Abstract
Sperm are specialized cells that require adenosine triphosphate (ATP) to support their function. Maintaining sperm energy homeostasis in vitro is vitally important to improve the efficacy of boar sperm preservation. Metformin can activate 5′-AMP-activated protein kinase (AMPK) to improve metabolic flexibility and maintain energy homeostasis. Thus, the aim of the present study was to investigate whether metformin can improve boar sperm quality through AMPK mediation of energy metabolism. Sperm motility parameters, membrane integrity, acrosome integrity, mitochondrial membrane potential (ΔΨm), ATP content, glucose uptake, and lactate efflux were analyzed. Localization and expression levels of AMPK and phospho-Thr172-AMPK (p-AMPK) were also detected by immunofluorescence and western blotting. We found that metformin treatment significantly increased sperm motility parameters, ΔΨm, and ATP content during storage at 17 °C. Moreover, results showed that AMPK was localized at the acrosomal region, connecting piece, and midpiece of sperm and p-AMPK was distributed at the post-acrosomal region, connecting piece, and midpiece. When sperm were incubated with metformin for 4 h at 37 °C, sperm motility parameters, ΔΨm, ATP content, p-AMPK, glucose uptake, and lactate efflux all significantly increased, whereas the addition of Compound C treatment, an inhibitor of AMPK, counteracted these positive effects. Together, our results suggest that metformin promotes AMPK activation, which contributes to the maintenance of energy hemostasis and mitochondrial activity, thereby maintaining boar sperm functionality and improving the efficacy of semen preservation.
Keywords: Metformin, AMPK, Sperm, Energy metabolism, Glycolysis
INTRODUCTION
The preservation of boar semen and artificial insemination (AI) are widely used in the pig industry (Waberski et al., 2019). Thus, developing effective strategies to improve sperm fertilizing capability is important. Mammalian sperm are specialized cells with high energy requirements for motility and fertilization (Rodriguez-Gil & Bonet, 2016). The biochemical and physiological changes that occur in sperm during preservation can adversely influence the restoration of energy-dependent sperm function (Nguyen et al., 2015). Thus, energy management plays an essential role in the modulation and maintenance of sperm function (Rodriguez-Gil, 2006). Glycolysis and oxidative phosphorylation (OXPHOS) are two crucial pathways for energy production in boar sperm (Zhu et al., 2019a). The precise equilibrium between glycolysis and mitochondrial oxidation differs among species (Rodriguez-Gil & Bonet, 2016). In boar sperm, this equilibrium is greatly unbalanced in favor of glycolysis, which is the major energy-obtaining pathway in the presence of sugars (Rodriguez-Gil & Bonet, 2016). Understanding the regulation of sperm metabolism could help improve the efficacy of semen storage (Martin-Hidalgo et al., 2018).
Previous studies have demonstrated that 5′-AMP-activated protein kinase (AMPK), a cellular energy sensor, plays a crucial role in restoring energy homeostasis during metabolic disorders (Hardie et al., 2012). Increased hepatic AMPK activity can contribute to the fed-to-fasted transition from anabolism to catabolism in the liver (Foretz & Viollet, 2011). Under low cellular energy circumstances, AMPK is activated and switches on alternative catabolic pathways, which generate ATP (Lin & Hardie, 2018). In somatic cells, AMPK is capable of restoring energy balance and metabolic flexibility (Vazirian et al., 2018). Moreover, AMPK plays an indispensable role in sustaining energy metabolism homeostasis for male fertility and acts as a crucial regulator of sperm function, including that of motility, membrane structure, acrosome integrity, and mitochondrial membrane potential (ΔΨm) (Nguyen, 2017). Maintaining AMPK activity within an appropriate range is necessary for sperm function (Martin-Hidalgo et al., 2018).
Metformin can activate AMPK and plays a key role in the modulation of glucose metabolism and mitochondrial function (Berstein, 2012; Vial et al., 2019). Previous research has also indicated that metformin can induce AMPK phosphorylation and improve motility, viability, acrosome reaction, and lactate production in chicken sperm during incubation at 35 °C (Nguyen et al., 2014). Similarly, in our previous study, we found that metformin enhances motility, membrane integrity, and acrosome reaction, and maintains ΔΨm, lactate content, and ATP content in goat sperm in vitro (Zhu et al., 2018). However, high metformin doses have been shown to reduce motility in mouse sperm incubated at 37 °C (Bertoldo et al., 2014) and block ΔΨm and inhibit boar sperm motility during incubation at 38.5 °C or preservation at 17 °C (Hurtado de Llera et al., 2018).
Therefore, the goal of the present study was to elucidate whether the addition of metformin at low concentration was beneficial for boar sperm survival, and if so, to elucidate the related underlining mechanism.
MATERIALS AND METHODS
Experimental design
Experiment 1 was designed to investigate whether metformin has beneficial effects on boar sperm functionality during preservation at 17 °C. Sperm were preserved in Modena extender with different concentrations of metformin (50, 100, 200, 500 µmol/L) at 17 °C for 13 d. Sperm motility was analyzed every 2 d. Plasma membrane integrity, acrosome integrity, ΔΨm, and cellular ATP levels were detected every 4 d.
Experiment 2 sought to confirm the expression and localization of AMPK protein and p-AMPK in boar sperm and to elucidate whether metformin was involved in the phosphorylation of AMPK. The expression and localization of AMPK and p-AMPK in boar sperm were detected by western blotting and immunofluorescence analysis. The levels of p-AMPK in the metformin and inhibitor (dorsomorphin dihydrochloride, Compound C) groups after 4 h of incubation at 37 °C or after 1, 5, 9, and 13 d at 17 °C were detected by western blot analysis. Samples for localization of AMPK and p-AMPK were from fresh semen.
Experiment 3 was devised to investigate whether metformin also protected boar sperm at 37 °C and whether it exerted its role through regulating AMPK activity. Sperm were preserved in Modena extender at 37 °C for 4 h with 200 µmol/L metformin in the presence or absence of Compound C. Sperm motility, membrane integrity, and ΔΨm were evaluated after 1 and 4 h of incubation at 37 °C. ATP content, glucose uptake capacity, lactate efflux, and lactate dehydrogenase (LDH) activity were detected after 4 h of incubation.
Reagents and media
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (USA). Compound C was obtained from MedChemExpress (China). 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) was purchased from the Cayman Chemical Company (USA) and 4',6-diamidino-2-phenylindole (DAPI) was obtained from the Beyotime Institute of Biotechnology (China).
Modena solution was used as the basic medium, which contained 152.8 mmol/L D-glucose, 26.7 mmol/L trisodium citrate, 11.9 mmol/L sodium hydrogen carbonate, 15.1 mmol/L citric acid, 6.3 mmol/L ethylenediamine tetraacetic acid disodium (EDTA-2Na), 46.6 mmol/L tris (hydroxymethyl)aminomethane (Tris), and 4 g/L bovine serum albumin (BSA) (pH=7.2). The Modena solution was supplemented with penicillin G sodium salt (1 000 IU/mL; Solarbio, China), streptomycin sesquisulfate (1 mg/mL; Solarbio, China), and polymyxin B (400 IU/mL; Amresco, USA), and filtered with a 0.22 μm filter to prevent bacterial contamination.
