Abstract
An asymptomatic 47-year-old woman was admitted with pleural effusion and pulmonary infiltrates 1 month after ingesting raw wild boar and deer meat. Both her blood and pleural fluid were eosinophilic. Thoracoscopy revealed multiple nodules of the pleura, and biopsy samples of the nodules showed necrosis with epithelioid cell granulomas. An enzyme-linked immunosorbent assay was positive for antibodies against Paragonimus westermani, and the patient was successfully treated with praziquantel. This is the first reported case of pulmonary or pleuropulmonary paragonimiasis where several pleural nodules were observed. The detection of pleural nodules on thoracoscopy can contribute to the prompt and accurate diagnosis of paragonimiasis.
Keywords: paragonimiasis, Paragonimus westermani, pleuricy, thoracoscopy, pleural nodules
Introduction
Paragonimiasis is a food-borne parasitic disease common in East and Southeast Asia (1). Pleural effusion is one of the major and typical characteristics of the early phase of paragonimiasis (2-5). Nevertheless, there have been few cases of pleuropulmonary paragonimiasis reported with thoracoscopic findings, most of which showed only diffuse fibrin deposition due to empyema (6-8); little is known about the visual features of the pleura of early-stage paragonimiasis.
We herein report a case of pleuropulmonary paragonimiasis with multiple nodules in the pleura at thoracoscopy.
Case Report
An asymptomatic 47-year-old Japanese woman presented to our hospital with abnormal computed tomography findings of the chest, including unilateral effusion of the right pleura with infiltrates of the upper lobe in the right lung (Fig. 1). Computed tomography was performed as a part of a comprehensive medical examination. One month prior to admission, the patient had ingested raw wild boar and deer meat. The patient was born and raised in Fukuoka Prefecture, Japan, and had never been abroad.
Figure 1.
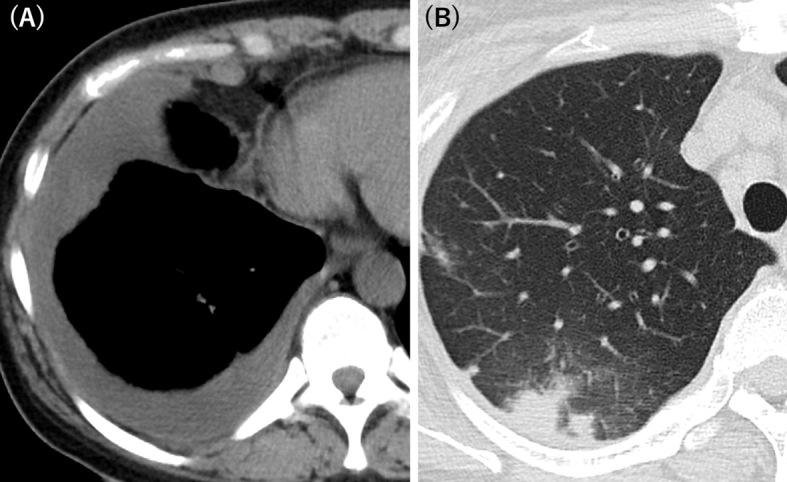
Computed tomography findings of the chest revealed (A) pleural effusion and (B) pulmonary infiltrates.
An examination of the blood revealed eosinophilia (3,046 /μL, 37.8% of the total white blood cell count) and elevated levels of serum immunoglobulin E (6,170 IU/mL). An interferon-gamma release assay for tuberculosis was negative. A multiple-dot enzyme-linked immunosorbent assay (ELISA) was weakly positive for Paragonimus westermani (P. westermani). An analysis of aspirated pleural fluid showed eosinophilia (21.0% of the total white blood cell count) with total protein levels of 6.9 g/dL, lactate dehydrogenase (LDH) levels of 4,521 IU/L, glucose levels of 3 mg/dL, and adenosine deaminase (ADA) levels of 116.4 IU/L. Gram staining, acid-fast staining, and culture of the pleural fluid were negative. The patient underwent bronchoscopy, but no pathogens were detected in the bronchial washing fluid. A transbronchial lung biopsy was not performed because of excessive cough during bronchoscopy.
We accordingly performed uniportal thoracoscopy, which revealed multiple pleural nodules (Fig. 2). Biopsy samples of the nodules showed necrosis with epithelioid cell granulomas (Fig. 3). Eosinophilic infiltrate was not conspicuous. Although there were no ova in the biopsy samples, the diagnosis of pleuropulmonary paragonimiasis was established based on a microplate ELISA, which detected antibodies against P. westermani. The patient underwent pleural fluid drainage followed by successful treatment with praziquantel at 75 mg/kg/day for 3 days, as generally recommended (9). Three months after treatment, both the effusion and lung infiltrates had improved, and her clinical condition remained stable.
Figure 2.
