Abstract
Objective
Carbazochrome sodium sulfonate (CSS) has been routinely used to treat bleeding; however, no study has examined the effect of CSS for gastrointestinal bleeding. Therefore, we aimed to investigate the effect of CSS for colonic diverticular bleeding.
Methods
We performed a nationwide observational study using the Japanese Diagnosis Procedure Combination inpatient database. We identified patients who were admitted for diverticular bleeding from July 2010 to March 2018. Patients who received CSS on the day of admission were defined as the CSS group, and those not receiving CSS were defined as the control group. The primary outcome was in-hospital mortality. Secondary outcomes were length of stay, total costs, and blood transfusion within 7 days of admission. Propensity score matching analyses were performed to compare outcomes between the two groups.
Results
A total of 59,965 patients met our eligibility criteria. Of these, 14,437 (24%) patients received CSS on the day of admission. One-to-one propensity score matching created 14,379 matched pairs. There was no significant difference in the in-hospital mortality between the CSS and control groups (0.6% vs. 0.5%, respectively; odds ratio: 0.96; 95% confidence interval: 0.72-1.29). The length of stay was longer in the CSS group than in the control group (11.4 vs. 11.0 days, respectively; difference: 0.44; 95% confidence interval: 0.14-0.73). There were no significant differences in the total costs or the proportion of patients receiving blood transfusion between the groups.
Conclusions
CSS may not reduce in-hospital mortality, length of stay, total costs, or the need for blood transfusion in patients with colonic diverticular bleeding.
Keywords: diverticular bleeding, carbazochrome, propensity score matching
Introduction
Colonic diverticular bleeding is a major cause of lower gastrointestinal bleeding in adults (1). In most cases, colonic diverticular bleeding stops spontaneously (2,3); however, some patients may require endoscopic, radiological, or surgical intervention, potentially resulting in in-hospital death.
Carbazochrome sodium sulfonate (CSS) is an oxidation substance of adrenaline. Theoretically, CSS decreases capillary permeability and increases capillary resistance (4), which may decrease the hemorrhaging time. The hemostatic effect of CSS has been suggested since at least the 1960s (5), and CSS has been used to treat bleeding in the gastrointestinal and respiratory tracts or during obstetrical surgery (6), orthopedic surgery (7), and abdominal surgery (8).
A recent randomized controlled trial by Luo et al. showed that CSS reduced perioperative blood loss in patients undergoing total knee arthroplasty, without thromboembolic complications (7). One small observational case-only study reported that CSS may have improved bleeding symptoms in patients with hereditary hemorrhagic telangiectasia (9). However, to our knowledge, no study has examined the effect of CSS in gastrointestinal bleeding.
In the present study, we investigated the effect and cost of diverticular bleeding treatment with or without CSS, using a Japanese nationwide inpatient database.
Materials and Methods
This was an observational study using routinely- collected data. The study was approved by the Institutional Review Board of The University of Tokyo. The requirement for informed consent from the patients was waived because of the anonymous nature of the data.
Data source
We used the Japanese Diagnosis Procedure Combination database, which is a national administrative claims and discharge abstract of acute-care inpatients in Japan. The database includes data for approximately half of inpatient admissions to acute-care hospitals in Japan and records patients' age, sex, body height, body weight, smoking history, whether the hospital is a teaching hospital, diagnoses, comorbidities on admission, complications after admission, examinations, procedures, prescriptions, intensive care unit admission, and discharge status. Diagnoses, comorbidities on admission, and complications after admission are coded using the International Classification of Diseases 10th revision (ICD-10) codes. The specificity of diagnoses was reported to exceed 96% with a sensitivity of 50-80%. In addition, procedures were coded with original Japanese operation codes, and their sensitivities and specificities exceeded 90% (10).
Patient selection
We extracted data for patients hospitalized from July 2010 to March 2018 from the database, for this study. We identified patients coded with diverticular diseases (ICD 10 code: K57.3) along with the text data “hemorrhaging” or “bleeding”. We excluded the following patients: 1) ≤17 years of age; 2) died on the day of admission; 3) discharged on the day of admission; 4) underwent colonic resection on the day of admission; and/or 5) received tranexamic acid on the day of admission. We excluded patients who received tranexamic acid because the impact of tranexamic acid for diverticular bleeding is controversial, and we needed to eliminate the effect of tranexamic acid to focus on evaluating the effect of CSS for hemorrhaging.
Patients who received CSS on the day of admission were defined as the CSS group, while those who did not receive CSS were defined as the control group.
