Abstract
As a transparent avascular tissue located at the front of the eyeball, the cornea is an important barrier to external damage. Both epithelial and endothelial cells of the cornea harbor primary cilia, which sense changes in the external environment and regulate intracellular signaling pathways. Accumulating evidence suggests that the primary cilium regulates corneal development in several ways, including participation in corneal epithelial stratification and maintenance of corneal endothelial cell morphology. In addition, the primary cilium has been implicated in the pathogenesis of several corneal diseases. In this review, we discuss recent findings that demonstrate the critical role of the primary cilium in corneal development. We also discuss the link between ciliary dysfunction and corneal diseases, which suggests that the primary cilium could be targeted to treat these diseases.
Keywords: Cornea, Development, Disease, Cilium, Epithelium, Endothelium, Treatment
INTRODUCTION
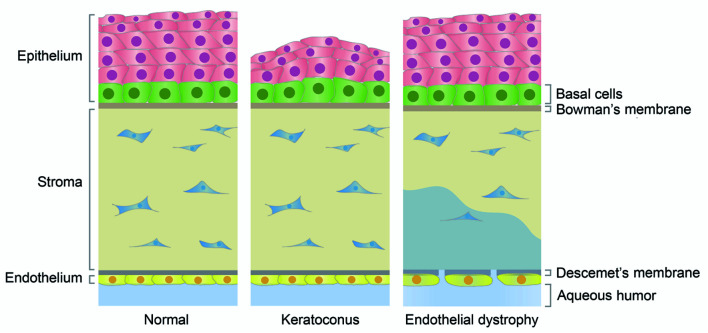
The transparent cornea, which is located in front of the eyeball, has both barrier and refractive functions and is very important for maintaining ocular health and visual acuity. The cornea is a multi-layered structure consisting of an epithelial layer, stroma, endothelial layer, Bowman’s membrane, and Descemet’s membrane (DelMonte & Kim, 2011). The corneal epithelium has six to eight layers, with correct layering maintaining the stability and transparency of the entire corneal structure. The corneal endothelium on the innermost side of the cornea is composed of a single layer of flat cells, which prevents the flow of aqueous humor to the stroma, thereby ensuring the fidelity of the object being viewed. The corneal stroma, which relies on keratocytes to maintain integrity, is located between the epithelium and endothelium and contains a trigeminal nerve plexus (DelMonte & Kim, 2011; Oliveira-Soto & Efron, 2001). Bowman’s membrane is located between the corneal epithelium and the stroma and is thought to play a role in protecting the stromal plexus. Descemet’s membrane lies between the endothelial layer and stroma and helps the endothelial layer maintain corneal hydration (Figure 1).
Figure 1. Structures of normal and diseased corneal tissues.
The cornea is composed of an epithelial layer, stroma, endothelial layer, Bowman’s membrane, and Descemet’s membrane. The epithelial layer is formed from multiple layers of cells, but only basal layer cells can divide. The endothelial layer is a monolayer and the cells are blocked in the G0/G1 phase of the cell cycle. In the normal cornea, the epithelial layer relies on a stable multilayered structure to prevent external damage, and endothelial cells are closely arranged together with Descemet’s membrane to prevent the flow of aqueous humor to the stroma and consequent corneal edema. Keratoconus is the most common corneal epithelial disease and is characterized by protrusion of the central cornea and thinning of the epithelial layer. Some abnormally expressed cilium-related molecules have been identified in keratoconus, such as up-regulation of TGF-β1 and TIMP3 (negatively correlated with cilia) and down-regulation of VEGF, COL1A1, and COL1A2 (positively correlated with cilia). Endothelial dystrophy is the most common retinal endothelial disease, in which endothelial cells are lost in large quantities, cells expand, and Descemet’s membrane is destroyed, causing aqueous humor to flow to the stroma, leading to corneal edema. Some abnormal cilium-related molecules have also been found in endothelial dystrophy, such as down-regulated ZEB1 expression, which is positively correlated with cilia, and TCF4 mutations that affect the Wnt/β-catenin pathway.
The precise molecular mechanisms underlying corneal development and pathology are incompletely understood, although the primary cilium has been implicated in both processes (Blitzer et al., 2011). The primary cilium is a specialized microtubule-based protrusion that emanates from the mother centriole and protrudes from the cell surface. It is composed of a basal body, transition zone, axoneme, and ciliary membrane. During organ development and tissue repair, cells precisely integrate extracellular signals to reconstruct or repair the complex tissue structure and function (Blitzer et al., 2011; Lyu & Zhou, 2017; Yang et al., 2014). Primary cilia allow cells to sense changes in their external environment, receive extracellular cues, participate in various signaling pathways, and mediate tissue development and homeostasis (Berbari et al., 2009; Yang et al., 2019; Yu et al., 2019). In mammals, primary cilia are present on the surfaces of most types of cells, including corneal epithelial and endothelial cells (Collin & Collin, 2004). A variety of signaling pathways, such as the Notch, Wnt, nuclear factor κB, and Hedgehog pathways, have been demonstrated to regulate ciliogenesis and thereby play important roles in tissue development (Pala et al., 2017; Reiter & Leroux, 2017). In this review, we discuss the specific role of primary cilia in corneal development and pathology and explore the possibility of using cilium-related molecular targets to treat corneal diseases.
