Abstract
Objective
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary hypertension caused by persistent thromboemboli of the pulmonary arteries, and one of its etiological factors may be inflammation. Sleep disordered breathing (SDB) is reportedly an important complication of pulmonary hypertension. However, the association between SDB and inflammation in CTEPH has been undefined. This prospective observational study analyzed the association between the severity of SDB, pulmonary hemodynamic parameters and the systemic inflammation level in patients with CTEPH.
Methods
CTEPH patients admitted for a right heart catheter (RHC) examination were consecutively enrolled from November 2017 to June 2019 at the pulmonary hypertension center in Chiba University Hospital. Patients with idiopathic pulmonary arterial hypertension (IPAH) were also enrolled as a control group. All patients underwent a sleep study using a WatchPAT 200 during admission.
Results
The CTEPH patients showed worse nocturnal hypoxemia, oxygen desaturation index (ODI), and apnea-hypopnea index than the IPAH patients. Among these factors, only the nocturnal mean percutaneous oxygen saturation (SpO2) was negatively correlated with the pulmonary hemodynamic parameters. The circulating tumor necrosis factor-alpha (TNF-α) level was also high in the CTEPH group, and a multivariate analysis showed that the nocturnal mean SpO2 was the most important predictive factor for a high TNF-α level.
Conclusion
We showed that CTEPH patients had high serum TNF-α levels and that the nocturnal mean SpO2 was a predictive factor for serum TNF-α levels. Further investigations focused on nocturnal hypoxemia and the TNF-α level may provide novel insight into the etiology and new therapeutic strategies for CTEPH.
Keywords: chronic thromboembolic pulmonary hypertension, nocturnal hypoxemia, sleep disordered breathing, tumor necrosis factor-alpha
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a type of pulmonary hypertension caused by persistent thromboemboli of the pulmonary arteries that fail to undergo complete thrombolysis after pulmonary thromboembolism (PTE) (1). Various etiological factors, including infection, inflammation and genetic susceptibility, have been discussed as important pathogenetic factors (2), and Chausheva et al. recently suggested the involvement of inflammation in the CTEPH disease phenotype (3).
Sleep disordered breathing (SDB) is common in congestive heart failure; however, data on SDB in pulmonary hypertension have been limited (4). Schulz et al. investigated nocturnal periodic breathing in idiopathic pulmonary hypertension and reported the effectiveness of nasal oxygen therapy (5). Similarly, Ulrich reported the existence of central sleep apnea (CSA) and obstructive sleep apnea (OSA) in pulmonary hypertension (6). Recently, Minic et al. reported the high prevalence of SDB in group 1 pulmonary arterial hypertension (PAH) (7), and Orr et al. reported the correlation between the apnea hypopnea index (AHI) and the cardiac index in CTEPH (8). Interestingly, SDB is reportedly associated with the inflammatory cytokine levels in systemic heart failure (9), but little is known concerning the levels in patients with CTEPH.
In this prospective observational study, we conducted a sleep study using a WatchPAT 200 and analyzed the association between the severity of SDB, pulmonary hemodynamics and the systemic inflammation level in patients with CTEPH.
Materials and Methods
Ethical statement
The protocol for the analysis of SDB was approved by the Local Ethical Review Board of the Chiba University Graduate School of Medicine. The protocol for the analysis of patient blood samples was also approved by the Local Ethical Review Board of the Chiba University Graduate School of Medicine. Written informed consent was obtained from all participating patients. The study was registered in UMIN-CTR (UMIN ID: UMIN000037446).
Study population
Patients with CTEPH admitted for right heart catheterization (RHC) were consecutively enrolled from November 2017 to June 2019 at the pulmonary hypertension center in Chiba University Hospital. Idiopathic PAH (IPAH) patients admitted for RHC were also enrolled for a comparison during the same period. The definition of CTEPH was described previously (10). IPAH was defined as follows: (1) a mean pulmonary arterial pressure (mPAP) of ≥25 mmHg and normal pulmonary arterial wedge pressure (PAWP) on RHC; (2) no perfusion defect on lung scintigraphy; and (3) no identifiable cause of pulmonary hypertension (connective tissue disease, congenital heart disease, portal hypertension, HIV infection, chronic lung disease) on further diagnostic work-up. During admission for RHC, written informed consent was obtained, and SDB screening using a WatchPAT 200 device (Itamar Medical, Caesarea, Israel) was performed during the same admission period.