Covariates and outcomes
Covariates were age category, sex, body mass index (BMI), smoking history (nonsmoker, current/past smoker, and missing data), Charlson Comorbidity Index score (11), hospital volume of patients with diverticular bleeding, whether the hospital was a teaching hospital, intensive care unit admission, examinations on the day of admission, and treatments on the day of admission. Age was categorized into the following six groups: ≤49, 50-59, 60-69, 70-79, 80-89, and ≥90 years old. The BMI was categorized as underweight (BMI <18.5), normal weight (>18.5 to <25), overweight (>25 to <30), and obese (≥30). Charlson Comorbidity Index scores were classified into five groups: 0, 1, 2, 3, and ≥4. Hospital volume was divided into quartiles and categorized as “low” (≤44 cases), “medium-low” (45-91 cases), “medium-high” (92-168 cases), and “high” (≥168 cases).
The primary endpoint was in-hospital mortality. Secondary endpoints were length of stay, total costs, and receiving blood transfusions (red blood cells, fresh-frozen plasma, or platelet concentrate) within seven days of admission.
Statistical analyses
We performed propensity score matching between the CSS and control groups to minimize the effect of selection bias and imbalances in patients' backgrounds between the groups (12). We estimated propensity scores with logistic regression using CSS as a dependent variable and all covariates as independent variables. One-to-one nearest-neighbor propensity score matching without replacement was performed with a caliper width of 0.2 of the standard deviation of the estimated propensity scores. We considered that imbalances in absolute standardized differences to be negligible when the absolute standardized differences between the CSS and control groups were less than 10% (12).
Crude outcomes were compared by the chi-square test for binary outcomes and the t-test for continuous outcomes. After propensity score matching, we used a generalized estimating equation approach to compare the primary and secondary outcomes accompanied by cluster-robust standard errors with individual hospitals as clusters. Odds ratios and their 95% confidence intervals were calculated for the binary outcomes, and absolute differences and their 95% confidence intervals were calculated for the continuous outcomes. These estimates were obtained by generalized estimating equation regression models with the logit link function for odds ratios and identity link function for absolute differences.
We conducted two sensitivity analyses. First, we performed a propensity score adjustment to confirm the robustness of the study findings. In the analysis, we created a multivariable regression model with generalized estimating equations accompanied by cluster-robust standard errors with hospitals used as the cluster variable for the overall cohort. Second, we included the patients who were administered CSS within the first two days of hospitalization as a CSS group. Accordingly, we defined all examinations and interventions mentioned above within two days of admission as covariates.
Primary and secondary outcomes were defined as the dependent variable, and we used CSS on the day of admission and the estimated propensity scores in the main analyses as covariates. Continuous variables were presented as means [standard deviation (SD)], and categorical variables were described as numbers (%). The two-sided significance levels for all tests were p <0.05. All analyses were performed using the Stata/MP version 16 software program (StataCorp, College Station, USA).
Results
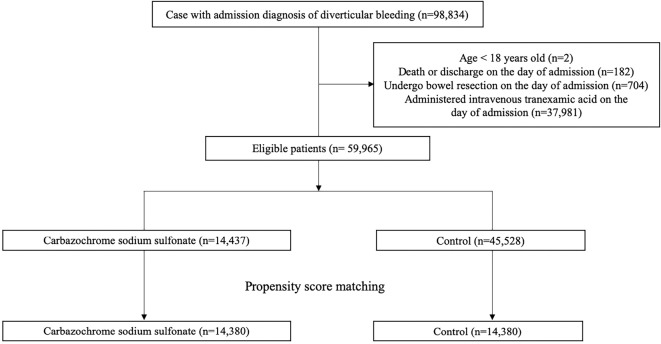
During the study period of 93 months, 59,965 patients met our eligibility criteria. Of these, 14,437 (24.1%) patients received CSS on the day of admission (Figure). There were 9,550 (16%) patients who received CSS after the second day of admission. Among the patients who received CSS during their hospitalization, the mean dose of CSS was 88 mg/day (SD: 37 mg/day), and the mean duration of administration was 5.1 days (SD: 3.5 days).
Figure.
Patient selection flow chart.
Table 1 shows patients' baseline characteristics before and after propensity score matching. Before propensity score matching, patients in the CSS group were more likely to receive computed tomography examinations and H2-receptor antagonists, while these patients were less likely to be admitted to the intensive-care unit than in the control group. After propensity score matching, the baseline characteristics in the two groups were well-balanced.