DEVELOPMENT OF CORNEAL EPITHELIUM AND ENDOTHELIUM
The corneal epithelium serves as the eyeball’s first barrier to the outside environment. Its integrity, stability, and correct stratification are essential for normal visual function and eye health. The 6–8 layers of the corneal epithelium are formed from an outer 3–4 layers of flat squamous cells and an inner 3–4 layers of wing cells. Cells are connected by tight junctions to prevent invasion by pathogenic microorganisms. The corneal epithelium regenerates rapidly, surviving for approximately 7–10 days before being replenished after apoptosis by limbal stem cell division and migration (DelMonte & Kim, 2011). During corneal epithelial development, limbal stem cells differentiate into transiently expanded cells (TACs) (Grisanti et al., 2016). These TACs migrate to the center of the cornea to form the basal layer of the corneal epithelium, and then the basal cells differentiate upwards and migrate into multilayer cells (Douvaras et al., 2013). The timing of corneal epithelial stratification differs between species. In humans, a corneal epithelium layer begins to form at about five weeks of gestation, after which the corneal epithelium develops in layers such that, at birth, a fully developed, multi-layered corneal epithelium is present. In mice, the corneal epithelium beings to form at embryonic day 12.5 (E12.5), at which time the corneal epithelial layer is composed of two layers of superficial flat cells and basal cuboid cells. However, the corneal epithelium is not fully developed at birth, only reaching full maturity after 70 days (P70) when it is formed from six layers of cells (Hoar, 1982; Lwigale, 2015).
Unlike the corneal epithelium, the corneal endothelium is a single layer derived from nerve cells (Cvekl & Tamm, 2004). In mice, the endothelial monolayer begins to form at E15.5 and is then maintained throughout development, becoming fully mature at about P70. Interestingly, during corneal endothelial development, cells appear as irregular polygons, whereas mature endothelial cells are regular hexagons (Blitzer et al., 2011). When the endothelium matures, the endothelial cells no longer divide and are arrested at the G0/G1 phase, meaning that the number of endothelial cells decreases with age (Joyce, 2003). Indeed, endothelial cell density is highest at birth (~6 000 cells/mm2) and then decreases by ~0.6% annually (Edelhauser, 2006). Endothelial cells rely on Na+/K+-ATPase pumps to regulate corneal hydration and maintain corneal transparency. Damaged endothelial layers are repaired by endothelial cell expansion. Under low cell density (i.e., <500 cells/mm 2), endothelial cells cannot prevent aqueous humor leakage to the corneal stroma, resulting in corneal edema (Morishige & Sonoda, 2013). Corneal endothelial cells are prone to endothelial to mesenchymal transition (EnMT) during proliferation, which can destabilize cell connections and cause edema (Roy et al., 2015). Therefore, endothelial health is critical for corneal function. In this regard, pituitary adenylate cyclase-activating polypeptide is a trophic factor that promotes tight junction protein expression, increases endothelial resistance, and protects the health of endothelial cells (Maugeri et al., 2018).
PRIMARY CILIA IN CORNEAL EPITHELIAL DEVELOPMENT
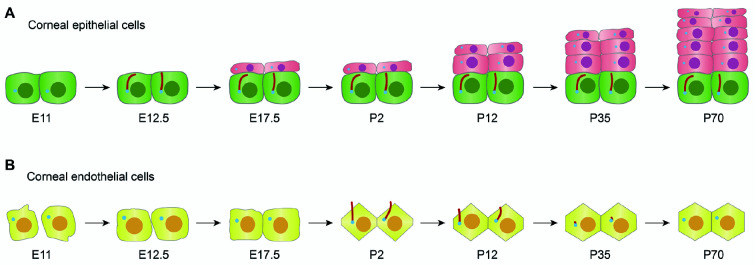
Multiple signaling pathways are involved in the development of the corneal epithelium. Primary cilia are specialized organelles that receive and transmit signals that play an important role in corneal epithelial development. Primary cilia only exist in the basal layer of the mature mammalian corneal epithelium. In mice, primary cilia can be detected in the single corneal epithelium layer that forms at E12.5, i.e., formation of primary cilia is synchronized with corneal epithelial development. The corneal epithelium then develops slowly from E17.5 to P12, after which it rapidly proliferates and develops in layers until full maturity at P70 (Figure 2A). Primary cilia in the peripheral area are longer than primary cilia in the central area, which suggests that primary cilia participate in the initial stages of corneal epithelial differentiation (Grisanti et al., 2016).
Figure 2. Patterns of primary cilia during corneal development.
A: In mice, at E11, the epithelial layer begins to develop from the ectoderm; at E12.5, the epithelial layer continues to form, at which time primary cilia can be detected; at E17.5, the epithelial layer slowly develops into two layers until the mouse opens its eyelids (around P12), after which it begins to develop rapidly. By P70, the epithelial layer matures, forming a stable structure of 6−8 layers of cells. Throughout development, primary cilia only exist on basal cells. B: At E11-12.5, neural crest cells begin to migrate to form endothelial cells; at E17.5, tight junctions form between the cells. Primary cilia are not detectable throughout the embryonic period, and cell morphology is irregular. From P2 to P12, the primary cilia begin to grow, regulating hexagonal cell morphology changes; at P35, most of the primary cilia depolymerize. By P70, the primary cilia completely disappear, cells are regular hexagons, and a mature endothelial layer has developed.