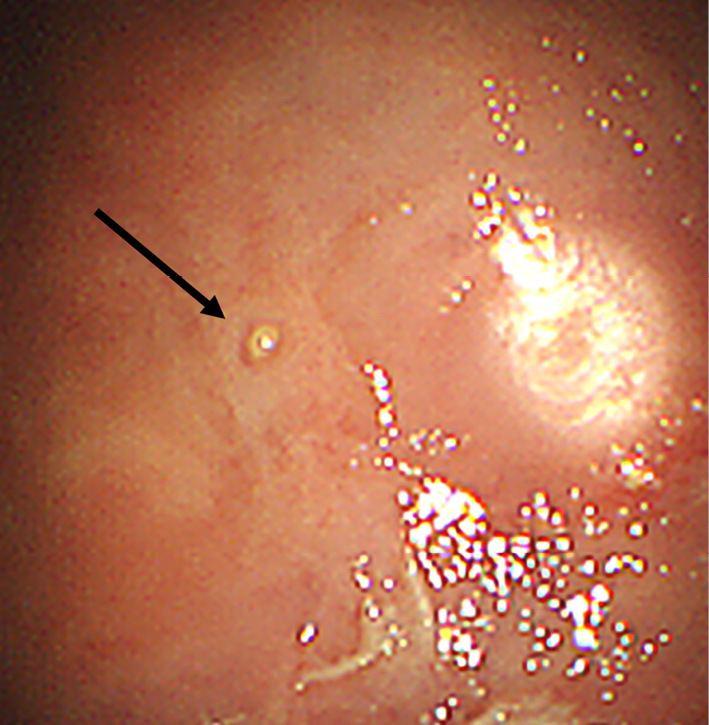
Thoracoscopic finding of the pleura. Several nodules were observed (arrow).
Figure 3.
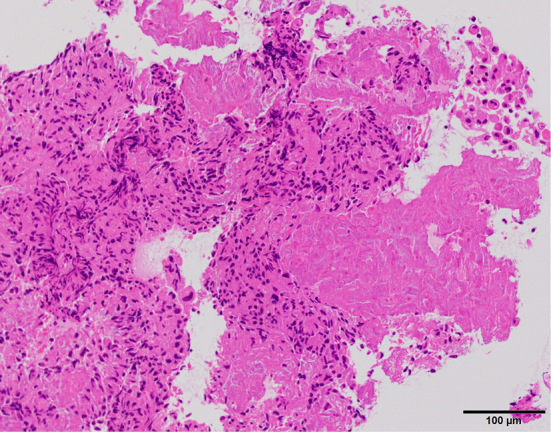
Histology of a biopsy sample of the pleural nodules (Hematoxylin and Eosin staining). Necrosis with epithelioid cell granulomas were seen.
Discussion
Paragonimiasis is a trematode infection caused by Paragonimus spp. Pleural effusion is one of the most common manifestations of paragonimiasis, whose frequency has been reported as high as about 70% (5,10,11). There have been four cases of paragonimiasis in which diagnostic or surgical thoracoscopy was performed; on thoracoscopy, three of them showed only a fibrous peel covering the pleura (6-8), and the other showed pleural thickening with some white dots (12). Whether or not the white dot lesions were the same as the nodules in our case is unclear, as there was no picture of the pleura in the literature (12). Contrary to macroscopic imaging findings, the histopathological findings of the pleural nodules in the present case were similar to those of previous reports (7,8), which demonstrated necrotizing granulomas. The differences in the visual characteristics of the pleura between previous cases and our own may be due to variations in the length of the clinical course; the present patient underwent thoracoscopy only one month after the ingestion of fluke-infested raw meat, whereas the time intervals of the others were at least several months. We speculate that the early phase of paragonimiasis tends to induce the development of nodules in the pleura, which might be similar to thoracoscopic findings of other pleuritis cases (e.g., tuberculous pleurisy). To our knowledge, the present case is the first reported case of pleuropulmonary paragonimiasis with nodular changes of the pleura.
It has been repeatedly demonstrated that pleural fluid of paragonimiasis is eosinophilic and shows increased levels of total protein, LDH, and ADA and decreased levels of glucose (7,8,13), which was consistent with the present findings. It is noteworthy that, for paragonimiasis, both the macroscopic and microscopic features of the pleura as well as the laboratory examinations of pleural effusion resemble those of tuberculosis (7,14,15). Among patients with paragonimiasis, the frequency of a misdiagnosis and/or treatment for tuberculosis has been found to be 44.4-59.0% (16-19). To reduce the rate of inadequately treated cases, careful attention and intensive efforts to make an accurate diagnosis of paragonimiasis are mandatory.
The definitive diagnosis of paragonimiasis can be achieved by detecting parasite eggs (2). However, a recent analysis indicated that the detection rate of eggs was only 11.7% in Japan (20). Generally, it takes at least two months for P. westermani to mature and lay eggs, since paratenic hosts (e.g. boars and deer) with P. westermani are ingested by definitive hosts (e.g., humans) (2). The rapid diagnosis was probably the reason why we were unable to detect any parasite ova in the present case. Eosinophilic necrosis was not evident either; pleural nodules might not be peculiar to paragonimiasis. We made the final diagnosis of paragonimiasis using an ELISA (10), although its results took over two weeks to obtain, so we proceeded with treatment before the conclusive diagnosis was made.