Table 1.
Patients’ Characteristics before and after Propensity Score Matching.
| Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|
| Carbazochrome sodium sulfonate (n=14,437) | Control (n=45,528) | ASD | Carbazochrome sodium sulfonate (n=14,379) | Control (n=14,379) | ASD | ||
| Age, years, mean (SD) | 74 (12) | 74 (12) | 74 (12) | 74 (12) | |||
| Age, years, n (%) | |||||||
| ≤49 | 620 (4.3) | 1,977 (4.3) | 0.2 | 619 (4.3) | 603 (4.3) | 0.1 | |
| 50–59 | 1,292 (8.6) | 3,937 (8.6) | 1.1 | 1,283 (8.9) | 1,226 (8.5) | 0.0 | |
| 60–69 | 2,758 (19) | 8,685 (19) | 0.1 | 2,747 (19) | 2,797 (20) | 1.8 | |
| 70–79 | 4,346 (30) | 14,191 (31) | 2.3 | 4,333 (30) | 4,476 (31) | 0.2 | |
| 80–89 | 4,517 (31) | 13,994 (31) | 1.2 | 4,495 (31) | 4,436 (31) | 1.0 | |
| ≥90 | 904 (6.3) | 2,774 (6.0) | 1.0 | 903 (6.3) | 842 (5.9) | 1.4 | |
| Male, n (%) | 8,875 (62) | 27,714 (61) | 1.2 | 8,840 (62) | 8,927 (62) | 1.6 | |
| Body mass index, n (%) | |||||||
| ≤18.5 | 1,138 (7.9) | 3,610 (7.9) | 0.2 | 1,134 (7.9) | 1,108 (7.7) | 1.2 | |
| 18.5–24.9 | 8,321 (58) | 26,315 (58) | 0.3 | 8,285 (58) | 8,416 (59) | 1.4 | |
| 25.0–29.9 | 3,224 (22) | 10,138 (22) | 0.2 | 3,212 (22) | 3,251 (23) | 0.8 | |
| ≥30.0 | 635 (4.4) | 1,868 (4.1) | 1.5 | 632 (4.4) | 578 (4.0) | 0.9 | |
| Missing data | 1,119 (7.8) | 3,597 (7.9) | 0.6 | 1,117 (7.8) | 1,027 (7.1) | 2.1 | |
| Smoking history, n (%) | |||||||
| Nonsmoker | 8,601 (60) | 28,025 (62) | 4.1 | 8,592 (60) | 8,623 (60) | 0.7 | |
| Current/past smoker | 3,913 (27) | 12,716 (28) | 1.8 | 3,906 (27) | 3,948 (28) | 1.3 | |
| Missing data | 1,923 (13) | 4787 (11) | 8.7 | 1,882 (13) | 1,809 (13) | 2.8 | |
| Charlson comorbidity index, n (%) | |||||||
| 0 | 8,477 (59) | 25,877 (57) | 3.8 | 8,435 (59) | 8,599 (60) | 2.3 | |
| 1 | 3,539 (25) | 10,997 (24) | 0.8 | 3,526 (24) | 3,457 (24) | 0.4 | |
| 2 | 1,573 (11) | 5,608 (12) | 4.4 | 1,572 (11) | 1,547 (11) | 1.1 | |
| 3 | 554 (3.8) | 1,987 (4.4) | 2.7 | 553 (3.8) | 530 (3.7) | 1.3 | |
| ≥4 | 294 (2.0) | 1,059 (2.3) | 2.0 | 294 (2.0) | 247 (1.7) | 2.6 | |
| Hospital volume, number of cases (%) | |||||||
| Low (1–44) | 3,671 (25) | 11,856 (26) | 1.4 | 3,665 (26) | 3,703 (26) | 0.6 | |
| Medium-low (45–91) | 3,669 (25) | 10,953 (24) | 3.7 | 3,653 (25) | 3,700 (26) | 0.6 | |
| Medium-high (92–168) | 3,566 (25) | 11,614 (26) | 1.9 | 3,562 (25) | 3,565 (25) | 0.0 | |
| High (168–510) | 3,531 (25) | 11,205 (25) | 0.4 | 3,500 (24) | 3,412 (24) | 1.2 | |
| Teaching hospital, n (%) | 11,923 (83) | 37,400 (82) | 1.2 | 11,872 (83) | 12,037 (84) | 2.5 | |