Primary cilia have also been found to affect corneal epithelial development in knockout mouse models. Primary cilia play a key role in controlling proliferation and differentiation signaling pathways, e.g., Notch signaling pathway (Berbari et al., 2009; Wood et al., 2013). Activation of Notch signaling helps to maintain corneal epithelial stratification (Djalilian et al., 2008). Knockout of intraflagellar transport (IFT) components of primary cilia decreases Notch signaling (Ezratty et al., 2011); in IFT88 knockout mice, primary cilia in the basal layer disappear and the lack of IFT components reduces Notch signaling but not cilium-related Hedgehog and Wnt signaling (Grisanti et al., 2016). In this model, the lack of primary cilia also increases the proliferation and vertical migration of corneal epithelial basal-layer cells, resulting in abnormal thickening of the corneal epithelium and structural abnormalities. Taken together, primary cilia regulate the proliferation of corneal epithelial cells by controlling Notch signaling and stabilizing the corneal epithelial layer. It should be noted that in addition to the regulation of corneal epithelial development, primary cilia also regulate epithelial development in many other tissues, such as retinal and renal tissues (May-Simera et al., 2018; Pazour et al., 2020).
PRIMARY CILIA IN CORNEAL ENDOTHELIAL DEVELOPMENT
In contrast to the epithelial layer, primary cilia are not always present in the corneal endothelium (Pitaval et al., 2010). As noted above, in mice at E12.5, the endothelial layer forms with irregular cellular morphology and an absence of tight junctions. At E17.5, the cells become tightly connected, although the morphology remains irregular. Throughout embryonic development in mice, corneal endothelial cells exhibit irregular morphology and primary cilia are absent. From P2 to P12, primary cilia begin to grow, during which time they increase to their maximal length and endothelial cell morphology gradually becomes regular. At P35, endothelial cells become hexagonal, the primary cilia begin to shorten, and some primary cilia depolymerize. From P45 to P75, i.e., full maturation of the endothelial layer, the primary cilia depolymerize, and cells become regular hexagons (Figure 2B). When adult endothelial cells are mechanically damaged, the primary cilia reassemble and then regulate endothelial cell expansion and repair (Blitzer et al., 2011). Therefore, the presence, absence, and reemergence (regeneration) of primary cilia may play important roles in the development of endothelial cells into regular hexagons as well as the maintenance of endothelial integrity.
Changes in the structure and function of primary cilia can lead to defects in the morphology of corneal endothelial cells. In IFT88 mutant mice and lentivirus-mediated IFT88-knockdown corneal endothelial cells (Lehman et al., 2008), ciliary defects can be detected, and corneal endothelial cells can become irregular (Blitzer et al., 2011). Primary cilia can control the morphology of endothelial cells and ensure their density, thus playing a vital role in corneal endothelial development. Primary cilia are also essential for the development and repair of the corneal endothelium. For example, in the early stages of embryonic development in zebrafish, the primary cilia located in the vascular endothelium are responsible for the recruitment of dorsal aortic cells (Grimes et al., 2016). In mice, however, the cilia are not essential for the development of the vascular endothelium (Dinsmore & Reiter, 2016). Taken together, these findings suggest a complex nature for the role of primary cilia in endothelial development in different tissues.
ALTERATION OF CILIUM-RELATED SIGNALLING PATHWAYS IN CORNEAL DISEASES
An increasing number of cilium-related signaling molecules have been implicated in corneal diseases. Corneal diseases can arise from dysregulation in corneal development or healing by primary cilia (Portal et al., 2019). Corneal diseases include conditions such as keratoconus and dry eye, which are characterized by alterations in the thickness of the corneal epithelial layer (Cui et al., 2014; Serrao et al., 2019). Keratoconus is the most common corneal epithelial disease and is characterized by forward bulging of the corneal tip and thinning of the central cornea (Figure 1) (Kanellopoulos, 2009; McMonnies, 2015). Endothelial dystrophy is the most common endothelial disease, and includes Fuchs endothelial corneal dystrophy (FECD), congenital vascular endothelial dystrophy (CHED), and posterior polymorphous corneal dystrophy (PPCD) (Ahn et al., 2017; Keller et al., 2015; Vedana et al., 2016). These diseases can result in massive loss of endothelial cells and damage to the tight junctions, leading to corneal edema (Figure 1). Changes in the thickness of the corneal epithelium and loss of corneal endothelial cells suggest that primary cilia play an important role in these diseases. The mechanisms of corneal disease pathogenesis are poorly understood, but changes in cilium-related molecules may be involved in their pathogenesis.
Genomic and proteomic studies have reported differentially expressed proteins in diseased corneal tissue compared to normal tissue, including cilium-related proteins. For example, transforming growth factor-β1 (TGF-β1) is significantly up-regulated in patients with keratoconus and can induce histone deacetylase 6 (HDAC6) expression, thereby stimulating cilium disassembly (Bykhovskaya et al., 2016; Ran et al., 2020; Yu et al., 2016). The TGF-β signaling pathway, which is an essential pathway in primary cilia, induces phosphorylation of a variety of proteins that regulate cilium-related vesicle transport (Mönnich et al., 2018). Furthermore, tissue inhibitor of metalloproteinases-3 (TIMP3) is significantly up-regulated in patients with keratoconus. When fibro/adipogenic progenitors (FAPs) are damaged, they are transformed into adipocytes via the Hedgehog signaling pathway through cilia (Kopinke et al., 2017; Loukovitis et al., 2019). In contrast, collagen 1A1 and 1A2 (COL1A1 and COL1A2) can inhibit HDAC expression and promote cilium growth and are down-regulated in keratoconus (Chaerkady et al., 2013; Marini et al., 2007; Xu et al., 2018). Furthermore, vascular endothelial growth factor (VEGF) is down-regulated in keratoconus (Saghizadeh et al., 2001), and its receptor can be found on primary cilia (Wang et al., 2017). Primary cilia also play a role in VEGF-regulated trophoblast cell invasion by activating mitogen activated protein kinase (MAPK) signaling and subsequently matrix metalloproteinase-2 (MMP2) to promote trophoblast invasion (Wang et al., 2017).