SDB testing by WatchPAT
The WatchPAT 200 is a portable monitoring device for sleep apnea testing that records peripheral arterial tonometry (PAT) signals, heart rate, oxygen saturation, and actigraphy. The WatchPAT calculates clinical parameters, such as the respiratory event index and 4% oxygen desaturation index (ODI), using an automated and proprietary algorithm (analyzed data reports are automatically made in the default setting). It is less burdensome for the patient than full polysomnography (PSG) and is the screening method recommended in the American Academy of Sleep Medicine (AASM) guideline for obstructive sleep apnea syndrome (OSAS) (11). Using the WatchPAT software program (ZZZ PAT version 4.4.64.4p, Itamar Medical), the resulting data were automatically analyzed to estimate respiratory events [e.g., AHI and respiratory disturbance index (RDI)] and sleep states. The analysis has already been described in greater detail elsewhere (12). The accuracy of WatchPAT for the diagnosis of OSA has also previously been validated (13,14). Nasal oxygen therapy was continued during the test under the usual condition for each patient for safety reasons.
RHC
A 7.5-Fr Swan-Ganz catheter (Edwards Lifesciences, Irvine, USA) was used for RHC. The PAWP and the pressure in the right atrium, right ventricle, main pulmonary artery and right or left pulmonary artery were evaluated. The cardiac output (CO) was measured according to the thermodilution method. The procedure has already been described in greater detail elsewhere (15).
Blood samples
Blood samples were collected from CTEPH and IPAH patients at the time of RHC, which was performed early in the morning. Control blood samples were also obtained from PTE survivors without evidence of pulmonary hypertension during a routine medical consultation more than three months after the acute PTE episode. The serum level of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 and the plasma level of pentraxin3 (PTX3) were measured by the enzyme immunoassay method (LSI Medience, Tokyo, Japan). The plasma level of P-selectin was measured using a sandwich enzyme linked immunosorbent assay kit (Proteintech Group, Rosemont, USA) in accordance with the manufacturer's instructions.
Statistical analyses
All statistical analyses were performed with the JMP software program (version 10.0.2, Japanese version; SAS Institute Tokyo, Japan). Gaussian distributions were analyzed by the Shapiro-Wilk test. Normally distributed variables are expressed as the mean ± standard deviation, and non-normally distributed data are described as median and interquartile range. Comparisons between two groups were performed by the Mann-Whitney U-test (continuous variables) or chi-squared test (categorical variable), and Spearman's correlation coefficients were determined to assess the correlation between two continuous variables. Differences among three groups were evaluated using a one-way analysis of variance (ANOVA) followed by Dunnett’s test for a post-hoc analysis. A multivariate logistic regression analysis was performed to evaluate the factors associated with the high inflammatory cytokine level.
Results
Patients' characteristics
Forty-one CTEPH patients, including six with residual pulmonary hypertension after pulmonary endarterectomy (PEA), were enrolled. There were no patients who had undergone balloon pulmonary angioplasty. During the same period, 12 IPAH patients were enrolled. There were no patients with acute right heart failure. A total of 31.7% and 91.6% of patients were receiving selective pulmonary vaso-dilators, and 73.1% and 58.3% of patients were receiving long-term oxygen therapy in the CTEPH and IPAH groups, respectively. There were 2 patients with a cancer history within the past 5 years, 1 with Crohn’s Disease and 2 with severe obesity (BMI >35) in the CTEPH group. Aside from those patients, however, there were no patients with other significant comorbidities (cancer, infection, or other inflammatory disease). The basic characteristics of the study population are summarized in Table 1. CTEPH patients were older and had higher systemic blood pressure than IPAH patients. There were no significant differences in the mean pulmonary artery pressure, pulmonary vascular resistance or WHO functional class. The cardiac index and 6-minute walking distance were slightly lower and the brain-type natriuretic peptide (BNP) level higher in the CTEPH patients than in the IPAH patients.
Table 1.
