancer-specific mortality. We did not identify any additional studies reporting innovative findings using the technology alone in preoperative staging.
Magnetic Resonance Imaging
MRI has been increasingly used for preoperative staging and offers advantages over conventional CT in terms of ionizing radiation dose as well as soft tissue resolution as shown in Fig. 1a, b. Similar to CT scan, MRI can also be performed with delayed urogram phases to evaluate the upper urinary tracts. Multiparametric MRI provides additional delineation and resolution of tissue with diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) [6]. DWI takes advantage of measurable differences in water diffusion between tissues and can be performed without administration of a contrast agent. Tissues with more tightly compacted cells and intact cell membranes (tumors) will demonstrate lower water diffusion compared with less cellular tissues with disrupted cell membranes [7]. DCE is the acquisition of images before, during, and after the administration of a contrast agent. These images reflect tissue perfusion, vessel permeability, and the volume of the extravascular and extracellular space. The wash-in and wash-out kinetics captured by multiparametric MRI are used to determine whether a tissue is benign or malignant [8]. The historical performance of MRI and multiparametric MRI for preoperative bladder cancer staging has been reviewed elsewhere and not the focus of this review [6, 9, 10].
Fig. 1.
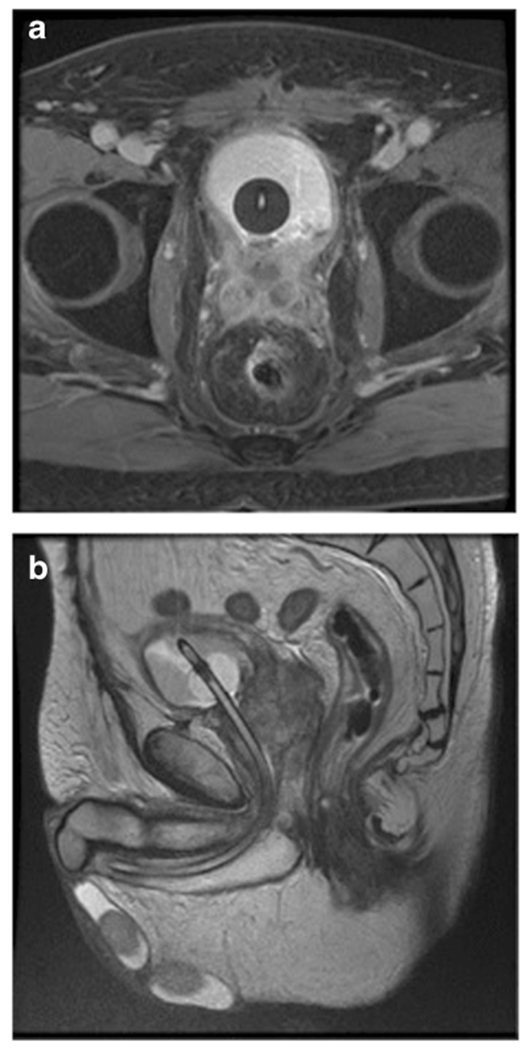
MRI images of a patient with a history of radiation therapy for prostate cancer. a Axial image demonstrates urothelial cell carcinoma invasion of the seminal vesicles and prostate and possible radiation proctitis. b Coronal image of the same patient
One advancement in MRI data analysis was the use of k-means clustering to predict neoadjuvant chemotherapy response at the mid-cycle point in patients undergoing neoadjuvant chemotherapy prior to radical cystectomy. Patients were classified as responders if no malignancy was found by pathology in the cystectomy specimen (complete responder), post-chemotherapy T stage was lower than prechemotherapy T stage and no increase in tumor volume (tumor down-staging), or the patient had a tumor volume reduction of >50 % but no decrease in stage (volume reduction). The changes in baseline MRI to mid-cycle MRI were then correlated with the tumor response to chemotherapy. Using these k-cluster data, the authors found an area under the curve of 0.97 with sensitivity of 96 %, specificity of 100 %, and accuracy of 97 % to predict response to chemotherapy [11]. The ability to predict response to chemotherapy is important as non-responders can change therapeutic strategy early if not responding and avoid the morbidity of non-beneficial chemotherapy.