Similarly, inflammatory molecules are highly expressed in dry eye disease and are negatively related to ciliogenesis (Baek et al., 2017). Compared to the normal eye, interleukin-1β (IL-1β) expression is significantly up-regulated in dry eye, which can induce toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling, with primary cilia affecting this process and ultimately mediating the inflammatory response (Baek et al., 2017; Na et al., 2012). In addition, intercellular adhesion molecule-1 (ICAM-1) and IL-6 are also significantly increased in patients with dry eye (Na et al., 2012; Zhong et al., 2012). When bronchial epithelial cell cilia decrease or disappear, the expression of some inflammatory factors, such as ICAM-1 and IL-6, increases (Chen et al., 2019; Shen et al., 2020). Therefore, cilia also play an important role in regulating the expression of inflammatory mediators, and cilium-related molecules may be used as diagnostic indicators in the treatment of dry eye. In contrast, transcription factor paired box 6 (PAX6) is down-regulated in dry eye, which can regulate the activity of outer dense fiber 2 (ODF2), a centrosomal protein, and participate in the assembly of mother centrioles (Chen et al., 2013; Tylkowski et al., 2015).
Multiple gene mutations and protein abnormalities in the Wnt signaling pathway have been implicated in corneal endothelial malnutrition (Wieben et al., 2019). As ciliary function is closely associated with Wnt signaling (Anvarian et al., 2019), we speculate that primary cilia may be involved in corneal endothelial dystrophy by affecting the Wnt signaling pathway. Clusters of regularly interspaced short palindromic repeats (CRISPR)-guided single-molecule real-time (SMRT) sequencing reveals that FECD patients harbor CTG trinucleotide repeats in the intron of transcription factor 4 (TCF4) (Hafford-Tear et al., 2019; Tang et al., 2016). As a downstream transcription factor in Wnt signaling, TCF4, together with β-catenin, promotes G1/S transition of the cell cycle, thereby promoting cell proliferation. Abnormal TCF4 trinucleotide repeats are likely to affect endothelial cell ciliary function (Stolz et al., 2015). Cilia can negatively regulate the Wnt/β-catenin pathway. This negative regulation may lead to alterations in TCF4. Sequencing of FECD patients also reveals that zinc finger E-box binding homeobox 1 (ZEB1) has missense mutations in p.Glu733Lys, p.Ala818Val, p.Gln840. These mutation sites are located in conserved regions that are probably related to the pathogenesis of this disease (Gupta et al., 2015). Moreover, ZEB1 expression is down-regulated in FECD (Tang et al., 2016). The absence of ZEB1 affects the ZEB1-ovo-like zinc finger 2 (OVOL2)-grainyhead-like transcription factor 2 (GRHL2) axis and impairs corneal endothelial cell morphology by abnormally activating Wnt signaling (Chung et al., 2019). In the highly malignant brain tumor glioblastoma multiforme (GBM), primary cilia play a role in GBM invasion by guiding tumor cell migration and cells with cilia demonstrate high ZEB1 expression (Sarkisian et al., 2014). Elucidation of whether primary cilia regulate the Wnt signaling pathway through ZEB1 may provide novel ideas for the treatment of FECD.
TARGETING CILIA FOR CORNEAL DISEASE TREATMENT
A commonly used treatment for keratoconus is corneal collagen cross-linking (CXL), which can enhance corneal hardness, thereby successfully inhibiting the progression of this disorder (O'Brart, 2014). However, CXL does not improve visual defects. To address this limitation, combining CXL with photorefractive keratectomy (PRK) can not only restore corneal stability but can also improve vision; however, the long-term safety and stability of this combined technique has yet to be established (Mohammadpour et al., 2017). In addition, cyclosporine A can be used to treat epithelial dysfunction in dry eye, but it can be expensive, and side-effects include eye burns (de Paiva et al., 2019). For endothelial cell dysfunction, the most common, currently available treatment is corneal transplantation, which can quickly restore vision but suffers from the possibility of transplant rejection, and donor corneas are very limited (Shen et al., 2017). Corneal endothelial cells can be expanded in vitro and then transplanted, but this approach is limited by low cell proliferation, cell senescence, and difficulty in transplantation (Feizi, 2018). These conditions can also be treated with topical drugs such as Y-27632, a selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK), but only for symptom relief (Okumura et al., 2011).
Therefore, existing treatments for corneal diseases have problems with instability, adverse reactions, and durability of effects. Gene therapy exhibits great potential for the treatment of corneal diseases (Williams & Irani, 2016). Given that primary cilia and their molecular abnormalities play an important role in corneal diseases, gene therapy could be used to restore the expression of dysfunctional molecules to prevent and treat corneal diseases. For cilium-related molecules up-regulated in corneal diseases, gene silencing can be performed by RNA interference (RNAi), such as with small interfering RNAs, microRNAs, or short hairpin RNAs (Toyono et al., 2016; Yang et al., 2016). RNAi-mediated inhibition of VEGFR1 can effectively reduce corneal angiogenesis and improve the survival rate of corneal grafts in mice (Cho et al., 2012). For cilium-related molecules with down-regulated expression, gene therapy could be performed by overexpressing these molecules (Das et al., 2014). These cilium-related genes could be transported by suitable carriers, such as nanoparticles or viral vectors, and delivered to eye tissues by topical or systemic medication or injection (Solinís et al., 2015). For example, the ability of adeno-associated viruses (AAVs) to deliver repair genes to the corneal epithelium or endothelium has been verified in animal experiments, although the uncertainty of inflammatory response limits the development of this technique (Lu et al., 2016). CRISPR-based gene editing technology could also be used to specifically modify target genes (Chen & Niu, 2019; Ma et al., 2018; Sun et al., 2019); however, this method is still in its infancy in regard to the treatment of ocular surface diseases (Torrecilla et al., 2018). Encouragingly, the CRISPR/Cas9 technique has been shown to target gRNAs to specific viral genes, effectively eliminating HSV-1-induced corneal inflammation (van Diemen et al., 2016). Thus, the potential of CRISPR/Cas9-based gene therapy in the treatment of cilium-related corneal diseases warrants further investigation.