The Basic Characteristics of the Patients.
| CTEPH n=41 | IPAH n=12 | p value | ||||
|---|---|---|---|---|---|---|
| Age (years) | 68 (55-73) | 50 (40-60) | 0.002‡ | |||
| Sex (M: F) | 18: 23 | 2: 10 | 0.086 | |||
| Body mass index (kg/m2) | 22.7 (20.4-25.4) | 21.5 (19.6-23.8) | 0.172 | |||
| Epworth sleepiness scale | 4.0±2.8 | 6.1±4.9 | 0.121 | |||
| Systolic blood pressure | 123.0±21.9 | 99.7±12.7 | 0.001‡ | |||
| Diastolic blood pressure | 70.0±12.4 | 61.4±10.2 | 0.033 | |||
| Treatment, N (%) | ||||||
| PH-specific therapy | 13 (31.7) | 11 (91.6) | <0.001‡ | |||
| Long-term oxygen | 30 (73.1) | 7 (58.3) | 0.324 | |||
| Pulmonary hemodynamics | ||||||
| mPAP (mmHg) | 41.7±12.8 | 35.0±10.2 | 0.106 | |||
| RAP (mmHg) | 5.4±3.1 | 5.0±3.6 | 0.674 | |||
| PVR (dyne·sec·cm-5) | 670 (403-882) | 412 (230-651) | 0.154 | |||
| Cardiac index (L/min/m2) | 2.46±0.37 | 2.85±0.66 | 0.012† | |||
| 6MWD(m) | 372.5±94.1 | 498.9±119.7 | <0.001‡ | |||
| BNP (pg/mL) | 72.9 (28.9-750) | 16.4 (8.2-84.6) | 0.015† | |||
| WHO-FC (I:II:III:IV) | 0:30:11:0 | 0:9:3:0 | 0.899 |
mPAP: mean pulmonary artery pressure, RAP: right atrial pressure, PVR: pulmonary vascular resistance, PaO2: arterial oxygen tension, PvO2: mixed venous oxygen tension, 6MWD: 6 minutes walking distance, BNP: brain natriuretic peptide. †, p<0.05; ‡, p<0.01
Respiratory variables in WatchPAT and the correlation between pulmonary hemodynamic parameters
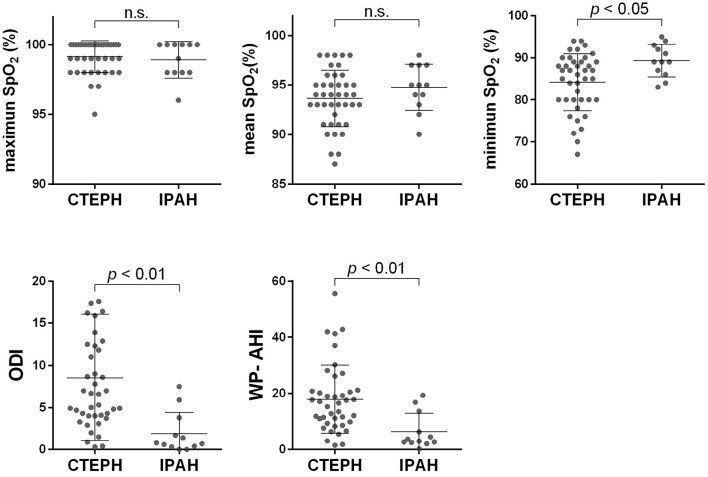
There was no marked difference in the maximum percutaneous oxygen saturation (SpO2), namely the baseline SpO2, between the CTEPH and IPAH groups [100 (98-100) and 99.5 (98-100), respectively]. However, the mean SpO2 was 94 (91.5-95.5) and 95 (93.2-97), and the minimum SpO2 was 86 (80-89) and 89 (86.2-92.7) in the CTEPH and IPAH groups, respectively. The median apnea-hypopnea index calculated by WatchPAT (WP-AHI) in the CTEPH and IPAH groups was 16.6 (9.65-21.05) and 3.3 (2.5-11.8), respectively. Likewise, the ODI was 6.6 (4.0-12.4) and 0.7 (0.3-3.2) in the CTEPH and IPAH groups, respectively (Fig. 1). The mean SpO2 tended to be lower, and the minimum SpO2 was significantly lower in the CTEPH group than in the IPAH group (p<0.05). The WP-AHI and ODI values were also significantly higher in the CTEPH group than in the IPAH group (p<0.01). The WP-AHI values of the CTEPH patients were higher than those in a large Japanese community-based cohort (16,17). The light sleep time, as estimated by the WatchPAT algorithm, was longer and the sleep efficacy lower in the CTEPH group than in the IPAH group. These results imply that CTEPH patients tend to have poor-quality sleep (Supplementary material 1).