Chemical Exchange Saturation Transfer MRI
Chemical exchange saturation transfer (CEST) MRI is an exciting technology in the early stages of development and may be used clinically in the future to stage bladder cancer. Generally, CEST is an MRI contrast created by the application of pulses to exchangeable protons and then detecting the subsequent changes in the magnetization of water [12]. Using this concept, Whyhard et al. have demonstrated that bladder cancer cells have increased glucose uptake compared with normal bladder epithelium. Therefore, development of glucose analogs to be used for CEST MRI will allow for detection of tumor tissue due to increased proton exchange in tumor cells giving MRI a cancer-specific contrast agent similar to positron emission tomography (PET) imaging while providing the soft tissue resolution inherent with MRI technology [13]. As this technology continues to develop, it will be interesting to compare its functional characteristics with PET/MRI.
Positron Emission Tomography/Computed Tomography
18F-Fluorodeoxyglucose
PET/CT is a combined imaging technique which superimposes metabolic activity detected using a glucose analog radiotracer with anatomic information obtained from CT and presented as a single image as shown in Fig. 2. Despite widespread use of PET scans in other cancer types and multiple studies reporting improved sensitivity compared with CT scans, PET/CT is infrequently utilized for preoperative bladder cancer staging. This may be due to the fact that early studies noted the accumulation of renally cleared (18)F-fluorodeoxyglucose (FDG) in the bladder can obscure the surrounding tissue leading to decreased diagnostic accuracy, particularly of the primary tumor [14].
Fig. 2.
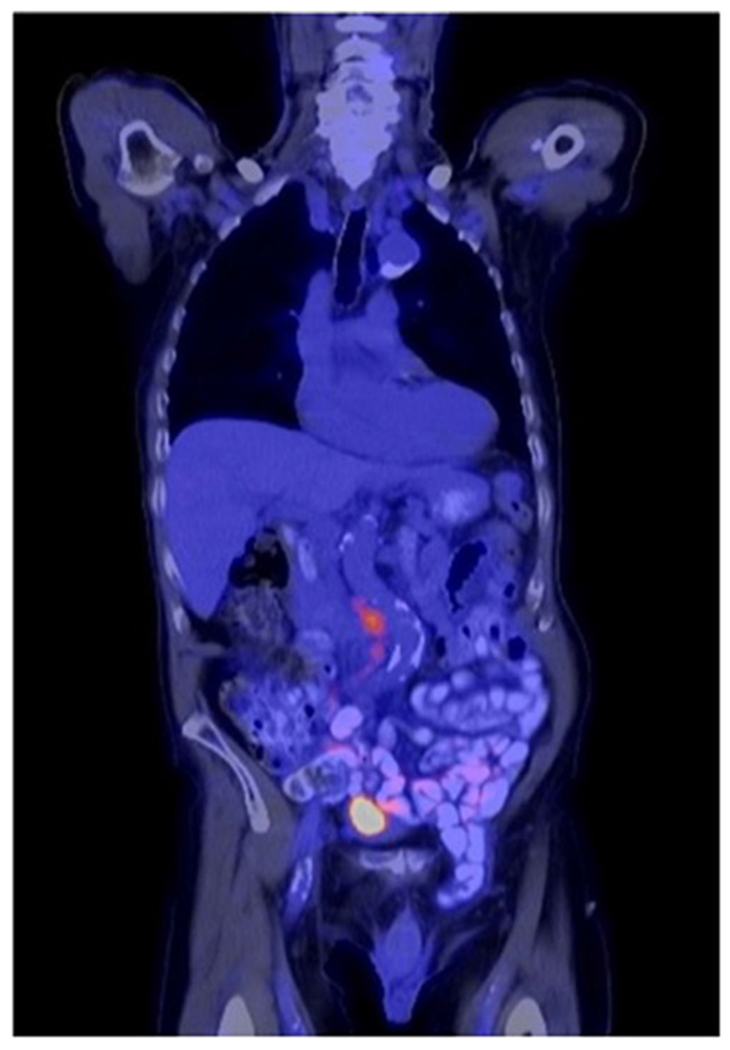
18F-fluorodeoxyglucose (FDG) PET/CT scan demonstrating interaortocaval lymph node metastasis as well as intense radiotracer accumulation in the bladder
Several studies were published recently focused on using FDG-PET/CT for preoperative staging. Jeong et al. published their experience using preoperative FDG-PET/CT to evaluate nodal staging in 61 patients treated with radical cystectomy and extended lymphadenectomy to the level of the inferior mesenteric artery. They compared the preoperative imaging of both FDG-PET/CT and conventional CT with pathologic results from the lymphadenectomy which were processed in 11 anatomic packets in each patient. They concluded that FDG-PET/CT did not improve diagnostic accuracy for lymph node staging compared to conventional CT [15].