| Intensive care unit admitted, n (%) | 547 (3.8) | 2,821 (6.2) | 11 | 68 (0.5) | 73 (0.5) | 1.0 | |
| Examination on the day of admission | |||||||
| Computed tomography | 9,811 (68) | 28,713 (63) | 10 | 9,754 (68) | 9,741 (68) | 0.6 | |
| Upper gastrointestinal endoscopy, only for examination | 565 (3.9) | 2,288 (5.0) | 5.4 | 563 (3.9) | 519 (3.6) | 2.2 | |
| Colonoscopy, only for examination | 4,055 (28) | 10,961 (24) | 9.1 | 4,009 (28) | 3,971 (28) | 0.0 | |
| Treatment on the day of admission | |||||||
| Oxygen administration | 1,051 (7.3) | 3,798 (8.3) | 4.0 | 1,048 (7.3) | 927 (6.4) | 3.4 | |
| Mechanical ventilation | 13 (0.1) | 69 (0.2) | 1.8 | 13 (0.1) | 11 (0.1) | 0.0 | |
| Renal replacement therapy | 111 (0.8) | 433 (1.0) | 2.0 | 111 (0.8) | 93 (0.6) | 0.0 | |
| Vasopressor | 262 (1.8) | 722 (1.6) | 1.8 | 260 (1.8) | 220 (1.5) | 2.3 | |
| Colonoscopy, for treatment | 2,223 (15) | 7,124 (16) | 0.7 | 2,216 (15) | 2,134 (15) | 2.3 | |
| Interventional radiology | 186 (1.3) | 865 (1.9) | 4.9 | 186 (1.3) | 162 (1.1) | 1.7 | |
| Red blood cell transfusion, mL, mean (SD) | 2,213 (15) | 7,508 (17) | 3.2 | 2,205 (15) | 2,034 (14) | 3.3 | |
| Fresh-frozen plasma transfusion, mL, mean (SD) | 53 (0.4) | 254 (0.6) | 2.8 | 52 (0.4) | 52 (0.4) | 0.7 | |
| Platelet concentrate transfusion, mL, mean (SD) | 10 (0.1) | 31 (0.1) | 0.0 | 10 (0.1) | 12 (0.1) | 0.5 | |
| Vitamin K | 372 (2.6) | 801 (1.8) | 5.6 | 357 (2.5) | 324 (2.3) | 1.7 | |
| Antiplatelet | 234 (1.6) | 998 (0.2) | 4.2 | 234 (1.6) | 196 (1.4) | 0.8 | |
| Anticoagulant | 69 (0.5) | 296 (0.7) | 2.3 | 69 (0.5) | 54 (0.4) | 0.9 | |
| Steroid use | 165 (1.1) | 671 (1.5) | 2.9 | 164 (1.1) | 149 (1.0) | 0.5 | |
| Nonsteroidal anti-inflammatory drug use | 187 (1.3) | 695 (1.5) | 2.0 | 187 (1.3) | 170 (1.2) | 1.1 | |
| Proton pump inhibitor use | 1,973 (14) | 6,371 (14) | 0.9 | 1,972 (14) | 1,846 (13) | 3.7 | |
| H2-receptor antagonist use | 1,487 (10) | 2,452 (5.4) | 18 | 1,430 (9.9) | 1,424 (9.9) | 0.2 | |
ASD: absolute standardized difference, SD: standard deviation, H2: type-2 histamine receptor
Table 2 shows a comparison of the crude outcomes between the groups. The in-hospital mortality was 0.6% in the CSS group and 0.7% in the control group, and the length of stay was 11.4 days in the CSS group and 11.2 days in the control group. The proportions of patients who received red blood cell, fresh-frozen plasma, and platelet concentrate transfusions were 32.7%, 1.3%, and 0.3% in the CSS group and 34.0%, 1.5%, and 0.2% in the control group, respectively.
Table 2.