Figure 1.
The WatchPAT parameters in patients with CTEPH and IPAH. The maximum SpO2 levels did not differ markedly between the two groups; however, the minimum SpO2 was lower and the mean SpO2 tended to be lower in the CTEPH group than in the IPAH group. The WP-AHI and ODI were both higher in the CTEPH group than in the IPAH group.
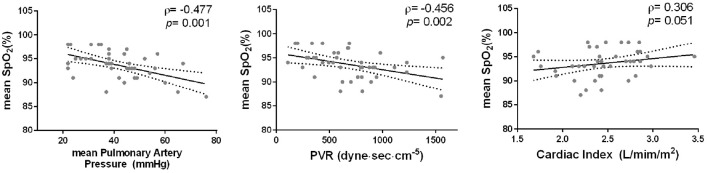
We then assessed the correlation between the variables in WatchPAT and the pulmonary hemodynamic parameters measured by RHC in the CTEPH group. Of those factors described in Fig. 1, only the nocturnal mean SpO2 had a negative correlation with the mean pulmonary artery pressure and pulmonary vascular resistance (Fig. 2). Other parameters did not have any marked correlation with pulmonary hemodynamic parameters (Supplementary material 2), and there was no particular correlation between the nocturnal mean SpO2 and the pulmonary hemodynamic parameters in IPAH patients (data not shown).
Figure 2.
The correlation between the nocturnal mean SpO2 and pulmonary hemodynamic parameters. Of the WatchPAT parameters described in Fig. 1, only the nocturnal mean SpO2 was correlated with pulmonary hemodynamic parameters (negative correlation with mean pulmonary artery pressure and pulmonary vascular resistance).
Inflammatory cytokine levels in patients with CTEPH
We measured the circulating TNF-α, IL-6, PTX3 and P-selectin levels in the CTEPH and IPAH groups as well as in the remitting PTE patients as a control group. The background characteristics of the remitting PTE patients are summarized in Supplementary material 3. They had no symptoms suggesting pulmonary hypertension or heart failure. Of those patients, one had a history of endometrial cancer.
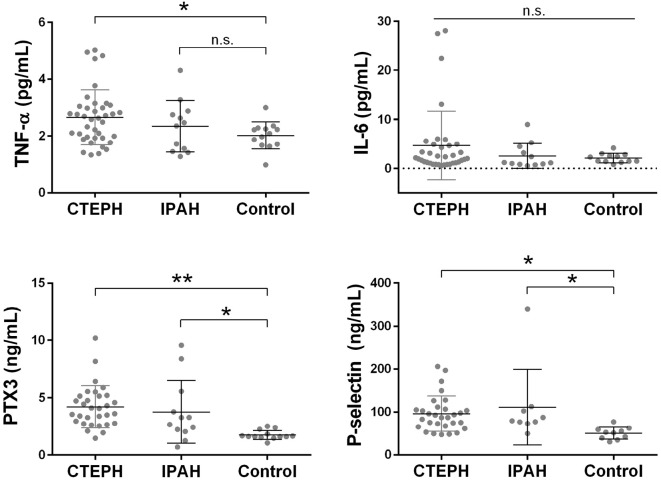
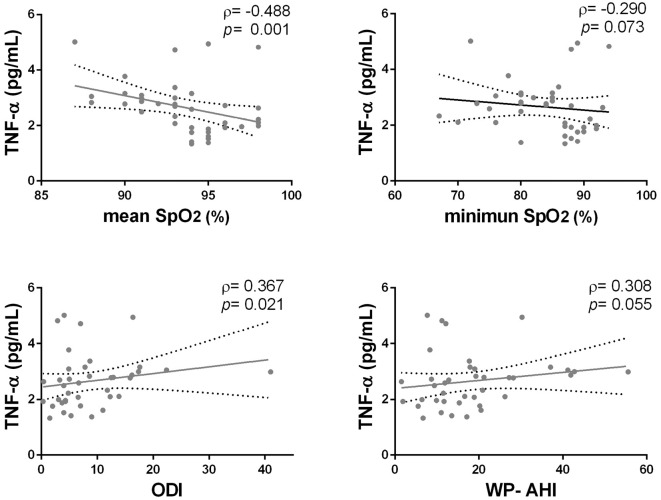
In the CTEPH group, the TNF-α, PTX3 and P-selectin levels were higher than those in the control group (Fig. 3). There were no significant differences in the IL-6 levels according to a one-way analysis of variance. We then assessed the correlation between these cytokine levels and the WatchPAT parameters. The circulating TNF-α level was significantly correlated with the mean SpO2 (ρ=-0.488, p<0.001) and ODI (ρ=0.367, p=0.010) in CTEPH patients (Fig. 4). The PTX3 and P-selectin levels were not significantly correlated with any of the WatchPAT parameters. There was no significant correlation between these cytokines, although the IL-6 levels and the P-selectin levels showed a weak tendency to correlate with the TNF-α levels. The circulating PTX3 and P-selectin levels were also elevated in the IPAH group; however, the levels of these cytokines were not significantly correlated with the WatchPAT parameters.
Figure 3.