Semen collection and processing
Seven mature and fertile Duroc boars (aged 15–28 months) were used in this study. The boars were housed individually, maintained under natural daylight, and provided with free access to food and water. The sperm-rich fraction was collected with the gloved hand technique twice a week, with fresh semen placed in a 37 °C bath and delivered to the laboratory within 15 min for the evaluation of sperm motility and concentration. Only semen samples with over 80% total motility were used for this study. The ejaculated semen was diluted by Modena solution containing metformin at a final concentration of 1×108 sperm/mL for the following processes. The liquid storage experiment was performed to determine whether metformin helped maintain sperm functionality during long-term preservation at 17 °C. Moreover, the diluted semen was also incubated at 37 °C. All experimental procedures involving the care and use of animals were approved by the Northwest A&F University Institutional Animal Care and Use Committee.
Motility
Sperm motility parameters were evaluated using computer-assisted sperm analysis (CASA; HVIEW, China), as per Zhu et al. (2018). Samples (1 mL) were incubated at 37 °C for 5 min before evaluation. The standard parameter settings were set at 30 frames/s. Total motility was defined as the percentage of sperm with curvilinear velocity (VCL)>10 μm/s, and progressive motility was defined as the percentage of sperm with straight line velocity (VSL)>25 μm/s and straightness of path (STR)≥75%. A minimum of 300 sperm were observed from at least five randomly selected fields with 20 μm CELL-VU® DRM-600 sperm count slides (Millennium Sciences, USA) and a microscopic stage warmer (KITAZATO, Japan).
Integrity of plasma membrane and acrosome
Based on previous study (Zhu et al., 2019b), sperm membrane integrity and intact acrosomes were determined using a Live/Dead Sperm Viability Kit (Invitrogen™, USA) and fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA; Sigma-Aldrich, USA), respectively. Spermatozoa were incubated at 37 °C for 10 min with propidium iodide (PI) and SYBR-14 at a final concentration of 4.8 μmol/L and 0.1 μmol/L, respectively. The spermatozoa were classified into two groups: Group A showing plasma membrane integrity, which only were stained green fluorescence with SYBR-14; Group B showing plasma membrane damage, which only were stained red fluorescence with PI. Furthermore, to evaluate acrosome integrity via FITC-PNA, sperm samples were fixed with absolute methanol and spread onto poly-L-lysine slides and air-dried at room temperature. The stain solution, which included FITC-PNA (100 μg/mL in phosphate-buffered saline (PBS)) and PI (4.8 μmol/L in PBS), was then spread over the slide. Spermatozoa with an intensively bright fluorescence of the acrosomal cap were deemed to have an intact outer acrosomal membrane; spermatozoa with a disrupted fluorescence of the acrosomal cap or no fluorescence of the outer acrosomal membrane were deemed to have a damaged acrosome membrane. The stained sperm were monitored and photographed with an epifluorescent microscope (Nikon 80i, Japan) with a set of filters (400×). A minimum of 1 000 sperm were observed from at least five randomly selected fields for each sample. All samples were identified and evaluated by one observer.
Mitochondrial membrane potential (ΔΨm)
Here, ΔΨm was evaluated using a Mitochondrial Membrane Potential Detection Kit with JC-1 (Beyotime Institute of Biotechnology, China; Zhu et al., 2019b). Briefly, sperm samples (5×106 sperm) were stained with 1×JC-1 (lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) solution at 37 °C for 30 min in the dark. The samples were then centrifuged at 1 000 g at 4 °C for 5 min and washed with JC-1 buffer. Fluorescence intensity of JC-1 (488 nm excitation and 525 nm emission for JC-1-monomer vs 525 nm excitation and 590 nm emission for JC-1-aggregates) was detected by a multi-detection microplate reader (BioTek, Synergy H1, USA). The ΔΨm of the sperm samples was calculated as the fluorescence ratio of JC-1-aggregates (red) to monomer (green). At least three technical replicates were evaluated for each sample.
ATP content
ATP content of sperm was measured using an ATP Assay Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. Briefly, 1 mL aliquots containing 5×107 sperm were centrifuged and re-suspended in ATP assay lysate to release intracellular ATP on ice. Sperm counts were performed for each sample to normalize ATP content to sperm number. Samples were centrifuged at 12 000 g for 10 min at 4 °C. The ATP standard solution (0.5 mmol/L) was diluted to concentrations of 10 nmol/L to 10 µmol/L in succession by ATP assay lysate. Either supernatants or standards (lysate at the same volume as the blank) were added to luciferin/luciferase reagent in opaque 96-wells, and the fluorescence intensity of samples was detected by a multi-detection microplate reader (BioTek, Synergy H1, USA). At least three technical replicates were evaluated for each sample.
Glucose uptake
Fluorescent 2-deoxy-D-glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG; Cayman Chemical Company, USA) was used to measure the glucose uptake capacity of sperm, as per Swegen et al. (2016). Briefly, sperm (2×107) were incubated with or without metformin and Compound C in specific Modena extender (contain 30 mmol/L glucose) with 100 µg/mL 2-NBDG at 37 °C for 4 h. The samples were centrifuged at 1 000 g for 3 min at room temperature and re-suspended with PI (1 μL/mL in specific Modena) for 15 min at 37 °C. Subsequently, the samples were analyzed by flow cytometry after a single wash with specific Modena. The geometric mean of fluorescence intensity (GMFI) of 2-NBDG (green fluorescence, FL-1) was used to indicate the cellular glucose up-take capacity measured after dead (red fluorescence positive, FL-2) cells were gated out of the analysis plot. As specified above, flow cytometry gates were set using a boiled sperm sample as a dead (red fluorescence) positive control.
Lactate efflux and lactate dehydrogenase activity
Lactate efflux was evaluated using a Lactate Content Assay Kit (Nanjing Jiancheng, China) following the manufacturer’s instructions. Briefly, the lactate concentration of the Modena medium was measured by indirectly detecting the NADH formed following lactate oxidation by lactate dehydrogenase (LDH) using a multi-detection microplate reader (BioTek, Synergy H1, USA) at 340 nm. A standard curve with increasing concentrations of lactic acid (0, 0.6, 0.9, 1.5, 3 mmol/L) was constructed before the measurement of samples. Sperm counts were performed for each sample to normalize lactate content to sperm number.
LDH activity was evaluated using an LDH activity assay kit (Beyotime Institute of Biotechnology, China) following the manufacturer’s instructions. Briefly, the sperm samples were lysed with LDH release reagent for 1 h at 37 °C. The LDH activity was measured by indirectly detecting the production of NAD+ with a multi-detection microplate reader (BioTek, Synergy H1, USA). The protein concentration of samples was determined using a BCA Protein Assay Kit (TaKaRa, Japan) for the normalization of LDH activity.