Crude Outcomes according to the Use of Carbazochrome Sodium Sulfonate.
| Carbazochrome sodium sulfonate (n=14,437) | Control (n=45,528) |
p value | |
|---|---|---|---|
| In-hospital mortality, n (%) | 88 (0.6) | 303 (0.7) | 0.52 |
| Length of stay, days (SD) | 11.4 (10.0) | 11.2 (11.5) | 0.012 |
| Total Costs, Japanese Yen (SD) | 495,354 (406,080) | 509,141 (477,296) | 0.002 |
| Blood transfusion within 7 days of admission | |||
| Red blood cell transfusion, n, mean (%) | 4,722 (32.7) | 15,471 (34.0) | 0.005 |
| Fresh-frozen plasma transfusion, n, mean (%) | 181 (1.3) | 689 (1.5) | 0.023 |
| Platelet concentrate transfusion, n, mean (%) | 41 (0.3) | 111 (0.2) | 0.40 |
n: number, SD: standard deviation
Table 3 shows the outcomes after propensity score matching. There was no significant difference between the two groups regarding in-hospital mortality (0.6% vs. 0.5%, respectively; odds ratio: 0.96; 95% confidence interval: 0.72-1.29). The length of stay was significantly longer in the CSS group than that in the control group (11.4 vs. 11.0 days, respectively; difference: 0.44; 95% confidence interval: 0.14-0.73). Total costs and the proportion of patients who received blood transfusions did not differ significantly between the two groups.
Table 3.
Results of the Outcome Analyses after Propensity Score Matching.
| Carbazochrome sodium sulfonate (n=14,379) | Control (n=14,379) | Odds Ratio (95% CI) | p value | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 88 (0.6) | 91 (0.5) | 0.96 (0.72-1.29) | 0.82 |
| Blood transfusion within 7 days of admission | ||||
| Red blood cell transfusion, n (%) | 4,705 (32.7) | 4,591 (31.9) | 1.02 (0.98-1.07) | 0.15 |
| Fresh-frozen plasma transfusion, n (%) | 180 (1.2) | 176 (1.2) | 0.99 (0.76-1.29) | 0.83 |
| Platelet concentrate transfusion, n (%) | 41 (0.3) | 33 (0.2) | 1.28 (0.75-2.20) | 0.35 |
| Carbazochrome sodium sulfonate (n=14,379) | Control (n=14,379) | Absolute Difference (95% CI) | p value | |
| Length of stay, days, mean (SD) | 11.4 (10.0) | 11.0 (10.2) | 0.44 (0.14-0.73) | <0.001 |
| Total Costs, Japanese Yen, mean (SD) | 495,512 (406,516) | 493,696 (437,940) | 1,816 (-10,145-13,777) | 0.72 |
CI: confidence interval, n: number, SD: standard deviation
Table 4 shows the results after propensity score adjustment as the sensitivity analysis. The results were similar to those in the main analysis.
Table 4.
Results of the Outcome Analyses after Propensity Score Adjustment.
| Odd Ratio (95% CI) | |
|---|---|
| In-hospital mortality, n (%) | 0.96 (0.76-1.21) |
| Blood transfusion within 7 days of admission | |
| Red blood cell transfusion, n (%) | 1.00 (0.95-1.06) |
| Fresh-frozen plasma transfusion, n (%) | 0.97 (0.77-1.21) |
| Platelet concentrate transfusion, n (%) | 1.30 (0.82-2.04) |
| Absolute Difference (95% CI) | |
| Length of stay, days, mean (SD) | 0.35 (0.07-0.64) |
| Total Costs, Japanese Yen, mean (SD) | -2,775 (-13,490-7940) |
CI: confidence interval, n: number, SD: standard deviation
We also conducted propensity score matching including the patients who received CSS within two days of admission, and the results are described in Table 5. Compared with the main analysis, the results were similar in terms of the mortality and length of stay, but the total costs and the proportion of patients who received blood transfusions were higher in the CSS group than the control group.
Table 5.
Results of the Outcome Analyses after Propensity Score Matching according to the Use of Carbazochrome Sodium Sulfonate within 2 Days of Admission.
| Odds Ratio (95% CI) | |
|---|---|
| In-hospital mortality, n (%) | 1.05 (0.81-1.39) |
| Blood transfusion within 7 days of admission | |
| Red blood cell transfusion, n (%) | 1.15 (1.07-1.23) |
| Fresh-frozen plasma transfusion, n (%) | 1.03 (0.79-1.36) |
| Platelet concentrate transfusion, n (%) | 1.95 (1.06-3.63) |
| Absolute Difference (95% CI) | |
| Length of stay, days, mean (SD) | 0.72 (0.41-1.03) |
| Total Costs, Japanese Yen, mean (SD) | 19,886 (8,135-31,638) |
CI: confidence interval, n: number, SD: standard deviation
Discussion
The present study examined the effect of CSS in patients with colonic diverticular bleeding, using a Japanese nationwide inpatient database. The results showed that CSS did not reduce the mortality, length of stay, medical costs, or proportions of patients who received blood transfusion. The sensitivity analyses showed almost the same results.