The inflammatory cytokine levels of CTEPH patients and IPAH patients compared to the control group (patients cured from acute PTE without evidence of pulmonary hypertension). We measured the serum levels of TNF-α and IL-6 and the plasma level of pentraxin3 (PTX3) and P-selectin. The TNF-α, PTX3 and P-selectin levels in the CTEPH group were higher than in the control group [TNF-α; 2.63 (1.93-3.03) and 2.09 (1.69-2.27) pg/mL, PTX3; 4.10 (2.81-5.20) and 1.62 (1.56-2.04) ng/mL, P-selectin; 90.0 (66.9-108.1) and 52.1 (38.3-60.2) ng/mL in the CTEPH and control groups, respectively]. Some CTEPH patients had high IL-6 levels; however, there were no significant differences in the IL-6 levels according to a one-way ANOVA. The plasma PTX3 and P-selectin levels were also elevated in the IPAH group, although the TNF-α and IL-6 levels did not differ to a statistically significant extent. *, p<0.05; **, p<0.01.
Figure 4.
The correlations between the serum TNF-α level and the WatchPAT parameters in CTEPH patients. The serum TNF-α level was significantly correlated with the nocturnal mean SpO2 values (ρ=-0.488, p=0.001) and oxygen desaturation index (ODI) (ρ=0.367, p=0.021).
To assess the predictive factors for the circulating TNF-α levels, we divided the CTEPH patients into a high TNF-α group and low TNF-α group using the median value (2.63 pg/mL) as the cut-off and developed multivariate logistic regression models. A univariate analysis identified the mean SpO2, ODI and WP-AHI as risk factors for a high TNF-α level. However, only the mean SpO2 was identified as an independent risk factor in the multivariate analysis (Table 2).
Table 2.
Predictors for High Serum TNF-α Level Analyzed by Multivariate Logistic Regression Analysis.
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| odds ratio | 95% CI | p value | odds ratio | 95% CI | p value | |||||||
| Age (year) | 0.997 | 0.935-1.064 | 0.950 | |||||||||
| m PAP (mmHg) | 0.970 | 0.916-1.020 | 0.240 | |||||||||
| CI (mL/min/m2) | 2.801 | 0.521-18.32 | 0.233 | |||||||||
| PVR (dyne∙sec∙cm-5) | 0.999 | 0.997-1.001 | 0.527 | |||||||||
| WP-AHI | 0.951 | 0.835-0.978 | 0.007‡ | 0.961 | 0.771-1.167 | 0.699 | ||||||
| ODI | 0.861 | 0.737-0.971 | 0.011† | 0.950 | 0.658-1.341 | 0.781 | ||||||
| Mean SpO2 (%) | 1.575 | 1.178-2.320 | 0.011† | 1.450 | 1.075-2.149 | 0.012† | ||||||
| Minimum SpO2 (%) | 1.032 | 0.938-1.142 | 0.515 | |||||||||
mPAP: mean pulmonary artery pressure, CI: cardiac index, PVR: pulmonary vascular resistance, WP-AHI: apnea hypopnea index measured by WatchPAT, ODI: oxygen desaturation index
†, p<0.05; ‡, p<0.01
Discussion
We showed that the CTEPH patients had worse nocturnal hypoxemia and a higher prevalence of SDB than the IPAH patients at our hospital (Fig. 1). Of those WatchPAT parameters, only the nocturnal mean SpO2 had a negative correlation with the mean pulmonary artery pressure and pulmonary vascular resistance (Fig. 2). CTEPH patients had also high circulating TNF-α levels (Fig. 3), and the multivariate logistic regression model showed that the nocturnal mean SpO2 was an independent risk factor for high TNF-α levels (Table 2).