Western blotting
Samples under different treatments were first centrifuged at 2 000 g at room temperature for 3 min, then washed with PBS and re-suspended with RIPA buffer containing 1% phenylmethyl sulfonylfluoride (PMSF) and phosphatase inhibitor (HAT, China) and 1% protease inhibitor cocktail (EDTA free, 100×; MedChemExpress, China) for 10 min at 4 °C. Given that the sperm membrane is relatively unbreakable, the samples were lysed by ultrasonication (20 KHz, 750 W, operating at 30% power, six cycles for 5 s on and 5 s off). After 30 min of lysis at 4 °C, the samples were centrifuged at 12 000 g for 10 min at 4 °C. A portion of the supernatant was used to analyze the concentration of total protein, with the rest mixed with 5×SDS loading buffer and boiled at 90 °C for 5 min. According to previous study (Lv et al., 2018), the lysates containing equivalent protein (30 μg) were determined by SDS-PAGE followed by western blotting in compliance with standard procedures using the following primary antibodies: anti-AMPKα rabbit polyclonal antibody detecting the α-1 and α-2 isoforms of the catalytic subunit (Cell Signaling Technology, 1∶1 000) and anti-p-AMPKα1/2 (Thr172) rabbit polyclonal antibody raised against a short amino acid sequence containing Thr172 phosphorylated AMPKα2 of human origin (Santa Cruz Biotechnology, 1∶1 000). The PVDF membranes were stripped and incubated with loading control antibodies overnight at 4 °C. Alpha-tubulin blotted with anti-alpha-tubulin rabbit polyclonal antibody (Proteintech, 1∶5 000) was used as a loading control.
Immunofluorescence
Aliquots of 100 μL (1×107) sperm samples were fixed with 4% paraformaldehyde for 10 min, followed by washing in PBS, permeabilization with 0.25% Triton X-100 in PBS for 10 min, and washing again in PBS. Samples were incubated with 10% BSA and 100 mmol/L glycine in PBS for 1 h at 37 °C and incubated with one of the primary antibodies for AMPK (Cell Signaling Technology, 1∶100) or p-AMPK (Santa Cruz Biotechnology, 1∶100) overnight at 4 °C. Negative control immunostaining was also performed by omitting the primary antibody. The samples were washed and re-suspended with secondary antibody (FITC conjugated goat anti-rabbit IgG from CWBIO, 1∶200) for 2 h at 37 °C. Finally, the samples were washed and re-stained with DAPI (Beyotime Institute of Biotechnology, 1∶1 000) for 10 min. Images were captured using confocal laser scanning microscopy (Leica TCS SP8, Germany). A minimum of 200 sperm were observed from at least five randomly selected fields for each sample.
Statistical analysis
All values are presented as mean±standard error of the mean (SEM). All data were tested for normality and variance homogeneity prior to statistical analysis. Data were transformed by arc-sin square root transformation when necessary. Data were analyzed by one-way ANOVA (with repeated measures) and the Duncan test was used to perform post hoc analyses. Statistical analysis was determined using the unpaired Student’s t-test for Table 1 and Figures 1, 2. All analyses were performed using SPSS v23.0 for Windows (SPSS Inc., USA). Significant differences among treatments were set at *: P<0.05 and**: P<0.01.
Table 1. Effects of metformin addition on sperm motility parameters during long-term preservation at 17 °C.
| Total motility (%) | |||||
| Control | Met - 50 μmol/L | Met - 100 μmol/L | Met - 200 μmol/L | Met - 500 μmol/L | |
| Sperm motility parameters were determined using the CASA system. Values are presented as mean±SEM. Different lower-case letters indicate significant difference (P<0.05) between treatments; asterisks represent significant difference from D0.*: P<0.05, determined by unpaired Student’st-test. n=5. Met: Metformin. | |||||
| 0 d | 87.37±1.88 | 86.65±1.76 | 86.74±2.75 | 87.65±0.96 | 88.90±1.60 |
| 1 d | 84.20±2.08 | 83.06±3.32 | 86.12±2.87 | 88.17±1.83 | 85.18±2.57 |
| 3 d | 82.17±3.11 | 84.70±3.09 | 85.63±2.29 | 85.30±2.09 | 84.51±2.22 |
| 5 d | 80.07±2.93* | 84.79±2.80 | 85.03±1.58 | 86.65±1.44 | 83.29±2.27 |
| 7 d | 77.63±3.47b* | 83.24±1.67ab | 84.26±2.14ab | 85.35±1.43a | 83.60±1.67ab |
| 9 d | 79.93±3.42b* | 82.16±2.15ab | 84.92±1.94ab | 86.31±1.93a | 81.35±2.61b |
| 11 d | 73.49±2.79c* | 77.80±3.17bc | 79.68±2.99ab | 86.11±2.00a | 82.19±2.69ab |
| 13 d | 66.82±3.90b* | 77.84±2.34a | 79.54±1.84a | 82.92±2.29a | 77.16±2.72a |
| Progressive motility (%) | |||||
| Control | Met - 50 μmol/L | Met - 100 μmol/L | Met - 200 μmol/L | Met - 500 μmol/L | |
| 0 d | 78.46±2.80 | 78.47±2.58 | 76.47±2.04 | 75.34±1.91 | 76.85±3.56 |
| 1 d | 72.29±1.97 | 67.71±4.33 | 74.03±3.84 | 73.88±2.76 | 67.86±3.66 |
| 3 d | 66.56±3.56* | 68.70±2.50 | 68.11±2.45 | 71.60±3.05 | 64.85±1.68 |
| 5 d | 62.35±2.79b* | 68.70±3.90ab | 69.53±2.46ab | 73.08±3.57a | 67.83±2.92ab |
| 7 d | 61.48±2.14b* | 69.13±2.27ab | 67.02±2.53ab | 73.25±3.42a | 66.34±4.30ab |
| 9 d | 55.33±4.81b* | 55.56±3.99b | 61.59±3.23ab | 67.35±3.41a* | 60.74±2.48ab |
| 11 d | 42.52±4.18c* | 49.23±4.13bc | 55.83±3.08ab | 61.70±3.21a* | 55.35±2.40ab |
| 13 d | 38.16±2.71c* | 43.44±3.10bc | 49.00±4.02b | 59.26±3.44a* | 45.94±2.78b |
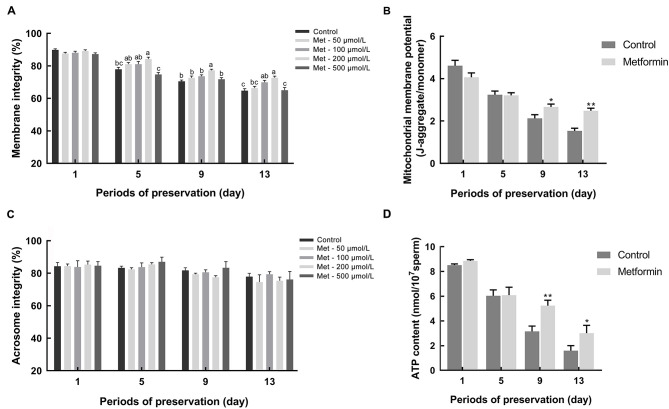
Figure 1. Effects of metformin addition on sperm plasma membrane integrity, acrosome membrane integrity, mitochondrial membrane potential (ΔΨm), and cellular ATP content during long-term preservation at 17 °C .