CONCLUDING REMARKS
There is increasing evidence that corneal development is regulated by primary cilia. Primary cilia play a vital role in corneal epithelial layer development (especially maintenance of epithelial cell layer thickness) and corneal endothelial morphogenesis (especially regulation of endothelial cell density). However, outstanding questions remain about the regulation of corneal development by primary cilia. For example, do primary cilia play a regulatory role in the corneal cell cycle? As corneal epithelial cells develop from limbal stem cells, do primary cilia affect epithelial cell proliferation by affecting the functions of limbal stem cells (such as cell division and migration)? Corneal wound healing is a complex process, including the regulation of coagulation factors and cell migration, so are primary cilia involved in regulating corneal epithelial repair by participating in these processes? Primary cilia are only present during corneal endothelial development and repair, so how do cilium-based cellular dynamics maintain the hexagonal shape of endothelial cells? When endothelial cells are stretched or damaged, how are primary cilia induced, and which cilium-related molecules and signaling pathways play a role? Solving these problems will help us to better understand corneal development and homeostasis.
While many studies have explored the effects of primary cilia in human diseases, relatively little research has been conducted on how primary cilia regulate corneal diseases. Some abnormal cilium-related molecules have been found in corneal diseases, but the mechanisms of how these cilium-related molecules participate in corneal diseases are not particularly clear. For instance, are cilium-related molecules cornea-specific? Are there defects in primary cilia in the pathogenesis of corneal diseases, and how are these defects caused? Are there any changes in cilium length and number in corneal diseases? What is the relationship between primary cilia and corneal inflammation markers? How do primary cilia participate in signaling pathways (such as Notch and Wnt signaling) in corneal diseases? Can we use gene therapy to restore normal expression in primary cilia for the purpose of disease treatment? The study of primary cilia is an ideal starting point for investigations on corneal diseases, and gene therapy holds promise for the development of new and effective drugs for the prevention and treatment of corneal diseases. Future research on these issues will help us to understand the role of primary cilia in corneal development and disease and provide new ideas for corneal disease therapies.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
T.S. wrote the manuscript and drew the figures. J.Z. conceived the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the Taishan Scholars Program of Shandong Province (20161201)
References
- 1.Ahn YJ, Choi SI, Yum HR, Shin SY, Park SH Clinical features in children with posterior polymorphous corneal dystrophy. Optometry and Vision Science. 2017;94(4):476–481. doi: 10.1097/OPX.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 2.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST Cellular signalling by primary cilia in development, organ function and disease. Nature Reviews Nephrology. 2019;15(4):199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek H, Shin HJ, Kim JJ, Shin N, Kim S, Yi MH, et al Primary cilia modulate tlr4-mediated inflammatory responses in hippocampal neurons. Journal of Neuroinflammation. 2017;14(1):189. doi: 10.1186/s12974-017-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK The primary cilium as a complex signaling center. Current Biology. 2009;19(13):R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2819–2824. doi: 10.1073/pnas.1016702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bykhovskaya Y, Gromova A, Makarenkova HP, Rabinowitz YS Abnormal regulation of extracellular matrix and adhesion molecules in corneas of patients with keratoconus. International Journal of Keratoconus and Ectatic Corneal Diseases. 2016;5(2):63–70. doi: 10.5005/jp-journals-10025-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaerkady R, Shao HJ, Scott SG, Pandey A, Jun AS, Chakravarti S The keratoconus corneal proteome: Loss of epithelial integrity and stromal degeneration. Journal of Proteomics. 2013;87:122–131. doi: 10.1016/j.jprot.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen XM, Li YP, Hua CF, Jia PJ, Xing YP, Xue BH, et al Establishment of rapid risk assessment model for cigarette smoke extract exposure in chronic obstructive pulmonary disease. Toxicology Letters. 2019;316:10–19. doi: 10.1016/j.toxlet.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Chen YT, Chen FY, Vijmasi T, Stephens DN, Gallup M, McNamara NA Pax6 downregulation mediates abnormal lineage commitment of the ocular surface epithelium in aqueous-deficient dry eye disease. PLoS One. 2013;8(10):e77286. doi: 10.1371/journal.pone.0077286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZZ, Niu YY Stem cell therapy for parkinson's disease using non-human primate models. Zoological Research. 2019;40(5):349–357. doi: 10.24272/j.issn.2095-8137.2019.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YK, Zhang XH, Uehara H, Young JR, Archer B, Ambati B Vascular endothelial growth factor receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Investigative Ophthalmology & Visual Science. 2012;53(13):8458–8471. doi: 10.1167/iovs.12-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung DD, Zhang WL, Jatavallabhula K, Barrington A, Jung JY, Aldave AJ Alterations in GRHL2-OVOL2-ZEB1 axis and aberrant activation of wnt signaling lead to altered gene transcription in posterior polymorphous corneal dystrophy. Experimental Eye Research. 2019;188:107696. doi: 10.1016/j.exer.2019.107696. [DOI] [PubMed] [Google Scholar]