The CTEPH patients had worse nocturnal hypoxia than the IPAH patients despite the fact that there were no significant differences in the mean pulmonary artery pressure or pulmonary vascular resistance. Many previous reports have described SDB, especially CSA, in cases of pulmonary hypertension (4,18). Prisco et al. reported the correlation between the severity of pulmonary hypertension and comorbid sleep-disordered breathing (19). These reports suggested that SDB, especially CSA, was a result of pulmonary hypertension. However, CTEPH patients have organized thrombi, which cause ventilation/perfusion mismatch. Furthermore, it has been reported that sleep itself worsens the ventilation/perfusion balance through various mechanisms (20). Ventilation/perfusion mismatch due to organized thrombus may thus worsen during sleep, which in turn worsens nocturnal hypoxemia in CTEPH patients, unlike in IPAH patients.
As another interesting result, the circulating TNF-α level was correlated with the SDB parameters, especially the nocturnal mean SpO2. TNF-α is an inflammatory cytokine that causes a thrombophilic condition (21,22). The high serum TNF-α level induced by the nocturnal hypoxemia might make CTEPH patients more thrombophilic, which can lead to the exacerbation of CTEPH from a long-term perspective. We suspect that nocturnal hypoxemia and a high serum TNF-α level may be involved in a vicious cycle in CTEPH.
There is no consensus as to whether or not the treatment of CTEPH ameliorates SDB. Rovere et al. reported that PEA reversed pulmonary hypertension but not SDB (23). In contrast, Kohno et al. reported that balloon pulmonary angioplasty ameliorated both pulmonary hypertension and SDB (24). We experienced two CTEPH cases in which remission from SDB was obtained after PEA (Supplementary material 4). We assume that this discrepancy depends on the ratio between OSA and CSA before CTEPH treatment. If a CTEPH patient has severe obesity and obstructive sleep apnea as background comorbidities, SDB might persist even after effective PEA.
In contrast, there are no data on whether or not treatment of SDB, such as with continuous positive airway pressure (CPAP), ameliorates CTEPH. Two previous studies reported that CPAP ameliorated thrombophilic conditions (25,26), and Yoshikawa et al. reported that the TNF-α level in OSA patients decreased after CPAP treatment (27). CPAP might ameliorate the thrombophilic condition in CTEPH as well; however, clarifying the effectiveness of CPAP for SDB accompanied by CTEPH should be addressed in a future study.
The present study was associated with several limitations. First, although the WatchPAT 200 is less burdensome for patients than full PSG and shows good cost-benefit performance, we did not conduct PSG examinations for all patients. Four patients in the CTEPH group underwent a full polysomnography examination outside of the study protocol, and respiratory events were mostly due to hypopnea (Supplementary material 5). Further investigations will be needed to elucidate the mechanism underlying the apnea itself. Second, there were differences in the background characteristics between CTEPH and IPAH patients, and the study population was small, especially for the control IPAH patients. Furthermore, the WatchPAT test was performed under the usual oxygen inhalation conditions for safety reasons, which might have been a factor leading to the underestimation of the actual SDB value. However, the present and previous findings strongly suggest that screening for SDB should be performed as the clinical routine in CTEPH patients, and those data should be analyzed in the near future to resolve the limitations mentioned above. Third, we were unable to clarify the precise origin of the elevated serum TNF-α levels in our patients. Minoguchi et al. reported that circulating monocytes in OSA patients produced TNF-α (28); therefore, monocytes may be an origin of the serum TNF-α in CTEPH.