A, C: Sperm were treated with 0, 50, 100, 200, and 500 µmol/L metformin, respectively, in Modena medium. Sperm membrane integrity was evaluated using SYBR-14/PI kit at D1, D5, D9, and D13 (n=5) (A). Sperm acrosome integrity was evaluated using a FITC-PNA kit at D1, D5, D9, and D13 (n=3) (C). B, D: Sperm were treated with or without 200 µmol/L metformin in Modena medium. Sperm ΔΨm was evaluated using a JC-1 kit at D1, D5, D9, and D13 (n=5) (B). Cellular ATP content was evaluated using an ATP bioluminescence assay kit at D1, D5, D9, and D13 (D). Before ATP extraction, sperm counts were executed to normalize ATP content (n=5). Graph bars represent mean±SEM. Different lower-case letters indicate significant difference (P<0.05). Asterisks represent significant difference from control.*: P<0.05,**: P<0.01, determined by unpaired Student'st-test; Met: Metformin.
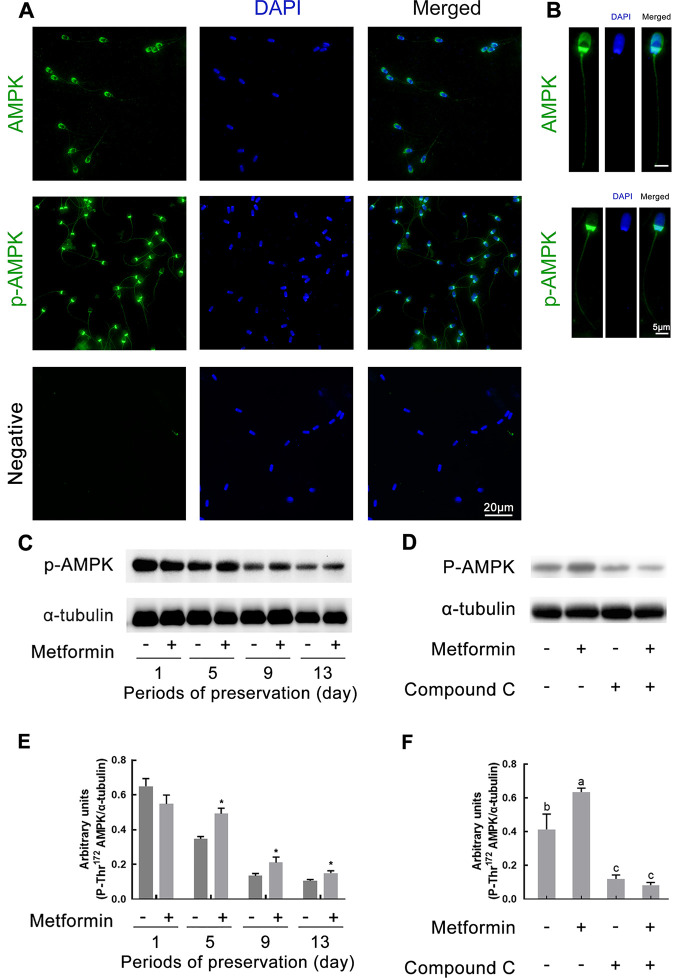
Figure 2. Subcellular localization of AMPK protein and phospho-Thr172 AMPK in boar sperm and AMPK phosphorylation analyzed by western blotting.
A, B: Fresh sperm were stained green with anti-AMPK antibody or anti-phospho-Thr172-AMPKα antibody, and sperm nuclei were stained with DAPI (blue). AMPK and p-AMPK proteins were detected with secondary antibody FITC conjugated goat anti-rabbit immunoglobulin G. Negative control: Primary antibody was not added. Images were visualized using confocal laser scanning microscopy. Serial images of right panels were obtained from a unique sperm. C, E: Sperm were treated with or without 200 µmol/L metformin at D1, D5, D9, and D13 of preservation at 17 °C. D, F: Sperm were incubated for 4 h at 37 °C with or without 200 µmol/L metformin in Modena medium, Modena+100 µmol/L AMPK inhibitor Compound C. C, D: Western blotting using anti-AMPK antibody and anti-phosphor (Thr172)-AMPKα antibody. Loading control was performed for each experiment in same membrane using anti-α tubulin antibody (n=5). E, F: Densitometric quantitation of phosphor (Thr172)-AMPKα bands obtained in C, D. Values obtained for phosphor (Thr172)-AMPKα bands were normalized with values of α-tubulin (n=3). Graph bars represent mean±SEM. Asterisks represent significant difference from control. *: P<0.05,**: P<0.01, determined by unpaired Student’st-test; Different lower-case letters indicate significant difference (P<0.05).
RESULTS
Metformin improves sperm motility
During preservation at 17 °C, the addition of 50–500 µmol/L metformin to the extender contributed to the maintenance of sperm motility (Table 1). Both total motility and progressive motility decreased with the increase in storage time, especially on day 5 (D5) and thereafter. Notably, the addition of metformin (200 µmol/L) maintained higher total motility and progressive motility from D7 to D13 (P<0.05). Thus, metformin demonstrated the capacity to attenuate the decline of motility during preservation.
Influence of metformin on plasma membrane and acrosome integrity
Higher membrane integrity was observed in the metformin groups (200 µmol/L) on D5 and D9 (P<0.05). Furthermore, the addition of metformin (100, 200 µmol/L) resulted in higher membrane integrity at D13 compared to the control (P<0.05;Figure 1A). However, acrosome integrity remained constant during preservation and no difference was detected between the control and metformin groups (P>0.05; Figure 1C). Therefore, the addition of metformin protected sperm membrane integrity during preservation.
Metformin improves sperm ΔΨm and ATP content
Regarding ΔΨm, no difference was observed between the control and metformin groups on either D1 or D5 (Figure 1B). However, the addition of metformin resulted in a higher ΔΨm on D9 (P<0.05) and D13 (P<0.01). Similarly, significantly higher ATP content was observed in the metformin group on D9 (P<0.01) and D13 (P<0.05;Figure 1D). Thus, the addition of metformin led to higher ΔΨm and ATP content during preservation.