Recently, Soubra et al. also published their single institution experience on the diagnostic accuracy of FDG-PET/CT in staging lymph nodes [16]. They utilized a forced diuresis protocol with saline and furosemide to decrease bladder uptake to the background. Of the 78 patients who underwent cystoprostatectomy and lymph node dissection, the sensitivity and specificity of preoperative FDG-PET/CT for positive lymph nodes compared with pathologic results were 56.3 and 98.4 %, respectively. By comparison, the accuracy of CT alone using a size cutoff of 8 mm in the pelvis and 10 mm in the retroperitoneum was 89.7 %. Of note, the FDG-PET/CT also identified eight patients with metastatic disease prior to cystectomy.
In the same publication, this group also included a systematic review and meta-analysis on the diagnostic accuracy of FDG-PET/CT in staging bladder cancer. This systematic review identified seven additional studies meeting their inclusion criteria [17–23]. They found that the pooled sensitivity of FDG-PET/CT for lymph node metastasis and specificity was 0.565 (95 % CI 0.473–0.654) and 0.954 (95 % CI 0.922–0.976), respectively. The positive likelihood ratio (LR) was 9.02 (95 % CI 4.48–18.16) while the negative LR was 0.5 (95 % CI 0.39–0.64). The pooled sensitivity of CT alone was lower at 0.35 (95 % CI 0.25–0.45), specificity of 0.95 (95 % CI 0.91–0.98), a positive LR of 4.91 (95 % CI 1.39–1735), and negative LR of 0.7 (95 %CI 0.48–1.03). They also compared studies using a forced diuresis protocol versus those that did not use forced diuresis and reported a diagnostic odds ratio (OR) of 4.42 (95 % CI 0.35–55.84, p = 0.2007).
The authors suggest that based on calculated likelihood ratios that a positive FDG-PET/CT for lymphatic metastasis may help change management of the patient, while a negative scan would have less of an impact. However, we would refine this even further to suggest that patients most likely to benefit from a preoperative FDG-PET/CT are patients with ≥cT2 bladder cancer who are not candidates for cisplatin-based neoadjuvant chemotherapy but could be considered for a clinical trial with another agent or in patients who completed neoadjuvant chemotherapy and the lymph node dissection template would be modified based on the results of the scan. Scanning all patients with ≥cT2 cancer prior to neoadjuvant chemotherapy is unlikely to change the management for patients already undergoing neoadjuvant chemotherapy. Additionally, the node positive rate for patients undergoing cystectomy for clinical non-muscle invasive bladder cancer (NMIBC) is 7.9 % [24] with even fewer cases of positive nodes outside an extended template and therefore scanning all NMIBC patients undergoing radical cystectomy with FDG-PET/CT may not be warranted.
A significant limitation of the literature to date has been the lack of FDG-PET/CT information with regard to primary tumor staging, which as mentioned earlier, may be secondary to the accumulation of radiotracer in the bladder. Neither Soubra [16] nor Lu et al. [25] in their systematic review and meta-analysis from 2012 were able to evaluate the use of FDG-PET/CT for primary tumor staging due to lack of published studies.