- 13.Collin SP, Collin HB Primary cilia in vertebrate corneal endothelial cells. Cell Biology International. 2004;28(2):125–130. doi: 10.1016/j.cellbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Cui XH, Hong JX, Wang F, Deng SX, Yang YJ, Zhu XY, et al Assessment of corneal epithelial thickness in dry eye patients. Optometry and Vision Science. 2014;91(12):1446–1454. doi: 10.1097/OPX.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cvekl A, Tamm ER Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26(4):374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das SK, Gupta I, Cho YK, Zhang XH, Uehara H, Muddana SK, et al Vimentin knockdown decreases corneal opacity. Investigative Ophthalmology & Visual Science. 2014;55(7):4030–4040. doi: 10.1167/iovs.13-13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Paiva CS, Pflugfelder SC, Ng SM, Akpek EK Topical cyclosporine a therapy for dry eye syndrome. Cochrane Database of Systematic Reviews. 2019;9(9):Cd010051. doi: 10.1002/14651858.CD010051.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DelMonte DW, Kim T Anatomy and physiology of the cornea. Journal of Cataract & Refractive Surgery. 2011;37(3):588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Dinsmore C, Reiter JF Endothelial primary cilia inhibit atherosclerosis. EMBO Reports. 2016;17(2):156–166. doi: 10.15252/embr.201541019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, et al Down-regulation of notch signaling during corneal epithelial proliferation. Molecular Vision. 2008;14:1041–1049. [PMC free article] [PubMed] [Google Scholar]
- 21.Douvaras P, Mort RL, Edwards D, Ramaesh K, Dhillon B, Morley SD, et al Increased corneal epithelial turnover contributes to abnormal homeostasis in the pax6+/- mouse model of aniridia . PLoS One. 2013;8(8):e71117. doi: 10.1371/journal.pone.0071117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelhauser HF The balance between corneal transparency and edema: the proctor lecture. Investigative Ophthalmology & Visual Science. 2006;47(5):1754–1767. doi: 10.1167/iovs.05-1139. [DOI] [PubMed] [Google Scholar]
- 23.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E A role for the primary cilium in notch signaling and epidermal differentiation during skin development. Cell. 2011;145(7):1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feizi S Corneal endothelial cell dysfunction: etiologies and management. Therapeutic Advances in Ophthalmology. 2018;10 doi: 10.1177/2515841418815802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimes DT, Boswell CW, Morante NFC, Henkelman RM, Burdine RD, Ciruna B Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science. 2016;352(6291):1341–1344. doi: 10.1126/science.aaf6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisanti L, Revenkova E, Gordon RE, Iomini C Primary cilia maintain corneal epithelial homeostasis by regulation of the notch signaling pathway. Development. 2016;143(12):2160–2171. doi: 10.1242/dev.132704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta R, Kumawat BL, Paliwal P, Tandon R, Sharma N, Sen S, et al Association of ZEB1 and TCF4 rs613872 changes with late onset fuchs endothelial corneal dystrophy in patients from northern india. Molecular Vision. 2015;21:1252–1260. [PMC free article] [PubMed] [Google Scholar]
- 28.Hafford-Tear NJ, Tsai YC, Sadan AN, Sanchez-Pintado B, Zarouchlioti C, Maher GJ, et al CRISPR/cas9-targeted enrichment and long-read sequencing of the fuchs endothelial corneal dystrophy-associated tcf4 triplet repeat. Genetics in Medicine. 2019;21(9):2092–2102. doi: 10.1038/s41436-019-0453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoar RM Embryology of the eye. Environmental Health Perspectives. 1982;44:31–34. doi: 10.1289/ehp.824431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce NC Proliferative capacity of the corneal endothelium. Progress in Retinal and Eye Research. 2003;22(3):359–389. doi: 10.1016/S1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Kanellopoulos AJ Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided prk for treatment of keratoconus. Journal of Refractive Surgery. 2009;25(9):S812–S818. doi: 10.3928/1081597X-20090813-10. [DOI] [PubMed] [Google Scholar]
- 32.Keller J, Giralt J, Alforja S, Casaroli-Marano RP Altering the clinical course of sorsby fundus dystrophy with the use of anti-vascular endothelial growth factor intraocular therapy. Retinal Cases and Brief Reports. 2015;9(2):104–105. doi: 10.1097/ICB.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 33.Kopinke D, Roberson EC, Reiter JF Ciliary hedgehog signaling restricts injury-induced adipogenesis. Cell. 2017;170(2):340–351. e312. doi: 10.1016/j.cell.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK The oak ridge polycystic kidney mouse: Modeling ciliopathies of mice and men. Developmental Dynamics. 2008;237(8):1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loukovitis E, Kozeis N, Gatzioufas Z, Kozei A, Tsotridou E, Stoila M, et al The proteins of keratoconus: a literature review exploring their contribution to the pathophysiology of the disease. Advances in Therapy. 2019;36(9):2205–2222. doi: 10.1007/s12325-019-01026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Ai JZ, Gessler D, Su Q, Tran K, Zheng Q, et al Efficient transduction of corneal stroma by adeno-associated viral serotype vectors for implications in gene therapy of corneal diseases. Human Gene Therapy. 2016;27(8):598–608. doi: 10.1089/hum.2015.167. [DOI] [PubMed] [Google Scholar]