Metformin enhances sperm AMPK phosphorylation
The immunofluorescent staining of AMPK and p-AMPK proteins is shown in Figure 2. Results showed that AMPK was mainly located at the apical part of the acrosome, post-acrosomal region of the sperm head, and the connecting piece and midpiece of the sperm tail. Occasional punctate staining of AMPK was also observed at the principle and end pieces of the sperm tail (Figure 2A, B). In addition, results showed that p-AMPK was mainly localized at the post-acrosomal region of the head and the connecting piece and midpiece of the tail. Weak p-AMPK staining was also observed at the principle and end pieces of the sperm tail (Figure 2A, B). Representative images of AMPK and p-AMPK staining in a single sperm are shown on the right side of Figure 2B. Furthermore, p-AMPK was also evaluated by western blotting. The level of p-AMPK in sperm exhibited a persistent decrease in the control and metformin treatment groups (Figure 2C, E). Specifically, p-AMPK in the metformin group was significantly higher than that in the control group from D5 to D13 during preservation (P<0.05;Figure 2C, E). Moreover, metformin significantly promoted p-AMPK, whereas the phosphorylation inhibitor (Compound C) completely repressed AMPK activation and attenuated the ability of metformin to activate AMPK during incubation for 4 h at 37 °C (P<0.05;Figure 2D, F).
Metformin maintains sperm metabolism homeostasis by activating AMPK
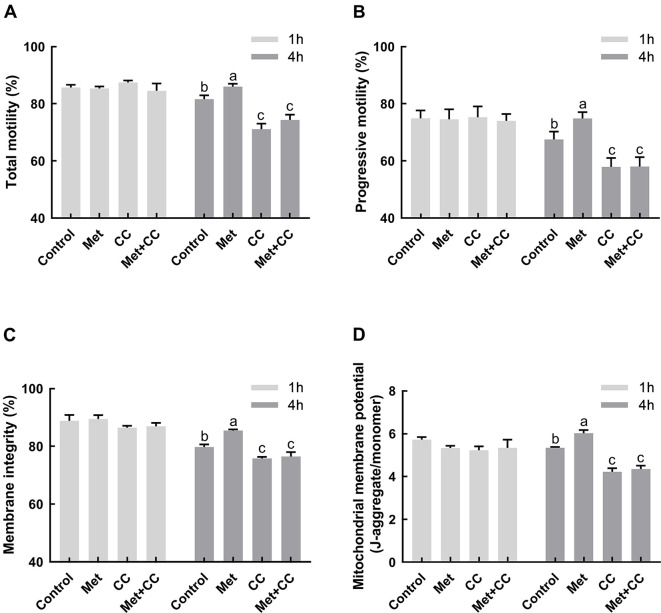
Compared to the control, significant increases in total motility, progressive motility, membrane integrity, and ΔΨm were observed in the metformin treatment group after incubation for 4 h (P<0.05;Figure 3). Consistent with the level of p-AMPK, the positive effects of metformin on sperm motility, membrane integrity, and ΔΨm were entirely abrogated by the addition of inhibitor Compound C.
Figure 3. Effects of metformin and Compound C on sperm motility, membrane integrity, and ΔΨm.
Sperm were incubated for 1 h and 4 h at 37 °C with or without metformin in Modena medium, Modena+100 µmol/L Compound C. A, B: Total motility and progressive motility were recorded using CASA system (n=3). C: Membrane integrity was evaluated using SYBR-14/PI kit (n=3). D: Mitochondrial membrane potential was evaluated using a JC-1 kit (n=5). Graph bars represent mean±SEM. Different lower-case letters indicate significant difference (P<0.05). Met: Metformin; CC: Compound C; Met+CC: Metformin+Compound C.
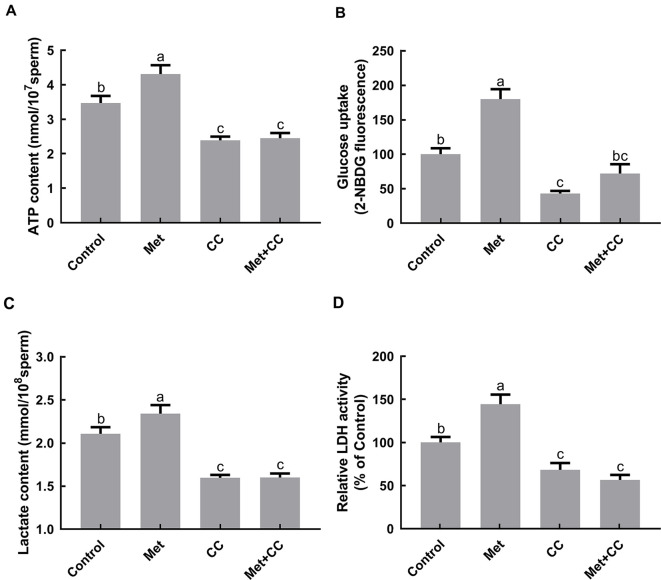
Sperm ATP content, glucose uptake, lactate efflux, and lactate dehydrogenase activity were also evaluated to investigate the relationship between metformin and energetic metabolism. ATP content in the metformin treatment group exhibited a significant increase in comparison with the control after 4 h of incubation (P<0.05;Figure 4A), but no differences were found between the control and the group treated with both metformin and Compound C. In addition, the ability of metformin to facilitate glucose uptake and lactate efflux was suppressed by Compound C (P<0.05;Figure 4B, C). Similarly, exposure to metformin led to an increase in LDH activity, whereas simultaneous exposure to both metformin and Compound C did not (P<0.05;Figure 4D). It is worth noting that ATP content, glucose uptake capacity, lactate efflux, and LDH activity decreased when Compound C was present.
Figure 4. Effects of metformin and Compound C on sperm ATP content, glucose uptake, extracellular lactate content, and lactate dehydrogenase activity.
Sperm were incubated for 4 h at 37 °C with or without metformin in Modena medium, Modena+100 µmol/L Compound C. A: Cellular ATP content was evaluated using an ATP bioluminescence assay kit. Before ATP extraction, sperm counts were executed to normalize ATP content (n=3). B: Glucose uptake capability was measured using green fluorescence D-glucose analogue, 2-NBDG, in glucose-free medium. Results are presented as geometric mean of fluorescence intensity of live cells as measured by flow cytometry (n=3). C: Extracellular lactate content was evaluated using a lactate content assay kit, and sperm counts were performed to normalize lactate content (n=3). D: Relative lactate dehydrogenase activity was evaluated using an LDH activity assay kit and results are shown as percentage of untreated control sample (n=3). Graph bars represent mean±SEM. Different lower-case letters indicate significant difference (P<0.05). Met: Metformin; CC: Compound C; Met+CC: Metformin+Compound C.