When making the differential diagnosis of eosinophilic pleural effusion, clinicians should consider the possibility of paragonimiasis. Especially in asymptomatic cases, the detection of parasite ova cannot be expected because of the likely short duration from the onset of infection. Furthermore, an ELISA takes anywhere from several days to weeks to complete, although it is the strongest tool available at present for making the clinical diagnosis of paragonimiasis aside from detecting ova. Under these circumstances, the demonstration of pleural nodules during thoracoscopy can contribute to the prompt diagnosis and treatment of paragonimiasis, although these findings might not be specific to paragonimiasis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Professor Haruhiko Maruyama and the staff of the Department of Infectious Diseases, Division of Parasitology, Faculty of Medicine, University of Miyazaki for making the serodiagnosis of paragonimiasis using an ELISA.
References
- 1. Yokogawa M. Paragonimus and paragonimiasis. Adv Parasitol 3: 99-158, 1965. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura-Uchiyama F, Mukae H, Nawa Y. Paragonimiasis: Japanese perspective. Clin Chest Med 23: 409-420, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Procop GW. North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev 22: 415-446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uchiyama F, Morimoto Y, Nawa Y. Re-emergence of paragonimiasis in Kyushu, Japan. Southeast Asian J Trop Med Public Health 30: 686-691, 1999. [PubMed] [Google Scholar]
- 5. Im J-G, Kong Y, Shin YM, et al. Pulmonary paragonimiasis: clinical and experimental studies. RadioGraphics 13: 575-586, 1993. [DOI] [PubMed] [Google Scholar]
- 6. Luo J, Wang M-Y, Liu D, et al. Pulmonary paragonimiasis mimicking tuberculous pleuritis. Medicine (Baltimore) 95: e3436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Hong G, Song J, et al. A case of pleural paragonimiasis confused with tuberculous pleurisy. Tuberc Respir Dis (Seoul) 76: 175-178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeFrain M, Hooker R. North American paragonimiasis: case report of a severe clinical infection. Chest 121: 1368-1372, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Mukae H, Nakamura-Uchiyama F, Nawa Y. Pulmonary paragonimiasis and its surgical complications. In: General Thoracic Surgery. Lippincott Williams & Wilkins, Philadelphia, PA, 2005: 1309. [Google Scholar]
- 10. Mukae H, Taniguchi H, Matsumoto N, et al. Clinicoradiologic features of pleuropulmonary Paragonimus westermani on Kyusyu Island, Japan. Chest 120: 514-520, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura-Uchiyama F, Onah DN, Nawa Y. Clinical features of paragonimiasis cases recently found in Japan: parasite-specific immunoglobulin M and G antibody classes. Clin Infect Dis 32: e171-e175, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Sudo S, Hara Y, Yamaguchi N, Kudo M, Kaneko T. A case of Paragonimus westermani diagnosed by thoracoscopic pleural biopsy. Nihon Kokyuki Gakkaishi (Ann Jpn Respir Soc) 8: 108-112, 2019(in Japanese). [Google Scholar]
- 13. Oh I-J, Kim Y-I, Chi S-Y, et al. Can pleuropulmonary paragonimiasis be cured by only the 1st set of chemotherapy? treatment outcome and clinical features of recently developed pleuropulmonary paragonimiasis. Intern Med 50: 1365-1370, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Hwang K-E, Song H-Y, Jung J-W, et al. Pleural fluid characteristics of pleuropulmonary paragonimiasis masquerading as pleural tuberculosis. Korean J Intern Med 30: 56-61, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Yu Y, An J, et al. A case of delayed diagnosis of pulmonary paragonimiasis due to improvement after anti-tuberculosis therapy. Tuberc Respir Dis (Seoul) 77: 178-183, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Translocat R Soc Trop Med Hyg 80: 967-971, 1986. [DOI] [PubMed] [Google Scholar]
- 17. Jeon K, Koh W-J, Kim H, et al. Clinical features of recently diagnosed pulmonary paragonimiasis in Korea. Chest 128: 1423-1430, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Johnson JR, Falk A, Iber C, Davies S. Paragonimiasis in the United States: a report of nine cases in Hmong immigrants. Chest 82: 168-171, 1982. [DOI] [PubMed] [Google Scholar]
- 19. Sharma DC. Paragonimiasis causing diagnostic confusion with tuberculosis. Lancet Infect Dis 5: 538, 2005. [Google Scholar]
- 20. Nagayasu E, Yoshida A, Hombu A, Horii Y, Maruyama H. Paragonimiasis in Japan: a twelve-year retrospective case review (2001-2012). Intern Med 54: 179-186, 2014. [DOI] [PubMed] [Google Scholar]