Another group selectively used FDG-PET/CT in patients presumed to be of high risk with somewhat promising results. Kollberg and colleagues [26] imaged only high-risk patients, defined as cT3, cT4, cT2 with hydronephrosis, or cT2 with a high-risk histology. Out of 103 patients in the study cohort, 48 (47 %) had findings suggestive of metastatic disease or additional malignancy not detected by CT scan alone. In 26 (25 %) of these patients, there was lymphatic involvement above the aortic bifurcation or distant metastasis. Overall, the treatment plan was altered in 28 (27 %) patients due to findings on the scan and 16 patients never underwent curative cystectomy as a result of the FDG-PET/CT findings, although two patients did later receive a palliative cystectomy for local symptoms. The percentage of patients with a change of management strategy was comparable to the 22 % reported in a similar 2013 study from Mertens et al. [27]. As Kollberg points out, a major limitation of their study is the lack of confirmation of metastasis which occurred in only 7 out of 28 patients. This study does point to the potential usefulness of obtaining FDG-PET/CT in select high-risk patients; however, confirmatory studies are needed.
11C-Choline PET/CT
While FDG is the most extensively studied imaging agent, other radiopharmaceuticals have been used including (11)C-choline and (11)C-methionine. Brunocilla et al. compared (11)C-choline PET/CT to conventional CT in 28 patients who underwent radical cystectomy and extended lymphadenectomy for the detection of primary tumor and lymph nodes. The accuracy for the detection of the primary tumor for (11)C-choline PET/CT and conventional CT was 52.5 and 50 % while the accuracy for lymph node metastasis was 31.7 and 27.7 %, respectively [28]. Similarly, this same group updated their results in a publication by Ceci et al. [29] which compared preoperative (11)C-choline PET/CT with pathologic results following radical cystectomy with extended lymphadenectomy in 39 patients as well as using PET scanning to evaluate for recurrence following cystectomy in an additional 20 patients. They detected the primary tumor in 38.4 % (15/39) of patients preoperatively and the lymph node sensitivity and specificity were 59 and 90 %, respectively. Unfortunately, there was no comparison with conventional CT in this second study to determine which modality performed more accurately We did not identify any recently published studies evaluating (11)C-methionine PET/CT for preoperative bladder cancer staging.
PET/MRI
While PET/CT has demonstrated improved staging capabilities compared to CT alone [16], the limits with soft tissue resolution using PET/CT has spurred the development of PET/MRI. While most PET/MRI images are created using images obtained from separately acquired PET and MRI scans, there is interest in using integrated systems allowing for the simultaneous capture of PET/MRI images which could improve co-registration [30]. Simultaneous capture could be important for decreasing co-registration errors in bladder cancer because of the fluctuating volume that occurs with filling and emptying. Unfortunately, financial limitations have limited the use of integrated PET/MRI systems and most centers fuse separately performed PET and MRI images.
One recent study did compare co-registration accuracy between sequential and simultaneous image acquisition for PET/MRI in patients with bladder cancer. Rosenkrantz et al. [31] evaluated six patients and compared bladder wall, bladder mass, and pelvic lymph nodes from fused images obtained from separate imaging acquisition using FDG-PET/MRI to images obtained using simultaneous acquisition. They found that the mean (±standard deviation) in-plane misregistration in millimeters for simultaneous acquisition versus sequential acquisition was 2.8 (3.1) versus 7.4 (9.1) for the bladder wall, 2.2 (1.4) versus 2.6 (1.9) for the bladder mass, and 1.1 (0.8) versus 2.5 (0.6) for pelvic lymph nodes. The through plane misregistration also favored simultaneous acquisition for the bladder wall and bladder mass but was similar for pelvic lymph nodes. This small pilot study demonstrates that simultaneous imaging acquisition leads to improved co-registration; however, it certainly requires further validation.
Clinical Trials
Several preoperative imaging clinical trials are open and enrolling patients to study various preoperative staging technologies. NCT02662166 is a prospective non-randomized study evaluating MRI in the preoperative setting. The primary outcome measure is the accuracy of multiparametric MRI for staging bladder cancer. Secondary outcome measures include using MRI to predict response to neoadjuvant chemotherapy and/or BCG. A similar study, NCT00938145, is evaluating the preoperative tumor and lymph node staging using 3 Tesla MRI by comparing clinical staging results to pathologic staging.
Two trials, NCT01655745 and NCT01918592, are evaluating the preoperative staging accuracy of PET/MRI. NCT01655745 is a pilot study whose primary outcome measures are the sensitivity and specificity of FDG-PET/MRI for preoperative staging of bladder cancer. These parameters are calculated by comparing the clinical staging determined by FDG-PET/MRI with pathologic staging results. These results will be compared with conventional CT. Similarly, NCT01918592 will also evaluate the accuracy of PET/MRI by comparing the imaging results to pathologic staging.