DISCUSSION
Preservation in vitro prolongs sperm lifespan, but physiological senescence occurs during preservation. Furthermore, oxidative stress and metabolic disorders can undermine the structural and functional integrity of sperm (Bielas et al., 2017; Fu et al., 2017). In the present study, we observed that metformin promoted AMPK activation, which facilitated glucose uptake and lactate efflux and maintained mitochondrial activity, thereby maintaining sperm functionality and improving the efficacy of boar semen preservation.
Sperm motility is a prerequisite factor for fertilization. The plasma membrane is also the only barrier between sperm cells and the environment. Therefore, motility and plasma membrane integrity are important indicators for assessment of fertilizing potential. In the present study, sperm were preserved at 17 °C and the relevant indicators were evaluated for 13 days because Modena is a long-term diluent and long-term preservation of sperm contributes to efficient AI outcome. We found that the addition of metformin (200 µmol/L) improved motility and membrane integrity, similar to that reported in chicken, goat, and mouse sperm (Bertoldo et al., 2014; Nguyen et al., 2014; Zhu et al., 2018). However, our results are inconsistent with those of Calle-Guisado et al. (2017) and Hurtado de Llera et al. (2018), who demonstrated that metformin exerts negative effects on human and boar sperm. These contradictions may be due to the following reasons. Firstly, the dose of metformin used in the present study was relatively low. It has been reported that millimolar levels of metformin severely inhibit mitochondrial respiratory complex I, whereas micromolar levels show protective effects in cells (Chandel et al., 2016; Detaille et al., 2005). Gravel et al. reported that the effect of metformin is also dependent on the metabolites in the culture medium (Gravel et al., 2014). In the present study, the concentration of glucose (152.8 mmol/L) in the Modena solution was higher than that in Tyrode’s basal medium (5.5 mmol/L; TBM) (Hurtado de Llera et al., 2013). In addition, the Modena solution contains 26.7 mmol/L trisodium citrate and 15.1 mmol/L citric acid, whereas TBM contains 1 mmol/L sodium pyruvate and 21.6 mmol/L sodium lactate.
Energy is essential to maintain motility and plasma membrane integrity in vitro. ATP synthesis via oxidative phosphorylation requires a normal ΔΨm, which is the major electrical component of the chemiosmotic proton gradient across the inner mitochondrial membrane. The present study indicated that sperm treated with metformin maintained a higher ATP level during preservation, in agreement with that reported in chicken and goat sperm (Nguyen et al., 2014; Zhu et al., 2018). Metformin also contributed to maintenance of ΔΨm, in agreement with previous study, despite the mild suppression of metformin on sperm ΔΨm during the early stage of preservation (Martin-Montalvo et al., 2013). This suppression may be related to mild inhibition of mitochondrial electron transport chain (ETC) complex I activity (Brunmair et al., 2004; El-Mir et al., 2000). Taken together, these data indicate that the addition of metformin prevents the decline in sperm motility and membrane integrity probably through restoration of energy homeostasis.
The regulation of energy is essential for the maintenance of structural and functional integrity. AMPK controls cellular metabolism homeostasis by switching off anabolic pathways and switching on metabolic pathways. AMPK is also involved in the maintenance of sperm function, such as motility, mitochondrial activity, plasma membrane fluidity, and lipid organization (Hurtado de Llera et al., 2012, 2013; Tartarin et al., 2012; Zhu et al., 2018). We found that metformin increased the level of AMPK phosphorylation, in accordance with that reported for goat and chicken sperm (Nguyen et al., 2015; Zhu et al., 2018). Importantly, metformin maintained AMPK activity within an appropriate range. In the present study, Compound C, a specific AMPK inhibitor, completely blocked AMPK phosphorylation, in accordance with that reported for stallion, chicken, goat, and human sperm (Calle-Guisado et al., 2017; Nguyen et al., 2014; Swegen et al., 2016; Zhu et al., 2018). Furthermore, Compound C abrogated the protective role on sperm motility parameters, membrane integrity, ΔΨm, and ATP level, in agreement with previous studies (Martin-Hidalgo et al., 2018). These data suggest that AMPK improves sperm functionality by regulating energy metabolism.
AMPK is involved in the regulation of glucose uptake in skeletal muscle cells, hepatocytes, and endometrial cancer cells (Han et al., 2015; Tsuda et al., 2017; Vlavcheski et al., 2017). Here, we found that metformin induced AMPK activation and facilitated cellular glucose uptake, consistent with that found in stallion sperm (Swegen et al., 2016). In addition, Compound C did not completely abrogate the increase in glucose uptake, suggesting that metformin likely facilitates glucose uptake in an AMPK-independent manner (Polianskyte-Prause et al., 2019; Ushiyama et al., 2019).
AMPK is well known for restoring cellular ATP levels through increasing glycolysis in somatic cells (Hardie et al., 2012). Moreover, excess lactate produced by anaerobic glycolysis can cause intracellular acidification, which reduces the glycolytic rate by inhibition of the rate-limiting enzyme phosphofructokinase (PFK) (Hardie, 2011). Therefore, as a glycolysis-dependent cell (Rodriguez-Gil & Bonet, 2016), boar sperm cells need to export excess lactate and H+ to avoid intracellular acidification. In addition, LDH is a crucial enzyme for sustaining anaerobic glycolysis by regeneration of NAD+ from NADH. In the present study, we observed that metformin induced AMPK activation, and increased both LDH activity and lactate efflux, as reported in previous studies on chicken and goat sperm (Nguyen, 2019; Zhu et al., 2018). These results suggest that p-AMPK increases cellular ATP levels probably via promoting anaerobic glycolysis. Hardie et al. (2012) reported that AMPK regulates glycolysis via phosphorylating 6-phosphofructo-2-kinase (PFKFB3), resulting in an increase in fructose-2, 6-bisphosphate to allosterically activate PFK in cardiac muscle and macrophages. Furthermore, Zhu et al. (2018) demonstrated that exposure of goat sperm to metformin enhances glycolytic enzyme activity, including that of pyruvate kinase and LDH. Interestingly, most glycolytic enzymes and AMPK are co-localized in the proximal region of the boar sperm tail, which further suggests that metformin-induced AMPK modulates the glycolysis pathway.
Activating glycolysis also shows protective effects against stress in somatic cells, such as endothelial cells and rat thymocytes (Brand & Hermfisse, 1997; Xu et al., 2018). Interestingly, glycolysis seems to be a protective strategy for sperm cells against biochemical and physiological stresses in vitro. Swegen et al. (2016) demonstrated that rosiglitazone-induced metabolic flexibility shifts from mitochondrial metabolism towards glycolytic pathways and reduced mitochondrial activity, which helps alleviate the deterioration of stallion sperm in vitro. Furthermore, squid sperm with higher fertility exhibit higher capacities of glucose uptake and lactate efflux (Hirohashi et al., 2016). Therefore, when oxygen concentrations are low, glucose availability is high, and energy demand is high, glycolysis may be a more efficient option for energy demand in boar sperm. Moreover, a metabolic shift toward glycolysis has also been proposed as a mechanism for protecting cells from oxidative damage induced by OXPHOS in mitochondria (Kondoh et al., 2005). Taken together, promotion of energy flux through the glycolytic pathway could be beneficial for the maintenance of boar sperm functionality.