- 37.Lwigale PY Corneal development: different cells from a common progenitor. Progress in Molecular Biology and Translational Science. 2015;134:43–59. doi: 10.1016/bs.pmbts.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Lyu R, Zhou J The multifaceted roles of primary cilia in the regulation of stem cell properties and functions. Journal of Cellular Physiology. 2017;232(5):935–938. doi: 10.1002/jcp.25683. [DOI] [PubMed] [Google Scholar]
- 39.Ma X, Wong ASY, Tam HY, Tsui SYK, Chung DLS, Feng B In vivo genome editing thrives with diversified CRISPR technologies . Zoological Research. 2018;39(2):58–71. doi: 10.24272/j.issn.2095-8137.2017.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Human Mutation. 2007;28(3):209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maugeri G, Longo A, D'Amico AG, Rasa DM, Reibaldi M, Russo A, et al Trophic effect of PACAP on human corneal endothelium. Peptides. 2018;99:20–26. doi: 10.1016/j.peptides.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 42.May-Simera HL, Wan Q, Jha BS, Hartford J, Khristov V, Dejene R, et al Primary cilium-mediated retinal pigment epithelium maturation is disrupted in ciliopathy patient cells. Cell Reports. 2018;22(1):189–205. doi: 10.1016/j.celrep.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMonnies CW Inflammation and keratoconus. Optometry and Vision Science. 2015;92(2):e35–e41. doi: 10.1097/OPX.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadpour M, Masoumi A, Mirghorbani M, Shahraki K, Hashemi H Updates on corneal collagen cross-linking: Indications, techniques and clinical outcomes. Journal of Current Ophthalmology. 2017;29(4):235–247. doi: 10.1016/j.joco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mönnich M, Borgeskov L, Breslin L, Jakobsen L, Rogowski M, Doganli C, et al CEP128 localizes to the subdistal appendages of the mother centriole and regulates TGF-β/bmp signaling at the primary cilium. Cell Reports. 2018;22(10):2584–2592. doi: 10.1016/j.celrep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 46.Morishige N, Sonoda KH Bullous keratopathy as a progressive disease: evidence from clinical and laboratory imaging studies. Cornea. 2013;32(Suppl 1):S77–S83. doi: 10.1097/ICO.0b013e3182a1bc65. [DOI] [PubMed] [Google Scholar]
- 47.Na KS, Mok JW, Kim JY, Rho CR, Joo CK Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Investigative Ophthalmology & Visual Science. 2012;53(9):5443–5450. doi: 10.1167/iovs.11-9417. [DOI] [PubMed] [Google Scholar]
- 48.O'Brart DPS Corneal collagen cross-linking: a review. Journal of Optometry. 2014;7(3):113–124. doi: 10.1016/j.optom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hirata K, et al Enhancement of corneal endothelium wound healing by rho-associated kinase (ROCK) inhibitor eye drops. British Journal of Ophthalmology. 2011;95(7):1006–1009. doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira-Soto L, Efron N Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Pala R, Alomari N, Nauli SM Primary cilium-dependent signaling mechanisms. International Journal of Molecular Sciences. 2017;18(11):2272. doi: 10.3390/ijms18112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pazour GJ, Quarmby L, Smith AO, Desai PB, Schmidts M Cilia in cystic kidney and other diseases. Cellular Signalling. 2020;69:109519. doi: 10.1016/j.cellsig.2019.109519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitaval A, Tseng Q, Bornens M, Théry M Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. The Journal of Cell Biology. 2010;191(2):303–312. doi: 10.1083/jcb.201004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portal C, Rompolas P, Lwigale P, Iomini C Primary cilia deficiency in neural crest cells models anterior segment dysgenesis in mouse. eLife. 2019;8:e52423. doi: 10.7554/eLife.52423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ran J, Liu M, Feng J, Li HX, Ma HX, Song T, et al ASK1-mediated phosphorylation blocks hdac6 ubiquitination and degradation to drive the disassembly of photoreceptor connecting cilia. Developmental Cell. 2020;53(3):287–299. doi: 10.1016/j.devcel.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Reiter JF, Leroux MR Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology. 2017;18(9):533–547. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy O, Leclerc VB, Bourget JM, Thériault M, Proulx S Understanding the process of corneal endothelial morphological change in vitro . Investigative Ophthalmology & Visual Science. 2015;56(2):1228–1237. doi: 10.1167/iovs.14-16166. [DOI] [PubMed] [Google Scholar]
- 58.Saghizadeh M, Chwa M, Aoki A, Lin B, Pirouzmanesh A, Brown DJ, et al Altered expression of growth factors and cytokines in keratoconus, bullous keratopathy and diabetic human corneas. Experimental Eye Research. 2001;73(2):179–189. doi: 10.1006/exer.2001.1028. [DOI] [PubMed] [Google Scholar]