Conclusions
Numerous imaging modalities exist for preoperative staging of bladder cancer. Unfortunately, to date, no technology has distinguished itself as the clear gold standard for preoperative imaging modality to determine primary tumor stage and lymph node involvement. For the time being, we continue to rely on histologic and molecular pathology techniques to stage primary tumor and identify positive lymph nodes following surgery [32]; however, we believe advances in technology and research will lead to the development of improved imaging for preoperative staging.
Footnotes
This article is part of the Topical Collection on New Imaging Techniques
Conflict of Interest Cory M. Hugen, Vinay Duddalwar, and Siamak Daneshmand each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Network NCC. Bladder Cancer (version 2.2015). http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed March 8 2016.
- 2.Bostrom PJ, van Rhijn BWG, Fleshner N, Finelli A, Jewett M, Thoms J, et al. Staging and staging errors in bladder cancer. Eur Urol Suppl. 2010;9(1):2–9. doi: 10.1016/j.eursup.2010.01.005. [DOI] [Google Scholar]
- 3.Horn T, Zahel T, Adt N, Schmid SC, Heck MM, Thalgott MK, et al. Evaluation of computed tomography for lymph node staging in bladder cancer prior to radical cystectomy. Urol Int. 2016;96(1):51–6. doi: 10.1159/000440889. [DOI] [PubMed] [Google Scholar]
- 4.Li QY, Tang J, He EH, Li YM, Zhou Y, Zhang X, et al. Clinical utility of three-dimensional contrast-enhanced ultrasound in the differentiation between noninvasive and invasive neoplasms of urinary bladder. Eur J Radiol. 2012;81(11):2936–42. doi: 10.1016/j.ejrad.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Schmid SC, Zahel T, Haller B, Horn T, Metzger I, Holzapfel K, et al. Prognostic value of computed tomography before radical cystectomy in patients with invasive bladder cancer: imaging predicts survival. World J Urol. 2015. doi: 10.1007/s00345-015-1654-9. [DOI] [PubMed] [Google Scholar]
- 6.McKibben MJ, Woods ME. Preoperative imaging for staging bladder cancer. Curr Urol Rep. 2015;16(4):22. doi: 10.1007/s11934-015-0496-8. [DOI] [PubMed] [Google Scholar]
- 7.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188(6):1622–35. doi: 10.2214/ajr.06.1403. [DOI] [PubMed] [Google Scholar]
- 8.Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2003;17(5):509–20. doi: 10.1002/jmri.10304. [DOI] [PubMed] [Google Scholar]
- 9.Rais-Bahrami S, Pietryga JA, Nix JW. Contemporary role of advanced imaging for bladder cancer staging. Urol Oncol. 2016;34(3):124–33. doi: 10.1016/j.urolonc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Daneshmand S, Ahmadi H, Huynh LN, Dobos N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: results from a prospective study. Urology. 2012;80(6):1313–8. doi: 10.1016/j.urology.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HT, Jia G, Shah ZK, Pohar K, Mortazavi A, Zynger DL, et al. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging. 2015;41(5):1374–82. doi: 10.1002/jmri.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Song X, Chan KW, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013;26(7):810–28. doi: 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyard T, Waltzer WC, Waltzer D, Romanov V. Metabolic alterations in bladder cancer: applications for cancer imaging. Exp Cell Res. 2016;341(1):77–83. doi: 10.1016/j.yexcr.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Kosuda S, Kison PV, Greenough R, Grossman HB, Wahl RL. Preliminary assessment of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with bladder cancer. Eur J Nucl Med. 1997;24(6):615–20. [DOI] [PubMed] [Google Scholar]
- 15.Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim CS. FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol. 2015;22(9):3150–6. doi: 10.1245/s10434-015-4369-7. [DOI] [PubMed] [Google Scholar]
- 16.Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol. 2016. doi: 10.1007/s00345-016-1772-z. [DOI] [PubMed] [Google Scholar]