In conclusion, we showed that CTEPH patients had worse SDB than IPAH patients. CTEPH patients had also high serum TNF-α levels, and the nocturnal mean SpO2 was the most important predictive factor. Further investigations focused on nocturnal hypoxemia and the TNF-α level may provide novel insight into the etiology and new therapeutic strategies for CTEPH.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was conducted as collaborative research with Teijin Pharma Ltd. (Tokyo, Japan), and partly funded by Teijin Pharma Ltd. The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary Materials
The deep sleep time in the CTEPH group seemed to be less than that in the IPAH group; however, the difference was not statistically significant (12.9%±6.1%, 15.3%±4.5%, respectively). In the CTEPH group, the light sleep time was greater and the REM sleep time less than in the IPAH group (light sleep time: 66.7%±9.8% and 59.9%±9.1%, REM sleep time: 20.2%±6.3% and 24.6%±7.1%, respectively). The sleep efficacy in CTEPH patients was worse than that in IPAH patients (83.2%±7.1%, 89.8%±4.16%, respectively). These results imply that CTEPH patients tend to have poor quality sleep.
WP-AHI, apnea-hypopnea index calculated by WatchPAT. ‡, p < 0.01
SpO2, percutaneous arterial oxygen saturation; BNP, brain natriuretic peptide. No patient received oxygen supplementation therapy
A) A 70-year-old woman. The patient had a mean pulmonary artery pressure (mPAP) of 49 mmHg, a cardiac index (CI) of 2.81 L/min/m2, a WP-AHI of 9.4 and a mean SpO2 of 91%. At 1 month after pulmonary endarterectomy, her mPAP level had decreased to 17 mmHg, CI had increased to 3.02 L/min/m2, WP-AHI had decreased to 0.6, and mean SpO2 had increased to 99%. B) A 53-year-old woman. She had an mPAP of 25 mmHg, a CI of 3.46 L/min/m2, a WP-AHI of 20.6 and a mean SpO2 of 95%. At 1 year after pulmonary endarterectomy, her mPAP level had decreased to 21 mmHg, CI had increased slightly to 3.66 L/min/m2, WP-AHI had decreased to 0.9, and mean SpO2 had increased to 98%.
Acknowledgement
We thank the technical staff for performing the WatchPAT analysis in Chiba University Hospital.
References
- 1. Witkin AS, Channick RN. Chronic thromboembolic pulmonary hypertension: the end result of pulmonary embolism. Curr Cardiol Rep 17: 63, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 41: 462-468, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Chausheva S, Naito A, Ogawa A, et al. . Chronic thromboembolic pulmonary hypertension in Austria and Japan. J Thorac Cardiovasc Surg 158: 604-614, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Randerath W, Verbraecken J, Andreas S, et al. . Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 5. Schulz R, Baseler G, Ghofrani HA, Grimminger F, Olschewski H, Seeger W. Nocturnal periodic breathing in primary pulmonary hypertension. Eur Respir J 19: 658-663, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Ulrich S, Fischler M, Speich R, Bloch KE. Sleep-related breathing disorders in patients with pulmonary hypertension. Chest 133: 1375-1380, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Minic M, Granton JT, Ryan CM. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med 10: 277-283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orr JE, Auger WR, DeYoung PN, Kim NH, Malhotra A, Owens RL. Usefulness of low cardiac index to predict sleep-disordered breathing in chronic thromboembolic pulmonary hypertension. Am J Cardiol 117: 1001-1005, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehra R, Wang L, Andrews N, et al. . Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 13: 1411-1422, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miwa H, Tanabe N, Jujo T, et al. . long-term outcome of chronic thromboembolic pulmonary hypertension at a single Japanese pulmonary endarterectomy center. Circ J 82: 1428-1436, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Kapur VK, Auckley DH, Chowdhuri S, et al. . clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med 13: 479-504, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinoshita T, Yahaba M, Terada J, et al. . impact of arterial stiffness on WatchPAT variables in patients with obstructive sleep apnea. J Clin Sleep Med 14: 319-325, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi JH, Kim EJ, Kim YS, et al. . Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol 130: 838-843, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Yuceege M, Firat H, Demir A, Ardic S. Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. J Clin Sleep Med 9: 339-344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jujo T, Sakao S, Ishibashi-Ueda H, et al. . evaluation of the microcirculation in chronic thromboembolic pulmonary hypertension patients: the impact of pulmonary arterial remodeling on postoperative and follow-up pulmonary arterial pressure and vascular resistance. PLoS One 10: e0133167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanigawa T, Tachibana N, Yamagishi K, et al. . Relationship between sleep-disordered breathing and blood pressure levels in community-based samples of Japanese men.. Hypertens Res 27: 479-484, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Cui R, Tanigawa T, Sakurai S, et al. . Associations of sleep-disordered breathing with excessive daytime sleepiness and blood pressure in Japanese women. Hypertens Res 31: 501-506, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Thurnheer R, Ulrich S, Bloch KE. Precapillary pulmonary hypertension and sleep-disordered breathing: is there a link? Respiration 93: 65-77, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Prisco DL, Sica AL, Talwar A, et al. . Correlation of pulmonary hypertension severity with metrics of comorbid sleep-disordered breathing. Sleep Breath 15: 633-639, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Marrone O, Salvaggio A, Insalaco G. Respiratory disorders during sleep in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 1: 363-372, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood 128: 753-762, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, Bester J, Pretorius E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci Rep 8: 1812, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. La Rovere MT, Fanfulla F, Taurino AE, et al. . Chronic thromboembolic pulmonary hypertension: reversal of pulmonary hypertension but not sleep disordered breathing following pulmonary endarterectomy. Int J Cardiol 264: 147-152, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Kohno T, Fukuoka R, Kawakami T, et al. . Balloon pulmonary angioplasty attenuates sleep apnea in patients with chronic thromboembolic pulmonary hypertension. Heart Lung 48: 321-324, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Hui DS, Ko FW, Fok JP, et al. . The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest 125: 1768-1775, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Oga T, Chin K, Tabuchi A, et al. . Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb 16: 862-869, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Yoshikawa M, Yamauchi M, Fujita Y, et al. . The impact of obstructive sleep apnea and nasal CPAP on circulating adiponectin levels. Lung 192: 289-295, 2014. [DOI] [PubMed] [Google Scholar]
- 28. Minoguchi K, Tazaki T, Yokoe T, et al. . Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest 126: 1473-1479, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The deep sleep time in the CTEPH group seemed to be less than that in the IPAH group; however, the difference was not statistically significant (12.9%±6.1%, 15.3%±4.5%, respectively). In the CTEPH group, the light sleep time was greater and the REM sleep time less than in the IPAH group (light sleep time: 66.7%±9.8% and 59.9%±9.1%, REM sleep time: 20.2%±6.3% and 24.6%±7.1%, respectively). The sleep efficacy in CTEPH patients was worse than that in IPAH patients (83.2%±7.1%, 89.8%±4.16%, respectively). These results imply that CTEPH patients tend to have poor quality sleep.
WP-AHI, apnea-hypopnea index calculated by WatchPAT. ‡, p < 0.01
SpO2, percutaneous arterial oxygen saturation; BNP, brain natriuretic peptide. No patient received oxygen supplementation therapy
A) A 70-year-old woman. The patient had a mean pulmonary artery pressure (mPAP) of 49 mmHg, a cardiac index (CI) of 2.81 L/min/m2, a WP-AHI of 9.4 and a mean SpO2 of 91%. At 1 month after pulmonary endarterectomy, her mPAP level had decreased to 17 mmHg, CI had increased to 3.02 L/min/m2, WP-AHI had decreased to 0.6, and mean SpO2 had increased to 99%. B) A 53-year-old woman. She had an mPAP of 25 mmHg, a CI of 3.46 L/min/m2, a WP-AHI of 20.6 and a mean SpO2 of 95%. At 1 year after pulmonary endarterectomy, her mPAP level had decreased to 21 mmHg, CI had increased slightly to 3.66 L/min/m2, WP-AHI had decreased to 0.9, and mean SpO2 had increased to 98%.