In the present study, AMPK was mainly located at the apical part of the acrosome, post-acrosomal region of the sperm head, and the connecting piece and midpiece of the sperm tail. Furthermore, p-AMPK was mainly localized at the post-acrosomal region of the head and the connecting piece and midpiece of the tail. The staining of AMPK was slightly different from that observed in previous studies. Hurtado de Llera et al. (2013) found AMPK to be localized at the entire acrosome of the spermatozoa head and at the midpiece of the flagellum with less intensity, and p-AMPK to be localized in the acrosome and sub-equatorial segment of the head of boar sperm. Similarly, Martin-Hidalgo et al. (2013) found AMPK to be present in the acrosome and midpiece of boar sperm. Other studies have also found the AMPK protein to be located in the sperm acrosome and midpiece in goats (Zhu et al., 2018), stallions (Swegen et al., 2016), chickens (Nguyen et al., 2014), and humans (Calle-Guisado et al., 2017; Shabani Nashtaei et al., 2017). Furthermore, p-AMPK is also reported to be localized at the midpiece of the sperm tail in the aforementioned species.
The main role of AMPK is the regulation of metabolism (Hardie, 2011; Lin & Hardie, 2018; Martin-Hidalgo et al., 2018; Nguyen, 2017). It is suggested that activated AMPK in the sperm tail could play a role in sperm motility and survival, and that AMPK located at the acrosome would participate in sperm-egg interactions (Nguyen, 2017). Therefore, it would be interesting to illuminate the role of AMPK localized in the acrosome in detail in future study.
CONCLUSIONS
In conclusion, metformin activates AMPK, increases glucose uptake and lactate efflux, and maintains ΔΨm and ATP level, resulting in improved sperm motility and membrane integrity in vitro (Figure 5). Therefore, maintenance of sperm energy metabolism and functionality by metformin could reveal novel prospects for improving AI fertilization in pigs and possibly other animals.
Figure 5. Proposed model of regulation of metformin on boar sperm function via activation of AMPK.
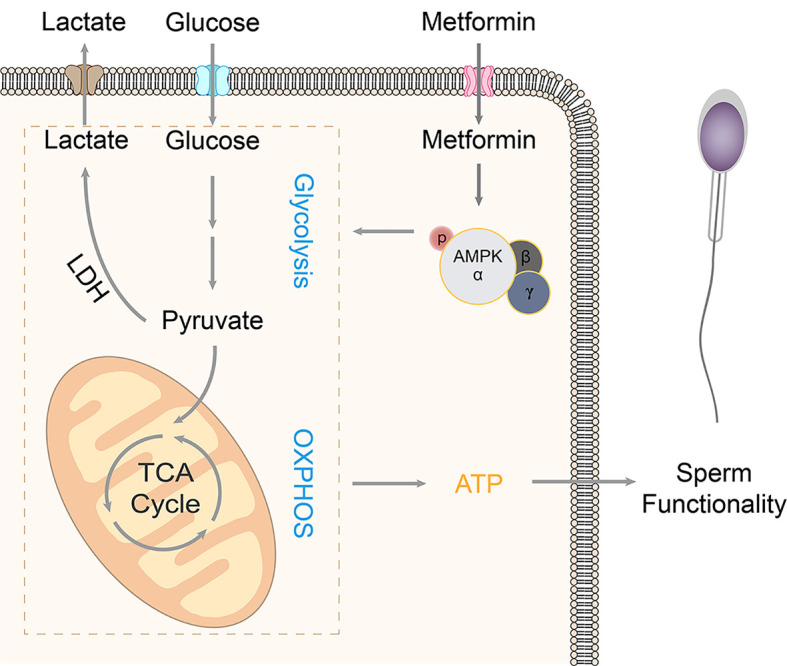
Metformin enters the intracellular space by the transporter, resulting in AMPK activation, which facilitates glucose uptake and lactate efflux and maintains mitochondrial activity, thereby maintaining sperm functionality and improving the efficacy of boar sperm preservation invitro.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
R.N.L., Y.J.W., and W.X.Z. conceived and designed the experiments. R.N.L., Z.D.Z., Y.J.W., and X.E.T. performed the experiments. R.N.L., Y.H.L., D.W., and Y.Z. analyzed the data. R.N.L. and W.X.Z. wrote the paper. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the Life Science Research Core Services of Northwest A&F University for providing the confocal microscope. We also thank Yan-Qing Wang for guidance in its use.
Funding Statement
This study was supported in part by the National Key R&D Program of China (2018YFD0501000) to W.X.Z.
Contributor Information
Yong-Jun Wang, Email: dkxywyj@163.com.
Wen-Xian Zeng, Email: zengwenxian2015@126.com.