- 59.Sarkisian MR, Siebzehnrubl D, Hoang-Minh L, Deleyrolle L, Silver DJ, Siebzehnrubl FA, et al Detection of primary cilia in human glioblastoma. Journal of Neuro-Oncology. 2014;117(1):15–24. doi: 10.1007/s11060-013-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serrao S, Lombardo G, Cali C, Lombardo M Role of corneal epithelial thickness mapping in the evaluation of keratoconus. Contact Lens and Anterior Eye. 2019;42(6):662–665. doi: 10.1016/j.clae.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 61.Shen HY, Zhou Y, Zhou QJ, Li MY, Chen J Mudskipper interleukin-34 modulates the functions of monocytes/macrophages via the colony-stimulating factor-1 receptor 1. Zoological Research. 2020;41(2):123–137. doi: 10.24272/j.issn.2095-8137.2020.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen L, Sun P, Zhang CW, Yang L, Du LQ, Wu XY Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Scientific Reports. 2017;7(1):13400. doi: 10.1038/s41598-017-13787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solinís MA, del Pozo-Rodríguez A, Apaolaza PS, Rodríguez-Gascón A Treatment of ocular disorders by gene therapy. European Journal of Pharmaceutics and Biopharmaceutics. 2015;95:331–342. doi: 10.1016/j.ejpb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 64.Stolz A, Neufeld K, Ertych N, Bastians H Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Reports. 2015;16(4):490–499. doi: 10.15252/embr.201439410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun YZ, Liu ST, Li XM, Zou K Progress in in vitro culture and gene editing of porcine spermatogonial stem cells . Zoological Research. 2019;40(5):343–348. doi: 10.24272/j.issn.2095-8137.2019.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang H, Zhang W, Yan XM, Wang LP, Dong H, Shou T, et al Analysis of SLC4A11, ZEB1, LOXHD1, COL8A2 and TCF4 gene sequences in a multi-generational family with late-onset fuchs corneal dystrophy. International Journal of Molecular Medicine. 2016;37(6):1487–1500. doi: 10.3892/ijmm.2016.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torrecilla J, Del Pozo-Rodríguez A, Vicente-Pascual M, Solinís MÁ, Rodriguez-Gascon A Targeting corneal inflammation by gene therapy: emerging strategies for keratitis. Experimental Eye Research. 2018;176:130–140. doi: 10.1016/j.exer.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Toyono T, Usui T, Villarreal G Jr, Kallay L, Matthaei M, Vianna LM, et al Microrna-29b overexpression decreases extracellular matrix mrna and protein production in human corneal endothelial cells. Cornea. 2016;35(11):1466–1470. doi: 10.1097/ICO.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tylkowski MA, Yang K, Hoyer-Fender S, Stoykova A Pax6 controls centriole maturation in cortical progenitors through odf2 . Cellular and Molecular Life Sciences. 2015;72(9):1795–1809. doi: 10.1007/s00018-014-1766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Diemen FR, Kruse EM, Hooykaas MJG, Bruggeling CE, Schürch AC, van Ham PM, et al CRISPR/cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathogens. 2016;12(6):e1005701. doi: 10.1371/journal.ppat.1005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vedana G, Villarreal G Jr, Jun AS Fuchs endothelial corneal dystrophy: current perspectives. Clinical Ophthalmology. 2016;10:321–330. doi: 10.2147/OPTH.S83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang CY, Tsai HL, Syu JS, Chen TY, Su MT Primary cilium-regulated EG-VEGF signaling facilitates trophoblast invasion. Journal of Cellular Physiology. 2017;232(6):1467–1477. doi: 10.1002/jcp.25649. [DOI] [PubMed] [Google Scholar]
- 73.Wieben ED, Baratz KH, Aleff RA, Kalari KR, Tang XJ, Maguire LJ, et al Gene expression and missplicing in the corneal endothelium of patients with a TCF4 trinucleotide repeat expansion without fuchs' endothelial corneal dystrophy . Investigative Ophthalmology & Visual Science. 2019;60(10):3636–3643. doi: 10.1167/iovs.19-27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams KA, Irani YD Gene therapy and gene editing for the corneal dystrophies. Asia-Pacific Journal of Ophthalmology. 2016;5(4):312–316. doi: 10.1097/APO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 75.Wood CR, Huang KY, Diener DR, Rosenbaum JL The cilium secretes bioactive ectosomes. Current Biology. 2013;23(10):906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Q, Liu WW, Liu XL, Otkur W, Hayashi T, Yamato M, et al Type I collagen promotes primary cilia growth through down-regulating HDAC6-mediated autophagy in confluent mouse embryo fibroblast 3T3-L1 cells. Journal of Bioscience and Bioengineering. 2018;125(1):8–14. doi: 10.1016/j.jbiosc.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Feng S, Yi G, Wu W, Yi R, Lu X, Xu W, Qiu H Inhibition of RelA expression via RNA interference induces immune tolerance in a rat keratoplasty model. Molecular Immunology. 2016;73:88–97. doi: 10.1016/j.molimm.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y, Hao HJ, Wu XF, Guo S, Liu Y, Ran J, et al Mixed-lineage leukemia protein 2 suppresses ciliary assembly by the modulation of actin dynamics and vesicle transport. Cell Discovery. 2019;5(1):33. doi: 10.1038/s41421-019-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang YF, Ran J, Liu M, Li DW, Li YY, Shi XJ, et al CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Research. 2014;24(11):1342–1353. doi: 10.1038/cr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu F, Guo S, Li T, Ran J, Zhao W, Li DW, et al Ciliary defects caused by dysregulation of O-GlcNAc modification are associated with diabetic complications. Cell Research. 2019;29(2):171–173. doi: 10.1038/s41422-018-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu F, Ran J, Zhou J Ciliopathies: does HDAC6 represent a new therapeutic target? Trends in Pharmacological Sciences. 2016;37(2):114–119. doi: 10.1016/j.tips.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Zhong M, Gadek TR, Bui M, Shen W, Burnier J, Barr KJ, et al Discovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eye. ACS Medicinal Chemistry Letters. 2012;3(3):203–206. doi: 10.1021/ml2002482. [DOI] [PMC free article] [PubMed] [Google Scholar]