There are several strengths associated with our study. One was that we investigated the effect of CSS in diverticular bleeding, which is one of the most common causes of lower gastrointestinal bleeding. This is because lower gastrointestinal bleeding is a common problem requiring hospitalization and often blood transfusion. Another strength was that we assessed in-hospital mortality using a large, nationwide dataset from approximately 1,000 hospitals across Japan. Using propensity score matching was also a strength in our study. Propensity score matching can adjust for baseline differences and reduce selection bias. After propensity score matching, we found no marked effectiveness of CSS in patients with diverticular bleeding.
There was no significant difference in the in-hospital mortality between the CSS and control groups. In contrast, the length of stay in the CSS group was longer than that in the control group; however, we believe this result is not clinically important because the difference between the groups was only 0.4 days. Our results also showed that the number of patients who received blood transfusions was not significantly different between the two groups. Colonic diverticular bleeding is likely to occur when the vasa recta, penetrating the colonic wall, are damaged (13). CSS is thought to enhance microcirculatory tone without affecting coagulation-fibrinolysis (8), but the actual mechanism of action is unknown (9). CSS may not be effective for managing arterial bleeding, such as in the vasa recta.
Several limitations associated with the present study warrant mention. First, the database used in our study lacked several clinical data, such as vital signs, blood loss volume, and laboratory data. Second, the generalizability is limited because we enrolled patients from only hospitals in Japan, and the prevalence of diverticulosis and the incidence of diverticular bleeding differs between patients of Asian descent and those of other ethnicities. Third, we were unable to evaluate the recurrence rate of diverticular bleeding because we could not identify readmissions to other hospitals in this database. Fourth, the patients in the control group included some who received CSS after the second day of admission, which may represent a misclassification of exposure. Therefore, we conducted a sensitivity analysis including patients who received CSS within 2 days of admission as the CSS group, and those results were similar to the findings of the main analysis. Finally, we did not analyze the effects of the dose or duration of CSS administration.
In conclusion, despite its potential pharmacological effect, the results of our nationwide study showed that CSS was not associated with a decreased in-hospital mortality, shorter length of stay, or lower total costs or proportion of patients receiving blood transfusions. Thus, the use of CSS may not be justified for treating patients with colonic diverticular bleeding.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (19AA2007 and H30-Policy-Designated-004) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04141).
References
- 1. Strate LL, Ayanian JZ, Kotler G, et al. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol 6: 1004-1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGuire HH., Jr. Bleeding colonic diverticula: a reappraisal of natural history and management. Ann Surg 220: 653-656, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis M NDSG. Bleeding colonic diverticula. J Clin Gastroenterol 42: 1156-1158, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto Y, Hayashi T, Hayakawa Y, et al. Carbazochrome sodium sulphonate (AC-17) decreases the accumulation of tissue-type plasminogen activator in culture medium of human umbilical vein endothelial cells. Blood Coagul Fibrinolysis 6: 233-238, 1995. [DOI] [PubMed] [Google Scholar]
- 5. Dykes ER, Anderson R. Carbazochrome salicylate as a systemic hemostatic agent in plastic operations: a clinical evaluation. JAMA 177: 716-717, 1961. [DOI] [PubMed] [Google Scholar]
- 6. Diefenbach EJ. Comparative blood loss in hysterectomies: an apparent effect of carbazochrome salicylate on postoperative infections. Obstet Gynecol 39: 357-361, 1972. [PubMed] [Google Scholar]
- 7. Luo Y, Zhao X, Releken Y, et al. Hemostatic and anti-inflammatory effects of carbazochrome sodium sulfonate in patients undergoing total knee arthroplasty: a randomized controlled trial. J Arthroplasty 35: 61-68, 2019. [DOI] [PubMed] [Google Scholar]
- 8. Hamada H, Senami M, Fujii K, et al. Prophylactic hemostatic drugs do not reduce hemorrhage: thromboelastographic study during upper abdominal surgery. J Anesth 9: 32-35, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Passali GC, De Corso E, Bastanza G, et al. An old drug for a new application: carbazochrome-sodium-sulfonate in HHT. J Clin Pharmacol 55: 601-602, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Yamana H, Moriwaki M, Horiguchi H, et al. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27: 476-482, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130-1139, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 39: 33-38, 1985. [Google Scholar]
- 13. Meyers MA, Alonso DR, Gray GF, et al. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology 71: 577-583, 1976. [PubMed] [Google Scholar]