References
- 1.Berstein LM Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY) 2012;4(5):320–329. doi: 10.18632/aging.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoldo MJ, Guibert E, Tartarin P, Guillory V, Froment P Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology. 2014;68(2):262–268. doi: 10.1016/j.cryobiol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Bielas W, Nizanski W, Partyka A, Rzasa A, Mordak R Effect of long-term storage in Safe Cell+ extender on boar sperm DNA integrity and other key sperm parameters. Acta Veterinaria Scandinavica. 2017;59(1):58. doi: 10.1186/s13028-017-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand KA, Hermfisse U Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB Journal. 1997;11(5):388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 5.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, et al Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53(4):1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 6.Calle-Guisado V, de Llera AH, Martin-Hidalgo D, Mijares J, Gil MC, Alvarez IS, et al AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian Journal of Andrology. 2017;19(6):707–714. doi: 10.4103/1008-682X.185848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, et al Are metformin doses used in murine cancer models clinically relevant? Cell Metabolism. 2016;23(4):569–570. doi: 10.1016/j.cmet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54(7):2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 9.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. Journal of Biological Chemistry. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 10.Foretz M, Viollet B Regulation of hepatic metabolism by AMPK. Journal of Hepatology. 2011;54(4):827–829. doi: 10.1016/j.jhep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Li Y, Wang L, Zhen L, Yang Q, Li P, et al Bovine serum albumin and skim-milk improve boar sperm motility by enhancing energy metabolism and protein modifications during liquid storage at 17 °C. Theriogenology. 2017;102:87. doi: 10.1016/j.theriogenology.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Gravel SP, Hulea L, Toban N, Birman E, Blouin MJ, Zakikhani M, et al Serine deprivation enhances antineoplastic activity of biguanides. Cancer Research. 2014;74(24):7521–7533. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- 13.Han JJ, Zhang L, Guo H, Wysham WZ, Roque DR, Willson AK, et al Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecologic Oncology. 2015;138(3):668–675. doi: 10.1016/j.ygyno.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie DG AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes and Development. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie DG, Ross FA, Hawley SA AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirohashi N, Tamura-Nakano M, Nakaya F, Iida T, Iwata Y Sneaker male squid produce long-lived spermatozoa by modulating their energy metabolism. Journal of Biological Chemistry. 2016;291(37):19324–19334. doi: 10.1074/jbc.M116.737494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurtado de Llera A, Martin-Hidalgo D, Garcia-Marin LJ, Bragado MJ Metformin blocks mitochondrial membrane potential and inhibits sperm motility in fresh and refrigerated boar spermatozoa. Reproduction in Domestic Animals. 2018;53(3):733–741. doi: 10.1111/rda.13164. [DOI] [PubMed] [Google Scholar]
- 18.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PloS One. 2012;7(6):e38840. doi: 10.1371/journal.pone.0038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurtado de Llera A, Martin-Hidalgo D, Rodriguez-Gil JE, Gil MC, Garcia-Marin LJ, Bragado MJ AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2013;1828(9):2143–2151. doi: 10.1016/j.bbamem.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, et al Glycolytic enzymes can modulate cellular life span. Cancer Research. 2005;65(1):177–185. [PubMed] [Google Scholar]
- 21.Lin SC, Hardie DG AMPK: Sensing glucose as well as cellular energy status. Cell Metabolism. 2018;27(2):299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Lv YH, Zhang PF, Guo JY, Zhu ZD, Li XL, Xu D, et al Melatonin protects mouse spermatogonial stem cells against hexavalent chromium-induced apoptosis and epigenetic histone modification. Toxicology and Applied Pharmacology. 2018;340:30–38. doi: 10.1016/j.taap.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Hidalgo D, Hurtado de Llera A, Calle-Guisado V, Gonzalez-Fernandez L, Garcia-Marin L, Bragado MJ AMPK function in mammalian spermatozoa. International Journal of Molecular Sciences. 2018;19(11):3293. doi: 10.3390/ijms19113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al Metformin improves healthspan and lifespan in mice. Nature Communications. 2013;4(1):2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen TMD Impact of 5′-AMP-activated protein kinase on male gonad and spermatozoa functions. Frontiers in Cell and Developmental Biology. 2017;5:25. doi: 10.3389/fcell.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TMD Role of AMPK in mammals reproduction: Specific controls and whole-body energy sensing. Comptes Rendus Biologies. 2019;342(1-2):1–6. doi: 10.1016/j.crvi.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TMD, Alves S, Grasseau I, Metayer-Coustard S, Praud C, Froment P, et al Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biology of Reproduction. 2014;91(5):121. doi: 10.1095/biolreprod.114.121855. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TMD, Seigneurin F, Froment P, Combarnous Y, Blesbois E The 5′-AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken sperm. PloS One. 2015;10(7):e0134420. doi: 10.1371/journal.pone.0134420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polianskyte-Prause Z, Tolvanen TA, Lindfors S, Dumont V, Van M, Wang H, et al Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB Journal. 2019;33(2):2858–2869. doi: 10.1096/fj.201800529RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Gil JE Mammalian sperm energy resources management and survival during conservation in refrigeration. Reproduction in Domestic Animals. 2006;41(S2):11–20. doi: 10.1111/j.1439-0531.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Gil JE, Bonet S Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology. 2016;85(1):4–11. doi: 10.1016/j.theriogenology.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Shabani Nashtaei M, Amidi F, Sedighi Gilani MA, Aleyasin A, Bakhshalizadeh S, Naji M, et al Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5' AMP-activated protein kinase activation. Andrology. 2017;5(2):313–326. doi: 10.1111/andr.12306. [DOI] [PubMed] [Google Scholar]
- 33.Swegen A, Lambourne SR, Aitken RJ, Gibb Z Rosiglitazone improves stallion sperm motility, ATP content, and mitochondrial function. Biology of Reproduction. 2016;95(5):107. doi: 10.1095/biolreprod.116.142687. [DOI] [PubMed] [Google Scholar]
- 34.Tartarin P, Guibert E, Touré A, Ouiste C, Leclerc J, Sanz N, et al Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology. 2012;153(7):3468–3481. doi: 10.1210/en.2011-1911. [DOI] [PubMed] [Google Scholar]
- 35.Tsuda Y, Iwasawa K, Yokoyama M, Yamaguchi M Trypsin-treated β-lactoglobulin improves glucose tolerance in C57BL/6 Mice by enhancing AMPK activation and glucose uptake in hepatocytes. Biological and Pharmaceutical Bulletin. 2017;40(11):1917–1922. doi: 10.1248/bpb.b17-00437. [DOI] [PubMed] [Google Scholar]
- 36.Ushiyama A, Priyadarshana C, Setiawan R, Miyazaki H, Ishikawa N, Tajima A, et al Membrane raft-mediated regulation of glucose signaling pathway leading to acrosome reaction in chicken sperm. Biology of Reproduction. 2019;100(6):1482–1491. doi: 10.1093/biolre/ioz015. [DOI] [PubMed] [Google Scholar]
- 37.Vazirian M, Nabavi SM, Jafari S, Manayi A Natural activators of adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) and their pharmacological activities. Food and Chemical Toxicology. 2018;122:69–79. doi: 10.1016/j.fct.2018.09.079. [DOI] [PubMed] [Google Scholar]
- 38.Vial G, Detaille D, Guigas B Role of mitochondria in the mechanism(s) of action of metformin. Frontiers in Endocrinology. 2019;10:294. doi: 10.3389/fendo.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlavcheski F, Naimi M, Murphy B, Hudlicky T, Tsiani E Rosmarinic acid, a rosemary extract polyphenol, increases skeletal muscle cell glucose uptake and activates AMPK. Molecules. 2017;22(10):1669. doi: 10.3390/molecules22101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waberski D, Riesenbeck A, Schulze M, Weitze KF, Johnson L Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology. 2019;137:2–7. doi: 10.1016/j.theriogenology.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 41.Xu L, Kong L, Wang J, Ash JD Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(41):10475–10480. doi: 10.1073/pnas.1802724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu ZD, Kawai T, Umehara T, Hoque SAM, Zeng WX, Shimada M Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radical Biology and Medicine. 2019a;141:159–171. doi: 10.1016/j.freeradbiomed.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Zhu ZD, Li RN, Fan XT, Lv YH, Zheng Y, Hoque SAM, et al Resveratrol improves boar sperm quality via 5'AMP-activated protein kinase activation during cryopreservation. Oxidative Medicine and Cellular Longevity. 2019b;2019:5921503. doi: 10.1155/2019/5921503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu ZD, Li RN, Ma GZ, Bai WJ, Fan XT, Lv YH, et al 5′-AMP-activated protein kinase regulates goat sperm functions via energy metabolism in vitro . Cellular Physiology and Biochemistry. 2018;47(6):2420–2431. doi: 10.1159/000491616. [DOI] [PubMed] [Google Scholar]