- 17.Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114(3):389–95. doi: 10.1111/bju.12608. [DOI] [PubMed] [Google Scholar]
- 18.Hitier-Berthault M, Ansquer C, Branchereau J, Renaudin K, Bodere F, Bouchot O, et al. 18 F-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: a prospective study. Int J Urol Off J Jpn Urol Assoc. 2013;20(8):788–96. doi: 10.1111/iju.12045. [DOI] [PubMed] [Google Scholar]
- 19.Jensen TK, Holt P, Gerke O, Riehmann M, Svolgaard B, Marcussen N, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: correlation with histopathology. Scand J Urol Nephrol. 2011;45(2):122–8. doi: 10.3109/00365599.2010.544672. [DOI] [PubMed] [Google Scholar]
- 20.Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging ofmuscleinvasive bladder carcinoma. J Clin Oncol. 2009;27(26):4314–20. doi: 10.1200/jco.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodde M, Lacombe L, Friede J, Morin F, Saourine A, Fradet Y. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int. 2010;106(5):658–63. doi: 10.1111/j.1464-410X.2010.09212.x. [DOI] [PubMed] [Google Scholar]
- 22.Nayak B, Dogra PN, Naswa N, Kumar R. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. 2013;40(3):386–93. doi: 10.1007/s00259-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 23.Swinnen G, Maes A, Pottel H, Vanneste A, Billiet I, Lesage K, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. 2010;57(4):641–7. doi: 10.1016/j.eururo.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Bruins HM, Skinner EC, Dorin RP, Ahmadi H, Djaladat H, Miranda G, et al. Incidence and location of lymph node metastases in patients undergoing radical cystectomy for clinical non-muscle invasive bladder cancer: results from a prospective lymph node mapping study. Urol Oncol. 2014;32(1):24.e13–9. doi: 10.1016/j.urolonc.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81(9):2411–6. doi: 10.1016/j.ejrad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Kollberg P, Almquist H, Blackberg M, Cronberg C, Garpered S, Gudjonsson S, et al. [(18)F]Fluorodeoxyglucose-positron emission tomography/computed tomography improves staging in patients with high-risk muscle-invasive bladder cancer scheduled for radical cystectomy. Scand J Urol. 2015;49(4):296–301. doi: 10.3109/21681805.2014.990053. [DOI] [PubMed] [Google Scholar]
- 27.Mertens LS, Fioole-Bruining A, Vegt E, Vogel WV, van Rhijn BW, Horenblas S. Impact of (18) F-fluorodeoxyglucose (FDG)-positron-emission tomography/computed tomography (PET/CT) on management of patients with carcinoma invading bladder muscle. BJU Int. 2013;112(6):729–34. doi: 10.1111/bju.12109. [DOI] [PubMed] [Google Scholar]
- 28.Brunocilla E, Ceci F, Schiavina R, Castellucci P, Maffione AM, Cevenini M, et al. Diagnostic accuracy of (11)C-choline PET/CT in preoperative lymph node staging of bladder cancer: a systematic comparison with contrast-enhanced CT and histologic findings. Clin Nucl Med. 2014;39(5):e308–12. doi: 10.1097/rlu.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 29.Ceci F, Bianchi L, Graziani T, Castellucci P, Pultrone C, Eugenio B, et al. 11C-choline PET/CT and bladder cancer: lymph node metastasis assessment with pathological specimens as reference standard. Clin Nucl Med. 2015;40(2):e124–8. doi: 10.1097/rlu.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 30.Partovi S, Kohan A, Rubbert C, Vercher-Conejero JL, Gaeta C, Yuh R, et al. Clinical oncologic applications of PET/MRI: a new horizon. Am J Nucl Med Mol Imaging. 2014;4(2):202–12. [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenkrantz AB, Balar AV, Huang WC, Jackson K, Friedman KP. Comparison of coregistration accuracy of pelvic structures between sequential and simultaneous imaging during hybrid PET/MRI in patients with bladder cancer. Clin Nucl Med. 2015;40(8):637–41. doi: 10.1097/rlu.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss B, Thoeny HC, Studer UE. Current status of lymph node imaging in bladder and prostate cancer. Urology. 2016. doi: 10.1016/j.urology.2016.02.014. [DOI] [PubMed] [Google Scholar]


